- 1Centre d’Etudes Biologiques de Chizé, UMR 7372, CNRS – Université de la Rochelle, Villiers-en-Bois, France
- 2Réserve Naturelle Nationale des Terres Australes Françaises (headquarters of the French Southern and Antarctic Lands), Saint-Pierre, Réunion
- 3PELAGIS Observatory UAR- 3562, CNRS-La Rochelle Université, La Rochelle, France
Southern elephant seals (Mirounga leonina) play a pivotal role in the Southern Ocean as wide-ranging marine predators and major prey consumers within Southern Ocean marine ecosystems. Due to their circumpolar distribution and the remoteness of their habitat, large uncertainties remain about their total population sizes. This is especially true for elephant seal populations in the French Southern Territories in the southern Indian Ocean (i.e. Crozet and Kerguelen Archipelagos) as many breeding sites are inaccessible for ground censuses. Here, we present a simple and efficient approach for estimating the total elephant seal populations of the Kerguelen and Crozet Archipelagos by using very high-resolution satellite imagery (<1m resolution). Twenty-eight satellite images taken during the breeding season to count female elephant seals in inaccessible areas were used and complemented the traditional annual ground counts in accessible areas. For Kerguelen Island sectors likely to host colonies and where no satellite images were available for the breeding season, a statistical predictive model was built to estimate the most likely number of breeding females to be present on a given beach according to its physiographic characteristics. Our results show the reliability of using very high-resolution satellite images, a relatively low-cost platform, to count pinniped populations and provide the first estimation of the total southern elephant seal population for both the Kerguelen 347,995 (s e = 4,950) and Crozet 13,065 (s e = 169) Archipelagos. The combined total represents over 35% of the global elephant seal population with the Kerguelen stock being numerically equivalent to the South Georgia stock. In addition, we re-examined the population trends since the last mid-century for Kerguelen and over the last five decades for Crozet. The demographic trends of the southern Indian Ocean populations show marked growth over the last decade (5.1% and 1.6% annual growth rate for Crozet and Kerguelen respectively), particularly on Crozet where the elephant seal population has more than tripled.
1 Introduction
Effective monitoring using appropriate biodiversity indicators is critical to the surveillance of the natural environment, to detect changes and assess threats associated with natural or human-driven environmental changes (Johnson et al., 2017). Total population size data are critically important to improve ecological or trophic modeling studies such as estimations of the overall amount of food consumed by seabirds and marine top mammals and their contribution to ocean carbon and nutrients fluxes (Guinet et al., 1996; Moore, 2008). As such, long-term monitoring of population size along with demographic studies are essential to define the conservation status of species and implement conservation responses. Beyond conservation issues, long-term surveys are also essential for research purposes.
In this context, monitoring of the southern elephant seal (Mirounga leonina), an abundant marine top predator with a large circumpolar distribution (Ling and Bryden, 1992), is of particular interest. Southern elephant seals are major deep diving predators within the Southern Ocean. Monitoring their population changes could provide insights into the changes affecting the mesopelagic and bathypelagic fishes and squids they consume (Guinet et al., 1996; Cherel et al., 2008).
Four genetically distinct populations are identified: the Valdés Peninsula population in Argentina, the South Georgia population in the South Atlantic Ocean, the Kerguelen and Heard populations including the Crozet and Prince Edward Archipelagos in the South Indian Ocean, and the Macquarie population in the South Pacific Ocean (Slade et al., 1998; Rus Hoelzel et al., 2001). The number of breeding females ashore provides a reliable indicator of the total population size (Hindell and Burton, 1987). Available land censuses suggest that the three largest elephant seal breeding populations located in South Georgia, Kerguelen and Macquarie, hosted approximately 100,000, 60,000 and 20,000 breeding females respectively in 2010 (Hindell et al., 2016).
Monitoring studies of elephant seal populations have reported different population trends according to the breeding localities over the last sixty years. South Georgia, in the Atlantic population of the Southern Ocean, was the only breeding location where elephant seal numbers were reported to be stable (McCann and Rothery, 1988; Boyd et al., 1996; McMahon et al., 2005). Elsewhere, in Argentina, population size increased rapidly over the 1982-1995 period (Campagna and Lewis, 1992; Lewis et al., 1998). In contrast, within the Pacific sector of the Southern Ocean, the number of elephant seal females breeding on Macquarie Island declined from a maximum of about 40,000 in the 1950s to a minimum of 18,300 in 2000, then increased slightly to 19,200 up to 2004 (van den Hoff et al., 2007) before initiating a new decline in recent years (Hindell et al., 2017).
Meanwhile, within the Southern Indian Ocean, large changes in elephant seal numbers have been observed since 1950 (Guinet et al., 1992; Guinet et al., 1999; Authier et al., 2011; Oosthuizen et al., 2015). On Marion Island, the population has declined by 83%, from approximately 3 700 breeding females in 1951, and an estimation of 1,200 females in 1970, to a low 450 breeding females in the late 1990s with the population declining by 5.8% per year between 1986 and around 1994 (McMahon et al., 2009; Oosthuizen et al., 2015). Since then the population has slightly increased (N. de Bruyn, personal communication and see https://www.marionseals.com/elephantseals). On the Crozet Archipelago, the Possession Island population has decreased at a rate of 5.4% per year between 1970 (with an estimation of ~1,700 breeding females) to 500 breeding females in 1990 (i.e. a decline of 73%) with no change in numbers detected between 1990 and 1997 (Barrat and Mougin, 1978; Guinet et al., 1992; Guinet et al., 1999). At the Kerguelen Islands, the number of breeding females censused on the Courbet Peninsula declined from about 70,000 breeding females in 1952 (Pascal, 1981) to 37,400 in 1987 (Guinet et al., 1992) before starting to increase at a 1% annual rate over the 1987-2009 period (Guinet et al., 1999; Authier et al., 2011). However, within the Crozet and Kerguelen Archipelagos, population trends were estimated from only about 20% of the total coastlines, the remaining 80% of coastlines being inaccessible. On the Crozet Archipelago, censuses of elephant seals colonies are conducted annually on Possession Island only even though colonies also inhabit Cochons Island and Est Island (Despin et al., 1972; Barrat and Mougin, 1978; Jouventin et al., 1982). Similarly, beaches located south, west and northeast of the Kerguelen Archipelago are inaccessible by land for censuses, resulting in annual censuses being conducted solely east of the Courbet Peninsula despite the fact that 1925-1931 sealing data revealed the harvesting of very large numbers of elephant seals south and west of Kerguelen Island (Savours, 2009).
Satellite images have been successfully used to census seabirds (Fretwell et al., 2017; Zhao et al., 2020), whales (Fretwell et al., 2014; Borowicz et al., 2019; Cubaynes et al., 2019; Guirado et al., 2019) and seals (LaRue et al., 2011; McMahon et al., 2014; Gonçalves et al., 2020). However, most of these studies were small-scale, proof-of-concept investigations except for some studies that have shown the ability of satellites to survey animals at more regional scales (Fretwell et al., 2014; Weimerskirch et al., 2018; LaRue et al., 2020), and global scales (Lynch and LaRue, 2014; LaRue et al., 2021).
In view of using very high-resolution satellite imagery to expand data on population estimates, the global objective of this present study is to provide the first exhaustive census of southern elephant seals (i.e. breeding female) for both the Kerguelen and Crozet Archipelagos. To achieve this goal, very high-resolution (VHR) satellite images obtained during the breeding season were acquired to complement ground censuses. VHR images provided by earth-observation satellites such as Pleiades PHR-1B (panchromatic 0.50 m resolution), WorldView (WV) 2 and 3 (panchromatic 0.50 m, and 0.30 m resolution respectively) and GeoEye1 (panchromatic 0.50 m resolution) offer a means to overcome the challenge of censusing colonies of large seabirds and pinnipeds in difficult-to-access areas while limiting the impact of human presence on protected sites (disturbance, invasive species or habitat degradation).
Building on the new horizons opened up by satellite images, this present study aims to: (1) use satellite images taken during the breeding season to count seals on selected favorable breeding beaches on the Kerguelen and Crozet Islands; (2) assess the accuracy of the satellite-image census method compared to (3) ground counts and when available, (4) complement satellite counts with ground censuses in order to (5) construct and validate a predictive statistical model for estimating the number of breeding females according to the physiography of beaches, and implement this model for beaches where no direct censuses and/or satellite images were available during the elephant seal breeding season; and (6) reevaluate the importance of the Crozet and Kerguelen southern elephant seal populations with respect to the species’ other populations. Finally, we aim to (7) assess recent population trends on the Kerguelen and Crozet Archipelagos.
2 Materials and methods
2.1 Study sites
This study was carried out in the Indian sector of the southern Indian Ocean, in the Crozet Archipelago (-46.4260°S, 51.7750°E) composed of five small main islands totaling 350 km², and the Kerguelen Archipelago (-49.3649°S, 69.3843°E, Figure 1) comprising more than 300 islands and islets covering a total area of more than 7,200 km². These islands are part of the National Nature Reserve of the French Southern Territories, listed as a UNESCO World Heritage area, and constitute a sanctuary for biodiversity, in particular for seabirds and marine mammals. Scientific monitoring studies have been performed in this sector for almost 70 years, with the first elephant seal census performed in 1952 on the Courbet Peninsula, Kerguelen Island (Pascal, 1981). On Possession Island within the Crozet Archipelago, the first census was conducted in 1966 and totaled about 2000 breeding females (Barrat and Mougin, 1978).
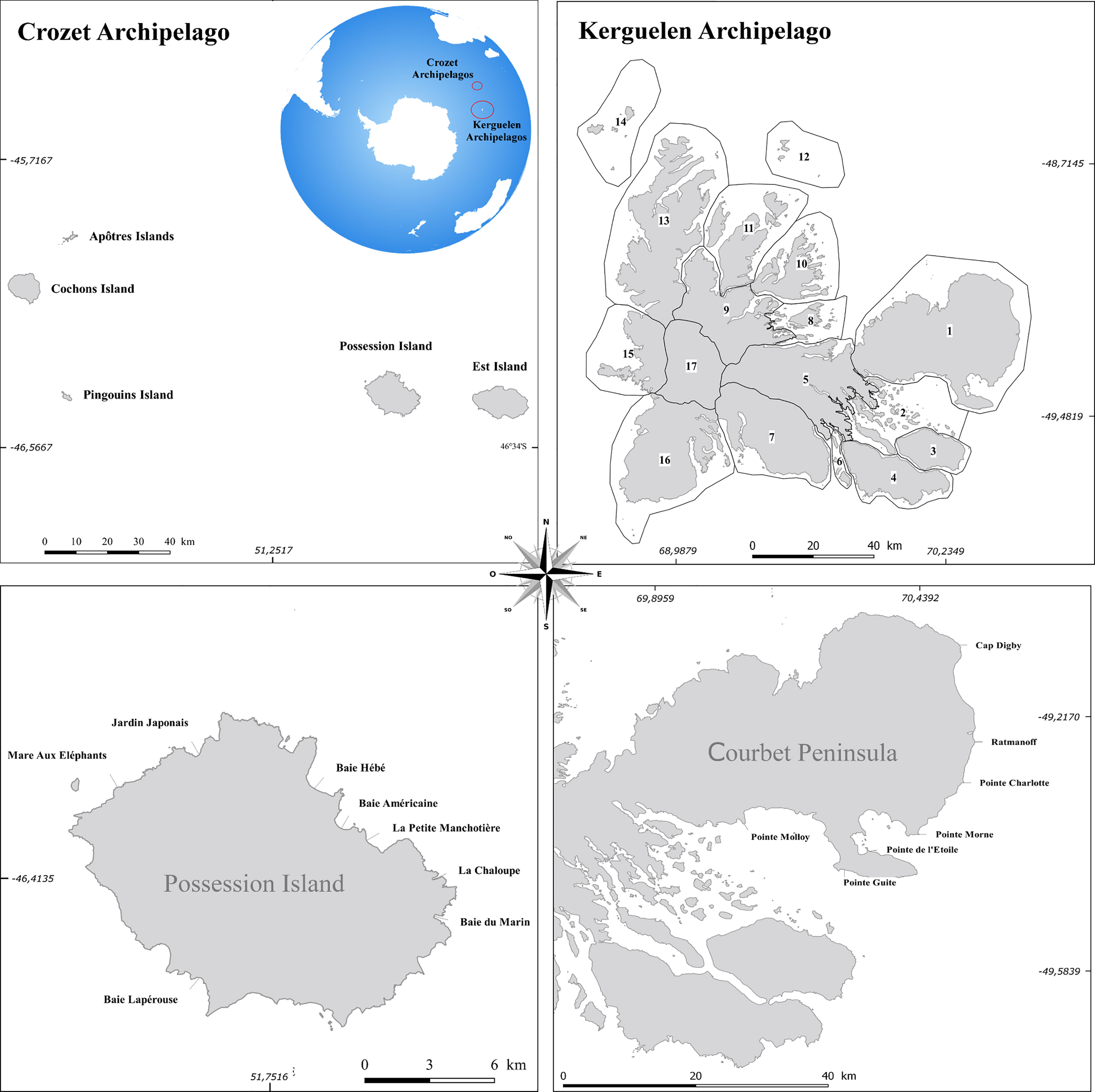
Figure 1 Location of the French Southern Islands with the Crozet (upper left panel) and Kerguelen Archipelagos divided into 17 sectors (upper right panel). For Kerguelen Island (top right), the count areas are 1) Péninsule Courbet, 2) Golfe du Morbilhan, 3) Presqu’île Ronarch, 4) Presqu’île Jeanne d’Arc, 5) Plateau central, 6) Ilots de la Baie des Swain, 7) Massif Galieni, 8) Ilots du Golfe des baleiniers, 9) Presqu’île de la Socièté de Géographie, 10) Presqu’île de Joffre, 11) Îles Foch, Howe, Saint-Lanne Grammont, 12) Îles Leygues, 13) Presqu’île du Loranchet, 14) Îles Nuageuses, Îles de l’Ouest, 16) Péninsule Ralier du Batty. The two lower panels represent the long-term monitoring study areas in Crozet and Kerguelen.
