- 1School of the Earth, Ocean and Environment, College of Arts and Sciences, University of South Carolina, Columbia, SC, United States
- 2Integrated Statistics Under Contract to the Northeast Fisheries Science Center, National Marine Fisheries Service, National Oceanic and Atmospheric Administration, Woods Hole, MA, United States
- 3Department of Marine Science, University of Otago, Dunedin, New Zealand
- 4Anderson Cabot Center for Ocean Life, New England Aquarium, Boston, MA, United States
The critically endangered North Atlantic right whale population (Eubalaena glacialis) has experienced multiple periods of decreased reproduction within its observable history, which have played a role in the overall decline of the species. In addition to this synchronized variation in reproduction across the population, there exists considerable individual variation in fecundity. To determine the impacts of family history and habitat use behavior on these individual variations in fecundity, photo identification data collected during four decades of visual monitoring were used to create a calving index for sexually mature females that could be used to evaluate matrilineal influence on fecundity. Reproductive life histories were analyzed to assess fecundity variation within matrilines over time. Individual variations in fecundity were also assessed with respect to a recent climate-driven habitat distribution shift by a loyal cohort of right whales that use the Gulf of St. Lawrence during the summer and autumn seasons. Lifetime fecundity in the oldest known living reproductive female, or matriarch, in a matriline was positively associated with the fecundity of her female progeny. Sexually mature females that have used the Gulf of St. Lawrence since 2015 were significantly more likely to give birth over this time period compared to individuals who did not use that habitat. Individuals of both sexes were significantly more likely to use the Gulf of St. Lawrence if their mothers did as well; however, this association declined as offspring aged. These results provide insight on the environmental, behavioral, and genetic factors that contribute to individual variation in fecundity. Low calving rates and increased dangers posed by habitat use shifts in the past decade have played a major role in the species’ decline, and these new insights into the mechanistic drivers of right whale reproduction and habitat use show that lineage guides progeny behavior and reproductive success. As anthropogenic climate change continues to disrupt right whale seasonal distributions through changing ocean circulation patterns, understanding the demographic consequences of novel habitat use patterns will be essential to updating protective policies.
Introduction
The North Atlantic right whale (Eubalaena glacialis) is one of the most critically endangered baleen whale species in the world, with an estimated population size of less than 350 individuals in 2020 (Kraus et al., 2001; Pettis et al., 2022). The species has struggled with dangerously low population numbers since the 20th century, if not longer (McLeod et al., 2008; Reeves, 2001), and the population is currently in a state of decline (Pettis et al., 2022). High rates of anthropogenic mortality from ship strikes and entanglements in fishing gear have played a dominant role in this decline (Knowlton and Kraus, 2001; Kraus et al., 2005; Knowlton et al., 2012; van der Hoop et al., 2015; Sharp et al., 2019), with mortalities rapidly increasing since 2013 (Meyer-Gutbrod et al., 2018; Pace et al., 2021).
In addition to the high mortality rates, decadal-scale fluctuations in reproduction, linked to climate induced changes in prey availability (Greene et al., 2003; Greene and Pershing, 2004; Meyer-Gutbrod and Greene, 2014; Meyer-Gutbrod and Greene, 2018), have contributed to depressed population growth. In the 1990s, a population-wide decline in reproduction was documented and later shown to be the result of limited prey availability (Caswell et al., 1999; Fujiwara and Caswell, 2001; Meyer-Gutbrod et al., 2015). Despite a rebound in reproduction in 2000, the population has once again entered a period of low reproduction as of 2010 (Meyer-Gutbrod et al., 2021; Pettis et al., 2022). Low reproductive rates and high calving intervals (the number of years that occur between subsequent births) have been observed in this species (Knowlton et al., 1994; Caswell et al., 1999; Meyer-Gutbrod et al., 2015). The average calving interval increased from 4.0 to 9.2 years between 2009–2021 (Pettis et al., 2022), and the frequency of high calving intervals (defined here as 6+ years, which is double the calving interval that females are biologically capable of) increased as well (Knowlton et al., 1994; Kraus et al., 2001). Individual and population-level variation in right whale reproduction occurs in part due to the high energetic requirements of pregnancy and lactation (Fortune et al., 2013). To satisfy these requirements, adult females require an adequate amount of body fat to successfully reproduce (Miller et al., 2011). In years when prey availability is low, many females are unable to build a blubber layer that is thick enough to sustain successful reproduction; therefore, reproduction is delayed until enough food can be consumed (Miller et al., 2011). Fluctuations in prey availability are exacerbated by chronic non-fatal entanglements which limit an individual’s ability to forage (Stewart et al., 2021).
Previous studies have shown that matrilineal family structure also has a significant impact on survival and reproduction rates in cetacean species, and this is best documented in odontocetes. For example, the presence of both reproductive and post-reproductive females has been shown to increase the survival of young offspring in resident killer whales (Foster et al., 2012). In addition, post-reproductive females are known to be important sources of ecological knowledge, including feeding and breeding information, in various cetacean species (Brent et al., 2015; Wright et al., 2016; Nattrass et al., 2019). Their knowledge and leadership are particularly important in periods of low prey years (Brent et al., 2015). The social structure of mysticetes is poorly understood and, while post-reproductive females are not known to exist in the right whale population, similar dynamics of maternally-influenced habitat use and matrilineal transmission of ecological and behavioral knowledge may exist within this species, which potentially impact the survivability and fecundity of specific kin groups.
Individual matrilines with characteristically distinct fecundities have already been observed in the Northwest Atlantic humpback whale population (Rosenbaum et al., 2002) which may have arisen, in part, because learned behavior and site fidelity are passed down by family members. Baleen whale species including southern right whales, humpbacks, and North Atlantic right whales have been documented exhibiting fidelity for specific sites (Schaeff et al., 1993; Palsbøll et al., 1995; Larsen et al., 1996; Malik et al., 1999; Stevick et al., 2006; Patenaude et al., 2007; Crowe et al., 2021), with direct evidence of maternally inherited feeding site fidelity being observed in southern right whales (Carroll et al., 2015). Southern right whale ecological knowledge of nursery and feeding grounds has been shown to be communicated to a calf by its mother during its first migration (Valenzuela et al., 2009). Similar carbon and nitrogen isotopic values found between specific southern right whale matrilines indicated that individual families follow the same migratory patterns, further supporting the idea of matrilineal site fidelity (Valenzuela et al., 2009).
North Atlantic right whales live largely within the northwest Atlantic Ocean (Winn et al., 1986). The spring and summer months are spent primarily foraging in the Great South Channel, Cape Cod and Massachusetts Bays, the Bay of Fundy, and the southern Scotian Shelf (Winn et al., 1986; Baumgartner & Mate, 2003; Mayo et al., 2018; Ganley et al., 2019). Most of these areas have been designated as critically important habitats (Department of Fisheries and Oceans Canada, 2007; Brown et al., 2009; National Marine Fisheries Service, 2016). Reproductive females in calving years spend the winter at their only known calving grounds in the Southeast United States, along the Georgia and Florida coasts (Winn et al., 1986), where they can be found through late spring (Kraus et al., 1986). The winter movement patterns of all other demographics are not well known, though a southward migration has been hypothesized, with some individuals spotted in the Gulf of Maine and off of Cape Cod (Winn et al., 1986; Brown et al., 2009). Despite these observations, movement and habitat occupancy patterns for large portions of the population and significant seasonal periods remain unknown.
Calves typically remain with their mothers for one year, and follow their mother’s migration patterns during that time (Hamilton et al., 1995). There is evidence that individuals display site fidelity for the habitats they were taken to during their weaning period as calves. One study reported that individuals who visited the Bay of Fundy as a nursery site were documented using it as a feeding ground in later life, while individuals who were not taken to the Bay of Fundy as calves did not subsequently migrate there to feed (Schaeff et al., 1993). Strong, but nonsignificant evidence was later found that the transmission of this site information is linked between mothers and daughters (Malik et al., 1999). Finally, recent genetic evidence from ancient whale bones revealed that maternally inherited site fidelity likely played a significant role in defining the habitat use patterns of historic right whale populations (Frasier et al., 2022).
In recent years, an occupancy and distribution shift has been observed for North Atlantic right whales throughout their range (Davis et al., 2017; Simard et al., 2019; Meyer-Gutbrod et al., 2021). This shift began in the early 2000s, when the proportion of the population observed using Cape Cod Bay increased significantly (Mayo et al., 2018; Ganley et al., 2019). Over the past decade, the Gulf of St. Lawrence has been used as a foraging habitat (Meyer-Gutbrod et al., 2021), and survey effort in recent years has indicated that a loyal subset of the population uses this habitat during the summer and autumn months (Crowe et al., 2021). While there is a long record of sporadic right whale sightings in the Gulf of St. Lawrence (McLeod et al., 2008; Brown et al., 2009; Reeves et al., 1999), the current group of users, comprising approximately 40% of the population, predominantly returns to this habitat annually (Crowe et al., 2021). This novel habitat use pattern is most likely caused by climate-driven declines in prey availability in traditional summertime right whale foraging habitats (Record et al., 2019; Meyer-Gutbrod et al., 2021).
With the population being regularly affected by low prey availability and resultantly low reproduction rates, it is critical to understand the more nuanced, underlying mechanisms driving female reproductive success as well as factors driving habitat use. While previous studies have confirmed that prey availability drives interannual variation in right whale reproduction (Greene et al., 2003; Meyer-Gutbrod et al., 2015; Gavrilchuk et al., 2021), some sexually mature females still fail to reproduce in high prey years, as evidenced by the continuously declining birth rate within the population despite concurrent spikes in the abundance of their primary prey, Calanus finmarchicus (Kraus et al., 2001; Meyer-Gutbrod and Greene, 2014). In years when prey is anomalously low and population-wide reproduction is stunted (ex: 1998–2000), some females still manage to birth healthy calves. What drives variations in fecundity outside of broad-scale environmental conditions? This study aims to determine whether lifetime fecundity patterns are consistent among kin groups (matrilines) and how these fecundity patterns are related to recent climate-induced habitat use behaviors. Determining drivers of fecundity variation between individuals could be used to advance our understanding of population dynamics and inform initiatives to support species recovery.
