- 1Department of Biology, “Tor Vergata” University of Rome, Rome, Italy
- 2National Inter-University Consortium for Marine Sciences (CoNISMa), Rome, Italy
- 3Department of Environmental Biology, Sapienza University of Rome, Rome, Italy
- 4Department of Science and Technology, University of Naples “Parthenope”, Naples, Italy
- 5CNR-IAS, National Research Council – Institute for the Study of Anthropic Impact and Sustainability in the Marine Environment, Torregrande, Oristano, Italy
- 6Bio.Co.Ré. Laboratory, Scurcola Marsicana, Italy
- 7Biology Laboratory, Istituto Centrale per il Restauro (ICR), Ministry of Culture (MIC), Rome, Italy
Historical traces of organisms on the seafloor, such as shells and tubes, constitute the ecological memory of ancient benthic assemblages and serve as an important resource for understanding the assembly of modern communities. Archeological shipwrecks are particularly interesting submerged substrata for both their archeological and biological implications. For the first time, we studied the species composition and life-history traits of dominant organisms in the benthic assemblage on a bronze Carthaginian naval ram, which sank more than two thousand years ago in the Southern Tyrrhenian Sea. By comparing the species composition of the ram assemblage with those of the surrounding habitats, we inferred possible colonization patterns for the ram and discussed the informative role of the shipwreck as a proxy of marine biodiversity. The ram assemblage was rich in species, including both sessile (bryozoans, serpulid polychaetes, and few bivalves) and motile (gastropods) species. Sexual reproduction with free-spawning fertilization and long-duration larvae characterized most species. The long submersion time of the ram, together with the reproductive strategies, growth forms, and motility of the dominant species were key factors shaping the community of the ram. The ram itself offers an archeological artifact of inestimable value, but our analysis revealed it to be an effective collector of fauna from the surrounding seabed. The ram community hosted species from a range of nearby natural habitats (mostly coralligenous, detritic bottoms, and zoosteracean meadows) and thus served as a proxy for marine biodiversity on the surrounding seabed. We conclude that the presence of many species on the ram that commonly occur in adjacent habitats of great environmental value was informative and highlight the important marine biodiversity in the area of the Aegadian archipelago.
Introduction
Paleontological investigations of geological remains primarily aim at reconstructing ancient communities (i.e., tanatocenosis). However, the traces of more recent ecosystems offer further valuable consideration in neontological investigations. In fact, such ecological memory provides not merely a passive legacy but rather as a primary driver in the assembly of modern communities (Balaguer et al., 2014). Given that the time-scales for limestone degradation agreattly exceed those for sedimentation, the accumulation of calcified remains such as skeletons and shells in marine sediments preserves the remnants of organisms that previously lived on a substratum. On the one hand, the analysis of historical traces of organisms trapped within a substratum provides the opportunity to describe ancient benthic assemblages over time (Bertolino et al., 2014). On the other hand, calcareous remains of organisms accumulated on a substratum have been used to study biodiversity over spatial scales (Albano and Sabelli, 2011).
Shipwrecks provide one type of substrata for the growth of benthic organisms that also hold interesting information on the development and dynamics of benthic communities. Archeological shipwrecks, the wreckages of ships sunk in the past, are considered highly valuable for both their archeological and biological implications. Most shipwreck studies have focused on archeological questions (for example, see Bass et al., 1989; Pulak, 2008; Foley et al., 2009; Petriaggi and Davidde, 2010; Leidwanger et al., 2021). In contrast, our study addresses ecological questions and focuses on the biological colonization of these substrata. Few published studies consider benthic communities colonizing shipwrecks. Some of them focus on ships sunk less than 100 years ago (Costa, 2016; Meyer et al., 2017), whereas others focus on archeological remains and the epilithic bioencrustations of ships’ cargos (e.g., the marble statues from Antikythera, Greece, and the bronze statue of Satyrus of Mazara del Vallo, Sicily, Italy) (Ricci and Bartolini, 2005; Davidde et al., 2017; Ricci et al., 2019). Studies in the last decade have reported on the biodeterioration of underwater remains on stone and organic materials, in particular epilithic and endolithic assemblages and their role in the biodeterioration of archeological submerged artifacts (Ricci et al., 2013, 2015, 2016a,b; Antonelli et al., 2015; Sacco Perasso et al., 2015; Calcinai et al., 2019; Casoli et al., 2019a; Gravina et al., 2019).
Ours is the first study on the benthic assemblage associated with a bronze artifact and offers a novel contribution to the assessment of biodiversity on the seabed surrounding the archeological shipwreck. The ram 13 sank with a punic ship in 241 BC during the Aegates battle between the Romans and the Carthaginians (Tusa and Royal, 2012). We analyze the calcified zoobenthic organisms, including gastropod and bivalve mollusks, serpulid polychaetes, and bryozoans, accumulated in the sediment inside the naval ram during the prolonged period lying on the seabed in order to: (i) document species composition of the benthic assemblage collected in the ram; (ii) compare the observed assemblage with those of other Mediterranean communities and recognize biocenotic affinities; (iii) hypothesize possible colonization patterns of the ram by the benthic biota; and (iv) infer the role of the wreck as “ecological memory” of marine biodiversity in the Aegates archipelago region.
Materials and Methods
Study Area
The Battle of the Aegates Islands marked the end of the First Punic War and took place on March 10th 241 BC, when the Carthaginian relieving fleet was defeated off of western Sicily.
The environmental context of the area off-shore of Trapani, Marsala, and Mazzara del Vallo (west and southwest of Sicily) that comprises the Aegates islands is a good example of a shelf affected by marine abrasion of a rocky, tectonized substratum, where scarce recent sediment deposits are mainly composed of bioclastic fragments (Colantoni et al., 1993).
The ram on which our study focuses work was discovered on the inner continental shelf that joins the islands of Favignana and Levanzo with the mainland (Figure 1). The shelf has a gentle slope with several shallow banks and minor islets that rise from the seafloor as erosional remnants. Depth minima occur on the shoals of Secca del Toro, 6 m; Secca dei Pesci, 21 m; and NW of Levanzo, 29 m (Colantoni et al., 1993).
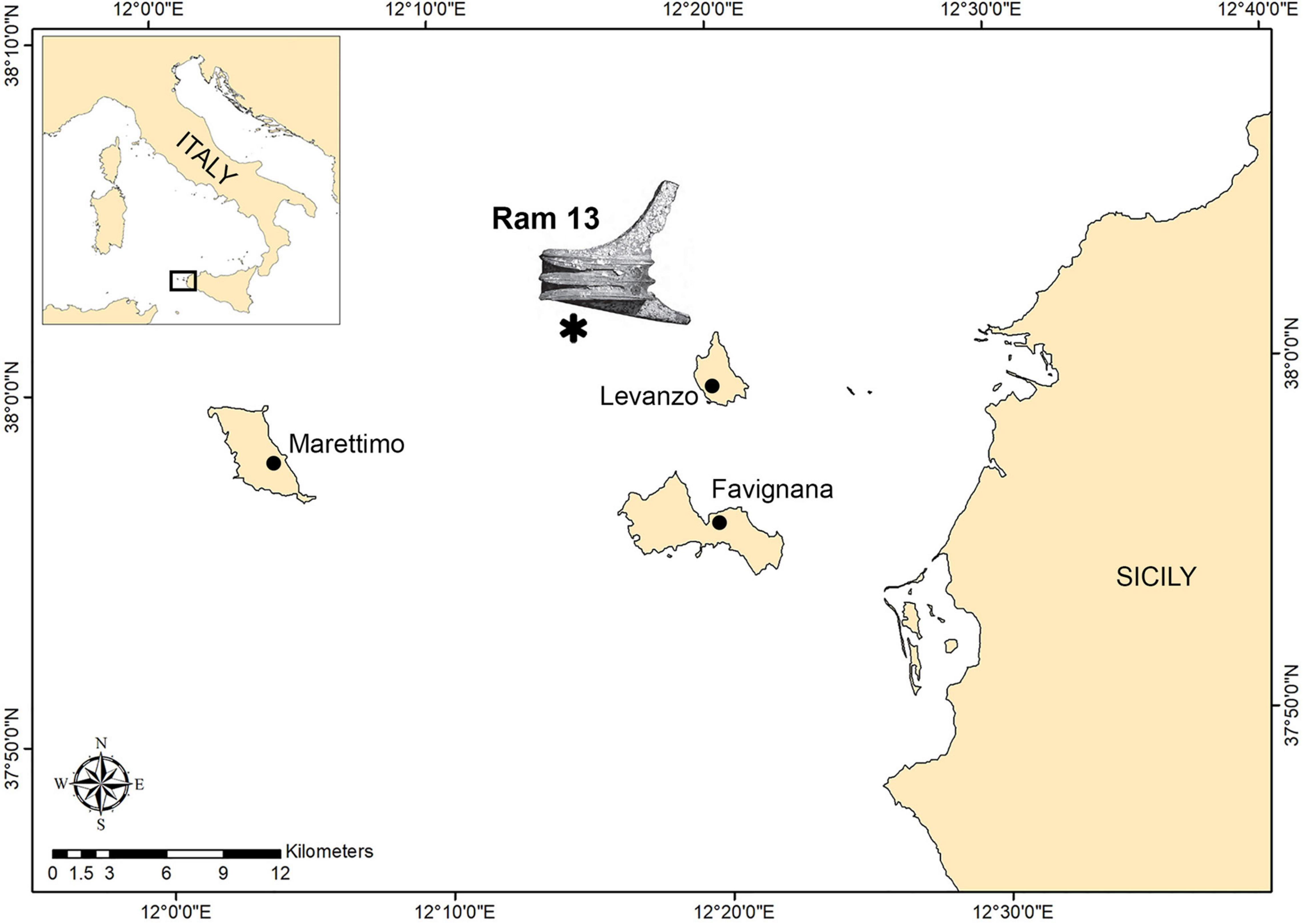
Figure 1. Map of the study area within the Aegadian archipelago. The asterisk indicates the area of ram recovery based on Tusa (2020).
The Ram Egadi 13
A naval ram (rostrum in Latin) is a breakthrough thrusting weapon that was fitted to the bow of ancient galleys to break the hull framing of enemy ships. The rams found in the Aegates Islands weigh 170 kg on average and vary in thickness from about 1.5 cm in the laminar region to 5 cm in the front. The artifact examined in this study, referred to as ram 13, is a trident rostrum characterized of three sharp and blunt cuts (Figure 2A). This type of ram was common among the main Mediterranean warships beginning in the 4th century BC (Tusa and Royal, 2012). Ram 13 is of great importance because since it shows a Punic inscription on the upper sheath. This is the second rostrum with a Punic inscription discovered to date (the other was the Egates 3) (Tusa, 2020).