2.2 Satellite image acquisition
Three different sources of satellite images were used in this study. Firstly, we acquired a Pleiades PHR-1B (AIRBUS Defence & Space satellite) image taken on a relatively cloud-free day (28th October 2015). This image, acquired in 2015, covers a small part of the Courbet Peninsula for which ground censuses were also performed (Figure 2). Image processing was performed by the French National Center for Space Research (CNES) to produce a pan-sharpened orthorectified image. The resolution (pixel size) of this image, 0.5 meter per pixel, provides a potential density of approximately 4 pixels/m² allowing the detection of adult elephant seals present on the beaches during this period. Secondly, satellite images perform by the MAXAR satellites WV-1, WV-2, WV-3 and GeoEye-1 and by the French satellite Pleiades PHR-1B, providing a ground resolution varying from 30 to 50 cm per pixel with panchromatic images, were bought or freely available from Google Earth and Bing Maps. 26 VHR images were acquired for this study to perform elephant seal counts at inaccessible locations (one on Crozet and 25 on Kerguelen; Table 1; Figures 2, 3).
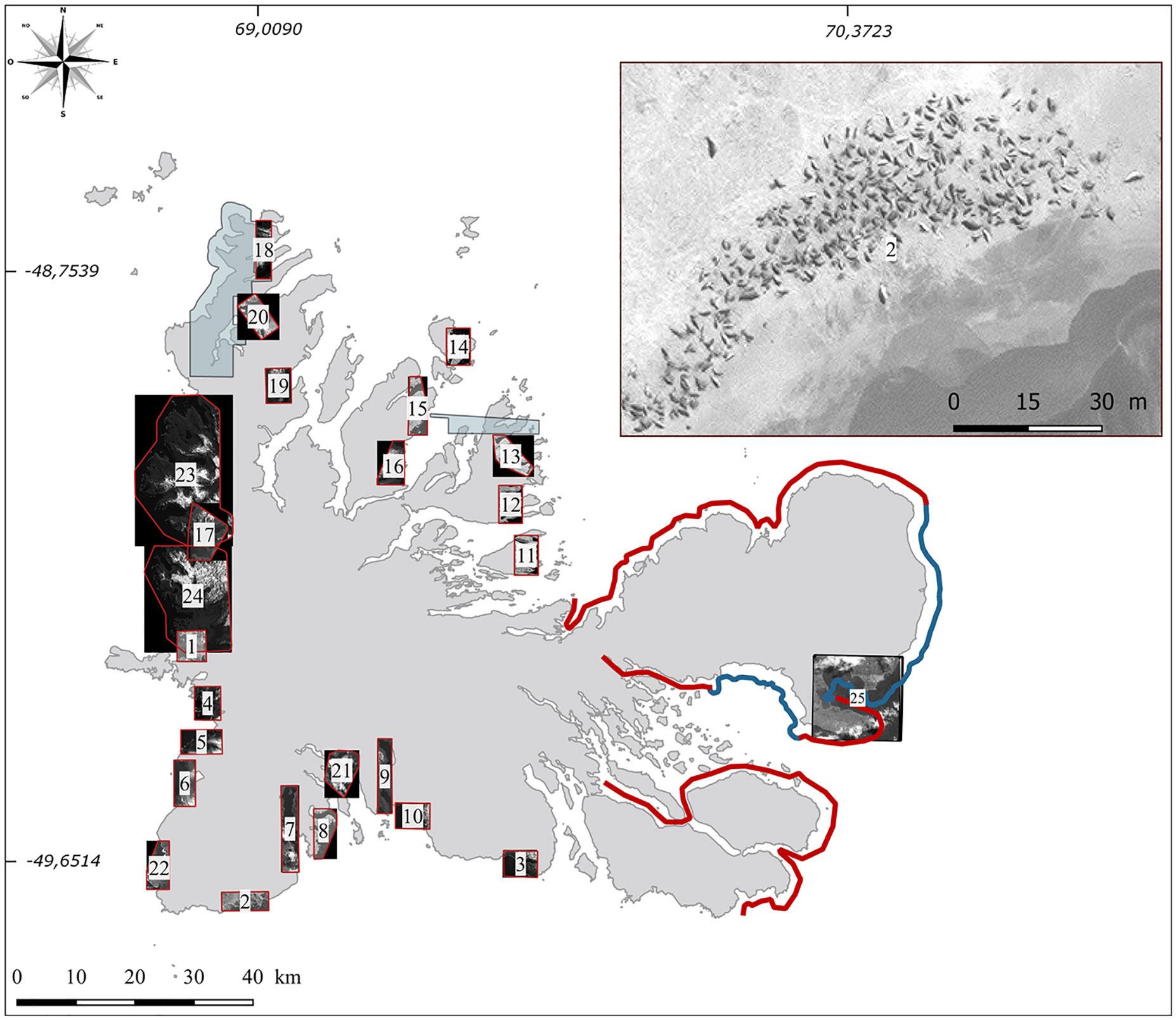
Figure 2 Locations of ground and satellite censuses in the Kerguelen Archipelago. Beaches annually censused are indicated with a blue line. The complementary ground census zone are indicated by a red line and encompass 37 breeding colonies. The black square corresponds to the validation satellite area. For all other areas (1 to 24), censuses were conducted on the basis of very high-resolution satellite images. The top right represents an example of a very high-resolution satellite image (0,30 m/pixel) of a beach. The blue areas represent the satellite images available in the opensource platform Google Earth during the peak presence of breeding females on land.
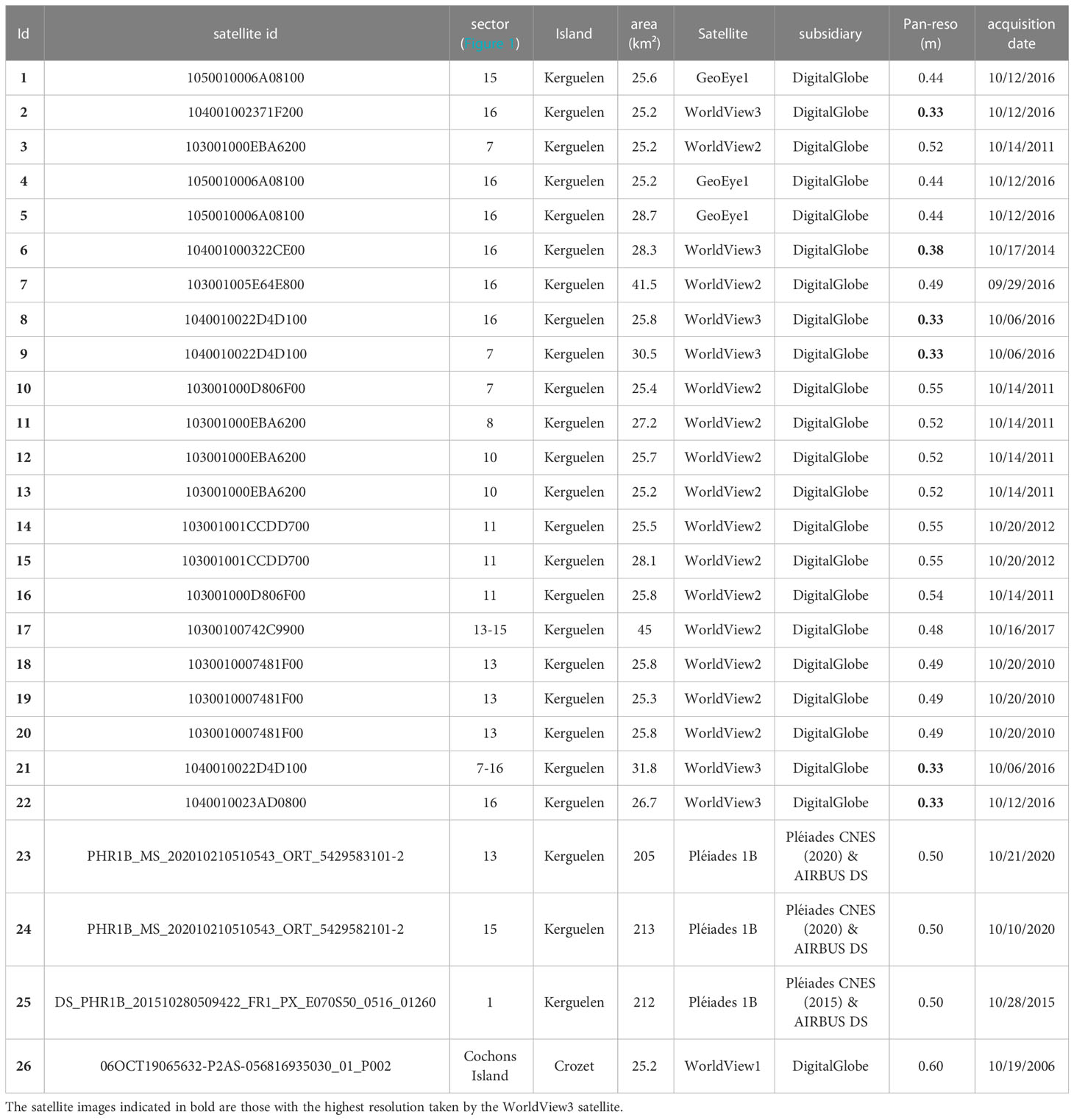
Table 1 Information from the 26 satellite images available for the estimation of the total population size of elephant seals in the Kerguelen and Crozet Archipelagos.
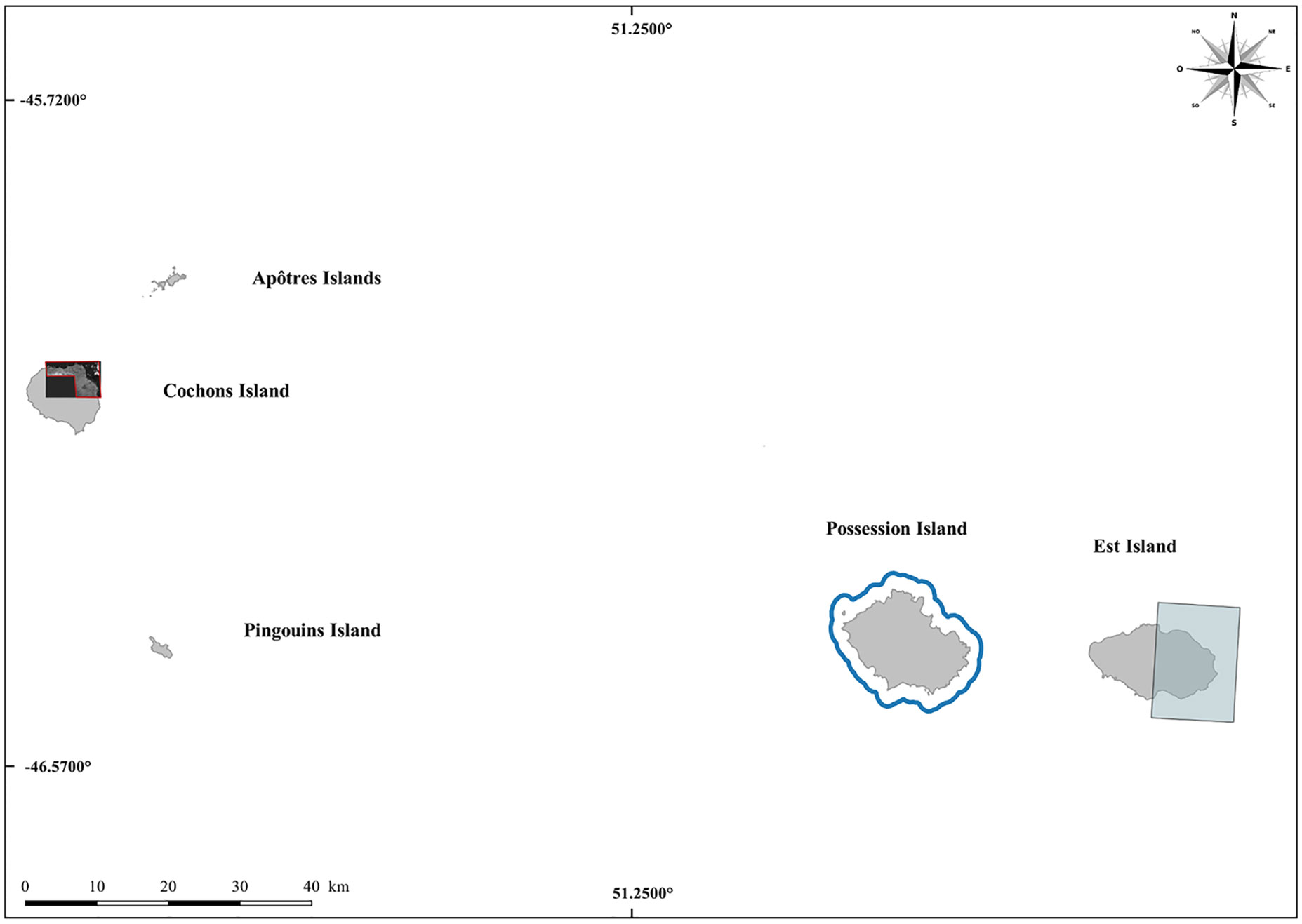
Figure 3 Locations of ground and satellite censuses in the Crozet Archipelago. Beaches annually censused are indicated by a blue line, and satellite images censuses for Cochons Island and Est Island are represented by red and blue polygons respectively.
