- 1MARE – Marine and Environmental Sciences Centre/ARNET - Aquatic Research Network, Agência Regional para o Desenvolvimento da Investigação Tecnologia e Inovação (ARDITI), Funchal, Madeira, Portugal
- 2Marine Biology Station of Funchal, Faculty of Life Sciences, University of Madeira, Madeira, Portugal
- 3Whale Watch Azores, Horta, Azores, Portugal
- 4Canary Islands Cetaceans Research Centre, Society for the Study of Cetaceans in the Canary Archipelago (SECAC), Lanzarote, Canary Islands, Spain
- 5CIIMAR-Interdisciplinary Centre of Marine and Environmental Research of the University of Porto, Matosinhos, Portugal
- 6cE3c/Azorean Biodiversity Group, Departamento de Biologia, Faculdade de Ciências e Tecnologia, Universidade dos Açores, Ponta Delgada, Portugal
Knowledge of the distribution and residency of pelagic marine megafauna, particularly deep-diving species, is scarce due to their high mobility over difficult-to-access oceanic areas and long periods underwater. However, the threatened status of many of these species, such as the sperm whale Physeter macrocephalus, increases the need to obtain quantitative data to support conservation measures. In the warm temperate waters of Macaronesia (Eastern North Atlantic), sperm whales occur year-round in a set of island systems (the Azores, Madeira, and the Canaries), mainly in social groups of females and juveniles with the occasional visits of mature males. Although it is known that they perform inter-archipelago movements, information on site fidelity and residency times is still scarce. Here, based on photographic-identification data, site fidelity and residency times of sperm whales were estimated for subareas of the Azores and the Madeira archipelagos, with a preliminary assessment for a subarea of the Canaries. The Azores and Madeira subareas presented similar proportions of individuals with recaptures (~25%), mainly inter-annual, while in the subarea of the Canaries, only <10% of the individuals were recaptured. Standardized Site Fidelity Indexes showed very low values (<0.01) for both the Azores and Madeira subareas. Lagged identification rates based on models including emigration and reimmigration estimated that an average of 44.8 individuals (SE=4.9) spent 12.9 days (SE=1.5) in the Azores before leaving for 99.1 days (SE=12.5), while 8.4 individuals (SE=16.1) spent 0.8 day (SE=6.6) in Madeira before leaving for 8.6 days (SE=6.9), with a very low mortality rate. This study i) indicates a degree of residency of about ¼ of the identified individuals for the Azores and Madeira subareas and ii) supports that these oceanic archipelagos constitute an important habitat for a Vulnerable species in the Atlantic. Moreover, it also highlights the importance of combining data from opportunistic and dedicated surveys and joint national and international efforts toward the conservation of marine megafauna.
Introduction
Research and conservation of top oceanic predators present unique challenges due to their high mobility over difficult-to-access areas, with costly and logistically complex data collection. Most pelagic marine megafauna is not easily seen and has large ranges extending to offshore areas (Tittensor et al., 2010; Kaschner et al., 2011). In the case of deep-diving species, there are increased difficulties associated with their long submersion periods (Aoki et al., 2012; Li & Rosso, 2021; Badenas et al., 2022). Moreover, many of these species are of significant conservation concern and represent an ecologically and functionally important part of marine biodiversity (Katona & Whitehead, 1988; Schipper et al., 2008; Pimiento et al., 2020; Alves et al., 2022; Braun et al., 2022). Thus, information on the distribution and movements of these species is valuable for planning practical conservation efforts.
The sperm whale Physeter macrocephalus, the largest deep-diver and toothed animal, is distributed worldwide. It ranges from the ice edge in both hemispheres to tropical waters (Whitehead, 2018). Its distribution is highly connected to social structure and sex, with social groups of females and immatures inhabiting low and mid-latitudes. On the other hand, males leave their maternal groups and aggregate in bachelors groups for a few years before living mainly solitary in high latitudes, returning to tropical and subtropical waters to mate (Cantor et al., 2019).
Sperm whales are globally classified as Vulnerable by the International Union for Conservation of Nature, with an unknown worldwide population trend (Taylor et al., 2019), with recent studies indicating a global population of 844 761 individuals (Whitehead & Shin, 2022). This species was extensively hunted worldwide since the 18th century, growing from a shore-based enterprise to industrial whaling that only ceased in the 1980s. This caused a decrease of 68% in the global population, with males being more heavily targeted (Whitehead, 2002; Whitehead, 2018). Due to the low reproduction rates of these long-lived mammals, the populations of sperm whales are still recovering. However, presently, they still face several threats, such as entanglement in fishing gear, ingestion of plastics, chemical pollution, or ship strikes (Schipper et al., 2008; Savery et al., 2013; Notarbartolo-Di-Sciara, 2014; Fais et al., 2016; Whitehead, 2018; Arregui et al., 2019).
The Macaronesian archipelagos of the Azores, Madeira, and Canaries (Eastern North Atlantic) are some of the most isolated oceanic habitats of the North Atlantic, surrounded by steep submarine canyons and deep waters due to their volcanic origin and lack of continental shelf (Carracedo & Troll, 2021), which offer easy access to study deep-divers and oceanic species. Here, social groups of females and immature sperm whales are present year-round, with the occasional presence of visiting males (André, 1997; Silva et al., 2014; Fernandez et al., 2021). This biogeographic region is known to be used by sperm whales for reproduction, besides feeding and calving (Clarke, 1956; André, 1997; Steiner et al., 2012; Correia-Fagundes & Romano, 2013; Silva et al., 2014; Alves et al., 2018; Mullin et al., 2022). The sperm whale was the target species of a whaling activity that killed around 26 000 individuals in the Azores and Madeira, while in the Canaries it was a residual activity. This resulted in a reduction of 55% of the population in this region (Cabral et al., 2005; Brito, 2008; Perez, 2011). Currently, these three archipelagos are important destinations for whale-watching, with as many as 30 cetacean species identified so far, where the sperm whale is one of the target species in the Azores and, to a lesser extent, in Madeira (Freitas et al., 2012; Silva et al., 2014; Ferreira et al., 2017; Alves et al., 2018; Cartagena‐Matos et al., 2021; Herrera et al., 2021; McIvor et al., 2022). In Macaronesia, and specifically in the Canaries, collision with ships is nowadays a relevant threat to the population of sperm whales, presenting one of the world’s highest rates of ship strikes, with an annual average of two stranded whales from ship-strikes (Fais et al., 2016). Due to the oceanic habits of sperm whales, many more events may go unreported in offshore waters, creating a high level of conservation concern. Therefore, the sperm whale is still vulnerable to human-induced disturbances in these remote archipelagos.
To understand population movement patterns and life history, it is essential to evaluate site fidelity and residency (Baird et al., 2008; Tschopp et al., 2018). Site fidelity, defined as the tendency of an animal to return to a previously occupied place, is a well-documented behavior in many taxonomic groups (e.g., birds, Hoover, 2003; Iverson & Esler, 2006; seals, Lunn & Boyd, 1991; Pomeroy et al., 2001; insects, Switzer, 1997). It is known to provide evolutionary benefits and may increase survival (Greenwood, 1980; Switzer, 1993; Bose et al., 2017). Sperm whales, like other mammalian species (e.g., deers, Bose et al., 2017; elephants, Archie et al., 2006), demonstrate female philopatry and male dispersal due to the higher dependency of females on local resources (Greenwood, 1980). Male sperm whales show limited site fidelity to their feeding grounds, with few possible resident individuals (Jaquet et al., 2000; Lettevall et al., 2002; Rødland & Bjørge, 2015; Somerford et al., 2021). On the other hand, females exhibit site fidelity across years in several locations (e.g., Caribbean, Gero et al., 2014; Mediterranean Sea, Drouot-Dulau & Gannier, 2007), which may lead to genetic differentiation of specific populations (Engelhaupt et al., 2009).
Studies exploring site fidelity and residency of sperm whales in the oceanic environment of the Eastern North Atlantic are limited to the archipelago of the Azores, where both photographic-identification and genetic studies indicate some degree of site fidelity in females, although there are no permanent resident individuals (Matthews et al., 2001; Silva et al., 2006; Pinela et al., 2009; van der Linde & Eriksson, 2020). The more than 40 individual photographic-identification matches within the Macaronesian archipelagos of the Azores, Madeira, and Canaries (Steiner et al., 2015; Steiner, 2022) indicate that these animals carry out inter-archipelago movements and support the existence of a single population in this region of the Atlantic. Nevertheless, quantitative information on site fidelity and residency times is limited (to one archipelago) or unavailable for Macaronesia.
Here, photographic data of sperm whales from three subareas of Macaronesian archipelagos were used to investigate and quantify this species’ habitat use, with a main focus on Azores and Madeira. More specifically, composite indexes and likelihood techniques were applied to i) calculate the site fidelity of sperm whales in subareas of the Azores and Madeira, and ii) estimate residency times to inform on the movements in and out of these areas. Filling these knowledge gaps regarding population habitat use will provide novel insights into future coordinated efforts between the countries involved (i.e., Portugal and Spain) to establish transborder conservation measures.
