- 1Greater Caribbean Energy and Environment Foundation, Miami, FL, United States
- 2Yale Center for Natural Carbon Capture, Yale University, New Haven, CT, United States
- 3School of Environmental and Conservation Sciences, Murdoch University, Murdoch, WA, Australia
- 4Oceanography Research Center, Indonesian Institute of Sciences, Jakarta, Indonesia
- 5Indonesian Institute of Sciences, South China Sea Institute for Oceanology, Jakarta, Indonesia
- 6Yayasan Lamun Indonesia (Lamina), Depok, Indonesia
- 7Seaweed and Seagrass Department, Prince of Songkla University, Kho Hong, Thailand
- 8Key Laboratory of Tropical Marine Bio Resources and Ecology, South China Sea Institute of Oceanology, Chinese Academy of Sciences (CAS), Guangzhou, Guangdong, China
- 9Institute for Marine and Antarctic Studies, College of Sciences and Engineering, University of Tasmania, Hobart, TAS, Australia
- 10Borneo Marine Research Institute, University Malaysia Sabah, Kota Kinabalu, Sabah, Malaysia
- 11Department of Marine Science, Faculty of Marine Science and Fisheries, Hasanuddin University, Makassar, South Sulawesi, Indonesia
- 12Seagrass Restoration and Ecosystem Services Research Group, Hasanuddin University, Makassar, Indonesia
- 13Facilities & Grounds, Raritan Valley Community College, Branchburg, NJ, United States
- 14Department of Biological Sciences, Southwestern Adventist University, Keene, TX, United States
- 15School of Forestry and Environmental Studies, Yale University, New Haven, CT, United States
The greater Southeast Asian region contains the largest global extent of tropical seagrass; however, anthropogenic degradation is estimated to be greater than 7% per year. Although the areal extent of seagrass is presently 36,765 km2, Fortes group estimates that 50% of the original seagrass has been degraded from a variety of impacts. One set of solutions to degradation is to restore tropical seagrass successfully, for which information from past results is needed to avoid failures. Van Katwijk, Thorhaug and others provided a global seagrass restoration review of 1,786 trials, but did not include the full Southeast Asian regional information. Thus, we review findings from 228 trials in the greater Southeast Asian region, involving 305,807 restored units with an extent of 372,649 m2. Seagrasses planted with varying successes include 13 tropical species and five subtropical or near-subtemperate species. We compare methodologies as well as key factors of light level, energetics, and depth. This review demonstrates the highest survival in seagrass restoration employing sprigs or plugs at medium depths (2–4 m) with adequate light levels in medium to low energetics planting one to several dominant species. Substrate anchors improved successful establishment. Information gaps occur in quantified monitoring of seagrass services reassembled with tropical-seagrass restoration; thus, fisheries’ nursery potentials are not provided. Future actions need national seagrass restoration policies and plans to restore degraded seagrasses. At present, such policies and plans are non-existent in most greater Southeast Asian regional nations, with the exceptions of Australia and the Philippines, although some nations have national plans for restoring corals or mangroves.
1 Introduction
We review the investigations of restoration of seagrasses within the greater Southeast Asian region as one of the sets of important solutions to maintain and bolster seagrass resources after seagrass degradation. Regional seagrass degradation level is presently estimated to be approximately 50% of the original seagrass extent (Ooi et al., 2011; Arias-Ortiz et al., 2018; Fortes et al., 2018). These authors indicate that anthropogenic impacts within the coastal zone have been an important cause of degradation. The seagrass importance in the greater Southeast Asian region is stated by these authors and others as based on containing the most seagrass species plus the largest seagrass biomass. The region has also been identified as a key global area for carbon sequestered by seagrass productivity (Orth et al., 2006; Alongi et al., 2016; Gallagher et al., 2019; Macreadie et al., 2019; Thorhaug et al., 2020a, 2020c), as well as an important global marine biodiversity region (Ooi et al., 2011; Nakaoka et al., 2014; Fortes et al., 2018; Langlois et al., 2023).
1.1 Southeast Asian seagrass present extent and degradation of extent
A dismal future for Southeast Asian nations has been predicted if seagrass degradation is not reversed. Ooi et al. (2011); Fortes et al. (2018) estimate a 30%–50% loss. The present extent of 36,765 km2, estimated by Fortes et al. (2018) and McKensie et al. (2020), is considered to be an underestimate, but did not include regional areas of Papua New Guinea (PNG) or western Australia. Green and Short (2003) estimated that the Indonesian seagrass extent alone is 30,000 km2. Indonesian mangroves, for comparison, in carefully documented measurements from satellite mapping, lost 250,000 ha in the last decade (Spalding et al., 2010; Giri et al., 2011). Clearly, anthropogenic impacts in coastal Indonesia are considerable (Giri et al., 2011), which implies impacts on the seagrasses adjacent to the mangroves. Langlois et al. (2020) estimated the extent of 235,261 km2 for the tropical Indo-Pacific (which includes a far greater area with the inclusion of the Indian Ocean and parts of Oceania as well as the Southeast Asian region). McKensie et al. (2024) emphasize the substantial ongoing extent debate due to deep seagrass being difficult to ascertain by aerial imagery.
This review raises the question, “Can the loss of a maximum of 7% per year of seagrass ecosystem services, predicted by Waycott et al. (2009), be tolerated socially (values for nutrition and fisheries employment) and economically in the Southeast Asian region”?
1.2 Objectives of this review
Our hypothesis is that various seagrass species can be restored with moderate survival in the greater Southeast Asian region. We analyze this by comparing a series of seagrass restoration efforts in various regional nations to delineate the state of seagrass restoration in the Southeast Asian region.
2 Methods
2.1 The hydrological setting of the review
The Southeast Asian region is rich in seagrasses (Figure 1), due to the underlying physical–chemical environment. Convergent equatorial ocean currents form a complex pattern, termed the “through flow” (Wyrtki, 1987) (Figure 2), flowing from the Equatorial Pacific past the Southeast Asian region southwestward into the Indian Ocean Waters. Partially driven by a hydrostatic head of 30 cm between the Equatorial Pacific and the Indian Oceans (Sprintall et al., 2009), oceans water pour over the underlying Sunda Plate, through island archipelagos. The Sunda Plate (Figure 3) consists of a large shallow shelf and lies between volcanically formed island archipelagos and the Asian mainland forming multiple estuaries and coastlines sheltering large seagrass meadows. Upwellings of nutrient-rich deeper water fertilizing upper layers occasionally punctuate coastal shelves near periodic deep trenches (Figures 2, 3). The northeast Indian Ocean water flow modulated by trade winds and the Equatorial Indian Ocean current patterns also have effects on the western nations of the greater Southeast Asian region (Conservation Biology Institute, 2010). Ashton (2015) points out that volcanic soils are particularly nutrient-rich, stimulating tropical forests’ growth rates. Our operational definition of “Greater Southeast Asia” begins at the Philippines’ northeastern corner running westward across the top of the Gulf of Tonkin to the Myanmar continental shelf in the Andaman Sea. From Myanmar, our perimeter moves southwest of Perth, Australia, with our southwestern corner facing the Indian Ocean (see Figure 4). The perimeter moves on a diagonal to the northeast from Perth, including the Northern Australia shoreline including estuaries to the southeastern shelf edge of PNG. From the southeast shelf of PNG, the perimeter goes north to the northeastern side of the Philippines.
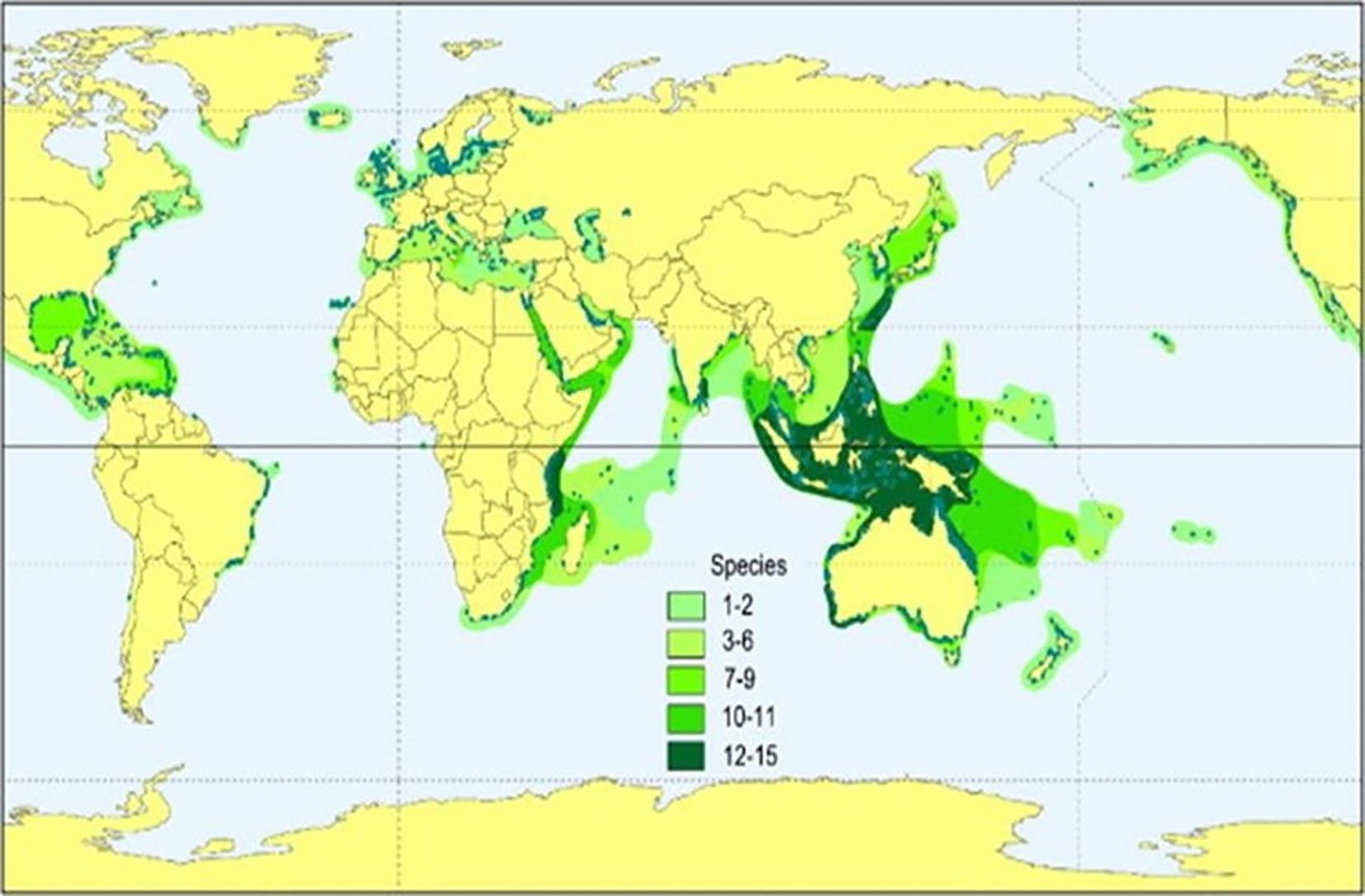
Figure 1. Map illustrating the distribution and diversity of seagrass species in coastal regions worldwide. Areas are shaded n varying green tones, indicating species diversity: 1-2,3-6,7-9,10-11, and12-15 species. The highest diversity is in Southeast Asia and Oceania (from Short 2017).