2.3 Satellite image counts
Counting on satellite images was carried out by visual inspection using GIMP 2.8 software (GIMP 2014) which allows counts to be made manually. Only females were counted during the breeding season. When, contrary to what might be observed during the molt, females tend to maintain some free space around themselves (Figure 2), reducing the probability of merging several females as one individual. Adult males and newborns, distinguishable from females by their size, were not counted, and the probability of confusion with females is low. Males (4 to 6 m) are two times longer and wider than females (2 to 3 m), while newborns and nearly weaned pups are much smaller (0.8 to 1.3 m) and have a black coat instead of the brown color of adults. McMahon et al. (2014) found one-to-one relationships with a R² = 0.9062, between harem counts performed by a naive observer on satellite images and those performed on the ground.
For each satellite image, three successive counts were performed by the same experienced observer at intervals of least 6 months to estimate any census errors and to avoid biases introduced by prior knowledge. The observer’s experience in land-based elephant seal surveys helped to facilitate detection of harems and to limit confusion with the different environmental elements of breeding sites (sexing, other species, rocks and vegetation). The mean of these three corrected counts was taken to represent the number of females per sector during the breeding season.
We prioritized the satellite image search for beaches with known historical sealing records (Savours, 2009) and for those where no ground census was available. Beaches were distinguished according to their physiographic characteristics (i.e. sandy versus boulder beaches, beach lengths, and beaches directly exposed to the open ocean versus beaches at the inner end of fjords). For this purpose, we used Geographic Information System (GIS) software QGIS (QGIS, 2020) with the OpenLayers extensions allowing access to Google Earth and Bing Maps public software for visualization of the Earth from space.
2.4 Ground counts
Males arrive in colonies earlier than females, and fight for control of harems upon their arrival (Jones, 1981). Large body size confers advantages in fighting, and the antagonistic relationships between bulls give rise to a dominance hierarchy, with access to harems and activity within harems being determined by rank (McCann, 1981). The dominant bulls (harem masters) establish harems of several dozen females.
During the reproduction haul-out when pinnipeds spend time on land, females aggregate in dense colonies to give birth to a single pup, weaned 3 weeks later, then mate before returning to sea. While the timing of female return varies from mid-September until late October, each female only stays ashore for about four weeks (van Aarde, 1980) with a maximum number of breeding females present ashore in mid-October (Galimberti and Boitani, 1999; Galimberti et al., 2001; Authier et al., 2011). For this reason, no census can comprehensively encompass all females that may have come ashore.
The method for counting elephant seals on beaches is carried out as part of a standardized long-term monitoring protocol for the breeding populations of these pinnipeds in the French Southern Territories. At Kerguelen Island, such ground counts have been carried out annually since 1986 on the eastern part of the Courbet Peninsula (Figure 1). The method relies on an exhaustive count of all individuals present ashore on dates centered on 15 October (i.e. date of maximum haul-out). The counts are performed while walking along the coast, separately counting all females and males ashore. For the breeding females, ideally three independent counts are conducted by different observers while one count is performed for both harem and peripheral males. If the difference between the 3 counts of females exceeds 10%, new counts are performed for this given area in order to limit detection bias by observers. A GPS point (latitude and longitude) is taken between each census sector’s start and end, generally between each harem, in order to minimize the risks of differences between counts, but also to facilitate the location of abundance areas and the comparison with counts by satellite image. These data are collected and archived at the CEBC-CNRS long-term population monitoring database (program no. 109 of the French Polar Institute Paul-Emile Victor) and the National Natural Reserve of the French Southern Territories.
The number of breeding females present ashore at any time during the breeding season varies according to a normal distribution model (McCann and Rothery, 1988). For both the Crozet and Kerguelen Archipelagos, the average date for the maximum haul-out is 15 October ± 2 days, (Barrat and Mougin, 1978; Pascal, 1981; Guinet et al., 1992), a date identical to that of the Marion and Heard Islands breeding colonies (Condy, 1979; Wilkinson, 1992; Slip and Burton, 1999) with no change over time in this maximum haul-out date (Barrat and Mougin, 1978; Guinet et al., 1992; Slip and Burton, 1999; Authier et al., 2011). Following Rothery and McCann (1987) and Authier et al. (2011), we applied a correction factor to the counts according to the census date. The calculation of this correction factor takes into account: i) measurement error by the various observers; and ii) the date of the counts compared to the peak of female presence on the ground evaluated on 15 October when approximately 90% of breeding females are estimated to be present ashore (Authier et al., 2011).
For each census, an estimation of the total number of females breeding (± confidence interval) was calculated by implementing a Bayesian statistical approach combined with a Generalized Additive Model (GAM) and “Penalized B-Splines” (Eilers and Marx, 2010).
2.5 Comparison between ground and satellite counts
A Pleiades PHR-1B (ID 25; Table 1) satellite image with a 50 cm ground resolution was used to compare the number of breeding females provided by ground counts and those counted on the image. To this end, both counts were corrected according to Authier et al. (2011) to estimate the total number of females giving birth in a specific area. A linear regression model was constructed to compare the number of breeding females for the 18 different beaches encompassed by both the satellite and ground counts. To assess any possible bias in detection by satellite image resulting from the substrate on which the elephant seals reproduce (Laliberte and Ripple, 2003), the habitat was described for each counting area during the ground counts fieldwork (i.e. two categories: sand beach or vegetated area) and we compared the performances of the regression models for both conditions.
2.6 Estimation of the total elephant seal population size
An unknown proportion of elephant seal populations is always at sea, making accurate assessments of total population size is difficult. For this reason, the number of females is often used as an index of population size. Males on land during the breeding season represent a small part of the population and therefore do not provide a reliable indicator, in contrast to females and pups. As pups are more difficult to count during the peak of their presence on land and are smaller than females, to estimate the total population size, the number of breeding females should be multiplied by a correction factor estimated from southern elephant seal life-table analyses (Laws, 1952; Harwood and Prime, 1978; McCann, 1985; McCann and Rothery, 1988). Based on Hindell and Burton (1987) and McCann and Rothery (1988), we chose a correction factor of 3.47.
2.7 Building a predictive model
In order to estimate a total elephant seal population size for the Kerguelen and Crozet Archipelagos and to overcome the absence of censuses for beaches likely to host elephant seal colonies according to their physiographic characteristics, a statistical predictive model was built to estimate the number of breeding females likely to be present on a given beach. VHR satellite images enabled characterization of each beach according to seven explanatory variables: 1) “Z” representing the 17 different geographical sectors of the Kerguelen Archipelago (see Figures 1, 2) “D” in order to make corrections according to the date of the counts of the known beaches used; 3) “SP” corresponding to the beaches’ surface area in m²; 4) “EP” corresponding to the beaches’ orientation (east, north, west, south); 5) “EM” corresponding to the orientation of the open sea area at the entrance of a fjord (east, north, west, south); 6) “DM” corresponding to the distance (m) of the beach from the entrance of a fjord; and 7) “DB50” corresponding to the distance (m) of the beach from the 50 m depth contour (i.e. shelf break, Table 2).
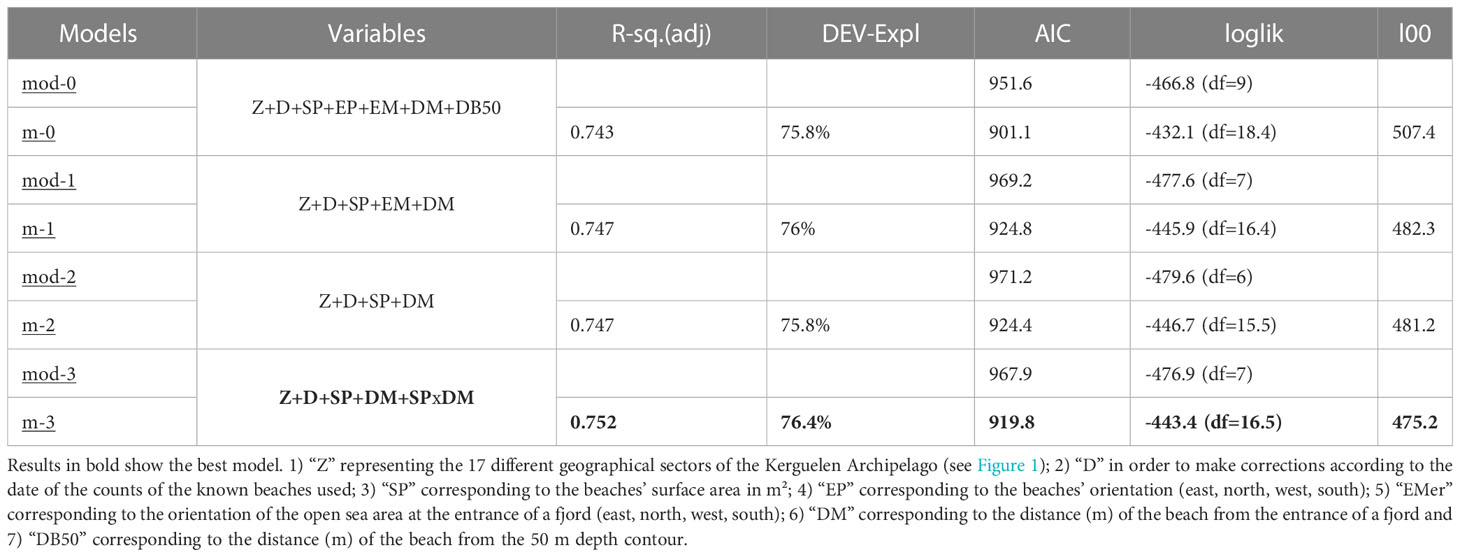
Table 2 Comparison of different linear mixed-effect (MOD) and generalized additive models (M) and selection of the best predictive model of the number of breeding females on the ranges.
We were unable to include the nature of the beach substrate (sand, pebbles, or blocks) from the satellite images and that information was not collected when ground censuses were performed as nearly all Courbet Peninsula beaches are made of sand.
Some variables such as the rough number of females (not corrected by date) or the “surface area” of the beaches were log-transformed, in order to facilitate analysis of the models and comparison of the effects between them.
A data correlation test was carried out in order to eliminate the correlated variables, for which we used the function GGally: ggcorr, with the “pearson” method. The residues were also tested on each model with a Shapiro test. The statistical models used were Mixed Effects Linear Models (lmer) and Generalized Additive Models (GAM). We started with a full model and followed a stepwise procedure of removing non-significant variables with the threshold set at p-value< 0.05 (Zuur et al., 2009). We followed the standard procedure for model validation (Zuur et al., 2009). Best model selection was made by comparing Akaike information criterion (AIC). In addition to the selection by AIC, we verified the predictive capacity of the models by performing a cross-validation with the Leave-one-out (Loo) package, which randomly chose a beach and ignored the number of females counted on this beach to predict the number of females with all the models (Tab. II). The model giving the closest estimate to reality was used to estimate the number of breeding females on the uncensused beaches.
The best model was used to predict the most likely number of breeding females present on the unobserved beaches at the time of the peak presence of breeding females (i.e. 15 October). then this value was corrected according to Authier et al. (2011) to estimate the total number of elephant seal females which went to that beach to breed.
All the processing and analysis of statistical data were realized with R version.3.6.0 (R Core Team, 2019) and required the use of different specific packages such as “lme4,” “mgcv,” “tidyverse,” “mvtnorm,” “rstan,” “coda,” “ggplot2,” “ggthemes”.
2.8 Population trends in the Kerguelen and Crozet Archipelagos
Population trends for the Kerguelen and Crozet Archipelagos were established by a series of calculations performed on yearly censuses. Inter-annual changes in breeding elephant seal females were determined using the formula for mean annual percentage change:
where e = (ln(Nt/N0))/t, and r = intrinsic rate of population change, Nt = population at time t, N0 = original population size, and t = time elapsed between the two population estimates (Caughley, 1977; Slip and Burton, 1999; Bester et al., 2006).
For Kerguelen, the population trend was calculated from 40 annual censuses conducted over a 63-year period [1956: 2019] (on the Courbet Peninsula, no censuses were conducted between 1960 and 1977). Furthermore, incomplete censuses (1952, 1970 and 1984) were excluded, so as not to distort the trajectory of the population trend that was calculated between 1956 and 2018. These censuses were used to assess the Kerguelen elephant seal population on the Eastern and southern part of Courbet peninsula over the 1956-2019 period and shown in Figure 2.
Furthermore, over the 2010-2018 period, the usual Courbet Peninsula census area on Kerguelen Island was extended to breeding colonies located in the northwestern part of the Courbet Peninsula and around the Ronarc’h Peninsula (Figure 2). Beaches that were surveyed for at least four years between 2010 and 2018 provided data to estimate an observed growth rate over the same period. This growth rate was then used and implemented on the latest census available for a given beach to estimate the number of breeding females on that beach for the 2018 reference year, in order to estimate the total breeding female population sizes.
In this way, the Crozet population trend was assessed for nine beaches on Possession Island, using annual censuses since 1980 (Figure 3).
3 Results
3.1 Validation of seal counts from satellite images
Comparison between satellite and grounds counts was conducted over a large sector, south-east of the Courbet Peninsula, shown in Figure 1. Ground counts yielded an estimated total number of 13,190 ± 2,120 breeding females compared to 13,540 ± 6,290 females counted on satellite images, i.e. a 2.6% difference between the two approaches.
The type of substrate on which the harems are located influenced the quality of the relationship (Figures 4A, B). On beaches, the linear regression between satellite and ground counts were highly correlated (R² = 0.98, Figure 4A). On vegetated areas, in particular in the presence of vegetated mounds, the quality of the relationship decreased (R² = 0.71, Figure 4B).
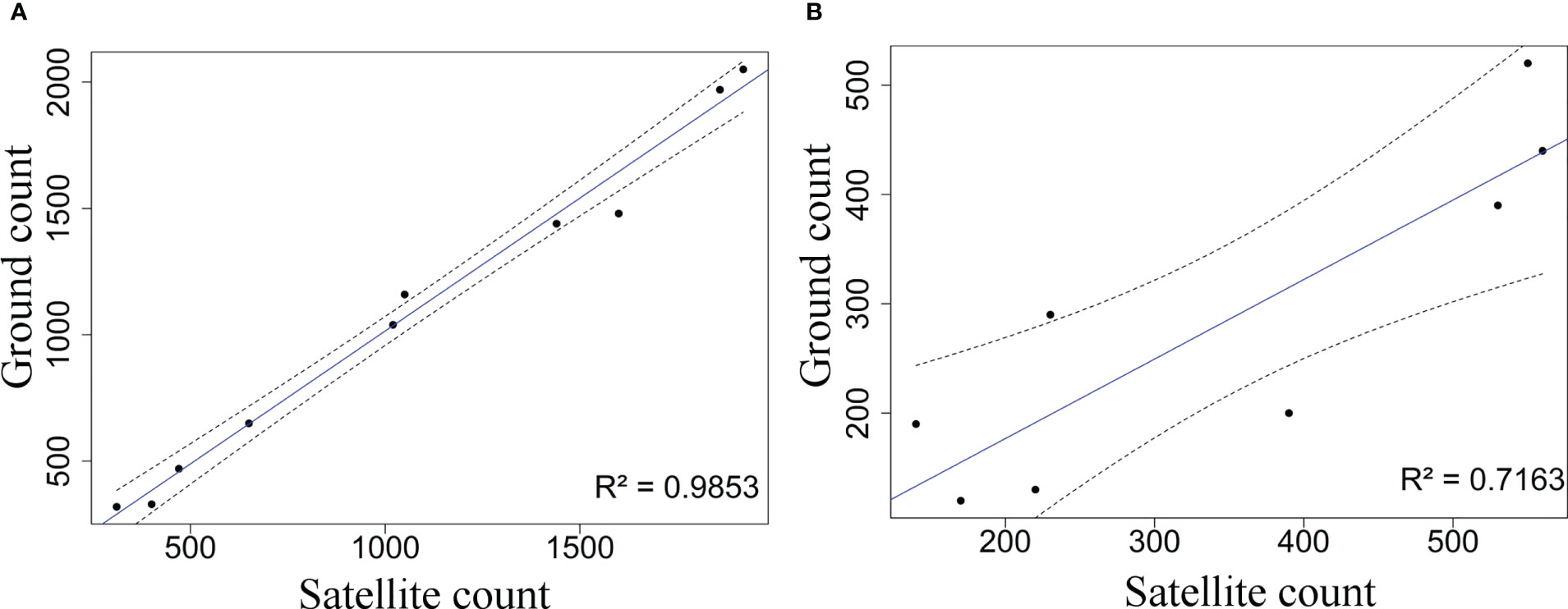
Figure 4 Correlation between counts performed based on very high-resolution satellite images and ground counts over the Baie Novégienne area, Kerguelen in 2015 (see Figure 1) over (A) sandy beaches or vegetated and (B) eroded areas with a presence of vegetation turradon. The black dots represent the relationship between the land-based counts and the satellite counts for each beach. The solid blue line represents the line of best fit and the dashed black lines indicate the 95% confidence limits of that line.
3.2 Estimating total elephant seal population sizes in the Kerguelen and Crozet Archipelagos
3.2.1 Explorations and counts from satellite images
Over the 2010-2016 period, 25 VHR satellite images of the south and southwest part of the Kerguelen Islands, taken between 29 September at the earliest and 20 October at the latest, were acquired over the various sectors of the islands, where historical sealing activities had taken place (Figure 2). These images covered 218 beaches, representing a coastal line of 572 km, in other words, more than 80% of beaches favorable for elephant seal breeding colonies not yet counted. However, due to cloud cover during the elephant seal breeding season, no satellite images were available for 46 beaches (approximately 20% of the favorable beaches). A total of 21,046 ± 2,470 females were counted directly on the satellite images available (Table 3). When applying: i) corrections for the census date versus the maximum haul-out date; and ii) a mean annual growth rate of 1.6% over the 2010-2018 period (corresponding to the growth rate estimated from ground censuses for all areas combined), and taking account of the time elapsed (in years) between each satellite picture and 2018, we estimated a total of 27,600 ± 5,300 females breeding on those beaches in 2018 (Figure 5; Table 3).
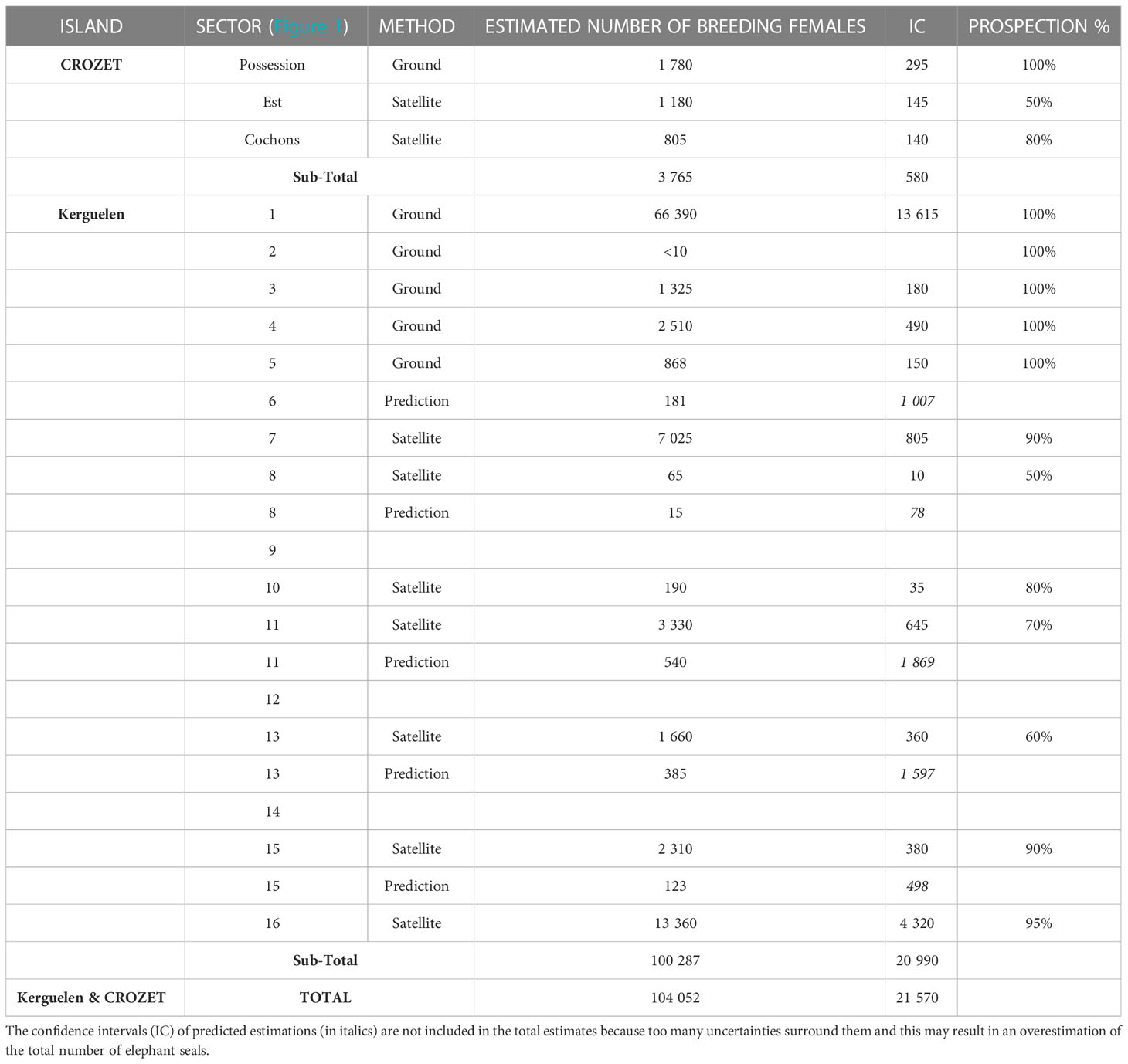
Table 3 The estimated population size (number of breeding females) of Kerguelen and Crozet Archipelagos elephant seals updated for the year 2018.
