- 1Department of Biological and Environmental Sciences, Qatar University, Doha, Qatar
- 2Department of Marine Biology, Texas A&M University at Galveston, Galveston, TX, United States
- 3Department of Ecology and Conservation Biology, Texas A&M University, College Station, TX, United States
The dugongs (Dugong dugon), one of the few marine herbivorous mammals, are classified as vulnerable species by the IUCN, and their population monitoring is critical for informed conservation efforts. Although limited research has confirmed that the Arabian Gulf, in the northwest Qatar, hosts the world’s largest dugong aggregations, studies on their exact numbers remain limited. We conducted boat-based drone surveys in 2019–2020 to estimate the magnitude of the sizable aggregations of dugongs in northwest Qatar. We conducted 14 surveys during 2019–2020 by employing Unmanned Aerial Vehicle (Drone) and photographic analysis techniques. Potential biotic and abiotic factors driving this gathering of dugongs were explored. Maximum dugong observed counts were 1108 in 2019 and 1209 in 2020. The percentage of cow-calf pairs in the total group was 5.8% in 2019, and 10.2% in 2020. Upon applying a detection probability of 0.96 and an availability probability range of 0.8-0.98, the adjusted estimated counts of dugongs ranged from 994 to 1574, with an overall estimated mean of 1248 ± 122 dugongs. We suggest that the significant dugong aggregations in the area during winter are primarily due to foraging on the abundant seagrass in this area, the relatively warmer waters (>18 °C) and the sheltering effect topography from turbulent waters caused by shamal winds during the winter months. This information is crucial for wildlife managers, stakeholders, and government agencies to facilitate informed decision-making concerning the management and protection of dugongs.
1 Introduction
The Arabian Gulf is home to the second largest dugong population, following Australia. Dugongs (Dugong dugon), first identified by Müller in 1776, are the only living species of the Dugongidae family, feeding primarily on seagrass (Cullen-Unsworth et al., 2018; Heinsohn, 1972; Lanyon, 2003; Shawky, 2019). Nevertheless, dugongs consume marine macroalgae and invertebrates, particularly during the winter, as noted in the Arabian Gulf (Marsh, 2018; Preen, 1992). They usually inhabit shallow warm marine coastal waters (Heinsohn et al., 1977). Dugongs are experiencing a significant population decline, which is driven by anthropogenic pressure including habitat alteration and degradation, boat strikes, and water pollution (Marsh, 2018).
Dugongs’ populations are now fragmented in several regions, and they are facing a serious decline in some areas such as in China (Lin et al., 2022). Robust and accurate monitoring for dugong populations is vital for effective conservation and management strategies. Traditional approaches including boat-based surveys and manned arial surveys have provided valuable information, but they are often costly, have limitations in accessibility and have scale and resolution limitations causing observer bias. Observers may miss individuals entirely (perception bias) or be unable to detect them if they are obscured by water or vegetation, or because the animals moved away (availability bias) (Edwards et al., 2021). Consequently, flawed detections frequently result in either an underestimation or an overestimation of the population sizes (Cleguer et al., 2021; Hammond et al., 2021). Recent advances in unmanned aerial vehicle(UAV) tools offers a promising resolution to several limitations (Cleguer et al., 2021; Hodgson et al., 2013; Marshall et al., 2018), and many studies have explored its application and advancements (Aniceto et al., 2018; Hensel et al., 2018; Williams et al., 2017). However, limited research has employed it to investigate dugongs (Cleguer et al., 2021; Hodgson et al., 2013). Unmanned aircraft (UAVs) or drone technology have revolutionized the methods of collecting data and tracking distribution for marine wildlife (Brack et al., 2018). They have better adaptability, and they are cost-effective. For the marine environment, this technology is still in its early stages.
Research has confirmed that Australia is home to the largest dugong population globally (Anderson, 1986; Hodgson, 2007; Lanyon, 2003; Marsh and Saalfeld, 1989; Preen and Marsh, 1995; Seddon et al., 2014), however, it is fragmented over various areas (Cullen-Unsworth et al., 2018). On the other hand, the Arabian Gulf, a shallow and primarily landbound extension of the Indian Ocean, has been identified as the location of the largest gathering of dugongs (Marshall et al., 2018). The Gulf is marked by unusually elevated temperatures reaching 36-38 °C in summer, and hyper-salinity (40–70 psu) resulting from intense evaporation and limited freshwater influx (Smith et al., 2007). Despite the harsh environmental conditions, the Gulf remains a home to diverse communities that exhibit a marked adaptations to survive under such extremes. Among the most adapted ecosystems is seagrass, which is critical habitat for diverse marine organisms including dugongs. The Gulf also harbor a sizable population of dugongs that is estimated to be ~6,000 dugongs in 1986 (Preen, 2004), while large aggregations of sizes of about 600 individual was observed between Bahrain and Qatar (Khamis et al., 2023). This highlights the significance of the region between Qatar and Bahrain in the viability and conservation of the dugongs in the Arabian Gulf (Marshall et al., 2018).
Although dugongs are classified as vulnerable species on the IUCN Red list and play a critical ecological role in the seagrass ecosystems, they remain understudied in the Gulf (Marshall et al., 2018; Preen, 2004). Investment in effective monitoring program utilizing technologies such as drones is essential. Drone-based monitoring allow for the detection of mammals’ population in different marine environments some of which are difficult to access (Hodgson et al, 2017). A comprehensive understanding of the dynamics of the dugongs of the Arabian Gulf and monitoring for their population size are crucial for guiding conservation efforts and policies, as well as for ensuring the long- term sustainability of the dugong population in a vulnerable marine basin (Marsh et al., 1999).
This research aimed to evaluate the size of the large gathering of dugongs along Qatar’s northwest coast in 2019 and 2020, employing an unmanned aerial vehicle (UAV) along with photographic analysis techniques. Both biotic and abiotic factors such as seagrass presence and water temperature, were explored in analyzing the environmental influences driving this largest recorded dugong aggregation in the northwest of Qatar in consecutive winters.
2 Methodology
2.1 Study area
The State of Qatar is a 11,586 km² peninsula located halfway along the western shore of the Gulf between latitudes 24.27 and 26.10° N and longitudes 50.45 and 51.40° E. It is bordered by Saudi Arabia to the south, and shares maritime borders with Bahrain, Iran, and the United Arab Emirates. Qatar is characterized by its hot, arid climate in the summer and relatively cool winters. Its coastal water is hypersaline, resulting from lack of freshwater input, excessive evaporation rates with an annual average of 2200 mm, and sparse rainfall, creating an extreme environment (Abu Sukar et al., 2007). Our study site is in the northwest of Qatar, south of Ras Eshairij and north of Dukhan; an area called Umm AlMaa. This site was selected based on historical data (Preen, 2004), which recognized the area between Qatar and Bahrain as one of the most significant dugong habitats in the region. Furthermore, initial data, indicated multiple observation of substantial dugong herds during winter, detected through boats and helicopter surveys (Marshall et al., 2018), along with various sightings of significant groups reported by local fishermen. Based on these published observations, and anecdotal information, it was evident that dugongs gathered between Qatar and Bahrain from December to March.
2.2 Survey design
Transect surveys were conducted by boats from the northern Hawar Islands to Ras Eshairij, during 2019-2020. The transect lines were predetermined, evenly spaced out at intervals of 2 km and input into a GPS device. Surveys proceeded along intended parallel transect lines that are perpendicular to the coastline and extended out to the 10m bathymetric contour of the Bahraini border (Figure 1). Two center console boats were used, each measuring 28 feet and able to accommodate 6 individuals. Similar to their Australian counterparts, dugongs in the Arabian Gulf are wary of people and frequently hide when loud or abrupt noises happen. As a result, the speed of the boat was continually checked to stay under 8 km/hr, usually fluctuating between 5.5 and 7.4 km/hr, with 2-minute pauses taken every 5 minutes to reduce engine noise as much as possible. Each observer employed the naked eye and Nikon 10x50 marine binoculars to scan directly from front to either port or starboard, depending on the side of the boat they were on. When dugongs were sighted, the GPS coordinates, the angle from the transect line, and an estimated distance to the group were noted. The boat remained at no less than half a kilometer while ideally keeping a speed close to that of the herd. Modern, innovative state-of-the-art UAVs (hexacopters) equipped with cutting-edge side-by-side infrared and high-definition cameras were deployed to capture images of entire groups and assess the counts of individuals and cow-calf pairs.
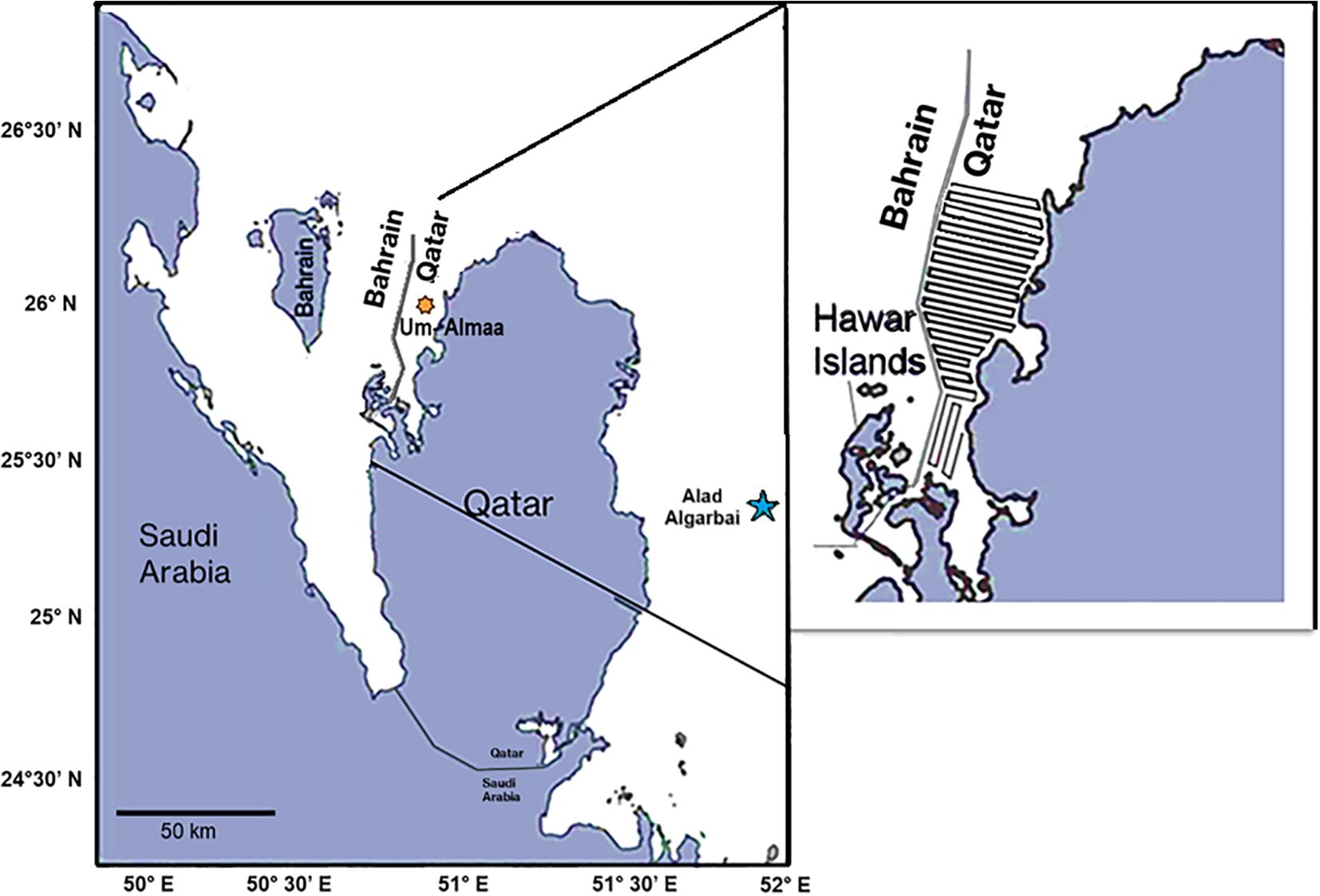
Figure 1. Study area map in the Northwest Coast of Qatar. Star on the west denotes the location where the dugong gathering was located. The two stars in the east and the west denote the locations where CTD-Divers® were installed. The enlarged inset highlights the zigzag path of the boat.
In 2019, three boat-based drone surveys were conducted between January and February, during which a large dugong herd was recorded only once, on the 7th of February (Table 1). In 2020, survey effort was increased to eleven surveys covering a wider time range including 9 surveys from February to March, one in August and one in November, to investigate if dugong aggregations happen outside the previously noted peak period. Surveys took place in the morning to prevent strong sun glare typically at its peak around noon and when wind speed was less than 6 knots. Moreover, to enhance water visibility, all surveys were conducted during moderate tide periods when the water’s turbidity was low, and the sea appeared calm and glassy. Each of these factors was considered to enhance the chances of spotting when the images were captured.
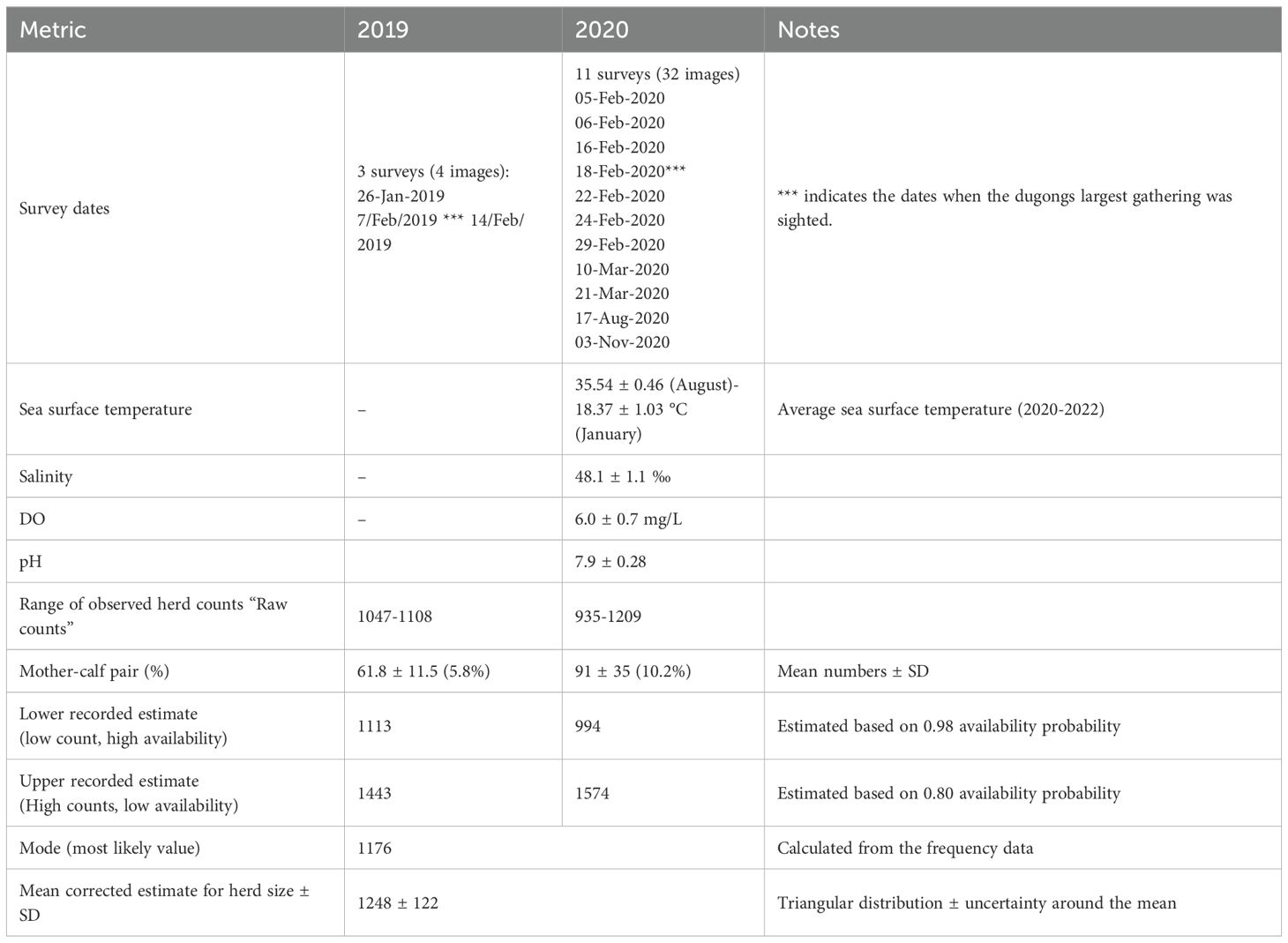
Table 1. Observed and bias-corrected estimates of dugong herd size from boat-based drone survey with associated survey and environmental information in the northwestern of Qatar.
Boat-based drone surveys were conducted with a DJI Mavic 2 Pro, featuring a 1-inch CMOS sensor and 28mm focal length. The drone utilized was equipped with a built in, high-resolution camera, recording a 4k video at 3840x2160 pixels. To reduce motion blur, the camera was adjusted to shutter speed priority with automatic exposure enabled. Additionally, flight information, such as GPS coordinates and altitude, was captured and noted. The UAV was deployed from height of the boat and steadily climbed, recording video during its journey. The entire herd became completely visible in the video frame at an elevation of about 80 meters. For five minutes, the UAV flew straight above the dugong group, recording continuous video with the camera directed downward. The drone’s time, location, and altitude were recorded during each flight using data from the GPS stream. Video recordings were examined manually to select frames that showcased the entire gathering in the footage. In 2019, merely 4 high-resolution images were obtained for examination and the groups count. In 2020, 32 high-resolution images of the dugong aggregation were acquired, processed and used for image analysis. These screenshots were not extracted at regular intervals; instead, they were deliberately taken only when the entire herd was visible within a single video frame.
2.3 Image analysis
All images and videos gathered during the drone surveys were analyzed on a 27-inch, LCD screen. To improve the detection of dugongs in drone images and enable more accurate group counts, multiple image adjustments were implemented. Color correction was applied in the images to enhance analysis and reduce the likelihood of misidentifying dugongs (Figures 2A, B). A magenta-purple color filter was created by modifying the white balance, effectively neutralizing the prevalent blue-green tones of the water. Saturation and contrast were enhanced to highlight the dugongs’ natural hues and enhance their separation from the background (Figure 2). Additionally, localized brightness adjustments were applied to brighten the central area with the highest density. These adjustments together enhance the subject visibility and counting accuracy.

Figure 2. Images from a boat-based drone of a dugong aggregation in northwest Qatar in 2019 (A) and 2020, demonstrating the use a magenta-purple filter and enhanced contrast for better visual detection (B).
Dugong individuals as well as proportion of dependent calves were counted manually in each image (Figure 3B). Mother-calf pairs were identified as 1) calves are significantly smaller than the mother, 2) the two dugongs swim in echelon position, in proximity of each other, 3) synchronous behavior in surfacing and direction and duration of association (Figures 3C, D). While calves are often undercounted in boat-based drone surveys, the accuracy of the count was greatly enhanced by the assurance that they were observed in clear water and good weather conditions Consequently, no adjustments were implemented for changing weather conditions. (Marsh et al., 1984) claims that vertical boat-based drone images of dugongs taken in ideal weather conditions that improve water visibility, yield the most precise calf counts. To facilitate counting, a grid and alphanumeric rows and columns were superimposed on each image (Figures 2B, 3A, B). Counts were noted on an analogous datasheet for each image. The criteria for counting dugongs (indicated with a dot) included: 1) dugongs surfacing in the water, 2) dugongs that were underwater showing a distinct elongated shape, with visible tail, 3) dugongs with part of their snout or tail visible above the surface, and 4) a submerged dugong exhibiting a clear contrast in color relative to the surroundings. The identification process was made easier by having several chronologically ordered images, with each second of the video depicted by one image. This approach allowed for tracking individuals over time to avoid false positives, like vague “dugong-like” forms, densely packed groups where individual identification was challenging, or erroneous benthic features. All images of the dugong aggregation were initially reviewed and annotated by a primary observer, who recorded the dugong counts and conducted a second review to verify their initial annotations. To ensure accuracy and consistency, the images were then independently analyzed by a second observer with extensive field experience in dugong identification and behavior.
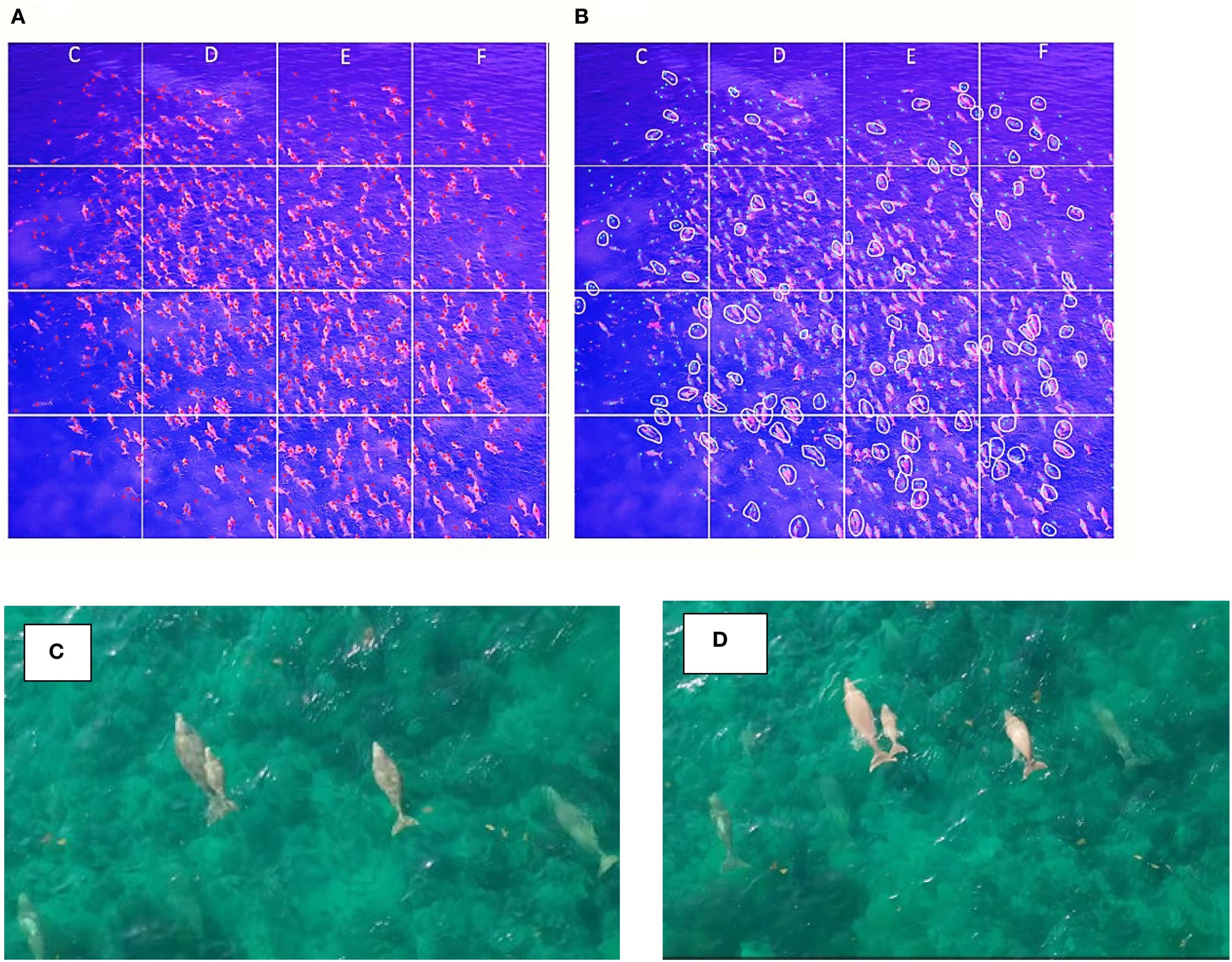
Figure 3. (A) Example of dot marking process to estimate dugong group size, (B) a screenshot of the mother-calf pair counting method shows pairs within the white circles. (C, D) mother-calf pair’s size difference and synchronous behavior.
2.4 Environmental data collection
Environmental parameters including temperature, salinity, dissolved oxygen (DO) and pH were measured in the dugong site. CTD-Diver® (manufactured by Van Essen Instruments, model DI273) was used to measure temperatures daily, with 6 hours reporting intervals. One device was deployed in the dugong location at the west of Qatar (Umm AlMaa) while another one was deployed in the east of Qatar at Alad Algarbai (Figure 1), to compare temperatures at the two locations. The CTD-Divers® were installed on-site for two years, from 2020 to 2022, recording monthly mean sea surface temperatures (SST°C). Additional environmental parameters, including salinity (ppt), dissolved oxygen (mg/L), and pH, were measured in the survey location using the EXO2 Sonde—a multiparameter water quality device. Measurements were taken during winter and summer at nine stations along the transect line. Data of wind speed (Km/hours) for the month of February in 2019 and 2020 was obtained from the Weather underground (2025), which was accessed on [July 31, 2025]. This data was used to understand the prevailing wind condition during the study period.
2.5 Estimating detection probability
Our surveys were conducted using vertical drone imagery, high-resolution cameras, and calm, clear waters ≈3.5m in depth, which enabled strong visual contrast between dugongs and the surrounding seafloor. Using the dugongs counts from two observers’ protocol in a set of frames, two observers conducted independent counting using colored marking on images. Following the counting, numbers of shared detections (nA) was determined and in addition to detections by observer B not seen by observer A (nB). Then with the assumption that the total (nA+nB) approximates the total number of animal present, the estimate of observer A’s detection probability (pd) will be:
To address the uncertainty in dugong availability, we utilized the depth-dependent framework suggested by Hagihara et al. (2018). While the Environmental Conditions Index (ECI1) assumes availability = 1.0 when the water is clear with a visible bottom, our survey circumstances did not consistently satisfy this criterion. Even though a significant portion of the bottom had favorable visibility, depths at the aggregation location often ranged from 3.5 to 5 m, exhibiting clear water but diminished bottom clarity. Consequently, we utilized the availability detection probabilities reported by Hagihara et al. (2018) for ECI3 conditions (p≈0.8-0.98), which represent environmental conditions and depth range (<5m) present in our study.
2.5.1 Estimating true herd size from observed counts
To estimate the actual number of dugongs in the gathering, we used the maximum number of individuals reported both in 2019 and 2020, and corrected them for detection and availability bias using the following equation (Nichols et al, 2000):
Where:
● N is the estimated herd size.
● C is the raw count of dugongs observed in the drone imagery by observer A.
● pa is the estimated availability probability.
● pd is the estimated detection probability.
Using Equation 2 and pa = 0.80, 0.98, along with detection probability calculated from Equation 1 we yielded estimated lower and upper bounds for the number of individuals detected. Using these numbers, we constructed a triangular distribution with lower bound corresponding to availability at 0.80 (L), and the upper bound corresponding to availability at 0.98 (U) and using a calculated mode (M). From this distribution we calculated the mean (µ) = (L+M+U)/3 and we calculated the standard deviation (σ)= . Data and graphical analysis were conducted using SPSS Statistics for Windows, Version 28.0 (IBM SPSS Statistics for Window, Version 28.0. IBM).
3 Results
3.1 Environmental conditions in the study area
Data of measured environmental parameters for the study area are showing in Table 1, all values are reported as mean ± SD. Temperatures in the study area peaked during the summer months (35.5 ± 0.46 °C in August), while the lowest mean monthly temperature (18.4 ± 1.04 °C) was in January (Figure 4A). Temperatures exhibited slightly higher values in the west during winter and slightly lower values in summer months compared to the eastern region (Figure 4A). For example, the average monthly temperature in January in the dugong location (18.52 ± 1.25) was slightly higher than the average monthly temperature in the east of Qatar (18.37 ± 1.03). Similarly, in February, the average temperature in the gathering site (19.2 ± 1.1) was slightly higher than the average values in Alad Algarbia (18.9 ± 1.0). Notably, the year’s lowest temperatures, between January and February, aligned with the largest dugong aggregation in the northwest of Qatar. A comprehensive assessment of key environmental parameters in the northwest region of Qatar, analyzing a total of 463 samples (N = 463) for water parameters indicated that the average salinity was 48.1 ± 1.1 ‰, dissolved oxygen (DO) levels averaged 6.0 ± 0.7 mg/L and the pH levels had an average of 7.9 ± 0.3 (Table 1). Additionally, the average depth recorded across sampling sites was 3.5 ± 1.4 meters, providing insight into the shallow nature of the dugong gathering area. These findings offer a baseline for understanding the environmental characteristics of this region.

Figure 4. Monthly mean sea surface temperature (SST) in the east of Qatar (Elad Algarbai) and the west (Um-Almaa) (2020-2022), (error bars = ± 2SD) (A), and the wind speed in Qatar in February of 2019 and 2020 (Weather underground, Philadelphia, PA Weather history, accessed July 31st, 2025) (B), the red and blue thick lines represent the days of dugong gathering in 2020 and 2019 respectively.
3.2 Dugong herd size estimation
From 14 surveys conducted in 2019 and 2020, only two encounters for the large gathering were identified while in the other encounters, a small number was found. In agreement with reports from local fishermen and research by (Marshall et al., 2018; Preen, 2004), dugongs were found approximately 5km off the coast near the region from Ras Eshairij to Dukhan. They moved collectively as a single, large herd across the area between latitudes 25.97 and 25.88° N and longitudes 50.97 and 50.89°E. The dugong herd displayed no noticeable behavioral changes in response to drone operations, even when the drone approached within 5 meters above them. Their swimming behavior remained steady throughout the observation period, and no indication of disturbance or avoidance actions were observed. Dugongs were moving very close to each other, alternating surfacing and diving. Their movements were in very large aggregations and during the diving, we reported scattered distinctive feeding plumes indicator of active foraging behavior.
There were no large marine fauna other than dugongs observed in the area. The double observer approach yielded detection probability of 0.96, consequently, we assumed an observer bias of <0.05.
Boat based drone surveys conducted in 2019 and 2020 recorded maximum observed dugongs of 1108 and 1209 respectively (Table 1). Corrected herd size estimates for dugongs were derived from the maximum observed counts after correcting for detection probability (0.96) and availability (range 0.80-0.98) estimated to herd sizes from 1113 to 1443in 2019 and from 994 to 1574 in 2020 (Table 1). We applied a triangular distribution using the upper (1574) and lower (994) bounds and a calculated mode of 1176, resulting a bias adjusted mean estimate of 1248 ± 122 dugongs in the two years (Table 1). The proportion of calves in 2019 was approximately 5.8% whereas the proportion of calves in 2020 were almost doubled, 10.2% (Table 1). Analysis for frequency distribution of dugong raw counts from the analyzed 36 images is shown in Figure 5. About 72% of the images (n=26) contained between 1100 and 1209 dugongs (1100> n < 1209). Our methodology does not allow for definitive determination of the sex of individual dugongs. Nonetheless, it is reasonable to assume that a dugong accompanied by a calf is a female.

Figure 5. Histogram showing frequency distribution of raw dugongs counts in 36 images in 2019 and 2020.
4 Discussion
4.1 Dugong herd size
The current research documented the largest single aggregation of dugongs recorded globally in the waters of northwest of Qatar between 2019 to 2020, with a maximum observed number of 1209 and an estimated herd size of 1248 ± 122, after correction for detection and availability biases. The observation was conducted employing UAVs in a shallow water, with a visible seabed (Pollock et al., 2006). Notably, Marshall et al. (2018) had previously observed 508 dugongs in the exact same area, suggesting the group may have comprised as many as 600 individuals. While this was already considered as an exceptionally large sighting, our results demonstrated aggregations more than twice that size. This discovery is twice the previously noted largest single group of 700 dugongs near Hawar Island in Bahrain based on UAV surveys (Khamis et al., 2023). It is also two times the figures reported by Preen (2004), which documented groups of 674 and 508 individuals in that region. On the other hand, our figures are considerably higher than a large group reported by Hodgson (2009) that consisted of about 50 individuals in a manned aerial survey, which estimated the total population size in this study to be 1164 individuals after applying correction factors (Table 2). The gathering of such large numbers of dugongs are exceptionally rare even in Australia. Recent studies reported aggregations of ≤700 but not above thousand individuals at a single time, highlighting the significance of this area for conservation efforts. Dugongs’ aggregation of >1000 dugongs in a shallow marginal extreme basin in the northwest of Qatar is remarkable. The largest actual sighting of a single dugong group that was not in the Arabian Gulf, was about 300 dugongs in Moreton Bay, Queensland (Lanyon, 2003).

Table 2. Comparative overview of dugong number estimates in the Middle East and in the northwest of Qatar.
Surveys on marine mammals usually use different tools including UAVs, manned aerial surveys and boat-based observations, each of these methods has its limitations. It is quite possible that these reported dugong gatherings in the Gulf are a part of one Gulf population. Although dugongs traverse territorial boundaries, there is no data that sheds light on whether the Qatar-Bahrain and UAE dugongs comprise one or more populations, or if these groups migrate to and from UAE along the southern coast. Wider and more coordinated efforts are crucial to understand elements driving these gatherings, including comparative evaluations of biotic and abiotic aspects such as seagrass biomass seasonality at the dugong aggregation location versus nearby areas and the hydrodynamics of the open water during the gathering time. Moreover, studies to investigate the genetic and demographic connectivity to other groups in the Gulf region, like those noted in Bahrain and UAE are essential for making conservation decisions.
Our surveys indicated that mother-calf pairs comprised 5.8% and 10.2% in 2019 and 2020, respectively. The observed difference in calves’ numbers between years is probably due to a reduced sample size in 2019 (4 images only) when compared to 2020 (n=32), or it can be due to reduced human activity in the coastal waters during COVID time which would lower the number of calves lost in boat strikes and fishing nets. The ratio of mother-calf pairs in our research is consistent with findings from other studies including ratios reported by Marshall et al. (2018) 9.9% and 5.8-5.4% of two groups reported in February and December (2015), respectively. The ratios of the calves in the study by Khamis et al. (2023) of 6.4% were close to the ratios we reported in 2019. However, Preen (2004) found higher ratios reaching 15.7% of the dugong population in the Arabian Gulf during winter. The variation in calf ratios between different studies likely reflects differences in the geographic coverage and the duration over which each study was conducted, with Preen’s research spanning a broader area and longer timeframe. Variation can also arise from differences in the criteria used to classify an animal as a calf, as well as observer experience and survey methods. Our findings are consistent with calf proportions reported in other regions, which generally range from around 1.3% to 14.8% in areas like New Caledonia, Moreton Bay, Torres Strait, Shark Bay, and Cape Bedford (Garrigue et al., 2008; Hodgson et al., 2013; Lanyon, 2003; Marsh and Saalfeld, 1990, 1991). Higher calf ratios have also been recorded from other regions including the Solomon Islands (17.3%), near Shark Bayand Ningaloo Reef (21.4%) and in Johor, Malaysia (24%) (Bass, 2010; Hodgson, 2007; Ponnampalam et al., 2015).
4.2 Importance of seagrass biomass and seasonal variation
There is no evidence that dugongs gather in large numbers for social hierarchy; instead, they probably come together to share resources like seagrass, find shelter, or seek suitable water temperatures (Marsh et al., 2011). This location in the northwest of Qatar encompasses a vast area of seagrass beds, ranging from 500 to 1,000 km², and provides a rich feeding ground for the Gulf’s dugongs (Erftemeijer and Shuail, 2012). A recent study found that a significant portion of this expanse contains dense seagrass beds (Butler et al., 2020), making it one of the largest seagrass habitats along the coastal waters of Qatar. It is considered the second-largest seagrass habitat in the Gulf after the UAE, making up about 1% of the total areas of seagrass around the world (Al-Mansoori and Das, 2024). It was established that dugongs actively feed on mixed seagrass habitats that include Halodule uninervis, Halophila stipulacea, and Halophila ovalis, as well as macroalgae (Marshall et al., 2018). These three opportunistic seagrass species thrive in the extreme environment of the Arabian Gulf due to their high tolerance for salinity and temperature fluctuations (Erftemeijer and Shuail, 2012). As pioneering species, they not only endure these harsh conditions but also exhibit rapid recovery, making them the most dominant seagrasses in the region. Additionally, they are among the most globally recognized primary food sources for dugongs (Al-Abdulrazzak and Pauly, 2017; Aragones, 1994; Bayliss and Freeland, 1989; D’Souza and Patankar, 2009; Lanyon, 2003; Ponnampalam et al., 2015). More specifically, the existence of Halophila uninervis was linked to a notable rise in dugong numbers in Australia (Said et al., 2025). Dugongs probably prefer these seagrass species due to their low fiber content, derived from low biomass strands. They are also abundant in readily available nutrients like starch and nitrogen, and they grow quickly (Marsh, 2018; Tol et al., 2016; Yamamuro et al., 2004).
Studies on the seasonal trends of seagrass in the Gulf are scarce, and their results are contradictory. Research along the coastlines of Saudi Arabia and Qatar indicated that seagrass experiences senescence or dieback during winter due to the powerful northwesterly Shamal wind that pushes surface cooler water toward Qatar eastern shores especially in February (Thoppil and Hogan, 2010), that disturb and break apart seagrass rhizomes, resulting in heightened water turbidity (Whitehead, 2015). On the other hand, research along the UAE coastline has demonstrated that seagrass vanishes from shallow areas (<5m) in summer, due to high temperatures (>34 °C) leading to leaf loss and forcing dugongs to seek food in deeper waters (Al-Mansoori and Das, 2024). Further studies showed no clear seasonal pattern in seagrass biomass and failed to link this pattern to changes in environmental elements such as temperature or wind patterns (Price and Coles, 1992). Preliminary findings from our ongoing research (not yet published) indicated that seagrass density in northwestern Qatar was high from November to March, with a peak biomass of approximately 55 g dry wt/m². This seasonal increase corresponds with the documented notable dugong aggregations in the area. In contrast, seagrass biomass decreased to around 17 g dry wt/m² at the onset of early summer (unpublished data, n.d). Previous studies have connected dugong aggregations to the peak growth seasons of seagrass (Aragones, 1994). Our novel observation of seagrass biomass in the aggregation site is similar to a maximum seagrass biomass of 54.2 g dry wt.m2 reported from a recognized dugong feeding area in Australia, highlighting the significance of this location in Qatar as a feeding ground during this seasonal period (Westlake et al., 2022).
Hence, to conserve and manage the large dugong herds in the Gulf, additional research is needed to understand seasonal seagrass dynamics and their relationship with water temperature and physicochemical conditions, especially given that the Gulf supports one of the largest numbers of dugongs reported outside Australia.
4.3 Influences of water physical parameters
In addition to the food availability indicated by seagrass biomass, other factors such as water temperature and water stability influence the presence and numbers of dugongs (Budiarsa et al., 2021). Dugongs possess slow metabolic rates and limited thermoregulation, making them sensitive to temperature changes (Heinsohn et al., 1977) and capable of large-scale movements when sea surface temperatures decline (Deutsch et al., 2022). In northwest Qatar, aggregations coincided with the coldest months (January–February), when coastal waters reached 18–20 °C. Dugongs generally prefer warmer waters, often avoiding temperatures below 18 °C (Marsh et al., 1994; Preen, 2004). For example, dugongs in New South Wales and Queensland were active at 18.9–24.6 °C and moved away from colder waters (~17–18 °C) (Allen et al., 2004; Sheppard et al., 2006). Similar patterns were reported in Moreton Bay (Lanyon, 2003), Japan (Nishiwaki et al., 1979), and Shark Bay (Anderson, 1986; Marsh et al., 1994). In Qatar, winter sea temperatures rarely fall below 18–21 °C due to the Gulf’s shallow morphology. This region exhibits a strong correlation between air and water temperatures, likely due to limited thermal inertia (Vasou et al., 2024). Comparable results from Egypt also show dugongs inhabiting waters from 22 °C in winter to 29 °C in summer (Hanafy et al., 2006). Seasonal shifts in distribution have been noted at the species’ northern range limits (Zeh et al., 2018). Preen (2004) observed summer groups near the UAE and a winter herd between Qatar and Bahrain, previously attributed to freshwater springs. However, this explanation is unlikely, as there have been no in-situ measurements to test this theory. Recent hydrogeological studies indicated that all land-based springs in the area have dried up and only a limited number of submarine springs remain; they are insufficient to support dugong aggregations (Rausch and Dirks, 2024).
The largest single aggregation of dugongs, globally, was observed in the northwest of Qatar after strong wind gusts in the open Gulf as shown in Figure 4B, during which wind speed exceeded 35 km/h. Nevertheless, no surveys were conducted following the peak wind event of February of 2019, during which wind speed surpassed 40 km/h between 17th and 19th of the same month. In the winter months especially from December to February, the Gulf experiences intense shamal winds driven by cold fronts, with speeds over 50 km/h and gusts exceeding 100 km/h (Thoppil and Hogan, 2010), peaking in strength during February (Owlad et al., 2022). These winds not only transport cold air leading to a decrease in surface temperature that can attain 15 °C (Burt and Paparella, 2023), but they also initiate downwelling or subsidence of surface water across the shallow southern banks (Al-Thani et al., 2023). This can generate relatively strong waves reaching up to 2.7 m in the southern Gulf (Kamranzad et al., 2013). Under these intense winter winds, the low temperatures of the open Gulf waters render some areas excessively cold for dugongs to access seagrasses, like in Ras Tanura near Saudi Arabia, prompting dugongs to relocate to warmer regions (Hodgson, 2009). Earlier studies indicated that wind speed and direction and the associated effects on the water dynamics might affect the distribution and the foraging behavior of the dugongs rendering seagrass unreachable and promoting movement to sheltered habitats during rough conditions (Budiarsa et al., 2021; Ng et al., 2022). Dugongs are generally sluggish and are unable to sustain activity for longer than a few minutes (Anderson, 1981). They maintain their optimal energy balance between the energy spent during dives and the energy gained while foraging by residing in shallow and sheltered waters (Lefebvre, 2023). Their dives turn energetically less efficient in deeper and rougher waters. Waves and currents caused by wind interfere with the feeding habits of dugongs, increasing the energy expenditure required and complicating the process of diving and reaching seagrass meadows. Dugongs are observed to reduce their travel distances in the winter months as a strategy for adjusting moving costs and displaying thermoregulatory behavior (Zeh et al., 2018). The resulting interference with their ability to feed effectively, likely compels them to relocate to calmer, more stable waters. The gathering site on the western shores of Qatar encounters significantly reduced wave heights, due to the shallow depths and the safeguarding topography of the Qatar and Bahrain peninsulas in the central Gulf (Aboobacker et al., 2021). Moreover, the current study noted that this area tends to have slightly cooler temperatures in summer and slightly warmer during January to March (Figure 4A). Besides, dugongs tend to exhibit less predictable migratory patterns, which are frequently influenced by periodic seagrass loss caused by extreme temperature events, leading them to undertake occasional long-range foraging excursions (Deutsch et al., 2022). We suggest that the plentiful seagrass along Qatar’s western coastline during winter, coupled with the protective impact of the land during winter shamal winds and the comparatively mild and warmer environment, creates ideal circumstances for the substantial aggregation of dugongs noted in February. The west of Qatar also has minimal human interference, creating an ideal habitat for these marine mammals. Therefore, it is likely that female dugongs not only seek this area because it supports open-sea grazing, but because it also serves as a crucial nursery ground, where they can give birth and nurture their calves without significant threats. It has been evidenced in literature that dugongs seek the seclusion of sheltered backwaters for calving, which further emphasizes the importance of the northwest of Qatar as a nursery area (Marsh et al., 1984).
The average salinity in the study area was 48.1 ± 1.1 ‰, reflecting a hypersaline condition. Preen (2004) indicated that dugong presence in the region does not appear to be constrained by high salinity. For instance, the salinity was determined to be 70‰ when dugongs were discovered in the southern Gulf of Salwa, Qatar, whereas it ranged from 50‰ to 60‰ where they were found in the northern Gulf of Salwa. Additionally, dugongs were discovered in water with salinities between 40‰ and 50‰ along the northern Saudi Arabian coast (Preen, 2004).
5 Threats and protection
Dugongs generally exhibit a low reproductive rate, extended generation times and high parental investment, making them highly vulnerable to additional pressures (Marsh et al., 2011; Marsh, 2018). The International Union for the Conservation of Nature (IUCN) has categorized dugongs as vulnerable to extinction, with about one third of their range assumed to be nearing extinction, and the condition of about half of their range remaining uncertain (Marsh and Sobtzick, 2019). In the Arabian Gulf, threats are amplified by extensive coastal development, oil and gas activities, and widespread habitat degradation, particularly in seagrass beds. Dredging and reclamation in the Gulf of Salwa between Saudi Arabia and Qatar have altered hydrography and salinity, endangering these habitats (Al-Abdulrazzak and Pauly, 2017).
Local observations suggest dugong populations are declining across the Gulf, largely due to bycatch, with strandings in Qatar frequently linked to entanglement in fishing gear (Marshall et al., 2018; Marshall pers. obs.). Comparable losses have been documented elsewhere in the region, including 50–150 dugong deaths annually in the UAE (Preen, 2004). Oil pollution is an additional risk; the 1983 Nowruz spill killed at least 38 dugongs (Marsh et al., 2002).
In the region, protection measures including bans on dugong hunting under Federal Law No. 19 in Qatar and No. 23 in the UAE have been enacted (State of Qatar, 2004; Al-Abdulrazzak and Pauly, 2017). In Qatar, substantial marine protected areas have been established, notably the designation of 1,650 km² in the western waters specifically for dugong and seagrass conservation (MOECC, 2025). This encourages neighboring countries to take similar action to establish zones where human induced impacts can be minimized. Among seven proposed Gulf conservation zones, three are considered critical dugong habitats: waters around Murawah Island (UAE), between Qatar and Bahrain, and between Qatar and the UAE (Preen, 2004). Our findings reinforce that Qatar’s waters support a substantial proportion of the Gulf’s dugong population, highlighting the urgent need for regional protection.
6 Conclusion
This research sought to assess the extent of the large groups of dugongs aggregated in the northwest coast of Qatar by employing UAVs (drones) and photographic analysis techniques. Our findings documented the presence of the largest single group of dugongs observed globally during February of two consecutive years (2019-2020). This location off Qatar was found to be of attraction to a group of about 1209 observed dugongs.
Mother-calf pairs constituted an average of 8.1% of all sightings, with average proportions of 5.8% in 2019 and 10.2% in 2020. The massive presence of dugongs in this area during winter, can be attributed to the abundant seagrass at this time of the year in addition to using this site as shelter from the cold turbulent waters in the eastern coast of Qatar which intensifies during February, that probably affects their accessibility to other seagrass beds around the area. The utilization of drone technology in this study has demonstrated its effectiveness as a transformative tool and promising approach, offering researchers unprecedented access to real time data for dugongs with minimal disturbance. Drones can cover broader as well as remote areas, which enhances monitoring strategies and improves our understanding of dynamics of marine mammal populations. The current study only addresses the aggregation number of dugongs at a specific time and in one place, giving the minimum number of dugongs in the area and at a certain time. However, a long-term and larger-scale aerial surveys need to be implemented. To fully understand the dynamics of the dugongs and their connectivity in the Gulf, satellite telemetry for tagged dugongs across space and time in addition to subsequent genetic analyses must be applied in for targeted conservation and risk assessment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because the data collection was purely observational.
Author contributions
DA: Formal analysis, Writing – original draft, Data curation, Writing – review & editing. YS: Writing – original draft, Conceptualization, Supervision, Formal analysis, Visualization, Investigation, Writing – review & editing. CM: Conceptualization, Writing – review & editing, Funding acquisition. MA: Project administration, Methodology, Formal analysis, Investigation, Writing – review & editing, Funding acquisition, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by a grant (NPRP No. 11S-0102-180177) from the Qatar National Research Fund (a member of Qatar Foundation) to Mohsin Alanasi, and Yousria Soliman.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The statements made here are solely the responsibility of the authors.
References
Aboobacker V. M., Samiksha S. V., Veerasingam S., Al-Ansari E. M. A. S., and Vethamony P. (2021). Role of shamal and easterly winds on the wave characteristics off Qatar, central Arabian Gulf. Ocean Eng. 236, 109457. doi: 10.1016/j.oceaneng.2021.109457
Abu Sukar H. K., Almerri F. H., and Almurekki A. A. (2007). Agro-hydro-meteorological data book for the State of Qatar (Department of Agricultural Water Research (DAWR). Available online at: https://openknowledge.fao.org/server/api/core/bitstreams/6d1c5804-a9b1-497c-99b7-10ad04a1bce1/content (accessed June 26, 2025).
Al-Abdulrazzak D. and Pauly D. (2017). Reconstructing historical baselines for the Persian/Arabian Gulf dugong, Dugong dugon (Mammalia: Sirenia). Zoology Middle East 63, 1–8. doi: 10.1080/09397140.2017.1315853
Allen S., Marsh H., and Hodgson A. (2004). Occurrence and conservation of the dugong (Sirenia: Dugongidae) in New South Wales. Proc. Linn. Soc. New South Wales 125, 211–216. Available at: https://researchportal.murdoch.edu.au/esploro/outputs/journalArticle/991005546005607891 (Accessed June 27, 2025).
Al-Mansoori N. and Das H. S. (2024). “Seagrasses of the United Arab Emirates,” in A natural history of the Emirates. Ed. Burt J. A. (Springer Nature, Cham, Switzerland), 267–285. doi: 10.1007/978-3-031-37397-8_9
Al-Thani J. A., Soliman Y., Al-Maslamani I. A., Yigiterhan O., and Al-Ansari E. M. A. S. (2023). Physical drivers of chlorophyll and nutrients variability in the southern-central Arabian Gulf. Estuarine Coast. Shelf Sci. 283, 108260. doi: 10.1016/j.ecss.2023.108260
Anderson P. K. (1981). The behavior of the dugong (Dugong dugon) in relation to conservation and management. Bull. Mar. Sci. 31 (3), 640–647. Available at: https://www.ingentaconnect.com/content/umrsmas/bullmar/1981/00000031/00000003/art00015 (accessed August 18, 2025).
Anderson P. K. (1986). Dugongs of Shark Bay, Australia – Seasonal migration, water temperature, and forage. Natl. Geographic Res. 2, 473–490.
Aniceto A. S., Biuw M., Lindstrøm U., Solbø S. A., Broms F., and Carroll J. (2018). Monitoring marine mammals using unmanned aerial vehicles: Quantifying detection certainty. Ecosphere 9, e02122. doi: 10.1002/ecs2.2122
Aragones L. (1994). Observations on dugongs at Calauit Island, Busuanga, Palawan, Philippines. Wildlife Res. 21, 709–717. doi: 10.1071/WR9940709
Bass D. K. (2010). Status of dugong (Dugong dugon) and Australian snubfin dolphin (Orcaella heinsohni) in the Solomon Islands. Pacific Conserv. Biol. 16, 133–143. doi: 10.1071/PC100133
Bayliss P. and Freeland W. J. (1989). Seasonal distribution and abundance of dugongs in the western Gulf of Carpentaria. Wildlife Res. 16, 141–149. doi: 10.1071/WR9890141
Brack I. V., Kindel A., and de Oliveira L. F. B. (2018). Detection errors in wildlife abundance estimates from unmanned aerial systems (UAS) surveys: Synthesis, solutions, and challenges. Methods in Ecology and Evolution 9 (8), 1864–1873. doi: 10.1111/2041-210X.13026
Budiarsa A. A., De Iongh H. H., Kustiawan W., and van Bodegom P. M. (2021). Dugong foraging behavior on tropical intertidal seagrass meadows: The influence of climatic drivers and anthropogenic disturbance. Hydrobiologia 848, 4153–4166. doi: 10.1007/s10750-021-04583-0
Burt J. A. and Paparella F. (2023). “The marine environment of the Emirates,” in A natural history of the Emirates. Ed. Burt J. A. (Springer Nature, Cham, Switzerland), 95–117. doi: 10.1007/978-3-031-37397-8_4
Butler J. D., Purkis S. J., Yousif R., Al-Shaikh I., and Warren C. (2020). A high-resolution remotely sensed benthic habitat map of the Qatari coastal zone. Mar. pollut. Bull. 160, 111634. doi: 10.1016/j.marpolbul.2020.111634
Cleguer C., Kelly N., Tyne J., Wieser M., Peel D., and Hodgson A. (2021). A novel method for using small unoccupied aerial vehicles to survey wildlife species and model their density distribution. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.640338
Cullen-Unsworth L. C., Jones B. L., Seary R., Newman R., and Unsworth R. K. F. (2018). Reasons for seagrass optimism: Local ecological knowledge confirms presence of dugongs. Mar. pollut. Bull. 134, 118–122. doi: 10.1016/j.marpolbul.2017.11.007
D’Souza E. and Patankar V. (2009). First underwater sighting and preliminary behavioural observations of dugongs (Dugong dugon) in the wild from Indian waters, Andaman Islands. J. Threatened Taxa 1, 49–53. doi: 10.11609/JoTT.o2002.49-53
Deutsch C. J., Castelblanco-Martínez D. N., Groom R., and Cleguer C. (2022). “Movement behavior of manatees and dugongs: I. Environmental challenges drive diversity in migratory patterns and other large-scale movements,” in Ethology and behavioral ecology of Sirenia. Ed. