- 1The Marine Science Institute, University of the Philippines Diliman, Quezon City, Philippines
- 2Department of Biology and Environmental Science, College of Science, University of the Philippines Cebu, Cebu City, Philippines
Octocorals are relatively understudied than other coral reef organisms despite their ecological and economic values. The Philippines is known to have high marine biodiversity, but information on octocorals is lacking. This study investigated spatial and temporal variations in the assemblage of octocorals in selected reef sites in the West Philippine Sea (WPS)- the Kalayaan Island Group (i.e., Pag-asa, Sabina, Lawak, and Northeast Investigator) and Ulugan in 2017 and 2019. Results showed high octocoral taxonomic richness (at least 10 families) in the study sites. Mean percent octocoral cover in WPS was 5.35% SE ± 0.55, with Sabina having the highest octocoral cover in both years. Significant differences in octocoral cover were observed among sites in both years, but among-station differences were only observed in 2017. Octocoral assemblage also differed among sites in both years (ANOSIM: R > 0.5, p < 0.05), wherein different octocoral taxa dominated in different sites. In particular, variations were driven by high cover of holaxonians, nephtheids, and coelogorgiids in Sabina, and clavulariids, tubiporiids, and xeniids in Northeast Investigator in 2017. In 2019, significant variations were driven by high cover of helioporiids in Pag-asa, while Sabina had higher abundance of holaxonians, nephtheids, alcyoniids, and xeniids. Short-term temporal variation on octocoral cover in monitoring stations in Pag-asa was not observed (Kruskal-Wallis, p > 0.05), although the overall mean octocoral cover increased from 1.23% ± SE 0.47 in 2017 to 2.09% SE ± 0.37 in 2019. Further, there was no significant change in the octocoral assemblage in Pag-asa between years (ANOSIM, R = 0.11, p = 0.07). This study highlights high octocoral taxonomic richness in the WPS relative to other sites in the Indo-Pacific Region and provides baseline information on the octocoral assemblages, which can be useful for future ecological studies and marine biodiversity conservation efforts.
Introduction
Octocorals are benthic marine organisms characterized by the presence of polyps bearing eight pinnate tentacles and have evolved a wide range of biomineralization strategies, including the production of skeletal structures composed of different calcium carbonate polymorphs (Fabricius and Alderslade, 2001; Aharonovich and Benayahu, 2012; Conci et al., 2021). Octocorals belong to the subclass Octocorallia, which comprises orders Helioporacea (blue corals), Pennatulacea (sea pens), and Alcyonacea (soft corals and gorgonians) that represents one of the most diverse macrobenthic group in tropical and subtropical shallow coral reefs and deep-sea environments (Lau et al., 2020). In addition, Octocorallia represents 64% of all extant coral species in the world (Williams and Cairns, 2018), providing a wide array of ecological benefits including coral reef development and structural complexity (Jones et al., 1994; Edmunds et al., 2016; Shoham et al., 2019), nutrition (Etnoyer et al., 2016; Garra et al., 2020), and refuge for reef organisms (Carvalho et al., 2014; Cúrdia et al., 2015; Dias et al., 2015; Epstein and Kingsford, 2019). As major macrobenthic components, octocorals play a significant role in coral reef community dynamics and may serve as indicators of water quality in reefs affected by various disturbances including climate change and pollution (Nörstrom et al., 2009; De’ath and Fabricius, 2010; Edmunds and Lasker, 2019). Apart from their ecological importance, octocorals are known to be valuable sources of natural products, which are utilized in the pharmaceutical industry (Zubair et al., 2018). In fact, aquaculture studies in soft corals are currently gaining attention due to their biomedical potential (Sella and Benayahu, 2010).
Studies on shallow water octocorals began in the Indo-Pacific (Red Sea) about 250 years ago (Benayahu et al., 2019). While octocoral distribution across the Red Sea, Great Barrier Reef (Australia), and Hong Kong has been widely documented, only limited octocoral taxonomic and distribution studies have been conducted in the Philippines, with majority from the early twentieth century (Lalas et al., 2021). Most recent octocoral studies in the archipelago have focused on the distribution of blue corals (e.g., Licuanan et al., 2019; Atrigenio et al., 2020) while information on other octocorals is limited (Lalas et al., 2021). Unlike scleractinians, octocoral assemblage dynamics have been overlooked and actual diversity and abundance analyses are often underestimated (Shoham and Benayahu, 2016; Benayahu et al., 2019; Lasker et al., 2020). This results in an overall lack of information relative to other taxa, such as scleractinians. Knowing overall biodiversity in an area can have great benefits on biogeography and conservation (Benayahu et al., 2004; Huang et al., 2014), especially in understudied regions, which are hotspots of biodiversity and of multiple disturbances.
The Philippines is located within the Coral Triangle, the epicenter of marine biodiversity (Sanciangco et al., 2013). One of the major biogeographic regions in the Philippines is the West Philippine Sea (WPS) (Aliño and Gomez, 1994) located in the South China Sea. This area accounts for approximately 30% of the total reef area in the Philippines. Considered to have the highest species richness and biomass of reef-associated fauna among the marine biogeographic regions of the Philippines, the WPS supports a great diversity of fish and invertebrate species including corals, gastropods, and bivalves (Huang et al., 2014).
Located within the WPS is the Kalayaan Island Group (KIG), a part of the Spratly Islands consisting of several islets, shoals, and reefs covering an area of 3,257.70 km2 (Asian Development Bank, 2014). Fisheries production and diversity in the WPS is relatively high, with the KIG potentially producing 3–5% of the total annual marine capture fisheries of the Philippines (Aliño et al., 1998; Arceo et al., 2020). Hypothesized to be a critical support system to reefs in nations bordering the South China Sea, KIG reefs serve as a potential source of pelagic propagules, replenishing populations of marine organisms such as fish and invertebrates (Aliño et al., 1998; Carpenter and Springer, 2005; Pata and Yñiguez, 2019). However, due to the geopolitical situation and remote distance of the KIG, conducting reef surveys and regular monitoring in the area is challenging (McManus, 1994; Aliño et al., 1998; Arceo et al., 2020). Most published studies in the KIG have focused on hard corals (i.e., Quibilan and Aliño, 2006; Quimpo et al., 2019). In fact, only the study of Dai and Fan (1996) on coral communities in Taiping Island has included a more detailed analysis of octocoral assemblages, leaving the ecological importance of these taxa underestimated (Epstein and Kingsford, 2019). This, and their major ecological and economic benefits on reefs, provide an impetus to conduct studies concerning this key taxon.
This study investigated the octocoral assemblage in shallow coral reefs in the West Philippine Sea (i.e., KIG: Pag-asa, Sabina, Lawak, and Northeast Investigator, and Ulugan). Through this study, (1) spatial differences among octocoral assemblages in this part of the WPS at different spatial scales were assessed and (2) changes in octocoral assemblage in selected stations in Pag-asa were evaluated through time. The results of this study will serve as baseline information on the octocoral assemblage of coral reefs in the Philippines and in the Indo-Pacific Region, which will highly benefit future coral reef monitoring and management efforts.
Materials and Methods
Study Area
Scientific expeditions to the West Philippine Sea were carried out in 2017 and 2019 to conduct octocoral assemblage surveys (Figure 1). In 2017, twelve stations in four sites located in the Kalayaan Island Group (KIG), which were Pag-asa (n = 7) (N 114.3 E 11.0), Sabina (n = 3) (N 116.4 E 9.8), Lawak (n = 1) (N 115.8 E 10.7), and Northeast Investigator (n = 1) (N 116.5 E 9.2) were surveyed. In 2019, surveys were conducted in Pag-asa (n = 5) and Sabina (n = 2), with the addition of stations in Ulugan (n = 3) on the western coast of mainland Palawan. In Pag-asa, four stations were established as monitoring stations in 2017 and re-surveyed in 2019 to determine temporal changes in octocoral assemblages.

Figure 1. Study sites and stations in the Kalayaan Island Group (Pag-asa, Sabina, Lawak and Northeast Investigator) and Ulugan in the West Philippine Sea surveyed in 2017 and 2019. Insets show stations in Pag-asa and Ulugan.
Octocoral Assemblage Survey
Two permanent 50-m transect lines were laid and established at a constant depth of 8–10 m in each survey station. Octocoral assemblage surveys were carried out using the photoquadrat method wherein a photograph was taken at every meter of the transect line using a digital camera (Sony RX100) with an underwater housing (Ikelite for Sony RX100) attached to a tetrapod covering a surface area of 1 m2. The photos were analyzed using the Coral Point Count with Excel extensions (CPCe) program (Kohler and Gill, 2006). Through the area analysis feature of the CPCe, the circumference of each octocoral colony was traced to obtain the total area (cm2) covered per transect. Percentage octocoral cover of each taxonomic group per transect was then computed.
Each octocoral colony was identified using coral taxonomic amalgamation units (TAUs) since it was not possible to differentiate the colonies to higher taxonomic resolutions. Coral TAUs are used to represent different closely related species that are hard to differentiate (Zvuloni et al., 2010). Given the current general taxonomic uncertainties among octocorals even at the generic level (Lau et al., 2020), the use of TAUs was necessary. The TAUs used in this study were generated based on the initial identification of inventories of octocoral specimens, which were systematically collected in the study stations in 2019. Soft coral TAUs used were mostly families, while gorgonians, which were colonies with obvious presence of axial skeleton, were identified as members of the suborder Holaxonia. Due to the difficulty in identifying these colonies to higher taxonomic resolutions basing on the photoquadrats, a Holaxonia TAU was created to represent colonies with such morphologies.
Data Analyses
A nested analysis was carried out to compare the mean octocoral cover (dependent variable) at different spatial scales (independent variable): among sites (250–500 km), among-stations (1–500 km), and within sites (1–10 km) in 2017 and 2019 (Figure 1). Each site was represented by the mean of the values representing the stations within the site, and a station was represented by the mean of the values representing each transect within the station. In 2017, Lawak and Northeast Investigator were not included in the among-sites and within-site analysis due to lack of replicate stations. Thus, the two sites, each represented by one station, were only compared at the among-stations analysis. Assumptions for normality and homogeneity of variance were tested using Shapiro-Wilk’s test and Levene’s test, respectively. One-way analysis of variance (ANOVA) was then used to compare variations in percent octocoral cover at different spatial scales. For datasets that were not normal or non-homogenous, the non-parametric Kruskal-Wallis test was performed. The Tukey’s HSD post hoc (parametric) test and the Mann-Whitney pairwise comparisons (non-parametric) were then used to determine which pairs of stations or sites were significantly different. All tests were performed using the Paleontological Statistics v3 software (PAST) (Hammer et al., 2001).
Differences of the octocoral assemblage at different spatial scales were determined through the analysis of similarity (ANOSIM) using the Bray-Curtis similarity index. However, at the among-sites analysis, Lawak and Northeast Investigator were only represented by percentage cover per TAU from one station, unlike the rest of the sites, which were represented by the means of cover per TAU of multiple stations. The lack of station replicates for Lawak and Northeast Investigator prevented us from doing a within-site analysis for the two sites. A pairwise ANOSIM, using Bonferroni-corrected values, was then carried out for spatial scales (i.e., among sites, within site, among stations) with significant overall differences. Prior to the analyses, percentage cover datasets were square root-transformed. The similarity of percentages (SIMPER) analysis was then carried out to determine which taxonomic groups contributed to the differences and similarities in the octocoral assemblage among sites. However, in the SIMPER analysis, Lawak and Northeast Investigator were represented by one station only, unlike the other sites that were represented by multiple stations. To visualize the similarities and differences among stations, a non-metric multidimensional scaling (nMDS) and cluster analysis was done. The ANOSIM, SIMPER analysis, and nMDS were performed using PRIMER-E v6.0 statistical software (Clarke and Gorley, 2006). Short-term temporal changes in the octocoral cover and assemblage in Pag-asa were also determined. The Kruskal-Wallis test was used to determine differences in the mean octocoral cover of four monitoring stations in 2017 and 2019. ANOSIM was then used to determine changes in octocoral assemblage in Pag-asa between years.
