Abstract
Due to its small size, large specific surface area and hydrophobicity, microplastics, and the adsorbed contaminants may together cause potential negative effects on ecosystems and human beings. In this study, kinetics and size effects on adsorption of Cu(II), Cr(III), and Pb(II) onto PE, PP and PET microplastic particles were explored. Results indicated that the PE and PET microplastics have the higher adsorption capacity for Cu(II), Cr(III), and Pb(II) than that for PP microplastic. The adsorption capacity was affected by microplastic types and metal species. Among the three metals, Pb(II) had the largest adsorption amount on microplastic particles, especially on PET particles. Moreover, the adsorption capacities of microplastics increase with the decrease of particle size. The metal adsorption capacity of <0.9 mm microplastics is greater than that of 0.9–2 mm and 2–5 mm microplastics. The size effect on metal adsorption was largest for PE microplastic. More attention should be paid in case of the coexistence of heavy metals and tiny PE and PET microplastics in the environment.
Introduction
Microplastics have already posed potentially risk for human health through transmission and accumulation in food chain (Yang et al., 2015; Xu et al., 2019) as they have already been widely detected in food (Liebezeit and Liebezeit, 2013, 2014; Yang et al., 2015). In the future, the environmental exposure risk of microplastic may be elevated as the plastic production is expected to increase to 318 million tons annually in 2050 (Neufeld et al., 2016). Furthermore, environmental microplastics could be a carrier for heavy metals transport from river to sea due to its small size, large specific surface area and hydrophobicity (Wang et al., 2017). As commonly detected pollutants in the environment (Zhang et al., 2018), heavy metals such as Cu, Cr, and Pb were also frequently detected in environmental microplastics (Selvam et al., 2021), and the metal concentration of microplastics was even similar or higher than that of the sediment phase (Ashton et al., 2010). It indicated that microplastics were able to enhance the mobility of heavy metal along river-coast-sea system. Once the metal-contained microplastics are ingested by aquatic organisms, these metals may be released in the organism, causing further damage to the function of the organism. Then threaten human health via gradual accumulation in food chain (Fries et al., 2013). Therefore, the potential risk of microplastics and the heavy metal to freshwater-marine ecosystems would be both intensified. Therefore, to investigate the affinity of heavy metals to microplastics is essential to estimate the coexisting toxicity of heavy metals and microplastics in aqueous environments (Xu et al., 2018).
Although microplastics have ability to adsorb heavy metals (Koelmans et al., 2016; Wang et al., 2019; Fu et al., 2021), the adsorption capacity varies with the type of microplastics and heavy metals because of the difference in physicochemical properties of various microplastics and heavy metals. For example, polystyrene and film microplastic have greater adsorption capacity for Cu(II) than polyvinyl chloride, polyethylene, fishing line fibers and bottle cap particles, due to the conducive physicochemical properties of film microplastic (Almeida et al., 2020; Gao et al., 2021a). Compared with Cu and Cd, Pb showed the higher affinity to microplastics, because it is more likely to efficiently bind to function group on microplastics to promote the adsorption (Gao et al., 2019, 2021b). In addition, particle size is the generally essential factor influencing adsorption. In the environments, microplastics will be further fragmented into smaller part due to environmental dynamics, thereby affecting the adsorption capacity of heavy metals on microplastics (Gao et al., 2019; Zhang et al., 2020).
Therefore, it is important to investigate how microplastic size and type affect the interaction with heavy metals, which heavy metal has the most potential to be absorbed onto microplastics, and how the interaction will change with time. Adsorption kinetics are a conventional method to identify the temporal change of adsorption process, and the model parameters would attribute to reveal the possible adsorption mechanism (Almeida et al., 2020; Purwiyanto et al., 2020). Here, polyethylene (PE), polypropylene (PP) and polyethylene terephthalate (PET), which are three mostly used and typical types of plastics in the world (The Essential Chemical Industry (ECI), 2016a,b, 2017), are selected to study the adsorption kinetics and size effect for the three typical metal ions of Cu(II), Cr(III), and Pb(II) to test the hypotheses: (a) different temporal change in metal adsorption for different microplastics; and (b) larger metal adsorption for smaller microplastic particles.
Materials and Methods
Chemicals and Materials
Cu(NO3)2, Cr(NO3)3, and Pb(NO3)2 were purchased from Aladdin Bio-Chem Technology Corporation (Shanghai, China). HNO3 was purchased from Bohua Chemical Reagent Corporation (Tianjin, China). All chemicals were analytical grade or higher purity. PE, PP, and PET pellets with particle size of 5mm were purchased from Yousuo Chemical Technology Corporation (Shandong, China). Before use, the PE, PP, and PET pellets were crushed using a high-speed crusher. The crushed microplastic particles were then sequentially sieved through 20-, 10-, and 4- mesh screens in order to separate the particle sizes 2–5, 0.9–2, and <0.9 mm. The morphology of PE, PP, and PET microplastics were observed with a scanning electron microscope (Tescan Mira 4). To prevent contamination, all the lab materials were soaked in 10% (v/v) HCl solution for at least 48h, rinsed at least three times with deionized water (conductivity < 0.1 mS cm–1) and dried in an oven at 50oC.
