- 1School of Geography Science and Geomatics Engineering, Suzhou University of Science and Technology, Suzhou, China
- 2Key Laboratory of Geographic Information Science (Ministry of Education), School of Geographic Sciences, East China Normal University, Shanghai, China
- 3School of Geographical Sciences, Fujian Normal University, Fuzhou, China
- 4Research Center of Geography and Ecological Environment, Fuzhou University, Fuzhou, China
- 5College of Marine Sciences, South China Agricultural University, Guangzhou, China
- 6Institute of Agricultural Resources and Environment, Guangdong Academy of Agricultural Sciences, Guangzhou, China
Intensive aquaculture in estuaries and coasts has resulted in several ecological and environmental problems. Among various nitrogen transformation pathway, dissimilatory nitrate (NO3-) reduction is considered to be highly important in regulating reactive nitrogen. However, there are relatively few studies on the processes and contribution of NOx- reduction in sediment during the shrimp pond culture period. Three sediment NO3- reduction processes, denitrification (DNF), anaerobic ammonium oxidation (ANA), and dissimilatory NO3- reduction to ammonium (DNRA), were surveyed in eight shrimp ponds across three subtropical estuaries using 15N isotope tracing experiments. The rates of DNF, ANA and DNRA ranged from 2.87–18.11, 0.10–1.92, and 0.21–1.25 nmol N g -1 h -1, respectively. DNF was responsible for 64.2–91.6% of the total NO3- reduction. Regarding environmental factors, C and N substrates, as well as salinity, significantly affected NO3- reduction. In general, the N losses were approximately 32.43–131.64 g N m-2 yr-1 for DNF and 2.38–15.85 g N m-2 yr-1 for ANA in this study, indicating that coastal reclamation is a nonnegligible way to remove nitrogen. Our results provide a scientific foundation for understanding the mechanism of nitrogen cycling in the artificial aquatic environment of shrimp ponds.
Introduction
With rapid economic development and the influence of human activities, large amounts of reactive nitrogen from upstream have been carried to estuarine and coastal systems by atmospheric transport and river runoff in recent years (Galloway et al., 2008; Canfield et al., 2010; Chen et al., 2016b). Reactive nitrogen mainly exists in the form of nitrate (NO3-), which has a significant influence on the ecology and functions of estuaries and coastal environments (Kennison and Fong, 2014; Macdonald et al., 2018). Such as eutrophication and algal blooms caused by increased NO3- concentrations, and even pose a potential threat to human heath (Birch and McCaskie, 1999; Wang et al., 2020). Thus, further understanding of the transformation processes in estuarine and coastal systems is required.
Dissimilatory NO3- reduction is an important pathway for removing reactive nitrogen and mainly includes three processes: denitrification (DNF), anaerobic ammonium oxidation (ANA), and dissimilatory NO3- reduction to ammonium (DNRA) (Thamdrup and Dalsgaard, 2002; Deng et al., 2015; Huang et al., 2021). Among these processes, DNF, which converts NO3-/NO2- to N2 or N2O, has long been considered the main pathway for NO3- removal progress (Seitzinger et al., 2006; Burgin and Hamilton, 2007). ANA oxidises ammonia (NH4+) into dinitrogen gas by reducing NO3-/NO2-, which has recently been thought to play an important role in the regulation of the sediment nitrogen cycle (Trimmer et al., 2003; Dale et al., 2009; Hou et al., 2015). While both DNF and ANA would remove NO3-/NO2- by conversion to gaseous nitrogen, DNRA converts NO3- to bioavailable NH4+, inducing the net retention of reactive N in the environment (Silver et al., 2005; Huygens et al., 2007; Dong et al., 2011). The contributions of the three NO3- reduction processes to nitrogen cycling differ depending on the type of ecosystem and sediment (Thamdrup and Dalsgaard, 2002; Laverman et al., 2007; Minick et al., 2016). Previous studies have shown that DNF was the main pathway of NO3- reduction processes in aquatic ecosystems (Deegan et al., 2012; Shan et al., 2016). However, studies have shown that DNRA plays an important role in mangrove systems (Cao et al., 2016). Studying the mechanism of NO3- reduction would help us to further understand the nitrogen transformation process in aquatic ecosystems.
As a key area of land-marine interaction, estuarine tidal flat wetlands are hotspots for the nitrogen cycle (Osburn et al., 2016; Hou et al., 2018). In recent years, because of the increasing demand for seafood products, large areas of tidal flat wetlands in China’s coastal estuaries have been reclaimed as artificial aquaculture ponds (Chen et al., 2016a; Yang et al., 2017b). Owing to the addition of feed, aquaculture significantly increases carbon and nitrogen-based substances, changing carbon and nitrogen cycle processes, such as CH4 and N2O emissions (Yang et al., 2017a; Gao et al., 2018). According to previous research, the NO3- reduction route and proportion changed after the reclamation of estuarine tidal flat wetlands into aquaculture ponds (Gao et al., 2019). The process of NO3- reduction were closely related to environmental factors such as NO3-, TOC, and NH4+ (Deng et al., 2015; Cheng et al., 2016; Damashek and Francis, 2018). However, the detailed process of dissimilatory NO3- reduction in aquaculture ponds during the culture period remains unclear. With the continuous expansion of farming scale, it is necessary to develop the understanding of this special ecosystem.
This study selected shrimp ponds in three different regions of the subtropical estuarine and analysed the dissimilatory NO3- reduction process during the culture period using nitrogen isotopic techniques. In addition, the primary environmental factors influence DNF, ANA, and DNRA processes were studied. Furthermore, we compared the relative contributions of the three processes following the culture period in shrimp ponds. Our study provides a deeper understanding of nitrogen dynamics and the progress of aquaculture ponds in estuaries and coasts.
Materials and methods
Study area and samples collection
Three main estuaries in Fujian Province were selected as the study area: From north to south, the Min River, Mulan River, and Jiulong River. The three estuaries all have a subtropical monsoon climate, with an average annual rainfall of more than 1300 mm and an average annual temperature of 19.6 to 21.0°C (Zhang et al., 2011; Tong et al., 2012; Luo et al., 2019). In August 2017, eight sampling points were selected for shrimp culture ponds near the three estuaries, and surface sediments were collected underwater using a Plexiglas tube (Figure 1). The shrimp ponds at the sampling points were all reclaimed from estuary swamp wetlands, with similar land-use transformation years (7–9 years), and all were muddy pond slopes and pond bottoms. The shrimp ponds contained white shrimp (Litopenaeus vannamei). Shrimp seedlings are put in at the end of May every year, and farming ends after all the shrimps are harvested in mid- to late October. Shrimp farming ponds use local river water as a source of farming water. Each shrimp farming pond is equipped with two impeller aerators with a power of 1.5 kW, and the startup time is about 20:00 to 2:00 the next morning. The switch of the aerator during the day depends on the weather, the growth of fish and shrimp, and the water quality. The feed was provided twice a day at 7:00 and 17:00, and the feeding amount was in accordance with the conventional requirements. The basic features of shrimp ponds are listed in Table 1.