Results
A total of 5 sites, 18 stations, 44 transects, and 2,200 photoquadrats were analyzed in this study. Overall, the mean percent octocoral cover in the West Philippine Sea was 5.35% SE ± 0.55 with a total of 10 taxonomic groups observed: Alcyoniidae, Briareidae, Clavulariidae, Coelogorgiidae, Nephtheidae, Helioporiidae, Nidaliidae, Tubiporiidae, Xeniidae, and Holaxonians. To compare values with recent studies that did not pool Helioporiidae cover with other octocorals (e.g., soft corals and gorgonians) (e.g., Licuanan et al., 2019), an overall average value was also computed without helioporiids, which resulted to an overall mean percent cover of 4.74% SE ± 0.18.
Octocoral Cover
Among the sites surveyed in 2017, Sabina had the highest mean octocoral cover (8.43% SE ± 1.61), followed by Northeast Investigator (8.2% SE ± 2.18), Lawak (2.18% SE ± 0.89) and Pag-asa (1.12% SE ± 0.27) (Figure 2A). The analysis of variance revealed a significant difference between Pag-asa and Sabina (Kruskal-Wallis, p < 0.01). Overall significant difference of mean octocoral cover was also observed at the station level (Kruskal-Wallis, p = 0.03) (Figure 3A). However, there were no significant within-site differences in Pag-asa (Kruskal-Wallis, p = 0.07) and Sabina (Kruskal-Wallis, p = 0.28).
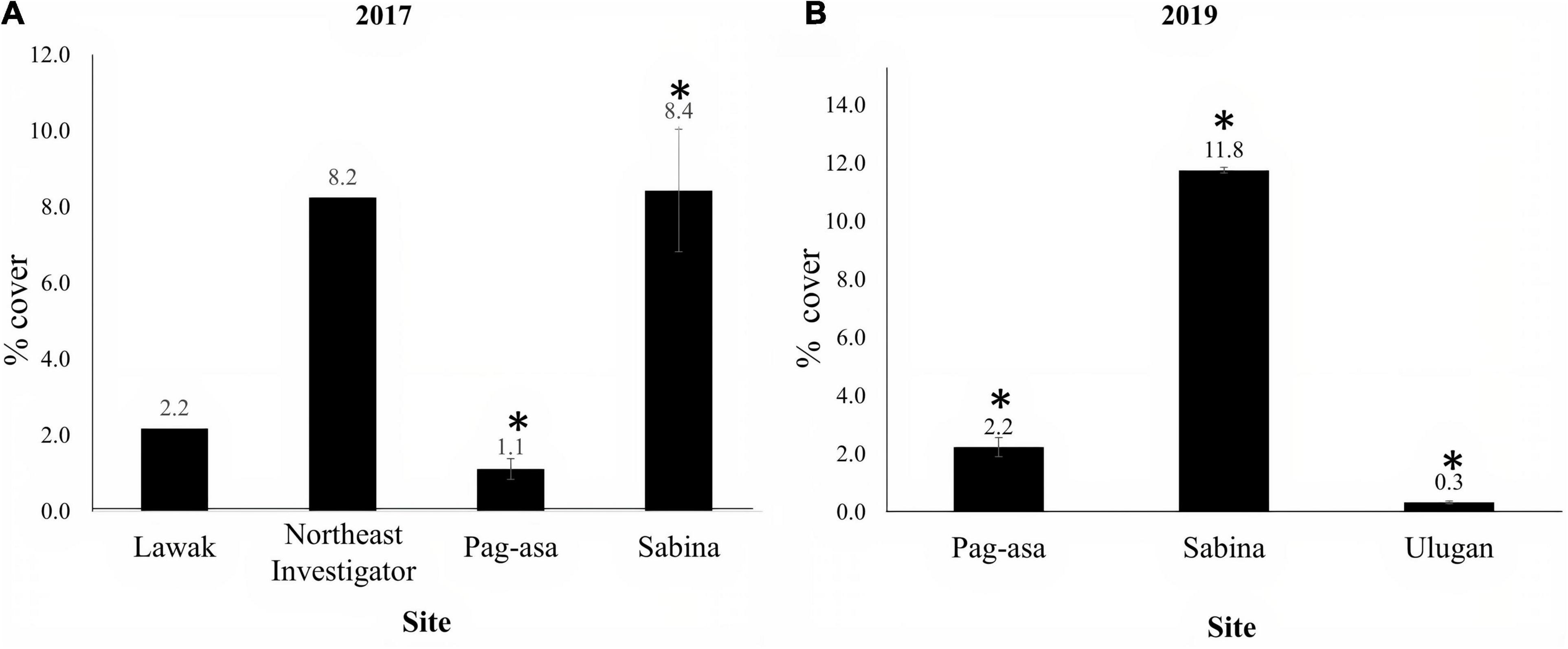
Figure 2. Octocoral cover (mean ± SE) of the sites in the Kalayaan Island Group (Pag-asa, Sabina, Lawak and Northeast Investigator) and Ulugan surveyed in (A) 2017 and (B) 2019. Asterisks (*) indicate significant difference (p-value < 0.05). Lawak and Northeast Investigator were not included in the among-sites analyses due to lack of replicates.
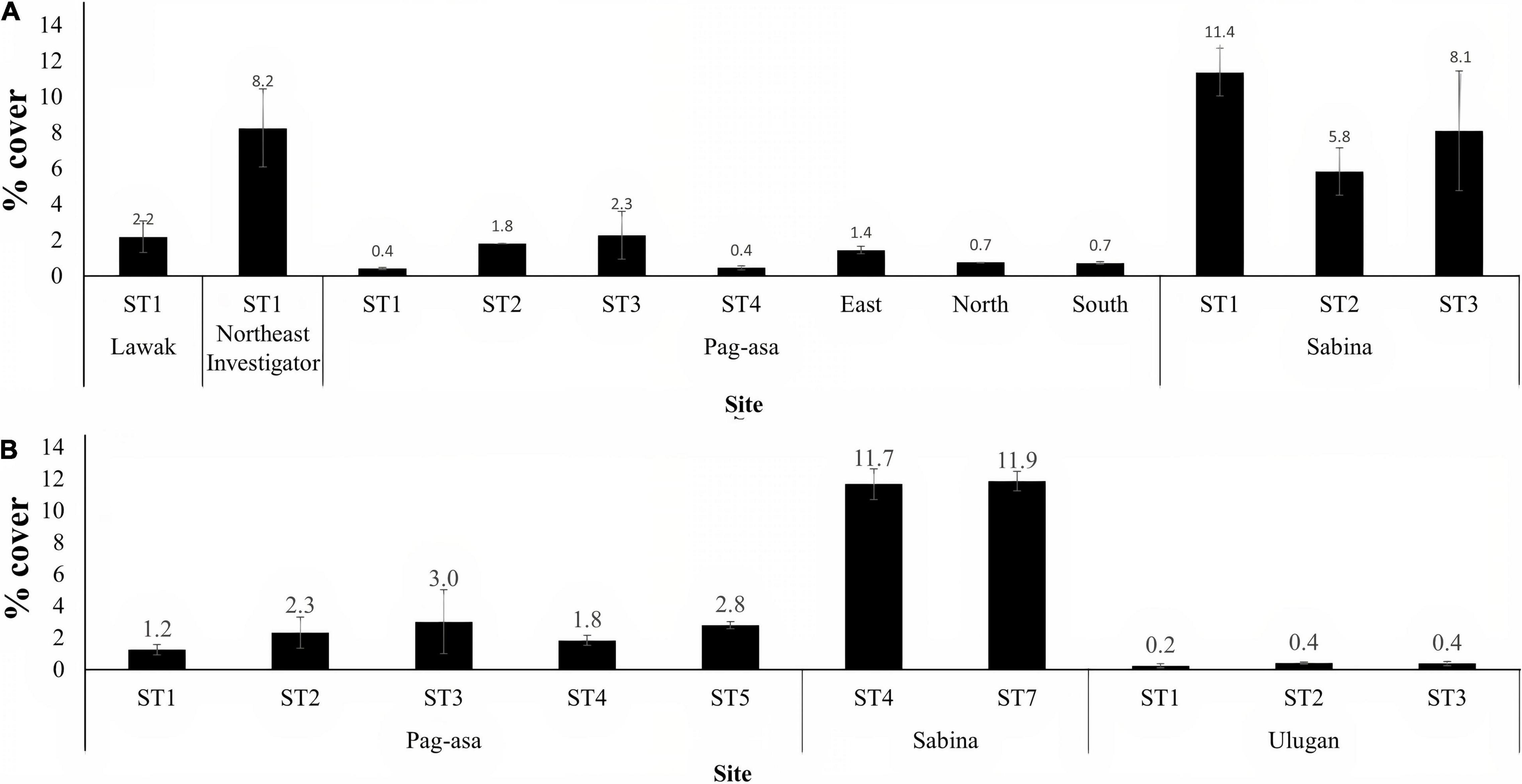
Figure 3. Octocoral cover (mean ± SE) of the stations in the Kalayaan Island Group (Pag-asa, Sabina, Lawak and Northeast Investigator) and Ulugan surveyed in (A) 2017 and (B) 2019. ST denotes station.
In 2019, Sabina still had the highest mean octocoral cover (11.76% SE ± 0.09) among the sites, while Ulugan, which was not surveyed in 2017, had the lowest (0.33% SE ± 0.05) (Figure 2B). Analysis of variance revealed significant differences among the three sites (ANOVA, p < 0.001). However, no significant difference was observed among the stations (Kruskal-Wallis, p = 0.05) and within the stations (Kruskal-Wallis, p > 0.05) (Figure 3B). Similar to the results in the 2017 surveys, this revealed greater among-site than within-site differences in terms of mean octocoral cover.
Octocoral Assemblage
Overall difference in the octocoral assemblage among the sites was observed in 2017 (ANOSIM: R = 0.57, p = 0.001) (Table 1). The pairwise comparisons between sites showed variations in the octocoral assemblage (ANOSIM: R > 0.50) except between Lawak and Pag-asa (ANOSIM: R = -0.14). Differences and similarities in the octocoral assemblage among the stations were also evident in the non-metric multidimensional scaling ordination (Figure 4A) and cluster analysis dendrogram (Figure 5A). Specifically, most stations from the same site grouped together while stations from different sites showed distinct separations in the nMDS and clusters analysis except for Lawak, which clustered together with stations in Pag-asa. The ANOSIM revealed overall significant difference (ANOSIM: R = 0.84, p = 0.001) among all the stations in terms of octocoral assemblage (Table 1). However, the stations within Pag-asa differed (ANOSIM: R = 0.67, p = 0.001), while stations within Sabina did not (ANOSIM: R = 0.83, p = 0.067). The similarity of percentages (SIMPER) analysis revealed that different octocoral taxa dominated the different survey sites (Table 2). In particular, alcyoniids dominated the octocoral assemblage in Pag-asa (Similarity contribution: 46.51%), Holaxonians in Sabina and Lawak (Similarity contribution: > 50.00%), and clavulariids in Northeast Investigator (Similarity contribution: 60.50%). Meanwhile, the dissimilarity percentages revealed which taxa contributed to the variations observed between sites (Table 3). Sabina mainly differed from Pag-asa and Lawak due to its higher holaxonian cover (Dissimilarity contribution: 43.93 and 36.64%, respectively). Northeast Investigator differed from the other sites due to higher presence of clavulariids [Dissimilarity contribution: Pag-asa (48.99%); Sabina (31.34%); Lawak (44.30%)]. Lastly, Lawak differed from Pag-asa due to higher abundance of Holaxonians (Dissimilarity contribution: 45.62%).