Adsorption Experiments
The first experiment was to investigate temporal change of metal adsorption onto microplastics. Three microplastics (PE, PP, and PET) with same particle size < 0.9 mm were mixed with 50 mL solutions of Cu(II) with concentration of 5 mg L–1 in centrifuge tubes. The adsorption of Cr(III) and Pb(II) were also conducted simultaneously at the same condition. The medium is the deionized water. Samples were shaken at 150 r/min in a constant temperature water bath shaker at room temperature (∼25oC). Sub-samples after 1, 2, 4, 8, 24, 72, 120, 168, 240, 312, and 384 h were taken, respectively.
The second experiment was to investigate the influence of microplastic particle size on adsorption. PE, PP, and PET microplastics with particle size 2–5, 0.9–2, and <0.9 mm were used. A series of centrifuge tubes, respectively containing 0.5 g microplastic with different size and 50 mL solutions of Cu(II) with concentration of 5 mg L–1, were shaken for 240 h at 150 r/min in a constant temperature water bath shaker at room temperature (∼25oC). At the same time, the adsorption of Cr(III) and Pb(II) were also conducted at the same condition. The medium is the deionized water.
At the terminal of shaking step, the mixture was immediately filtered with filter paper with a pore size of 15 to 20 μm. The trapped microplastics were collected, then were dried and transferred to a series of 10 mL centrifuge tubes. Then, 5 mL 2% HNO3 was added to these tubes and ultrasound for 10 min to extract metal ions from microplastics. Finally, the mixture after ultrasonic was filtered with a syringe filter and the filtered solution was transferred to a clean PP centrifuge tube for quantification analysis. An inductively coupled plasma mass spectrometry (ICP-MS, Elan DRC-e, PerkinElmer) was used to analyze the heavy metal contents using the certified reference material (CRM). The detection limits for the three metals are 1 ppt and the recoveries for all are above 90%. All the treatments were in duplicate. The amount of heavy metal adsorbed by per unit mass of microplastic (q) could be calculated by Eq. (1).
where, m (g) was the mass of microplastics used in adsorption, V (L) was the volume of the added solution with 2% HNO3, C (μg L–1) was the concentration of heavy metals after ultrasonic, respectively.
Kinetic Models
Four kinetic models were used to describe the kinetic adsorption of Cu(II), Cr(III), Pb(II) onto PE, PP, and PET microplastics.
The pseudo-second-order kinetic model:
The pseudo-first-order kinetic model:
The Elovich kinetic model:
The intra-particle diffusion model:
where, qt (μg g–1) is the adsorption amount at the time of t (h); qe (μg g–1) is the saturated adsorption capacity of heavy metals at equilibrium; k1 (h –1) is the reaction rate constant of pseudo-first-order equation at equilibrium; k2 (g μg–1 h –1) is the reaction rate constant of the pseudo-second-order equation at equilibrium; a (μg g–1) and b (μg g–1 h–1) are the parameters of the Elovich equation; kp (μg g–1 h–0.5) is the constant of intra-particle diffusion model, C (μg g–1) represents a conception about the thickness of boundary layer, describing the influence of thickness of boundary layer on adsorption.
Results and Discussion
Metal Adsorption Kinetics
The kinetics experiments results were shown in Figure 1. The maximum of Cu(II) and Cr(III), Pb(II) adsorption were 0.51, 0.64, and 4.78 mg g–1 for PET, PE and PE, respectively. For all the three metals, the adsorption capacity on PP particles was the lowest. The adsorption of PE, PP, and PET particles increased rapidly in the initial 24 h, and then changed slowly. In general, the adsorption rates and adsorption capacities followed the orders of PET > PE > PP for Cu(II), and PE > PET > PP for Cr(III) and Pb(II).
