- School of Marine Science, Ningbo University, Ningbo, China
Silver pomfret (Pampus argenteus) is an economically important mariculture species. However, little is known about the genetic parameters of its commercially important traits. In order to determine whether continuous progress can be achieved by selection for growth, we took the body weight trait of East China Sea P. argenteus as the target trait of mass selection. The realized heritability of P. argenteus from the selected group and control group was analyzed at the three growth times (60, 90, and 120 days). The results showed that the growth performance parameters of the selected group in the third month post hatching were higher than those in the control group, but the opposite results were found in the fourth month. The morphological traits highly correlated with the body weight of P. argenteus were found by Pearson correlation analysis and path analysis, which were body length and tail length, respectively. On the 60th, 90th, and 120th days after hatching, the genetic gains of body weight trait in the selected group were 9.44%, 17.64%, and 15.18%, respectively, and the mean values of realized heritability were at moderate level. Nevertheless, in the selected group, the genetic variation values of the two morphological traits significantly related to body weight were all below 10%, and the genetic gains were relatively low and stable, but the genetic variation values of body weight trait in the selected group were above 20%, and genetic gains were relatively high. These implied that it is possible to obtain considerable genetic gains by selecting for body weight trait, and the results provide supportive evidence for the continuity of the P. argenteus-selective breeding program.
Introduction
Silver pomfret (Pampus argenteus) is an inshore species, which belongs to the family Stromateidae and is endemic to the Indian Ocean and the western Pacific Ocean, from China’s coastal areas, North Korea to Western Japan, the Bay of Bengal, and the Persian Gulf of India (Davis and Wheeler, 1985; Zhao et al., 2011; Mohitha et al., 2015; AlMomin et al., 2016). It is known for its nutritive value and medicinal applications (Xu et al., 2012; Zhang et al., 2014). P. argenteus has become one of highly marketed fish species in the aquaculture industry of Kuwait, India, and China (Parsamanesh et al., 1998; Duremdez et al., 2004; Wang and Wu, 2021). However, the broodstock used today remains largely not from selective breeding programs, and wild brood P. argenteus is commonly used in many hatcheries. Undoubtedly, genetic improvement in economically important traits (such as growth-related traits) of P. argenteus would be of great benefit to fish culture and farmers.
Traditionally, selective breeding has been used to address efficiency and sustainability problems in the aquatic animal industry; mass selection is one of the most common and effective approaches (Yuan et al., 2015). The potential for genetic improvement through mass selection is well documented in many aquaculture species during the past decades, such as brown trout (Salmo trutta fario) (Chevassus et al., 2005), channel catfish (Ictalurus punctatus) (Rezk et al., 2003), snakeskin gourami (Trichopodus pectoralis) (Chatchaiphan et al., 2019), Pacific oyster (Crassostrea gigas) (Wang et al., 2012), bay scallop (Argopecten irradians) (Wang et al., 2020), and Pacific oyster (Crassostrea gigas) (Zhang et al., 2019). Majority of selective breeding experiments were effective and encouraging in altering the selected traits. For example, Chatakondi and Yant (2005) studied the growth traits of four Gold Kist strains (Gk1, Gk2, Gk3, and Gk4) of Ictalurus punctatus and found that the average realized heritability values for the select strains ranged from 0.20 to 0.40; the average body weight of four strains of 6-month-old I. punctatus increased by 50.2% to 153.4% compared with the control group. Wang et al. (2020) estimated the realized heritability of total weight in two shell color lines of Argopecten irradians and found that the mean estimates of the realized heritability () of total weight at the harvest stage (days 210) were 0.51 and 0.53 for RS2 and BS2, respectively, which were all at a high level. These provide guidance for their genetic selection and systematic breeding. In addition, as far as China is concerned, it has launched the vitalization program of the aquaculture seed industry, focusing on innovation in genetic breeding and improving the production capacity of superior varieties, to make contributions to ensuring the safe and effective supply of aquatic products and promoting the high-quality development of fishery (Tang, 2021). Therefore, in order to improve the productivity traits of P. argenteus and satisfy the high demand of the market, in 2019, a selective breeding program for improving the growth performance of P. argenteus was initiated in China.
In this study, the growth performance and the realized heritability for fast growth of P. argenteus were examined to evaluate the potential of the growth improvement in the selective breeding program.
Materials and methods
Establishment of the lines
From May to June 2016, we caught 20,000 P. argenteus larvae in the coastal waters of Taizhou, Zhejiang Province (Fishing Area No. 209 of China), which were farmed in the Breeding Base of Xiangshan Bay Aquatic Offspring Seed Co., Ltd., Ningbo City, Zhejiang Province. P. argenteus can reach sexual maturity at 1 year and then reproduce normally. In 2019, we randomly selected 3,000 fish fry from the third generation of artificial breeding, which were transferred to the breeding base of the Zhejiang Marine Fisheries Research Institute in Zhoushan City, Zhejiang Province (28°31′56′′N, 122°12′24′′E); they were cultured in three adjacent rearing cement ponds (5 × 5 × 1.8 m) with the same density, which were mixed regularly to reduce the pond effect. After the fourth generation of P. argenteus was normally propagated and cultured to sexual maturity, we randomly selected 2,200 high-quality P. argenteus as the basic group for mass selection, and 178 individuals were randomly weighed (the weighted mean of body weight: 48.93 ± 22.75 g). Selective breeding of P. argenteus was carried out by the rough truncation point, and the seed retention rate was set at 10%, while the selection intensity was 1.755 (Yuan et al., 2015). Among them, we randomly selected 200 individuals from the basic population as the parents (PC0) of the control group (average body weight 36.15 ± 20.87 g, average body length 104.48 ± 10.43 mm; 76 sires, 124 dams) and then selected 200 individuals with the highest body weight as the parents (PS0) of the breeding group (average body weight 66.50 ± 21.41 g, average body length 127.33 ± 12.84 mm; 72 sires, 128 dams), and these two parent groups were cultured in two 35-t mating tanks with the same infrastructure and randomly mated within their respective ponds, and the sperm and eggs were naturally fertilized. The corresponding offspring of PC0 and PS0 were PC1 and PS1, respectively. In the two mating ponds, fertilized eggs were collected with a 100-mesh screen, and these tools were not mixed to avoid cross-contamination between ponds. During the peak period of spawning, the high-quality fertilized eggs were collected in 10-t hatching buckets for hatching and rearing. When the total length of P. argenteus seedlings reached 3 cm, the seedlings were evenly distributed in 35-t cement ponds, and the stocking densities were initially set to about 143 larvae/t and decreased with larval growth. In order to retain all the genetic variability, no culling of small individuals was employed.