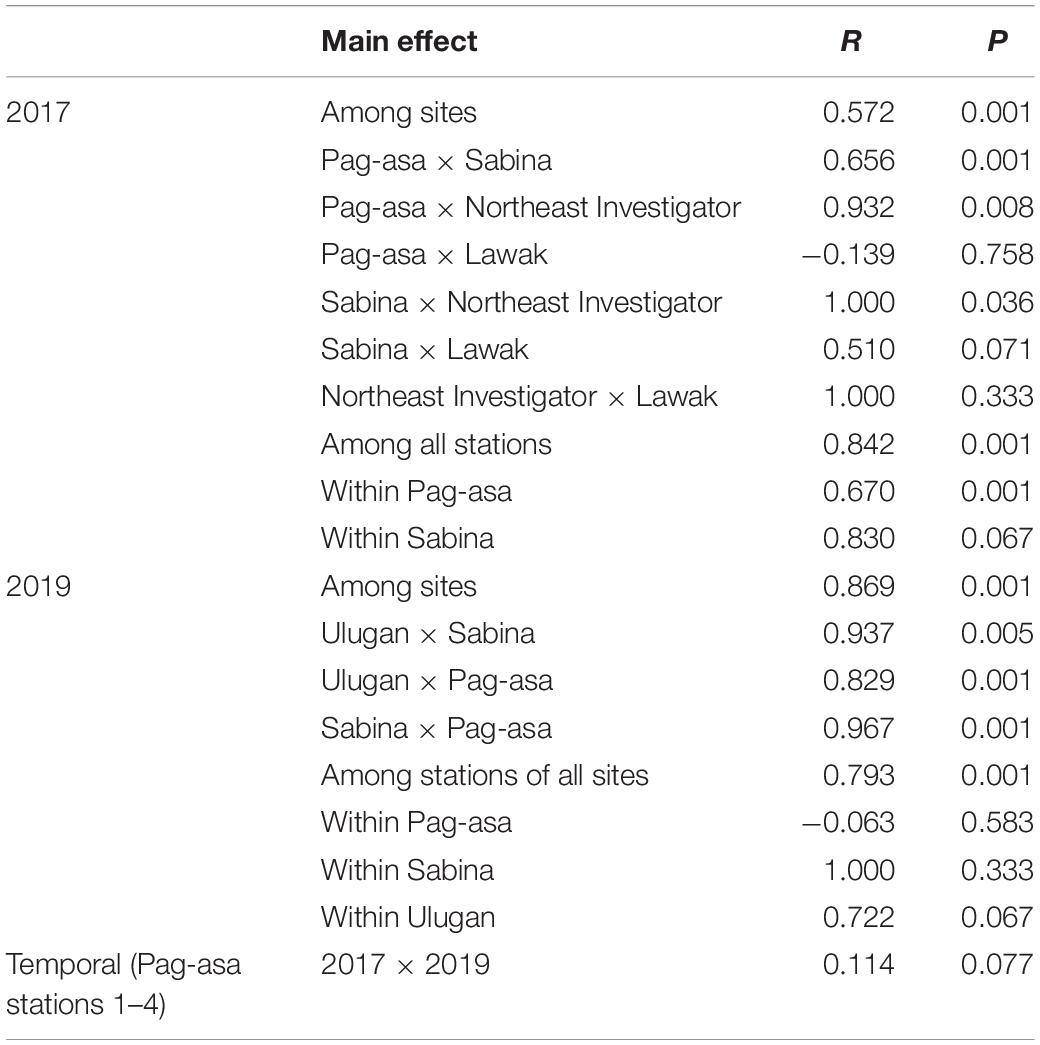
Table 1. Summary result of the ANOSIM comparing octocoral assemblages at different spatial scales (among sites, stations and within site) in the Kalayaan Island Group and Ulugan and short-term temporal variation in Pag-asa in 2017 and 2019.
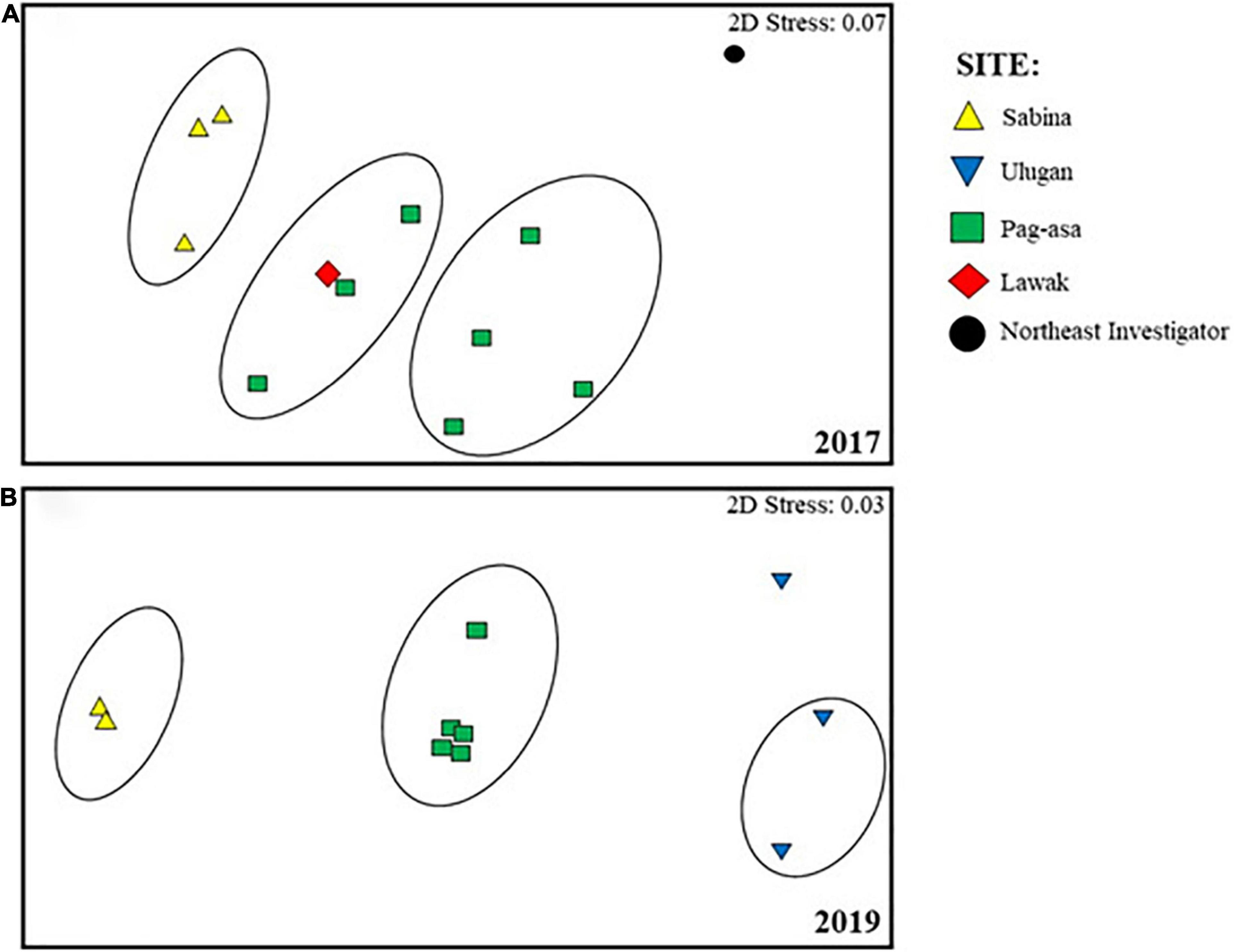
Figure 4. Non-multidimensional scaling ordination based on the Bray-Curtis similarity index, showing differences in the octocoral assemblages in different stations in Kalayaan Island Group (Pag-asa, Sabina, Lawak and Northeast Investigator) and Ulugan, surveyed in (A) 2017 and (B) 2019. Eclipses indicate stations with more than 40% similarity.
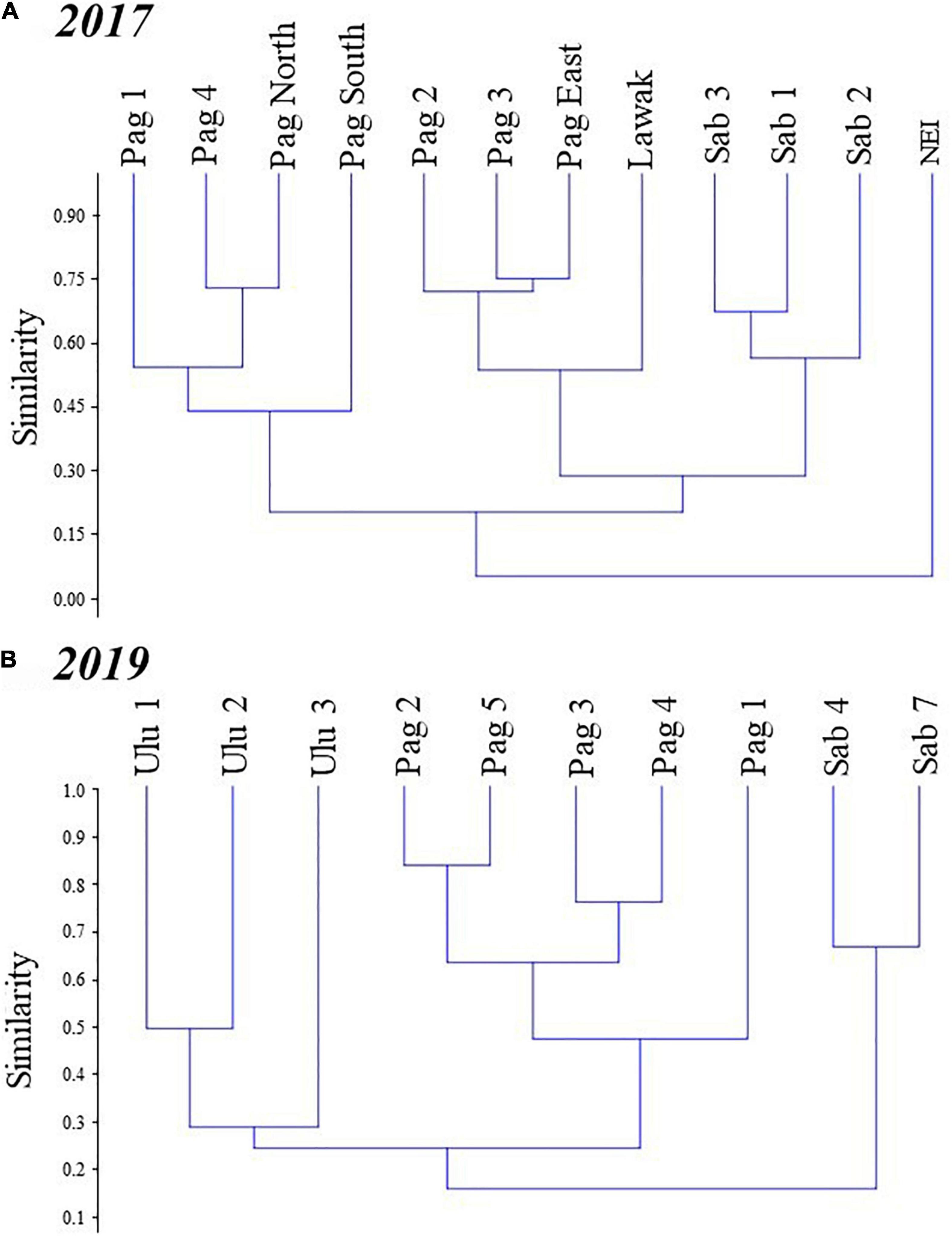
Figure 5. Cluster analysis dendrogram based on the Bray-Curtis similarity index, showing differences in the octocoral assemblages in different stations in the Kalayaan Island Group [Pag-asa (Pag), Sabina (Sab), Lawak, and Northeast Investigator (NEI)] and Ulugan (Ulu), surveyed in (A) 2017 and (B) 2019.
