- 1College of Animal Science and Technology, Guangxi University, Nanning, China
- 2Key Laboratory of Tropical Marine Ecosystem and Bioresource, Fourth Institute of Oceanography, Ministry of Natural Resources, Beihai, China
- 3Institute of Marine Drugs, Guangxi University of Chinese Medicine, Nanning, China
- 4Fisheries College, Key Laboratory of Healthy Mariculture for the East China Sea, Ministry of Agriculture, Jimei University, Xiamen, China
- 5Health and Environmental Research Center, Faculty of Environmental Management, Prince of Songkla University, Hat Yai, Songkhla, Thailand
Portunion (Isopoda, Epicaridea, Entoniscidae) is an extremely rare endoparasitic isopod genus that is found mainly in the blood cavity of decapod crustaceans, such as true crabs and hermit crabs. The mud crab (Scylla paramamosain) is one of the most economically important crab species in south China and Asia. In this study, we identified a new endoparasitic isopod species, Portunion sinensis sp. nov., from the hemocoel of S. paramamosain. Portunion sinensis sp. nov. is similar to Portunion conformis in having the anterior vertical to thorax, the posterior directed backwards for Female’s ventral processes. However, the new species can be distinguished from Portunion conformis by the rudimentary abdominal spines present on segment III-IV in males. Herein we describe a detailed morphological description of this new species and conduct an extensive comparison with related species. This study marks the first description Portunion sinensis sp. nov. of an endoparasitic isopod species found in Scylla paramamosain in China. The findings expand the known biodiversity of parasitic isopods and contribute to understanding the host-parasite associations within this commercially significant crab species in the region.
1 Introduction
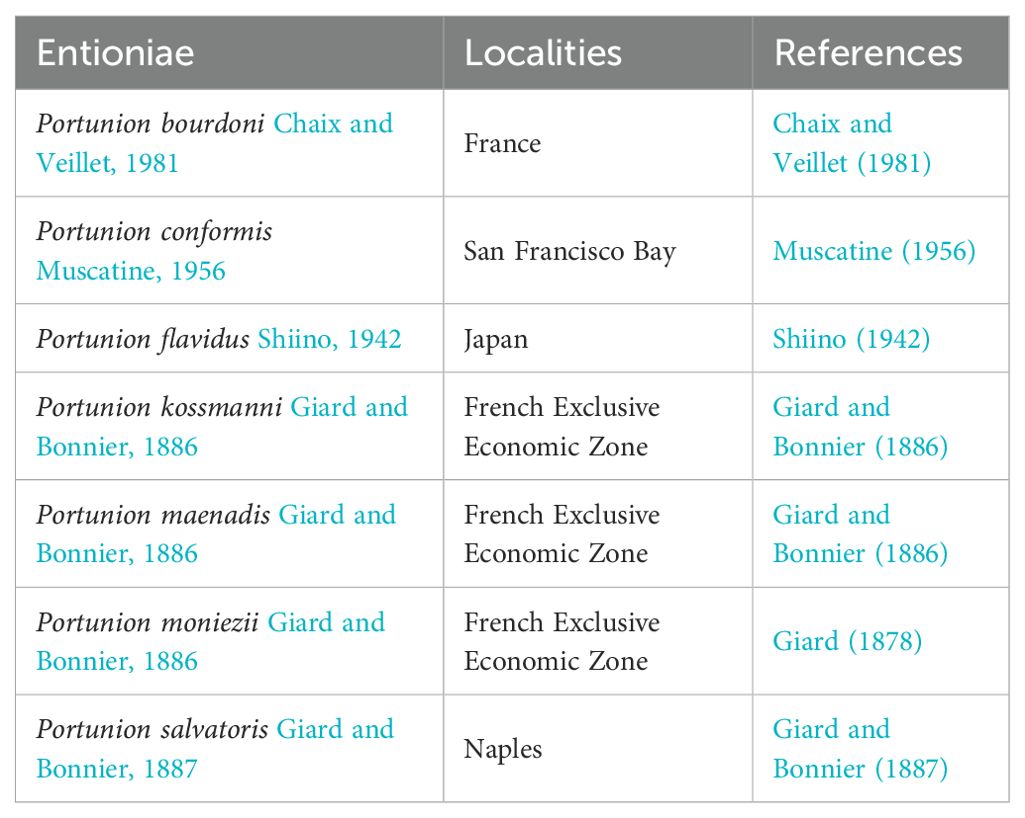
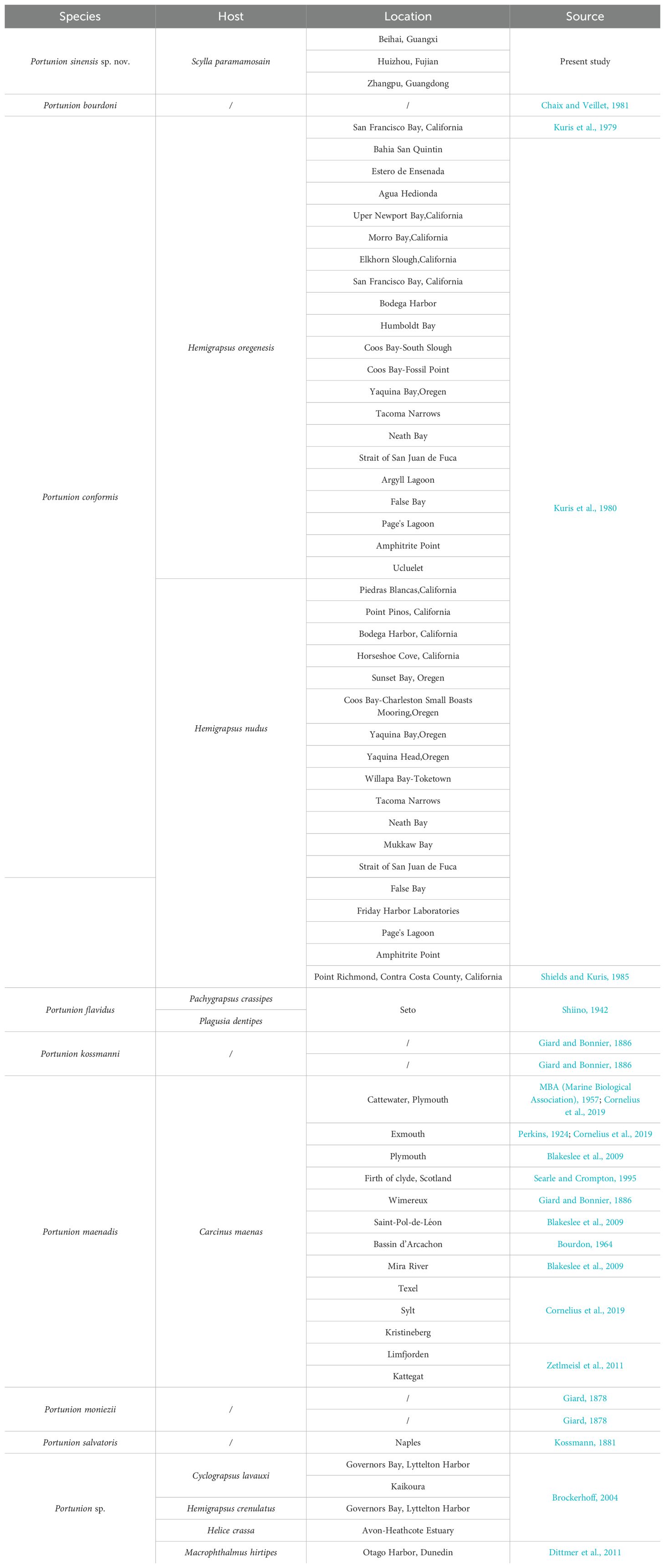
Bopyrid isopods infesting Scylla paramamosain belong to the superfamily Bopyroidea, family Entoniscidae, subfamily Entioninae. Species in the superfamily Bopyroidea are known for their holoparasitic behaviors. They mainly parasitize the gill cavity or abdomen of crustaceans (Jianmei, 2011) and can affect the reproduction and development of the host (Williams and Boyko, 2012). One member of this superfamily is the family Entoniscidae, which contains 53 species and includes the genus Portunion. These are rare isopod parasites that inhabit the hemocoel of decapod crabs. Only seven valid Portunion species were recognized in the latest WoRMS list (Table 1). Species of Portunion have been reported in various areas, including the North Atlantic (Association, 1957; Blakeslee et al., 2009; Cornelius et al., 2019; Perkins, 1925; Zetlmeisl et al., 2011), the northeast Pacific (Kuris et al., 1979; Shields and Kuris, 1985), and the southwest Pacific (Brockerhoff, 2004; Koehler and Poulin, 2010) (Table 2). We summarize the characteristics that differentiate the new species from other Portunion species. A key to the genera of the Entoniscidae is also provided. Prior to this study, however, there was no description of Portunion spp. from the mud crab S. paramamosain.
2 Materials and methods
2.1 Sample collection and prevalence survey
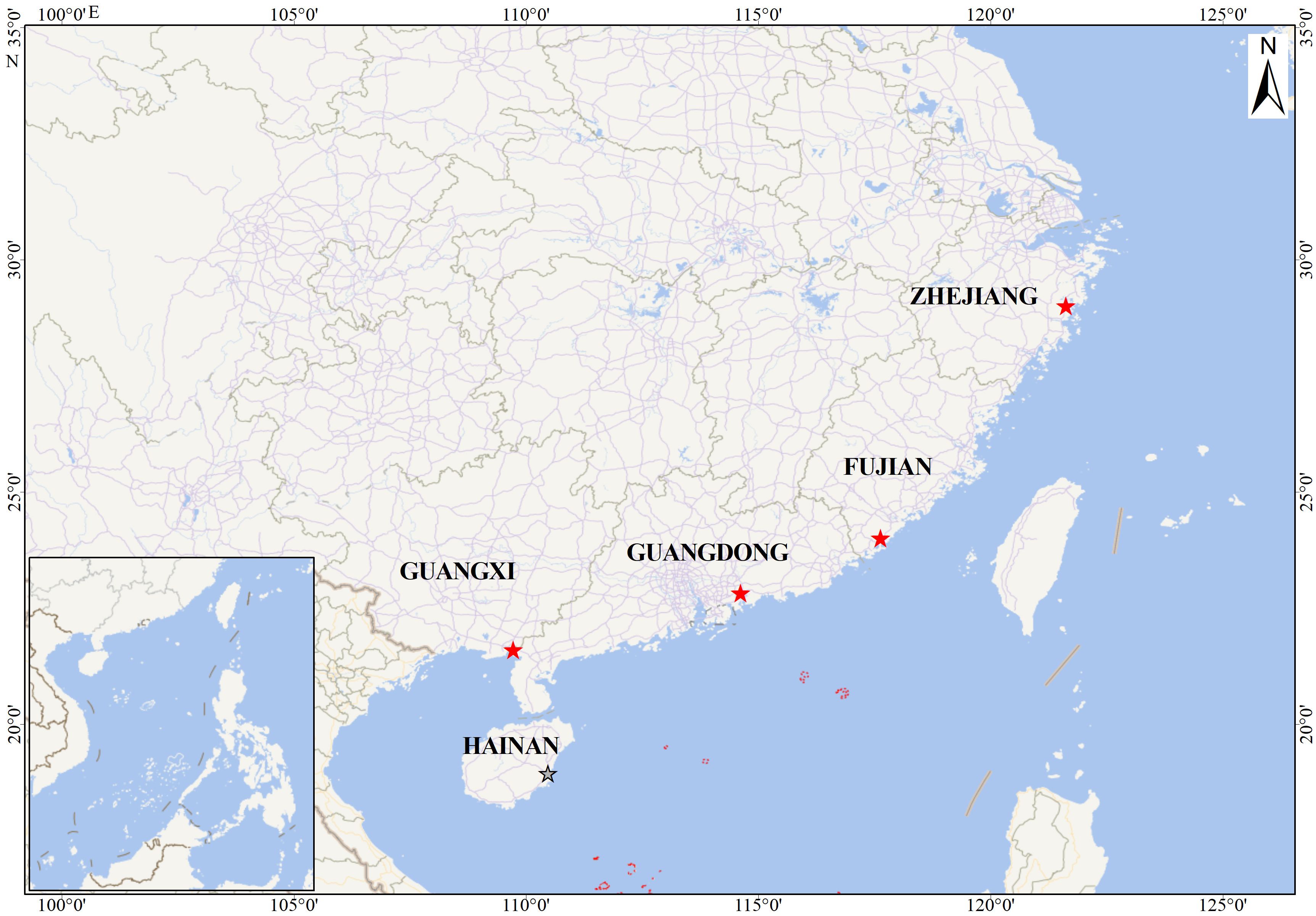
We collected specimens of S. paramamosain from 2019 to 2024 from five provinces in China: Beihai, Guangxi Zhuang Autonomous Region (21° 33’ 10.86” N, 109° 40’ 16.11” E); Huizhou, Guangdong province (22° 40’ 4.38” N, 114° 44’ 17.12” E); Zhangpu, Fujian province (23° 52’ 58.15” N, 117° 33’ 43.39” E); Taizhou, Zhejiang province (29° 06’ 49” N,121° 23’ 13” E); and Haikou, Hainan province (18° 54’ 6.82” N, 110° 31’ 27.37” E) (Figure 1). Crab specimens were collected from natural aquatic environments and transported to the laboratory within a period not exceeding 2 days. Upon arrival at the laboratory, the crabs were humanely euthanized by exposure to freezing conditions at 0°C, achieved through the use of an ice-water mixture, prior to dissection. The carapace of each S. paramamosain specimen was meticulously removed by making precise incisions along the suture with a medical scalpel. In instances where present, the female parasite was examined and delicately dissected to separate it from the host’s visceral mass. Marsupium dissection of the females yielded eggs, embryos, epicaridium larvae, and dwarf males.
2.2 Morphological analysis
The morphological characteristics of the parasites were documented, and specimens were imaged using a stereoscopic microscope (SteREO Discovery.V12, Zeiss, Oberkochen, Germany). For scanning electron microscopy (SEM) analysis, adult parasites were fixed in SEM fixation buffer (G1102, Servicebio, Wuhan, China), meticulously washed, and dehydrated for 10 minutes, followed by critical point drying. Parasite embryos and epicaridium larvae were similarly fixed in SEM fixation buffer, washed, and post-fixed with osmium tetroxide for 30 to 60 minutes, and then dehydrated for 5 minutes prior to critical point drying. Upon completion of sample preparation, the parasites were examined and photographed using a FESEM SU 8100 SEM (Hitachi, Tokyo, Japan).
3 Results and Discussion
3.1 Taxonomy
Order Isopoda Latreille, 1817.
Suborder Cymothoida Wägele, 1989.
Superfamily Bopyroidea Rafinesque, 1815.
Family Entoniscidae Kossmann, 1881.
Subfamily Entioninae Codreanu, Codreanu and Pike, 1960.
3.2 Genus Portunion Giard and Bonnier, 1886
Diagnosis. The description below follows the terminology of the Shiino system (Shiino, 1942).
Female. Females were categorized into five developmental stages based on external morphology: the C-shaped larva (first stage), the straight juvenile (Figure 2A), the V-shaped large juvenile (Figure 2B), the mature adult (Figure 2C), and the ovigerous adult (Figure 2D).
Adult Female. The body is highly modified due to their parasitic lifestyle. Their deformed sac-like bodies have few distinguishing morphological characteristics, except for faint segmentations. The entire body is intricately intertwined with the hepatopancreas and gonads of its host. In mature and ovigerous adults, all oostegites meet each other on the opposite side and form the marsupium. The pleopods are uniramous lamellae with highly crimped margins. The first pair is much larger than the others and the pleopods get gradually smaller from front to back. Ovigerous adults have a whitish to yellowish ovary and an abdomen that is whitish to translucent. The marsupium is composed of several pairs of oostegites and the host response sheath. It is exceedingly inflated, extending dorsally and covering the spherical cephalogaster. It is pale yellow when full of extruded eggs and developing embryos; when full of epicaridium larvae, it is brown to dark brown.
C-shaped larva. According the description in Shiino’s study, it has an appearance of a caterpillar and is strongly curved ventrally, so that the cephalon almost touches the pygidium. No clear specific difference is discernible in this stage.
Straight juvenile. The body is dorsally folded similar to the adult form, but it straightens when the parasite is extracted from the host, except for a slight ventral curvature in the thoraco-abdominal region. The oostegites are somewhat expanded, with the first pair extending anteriorly beyond the cephalon, forming a rudimentary “hood.” The last two pairs of peraeopods are somewhat enlarged into lateral protuberances. The pleopods have begun to overlap each other, and the pleural lamellae have developed crispate margins.
V-shaped large juvenile. At this stage, all major bodily organs are substantially developed. The stage is characterized by the protrusion of ovarian processes, the enlargement of oostegites, and the development of pleural folds.
Male. The male, without having any membrane enclosing it, has more or less the typical constitution of an isopod, although it has degenerated in many points; its body is normally curved ventrally. All segments are distinct. Thorax gradually narrows from the 5th segment forwards, the 1st being half as wide as the 5th; lateral parts are attenuated except in the last segment, which has a truncated margin. The limbs of the first six thoracic segments are all similar, only increasing in size posteriorly. They are scarcely prehensile in all cases. The last thoracic segment is limbless, but is provided with protuberances bearing gonopores.
Epcaridium larvae. The Epicaridea stage has a strong dorsal convexity and scattered pigment patterns. The cephalon is evenly rounded in front, bearing, on either side, a few crystallines surrounded by chromatophores. The antennules are short and two-jointed, while the antennas are long and composed of six joints of which the last two form a comparatively short flagellum. The oral cone encloses the usual piercing mandibles. Of the peraeopoda, the seventh pair of lacking. The sixth peraeopod differs in structure from others. The propodus bears a short process at the lateral end of its distal margin and merus with a very small seta on the external margin. This pair is slightly smaller than the foregoing pairs, the dactylopodite at the same time being highly degenerated, and the propodus ending in a short-pointed process.
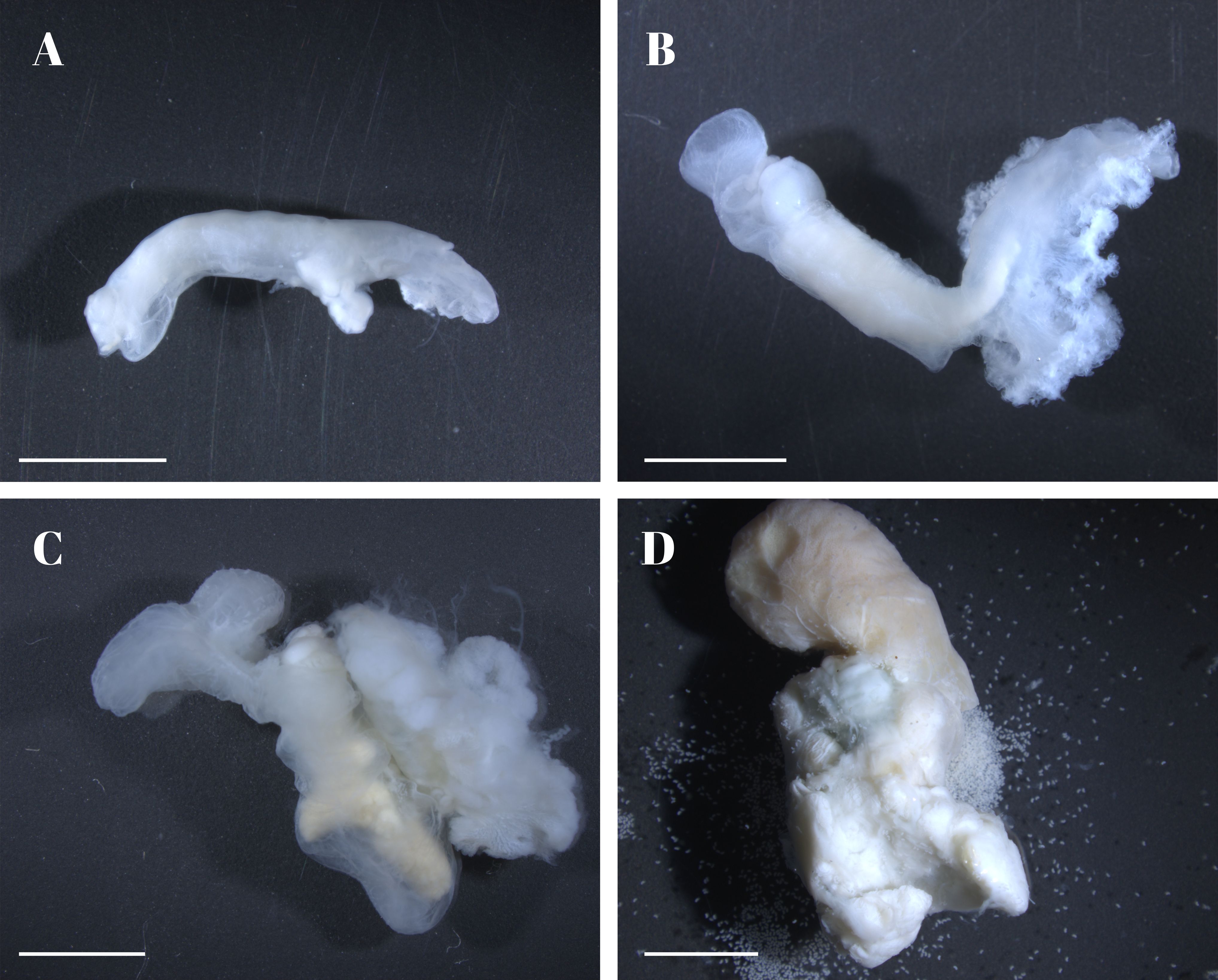
Figure 2. Illustration of different stages of the female Portunion sinensis sp. nov. (A) straight juvenile; (B) V-shaped juvenile; (C) mature adult not yet spawning; (D) ovigerous adults with eggs and developing embryos in the marsupium. Scale: 2 mm.
3.3 Portunion sinensis sp. nov.
https://zoobank.org/D5BB6083-114B-4D52-AF54-F79CD5604055.
Material examined. Infesting S. paramamosain. Key Laboratory of Tropical Marine Ecosystem and Bioresource, Fourth Institute of Oceanography, Ministry of Natural Resources, Beihai, China. Holotype, female; Zhang pu, Fujian China. Allotype, male; Zhang pu, Fujian China. Paratype, epcaridium larvae, Zhang pu, Fujian China.
Description
Holotype, adult female (Figure 2C)
Body (Figure 3A) of mature adult is curved in a U-shape, from hood to tip of posterior ventral process ca. 8.1 mm, abdomen ca. 8.8 mm in the largest specimen.
Cephalon is swollen into a double-spherical shape and is distinctly demarcated from the thorax.
Antennae, two parallel pairs of antennae, both external and internal, are inserted at the anterior part of the cephalogaster.
Maxillipeds are situated at the ventral border between the cephalon and the thorax. The exopodite of the maxilliped is broad and features a frilled surface, while the endopodite is oval-shaped and considerably smaller in comparison.
Ovarian processes. There are two ventral processes on the chest and a pair of dorsal ovarian processes. The dorsal ovarian processes arise from the anterior end of the thorax; of the two ventral processes, the anterior is shorter and makes a right angle with the posterior which projects backward.
Oostegite 1 (Figure 3B) is well developed, composed of three uniramous lobes: ascendant lobe covering the cephalogaster; transverse lobes short and blunt; recurrent lobe largest and appears slightly wrinkled, extending the entire length of pereon. Oostegites 2 to 5 are typically distinctly separated, adhering to the host membrane, enclosing a marsupium; it may adhere to the host membrane and be removed together during dissection.
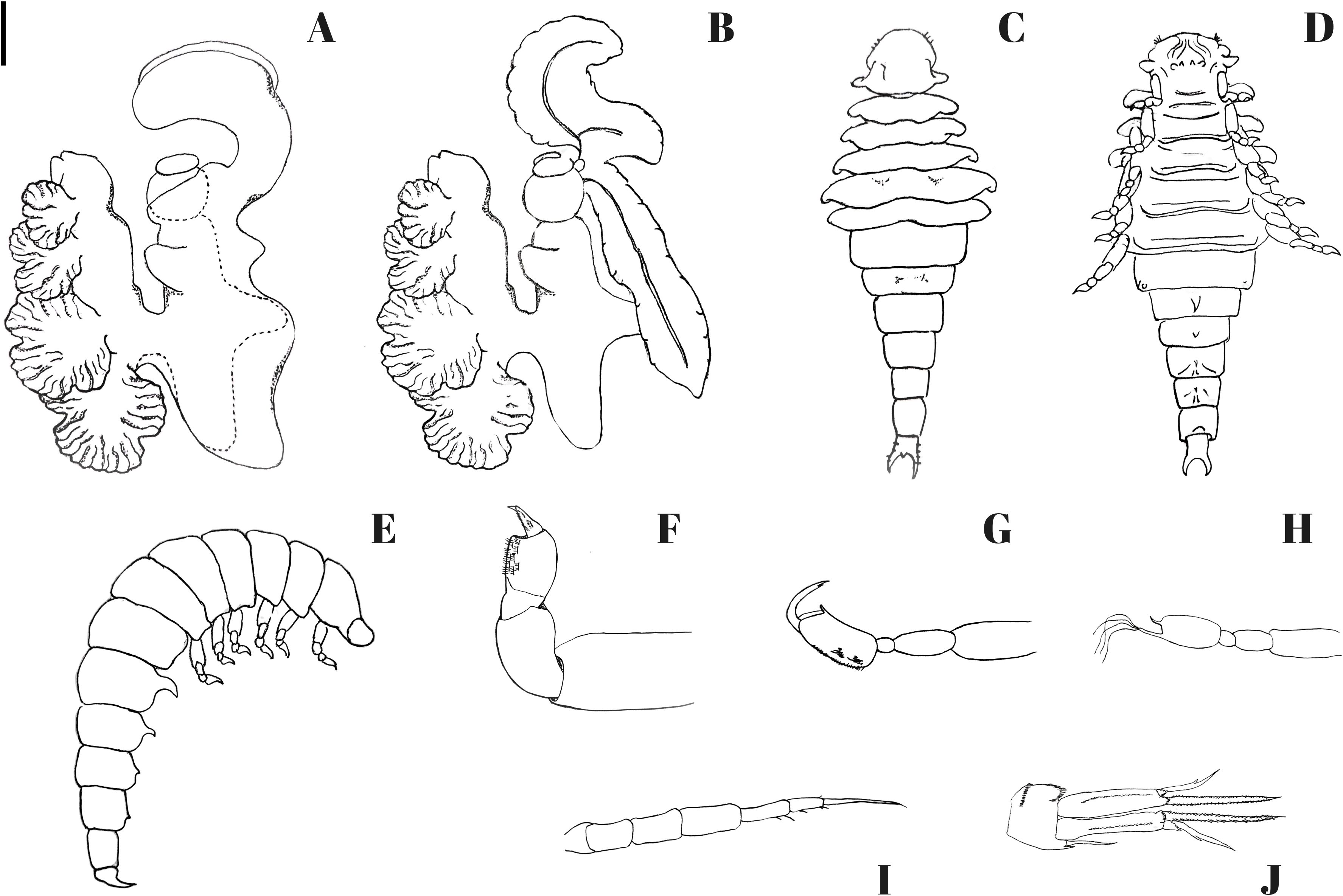
Figure 3. Portunion sinensis sp. nov. (A, B) holotype female; (C–F) allotype male; (G–J) Paratype, epcaridium larvae. (A) female enclosed in host membrane; (B) female with membrane removed; (C) male, dorsal view; (D) male, ventral view; (E) male, side view; (F) male, side view of pereopods; (G) epcaridium, paraeopod I; (H) epcaridium, paraeopod VI; (I) epcaridium, antenna II; (J) epcaridium, uropod. Scale: 2000 μm (A, B); 200 μm (C–E); 55μm (F); 20 μm (G–I); 10 μm (J).
Marsupium is full of ova yellowish brown to dark brown, ovary lemon-yellow, abdomen whitish.
Heart. An oval, bulbous heart is present on the dorsal surface of the pleomere.
Pleural lamellae of the initial four segments are intricately folded and exhibit crispate margins, resembling a bouquet. As one progresses posteriorly, these lamellae progressively diminish in both size and fold complexity, ultimately adopting a simple triangular form by the fifth segment.
Description.
Allotype, male (Figure 4).

Figure 4. Scanning electron micrographs depicting the male of Portunion sinensis sp. nov. (A) ventral view of the male; (B) side view of pereopods; (C) pereopod VI; (D) close-up view of posterior region of the male showing two ventromedian hooks (white arrowhead); (E) close-up view of ventromedian hook with protuberances; (F) close-up view of telson showing anal cone and irregularly arranged protuberances.
Body (Figures 3C-E) of male 1.1 mm long, 0.3 mm wide at 4th thoracic segment; with scattered pigment patches, the body typically assumes a ventrally curved posture.
Cephalon is fused with the first thoracic segment; however, they can be distinguished in the lateral regions, and there is also a shallow surface groove indicating the boundary.
Antenna II absent.
Mandibles missing, maxillae and maxillipeds degenerate.
Thorax gradually narrows from the 5th segment forwards, the 1st being half as wide as the 5th; lateral parts attenuated except in the last segment which has a truncated margin. The last thoracic segment is limbless, but provided with protuberances bearing gonopores. The pleura of the thoracic segments are characterized by their abbreviated, narrow, and contorted structure, with their inferior surfaces slightly posteriorly oriented. The seventh segment is distinguishable by its absence of pleura and cylindrical morphology.
Peraeopoda (Figure 3F) are 5-jointed, with carpo-produs as well as dactylus clad with many spinule-rows.
Ventral spine. The 1st and 2nd abdominal segments are hook-like and with a pointed tip. The spine of the 3rd and 4th segments is very rudimentary but still distinguishable.
Dot-like protrusions are present on the first four ventral spines and they also encircle segment V.
Description.
Paratype, Epcaridium larvae (Figures 5, 6).

Figure 5. Scanning electron micrographs depicting the epicaridan of Portunion sinensis sp. nov. (A) extruded egg; (B, C) developing embryos; (D) side view of epicaridium larva showing 2nd antennae (white arrowhead); (E) ventral view of epicaridium larva; (F) dorsal view of epicaridium larva.
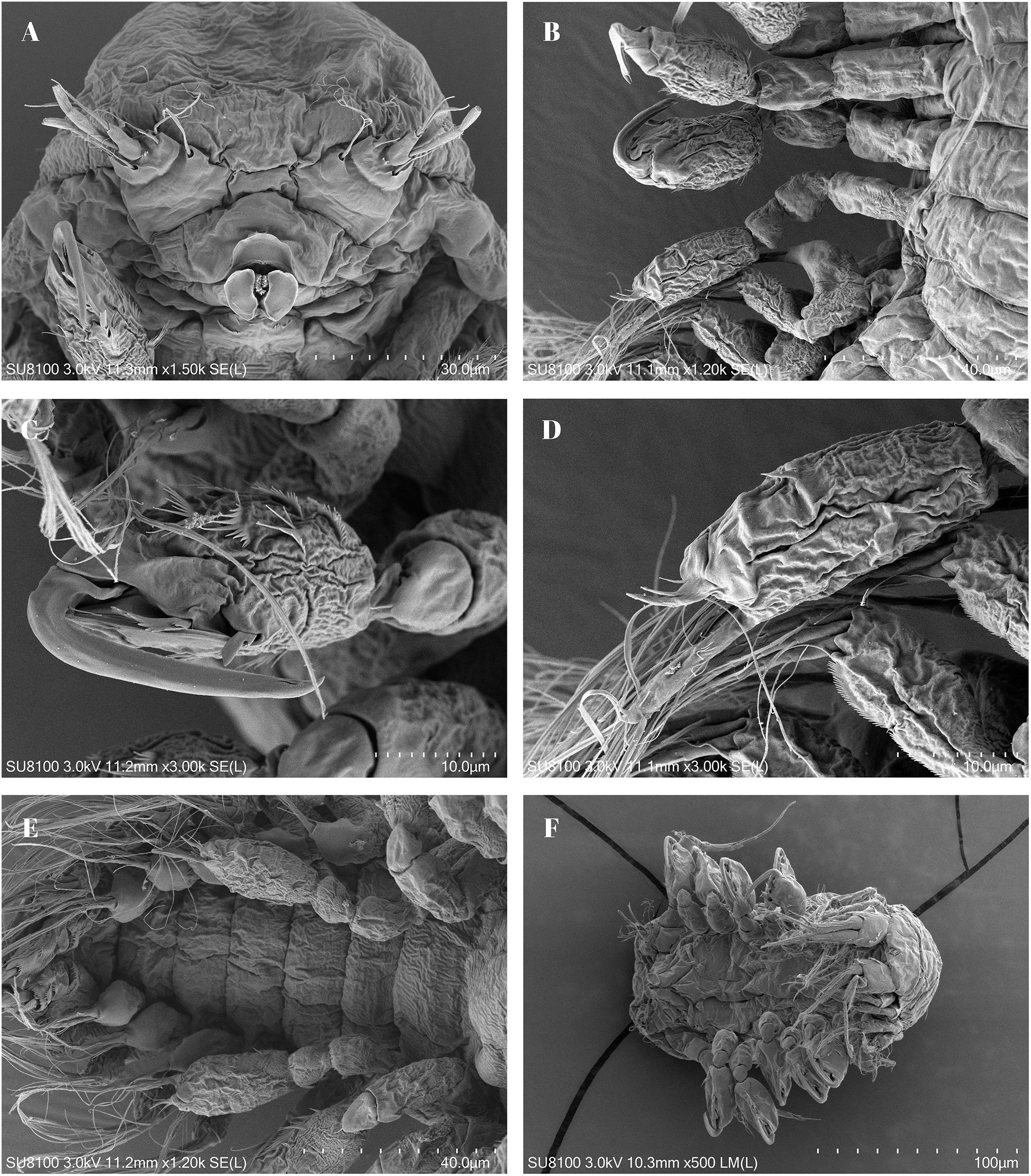
Figure 6. Scanning electron micrographs depicting the epicaridan of Portunion sinensis sp. nov. (A) cephalon, showing antennules mouthparts; (B) side ventral view, showing pereopods; (C) pereopod V; (D) pereopod VI, close-up of dactyl and propodus, with seto; (E) ventral view of posterior, showing pleopods; (F) pleon.
Body is approximately 208–238 µm in length (anterior margin of head to end of telson) and has a strong dorsal convexity and scattered pigment patterns.
Cephalon presents a uniformly rounded anterior margin, with each lateral aspect bearing a small number of crystalline cones encompassed by chromatophores.
Antennule of three rounded articles, the basal article is the largest, with the second article rounded with thin seta anterior and posterior to the terminal article; the terminal article is digitiform with two long, thick setae at the terminus and two long, thick setae extending from the base.
Antennae (Figure 3I) are elongated and approximately half the length of the body; composed of six articles (four peduncular, two flagellar), the basal article the smallest, articles 2–4 progressively longer, article 4 expanded, with two setae on distolateral margin; first flagellar article slightly greater than half the width of the terminal peduncular article, second flagellar article with a thick, long seta (subequal in length to the combined length of the first and second flagellar articles).
Mouthparts consist of an oral cone with a sucker-shaped structure at the terminus, composed of three plates (one anterior, two lateral). Maxilliped is manifested as diminutive, unsegmented lamellae.
Peraeopoda, six pairs of pereopods. Five anterior pairs of gnathopodal pereopods, subequal in size, each with a slender, slightly curved dactylus extending approximately half the length of the propodus, tip of the dactylus is hooked (Figure 3G). Pereopod 6 differs in structure from others by lacking a hook-shaped dactylus, but the end of the dactylus has a seta that is the same length as the propodus (Figure 3H).
Pleopoda, the five pairs of uniramous pleopoda are all similar in structure; the protopodite is triangular, bears two long stiff setae at its inner distal angle, and at the outer angle a quadrangular exopodite terminating in three setae.
Uropod (Figure 3J), biramous, stout cylindrical peduncle, distally with 2 slender rami.
3.4 Remarks
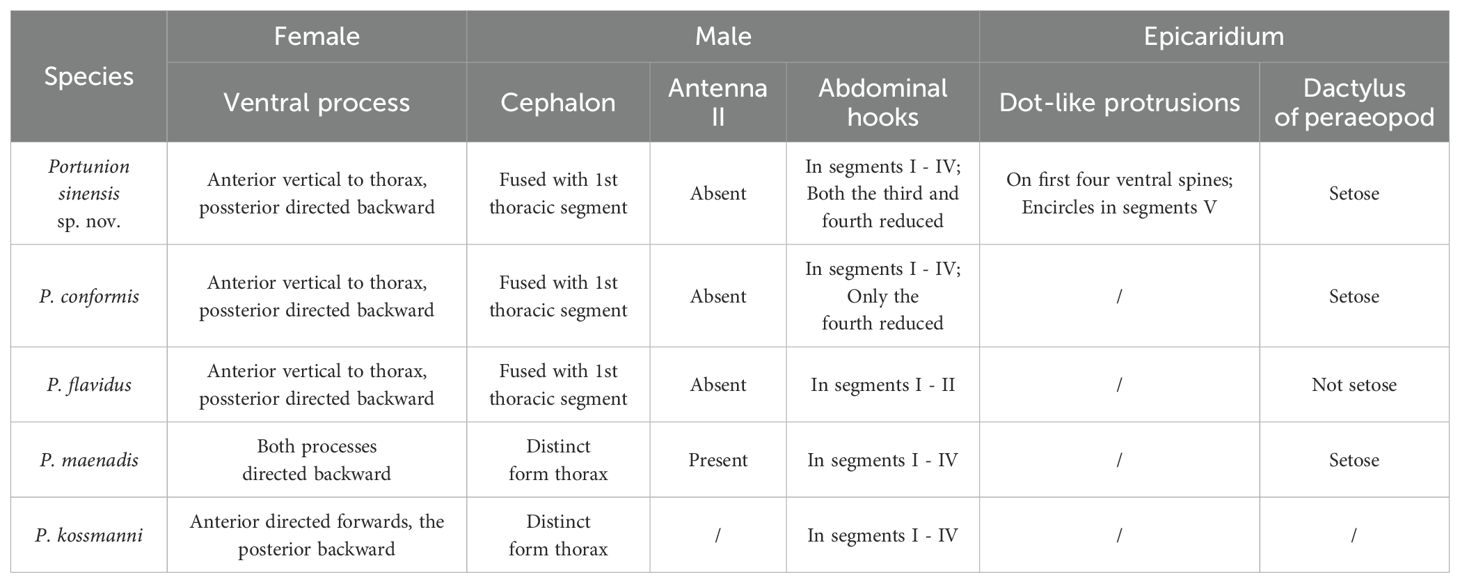
The Portunion sp. nov. differs from other closely similar species P. maenadis, P. kossmanni, P. flavidus, and P. conformis in the following way (Table 3): Female, the anterior ventral process is oriented vertically to the thorax, while the posterior process is directed backward. In contrast, both processes in P. maenadis are directed backward, whereas in P. kossmanni, the anterior process is directed forward and the posterior backward. Male, the cephalon is fused with the first thoracic segment, the second antenna is absent, abdominal hooks are present in segments I-IV, the first two hooks with pointed tips, the spine of the 3rd and 4th segments is very rudimentary. However, in both P. maenadis and P. kossmanni, the cephalon is distinct from the thorax, and the antennae are present. In P. flavidus, abdominal hooks are only found in segments I-II, with the 2nd rudimentary. In P. conformis, abdominal spines are present in segments I-IV, the first three hooks with pointed tips, and only the spine of the 4th segment is reduced. Epicaridium, the dactylus of peraeopod VI exhibits setose characteristics, whereas in Flavidus species, the dactylus lacks setae.
Etymology. The species name is derived from the locality, Zhangpu, located in Fujian, China.
Distribution. China (Beihai, Huizhou, Zhangpu).
3.5 Key to the genera of the Entoniscidae
I. Female marsupium formed by host membrane alone, with all oostegites not adhering to it, and freely movable; 1st oostegite not differentiated from others; pleopoda lanceolate and pleural lamellae missing. Male peraeopoda unsegmented, tuberculiform.
A. Female without maxilliped; terminal segment of male undivided.
1. Antenna of male projected laterally; 5th pleopod of Epicaridium dissimilar to others. (Parasitic on Anomura)
……… Entoniscus F. MÜLLER (5 spp.)
2. Antenna of male not projected; 5th pleopod of Epicaridium similar to others. (Parasitic on Macrura)
………. Synalpheion COUTIÈRE (1 sp.)
B. Female with maxilliped; terminal segment of male bifurcated. (Parasitic on Brachyura)
3. .….….…….Entoniscoides MIYASHITA (1 sp.)
II. Female marsupium formed by close adhesion of oostegites to host membrane; 1st oostegite differentiated into three parts; pleopoda lamellar and pleural lamellae well developed. Male peraeopoda jointed. (Parasitic on Brachyura)
A. Marsupium complete, with 2nd pair of oostegites enclosing ascendant lamellae of 1st pair; two ventral ovarian processes present,
a. Last peraeopod of Epicaridium shorter than others, with a simple propodite and rudimentary dactylopodite.
4. Female without dorsal ovarian process.
.….Pinnotherion G. & B. (2 spp.)
5. Female with a pair of dorsal processes.
…… Portunion G. & B. (5 spp.)
b. Last peraeopod of Epicaridium much longer than others, with a distally expanded propodite and well-developed dactylopodite.
i. Female with two pairs of dorsal ovarian processes.
6. Male without penis…………………………………….Grapsion G. & B. (1 sp.)
7. Male with penis………………………………………. Priapion G. & B. (1 sp.)
ii. Female with an unpaired dorsal ovarian process.
8. ………………. Micippion n. gen. (1 sp.)
B. Marsupium partly uncovered, with the 2nd pair of oostegites overlapping only the basal part of the 1st pair; ventral ovarian processes present or absent. (Last peraeopod of Epicaridium as in II, A, b)
a. Female with a ventral ovarian process, but without a dorsal one.
9. With two ventral ovarian processes.
.….….…. Tiarinionn. gen. (1 sp.)
10. With one ventral ovarian process.
.….……. Xanthion n. gen. (1 sp.)
b. Female with two pairs of dorsal ovarian processes, but without ventral one.
11. ……………. Cancrion G. & B. (4 spp.)
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by ethics committee of Fourth Institute of Oceanography, Ministry of Natural Resources. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
TH: Investigation, Writing – original draft. XM: Investigation, Writing – original draft. SZ: Investigation, Methodology, Writing – review & editing. XC: Formal analysis, Investigation, Methodology, Writing – review & editing. DY: Investigation, Methodology, Resources, Writing – review & editing. JG: Formal analysis, Investigation, Methodology, Resources, Writing – review & editing. LH: Formal analysis, Investigation, Methodology, Writing – review & editing. WL: Formal analysis, Investigation, Methodology, Writing – review & editing. TP: Conceptualization, Writing – review & editing. YQ: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by National Parasitic Resource Center (Grant No. 2019-194-30); the General Program of Guangxi Zhuang Autonomous Region (Grant No. 2021GXNSFAA220030); the Youth Foundation of Guangxi Zhuang Autonomous Region (Grant No. 2021JJB150073); Guangxi Science and Technology Major Program (Grant Number:桂科AA24206044); China Postdoctoral Scientific Foundation (Grant No. 2021M701798); Ministry of Science and Technology of the People´s Republic of China (Grant Number: 2023YFD2402600); Science & Technology Fundamental Resources Investigation Program (Grant Number: 2023FY100800); The Fund of Hainan Provincial Key Laboratory of Tropical Maricultural Technologies (Grant No. TMTOF202104 & TMTOF202205); the Guangxi Key Laboratory of Beibu Gulf Marine Biodiversity Conservation, Beibu Gulf University(Grant No. 2022KA04); Guangxi University of Chinese Medicine “GuiPai Traditional Chinese Medicine inheritance and innovation team” Project (2022A007); Development Program of High-level Talent Team under Qihuang Project of Guangxi University of Chinese Medicine (2021004); Science and Technology Planning Projects of Beihai (No. 202082035 & 2021158007).
Acknowledgments
We are grateful to Prof. Armand Kuris, Jianmei An, Christopher Boyko, and Jason Williams for their valuable and constructive advice for the morphology description of the new specie.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Association M (1957). Plymouth marine fauna (Plymouth: Marine Biological Association of the United Kingdom).
Blakeslee A. M., Keogh C. L., Byers J. E., Kuris A. M., Lafferty K. D., Torchin M. E. (2009). Differential escape from parasites by two competing introduced crabs. Mar. Ecol. Prog. Ser. 393, 83–96. doi: 10.3354/meps08225
Brockerhoff A. M. (2004). Occurrence of the internal parasite Portunion sp. (Isopoda: Entoniscidae) and its effect on reproduction in intertidal crabs (Decapoda: Grapsidae) from New Zealand. J. Parasitol. 90, 1338–1344. doi: 10.1645/GE-295R
Bourdon R. (1964). Epicarides et rhizocéphales du bassin D'Arcachon Vol. 101 (Procés-Verbaux de la Société Linnéenne de Bordeaux), 1–7.
Chaix J. C., Veillet A. (1981). Entonisciens nouveaux des cotes de France II. Portunion bourdoni sp. nov. parasite d'Acanthonyx lunulatus Risso. Bull. l'Academié la Societe Lorraines Des. Sci. 20, 71–81.
Cornelius A., Waser A. M., Buschbaum C., Thieltges D. W. (2019). First record of the endoparasitic isopod Portunion maenadis (Giard 1886) (Epicaridea: Entoniscidae) in shore crabs in the Wadden Sea and a review of its distribution in Europe. Mar. Biodiversity 49, 2931–2936. doi: 10.1007/s12526-019-01012-3
Dittmer J., Koehler A. V., Richard F. J., Poulin R., Sicard M. (2011). Variation of parasite load and immune parameters in two species of new zealand shore crabs. Parasitol. Res. 109 (3), 759–767. doi: 10.1007/s00436-011-2319-2
Giard A. (1878). Sur les isopodes parasites de genre Entoniscus. Comptes Rendus Hebdomadaires Des. Séances l'Académie Des. Sci. 87, 299–301.
Giard A., Bonnier J. (1886). Nouvelles remarques sue les Entoniscus. Comptes Rendus Hebdomadaires Des. Séances l'Académie Sci. 103, 1173–1176.
Giard A., Bonnier J. (1887). Contributions a l'etude des bopyriens. Travaux l'Institut Zoologique Lille du Laboratoire Mar. Wimereux 5, 1–272.
Koehler A. V., Poulin R. (2010). Host partitioning by parasites in an intertidal crustacean community. J. Parasitol. 96, 862–868. doi: 10.1645/GE-2460.1
Kossmann R. (1881). Die entonisciden Vol. 3 (Mittheilungen aus der Zoologischen Station zu Neapel), 149–169, pls. 8, 9.
Kuris A., Poinar G., Hess R. (1980). Post-larval mortality of the endoparasitic isopod castrator Portunion conformis (Epicaridea: Entoniscidae) in the shore crab, Hemigrapsus oregonensis, with a description of the host response. Parasitology 80, 211–232. doi: 10.1017/S003118200000069X
Kuris A., Poinar G., Hess R., Morris T. J. (1979). Virus particles in an internal parasite, Portunion conformis (Crustacea: Isopoda: Entoniscidae), and its marine crab host, Hemigrapsus oregonensis. J. Invertebrate Pathol. 34, 26–31. doi: 10.1016/0022-2011(79)90050-8
Muscatine L. (1956). A new entoniscid (Crustacea: Isopoda) from the Pacific coast. J. Washington Acad. Sci. 46, 122–126.
MBA (Marine Biological Association) (1957). Plymouth marine fauna, 3rd edn. Marine Biological Association of the United Kingdom, Plymouth.
Perkins M. (1924). Two abnormal chelae of Carcinus maenas, pennant. Ann. Mag. Nat. Hist. 14, 136–138. doi: 10.1080/00222932408633098
Perkins M. (1925). XVIII.—Abnormal chelæ of Carcinus mœnas, pennant, from Wimereux. Ann. Magazine Natural History 16 (91), 182–187. doi: 10.1080/00222932508633287
Searle D. W., Crompton D. W. T. (1995). Observations on portunion maenadis (Isopoda, epicaridea, entoniscidae), parasitic in carcinus maenas (Decapoda, reptantia, portunidae) from the firth of clyde, scotland. In: Crustaceana (JSTOR), 68, 403-5. Available at: http://www.jstor.org/stable/20105059 (Accessed April 28, 2025).
Shields J. D., Kuris A. M. (1985). Ectopic infections of Portunion conformis (Isopoda: Entoniscidae) in Hemigrapsus spp. J. Invertebrate Pathol. 45, 122–124. doi: 10.1016/0022-2011(85)90060-6
Shiino S. M. (1942). On the parasitic isopods of the family Entoniscidae, especially those found in the vicinity of Seto. Memoirs Coll. Science Kyoto Imperial University Ser. B 18, 37–76.
Williams J. D., Boyko C. B. (2012). The global diversity of parasitic isopods associated with crustacean hosts (Isopoda: Bopyroidea and Cryptoniscoidea). PLoS One 7, e35350. doi: 10.1371/journal.pone.0035350
Keywords: endoparasite, Isopoda, Portunion sinensis sp. nov., Scylla paramamosain, taxonomy
Citation: Huang T, Ma X, Zhong S, Chen X, Yang D, Guo J, Huang L, Li W, Pengsakul T and Qiao Y (2025) A new species of endoparasitic isopod, Portunion sinensis sp. nov. (Isopoda: Entoniscidae), found in the mud crab Scylla paramamosain in China. Front. Mar. Sci. 12:1565361. doi: 10.3389/fmars.2025.1565361
Received: 23 January 2025; Accepted: 09 April 2025;
Published: 16 May 2025.
Edited by:
Huan Wang, Ningbo University, ChinaReviewed by:
Ajit Kumar Mohanty, Indira Gandhi Centre for Atomic Research (IGCAR), IndiaKhaled Mohammed Geba, Menoufia University, Egypt
Copyright © 2025 Huang, Ma, Zhong, Chen, Yang, Guo, Huang, Li, Pengsakul and Qiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Qiao, cWlhb3lpbmcwNjE4QGhvdG1haWwuY29t; Theerakamol Pengsakul, dGhlZXJha2Ftb2wucEBwc3UuYWMudGg=
†These authors have contributed equally to this work
 Teng Huang
Teng Huang Xiaowan Ma
Xiaowan Ma Shengping Zhong
Shengping Zhong Xuyang Chen1
Xuyang Chen1 Lixing Huang
Lixing Huang Theerakamol Pengsakul
Theerakamol Pengsakul Ying Qiao
Ying Qiao