- Red Sea Research Center (RSRC) and Computational Bioscience Research Center (CBRC), King Abdullah University of Science and Technology (KAUST), Thuwal, Saudi Arabia
Eutrophication-induced hypoxic sites are increasingly reported in coastal regions. At the same time, ocean warming, water column stratification, and changing circulation lead to open-ocean deoxygenation. In coastal areas and reefs with dense vegetation, aquatic organisms can be exposed to oxygen limitation stress where oxygen concentration reaches extremely low levels, particularly during nighttime once photosynthetic O2 production has ceased. Despite scientists being aware of this for decades, little is known about the impact of deoxygenation on the physiology of marine primary producers, such as macroalgae. In the Red Sea, in particular, the physiological adaptations of macroalgae under future climate scenarios are nonexistent. Here, we investigate the impact of different oxygen levels (6.5, 2.5, and 1.3 mg O2 L-1) at night for three conspicuous Red Sea macroalgae species Halimeda opuntia and Padina boryana (calcareous) and the brown algae Sargassum latifolium (noncalcifying). We monitored algal physiological responses during a 12-hour nighttime (dark) period at 32°C by measuring photochemical efficiency (Fv/Fm), respiration rates, and cellular viability. No lethal thresholds were detected. However, both deoxygenation treatments decreased respiration rates and induced changes in cellular activity, and only under severe hypoxia was a decrease in photochemical efficiency observed in all species. We calculated sublethal O2 thresholds SLC(50) of 1.2 ± 0.1, 1.5 ± 0.1, and 1.7 ± 0.1 mg O2 L-1 for H. opuntia, P. boryana, and S. latifolium, respectively. Therefore, the effects of nighttime hypoxia are evident over short timescales and may impact ecosystems via reduced primary production. Future consequences of persistent hypoxia and subsequent performance in multifaceted stressor exposures will provide a fundamental understanding of hypoxia’s threat to biodiversity and ecosystems.
1 Introduction
Macroalgal forests cover 7.5 million Km2 and support 1.5 Pg C year-1 net primary production, and have been, therefore, equated to a marine Amazonia (Duarte et al., 2022), with reef algae occupying an estimated cover of 0.038 million Km2. As reef formers, macroalgae provide habitat for many aquatic species (Mejia et al., 2012). However, they can also contribute to reef degradation, as they can out-compete reef-building corals (McCook, 1999), especially when overfishing eliminates herbivores, adding less control to algal abundance (Graham et al., 2013; Jessen et al., 2013), or when excess nutrient inputs or coral bleaching favor macroalgal growth and exhibit coral-algal phase shifts (Ellis et al., 2017; Anton et al., 2020). Besides their ecological functions, macroalgae provide emerging potentials with many eco-opportunities and are sources of natural products for multiple applications, including sustainable food production (Duarte et al., 2021). Moreover, they play an essential role in biogeochemical cycles, the production and release of organic matter (Haas et al., 2010), and contribute significantly to nitrogen fixation (El-Khaled et al., 2020) and carbon sequestration (Hill et al., 2015; Krause-Jensen and Duarte, 2016; Ortega et al., 2019).
Hypoxic events have been reported in many marine ecosystems worldwide (Altieri et al., 2017; Breitburg et al., 2018). However, short-term (<12h) hypoxia is less documented and may go undiscovered. In coastal regions, hypoxic events are defined by a reduction in dissolved oxygen (DO) to less than 2 ml O2 L-1 (= 2.8 mg O2 L-1) (Diaz and Rosenberg, 1995; Diaz and Rosenberg, 2008; Klein et al., 2020), and these events can lead to mass mortality and, when persistent, dead zones, with growing evidence that they may play a role in driving coral bleaching (Altieri et al., 2017; Alderdice et al., 2021). Whereas macroalgae photosynthetic oxygen production may shelter them from hypoxia during the daytime, they may be vulnerable at night, when respiratory demands must be made through the uptake of DO (Berthold and Paar, 2021). Hence, the extreme variation in O2 concentrations during diel cycles, especially in productive habitats, wherein high respiratory demands coupled with a lack of photosynthetic oxygen production can lead to oxygen deprivation and anoxia just before dawn (Andersen et al., 2017; Dubuc et al., 2017). A meta-analysis by Sampaio et al. (2021) reveals that hypoxic events reflected far greater consequences than future warming and ocean acidification (OA), and the combined effect is expected to exacerbate climate change consequences. Therefore, it is critical to understand the fate of primary production and macroalgae adaptation in response to ocean deoxygenation.
The Red Sea is inherently vulnerable to hypoxia due to its extreme maximum temperature (35 °C) and high salinity, which enhance respiratory demands while reducing oxygen solubility. Low DO has been observed near coastal cities along the coast of Saudi Arabia, such as two lagoons in Jeddah, Al Shabab, and Al Arbaeen, which record hypoxic and anoxic events (Orif, 2020). Extreme diel fluctuations of oxygen concentration have also been observed in major Red Sea ecosystems, including coral reefs, mangroves, and seagrass meadows, ranging from 0.1 to 8.9 mg O2 L-1 (Omar, 2013; Harabi and Affan, 2016; Roik et al., 2016; Giomi et al., 2019; Masoud et al., 2019) with extremely low DO levels occurring at nighttime. At the central Red Sea, a tidal node (tidal amphidrome) leading to minimal tidal ranges ~ 0.2 m (Madah et al., 2015; Gharbi et al., 2018), localized nutrient enrichments (Dunne et al., 2021) and coastal eutrophication-induced hypoxia (Duarte, 1995; Ansari et al., 2015) can all lead to increased fluctuations in oxygen availability by fueling respiratory oxygen demand. Therefore, complex ecological patterns can emerge as a result of short-term oxygen availability with increasing respiratory demands during nighttime, even over a few hours (Altieri et al., 2021; Dubuc et al., 2021; Johnson et al., 2021) or at very shallow water despite the influence of tidal change (Truchot and Duhamel-Jouve, 1980; DeCarlo et al., 2017). The macroalgae species studied here inhabit many different habitats in the central Red Sea. For instance, Padina sp. is reported to inhabit rocky shores, shallow reefs, and sandy sediments. Halimeda sp. can be also found on shores, coral reefs, and seagrass meadows, and Sargassum sp. inhabit the subtidal zone and is often attached to coastal rocks and pebble areas. The extent to which these habitats experience nighttime hypoxia is comparable among macroalgae assemblages and is influenced by the community oxygen consumption rate (Long et al., 2019; Wallace et al., 2021). For example, on shallow vegetative coral reefs in the central Red Sea DO varies from 0.1 mg O2 L-1 in the nighttime when community respiration dominates and increases to more than 10 mg O2 L-1 during the daytime, owing to community productivity (Giomi et al., 2019). Our study species grow in abundant aggregated clusters and dominate the Red Sea in coastal reefs, mangroves, and seagrass meadows habitats (Ortega et al., 2020), which further fuel the respiratory oxygen demand at nighttime.
For macroalgae, low DO during the day may provide some benefit since O2 shortage can reduce Rubisco’s affinity for oxygen, eliminating the energy waste of photorespiration and enhancing photosynthetic carbon fixation and growth (Crowder et al., 2019; Nelson and Altieri, 2019; Ji and Gao, 2021). Nonetheless, during the nighttime, algae derive energy through cellular respiration, primarily by O2-dependent (aerobic) energy metabolism. Hence, in an oxygen-deprived dark environment, algal viability is likely compromised. Many plants and algae have developed oxygen-sensing mechanisms to effectively compensate for oxygen shortage (Yang et al., 2015), including Nitric Oxide (NO), Reactive Oxygen Species (ROS) signaling, and altered gene expression (Bailey-Serres and Chang, 2005; Van Alstyne and van Hees, 2013; Astier et al., 2021; Pucciariello and Perata, 2021), whereby algae can reduce energy demands or alter metabolic pathways (Dromgoole, 1978). In the same context, dark calcification in calcareous algae can be moderated in response to oxygen concentration and energy limits (Beer and Larkum, 2001; Nelson and Altieri, 2019). Yet, current understanding of the impacts of nighttime hypoxia on macroalgae is limited.
The aim of this study is to explore the effects of short-term exposure to nighttime hypoxia on common Red Sea macroalgae at summer temperatures. We do so by comparing the responses of Halimeda opuntia, Padina boryana, and Sargassum latifolium fragments to one control, normoxic treatment (6.5 mg O2 L-1), and two reduced, hypoxic, oxygen treatments (2.5, and 1.3 mg O2 L-1) over a 12 hour dark experiment conducted for each species. Biological responses, such as photochemical efficiency, dark respiration, and cellular viability were investigated to determine nighttime oxygen thresholds, and the effects of short-term hypoxia on the physiological performance.