Analysis of sediment properties
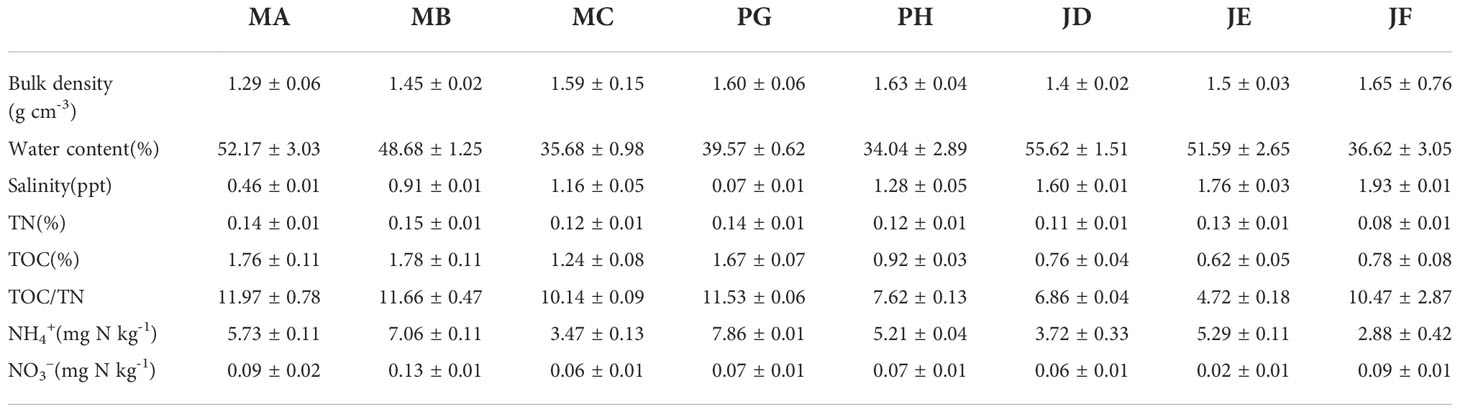
Sediment density was measured using the syringe method (Percival and Lindsay, 1997), and the moisture content was dried using the weight loss method at 80°C to a constant weight. After removing sedimentary carbonate using 0.1 M HCl, the concentraction of total organic carbon (TOC) and total nitrogen (TN) in the sediments were determined using a carbon-hydrogen-nitrogen elementary analyser (VVarioELIII, Elementary, Germany) and C N elemental analyser (Elementar Vario MAX CN, Germany), respectively (Lin et al., 2017; Hu et al., 2022). The sediment concentrations of NH4+ and NO3- were extracted with 2 M KCl solution and then determined using a continuous flow analyser (SAN Plus, Skalar Analytical B.V., The Netherlands) (Sun et al., 2022).
Determination of potential DNF, ANA, and DNRA rates
The potential DNF, ANA, and DNRA rates were measured using the nitrogen isotope tracing method (Yin et al., 2014; Deng et al., 2015; Gao et al., 2017). In brief, the slurry was prepared by mixing sediment into helium-purged water with a sediment/water volume ratio of 1:7 and then transferring to a helium-purged 12-mL vial (Labco Exetainers) (Hou et al., 2015). The vials were then pre-incubated for 36 h to remove surplus NO3-, NO2-, and O2 at in situ temperatures. After pre-incubation, sterile anoxic solutions of 15NO3- (15N at 99%) was added to all vials via the septa, with the final content of 15N being approximately 100 μM (Hou et al., 2013). Then, 200 μL of ZnCl2 solution (50%) was added to half of the replicates (as initial samples). Then, half of the slurries were incubated for 8 h, and 200 μL of ZnCl2 solution (50%) was added at the end of incubation to terminate the reaction (Lin et al., 2017). Both of the contents of 29N2 and 30N2 were measured by membrane inlet mass spectrometry (MIMS) during incubation, to calculate the DNF and ANA rates based on the difference in 29N2 and 30N2 produced among the final and initial results (Gao et al., 2019).
The DNF and ANA rates were approximated based on the accumulations of 29N2 and 30N2, respectively (Deng et al., 2015). The contributions of DNF and ANA to 29N2 production were calculated using Equation (1).
Here, P29, D29, and A29 (nmol N g−1 h−1) denote the total 29N2 production rate and the production rate of 29N2 from DNF and ANA during the slurry experiments, respectively. The ratio of 14N and 15N produced by 14NO3- or 15NO3- with random isotope pairing and D29 was calculated using equation (2) (Risgaard-Petersen et al., 2003).
Here, P30 (nmol N g−1 h−1) represents the production rate of total 30N2, and FN (%) denotes the proportion of 15N in NO3-, which was estimated from the measured content of added 15NO3- and surplus NO3- (Shan et al., 2016). Finally, the DNF potential rates were calculated using Equation (3), and the ANA was calculated using Equation (4).
Here, Dtotal and A29 (nmol N g−1 h−1) denote DNF and ANA rates, respectively.
The DNRA rate was calculated using the 15NH4+ oxidation and MIMS analysis (OX/MIMS) method (Yin et al., 2014). First, the sediment slurry was pre-incubated. After the preincubation, 100 μL of 15NO3 (15N at 99.6% and a final content of approximately 100 μM 15N) was added to all the vials. Immediately, half of the slurry was saved (as initial sample) with 200 μL of ZnCl2 solution (50%). The rest of the vials (as final samples) were further incubated 8 h before adding 200 μL of ZnCl2 solution (50%). The potential rates of DNRA were calculated using Equation (5).
Here, RDNRA (nmol N g-1 h-1) is the total potential rate of DNRA; (15NH4+)Final and (15NH4+)Initial (nmol N L-1) are the content of 15NH4+ in the final and initial sample, respectively; and V (L), W (g), and T (h) denote the volume of the vial, dry weight of the sediment, and time, respectively (Gao et al., 2019).
Statistical analyses
The differences between the NO3- reduction rates among all points were analysed by one-way analysis of variance (ANOVA) (homogeneity of variance was tested by the LSD test, and Dunnett’s T3 was used to test for heterogeneity of variance). The relationships between the environmental variables and DNF, ANA, and DNRA rates were revealed by Pearson correlation analyses. SPSS 22.0 was used for one-way ANOVA and pearson analysis. While Redundancy analysis (RDA) was used to evaluate variations in NO3- reduction rates with respect to environmental variables using software Canoco 4.5 (Sun et al., 2020). The significance level was set at 0.05.
Results
Physiochemical characteristics of the site
The physicochemical characteristics of each site are presented in Table 2. Sediment bulk density varied from 1.27 to 1.78 g·cm-3 in the study area, and the water content in the sediment varied from 31 to 59%. The salinities of the shrimp ponds ranged from 0.06 to 1.93 ppt. The TOC, TN, and TOC/TN ratios in the sediment varied between 0.58 to 1.97%, 0.07 to 0.16%, and 4.51 to 13.75, respectively. The concentration of NH4+ and NO3- in sediments varied from 2.42 to 8.41 and 0.02 to 0.14 μmol·g-1, respectively. In addition to the physicochemical characteristics of the sediments, the area and average depth of each shrimp pond were also different, and the largest area (1.113–1.255 ha) and average depth (2.1–2.8 m) of shrimp ponds were found in the Mulan estuary. The unit water output varied between 9.77–10.6 kg ha−1, and the food coefficient ranged from 1.38–1.86 in the shrimp pond (Table 1).
Dissimilatory NO3- reduction processes
The sediment potential rates of DNF ranged from 2.87 to 18.11 nmol N g-1 h-1, and there were significant differences between the all sites (n = 24, p < 0.05). The highest DNF rate was found at site MA, while the lowest DNF rate was at site PH (Figure 2). DNF contributed 64.2–91.5% of the total NO3- reduction rate (Figure 3). The rates of ANA varied from 0.10 to 1.92 nmol N g-1 h-1 in the study area, and a distinct spatial difference in the ANA rates was observed among sites (n = 24, p < 0.05). The highest ANA rate occurred at site PH, whereas the lowest ANA rate occurred at site JF. Compared to DNF, ANA had less effect on NO3- reduction and contributed 1.2–31.4% to total nitrogen loss. Potential DNRA rates varied from 0.21 to 1.25 nmol N g-1 h-1. Significant differences were observed among the sites in the study area (n = 24, p < 0.05). DNRA and DNF showed the same distribution, with the highest value appearing at the MA site and the lowest value appearing at the PH site. The contribution of DNRA accounted for 4.38–12.35% of the total NO3- reduction.