Material and methods
Study area
This study was conducted in subareas of three oceanic archipelagos of Macaronesia: around Pico and Faial islands in the Azores (approximately 3 500 km2), south and southeast of Madeira island (approximately 800 km2), and along the eastern coast of Lanzarote and Fuerteventura in the Canaries (approximately 6 500 km2) (Figure 1). The biogeographical unit of Macaronesia, by definition, also includes Cabo Verde islands; however, recent studies support the exclusion of the latter due to considerable differences, specifically regarding marine biodiversity, and aggregates the three remaining archipelagos in one province within the Lusitanian ecoregion (Spalding et al., 2007; Freitas et al., 2019). These warm-temperate archipelagos are located in the Eastern North Atlantic Ocean, between latitudes 28 and 39°N, and share natural, geological, oceanographic, and biogeographical features (Freitas et al., 2019). The Azores archipelago is located approximately 1 800 km west of Lisbon (Portugal), around the Mid-Atlantic Ridge, and is surrounded by very narrow shelves and steep slopes, with the frequent presence of seamounts, and a mean depth of about 3 000 m (Morato et al., 2008). The Madeira archipelago is located approximately 1 000 km off the European continent and 500 km off the African coast, being also surrounded by steep submarine canyons and deep waters (approximately 1 500 m in depth) very close to the coast, due to the lack of a continental shelf (Geldmacher et al., 2000). The Canaries archipelago is located 100 km off the African coastline and is formed by seven main islands, that extend over 500 km. The average depth increases towards the west, from depths of 1 200 m in Lanzarote and Fuerteventura (the most eastern islands) to 4 000 m in La Palma and Hierro (the most western islands) (Valdés & Déniz-González, 2015).
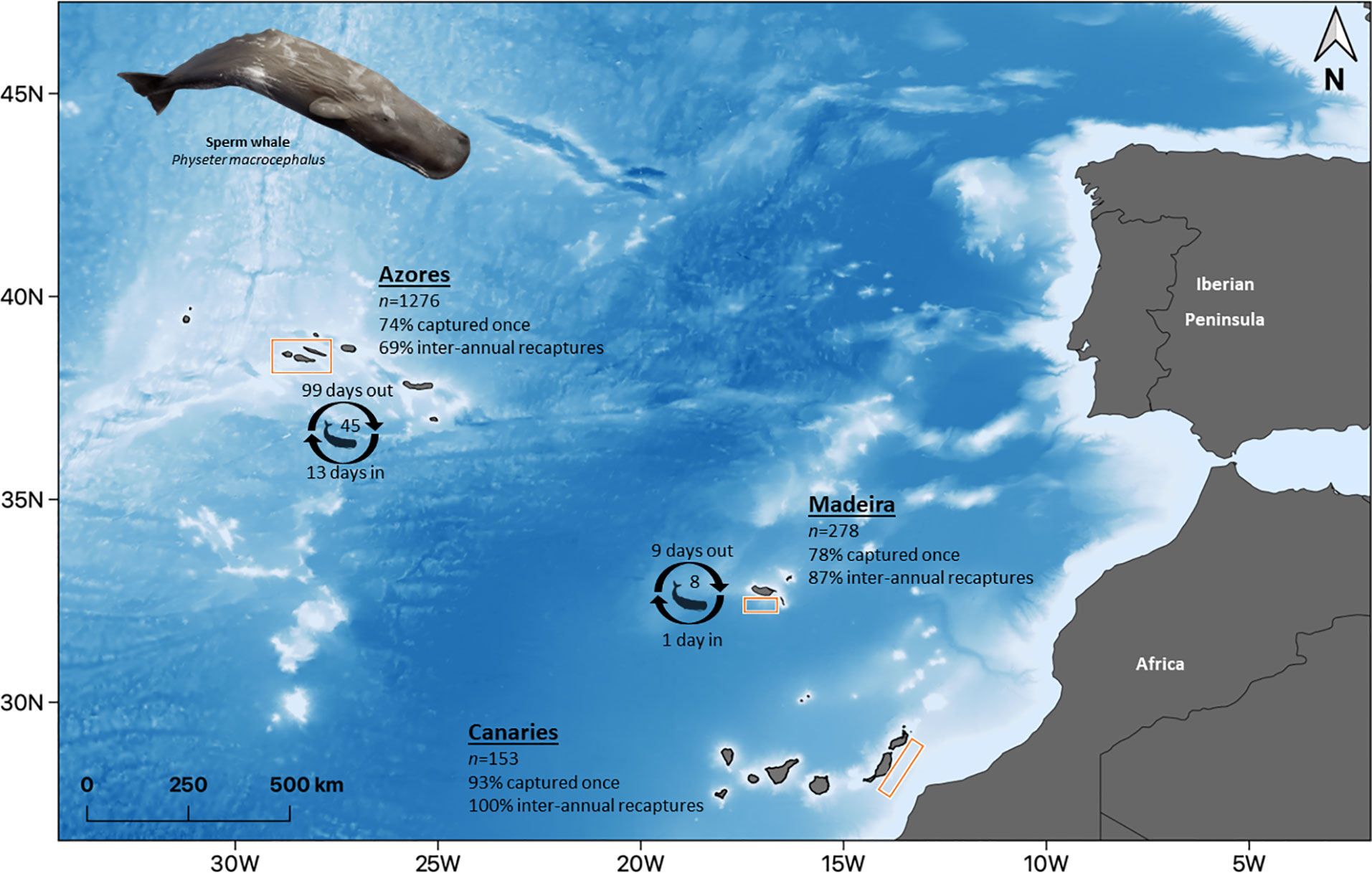
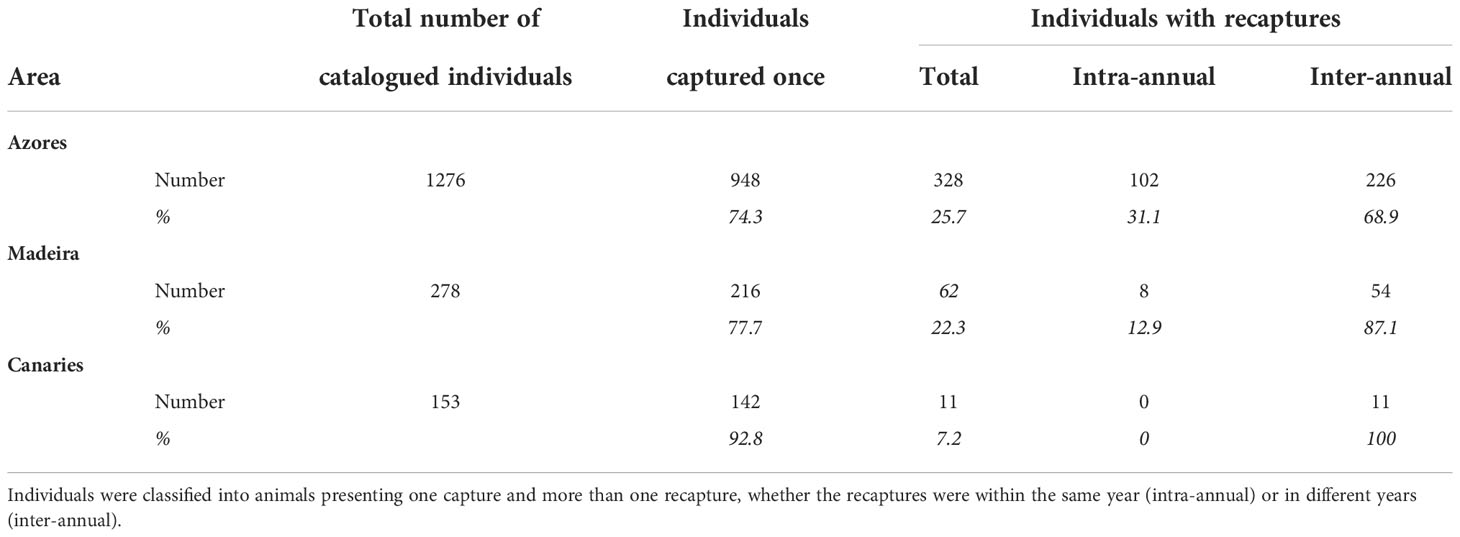
Figure 1 Location of the study area in the Eastern North Atlantic, Macaronesia, formed by the archipelagos of the Azores, Madeira, and the Canaries. Bathymetry ranging from approximately 1 000 to 4000 m, from white to blue, respectively. n indicates the number of identified individuals in each archipelago. Percentages refer to the proportion of individuals captured once and of individuals recaptured inter-annually. Number of individuals and time spent in and out of the area refer to the estimates of the best model of lagged identification rates (Table 2). Illustration by E. Berninsone © ARDITI.
Data collection and photographic analysis
Photographic-identification (hereafter, photo-id) data from sperm whales were collected in the three subareas. In the Azores, data was collected from April to October, from 2014 to 2019, during dedicated research and opportunistic surveys (whale-watching trips). In Madeira, data was collected year-round from 2007 to 2019 during dedicated research and opportunistic surveys (whale-watching trips). In the Canaries, data was collected year-round in 2009, 2011 and 2012 during dedicated research surveys.