2 Material and methods
2.1 Sample collection and acclimation
Three conspicuous species of macroalgae were selected for this study: (1) the calcareous green algae Halimeda opuntia, (2) the calcareous brown algae Padina boryana, and (3) the noncalcifying brown algae Sargassum latifolium. All species were collected from the Central Red Sea in late July 2021 at 0.5-1 m depth and water temperature (at noon) ranging from 29 to 33°C. H. opuntia was collected from a mangrove forest at King Abdullah Economic City lagoon (22.376606N, 39.134341E), P. boryana was collected from a seagrass meadow at Alkharrar lagoon (22.861072N, 38.932579E), and S. latifolium was collected from Rabigh beach (22.812461N, 38.940420E). The specimens were visually identified at the genus level according to Lieske and Myers (2004), Richmond (2002), and AlgaeBase (Guiry, 2013).
Macroalgae samples were collected by carefully detaching rhizomes from the substrate and transferred to the aquaria facility in a container equipped with continuous air bubbling flow. The samples were immediately transported to the Coastal and Marine Operations Laboratory Facility (CMOR) at KAUST, where they were fragmented and gently washed with filtered seawater to remove epiphytes without damaging the thalli. Fragments used in the experiments for P. boryana, S. latifolium, and H. opuntia (Figure 1) were 0.23 ± 0.07, 0.22 ± 0.08, and 0.37 ± 0.08 grams dry weigh ±1SD, respectively.

Figure 1 Upper panel (left to right): experimental samples, fragments of Sargassum latifolium, Halimeda opuntia, and Padina boryana. Lower panel: oven-dried samples after the experiments.
All samples were kept in continuous flow-through outdoor tanks (i.e., on a natural photoperiod) supplied with fresh filtered seawater (20 µm pore size) from an adjacent Red Sea basin for at least a week prior to the beginning of the experiments. Then, the fragments were acclimated to indoor laboratory conditions for at least five days before conducting the experiments. Indoors, specimens were maintained in 2L glass aquaria filled with fresh filtered seawater and placed in three 70L tanks that were used as temperature-controlled water baths using 300-watt titanium heaters (Schego Teichheizer, Germany) connected to dual heating and cooling controllers (D-D The Aquarium Solution Ltd) and water pumps were used to maintain homogenous temperature. Aquaria were illuminated by Radion XR G4 PRO LED lights 40 cm above each tank, providing a constant light intensity of 150-200 μmol photons m-2 s-1 on a 12:12-h dark:light cycle. All indoor acclimation aquaria were equipped with plastic lids to reduce water evaporation and air-bubbling tubes, thus the oxygen concentration was maintained in relative equilibrium with air. During the indoor acclimation period, temperature was increased at a rate of 1°C day-1 until temperature reached the mean maximum Red Sea surface temperature for the region at 32°C, which was maintained throughout the experiments (Chaidez et al., 2017).
2.2 Experimental approach and gas mixtures
We tested each species individually in three identical experiments. For each species, 60 fragments were used (n=20 per oxygen treatment). Each specimen was placed in 2L aquaria filled with fresh filtered (20 µm pore size) Red Sea water. Replicates belonging to each oxygen treatment were evenly distributed among the eight 300L temperature-controlled water baths at 32°C to prevent confounding influence (temperature control and light condition are the same as explained in the acclimation stage). Algae fragments were exposed to three experimental treatments: 1) Control treatment henceforth referred to as the normoxia treatment, defined as 6.5 mg O2 L-1 (i.e., corresponding to atmospheric equilibrium with ambient oxygen saturation); 2) Moderate hypoxia, defined as 2.5 mg O2 L-1 (i.e., low DO; (Vaquer-Sunyer and Duarte, 2008; Hughes et al., 2020; Klein et al., 2020)); and 3) Severe hypoxia, defined as 1.3 mg O2 L-1 (i.e., corresponding to extreme oxygen reduction). Oxygen target levels were chosen according to oxygen levels in diel fluctuation from data collected in the central Red Sea (i.e., 0.1 to 8.9 mg O2 L-1; Roik et al., 2016; Giomi et al., 2019). The normoxic treatment mimics the conditions in well-mixed and more exposed environments, while the hypoxia treatment corresponds to nighttime sheltered backreef or low water flow areas and mangroves swamps. The severe hypoxia treatment corresponds to dense algal mats, coral-algae interfaces or interstices, and decaying algal blooms environments. In all treatments, pH was kept consistent at 8.3 throughout the experiments to isolate the effect of hypoxia from changes in pH.
Treatment levels were obtained by mixing pure CO2, O2, N2 (Air Liquide, Jeddah) gases via mass flow controllers (Omega Engineering Inc., USA) connected to a modified version of the LabVIEW software via an Omega Data Acquisition system (sensu Klein et al., 2017). Gas mixtures were delivered using plastic air stones, and clear plastic lids were used to minimize evaporation. Night experiments were conducted in a completely dark environment, and when necessary red headlights were used. Before the start of the experiment, we linearly transitioned from ambient (normoxia) to treatment conditions over a 7.5-hours window. Hence, the rate of decline over this timeframe differed among the treatments tested, wherein normoxia control treatment was kept stable, the moderate and severe hypoxia declined at 0.52 and 0.71 mg O2 L-1 h-1, respectively, reaching the target oxygen concentration by the start of the experiments (18:00 hours). pH 8.3 was held consistent for the duration of the experiments, a total of 12 hours during nighttime. Since each experimental aquaria was supplied with a continuous gas-mixture bubbling tube, which is connected to a mass-flow-controller that is controlled digitally to target treatment levels, we monitored manually the different parameters every hour to assess, adjust, and account for any variation (Table 1).
2.2.1 Water chemistry
Water chemistry was effectively maintained for the duration of each experiment (Table 1). DO, pH, and temperature were measured every three hours using a combined portable unit optic DO sensor (OptiOx, Mettler Toledo Ltd), and water salinity was monitored at the beginning and end of each experiment and maintained at 43 ppt. DO probe was calibrated with oxygen air saturation at 100% calibration, and the pH electrode was calibrated using a 3-point calibration (4.0, 7.0, and 10.0).
To evaluate the carbonate system, water samples (n=3 per treatment) were collected at the beginning and end of each experiment for total alkalinity (TA) analysis. Water samples were collected in 100 ml Borosilicate bottles by using a drawing tube, fixed with 50 µl mercuric chloride, then stored at room temperature, and analyzed within four weeks from sampling. TA was quantified by open-cell titration with 0.1 M hydrochloric acid using an Alkalinity Titrator (MODEL AS-ALK2, Apollo SciTech) with two pumps (VersaPump6, KLOHN) and pH meter (Orion Star A2II, ThermoFisher) using pH sensor (SI Analytics), method retrieved from (Dickson et al., 2007). TA standard provided by A.G. Dickson was used as certified reference material (Batch #172).
TA, pH, temperature, and salinity were used to calculate the partial pressure of CO2 (pCO2) and its standard error by using the “carb” and “errors” functions of the seacarb package 3.3.0v in R software (Gattuso et al., 2021).
2.3 Physiological response variables
2.3.1 Dark respiration measurements
Measurements for dark respiration were taken at three time points corresponding to the start, middle, and end of each experiment (19:00, 24:00 and 05:30 hours; n=3 per treatment for each time point). These timepoints are henceforth referred to as phase 1, phase 2, and phase 3. Real-time dark oxygen flux for macroalgae samples was measured every 10 seconds by incubating the samples in closed 50 ml chambers connected to fiber-optic oxygen sensors through contactless OXSP5 oxygen sensors spots (FireSting Pyroscience, Germany) attached inside the chambers for at least one hour. Stirring rods were used in each chamber to ensure homogenous mixing of the water column. Measurements were conducted inside a dark temperature-controlled (32°C) growth chamber (Percival Scientific, Inc). Sensors were calibrated at 0% DO using nitrogen bubbling and 100% DO saturation using air bubbling.
Only-water blank samples from each algal aquaria were taken to account for any microbial respiration in the seawater. After the incubation, samples’ dry weight (DW) was measured by drying the macroalgae specimens in a dry oven at 60°C for at least 24 h to remove any moisture. Respiration rates were calculated from the slope of the oxygen fluxes, and oxygen consumption rates were normalized to DW and calculated as mg O2 h-l g DW-l.
2.3.2 Maximum potential quantum efficiency of photosystem II
Changes in maximum photochemical efficiency yield (Fv/Fm) of photosystem II (PSII) were measured using pulse amplitude modulated fluorometry, MINI-PAM (Waltz, Germany). Measurements were taken every hour starting at 18:30 until 05:30 hours for all available samples during each time point. PAM readings were taken in triplicate for each specimen at three different areas across the sample. The average reading for each sample was used as the biological replicate for the analysis.