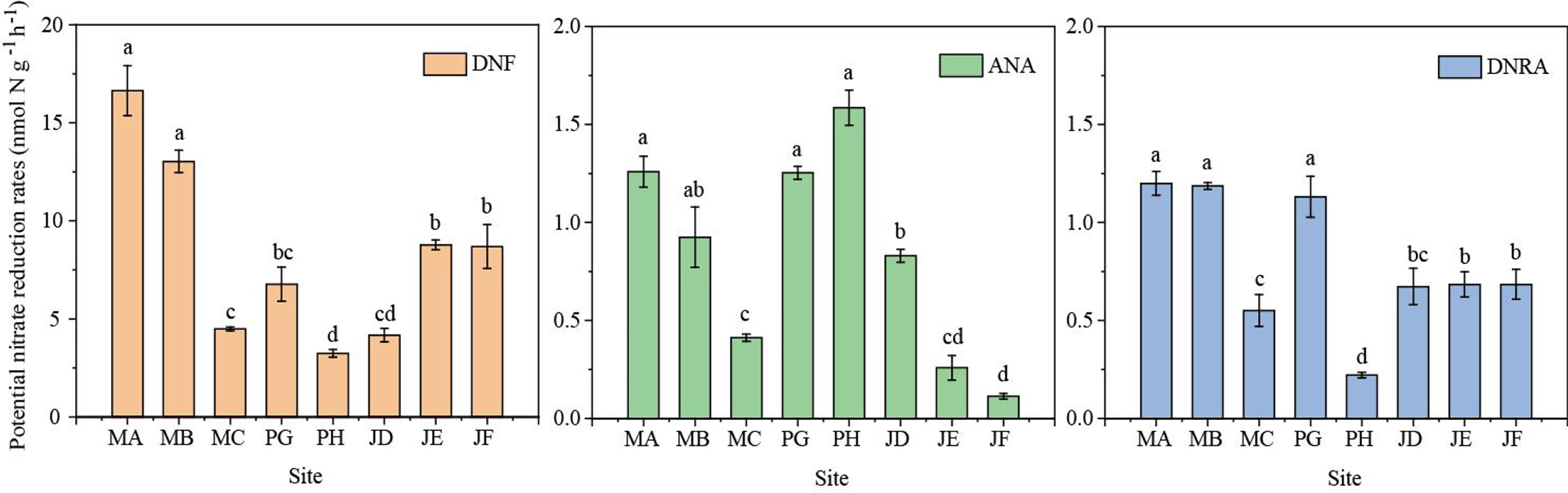
Figure 2 Sediment potential DNF, ANA and DNRA rates in the shrimp ponds. Different letters indicate significant differences (p < 0.05) among different sites. Error bars represent the standard deviation(n = 3).
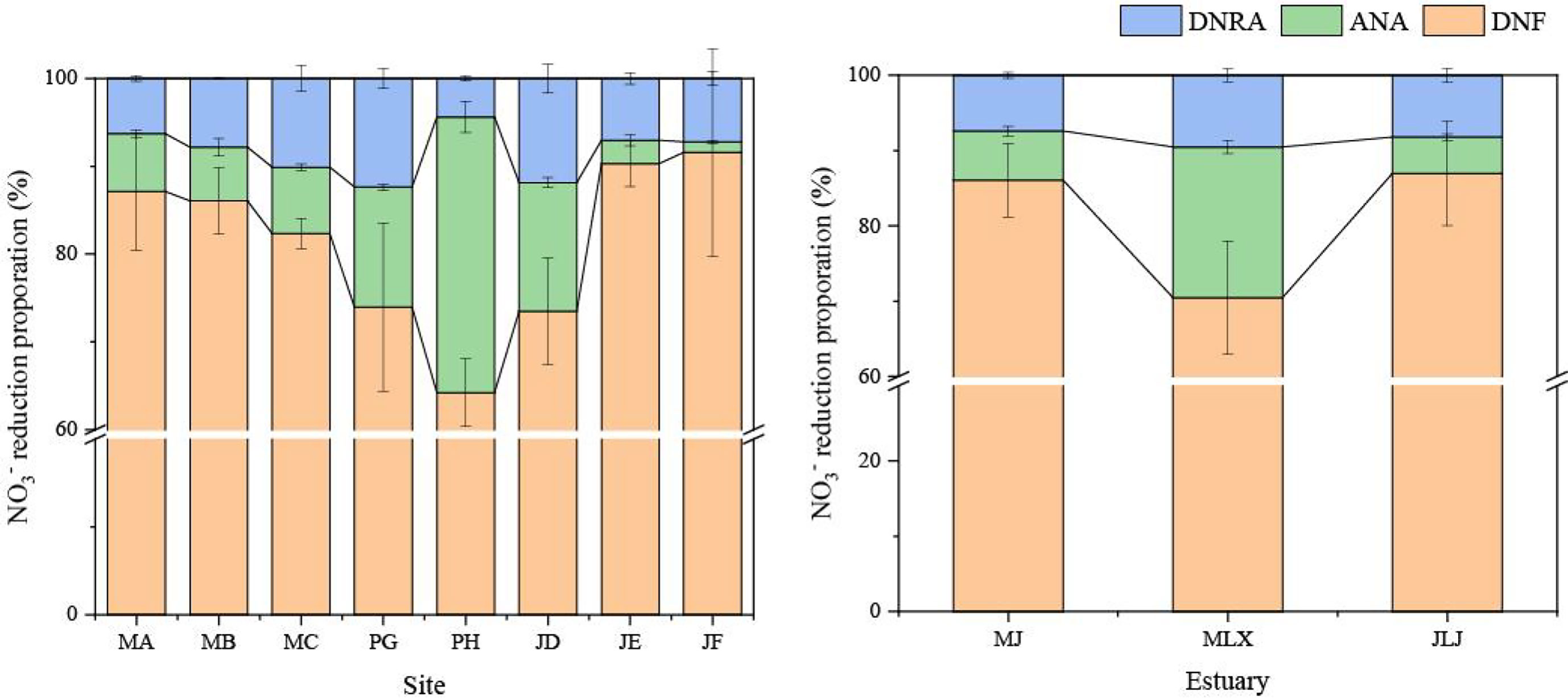
Figure 3 Relative contributions of DNF, ANA and DNRA to total NO3- reduction in the shrimp ponds. Error bars represent the standard deviation.
Influences of physiochemical characteristics on NO3- reduction rates
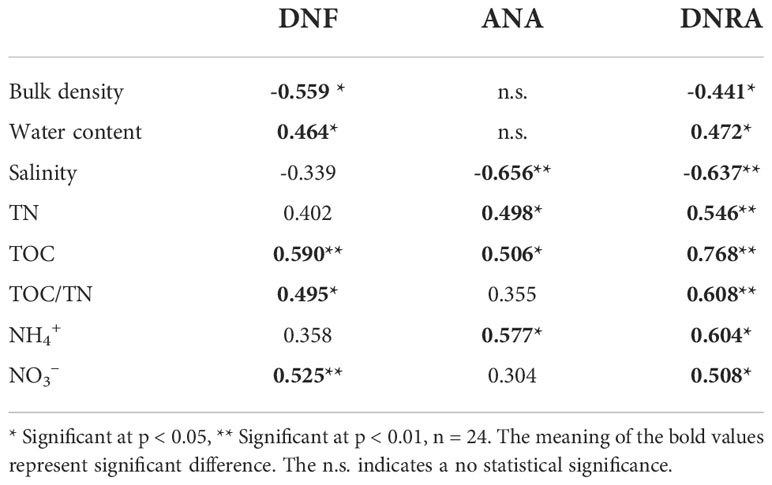
The RDA was implemented to evaluate variations in the NO3- reduction rate with respect to environmental variables. The first two RDA dimensions were found to account for 70.56% of the cumulative variance, and the first and second axes contributed 55.12 and 15.44%, respectively (Figure 4). The RDA results showed that salinity and TOC were significantly associated with various NO3- reduction process. The DNF rates were significantly and negatively correlated with bulk density (r = - 0.559, p < 0.05, n = 24) and positively correlated with water content (r = 0.464, p < 0.05, n =24), TOC (r = 0.590, p < 0.01, n = 24), TOC/TN (r = 0.495, p < 0.05, n = 24), and NO3- (r = 0.525, p < 0.01, n = 24) (Table 3). Of the detected environmental factors, salinity and the ANA rate showed a significant negative correlation (r = - 0.656, p < 0.01, n = 24), and there was a positive correlation with TN (r = 0.498, p < 0.05, n =24), TOC (r =0.506, p < 0.05, n = 24) and NH4+ (r = 0.577, p < 0.05, n = 24). The sediment potential rate of DNRA was negatively associated with bulk density (r = 0.441, p < 0.05, n = 24) and salinity (r = - 0.637, p < 0.01, n = 24) and positively related to water content (r = 0.472, p < 0.05, n = 24), TOC (r = 768, p < 0.01, n = 24), TN (r = 0.546, p < 0.01, n = 24), TOC/TN (r = 0.608, p < 0.01, n = 24), NH4+ (r = 0.604, p < 0.05, n = 24), and NO3- (r = 0.508, p < 0.05, n = 24) (Table 3). There are positive correlations of most of the dissimilatory NO3- reduction processes with TOC, TOC/TN, NH4+, and NO3- were found (Table 3).