Hatching and rearing
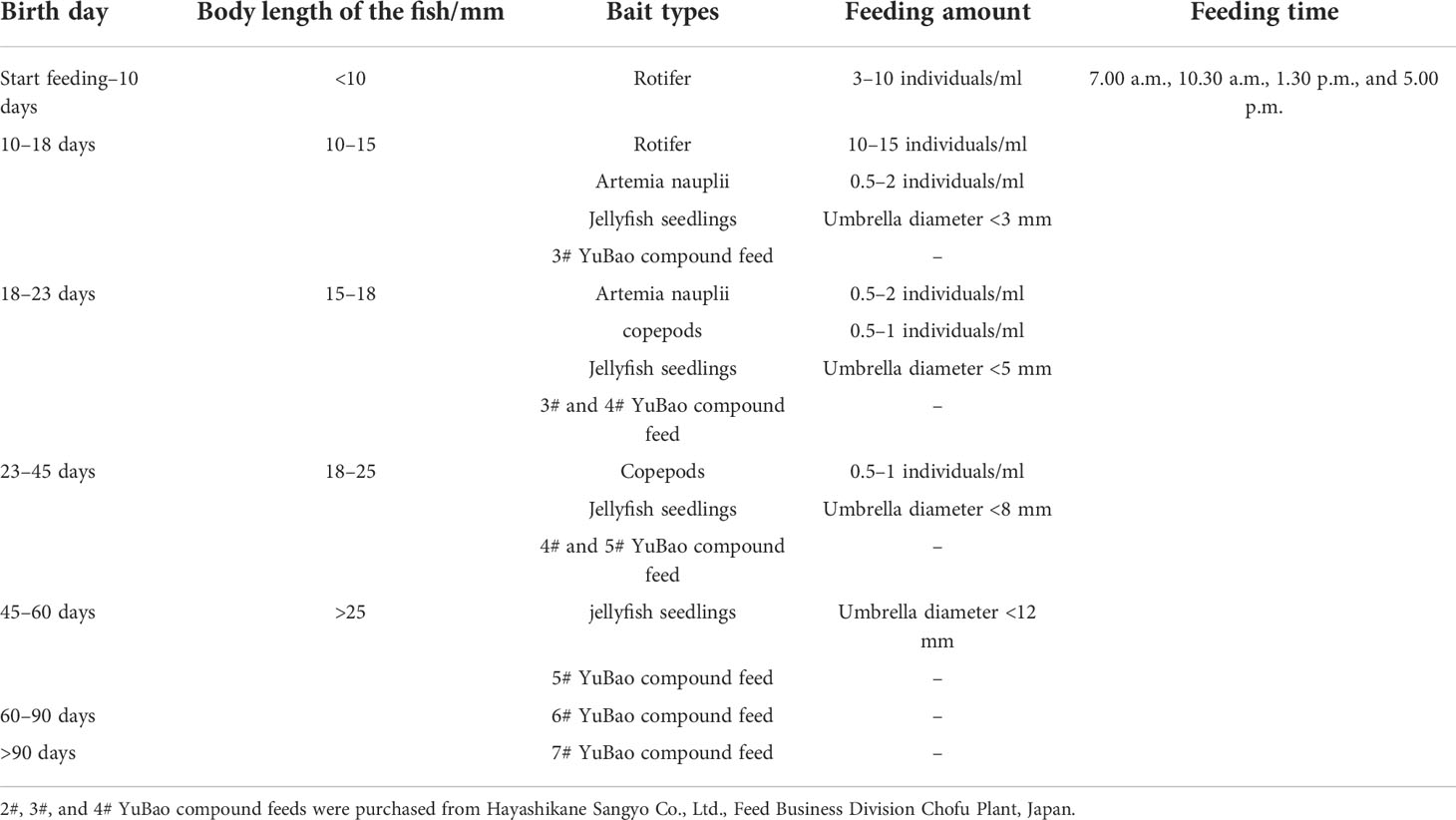
The specific artificial culture technology of P. argenteus followed the usual protocol described by Tian (2014), and the feeding information of P. argenteus from larval to weaning, pre-growth, and growing is described in Table 1. In the culture of P. argenteus, the experimental samples of the selected group and control group were cultured in an indoor cement pond of 25 m2, with a water depth of 1.4 m. The daily water exchange rate reached 100%–220%, and the micro-flow water was kept; all the water came from the same reservoir. The feeding amounts accounted for about 3%–5% of fish body weight, the pH value ranged from 7.8 to 8.2, the water temperature ranged from 15°C to 28°C, the salinity remained at about 25‰, and the dissolved oxygen was kept at 7 mg/l. P. argenteus was sensitive to light, so it was necessary to control the light intensity with an adjustable LED lamp, which was adjusted to 100 lx at 6:30, 200 lx at 10:30–16:30, 50 lx at 16:30–22:30, and the lowest 5 lx after 22:30 in the evening (Tian, 2014). During the whole culture stage, the hatching and breeding conditions of the selected group along with the control group were basically the same.