2.3.3 MTT viability assay
To assess cell viability, we used a colorimetric assay based on reducing the yellow MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma-Aldrich) in living cells by mitochondrial dehydrogenases to water-insoluble purple formazan (Mosmann, 1983). The viability assay was conducted three times during each experiment at phase 1, phase 2, and phase 3 (n=3 per treatment at each time point). For each sample, the whole specimen was submerged in a 50 ml vial containing the test medium: sterilized seawater and 0.2 mg ml-1 MTT (MTT stock solution 5 mg ml-1, prepared with PBS). Vials were incubated in a temperature-controlled water bath at 37°C for two hours. Algal specimens were then washed with distilled water and transferred to vials containing dimethyl sulfoxide (DMSO, Sigma-Aldrich) at the same volume of the incubation medium and gently vortexed for 10 minutes. 250 µL aliquots of the DMSO solutions were loaded in 96-well plates, and absorbance at 570 nm was measured using Spectramax Paradigm microplate reader (Molecular Devices). Modified protocol from (Mendes et al., 2013).
All assay samples were placed in a dry oven at 60°C for at least 24 h to obtain DW, optical density (OD) readings were normalized to sample DW and calculated as OD per g DW (Comas et al., 2000). Subsequently, we measured percent MTT reduction in treatment conditions (moderate hypoxia and severe hypoxia) relative to our control condition (normoxia) as follow (Equation 1):
2.4 Statistical analyses
Three technical replicates were taken for each PAM reading. PAM readings were taken every hour for all available samples; thus, we have repeated measure on the same sample as per every hour. Considering other sacrificial analyses, e.g., respiration rates, we have fewer samples for photochemical efficiency measurement as we go on time. The data were analyzed using linear mixed models (LMMs). The fixed factors were species (Halimeda sp., Padina sp., Sargassum sp.), oxygen treatments (control, hypoxia, and severe hypoxia), and time (hourly time scale 1-12) which was treated as a repeated measure. Different covariance structures (AR(1), AR(1) heterogeneous, and CS) were investigated to test the model of best fit through comparing goodness of fit statistics (-2 restricted log-likelihood, Akaike’s information criteria [AIC], and Bayesian information criterion [BIC]). Estimated marginal means were used to calculate pair-wise comparisons.
Sacrificial measurements of respiration rates and MTT reduction were analyzed independently for each species using two-way ANOVA type III followed by pairwise comparisons with bonferroni adjustment, with time (1h, 6h, 12h) and oxygen treatments (control, moderate and severe deoxygenation) as categorical variables with three levels each. Data were checked for normality and homoscedasticity using standardized residual plots and Q-Q plots.
Estimated sublethal concentrations for SLC(50) response in respiration rates under the three different oxygen concentrations were calculated using 2-parameter log-logistic dose-response models from the drc package in R (Ritz et al., 2015). All statistical data analyses and figures were conducted using IBM SPSS statistics (v28) and R Studio (v2021.09.0). Data are reported as mean ± one standard deviation (1SD), unless otherwise indicated (e.g., standard error as 1SE).
3 Results
3.1 MTT viability assay
We did not observe any tissue mortality for all tested species across treatments, MTT was reduced by viable cells in all tissue fragments under all treatment conditions in more than 50% reduction capacity relative to control treatment (Figure 2). No reduction inhibition or cell death was detected in all species, in fact, we detected increased reduction capacity under hypoxia stress for two of our tested species, P. boryana and S. latifolium. There was no significant interaction between time and treatment in MTT reduction for H. opuntia, however, P. boryana and S. latifolium showed a significant interaction between time and treatment (P-value< 0.05, Table 2). Relevant pairwise comparison showed that P. boryana, had higher MTT reduction capacity under severe hypoxia compared to the control treatment at midnight (6h) (P-value = 0.004), and S. latifolium had higher MTT reduction capacity under hypoxia treatment at the beginning of treatment (1h) (P-value = 0.013).
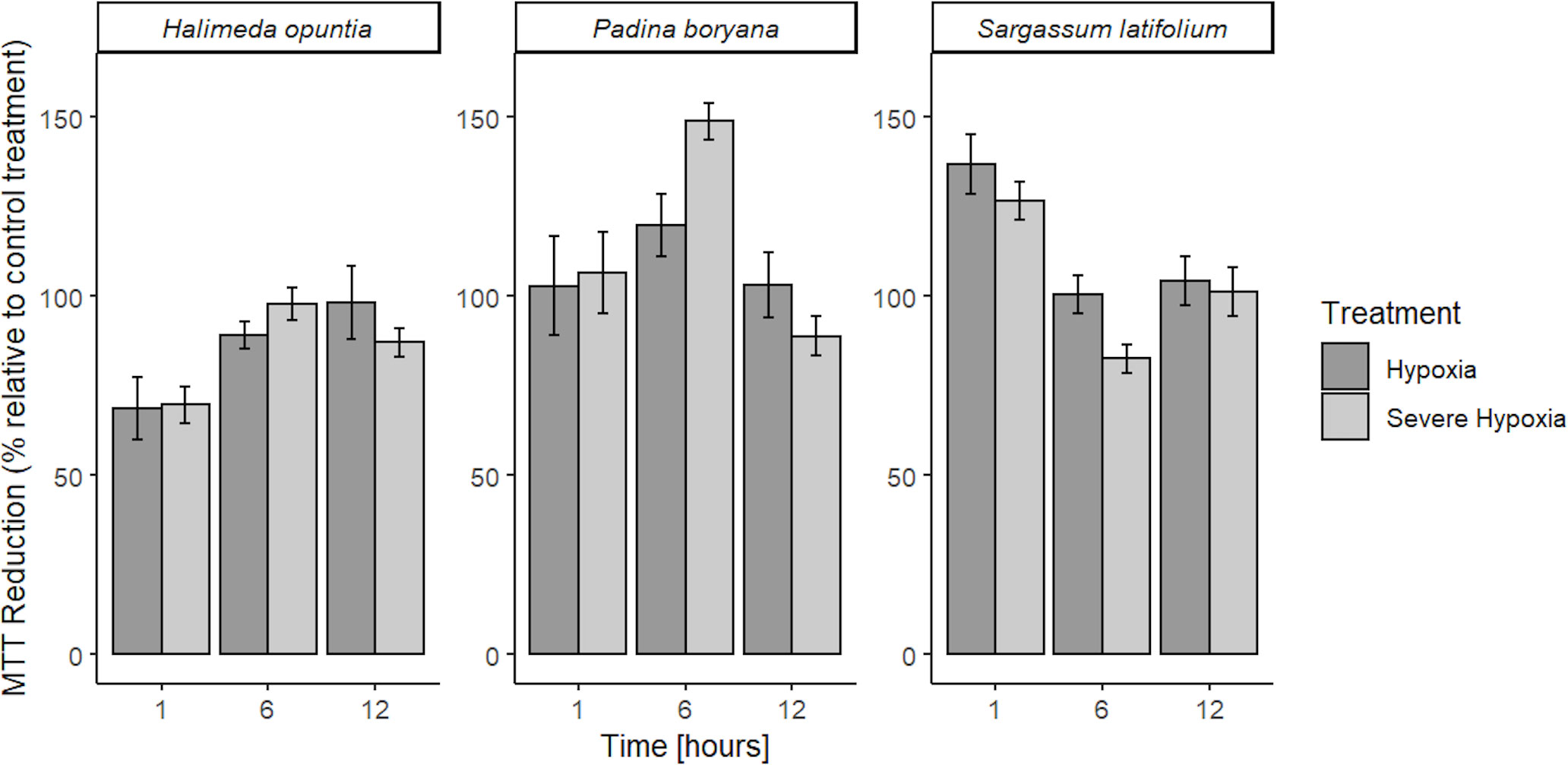
Figure 2 Mean % MTT reduction capacity in low DO treatments (OD gDW-1) relative to control treatment ± SE (n=3) for Halimeda opuntia, Padina boryana, and Sargassum latifolium. No lethal results were detected, all tissue fragments were viable throughout the experimental duration (12h) and had sufficient MTT reduction capacity.
3.2 Maximum photochemical efficiency
There was no significant three-way interaction among species, time, and treatment for Fv/Fm (Table 3). The different macroalgae P. boryana, S. latifolium, and H. opuntia exhibited the same Fv/Fm response pattern (Figure 3), where measurements under normoxia and moderate hypoxia treatments were similar. Although some lower values (0.4-0.5) were detected for moderate hypoxia, a significant decrease in performance was only detected under severe hypoxia treatment regardless of time and species (P-value< 0.01).