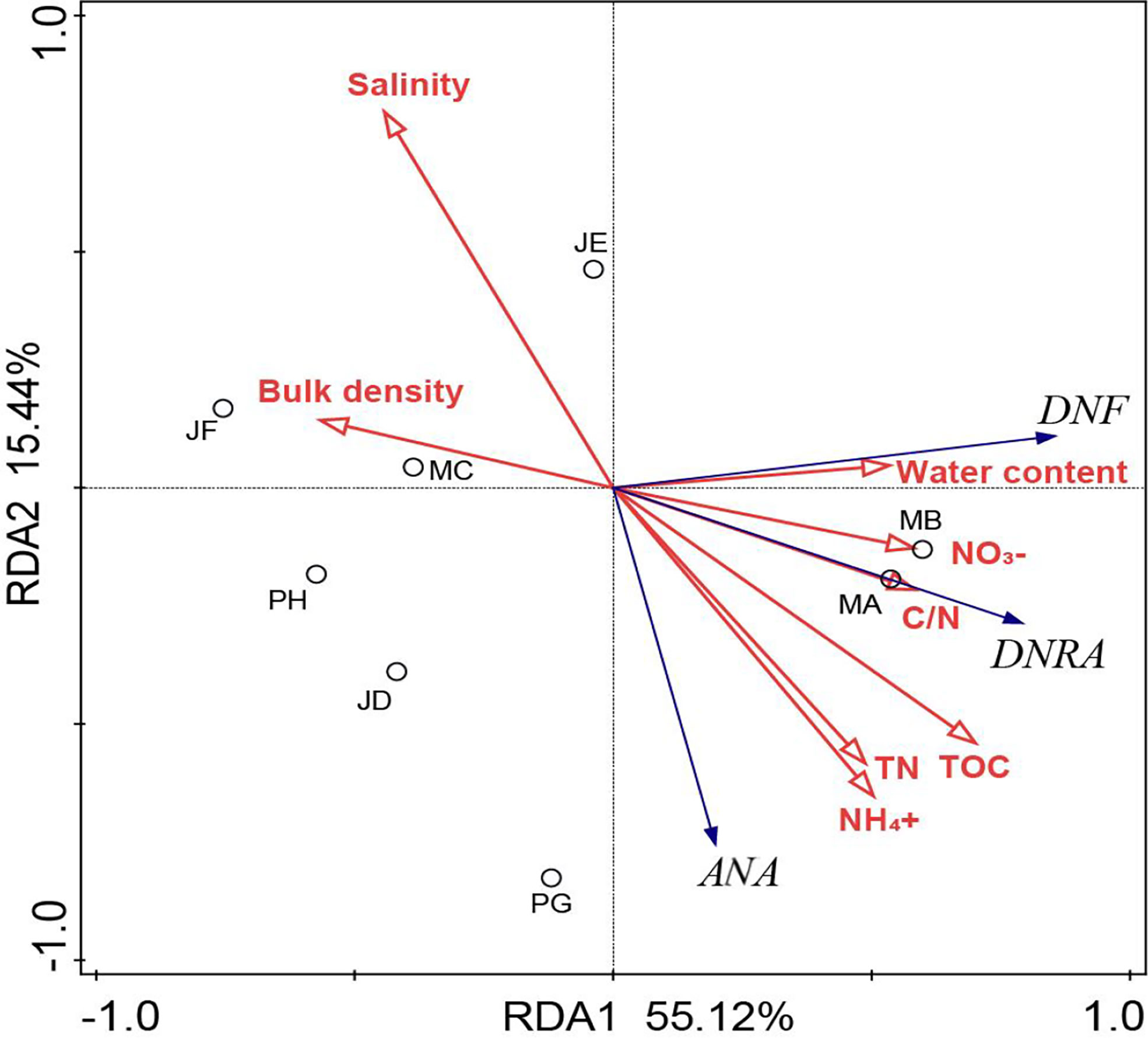
Figure 4 Ordination diagram showing the results of RDA of NO3- reduction processes and soil physicochemical characteristics. The hollow circles represent individual sediment samples from the eight shrimp ponds in the subtropics Estuary. The red arrows represent soil physicochemical characteristics, and blue arrows represent NO3- reduction processes.
Discussion
In coastal wetlands, reclaiming natural wetlands for aquaculture activities is a major anthropogenic disturbance that threatens intrinsic N balance. The addition of feed and the growth and excretion of shrimp during the breeding process increased C and N substrates in the sediment of shrimp ponds; at the same time, the rates of the dissimilatory NO3- reduction process were promoted (Wu et al., 2014; Chen et al., 2016b; Gao et al., 2019). The addition of bait to coastal wetlands can change the microbial community structure in sediments and contribute to greenhouse gas (GHGs) emissions (Lin and Lin, 2022). DNF, ANA, and DNRA are the most crucial processes in dissimilar NO3- reduction processes in aquatic environments (Rysgaard et al., 2004; Song et al., 2013; Yang et al., 2022). We researched the distribution of dissimilatory NO3- reduction processes in shrimp ponds and analysed the main influencing factors controlling the process. In the present study, most of the dissimilatory NO3- reduction processes with TOC, TOC/TN, NH4+, and NO3- were showed a positive correlations. Therefore, the contribution of these environmental factors to NO3- reduction in shrimp ponds requires further elucidation.
NO3- reduction process and the influence of environmental factors
DNF rate were measured by 15N tracer techniques according to the assumption of N2, because of the ratio of N2O to N2 from DNF in aquatic ecosystems is very low (Dong et al., 2002). Salinity affected the DNF rate to some extent, although no statistically significant correlation was observed, and a decrease in denitrification activity associated with higher salinity was observed. RDA showed a negative correlation between salinity and DNF rate. Salinity may result in physiological stress on DNF, which in turn affects the DNF rate, which generally decreases with an increase in salinity (Rysgaard et al., 1999; Seo et al., 2008). In general, the DNF rate was positively related to the TOC content in previous studies, such as in lakes, rice fields, estuaries, and coastal environments (Vymazal, 2007; Deng et al., 2015; Yang et al., 2022). Our study showed that the DNF rate showed a positive relationship with the TOC content in the shrimp ponds, which is similar to previous research (Gao et al., 2019). Because shrimp ponds have been in a high organic carbon environment for a long time, they are beneficial for the growth of denitrifying-associated bacteria and promote the DNF rate (Canion et al., 2014; Plummer et al., 2015; Yang et al., 2017b). A remarkable relationship between the DNF rate and NO3- was observed in our study (Table 3), and it was confirmed that NO3- is a major driver of the rate of NO3- reduction in shrimp ponds. DNF microorganisms use NO3- as an electron acceptor and substrate, which is strongly dependent on the NO3- concentration (Giles et al., 2012; Shan et al., 2016; Palacin-Lizarbe et al., 2020).
Spatial variations in ANA rates have also been found, and numerous studies have reported that salinity is a crucial environmental factor affecting ANA (Wang et al., 2012; Hou et al., 2015). Salinity was significantly correlated with the ANA rate in this study, suggesting that high salinity might inhibit the rate of ANA activity. It has been reported that ANA activity is not directly caused by an energy source, but is influenced by C and N substrates in sediments (Hou et al., 2015). A strong correlation was found between ANA and the C and N substrates in our study; this C and N matrix mainly comes from the decomposition of a large amount of feed and manure residues in the culture period (Wu et al., 2014). Studies have shown that TOC and NH4+ concentrations at suitable concentrations can promote the ANA rate (Cheng et al., 2016; Damashek and Francis, 2018), as well as a significant positive correlation between TOC, NH4+, and ANA. However, it should be noted that high TOC concentrations may inhibit ANA activity (Bettazzi et al., 2010).