FIGURE 1
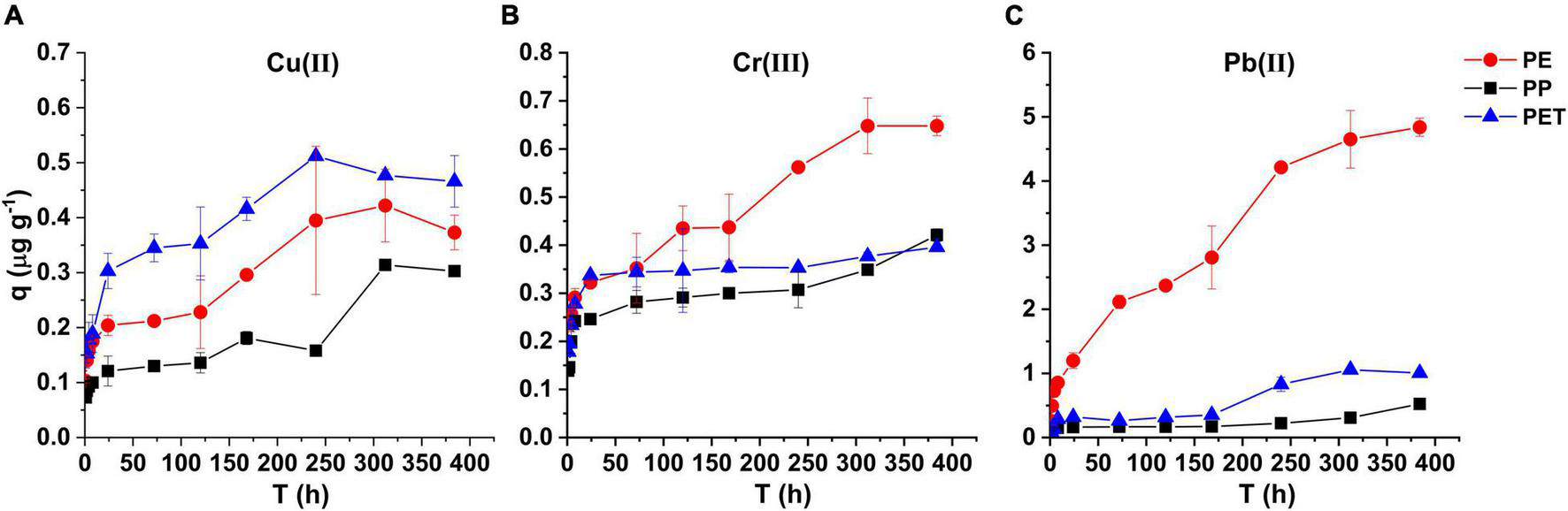
(A) The Cu(II), (B) Cr(III), and (C) Pb(II) adsorption of PE, PP, and PET microplastics with a particle size of <0.9 mm at different adsorption time.
As shown in Figure 2, the kinetics of Cu(II) and Cr(III) adsorption onto the PE, PP, and PET microplastics were well regressed by the pseudo-second-order model (Table 1). The derived equilibrium adsorption capacities (qe) of Cu(II) and Cr(III) for PP microplastic were the lowest, which was the same with the experimental results in Figure 1. It may be attributed to no functional group on PP microplastic compared with PET microplastic and the smoother surface of PP microplastic than PE microplastic (Figure 3). The adsorption capacity of Cu(II) for PET microplastic is greater than that for PE microplastic, while the adsorption capacities of Cr(III) for PET microplastic is smaller than that for PE microplastic. However, other researchers found that sequence of adsorption capacity was PE > PP > PET for both Cu(II) and Cr(III) (Godoy et al., 2019). The differences may be because the microplastics they used were from daily objects and may be aged. This may suggest that the adsorption capacity of heavy metals on microplastic greatly varies with the change of microplastics surface. In Table 1, the values of k2 were lower than 0.01 g (μgh) –1 for Cu(II) and Cr(III) adsorption. It did not only indicate that the adsorption rate was proportional to the number of unoccupied sites (Fan et al., 2021), but also revealed the adsorption of Cu(II) and Cr(III) onto the microplastics were a slow process, especially for the virgin microplastics with relatively homogeneous smooth surface (Li et al., 2019; Oz et al., 2019; Wang et al., 2020). Turner and Holmes (2015) and Wang et al. (2020) also found that the interaction between metals and microplastics was a long-term process even for the aged microplastics. It indicated that the microplastics might continue to accumulate heavy metals when the interaction time is long. However, further evidence is needed.