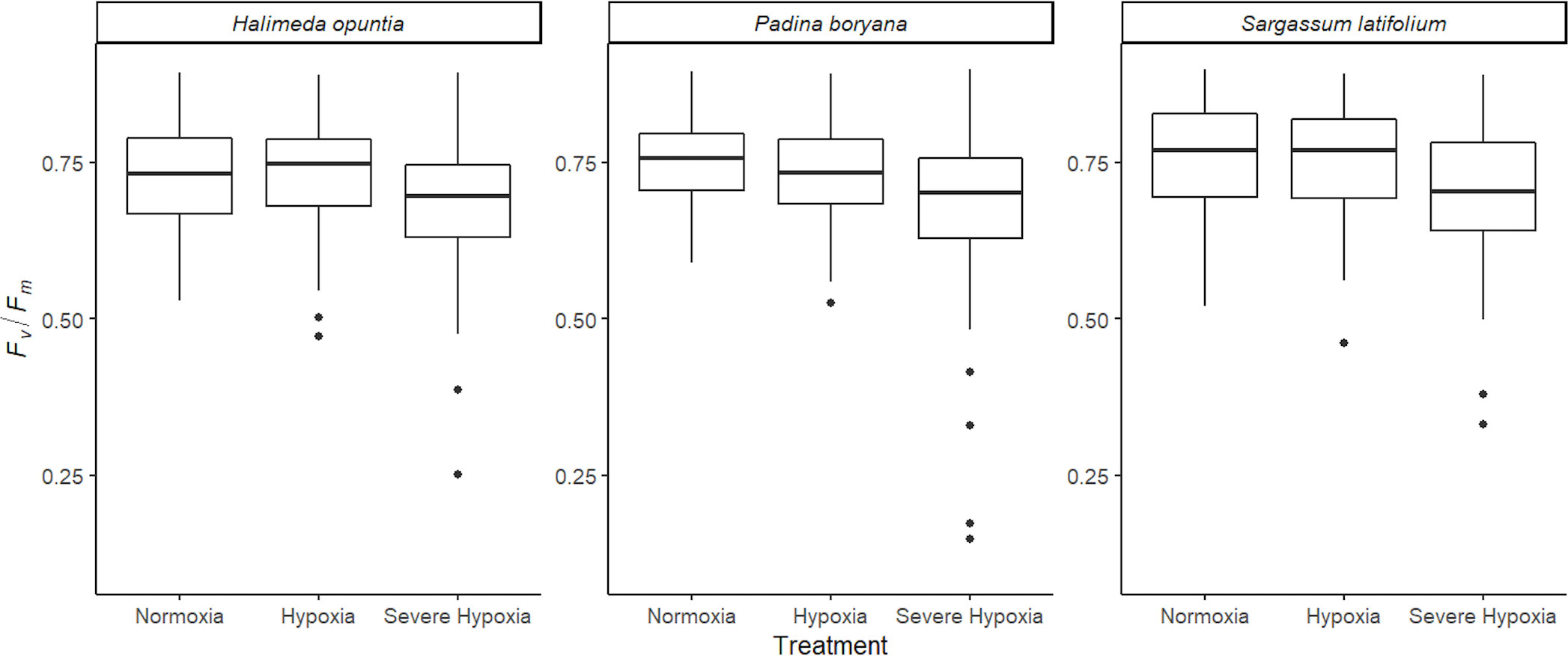
Figure 3 Average photochemical efficiency (Fv/Fm) for the different species tested Halimeda opuntia, Padina boryana, and Sargassum latifolium across the different experimental treatments. Figure represents all photochemical efficiency (PAM) measurements that were taken every hour during the entire duration of the experiments of 12 hours combined (at least 130 observations per treatment for each species). Whiskers represent standard error, and the black line is the average.
3.3 Respiration rates
The three species tested showed different response patterns in respiration rates under hypoxia treatments (Figure 4; Table 4). There was significant interaction between time and treatment for both H. opuntia and P. boryana (P-value< 0.05), and a significant treatment effect for S. latifolium (P-value< 0.001).
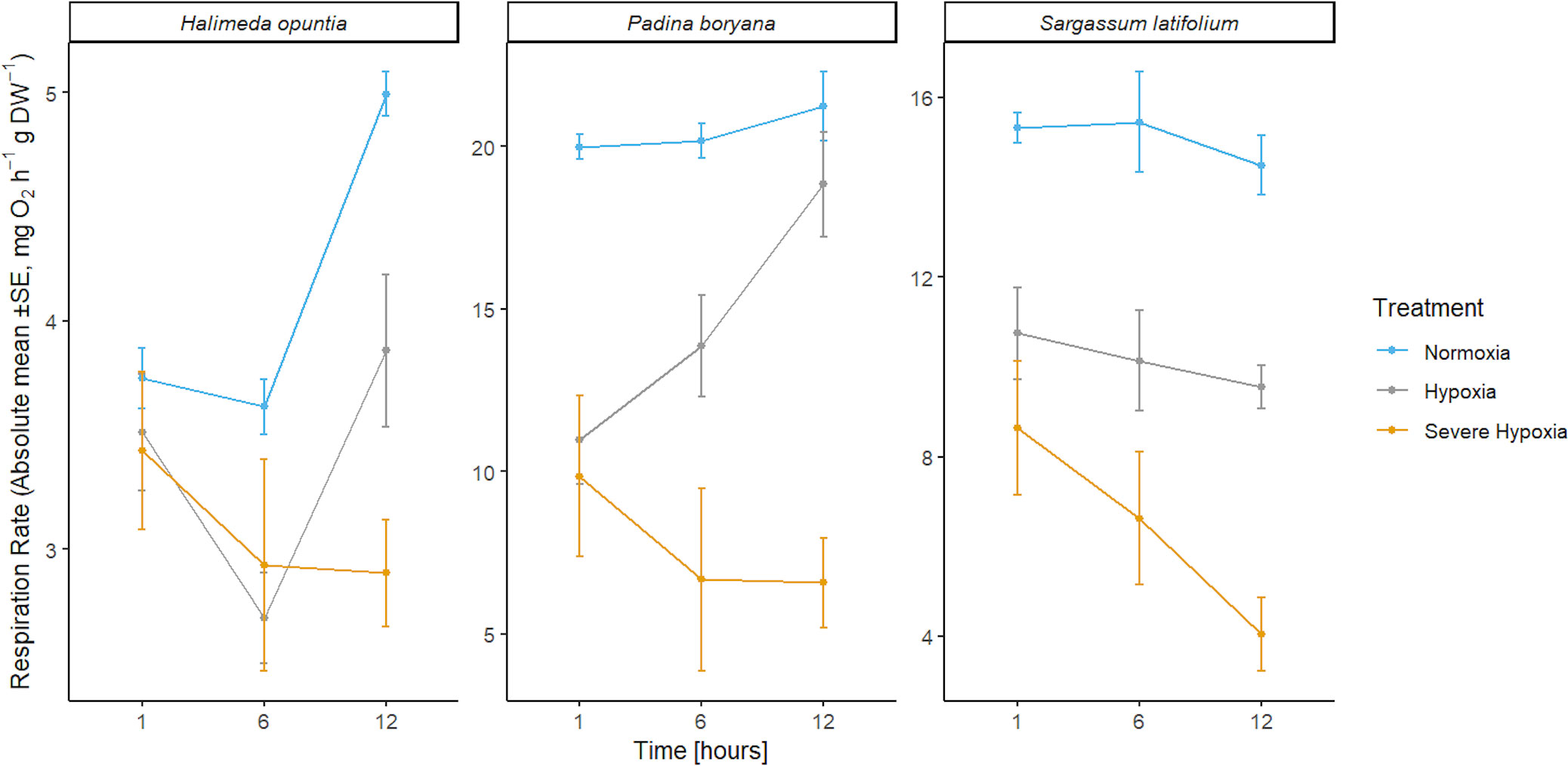
Figure 4 Mean absolute values of respiration rates ± SE (n=3) for the three different species Halimeda opuntia, Padina boryana, and Sargassum latifolium at the different time phases of the experimental duration.
In S. latifolium, respiration rates were significantly different under the different treatments, resulting in three distinct responses corresponding to the three different treatments (P-value< 0.01). The magnitude of the decline in respiration rate increased with increased deoxygenation regardless of exposure time.
In H. opuntia, only long exposure to severe oxygen reduction caused a decline in respiration rates. There was no significant difference between moderate hypoxia and control treatments, and under severe hypoxia treatment significant result was shown after 12 hours of treatment (P-value = 0.002).
In P. boryana, although both moderate and severe hypoxia treatments showed significant reduction in respiration rates at the first hour of treatments (P-value< 0.05), fragments exposed to moderate hypoxia were able to acclimate and recover to normal respiration rates similar to those of control treatment after 12 hours of exposure. However, respiration rates under the severe hypoxia treatment remained significantly lower compared to control treatment at all time phases (P-value< 0.007).
Based on respiration rate reduction under the hypoxia treatments which are associated with reduced species fitness and reduction in photochemical efficiency and subsequently growth, we calculated a sublethal O2 threshold SLC(50) of 1.2 ± 0.1, 1.5 ± 0.1, and 1.7 ± 0.1 mg O2 L-1 for H. opuntia, P. boryana, and S. latifolium responses, respectively.
4 Discussion
Our results show that exposure to decreased oxygen concentrations affects several biological processes in the tested macroalgae species. Across the three species, maximum photochemical efficiency (Fv/Fm) declined under severe hypoxia (1.3 mg O2 L-1), while moderate hypoxia (2.5 mg O2 L-1) had no effect on Fv/Fm.