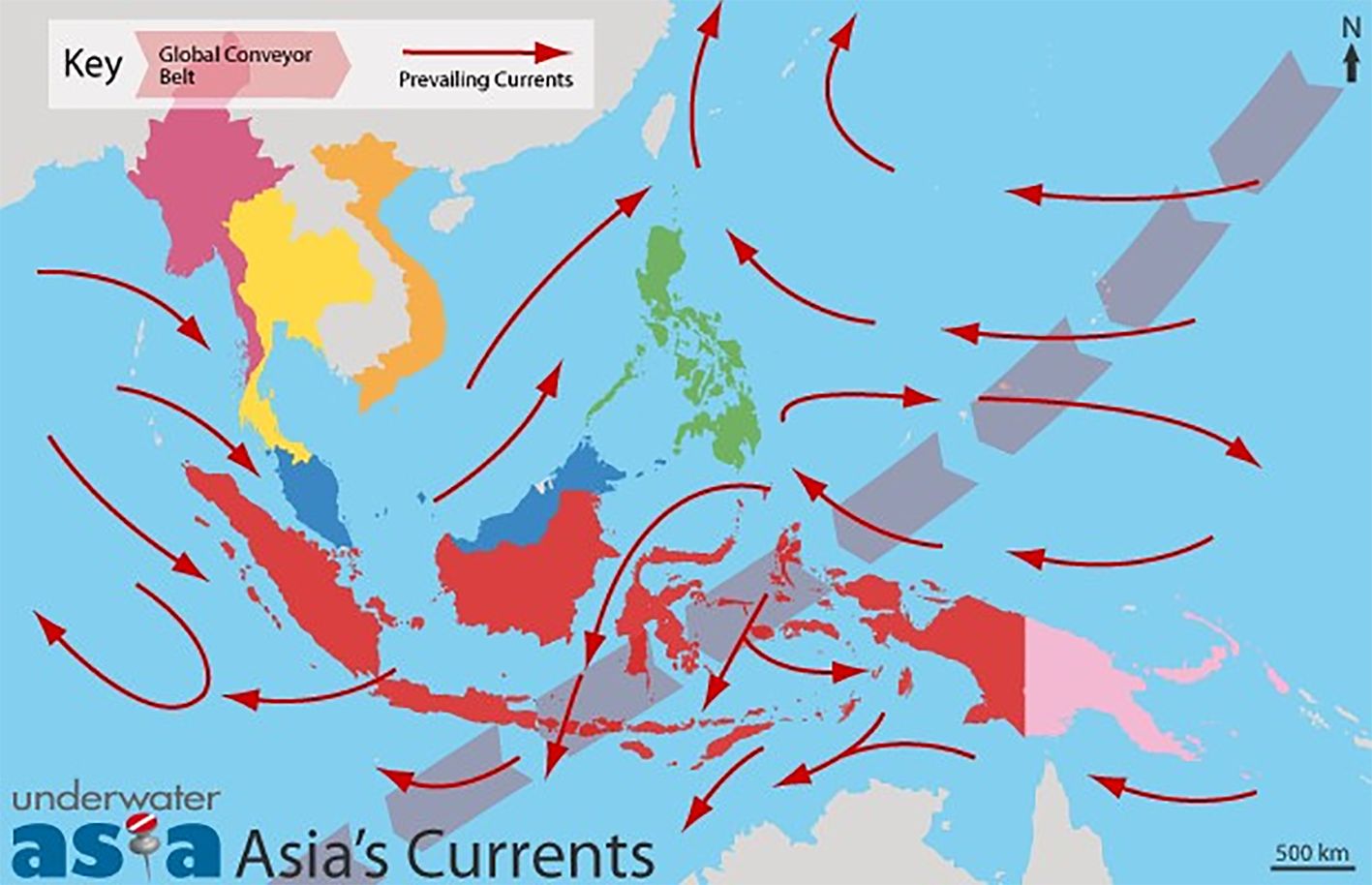
Figure 2. Map highlighting ocean currents in Asia, showing the Global Conveyor Belt and prevailing currents with red arrows. Countries are color-coded with a distance scale of 500 kilometers. (from KGR Oceans,2009).
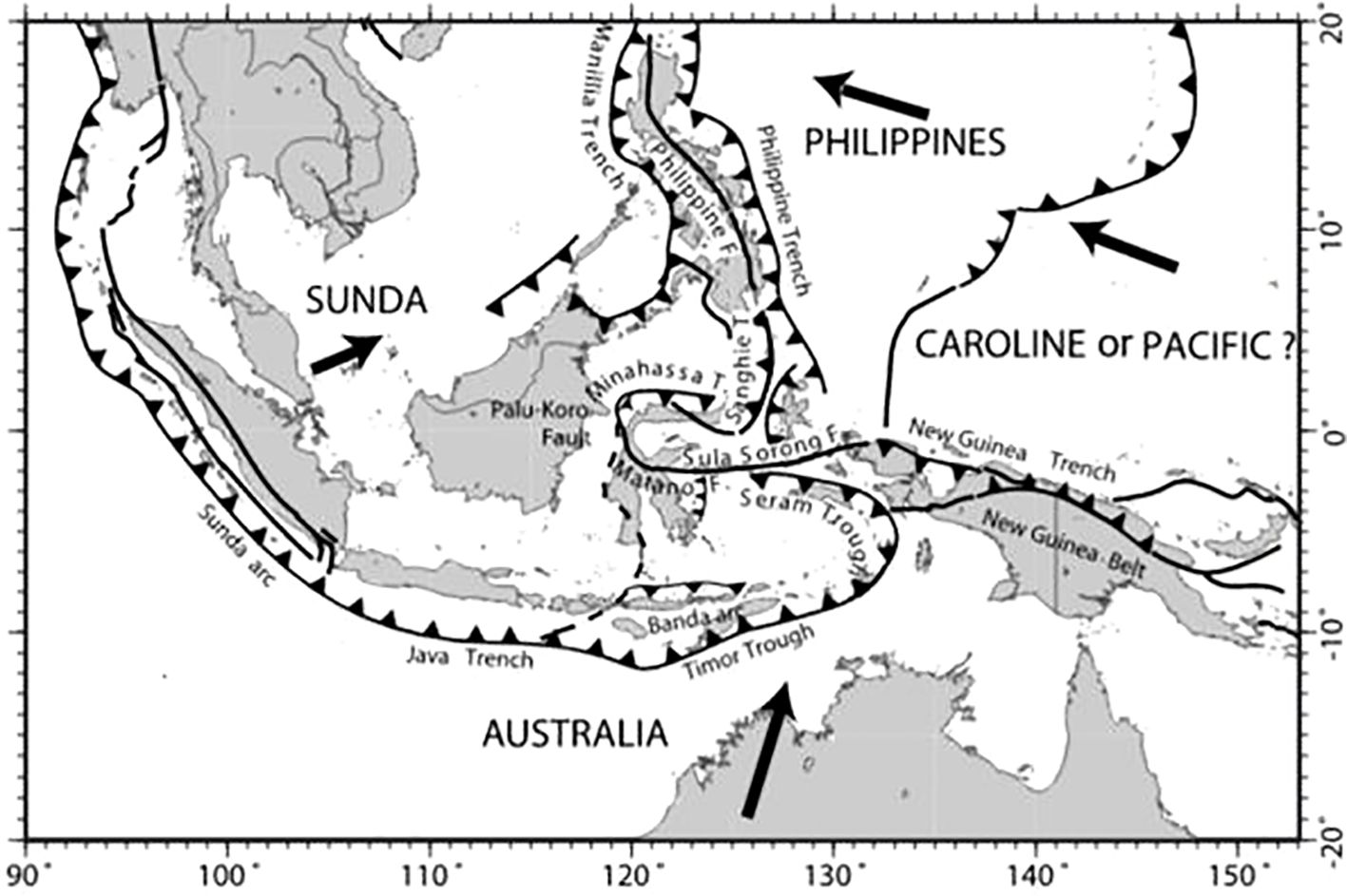
Figure 3. Map depicting tectonic plates and trenches in Southeast Asia. Labels include Sunda, Philippines, and Australia. Key features are the Sunda Arc, Java Trench, Philippine Trench , and Timor Trough. Arrows indicate plate movements (from Baroux et al, 1998).
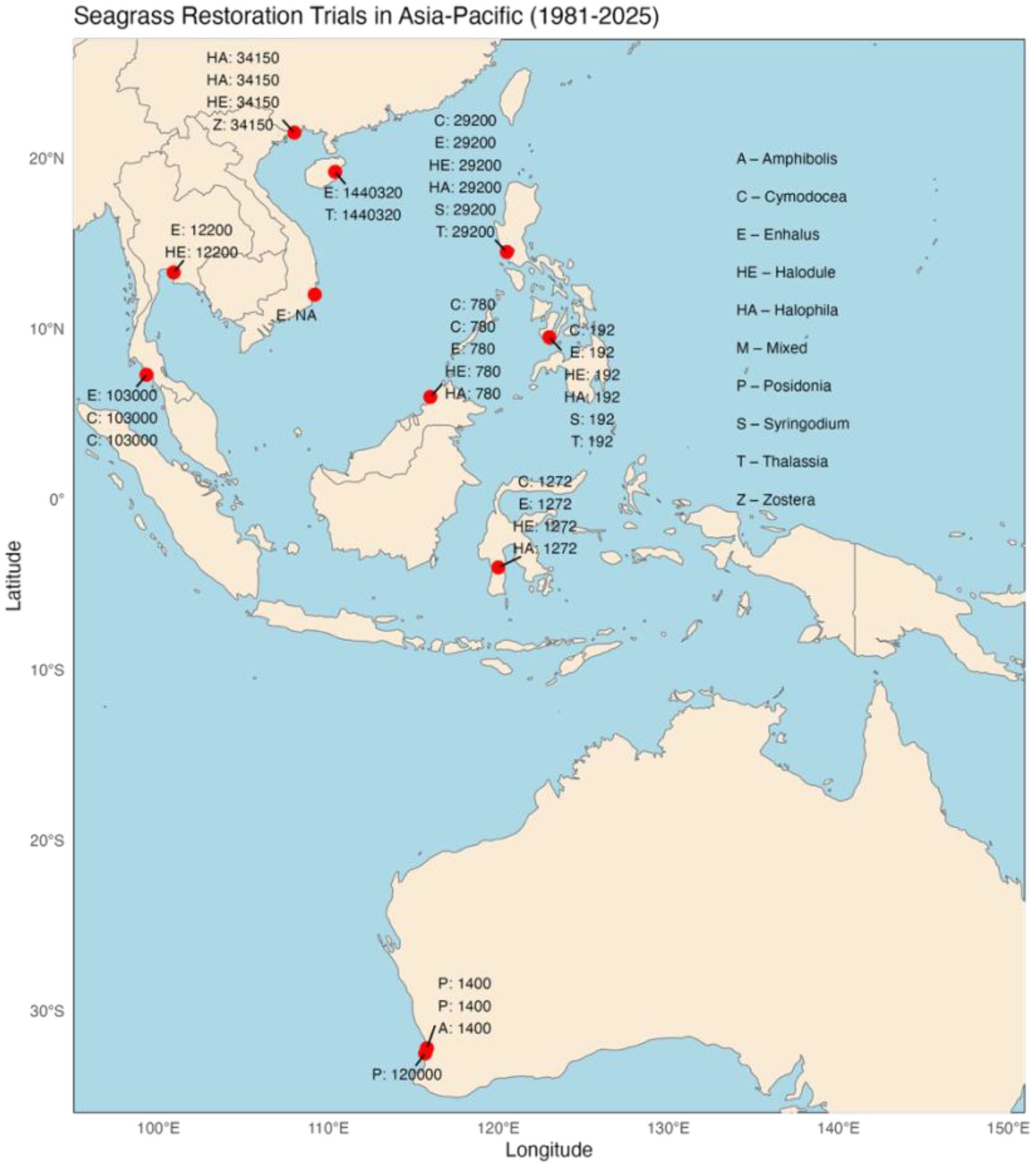
Figure 4. Map of the Asia-Pacific region showing seagrass restoration trials from 1981 to 2025. Red dots indicate various locations with annotations for seagrass species, such as Amphibolis, Cymodocea, and Enhalus. Latitude and longitude are marked, with specific codes and numbers denoting trial data for each site (composed by SBZ from earlier fig. by TMY).