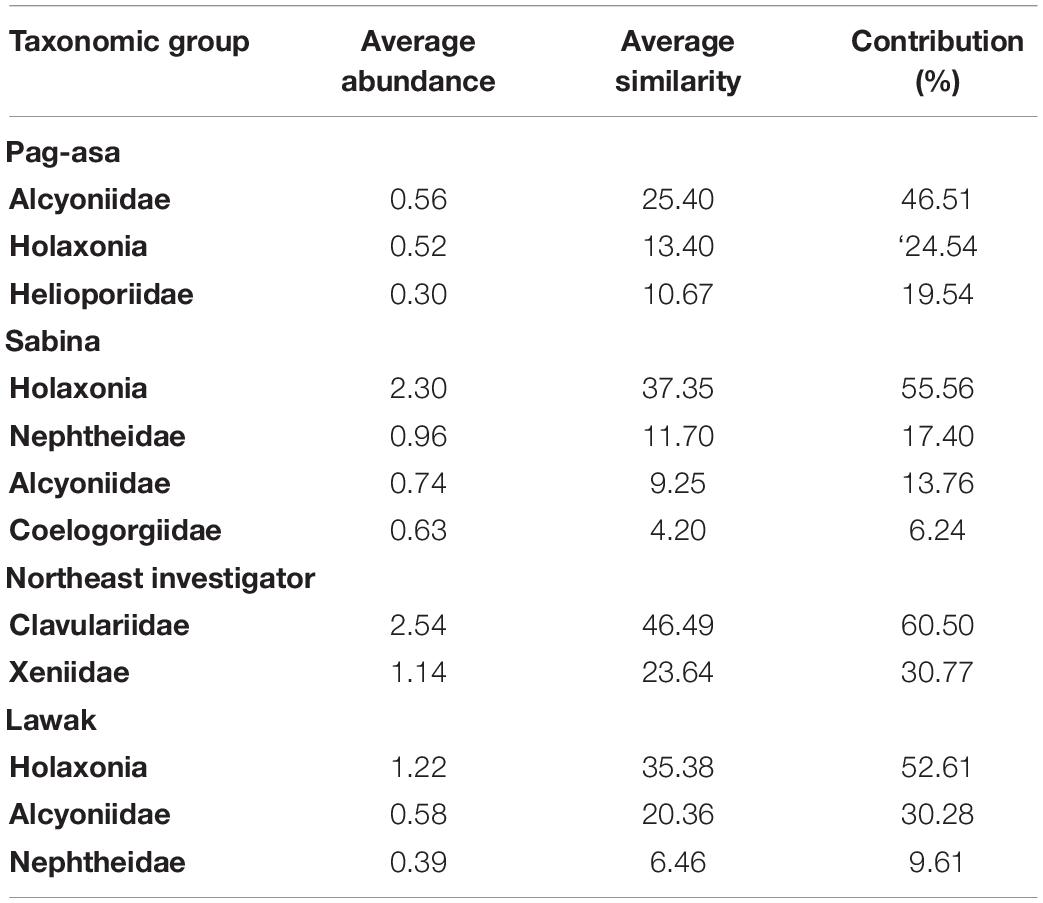
Table 2. Summary result of the Similarity of Percentages (SIMPER) analysis among the sites in the Kalayaan Island Group in 2017.
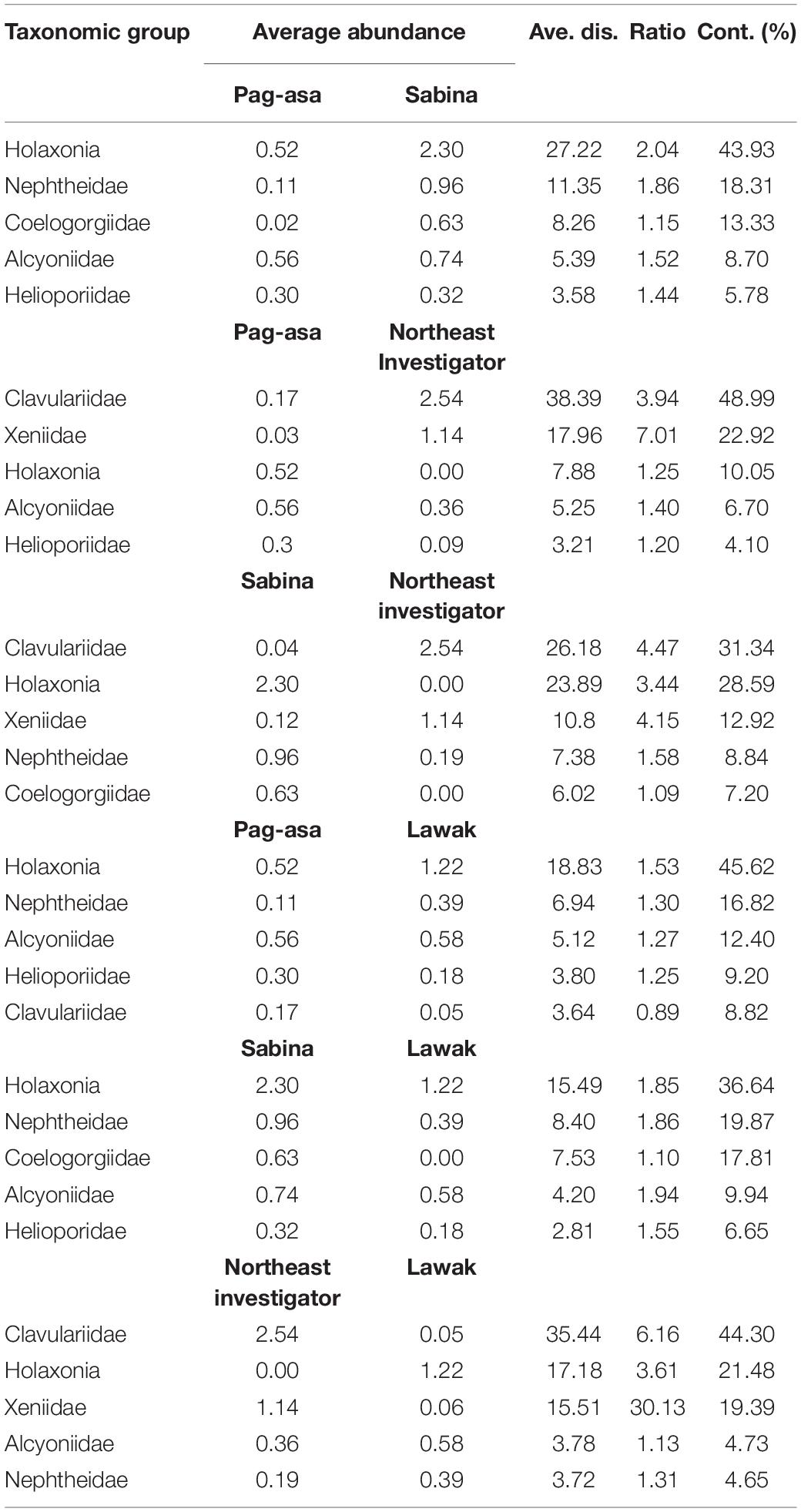
Table 3. Dissimilarity percentages among sites from the SIMPER analysis among sites in the Kalayaan Island Group in 2017.
Significant overall among-site differences in the octocoral assemblage were also observed in 2019 (ANOSIM: R = 0.87, p = 0.001) (Table 1). Pairwise comparisons showed variations in the octocoral assemblage in all pairings (ANOSIM: R > 0.80). Among stations, overall significant differences were also observed (ANOSIM: R = 0.79, p = 0.001). Comparisons of the stations within each site showed no significant difference (ANOSIM: p > 0.05). The nMDS ordination (Figure 4B) and cluster analysis dendrogram (Figure 5B) also showed distinct clustering and separation of the different stations of the three sites surveyed in 2019. The SIMPER analysis revealed similar dominant octocoral taxa (i.e., holaxonians and alcyoniids) in the stations surveyed in Sabina in 2017 (Table 2) and 2019 (Table 4). Similar to results in 2017, holaxonians (Similarity contribution: 33.09%), alcyoniids (Similarity contribution: 29.73%), and helioporiids (Similarity contribution: 27.98%) also dominated the assemblage in Pag-asa in 2019. Ulugan, which was only surveyed in 2019, was dominated by holaxonians (Similarity contribution: 43.07%) and alcyoniids (Similarity contribution: 24.81%). Sabina differed from the other sites due to higher abundance of nephtheids and holaxonians (dissimilarity contribution:>20.00%), whereas Pag-asa differed from Ulugan due to higher abundance of helioporiids (dissimilarity contribution: 26.85%) (Table 5).