DNRA is often considered to be closely related to salinity, and previous studies have shown that increasing salinity accelerates the DNRA rate (Gardner et al., 2006; Giblin et al., 2010). However, some studies have found that the abundance of the nrfA gene associated with DNRA rates is not significantly affected by salinity (Yin et al., 2017). Interestingly, our results showed that DNRA and ANA rates declined significantly with increasing salinity, as both showed similar results (Table 3). RDA also showed a negative correlation between salinity and ANA, DNRA rates. TOC and NH4+ could be important factors influencing DNRA in sediments (Bu et al., 2017), which was also proven by the significant positive correlation between the rate of DNRA and TOC in our study. High TOC concentrations may provide adequate organic substrates for the growth of DNRA-associated microorganisms (Deng et al., 2015). DNRA can more efficiently utilise NO3- as an electron acceptor in a carbon-rich environment, thereby increasing the DNRA rate (Kraft et al., 2014; Cheng et al., 2016).
Relationship between different NO3- reduction processes
The DNF rate was compared with the ANA and DNRA rates to identify potential connections among them (Figure 5). These correlation analyses show that DNF is closely related to ANA and DNRA in the study area. During the interaction between ANA and DNF, DNF is a major source of NO2- for ANA in coastal wetland sediments (Meyer et al., 2005), and there was a significant relationship found between DNF and ANA rates in the shrimp pond (R2 = 0.34, p < 0.05). Studies have also found a coupling related process between ANA and DNF (Hou et al., 2015). The ANA rates were significantly associated with DNRA rates (R2 = 0.66, p < 0.01), indicating that DNRA can produce NO2- as an intermediate product, which is an alternative substrate for ANA (Lam et al., 2009). DNF and DNRA compete for NO3- under hypoxic or anaerobic conditions. Our results showed that the DNF rates were significantly related with the DNRA rates in the sediment (R2 = 0.61, p < 0.05) (Figure 5), indicating that the growth of NO3- substrate promoted both of them.
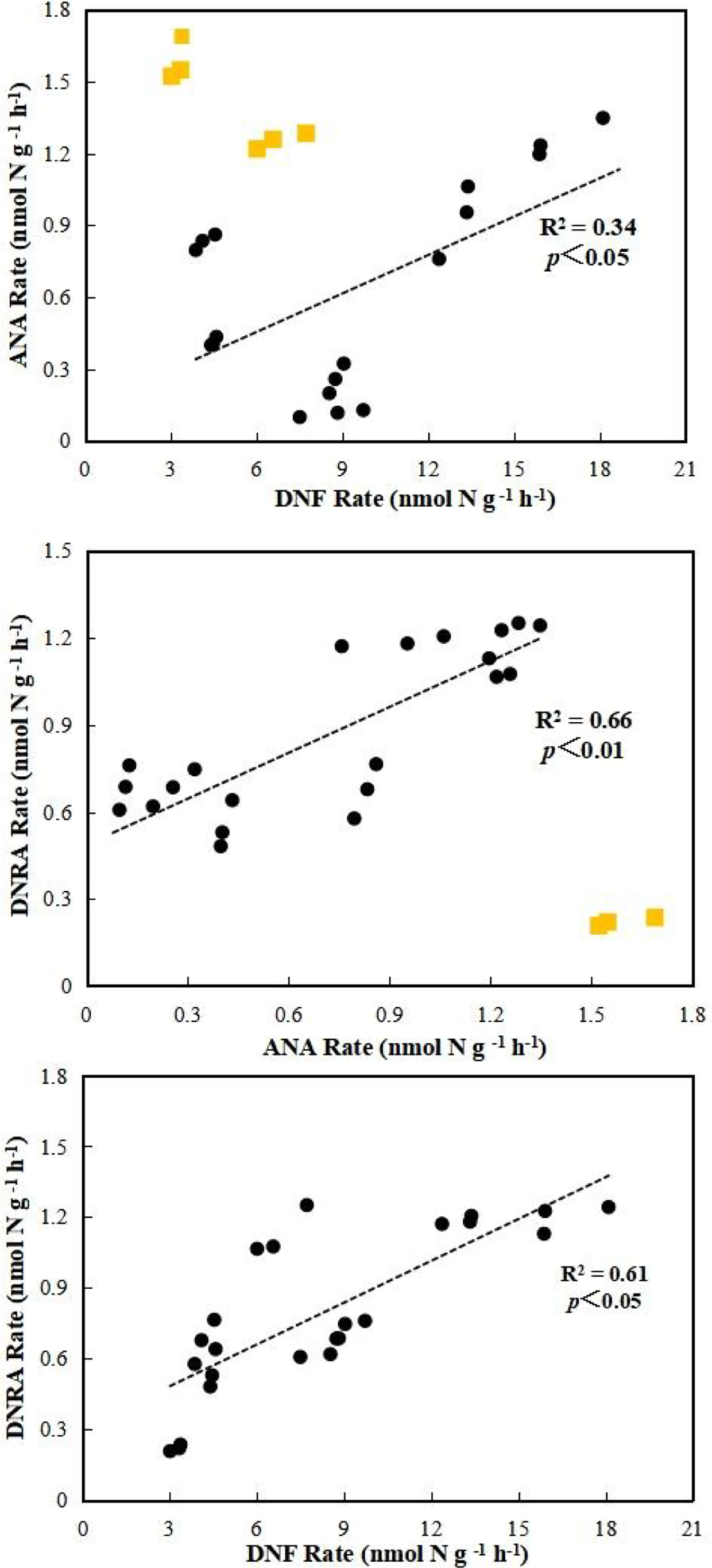
Figure 5 Correlations between DNF, ANA and DNRA rate. The linear regression is shown for the relation between DNF, ANA and DNRA rate. R2 represents regression coefficient, and Yellow points represents not included in the statistics.The significance level was set at 0.05 and 0.01.
Competition among DNF, ANA, and DNRA can determine the fate of NO3- owing to their diverse roles in dissimilatory NO3- reduction. DNF was the dominant route contributing 64.2–91.6% (81.14%) of the total the dissimilatory NO3- reduction processes in the shrimp pond, while the contributions of ANA and DNRA were 1.2–31.4% (10.48%) and 4.34–12.4% (8.38%), respectively (Figure 6). According to several studies, DNF plays a major role in removing nitrogen from various aquatic systems, while the proportions of ANA and DNRA differ between ecosystems (Deng et al., 2015; Shan et al., 2016; Yang et al., 2022). Different aquatic environments contribute different amounts of dissimilatory NO3- reduction, as shown in Table 4. Studies have shown that the DNF, ANA, and DNRA rates in urban rivers and seas are higher than those in shrimp ponds (Song et al., 2013; Cheng et al., 2016). However, the rate of DNF in a paddy field was between 2.37 and 8.30 nmol N g-1 h-1, and ANA and DNRA were also relatively low (Shan et al., 2016). The DNF rate in estuarine and lake regions ranges from 0.09 to 11.47 nmol N g-1 h-1, which is lower than that in shrimp ponds (Deng et al., 2015; Yang et al., 2022). Compared with the estuary wetland, the dissimilation NO3- reduction rate increased after reclamation for shrimp ponds, showing an increasing trend with increasing reclamation years (Gao et al., 2019). It was found that the biggest difference between the shrimp ponds system and other systems may be more dependent on the difference caused by the high concentration of C and N matrix input. We found that the rate of DNF ranged from 3.24 to 16.64 nmol N g-1 h-1 in shrimp ponds, while the rate of ANA was slightly higher than that of DNRA (Figure 2). Further analysis showed that DNRA was higher than ANA in aquaculture ponds in the Min and Jiulong River estuaries, indicating that the contribution of DNRA to the total NO3- reduction was still higher than that of natural wetlands (Gao et al., 2019). ANA was found to be much higher than DNRA in the Mulan River estuary, especially the PH site at 31.4% (Figure 3). This may be related to the large size and depth of shrimp ponds in the estuary of the Mulan River, however, specific causes need to be identified.

Figure 6 The conceptual map on NO3− reduction processes in the shrimp pond sediment. The unit of DNF, ANA, and DNRA rates were nmol N g-1 h-1.