Sampling and growth measurement
During growth periods, the body weight of 50 P. argenteus per group was randomly measured using an electronic balance (0.01 g accuracy) on days 60, 90, and 120. Feeding was stopped 24 h in advance before each morphological measurement. Then, a digital camera was used to take pictures of the measured objects (total length, fork length, body length, body height, head length, snout length, eye diameter, trunk length, caudal length, caudal height, and tail length), and Digimizer Version 5.4.4 software was used to measure the morphometric traits of P. argenteus (accurate to 0.01 mm). The mean and standard deviation were collected.
Statistics of fertilization rate and hatching rate
From April to June each year, high-quality fertilized eggs of P. argenteus were collected by the egg-collecting device of the breeding base. After weighing the total eggs (in actual statistics, the wet weight of 650 fertilized eggs is about 1 g), they were placed in a bucket for 10 min; the high-quality fertilized eggs floating in the upper layer were collected and weighed by electronic balance (0.01 g accuracy). Then, the fertilized eggs were washed and transferred to a 10-t incubation pond, with the operating temperature difference less than 0.5°C. After all fertilized eggs were hatched, the number of hatched fishes was counted.
where F is the fertilization rate, and N1 and N2 are the total number of fertilized eggs and the total number of eggs, respectively.
where H is the hatching rate and n1 and n2 are the actual number of hatchlings and the number of fertilized eggs, respectively.
Growth performance parameters
The survival rate (TSR), weight gain rate (WGR), specific growth rate (SGR), and condition factor (CF) were selected as the growth performance index, which were calculated according to the following formulas: TSR (%) = 100 × (terminal mantissa/initial mantissa); WGR (%) = 100 × (final total weight - initial total weight)/initial total weight; SGR (%/d) = 100 × ((ln (final body weight) – ln (initial body weight))/feeding days; CF (g/cm3) = 100 × (body weight/body length3).
Estimation of genetic parameters
The realized heritability () was calculated as the difference between selected and control means (Chen, 2009):
where XS and XC are the mean sizes of the offspring in the selected group and control group, respectively; σc is the standard deviation of control offspring; and i is the intensity of selection. The standard response to selection (SR) was calculated using the following:
Genetic gain (GG) was defined as the proportional increment in the morphological values achieved by selection:
Statistical analyses
Prior to do the genetic parameter estimation, all quantitative data were analyzed for normality and variance homogeneity. The significance level for all analyses was set to P < 0.05.
Results
Comparative analysis of fertilization rate and hatching rate
The statistical results of breeding data showed that the number of eggs laid, the number of high-quality fertilized eggs, and the number of hatchlings of P. argenteus in the selected group (PS0) were 89.25 × 104, 14.17 × 104, and 4.15 × 104, respectively, which were higher than those in the control group (PC0) and the corresponding parameters of the control group were 29.76 × 104, 4.35 × 104, and 1.21 × 104, respectively. In the selected group, the fertilization rate and hatching rate were 15.88% and 29.28%, respectively, while those in the control group were 14.62% and 27.81%, respectively (Table 2).

Table 2 The egg-laying information summary of Pampus argenteus from the selected group and control group.
Growth performance comparison
In this study, the survival rates of P. argenteus were at high levels in the whole breeding process, and the specific growth rate and weight gain rate of 60- to 90-day-old PS1 were higher than those of PC1, but the opposite trends were observed in 90- to 120-day-old P. argenteus. In addition, in the same growth period, the condition factors of PS1 and PC1 were basically the same, and there was no significant difference. Among them, the condition factor of 60- to 90-day-old PS1 (0.0329 ± 0.0052) was slightly higher than that of PC1 (0.0305 ± 0.0101). At the age of 90–120 days, the condition factor of 90- to 120-day-old PS1 (0.0235 ± 0.0083) was slightly lower than that of PC1 (0.0252 ± 0.0085) (Figure 1). The correlation analysis of morphological traits found that the body weight traits of 60-day-old PS1 (16.76 ± 3.39 g) and PC1 (15.32 ± 7.13 g) were significantly different (P < 0.05). Except for eye diameter and trunk length traits, the other nine morphological traits were significantly different between the two groups. On the 90th day, the body weight of PS1 (21.38 ± 4.32 g) was significantly higher than that of PC1 (19.67 ± 7.17 g). Among the other 11 morphological traits, there was no significant difference (P > 0.05) in body height, head length, eye diameter, trunk length, and caudal length traits, but there were significant differences among the remaining six morphological traits. On the 120th day, the body weight of PS1 (27.74 ± 7.80 g) was significantly higher than that of PC1 (24.08 ± 11.38 g), and the results of significant differences in morphological traits were similar to those at 90 days, except for body height trait. In terms of coefficient of variation (CV), in the three growth periods, the CV values for body weight were greater than those of all other morphological traits in the two groups, and the CV values of body weight of PS1 ranged from 20.21% to 28.12%, while those of PC1 ranged from 30.60% to 47.06%. Meanwhile, except for snout length, trunk length, and caudal length traits, the CVs of the remaining morphological traits of PS1 were less than 10% during the three growth periods, and the CVs of PC1 were higher than those of PS1, ranging from 7.51% to 19.68% (Table 3). During the three growth periods, the survival rates of PS1 and PC1 were at a high level. In the third month post hatching, the weight gain rate, specific growth rate, and condition factor of PS1 were 27.57%, 0.81%, and 3.29%, respectively. They were all higher than those of PC1 (18.60%, 0.57%, and 3.05%). However, at 4 months after hatching, the three growth performances of P. argenteus in the selected group were lower than those in the control group; the specific information is found in Figure 1.