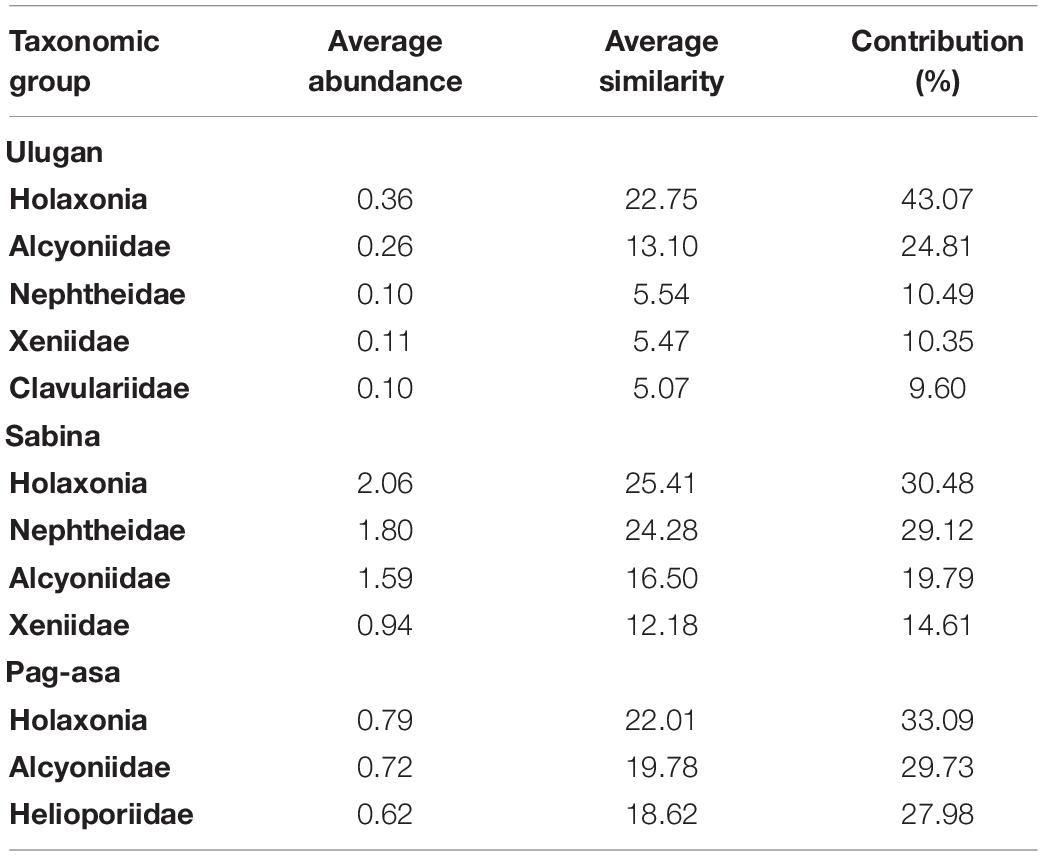
Table 4. Summary result of the Similarity of Percentages (SIMPER) analysis among sites in the Kalayaan Island Group and Ulugan in 2019.
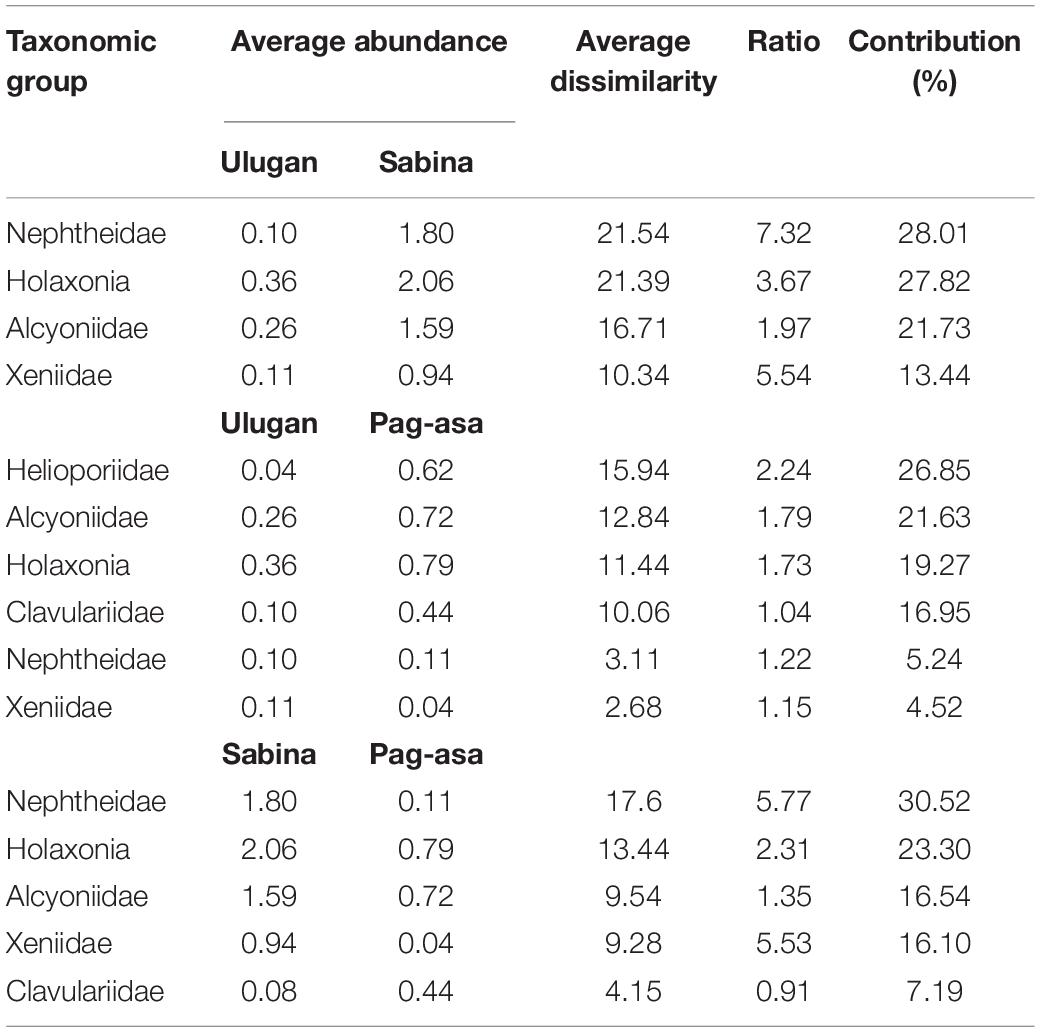
Table 5. Dissimilarity percentages among sites from the SIMPER analysis among sites in the Kalayaan Island Group and Ulugan in 2019.
Temporal Patterns of Octocoral Assemblage in Pag-asa
Mean octocoral cover of all monitoring stations in Pag-asa increased in 2019 (Figure 6). This resulted to an increase in the overall mean octocoral cover in Pag-asa from 1.23% SE ± 0.47 in 2017 to 2.09% SE ± 0.37 in 2019, although the difference was not significant (Kruskal-Wallis, p > 0.05). The ANOSIM revealed no significant change in the octocoral assemblage in Pag-asa between years (ANOSIM, R = 0.11, p = 0.07).
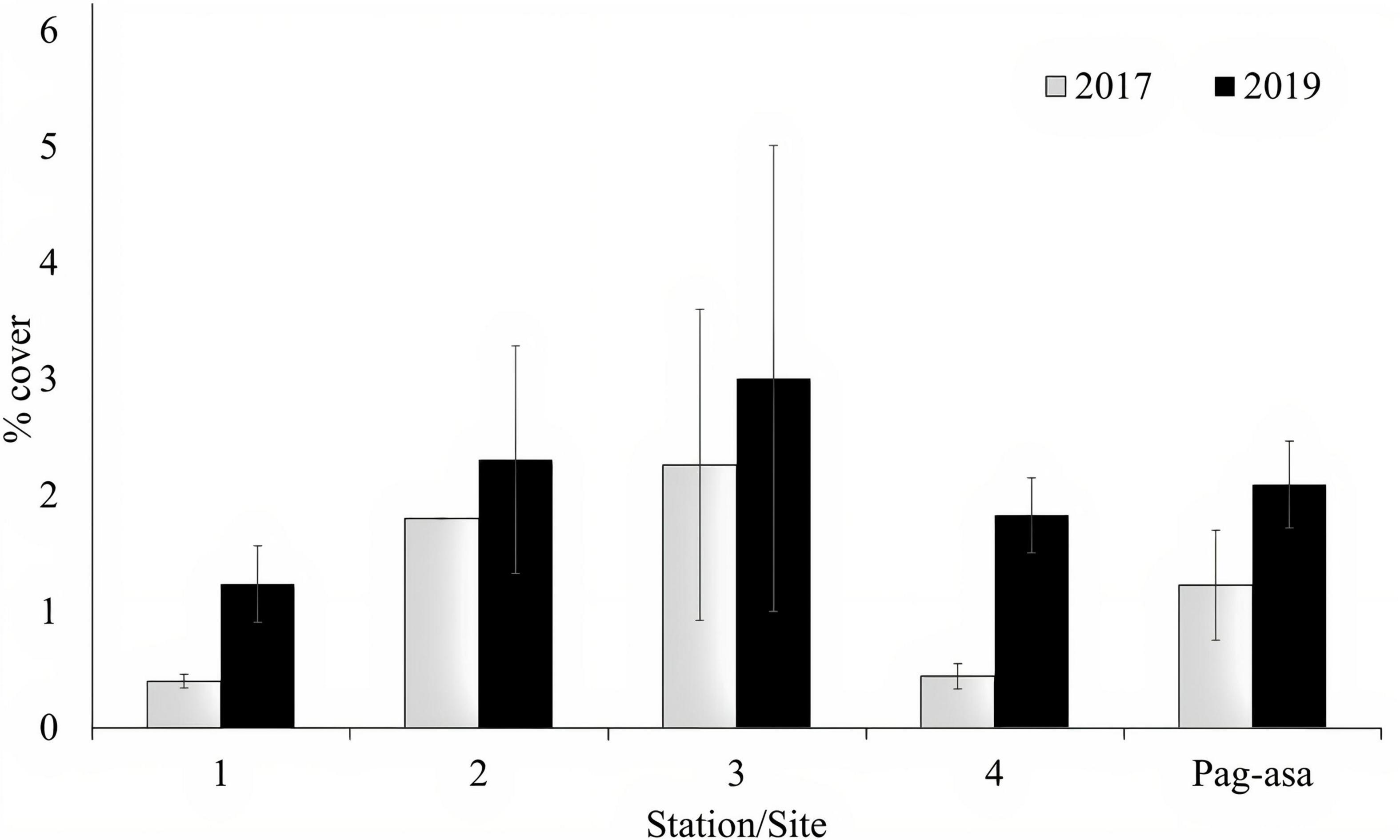
Figure 6. Octocoral cover (mean ± SE) of the sampling stations in Pag-asa (n = 4) monitored in 2017 and 2019. The last two bars represent means of the means (mean ± SE) of the four monitoring stations in different years.
Discussion
This study is one of the few studies focused on the octocoral assemblage in reefs in the South China Sea (SCS) and one of the first in the Philippines. The overall mean octocoral cover of the area (5.35% with helioporiids, 4.74% without helioporiids) is slightly higher than the 3.2% overall octocoral cover (excluding helioporiids) of the coastal fringing reefs of northwestern Philippines (Lalas et al., 2021) and the national mean soft coral cover (3.2% excluding helioporiids) reported by Licuanan et al. (2019). However, it is generally lower compared with covers recorded from other regions, with some reaching up to 50% soft coral cover (e.g., Red Sea: Benayahu and Loya, 1977, 1981; Great Barrier Reef, Australia: Dinesesn, 1983; Papua New Guinea: Tursch and Tursch, 1982). Most of the studies in other regions that measured octocoral cover using the similar techniques as the present study, however, were conducted decades ago. Revisiting these areas will benefit comparisons at a global scale.
Several factors may influence the living coverage of octocorals in a reef. In this study, reefs located near coastal communities (i.e., Pag-asa, Ulugan) and inside an embayment, which had limited water movement (i.e., Ulugan), tended to have lower octocoral cover compared with offshore reefs that had relatively high wave exposure (i.e., Sabina, Lawak, Northeast Investigator). This might be attributed to poor water quality due to anthropogenic disturbances, which is also consistent with findings of studies in other regions. Remotely sensed data show that among the sites, Ulugan has the higher chlorophyll a content and the lowest Secchi depth (Supplementary Table 1), both of which are proxies for water quality (Hughes et al., 2017). Low water quality in Ulugan can be explained by its proximity to a river mouth (Supplementary Table 1). Yeung et al. (2014) reported that limited water movement and poor water quality due to anthropogenic disturbances contributed to low abundance of octocorals in Hong Kong. This pattern in octocoral cover is similar to earlier studies in other regions (e.g., Dinesesn, 1983; Great Barrier Reef, Australia) where it was reported that increased octocoral cover in offshore more than nearshore reefs was likely due to clearer waters and stronger currents.