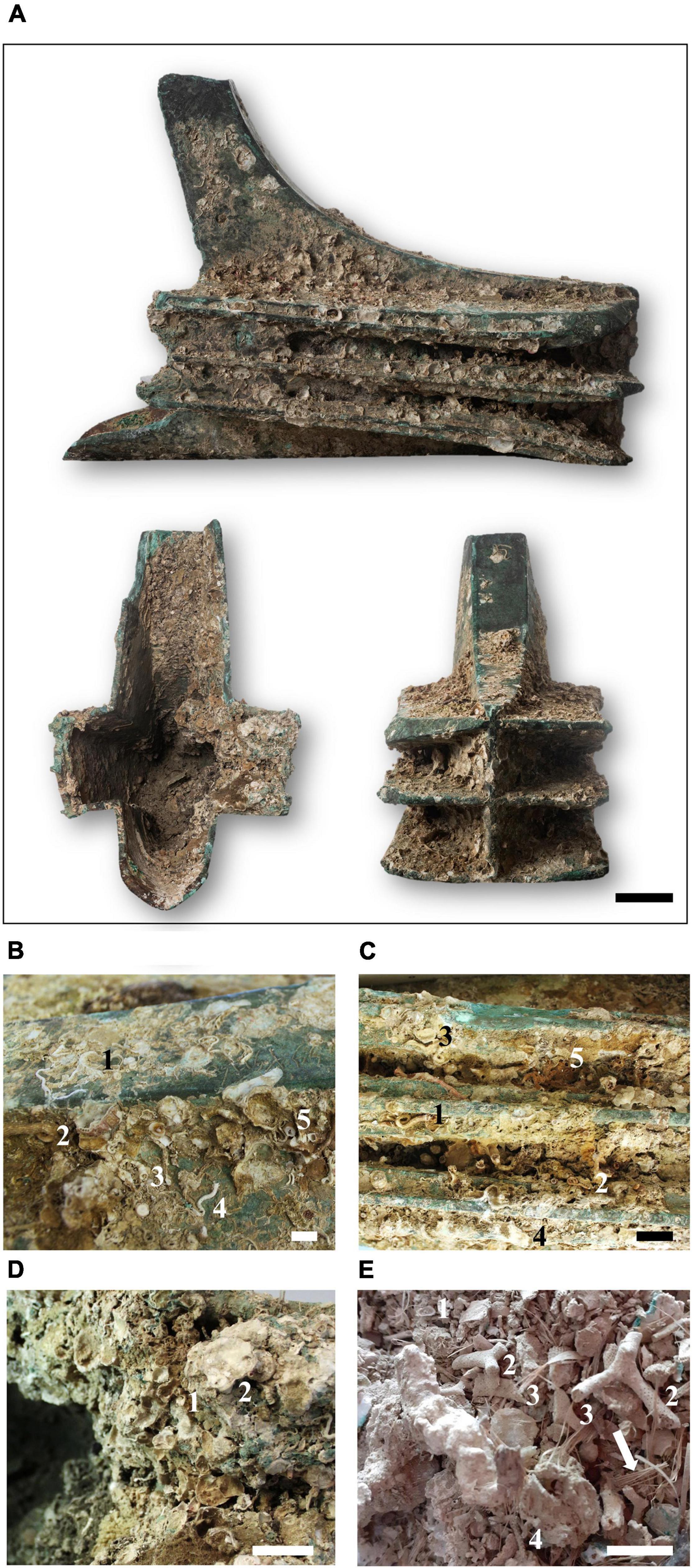
Figure 2. The ram Egadi 13 (A) and details of the biological colonization. Numbers mark calcareous tubes of serpulids: 1, Spirobranchus triqueter; 2, Serpula vermicularis; 3, Vermiliopsis monodiscus; 4, Metavermilia multicristata; and an oyster valve, 5 (B); tubes of serpulids: 1, Serpula vermicularis; 2, Spiraserpula massiliensis; 3, Vermiliopsis monodiscus; 4, an oyster valve; 5, erect colony of the bryozoan Cellaria fistulosa (C); examples of engineering of encrusting organisms: 1, a bryozoan laminar colony growing around a serpulid tube; 2, a large encrusting bryozoan bindering some tubes of serpulids (D); examples of calcareous remains inside the ram: 1, valve of Asperarca sp.; 2, colonies of the bryozoan Myriapora truncata, 3, Pentapora fascialis, 4, Hornera sp.; the arrow marks vegetable fibers of Posidonia (E). Scale bars: (A) = 10 cm; (B,D,E) = 1 cm; (C) = 2 cm.
The discovery of these remains of significant scientific value occurred during several research campaigns as part of the Egadi Project. Exploration was carried out by the Soprintendenza del Mare della Regione Sicilia in collaboration with technical SCUBA divers from the organization GUE (Global Underwater Explorers). The project was conceived and directed through the cooperative efforts of Prof. Sebastiano Tusa and Jeffrey Royal and their institution, the Soprintendenza del Mare, Regione Siciliana, and the RPM Nautical Foundation, from 2005 to 2018. The ram in this study was recovered in October 2017 between 75 and 95 m depth, about 7 km north-west of the island of Levanzo, together with rostrum 12 and 10 bronze Montefortino helmets (Tusa, 2020).
Sampling Analysis
The ram was restored in 2019 using metals and alloys by Stefano Ferrari and Antonella Di Giovanni of the ICR Restoration Laboratory. The first stage of restoration involved sampling and documentation of the sediment blocks and biogenic materials collected inside the inlet of the artifact. Images of the concretions and the material accumulated in the ram were collected using a digital camera. The sediment blocks compacted with Posidonia fibers and bioconcretions were separated from the sandy sediment. Every block was wetted with water, cleaned with a brush to remove the sediment, dried, and sieved with a 0.5 mm mesh. Subsequently, samples were observed under a stereomicroscope. All the biogenic fragmented remains, shells and tubes, were sorted by higher taxon and preserved in Petri dishes.
Data Analysis
Benthic specimens collected inside the entire ram were identified to species level whenever possible, and all data were used to construct a binary presence/absence matrix. The species richness of total benthos and of mollusks, polychaetes, and bryozoans, respectively, was considered a measure of α-diversity. All faunal data were analyzed by means of multivariate ordination technique non-metric multidimensional scaling (nMDS) using the Jaccard index. A clustering analysis based on Ward’s minimum variance method compared the similarity between the faunal assemblage found in the ram and those of common shallow (infralittoral) and deep (circalittoral and bathyal) habitats. Analysis of similarities (ANOSIM) based on Bray–Curtis similarity matrix assessed significant differences between grouping of habitats. A non-parametric SIMPER (Similarity Percentage) test identified those species that contributed most to the distinction among groups of habitats. Published literature sources provided information on species composition in each natural habitat and functional traits of each species on the ram (Table 2).
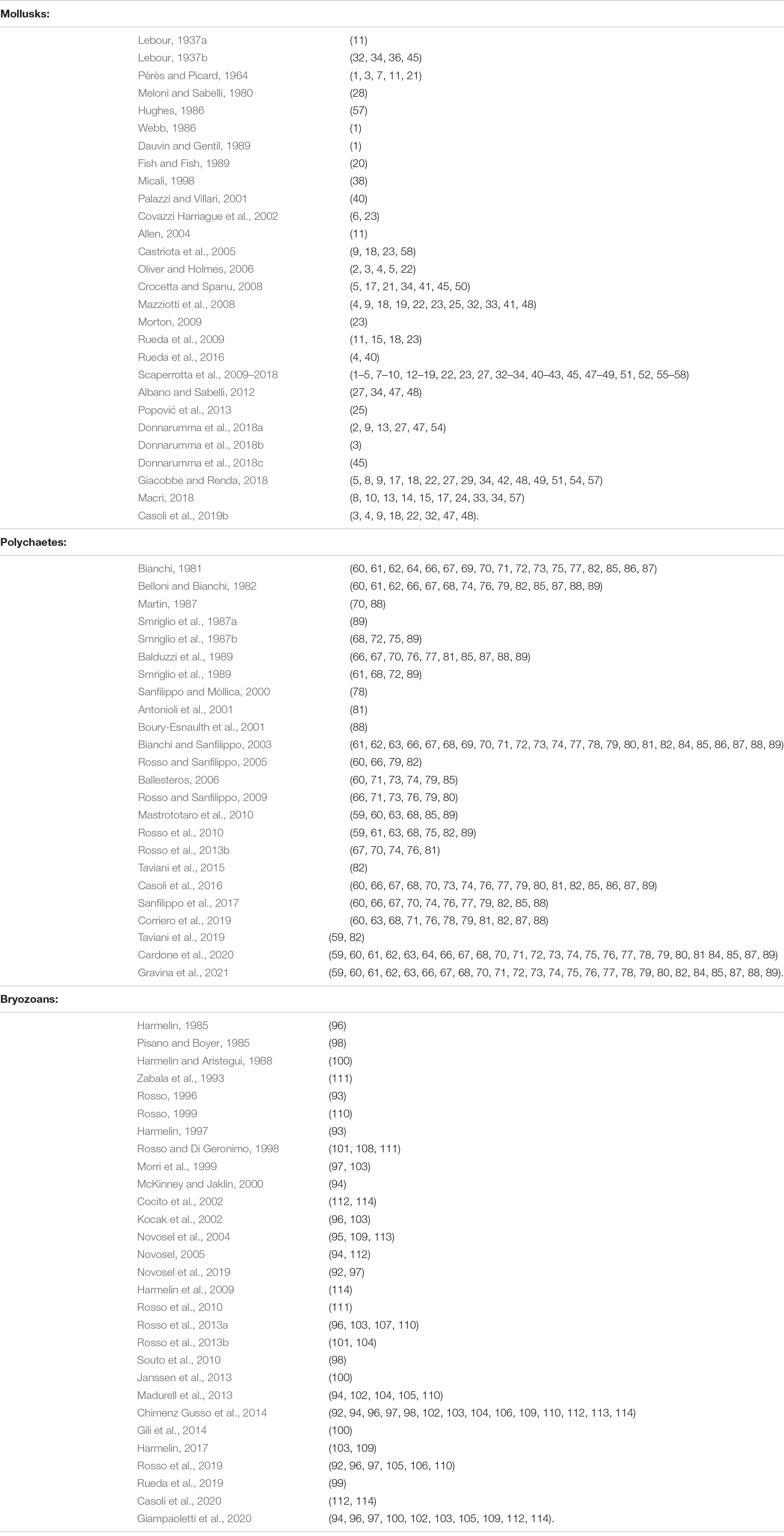
Table 2. List of the references providing information on the functional traits and ecological affinities of each species of mollusks, polychaetes, and bryozoans found in the ram; species are marked in brackets with the same numbers as Table 1.