Materials And Methods
Data Collection and Life History
Right whale demographic and sightings data were obtained from the North Atlantic Right Whale Consortium Identification database and Sightings database (North Atlantic Right Whale Consortium, 2021a; 2021b). These databases contain right whale sightings data occurring from 1935 to 2021, and were collected through aerial surveys, boat surveys, and opportunistic sources, with dedicated survey effort beginning in the late 1970s (Kenney, 2018). Survey effort occurs along the eastern seaboard of the United States and Canada to monitor the population, though the effort is not comprehensive or consistent in all regions annually. In the past decade of published data (2009–2019), the total survey effort fell between 141,000 km and 271,000 km surveyed annually, with 57–93% of the presumed alive population observed each year (Pettis et al., 2022). Individuals are presumed alive if they have been seen within five years of their last sighting (Knowlton et al., 1994).
Individual right whales are primarily identified by unique callosity patterns, rough patches of skin on their heads that are colonized by whale lice (Payne et al., 1983; Kraus et al., 1986), though scars, natural variations in pigmentation, and DNA can also be used for identification (Hamilton et al., 2007). Age, matriline, calving history, and sightings location data were used to observe patterns in individual fecundity variations. This study utilized complete demographic data through 2019; however, calving data for 2020 and 2021 were obtained from the National Oceanic and Atmospheric Administration and the North Atlantic Right Whale Consortium to ensure up-to-date calving analysis (National Oceanic and Atmospheric Administration, 2021; Right Whale News, 2021; Pettis et al., 2022). R Statistical Software Version 1.4.1103 was used for all data visualization and statistical analysis (R Core Team, 2020).
Calf birth data were analyzed to assess individual variations in fecundity. Females were classified as sexually mature if they were 1) at least 9 years old, 2) had an observation history of 8 or more years, or 3) had been previously sighted with a calf (Hamilton et al., 1998). Since not all sexually mature females have given birth, we separately examined the age distribution of females that have not yet produced a calf. Only females with known ages from the sighting record and that were at least 10 years old in 2021 were used in this analysis.
Two case studies of matrilines that had highly fecund matriarchs but produced substantially different numbers of progeny were presented. These case studies highlight the differences in matrilineal reproductive success that can occur. One showcased a highly productive matriline, while the other represented a matriline that was far less productive. Birth years, death years, last sighting years, reproduction events, and family patterns were documented and analyzed for both case studies to qualitatively assess factors that contribute to matrilineal fecundity. Individuals classified as “presumed dead” within these case studies have not been sighted for at least five years (Knowlton et al., 1994), while individuals classified as “confirmed dead” are those whose carcasses have been observed and identified.
Matrilineal Influence on Fecundity
Matrilineal influence on fecundity was analyzed by developing a calving index based on sighting histories to compare adult female reproductive history regardless of age. Individual calving indices were calculated by dividing a female’s total number of known calves by her total number of years spent as a sexually mature adult. For example, a calving index of 0.25 corresponds to a female that has given birth, on average, once every four years since becoming sexually mature. Notably, births are determined on an observational basis; therefore, unseen calves, including those that died during birth or soon after, could not be included in this analysis.
Fecundity patterns were examined within matrilines to assess potential genetic or learned behavioral influences on fecundity. For each matriline considered in this study, the matriarch was determined from the life history data available through the North Atlantic Right Whale Consortium database, and the matriarch is defined here as the oldest known reproductive female in a line of direct descendants. The calving indices of individual sexually mature females were compared to the calving indices of their oldest living matriarch to determine whether fecundity is linked within matrilines. Kendall’s tau rank order correlation was used to determine whether there was a significant relationship between matriarch fecundity and the fecundity of their descendants.
Influence of Habitat Use on Fecundity
To assess whether recent shifts in habitat use impact fecundity, individual demographics and recent calving success were compared between individuals grouped into one of two habitat use patterns: those sighted in the Gulf of St. Lawrence between 2015 and 2019 (see Crowe et al., 2021 supplementary for the list of these whales), and those that were not sighted in the Gulf of St. Lawrence during that time period. Calving success in sexually mature females was compared between whales who did and did not go to the Gulf of St. Lawrence to determine the influence of this habitat use pattern on reproduction. The right whale calving year begins one month before the calendar year to accurately account for the calving season (Pace et al., 2017), and we analyzed calf births from 1 December 2015 through 30 November 2021 to encompass all calving years that were potentially impacted by the distribution shift described in Crowe et al. (2021). Reproduction was coded as a binary variable, where sexually mature females were classified as “Reproductive” if they had one or more calves or “Non-Reproductive” if they had zero calves during that period. A chi-square test was used to assess the relationship between reproductive status and habitat use pattern.
Matrilineal Influence on Habitat Use
We examined matrilineally directed habitat use between the group of whales that use the Gulf of St. Lawrence and the remainder of the population. For all analyses, the type of habitat use exhibited by each whale was defined by whether an individual was seen in the Gulf of St. Lawrence at least once between 2015–2019. The high site fidelity noted in whales who use this habitat (Crowe et al., 2021) supports the assumption that an individual sighted there once will likely return to forage there again. Only whales sighted since 2015 were used so that our analyses exclusively accounted for habitat use patterns that occurred during the current distribution shift. Two chi-square tests were performed to assess whether an individual was more likely to use the Gulf of St. Lawrence if its mother or matriarch did as well. Two additional chi-square tests were conducted to assess whether the habitat use patterns of individual sexes were individually more likely to match their mothers’. Finally, we examined whether an individual’s age impacted their tendency to match the habitat use behavior of their mother. All individuals with known birth years were sorted into two classes based on their age in 2019: “Age <5” include individuals born in or after the year 2015, and thus had the chance to use the Gulf of St. Lawrence as calves with their mothers during the 2015–2019 survey period; “Age 5+” is defined as individuals born before the year 2015, who did not have the chance to use the Gulf of St. Lawrence as calves with their mothers while the distribution shift was taking place. The number of individuals who match their mothers’ habitat use patterns was analyzed between each age class, parsed by the habitat usage pattern of their mothers, using Fisher exact tests (Fisher, 1934).
Results
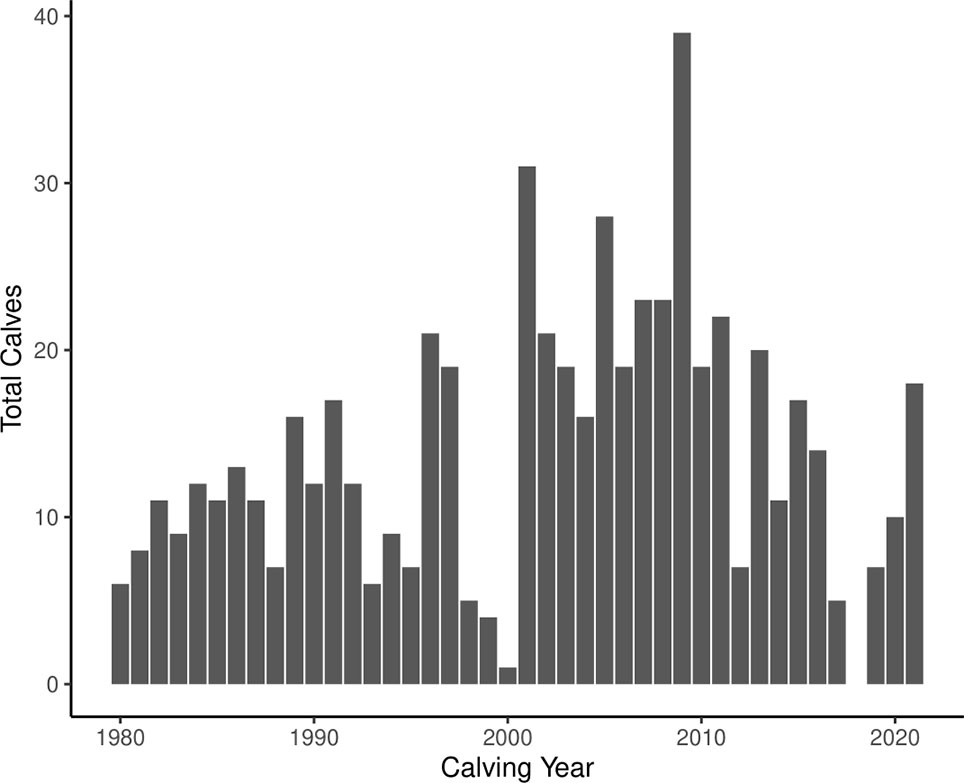
The life histories of 766 individually identified right whales were examined and 260 sexually mature females were identified since 1980. There were 586 calf births documented over the same time period (Figure 1). The calving index of sexually mature females ranged from 0 to 0.286 calf births over their full lifetime reproductive period observed to date. As of 2021, there were 49 females in the database who were presumed alive and reached sexual maturity, but were never observed with a calf. Of those with known ages (n = 43), these non-reproductive females ranged in age from 10 years old to 34 years old, but tended to be relatively young (Figure 2), with the majority falling at or below the age of 13 (67.4%). Only four of the females, or 9.30% of the demographic, were above the age of 20.
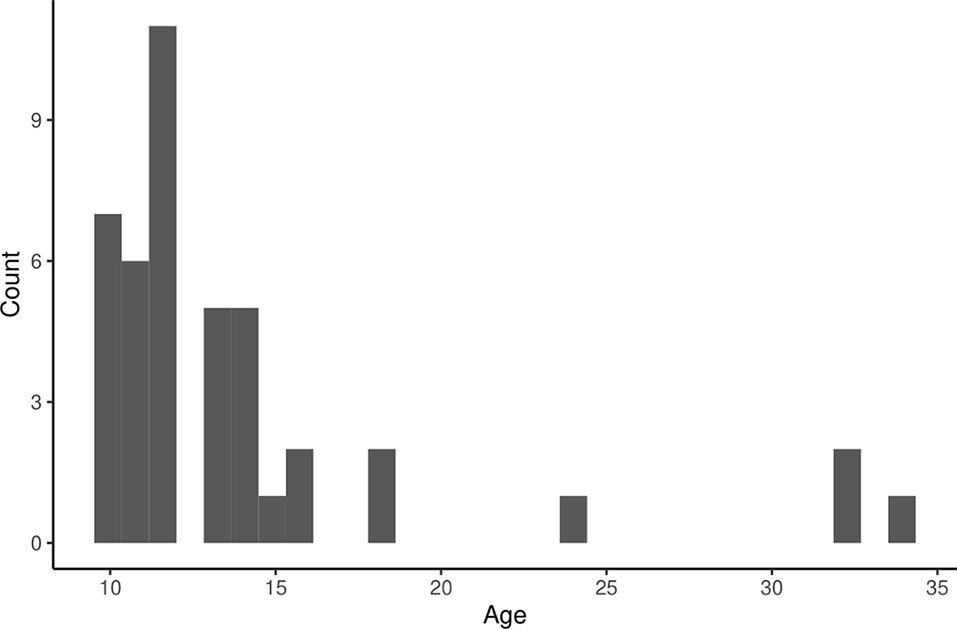
Figure 2 Histogram showing the age frequencies of known-age, sexually mature females that have never been observed with a calf and were presumed alive as of 2021.
Matrilineal Influence on Fecundity
There were 580 individual calves visually observed in a mother/calf pair, thereby confirming their maternal lineage and allowing them to be assigned to distinct matrilines. There were 97 matrilines determined, consisting of two to four observed generations (2 generations: 58.8%; 3 generations: 28.9%; 4 generations: 12.4%). The longevity of the matrilines ranged from 3–53 years (calculated by finding the difference between the earliest year and latest year a member from each matriline was sighted), with some matriline observation periods spanning over 30 years in all generational categories (two, three, and four generation matrilines). Periods of low reproduction were most apparent in 1998–2000 and 2017–2019 (Figure 1). There was a significant positive association between the calving index of individual females and the calving index of their matriarchs (p = 0.01, τ = 0.22), therefore supporting that fecundity is matrilineally linked.
The case studies shown in Figure 3 provide specific examples of matrilines who differed in their reproductive success over the same time period. The matriline of matriarch #1140, also known as “Wart”, produced four observed generations in 40 years, and included 29 individuals total (Figure 3A). This matriline maintained high fecundity throughout its generations; matriarch #1140 had a calving index of 0.175 that fell within the highest 50% of the overall calving index range of the population (0–0.286), and five of her progeny’s calving indices fell within this range as well. In contrast, matriarch #1281, also known as “Punctuation”, had a higher calving index of 0.229, but fewer total progeny; three generations were observed in 38 years, which only included ten individuals total (Figure 3B). Factors beyond the individual fecundity of each sexually mature female contributed to the differences in matriline size in these two case studies, including premature death and offspring sex ratio. Matriarch #1140 had a higher ratio of female calves compared to matriarch #1281, which created higher potential for growth within the matriline. Both matrilines included female descendants that died prematurely, including four of the 11 known female descendants of #1140 as well as the only two known female descendants of #1281. These case studies illustrate how high anthropogenic mortality rates and associated premature mortality reduce population fecundity.
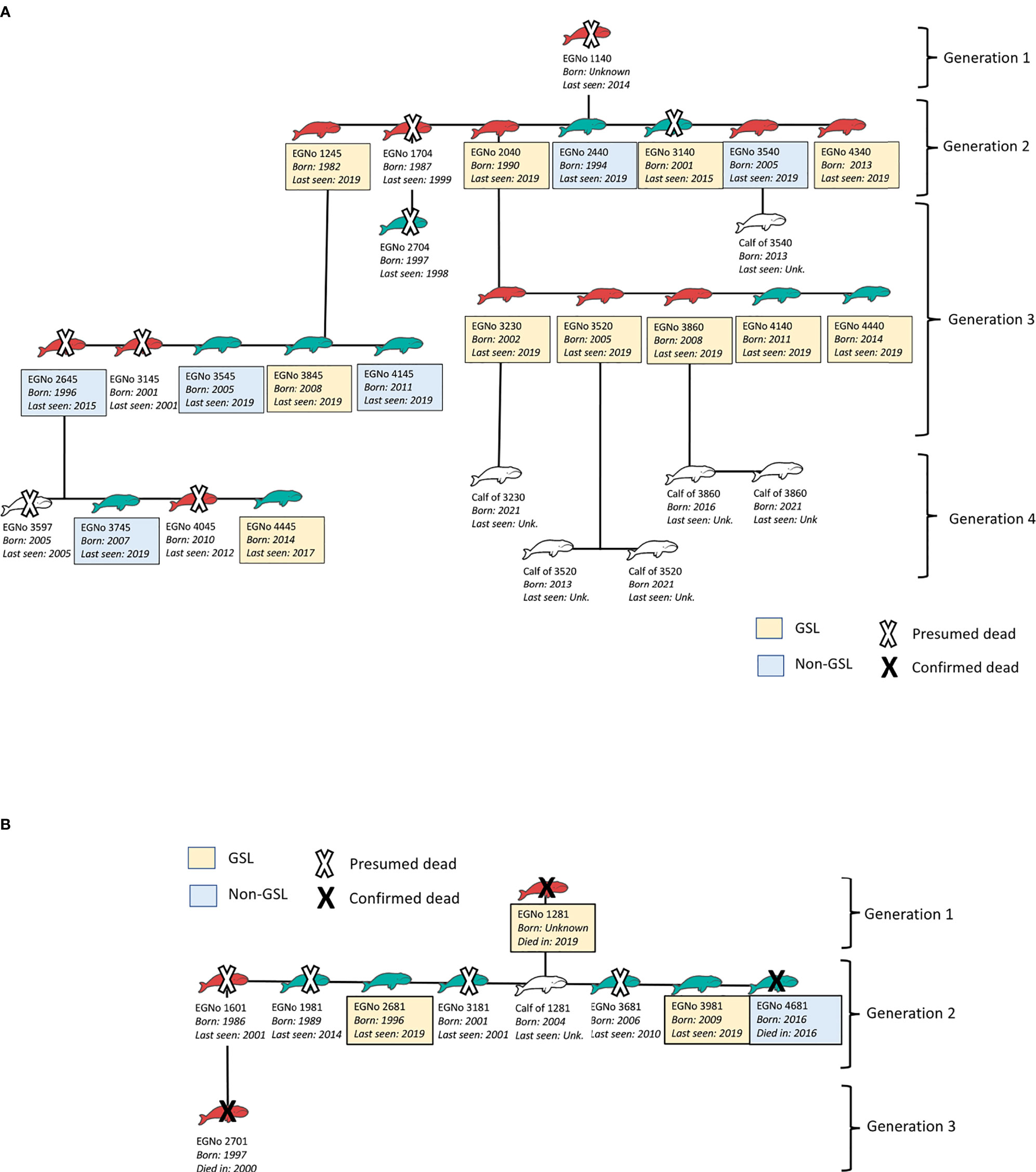
Figure 3 Schematics showing the direct descendants of two right whale matriarchs and their progeny. (A) shows the matriline produced by matriarch #1140, and (B) shows the matriline produced by matriarch #1281. Blue icons indicate males, red icons indicate females, and white icons are individuals of unknown sex. Text below each icon lists the individual identification number, birth year, and year last sighted or year of death. Complete sightings data in this study were only assessed through 2019, so individuals last seen in 2019 may have been seen more recently, but complete calving data is shown through the 2021 birthing season. Text boxes with a yellow background indicate individuals that have been seen in the Gulf of St. Lawrence since 2015, and text boxes with a blue background indicate individuals that have been sighted since 2015, but not in the Gulf of St. Lawrence. Text boxes without a colored background indicate individuals that have not been sighted in 2015 or after. Whale icons with a white X are presumed to have died, and those with a black X have had their death visually confirmed.
Influence of Habitat Use on Fecundity
Reproductive success between GSL and non-GSL females was significantly different (x2 = 4.45, p = 0.035; Figure 4). Of the sexually mature females who used the Gulf of St. Lawrence (n = 44), 47.7% gave birth at least once between 2016 and 2021, while out of the sexually mature females who did not use the Gulf of St. Lawrence (n = 100) only 28.0% gave birth within the same period. Notably, four females gave birth twice within this time span, and all four mothers used the Gulf of St. Lawrence. From the 2021 calving year alone, there were 18 new calves (National Oceanic and Atmospheric Administration, 2021; Right Whale News, 2021; Pettis et al., 2022), and half of their mothers were recorded in the Gulf of St. Lawrence between 2015–2019. The pattern of high Gulf of St. Lawrence usage amongst fecund females was also seen in our case studies: of the individuals in #1140’s matriline with known habitat use patterns (n = 17), 64.7% visited the habitat (Figure 3A), while 75.0% of #1281’s matriline who had known habitat use patterns (n = 4) did the same (Figure 3B).