2.2 Methods of review
We first recognized the need for this summary of results in tropical Southeast Asia at our Seagrass Restoration workshop during the World Seagrass Association Conference and Workshops in Singapore. We generally followed the same protocol we had used in van Katwijk et al. (2016). We compiled data from restoration trials conducted from published articles listed in Web of Science. A trial consists of one or more shoots or seeds that have the same “treatment”, i.e., they are planted at the same location, with similar techniques and treatments in the same year and season, using the same species and plant material. The study is not a traditional meta-analysis (e.g., Harrison and Kaufman, 2011); first, we aimed not to exclude any reported trial (resulting in many missing values of factors key to growth and survival); second, since the recorded characteristics frequently had no controls, effect sizes can only be estimated relatively between categories (as an example, plant material has the following categories: seeds, sods, rhizome fragments, seedlings, or plugs); and third, the data did not allow for assignment of a nesting factor like sources.
van Katwijk et al.’s (2016) global review included 1,786 trials. Our review herein replicated less than seven from van Katwijk’s Southeast Asian locations in our almost 40 investigations. The conceptual approach of van Katwijk et al. (2016) used each specific site per species as one trial with a single survival rate for that trial, disregarding the number of planting units (PUs) carried out at that site, whereas, throughout this review, we refer to each PU (the singular unit that was planted at each site) as the fundamental unit for calculating survival rate. Hence, the trial data sets are handled separately for the survival of individual PUs using the total number of PUs planted as the basis for “survival”. Thus, our method allots large-scale plantings with more weight reflected as percentages of PUs being larger than small-scale plantings. In various trials, the PU consisted of one of the following donor material: a seed; a germinated seed (termed a seedling); a sprig (containing at least one rhizome fragment with roots, a meristem, and blades); a sod (large plant mass containing roots, rhizomes, sediment, stems, and blades with some underlying sediment, which could measure to a meter square); or a plug (like a sod, but much smaller, usually approximately 8–14 cm in diameter).
Environmental factors were generally recorded by investigators either descriptively or quantitatively to include the following: planting date, published citation date, geo-reference, planting season, plot size, energy level at site, estimated light level, depth, and salinity. All data did not have complete environmental and biological factors for each trial and site. In some studies, the investigators could estimate data from ongoing corollary studies at the same site. The biological factors monitored included species, number of PUs, survival percent, interval of monitoring in months, final density of donor site (blades m−2), and planting methodology. Other factors included depth, approximate light level split into high, medium, and low defined under the table itself from surface light levels, approximate energetic level, general sediment composition, anchor types, and use of growth substances. Diverse monitoring techniques occurred among the numerous investigators. The restoration investigator team was always the monitoring investigator. We compare metrics for various techniques, species, and treatments of PUs to produce data that characterize the survival of restored plants. We chose to compare PU survival as a function of time as the success metric. A monitoring discrepancy among the 228 trials occurred due to investigator choice of amount of PU measured. Some studies measured all units at all planted areas and some measured only sub-sections of planted areas. The conceptual error may occur when just a sub-section is monitored and if a segment of the planted areas did not survive and the reporter did not take the non-surviving segment into account as zeros for missing PUs. (Note: this affected larger plantings only; in very small plantings, all PUs were consistently measured.) This conceptual error of taking a percentage from surviving PUs and not using zeros for non-surviving PUs would skew results with elevated percentage survival rates. For this reason, we report the subsample population of PUs from which the survival was calculated as well as the original number of PUs.
2.3 Methods for comparison: are the data adequate for a quantitative analysis?
Upon examination of data, it was found that simple summing, percentages, and means could fulfill the general objectives to compare results among studies in a variety of the greater Southeast Asian locations with multiple species tested, multiple methodologies, and key environmental factors all affecting the survival rates. The list with the investigators’ per nation and per author is found in Table 1. Original data are found in an array of publications and reports listed in Table 1 and the literature cited (publications in multiple languages, but the majority in English). Seagrass plants, planting data, and methodologies are organized by factor in Table 2 and by species in Table 3.
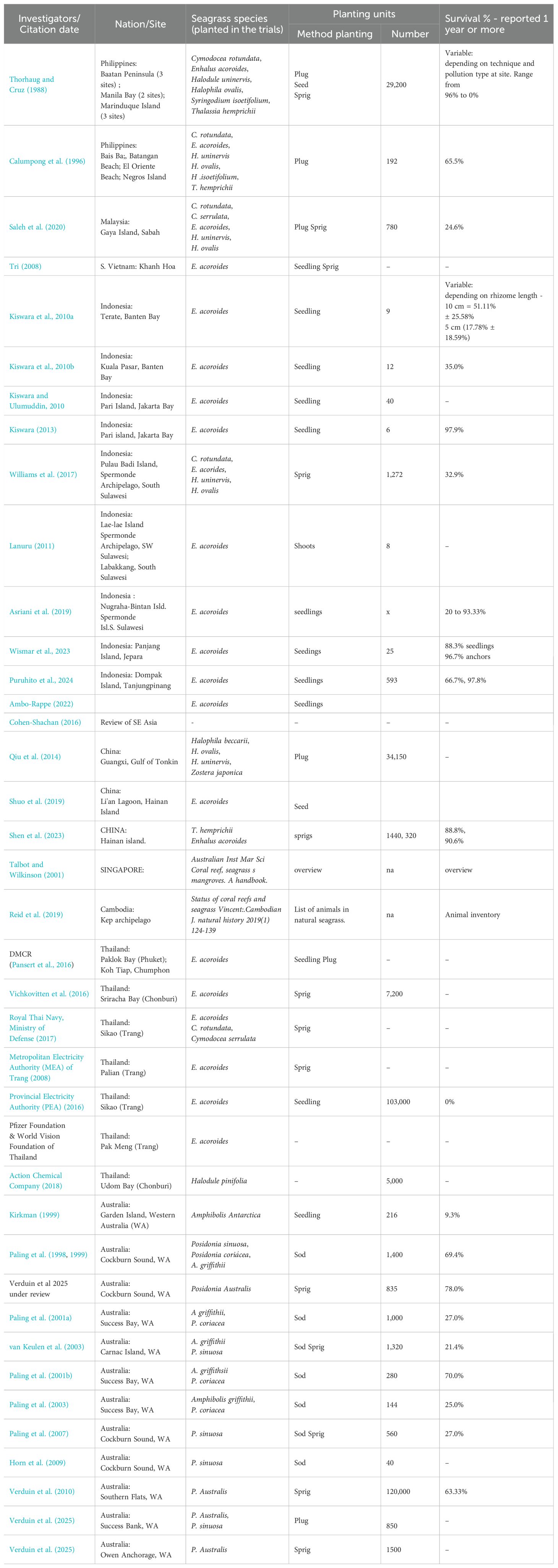
Table 1. Overview of seagrass restoration baseline data in Southeast Asia 1974-2019 listed in order of date of trials seen in columns: Investigators and citation date; Nation and site names; Seagrass species; Method of planting; Number of planting units; Survival in year 1 & percentage & remarks.
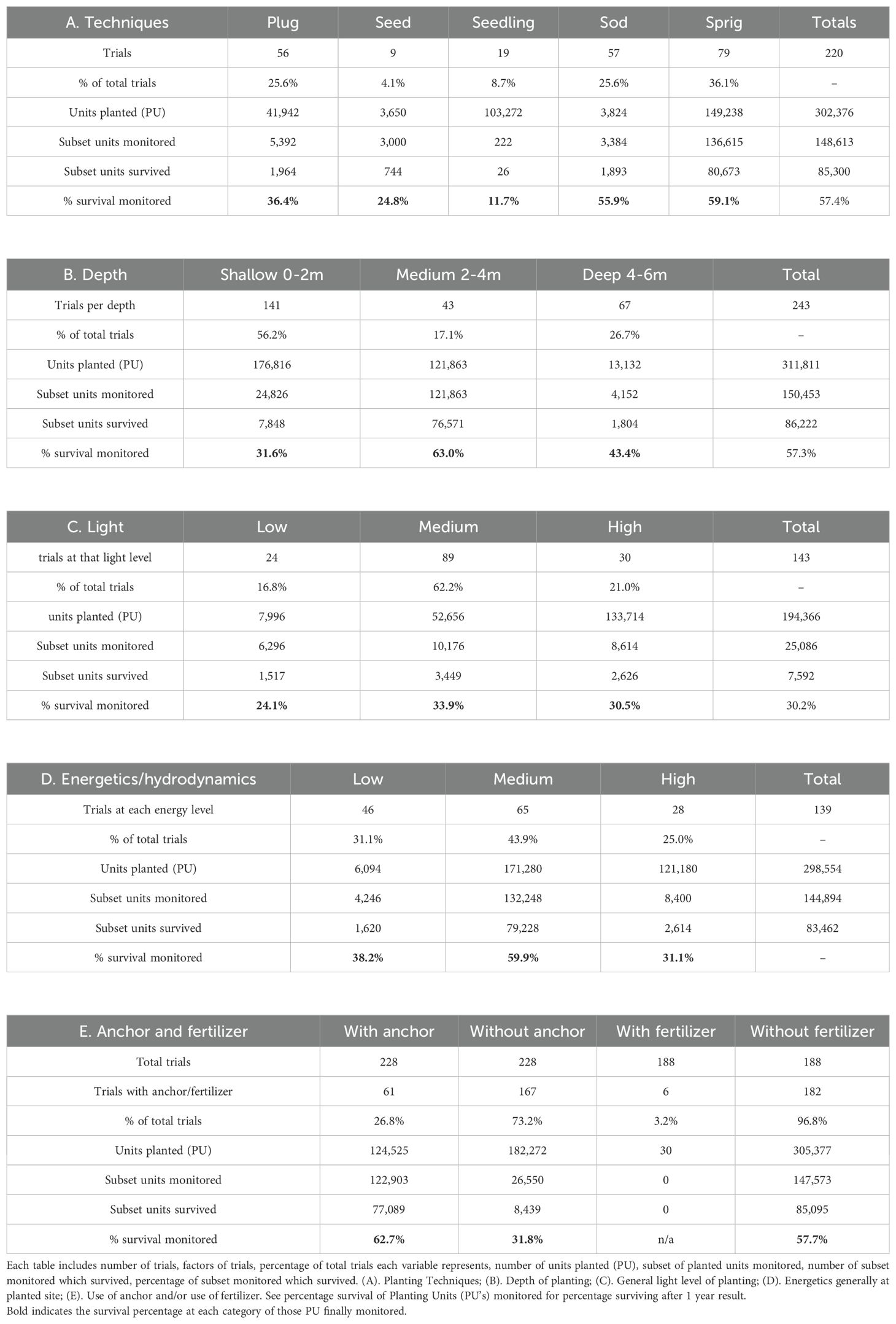
Table 2. For seagrass restoration in the greater Southeast Asia region, variables of restoration planted units measured for total planted units from all cited investigators. (Each table includes Bold Font which indicates average of the PU’s finally monitored).
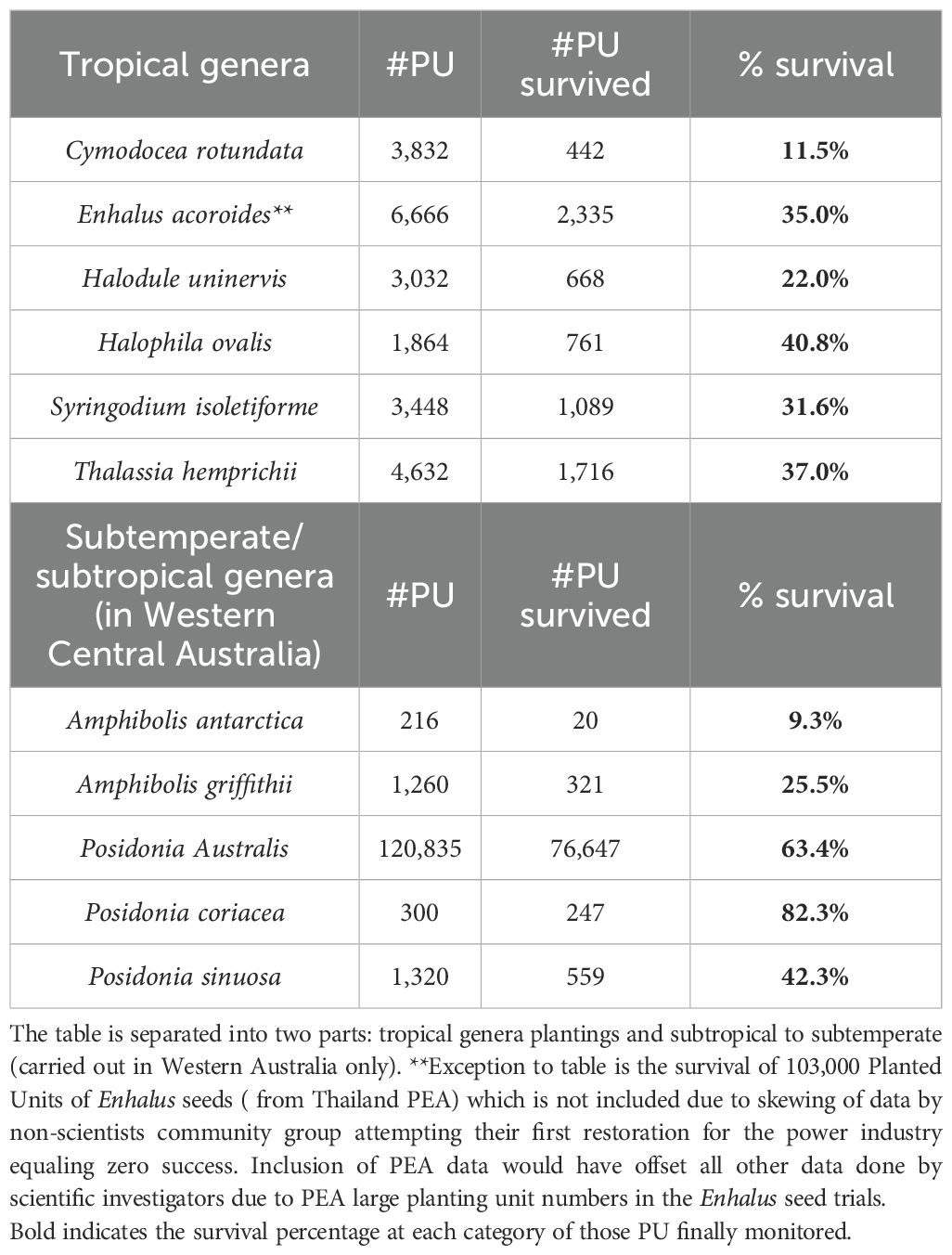
Table 3. For seagrass restoration in greater Southeast Asia region, number of total planted units per genera and species, planted units, survival number, percentage survival of planted units. Bold font indicates survival percentage of each category of those PU was finally monitored.
3 Results
3.1 Overall results
In 10 of 14 nations, namely, the Philippines, Malaysia, Vietnam, Indonesia, tropical China, Australia, Cambodia, Vietnam, Singapore, and Thailand (Table 1), 38 investigator groups worked on seagrass restoration. Many of the 228 trials were small studies (tens to hundreds of PUs). These investigators placed a total of 305,807 PUs into a space of 372,649 m2 (Table 1). Table 1 shows that most large-scale plantings were executed by knowledgeable seagrass scientists (e.g., Paling, Verduin, van Keulen, Kiswara, Calumpong, Phillips, Williams, Ambo-Rappe, Huang, Thorhaug, and Cruz). However, some trials were carried out by unskilled community volunteers, as indicated in Table 1, which generally showed far less survival.
3.2 Successful results with various planting techniques
The survival rate differed among the four major planting techniques. We rank them from the highest to the least: sprigs (35.5% survival of 150,678 PUs within 81 trials); sods (25.0% survival of 3,824 PUs with many fewer PU plantings, with 57 trials); plugs (24.6% survival of 41,942 PUs within 56 trials); and seedlings 1 [0.7% if all seedlings planted were considered but with far higher survival (11%) if the PEA (Thailand Electric company) planting was excluded from the 105,713 PUs within 25 trials] (Table 2). A gap in some subsets of data exists, since the monitoring period of some trials ended after the first year. We consider 1 year as an inadequate monitoring period to evaluate long-term success. Table 2 shows that the amount of usage of various restoration techniques is the following at 12 months post-planting: 35.5% of trials utilized sprigs; 25.6%, sods; 24.6%, plugs; 11.0%, seedlings; and 3.9%, seeds. 2 The most frequently monitored time was 12 months. Note that three-fourths of the sites were measured additionally at 24 months.
3.3 Results of the effect of depth on restoration success
The majority, almost 56.5% of PUs (176,816), were planted at depths of 2 m or less seen in Table 2. At medium depths of 2–4 m, there were 121,863 PUs from 45 trials (but predominantly—120,835 PUs—from the Australian planting). At depths of over 4 m, Australian investigators planted a total of 13,132 PUs using scuba gear. The medium-depth plantings result in higher survival (64.2%) than shallower (6.2% survival) or deeper (43.4% survival) plantings (Table 2). The lowest survival rate occurred as a subset within the shallow (0–2 m) depth group, which was the “very” shallow cohort (planted at or less than 0.3 m). Intertidal plantings had little to no success in the results reported herein. In a comparative depth planting investigation globally reported by Verduin et al. (2010), plantings at 2 m showed substantially higher survival (70%) than at 4 m or deeper (37%).
3.4 Results of light intensity on restoration success
Depth is related to both light intensity and light quality in shallow marine and estuarine environments. Restoration survival percentages of planted units were slightly greater in medium light (33.9%) than in high light intensity (30.5%), or in low light intensity (24.1%) (Table 2). These results did not show as great a difference among light levels as survival for depth results. The large numbers planted in Australia in high light may be a factor here. Obviously, multiple simultaneous factors were influencing the seagrass light requirement such as energetics, or perhaps pulses of turbidity lessening light intensity. The number of planted units in trials showed that plantings in medium light (63%) were more abundant than at high light (23.8%) or at low light (15.9%) (Table 2). 3 Importantly, no measurements of the duration of low light intensity were included, or of other light changes such as caused by diurnal pulses or variation of riverine turbidity. In a number of trials, the light data were by investigators’ estimates or by Secchi-disc measurements, not direct photometer measurements. Thus, these light data do not comprise a set of statistically accurate metrics. Some detailed light measurements with photometers are given in seagrass-restoration studies by Thorhaug and Cruz (1987); Paling et al. (1998; 1999), Verduin et al. (2010); Kendrick and Verduin, (2025), and Williams et al. (2017).
3.5 Results of energetics such as wave energy and currents on restoration success
Clearly, in Table 2, the low-energy plantings (38.2%) and medium-energy plantings (60.6%) were greater than survival rates of higher-energy sites (27.8%). 4 Estuarine energy regimes can be relatively calm and experience periods during which the PU roots attach themselves into the sediment structure. High wave energy within seasonal monsoon events (rather than daily) are present in many Southeast Asian sites. In the Intertropical Convergence Zone (ITCZ), monsoon winds occur far less than in other Southeast Asian regional nations such as the Philippines. Experienced investigators related that they chose not to plant on the seaward side of barrier islands to avoid the disruptive effects of waves. Likewise, some did not plant in high energetic seasons. Some investigators stated in their multiple test studies that they attempt to find a balance between sufficient depth to overcome disruptive energetics and sufficient light penetration for seagrass growth requirements.
3.6 Results of anchors on planting success
To improve survival, investigators of 61 trials used anchors to stabilize planted units, mostly within medium- and higher-energy regimes (Table 2). They anchored 125,285 PUs in 61 trials. Anchored PUs showed higher survival (62.7%) than the 167 non-anchored PUs (30%) (Table 2). Anchor types varied: hand-made bamboo frames onto which sprigs were tied (Kiswara, 2018); clips; stakes; and metal frames pinned and buried in the sediment with sprigs firmly attached (Verduin et al., 2010) (Table 2). As an example, the anchors of Verduin and Sinclair (2013) were the most complex and had high survival rates. Thalassia, Cymodocea, Enhalus, and Halodule sprigs, sods, and plugs were established without anchors with moderate to high survival at medium- to low-energy sites.
3.7 Results of fertilizers and growth-stimulating additives on success
Only a small number (6) of trials in Southeast Asia used growth stimulators such as fertilizers for improved initial growth (Table 2). The larger-scale trials did not use any growth stimulants. Without any growth additives, the large-scale survival rate was 57.7%.
3.8 Results of species employed as restoration material
Southeast Asian seagrass restoration trials used 13 species (Tables 3, 1). Eight tropical species (of 21 regional tropical species) and five subtemperate species were planted. From 305,807 PUs, the most intensively planted were the following: Enhalus acoroides (105,438 PUs), Posidonia australis (122,995 PUs), and two species of Halodule (H. uninervis and H. pinifolis; 18,207 PUs in total). The group of medium numbers of planted seagrasses included the following: Halophila (H. ovalis and H. becarii) (3,023 PUs in total); Thalassia hemprichii (5,264 PUs), two species of Cymodocea (C. rotendata and C. serrulata; 6,072 PUs), and Syringodium isoetifolium (3,832 PUs).
3.9 Results of various seagrass species survival in restoration trials
Over the entire Southeast Asian region, the restoration survival rates of plantings in multiple locations by various methods were as follows: E. acoroides, 35.0% (this percentage does not include the PEA plantings); Halophila ovalis, 40.8%; T. hemprichii, 49.3%; Syringodium isoletiforme, 31.6%; H. uninervis, 22.4%; and Cymodocea rotundata, 11.7% (Tables 3, 1; Figure 4) (Wismar et al., 2023). In western Australia, in the subtropical/subtemperate part of the region, the highest survival rate was seen in large-scale restoration where the genus Posidonia coriacea demonstrated higher survival (82.3%) than the other species planted as demonstrated in the following results: P. coriacea, 82.3%; P. australis, 63.4% (2 ha at 80% plus 1 ha at 30%), plus P. sinuosa at 42.3%. Other species that showed lower survival were Amphibolis griffithii (25.5%) and A. antarctica (9.3%) planted within the same area of western Australia. Thus, summing from the total results of the almost 40 investigations, the seagrasses species demonstrating the highest survival were Enhalus acordoides, T. hemprichii, H. ovalis, and three species of Posidonia: P. coriacea, P. australis, and P. sinuosa.
The success criterion for species included survival and lateral growth. Investigators generally ascertained from test plots or observational knowledge the most locally appropriate species for the trials. Control sites comprised proximate naturally occurring seagrass area, which investigators compared to restored seagrass in terms of blade density, blade characteristics, etc (Verduin et al., 2012).
3.10 Results of stated objectives for seagrass planting and monitoring actions
The highest trial numbers were stated by the authors to be restored for “experimental” purposes, in which investigators sought information about the factors allowing successfully seagrass growth at a multiple given sites and which method and seagrass species was most suited in various sites. “Mitigation” purposes comprised the largest volume of PU deployment (particularly in Australia, Thailand, and the Philippines where government mitigation policies were in effect) (Table 1; Figure 4).
4 General discussion
4.1 Summary of main findings
For the greater Southeast Asian region of tropical and subtropical to subtemperate seagrass restoration investigations, we assembled an updated, unbiased review supplementing the larger global seagrass restoration (1,786 trials) review of van Katwijk et al. (2016) where only seven southeast Asian investigations were used. The summary results of almost 40 trials from 10 of the 14 regional nations are seen in the conclusions below. We have attempted to enumerate environmental and biological factors plus methodologies allowing various survival. Below, we point out gaps in the data. In these attempts to restore, investigators found a wide variety of results. This set of varying survival metrics (ranging from 0% to 83%) can be compared to van Katwijk et al.’s (2016) global study that found a global average of 37% overall survival for 1,738 investigations when combining small- and large-scale plantings. van Katwijk et al. (2016) attributed this apparently moderate survival percentage to many small efforts that lowered survival rates compared to the larger-scale plantings. The general results for the greater Southeast Asian region demonstrate that some tropical seagrass species were more successfully restored, including E. acoroides near 40% and T. hemprichii at 49.3%, while other species demonstrated an overall lower survival rate (C. rotundata at 11%). For the subtropical/subtemperate regions, Posidonia had species survival differences with P. coriacea at 82.3% and P. australis at 63.4%, with other Posidonia species far lower. Our recommended techniques and species (discussed below), when well executed, should be useful in initial large-scale attempts to restore and mitigate such seagrass regional losses as reported by Fortes et al. (2018). The entire region is in urgent need of seagrass restoration from large-scale projects such as those executed by Verduin and Paling and their groups. These Australian large-scale plantings serve as examples to move forward in planning and execution for governments at multiple levels, philanthropic foundations, and NGOs throughout the region (Buelow et al., 2022). Large-scale plans, securing funding, and prodigious work should form the future of seagrass restoration in the greater Southeast Asian region.
The summary of the best survival results indicates the following: (1) species in tropical areas (E. acoroides, T. hemprichii, Halophila sp., and H. uninervis) and in subtemperate/subtropical regions like Australia (P. coriacea and P. australis); (2) planting by methods of sprigs or plugs; (3) planting at medium depth (2–4 m) in moderate- to low-energy areas of sufficient light, possibly with anchoring devices; (4) growth stimulants do not appear to be needed; and (5) site selection needs to be carefully carried out by a knowledgeable seagrass scientist to produce the best survival results.
Our hypothesis that seagrass restoration is viable for multiple seagrass species in the Southeast Asian region has been supported by these reviewed data.
Within this region, there are 14 nations, each with differing environmental assets, histories, legal systems, and government attitudes toward environmental conservation of coastal resources, resulting in differing environmental policies. Most of these nations have extensive seagrass resources. Natural resource management policies, regulations, and enforcement have created a patchwork of seagrass habitats with extensive seagrass loss (Ooi et al., 2011; Fortes et al., 2018), which needs enhancing. Restored seagrasses were found to be sustainable over many decades of continual growth in other parts of the world (van Katwijk et al., 2016; Nordlund et al., 2017; Thorhaug et al., 2020c; Seraphim et al., 2020; Kiswara, 2018; Kendrick et al., 2025).
4.2 Fundamental concepts and problems
This review suggests that the ecological and physical conditions can be managed for large-scale seagrass transplantation survival in the greater Southeast Asian region. The prime example of large-scale success is the approach applied in subtropical to subtemperate regions of western central Australia (Paling et al., 2007; Verduin et al., 2010; Verduin and Sinclair, 2013, and Kendrick et al., 2025). This Australian Indian Ocean study bears resemblance to large-scale projects that have been carried out in other areas of the world in terms of survival results (60%–85%). These large-scale efforts by Verduin and Paling groups were built on a series of trials showing survival of species and methods that could be applied to a degraded estuary to restore a partial seagrass meadow, the area of which ameliorated the meadow’s long-term absence post-degradation. The total efforts were monitored over 8 years (Verduin et al., 2025), demonstrating longevity in the species of restored seagrass there.
Generally, in the reviewed trials, many regionally diverse groups found higher success when sprigs were affixed to buried frames as the use of anchors or other materials. Our review demonstrated key environmental factors influencing the best survival with a combination of adequate light for photosynthesis and adequate depth to avoid uprooting. Approximate light level split into high, medium and low (defined under the table itself from surface light levels), (Kendrick et al., 2025) were higher survival.
A major problem encountered by most seagrass restoration regional practitioners included inadequate funding to carry out longer-term monitoring for survival and growth over multiple years. This included funds to obtain instrumentation to measure environmental factors such as light intensity, oxygen and dissolved carbon dioxide water content, and animal recolonization rates. They also lacked funds to monitor services provided by restored seagrasses over time scales.
A second important problem encountered was the lack of awareness of many governments to the importance of ecosystem services provided by seagrass as compared to those provided by coral reefs or mangroves. This led to governments not assessing and managing the social benefits of seagrass restoration to their citizens. It also created a low number of policies and/or regulations, leading to greater seagrass protection and enhancement.
A third problem with the review is that the restoration investigations were chiefly small-scale, not large-scale studies. This did not allow the type of large-scale process discussed in van Katwijk et al. (2016) to occur so as to influence the survival percentage of the total projects. In the van Katwijk review, the average survival of transplanted units was 37%, and in our review, the average survival was in this same range. The large-scale survival rate was far higher in both reviews. The van Katwijk review had many large-scale projects from multiple ocean basins, mainly the Atlantic Ocean, although our review had fewer than 20% of almost 40 studies.
The overlap in studies cited by both van Katwijk et al. (2016) and this review was chiefly some of the larger studies: Paling et al. (1998, 2001), Verduin et al. (2010), Thorhaug and Cruz, (1987, 1990), Calumpong et al. (1996), and Qui (2014). The repeated use of these studies is definitely important since they confirmed the feasibility of large-scale restoration and showed species tolerance to various types of degradation generally found regionally.
4.3 Different schools of thought or concepts
Most seagrass investigators agree on the Southeast Asian regional degradation (Todd et al., 2010; Ooi et al., 2011; Nakaoka et al., 2014; Fortes et al., 2018; Thorhaug et al., 2020a, 2020b). Fortes et al. (2018) estimated that at least 50% of the original seagrass have been decimated from a variety of impacts. One school of thought is that degrading activities must be corrected first, prior to restoring seagrass. The second school of thought is that some level of the degrading effects can be physiologically tolerated by various dominant species of seagrasses at variable distances from the degradation source. Usually, these species’ tolerances are ascertained after pilot testing, which has shown some dominant seagrass species having higher tolerances to diverse pollutants than others. [For example, this principle is long-established in the Atlantic tropics/subtropics with the dominant habitat species Thalassia testudinum and Halodule wrightii being far more tolerant of multiple degrading factors than Syringodium filiforme (Thorhaug et al., 1985; Thorhaug, 1985, 1987, 2001)]. This second school of thought is widespread among the “Restore America’s Estuaries” group of 1,500 members and 300,000 volunteers in 800 scientific and government management projects, holding biannual meetings of thousands of restoration practitioners over the past 25 years, all working to restore coastal vegetation in damaged estuaries. The basic estuarine problems still remain, although effluents are sometimes reduced substantially. A number of investigations report on restoring specific types of areas of degradation. In the greater Southeast Asian region, survival in damaged estuaries and subsequent growth of seagrass have also been tested in the face of various types of degradation. A variety of seagrass species were tested in areas degraded by urban waste, dredge channels and artificial land fill, thermal effluents, mining wastes, and non-degraded controls. The focus was to find types of degraded habitats that could be restored by some species. Results clearly showed some dominant species (E. acoroides, T. hemprichii, H. ovalis, H. uninervis, P. australis, P. sinuosa, and A. griffithii) that tolerated a variety of impacts (in historical order, Thorhaug and Cruz, 1987, 1988; Calumpong et al., 1996; Paling et al., 1998, 2001a, 2001b, 2003; Verduin et al., 2010, 2024). That various seagrass species have a range of tolerance to degraded habitat has been shown in over a wide range of multiple seagrass restorations in the Atlantic, such as Chesapeake Bay (Orth et al., 2020), and in Atlantic tropical areas such as Jamaica (Thorhaug et al., 1985) and Biscayne Bay, Florida (Thorhaug and Hixon, 1975; Thorhaug, 1985), Texas coastal waters (Thorhaug, 2001; Thorhaug et al., 2020c), as well as in the Pacific, in Vancouver Bay, British Columbia, Canada (Durance, 2001).
4.4 Gaps and limitations in the studies reviewed
In the almost 40 studies and 288 trials reviewed, there were some notable gaps. First, many studies did not use two controls to ascertain seagrass transplantation survival: (1) naturally occurring seagrasses and (2) areas barren of seagrass to compare transplantation survival and growth. Both controls are essential to understand natural changes that occur over time in estuarine or coastal sites. Usually, the changes are discovered in time-sequential monitoring. Second, there was no single monitoring methodology, especially for environmental data and for biological data on lateral growth and density of blades of transplanted seagrass. The data measured were dependent on the capability of the investigators, who frequently measured survival only, without a standard environmental monitoring protocol. This gap created difficulties in making statistical comparisons among trials.
Third, few investigators measured seagrass ecosystem services over the period of maturation of seagrass transplantation, including increased biodiversity (fish, invertebrates, reptiles, mammals, and, at some sites, endangered species), improved fish nursery habitat, sublittoral sediment stability, shoreline stability, water clarity, and organic carbon sequestration in sediment under the seagrass. Exceptions are studies on restored-seagrass services including Marbà et al. (2015) on sedimentary organic carbon under restored seagrass in western Australia and Ambo-Rappe (2022) on recolonization of invertebrates in restored Enhalus sites in northern Indonesia.
Fourth, planting attempts by the non-scientific community stand out as having very different results from plantings led by experienced seagrass scientists. Seagrass restorations at very shallow sites had already been demonstrated to be disrupted by wave energy compared to plantings deeper than 2 m in the 1,786 trials reviewed by van Katwijk et al. (2016). 5 While we appreciate the PEA Thailand community groups’ enthusiasm and concern for the environment, they failed completely. It is our opinion that community efforts should be led by someone with scientific seagrass experience. Data from community plantings created negative results due to their large numbers of PUs not surviving. This lowered the overall survival rate for that species and methodology. Our intuition tells us that community plantings were carried out in very shallow waters, because the groups thought that the depths for mangrove planting would also be appropriate for seagrass planting. Their methods may have followed previous community mangrove planting methods that included planting in dry intertidal areas or in very shallow depths where non-water-skilled community members would be comfortable working. These depths are known to produce very low survival areas for seagrass transplantation, clearly discussed in van Katwijk et al. (2016).
4.5 Recommendations for future seagrass restoration
To improve future success in larger-scale seagrass restoration, based on this review, recommendations for future investigations include the following: (1) Monitoring duration should last at least 3–4 years post-restoration; (2) monitoring should include key environmental factors (listed above), seagrass survival, lateral rhizomal growth expansion, blade length and density conducted across pre- and post-restoration sites, and the nearby naturally occurring seagrass and control areas barren of seagrass; (3) it is optimal to measure animal communities for restoration studies including barren controls [McLaughlin et al. (1983) is an example]; (4) it is highly advisable for materials and funding to be made available to measure organic carbon in sediments at 5-, 10-, and 15-cm depths in restored seagrass at 1 and 2 years and after to build a global database on carbon sequestration associated with seagrass restoration; this can add into an “offset” revenue stream; (5) to prepare for large-scale projects, dominant species in each nation or general coastal region should be tested for tolerance limits to ambient degrading factors at the site such as urban wastes, dredging and filling, agricultural run-off, and other pollutants; and (6) large-scale projects should be funded and carefully managed, planned, and executed as demonstrations to governments, citizens, and organizations that may finance large-scale funding.
4.6 Final concepts
On a much broader scale, this review raises the question, “Can the maximum loss of 7% per year of seagrass ecosystems and their services, predicted by Waycott et al. (2009), be allowed socially (health-wise) and economically (for village employment for planting and enhanced fisheries) in the greater Southeast Asian region?” If not, should governments at the national and international scale as well as other philanthropic groups now begin to conserve and restore seagrasses in large scale?
For those who doubt restoration can catch up with degradation, an example of how rapidly a new technology can be spread throughout the greater Southeast Asian region is the increased mariculture of seaweed and its resulting employment and production in villages throughout Southeast Asia. The type of large-scale restoration we suggest will require national policies focusing on seagrass and coastal habitats to mitigate and restore seagrass. Funding from government agencies and other philanthropic sources as well as training courses are needed.
5 Conclusions
Southeast Asia is estimated to presently have 36,762.6 km2 of seagrass (Fortes et al., 2018), and the greater Southeast Asian region includes additional seagrass extents if PNG, tropical to subtemperate west Australia, and south China are included. The largest global tropical seagrass region is found in this greater Southeast Asian region (Fortes et al., 2018). Minimal estimates are that 50% of the seagrass stock has been degraded over the last century (Ooi et al., 2011; Alongi et al., 2016; Fortes et al., 2018). These losses highlight the challenging need for seagrass restoration. In our review of 228 trials in almost 40 investigations, we found that seagrass was restored with 305,807 PUs, covering an extent of 372,649 m2. Restoration investigations have led to survival in large restoration projects in central western Australia with smaller projects in Indonesia, the Philippines, Thailand, Malaysia, Vietnam, and tropical China (Figure 4). For nine tropical species, E. acoroides at 35% and T. hemprichii at 49.3% survived at higher levels than the more moderate survival of H. ovalis and H. uninervis. For four subtemperate or subtropical species, high to medium survival occurred [P. coriacea (82.3%) and P. australis (63.4%) were the most successful]. Few seagrass services were reported from the restorations: (1) sedimentary carbon measurement under restored seagrass in west Australia (Marbà et al., 2015) and (2) recolonization of invertebrates in restored Enhalus sites in northern Indonesia (Ambo-Rappe, 2022). Other seagrass services were not included in the restoration reports we examined, so we cannot draw actionable conclusions. However, based on comparative data with the Atlantic subtropical and tropical zones, there are indications that restored seagrass meadows appear to be important for the return of lost ecosystem services. This must be fully investigated.
Author contributions
AT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JV: Writing – review & editing, Data curation, Investigation, Methodology, Validation, Visualization. WK: Writing – review & editing, Data curation, Formal analysis, Investigation, Methodology, Visualization. AP: Writing – review & editing, Data curation, Methodology, Validation. XH: Writing – review & editing, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization. JG: Writing – review & editing, Formal analysis, Investigation, Methodology, Validation, Visualization, Conceptualization, Data curation, Writing – original draft. T-KY: Writing – review & editing, Conceptualization, Data curation, Investigation, Validation, Visualization, Methodology. RA-R: Writing – review & editing, Data curation, Investigation, Methodology, Resources, Validation, Visualization. SD: Writing – review & editing, Data curation, Formal analysis, Methodology, Validation, Visualization. AS: Writing – review & editing, Data curation, Investigation, Methodology, Validation, Visualization, Supervision, Writing – original draft. GB: Writing – review & editing, Data curation, Validation, Visualization, Methodology, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Thorhaug and Berlyn partial time from Yale Center for Natural Carbon Capture grant # 7782674. Thorhaug and Schwarz partial time from Texas General Land Office Grant # GLO-23-020-013-D607 and through the TX CCAC as partner federal 76-0491-491.
Acknowledgments
The authors appreciate the following groups for hosting previous presentations and initial International Seagrass Workshop in Singapore and in Annapolis periods of data assembly and writing. We thank other authors with whose permission we cited their data: R. Cruz, E. I. Paling, M. Van Keulen, F. Short (the latter for his map of seagrass global extent), and Y. Shuo. These investigators carried out excellent field work on the original plantings along with the present authors. We cite acknowledgments to project-specific funding sources within cited publications of co-authors. We gratefully acknowledge the multiple funding sources who funded portions of the various reviewed data. Shiran Ben Zeev has contributed to statistical updates to all the tables, to perusing new information, to redrafting figures, to editing the proofs, and other tasks of final preparation. It is with great respect for a most distinguished career in plant sciences we pay tribute to our deceased co-author Professor Graeme P. Berlyn, Harriman Professor of Yale School of Environment who passed away after adding much wisdom to our thoughts.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ Seedling data in this Southeast Asian region were skewed by a single failed trial by a Provincial Electricity Authority in Thailand, where Enhalus acoroides was planted without expert guidance, using the seedling technique to plant 103,000 PUs. These were apparently planted in very shallow depths just before a monsoon by community participation, not by knowledgeable scientists.
- ^ The four types of seagrass seeds (Kuo and den Hartog, 2006) are the following: (1) hard-exterior seeds that bury in sediment and germinate later; (2) fleshy fruit that floats during dispersal, then sink to the bottom (e.g., Enhalus and Thalassia); (3) viviparous seedlings (e.g., Syringodium) dispersed by flotation of fruit or fruit-bearing blades; and (4) floating seeds (Ruppia).
- ^ The terms “low light” is ≤15% of surface light, “medium light” is 16%–50% of surface light, and “high light” is above 50% surface light.
- ^ Our use of qualitative energy terms was as follows: Low-energy sites are highly protected, experiencing calm water, with exposure to low currents and low tidal flow. Medium-energy sites are associated with occasional strong waves and currents during storms and moderately protected from prevailing or occasional high storm winds. High-energy sites are far less protected from prevailing winds and can experience the effect of fetch, open ocean waves, and occasionally major currents.
- ^ We offer the caveat that seagrass restoration is more difficult than mangrove or marsh restoration and should not be thought of as using similar methods. Those experienced in seagrass restoration have repeatedly found that it does not have similar success rates as mangrove or marsh restoration.
References
Action Chemical Company (2018). Seagrass Restoration for a better Gulf of Thailand. Available online at: http://www.acc1976.com/news_june_60_N2.php (Accessed 12 March 2020).
Alongi D. M., Murdiyarso D., Fourqurean J. W., Kauffman J. B., Hutahaean A., Crooks S., et al. (2016). Indonesia’s blue carbon: a globally significant and vulnerable sink for seagrass and mangrove carbon. Wetlands Ecol. Manage. 24, 3–13. doi: 10.1007/s11273-015-9446-y
Ambo-Rappe R. (2022). The success of seagrass restoration using Enhalus acoroides seeds is correlated with substrate and hydrodynamic conditions. J. Environ. Manage. 310, 114692. doi: 10.1016/j.jenvman.2022.114692
Arias-Ortiz A., Serrano O., Masqué P., Lavery P. S., Mueller U., Kendrick G. A., et al. (2018). A marine heatwave drives massive losses from the world’s largest seagrass carbon stocks. Nat. Climate Change 8, 338–344. doi: 10.1038/s41558-018-0096-y
Ashton P. S. (2015). On Forests of Tropical Asia: Lest the Memories Fade (Richmond, Surrey, UK: Kew Publishing).
Asriani N., Ambo-Rappe R., Lanuru M., and Williams S. L.. (2019). Macrozoobenthos community structure in restored seagrass, natural seagrass and seagrassless areas around Badi Island, Indonesia. Earth Environ. Sci. 253, 812034. doi: 10.1088/1755-1315/253/1/012034
Buelow C. A., Connolly R. M., Turschwell M. P., Sousa A. I., Worthington T. A., and Brown C. J.. (2022). Ambitious global targets for mangrove and seagrass recovery. Curr. Biol. 32 (7), 1641–1649.
Calumpong H. P., Menez E. G., and Phillips R. C. (1996). “Factors affecting survival and growth of reciprocal seagrass transplants on Negros Island, Central Philippines,” in Seagrass Biology: Scientific Discussion from an International Workshop. Eds. Kuo J., Walker D. I., and Kirkman H. (University of Western Australia, Rottnest Island, Western Australia), 59–64.
Conservation Biology Institute (2010). Water sheds of Southeast Asia. Available online at: http://patonggho.blogspot.com/2014/08/blog-post.html (Accessed 22 May 2019).
Cohen-Shacham E., Walters G., Janzen C., and Maginnis S. (2016). Nature-based solutions to address global societal challenges. IUCN: Gland, Switzerland 97, 2036.
Durance C. (2001). “A review and assessment of eelgrass transplant projects in British Columbia, document prepared for Fisheries and Oceans Canada, South Coast Area, Nanaimo: 37.Ejatlas. 2017. Montara Oil Spill,” in Atlas of Environmental Justice. Available online at: https://ejatlas.org/conflict/montara-oil-sp.
Fortes M. D., Ooi J. L. S., Tan Y. M., Prathep A., Bujang J. S., and Yaakub S. M. (2018). Seagrass in Southeast Asia: a review of status and knowledge gaps, and a road map for conservation. Botanica Marina 61, 269–288. doi: 10.1515/bot-2018-0008
Gallagher J. B., Chuan C. H., Yap T.-K., and Fredelina Dona W. F. (2019). Carbon stocks of coastal seagrass in Southeast Asia may be far lower than anticipated when accounting for black carbon. Biol. Lett. 15, 20180745. doi: 10.1098/rsbl.2018.0745
Giri C., Ochieng E., Tieszen L. L., Zhu Z., Singh A., Loveland T., et al. (2011). Status and distribution of mangrove forests of the world using earth observation satellite data. Global Ecol. Biogeography 20, 154–159. doi: 10.1111/j.1466-8238.2010.00584.x
Green E. P. and Short F. T. (2003). World Atlas of Seagrasses (Berkeley, USA: University of California Press).
Harrison M. and Kaufman R. (2011). “Western pinellas county. Florida seagrass integrated mapping and monitoring program,” in Seagrass Integrated Mapping and Monitoring for the State of Florida Mapping and Monitoring Report No. Eds. Yarbro L. A. and Carlson P. R. (Florida Fish and Wildlife Conservation Commission Fish and Wildlife Research Institute, St. Petersburg, Florida).
Horn L. E., Paling E. I., and van Keulen M. (2009). Photosynthetic recovery of transplanted Posidonia sinuosa, Western Australia. Aquat. Bot. 90, 149–156. doi: 10.1016/j.aquabot.2008.08.002
Kendrick G. A., Austin R., Ferretto G., van Keulen M., Verduin J. J., et al (2025). Lessons learnt from revisiting decades of seagrass restoration projects in Cockburn Sound, southwestern Australia. Restoration Ecology 23 (4), 70040. doi: 10.1111/rec.70040
Kirkman H. (1999). Pilot experiments on planting seedlings and small seagrass propagules in Western Australia. Marine Pollution Bulletin 37 (8-12), 60–467. Provincial Electricity Authority (PEA). 2016. PEA Seagrass restoration in PEA for a better Thai sea. doi: 10.1016/S0025-326X(99)00146-0
Kiswara W. (2013). Tehnik transplantasi lamun yang mudah dan murah: Enhalus acoroides dan kumpulan tunas Thallasia hemprichii Pulau Pari, Jakarta (Yogyakarta: Seminar Nasional Tahunan. X Hasil Penelitian Kelautan dan Perikanan, Universitas Gajahmada).
Kiswara W. (2018). Seagrass and Carbon studies in Indonesian Waters (Jakarta, Indonesia: Presentation at the International Workshop on Indonesian Carbon).
Kiswara W., Bouma T. J., Huiskes A. H. L., and Herman P. M. J. (2010b). “Survival and development transplant single shoots of Enhalus acoroides L.f. Royle in different morphological International Seagrass types at Banten Bay, Indonesia,” in World Seagrass Conference and 9 Biology Workshop (ISBW9)(Trang, Thailand), 77–84.
Kiswara W., Kumoro E. D., Kawaroe M., and Rahadian N. P. (2010a). Transplanting Enhalus Acoroides (l.f) royle with different length rhizome on the muddy substrate and high water dynamic at Banten Bay, Indonesia. Mar. Res. Indonesia 35, 1–7. doi: 10.14203/mri.v35i2.472
Kiswara W. and Ulumuddin Y. I. (2010). “Transplantasi lamun Enhalus acoroides II: Pengaruh kompos terhadap pertumbuhan bibit di Pulau Pari, Teluk Jakarta,” in Dinamika Ekosistem Perairan Kepulauan Seribu. Eds. Muchtar D., Pramudji M., Sulistyo, and Fahmi(Teluk Jakarta), 118–122.
Kuo J. and den Hartog C. (2006). “Seagrass morphology, anatomy, and ultrastructure,” in Seagrasses: biology, ecology and conservation. Eds. Larkum A. W. D., Orth R. J., and Duarte C. M. (Springer, Dordrecht, Netherlands), 51–87.
Lanuru M. (2011). “Bottom sediment characteristics affecting the success of seagrass (Enhalus acoroides) transplantation in the west coast of South Sulawesi (Indonesia),” in 3rd International Conference on Chemical, Biological and Environmental Engineering IPCBEE, vol. 20. (IACSIT Press, Singapore), 97–102.
Langlois L. A., Collier C. J., and McKenzie L. J. (2023). Subtidal seagrass detector: development of a deep learning seagrass detection and classification model for seagrass presence and density in diverse habitats from underwater photoquadrats. Frontiers in Marine Science 10, 97695.
Macreadie P. I., Anton A., Raven J. A., Beaumont N., Connolly R. M., Friess D. A., et al. (2019). The future of Blue Carbon science. Nat. Commun. 10, 1–13. doi: 10.1038/s41467-019-11693-w
Marbà N., Arias-Ortiz A., Masqué P., Kendrick G. A., Mazarrasa I., Bastyan G. R., et al. (2015). Impact of seagrass loss and subsequent revegetation on carbon sequestration and stocks. J. Ecol. 103, 296–302. doi: 10.1111/jec.2015.103.issue-2
McKenzie L. J., Nordlund L. M., Jones B. L., Cullen-Unsworth L., Roelfsema C., and Unsworth R. K. F.. (2020). Global extent of seagrass meadow. Environ. Res. Lett. 15, 074041. doi: 10.1088/1748-9326/ab7d06
McLaughlin P., Treat S., Thorhaug A., and Lemaitre R. (1983). A restored seagrass (Thalassia) bed and its animal community. Environ. Conserv. 10, 247–254. doi: 10.1017/S0376892900012662
Metropolitan Electricity Authority (MEA) of Trang (2008). Report on seagrass restoration. Pers. communication.
Nakaoka M., Lee K. S., Huang X., Almonte T., Bujang J. S., Kiswara W., et al. (2014). “Regional comparison of the ecosystem services from seagrass beds in Asia,” in Integrative Observations and Assessments. Ecological Research Monographs. Eds. Nakano S., Yahara T., and Nakashizuka T. (Springer, Tokyo, Japan), 367–391.
Nordlund L. M., Koch E. W., Barbier E. B., and Creed J. C. (2017). Seagrass ecosystem services and their variability across genera and geographical regions. PloS One 12, e0169942. doi: 10.1371/journal.pone.0169942
Ooi J. L. S., Kendrick G., Van Niel K., and Affendi A. (2011). Knowledge gaps in tropical Southeast Asian seagrass systems. Estuarine Coast. Shelf Sci. 92, 118–131. doi: 10.1016/j.ecss.2010.12.021
Orth R. J., et al. (2020). Restoration of seagrass habitat leads to rapid recovery of coastal ecosystem services. Science 6, 643. doi: 10.1126/sciadv.abc643
Paling E. I., van Keulen M., and Tunbridge D. J. (2007). Seagrass transplanting in Cockburn Sound, Western Australia: A comparison of manual transplantation methodology using Posidonia sinuosa Cambridge et Kuo. Restor. Ecol. 15, 240–249. doi: 10.1111/j.1526-100X.2007.00207.x
Paling E. I., van Keulen M., and Wheeler K. D. (1998). Seagrass Rehabilitation in Owen Anchorage, Western Australia (Murdoch, Australia: Murdoch University). Report no. MAFRA 98/4.
Paling E. I., van Keulen M., Wheeler K. D., Phillips J., and Dyhrberg R. (2001a). Mechanical seagrass transplantation in Western Australia. Ecol. Eng. 16, 331–339. doi: 10.1016/S0925-8574(00)00119-1
Paling E. I., van Keulen M., Wheeler K. D., Phillips J., and Dyhrberg R. (2003). Influence of spacing on mechanically transplanted seagrass survival in a high wave energy regime. Restor. Ecol. 11, 56–61. doi: 10.1046/j.1526-100X.2003.00072.x
Paling E. I., van Keulen M., Wheeler K. D., Phillips J., Dyhrberg R., and Lord D. A. (2001b). Improving mechanical seagrass transplantation. Ecol. Eng. 18, 107–113. doi: 10.1016/S0925-8574(01)00065-9
Paling E. I., van Keulen M., Wheeler K. D., and Walker C. (1999). Effects of depth on manual transplantation of the seagrass Amphibolis griffithii (J. M. Black) den Hartog on Success Bank, Western Australia. Pacific Conserv. Biol. 5, 314–320. doi: 10.1071/PC000314
Pansert T., et al. (2016). Seagrass Restoration: An Update from Trang Province, Southwestern Thailand. https://academuspub.com/en/nauka/conference/section/30/view. COASTAL AND MARINE ECOSYSTEMS MONITORING AND MODELINGProceedings: https://academuspub.com/en/nauka/collection/46/view. MATERIALS OF XXVI INTERNATIONAL COASTAL CONFERENCE "MANAGINAG RISKS TO COASTAL REGIONS AND COMMUNITIES IN A CHANGINAG WORLD" CSCSTI 34.35.
Provincial Electricity Authority (PEA) (2016). PEA Seagrass restoration in PEA for a better Thai sea.
Puruhito H. N. F., Addini I., and Nugraha A. H. (2024). The influence of planting distance on seagrass (Enhalus acoroides) seedling growth Dipak. Jurnal Ilmu-Ilmu Perairan Pesisir dan Perikanan 13, 274–280.
Qiu G., Zhou Y., Liu P., Liu B., and Liu X.. (2014). Transplantation techniques for restoring the intertidal seagrasses in Guangxi, China. Mar. Sci. 38, 24–30. doi: 10.11931/guihaia.gxzw202401056
Reid A. E. A., Haissoune A., and Ferber P. I.. (2019). Status of coral reefs and seagrass Indonesia rep Cambodian natural history. Cambodian Journal of Natural History. 2019, 24–39.
Royal Thai Navy, Ministry of Defense (2017). Seagrass Research in Sikao (Trang). Available online at: http://www.tis-museum.org/research/08.pdf.
Saleh E., Yap T. K., and Gallagher J. B. (2020). Seagrass coverage and associated fauna at Gaya Island, Sabah, Malaysia: A pilot seagrass transplantation. Borneo J. Mar. Sci. Aquaculture 4, 14–19. doi: 10.51200/bjomsa.v4i1.1786
Seraphim M. J., Ambo-Rappe R., et al. (2020). Interactions between coral restoration and fish assemblages: implications for reef management. Journal of Fish Biology 97 (3), 633–655.
Shen J., Yin L., Zhang J., Shuwen J., Wang Y., Wang A., et al. (2023). Thalassia & Enhalus. Front. Mar. Sci. 10, 3389. doi: 10.3389/fmars.2023.1294779
Shuo Y. U., Zhang J., Cui L., Jiang Z., Zhang L., and Huang X. (2019). Preliminary study on seedbased restoration for Enhalus acoroides meadow. J. Trop. Oceanography 38, 49–54. doi: 10.1016/j.marpolbul.2025.118205
Spalding M., Kainuma M., and Collins L. (2010). World atlas of mangroves (London, United Kingdom: A collaborative project of ITTO, ISME, FAO, UNEP-WCMC, UNESCO-MAB, UNU-INWEH and TNC. Earthscan).
Sprintall J., Wijffels S. E., Molcard R., and Jaya I. (2009). Direct estimates of the Indonesian Throughflow entering the Indian Ocean: 2004–2006. J. Geophysical Research: Oceans 114. doi: 10.1029/2008JC005257
Talbot F. and Wilkinson C. R. (2001). Coral reef, mangroves, and seagrass: A source book for managers (Australian Institute Marine Science).
Thorhaug A. (1985). Large-scale seagrass restoration in a damaged estuary. Mar. pollut. Bull. 16, 55–62. doi: 10.1016/0025-326X(85)90124-9
Thorhaug A. (1987). Large-scale seagrass restoration in a damaged estuary. Mar. pollut. Bull. 18, 442–446. doi: 10.1016/0025-326X(87)90621-7
Thorhaug A. (2001). “Petroleum industry’s use of seagrass restoration as a mitigation for construction and as a potential cleanup tool,” in Proceedings International Oil Spill Conference (APT/EPA/USCG), Washington, DC, USA. 386–391.
Thorhaug A., Belaire C., Verduin J. J., Schwarz A., Kiswara W., Prathep A., et al. (2020b). Longevity and sustainability of tropical and subtropical restored seagrass beds among Atlantic, Pacific, and Indian Oceans. Mar. pollut. Bull. 160, 111168–111175. doi: 10.1016/j.marpolbul.2020.111544
Thorhaug A. and Cruz R. T. (1987). Restoration of seagrass in a series of polluted sites in the Philippines (Rome Italy: FAO). FAO report FAO-FI-TCP/PHI/4511.
Thorhaug A. and Cruz R. T. (1988). “Seagrass restoration in the Pacific tropics,” in Proceedings of the 6th International Coral Reef Symposium, vol. 2 . Eds. Choat J. H., Barnes D., Borowitzka M. A., Coll J. C., Davies P. J., Flood P., Hatcher B. G., et al (Townsville, Australia), 415–419.
Thorhaug A., Gallagher J. B., Kiswara W., Prathep A., Huang X., Yap T. K., et al. (2020a). Coastal and estuarine blue carbon stocks in the greater Southeast Asia region: seagrasses and mangroves per nation and sum of total. Mar. pollut. Bull. 160, 1–17. doi: 10.1016/j.marpolbul.2020.111168
Thorhaug A., Gallagher J. B., Lopez-Portillo J., Poulos H. M., Berlyn G. P., Schwarz A., et al. (2020c). “Emerging constraints of carbon and influence of water in regional blue carbon tropical estuarine/coastal hot spots: policies, climate mitigation planning,” in Carbon monitoring Systems and Applications (American Geophysical Union, San Francisco, CA, USA). Abstract B108-0021.
Thorhaug A. and Hixon R. (1975). “Revegetation of Thalassia testudinum in a multiple stressed estuary, North Biscayne Bay, Florida,” in Proceedings of the Second Annual Conference on restoration of coastal vegetation in Florida. Ed. Lewis R. R. (Hillsborough Community College, Tampa, Florida, USA), 12–27.
Thorhaug A., Miller B. A., Jupp B., and Booker F. (1985). Effects of a variety of impacts on seagrass restoration in Jamaica. Mar. pollut. Bull. 16, 355–360. doi: 10.1016/0025-326X(85)90086-4
Todd P. A., Ong X., and Chou L. M. (2010). Impacts of pollution on marine life in Southeast Asia. Biodiversity Conserv. 19, 1063–1082. doi: 10.1007/s10531-010-9778-0
Tri P. H. (2008). Rehabilitation and Conservation the Seagrass Meadows At Cam Hai Dong, Cam Ranh Bay, Khanh Hoa Province, Central Vietnam (Nhatrang, Vietnam: Institute of Oceanography).
van Katwijk M. M., Thorhaug A., Marbà N., Orth R. J., Duarte C. M., Kendrick G. A., et al. (2016). Global analysis of seagrass restoration: The importance of large-scale planting. J. Appl. Ecol. 53, 567–578. doi: 10.1111/jpe.2016.53.issue-2
van Keulen M., Paling E. I., and Walker C. J. (2003). Effect of planting unit size and sediment stabilization on seagrass transplants in Western Australia. Restor. Ecol. 11, 50–55. doi: 10.1046/j.1526-100X.2003.00036.x
Verduin J. J., Paling E., and van Keulen M. (2010). “Seagrass rehabilitation; requisites to successful, long term, mitigation outcomes,” in 9th International Seagrass Biology Workshop (ISBW9), Trang, Thailand.
Verduin J. J., Paling E. I., van Keulen M., and Rivers L. E. (2012). Recovery of donor meadows of Posidonia sinuosa and Posidonia australis contributes to sustainable seagrass transplantation. Int. J. Ecol. 56, 42–43. doi: 10.1155/2012/837317
Verduin J. J. and Sinclair E. A. (2013). Seagrass meadow restoration trial using transplants – Cockburn Sound, Western Australia. Available online at: https://site.emrprojectsummaries.org/2013/03/08/seagrass-meadowrestoration-trial-usingtransplants-cockburn-sound-western-Australia/.
Verduin J. J., van Keulen M., and Rivers M. (2024). Success of a large scale, long term seagrass restoration trial of Posidonia australis using mature shoots. Manuscript submitted for publication. doi: 10.1111/rec.70040
Verduin J. J., Paling E. I., Pedrettie Y., Rivers L., and van Keulen M. (2025). Adaptive Management and Long-Term Monitoring Approaches Achieve First Functional Seagrass Meadow Restoration in Cockburn Sound, Western Australia. under review: Estuarine, Shelf and Coastal Sciences.
Vichkovitten T., Intarachart A., Khaodon K., and Rermdumri S. (2016). Transplantation of tropical seagrass Enhalus acoroides (L.) in Thai Coastal Water. GMSARN Int. J. 10, 113120. doi: 10.1515/BOT.2016.009
Waycott M. W., Duarte C. M., Carruthers T. J. B., Orth R. J., Dennison W. C., Olyarnik S., et al. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. PNAS 106, 12377–12381. doi: 10.1073/pnas.0905620106
Williams S. ,. L., Ambo- Rappe R., Sur C., Abbott J. M., and Limbong S. R. (2017). Species richness accelerates marine ecosystem restoration in the Coral Triangle. PNAS 114, 11986–11991. doi: 10.1073/pnas.1707962114
Wismar J. E., Ambarinyanto A., and Widingsighne. (2023). Seagrass (Enhalus acoroides) Restoration Performance with Two Different Methods (Anchor and Seed) in Panjang Island, Jepara, Indonesia. Jurnal Ilmiah Perikanan dan Kelautan. 15 (1), 84–94. doi: 10.20473/jipk.v15i1.35836
Keywords: seagrass restoration, seagrass restoration in Souteast Asian region, seagrass restoration Enhalus acoroides, seagrass restoration Thalassia hemprechii, seagrass restoration southeast Asia survival and success, seagrass restoration Halophila ovalis
Citation: Thorhaug A, Verduin JJ, Kiswara W, Prathep A, Huang X, Gallagher JB, Yap T-K, Ambo-Rappe R, Dorward SE, Schwarz A and Berlyn GP (2025) Seagrass restoration in the greater Southeast Asia region: techniques, species, survival and comparisons among investigations. Front. Mar. Sci. 12:1505222. doi: 10.3389/fmars.2025.1505222
Received: 02 October 2024; Accepted: 08 July 2025;
Published: 31 October 2025.
Edited by:
Jutta Papenbrock, Leibniz University Hannover, GermanyReviewed by:
W. Judson Kenworthy, Independent Researcher, Beaufort, NC, United StatesKapuli Gani Mohamed Thameemul Ansari, C. Abdul Hakeem College, India
Copyright © 2025 Thorhaug, Verduin, Kiswara, Prathep, Huang, Gallagher, Yap, Ambo-Rappe, Dorward, Schwarz and Berlyn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anitra Thorhaug, YXRob3JoYXVnQG1zbi5jb20=
†Deceased
 Anitra Thorhaug
Anitra Thorhaug Jennifer Joan Verduin
Jennifer Joan Verduin Wawan Kiswara4,5,6
Wawan Kiswara4,5,6 Anhana Prathep
Anhana Prathep Xiaoping Huang
Xiaoping Huang Rohani Ambo-Rappe
Rohani Ambo-Rappe