To infer possible colonization patterns of the artifact, we considered the main functional traits (Table 2) of the dominant species associated with the ram. We considered seven traits to describe the species’ niches: (1) reproductive mode (sexual, asexual), (2) development strategy (brooding, free spawning, eggs laid in capsules or masses), (3) larval type (planktotrophic or lecithotrophic, for species with pelagic larvae), (4) modularity (solitary, aggregation, aggregation of a few individuals, colonial), (5) adult motility (sessile, motile), and (6) engineering (primary constructor, binder, dweller), (7) size (large, medium, small, according the details described in the caption of Table 1). Information on the engineering role of each species was based on Fagerstrom (1988). Specifically, primary constructors refer to erect well-skeletonized builders that provide volume and rigidity to the concretion; binders are encrusters that expand and connect the organic structures; and dwellers are mostly motile (rarely sessile) organisms that are not strictly builders but inhabit cavities and crevices of the concretion. Moreover, the ecological affinity of each species was assigned by combining personal observations and literature data reported in Table 2.
Results
Species Composition, Ecology, and Life-Traits
The faunal assemblage in the ram included 114 species, including 58 species of mollusks (51%), 33 species of gastropods, 25 species of bivalves, 33 species of polychaetes (29%), and 23 species of bryozoans (20%). Table 1 provides a complete list of species identified from the ram and their functional traits and ecological affinities. Sessile species, i.e., bryozoans, serpulids, and a few bivalves, colonized the ram surface extensively and grew in epibiosis on calcareous surfaces of other organisms. Motile species, mostly mollusks, occupied the inside of the ram in large numbers together with the remains of sessile species (colony fragments, tubes) (Figures 2B–E). The percentage of the species sharing each of the functional traits is reported in Figure 3.
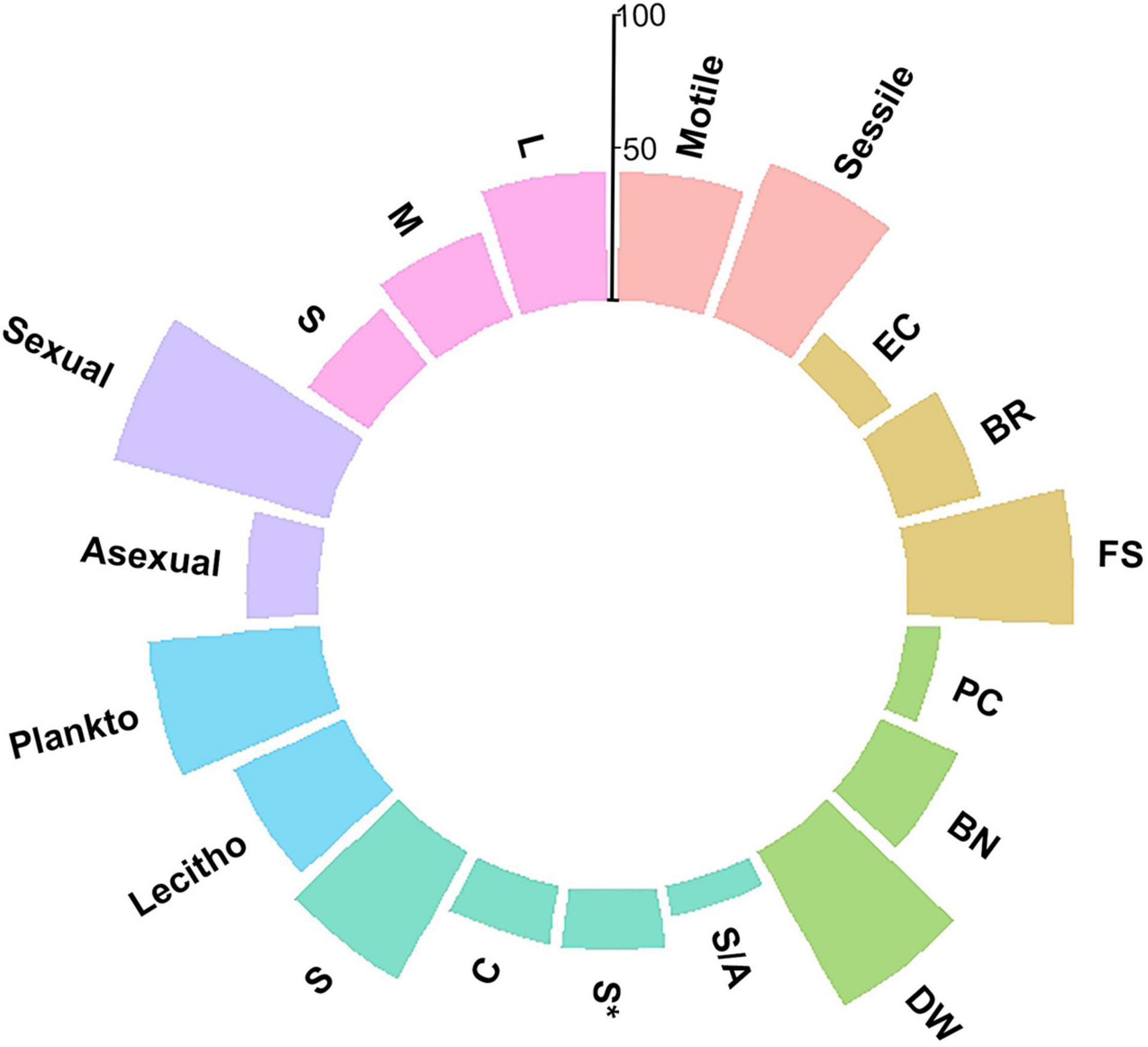
Figure 3. Percentage of the species characterized by functional traits taken into account in the description of the ram assemblage structure. Colors are used to distinguish among functional traits, following the classification in Table 1. Pink: Adult motility; Orange: Development strategy (BR = brooding; FS = free spawning; EC = egg capsules/masses); Green: Engineering (PC = primary constructors; BN = binders; DW = dwellers); Cyan: Modularity (S = solitary; S* = solitary/few individuals; S/A = solitary/aggregation; C = colonial); Blue: Larval type (Plankto = planktotrophic larvae; Lecitho = lecitotrophic larvae); Purple: Reproduction mode; Light purple: Size (S = small; M = medium; L = large).
Sexual reproduction, pelagic spawning, and extended pelagic larval duration characterized most of the mollusks. Embryonic development in masses or capsules with short pelagic larval duration characterized about half of the gastropods, e.g., Alvania spp., Bittium reticulatum, Chauvetia giunchiorum, Jujubinus exasperates, and Turritella turbona. Almost all of the species were solitary and motile. A few bivalves were sessile and primarily contributed to the concretion, such as Chama gryphoides and Ostraeidae. In contrast, other sessile bivalves, i.e., Acar clathrata, Arca tetragona, Asperarca secreta, Pteria hirundo, and Striaca lactea, and all the motile mollusks were concretion dwellers. As for polychaetes, all species reproduced sexually, except for Filograna sp., Filogranula spp., and Josephella marenzelleri, which reproduced asexually. Most polychaetes were pelagic spawners with external fertilization and planktotrophic larvae and long pelagic duration, while only Filograna sp. and five Spirorbinae species were brooders that produced lecithotrophic, short-duration larvae. All polychaetes were sessile in the adult stage, and most were solitary. Among the serpulids, only Filograna sp., J. marenzelleri, and Spiraserpula massiliensis formed aggregations: Filograna sp. formed dense aggregations of small tubes in the ram. Spirorbid species occurred in a specific epibiothic association on mollusk shells, bryozoans, and tubes of other serpulids. Gregarious settlement occurred in the spirorbid Janua. Based on the tube size and their role in building the concretion, we considered serpulids important encrusting species that played the role of binders. This group included 20 species with medium and large tubes. We considered an additional 11 small-sized species from the genera Filogranula and Semivermilia and subfamily Spirorbinae as dwellers because they colonized crevices and interstices among calcareous surfaces and settled on top of shells and skeletons of other organisms. Bryozoans on the ram included species with sexual and asexual reproduction. Their colonies grew by asexual fragmentation and budding. Lecithotrophic larvae produced by sexual reproduction developed into new colonies. In the identified species, individuals placed eggs either into external brood chambers or retained them in the body cavity. Almost half of the bryozoan species grew on the substrate with erect-rigid colonies: Myriapora truncata, Pentapora fascialis, and Schizoretepora imperati with large colonies (more than 20 mm), and Adeonella pallasii, Cellaria fistulosa, Diporula verrucosa, Frondipora verrucosa, Hippellozoon mediterraneum, Hornera frondiculata, H. lichenoides, and Smittina cervicornis with medium-sized colonies (11–20 mm). These bryozoans formed new colonizable substrata and increased habitat heterogeneity with their three-dimensional structures. The other half of the bryozoan species formed monolaminar and multilaminar colonies encrusting the substratum, such as Cribilaria hinckinsii, Escharella variolosa, Onychocella marioni, and Palmiskenea skenei, firmly attached to the substratum with their large multilaminar colonies. Other species, e.g., Celleporina spp., formed cellepoliform colonies and, together with species with small-sized (less than 10 mm) monolaminar encrusting colonies, e.g., Cribellopora trichotoma and Escharoides mamillata, colonized mostly fissures and interstices.
Faunal Assemblage of Ram and Similarity With Other Habitats
The nMDS ordination analysis (Figure 4A) highlighted differences between benthic assemblages in three groups. The ram assemblage most closely resembled the assemblages in coralligenous and detritic habitats in group 1. This group separated from shallow photophilic habitats and zoosteracean meadows (group 2) on one side of the nMDS plot and deeper mesophotic and bathyal habitats and caves (group 3) formed a second group on the other side of the nMDS plot (Figure 4A). The cluster analysis (Figure 4B) supported the nMDS ordination by yielded three main clusters. The assemblage on the ram grouped together with coralligenous and detritic habitats in group 1, while the shallower habitats and the deeper habitats in groups 2 and 3 formed separate clusters. The distinction between groups occurred at about 82% similarity. A total of 67 and 54 species on the ram, respectively, had affinities for coralligenous reefs (COR) and detritic bottoms (DET); meanwhile, bathyal habitats were less represented on the ram, with 30 species (Figure 4C).
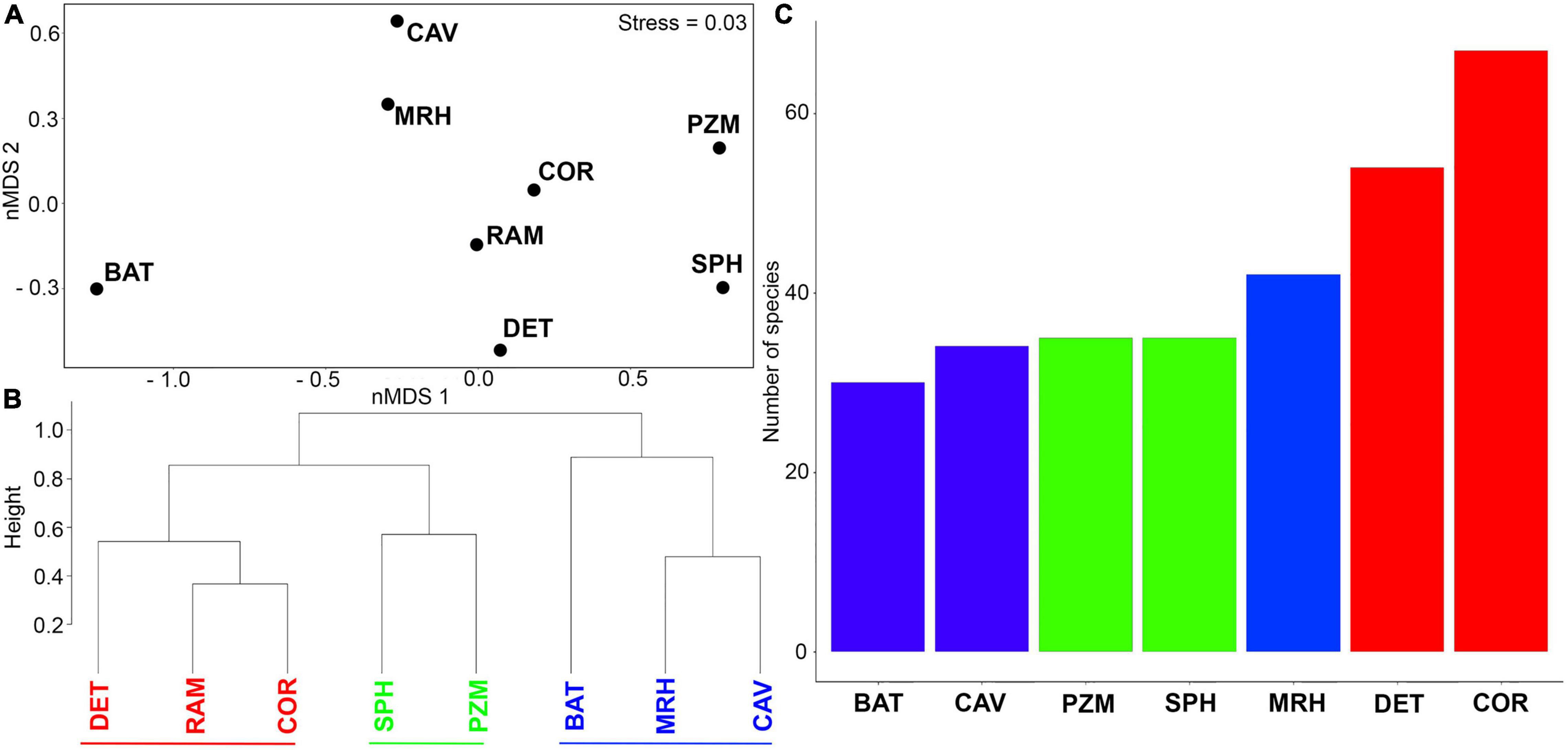
Figure 4. (A) nMDS ordination plot based on presence/absence species data in relation to species habitat affinities. (B) Cluster analysis highlighting the identification of three different groups. (C) Number of species found on the ram assemblage with different ecological affinities. Colors have been used to distinguish among groups (Red: group1; Green: group2; Blue: group3). Abbreviations are defined as follows: BAT, bathyal habitats; CAV, caves; COR, coralligenous reefs; DET, detritic bottoms; MRH, mesophotic reef habitats; PZM, Posidonia and Zosteracea meadows; RAM, ram 13; SPH, shallow shelf photophilic habitats.
A between-habitat ANOSIM comparison confirmed significant differences among these groups (Global R = 0.857, mean rank within group = 5.5, mean rank between groups = 17.5, and p < 0.005). Simper identified the species contributing most (>50%) to dissimilarity among habitats, including 22 species responsible for separating groups 1 and 2 (average dissimilarity 59%) and another 28 species that separated groups 1 and 3 (average dissimilarity 67.8%) (Table 3). A mostly sessile group of 12 species of bryozoans, eight species of serpulids, and two species of gastropods differentiated groups 1 and 2; bryozoans that form erect rigid colonies (66.7% of the species) contributed 54.5% to dissimilarity, with encrusting species comprising the remaining 33.3%. Large- or medium-sized serpulids (except S. crenata) accounted for 36% of dissimilarity. In contrast, 16 species of mollusks, all gastropods, were primarily responsible for the dissimilarity between groups 1 vs. 3 (57.2%). Different shell sizes and a wide range of ecological affinities characterized these gastropods, including sciaphilic circalittoral and photophilous infralittoral habitats (except for Volvarina mitrella, Alvania subareolata, and C. giunchiorum, which show specific preference for coralligenous and detritic habitats). Ten species of bryozoans contributed to the dissimilarity (35.7%), half of which form erect colonies and the other half form encrusting colonies.
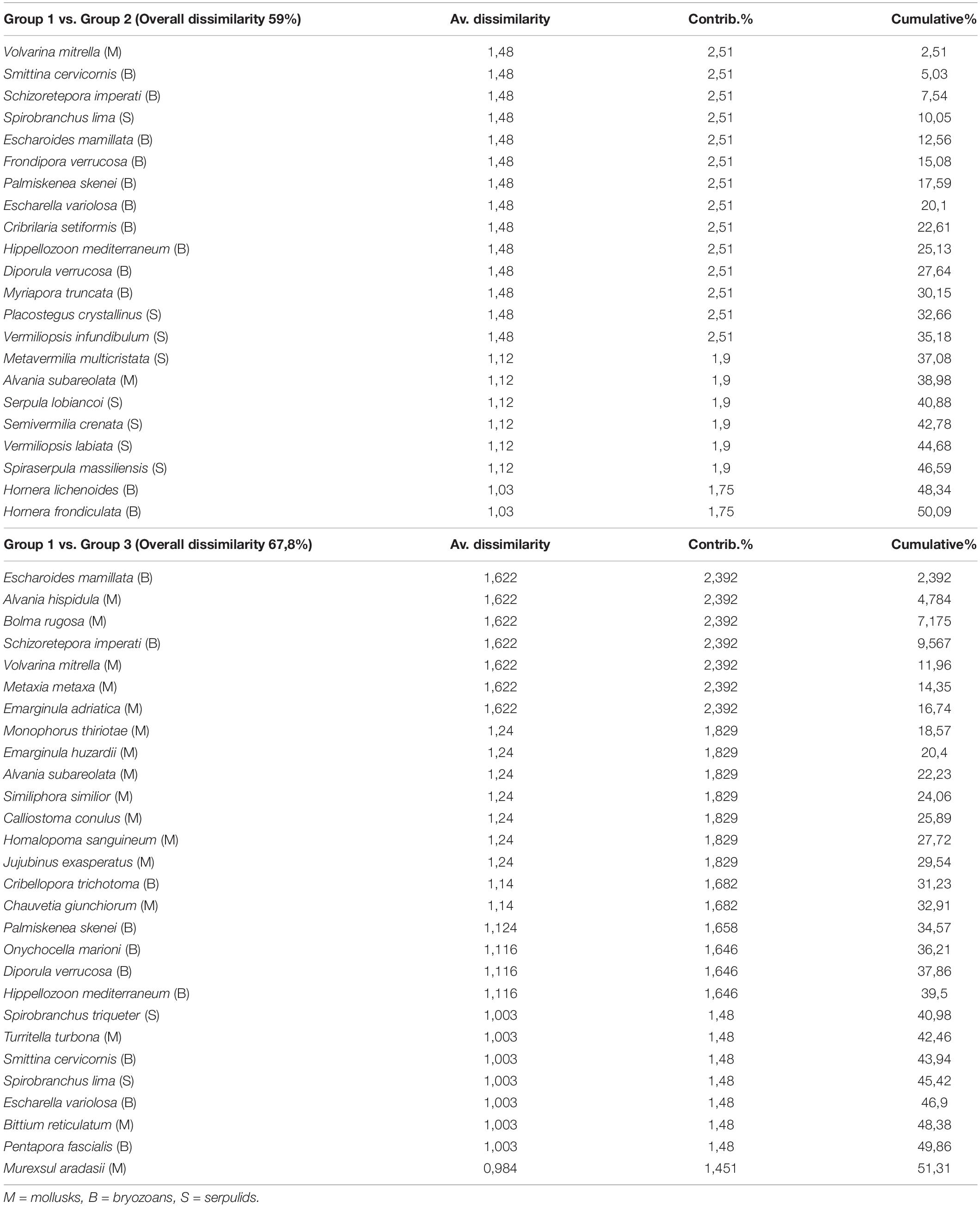
Table 3. Results of SIMPER analysis for identification of species contributing the most to faunal dissimilarity between habitats.
Discussion
Ours is the first study on the macrobenthic assemblage that colonized an artifact of cultural interest that remained submerged for two millennia in the Mediterranean Sea. The bronze naval ram, from a Carthaginian ship sunk during the battle of Aegates in 241 BC, collected colonizing fauna from surrounding habitats over a 2000-year period. The marine organisms settled, overgrew, and encrusted all available surfaces and, given the long time of immersion, we infer that this cultural artifact has become a suitable substratum for the development of a benthic community with ecological connectivity to natural communities in adjacent habitats. Indeed, duration of submersion and functional traits of the dominant species play major roles in shaping community structure on artificial reefs and their similarities (or dissimilarities) with natural reefs (Perkol-Finkel et al., 2005, 2006).