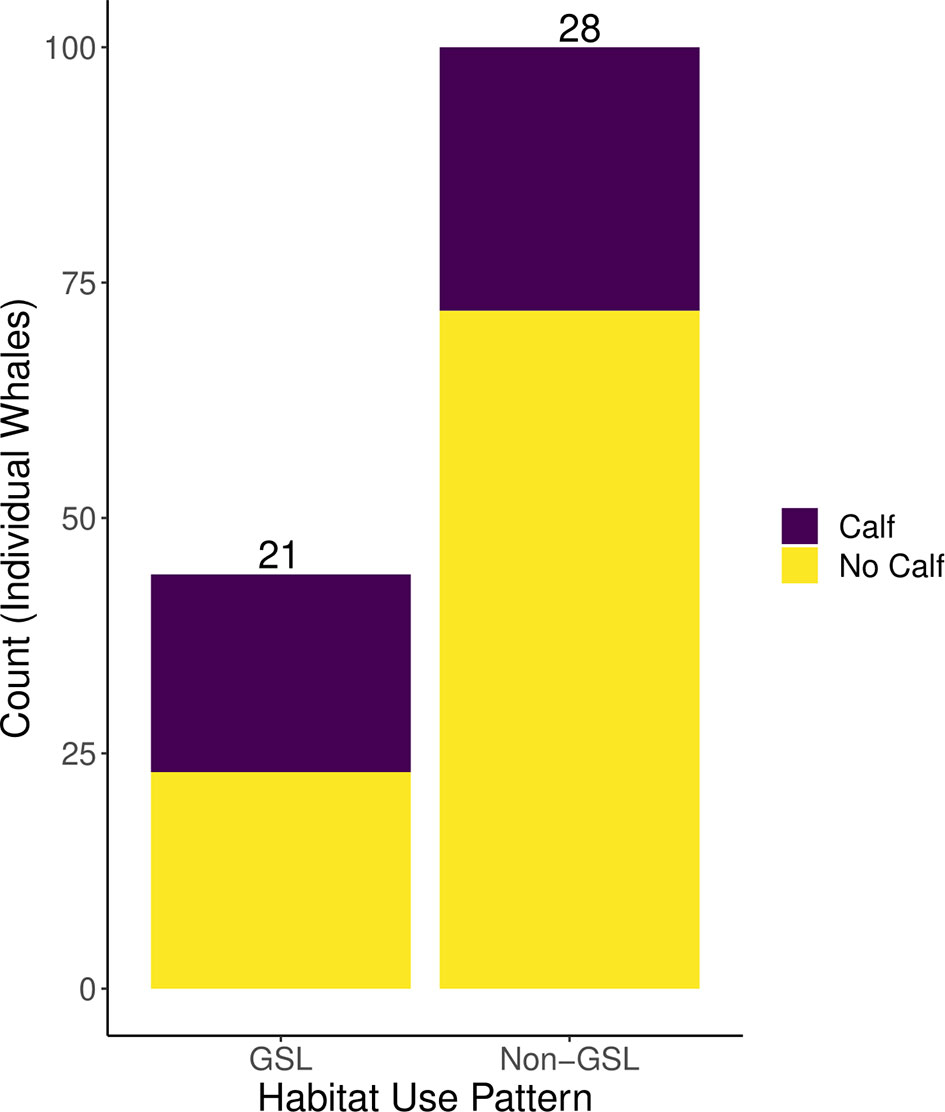
Figure 4 A comparison of the number of females who were sexually mature by 2016 that did and did not have a calf between the years 2016–2021, parsed by habitat use. “GSL” indicates individuals that used the Gulf of St. Lawrence at least once during the years 2015–2019. “Non-GSL” indicates individuals that were not seen in the Gulf of St. Lawrence during the same period. The numbers at the top of the bars show the total number of mothers who gave birth in each habitat category.
Matrilineal Influence on Habitat Use
Use of the Gulf of St. Lawrence was significantly associated between an individual and its mother (x2 = 14.46, p = 1.43x10-4; Figure 5), but not between matriarchs and their progeny, which encompasses multiple generations within a matriline. Of the 187 individuals that used the Gulf of St. Lawrence in the years 2015–2019 (Crowe et al., 2021), 78 had a mother that was observed at some point during that time. Of the 54 mothers who used the Gulf of St. Lawrence, the majority of their offspring used the habitat as well (64.8%). Similarly, of the 109 mothers who did not use the Gulf of St. Lawrence, the majority of their offspring also did not use it (67.9%). It was found that both female and male offspring were significantly likely to follow their mother’s habitat use patterns (females: x2 = 7.05, p = 7.94x10-3; males: x2 = 6.07, p = 0.01), with 68.0% of female and 65.1% of male habitat use matching that of their mothers.
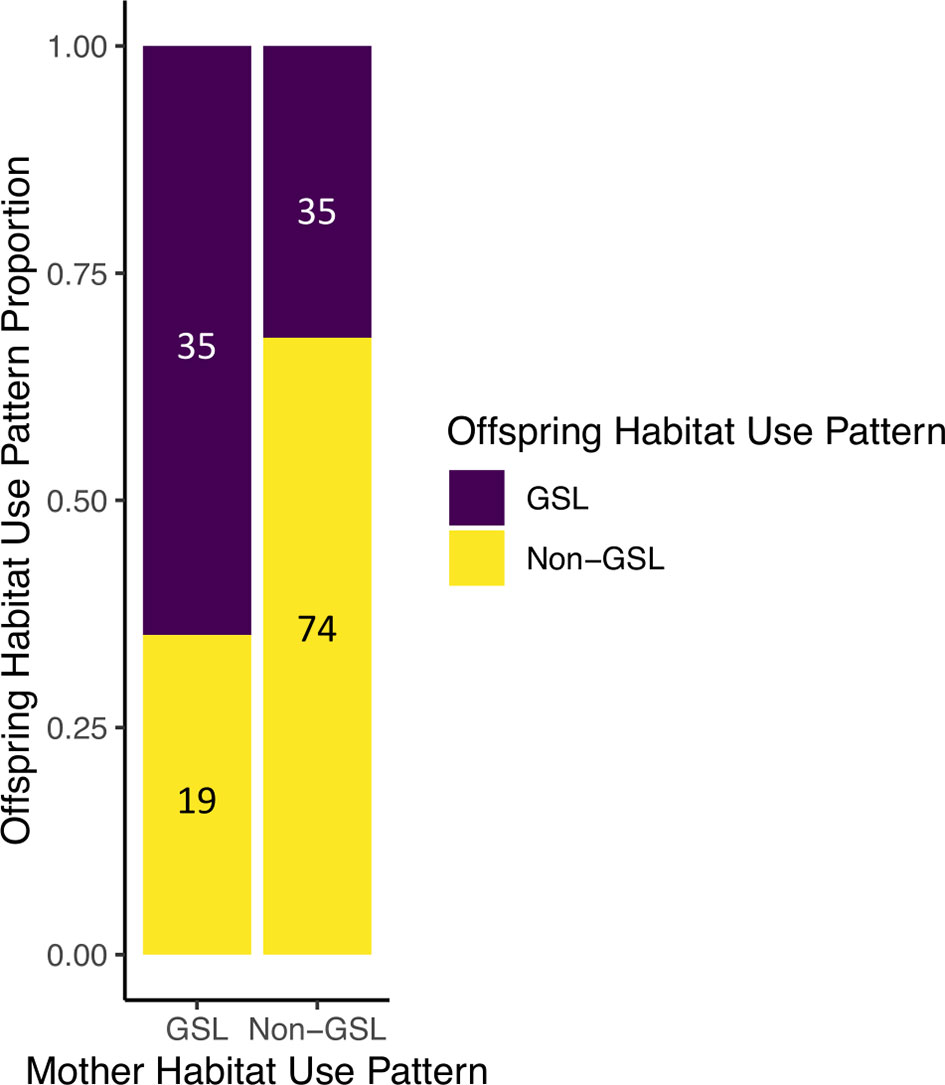
Figure 5 The proportions of individuals' observed habitat use patterns relative to the habitat use patterns of their mothers. “GSL” indicates individuals that used the Gulf of St. Lawrence at least once during the years 2015–2019. “Non-GSL” indicates individuals that were not seen in the Gulf of St. Lawrence during the same period. The numbers within the bars are the total number of offspring represented by the respective bar/color coding.
Finally, our analysis showed that offspring of mothers who used the Gulf of St. Lawrence between 2015–2019 were less likely to match their mother’s habitat use patterns as they aged. In contrast, no such tendency existed within offspring of mothers who did not use the Gulf of St. Lawrence (GSL: p = 0.02; Non-GSL: p = 0.1, Figure 6). It was found that 92.3% of offspring born in or after 2015 matched the habitat use patterns of their mothers who used the Gulf of St. Lawrence, while 56.1% of offspring born before 2015 did the same. In contrast, 68.8% of offspring born in or after 2015 matched the habitat use patterns of their mothers who did not use the Gulf of St. Lawrence, while 67.7% born before 2015 did the same.
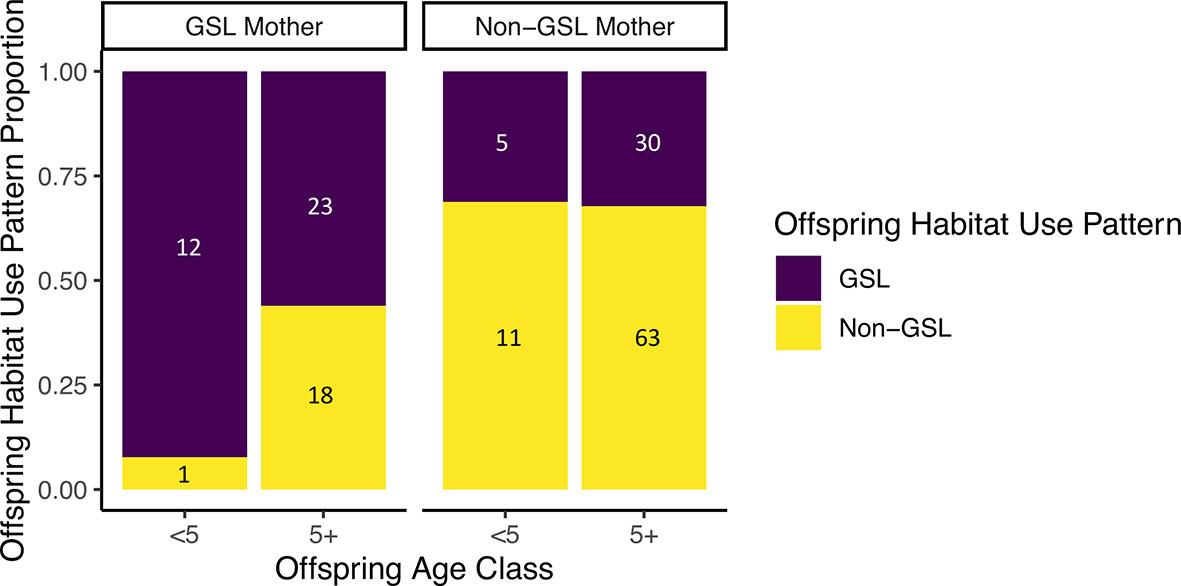
Figure 6 Comparison of habitat use patterns between mothers and their offspring, with offspring categorized by age and parsed by the habitat usage pattern of their mother. The x-axis labels correspond to the individual whale’s age in 2019, where “Age < 5” represents offspring younger than five, and “Age 5+” represents offspring that were at least five years old.
Discussion
Matriarchs with higher lifetime fecundity were more likely to produce more fecund female offspring. A matrilineal predisposition for high fecundity could be an underlying driver of reproductive resilience, even during periods of low fecundity at the scale of the population. Fecundity within a matriline is also heavily impacted by premature deaths (primarily caused by vessel strikes and fishing gear entanglements) and offspring sex ratio. Females who used the Gulf of St. Lawrence during or after 2015 have been more reproductively successful than their counterparts who did not use the habitat during that time. Finally, a significant number of offspring emulate their mother’s habitat use patterns. However, the likelihood of offspring matching their mother’s habitat use behavior decreases with age. These habitat use patterns provide evidence that learned behavior is maternally transmitted, which could contribute to differences in reproductive success that have been observed between matrilines.
Matrilines and Their Fecundity
We chose the case studies of matriarchs #1140 and #1281 to highlight the factors that can contribute to a matriline’s reproductive success, as well as how these factors converge to determine the matriline’s growth and longevity. The matriarchs in both case studies were highly fecund, having given birth to at least seven offspring each. Despite this, key differences contributed to the fate of their respective matrilines, and revealed the impact that sex ratio and survivability of offspring can have on reproductive success. When considering the results of this analysis, it is important to note that our matrilineal design did not account for the reproductive success of male progeny. Out of #1140’s seven offspring, five were female which allowed for more opportunities for reproduction than in #1281’s matriline. The descendants of #1140 followed in their matriarch’s highly fecund footsteps: all of #1140’s female descendants that lived long enough to reach sexual maturity gave birth at least once, and five of these females have given birth multiple times at a substantial rate. The matriline of #1140 shows that having many female offspring, combined with high survivability, can lead to a more successful matriline that exponentially contributes to the overall species’ growth. However, reproductive potential was lost in this matriline with the early deaths of two females, #1704 and #2645.
In contrast, #1281 was also highly fecund with eight offspring, but only one of these individuals was female. As a result, there was less opportunity for the matriline to expand. The sole female offspring, #1601, demonstrated the potential to be highly fecund since she gave birth to her first calf at 11 years old. Both #1601 and her female calf, #2701 have presumably died since they have not been sighted in several years. Moreover, the matriarch, #1281, died of a vessel strike in the Gulf of St. Lawrence in 2019 (Pettis et al., 2020), so it appears this matriline has ended. These case studies show that growth of a matriline depends on individual fecundity, sex ratio and timing of male vs. female calf births, and the occurrence of premature mortality.
Comparing the births that occurred within matrilines highlights missed opportunities for reproduction, and provides detailed insight into the generally low birth rates of North Atlantic right whales (Kraus et al., 2001). The considerably higher birth rates of southern right whale populations (Best, 1990; Payne et al., 1990; Kraus et al., 2001; Corkeron et al., 2018) indicate that North Atlantic right whales should be biologically capable of higher reproduction. These low birth rates are particularly troubling when considered with the fact that there are currently less than 100 breeding females in the population (Pettis et al., 2022). This study finds that the fecundity of an individual female is positively linked to the fecundity of her matriarch, and the premature losses of females in successful matrilines from vessel strike or entanglement therefore disproportionately reduce population growth potential. For this reason, the high level of documented mortality in Gulf of St. Lawrence in 2017 and 2019 (Daoust et al., 2018; Bourque et al., 2020) and the fact that mothers that use Gulf of St. Lawrence are more productive than other cohorts of the population is particularly concerning. Our case studies show that a large number of females with high survivability are critical for a productive population, yet female survival rates are currently declining species-wide, and there are fewer females than males in the population as a result (Pace et al., 2017). Anthropogenic pressures, including vessel strikes and fishing gear entanglements, are causes of these early deaths (Knowlton & Kraus, 2001; van der Hoop et al., 2013; Sharp et al., 2019), but they can also contribute to non-lethal health impacts that hinder reproductive potential (van der Hoop et al., 2016). The recovery of the population largely depends on the ability of these females to survive and successfully reproduce.
Gulf of St. Lawrence Usage and Fecundity
Gulf of St. Lawrence users were significantly more likely to give birth during the 2016–2021 period than their counterparts, and this may have occurred because prey conditions are more favorable in that habitat. Sightings data show that most of the population does not use the Gulf of St. Lawrence (Crowe et al., 2021), and the prey quality and location of the summertime foraging grounds for 60% of the population is largely unknown. Because of this gap in knowledge, a comparison between prey fields is not currently possible, but the reproductive rates of females who did and did not use the Gulf of St. Lawrence in recent years give us a proxy for how supportive the habitat is compared to these unknown locations. Gaining better insight into the drivers of habitat usage will be useful for predicting the capacity that the Gulf of St. Lawrence, and other habitats, will be used in the future. This is especially important since climate-driven shifts in prey distributions are projected to continue (Reygondeau & Beaugrand, 2011; Ross et al., 2021), causing right whales to seek out new foraging areas.
Although the Gulf of St. Lawrence seemingly played a significant role in supplying enough energy to support these pregnant females, the energetic costs associated with traveling there must be noted when considering the habitat’s ability to support the population. Usage of the Gulf of St. Lawrence occurs during peak foraging season (Baumgartner & Mate, 2003; Crowe et al., 2021), and while it is unknown whether foraging occurs during travel to the area, this consistent and extended transit could impact their seasonal energy budget. Within the Gulf of St. Lawrence, individuals aggregate on the western side (Crowe et al., 2021) which is about 1000 km swimming distance farther north than the traditional summertime foraging areas in the Gulf of Maine (Winn et al., 1986; Greene and Pershing, 2004). Using 3.5 km/hr as an estimated migratory speed (Mate et al., 1997; Zerbini et al., 2016; Mackay et al., 2020), it would take about 11–12 days of swimming 24 hr/day for a whale to travel the distance from the Gulf of Maine to Shediac Valley in the Gulf of St. Lawrence. Further study is warranted to determine the energetic budget and foraging opportunities associated with this transit. It is possible that the remaining 60% of the population that have not been recently sighted in the Gulf of St. Lawrence incurs similar energetic costs during their transits, but the increased reproductive success of the Gulf of St. Lawrence-users indicates there may be energetic differences existing between the two groups that should be explored. Furthermore, while the GSL may become a regular foraging habitat, there’s evidence that the effort to find and establish this new habitat costs them energy and foraging success (Pershing and Pendleton, 2021). Higher reproductive success in Gulf of St. Lawrence users may reflect a behavioral dichotomy between whales with good health and fitness that are able to manage the challenge of consistently traveling farther north compared to whales in poorer health. However, if the Gulf of St. Lawrence can provide adequate nutrition for all demographics that compensates for the potential energy expended, the habitat may benefit the population as a whole.
While a large, consistent number of individuals going to the Gulf of St. Lawrence is an extension of the whales’ typical habitat, historical documents indicate that right whales visited this area regularly in past centuries (Reeves et al., 1999; McLeod et al., 2008; Brown et al., 2009). Studies have also shown that the species has a precedent for travelling outside of its typical habitat, likely motivated by a need to forage (Knowlton et al., 1992; Jacobsen et al., 2004; Smith et al., 2006; Mellinger et al., 2011; Fortune et al., 2013). Given that the whales’ energetic needs were likely not being met at their typical feeding grounds due to climate-driven declines in prey abundance (Record et al., 2019; Meyer-Gutbrod et al., 2021), it is likely that they are traveling to the Gulf of St. Lawrence for the same reason.
Low birth rates in 2017–2020 (Meyer-Gutbrod et al., 2021; Pettis et al., 2022) and an analysis of Calanus spp. densities in the southern Gulf of St. Lawrence (Gavrilchuk et al., 2021) indicated that the habitat may not be supporting successful reproduction. However, the significant reproductive success of mothers that used the Gulf of St. Lawrence that we found in our study contradicts this idea, and indicates that the prey supply has been suitable. Though it is unknown whether individuals who forage in the Gulf of St. Lawrence remain there exclusively, the success of the 2016–2021 mothers who used it shows that the habitat likely played an important role in supporting their nutritional needs. The high rate of inter-annual return exhibited by the whales who use the Gulf of St. Lawrence (Crowe et al., 2021) underscores the likelihood that these individuals are being reinforced to return due to favorable conditions. However, reproductive success is only an indirect assessment of potential habitat quality. Coupled with the fact that the distribution shift is a relatively new phenomenon, further research is essential to determine long-term suitability of this habitat.
Maternally Influenced Habitat Use
Analysis of habitat use behaviors of kin shows that individuals are more likely to use the Gulf of St. Lawrence if their mother does as well, mirroring maternally influenced migratory and foraging site fidelity documented in southern right whales (Valenzuela et al., 2009; Carroll et al., 2015), and providing further support for matrilineally directed site fidelity in North Atlantic right whales that has been previously observed in both current and historical populations (Malik et al., 1999; Frasier et al., 2022). This result, as well as the size of the proportion of the population that uses the Gulf of St. Lawrence, suggests that learned behavior exists within family groups, including older juveniles and sexually mature whales. This behavior could indicate that some matrilines pass down knowledge of more productive foraging grounds than others, leading to superior reproductive success and higher fecundity. However, the strength of this pattern decreases with age. Could older whales, who were likely brought to other foraging grounds as calves that are now far less productive (Meyer-Gutbrod et al., 2021), be diverging from their learned habitat use patterns in favor of more productive areas? Given the increase in Gulf of St. Lawrence users over the 2015–2019 period (Crowe et al., 2021), this seems likely, but further research regarding the age and generational distribution of Gulf of St. Lawrence users relative to their family lines would be valuable.
While the patterns in fecundity and habitat use among kin shown in this analysis suggest that learned behavior likely impacts reproduction, it is unclear if genetics are also a component. Investigating the genetics of matriline members would provide valuable understanding of family dynamics that may not be accessible by reviewing traditional photographic data (Hamilton et al., 2022). There may also be patterns in individual size and health history that are consistent between the Gulf of St. Lawrence and non-Gulf of St. Lawrence sub-populations that contribute to the difference in fecundity between these two groups. Further analysis is required to understand the mechanistic drivers between these complex demographic and behavioral phenomena.
Limitations and Future Work
Dedicated monitoring of the right whale population and individual life histories largely began in the early 1980s, which limits our designations of matrilines and their founding matriarchs. As a result, some matrilines which were classified as distinct may share a common ancestor who was not documented, and the number of distinct matrilines may be overestimated. Furthermore, although the survey effort dedicated to this population is unparalleled relative to other baleen whale species in the North Atlantic, visual surveys are an imperfect method of detecting births. It is possible that some calves have been missed within the survey range, particularly those who died at birth. Therefore, our calving index may be underestimated for some individuals.