FIGURE 2
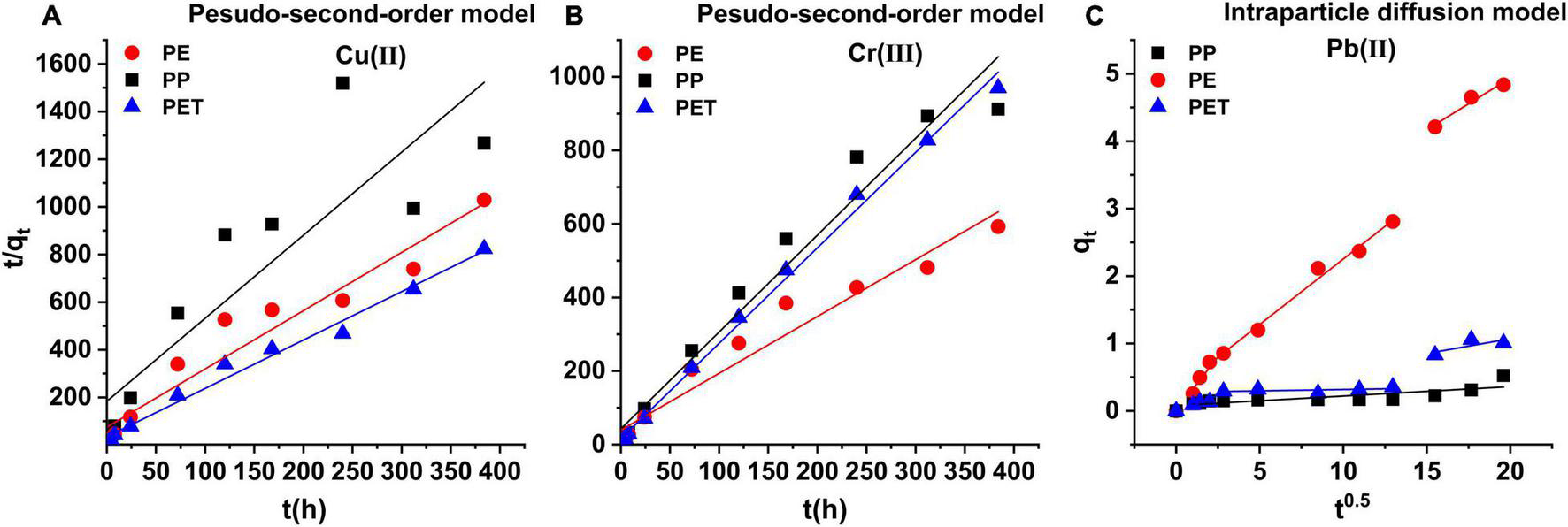
(A) The pseudo-second-order kinetic model for Cu(II), (B) Cr(III) adsorption on PE, PP, and PET microplastics, and (C) the intra-particle diffusion model for Pb(II) adsorption on PE, PP and PET microplastics.
TABLE 1
| Cu(II) |
Cr(III) |
Pb(II) |
||||||||
| PE | PP | PET | PE | PP | PET | PE | PP | PET | ||
| Pesudo-second-order model | q e (μg g–1) | 0.402 | 0.278 | 0.488 | 0.649 | 0.380 | 0.385 | 5.128 | 0.370 | 1.04 |
| k 2 (g/(μg⋅h)–1) | 0.0025 | 0.0005 | 0.0081 | 0.0107 | 0.0034 | 0.0094 | 2.217 | 0.0009 | 0.0123 | |
| R 2 | 0.94 | 0.78 | 0.98 | 0.95 | 0.96 | 0.99 | 0.88 | 0.67 | 0.52 | |
| Pesudo-first-order model | q e (μg g–1) | 0.303 | 0.287 | 0.147 | 1.71 | 0.267 | 0.151 | 6.97 | 0.542 | 1.21 |
| k 1 (h–1) | 0.0072 | 0.0076 | 0.007 | 0.014 | 0.006 | 0.0076 | 0.015 | 0.0066 | 0.104 | |
| R 2 | 0.69 | 0.67 | 0.61 | 0.82 | 0.71 | 0.82 | 0.76 | 0.48 | 0.60 | |
| Elovich model | a (μg g–1) | 21.8 | 32.3 | 16.5 | 15.7 | 27.4 | 29.8 | 1.09 | 26.7 | 7.63 |
| B (μg g–1 h–1) | 0.046 | 0.031 | 0.061 | 0.065 | 0.037 | 0.034 | 0.73 | 0.038 | 0.131 | |
| R 2 | 0.80 | 0.60 | 0.93 | 0.79 | 0.88 | 0.94 | 0.84 | 0.39 | 0.59 | |
| Intraparticle diffusion model | k p,1 (μg g–1 h–0.5) | 0.111 | 0.015 | 0.019 | 0.022 | 0.0602 | 0.056 | 0.320 | 0.0464 | 0.014 |
| C 1 (μg g–1) | 0.112 | 0.058 | 0.154 | 0.209 | 0.0727 | 0.120 | 0.0042 | 0.0252 | 0.078 | |
| R 2 | 0.90 | 0.79 | 0.92 | 0.96 | 0.95 | 0.99 | 0.89 | 0.80 | 0.76 | |
| k p,2 (μg g–1 h–0.5) | 0.0062 | 0.002 | 0.194 | 0.0018 | ||||||
| C 2 (μg g–1) | 0.222 | 0.327 | 0.311 | 0.149 | ||||||
| R 2 | 0.95 | 0.95 | 0.98 | 0.83 | ||||||
| k p,3 (μg g–1 h–0.5) | 0.0276 | 0.0105 | 0.153 | 0.0727 | ||||||
| C 3 (μg g–1) | −0.126 | 0.191 | 1.87 | −0.925 | ||||||
| R 2 | 0.93 | 0.99 | 0.93 | 0.85 | ||||||
The fitting parameters of different models of Cu(II), Cr(III), and Pb(II) adsorbed onto PE, PP, and PET microplastics, respectively.