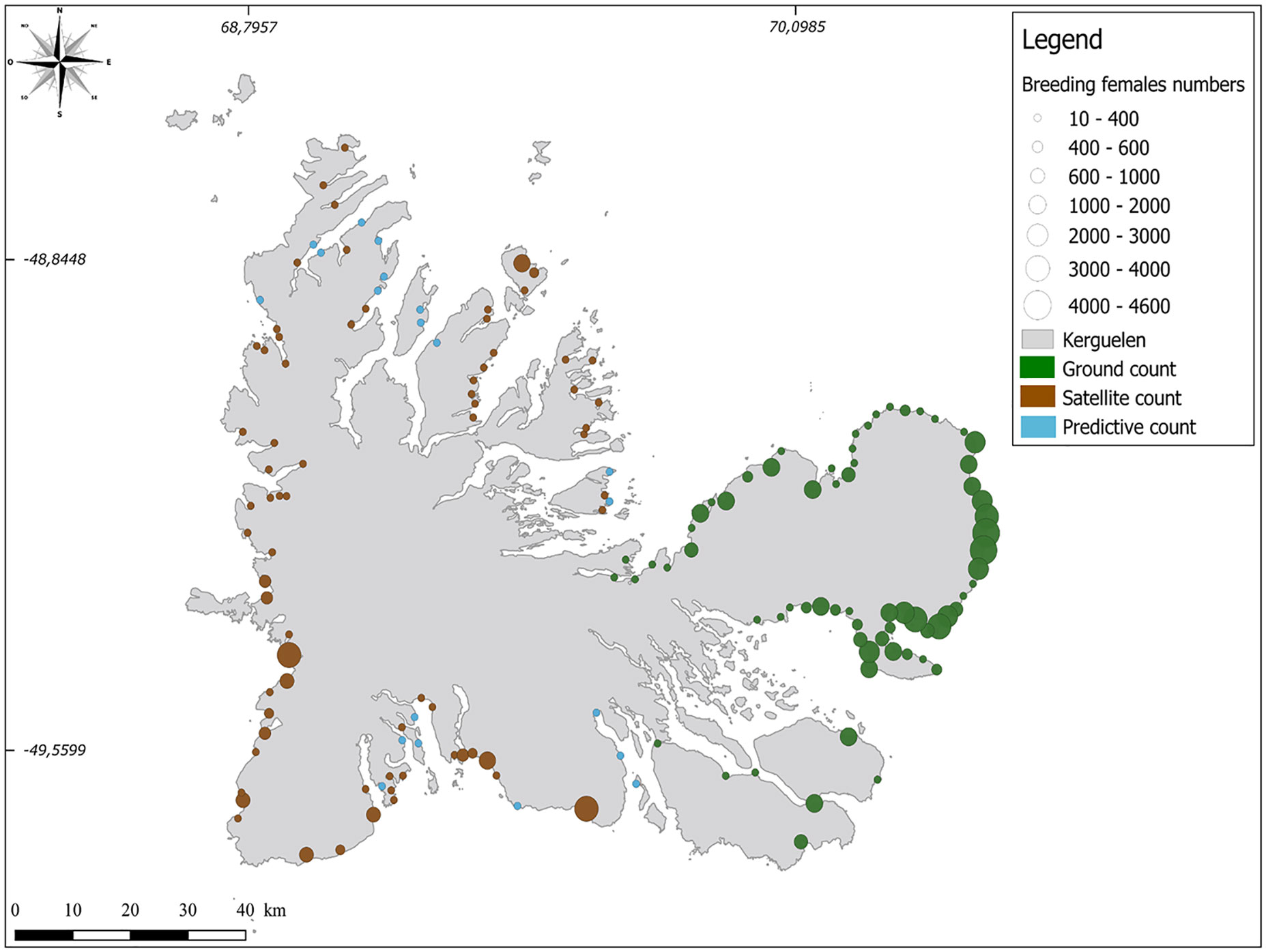
Figure 5 Update of the current distribution of the elephant seal population (breeding females) throughout the Kerguelen Archipelago. The scale is the same for all counting points. The light brown dots indicate the ground counts, the orange dots are the counts carried out by the satellite images, and the blue dots correspond to the estimations from the predictive model.
In the Crozet Archipelago, four beaches were thus counted (two on each island) giving a total number of females of 1,370 ± 160 from rough censuses (Figure 6; Table 3). Application of the mean 5% annual growth rate from Possession Island considering the time elapsed (in years) between the date of the satellite picture and 2018 provided an estimate of 1,986 ± 240 breeding females.
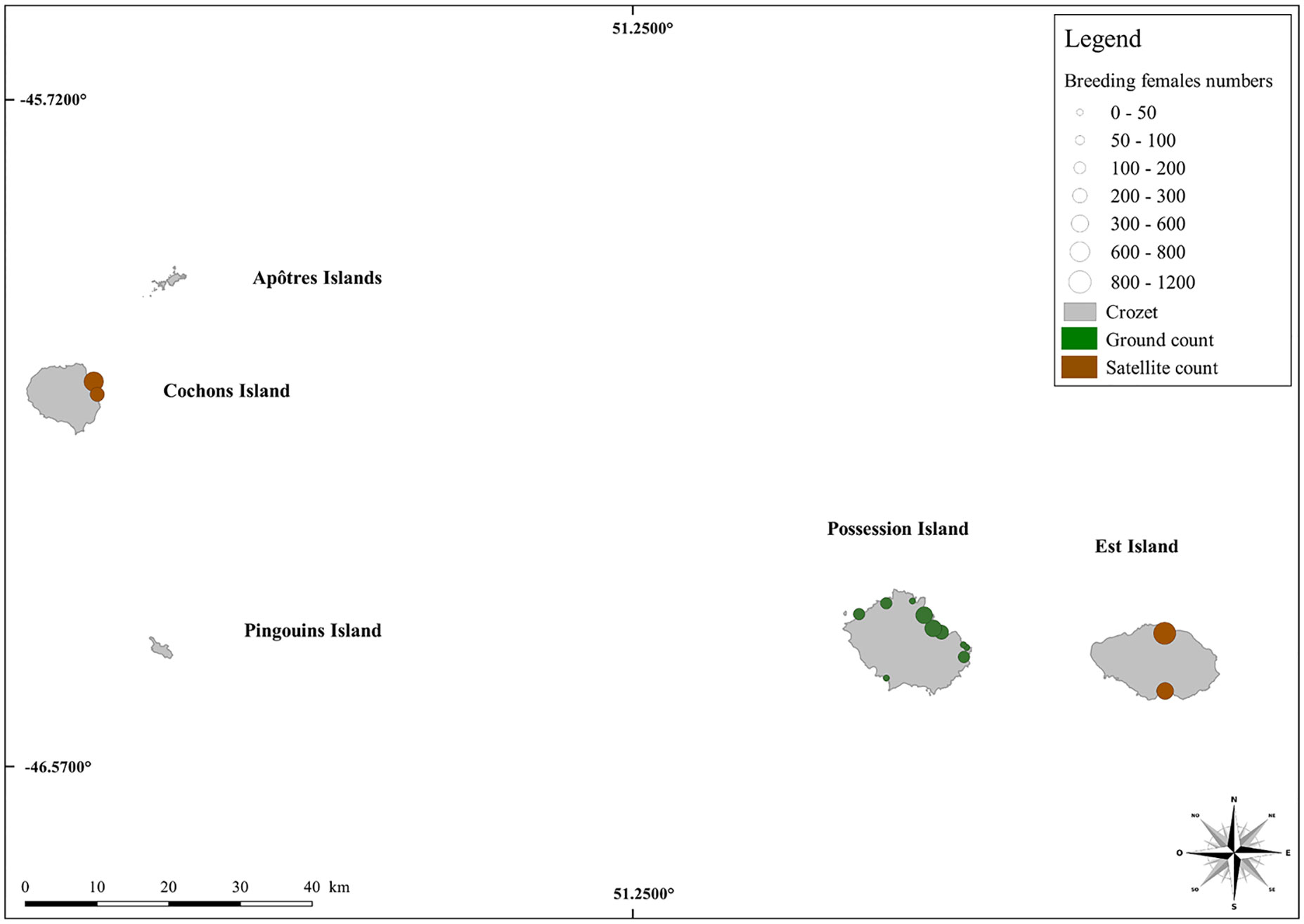
Figure 6 Update of the current distribution of the elephant seal population (breeding females) throughout the Crozet Archipelago. The colour codes and scale of the counting points and images are the same as those in Figure 7.
3.2.2 Predictive models
Eight models were fitted with the different explanatory variables, but due to missing values for the distance (km) of the beach from the 500 m depth contour (Dist_Bathy_50), the first two models (mod0 and M0) could not be compared with the others using AIC. The number of breeding females on the beaches was best explained by four variables: the zone, the beaches’ surface area, and the distance to the sea (best model selected (M3); Table 2) and an interaction between the surface area and the beaches’ distance to the sea. The model also revealed high variability between the different zones amongst the selected beaches, with the uncertainty of prediction much higher for new areas than for those used by the model. The selected model estimated approximately 1,250 breeding females [6: 14,000] on the remaining 46 beaches of the Kerguelen Archipelago for which no ground or satellite image censuses are available.
3.2.3 Estimation of the total population size
When adding together ground censuses, satellite censuses and predictive model estimates, the total number of breeding females for the whole of the Kerguelen Archipelago in 2018 is about 100,300 ± 20,840 (Table 3).
For the Crozet Archipelago in 2018, ground and satellite censuses total 3,765 ± 690 breeding females, which is likely to be an underestimation as some beaches are not yet censused (Table 3).
According to these new counts, the total southern elephant seal population sizes in 2018 for the Kerguelen and Crozet Archipelagos respectively are estimated to be about 347,995 (s e = 4,950) and 13,064 (s e = 169) individuals.
3.3 Population trends for long-term monitoring areas
At Kerguelen, censuses conducted annually between Cap Digby and Pointe Molloy on the Courbet Peninsula (Figures 1, 2) reveal that the number of breeding females increased by about 13,000 females (Figure 7) from the minimum estimate of 37,000 breeding females in 1987 (Guinet et al., 1992) to a maximum of 50,020 ± 11,050 (95% confidence index) females in 2018. All ground counts (long-term monitoring and additional counts) led to a total estimated number of approximately 66,400 ± 14,000 breeding females in 2018 (Table 3).
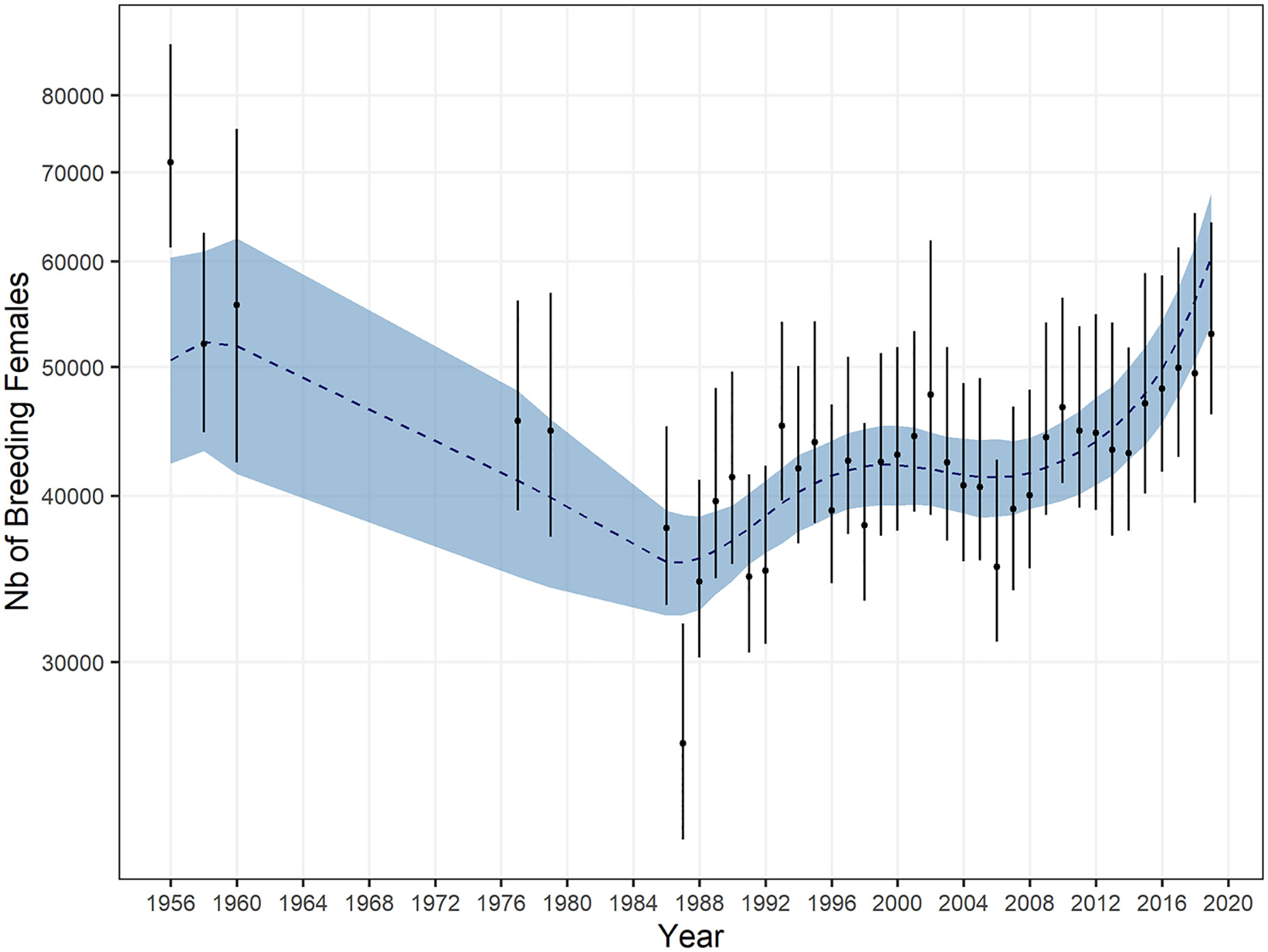
Figure 7 Population trend of female southern elephant seals breeding on the Courbet Peninsula, Kerguelen since the 1950s. Posterior medians of breeding female counts, corrected for census dates, along with their 95% CI, are depicted by the black dots and bars. The blue envelope and the dotted blue line represent a 95% CI and the posterior median value of the trend, respectively.