Faunal Assemblage Associated With Ram
The benthic fauna associated with the ram included a highly diverse assemblage primarily dominated by mollusks and secondarily dominated by polychaete serpulids and bryozoans. The analysis of functional traits revealed that the majority of species in the assemblage were large, sessile, solitary, or colonial invertebrates, i.e., all the bryozoans, the serpulids, and only nine bivalves. Among them, the bryozoans with erect colonies played the role of primary constructors. This role was filled by very few species (H. mediterraneum, M. truncata, P. fascialis, and S. cervicornis), similar to what occurs on hard substrata in shallow photophilic and deeper habitats, as well as on coralligenous reefs, semi-dark caves, and mesophotic bioconstuctions. Bryozoans often act as frame builders with erect colonies resulting in distinct conditions, such as the facies of large arborescent bryozoans on coastal detritic bottoms or structures built by just a few species (Cocito, 2004; Bianchi, 2009). In contrast, in many bioconcretions, bryozoans play a role as secondary constructors (encrusters) and produce crusts rather than erect layers (Cocito, 2009). The ram exhibited this latter colonization pattern, where the bryozoans largely encrusted the substatum and played the role of binders. Species such as Escharina vulgaris, Onychecella marioni, E. variolosa, Cribrilaria setiformis, Cribrilaria hincksii, and P. skenei, as well as other small-sized colonies, e.g., Celleporina caminata, Celleporina lucida, and C. trichotoma bound together the concretion as well as detrital material. They expanded their mineralized colonies both on the ram (as primary substratum) and on organic calcareous surfaces (as secondary substrata), overgrowing colonies, shells, and tubes of other organisms. They cemented the underlying species and, in turn, were colonized by epibiontic organisms. Similarly, several medium- to large-sized serpulids (e.g., Serpula vermicularis, Serpula concharum, Protula tubularia, Spirobranchus triqueter, Vermilipsis labiata, Vemiliopisis infundibulum, Vermiliopsis monodiscus, and Placostegus tridentatus) contributed to build the basal framework of the concretion, settling with their calcareous tubes directly on the bronze substrate. Many serpulids grew as epibionts on calcareous skeletons and valves of other sessile organisms, thus acting as secondary constructors together with the bryozoans. In this way, serpulids and bryozoans enhanced the small-scale spatial heterogeneity and increased the three-dimensionality of the ram’s surface. They created new microhabitas, from laminar crusts to massive crusts rich in interstices, pits, and crevices. These microhabitats provided cryptic refuges that served as high-quality habitat for many dweller species.
The engineering category of dwellers constituted the majority of the ram assemblage, including the inhabitants of the interstitial spaces of the concretion and of the detritus collected inside the ram. Small-/medium-sized species formed a conspicuous component of this functional group because of their small dimensions, an effective adaptation to cryptic habitats. Serpulids (Semivermilia cribrata, Semivermilia crenata, Filogranula annulata, F. calyculata, F. gracilis, Janua eterostropha, and Pileolaria militaris) with their small tubes and bryozoans (Celleporina spp., C. trichotoma, and Escharoides mamillata) with small-sized colonies both settled in clusters on interstitial surfaces. Similarly, many small gastropods typically occur in shaphilic coralligenous (Alvania subaereolata, Stricteulima jeffreysiama, and Volvarina mitrella) and bathyal habitats (Anatoma umbilicata, Epitonium tiberii, Raphitoma pseudohystrix, and Talassia degueneti) and colonize interstices and crypts where they find refuge from currents, irradiance, and predation. In contrast, most mollusks of medium to large size colonized both the concretion and the detritus accumulated inside the ram. Here, many bathyal and cave-dwelling species (Asperarca nodulosa and Venus nux) co-occurred with other species typical of shaphilic detritic and coralligenous habitats (Abra prismatica, Astarte sulcata, Cardiomya costellata, Pecten jacobaeus, Mimachlamys varia, Palliolum incomparabile, Papillicardium papillosum, and S. lactea).
Our analysis of the reproductive and life-history traits of the species in the ram supports conclusions from previous studies on the colonization of artifacts. Most species (60%) reproduced sexually through pelagic fertilization and produced larvae with long pelagic durations that may undergo long-range dispersal in the intense deep currents in our study area (Suriano et al., 1992). Thus, we consider the ram an island-like habitat (Meyer et al., 2017) of solid substrate surrounded by a soft seabed. In fact, the ram interrupted the continuity of the soft seafloor and offered a surface suitable for larval settlement of hard-bottom species as well as adequate elevation above the bottom to expose organisms to stronger bottom currents and associated particulate food sources. Moreover, planktonic larvae advected by oceanographic currents were not the only dispersal mechanism for the ram fauna. The presence of a remarkably high percentage (40%) of brooding species on the ram (i.e., all the bryozoans, a few serpulids, and half of the gastropods with embryonic development inside attached capsules) suggested that recruitment on the ram also took place via short-dispersal planktonic propagules, small-sized adults, and fragments of colonies arriving from source populations in the area by passive drift. Moreover, we hypothesize the arrival of some species by migration from nearby habitats, particularly for large-sized motile gastropods from coralligenous habitats (e.g., Murexsul aradasii and Calliostoma conulus) and detritic bottoms (e.g., T. turbona and Bolma rugosa).
Ram Assemblage and Relationship With Surrounding Habitats
Based on the ecological affinity of the species, the benthic assemblage on the ram differed in similarity from those in infralittoral, circalittoral, and bathyal habitats. All these habitats used as comparisons have been reported in the waters of the Aegadian archipelago. The Aegates Islands seabeds represent an area of great ecological value, with several endemic habitats protected by EU regulations and effectively managed through the designation of the largest Italian MPA (Aegates Islands MPA. Nevertheless, knowledge gaps remain regarding the distribution, biodiversity, and ecological status of benthic communities in the area. Uniformly distributed Posidonia oceanica meadows in the infralittoral zone cover the sandy seabed to depths over 30 m. Conversely, algae-dominated (i.e., brown algae belonging to the genus Cystoseira) belts develop at shallow depths and characterize photophilic rocky floors (Catra et al., 2006). The presence of the Cystoseira spp. thalli shapes environmental features and creates suitable conditions for invertebrate settlement (Sanfilippo et al., 2017). Coralligenous reefs occur mainly on steep walls, hard bottoms below 40 m depth, and shoals around the whole archipelago and represent the most attractive seascape for diving tourists (Cocito et al., 2014). Among the 200 Rhodophyta species reported in the algal checklist of the area (Catra et al., 2006 and the references therein), widespread distributions across all islands of the archipelago characterize the Corallinales and Peyssonneliales that form the basal layer of coralligenous reefs. Recent distribution patterns of encrusting red algae likely reflect the spread of coralligenous reefs in the study area. The carbonatic lithology of the islands supports several submerged caves that originated through karstic phenomena (Gerovasileiou and Bianchi, 2021). Furthermore, organogenic detritus composed of animal debris enriches the carbonate sandy bottom sediments of the Aegadian archipelago, likely stemming from the erosion of coralligenous reefs and marine cave communities. Recent work described deep and cold-water coral assemblages in seamounts and banks surrounding the Aegetes islands between 240 and 300 m depth (Bo et al., 2014; Angiolillo et al., 2021). Although this previous work reported Porifera and Cnidaria as the most frequent taxa, our study did not find them. Angiolillo et al. (2021) highlighted the importance of hard bottoms (sparse boulders and wrecks) for the richness and diversity of the megabenthic assemblages, resulting in high ecological value and conservation interest for the whole area.
All of these factors play a key role in understanding the composition of the ram assemblage. Indeed, the distribution and bathymetric range of the benthic communities in the region explain the higher affinity in species composition of the ram assemblage with coralligenous reefs and detritic bottoms, which form the group 1 in the nMDS plot and in the cluster analysis. On the one hand, both coralligenous and detritic habitats thus represent the main source populations that would have provided the larval supply necessary for colonization of the ram; in fact, pelagic spawning species with long-lived pelagic larvae dominated. On the other hand, the strong regional hydrodynamic regime, which presumably promoted the transport of both propagules and fragments or mineralized remains inside the ram, can partially the presence of species whose affinity links shallow habitats, such as Posidonia meadows. In support of this hypothesis, the facies dominated by Laminaria rodriguezii reported in the region (Suriano et al., 1992; Araújo et al., 2016) indicate the presence of high-speed bottom currents on the surrounding seabed.
These results offer insights regarding the expected timeframe for a submerged wreck to match natural habitats. Previous research showed that the benthic invertebrate communities on shipwrecks up to more than a century old do not match the background community (Perkol-Finkel and Benayahu, 2004; Perkol-Finkel et al., 2005, 2006). Indeed, wrecks generally have lower functional diversity than natural habitats and are dominated by species with long pelagic larval duration and/or asexual reproduction by fission. Our study showed that a ram that has accumulated biota over many centuries hosts a community with high functional diversity and species that occur in a range of surrounding natural habitats.
Conclusion
Ram 13, which remained on the sedimentary seafloor for more than 2000 years, has had sufficient time to establish a long-term stable community composed of both hard- and soft-bottom benthic organisms. The ram has trapped mineral structures and fragments (i.e., tubes and shells) of species living in the surrounding habitats transported by bottom current. Therefore, together with its inestimable value as an archeological artifact, the ram represents a novel and effective sampling tool. The ram highlights the dynamics of biological colonization on a large spatial scale and serves as a relevant proxy for the study of marine biodiversity.
Our study highlighted the high species richness of the benthic assemblage associated with the ram, whose composition showed strong similarity with coralligenous reefs and detritic circalittoral habitats, with Posidonia beds and photophilic rocky bottoms, and to a lesser degree with the deeper bathyal habitats and caves. All these habitats have great environmental value and are considered hotspots of biodiversity in different depth ranges. Thus, the presence of species in the ram assemblage that are common to different habitats serves as “ecological memory” of the occurrence of such habitats in the surrounding seabed and highlights the high marine biodiversity in the Aegadian archipelago region. In this way, the benthic assemblage of the ram served as a remarkable proxy for marine biodiversity over a large spatial scale. The present study may act as a crucial baseline for future investigations in the Battle of the Aegates Islands region, which is of great interest in ecology and in archeology.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
MG: supervision, conceptualization, data curation, investigation, taxonomical and formal analysis, methodology, writing—original draft, and writing—review and editing. EC: data curation, investigation, formal analysis, methodology, and writing—review and editing. LD and JG: data curation, investigation, taxonomical and formal analysis, methodology, and writing—review and editing. FA and CS: data curation, investigation, laboratory and formal analysis, methodology, and writing—review and editing. SR: supervision, conceptualization, data curation, investigation, formal analysis, writing—original draft, and writing—review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors express their most sincere gratitude to Valeria Li Vigni Tusa, Director of the Soprintendenza del Mare (Sicilian Region) for giving us the opportunity to publish the present work on the Egadi 13 ram and her staff who have worked for more than a decade on the Egadi project. The authors also thank Barbara Davidde Petriaggi, Superintendent of the Soprintendenza Nazionale per il Patrimonio Culturale Subacqueo (Ministry of Culture), who directed the restoration works at the Central Institute for Restoration. The authors also thank Kirstin Mayer-Kaiser for her careful review and editorial suggestions that significantly improved the manuscript.
References
Albano, P., and Sabelli, B. (2011). Comparison between death and living molluscs assemblages in a Mediterranean infralittoral off-shore reef. Palaeogeogr. Palaeoclimatol. Palaeoecol. 310, 206–215. doi: 10.1016/j.palaeo.2011.07.012
Albano, P. G., and Sabelli, B. (2012). The molluscan assemblages inhabiting the leaves and rhizomes of a deep water Posidonia oceanica settlement in the central Tyrrhenian Sea. Sci. Mar. 76, 721–732.