FIGURE 3
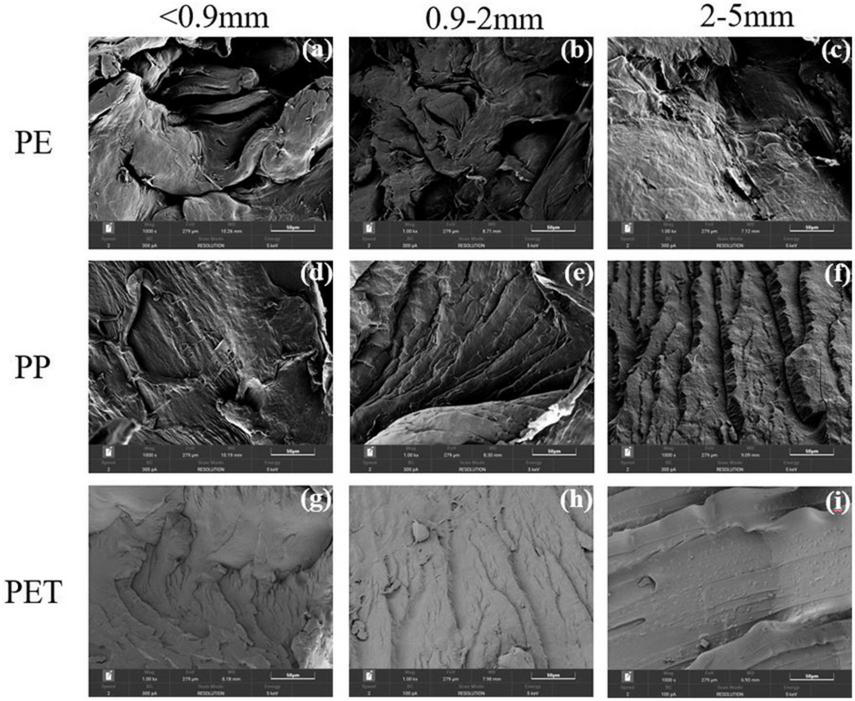
(a–i) The morphology of PE, PP and PET microplastics.
There was obvious discrepancy in Pb(II) adsorbed on PE, PP, and PET microplastics (Figures 1, 2). The adsorption amount of Pb(II) on PE microplastic was the highest, which may attribute to the higher crystallinity, high pore volume and rough surfaces of PE microplastic (Wang and Wang, 2018; Zou et al., 2020). It indicated that the crystallinity of microplastic may be one of the essential factors influencing Pb(II) adsorption, even more important than function group for the virgin microplastics. The kinetics of Pb(II) adsorption on PE, PP, and PET microplastics were well fitted by the intra-particle diffusion model (R2 ≥ 0.76 in Table 1), implying the inter-particle diffusion process was the rate-controlling step. The negative influence of the boundary layer on adsorption over time decreased to the lowest (C3 < 0) explained the keep growing in amount of adsorbed Pb(II) on PET microplastic. Although the order of C on PE microplastic was C1 > C2 > C3, the adsorption amount continuously increased with time. Turner and Holmes (2015) also found the same trend when the added Pb(II) concentration was 5 mg L–1. It implied that Pb(II) had strong prosperity of affinity and temporal accumulation on PE microplastic. However, the adsorption can quickly achieve the equilibrium at about 48h in the seawater medium (Holmes et al., 2012), because the ions existence would fasten the adsorption process and change the temporal procedure of adsorption.
Size Effect on Metal Adsorption
The results shown in Figure 4 validated the heavy metal absorption on microplastics decreased with increasing particle size. With the decrease of microplastic size from 2–5 mm to <0.9 mm, the adsorption amount increased about 1.8–2.2, 1.3–1.5, and 1.94–2.83 times for Cu(II), Cr(III), and Pb(II), respectively. In addition, the amount of adsorbed Cr(III) varied more slightly with the particle size. Namely, the effect of particle size of PP microplastic on metal adsorption was relatively low.