The results also highlight the variable spatial patterns in octocoral coverage and assemblage in the SCS. Significant variation observed in the octocoral assemblage among sites but not within sites may indicate higher variation in the environmental conditions at the site scale than at the station scale. This is in contrast with earlier observations in northwestern Philippines wherein variations in octocoral assemblage were observed within sites but not among sites (Lalas et al., 2021). This may indicate variabilities in different factors, both biological (e.g., interactions with other benthic organisms) and environmental (e.g., water quality, exposure monsoons). However, the lack of information on the different prevailing factors in this study limits the inferences on the causality of the observed variations. This warrants the need to include environmental data collection during reef surveys to elucidate octocoral-environment relationships.
Overall, octocoral assemblages in the study sites were dominated by holaxonians. Initial identification of collected samples, through analysis of morphological characters, indicate the presence of colonies belonging to the genera Isis and Pinnigorgia (Supplementary Material), which are both zooxanthellate and are usually abundant in flow-exposed areas (Fabricius and Alderslade, 2001). Northeast Investigator was dominated by clavulariids and xeniids. In the study of Lalas et al. (2021), clavulariids were positively correlated with water clarity and wave exposure. The relatively higher dominance of xeniids may also be an indication of a current-exposed and well-lit environment, similar to what was observed in inshore reefs of the central Great Barrier Reef (Dinesesn, 1983). Such inference may explain their abundance in our study sites, which are all exposed and non-inshore, except for Ulugan. Thus, the dominance of these taxa in the area may indicate generally high current conditions, which is a characteristic of offshore reefs.
The octocoral taxonomic composition in the study area is similar to observations in other areas in the SCS and in the Indo-Pacific. For example, all of the taxonomic groups reported here were also observed from Palau (Fabricius et al., 2007; McFadden et al., 2014) to as far as the Red Sea (Benayahu and Loya, 1977). The presence of these taxa was also reported in the Great Barrier Reef, Australia (Dinesesn, 1983). Many taxonomic groups reported in the present study were also reported in other areas such as in Southern Japan (Benayahu, 2002), Taiwan (Benayahu et al., 2004, 2012), and East Africa (Benayahu et al., 2003). Despite the taxonomic identification limitations of this study, information gained here may improve our knowledge on the biogeography of octocorals.
The taxonomic richness (at least 10 families) is relatively higher in this study compared with other quantitative studies in other regions (3–5 families) that assessed octocoral assemblages. In Thailand waters, only 4 families were reported by Chanmethakul et al. (2010) despite the larger study area covered. In the southern islands of Singapore, Goh et al. (2009) and Seah et al. (2015) reported genera from a total of 3 families only (i.e., Alcyoniidae, Nephtheidae, and Clavulariidae). In Hong Kong, which is generally characterized by turbid waters, only 5 of the 9 soft coral families observed in the present study were documented (Yeung et al., 2014). All taxonomic groups observed in the present study were also observed in northwestern Philippines (Lalas et al., 2021). This suggests that octocoral diversity may be higher in Philippine waters compared with other areas surrounding the South China Sea. It is also likely that patterns in octocoral diversity in the region is similar to patterns in scleractinian diversity, where species richness tends to be higher in the western Philippines (Huang et al., 2014). In an earlier study in adjacent reefs in the Spratly Islands, Dai and Fan (1996) reported only three families of soft corals that were limited to depths below 15 m. Dai and Fan (1996) also reported four gorgonian families. However, gorgonians in the present study were pooled into one taxonomic unit, thus, preventing comparisons of gorgonian taxonomic richness to other studies. It should be noted that the present study is not directly comparable with studies that generated taxonomic inventories of octocoral species (e.g., Benayahu et al., 2012; McFadden et al., 2014). Thus, identifying octocoral species at the highest possible taxonomic identification (i.e., genus, species) merits attention and is expected to provide better understanding of the diversity and assemblage of octocorals in the Philippines and in the Indo-Pacific.
In the context of the Coral Triangle, information on octocorals is still very poor (Pérez et al., 2016). Studies on octocorals in Indonesia (e.g., Janes, 2013; McFadden et al., 2014; Rowley, 2018), Malaysia (e.g., Mohammad et al., 2016), and Papua New Guinea (e.g., Tursch and Tursch, 1982; Mana, 2011) focused on establishing taxonomic inventories and information on the contribution of different taxa to benthic assemblages is lacking. In general, the lack of data on the abundance of different octocoral taxa in coral reef studies is common among nations in the Coral Triangle (e.g., Asian Development Bank, 2014). Janes (2013) highlighted that the Philippines has the highest diversity of xeniids, which may indicate high octocoral diversity. The lack of a finer taxonomic resolution used in this study limits comparisons in terms of diversity. However, taxonomic richness observed in this study and a study by Lalas et al. (2021) in the northwestern Philippines (using similar methodologies and taxonomic resolutions) was higher compared to other areas in the Coral Triangle (e.g., Verseveldt and Tursch, 1979; Tursch and Tursch, 1982). This may indicate high octocoral diversity in the Philippines. However, the very limited number of areas studied and different methods employed make it difficult to identify spatial patterns in diversity within the Coral Triangle. This highlights the need to incorporate taxonomic inventories with abundance surveys to improve our understanding on coral reef communities in the Coral Triangle.
Increasing trend in the mean octocoral cover was observed in Pag-asa from 2017 to 2019, suggesting possible shifts in the coral community structure due to changing environmental conditions (Nörstrom et al., 2009). In the Caribbean, increase in gorgonian abundance in the last decade has been associated with increasing sea surface temperature (Edmunds and Lasker, 2019). In Indonesia, rapid dominance of soft corals has been associated with degradation caused by destructive fishing practices (Fox et al., 2003). Hence, an alternative state is hypothesized wherein octocorals dominate coral reefs after a disturbance (Nörstrom et al., 2009), although this may not always be the case. For example, soft corals did not dominate a reef in the Great Barrier Reef, which had available substrate space following a crown-of-thorns starfish outbreak (Fabricius, 1995). Such case is possible as responses of octocorals to disturbances may be taxon- or site- specific. In areas where octocorals dominated after a disturbance, octocoral assemblage would usually be composed of fast-growing species (e.g., xeniids, nephtheids) (Fox et al., 2003). This highlights the need for long-term monitoring to observe how octocorals can affect community structure of reefs in the future.
The Spratly Islands harbor ecologically and economically important reef systems to countries bordering the South China Sea (SCS) (McManus and Meñez, 1997; Ablan et al., 2002). Through simulated particle dispersal models, the islands have been inferred to be important sources of larvae that can replenish populations of different coral reef organisms in the SCS and in the Coral Triangle Region (Kool et al., 2011). However, the reefs of the SCS are generally threatened by habitat degradation caused by anthropogenic factors (Rosenberg, 1999; Vo et al., 2013) such as military activities (Song, 2008; Asner et al., 2017), reclamation (Larson, 2015; Mora et al., 2016), unsustainable fishing practices (McManus, 1994), and oil and gas exploration (Song, 2008).
Natural perturbations (e.g., monsoons, typhoons; Quibilan and Aliño, 2006) and climate change-driven disturbances such as coral bleaching (Li et al., 2011; Tkachenko et al., 2020) may also have an influence on the structuring of coral reef communities. Although monitoring of environmental parameters was not done, remotely sensed data show that Sabina has the highest exposure to positive thermal anomaly events that causes bleaching and mass morality (Supplementary Table 1). In the Carribean, increased SST and the occurrence of bleaching events have been attributed to cause the decline of hard corals while gorgonian abundance increased (Edmunds and Lasker, 2019). Arceo et al. (2001) conducted one of the earliest comprehensive records of mass coral bleaching events in the Philippines and found that there were no significant changes in the soft coral cover in the Spratly Islands post bleaching. Although, it was emphasized that their report was not designed to detect small changes in the soft coral assemblages. Besides the 1998 bleaching event, coral reefs in the Philippines experienced sustained heat stress that led to mass bleaching in 2010, and 2016–2017 (Tun et al., 2010; Hughes et al., 2018; Licuanan et al., 2019). Unfortunately, due to geopolitical tensions and logistical constraints, studying the coral reef dynamics in the Spratly Islands has remained a challenge. This has resulted to patchy and sparse information on the area that limits our understanding on the changes or shifts of the octocoral assemblage brought about by climate change.
The ecological roles of octocorals remain greatly understudied. In areas where scleractinian corals do not dominate, such as in deep reefs, gorgonians may contribute to structural complexity providing potential habitats for other marine organisms (Slattery and Gochfeld, 2016). Conversely, increase in soft coral cover has been associated with degraded reefs (see Epstein and Kingsford, 2019). Soft corals are also considered non-reef builders, but some species, mostly from the genus Sinularia, are known to contribute to reef development through deposition of their sclerites (Jeng et al., 2011). Increased octocoral dominance has also been linked with by the decrease in diversity or biomass of associated organisms such as fishes and arthropods, but more recent studies report otherwise. For example, Syms and Jones (2001) reported lower coral reef fish biomass among soft coral-dominated areas compared to hard coral-dominated areas, but Epstein and Kingsford (2019) reported similar fish assemblages between hard coral- and soft coral-dominated reefs and an increase in fish species richness with increasing soft coral cover. Epstein and Kingsford (2019) suggested that the importance of soft corals as a habitat may have been underestimated. The contrasting results of different studies in different areas highlight the need to conduct more investigations related to the ecological roles of alternative state organisms.
This study has demonstrated the spatial patterns in the octocoral assemblage of an ecologically and economically important area in the South China Sea. Although factors that contributed to the variations were not identified, results of this study serve as baseline information on the contribution of octocorals in coral reefs. With the generally lacking information on octocoral assemblages in different marine regions, we recommend the inclusion of octocoral variables in the long-term monitoring of coral reef community structures, complemented with measurements of important environmental variables. This can further improve our knowledge on the mechanisms that affect coral reef community structure in the area, which will provide effective frameworks for the management of these ecologically and economically important habitats.
Data Availability Statement
Data inquiries can be directed to the corresponding author.
Ethics Statement
The research expeditions in the West Philippine Sea were conducted with the approval of various national government departments and agencies in the Philippines thru Gratuitous Permits #2017-09 and #2019-09 issued by the Palawan Council for Sustainable Development.
Author Contributions
MVBR and HOA secured the funding, designed the study, and provided supervision. JAAL, RTSL, JPC, CSS, RMAL, and DAMV conducted the fieldworks and analyzed the photoquadrats. JAAL, DAMV, and MRJ performed the data analysis. All authors contributed to the writing of this article.