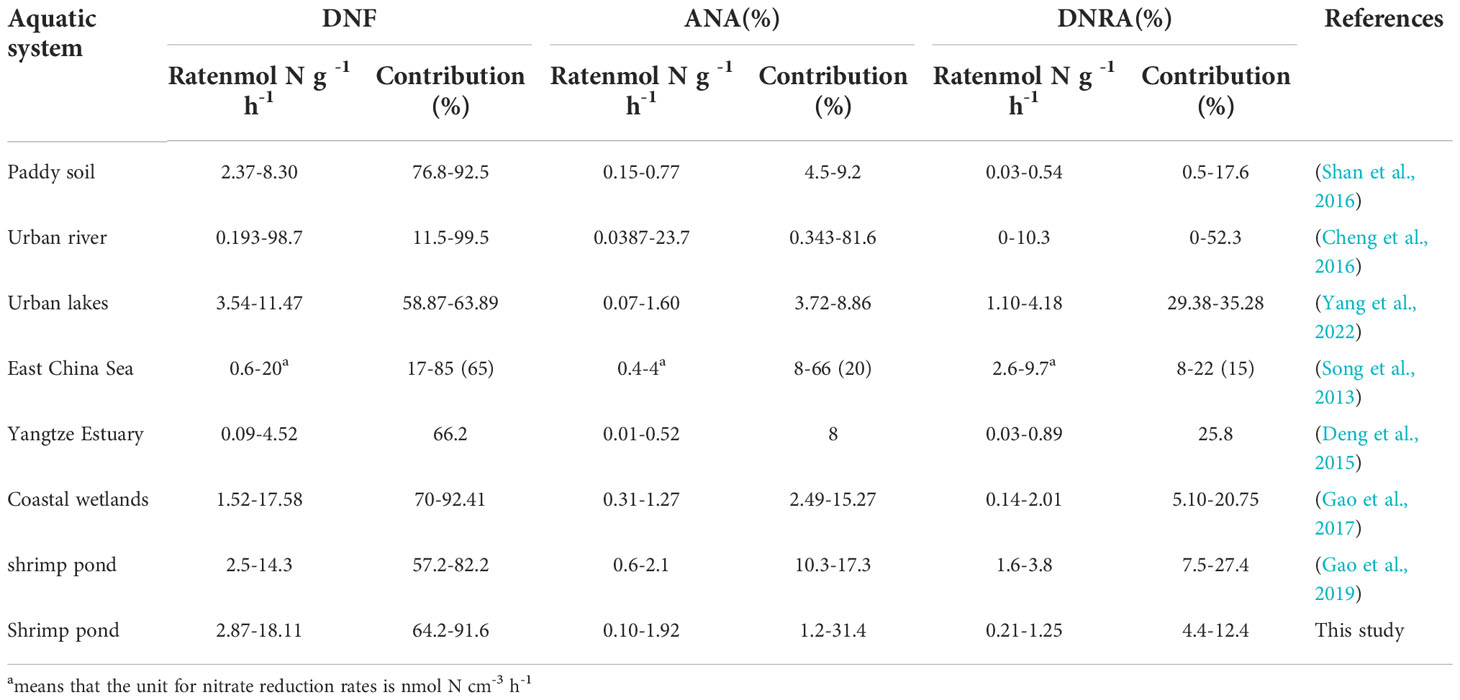
Table 4 Contributions of Denitrification, ANAMMOX, and DNRA to total nitrate reduction in our study and other aquatic ecosystems.
If the average DNF and ANA rates were extrapolated to the study area, the N losses were approximately 32.43–131.64 g N m-2 yr-1 for DNF and 2.38–15.85 g N m-2 yr-1 for ANA. According to the area of aquaculture ponds in China’s coastal wetland (2.6 ×106 ha) (Chen et al., 2016a; Chen et al., 2016b), approximately 2.18 × 106 t N can be removed from shrimp ponds by this process annually, indicating that coastal reclamation is a considerable way to remove nitrogen. Recent results suggest that if natural wetlands are gradually converted into shrimp ponds, reactive nitrogen may be retained (Murphy et al., 2016). Shrimp ponds may be more important than nitrogen loss (Gao et al., 2019). Consequently, sediment N loads increase, resulting in decreased water quality and shrimp disease outbreaks (Castillo Soriano et al., 2013).
Conclusion
This study investigated the process of NO3- dissimilation and reduction in shrimp pond sediments in a subtropical estuary area and identified the main factors influencing the process. Studies have shown that DNF is the main pathway, and that ANA and DNRA also play important roles. The feed added during the culture promoted the C and N matrices in the sediment, which in turn promoted the reduction of various NO3-. This process of nitrogen removal under intensive aquaculture in estuarine and coastal areas should receive more attention in future studies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
DS: Investigation, Formal analysis, Writing and editing. JH: Conceptualization, Methodology, Funding acquisition. ML: Methodology. XL, CC, WH: Formal analysis. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation of China (grant numbers: 41601102 and 32071598). And Funded by Fujian Forestry Science and Technology Project (No. 2021FKJ30), Public Welfare Project of Fujian Science and Technology Department (No. 2022R1002007) and the Starting Research Program of Suzhou University of Science and Technology (No. 332214803).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bettazzi E., Caffaz S., Vannini C., Lubello C. (2010). Nitrite inhibition and intermediates effects on anammox bacteria: A batch-scale experimental study. Process Biochem. 45, 573–580. doi: 10.1016/j.procbio.2009.12.003
Birch S., McCaskie J. (1999). Shallow urban lakes: a challenge for lake management. Hydrobiologia 395–396, 365–378. doi: 10.1023/A:1017099030774
Burgin A. J., Hamilton S. K. (2007). Have we overemphasized the role of denitrification in aquatic ecosystems? a review of nitrate removal pathways. Front. Ecol. Environmen. 5, 89–96. doi: 10.1890/1540-9295(2007)5[89:HWOTRO]2.0.CO;2
Bu C., Wang Y., Ge C., Ahmad H. A., Gao B., Ni S.-Q. (2017). Dissimilatory nitrate reduction to ammonium in the yellow river estuary: Rates, abundance, and community diversity. Sci. Rep. 7, 6830–6811. doi: 10.1038/s41598-017-06404-8
Canfield D. E., Giazer A. N., Falkowski P. G. (2010). The evolution and future of earth's nitrogen cycle. Science 330, 192–196. doi: 10.1126/science.1186120
Canion A., Overholt W. A., Kostka J. E., Huettel M., Lavik G., Kuypers M. M. M. (2014). Temperature response of denitrification and anaerobic ammonium oxidation rates and microbial community structure in Arctic fjord sediments: Temperature and n cycling in Arctic sediments. Environ. Microbiol. 16, 3331–3344. doi: 10.1111/1462-2920.12593
Cao W., Yang J., Li Y., Liu B., Wang F., Chang C. (2016). Dissimilatory nitrate reduction to ammonium conserves nitrogen in anthropogenically affected subtropical mangrove sediments in southeast China. Mar. pollut. Bull. 110, 155–161. doi: 10.1016/j.marpolbul.2016.06.068
Castillo Soriano F. A., Ibarra Junquera V., Escalante Minakata P., Mendoza Cano O., Ornelas Paz J., d. J., et al. (2013). Nitrogen dynamics model in zero water exchange, low salinity intensive ponds of white shrimp, litopenaeus vannamei, at colima, Mexico. Latin Am. J. Aquat. Res. 41, 68–79. doi: 10.3856/vol41-issue1-fulltext-5
Chen Y., Dong S., Wang F., Gao Q., Tian X. (2016b). Carbon dioxide and methane fluxes from feeding and no-feeding mariculture ponds. Environ. pollut. 212, 489–497. doi: 10.1016/j.envpol.2016.02.039
Cheng L., Li X., Lin X., Hou L., Liu M., Li Y., et al. (2016). Dissimilatory nitrate reduction processes in sediments of urban river networks: Spatiotemporal variations and environmental implications. Environ. pollut. 219, 545–554. doi: 10.1016/j.envpol.2016
Chen F., Hou L., Liu M., Zheng Y., Yin G., Lin X., et al. (2016a). Net anthropogenic nitrogen inputs (NANI) into the Yangtze river basin and the relationship with riverine nitrogen export. J. geophysical Res. Biogeosciences 121, 451–465. doi: 10.1002/2015JG003186
Dale O. R., Tobias C. R., Song B. (2009). Biogeographical distribution of diverse anaerobic ammonium oxidizing (anammox) bacteria in cape fear river estuary. Environ. Microbiol. 11, 1194–1207. doi: 10.1111/j.1462-2920.2008.01850.x
Damashek J., Francis C. A. (2018). Microbial nitrogen cycling in estuaries: From genes to ecosystem processes. Estuaries coasts 41, 626–660. doi: 10.1007/s12237-017-0306-2
Deegan L. A., Johnson D. S., Warren R. S., Peterson B. J., Fleeger J. W., Fagherazzi S., et al. (2012). Coastal eutrophication as a driver of salt marsh loss. Nature 490, 388–392. doi: 10.1038/nature11533
Deng F., Hou L., Liu M., Zheng Y., Yin G., Li X., et al. (2015). Dissimilatory nitrate reduction processes and associated contribution to nitrogen removal in sediments of the Yangtze estuary. J. geophysical Res. Biogeosci. 120, 1521–1531. doi: 10.1002/2015JG003007
Dong L. F., Nedwell D. B., Underwood G. J., Thornton D. C., Rusmana I. (2002). Nitrous oxide formation in the colne estuary, England: the central role of nitrite. Appl. Environ. Microbiol. 68 (3), 1240e1249. doi: 10.1128/AEM.68.3.1240-1249.2002
Dong L. F., Sobey M. N., Smith C. J., Rusmana I., Phillips W., Stott A., et al. (2011). Dissimilatory reduction of nitrate to ammonium, not denitrification or anammox, dominates benthic nitrate reduction in tropical estuaries. Limnology oceanogr. 56, 279–291. doi: 10.4319/lo.2011.56.1.0279
Galloway J. N., Townsend A. R., Erisman J. W., Bekunda M., Cai Z., Freney J. R., et al. (2008). Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 320, 889. doi: 10.1126/science.1136674