FIGURE 4
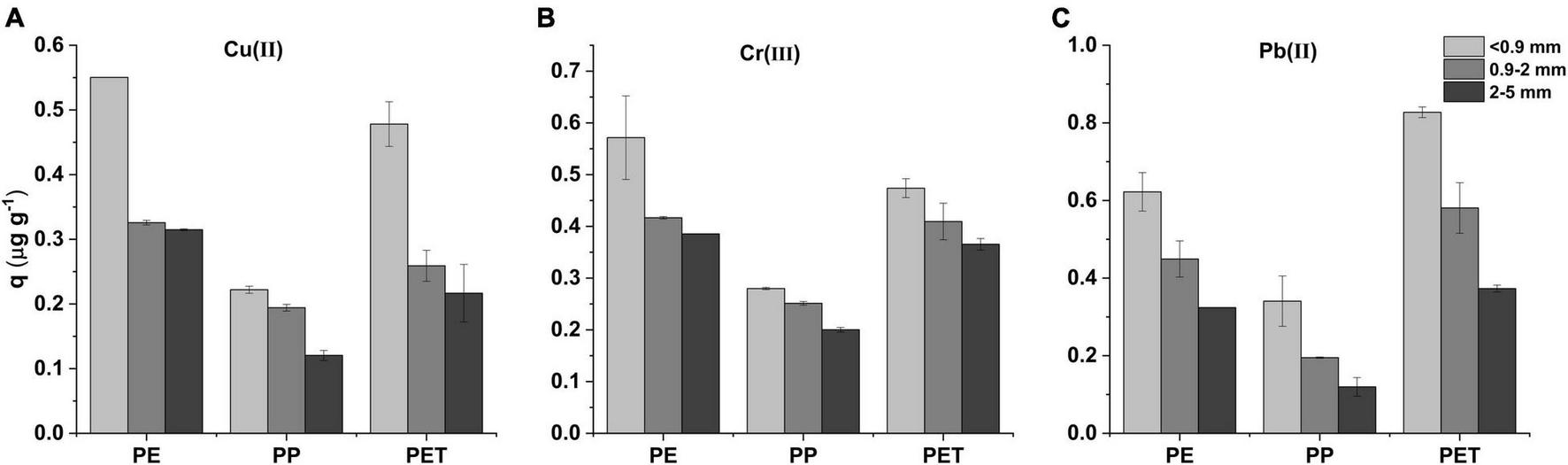
(A) The Cu(II), (B) Cr(III), and (C) Pb(II) adsorption capacities of PP, PE, and PET microplastics with different particle sizes in 240 h.
The phenomenon may attribute to the more complex morphology and higher specific area with decrease of particle size (Figure 3), which can lead more unoccupied site for adsorption. For Cu(II) and Pb(II), the observed adsorption variations with particle size implied that the adsorption on microplastic was considerably related to the porosity. Compared with Cu(II) and Pb(II), the influence of particle size on Cr(III) adsorption was relatively small, which is similar to the tendency observed in other studies (e.g., Zhang et al., 2021). It may be attributable to the insensitive response of Cr(III) adsorption to the stratification variation of the microplastic surface. The relatively low influence of particle size on PP microplastic adsorption profitably emphasized the significance of crystallinity and function group.
According to the experimental results above, it may be inferred that the adsorption amount of microplastics to other metals may also possibly increase with decrease of particle size. Namely, microplastics with a smaller particle size in the environment may cause higher environmental risks as a carrier of heavy metals (Thompson et al., 2004; Zhang et al., 2020). With the aging process of microplastics in natural environment, such as UV-irradiation, acid and alkali corrosion, particle crushing, biofouling, it would become more toxic to the environment. This means the results in this study may be regarded as the lowest metal amounts absorbed by microplastics in the natural environment.
All the three heavy metals can be accumulated increasingly with time onto the three microplastics. The PET microplastic has the relatively rapid and strong ability to adsorb Cu(II) and PE microplastic has the relatively rapid and strong ability to adsorb Cr(III) and Pb(II). It means that the virgin microplastic PE and PET can be a conducive carrier for heavy metal transport in the environment and their environmental toxicity would be magnified, especially for the combination of Pb(II) and PE. The risk to environmental security would be further elevated due to the aging process of PP and PET in the environment (Han et al., 2021). Therefore, more attention should be paid to PE and PET microplastics if metal contaminants exist in the aqueous system.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
XH and SW: investigation, data curation, validation, and writing-original draft preparation. XY: writing, reviewing, and editing. RV: conceptualization, writing, reviewing, and editing. JF, LZha, WM, and LZhu: resources, writing, reviewing, and editing. XL: conceptualization, supervision, writing, reviewing, and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by the projects from the National Key Research and Development Program of China (2018YFC1406403 and 2020YFC1909500).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Almeida C. M. R. Manjate E. Ramos S. (2020). Adsorption of Cd and Cu to different types of microplastics in estuarine salt marsh medium.Mar. Pollut. Bull.151:110797. 10.1016/j.marpolbul.2019.110797
2
Ashton K. Holmes L. Turner A. (2010). Association of metals with plastic production pellets in the marine environment.Mar. Pollut. Bull.602050–2055. 10.1016/j.marpolbul.2010.07.014
3
Fan X. L. Ma Z. X. Zou Y. F. Liu J. Q. Hou J. (2021). Investigation on the adsorption and desorption behaviors of heavy metals by tire wear particles with or without UV ageing processes.Environ. Res.195:110858. 10.1016/j.envres.2021.110858
4
Fries E. Dekiff J. H. Willmeyer J. Nuelle M. T. Ebert M. Remy D. (2013). Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy.Environ. Sci. Process. Impacts151949–1956. 10.1039/c3em00214d
5
Fu Q. M. Tan X. F. Ye S. J. Ma L. L. Gu Y. L. Zhang P. et al (2021). Mechanism analysis of heavy metal lead captured by natural-aged microplastics.Chemosphere270:128624. 10.1016/j.chemosphere.2020.128624
6
Gao F. L. Li J. X. Sun C. J. Zhang L. T. Jiang F. H. Cao W. et al (2019). Study on the capability and characteristics of heavy metals enriched on microplastics in marine environment.Mar. Pollut. Bull.14461–67. 10.1016/j.marpolbul.2019.04.039
7
Gao L. Fu D. Zhao J. Wu W. Wang Z. Su Y. et al (2021a). Microplastics aged in various environmental media exhibited strong sorption to heavy metals in seawater.Mar. Pollut. Bull.169:112480. 10.1016/j.marpolbul.2021.112480
8
Gao X. Hassan I. Peng Y. T. Huo S. L. Ling L. (2021b). Behaviors and influencing factors of the heavy metals adsorption onto microplastics: a review.J. Clean. Prod.319:128777. 10.1016/j.jclepro.2021.128777
9
Godoy V. Blazquez G. Calero M. Quesada L. Martin-Lara M. A. (2019). The potential of microplastics as carriers of metals.Environ. Pollut.255(Pt 3):113363.
10
Han X. Vogt R. D. Zhou J. Zheng B. Yu X. Feng J. et al (2021). Increased Cu(II) adsorption onto UV-aged polyethylene, polypropylene, and polyethylene terephthalate microplastic particles in seawater.Front. Mar. Sci.8:770606. 10.3389/fmars.2021.770606
11
Holmes L. A. Turner A. Thompson R. C. (2012). Adsorption of trace metals to plastic resin pellets in the marine environment.Environ. Pollut.16042–48. 10.1016/j.envpol.2011.08.052
12
Koelmans A. A. Bakir A. Burton G. A. Janssen C. R. (2016). Microplastic as a vector for chemicals in the aquatic environment: critical review and model-supported re-interpretation of empirical studies.Environ. Sci. Technol.503315–3326. 10.1021/acs.est.5b06069
13
Li X. Mei Q. Chen L. Zhang H. Dong B. Dai X. et al (2019). Enhancement in adsorption potential of microplastics in sewage sludge for metal pollutants after the wastewater treatment process.Water Res.157228–237. 10.1016/j.watres.2019.03.069
14
Liebezeit G. Liebezeit E. (2013). Non-pollen particulates in honey and sugar.Food Addit. Contam.302136–2140. 10.1080/19440049.2013.843025
15
Liebezeit G. Liebezeit E. (2014). Synthetic particles as contaminants in German beers.Food Addit. Contam.311574–1578. 10.1080/19440049.2014.945099
16
Neufeld L. Stassen F. Sheppard R. Gilman T. (2016). In the New Plastics Economy: Rethinking the Future of Plastics.Cologny: World Economic Forum.
17
Oz N. Kadizade G. Yurtsever M. (2019). Investigation of heavy metal adsorption on microplastics.Appl. Ecol. Environ. Res.17:4. 10.15666/aeer/1704_73017310
18
Purwiyanto A. I. S. Suteja Y. Trisno N. P. Putri W. Rozirwan R. Agustriani F. et al (2020). Concentration and adsorption of Pb and Cu in microplastics: case study in aquatic environment.Mar. Pollut. Bull.158:111380. 10.1016/j.marpolbul.2020.111380
19
Selvam S. Jesuraja K. Venkatramanan S. Roy P. D. Jeyanthi Kumari V. (2021). Hazardous microplastic characteristics and its role as a vector of heavy metal I groundwater and surface water of coastal south India.J. Hazard. Mater.402:123786. 10.1016/j.jhazmat.2020.123786
20
The Essential Chemical Industry (ECI) (2016a). Poly(propene)(Polypropylene) [OL]. Available online at: http://www.essentialchemicalindustry.org/polymers/polypropene.html. (accessed August 21, 2016).
21
The Essential Chemical Industry (ECI) (2016b). Polyesters[OL]. [2018-09-06]. Available online at: http://www.essentialchemicalindustry.org/polymers/polyesters.html. (accessed August 25, 2016).