There is little research studying macroalgae response to deoxygenation, however, our results are comparable to those reported for different photosynthetic organisms. In submerged mosses, a decrease in Fv/Fm was observed within 30 minutes of immersion (Rzepka et al., 2005). However, in the temperate seagrass Zostera marina and the tropical seagrass Thalassia hemprichii, reduction in Fv/Fm was a function of increasing temperature under low oxygen conditions (Rasmusson et al., 2020). On the other hand, the macroalgae Bryopsis sp. exposed to mild nighttime reduction in DO (4 mg O2 L-1) showed no changes in Fv/Fm (Haas et al., 2014).
In our analysis, respiration rates of both H. opuntia and P. boryana declined under the severe hypoxia treatment (1.3 mg O2 L-1) and showed only an initial decrease followed by a recovery to normal respiration rates under moderate hypoxia treatment (2.5 mg O2 L-1), exhibiting higher oxygen regulation capacity. While respiration rates of S. latifolium declined under both levels of hypoxia treatments, exhibiting characteristics of an oxyconformer. Oxyregulation in which an organism maintains oxygen consumption despite changes in oxygen availability is commonly observed in organisms living in environments subject to frequent and unpredictable changes in DO concentration. On the other hand, oxyconformity in which oxygen uptake decrease linearly with oxygen availability usually found in organisms living in an environment that seldom fall very low in DO concentration or do so in a regular predictable way (Hughes et al., 2022). Both Halimeda sp. and Padina sp. are attached closely to the benthic floor and grow in very dense aggregated masses, which requires higher metabolic demands. Hence, they might be more frequently subjected to lower levels of oxygen during nighttime. However, Sargassum sp., floating while attached to the benthic floor with longer thalli that move with minimal wave action, might be more adapted to higher levels of oxygen with less frequent severe hypoxia stress events.
Similar to our different responses, at severe hypoxia (1 mg O2 L-1), respiration rates in Cladophora sp. was reported to show no change compared to normal condition, while Gracilaria sp., respiration rates significantly declined (Peckol and Rivers, 1995). In Ulva. sp, anoxic conditions caused a gradual increase in dark respiration rate, especially following light periods (Vermaat and Sand-Jensen, 1987). In Bryopsis sp., moderate hypoxia caused an initial decrease before acclimating to normal respiration rates (Haas et al., 2014). In addition, seagrasses Zostera marina, Thalassia hemprichii, and Zostera muelleri show lower respiration rates in response to low oxygen conditions similar to that in macroalgae (Kim et al., 2018; Rasmusson et al., 2020). In fact, reduced respiration rate under dark hypoxia in seagrass Enhalus acoroides caused damage to PSII (Che et al., 2021). In our analysis, there was a significant decrease in Fv/Fm under severe hypoxia treatment, which was also associated with the lowest respiration rates. This warrants further investigation if the significant decline in respiration rates and Fv/Fm under severe hypoxia potentially implies an effect of these nighttime conditions on the photosynthetic system and subsequent daytime productivity.
Despite reduced respiration and photochemical efficiency, we did not observe evidence of mortality for the species tested under severe deoxygenation. All our results showed viable tissue throughout the experimental duration, consistent with results reported for Ulva sp., where thalli exposed to hypoxia for seven days in the dark were able to recover and produce gametes upon retrieval to normal condition and exposure to light (Corradi et al., 2006).
Along with viability assessment, our MTT viability assay provides a first screening tool of the reduction capacity in our samples. The MTT assay has been offered as a tool for the rapid assessment of plant antioxidant activity (Muraina et al., 2009). Hence, we hypothesize that higher MTT reduction capacity, as observed for P. boryana and S. latifolium is associated with higher antioxidant capacity. Similar to others, higher MTT reduction attributed to antioxidant activity was observed for Sargassum sp. subjected to heavy metals (Costa et al., 2017). Here, H. opuntia showed lower averages of MTT reduction relative to control among the three species. Future work is needed to verify if higher sensitivity and more vigorous antioxidant defense are associated with P. boryana and S. latifolium than H. opuntia.
Ultimately, possible limitations arise as a result of different abiotic stressors. Considering water flow and boundary layer, specimens could experience a further decrease in pH and oxygen concentration that could understate the hypoxic threshold or induce a synergic stress response. For instance, Halimeda sp. microsensor boundary layer measurements in the dark showed a slight decrease in oxygen at ambient level but a significant decrease in pH when photosynthesis is inhibited (Beer and Larkum, 2001). Moreover, the optimum thermal capacity reported for Halimeda tuna from the central Red Sea was 31.97°C for net primary production (Anton et al., 2020), which is around our experimental temperature (32°C), and that could have aided the moderate hypoxia acclimation capacity of the Halimeda sp.
Another factor adding to the complexity of examining hypoxia in macroalgae is the influence of previous exposure to other climate change factors such as acidification and warming on macroalgae response to deoxygenation. Al-Janabi et al. (2019) found that germlings of macroalgae Fucus sp. survival in hypoxia (2.75 mg O2 L-1) and survival in acidification and warming are anti-correlated. Different families of the macroalga exhibited different survival responses, whereas families who survived acidification and warming in spring and summer could not subsequently withstand three days of hypoxic autumn upwelling. They attribute their results to species-specific genotype-environment interactions.
Our experimental results show that nighttime hypoxia can induce a significant decrease in photosynthetic efficiency and respiration rates with changes in cellular biological pathways. According to definitions of sublethal and lethal oxygen thresholds, we did not observe a lethal threshold in any tested species (Vaquer-Sunyer and Duarte, 2008; Hughes et al., 2020). However, for the duration of our experiments (12h), all species show a significant reduction in respiration rates and photochemical efficiency under severe hypoxia (~1.3 mg O2 L-1). While under moderate hypoxia (~2.5 mg O2 L-1), there were no significant changes in photochemical efficiency for all tested species. P. boryana and H. opuntia were able to acclimate to normal respiration rates (after 12h of treatments), and S. latifolium kept a steady low respiration rate with respect to each hypoxia treatment.
Overall, a comparison between macroalgae responses with those of benthic invertebrates (Vaquer-Sunyer and Duarte, 2008) provides evidence of more resistance to hypoxia, with the experimentally determined for the Red Sea macroalgae studied here (1.2 to 1.7 ± 0.1 mg O2 L-1) being well below the median value for marine benthic invertebrates of 2.25 mg O2 L-1. Hence, higher resistance to nighttime hypoxia may also help explain regime shifts from corals to macroalgae observed in the Red Sea (Anton et al., 2020) and elsewhere.
5 Conclusion and future considerations
Our findings show that nighttime hypoxia can affect the physiological responses in subtropical macroalgae. However, the magnitude and duration of deoxygenation and the role of repetitive exposure to hypoxia need to be investigated to determine the terminal effects of hypoxia on primary producers. In addition, macroalgae hypoxic stress occurrence simultaneously or sequentially with other climate change factors, such as acidification and warming, needs to be further investigated. For instance, low pH and low DO co-occurrence due to nighttime respiratory demands can elicit additive, synergistic, or antagonistic effects. Addressing this knowledge gap will require isolated stressor experiments similar to our approach combined with multi-stressor approaches in factorial experiments to develop a potential predictive framework for a holistic approach to understanding species tolerance to hypoxia.
Furthermore, the ecological implications of the effect of nighttime hypoxia and its influence on subsequent daytime algal performance need to be examined. In future studies, changes in respiration rates, a decline in Fv/Fm, or an increase in oxidative stress should be monitored during the daytime when productivity drives back high oxygen levels. This will help understand to what extent acute or prolonged nighttime hypoxia has altered algal physiological have implications on species survival and fitness.
Diurnal changes in oxygen concentration and photosynthetic performance are linked to calcification rate for calcifiers, as low pH due to inhibited photosynthesis under darkness inflicts more negative effects on calcifying species like Halimeda sp. and Padina sp. (Buapet and Sinutok, 2021). Dark calcification rate and subsequent light calcification rate need to be measured in future studies.
Moreover, in plants, the loss of O2 as the final electron acceptor in hypoxia events suppresses mitochondrial respiration and activates coping metabolic mechanisms like fermentation and glycolysis (Klecker et al., 2014). In the microalgal model Chlamydomonas, short-term hypoxia (~2h) induced ROS-based and NO signaling response that activates gene transcriptions related to anaerobic pathways (Hemschemeier et al., 2013a; Hemschemeier et al., 2013b). Moreover, macroalgae Porphyra sp. increased ROS production and antioxidant capacity within one hour of desiccation stress (Contreras-Porcia et al., 2011). The concentration of oxidants can also accumulate in the surrounding environment. Ulva sp. and Ulvaria sp. show distinct extracellular oxidant concentrations in response to different abiotic stresses (Van Alstyne and van Hees, 2013). To counteract oxidative stress, algae stimulate the production of antioxidants (Mallick and Mohn, 2000). Similarly, in aquatic plants, the synergic effect of hypoxia and hydrogen sulfide triggered the production of ROS scavenging enzymes (Parveen et al., 2017). Indeed, the observed Fv/Fm decline in our severe hypoxia treatment results might be due to oxidative stress. Even in darkness, the accumulation of ROS and the disturbed cyclic electron flow can impair the photosynthetic process (Marutani et al., 2012). Future work is needed to investigate the influence of oxidative stress and dark hypoxia on macroalgae physiology.
In addition, the observed reduction in respiration rate in our analysis can be attributed to energy conservation strategy and as a mechanism to lower toxic end-products associated with anoxic metabolism (Kamermans et al., 1998). In fact, Ulva sp. incubated in an oxygen-free environment shows a rapid accumulation of acetate, which is attributed to a switch to the fermentation mode of metabolism (Nedergaard et al., 2002). This alternation in metabolism in response to abiotic stressors is mediated by NO and ROS signaling, a biological pathway widely known for terrestrial plants, aquatic plants, and algae (e.g., (Mallick and Mohn, 2000; Contreras-Porcia et al., 2011; Kottuparambil et al., 2012; You and Chan, 2015; Aljbour et al., 2019; Astier et al., 2021; Solé et al., 2021)). However, detailed descriptions of oxidative defense mechanisms for different macroalgae species are still lacking, particularly those induced by hypoxia stress.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
CD, TA, AS, and SK conceived the initial concept and designed the experiments. TA performed sampling of the specimens. TA, AS, SK, JG, AP, and SA conducted the experiments and performed the samples analyses. TA and SK conducted the data analyses. TA wrote the first draft of the manuscript with major contribution from CD, AS, and SK. All authors contributed to the article and approved the submitted version.
Funding
Funding supporting this research was provided by King Abdullah University of Science and Technology through baseline research funds awarded to Carlos M. Duarte.
Acknowledgments
We thank the team of KAUST Coastal and Marine Resources Core Labs (CMOR) for their support and assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alderdice R., Suggett D. J., Cárdenas A., Hughes D. J., Kühl M., Pernice M., et al. (2021). Divergent expression of hypoxia response systems under deoxygenation in reef-forming corals aligns with bleaching susceptibility. Glob. Change Biol. 27, 312–326. doi: 10.1111/gcb.15436
Al-Janabi B., Wahl M., Karsten U., Graiff A., Kruse I. (2019). Sensitivities to global change drivers may correlate positively or negatively in a foundational marine macroalga. Sci. Rep. 9, 14653. doi: 10.1038/s41598-019-51099-8
Aljbour S. M., Zimmer M., Al-Horani F. A., Kunzmann A. (2019). Metabolic and oxidative stress responses of the jellyfish cassiopea sp.to changes in seawater temperature. J. Sea Res. 145, 1–7. doi: 10.1016/j.seares.2018.12.002
Altieri A. H., Harrison S. B., Seemann J., Collin R., Diaz R. J., Knowlton N. (2017). Tropical dead zones and mass mortalities on coral reefs. Proc. Natl. Acad. Sci. 114, 3660–3665. doi: 10.1073/pnas.1621517114
Altieri A. H., Johnson M. D., Swaminathan S. D., Nelson H. R., Gedan K. B. (2021). Resilience of tropical ecosystems to ocean deoxygenation. Trends Ecol. Evol. 36, 227–238. doi: 10.1016/j.tree.2020.11.003
Andersen M. R., Kragh T., Sand-Jensen K. (2017). Extreme diel dissolved oxygen and carbon cycles in shallow vegetated lakes. Proc. R. Soc B Biol. Sci. 284, 20171427. doi: 10.1098/rspb.2017.1427
Ansari A. A., Ghanim S. A., Trivedi S., Rehman H., Abbas Z. K., Saggu S. (2015). Seasonal dynamics in the trophic status of water, floral and faunal density along some selected coastal areas of the red Sea, tabuk, Saudi Arabia. Int. Aquat Res. 7, 337–348. doi: 10.1007/s40071-015-0118-6
Anton A., Randle J. L., Garcia F. C., Rossbach S., Ellis J. I., Weinzierl M., et al. (2020). Differential thermal tolerance between algae and corals may trigger the proliferation of algae in coral reefs. Glob. Change Biol. 26, 4316–4327. doi: 10.1111/gcb.15141
Astier J., Rossi J., Chatelain P., Klinguer A., Besson-Bard A., Rosnoblet C., et al. (2021). Nitric oxide production and signalling in algae. J. Exp. Bot. 72, 781–792. doi: 10.1093/jxb/eraa421
Bailey-Serres J., Chang R. (2005). Sensing and signalling in response to oxygen deprivation in plants and other organisms. Ann. Bot. 96, 507–518. doi: 10.1093/aob/mci206
Beer D. D., Larkum A. W. D. (2001). Photosynthesis and calcification in the calcifying algae halimeda discoidea studied with microsensors. Plant Cell Environ. 24, 1209–1217. doi: 10.1046/j.1365-3040.2001.00772.x
Berthold M., Paar M. (2021). Dynamics of primary productivity in relation to submerged vegetation of a shallow, eutrophic lagoon: A field and mesocosm study. PloS One 16, e0247696. doi: 10.1371/journal.pone.0247696
Breitburg D., Levin L. A., Oschlies A., Grégoire M., Chavez F. P., Conley D. J., et al. (2018). Declining oxygen in the global ocean and coastal waters. Science 359, eaam7240. doi: 10.1126/science.aam7240
Buapet P., Sinutok S. (2021). Calcification in three common calcified algae from phuket, Thailand: potential relevance on seawater carbonate chemistry and link to photosynthetic process. Plants 10, 2537. doi: 10.3390/plants10112537
Chaidez V., Dreano D., Agusti S., Duarte C. M., Hoteit I. (2017). Decadal trends in red Sea maximum surface temperature. Sci. Rep. 7, 8144. doi: 10.1038/s41598-017-08146-z
Che X., Zhang T., Li H., Zhang L., Liu J. (2021). Effect of hypoxia on photosystem II of tropical seagrass enhalus acoroides in the dark. Photochem. Photobiol. 98, 421–428. doi: 10.1111/php.13522
Comas L. H., Eissenstat D. M., Lakso A. N. (2000). Assessing root death and root system dynamics in a study of grape canopy pruning. New Phytol. 147, 171–178. doi: 10.1046/j.1469-8137.2000.00679.x
Contreras-Porcia L., Thomas D., Flores V., Correa J. A. (2011). Tolerance to oxidative stress induced by desiccation in porphyra columbina (Bangiales, rhodophyta). J. Exp. Bot. 62, 1815–1829. doi: 10.1093/jxb/erq364
Corradi M. G., Gorbi G., Zanni C. (2006). Hypoxia and sulphide influence gamete production in ulva sp. Aquat Bot. 84, 144–150. doi: 10.1016/j.aquabot.2005.08.007
Costa G. B., Simioni C., Pereira D. T., Ramlov F., Maraschin M., Chow F., et al. (2017). The brown seaweed sargassum cymosum: changes in metabolism and cellular organization after long-term exposure to cadmium. Protoplasma 254, 817–837. doi: 10.1007/s00709-016-0992-9
Crowder L., Ng C., Frawley T., Low N., Micheli F. (2019). “The significance of ocean deoxygenation for kelp and other macroalgae. chapter 8.3,” in Ocean deoxygenation: Everyone’s problem–causes, consequences and solutions. Eds. Laffoley D., Baxter J. M. (Gland, Switzerland: IUCN), 309–322.
DeCarlo T. M., Cohen A. L., Wong G. T. F., Shiah F.-K., Lentz S. J., Davis K. A., et al. (2017). Community production modulates coral reef pH and the sensitivity of ecosystem calcification to ocean acidification. J. Geophys. Res. Oceans 122, 745–761. doi: 10.1002/2016JC012326
Diaz R., Rosenberg R. (1995). Marine benthic hypoxia: A review of its ecological effects and the behavioural response of benthic macrofauna. Oceanogr Mar. Biol. Annu. Rev. Ocean Mar. Biol. Annu. Rev. 33, 245–303.
Diaz R. J., Rosenberg R. (2008). Spreading dead zones and consequences for marine ecosystems. Science 321, 926–929. doi: 10.1126/science.1156401
Dickson A.G., Sabine C.L., Christian J.R. (2007). Guide to best practices for ocean CO2 measurements (Sidney, British Columbia: North Pacific Marine Science Organization). pp 191. doi: 10.25607/OBP-1342
Dromgoole F. I. (1978). The effects of oxygen on dark respiration and apparent photosynthesis of marine macro-algae. Aquat Bot. 4, 281–297. doi: 10.1016/0304-3770(78)90025-6
Duarte C. M. (1995). Submerged aquatic vegetation in relation to different nutrient regimes. Ophelia 41, 87–112. doi: 10.1080/00785236.1995.10422039
Duarte C. M., Bruhn A., Krause-Jensen D. (2021). A seaweed aquaculture imperative to meet global sustainability targets. Nat. Sustain, 5, 1–9. doi: 10.1038/s41893-021-00773-9
Duarte C. M., Gattuso J.-P., Hancke K., Gundersen H., Filbee-Dexter K., Pedersen M. F., et al. (2022). Global estimates of the extent and production of macroalgal forests. Glob. Ecol. Biogeogr. 31, 1422–1439. doi: 10.1111/geb.13515
Dubuc A., Collins G. M., Coleman L., Waltham N. J., Rummer J. L., Sheaves M. (2021). Association between physiological performance and short temporal changes in habitat utilisation modulated by environmental factors. Mar. Environ. Res. 170, 105448. doi: 10.1016/j.marenvres.2021.105448
Dubuc A., Waltham N., Malerba M., Sheaves M. (2017). Extreme dissolved oxygen variability in urbanised tropical wetlands: The need for detailed monitoring to protect nursery ground values. Estuar. Coast. Shelf Sci. 198, 163–171. doi: 10.1016/j.ecss.2017.09.014
Dunne A., Carvalho S., Morán X. A. G., Calleja M., Jones B. (2021). Localized effects of offshore aquaculture on water quality in a tropical sea. Mar. pollut. Bull. 171, 112732. doi: 10.1016/j.marpolbul.2021.112732
El-Khaled Y. C., Roth F., Tilstra A., Rädecker N., Karcher D. B., Kürten B., et al. (2020). In situ eutrophication stimulates dinitrogen fixation, denitrification, and productivity in red Sea coral reefs. Mar. Ecol. Prog. Ser. 645, 55–66. doi: 10.3354/meps13352
Ellis J., Anlauf H., Kürten S., Lozano-Cortés D., Alsaffar Z., Cúrdia J., et al. (2017). Cross shelf benthic biodiversity patterns in the southern red Sea. Sci. Rep. 7, 437. doi: 10.1038/s41598-017-00507-y
Gattuso J.-P., Epitalon J.-M., Lavigne H., Orr J., Gentili B., Hagens M., et al. (2021) Seacarb: Seawater carbonate chemistry. Available at: https://CRAN.R-project.org/package=seacarb.
Gharbi S. H., Albarakati A. M., Alsaafani M. A., P.p. S., Alraddadi T. M. (2018). Simulation of tidal hydrodynamics in the red Sea using COHERENS model. Reg Stud. Mar. Sci. 22, 49–60. doi: 10.1016/j.rsma.2018.05.007
Giomi F., Barausse A., Duarte C. M., Booth J., Agusti S., Saderne V., et al. (2019). Oxygen supersaturation protects coastal marine fauna from ocean warming. Sci. Adv. 5, eaax1814. doi: 10.1126/sciadv.aax1814
Graham N. A., Bellwood D. R., Cinner J. E., Hughes T. P., Norström A. V., Nyström M. (2013). Managing resilience to reverse phase shifts in coral reefs. Front. Ecol. Environ. 11, 541–548. doi: 10.1890/120305
Guiry M. D. (2013). AlgaeBase (Worldwide electronic publication). Available at: http://www.algaebase.org.
Haas A. F., Naumann M. S., Struck U., Mayr C., el-Zibdah M., Wild C. (2010). Organic matter release by coral reef associated benthic algae in the northern red Sea. J. Exp. Mar. Biol. Ecol. 389, 53–60. doi: 10.1016/j.jembe.2010.03.018
Haas A. F., Smith J. E., Thompson M., Deheyn D. D. (2014). Effects of reduced dissolved oxygen concentrations on physiology and fluorescence of hermatypic corals and benthic algae. PeerJ 2. doi: 10.7717/peerj.235
Harabi S. M., Affan M. (2016). Seasonal dynamics of epiphytic microalgae and their host seawoods florideophyceae at jeddah coast, the red sea, saudi arabia. Pak. J. Bot. 48, 1289–1298.
Hemschemeier A., Casero D., Liu B., Benning C., Pellegrini M., Happe T., et al. (2013a). COPPER RESPONSE REGULATOR1–dependent and —independent responses of the chlamydomonas reinhardtii transcriptome to dark anoxia. Plant Cell 25, 3186–3211. doi: 10.1105/tpc.113.115741
Hemschemeier A., Düner M., Casero D., Merchant S. S., Winkler M., Happe T. (2013b). Hypoxic survival requires a 2-on-2 hemoglobin in a process involving nitric oxide. Proc. Natl. Acad. Sci. 110, 10854–10859. doi: 10.1073/pnas.1302592110
Hill R., Bellgrove A., Macreadie P. I., Petrou K., Beardall J., Steven A., et al. (2015). Can macroalgae contribute to blue carbon? an Australian perspective. Limnol Oceanogr 60, 1689–1706. doi: 10.1002/lno.10128
Hughes D. J., Alderdice R., Cooney C., Kühl M., Pernice M., Voolstra C. R., et al. (2020). Coral reef survival under accelerating ocean deoxygenation. Nat. Clim. Change 10, 296–307. doi: 10.1038/s41558-020-0737-9
Hughes D. J., Alexander J., Cobbs G., Kühl M., Cooney C., Pernice M., et al. (2022). Widespread oxyregulation in tropical corals under hypoxia. Mar. pollut. Bull. 179, 113722. doi: 10.1016/j.marpolbul.2022.113722
Jessen C., Roder C., Lizcano J. F. V., Voolstra C. R., Wild C. (2013). In-situ effects of simulated overfishing and eutrophication on benthic coral reef algae growth, succession, and composition in the central red Sea. PloS One 8, e66992. doi: 10.1371/journal.pone.0066992
Ji Y., Gao K. (2021). “Chapter two - effects of climate change factors on marine macroalgae: A review,” in Advances in marine biology. Ed. Sheppard C. (Academic Press), 91–136. doi: 10.1016/bs.amb.2020.11.001
Johnson M. D., Scott J. J., Leray M., Lucey N., Bravo L. M. R., Wied W. L., et al. (2021). Rapid ecosystem-scale consequences of acute deoxygenation on a Caribbean coral reef. Nat. Commun. 12, 4522. doi: 10.1038/s41467-021-24777-3
Kamermans P., Malta E., Verschuure J., Lentz L., Schrijvers L. (1998). Role of cold resistance and burial for winter survival and spring initiation of an ulva spp. (Chlorophyta) bloom in a eutrophic lagoon (Veerse meer lagoon, the Netherlands). Mar. Biol. 131, 45–51. doi: 10.1007/s002270050295
Kim M., Brodersen K. E., Szabó M., Larkum A. W. D., Raven J. A., Ralph P. J., et al. (2018). Low oxygen affects photophysiology and the level of expression of two-carbon metabolism genes in the seagrass zostera muelleri. Photosynth Res. 136, 147–160. doi: 10.1007/s11120-017-0452-1
Klecker M., Gasch P., Peisker H., Dörmann P., Schlicke H., Grimm B., et al. (2014). A shoot-specific hypoxic response of arabidopsis sheds light on the role of the phosphate-responsive transcription factor PHOSPHATE STARVATION RESPONSE11. Plant Physiol. 165, 774–790. doi: 10.1104/pp.114.237990
Klein S. G., Pitt K. A., Nitschke M. R., Goyen S., Welsh D. T., Suggett D. J., et al. (2017). Symbiodinium mitigate the combined effects of hypoxia and acidification on a noncalcifying cnidarian. Glob. Change Biol. 23, 3690–3703. doi: 10.1111/gcb.13718
Klein S. G., Steckbauer A., Duarte C. M. (2020). Defining CO 2 and O 2 syndromes of marine biomes in the anthropocene. Glob. Change Biol. 26, 355–363. doi: 10.1111/gcb.14879
Kottuparambil S., Shin W., Brown M. T., Han T. (2012). UV-B affects photosynthesis, ROS production and motility of the freshwater flagellate, euglena agilis carter. Aquat Toxicol. 122–123, 206–213. doi: 10.1016/j.aquatox.2012.06.002
Krause-Jensen D., Duarte C. M. (2016). Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 9, 737–742. doi: 10.1038/ngeo2790
Lieske E., Myers R. F., Collins (2004). “Coral reef guide red Sea,” in The definitive guide to over 1200species of underwater life(London), 384.
Long M. H., Rheuban J. E., McCorkle D. C., Burdige D. J., Zimmerman R. C. (2019). Closing the oxygen mass balance in shallow coastal ecosystems. Limnol Oceanogr 64, 2694–2708. doi: 10.1002/lno.11248
Madah F., Mayerle R., Bruss G., Bento J. (2015). Characteristics of tides in the red Sea region, a numerical model study. Open J. Mar. Sci. 5, 193–209. doi: 10.4236/ojms.2015.52016
Mallick N., Mohn F. H. (2000). Reactive oxygen species: response of algal cells. J. Plant Physiol. 157, 183–193. doi: 10.1016/S0176-1617(00)80189-3
Marutani Y., Yamauchi Y., Kimura Y., Mizutani M., Sugimoto Y. (2012). Damage to photosystem II due to heat stress without light-driven electron flow: involvement of enhanced introduction of reducing power into thylakoid membranes. Planta 236, 753–761. doi: 10.1007/s00425-012-1647-5
Masoud M. S., Abdel-Halim A. M., El Ashmawy A. A. (2019). Seasonal variation of nutrient salts and heavy metals in mangrove (Avicennia marina) environment, red Sea, Egypt. Environ. Monit. Assess 191, 425. doi: 10.1007/s10661-019-7543-8
McCook L. J. (1999). Macroalgae, nutrients and phase shifts on coral reefs: scientific issues and management consequences for the great barrier reef. Coral Reefs 18, 357–367. doi: 10.1007/s003380050213
Mejia A. Y., Puncher G. N., Engelen A. H. (2012). “Macroalgae in tropical marine coastal systems,” in Seaweed biology: Novel insights into ecophysiology, ecology and utilization ecological studies. Eds. Wiencke C., Bischof K. (Berlin, Heidelberg: Springer), 329–357. doi: 10.1007/978-3-642-28451-9_16
Mendes L. F., Zambotti-Villela L., Colepicolo P., Marinho-Soriano E., Stevani C. V., Yokoya N. S. (2013). Metal cation toxicity in the alga gracilaria domingensis as evaluated by the daily growth rates in synthetic seawater. J. Appl. Phycol 25, 1939–1947. doi: 10.1007/s10811-013-0036-1
Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: application toproliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63. doi: 10.1016/0022-1759(83)90303-4
Muraina I., Suleiman M. M., Eloff J. (2009). Can MTT be used to quantify the antioxidant activity of plant extracts? Phytomedicine Int. J. Phytother Phytopharm 16, 665–668. doi: 10.1016/j.phymed.2008.11.005
Nedergaard R. I., Risgaard-Petersen N., Finster K. (2002). The importance of sulfate reduction associated with ulva lactuca thalli during decomposition: a mesocosm experiment. J. Exp. Mar. Biol. Ecol. 275, 15–29. doi: 10.1016/S0022-0981(02)00211-3
Nelson H. R., Altieri A. H. (2019). Oxygen: the universal currency on coral reefs. Coral Reefs 38, 177–198. doi: 10.1007/s00338-019-01765-0
Omar H. H., Abdullatif B. M., El-Kazan M. M., El-Gendy A.M. (2013). Red Sea water and biochemical composition of seaweeds at southern coast of jeddah, Saudi Arabia. Life Sci. J. 10, 1073–1080.
Orif M. I. (2020). Environmental aspects of Al-shabab and Al-arbaeen, two coastal lagoons in the eastern red Sea coast. Reg Stud. Mar. Sci., 40, 101401. doi: 10.1016/j.rsma.2020.101401
Ortega A., Geraldi N., Alam I., Kamau A., Acinas S., Logares R., et al. (2019). Important contribution of macroalgae to oceanic carbon sequestration. Nat. Geosci. 12, 748–754. doi: 10.1038/s41561-019-0421-8
Ortega A., Geraldi N. R., Duarte C. M. (2020). Environmental DNA identifies marine macrophyte contributions to blue carbon sediments. Limnol Oceanogr. 65, 3139–3149. doi: 10.1002/lno.11579
Parveen M., Asaeda T., Rashid M. H. (2017). Biochemical adaptations of four submerged macrophytes under combined exposure to hypoxia and hydrogen sulphide. PloS One 12, e0182691. doi: 10.1371/journal.pone.0182691
Peckol P., Rivers J. S. (1995). Physiological responses of the opportunistic macroalgae cladophora vagabunda (L.) van den hoek and gracilaria tikvahiae (McLachlan) to environmental disturbances associated with eutrophication. J. Exp. Mar. Biol. Ecol. 190, 1–16. doi: 10.1016/0022-0981(95)00026-N
Pucciariello C., Perata P. (2021). The oxidative paradox in low oxygen stress in plants. Antioxidants 10, 332. doi: 10.3390/antiox10020332
Rasmusson L. M., Buapet P., George R., Gullström M., Gunnarsson P. C. B., Björk M. (2020). Effects of temperature and hypoxia on respiration, photorespiration, and photosynthesis of seagrass leaves from contrasting temperature regimes. ICES J. Mar. Sci. 77, 2056–2065. doi: 10.1093/icesjms/fsaa093
Richmond M. D. (2002). A field guide to the seashores of Eastern Africa and the Western IndianOcean islands (Stockholm, Sweden: SIDA/Department for Research Cooperation SAREC).
Ritz C., Baty F., Streibig J. C., Gerhard D. (2015). Dose-response analysis using r. PloS One 10 (12), e0146021. doi: 10.1371/journal.pone.0146021
Roik A., Röthig T., Roder C., Ziegler M., Kremb S. G., Voolstra C. R. (2016). Year-long monitoring of physico-chemical and biological variables provide a comparative baseline of coral reef functioning in the central red Sea. PloS One 11, e0163939. doi: 10.1371/journal.pone.0163939
Rzepka A., Krupa J., lesak I. (2005). Effect of hypoxia on photosynthetic activity and antioxidative response in gametophores of mnium undulatum. Acta Physiol. Plant 27, 205–212. doi: 10.1007/s11738-005-0024-4
Sampaio E., Santos C., Rosa I. C., Ferreira V., Pörtner H.-O., Duarte C. M., et al. (2021). Impacts of hypoxic events surpass those of future ocean warming and acidification. Nat. Ecol. Evol. 5, 311–321. doi: 10.1038/s41559-020-01370-3
Solé M., Lenoir M., Durfort M., Fortuño J.-M., van der Schaar M., De Vreese S., et al. (2021). Seagrass posidonia is impaired by human-generated noise. Commun. Biol. 4, 1–11. doi: 10.1038/s42003-021-02165-3
Truchot J.-P., Duhamel-Jouve A. (1980). Oxygen and carbon dioxide in the marine intertidal environment: Diurnal and tidal changes in rockpools. Respir. Physiol. 39, 241–254. doi: 10.1016/0034-5687(80)90056-0
Van Alstyne K., van Hees D. (2013). Effects of emersion, temperature, dopamine, and hypoxia on extracellular oxidant accumulations surrounding the bloom-forming seaweeds ulva lactuca and ulvaria obscura. J. Exp. Mar. Biol. Ecol. 448, 207–213. doi: 10.1016/j.jembe.2013.07.013
Vaquer-Sunyer R., Duarte C. M. (2008). Thresholds of hypoxia for marine biodiversity. Proc. Natl. Acad. Sci. 105, 15452–15457. doi: 10.1073/pnas.0803833105
Vermaat J. E., Sand-Jensen K. (1987). Survival, metabolism and growth of ulva lactuca under winter conditions: a laboratory study of bottlenecks in the life cycle. Mar. Biol. 95, 55–61. doi: 10.1007/BF00447485
Wallace R. B., Peterson B. J., Gobler C. J. (2021). Ecosystem metabolism modulates the dynamics of hypoxia and acidification across temperate coastal habitat types. Front. Mar. Sci. 8 doi: 10.3389/fmars.2021.611781
Yang W., Catalanotti C., Wittkopp T. M., Posewitz M. C., Grossman A. R. (2015). Algae after dark: mechanisms to cope with anoxic/hypoxic conditions. Plant J. 82, 481–503. doi: 10.1111/tpj.12823
Keywords: hypoxia, macroalgae, nighttime, red sea, Halimeda sp., Padina sp., Sargassum sp.
Citation: Alamoudi T, Steckbauer A, Klein SG, Alva García JV, Arossa S, Parry AJ and Duarte CM (2022) Impacts of nighttime hypoxia on the physiological performance of Red Sea macroalgae under peak summer temperature. Front. Mar. Sci. 9:1034436. doi: 10.3389/fmars.2022.1034436
Received: 01 September 2022; Accepted: 20 October 2022;
Published: 02 November 2022.
Edited by:
Gang Li, South China Sea Institute of Oceanology (CAS), ChinaCopyright © 2022 Alamoudi, Steckbauer, Klein, Alva García, Arossa, Parry and Duarte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taiba Alamoudi, dGFpYmEuYWxhbW91ZGlAa2F1c3QuZWR1LnNh
 Taiba Alamoudi
Taiba Alamoudi Alexandra Steckbauer
Alexandra Steckbauer Shannon G. Klein
Shannon G. Klein Jacqueline V. Alva García
Jacqueline V. Alva García Silvia Arossa
Silvia Arossa Anieka J. Parry
Anieka J. Parry Carlos M. Duarte
Carlos M. Duarte