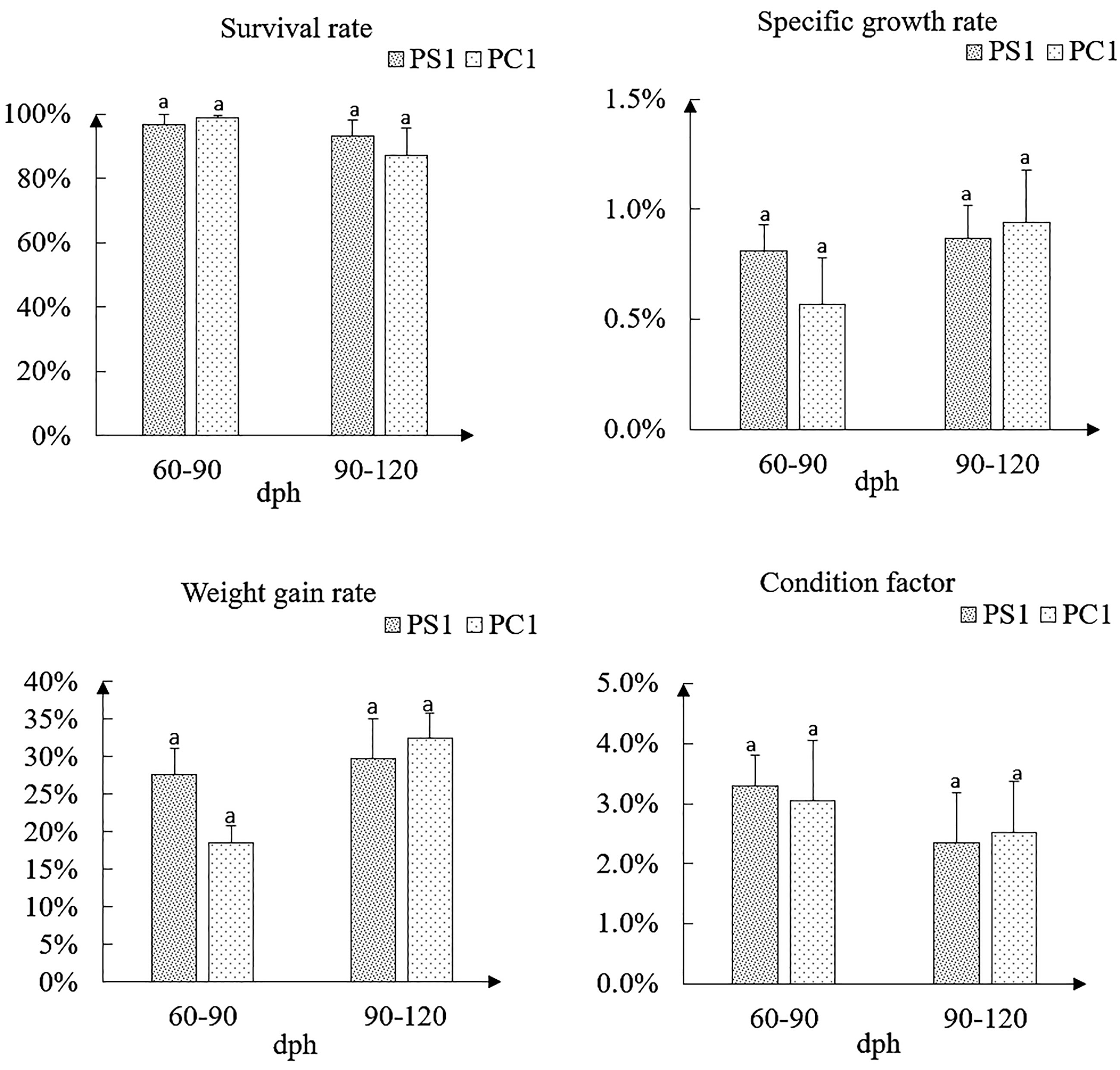
Figure 1 Growth performance parameters of Pampus argenteus from selected group and control group at different ages.
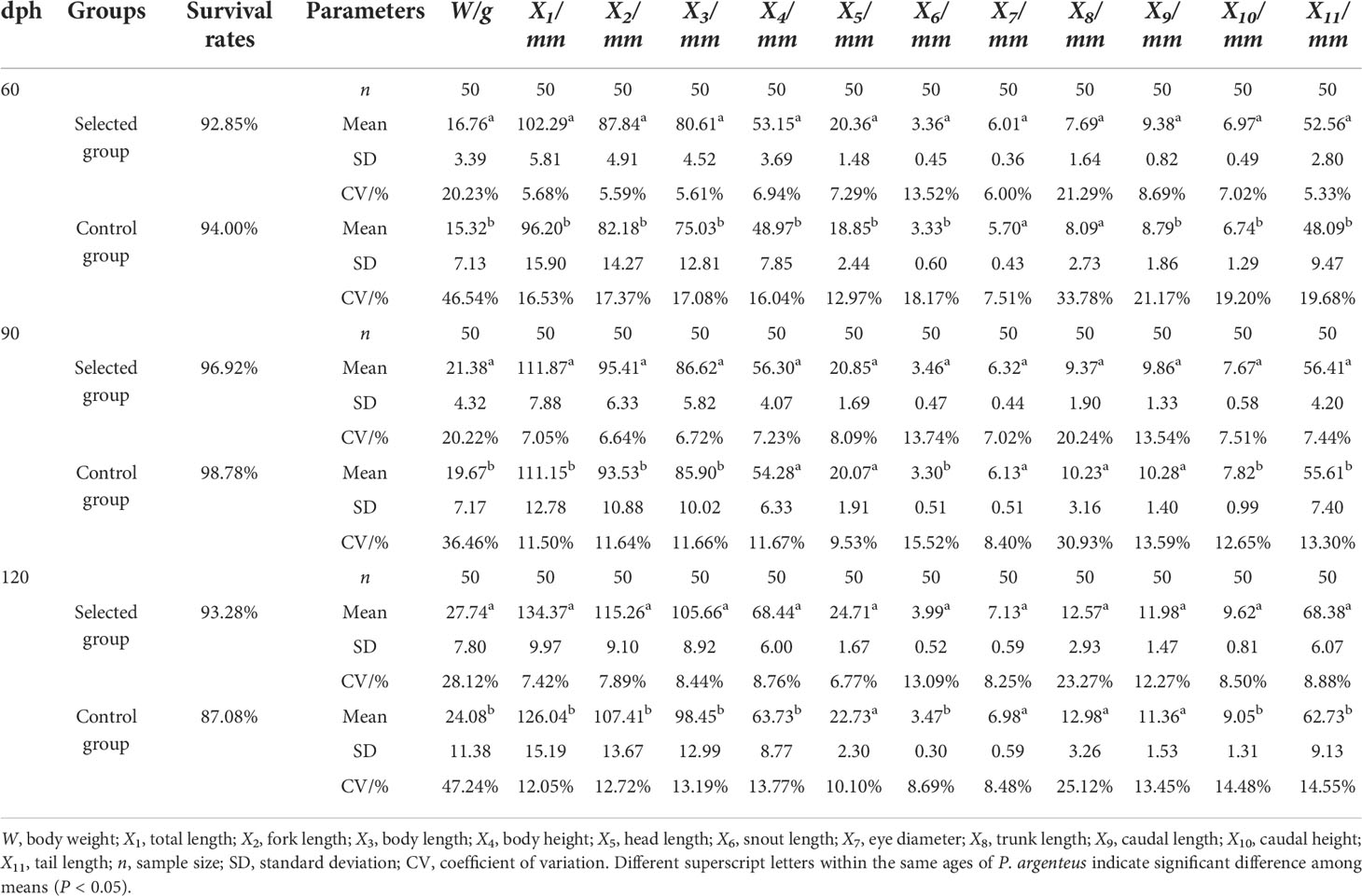
Table 3 Descriptive statistics of various morphological traits of P. argenteus from the selected group and control group at 60, 90, and 120 days post-hatching (dph).
Genetic parameters
Firstly, the morphological traits significantly correlated with the body weight of P. argenteus were found by Pearson correlation analysis and path analysis, which were body length and tail length, respectively. Then, we analyzed the genetic gains (GG), standardized response to selection (SR), and realized heritability () of the three morphological traits in the selected group of P. argenteus at 60, 90, and 120 days. The results showed that the GG, SR, and of body weight at 90 days were higher than those at 60 and 120 days, which were 17.64%, 0.58, and 0.33, respectively, and the of body weight was at a moderate heritability level (90 d). The three genetic parameters of body length and tail length traits at 90 days were lower than those at 60 and 120 days. Among them, the GG of body length were 