In each subarea, photographs were collected and classified into a catalogue following standard photo-id procedures (Arnbom, 1987; Würsig & Jefferson, 1990). Sperm whale individuals were identified using photographs of the ventral or dorsal side of the fluke based on natural or acquired markings on the trailing edge. Scars and pigmentation patterns on the fluke and peduncle were used to confirm matches. Each photograph was graded for quality (from 1=poor to 4=excellent) and distinctiveness (from 1=non-distinctive to 4=very distinctive) (Alves et al., 2013). To maximize the reliability of each of the three catalogues (one per subarea), the analysis was limited to photographic quality and distinctiveness ratings from 2 to 4. Each catalogue was compiled visually by a single researcher and verified whenever needed by experienced secondary researchers.
For the three subareas, catalogues were analyzed to determine the number of individuals captured only once and of individuals that presented recaptures. Recaptured individuals were then classified taking into consideration if the recaptures were intra-annual (i.e. all the recaptures of the individual occurred within the same year) or inter-annual (i.e. at least one of the recaptures occurred in a different year). Percentages of the individuals captured once and with intra and inter-annual recaptures were then calculated, and the capture frequency histograms were plotted. Discovery curves were created by plotting the cumulative number of identifications against the number of identified individuals throughout the study period. When the population is fully identified, the curve reaches a plateau; but if the curve is continuously growing and no stabilization occurs, it means that there are still new individuals being added to the catalogue. This analysis was performed with Socprog 2.9 (Whitehead, 2009).
Site fidelity and residency analysis
Evaluation of site fidelity and residency were only conducted for the Azores and Madeira datasets, since the dataset from the Canaries presented very few recaptures, which did not allow further analysis. A truncated dataset was used for Madeira to homogenize the effort, restricting to the years with the highest effort, i.e. from 2014 to 2019.
Site fidelity of sperm whales was assessed using the Standardized Site Fidelity Index (SSFI), a composite site fidelity index developed by Tschopp et al. (2018). Definition and quantification of site fidelity varies greatly among research studies and is largely dependent on species behaviour, life cycle and research objectives, among others (Tschopp et al., 2018). Also, is usually done at an individual level. Therefore, the development of a standardized index that provided information of site fidelity at a populational level and allowed for comparison between studies was needed. SSFI was the index that had the best performance in all of the evaluated scenarios (both theoretical and with real data) and was calculated based on the indicators of permanence and periodicity.
Permanence (IT) is the proportion of time in the study area given by the time between the capture and last recapture (Fi), over the sampling period (F):
Periodicity (It) is the recurrence of an individual, determined by the inverse of the average time between successive recaptures:
where cij indicates a capture (one) or an absence to capture (zero) of an individual i on the sampling occasion j, and T is the number of sampling occasions.
SSFI is therefore defined as:
SSFI quantifies site fidelity at a populational level using capture-recapture data and varies between zero (population without site fidelity) and one (resident population). This index works when effort is not constant and when the detection of the subject presents difficulties. This is the case with cetaceans in general and sperm whales in particular, due to their long diving periods associated with feeding (Cantor et al., 2019).
Likelihood techniques were used to estimate parameters of residency models (Whitehead, 2001). These techniques use datasets where animals are identified individually, but the identifications are distributed neither randomly nor systematically in space or time, and where the identifications themselves are used as a measure of effort. To estimate residency times, we applied the models developed by Whitehead (2001), that evaluate the estimated population size in the study area, the amount of time an individual spends within an area and the movements into and out of that area. Lagged identification rates (LIR) were calculated, which estimate the probability that an individual identified in the study area at any given time will be identified again in the study area some time lag after (Whitehead, 2001). Due to overdispersion (when the variance inflation factor >3, which may represent fundamental problems with the data; Lebreton et al., 1992), data from the Azores was limited to the months with the most homogeneous number of identifications (June to September). Since overdispersion for the Madeira dataset <3, the entire year was used in the analysis. The sampling period was defined as day for both archipelagos. Estimated LIRs were compared to expected LIRs from exponential mathematical models of residency established by Whitehead (2001) and fitted using maximum-likelihood methods. The model with the lowest quasi-Akaike information criterion (QAIC) was selected as providing the best fit to the data (Whitehead, 2009). Precision (SE) was estimated using a bootstrap method. The analysis was performed with Socprog 2.9 (Whitehead, 2009).
Results
Photographic analysis
Information on the photographic analysis for the three archipelagos is presented in Table 1. The number of individuals identified in the Azores is higher than in Madeira and the Canaries. However, Azores and Madeira showed similarities in the percentages of individuals captured only once (74.3 and 77.7%, respectively) and, consenquently, of individuals with recaptures (25.7 and 22.3%, respectively). These two archipelagos also presented a higher prevalence of individuals recaptured in more than one year (68.9% for the Azores and 87.1% for Madeira). In the Canaries, only 11 individuals presented recaptures (maximum two recaptures), all captured on the same two dates in 2009 and 2011. In Madeira, there was a maximum of 14 inter-annual recaptures, while in the Azores, the maximum was 27 (Figure 2A). The discovery curves indicated that, for all archipelagos, the number of individuals identified has not stabilized, and therefore the whole population is yet to be sampled (Figure 2B). Nevertheless, the curves for the Azores and Madeira were very similar in shape, despite the differences in the number of identified individuals, and presented an initial tendency for stabilization. The Canaries curve was still in linear growth with no signs of stabilization.
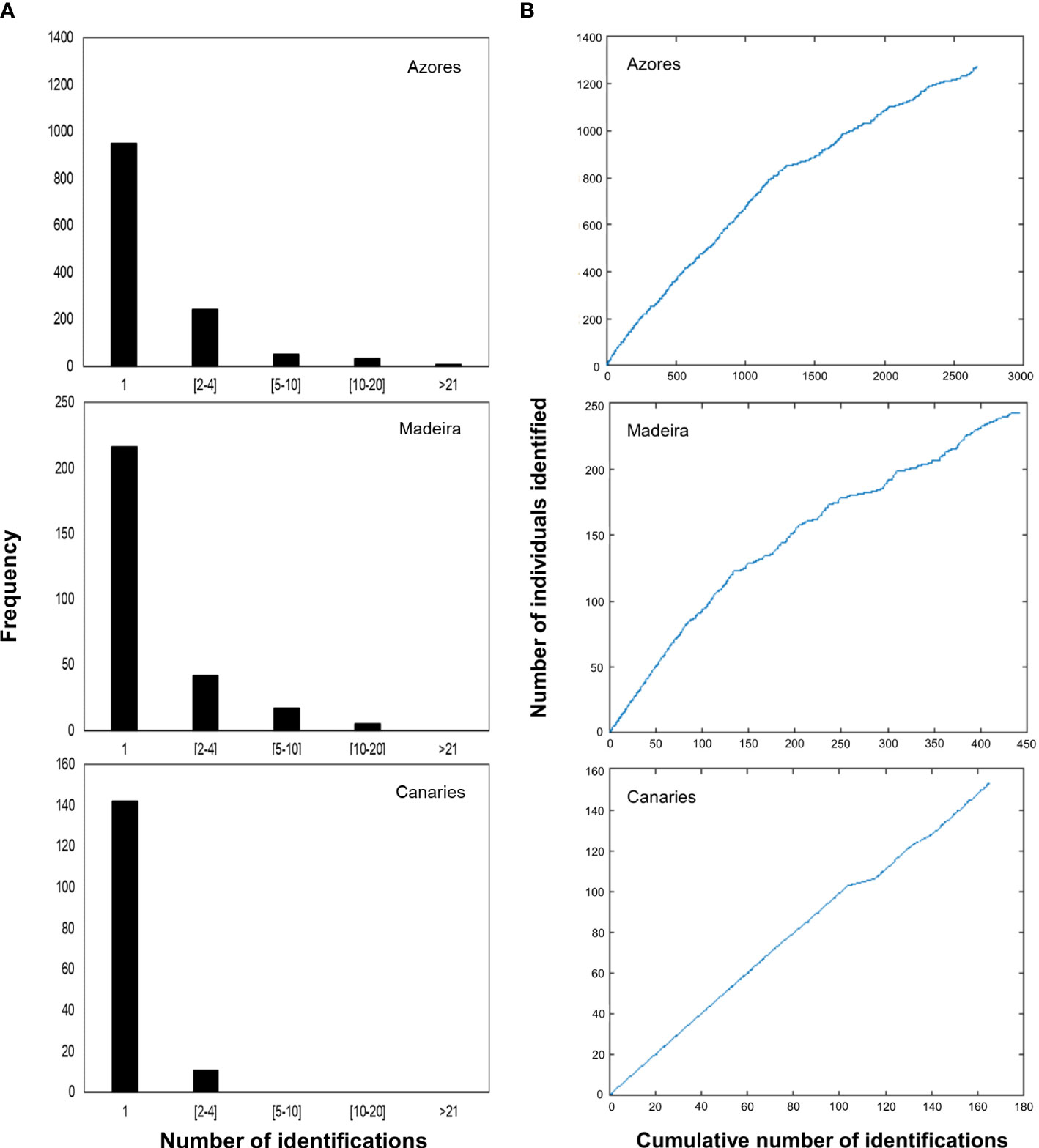
Figure 2 (A) Capture frequency histograms for individual sperm whales for the three subareas of the Azores, Madeira and the Canaries. Most of the individuals of the three subareas were captured only once. Captures were aggregated in categories to facilitate visualization. (B) Discovery curves for individual sperm whales in the three subareas, based on the cumulative number of identifications concerning the number of identified individuals throughout the study period.
Site fidelity and residency analysis
For the subarea of the Azores, the SSFI showed a median of 0.0067 (SD=0.0093, range 0.0056-0.0078; IT median=0.3207, SD=0.2818; It median = 0.0045, SD=0.1946). For the subarea of Madeira, SSFI presented a median of 0.0094 (SD=0.0069, range 0.0076-0.0112; IT median=0.3713, SD=0.2388; It median=0.0056; SD=0.1928) (Figure 3).
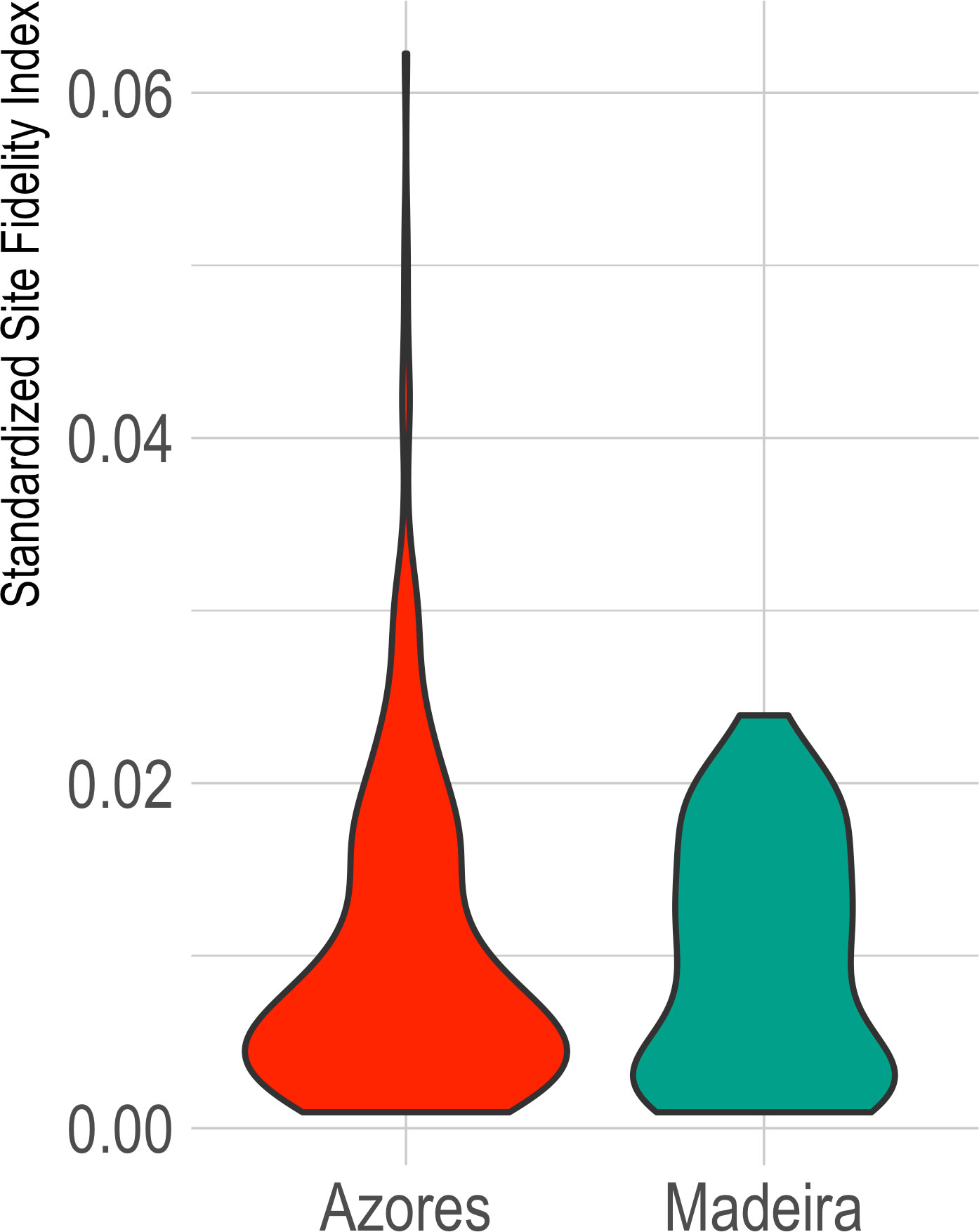
Figure 3 Violin chart for the Standardized Site Fidelity Index (SSFI) for the archipelagos of Azores and Madeira. SSFI varies between 0 and 1, with zero being a population without site fidelity and one for a resident population.
Four residency models were fitted to the lagged identification rate: “closed” (no changes in the individuals present in the area), “emigration/mortality” (individuals leave the area and never return), “emigration + reimmigration” (individuals leave the area and may return), and “emigration + reimmigration + mortality” (individuals leave the area and may or not return due to emigration or mortality) (Table 2). The model that best fitted the LIR for the Azores subarea was Emigration + reimmigration and for Madeira subarea was Emigration + reimmigration + mortality (Table 2, Figure 4). For the Azores subarea, from June to September, there was an average of 44.8 individuals (SE = 4.9) at any given time and individuals resided in the area for 12.9 days (SE = 1.5), before leaving for 99.1 days (SE = 12.5); goodness of fit x2 = 1643.563, df = 455, P = 0. For the Madeira subarea, there was an average of 8.4 individuals (SE = 16.1) at any given time and individuals resided in the area for 0.8 days (SE = 6.6) before leaving for 8.6 days (SE = 6.9), with a very low mortality rate of 0.0008 (SE = 0.0002); goodness of fit x2 = 91.534, df = 58, P = 0.0033.
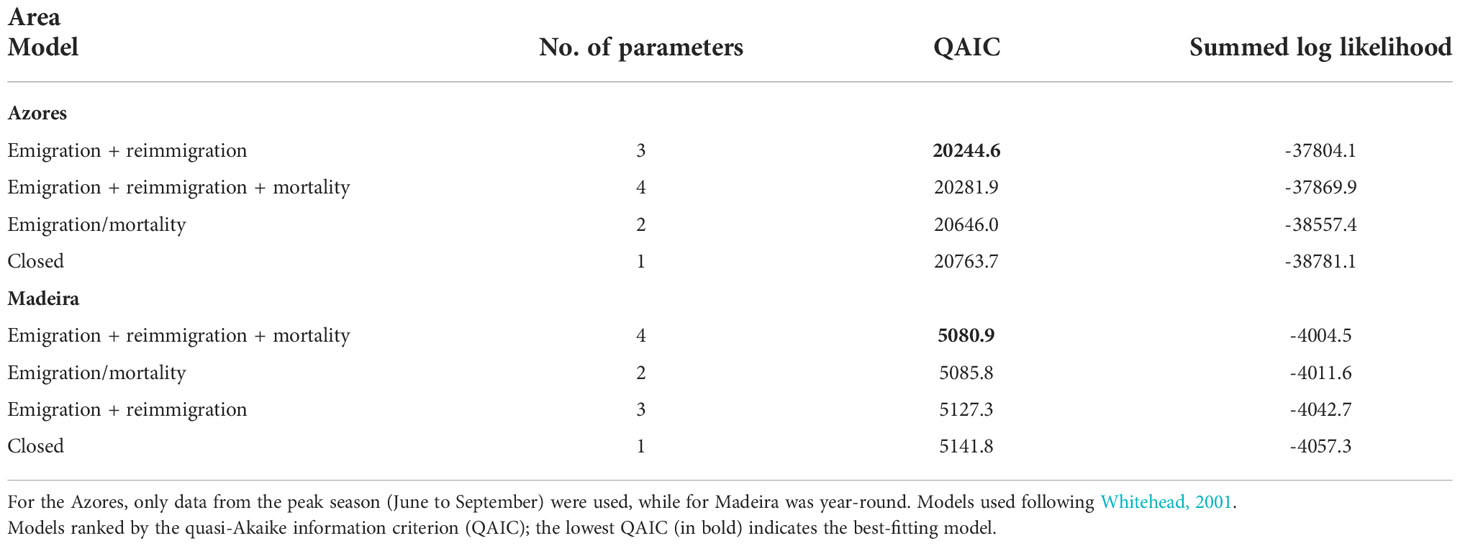
Table 2 Models fitted to lagged identification rates (LIRs) for sperm whales in the archipelagos of the Azores and Madeira from 2014 to 2019.
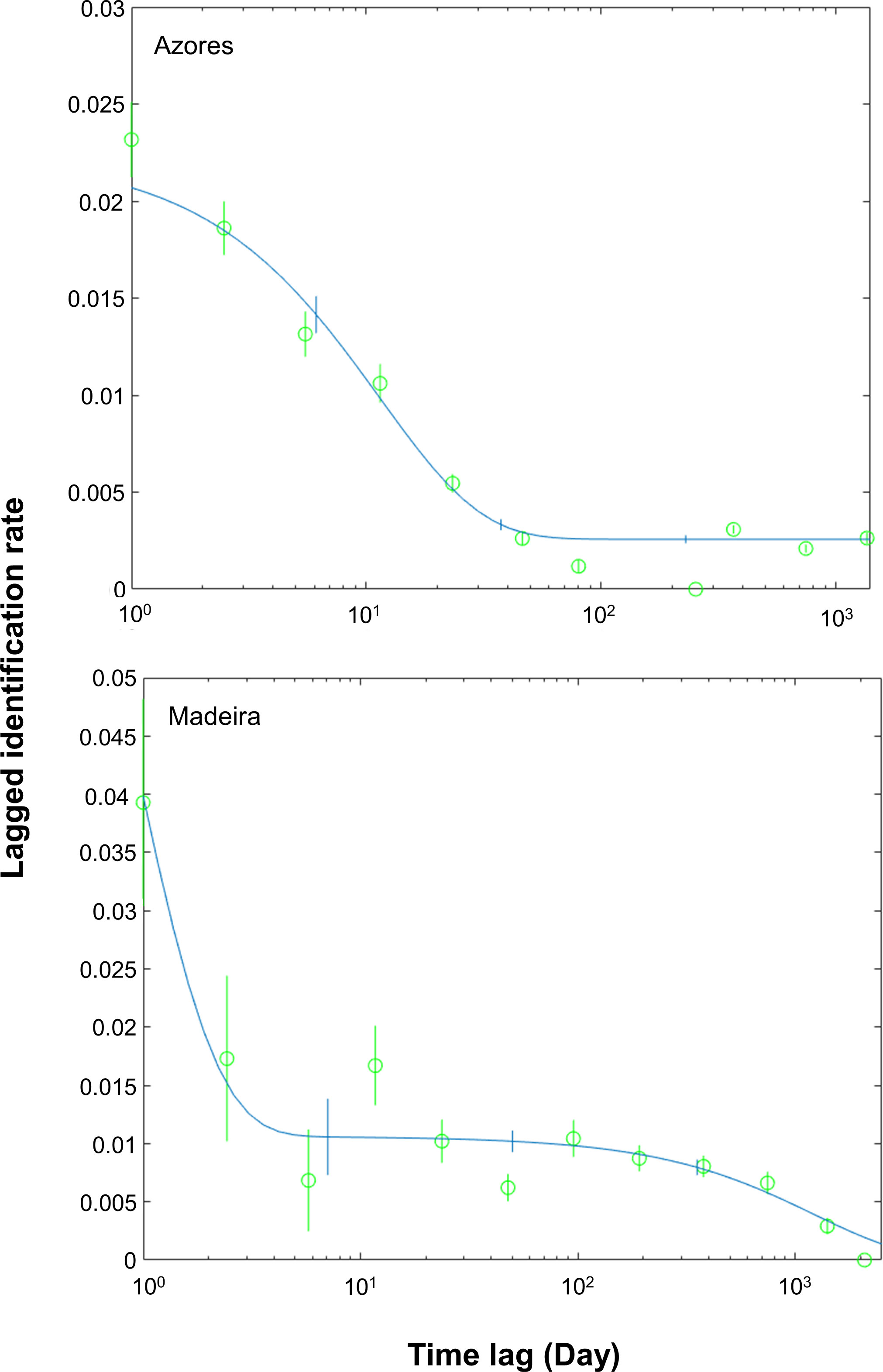
Figure 4 Lagged identification rates (LIRs) for sperm whales in the archipelagos the Azores (Silva et al., 2013; Prieto et al., 2014; González-Garcia et al., 2022) and Madeira from 2014 to 2019. For the Azores, analysis was restricted to the peak season, from June to September, while for Madeira was year-round. The figures show the probability that an individual identified in the study area at any time will be identified again in the study area some time lag after. The line represents the best-fitting model according to Table 2, and the vertical bars indicate standard errors calculated using the bootstrap method.
Discussion
This study provides the first assessment of site fidelity and residency of sperm whales in a remote oceanic environment in the Eastern North Atlantic. It brings forth valuable insights for a threatened species population whose offshore habitat and deep-diving behavior impair data collection. Through the collaborative effort from national and international teams, it was possible to identify areas in Macaronesia as important habitats for a portion of the population of sperm whales inhabiting the North Atlantic. Moreover, it is shown that individuals used this region intra- and inter-annually. Although this study brings forth important scientific knowledge, it is nonetheless a preliminary approach due to, among other factors, its geographic limitation that impairs the extrapolation of these conclusions to the whole Macaronesia. This first characterization allows to identify existing data gaps in Macaronesia and highlights the increasing need to obtain reliable quantitative data from more extended areas to obtain a solid assessment of sperm whales in this area of the Eastern North Atlantic. For the Canaries, the dataset did not allow for more than preliminary results, and therefore the main core of this study was conducted in subareas of the Azores and Madeira archipelagos. This study also highlighted the importance of using both opportunistic and dedicated effort when working with species displaying pelagic habits, such as the sperm whale. This contributed to a more profound knowledge that will allow implementing appropriate conservation measures.
The findings of this study are inferred from a combination of different analyses that support three broad main results. First, there is heterogeneity in capture probability, given that approximately ¼ of the identified individuals of the Azores and the Madeira subareas (25.7% and 22.3%, respectively) were captured more than once, with most of these (68.9% for the Azores and 87.1% for Madeira) presenting inter-annual recaptures. This result strongly indicates the importance of these subareas for a portion (¼) of the population that uses it on a regular basis, supporting previous studies (Silva et al., 2006; Boys et al., 2019; van der Linde & Eriksson, 2020). The Canaries dataset presents individuals captured mainly once (92.8%), which, together with the linear growth demonstrated by the discovery curve, indicates that the entire population is still far from being captured. This is most likely due to two reasons: i) low sampling effort, with the dataset covering only three years with homogenous effort and with a relative low number of identified individuals, and ii) geographic limitation (already a limitation for this study in general), with previous studies reporting a higher presence of sperm whales in other areas of the Canaries archipelago unsampled in this study (André, 1997; Fais et al., 2016; Correia et al., 2020; Herrera et al., 2021). Broader and more systematic research on sperm whales is needed for the Canaries, especially considering that this area could work as an sink habitat due to the high mortality associated with ship strikes (Fais et al., 2016). Taking into consideration the existing connection between Macaronesian archipelagos already demonstrated by photo-id and genetics (Pinela et al., 2009; Steiner et al., 2015; Steiner, 2022), this could be causing a decrease in the Macaronesian population (as demonstrated with the stranding in the Canaries in 2019 of an individual already sighted in the Azores, with signs of ship strike; Vidal Martín and Lisa Steiner own data). This impact could include the whole North Atlantic population if we consider the movement of males between Macaronesia, Norway, and the Bahamas (Steiner et al., 2012; Mullin et al., 2022).
Second, the site fidelity index values for the Azores and Madeira subareas are similarly low (0.0067 ± 0.0093 and 0.0094 ± 0.0069, respectively; SSFI varies between 0 and 1). This follows the results of the photo-id analysis and supports that only a minor part of the population presents site fidelity to these subareas, while the majority uses them as passage. Studies focusing on site fidelity of sperm whales in this area of the Atlantic are limited to the Azores archipelago and indicate a lack of geographical and genetic structure, providing indirect evidence of site fidelity over short periods as well as between years from part of a larger oceanic population (Matthews et al., 2001; Pinela et al., 2009). Sperm whales are known as ocean nomads, with both solitary males and social groups of females and juveniles traveling thousands of kilometers regularly (Cantor et al., 2019), although recent studies have identified populations with solid site fidelity (e.g., Gero et al., 2014; Vachon et al., 2022). The complex social structure and the large spatial and temporal scales in which sperm whales occur are challenging for understanding their populations and ecology (Kaschner et al., 2012). Differences arise not only between populations but also between oceans, with the North Atlantic populations of sperm whales being more geographically and genetically structured than the Pacific, demonstrating shorter range movements and smaller group sizes, together with a higher number of calves (Whitehead et al., 2012). Therefore, extrapolating results across geographical areas without corroborating them with regional observations could provide incorrect conclusions (Kaschner et al., 2011; Vachon et al., 2022).
Third, the LIR estimates for the Azores and Madeira subareas support the previous results, with individuals spending more extended periods out of the sampled areas than within. For each area, the best model presented differences in QAIC that vastly surpassed the minimum value of two required for the model choice, reinforcing the selection of the best-fitting model as the most appropriate one (Burnham & Anderson, 2002). This is also in agreement with the model selected from an ecological viewpoint, given the high levels of emigration and reimmigration expected from highly mobile species inhabiting vast oceanic areas, as also shown by other cetaceans in the region (Silva et al., 2013; Prieto et al., 2014; Dinis et al., 2016; Alves et al., 2019; Ferreira et al., 2021; Badenas et al., 2022; González-Garcia et al., 2022). Moreover, previous studies on the target species for the Azores Archipelago support these results (Silva et al., 2006; Boys et al., 2019; van der Linde & Eriksson, 2020), while for Madeira, this is the first study to conduct such analysis.
This study presents inevitable limitations associated with data collection, by joining information from multiple platforms across several areas, that covered only a small part of each archipelago. This invalidates the comparison between archipelagos, providing instead a characterization for each of the surveyed subareas: Pico and Faial islands in the Azores, south and southeast of Madeira island, and the eastern coast of Lanzarote and Fuerteventura in the Canaries. Also, while in Madeira and the Canaries the surveys took place year-round, in the Azores the weather conditions in the Winter invalidated such temporal scale, and data does not cover the entire year. However, the extended data collection period, together with the use of only good quality pictures and distinctive individuals, helped minimizing biases. In the Canaries, the smaller dataset hindered part of the analysis, and therefore more effort is needed for conclusions to be made regarding this area. This is already taking place with an ongoing project dedicated to the sperm whales in the Canaries. Opportunistic data is increasingly being used in cetacean research (e.g., Moura et al., 2012; Hupman et al., 2015; Alves et al., 2018; Fernandez et al., 2021). Although it presents limitations, those can be surpassed with adequate data analysis. The chosen index for this study, SSFI (Tschopp et al., 2018), is appropriate for situations where detection is not perfect, and the effort is heterogeneous, as in our study, thus providing robust quantifications of site fidelity at a populational level. This index accounts for the behavioral aspects of the target species and the characteristics of the sampling effort, which significantly improved the reliability of these results. Moreover, the use of likelihood techniques for residency parameters takes into consideration heterogeneous effort (Whitehead, 2001; Vachon et al., 2022).
Knowledge of biogeographical movement patterns is still limited for most pelagic species. Nevertheless, it is pivotal since many animals may encompass large geographical ranges within and beyond national waters (Dunn et al., 2019). The sperm whale is a cosmopolitan species with a complex differentiated behavior between sexes and populations. Yet, although having been the target of several studies worldwide (e.g., Drouot-Dulau & Gannier, 2007; Engelhaupt et al., 2009; Whitehead et al., 2012; Boys et al., 2019; Cantor et al., 2019), information on movements at the individual level is scarce for many populations. Its global threatened statuses require dedicated effort to establish conservation measures; however, its oceanic habitat hinders data collection and the coordination between stakeholders and governments. Conservation measures should include not only the core-used areas where social groups spend most of their time, exhibiting higher degrees of philopatry, but also the corridors used by males during their migrations between feeding and breeding grounds (Gero et al., 2014; Sahri et al., 2022). Remote islands such as the ones in Macaronesia provide an excellent location for studying this marine predator and/or the effects of anthropogenic threats, but surveillance of the open ocean is paramount since only a small part of the population approaches the islands regularly. For example, recent assessments of the cetaceans’ vulnerability to climate change in the biogeographic region of Macaronesia showed that the sperm whale presented a moderate to high vulnerability score (Sousa et al., 2019; Sousa et al., 2021). All combined, identifying the critical habitats for sperm whales, both offshore and closer to islands, as well as quantifying parameters of fidelity and residency at the individual level, is a crucial issue in the conservation of populations that may show considerable variability in their habitat use (Vachon et al., 2022).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RF, FA conceived the study design. RF, MF analyzed the data. RF wrote the original draft of the manuscript. RF, LS, VM, FFP, AD, FA supported the data collection and organized the databases. MK, FA supervised the work. All authors contributed to the article and approved the submitted version.
Funding
This study had the support of FCT through the strategic project UIDB/04292/2020 awarded to MARE and through the project LA/P/0069/2020 granted to the Associate Laboratory ARNET. It was also supported through the strategic project M1420-01-0142-FEDER-000001 for the Oceanic Observatory of Madeira and from Oceanário de Lisboa and Oceano Azul Foundation through Whale Tales Project (ODL/2019/003). RF was supported by the FCT grant SFRH/BD/147225/2019, MF by the MAC2/1.1a/385 in the framework of INTERTAGUA (MAC INTERREG 2014-2020), AD by ARDITI throughout the project M1420-09-5369-FSE-000002 and FA by the FCT project UIDP/04292/2020 granted to MARE.
Acknowledgments
We thank all researchers, volunteers, whale-watching operators and lookouts, and photographers who contributed with data. Thanks to Biosphere Expeditions for supporting research in the Spring in the Azores. In the Canary Islands, this research has been carried out with the scientific permission of the Ministerio de Transición Ecológica of the Government of Spain.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alves F., Alessandrini A., Servidio A., Mendonça A. S., Hartman K. L., Prieto R., et al. (2019). Complex biogeographical patterns support an ecological connectivity network of a large marine predator in the northeast Atlantic. Divers. Distrib. 25 (2), 269–284. doi: 10.1111/ddi.12848
Alves F., Ferreira R., Fernandes M., Halicka Z., Dias L., Dinis A. (2018). Analysis of occurrence patterns and biological factors of cetaceans based on long-term and fine-scale data from platforms of opportunity: Madeira island as a case study. Mar. Ecol. 39 (2), e12499. doi: 10.1111/maec.12499
Alves F., Quérouil S., Dinis A., Nicolau C., Ribeiro C., Freitas L., et al. (2013). Population tructure of short-finned pilot whales in the oceanic archipelago of Madeira based on photo-identificationcation and genetic analyses: Implications for conservation. Aquat. Conserv.: Mar. Freshw. Ecosyst. 23 (5), 758–776. doi: 10.1002/aqc.2332
Alves F., Rosso M., Li S., Nowacek D. P. (2022). A sea of possibilities for marine megafauna. Science 375 (6579), 391–392. doi: 10.1126/science.abn6022
André M. (1997). Distribución y conservación del cachalote (Physeter macrocephalus) en las islas canarias. PhD thesis (Gran Canaria, Canaries, Spain: University of Las Palmas de Gran Canaria).
Aoki K., Amano M., Mori K., Kourogi A., Kubodera T., Miyazaki N. (2012). Active hunting by deep-diving sperm whales: 3D dive profiles and maneuvers during bursts of speed. Mar. Ecol. Prog. Ser. 444, 289–301. doi: 10.3354/meps09371
Archie E. A., Moss C., Alberts S. C. (2006). The ties that bind: genetic relatedness predicts the fission and fusion of social groups in wild African elephants. Proc. R. Soc B 273, 513e522. doi: 10.1098/rspb.2005.3361
Arnbom T. (1987). Individual identification of sperm whales. Rep. Int. Whal. Comm 37 (SC/38/Sp4), 201–204.
Arregui M., Quirós Y., Saavedra P., Sierra E., Suárez-Santana C., Arbelo M., et al. (2019). Fat embolism and sperm whale ship strikes. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00379
Badenas A., Dinis A., Ferreira R., Sambolino A., Hamard E., Berninsone L., et al. (2022). Behavioural ecology traits of elusive deep-diver whales unravel a complex social structure influenced by female philopatry and defence polygyny. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.809902
Baird R., Webster D., Mahaffy S., McSweeney D., Schorr G., Ligon A. (2008). Site fidelity and association patterns in a deep-water dolphin: Rough-toothed dolphins ( Steno bredanensis ) in the Hawaiian archipelago. Mar. Mamm. Sci. 24 (3), 535–553. doi: 10.1111/j.1748-7692.2008.00201.x
Bose S., Forrester T., Brazeal J., Sacks B., Casady D., Wittmer H. (2017). Implications of fidelity and philopatry for the population structure of female black-tailed deer. Behav. Ecol. 28 (4), 983–990. doi: 10.1093/beheco/arx047
Boys R., Oliveira C., Pérez-Jorge S., Prieto R., Steiner L., Silva M. (2019). Multi-state open robust design applied to opportunistic data reveals dynamics of wide-ranging taxa: The sperm whale case. Ecosphere 10 (3), e02610. doi: 10.1002/ecs2.2610
Braun C., Arostegui M., Thorrold S., Papastamatiou Y., Gaube P., Fontes J., et al. (2022). The functional and ecological significance of deep diving by large marine predators. Ann. Rev. Mar. Sci. 14 (1), 129–159. doi: 10.1146/annurev-marine-032521-103517
Brito C. (2008). Assessment of catch statistics during the land-based whaling in Portugal. Mar. Biodiver. Rec. 1, e92. doi: 10.1017/S175526720700930X
Burnham K. P., Anderson D. R. (2002). Model selection and multimodel inference: a practical information theoretic approach (New York: Springer Verlag).
Cabral M. J., Almeida J., Almeida P. R., Dellinger T., Almeida N. F., Oliveira M. E., et al. (2005). Livro vermelho dos vertebrados de Portugal (Lisboa: Instituto de Conservação da Natureza).
Cantor M., Gero S., Whitehead H., Rendell L. (2019). “Sperm Whale: The Largest Toothed Creature on Earth”, in Ethology and Behavioral Ecology of Odontocetes. Eds. Würsig B.. Ethology and behavioral ecology of odontocetes (Springer, Cham). doi: 10.1007/978-3-030-16663-2_12
Carracedo J., Troll V. (2021). “North-East Atlantic islands: The macaronesian archipelagos,” in Encyclopedia of geology, 2nd Edition. Eds. Alderton D., Elias S. A. (Academic Press), 674–699. doi: 10.1016/B978-0-08-102908-4.00027-8
Cartagena-Matos B., Lugué K., Fonseca P., Marques T., Prieto R., Alves F. (2021). Trends in cetacean research in the Eastern north Atlantic. Mamm. Rev. 51 (3), 436–453. doi: 10.1111/mam.12238
Correia-Fagundes C., Romano H. (2013). Observation of a birth of a sperm whale Physeter macrocephalus at Madeira (NE Atlantic). Bocagiana 236, 1–3.
Correia M., Gil A., Valente R., Rosso M., Sousa-Pinto I., Pierce G. (2020). Distribution of cetacean species at a large scale - connecting continents with the macaronesian archipelagos in the Eastern north Atlantic. Divers. Distrib. 26 (10), 1234–1247. doi: 10.1111/ddi.13127
Dinis A., Alves F., Nicolau C., Ribeiro C., Kaufmann M., Cañadas A., et al. (2016). Bottlenose dolphins Tursiops truncatus group dynamics, site fidelity, residency and movement patterns in the Madeira archipelago (North-East Atlantic). Afr. J. Mar. Sci. 38 (2), 151–160. doi: 10.2989/1814232X.2016.1167780
Drouot-Dulau V., Gannier A. (2007). Movements of sperm whale in the Western Mediterranean Sea: Preliminary photo-identification results. J. Mar. Biolog. Assoc. U.K. 87 (1), 195–200. doi: 10.1017/S0025315407054860
Dunn D., Harrison A., Curtice C., DeLand S., Donnelly B., Fujioka E., et al. (2019). The importance of migratory connectivity for global ocean policy. Proc. R. Soc B 286 (1911), 20191472. doi: 10.1098/rspb.2019.1472
Engelhaupt D., Hoelzel A., Nicholson C., Frantzis A., Mesnick S., Gero S., et al. (2009). Female philopatry in coastal basins and male dispersion across the north Atlantic in a highly mobile marine pecies, the sperm whale (Physeter macrocephalus): Sperm whale female philopatry and male dispersal. Mol. Ecol. 18 (20), 4193–4205. doi: 10.1111/j.1365-294X.2009.04355.x
Fais A., Lewis T., Zitterbart D., Álvarez O., Tejedor A., Soto N. A. (2016). Abundance and distribution of sperm whales in the canary islands: can sperm whales in the archipelago sustain the current level of ship-strike mortalities? PLos One 11 (3), e0150660. doi: 10.1371/journal.pone.0150660
Fernandez M., Alves F., Ferreira R., Fischer J. C., Thake P., Nunes N., et al. (2021). Modeling fine-scale cetaceans' distributions in oceanic islands: Madeira archipelago as a case study. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.688248
Ferreira R., Alves F., Gomes C., Jardim D., Kok J., Dinis A. (2017). First confirmed record of northern bottlenose whale (Hyperoodon ampullatus) in Madeira archipelago, northeast Atlantic. Aquat. Mamm. 43 (5), 474–478. doi: 10.1578/AM.43.5.2017.474
Ferreira R., Dinis A., Badenas A., Sambolino A., Marrero-Pérez J., Crespo A., et al. (2021). Bryde's whales in the north-East Atlantic: New insights on site fidelity and connectivity between oceanic archipelagos. Aquat. Conserv: Mar. Freshw. Ecosyst. 31 (10), 2938–2950. doi: 10.1002/aqc.3665
Freitas L., Dinis A., Nicolau C., Ribeiro C., Alves F. (2012). New records of cetacean species for Madeira archipelago with an updated checklist. Bol. Mus. Mun. Funchal 62, 25–43.
Freitas R., Romeiras M., Silva L., Cordeiro R., Madeira P., González J., et al. (2019). Restructuring of the 'Macaronesia' biogeographic unit: a marine multi-taxon biogeographical approach. Sci. Rep. 9 (1), 15792. doi: 10.1038/s41598-019-51786-6
Geldmacher J., Van Den Bogaard P., Hoernle K., Schmincke H. U. (2020). The 40Ar/39Ar age dating of the Madeira Archipelago and hotspot track (eastern North Atlantic). Geochemistry, Geophysics, Geosystems 1 (2), 1999GC000018. doi: 10.1029/1999GC000018
Gero S., Milligan M., Rinaldi C., Francis P., Gordon J., Carlson C., et al. (2014). Behavior and social structure of the sperm whales of Dominica, West indies. Mar. Mamm. Sci. 30 (3), 905–922. doi: 10.1111/mms.12086
González Garcia L., Pierce G. J., Autret, Palenzuela J. M. T. (2022). Alongside but separate: Sympatric baleen whales choose different habitat conditions in São Miguel, Azores. Deep Sea Res. 184, 103766. doi: 10.1016/j.dsr.2022.103766
Greenwood P. (1980). Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162. doi: 10.1016/S0003-3472(80)80103-5
Herrera I., Carrillo M., Esteban M. C., Haroun R. (2021). Distribution of cetaceans in the canary islands (Northeast Atlantic ocean): implications for the natura 2000 network and future conservation measures. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.669790
Hoover J. (2003). Decision rules for site fidelity in a migratory bird, the prohonotary warbler. Ecology 84 (3), 416–430. doi: 10.1890/0012-9658(2003)084[0416:DRFSFI]2.0.CO;2
Hupman K., Visser F., Martinez E., Stockin K. (2015). Using platforms of opportunity to determine the occurrence and group characteristics of orca (Orcinus orca) in the hauraki gulf, new Zealand. N. Z. J. Mar. Freshw. Res. 49 (1), 132–149. doi: 10.1080/00288330.2014.980278
Iverson S., Esler D. (2006). Site fidelity and the demographic implications of winter movements by a migratory bird, the harlequin duck Histrionicus histrionicus. J. Avian Biol. 37 (3), 219–228. doi: 10.1111/j.2006.0908-8857.03616.x
Jaquet N., Dawson S., Slooten E. (2000). Seasonal distribution and diving behaviour of male sperm whales off kaikoura: foraging implications. Can. J. Zool. 78 (3), 407–419. doi: 10.1139/z99-208
Kaschner K., Quick N., Jewell R., Williams R., Harris C. (2012). Global coverage of cetacean line-transect surveys: status quo, data gaps and future challenges. PLos One 7 (9), e44075. doi: 10.1371/journal.pone.0044075
Kaschner K., Tittensor D., Ready J., Gerrodette T., Worm B. (2011). Current and future patterns of global marine mammal biodiversity. PLos One 6 (5), e19653. doi: 10.1371/journal.pone.0019653
Katona S., Whitehead H. (1988). Are cetacea ecologically important? Oceanogr. Mar. Biol. 26, 553–568.
Lebreton J. D., Burnham K. P., Clobert J., Anderson D. R. (1992). Modelling survival and testing biological hypotheses using marked animals: A unified approach with case studies. Ecol. Monog. 62, 67–118. doi: 10.2307/2937171
Lettevall E., Richter C., Jaquet N., Slooten E., Dawson S., Whitehead H., et al. (2002). Social structure and residency in aggregations of male sperm whales. Can. J. Zool. 80 (7), 1189–1196. doi: 10.1139/z02-102
Li S., Rosso M. (2021). Lack of knowledge threatens beaked whales. Science 371 (6531), 791. doi: 10.1126/science.abg892
Lunn N., Boyd I. (1991). Pupping-site fidelity of Antarctic fur seals at bird island, south Georgia. J. Mammal. 72 (1), 202–206. doi: 10.2307/1381999
Matthews J. N., Steiner L., Gordon J. (2001). Mark-recapture analysis of sperm whale (Physeter macrocephalus) photo-id data from the azores, (1987–1995). J. Cetacean Res. Manage. 3 (3), 219–226.
McIvor A., Williams C., Alves F., Dinis A., Pais M., Canning-Clode J. (2022). The status of marine megafauna research in macaronesia: a systematic review. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.819581
Morato T., Machete M., Kitchingman A., Tempera F., Lai S., Menezes G., et al. (2008). Abundance and distribution of seamounts in the Azores. Mar. Ecol. Prog. Ser. 357, 17–21. doi: 10.3354/meps07268
Moura A., Sillero N., Rodrigues A. (2012). Common dolphin (Delphinus delphis) habitat preferences using data from two platforms of opportunity. Acta Oecol. 38, 24–32. doi: 10.1016/j.actao.2011.08.006
Mullin K., Steiner L., Dunn C., Claridge D., González L., Gordon J., et al. (2022). Long-range longitudinal movements of sperm whales (Physeter macrocephalus) in the north Atlantic ocean revealed by photo-identification. Aquat. Mamm. 48 (1), 3–8. doi: 10.1578/AM.48.1.2022.3
Notarbartolo-Di-Sciara G. (2014). Sperm whales, Physeter macrocephalus, in the Mediterranean Sea: a summary of status, threats, and conservation recommendations. Aquat. Conserv: Mar. Freshw. Ecosyst. 24 (S1), 4–10. doi: 10.1002/aqc.2409
Perez J. (2011). The cetacean fishing in the canary islands. Anuario Estudos Atlânticos 57, 277–300.
Pimiento C., Leprieur F., Silvestro D., Lefcheck J. S., Albouy C., Rasher D. B., et al. (2020). Functional diversity of marine megafauna in the anthropocene. Sci. Adv. 6 (16), eaay7650. doi: 10.1126/sciadv.aay7650
Pinela A., Quérouil S., Magalhães S., Silva M., Prieto R., Matos J. A., et al. (2009). Population genetics and social organization of the sperm whale (Physeter macrocephalus) in the Azores inferred by microsatellite analyses. Can. J. Zool. 87 (9), 802–813. doi: 10.1139/Z09-066
Pomeroy P., Twiss S., Redman P. (2001). Philopatry, site fidelity and local kin associations within grey seals breeding colonies. Ethology 106 (10), 899–919. doi: 10.1046/j.1439-0310.2000.00610.x
Prieto R., Silva M. A., Waring G. T., Gonçalves J. M. A. (2014). Sei whale movements and behaviour in the North Atlantic inferred from satellite telemetry. Endang. Species Res. 26, 103–113. doi: 10.3354/esr00630
Rødland E., Bjørge A. (2015). Residency and abundance of sperm whales (Physeter macrocephalus) in the bleik canyon, Norway. Mar. Biol. Res. 11 (9), 974–982. doi: 10.1080/17451000.2015.1031800
Sahri A., Jak C., Putra M., Murk A., Andrews-Goff V., Double M., et al. (2022). Telemetry-based home range and habitat modelling reveals that the majority of areas important for pygmy blue whales are currently unprotected. Biol. Conserv. 272, 109594. doi: 10.1016/j.biocon.2022.109594
Savery L., Evers D., Wise S., Falank C., Wise J., Gianios C., et al. (2013). Global mercury and selenium concentrations in skin from free-ranging sperm whales (Physeter macrocephalus). Sci. Total Environ. 450–451, 59–71. doi: 10.1016/j.scitotenv.2013.01.070
Schipper J., Chanson J., Chiozza F., Cox N., Hoffmann M., Katariya V., et al. (2008). The status of the world's land and marine mammals: diversity, threat, and knowledge. Science 322 (5899), 225–230. doi: 10.1126/science.1165115
Silva M., Magalhães S., Prieto R., Quérouil S., Pinela A., Seabra M. I., et al. (2006). Ecologia e estrutura populacional dos roazes e cachalotes nos açores: relação com as características do habitat. Arquivos Do DOP Série Estudos 4, 1–30.
Silva M., Prieto R., Jonsen I., Baumgartner M. F., Santos R. S. (2013). North Atlantic blue and fin whales suspend their spring migration to forage in middle latitudes: building up energy reserves for the journey? Plos One 8 (10), e76507. doi: 10.1371/journal.pone.0076507
Silva M., Prieto R., Cascão I., Seabra M., Machete M., Baumgartner M., et al. (2014). Spatial and temporal distribution of cetaceans in the mid-Atlantic waters around the Azores. Mar. Biol. Res. 10 (2), 123–137. doi: 10.1080/17451000.2013.793814
Somerford T., Dawson S., Slooten E., Guerra M., Childerhouse S., Richter C., et al. (2021). Long-term decline in abundance of male sperm whales visiting kaikōura, new Zealand. Mar. Mamm. Sci. 38 (2), 606–625. doi: 10.1111/mms.12886
Sousa A., Alves F., Arranz P., Dinis A., Fernandez M., Gonzalez L., et al. (2021). Climate change vulnerability of cetaceans in macaronesia: insights from a trait-based assessment. Sci. Total Environ. 795, 148652. doi: 10.1016/j.scitotenv.2021.148652
Sousa A., Alves F., Dinis A., Bentz J., Cruz M. J., Nunes J. P. (2019). How vulnerable are cetaceans to climate change? developing and testing a new index. Ecol. Indic. 98, 9–18. doi: 10.1016/j.ecolind.2018.10.046
Spalding M., Fox H., Allen G., Davidson N., Ferdaña Z., Finlayson M., et al. (2007). Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. BioScience 57 (7), 573–583. doi: 10.1641/B570707
Steiner L. (2022). “Sperm whale movements around the north Atlantic and a glimpse at the new fluke matching algorithm,” in Cachalote Consortium Workshop, 24th Biennial Conference on the Biology of Marine Mammals, Palm Beach, USA, 1-5 August.
Steiner L., Lamoni L., Plata M., Jensen S., Lettevall E., Gordon J. (2012). A link between male sperm whales, Physeter macrocephalus, of the Azores and Norway. J. Mar. Biolog. Assoc. U.K. 92 (8), 1751–1756. doi: 10.1017/S0025315412000793
Steiner L., Pérez M., van der Linde M., Freitas L., Santos R. P., Martin V., et al. (2015). “Long distance movements of female/immature sperm whales in the north atlantic [Poster],” in 21st Biennial Conference of Society of Marine Mammalogy, San Francisco, USA, 13-18 December.
Switzer P. V. (1993). Site fidelity in predictable and unpredictable habitats. Evol. Ecol. 7, 533–555. doi: 10.1007/BF01237820
Switzer P. (1997). Factors affecting site fidelity in a territorial animal, Perithemis tenera. Anim. Behav. 53 (4), 865–877. doi: 10.1006/anbe.1996.0352
Taylor B. L., Baird R., Barlow J., Dawson S. M., Ford J., Mead J. G., et al (2019). Physeter macrocephalus (amended version of 2008 assessment). The IUCN Red List of Threatened Species. 2019, e.T41755A160983555. doi: 10.2305/IUCN.UK.2008.RLTS.T41755A160983555.en. Accessed on 28 November 2022.
Tittensor D. P., Mora C., Jetz W., Lotze H., Ricard D., Berghe E., et al. (2010). Global patterns and predictors of marine biodiversity across taxa. Nature 466 (7310), 1098–1101. doi: 10.1038/nature09329
Tschopp A., Ferrari M., Crespo E., Coscarella M. (2018). Development of a site fidelity index based on population capture-recapture data. PeerJ 6, e4782. doi: 10.7717/peerj.4782
Vachon F., Hersh T., Rendell L., Gero S., Whitehead H. (2022). Ocean nomads or island specialists? culturally driven habitat partitioning contrasts in scale between geographically isolated sperm whale populations. R. Soc Open Sci. 9 (5), 211737. doi: 10.1098/rsos.211737
Valdés L., Déniz-González I. (Eds.) (2015). Oceanographic and biological features in the canary current Large marine ecosystem (Paris: IOC-UNESCO), 383.
van der Linde M., Eriksson I. (2020). An assessment of sperm whale occurrence and social structure off são Miguel island, Azores using fluke and dorsal identification photographs. Mar. Mamm. Sci. 36 (1), 47–65. doi: 10.1111/mms.12617
Whitehead H. (2001). Analysis of animal movement using opportunistic individual identifications: application to sperm whales. Ecology 82 (5), 1417–1432. doi: 10.2307/2679999
Whitehead H. (2002). Current global population size, post-whaling trend and historical trajectory of sperm whales. Sci. Rep. 12: 19468. doi: 10.1038/s41598-022-24107-7
Whitehead H. (2009). SOCPROG programs: analysing animal social structures. Behav. Ecol. Sociobiol. 63 (5), 765–778. doi: 10.1007/s00265-008-0697-y
Whitehead H. (2018). “Sperm whale,” in Encyclopedia of marine mammals, 3rd Editions. Eds. Würsig B., Thewissen J. G. M., Kovacs K. M. (London, UK: Academic Press), 919–924.
Whitehead H., Antunes R., Gero S., Wong S., Engelhaupt D., Rendell L. (2012). Multilevel societies of female sperm whales (Physeter macrocephalus) in the Atlantic and pacific: why are they so different? Int. J. Primatol. 33 (5), 1142–1164. doi: 10.1007/s10764-012-9598-z
Whitehead H., Shin M. (2022). Current global population size, post-whaling trend and historical trajectory of sperm whales. Sci. Rep. 12, 19468. doi: 10.1038/s41598-022-24107-7
Keywords: marine megafauna, philopatry, transnational conservation, Atlantic, photographic-identification, capture-recapture, habitat use
Citation: Ferreira R, Steiner L, Martín V, Fusar Poli F, Dinis A, Kaufmann M, Fernandez M and Alves F (2022) Unraveling site fidelity and residency patterns of sperm whales in the insular oceanic waters of Macaronesia. Front. Mar. Sci. 9:1021635. doi: 10.3389/fmars.2022.1021635
Received: 17 August 2022; Accepted: 23 November 2022;
Published: 08 December 2022.
Edited by:
Lars Bejder, University of Hawaii at Manoa, United StatesReviewed by:
Massimiliano Rosso, CIMA Research Foundation, ItalyBruno Díaz López, Bottlenose Dolphin Research Institute (BDRI), Spain
Copyright © 2022 Ferreira, Steiner, Martín, Fusar Poli, Dinis, Kaufmann, Fernandez and Alves. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rita Ferreira, cml0YS5mZXJyZWlyYUBtYXJlLWNlbnRyZS5wdA==
 Rita Ferreira
Rita Ferreira Lisa Steiner3
Lisa Steiner3 Vidal Martín
Vidal Martín Francesca Fusar Poli
Francesca Fusar Poli Ana Dinis
Ana Dinis Manfred Kaufmann
Manfred Kaufmann Marc Fernandez
Marc Fernandez Filipe Alves
Filipe Alves