Funding
This study was funded by the Biodiversity Management Bureau of the Department of Environment and Natural Resources (DENR-BMB) of the Philippines through the (1) Coastal Assessment for Rehabilitation Enhancement: Capability Development and Resiliency of Ecosystems (CARE-CaDRES) project and the (2) Predicting Responses between Ocean Transport and Ecological Connectivity of Threatened Ecosystems in the West Philippines Sea (PROTECT WPS) project, which were implemented by the Marine Science Institute of the University of the Philippines (UP MSI).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the Department of Agriculture-Bureau of Fisheries and Aquatic Resources (DA-BFAR), Philippine Coast Guard (PCG), the Municipality of Kalayaan, and the Philippine Navy for their assistance and support during the research expeditions in the West Philippine Sea. We also thank Lovely Joy Heyres for assisting in the field collection and image analysis, and Kevin Yatco and Socorro Rodrigo for assisting in the satellite product processing. We also thank the valuable insights and suggestions given by the editor and reviewers of this journal that helped improve this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.782977/full#supplementary-material
References
Ablan, M. C. A., McManus, J. W., Chen, C. A., Shao, K. T., Bell, J., Cabanban, A. S., et al. (2002). Meso-scale transboundary units for the management of coral reefs in the South China Sea. Naga World Fish Center Q. 25, 3–4.
Aharonovich, D., and Benayahu, B. (2012). Microstructures of octocoral sclerites for diagnosis. Mar. Biodivers. 42, 173–177. doi: 10.1038/s41598-018-37696-z
Aliño, P. M., and Gomez, E. D. (1994). “Philippine coral reef conservation: its significance to the South China Sea,” in Proceedings of Regional Conference of the Development and Conservation in the Asia-Pacific Region, eds K. Yamamoto, S. Ishijima, S. Sakihara, H. Taira, Z. Shimabukuro, F. Teruya, et al. (Honolulu, HI: East West Center Association), 222–229.
Aliño, P. M., Nañola, C. L. Jr., Ochavillo, D. G., and Rañola, M. C. (1998). “The fisheries potential of the Kalayaan Island group, South China Sea,” in Proceedings of the International Conference on the Marine Biology of the South China Sea; 1996 Oct 28 – Nov 1, (Hong Kong: Hong Kong University Press), 219–226.
Arceo, H. O., Cabasan, J. P., Luciano, R. M. A., Heyres, L. J. D., Mamauag, S. S., and Aliño, P. M. (2020). Estimating the potential fisheries production of three offshore reefs in the West Philippine Sea, Philippines. Philipp. J. Sci. 149, 647–658.
Arceo, H. O., Quibilan, M. C., Aliño, P. M., Lim, G., and Licuanan, W. Y. (2001). Coral bleaching in Philippine reefs: coincident evidences with mesoscale thermal anomalies. Bull. Mar. Sci. 69, 579–593.
Asian Development Bank (2014). State of the Coral Triangle: Philippines. Mandaluyong: Asian Development Bank.
Asner, G. P., Martin, R. E., and Mascaro, J. (2017). C66oral reef atoll assessment in the South China Sea using Planet Dove satellites. Remote Sens. Ecol. Conserv. 3, 57–65.
Atrigenio, M. P., Conaco, C., Guzman, C., Yap, H. T., and Aliño, P. M. (2020). Distribution and abundance of Heliopora coerulea (Cnidaria: Coenothecalia) and notes on its aggressive behavior against scleractinian corals: temperature mediated? Reg. Stud. Mar. Sci. 40:101502. doi: 10.1016/j.rsma.2020.101502
Benayahu, Y. (2002). Soft Corals (Octocorallia:Alcyonacea) of southern Ryuku Archipelago: the families Tubiporiidae, Clavulariidae, Alctoniidae, and Briareidae. Galaxae. JCRS 4, 11–32.
Benayahu, Y., and Loya, Y. (1977). Space partitioning by stony corals, soft corals, and benthic algae in the coral reefs of the northern Gulf of Eilat (Red Sea). Helgol~inder wiss. Meeresunters 30, 362–382.
Benayahu, Y., and Loya, Y. (1981). Competition for space among coral-reef sessile organisms at Eilat, Red Sea. Bull. Mar. Sci. 31, 514–522.
Benayahu, Y., Bridge, T. C., Colin, P. L., Liberman, R., McFadden, C. S., Pizarro, O., et al. (2019). “Octocorals of the Indo-Pacific,” in Mesophotic Coral Ecosystems, eds Y. Loya, K. Puglise, and T. Bridge (Cham: Springer), 709–728. doi: 10.11646/zootaxa.3617.1.1
Benayahu, Y., Jeng, M. S., Perkol-Finkel, S., and Dai, C. F. (2004). Soft coral (Octocorallia: Alcyonacea) from southern Taiwan. II. Species diversity and distributional patterns. Zool. Stud. 45, 548–560.
Benayahu, Y., Shlagman, A., and Schleyer, M. H. (2003). Corals of the south-west Indian Ocean: VI. The Alcyonacea (Octocorallia) of Mozambique, with a discussion on soft coral distribution on south equatorial east African reefs. Zool. Verh. Leiden 345, 49–57.
Benayahu, Y., van Ofwegen, L. P., Dai, C., Jeng, M., Soong, K., Shlagman, A., et al. (2012). Diversity, distribution, and molecular systematics of octocorals (Coelenterata: Anthozao) of the Penghu Archipelago, Taiwan. Zool. Stud. 51, 1529–1548.
Carpenter, K. E., and Springer, V. G. (2005). The center of the center of marine shore fish biodiversity: the Philippine Islands. Environ. Biol. Fishes 72, 467–480. doi: 10.1007/s10641-004-3154-4
Carvalho, S., Cúrdia, J., Pereira, F., Guerra-García, J. M., Santos, M. N., and Cunha, M. R. (2014). Biodiversity patterns of epifaunal assemblages associated with gorgonians Eunicella gazella and Leptogorgia lusitanica in response to host, space and time. J. Sea Res. 85, 37–47. doi: 10.1016/j.seares.2013.10.001
Chanmethakul, T., Chansang, H., and Watanasit, S. (2010). Soft coral (Cnidaria: Alcyonacea) distribution patterns in Thai waters. Zool. Stud. 49, 72–84.
Clarke, K. R., and Gorley, R. N. (2006). PRIMER v6: User Manual/Tutorial (Plymouth Routines in Multivariate Ecological Research). Plymouth: PRIMER-E.
Conci, N., Vargas, S., and Wörheide, G. (2021). The biology and evolution of calcite and aragonite mineralization in Octocorallia. Front. Ecol. Evol. 9:623774. doi: 10.3389/fevo.2021.623774
Cúrdia, J., Carvalho, S., Fabio, P., Guerra-Garcia, J. M., Santos, M. N., and Cunha, M. R. (2015). Diversity and abundance of invertebrate epifaunal assemblages with gorgonians are driven by colony attributes. Coral Reefs 34, 611–624. doi: 10.1007/s00338-015-1283-1
Dai, C., and Fan, T. (1996). Coral fauna of Taiping island (Itu Aba island) in the Spratly’s of the South China Sea. Atoll Res. Bull. 436, 1–21. doi: 10.1163/9789004323445_002
De’ath, G., and Fabricius, K. (2010). Water quality as a regional driver of coral biodiversity and macroalgae on the Great Barrier Reef. Ecol. Appl. 20, 840–850. doi: 10.1890/08-2023.1
Dias, I. M., Cúrdia, J., Cunha, M. R., Santos, M. N., and Carvalho, S. (2015). Temporal variability in epifaunal assemblages associated with gorgonian gardens. Mar. Environ. Res. 112, 140–151. doi: 10.1016/j.marenvres.2015.10.006
Dinesesn, Z. D. (1983). Patterns in the distribution of soft corals across the central Great Barrier Reef. Coral Reefs 1, 229–236. doi: 10.1007/bf00304420
Edmunds, P. J., and Lasker, H. R. (2019). Regulation of population size of arborescent octocorals on shallow Caribbean reefs. Mar. Ecol. Prog. Ser. 615, 1–14. doi: 10.3354/meps12907
Edmunds, P. J., Tsounis, G., and Lasker, H. R. (2016). Differential distribution of octocorals and scleractinians around St. John and St. Thomas, US Virgin Islands. Hydrobiologia 767, 347–360. doi: 10.1007/s10750-015-2555-z
Epstein, H. E., and Kingsford, M. J. (2019). Are soft coral habitats unfavourable? A closer look at the association between reef fishes and their habitat. Environ. Biol. Fishes 102, 479–497. doi: 10.1007/s10641-019-0845-4
Etnoyer, P. J., Wickes, L. N., Silva, M., Dubick, J. D., Balthis, L., Salgado, E., et al. (2016). Decline in condition of gorgonian octocorals on mesophotic reefs in the northern Gulf of Mexico: before and after the Deepwater Horizon oil spill. Coral Reefs 35, 77–90. doi: 10.1007/s00338-015-1363-2
Fabricius, K. E. (1995). Slow population turnover in the soft coral genera Sinularia and Sarcophyton on mid- and outer-shelf reefs of the Great Barrier Reef. Mar. Ecol. Prog. Ser. 126, 145–152. doi: 10.3354/meps126145
Fabricius, K. E., Alderslade, P., Williams, G. C., Colin, P. L., and Golbuu, Y. (2007). Octocorallia in Palau, Micronesia: effects of biogeography and coastal influences on local and regional biodiversity. Palau Int. Coral Reef Centre 7, 79–92.
Fabricius, K., and Alderslade, P. (2001). Soft Corals and Sea Fans: A Comprehensive Guide to the Tropical Shallow Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea. Townsville, QLD: Australian Institute of Marine Science.
Fox, H. E., Pet, J. S., Dahuri, R., and Caldwell, R. L. (2003). Recovery in rubble fields: long-term impacts of blast fishing. Mar. Pollut. Bull. 46, 1024–1031. doi: 10.1016/S0025-326X(03)00246-7
Garra, S., Hall, A., and Kingsford, M. J. (2020). The effects of predation on the condition of soft corals. Coral Reefs 39, 1329–1343. doi: 10.1007/s00338-020-01967-x
Goh, B. P. L., Tan, G. E., and Tan, L. T. (2009). Diversity, distribution and biological activity of soft corals (Octocorallia, Alcyonacea) in Singapore. J. Coast. Dev. 12, 89–98.
Hammer, O., Harper, D. A. T., and Ryan, P. D. (2001). PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4:9.