7.43%, 2.98%, and 7.33% for 60, 90, and 120 days, respectively, while the corresponding SRs were 0.43, 0.29, and 0.56, respectively. The values of body length were at low to moderate heritability levels during the three growth periods, which were 0.25, 0.16, and 0.32 for 60, 90, and 120 days, respectively. Similarly, the GG values of tail length were 9.29%, 4.21%, and 9.00% for 60, 90, and 120 days, respectively, and the corresponding SRs were 0.47, 0.38 and 0.62, and the corresponding were 0.27, 0.22 and 0.35, respectively. As for the three morphological traits, the order of mean values of GG was body weight (14.08 ± 4.21), tail length (7.50 ± 2.85), and body length (5.91 ± 2.54). The order of mean values of SR was tail length (0.49 ± 0.12), body length (0.43 ± 0.14), and body weight (0.37 ± 0.19). The order of mean values of was tail length (0.28 ± 0.07), body length (0.24 ± 0.08), and body weight (0.21 ± 0.11), and they were at moderate level (Table 4).
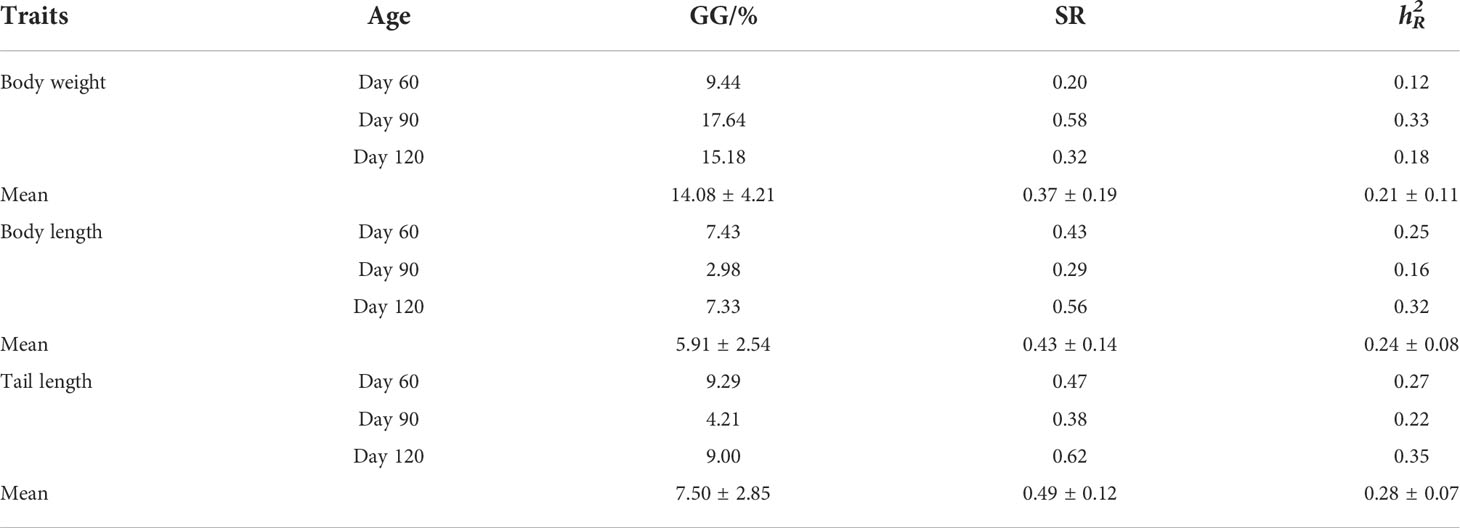
Table 4 Genetic gains (GG), standardized response to selection (SR), and realized heritability () of the growth traits in the selected group of P. argenteus at different ages.