Ground count areas were extended at Kerguelen Island with 37 new beaches over 400 km of coastline, located north-west and north of the Courbet Peninsula, Prince de Galles Peninsula, Ronarc’h Peninsula, and Jeanne d’Arc Peninsula, censused punctually over the 2010-2018 period for multiple years (Figure 2). For those additional beaches with multiple census years over that period, an overall growth rate of 2.7%.yr-1 was found, leading to an estimation of 16,500 ± 4,000 breeding females in 2018 for these 37 beaches. Meanwhile, over the 2010-2018 period, colonies located between Cap Digby and Pointe Molloy on the Courbet Peninsula (Figures 1, 2) grew at an annual growth rate of 0.9%.
At Crozet, on Possession Island, the minimum number of breeding females was observed over the 1990-1994 period with about 520 individuals coming ashore. In 2018, a total of 1,780 ± 450 females came to breed (Figure 8; Table 3), which represents a 5.1% annual growth rate over the 1994-2018 period.
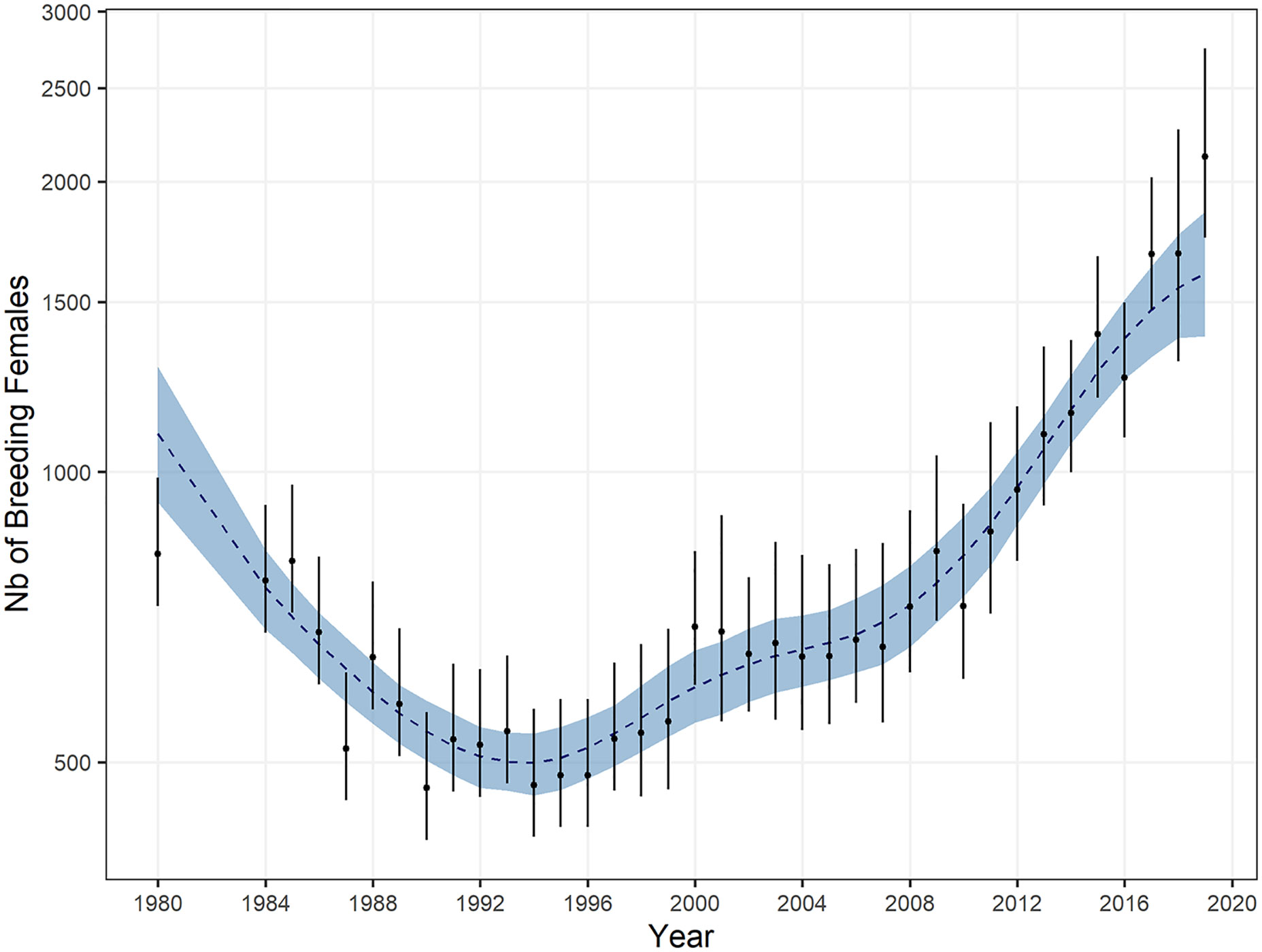
Figure 8 Population trend of female southern elephant seals breeding on Possession Island, Crozet since 1980. Posterior medians of breeding female count, corrected for census dates, along with their 95% CI are depicted by the black dots and bars. The blue envelope and the dotted blue line represent a 95% CI and the posterior median value of the trend, respectively.
4 Discussion
Here, we sought to extend the census method from space to all areas that are inaccessible on foot for ground counts and not previously counted on both the Kerguelen and Crozet Archipelagos. We provide the first estimates of the total breeding population of southern elephant seals at both these locations, which highlights the potential to conduct long-term population size monitoring. This study, thanks to satellite imagery, shows the importance of these two French Archipelagos at a global level for the southern elephant seal population. At both locations, the elephant seal populations have been increasing over the last two decades, particularly on the Crozet Archipelago.
4.1 Use of very high-resolution satellite images and estimate of total number of breeding elephant seal females
Satellite images have been successfully used to census wildlife(Fretwell et al., 2014; Fretwell et al., 2017; Weimerskirch et al., 2018; Cubaynes et al., 2019; Gonçalves et al., 2020; LaRue et al., 2020) Some more recent studies have also shown the ability of satellites to survey animals at more global scales (Lynch and LaRue, 2014; LaRue et al., 2021). As this field is still developing, most studies on southern elephant seal populations have been small-scale, proof-of-concept investigations. McMahon et al. (2014) pioneered in showing that VHR satellite images could be used to accurately census elephant seals, indicating that good precision counts could be conducted despite the low color contrast between beaches and elephant seals. Similarly to McMahon et al. (2014), we found no difference in average between satellite and ground counts for the total number of seals on the beaches (but associated errors are different), demonstrating that remotely sensed images can be used reliably to census elephant seals on remote sub-Antarctic islands. However, Dickens et al. (2021) showed variation between ground and unmanned aerial vehicles (UAV) counts, aerial counts consistently recording higher numbers of animals, particularly on beaches with higher densities of animals, and confirms that the aerial method (satellite and UAV) provides reliable or better results for population monitoring. A recent study by LaRue et al. (2021) showed that Weddell seals (Leptonychotes weddellii) could also be accurately counted from space on a global population study area.
The present study yields similar results from ground and satellite image censuses, and allows distinguishing between females, males and pups. However, the substrate on which elephant seals were found tended to be a limiting factor in the method using panchromatic resolution, and detection appeared to be more difficult in vegetated areas, in contrast to ice, as shown in LaRue’s study in 2011, and sand in Hindell’s study in 2014. Furthermore, due to the lack of successive satellite images, difference in resolution (30 vs 50cm/px) and/or consecutive ground counts for a given beach, we could not assess how the census error varied depending on the resolution of images and/or when departing from the optimal census date (i.e. 15 October).
This study joins others (Fretwell et al., 2017; LaRue et al., 2019) that demonstrate the huge potential of VHR satellite images (i.e. up to 0.30 m), now readily available, to census and monitor large seal and seabird species breeding in very isolated and inaccessible sectors of the planet. Such an approach may be even more competitive and efficient than the use of new aerial images by kite aerial photography (Delord et al., 2015) or by UAVs (Hodgson et al., 2018; Fudala and Bialik, 2020; Dickens et al., 2021; Edney and Wood, 2021), which often remain confined to restricted areas. The main constraint of satellite image censuses (particularly acute for subantarctic islands) lies in cloud cover, which limits visibility and hence prevents counts. We suggest experimenting with new accessible technologies imageries such as ICEYE satellite, based on X-band SAR sensors suitable for all-weather day and night Earth Observations with a resolution of 0.25 m. Although counts with standard resolution images (0.50 m) are relatively accurate, we recommend using the highest resolution (0.30 m) whenever possible, as the latter makes counts much easier to perform and can limit detectability errors on vegetated areas.
4.2 Total elephant seal population size estimates for the Crozet and Kerguelen Archipelagos
By combining ground counts with censuses on satellite images, and predictions for beaches for which no data were available, this study allowed us to provide the first estimate of the total elephant seal population for the Kerguelen Archipelago.
Prior to this study, the Kerguelen Archipelago elephant seal population was considered the second largest in the world (~45,000 breeding females), after South Georgia Island (~110,000 breeding females). Our study shows that the Kerguelen stock share of the total elephant seal population has been underestimated. In fact Kerguelen hosts about 100,000 breeding females, which is more or less equivalent to the South Georgia figure (Boyd et al., 1996). Based on the updated counts from this study, the Kerguelen and South Georgia populations (i.e. totaling about 740,000 individuals) could represent as much as 80% of the global southern elephant seal population (Hindell et al., 2016).
With a minimum estimate of about 4,000 breeding females, the Crozet Archipelago remains a relatively small population (Figure 6). Supplementary census efforts should be deployed to fill the gap on other beaches where additional seal colonies are likely to be present but where no satellite images are available to date due to cloud coverage. The use of a predictive model is however impossible on Crozet, as the beach characterization data are insufficient to be implemented.
These new total population size estimates have significant consequences for estimates of the biomass of marine organisms consumed by these predators, and their role in ecosystem models. In this way, the biomass consumed by these predators is very significantly higher than previously estimated for the area (Guinet et al., 1996), underlining the considerable ecological role that southern elephant seals may play in the marine ecosystem within the Indian sector of the Southern Ocean. The contribution of southern elephant seal predation to the ecosystem, using ecosystem models such as Ecopath (Christensen and Pauly, 1992; Whipple et al., 2000; Coll et al., 2015), will have to be revised in order to best sustainably manage the biological resources within those ecosystems as part of the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) area.
4.3 Reassessment of Crozet and Kerguelen population trends over the last four decades
Another major result of this study is to highlight the changes in southern elephant seal population trends for both the Crozet and Kerguelen Archipelagos. Population trends provided by the current study complement those previously performed for the Crozet and Kerguelen Islands (Barrat and Mougin, 1978; Pascal, 1981; Guinet et al., 1992; Guinet et al., 1999; Authier et al., 2011) but also the Marion and Heard Islands (van den Hoff et al., 2007; McMahon et al., 2009; Pistorius et al., 2011). These studies show that elephant seal foraging and breeding throughout the Indian sector of the Southern Ocean underwent a significant decline starting in the 1950s before stabilizing in the late 1980s-1990s and subsequently increasing. This common general pattern in the area’s population trends suggests similar processes driving those demographic changes for at least the Marion, Crozet and Kerguelen Islands. However, large differences are observed between the Crozet and Kerguelen populations, both in terms of the early rate of decrease and the current rate of increase. The annual rate of decrease as well as the overall decrease in percentage of the Crozet elephant seal population size were steeper in the Crozet Archipelago (i.e. ~80% of the population size was lost at Possession Island; Guinet et al., 1992; Guinet et al., 1999) compared to the Kerguelen Archipelago (i.e. 44%; Guinet et al., 1992). Population stabilized in the late 1980s on Kerguelen and about ten years later on Crozet. Since then, the Crozet population has been growing at a much faster rate (+5.1%.yr-1) compared to the Kerguelen one (+1.6%.yr-1, all ground censuses combined).
This general pattern, which is also shared by Marion Island’s population (Oosthuizen, pers. comm), is most likely to be driven by a global change in productivity within the Indian sector of the Southern Ocean (McMahon et al., 2009). However, the differences in the extent of decline and growth rates between Crozet and Kerguelen suggest that local factors are also at work.
Elephant seal weaning mass, a strong predictor of juvenile survival (McMahon et al., 2000; Mcmahon et al., 2003), was found to be positively related to chlorophyll-a concentration within the main foraging region of adult female seals (Authier et al., 2012; Oosthuizen et al., 2015). This suggests that despite the indirectness of the link between chlorophyll-a and elephant seal prey, phytoplankton biomass is a reasonable predictor of the foraging performances of elephant seals. Interestingly, chlorophyll-a concentration has been found to be increasing significantly over the whole Southern Ocean and in particular within the Indian sector (Castillo et al., 2019; Pinkerton et al., 2021), suggesting that primary production, and elephant prey availability, have been rising over the last two to three decades.
Local factors are also likely to drive the local differences in growth rates between the Crozet and Kerguelen pups. For instance, Mestre et al. (2020) showed that over the 2004-2018 period, post-molting females improved their body conditions more rapidly when foraging west of Kerguelen compared to the east, suggesting that foraging conditions varied according to longitude, and were more favorable over the Crozet and Marion sector than east of Kerguelen. Kerguelen females foraging westward overlap with the Marion Island females (Oosthuizen et al., 2015; Mestre et al., 2020), but unfortunately no tracking data are available so far for the Crozet individuals.
Currently, it is unknown if different population trends are observed within the Kerguelen Archipelago depending on the location of the breeding colonies. However, significant differences in the foraging locations of females have been found between colonies, with females breeding northwest of the Courbet Peninsula foraging, to a very large extent, west of Kerguelen, while females breeding east tend to forage mainly eastward (Mestre et al., 2020). The foraging locations of females breeding south and west of Kerguelen remain unknown. However, we can expect that at-sea distribution differences according to the location of the breeding colonies translate into local differences in population growth rate. This is suggested by the higher 2.7% annual growth rate observed for colonies located northwest of the Courbet, Ronarc’h and Jeanne d’Arc Peninsula compared to the 0.9% annual growth rate observed for colonies located east of Courbet Peninsula over the 2010-2018 period.
Changes in predation pressure, might also contribute to the differences in the rate of decrease and growth between Crozet and Kerguelen. Elephant seal population demographics could also be sensitive to killer whale predation (Reisinger et al., 2011). Killer whale (Orcinus orca) predation has been hypothesized to be the main factor driving the faster rate of decline of the Crozet compared to the Kerguelen population over the 1970-1980 period (Guinet et al., 1992). Killer whales preyed on as much as 20% of pups born on one beach and that figure was likely to be an underestimation (Guinet et al., 1992). However, due to the incidental mortality of Crozet killer whales interacting with illegal Patagonian toothfish (Dissostichus eleginoides) fisheries, the killer whale population was reduced by half from an estimated population size of 180 to 90 individuals over the 1996-2003 period (Poncelet et al., 2010; Guinet et al., 2015; Tixier et al., 2017). Therefore, the predation pressure exercised by killer whales on southern elephant seals was likely relaxed, and could have contributed to a higher population growth rate for the Crozet Archipelago colonies compared to the Kerguelen ones. It follows that the high Crozet elephant seal population growth rate could be explained by the combination of improved foraging conditions and the easing of predation pressure exercised by the Crozet killer whale population, with a more relaxed density-dependence effect (i.e. better foraging conditions) compare to Kerguelen Island. We cannot however exclude that the emigration of seals from Kerguelen to Crozet could also contribute to this high growth rate, as elephant seals movements have been observed between Kerguelen, Crozet and Marion (Oosthuizen et al., 2011).
The growth rate exhibited by the Crozet population is among the highest observed for any southern elephant seal population in the world, with the exception of Valdés Peninsula in Patagonia (Ferrari et al., 2013). At the same time, the change in the Kerguelen elephant seal population, which does not appear to be significantly affected by killer whale predation (Guinet et al., 1999), suggests that trophic conditions are improving for elephant seals over the whole Indian sector of the Southern Ocean.
Conclusion
Our study shows the reliability of using VHR satellite images to count pinniped populations in a remote and inaccessible areas of the planet, at reasonable costs if these operations are repeated a few times every decade. Following the validation of this counting method, we found that the previous estimates of elephant seal populations breeding in the French Southern Territories were largely underestimated due to a deficit of counts in hard-to-access areas. This study confirms the major status of these populations, as they emerge as representing around 35% of the world southern elephant seal population.
In addition to conventional methods, VHR satellite images would be useful to define the locations of new breeding colonies of relatively large southern elephant seal aggregations similar to what has been done for emperor penguins (Fretwell et al., 2012). Such work is key to improving assessments of the impact of top marine predators on marine food webs and to understanding how these fauna are responding to the ongoing changes of the Southern Ocean environmental conditions (Weimerskirch et al., 2018).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the animal study because the satellite studies did not qualify for ethical review, given that the research did not involve the direct handling of animals. The project was approved by the head office of the French Southern and Antarctic Lands.
Author contributions
JL, CG and AC designed the study protocol and collected the data. JL and MA performed the analyses. JL and CG wrote the manuscript. AC, KD and HW significantly contributed to and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
TAAF Natural Reserve (Satellite images, Funding of the Master II of JL) - Zone Atelier Antarctique (Satellite images) - CNES, (Satellite images).
Acknowledgments
We are grateful to B. Delesalle for enabling this study to be part of my Masters Internship project with PSL University, Ecole Pratique des Hautes Etudes (EPHE). We thank all the volunteers from the Crozet and Kerguelen missions for their data collection since the beginning of the program, and especially those who assisted us in the fieldwork at Kerguelen in 2015 and 2017: M.A. Colleu, M. Delpuech, B. Gineste, C. Brunet, C. Lin, E. Debenest, A. Fromant, S. Peroteau, P. Parentoine, A. Tavernier, B. Ginolin and A. Pimor for logistical and fieldwork assistance. We also warmly thank D. Fourcy and the CNES for acquiring Pleiades PHR-1B satellite images of the west of Kerguelen with the Zone Atelier Antarctique et Subantarctique (ZATA; CNRS-INEE) funding. Logistical support was provided by the French Polar Institute (IPEV, research program no. 109, PI: H. Weimerskirch and C. Barbraud, and no. 1201, PI: C. Gilbert and C. Guinet) and the French Southern and Antarctic Territories (TAAF). Fieldwork and data collection were undertaken with approval from the IPEV and TAAF Environmental committees and legislations. We would also like to thank E. Gaget, F. Orgeret and K. Heerah for their assistance and advice with statistical analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Authier M., Delord K., Guinet C. (2011). Population trends of female elephant seals breeding on the courbet peninsula, îles kerguelen. Polar Biol. 34, 319–328. doi: 10.1007/s00300-010-0881-1
Authier M., Dragon A.-C., Richard P., Cherel Y., Guinet C. (2012). O’ mother where wert thou? maternal strategies in the southern elephant seal: a stable isotope investigation. Proc. R. Soc B Biol. Sci. 279, 2681–2690. doi: 10.1098/rspb.2012.0199
Barrat A., Mougin J. L. (1978). L’Eléphant de mer mirounga leonina de l’île de la possession, archipel crozet (46°25’ s, 51°45’ e). Mammalia 42, 143–174. doi: 10.1515/mamm.1978.42.2.143
Bester M., Wilson J., Burle M.-H., Hofmeyr G. (2006). Population trends of subantarctic fur seals at Gough island. South Afr. J. Wildl. Res. 36, 191–194. Available at: https://hdl.handle.net/10520/EJC117239.
Borowicz A., Le H., Humphries G., Nehls G., Höschle C., Kosarev V., et al. (2019). Aerial-trained deep learning networks for surveying cetaceans from satellite imagery. PloS One 14, e0212532. doi: 10.1371/journal.pone.0212532
Boyd I. L., Walker T. R., Poncet J. (1996). Status of southern elephant seals at south Georgia. Antarct. Sci. 8, 237–244. doi: 10.1017/S0954102096000338
Campagna C., Lewis M. (1992). Growth and distribution of a southern elephant seal colony. Mar. Mammal Sci. 8, 387–396. doi: 10.1111/j.1748-7692.1992.tb00053.x
Castillo C. E. D., Signorini S. R., Karaköylü E. M., Rivero-Calle S. (2019). Is the southern ocean getting greener? Geophys. Res. Lett. 46, 6034–6040. doi: 10.1029/2019GL083163
Caughley G. (1977). Analysis of vertebrate populations. London: John Wiley and Sons Anal. Vertebr. Popul. 234 pp.
Cherel Y., Ducatez S., Fontaine C., Richard P., Guinet C. (2008). Stable isotopes reveal the trophic position and mesopelagic fish diet of female southern elephant seals breeding on the kerguelen islands. Mar. Ecol. Prog. Ser. 370, 239–247. doi: 10.3354/meps07673
Christensen V., Pauly D. (1992). ECOPATH II — a software for balancing steady-state ecosystem models and calculating network characteristics. Ecol. Model. 61, 169–185. doi: 10.1016/0304-3800(92)90016-8
Coll M., Akoglu E., Arreguín-Sánchez F., Fulton E. A., Gascuel D., Heymans J. J., et al. (2015). Modelling dynamic ecosystems: venturing beyond boundaries with the ecopath approach. Rev. Fish Biol. Fish. 25, 413–424. doi: 10.1007/s11160-015-9386-x
Condy P. R. (1979). Annual cycle of the southern elephant sealMirounga leonina (Linn.) at Marion island. Afr. Zool. 14, 95–102. doi: 10.1080/02541858.1979.11447655
Cubaynes H. C., Fretwell P. T., Bamford C., Gerrish L., Jackson J. A. (2019). Whales from space: four mysticete species described using new VHR satellite imagery. Mar. Mammal Sci. 35, 466–491. doi: 10.1111/mms.12544
Delord K., Roudaut G., Guinet C., Barbraud C., Bertrand S., Weimerskirch H. (2015). Kite aerial photography: a low-cost method for monitoring seabird colonies. J. Field Ornithol. 86, 173–179. doi: 10.1111/jofo.12100
Despin B, Mougin JL, Segonzac M (1972). Les oiseaux et les mammifères de l’ile de l’Est. Comité National Français des Recherche Antarctiques. 31, 106.
Dickens J., Hollyman P. R., Hart T., Clucas G. V., Murphy E. J., Poncet S., et al. (2021). Developing UAV monitoring of south Georgia and the south sandwich islands’ iconic land-based marine predators. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.654215
Edney A. J., Wood M. J. (2021). Applications of digital imaging and analysis in seabird monitoring and research. Ibis 163, 317–337. doi: 10.1111/ibi.12871
Eilers P. H. C., Marx B. D. (2010). Splines, knots, and penalties. WIREs Comput. Stat. 2, 637–653. doi: 10.1002/wics.125
Ferrari M. A., Campagna C., Condit R., Lewis M. N. (2013). The founding of a southern elephant seal colony. Mar. Mammal Sci. 29, 407–423. doi: 10.1111/j.1748-7692.2012.00585.x
Fretwell P. T., LaRue M. A., Morin P., Kooyman G. L., Wienecke B., Ratcliffe N., et al. (2012). An emperor penguin population estimate: the first global, synoptic survey of a species from space. PloS One 7, e33751. doi: 10.1371/journal.pone.0033751
Fretwell P. T., Scofield P., Phillips R. A. (2017). Using super-high resolution satellite imagery to census threatened albatrosses. Ibis 159 (3), 481–490. doi: 10.1111/ibi.12482
Fretwell P. T., Staniland I. J., Forcada J. (2014). Whales from space: counting southern right whales by satellite. PloS One 9, e88655. doi: 10.1371/journal.pone.0088655
Fudala K., Bialik R. J. (2020). Breeding colony dynamics of southern elephant seals at patelnia point, king George island, Antarctica. Remote Sens. 12, 2964. doi: 10.3390/rs12182964
Galimberti F., Boitani L. (1999). Demography and breeding biology of a small, localized population of southern elephant seals (mirounga leonina). Mar. Mammal Sci. 15, 159–178. doi: 10.1111/j.1748-7692.1999.tb00787.x
Galimberti F., Sanvito S., Boitani L., Fabiani A. (2001). Viability of the southern elephant seal population of the Falkland islands. Anim. Conserv. 4, 81–88. doi: 10.1017/S1367943001001093
Gonçalves B. C., Spitzbart B., Lynch H. J. (2020). SealNet: a fully-automated pack-ice seal detection pipeline for sub-meter satellite imagery. Remote Sens. Environ. 239, 111617. doi: 10.1016/j.rse.2019.111617
Guinet C., Cherel Y., Ridoux V., Jouventin P. (1996). Consumption of marine resources by seabirds and seals in crozet and kerguelen waters: changes in relation to consumer biomass 1962–85. Antarct. Sci. 8, 23–30. doi: 10.1017/S0954102096000053
Guinet C., Jouventin P., Weimerskirch H. (1992). Population changes, movements of southern elephant seals on crozet and kerguelen archipelagos in the last decades. Polar Biol. 12, 349–356. doi: 10.1007/BF00243106
Guinet C., Jouventin P., Weimerskirch H. (1999). Recent population change of the southern elephant seal at Îles crozet and Îles kerguelen: the end of the decrease? Antarct. Sci. 11, 193–197. doi: 10.1017/S0954102099000255
Guinet C., Tixier P., Gasco N., Duhamel G. (2015). Long-term studies of crozet island killer whales are fundamental to understanding the economic and demographic consequences of their depredation behaviour on the Patagonian toothfish fishery. ICES J. Mar. Sci. 72, 1587–1597. doi: 10.1093/icesjms/fsu221
Guirado E., Tabik S., Rivas M. L., Alcaraz-Segura D., Herrera F. (2019). Whale counting in satellite and aerial images with deep learning. Sci. Rep. 9, 14259. doi: 10.1038/s41598-019-50795-9
Harwood J., Prime J. H. (1978). Some factors affecting the size of British grey seal populations. J. Appl. Ecol. 15, 401–411. doi: 10.2307/2402600
Hindell M. A., Burton H. R. (1987). Past and present status of the southern elephant seal (Mirounga leonina) at macquarie island. J. Zool. 213, 365–380. doi: 10.1111/j.1469-7998.1987.tb03712.x
Hindell M. A., McMahon C. R., Bester M. N., Boehme L., Costa D., Fedak M. A., et al. (2016). Circumpolar habitat use in the southern elephant seal: implications for foraging success and population trajectories. Ecosphere 7, e01213. doi: 10.1002/ecs2.1213
Hindell M. A., Sumner M., Bestley S., Wotherspoon S., Harcourt R. G., Lea M.-A., et al. (2017). Decadal changes in habitat characteristics influence population trajectories of southern elephant seals. Glob. Change Biol. 23, 5136–5150. doi: 10.1111/gcb.13776
Hodgson J. C., Mott R., Baylis S. M., Pham T. T., Wotherspoon S., Kilpatrick A. D., et al. (2018). Drones count wildlife more accurately and precisely than humans. Methods Ecol. Evol. 9, 1160–1167. doi: 10.1111/2041-210X.12974
Johnson C. N., Balmford A., Brook B. W., Buettel J. C., Galetti M., Guangchun L., et al. (2017). Biodiversity losses and conservation responses in the anthropocene. Science 356, 270–275. doi: 10.1126/science.aam9317
Jones E. (1981). Age in relation to breeding to status of the Male southern elephant seal, mirounga leonina (L.), at macquarie island. Wildl. Res. 8, 327–334. doi: 10.1071/wr9810327
Jouventin P., Stahl J. C., Weimerskirch H. (1982). La recolonisation des îles crozet par les otaries (Arctocephalus tropicalis et a. gazella). Mammalia 46, 505–514. doi: 10.1515/mamm.1982.46.4.505
Laliberte A. S., Ripple W. J. (2003). Automated wildlife counts from remotely sensed imagery. Wildl. Soc Bull. 31 (2), 362–371.