Gao D., Chen G., Li X., Lin X., Zeng C. (2018). 'Reclamation culture alters sediment phosphorus speciation and ecological risk in coastal zone of southeastern China. Clean soil air Water 46, 1700495–n/a. doi: 10.1002/clen.201700495
Gao D., Li X., Lin X., Wu D., Jin B., Huang Y., et al. (2017). Soil dissimilatory nitrate reduction processes in the spartina alterniflora invasion chronosequences of a coastal wetland of southeastern China: Dynamics and environmental implications. Plant Soil. 421, 383–399. doi: 10.1007/s11104-017-3464-x
Gao D., Liu M., Hou L., Derrick Y. F. L., Wang W., Li X., et al. (2019). Effects of shrimp-aquaculture reclamation on sediment nitrate dissimilatory reduction processes in a coastal wetland of southeastern China. Environ. pollut. 255, 113219–113219. doi: 10.1016/j.envpol.2019.113219
Gardner W. S., McCarthy M. J., An S., Sobolev D., Sell K. S., Brock D. (2006). Nitrogen fixation and dissimilatory nitrate reduction to ammonium (DNRA) support nitrogen dynamics in Texas estuaries. Limnology oceanogr. 51, 558–568. doi: 10.4319/lo.2006.51.1_part_2.0558
Giblin A. E., Weston N. B., Banta G. T., Tucker J., Hopkinson C. S. (2010). The effects of salinity on nitrogen losses from an oligohaline estuarine sediment. Estuaries coasts 33, 1054–1068. doi: 10.1007/s12237-010-9280-7
Giles M., Morley N., Baggs E. M., Daniell T. J. (2012). Soil nitrate reducing processes - drivers, mechanisms for spatial variation, and significance for nitrous oxide production. Front. Microbiol. 3. doi: 10.3389/fmicb.2012.00407
Hou L., Wang R., Yin G., Liu M., Zheng Y. (2018). Nitrogen fixation in the intertidal sediments of the Yangtze estuary: Occurrence and environmental implications. J. geophysical Res. Biogeosciences 123, 936–944. doi: 10.1002/2018JG004418
Hou L., Zheng Y., Liu M., Gong J., Zhang X., Yin G., et al. (2013). Anaerobic ammonium oxidation (anammox) bacterial diversity, abundance, and activity in marsh sediments of the Yangtze estuary. journal of geophysical research. Biogeosciences 118, 1237–1246. doi: 10.1002/JGRG.20108
Hou L., Zheng Y., Liu M., Li X., Lin X., Yin G., et al. (2015). Anaerobic ammonium oxidation and its contribution to nitrogen removal in china's coastal wetlands. Sci. Rep. 5, 15621. doi: 10.1038/srep15621
Huang F., Lin X., Hu W., Zeng F., He L., Yin K. (2021). Nitrogen cycling processes in sediments of the pearl river estuary: Spatial variations, controlling factors, and environmental implications. Catena 206, 105545. doi: 10.1016/j.catena.2021.105545
Hu M., Sardans J., Le Y., Yan R., Zhong Y., Huang J., et al. (2022). Biogeochemical behavior of p in the soil and porewater of a low-salinity estuarine wetland: Availability, diffusion kinetics, and mobilization mechanism. Water Res. 219, 118617–118617. doi: 10.1016/j.watres.2022.118617
Huygens D., Rütting T., Boeckx P., Van Cleemput O., Godoy R., Müller C. (2007). Soil nitrogen conservation mechanisms in a pristine south Chilean nothofagus forest ecosystem. Soil Biol. Biochem. 39, 2448–2458. doi: 10.1016/j.soilbio.2007.04.013
Kennison R. L., Fong P. (2014). Extreme eutrophication in shallow estuaries and lagoons of California is driven by a unique combination of local watershed modifications that trump variability associated with wet and dry seasons. Estuaries coasts 37, S164–S179. doi: 10.1007/s12237-013-9687-z
Kraft B., Tegetmeyer H. E., Sharma R., Klotz M. G., Ferdelman T. G., Hettich R. L., et al. (2014). The environmental controls that govern the end product of bacterial nitrate respiration. Science 345, 676–679. doi: 10.1126/science.1254070
Lam P., Lavik G., Jensen M. M., van de Vossenberg J., Schmid M., Woebken D., et al. (2009). Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proc. Natl. Acad. Sci. 106, 4752–4757. doi: 10.1073/pnas.0812444106
Laverman A. M., Canavan R. W., Slomp C. P., Cappellen P. V. (2007). Potential nitrate removal in a coastal freshwater sediment (Haringvliet lake, the Netherlands) and response to salinization. Water Res. 41, 3061–3068. doi: 10.1016/j.watres.2007.04.002
Lin G., Lin X. (2022). Bait input altered microbial community structure and increased greenhouse gases production in coastal wetland sediment. Water Res. 218, 11850. doi: 10.1016/j.watres.2022.118520
Lin X., Liu M., Hou L., Gao D., Li X., Lu K., et al. (2017). Nitrogen losses in sediments of the East China Sea: Spatiotemporal variations, controlling factors, and environmental implications. J. Geophysical Research: Biogeosci. 122, 2699–2715. doi: 10.1002/2017JG004036
Luo M., Zhu W., Huang J., Liu Y., Duan X., Wu J., et al. (2019). Anaerobic organic carbon mineralization in tidal wetlands along a low-level salinity gradient of a subtropical estuary: Rates, pathways, and controls. Geoderma 337, 1245–1257. doi: 10.1016/j.geoderma.2018.07.030
Macdonald D. M. J., Dixon A. J., Gooddy D. C. (2018). Water and nitrate exchange between a managed river and peri-urban floodplain aquifer: Quantification and management implications. Ecol. engineer. 123, 226–237. doi: 10.1016/j.ecoleng.2018.09.005
Meyer R. L., Risgaard-Petersen N., Allen D. E. (2005). Correlation between anammox activity and microscale distribution of nitrite in a subtropical mangrove sediment. Appl. Environ. Microbiol. 71, 6142–6149. doi: 10.1128/AEM.71.10.6142-6149.2005
Minick K. J., Pandey C. B., Fox T. R., Subedi S. (2016). Dissimilatory nitrate reduction to ammonium and N2O flux: effect of soil redox potential and n fertilization in loblolly pine forests. Biol. fertility soils. 52, 601–614. doi: 10.1007/s00374-016-1098-4
Murphy A. E., Anderson I. C., Smyth A. R., Song B., Luckenbach M. W. (2016). Microbial nitrogen processing in hard clam (Mercenaria mercenaria) aquaculture sediments: the relative importance of denitrification and dissimilatory nitrate reduction to ammonium (DNRA). Limnology oceanogr. 61, 1589–1604. doi: 10.1002/lno.10305
Osburn C. L., Handsel L. T., Peierls B. L., Paerl H. W. (2016). Predicting sources of dissolved organic nitrogen to an estuary from an agro-urban coastal watershed. Environ. Sci. tech. 50, 8473–8484. doi: 10.1021/acs.est.6b00053
Palacin-Lizarbe C., Camarero L., Hallin S., Jones C. M., Catalan J., Sveriges L. (2020). Denitrification rates in lake sediments of mountains affected by high atmospheric nitrogen deposition. Sci. Rep. 10, 3003–3003. doi: 10.1038/s41598-020-59759-w
Percival J., Lindsay P. (1997). Measurement of physical properties of sediments (Boca Raton, Florida USA: CRC Press, Lewis Publishers), 287.
Plummer P., Tobias C., Cady D. (2015). Nitrogen reduction pathways in estuarine sediments: Influences of organic carbon and sulfide. J. geophysical Res. Biogeosci. 120, 1958–1972. doi: 10.1002/2015JG003057
Risgaard-Petersen N., Nielsen L. P., Rysgaard S., Dalsgaard T., Meyer R. L. (2003). Application of the isotope pairing technique in sediments where anammox and denitrification coexist. Limnology oceanogr. Methods 1, 63–73. doi: 10.1002/lom3.10127
Rysgaard S., Glud R. N., Risgaard-Petersen N., Dalsgaard T. (2004). Denitrification and anammox activity in Arctic marine sediments. Limnology Oceanogr. 49, 1493–1502. doi: 10.4319/lo.2004.49.5.1493
Rysgaard S., Thastum P., Dalsgaard T., Christensen P. B., Sloth N. P. (1999). Effects of salinity on NH4+ adsorption capacity, nitrification, and denitrification in Danish estuarine sediments. Estuaries 22, 21–30. doi: 10.2307/1352923
Seitzinger S., Harrison J. A., Böhlke J. K., Bouwman A. F., Lowrance R., Peterson B., et al. (2006). Denitrification across landscapes and waterscapes: A synthesis. Ecol. applications 16, 2064–2090. doi: 10.1890/1051-0761(2006)016[2064:DALAWA]2.0.CO;2
Seo D. C., Yu K., Delaune R. D. (2008). Influence of salinity level on sediment denitrification in a Louisiana estuary receiving diverted Mississippi river water. Archiv für Acker- und Pflanzenbau und Bodenkunde 54, 249–257. doi: 10.1080/03650340701679075
Shan J., Zhao X., Sheng R., Xia Y., ti C., Quan X., et al. (2016). Dissimilatory nitrate reduction processes in typical Chinese paddy soils: Rates, relative contributions, and influencing factors. Environ. Sci. Technol. 50, 9972–9980. doi: 10.1021/acs.est.6b01765
Silver W. L., Thompson A. W., Reich A., Ewel J. J., Firestone M. K. (2005). Nitrogen cycling in tropical plantation forests: Potential controls on nitrogen retention. Ecol. applications 15, 1604–1614. doi: 10.1890/04-1322
Song G. D., Liu S. M., Marchant H., Kuypers M. M. M., Lavik G. (2013). Anammox, denitrification and dissimilatory nitrate reduction to ammonium in the East China Sea sediment. Biogeosciences 10, 6851–6864. doi: 10.5194/bg-10-6851-2013
Sun D., Tang X., Li J., Liu M., Hou L., Yin G., et al. (2022). Chlorate as a comammox Nitrospira specific inhibitor reveals nitrification and N2O production activity in coastal wetland. Soil Biol. Biochem. 173, 108782. doi: 10.1016/j.soilbio.2022.108782
Sun D., Tang X., Zhao M., Zhang Z., Hou L., Liu M., et al. (2020). Distribution and diversity of comammox Nitrospira in coastal wetlands of China. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.589268
Thamdrup B., Dalsgaard T. (2002). Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl. Environ. Microbiol. 68, 1312–1318. doi: 10.1128/AEM.68.3.1312-1318.2002
Tong C., Wang W.-Q., Huang J.-F., Gauci V., Zhang L.-H., Zeng C.-S. (2012). Invasive alien plants increase CH4 emissions from a subtropical tidal estuarine wetland. Biogeochemistry 111, 677–693. doi: 10.1007/s10533-012-9712-5
Trimmer M., Nicholls J. C., Deflandre B. (2003). Anaerobic ammonium oxidation measured in sediments along the Thames estuary, united kingdom. Appl. Environ. Microbiol. 69, 6447–6454. doi: 10.1128/AEM.69.11.6447-6454.2003
Vymazal J. (2007). Removal of nutrients in various types of constructed wetlands. Sci. total environ. 380, 48–65. doi: 10.1016/j.scitotenv.2006.09.014
Wang S., Pi Y., Jiang Y., Pan H., Wang X., Wang X., et al. (2020). Nitrate reduction in the reed rhizosphere of a riparian zone: from functional genes to activity and contribution. Environ. Res. 180, 108867. doi: 10.1016/j.envres.2019.108867
Wang S., Zhu G., Peng Y., Jetten M. S. M., Yin C. (2012). Anammox bacterial abundance, activity, and contribution in riparian sediments of the pearl river estuary. Environ. Sci. tech. 46, 8834–8842. doi: 10.1021/es3017446
Wu H., Peng R., Yang Y., He L., Wang W., Zheng T., et al. (2014). Mariculture pond influence on mangrove areas in south China: Significantly larger nitrogen and phosphorus loadings from sediment wash-out than from tidal water exchange. Aquaculture 426-427, 204–212. doi: 10.1016/j.aquaculture.2014.02.009
Yang P., Bastviken D., Lai D. Y. F., Jin B. S., Mou X. J., Tong C., et al. (2017a). Effects of coastal marsh conversion to shrimp aquaculture ponds on CH4 and N2O emissions. Estuarine Coast. shelf Sci. 199, 125–131. doi: 10.1016/j.ecss.2017.09.023
Yang P., Lai D. Y. F., Jin B., Bastviken D., Tan L. (2017b). Dynamics of dissolved nutrients in the aquaculture shrimp ponds of the Min river estuary, China: Concentrations, fluxes and environmental loads. Sci. total environ. 603-604, 256–267. doi: 10.1016/j.scitotenv.2017.06.074
Yang Z., Lu L., Cheng Z., Xian J., Yang Y., Liu L., et al. (2022). Dissimilatory nitrate reduction in urban lake ecosystems: A comparison study between closed and open lakes in chengdu, China. Water Res. 214, 118218–118218. doi: 10.1016/j.watres.2022.118218
Yin G., Hou L., Liu M., Liu Z., Gardner W. S. (2014). A novel membrane inlet mass spectrometer method to measure 15NH4+ for isotope-enrichment experiments in aquatic ecosystems. Environ. Sci. Technol. 48, 9555–9562. doi: 10.1021/es501261s
Yin G., Hou L., Liu M., Li X., Zheng Y., Gao J., et al. (2017). DNRA in intertidal sediments of the Yangtze estuary. J. geophysical Res. Biogeosci. 122, 1988–1998. doi: 10.1002/2017JG003766
Keywords: denitrification, anammox, DNRA, shrimp ponds, sediment
Citation: Sun D, Huang J, Luo M, Chen C, Lan X and Hu W (2022) Dissimilatory nitrate reduction processes in surface sediments of shrimp ponds during the culture period. Front. Mar. Sci. 9:1082768. doi: 10.3389/fmars.2022.1082768
Received: 28 October 2022; Accepted: 28 November 2022;
Published: 08 December 2022.
Edited by:
Jing Wei, Sun Yat-sen University, ChinaReviewed by:
Zucheng Wang, Northeast Normal University, ChinaLishan Tan, The Chinese University of Hong Kong, China
Copyright © 2022 Sun, Huang, Luo, Chen, Lan and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiafang Huang, d2FodWdlb0Bmam51LmVkdS5jbg==
 Dongyao Sun
Dongyao Sun Jiafang Huang
Jiafang Huang Min Luo
Min Luo Cheng Chen2
Cheng Chen2 Weifang Hu
Weifang Hu