Discussion
In aquaculture species improvement programs, a classic example that is of great interest to breeders is body weight, which not only brings considerable economic benefits but also has considerable potential for genetic improvement (Gjedrem and Rye, 2018). Recently, Chatchaiphan et al. (2019) observed the significant genetic variation in the growth traits of the fish pacu (Piaractus mesopotamicus) and found that the selection made on total length trait can lead to weight gain. Wang and Ma (2020) found that the body weight/body length ratio could be used as a morphological marker of fast-growing turbot (Scophthalmus maximus) to boost the genetic breeding of S. maximus with large specifications. Qiu et al. (2017) estimated the genetic parameters of nine morphological traits of large yellow croaker (Larimichthys crocea) and found the high genetic correlations and genetic variation between the pairs of body weight, body length, body height, body width, and eviscerated weight. Similarly, in this study, two morphological traits significant correlated with the body weight of P. argenteus were found by Pearson correlation analysis and path analysis, which were body length and tail length, respectively (P < 0.05), and there might be some genetic correlation between these morphological traits (Trygve, 2005). In addition, we also found that the CVs of body weight were the highest in all three growth periods. These results were consistent with the results of most other Perciformes species (Garcia et al., 2017; Qiu et al., 2017). High CVs indicate that the trait might have sufficient genetic diversity to support selective breeding (Gjedrem and Baranski, 2009), indicating that the body weight trait of P. argenteus might be beneficial to selective breeding. However, there was a phenomenon in this paper that the fertilization rate and hatching rate of P. argenteus were relatively lower than those of other fish (Aya et al., 2022; Vasconcelos et al., 2022). Long reproductive cycle, unsynchronized gonad development, and different peak periods of fertilization and spawning may be the reasons for the low fertilization rate of P. argenteus. The low hatching rate was probably due to the poor quality of fertilized eggs or the mutual encroachment of seedlings, which indirectly reduced the hatching rate. These questions pointed out part of the follow-up work for us. For example, we can add gonad enhancer, fresh jellyfish, multivitamins, and multi-minerals to the feed of P. argenteus at the beginning of March every year, so as to promote the rapid and synchronous development of gonads of P. argenteus to phase V and improve the health of the parents; these are conducive to improving the quality and quantity of sperm eggs of P. argenteus. Then, we can try our best to ensure synchronous spawning and sperm excretion by exploring the optimum conditions of oxytocin in P. argenteus, which can indirectly improve fertilization rate and hatching rate of P. argenteus. In addition, we can reduce the mutual biting of seedlings and improve the survival rate of P. argenteus by reducing the breeding density and appropriately increasing the feeding amount and frequency of bait.
Heritability is an extremely important parameter in genetic breeding. It is probably the most important statistic when developing a selective breeding program, as it is used to estimate breeding values and to predict response to selection (Gjedrem and Baranski, 2009). In this study, we studied the genetic parameters of body weight, body length, and tail length traits and found that the values of body weight trait were low (60 and 120 days), but the value was moderate at 90 days, and the mean values of realized heritability of the three morphological traits were at moderate level. Similarly, in many Perciformes fish, the of body weight trait ranged from 0.20 to 0.53 (Nguyen Huu et al., 2014; Garcia et al., 2017; Yoshida et al., 2021; Gonzalez et al., 2022), and most of them were at moderate level. Moderate and high heritability on the traits demonstrates promising effects on genetic improvement in specific selective breeding programs, and improvement of the traits would be achieved through selection (Gjedrem and Baranski, 2009). It can be seen that the three morphological traits of the selected group had the potential of selective breeding. At 90 days after hatching, it might be more appropriate to screen with body weight trait as the main objective trait, which might be more conducive to the retention of growth-related genes or SNPs. The estimates the proportion of the morphological variation attributable to the additive effect of genes, and it allows inferring the potential selective response for a specific trait in a population (Falconer and Mackay, 1996). Among the three morphological traits, the mean values of selective response and realized heritability of tail length trait were the highest, while the mean value of the highest genetic gain was observed in body weight trait. These indicated that the response to selection was asymmetrical, and the asymmetrical response to selection could be attributed to multiple factors, such as genetic asymmetry, scalar asymmetry maternal effects, genetic drift, selection differential, inbreeding depression, and major gene effects (Falconer and Mackay, 1996). In addition, genetic gain studies in a selective breeding program are very important for measuring the efficiency of the program over a specific period. Compared with the control group, the genetic gains of body weight trait in the selected group at the three growth periods (60, 90, and 120 days) were 9.44%, 17.64%, and 15.18%, respectively. Genetic gain values of the other two morphological traits had little change, indicating that the improvement for body weight trait of the selected group might have a certain prospect to produce P. argenteus. For example, Campos et al. (2020) reported that the genetic gain for the growth traits of Colossoma macropomum was 15.6% at 12 months. Teodozio de Araujo et al. (2020) found that genetic gains ranging from 9.1% to 35.6% for weight and 5.6% to 10.6% for trunk length were obtained in the family-selected O. niloticus. Genetic gain was estimated for Atlantic salmon in the Norwegian Breeding Programme by using selection differentials, and the genetic gain of the growth rate trait was estimated to be 11% per generation (Gjøen and Gjerde, 1997). In many fish species, when the growth rate of each generation increases by 10%–15% through selection of programs, the encouraging results of genetic gain can be obtained (Gjedrem, 2000). These findings indicated that it was possible to obtain considerable genetic gains in the growth aspect by selective breeding for growth traits of P. argenteus. In summary, we found that the selected populations for mass selection successfully improved the growth performance of P. argenteus. The method of mass selection is helpful to provide a large number of excellent seedlings for the P. argenteus industry and alleviate the current status of breeding demand. Of course, if we want to increase the breeding industry of P. argenteus, we should pay special attention to improving the fertilization rate and hatching rate of P. argenteus, as well as improving the performance of commodity traits. Therefore, it is necessary to continue to carry out more experiments and investigations, so as to provide complete theoretical and technical support for the selective breeding of P. argenteus.