Allen, J. A. (2004). The Recent species of the genera Limatula and Limea (Bivalvia, Limacea) present in the Atlantic, with particular reference to those in deep water. J. Nat. Hist. 38, 2591–2653. doi: 10.1080/00222930310001647442
Angiolillo, M., La Mesa, G., Giusti, M., Salvati, E., Di Lorenzo, B., Rossi, L., et al. (2021). New records of scleractinian cold-water coral (CWC) assemblages in the southern Tyrrhenian Sea (western Mediterranean Sea): human impacts and conservation prospects. Prog. Oceanogr. 102656. doi: 10.1016/j.pocean.2021.102656
Antonelli, F., Sacco Perasso, C., Ricci, S., and Davidde Petriaggi, B. (2015). Impact of the sipunculan Aspidosiphon muelleri Diesing, 1851 on calcareous Underwater Cultural Heritage. Int. Biodeterior. Biodegrad. 100, 133–139. doi: 10.1016/j.ibiod.2015.02.025
Antonioli, F., Silenzi, S., and Frisia, S. (2001). Tyrrhenian Holocene palaeoclimate trends from spelean serpulids. Quat. Sci. Rev. 20, 1661–1670. doi: 10.1016/S0277-3791(01)00012-9
Araújo, R. M., Assis, J., Aguillar, R., Airoldi, L., Bárbara, I., Bartsch, I., et al. (2016). Status, trends and drivers of kelp forests in Europe: an expert assessment. Biodivers. Conserv. 25, 1319–1348. doi: 10.1007/s10531-016-1141-7
Balaguer, L., Escudero, A., Martìn-Duque, J. F., Mola, I., and Aronson, J. (2014). The historical reference in restoration ecology: re-defining a cornerstone concept. Biol. Conserv. 176, 12–20. doi: 10.1016/j.biocon.2014.05.007
Balduzzi, A., Bianchi, C. N., Boero, F., Cattaneo Vietti, R., Pansini, M., and Sarà, M. (1989). The suspension-feeder communities of a Mediterranean sea cave. Sci. Mar. 53, 387–395.
Ballesteros, E. (2006). Mediterranean coralligenous assemblages: a synthesis of present knowledge. Oceanogr. Mar. Biol. Ann. Rev. 44, 123–195. doi: 10.1201/9781420006391.ch4
Bass, G. F., Pulak, C., Collon, D., and Weinstein, J. (1989). The Bronze Age Shipwreck at Ulu Burun: 1986 Campaign. Am. J. Archaeol. 93, 1–29.
Belloni, S., and Bianchi, C. N. (1982). Policheti di alcune grotte marine della penisola Sorrentina (Golfo di Napoli). Boll. Mus. Ist. Biol. Univ. Genova 50, 118–127.
Bertolino, M., Calcinai, B., Cattaneo-Vietti, R., Cerrano, C., Lafratta, A., Pansini, M., et al. (2014). Stability of the sponge assemblage of Mediterranean coralligenous concretions along a millennial time span. Mar. Ecol. 35, 149–158. doi: 10.1111/maec.12063
Bianchi, C. N. (1981). Guide per il Riconoscimento delle Specie Animali delle Acque Lagunari e Costiere Italiane. AQ/1/96. (Roma: CNR), 1–187.
Bianchi, C. N. (2009). “Facies a grandi Briozoi,” in Gli Habitat Prioritari del Protocollo SPA/BIO (Convenzione di Barcellona) Presenti in Italia, eds G. Relini and G. Giaccone (Genova, IT: Erredi Grafiche Editoriali), 209–212.
Bianchi, C. N., and Sanfilippo, R. (2003). “Policheti Serpuloidei,” in Grotte Marine: Cinquant’anni di Ricerca in Italia, eds F. Cicogna, C. N. Bianchi, G. Ferrari, and P. Forti (Rome: Ministero dell’Ambiente e della Tutela del Territorio), 175–185.
Bo, M., Cerrano, C., Canese, S., Salvati, E., Angiolillo, M., Santangelo, G., et al. (2014). The coral assemblages of an off-shore deep Mediterranean rocky bank (NW Sicily, Italy). Mar. Ecol. 35, 332–342. doi: 10.1111/maec.12089
Boury-Esnaulth, N., Harmelin, J. G., Ledoyer, M., Saldanha, L., and Zibrowius, H. (2001). Peuplement benthique des grottes sous-marines de Sagres (Portugal, Atlantique Nord-Oriental). Bol. Mus. Munic. Funchal 6, 15–38.
Calcinai, B., Sacco Perasso, C., Davidde Petriaggi, B., and Ricci, S. (2019). Endolithic and epilithic sponges of archaeological marble statues recovered in the Blue Grotto, Capri (Italy) and in the Antikythera shipwreck (Greece). Facies 65, 1–21. doi: 10.1007/s10347-019-0562-7
Cardone, F., Corriero, G., Longo, C., Mercurio, M., Tarantini, S. O., Gravina, M. F., et al. (2020). Massive bioconstructions built by Neopycnodonte cochlear (Mollusca, Bivalvia) in a mesophotic environment in the central Mediterranean Sea. Sci. Rep. 10:6337. doi: 10.1038/s41598-020-63241-y
Casoli, E., Ricci, S., Antonelli, F., Sacco Perasso, C., Ardizzone, G., et al. (2019a). Biotic interaction between boring polychaetes and sessile epibionts on experimental limestone substrata. Hydrobiologia 842, 101–112. doi: 10.1007/s10750-019-04028-9
Casoli, E., Bonifazi, A., Ardizzone, G., Gravina, M. F., Russo, G. F., Sandulli, R., et al. (2019b). Comparative analysis of mollusc assemblages from different hard bottom habitats in the Central Tyrrhenian Sea. Diversity 11, 1–74. doi: 10.3390/d11050074
Casoli, E., Bonifazi, A., Ardizzone, G. D., and Gravina, M. F. (2016). How algae influence sessile marine organisms: the tube worms case of study. Estuar. Coast. Shelf Sci. 178, 12–20. doi: 10.1016/j.ecss.2016.05.017
Casoli, E., Piazzi, L., Nicoletti, L., Jona-Lasinio, G., Cecchi, E., Mancini, G., et al. (2020). Ecology, distribution and demography of erect bryozoans in Mediterranean coralligenous reefs. Estuar. Coast. Shelf Sci. 235:106573. doi: 10.1016/j.ecss.2019.106573
Castriota, L., Agamennone, F., and Sunseri, G. (2005). The mollusc community associated with maerl beds of Ustica Island (Tyrrhenian Sea). Cah. Biol. Mar. 46, 289–297.
Catra, M., Alongi, G., Serio, D., Cormaci, M., and Furnari, G. (2006). The benthic algal flora on rocky substrata of the Egadi islands, a marine protected archipelago off the western coast of Sicily (Italy, Mediterranean Sea). Nova Hedwigia 82, 489–538. doi: 10.1127/0029-5035/2006/0082-0489
Chimenz Gusso, C., Nicoletti, L., and Bondanese, C. (2014). Briozoi. Biol. Mar. Mediterr. 21, 1–336.
Cocito, S. (2004). Bioconstruction and biodiversity: their mutual influence. Sci. Mar. 68, 137–144. doi: 10.3989/scimar.2004.68s1137
Cocito, S. (2009). Bryozoan bioconstructions. Biol. Mar. Mediterr. 16, 19–30. doi: 10.1007/978-94-007-6704-1_21
Cocito, S., Barsanti, M., Delbono, I., Lombardi, C., and Peirano, A. (2014). Itinerari Sommersi Nelle Isole di Marettimo e Levanzo, Isole Egadi. Sarzana: RES-Edizioni, 1–72.
Cocito, S., Bedulli, D., and Sgorbini, S. (2002). Distribution patterns of the sublittoral epibenthic assemblages on a rocky shoal in the Ligurian Sea (NW Mediterranean). Sci. Mar. 66, 175–118. doi: 10.3989/scimar.2002.66n2175
Colantoni, P., Ligi, M., Morsiani, M. P., and Penitenti, D. (1993). Morphology and recent sedimentary evolution of the Western Sicilian continental shelf. Geological development of the sicilian-tunisian platform. UNESCO Rep. Mar. Sci. 58, 93–98.
Corriero, G., Pierri, C., Mercurio, M., Nonnis Marzano, C., Tarantini, S. O., Gravina, M. F., et al. (2019). A Mediterranean mesophotic coral reef built by non-symbiotic scleractinians. Sci. Rep. 9:3601.
Costa, M. (2016). Benthic Communities in Shipwrecks Along the Portuguese Continental Coast. Ph.D. thesis. Faro: Universidade do Algarve.
Covazzi Harriague, A., Schiaparelli, S., Panciroli, H., and Albertelli, G. (2002). Soft bottom mollusc communities of four south Tyrrhenian archipelagos and Ustica island (NW Mediterranean). P. Italian Assoc. Oceanol. Limnol. 15, 63–74.
Crocetta, F., and Spanu, M. (2008). Molluscs associated with a Sardinian deep water population of Corallium rubrum (Linné, 1758). Mediterr. Mar. Sci. 9, 63–86.
Dauvin, J. C., and Gentil, F. (1989). Long-term changes in populations of subtidal bivalves (Abra alba and A. prismatica) from the Bay of Morlaix (Western English Channel). Mar. Biol. 103, 63–73. doi: 10.1007/BF00391065
Davidde, B., Petriaggi, R., Ricci, S., Sacco Perasso, C., Antonelli, F., and Vlachogianni, E. (2017). An overview of the state of conservation of the marble artefacts from the Antikythera shipwreck. Archaeol. Marit. Mediterr. 14, 13–74. doi: 10.19272/201704501002
Donnarumma, L., Sandulli, R., Appolloni, L., and Russo, G. F. (2018a). Assessing molluscs functional diversity within different coastal habitats of Mediterranean marine protected areas. Ecol. Quest. 29, 35–51. doi: 10.12775/EQ.2018.021
Donnarumma, L., Sandulli, R., Appolloni, L., Sánchez-Lizaso, J. L., and Russo, G. F. (2018b). Assessment of structural and functional diversity of mollusc assemblages within vermetid bioconstructions. Diversity 10, 1–96. doi: 10.3390/d10030096
Donnarumma, L., Bruno, R., Terlizzi, A., and Russo, G. F. (2018c). Population ecology of Jujubinus striatus and Jujubinus exasperatus (Gastropoda: Trochidae) in a Posidonia oceanica seagrass bed. Eur. Zool. J. 85, 17–25. doi: 10.1080/24750263.2017.1420828
Fagerstrom, J. A. (1988). A structural model for reef communities. Palaios 3, 217–220. doi: 10.2307/3514531
Fish, J. D., and Fish, S. (1989). “Mollusca,” in A Student’s Guide to the Seashore, ed. J. I. D. I. Fish (Dordrecht: Springer), 183–281.
Foley, B. P., Dellaporta, K., Sakellariou, D., Bingham, B. S., Camilli, R., Eustice, R. M., et al. (2009). The 2005 chios ancient shipwreck survey: new methods for underwater archaeology. Hesperia 78, 269–305.
Gerovasileiou, V., and Bianchi, C. N. (2021). Mediterranean marine caves: a synthesis of current knowledge. Oceanogr. Mar. Biol. Annu. Rev. 1–59.
Giacobbe, S., and Renda, W. (2018). Infralittoral molluscs from the Scilla cliff (Strait of Messina, Central Mediterranean). Biodivers. J. 9, 255–270. doi: 10.31396/Biodiv.Jour.2018.9.3.255.270
Giampaoletti, J., Cardone, F., Corriero, G., Gravina, M. F., and Nicoletti, L. (2020). Sharing and Distinction in Biodiversity and Ecological Role of Bryozoans in Mediterranean Mesophotic Bioconstructions. Front. Mar. Sci. 7:581292. doi: 10.3389/fmars.2020.581292
Gili, J. M., Grinyó, J., Requena, S., Madurell, T., Gori, A., Ambroso, S., et al. (2014). Caracterización Ecológica del Área Marina del Canal de Menorca: Zonas Profundas y Semiprofundas (100-400 m). Informe Final Área LIFE+ INDEMARES (LIFE07/NAT/E/000732). (Barcelona: Instituto de Ciencias del Mar), 1–217.
Gravina, M. F., Antonelli, F., Sacco Perasso, C., Cesaretti, A., Casoli, E., et al. (2019). The role of polychaetes in bioerosion of submerged mosaic floors in the Underwater Archaeological Park of Baiae (Naples, Italy). Facies 65, 1–19. doi: 10.1007/s10347-019-0563-6
Gravina, M. F., Pierri, C., Mercurio, M., Nonnis Marzano, C., and Giangrande, A. (2021). Polychaete Diversity Related to Different Mesophotic Bioconstructions along the Southeastern Italian Coast. Diversity 13:239. doi: 10.3390/d13060239
Harmelin, J. G. (1985). “Bryozoan dominated assemblages in Mediterranean cryptic environments,” in Bryozoa: Ordovician to Recent, eds C. Nielsen and G. P. Larwood (Fredensborg: Olsen & Olsen), 135–143.
Harmelin, J. G. (1997). Diversity of bryozoans in a Mediterranean sublittoral cave with bathyal-like conditions: role of dispersal processes and local factors. Mar. Ecol. Prog. Ser. 153, 139–152. doi: 10.3354/meps153139
Harmelin, J. G. (2017). Bryozoan facies in the coralligenous community: two assemblages with contrasting features at port-cros archipelago (Port-Cros National Park, France, Mediterranean). Sci. Rep. Port Cros Natl. Park 31, 105–123.
Harmelin, J. G., and Aristegui, J. (1988). New Cribrilinidae (Bryozoa, Cheilostomata) from the upper bathyal of the Atlanto-Mediterranean region. J. Nat. Hist. 22, 507–535. doi: 10.1080/00222938800770351
Harmelin, J. G., Bitar, G., and Zibrowius, H. (2009). Smittinidae (Bryozoa, Cheilostomata) from coastal habitats of Lebanon (Mediterranean sea), including new and non-indigenous species. Zoosystema 31, 163–187. doi: 10.5252/z2009n1a9
Hughes, R. N. (1986). A Functional Biology of Marine Gastropods. (Baltimore, MD: The John Hopkins University Press), 1–245.
Janssen, A., Chevaldonné, P., and Arbizu, P. M. (2013). Meiobenthic copepod fauna of a marine cave (NW Mediterranean) closely resembles that of deep-sea communities. Mar. Ecol. Prog. Ser. 479, 99–113. doi: 10.3354/meps10207
Kocak, F., Balduzzi, A., and Benli, H. A. (2002). Epiphytic bryozoan community of Posidonia oceanica (L.) Delile meadow in the northern Cyprus (Eastern Mediterranean). Indian J. Mar. Sci. 31, 235–238.
Lebour, M. V. (1937a). Larval and post-larval Lima from Plymouth. J. Mar. Biolog. Assoc. U.K. 21, 705–710. doi: 10.1017/S0025315400053820
Lebour, M. V. (1937b). The eggs and larvae of the British prosobranchs with special reference to those living in the plankton. J. Mar. Biolog. Assoc. U.K. 22, 105–166.
Leidwanger, J., Greene, E. S., and Donnelly, A. (2021). The Sixth-Century CE Shipwreck at Marzamemi. Am. J. Archaeol. 125, 283–317.
Macrì, G. (2018). Secondo contributo alla conoscenza della malacofauna di Cava Signorella (Lecce). Boll. Malacol. 54, 139–155.
Madurell, T., Zabala, M., Dominguez-Carrió, C., and Gili, J. M. (2013). Bryozoan faunal composition and community structure from the continental shelf off Cap de Creus (Northwestern Mediterranean). J. Sea Res. 83, 123–136. doi: 10.1016/j.seares.2013.04.013
Martin, D. (1987). La comunidad de Anélidos Poliquetos de las concreciones de algas calcáreas del litoral catalán. Caracterización de las especies. P. Dept. Zool. Barcelona 13, 45–54.
Mastrototaro, F., D’Onghia, G., Corriero, G., Matarrese, A., Maiorano, P., Panetta, P., et al. (2010). Biodiversity of the white coral bank off cape Santa Maria di Leuca (Mediterranean Sea): an update. Deep Sea Res. Part II Top. Stud. Oceanogr. 57, 412–430. doi: 10.1016/j.dsr2.2009.08.021
Mazziotti, C., Agamennone, F., and Tisselli, M. (2008). Checklist della malacofauna delle Isole Tremiti (Medio Adriatico). Boll. Malacol. 44, 71–86.
McKinney, F. K., and Jaklin, A. (2000). Spatial niche partitioning in the cellaria meadow epibiont association, northern Adriatic Sea. Cah. Biol. Mar. 41, 1–18.
Meloni, G., and Sabelli, B. (1980). Ritrovamento di Alvania subareolata Monterosato, 1869. Boll. Malacol. 16, 361–366.
Meyer, K. S., Brooke, S. D., Sweetman, A. K., Wolf, M., and Young, C. M. (2017). Invertebrate communities on historical shipwrecks in the western Atlantic: relation to islands. Mar. Ecol. Prog. Ser. 566, 17–29. doi: 10.3354/meps12058
Micali, P. (1998). Note sulle specie di Chauvetia dell’Atlantico nord-orientale. Boll. Malacol. 34, 53–68.
Morri, C., Bianchi, C., Cocito, S., Peirano, A., De Biase, A. M., Aliani, S., et al. (1999). Biodiversity of marine sessile epifauna at an Aegean island subject to hydrothermal activity: Milos, eastern Mediterranean Sea. Mar. Biol. 135, 729–739. doi: 10.1007/s002270050674
Morton, B. (2009). Aspects of the biology and functional morphology of Timoclea ovata (Bivalvia: Veneroidea: Venerinae) in the Azores, Portugal, and a comparison with Chione elevata (Chioninae). Açoreana 6, 105–119.
Novosel, M., Hageman, S. J., Mihanovic, H., and Novosel, A. (2019). “Bryodiversity along the Croatian coast of the Adriatic Sea,” in Bryozoan Studies, eds P. N. Wyse Jackson and K. Zágoršek (Prague: Geological Survey), 99–109.
Novosel, M., Požar-Domac, A., and Pasari’c, M. (2004). Diversity and distribution of the Bryozoa along underwater cliffs in the Adriatic Sea with special reference to thermal regime. Mar. Ecol. 25, 155–170. doi: 10.1111/j.1439-0485.2004.00022.x
Oliver, P. G., and Holmes, A. M. (2006). The Arcoidea (Mollusca: Bivalvia): a review of the current phenetic-based systematics. Zool. J. Linn. Soc. 148, 237–251. doi: 10.1111/j.1096-3642.2006.00256.x
Palazzi, S., and Villari, A. (2001). Molluscs and Brachiopods from the Submarine Caves of Taormina, Sicily. La Conch. 32, 1–56.
Pérès, J. M., and Picard, J. (1964). Nouveau manuel de bionomie benthique de la Mer Méditerranée. Rec. Trav. St. Mar. Endoume 31:137.
Perkol-Finkel, S., and Benayahu, Y. (2004). Community structure of stony and soft corals on vertical unplanned artificial reefs in Eilat (Red Sea): comparison to natural reefs. Coral Reefs 23, 195–205. doi: 10.1007/s00338-004-0384-z
Perkol-Finkel, S., Shashar, N., Barneah, O., Ben-David-Zaslow, R., Oren, U., Reichart, T., et al. (2005). Fouling reefal communities on artificial reefs: Does age matter? Biofouling 21, 127–140. doi: 10.1080/08927010500133451
Perkol-Finkel, S., Shashar, N., and Benayahu, Y. (2006). Can artificial reefs mimic natural reef communities? The roles of structural features and age. Mar. Environ. Res. 61, 121–135.
Petriaggi, R., and Davidde, B. (2010). The sarcophagi from the wreck of San Pietro in Bevagna (Taranto): the subject of new works by the Istituto Superiore per la 1673 Conservazione ed il restauro. Archaeol. Marit. Mediterr. 7, 1000–1007.
Pisano, E., and Boyer, M. (1985). Development pattern of an infralittoral bryozoan community in the western Mediterranean Sea. Mar. Ecol. Prog. Ser. 27, 195–202.
Popović, Z., Mladineo, I., Ezgeta-Balić, D., Trumbić, Ž., Vrgoč, N., and Peharda, M. (2013). Reproductive cycle and gonad development of Venus verrucosa L. (Bivalvia: Veneridae) in Kaštela Bay, Adriatic Sea. Mar. Biol. Res. 9, 274–284. doi: 10.1080/17451000.2012.731690
Pulak, C. (2008). “The Uluburun shipwreck and late bronze age trade,” in Beyond Babylon: Art, Trade, and Diplomacy in the Second Millennium BC, eds J. Aruz, K. Benzel, and J. M. Evans (New York, NY: Metropolitan Museum of Art), 289–310.
Ricci, S., Antonelli, F., Sacco Perasso, C., Poggi, D., and Casoli, E. (2016b). Bioerosion of submerged lapideous artefacts: role of endolithic rhizoids of Acetabularia acetabulum (Dasycladales, Chlorophyta). Int. Biodeterior. Biodegrad. 107, 10–16. doi: 10.1016/j.ibiod.2015.10.024
Ricci, S., Antonelli, F., Davidde Petriaggi, B., Poggi, D., and Sacco Perasso, C. (2016a). Observations of two mosaic fragments from the Underwater City of Baiae (Naples, Italy): archaeological, geological and biological investigations. Int. J. Conserv. Sci. 7, 415–430.
Ricci, S., and Bartolini, M. (2005). “Il biodeterioramento del Satiro,” in Il Satiro Danzante di Mazara del Vallo: il Restauro e L’immagine, ed. R. Petriaggi (Napoli: Electa Napoli Edizioni).
Ricci, S., Pietrini, A. M., Bartolini, M., and Sacco Perasso, C. (2013). Role of the microboring marine organisms in the deterioration of archaeological submerged lapideous artifacts (Baia, Naples, Italy). Int. Biodeterior. Biodegrad. 82, 199–206. doi: 10.1016/j.ibiod.2013.03.016
Ricci, S., Sacco Perasso, C., Antonelli, F., and Davidde Petriaggi, B. (2015). Marine Bivalves colonizing roman artefacts recovered in the Gulf of Pozzuoli and in the Blue Grotto in Capri (Naples, Italy): boring and nestling species. Int. Biodeterior. Biodegrad. 98, 89–100. doi: 10.1016/j.ibiod.2014.12.001
Ricci, S., Sanfilippo, R., Basso, D., Sacco Perasso, C., Antonelli, F., et al. (2019). Benthic Community Formation Processes of the Antikythera Shipwreck Statues Preserved in the National Archaeological Museum of Athens (Greece). J. Marit. Archaeol. 14, 81–106. doi: 10.1007/s11457-018-9205-3
Rosso, A. (1996). Valutazione della biodiversità in mediterraneo: l’esempio dei popolamenti a briozoi della biocenosi del detritico costiero. Biol. Mar. Mediterr. 3, 58–65.
Rosso, A. (1999). Recent and fossil species of Characodoma Maplestone, 1900 (Bryozoa) from the Mediterranean with description of two new species. J. Nat. Hist. 33, 415–437. doi: 10.1080/002229399300326
Rosso, A., and Di Geronimo, I. (1998). Deep-sea Pleistocene Bryozoa of Southern Italy. Geobios 30, 303–317. doi: 10.1016/S0016-6995(98)80014-4
Rosso, A., Di Martino, E., Sanfilippo, R., and Di Martino, V. (2013a). “Bryozoan communities and thanatocoenoses from submarine caves in the Plemmirio marine protected area (SE Sicily),” in Bryozoan Studies 2010, eds A. Ernst, P. Schäfer, and J. Scholz (Berlin: Springer), 251–269. doi: 10.1007/978-3-642-16411-8_17
Rosso, A., Sanfilippo, R., Taddei Ruggieri, E., and Di Martino, E. (2013b). Faunas and ecological groups of serpuloidea, bryozoa and brachiopoda from submarine caves in sicily (Mediterranean Sea). Boll. Soc. Paleontol. Ital. 52, 167–176. doi: 10.4435/BSPI.2013.18
Rosso, A., Gerovasileiou, V., Sanfilippo, R., and Guido, A. (2019). Bryozoan assemblages from two submarine caves in the Aegean Sea (Eastern Mediterranean). Mar. Biodivers. 49, 707–726. doi: 10.1007/s12526-018-0846-0
Rosso, A., and Sanfilippo, R. (2005). Bryozoans and serpuloideans in skeletobiont communities from the Pleistocene of Sicily: spatial utilization and competitive interactions. Sezione Museol. Sci. Nat. 1, 115–124.
Rosso, A., and Sanfilippo, R. (2009). “The contribution of Bryozoans and serpuloideans to coralligenous concretions from SE sicily,” in Proceedings of the 1st Mediterranean Symposium on the Conservation of the Coralligenous and other Calcareous Bio-Concretions, eds C. Pergent Martini and M. Brichet (Tabarka: RAC/SPA publ), 123–128.
Rosso, A., Vertino, A., Di Geronimo, I., Sanfilippo, R., Sciuto, F., Di Geronimo, R., et al. (2010). Hard-and soft-bottom thanatofacies from the Santa Maria di Leuca deep-water coral province, Mediterranean. Deep Sea Res. Part II Top. Stud. Oceanogr. 57, 360–379. doi: 10.1016/j.dsr2.2009.08.024
Rueda, J. L., Gofas, S., Urra, J., and Salas, C. (2009). A highly diverse molluscan assemblage associated with eelgrass beds (Zostera marina L.) in the Alboran Sea: micro-habitat preference, feeding guilds and biogeographical distribution. Sci. Mar. 73, 679–700. doi: 10.3989/scimar.2009.73n4679
Rueda, J. L., González-García, E., Krutzky, C., López-Rodriguez, F. J., Bruque, G., López-González, N., et al. (2016). From chemosynthesis-based communities to cold-water corals: vulnerable deep-sea habitats of the Gulf of Cádiz. Mar. Biodivers. 46, 473–482. doi: 10.1007/s12526-015-0366-0
Rueda, J. L., Urra, J., Aguilar, R., Angeletti, L., Bo, M., García-Ruiz, C., et al. (2019). “29 cold-water coral associated fauna in the Mediterranean Sea and adjacent areas,” in Mediterranean cold-Water Corals: Past, Present and Future, eds C. Orejas and C. Jiménez (Cham: Springer), 295–333. doi: 10.1007/978-3-319-91608-8_29
Sacco Perasso, C., Ricci, S., Davidde Petriaggi, B., and Calcinai, B. (2015). Marine bioerosion of lapideous archaeological artifacts found in the Grotta Azzurra (Capri, Naples, Italy): role of microbiota and boring Porifera. Int. Biodeterior. Biodegrad. 99, 146–156. doi: 10.1016/j.ibiod.2014.08.010
Sanfilippo, R., and Mòllica, E. (2000). Serpula cavernicola Fassari & Mòllica, 1991 (Annelida Polychaeta); diagnostic features of the tube and new Mediterranean records. Mar. Life 10, 27–32.
Sanfilippo, R., Rosso, A., Sciuto, F., Serio, D., Catra, M., Alongi, G., et al. (2017). Serpulid polychaetes from Cystoseira communities in the Ionian Sea, Mediterranean. Vie Mil. Life Environ. 67, 217–226.
Scaperrotta, M., Bartolini, S., and Bogi, C. (2009–2018). Stages of Growth of Marine Molluscs of the Mediterranean Sea, Vol. 1-9. Harxheim: L’informatore Piceno.
Smriglio, C., Mariottini, P., and Gravina, M. F. (1987a). Molluschi del Mar Tirreno Centrale: ritrovamento di Typhlomangelia nivalis (Lovén, 1846). Contributo I. Boll. Malacol. 23, 47–52.
Smriglio, C., Mariottini, P., and Gravina, M. F. (1987b). Molluschi del Mar Tirreno Centrale: segnalazione di alcuni Turridi provenienti da una Biocenosi a Coralli Bianchi. Contributo II. Boll. Malacol. 23, 381–390.
Smriglio, C., Mariottini, P., and Gravina, M. F. (1989). Molluschi del Mar Tirreno Centrale: ritrovamento di Putzèysia wiseri (Calcara, 1892), Ischnochiton vanbellei Kaas, 1985 e Neopilina zografi (Dautzenberg & Fisher, 1896). Contributo VI. Boll. Malacol. 25, 125–132.
Souto, J., Reverter-Gil, O., and Fernandez-Pulpeiro, E. (2010). Bryozoa from detritic bottoms in the Menorca Channel (Balearic Islands, western Mediterranean), with notes on the genus Cribellopora. Zootaxa 2536, 36–52. doi: 10.11646/zootaxa.2536.1.2
Suriano, C., Mazzola, S., Levi, D., and Giusto, G. B. (1992). La biocenosi dei substrati duri circalitorali a grandi Phaeophyceae (Laminaria rodriguezii Bornet, 1988) nel Canale di Sicilia e nel Canale Maltese. Oebalia 17, 429–432.
Taviani, M., Angeletti, L., Canese, S., Cannas, R., Cardone, F., Cau, A., et al. (2015). The “Sardinian cold-water coral province” in the context of the Mediterranean coral ecosystems. Deep Sea Res. Part II Top. Stud. Oceanogr. 145, 61–78.
Taviani, M., Angeletti, L., Cardone, F., Montagna, P., and Danovaro, R. (2019). A unique and threatened deep water coral-bivalve biotope new to the Mediterranean Sea offshore the Naples megalopolis. Sci. Rep. 9:3411. doi: 10.1038/s41598-019-39655-8
Tusa, S. (2020). “Archaeological finds as true evidence of the Egadi battle,” in The Site of the Battle of the Aegates Islands at the end of the First Punic War, Vol. 60, eds J. Royal and S. Tusa (Roma: “L’Erma” di Bretschneider), 17–22.
Tusa, S., and Royal, J. (2012). The landscape of the naval battle at the Egadi Islands (241 B.C.). J. Rom. Archaeol. 25, 7–48. doi: 10.1017/S1047759400001124
Webb, C. M. (1986). Post-larval development of the tellinacean bivalves Abra alba, Tellina fabula and Donax vittatus (Mollusca: Bivalvia), with reference to the Late Larva. J. Mar. Biolog. Assoc. U.K. 66, 749–762. doi: 10.1017/S0025315400042338
Keywords: underwater cultural heritage, historical shipwrecks, submerged archeological artifacts, benthic community, marine biodiversity, Aegates archipelago, Mediterranean Sea
Citation: Gravina MF, Casoli E, Donnarumma L, Giampaoletti J, Antonelli F, Sacco Perasso C and Ricci S (2021) First Report on the Benthic Invertebrate Community Associated With a Bronze Naval Ram From the First Punic War: A Proxy of Marine Biodiversity. Front. Mar. Sci. 8:772499. doi: 10.3389/fmars.2021.772499
Received: 08 September 2021; Accepted: 15 November 2021;
Published: 10 December 2021.
Edited by:
Paul Snelgrove, Memorial University of Newfoundland, CanadaReviewed by:
Mauro Sinopoli, Stazione Zoologica Anton Dohrn Napoli, ItalyAlexander Tzetlin, Lomonosov Moscow State University, Russia
Kirstin Meyer-Kaiser, Woods Hole Oceanographic Institution, United States
Copyright © 2021 Gravina, Casoli, Donnarumma, Giampaoletti, Antonelli, Sacco Perasso and Ricci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Flavia Gravina, bWFyaWEuZmxhdmlhLmdyYXZpbmFAdW5pcm1hMi5pdA==
 Maria Flavia Gravina
Maria Flavia Gravina Edoardo Casoli
Edoardo Casoli Luigia Donnarumma2,4
Luigia Donnarumma2,4 Federica Antonelli
Federica Antonelli