Huang, D., Licuanan, W. Y., Hoeksema, B. W., Chen, C. A., Ang, P. O., Huang, H., et al. (2014). Extraordinary diversity of reef corals in the South China Sea. Mar. Biodivers. 45, 157–168. doi: 10.1038/s41597-020-00793-8
Hughes, T. P., Andesron, K. D., Connolly, S. R., Heron, S. F., Kerry, J. T., Lough, J. M., et al. (2018). Spatial and temporal patterns of mass coral bleaching of corals in the Anthropocene. Science 359, 80–83. doi: 10.1126/science.aan8048
Hughes, T. P., Kerry, J. T., and Alvarex-Noriega, M. (2017). Global warming and recurrent mass bleaching of corals. Nature 543, 373–385. doi: 10.1038/nature21707
Janes, M. P. (2013). Distribution and diversity of the soft coral family Xeniidae (Coelenterata: Octocorallia) in Lembeh Strait, Indonesia. Galaxea J. Coral Reef Stud. 15, 195–200. doi: 10.3755/galaxea.15.195
Jeng, M. S., Huang, H. D., Dai, C. F., Hsiao, Y. C., and Benayahu, Y. (2011). Sclerite calcification and reef-building in the fleshy octocoral genus Sinularia (Octocorallia: Alcyonacea). Coral Reefs 30, 925–933. doi: 10.1007/s00338-011-0765-z
Jones, C. G., Lawton, J. H., and Shachak, M. (1994). “Organisms as ecosystem engineers,” in Ecosystem Management (New York, NY: Springer). doi: 10.1007/978-1-4612-4018-1_14
Kohler, K. E., and Gill, S. M. (2006). Coral point count with excel extensions (CPCe): a visual basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 32, 1259–1269. doi: 10.1016/j.cageo.2005.11.009
Kool, J. T., Paris, C. B., Barber, P. H., and Cowen, R. K. (2011). Connectivity and the development of population genetic structure in Indo-West Pacific coral reef communities. Glob. Ecol. Biogeogr. 20, 695–706. doi: 10.1111/j.1466-8238.2010.00637.x
Lalas, J. A. A., Benayahu, Y., and Baria-Rodriguez, M. V. (2021). Community structure and size-frequency distribution of soft corals in a heavily disturbed reef system in northwestern Philippines. Mar. Pollut. Bull. 162:111871. doi: 10.1016/j.marpolbul.2020.111871
Larson, C. (2015). China’s island building is destroying reefs. Science 349:1434. doi: 10.1126/science.349.6255.1434
Lasker, H. R., Martinez-Quintana, A., and Edmunds, P. J. (2020). Resilience of octocoral forests to catastrophic storms. Sci. Rep. 10:4286. doi: 10.1038/s41598-020-61238-1
Lau, W. Y., Poliseno, A., Kushida, Y., Quere, G., and Reimer, J. D. (2020). The classification, diversity and ecology of shallow water octocorals. Encycl. World’s Biomes 4, 597–611. doi: 10.1016/B978-0-12-409548-9.12109-8
Li, S., Yu, K., Chen, T., Shi, Q., and Zhang, H. (2011). Assessment of coral bleaching using symbiotic zooxanthellae density and satellite remote sensing data in the Nansha Islands, South China Sea. Chin. Sci. Bull. 56, 1031–1037. doi: 10.1007/s11434-011-4390-6
Licuanan, W. Y., Robles, R., and Reyes, M. (2019). Status and recent trends in coral reefs of the Philippines. Mar. Pollut. Bull. 142, 544–550. doi: 10.1016/j.marpolbul.2019.04.013
Mana, R. R. (2011). BIOPAPUA Cruise: Highlighting the Deep-Sea Benthic Biodiversity of Papua New Guinea. A Report Submitted to School of Natural and Physical Sciences. Port Moresby, PG: University of Papua New Guinea.
McFadden, C. S., Brown, A. S., Brayron, C., Hunt, C. B., and van Ofwagen, L. P. (2014). Application of DNA barcoding in biodiversity studies of shallow-water octocorals: molecular proxies agree with morphological estimates of species richness in Palau. Coral Reefs 33, 275–286. doi: 10.1007/s00338-013-1123-0
McManus, J. W., and Meñez, L. A. B. (1997). “The proposed international Spratly island marine park: ecological considerations,” in Proceedings of the 8th Coral Reef Symposium, Vol. 2, (Panama: Smithsonian Tropical Research Institute), 1943–1948.
Mohammad, M., Apandi, Z. N., Marican, H. A. W., Kamphol, N., Mubin, N. A. A. A., Salleh, S., et al. (2016). The identification of octocorals from the northern regions of Straists of Malacca. Trop. Life Sci. Res. 27, 87–93. doi: 10.21315/tlsr2016.27.3.12
Mora, C., Caldwell, I. R., Birkeland, C., and McManus, J. W. (2016). Dredging in the Spratly Islands: gaining land but losing reefs. PLoS Biol. 14:e1002422. doi: 10.1371/journal.pbio.1002422
Nörstrom, A. V., Nyström, M., Lokrantz, J., and Folke, C. (2009). Alternative states on coral reefs: beyond coral-macroalgal phase shifts. Mar. Ecol. Prog. Ser. 376, 295–306. doi: 10.3354/meps07815
Pata, P. R., and Yñiguez, A. T. (2019). Larval connectivity patterns of the North Indo-West Pacific coral reefs. PLoS One 14:e0219913. doi: 10.1371/journal.pone.0219913
Pérez, C. D. de Moura Neves, B., Cordeiro, R. T., Williams, G. C., and Cairns, S. D. (2016). “Diversity and distribution of Octocorallia,” in The Cnidaria, Past, Present and Future, eds S. Goffredo and Z. Dubinsky (Cham: Springer). doi: 10.1007/978-3-319-31305-4_8
Quibilan, M. C., and Aliño, P. M. (2006). “Coral community structure of western Philippine reefs I: spatial patterns,” in Proceedings of 10th International Coral Reef Symposium, (Okinawa), 341–350. doi: 10.1007/s10661-014-4089-7
Quimpo, T. J. R., Cabaitan, P. C., Go, K. T. B., Dumalagan, E. E. Jr., Villanoy, C. L., and Siringan, F. P. (2019). Similarity in benthic habitat and fish assemblages in the upper mesophotic and shallow water reefs in the West Philippine Sea. J. Mar. Biol. Assoc. U. K. 99, 1507–1517. doi: 10.1017/S0025315419000456
Rosenberg, D. (1999). Environmental pollution around the South China Sea: developing a regional response. Contemp. Southeast Asia 21, 119–145. doi: 10.1016/j.ecoenv.2019.01.116
Rowley, S. J. (2018). Environmental gradients structure gorgonian assemblages on coral reefs in SE Sulawesi, Indonesia. Coral Reefs 37, 609–630. doi: 10.1007/s00338-018-1685-y
Sanciangco, J. C., Carpenter, K. E., Etnoyer, P. J., and Moretzsohn, F. (2013). Habitat availability and heterogeneity and the Indo-Pacific warm pool as predictors of marine species richness in the tropical Indo-Pacific. PLoS One 8:e56245. doi: 10.1371/journal.pone.0056245
Seah, J. Z. S., Yap, N. W. L., Tan, L. T., and Goh, B. P. L. (2015). Distribution and abundance of octocoral (Octocorallia, Alcyonacea) communities at three southern islands of Singapore. Ocean Sci. J. 50, 299–306. doi: 10.1007/s12601-015-0027-z
Sella, I., and Benayahu, Y. (2010). Rearing cuttings of the soft coral Sarcophyton glaucum (Octocorallia, Alcyonacea): towards mass production in a closed seawater system. Aquac. Res. 41, 1748–1758. doi: 10.1111/j.1365-2109.2009.02475.x
Shoham, E., and Benayahu, Y. (2016). Higher species richness of octocorals in the upper mesophotic zone in Eilat (Gulf of Aqaba) compared to shallower reef zones. Coral Reefs 36, 71–81. doi: 10.1007/s00338-016-1528-7
Shoham, E., Prohaska, T., Barkay, Z., Zitek, A., and Benayahu, Y. (2019). Soft corals form aragonite-precipitated columnar spiculite in mesophotic reefs. Sci. Rep. 9:1241. doi: 10.1038/s41598-018-37696-z
Slattery, M., and Gochfeld, D. J. (2016). Butterflyfishes exhibit species-specific responses to changes in Pacific coral benthic communities. Mar. Biol. 163:246. doi: 10.1007/s00227-016-3025-5
Song, Y. (2008). The potential threat from oil and gas development activities in the disputed South China Sea/Spratly Area: a role that Taiwan can play. Ocean Dev. Int. Law 39, 150–177. doi: 10.1080/00908320802013768
Syms, C., and Jones, G. P. (2001). Soft corals exert no direct effects on coral reef fish assemblages. Ecologia 127, 560–571. doi: 10.1007/s004420000617
Tkachenko, S., Hoang, D. T., and Dang, H. N. (2020). Ecological status of coral reefs in the Spratly Islands, South China Sea (East Sea) and its relation to thermal anomalies. Estuarine Coast. Shelf Sci. 238:106722. doi: 10.1016/j.ecss.2020.106722
Tun, K., Chou, L. M., Yeemin, T., Phongsuwan, N., Setiasih, N., Wilson, J., et al. (2010). “A regional overview on the 2010 coral bleaching event in Southeast Asia,” in Status of Coral Reefs in East Asian Seas Region: 2010 (Townsville, QLD: Global Coral Reef Monitoring Network), 9–27.
Tursch, B., and Tursch, A. (1982). The soft coral community on a sheltered reef quadrat at Laing Island (Papaua New Guinea). Mar. Biol. 68, 321–332. doi: 10.1007/bf00409597
Verseveldt, J., and Tursch, A. (1979). Octocorallia from the Bismarck Sea. Zool. Meded. 54, 133–148. doi: 10.11646/zootaxa.4652.2.1
Vo, S. T., Pernetta, J. C., and Paterson, C. J. (2013). Status and trends in coastal habitats of the South China Sea. Ocean Coast. Manage. 85, 153–163. doi: 10.1016/j.ocecoaman.2013.02.018
Williams, G. C., and Cairns, S. D. (2018). Biodiversity Myth Busters. Octocoral Research Center. Available online at: https://researcharchive.calacademy.org/research/izg/Biodiversity%20Myth%20Busters.html
Yeung, C. W., Cheang, C. C., Lee, M. W., Fung, H. L., Chow, W. K., and Ang, P. (2014). Environmental variabilities and the distribution of octocorals and black corals in Hongkong. Mar. Pollut. Bull. 85, 774–782. doi: 10.1016/j.marpolbul.2013.12.043
Zubair, M. S., Lallo, S., Rusmiyanti, R., Nugrahani, A. W., and Jantan, I. (2018). Screening of antibacterial and anticancer activity of soft corals from Togean Islands, Indonesia. Indones. J. Pharm. 29:173. doi: 10.14499/indonesianjpharm29iss4pp173
Keywords: octocorallia, soft corals, gorgonians, coral reef, Philippines, South China Sea, Spratly Islands
Citation: Lalas JAA, Lim RTS, Cabasan JP, Segumalian CS, Luciano RMA, Valino DAM, Jacinto MR, Arceo HO and Baria-Rodriguez MV (2022) Spatial and Short-Term Temporal Patterns of Octocoral Assemblages in the West Philippine Sea. Front. Mar. Sci. 8:782977. doi: 10.3389/fmars.2021.782977
Received: 25 September 2021; Accepted: 06 December 2021;
Published: 06 January 2022.
Edited by:
Susana Carvalho, King Abdullah University of Science and Technology, Saudi ArabiaReviewed by:
Erik Cordes, Temple University, United StatesHin Boo Wee, National University of Malaysia, Malaysia
Yuka Kushida, Kagoshima University, Japan
Copyright © 2022 Lalas, Lim, Cabasan, Segumalian, Luciano, Valino, Jacinto, Arceo and Baria-Rodriguez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Vanessa Baria-Rodriguez, dnJvZHJpZ3VlekBtc2kudXBkLmVkdS5waA==
 Jue Alef A. Lalas
Jue Alef A. Lalas Romina Therese S. Lim
Romina Therese S. Lim Joey P. Cabasan1
Joey P. Cabasan1 Christine S. Segumalian
Christine S. Segumalian Rhea Mae A. Luciano
Rhea Mae A. Luciano Darryl Anthony M. Valino
Darryl Anthony M. Valino Melchor R. Jacinto
Melchor R. Jacinto Maria Vanessa Baria-Rodriguez
Maria Vanessa Baria-Rodriguez