Data availability statement
The authors declare that the original data of this study are available from the corresponding author.
Ethics Statement
The animal study was reviewed and approved by Ningbo Experimental Animal Ethics Committee belongs to Ningbo University.
Author contributions
CZ drafted the manuscript and participated in the experiment. KJ checked the language and grammar of the paper. SZ performed sample preparation. SX helped to analyzed data. YW interpreted results and sample preparation. DW conceived and designed the study. All authors read and gave final approval of the final manuscript.
Funding
This work was funded by the Ningbo 2025 Major Project of Science Technology and Innovation (2021Z003), the Zhejiang Major Science Project (2019C02059), the Natural Science Foundation of Zhejiang (LY18C190008 and LY18C1900013), the National Natural Science Foundation of China (Nos. 31772869, 31872586, 42076118, and 31872195), and the K. C. Wong Magna Fund in Ningbo University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
AlMomin S., Kumar V., Al-Amad S., Al-Hussaini M., Dashti T., Al-Enezi K., et al. (2016). Draft genome sequence of the silver pomfret fish, Pampus argenteus. Genome 59, 51–58. doi: 10.1139/gen-2015-0056
Aya F. A., Sayco M. J. P., Garcia L. M. B. (2022). Effects of exogenous hormones and broodstock age on the spawning response of captive silver therapon Leiopotherapon plumbeus. J. Appl. Ichthyol. 38, 232–240. doi: 10.1111/jai.14297
Campos E. C., Oliveira C. A. L., Araujo F. C. T., Todesco H., Souza F. N., Rossi R. M., et al. (2020). Genetic parameters and response to selection for growth in tambaqui. Animal 14, 1777–1785. doi: 10.1017/s1751731120000488
Chatakondi N. G., Yant D. R. (2005). Three generations of mass selection for growth rate in harvest select (Gold kist) strains of channel catfish, Ictalurus punctatus, under commercial conditions. Aquaculture 247, 8–8.
Chatchaiphan S., Thaithungchin C., Koonawootrittriron S., Na-Nakorn U. (2019). Responses to mass selection in a domesticated population of snakeskin gourami, Trichopodus pectoralis, Regan 1910, and confounding effects from stocking densities. Aquaculture 498, 181–186. doi: 10.1016/j.aquaculture.2018.08.029
Chen Z. H. (2009). Population and quantitative genetics (Guiyang, China: Guizhou Science and Technology Publishing House Co.,Ltd.), 215–230.
Chevassus B., Quillet E., Krieg F., Hollebecq M. G., Mambrini M., Faure A., et al. (2005). Improved mass selection for growth rate in brown trout (Salmo trutta fario): the "PROSPE" process. Aquaculture 247, 8–8. doi: 10.1016/j.aquaculture.2005.02.001
Davis P., Wheeler A. (1985). The occurrence of Pampus argenteus (Euphrase), (Osteichthyes, perciformes, stromateoidei, stromateidae) in the north Sea. J. Fish. Biol. 26, 105–109. doi: 10.1111/j.1095-8649.1985.tb04247.x
Duremdez R., Al-Marzouk A., Qasem J. A., Al-Harbi A., Gharabally H. (2004). Isolation of streptococcus agalactiae from cultured silver pomfret, Pampus argenteus (Euphrasen), in Kuwait. J. Fish. Dis. 27, 307–310. doi: 10.1111/j.1365-2761.2004.00538.x
Falconer D. S., Mackay T. F. C. (1996). Introduction to quantitative genetics. Longman. Edinburgh. Gate. England. pp, 184–204.
Garcia A. L. S., de Oliveira C. A. L., Karim H. M., Sary C., Todesco H., Ribeiro R. P. (2017). Genetic parameters for growth performance, fillet traits, and fat percentage of male Nile tilapia (Oreochromis niloticus). J. Appl. Genet. 58, 527–533. doi: 10.1007/s13353-017-0413-6
Gjøen H. M., Gjerde B. (1997). Selection differentials for Atlantic salmon. year classes 1980–92 (Seleksjonsdifferanser for laks. Årsklasse 1980–92) Great Clarendon St, Oxford Ox2 6dp, England Oxford Univ Press. 31/97. AKVAFORSK.
Gjedrem T. (2000). Genetic improvement of cold-water fish species. Aquacul. Res. 31, 25–33. doi: 10.1046/j.1365-2109.2000.00389.x
Gjedrem T., Baranski M. (2009). Selective breeding in aquaculture: An introduction (Dordrecht, the Netherlands: Springer), 26–62.
Gjedrem T., Rye M. (2018). Selection response in fish and shellfish: a review. Rev. Aquacul. 10, 168–179. doi: 10.1111/raq.12154
Gonzalez C., Jousepth G. H., Yanez J. M. (2022). Genotype-by-environment interaction for growth in seawater and freshwater in Atlantic salmon (Salmo salar). Aquaculture 548, 737674. doi: 10.1016/j.aquaculture.2021.737674
Mohitha C., Joy L., Divya P. R., Gopalakrishnan A., Basheer V. S., Koya M., et al. (2015). Characterization of microsatellite markers in silver pomfret, Pampus argenteus (Perciformes: Stromateidae) through cross-species amplification and population genetic applications. J. Genet. 94, E89–E93. doi: 10.1007/s12041-014-0416-6
Nguyen Huu N., Ngo Phu T., Wayne K., Nguyen Hong N. (2014). Selection for enhanced growth performance of Nile tilapia (Oreochromis niloticus) in brackish water (15-20 ppt) in Vietnam. Aquaculture 428, 1–6. doi: 10.1016/j.aquaculture.2014.02.024
Parsamanesh A., Shalbaf M., Najafpour N. (1998). Status of Pampus argenteus fisheries in khoozestan waters (North-West Persian gulf), I.R.Iran. Indian J. Anim. Sci. 68, 407–409. doi: 10.1017/S175526721000093X
Qiu C. L., Dong L. S., Xiao S. J., Xu S. B., Fang M., Wang Z. Y. (2017). Genetic parameter estimation of nine quantitative traits by a marker-based method in Large yellow croaker, Larimichthys crocea (Richardson). Aquacul. Res. 48, 5892–5900. doi: 10.1111/are.13412
Rezk M. A., Smitherman R. O., Williams J. C., Nichols A., Kucuktas H., Dunham R. A. (2003). Response to three generations of selection for increased body weight in channel catfish, Ictalurus punctatus, grown in earthen ponds. Aquaculture 228, 69–79. doi: 10.1016/s0044-8486(03)00216-3
Tang R. J. (2021). Fully promote the revitalization of the seed industry. China Seed. Indust., 1–2. doi: 10.19462/j.cnki.1671-895x.2021.10.001
Teodozio de Araujo F. C., Lopes de Oliveira C. A., Campos E. C., Massako Yoshida G., Lewandowski V., Todesco H., et al. (2020). Effects of genotype x environment interaction on the estimation of genetic parameters and gains in Nile tilapia. J. Appl. Genet. 61, 575–580. doi: 10.1007/s13353-020-00576-2
Tian Z. (2014). A study on artificial breeding technology of pampus argenteus. [dissertation/master’s thesis] ([Ningbo]: Ningbo Uinversity).
Trygve G. (2005). Selected and breeding programs in aquaculture. Springer. Dordrecht. Netherlands. pp, 59–65.
Vasconcelos L. M., Farias R., Guimaraes I. M., Santos A. J. G., Andrade H. A., Coimbra M. R. M. (2022). Triploidy induction by cold shock in curimba (Prochilodus argenteus). J. Appl. Ichthyol. 38, 247–251. doi: 10.1111/jai.14296
Wang X., Ding S., Yin D., Song J., Chang Y. (2020). Response to selection for growth in the second generation of two shell color lines of the bay scallop Argopecten irradians. Aquaculture 528, 735536. doi: 10.1016/j.aquaculture.2020.735536
Wang Q., Li Q., Kong L., Yu R. (2012). Response to selection for fast growth in the second generation of pacific oyster (Crassostrea gigas). J. Ocean. Univ. China. 11, 413–418. doi: 10.1007/s11802-012-1909-7
Wang X. a., Ma A. (2020). Dynamic genetic analysis for body weight and main length ratio in turbot Scophthalmus maximus. Acta Oceanol. Sinica. 39, 22–27. doi: 10.1007/s13131-020-1551-y
Wang D., Wu F. X. (2021). China Fishery ststistical yearbook in 2020 (Beijing, China: Beijing Agriculture Press), 15–46.
Xu S. L., Wang D. L., Xu J. L., Yan X. J., Hu X. Y. (2012). Comparative study on fatty acid composition of different tissue in three kinds of wild pomfret. J. Biol. 29, 53–58. doi: 10.1007/s11783-011-0280-z
Yoshida G. M., Oliveira C. A. L., Campos E. C., Todesco H., Araujo F. C. T., Karin H. M., et al. (2021). A breeding program for Nile tilapia in Brazil: Results from nine generations of selection to increase the growth rate in cages. J. Anim. Breed. Genet. 00, 1–9. doi: 10.1111/jbg.12650
Yuan Z. F., Chang Z. J., Guo M. C., Sun S. Z. (2015). Genetic analysis of quantitative traits (Beijing, China: Science Press), 109–234.
Zhang M. Q., Bai H., Wang S., Zhou F. Q., Wu Y. F., Qian S. B., et al. (2014). Investigation on the varieties of marine traditional Chinese medicines in China. Chin. J. Mar. Drugs 33, 39–46. doi: 10.13400/j.cnki.cjmd.2014.06.008
Zhang J., Li Q., Xu C., Han Z. (2019). Response to selection for growth in three selected strains of the pacific oyster Crassostrea gigas. Aquaculture 503, 34–39. doi: 10.1016/j.aquaculture.2018.12.076
Keywords: Pampus argenteus, growth performance, realized heritability, selection intensity, mass selection
Citation: Zhang C, Jacques KJ, Zhang S, Xu S, Wang Y and Wang D (2022) Analyses of growth performance and realized heritability of Pampus argenteus in a breeding program in China. Front. Mar. Sci. 9:935924. doi: 10.3389/fmars.2022.935924
Received: 04 May 2022; Accepted: 12 July 2022;
Published: 04 August 2022.
Edited by:
Baojun Tang, Chinese Academy of Fishery Sciences, ChinaReviewed by:
Yaoyao Zhan, Dalian Ocean University, ChinaShengjie Ren, Queensland University of Technology, Australia
Copyright © 2022 Zhang, Jacques, Zhang, Xu, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danli Wang, d2FuZ2RhbmxpQG5idS5lZHUuY24=
 Cheng Zhang
Cheng Zhang Kimran Jean Jacques
Kimran Jean Jacques Shanliang Xu
Shanliang Xu Yajun Wang
Yajun Wang