The scope of our study is also limited by the lack of sighting data corresponding to the foraging grounds of individuals who do not use the Gulf of St. Lawrence as well as the limited sightings data from the Gulf of St. Lawrence prior to 2015. Without knowing the recent summertime foraging habitats of this large subset of the population, it is difficult to determine the specific benefits of Gulf of St. Lawrence usage and its relative impact on reproduction. For instance, it is possible that the more reproductively successful mothers who used the Gulf of St. Lawrence are not exclusively feeding there, and are traveling to other foraging grounds as well. Identifying where the rest of the population is feeding would provide valuable insight into the exclusive benefits and/or limitations that the Gulf of St. Lawrence provides, specifically regarding the extent of the role it plays in fecundity and successful reproduction.
When considering these results, it should be noted that the threat posed to reproductive females by anthropogenic pressures is significant (Corkeron et al., 2018). Recent studies have found that the stress of entanglement and vessel-strike injuries causes poor body condition within the population (Stewart et al., 2021), which negatively impacts the body condition and survival of offspring and decreases the female’s capacity to reproduce (Kraus et al., 2001; van der Hoop et al., 2016; Christiansen et al., 2018). We did not factor the impact of anthropogenic pressures or maternal condition into our assessment of female fecundity, and future work should consider the impact of these factors. This study assumed that whales had equal fecundity potential throughout their entire adult life, which was supported by a lack of correlation between age and calving index within females who had given birth at least once. However, further analysis to examine fluctuations in fecundity with age would be valuable. This relationship is difficult to explore because the high rate of anthropogenic mortalities within the population results in widespread premature death, and thus older reproductive females are relatively rare.
Implications
The results of this study illustrate a complex ecological dichotomy that has been emerging over the past few years regarding whether the distribution shift to the Gulf of St. Lawrence is beneficial. Usage of this habitat may be driven by anomalously low prey densities in the traditional foraging grounds consistent with anthropogenic climate change (Meyer-Gutbrod et al., 2021). Analysis of the densities of calanoid copepods in the southern Gulf of St. Lawrence have identified the region as a substantial foraging area, with its shallow bottom contributing to accumulations of copepods that provide notable foraging potential (Plourde et al., 2019; Sorochan et al., 2021). Our study revealed evidence that the foraging opportunity provided by the area supported increased reproduction, and these factors could possibly reinforce individuals to return to the Gulf of St. Lawrence each year as a result.
While communication between individuals and shared habitat use behaviors can benefit whales by improving foraging success, they can also harm the population when the habitat presents ecological risks. The Gulf of St. Lawrence is hazardous, causing the whales to pass through a heavily trafficked area that includes various fixed gear fishing grounds and shipping lanes that serve as the economic highway to the U.S. and Canada (Daoust et al., 2018; Crowe et al., 2021). The dangers of using this area were realized in 2017, when an unprecedented 17 mortalities were recorded, including 12 mortalities in the Gulf of St. Lawrence (Daoust et al., 2018; Meyer-Gutbrod et al., 2018). An unusual mortality event was declared as a result, and another mass mortality was recorded in 2019 that included ten mortalities, seven of which occurred in the Gulf of St. Lawrence (Pettis et al., 2020). The high mortality and injury rates associated with use of this habitat poses a significant threat to the species. These risks are compounded by our findings that female fecundity is positively associated with her matriarch’s fecundity, and learned habitat use behavior is communicated between mother and calf. With more fecund females utilizing the Gulf of St. Lawrence, and passing this habitat use pattern onto highly fecund offspring, future population growth may hinge on adequate protection of this habitat.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The datasets used for our study are curated and managed by the North Atlantic Right Whale Consortium. A written proposal must be submitted and approved in order to access any of the Consortium’s datasets. Instructions for accessing these datasets can be found at https://www.narwc.org/.
Ethics Statement
Ethical review and approval was not required for the animal study because no physical experiments were done during this project. This project exclusively utilized pre-existing aerial, boat, and opportunistic survey data.
Author Contributions
EM-G conceptualized this research. AB and EM-G performed the data analyses. AB wrote the manuscript, with significant contribution from EM-G. LC and PH curated the data, and provided additional reviews and edits for this manuscript. All authors contributed to the article and approved the submitted version.
Funding
Support for this work was provided by the University of South Carolina’s SURF and Magellan Scholar grants.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Many thanks to the North Atlantic Right Whale Consortium for granting the data used for this project and particular thanks to the New England Aquarium Catalog team for their impeccable curation, the field teams for collecting these data over a long time series, and the geneticists whose work have helped reveal maternal lineages. We thank Heather Pettis for assistance with these data.
References
Right Whale News. Available at: https://www.narwc.org/uploads/1/1/6/6/116623219/rwn_aug21.pdf (Accessed February 4, 2022).
Baumgartner M. F., Mate B. R. (2003). Summertime Foraging Ecology of North Atlantic Right Whales. Mar. Ecol. Prog. Ser. 264, 123–135. doi: 10.3354/meps264123
Best P. B. (1990). Natural Markings and Their Use in Determining Calving Intervals in Right Whales Off South Africa. S. Afr. J. Zool. 25, 114–123. doi: 10.1080/02541858.1990.11448199
Bourque L., Wimmer T., Lair S., Jones M., Daoust P.-Y. (2020) Incident Report: North Atlantic Right Whale Mortality Event in Eastern Canada, 2019. Collaborative Report Produced by: Canadian Wildlife Health Cooperative and Marine Animal Response Society. Available at: http://www.cwhc-rcsf.ca/docs/2019%20NARW%20incident%20report_June%202020.pdf (Accessed February 20, 2022).
Brent L. J. N., Franks D. W., Foster E. A., Balcomb K. C., Cant M. A., Croft D. P. (2015). Ecological Knowledge, Leadership, and the Evolution of Menopause in Killer Whales. Curr. Biol. 25, 746–750. doi: 10.1016/j.cub.2015.01.037
Brown M. W., Fenton D., Smedbol K., Merriman C., Robichaud-LeBlanc K., Conway J. (2009). Recovery Strategy for the North Atlantic Right Whale (Eubalaena Glacialis) in Atlantic-Canadian Waters. In: Species at Risk Act Recovery Strategy Series (Ottowa, ON: Fisheries and Oceans Canada). Available at: https://www.sararegistry.gc.ca/virtual_sara/files/plans/rs_north_atl_right_whale_0609_e.pdf (Accessed January 11, 2022).
Carroll E. L., Baker C. S., Watson M., Alderman R., Bannister J., Gaggiotti O. E., et al. (2015). Cultural Traditions Across a Migratory Network Shape the Genetic Structure of Southern Right Whales Around Australia and New Zealand. Sci. Rep. 5, 1–13. doi: 10.1038/srep16182
Caswell H., Fujiwara M., Brault S. (1999). Declining Survival Probability Threatens North Atlantic Right Whale. Proc. Natl. Acad. Sci. U.S.A. 96, 3308–3313. doi: 10.1073/pnas.96.6.3308
Christiansen F., Vivier F., Charlton C., Ward R., Amerson A. B., Burnell S., et al. (2018). Maternal Body Size and Condition Determine Calf Growth Rates in Southern Right Whales. Mar. Ecol. Prog. Ser. 592, 267–281. doi: 10.3354/meps12522
Corkeron P., Hamilton P., Bannister J., Best P., Charlton C., Groch K. R., et al. (2018). The Recovery of North Atlantic Right Whales, Eubalaena Glacialis, has Been Constrained by Human-Caused Mortality. R. Soc Open Sci. 5, 11. doi: 10.1098/rsos.180892
Crowe L. M., Brown M., Corkeron P. J., Hamilton P. K., Ramp C., Ratelle S., et al. (2021). In Plane Sight: A Mark-Recapture Analysis of North Atlantic Right Whales in the Gulf of St. Lawrence. Endang. Species Res. 46, 227–251. doi: 10.3354/esr01156
Daoust P.-Y., Couture E. L., Wimmer T., Bourque L. (2018). Incident Report: North Atlantic Right Whale Mortality Event in the Gulf of St. Lawrence, 2017. Collaborative Report Produced by: Canadian Wildlife Health Cooperative, Marine Animal Response Society, and Fisheries and Oceans Canada. Available at: http://www.cwhc-rcsf.ca/docs/technical_reports/NARW_Incident_Report-%2020180405%20MD.pdf (Accessed January 11, 2022).
Davis G. E., Baumgartner M. F., Bonnell J. M., Bell J., Berchok C., Thornton J. B., et al. (2017). Long-Term Passive Acoustic Recordings Track the Changing Distribution of North Atlantic Right Whales (Eubalaena Glacialis) From 2004 to 2014. Sci. Rep. 7, 1–12. doi: 10.1038/s41598-017-13359-3
Department of Fisheries and Oceans Canada (2007). Recovery Potential Assessment for Right Whale (Western North Atlantic Population). In: Can. Sci. Advis. Sec. Sci. Advis. Rep. 2007/027 (Ottawa: Fisheries and Oceans Canada). Available at: https://files.pca-cpa.org/pcadocs/bi-c/2.%20Canada/3.%20Exhibits/R-0828.PDF (Accessed January 20, 2022).