22
The Essential Chemical Industry (ECI) (2017). Poly(ethene)(Polyethylene)[OL]. Available online at: http://www.essentialchemicalindustry.org/polymers/polyethene.html. (accessed April 27, 2017).
23
Thompson R. C. Ylva O. Mitchell R. P. Anthony D. Rowland S. J. John A. W. G. et al (2004). Lost at sea: where is all the plastic?Science304:838.
24
Turner A. Holmes L. A. (2015). Adsorption of trace metals by microplastic pellets in fresh water.Environ. Chem.12600–610.
25
Wang F. Yang W. Cheng P. Zhang S. Zhang S. Jiao W. et al (2019). Adsorption characteristics of cadmium onto microplastics from aqueous solutions.Chemosphere2351073–1080. 10.1016/j.chemosphere.2019.06.196
26
Wang J. Peng J. Tan Z. Gao Y. Zhan Z. Chen Q. et al (2017). Microplastics in the surface sediments from the Beijiang River littoral zone: composition, abundance, surface textures and interaction with heavy metals.Chemosphere171248–258. 10.1016/j.chemosphere.2016.12.074
27
Wang Q. Zhang Y. Wangjin X. Wang Y. Meng G. Chen Y. (2020). The adsorption behavior of metals in aqueous solution by microplastics effected by UV radiation.J. Environ. Sci. (China)87272–280. 10.1016/j.jes.2019.07.006
28
Wang W. Wang J. (2018). Comparative evaluation of sorption kinetics and isotherms of pyrene onto microplastics.Chemosphere193567–573. 10.1016/j.chemosphere.2017.11.078
29
Xu P. Peng G. Y. Su L. Gao Y. Q. Gao L. Li D. J. (2018). Microplastic risk assessment in surface waters: a case study in the Changjiang Estuary.China. Mar. Pollut. Bull.133647–654. 10.1016/j.marpolbul.2018.06.020
30
Xu S. Ma J. Ji R. Pan K. Miao A. J. (2019). Microplastics in aquatic environments: occurrence, accumulation, and biological effects.Sci. Total Environ.703:134699. 10.1016/j.scitotenv.2019.134699
31
Yang D. Shi H. Li L. (2015). Microplastic pollution in table salts from China.Environ. Sci. Technol.49:13622. 10.1021/acs.est.5b03163
32
Zhang J. Zhou F. Chen C. Sun X. Shi Y. Zhao H. et al (2018). Spatial distribution and correlation characteristics of heavy metals in the seawater, suspended particulate matter and sediments in Zhanjiang Bay, China.PLoS One13:e0201414. 10.1371/journal.pone.0201414
33
Zhang L. Li Y. Wang W. Zhang W. Zuo Q. Abdelkader A. et al (2021). Heynderickx PM, Kim KH. The potential of microplastics as adsorbents of sodium dodecyl benzene sulfonate and chromium in an aqueous environment.Environ. Res.197:111057. 10.1016/j.envres.2021.111057
34
Zhang S. W. Han B. Sun Y. H. Wang F. Y. (2020). Microplastics influence the adsorption and desorption characteristics of Cd in an agricultural soil.J. Hazard. Mater.388:121775. 10.1016/j.jhazmat.2019.121775
35
Zou J. Liu X. Zhang D. Yuan X. (2020). Adsorption of three bivalent metals by four chemical distinct microplastics.Chemosphere248:126064. 10.1016/j.chemosphere.2020.126064
Summary
Keywords
microplastics, kinetics, metal, adsorption, size effect
Citation
Han X, Wang S, Yu X, Vogt RD, Feng J, Zhai L, Ma W, Zhu L and Lu X (2021) Kinetics and Size Effects on Adsorption of Cu(II), Cr(III), and Pb(II) Onto Polyethylene, Polypropylene, and Polyethylene Terephthalate Microplastic Particles. Front. Mar. Sci. 8:785146. doi: 10.3389/fmars.2021.785146
Received
28 September 2021
Accepted
09 December 2021
Published
24 December 2021
Volume
8 - 2021
Edited by
Lincoln Fok, The Education University of Hong Kong, Hong Kong SAR, China
Reviewed by
Xuegang Li, Institute of Oceanology, Chinese Academy of Sciences (CAS), China; Muhammad Reza Cordova, Center for Oceanographic Research, Indonesian Institute of Sciences, Indonesia
Updates
Copyright
© 2021 Han, Wang, Yu, Vogt, Feng, Zhai, Ma, Zhu and Lu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lifang Zhai, zhailifang01@163.comXueqiang Lu, luxq@nankai.edu.cn
†These authors have contributed equally to this work and share first authorship
This article was submitted to Marine Pollution, a section of the journal Frontiers in Marine Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.