LaRue M. A., Ainley D. G., Pennycook J., Stamatiou K., Salas L., Nur N., et al. (2020). Engaging ‘the crowd’ in remote sensing to learn about habitat affinity of the weddell seal in Antarctica. Remote Sens. Ecol. Conserv. 6, 70–78. doi: 10.1002/rse2.124
LaRue M. A., Rotella J. J., Garrott R. A., Siniff D. B., Ainley D. G., Stauffer G. E., et al. (2011). Satellite imagery can be used to detect variation in abundance of weddell seals (Leptonychotes weddellii) in erebus bay, Antarctica. Polar Biol. 34, 1727. doi: 10.1007/s00300-011-1023-0
LaRue M. A., Salas L., Nur N., Ainley D. G., Stammerjohn S., Barrington L., et al. (2019). Physical and ecological factors explain the distribution of Ross Sea weddell seals during the breeding season. Mar. Ecol. Prog. Ser. 612, 193–208. doi: 10.3354/meps12877
LaRue M., Salas L., Nur N., Ainley D., Stammerjohn S., Pennycook J., et al. (2021). Insights from the first global population estimate of weddell seals in Antarctica. Sci. Adv. 7, eabh3674. doi: 10.1126/sciadv.abh3674
Laws R. M. (1952). A new method of age determination for mammals. Nature 169, 972–973. doi: 10.1038/169972b0
Lewis M., Campagna C., Quintana F., Falabella V. (1998). Estado actual y distribución de la población del elefante marino del sur en la Península Valdés, Argentina. Mastozoología Neotropical 5 (1), 29–40.
Lynch H. J., LaRue M. A. (2014). First global census of the adélie penguin. Auk 131, 457–466. doi: 10.1642/AUK-14-31.1
McCann T. S. (1981). Aggression and sexual activity of male southern elephant seals, mirounga leonina. J. Zool. 195, 295–310. doi: 10.1111/j.1469-7998.1981.tb03467.x
McCann T. S. (1985). “Size, status and demography of southern elephant seal (Mirounga leonina) populations,” in Studies of Sea mammals in south latitudes. proceedings of a symposium of the 52nd. ANZAAS congress in Sydney, may 1982. Eds. Ling J. K., Bryden M. M. (Adelaide: South Australian Museum), 1–17.
McCann T. S., Rothery P. (1988). Population size and status of the southern elephant seal (Mirounga leonina) at south georgia 1951–1985. Polar Biol. 8, 305–309. doi: 10.1007/BF00263179
McMahon C. R., Bester M. N., Burton H. R., Hindell M. A., Bradshaw C. J. A. (2005). Population status, trends and a re-examination of the hypotheses explaining the recent declines of the southern elephant seal mirounga leonina. Mammal Rev. 35, 82–100. doi: 10.1111/j.1365-2907.2005.00055.x
McMahon C. R., Bester M. N., Hindell M. A., Brook B. W., Bradshaw C. J. A. (2009). Shifting trends: detecting environmentally mediated regulation in long-lived marine vertebrates using time-series data. Oecologia 159, 69–82. doi: 10.1007/s00442-008-1205-9
McMahon C. R., Burton H. R., Bester M. N. (2000). Weaning mass and the future survival of juvenile southern elephant seals,<span class=“italic”>Mirounga leonina, at macquarie island. Antarct. Sci. 12, 149–153. doi: 10.1017/S0954102000000195
Mcmahon C. R., Burton H. R., Bester M. N. (2003). A demographic comparison of two southern elephant seal populations. J. Anim. Ecol. 72, 61–74. doi: 10.1046/j.1365-2656.2003.00685.x
McMahon C. R., Howe H., van den Hoff J., Alderman R., Brolsma H., Hindell M. A. (2014). Satellites, the all-seeing eyes in the sky: counting elephant seals from space. PloS One 9 (3), e92613. doi: 10.1371/journal.pone.0092613
Mestre J., Authier M., Cherel Y., Harcourt R., McMahon C. R., Hindell M. A., et al. (2020). Decadal changes in blood δ13C values, at-sea distribution, and weaning mass of southern elephant seals from kerguelen islands. Proc. R. Soc B Biol. Sci. 287, 20201544. doi: 10.1098/rspb.2020.1544
Moore S. E. (2008). Marine mammals as ecosystem sentinels. J. Mammal. 89, 534–540. doi: 10.1644/07-MAMM-S-312R1.1
Oosthuizen W. C., Bester M. N., Altwegg R., McIntyre T., de Bruyn P. J. N. (2015). Decomposing the variance in southern elephant seal weaning mass: partitioning environmental signals and maternal effects. Ecosphere 6, art139. doi: 10.1890/ES14-00508.1
Oosthuizen W. C., Wessel C., Bester M. N., Tosh C. A., Guinet C., Besson D., et al. (2011). Dispersal and dispersion of southern elephant seals in the kerguelen province, southern ocean. Antarct. Sci. 23, 567–577. doi: 10.1017/S0954102011000447
Pascal M. (1981). Évolution numérique de la population d’éléphants de mer (Mirounga leonina (L.)) de l’Archipel des kerguelen au cours des 30 dernières années. Publications 51, 21.
Pinkerton M. H., Boyd P. W., Deppeler S., Hayward A., Höfer J., Moreau S. (2021). Evidence for the impact of climate change on primary producers in the southern ocean. Front. Ecol. Evol. 9. doi: 10.3389/fevo.2021.592027
Pistorius P. A., Bruyn P., Bester M. N. (2011). Population dynamics of southern elephant seals: a synthesis of three decades of demographic research at Marion island. Afr. J. Mar. Sci. 33, 523–534. doi: 10.2989/1814232X.2011.637357
Poncelet E., Barbraud C., Guinet C. (2010). Population dynamics of killer whales in crozet archipelago, southern Indian ocean: exploiting opportunistic and protocol-based photographs in a mark-recapture study. J. Cetacean Res. Manage. 11, 41–48. doi: 10.47536/jcrm.v11i1.629
R Development Core Team. (2019). R: a language and environment for statistical computing, reference index version 2.12.2. Available at: http://www.R-project.org.
Reisinger R. R., Bruyn P. J. N., Bester M. N. (2011). Predatory impact of killer whales on pinniped and penguin populations at the subantarctic prince Edward islands: fact and fiction. J. Zool. 285, 1–10. doi: 10.1111/j.1469-7998.2011.00815.x
Rothery P., McCann T. S. (1987). “Estimating pup production of elephant seals at south Georgia,” in Mammal population studies. the proceedings of a symposium held at the zoological society of London on 28th and 29th November 1986. Ed. Harris S. (Oxford: Clarendon Press), 211–223.
Rus Hoelzel A., Campagna C., Arnbom T. (2001). Genetic and morphometric differentiation between island and mainland southern elephant seal populations. Proc. R. Soc Lond. B Biol. Sci. 268, 325–332. doi: 10.1098/rspb.2000.1375
Savours A. (2009). “phoquiers de la dÉsolation: la chasse aux ÉLÉphants de mer aux Îles kerguelen par les navires–usines franÇais, (1925–1931). Patrick arnaud, Jean beurois, Pierre couesnon, and Jean-françois le mouël. 2007. vachères: privately published. 268 p, illustrated, soft cover. ISBN 978-2-9530233-0-5. €21.00,” in Polar Record, vol. 45. , 277–278. doi: 10.1017/S0032247409008225
Slade R. W., Moritz C., Hoelzel A. R., Burton H. R. (1998). Molecular population genetics of the southern elephant seal mirounga leonina. Genetics 149, 1945–1957. doi: 10.1093/genetics/149.4.1945
Slip D. J., Burton H. R. (1999). Population status and seasonal haulout patterns of the southern elephant seal (<span class=“italic”>Mirounga leonina) at heard island. Antarct. Sci. 11, 38–47. doi: 10.1017/S0954102099000061
Tixier P., Barbraud C., Pardo D., Gasco N., Duhamel G., Guinet C. (2017). Demographic consequences of fisheries interaction within a killer whale (Orcinus orca) population. Mar. Biol. 164, 170. doi: 10.1007/s00227-017-3195-9
van Aarde R. J. (1980). Fluctuations in the population of southern elephant seals mirounga leonina at kerguelen island. South Afr. J. Zool. 15, 99–106. doi: 10.1080/02541858.1980.11447694
van den Hoff J., Burton H., Raymond B. (2007). The population trend of southern elephant seals (Mirounga leonina l.) at macquarie island, (1952–2004). Polar Biol. 30, 1275–1283. doi: 10.1007/s00300-007-0288-9
Wilkinson I. S. (1992). Factors affecting reproductive success of Southern elephant seals, Mirounga, at Marion Island (Doctoral dissertation, University of Pretoria).
Weimerskirch H., Bouard F. L., Ryan P. G., Bost C. A. (2018). Massive decline of the world’s largest king penguin colony at ile aux cochons, crozet. Antarct. Sci. 30, 236–242. doi: 10.1017/S0954102018000226
Whipple S. J., Link J. S., Garrison L. P., Fogarty M. J. (2000). Models of predation and fishing mortality in aquatic ecosystems. Fish Fish. 1, 22–40. doi: 10.1046/j.1467-2979.2000.00007.x
Zhao P., Liu S., Zhou Y., Lynch T., Lu W., Zhang T., et al. (2020). Estimating animal population size with very high-resolution satellite imagery. Conserv. Biol. 00, 1–9. doi: 10.1111/cobi.13613
Keywords: marine mammals, pinnipeds, southern Elephant seals, Kerguelen and Crozet Islands, remote sensing, population size, census
Citation: Laborie J, Authier M, Chaigne A, Delord K, Weimerskirch H and Guinet C (2023) Estimation of total population size of southern elephant seals (Mirounga leonina) on Kerguelen and Crozet Archipelagos using very high-resolution satellite imagery. Front. Mar. Sci. 10:1149100. doi: 10.3389/fmars.2023.1149100
Received: 21 January 2023; Accepted: 18 April 2023;
Published: 12 June 2023.
Edited by:
Michele Thums, Australian Institute of Marine Science (AIMS), AustraliaReviewed by:
Peter Fretwell, British Antarctic Survey (BAS), United KingdomHannah Cubaynes, British Antarctic Survey (BAS), United Kingdom
Copyright © 2023 Laborie, Authier, Chaigne, Delord, Weimerskirch and Guinet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joris Laborie, am9yaXMubGFib3JpZUBnbWFpbC5jb20=
 Joris Laborie
Joris Laborie Matthieu Authier
Matthieu Authier Adrien Chaigne
Adrien Chaigne Karine Delord
Karine Delord Henri Weimerskirch1
Henri Weimerskirch1 Christophe Guinet
Christophe Guinet