Fortune S., Trites A., Mayo C., Rosen D., Hamilton P. (2013). Energetic Requirements of North Atlantic Right Whales and the Implications for Species Recovery. Mar. Ecol. Prog. Ser. 478, 253–272. doi: 10.3354/meps10000
Foster E. A., Franks D. W., Mazzi S., Darden S. K., Balcomb K. C., Ford J. K. B., et al. (2012). Adaptive Prolonged Postreproductive Life Span in Killer Whales. Science 337 (6100), 1313. doi: 10.1126/science.1224198
Frasier B. A., Springate L., Frasier T. R., Brewington S., Carruthers M., Edvardsson R., et al. (2022). Genetic Examination of Historical North Atlantic Right Whale (Eubalaena Glacialis) Bone Specimens From the Eastern North Atlantic: Insights Into Species History, Transoceanic Population Structure, and Genetic Diversity. Mar. Mam. Sci., 1–20. doi: 10.1111/mms.12916
Fujiwara M., Caswell H. (2001). Demography of the Endangered North Atlantic Right Whale. Nature 414, 537–541. doi: 10.1038/35107054
Ganley L. C., Brault S., Mayo C. A. (2019). What We See is Not What There Is: Estimating North Atlantic Right Whale Eubalaena Glacialis Local Abundance. Endang. Species Res. 38), 101–113. doi: 10.3354/esr00938
Gavrilchuk K., Lesage V., Fortune S. M., Trites A. W., Plourde S. (2021). Foraging Habitat of North Atlantic Right Whales has Declined in the Gulf of St. Lawrence, Canada, and may be Insufficient for Successful Reproduction. Endang. Species Res. 44, 113–136. doi: 10.3354/esr01097
Greene C. H., Pershing A. J. (2004). Climate and the Conservation Biology of North Atlantic Right Whales: The Right Whale at the Wrong Time? Front. Ecol. Environ. 2 (1), 29–34. doi: 10.2307/3868292
Greene C. H., Pershing A. J., Kenney R. D., Jossi J. W. (2003). Impact of Climate Variability on the Recovery of Endangered North Atlantic Right Whales. Oceanography 16, 98–103. doi: 10.5670/oceanog.2003.16
Hamilton P. K., Frasier B. A., Conger L. A., Clay George R., Jackson K. A., Frasier T. R. (2022). Genetic Identifications Challenge Our Assumptions of Physical Development and Mother–Calf Associations and Separation Times: A Case Study of the North Atlantic Right Whale (Eubalaena Glacialis). Mamm Biol. doi: 10.1007/s42991-021-00177-4
Hamilton P. K., Knowlton A. R., Marx M. K. (2007). “Right Whales Tell Their Own Stories: The Photo- Identification Catalog,” in The Urban Whale: North Atlantic Right Whales at the Crossroads. Eds. Kraus S., Rolland R. (Cambridge, MA: Harvard University Press), 75–104.
Hamilton P. K., Knowlton A. R., Marx M. K., Kraus S. D. (1998). Age Structure and Longevity in North Atlantic Right Whales Eubalaena Glacialis and Their Relation to Reproduction. Mar. Ecol. Prog. Ser. 171, 285–292. doi: 10.3354/meps171285
Hamilton P. K., Marx M. K., Kraus S. D. (1995). Weaning in North Atlantic Right Whales. Mar. Mam. Sci. 11, 386–390. doi: 10.1111/j.1748-7692.1995.tb00293.x
Jacobsen K. O., Marx M., ØIen N. (2004). Two-Way Trans-Atlantic Migration of a North Atlantic Right Whale (Eubalaena Glacialis). Mar. Mam. Sci. 20 (1), 161–166. doi: 10.1111/j.1748-7692.2004.tb01147.x
Kenney R. D. (2018)The North Atlantic Right Whale Consortium Database: A Guide for Users and Contributors. In: North Atlantic Right Whale Consortium Reference Document 2018-01. Available at: https://www.narwc.org/uploads/1/1/6/6/116623219/kenney_2018_narwc_users_guide:v_4_.pdf (Accessed May 5, 2021).
Knowlton A. R., Hamilton P. K., Marx M. K., Pettis H. M., Kraus S. D. (2012). Monitoring North Atlantic Right Whale Eubalaena Glacialis Entanglement Rates: A 30 Yr Retrospective. Mar. Ecol. Prog. Ser. 466, 293–302. doi: 10.3354/meps09923
Knowlton A., Kraus S. (2001). Mortality and Serious Injury of Northern Right Whales (Eubalaena Glacialis) in the Western North Atlantic Ocean. J. Cetacean Res. Manage. Special Issue) 2 (7), 193–208. doi: 10.47536/jcrm.vi.288
Knowlton A. R., Kraus S. D., Kenney R. D. (1994). Reproduction in North Atlantic Right Whales (Eubalaena Glacialis). Can. J. Zool. 72 (7), 1297—1305. doi: 10.1139/z94-173
Knowlton A. R., Sigurjoínsson J., Ciano J. N., Kraus S. D. (1992). Long-Distance Movements of North Atlantic Right Whales (Eubalaena Glacialis). Mar. Mam. Sci. 8 (4), 397–405. doi: 10.1111/j.1748-7692.1992.tb00054.x
Kraus S. D., Brown M., Caswell H., Clark C., Fujiwara M., Hamilton P., et al. (2005). North Atlantic Right Whales in Crisis. Science 309 (5734), 561–562. doi: 10.1126/science.1111200
Kraus S. D., Hamilton P., Kenney R. D., Knowlton A. R., Slay C. K. (2001). Reproductive Parameters of the North Atlantic Right Whale. J. Cetacean Res. Manage. 2, 231–236. doi: 10.47536/jcrm.vi.285
Kraus S. D., Moore K. E., Price C. A., Crone M. J., Watkins W. A., Winn H. E., et al. (1986). The Use of Photographs to Identify Individual North Atlantic Right Whales (Eubalaena Glacialis). Rep. Int. Whal. Commn (Special Issue) 10, 145–151.
Larsen A. J., Sigurjonsson J., Olen N., Vikingsson G., Palsbøll P. (1996). Populations Genetic Analysis of Nuclear and Mitochondrial Loci in Skin Biopsies Collected From Central and Northeastern North Atlantic Humpback Whales (Megaptera Novaeangliae): Population Identity and Migratory Destinations. Proc. R. Soc Lond. B. 263, 1611–1618. doi: 10.1098/rspb.1996.0236
Mackay A. I., Bailleul F., Carroll E. L., Andrews-Goff V., Baker C. S., Bannister J., et al. (2020). Satellite Derived Offshore Migratory Movements of Southern Right Whales (Eubalaena Australis) From Australian and New Zealand Wintering Grounds. PloS One 15 (5), e0231577. doi: 10.1371/journal.pone.0231577
Malik S., Brown M. W., Kraus S. D., Knowlton A. R., Hamilton P. K., White B. N. (1999). Assessment of Mitochondrial DNA Structuring and Nursery Use in the North Atlantic Right Whale (Eubalaena Glacialis). Can. J. Zool. 77 (8), 1217–1222. doi: 10.1139/z99-073
Mate B. R., Nieukirk S., Kraus S. D. (1997). Satellite-Monitored Movements of the Northern Right Whale. J. Wildl. Manage. 61 (4), 1393–1405. doi: 10.2307/3802143
Mayo C., Ganley L., Hudak C., Brault S., Marx M., Burke E., et al. (2018). Distribution, Demography, and Behavior of North Atlantic Right Whales (Eubalaena Glacialis) in Cape Cod Bay, Massachusett-2013: Right Whales in Cape Cod Bay. Mar. Mam. Sci. 34 (4), 979–996. doi: 10.1111/mms.12511
McLeod B., Brown M., Moore M. J., Stevens W., Barkham S. H., Barkham M., et al. (2008). Bowhead Whales, and Not Right Whales, Were the Primary Target of 16th- to 17th-Century Basque Whalers in the Western North Atlantic. Arctic 61 (1), 61–75. doi: 10.14430/arctic7
Mellinger D. K., Nieukirk S. L., Klinck K., Klinck H., Dziak R. P., Clapham P. J., et al. (2011). Confirmation of Right Whales Near a Historic Whaling Ground East of Southern Greenland. Biology Letters 7 (3), 411–413. doi: 10.1098/rsbl.2010.1191
Meyer-Gutbrod E. L., Greene C. H. (2014). Climate-Associated Regime Shifts Drive Decadal-Scale Variability in Recovery of North Atlantic Right Whale Population. Oceanography 27 (3), 148–153. doi: 10.5670/oceanog.2014.64
Meyer-Gutbrod E. L., Greene C. H. (2018). Uncertain Recovery of the North Atlantic Right Whale in a Changing Ocean. Glob. Change Biol. 24 (1), 455–464. doi: 10.1111/gcb.13929
Meyer-Gutbrod E. L., Greene C. H., Davies K. T. A. (2018). Marine Species Range Shifts Necessitate Advanced Policy Planning: The Case of the North Atlantic Right Whale. Oceanography 31, 2, 19–23. doi: 10.5670/oceanog.2018.209
Meyer-Gutbrod E. L., Greene C. H., Davies K. T. A., Johns D. G. (2021). Ocean Regime Shift is Driving Collapse of the North Atlantic Right Whale Population. Oceanography 34 (3), 22–31. doi: 10.5670/oceanog.2021.308
Meyer-Gutbrod E. L., Greene C. H., Sullivan P. J., Pershing A. J. (2015). Climate-Associated Changes in Prey Availability Drive Reproductive Dynamics of the North Atlantic Right Whale Population. Mar. Ecol. Prog. Ser. 535, 243–258. doi: 10.3354/meps11372
Miller C. A., Reeb D., Best P. B., Knowlton A. R. (2011). Blubber Thickness in Right Whales Eubalaena Glacialis and Eubalaena Australis Related With Reproduction, Life History Status and Prey Abundance. Mar. Ecol. Prog. Ser. 438, 267–283. doi: 10.3354/meps09174
National Marine Fisheries Service (2016). Endangered and Threatened Species; Critical Habitat for Endangered North Atlantic Right Whale. Available at: https://www.govinfo.gov/content/pkg/FR-2016-01-27/pdf/2016-01633.pdf (Accessed January 15, 2022).
National Oceanic and Atmospheric Administration (2021). North Atlantic Right Whale Calving Season. Available at: https://www.fisheries.noaa.gov/national/endangered-species-conservation/north-atlantic-right-whale-calving-season-2021 (Accessed December 20, 2021).
Nattrass S., Croft D. P., Ellis S., Cant M. A., Weiss M. N., Wright B. M., et al. (2019). Postreproductive Killer Whale Grandmothers Improve the Survival of Their Grandoffspring. Proc. Natl. Acad. Sci. U.S.A. 1 16 (52), 26669–26673. doi: 10.1073/pnas.1903844116
North Atlantic Right Whale Consortium (2021a). North Atlantic Right Whale Consortium Sightings Database 05/03/2021 (Boston, MA, U.S.A: Anderson Cabot Center for Ocean Life at the New England Aquarium).
North Atlantic Right Whale Consortium (2021b). North Atlantic Right Whale Consortium Identification Database 05/03/2021 (Boston, MA, U.S.A.: Anderson Cabot Center for Ocean Life at the New England Aquarium).
Pace R. M., Corkeron P. J., Kraus S. D. (2017). State–Space Mark–Recapture Estimates Reveal a Recent Decline in Abundance of North Atlantic Right Whales. Ecol. Evol. 7 (21), 8730–8741. doi: 10.1002/ece3.3406
Pace R. M., Williams R., Kraus S. D., Knowlton A., Pettis H. M. (2021). Cryptic Mortality of North Atlantic Right Whales. Conserv. Sci. Pract. 3, 2. doi: 10.1111/csp2.346
Palsbøll P. J., Clapham P. J., Mattila D. K., Larsen F., Sears R., Siegismund H. R., et al. (1995). Distribution of mtDNA Haplotypes in North Atlantic Humpback Whales: The Influence of Behaviour on Population Structure. Mar. Ecol. Prog. Ser. 116, 1–10. doi: 10.3354/meps116001
Patenaude N., Portway V., Schaeff C., Bannister J., Best P., Payne R., et al. (2007). Mitochondrial DNA Diversity and Population Structure Among Southern Right Whales (Eubalaena Australis). J. Hered. 98, 147–157. doi: 10.1093/jhered/esm005
Payne R., Brazier O., Dorsey E. M., Perkins J. S., Rowntree V. J., Titus A. (1983). “External Features in Southern Right Whales (Eubalaena Australis) and Their Use in Identifying Individuals,” in Communication and Behavior of Whales, Ed. R. Payne. AAAS Selected Symposia Series 76 (Colorado: Westview Press), 371–445.
Payne R., Rowntree V., Perkins J. S., Cooke J., Lankester K. (1990). Population Size, Trends and Reproductive Parameters of Right Whales (Eubalaena Australis) Off Peninsula Valdes, Argentina. Rep. – Int. Whal. Commn. (Special Issue 12), 271–278.
Pershing A. J., Pendleton D. E. (2021). Can Right Whales Out-Swim Climate Change? Can We? Oceanography 34 (3), 19–21. doi: 10.5670/oceanog.2021.315
Pettis H. M., Pace R. M. III, Hamilton P. K. (2020) North Atlantic Right Whale Consortium 2019 Annual Report Card (Boston, MA: North Atlantic Right Whale Consortium). Available at: https://www.narwc.org/report-cards.html (Accessed October 9, 2020).
Pettis H. M., Pace R. M. III, Hamilton P. K. (2022) North Atlantic Right Whale Consortium 2021 Annual Report Card (Boston, MA: North Atlantic Right Whale Consortium). Available at: https://www.narwc.org/report-cards.html (Accessed January 20, 2022).
Plourde S., Lehoux C., Johnson C. L., Perrin G., Lesage L. (2019). North Atlantic Right Whale () and Its Food: (I) a Spatial Climatology of Biomass and Potential Foraging Habitats in Canadian Waters. J. Plankton Res. 41, 667–685. doi: 10.1093/plankt/fbz024
R Core Team (2020). R: A Language and Environment for Statistical Computing (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Record N. R., Runge J. A., Pendleton D. E., Balch W. M., Davies K. T., Pershing A. J., et al. (2019). Rapid Climate-Driven Circulation Changes Threaten Conservation of Endangered North Atlantic Right Whales. Oceanography 32 (2), 162–169. doi: 10.5670/oceanog.2019.201
Reeves R. R. (2001). Overview of Catch History, Historic Abundance and Distribution of Right Whales in the Western North Atlantic and in Cintra Bay, West Africa. J. Cetacean Res. Manage. (Special Issue) 2, 187–192. doi: 10.47536/jcrm.vi.289
Reeves R. R., Breiwick J. M., Mitchell E. D. (1999). History of Whaling and Estimated Kill of Right Whales, Balaena Glacialis, in the Northeastern United State–1924. Mar. Fish. Rev. 61 (3), 1–36.
Reygondeau G., Beaugrand G. (2011). Future Climate-Driven Shifts in Distribution of Calanus Finmarchicus. Glob. Change Biol. 17 (2), 756–766. doi: 10.1111/j.1365-2486.2010.02310.x
Right Whale News. (2021). 29 (3), 1–2. Available at: https://www.narwc.org/uploads/1/1/6/6/116623219/rwn_aug21.pdf. [Accessed February 4, 2022].
Rosenbaum H. C., Weinrich M. T., Stoleson S. A., Gibbs J. P., Baker C. S., DeSalle R. (2002). The Effect of Differential Reproductive Success on Population Genetic Structure: Correlations of Life History With Matrilines in Humpback Whales of the Gulf of Maine. J. Hered. 93 (6), 389–399. doi: 10.1093/jhered/93.6.389
Ross C. H., Pendleton D. E., Tupper B., Brickman D., Zani M. A., Mayo C. A., et al. (2021). Projecting Regions of North Atlantic Right Whale, Eubalaena Glacialis, Habitat Suitability in the Gulf of Maine for the Year 2050. Elem. Sci. Anth. 9, 1. doi: 10.1525/elementa.2020.20.00058
Schaeff C. M., Kraus S. D., Brown M. W., White B. N. (1993). Assessment of the Population Structure of Western North Atlantic Right Whales (Eubalaena Glacialis) Based on Sighting and mtDNA Data. Can. J. Zool. 71 (2), 339–345. doi: 10.1139/z93-047
Sharp S. M., McLellan W. A., Rotstein D. S., Costidis A. M., Barco S. G., Durham K., et al. (2019). Gross and Histopathologic Diagnoses From North Atlantic Right Whale Eubalaena Glacialis Mortalities Between 2003 and 2018. Dis. Aquat. Org. 135, 1–31. doi: 10.3354/dao03376
Simard Y., Roy N., Giard S., Aulanier F. (2019). North Atlantic Right Whale Shift to the Gulf of St. Lawrence in 2015, Revealed by Long-Term Passive Acoustics. Endanger. Species Res. 40, 271–284. doi: 10.3354/esr01005
Smith T. D., Barthelmess K., Reeves R. R. (2006). Using Historical Records to Relocate a Long-Forgotten Summer Feeding Ground of North Atlantic Right Whales. Mar. Mam. Sci. 22 (3), 723–734. doi: 10.1111/j.1748-7692.2006.00067.x
Sorochan K. A., Plourde S., Baumgartner M. F., Johnson C. L. (2021). Availability, Supply, and Aggregation of Prey (Spp.) in Foraging Areas of the North Atlantic Right Whale. ICES J. Mar. Sci. 78, 10, 3498–3520. doi: 10.1093/icesjms/fsab200
Stevick P. T., Allen J., Clapham P. J., Katona S. K., Larsen F., Lien J., et al. (2006). Population Spatial Structuring on the Feeding Grounds in North Atlantic Humpback Whales (Megaptera Novaeangliae). J. Zool. 270, 244–255. doi: 10.1111/j.1469-7998.2006.00128.x
Stewart J. D., Durban J. W., Knowlton A. R., Lynn M. S., Fearnbach H., Barbaro J., et al. (2021). Decreasing Body Lengths in North Atlantic Right Whales. Curr. Biol. 31, 3174–3179. doi: 10.1016/j.cub.2021.04.067
Valenzuela L. O., Sironi M., Rowntree V. J., Seger J. (2009). Isotopic and Genetic Evidence for Culturally Inherited Site Fidelity to Feeding Grounds in Southern Right Whales (Eubalaena Australis). Mol. Ecol. 18 (5), 782–791. doi: 10.1111/j.1365-294X.2008.04069.x
van der Hoop J., Corkeron P., Moore M. (2016). Entanglement is a costly life-history stage in large whales. Ecol. Evol. 7, 92–106. doi: 10.1002/ece3.2615
van der Hoop J. M., Moore M. J., Barco S. G., Cole T. V. N., Daoust P.-Y., Henry A. G., et al. (2013). Assessment of Management to Mitigate Anthropogenic Effects on Large Whales. Conserv. Biol. 27, 121–133. doi: 10.1111/j.1523-1739.2012.01934.x
van der Hoop J. M., Vanderlaan A. S., Cole T. V., Henry A. G., Hall L., Mase-Guthrie B., et al. (2015). Vessel Strikes to Large Whales Before and After the 2008 Ship Strike Rule. Conserv. Lett. 8 (1), 24–32. doi: 10.1111/conl.12105
Winn H. E., Price C. A., Sorensen P. W. (1986). The Distributional Biology of the Right Whale (Eubalaena Glacialis) in the Western North Atlantic. Reports - International Whaling Commission, Special Issue 10, 129–138.
Wright B. M., Stredulinsky E. H., Ellis G. M., Ford J. K. (2016). Kin-Directed Food Sharing Promotes Lifetime Natal Philopatry of Both Sexes in a Population of Fish-Eating Killer Whales, Orcinus Orca. Anim. Behav. 115, 81–95. doi: 10.1016/j.anbehav.2016.02.025
Zerbini A., Rosenbaum H., Mendez M., Sucunza F., Andriolo A., Harris G., et al. (2016). Tracking Southern Right Whales Through the Southwest Atlantic: An Update on Movements, Migratory Routes and Feeding Grounds. Available at: https://ballenas.org.ar/descargas/publicaciones-cientificas/2016/90.%20Tracking%20southern%20right%20whales%20through%20the%20southwest%20Atlantic_%20An%20update%20on%20movements%2C%20migratory%20routes%20and%20feeding%20grounds.pdf (Accessed April 9, 2022).
Keywords: fecundity, matriline, reproduction, habitat, climate, right whale, mother-calf
Citation: Bishop AL, Crowe LM, Hamilton PK and Meyer-Gutbrod EL (2022) Maternal Lineage and Habitat Use Patterns Explain Variation in the Fecundity of a Critically Endangered Baleen Whale. Front. Mar. Sci. 9:880910. doi: 10.3389/fmars.2022.880910
Received: 22 February 2022; Accepted: 02 May 2022;
Published: 09 June 2022.
Edited by:
Eduardo Secchi, Federal University of Rio Grande, BrazilReviewed by:
Victoria Rowntree, University of Utah, United StatesPeter Owen Thomas, Marine Mammal Commission, United States
Copyright © 2022 Bishop, Crowe, Hamilton and Meyer-Gutbrod. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana L. Bishop, Y2IzMkBlbWFpbC5zYy5lZHU=
 Ana L. Bishop
Ana L. Bishop Leah M. Crowe
Leah M. Crowe Philip K. Hamilton
Philip K. Hamilton Erin L. Meyer-Gutbrod
Erin L. Meyer-Gutbrod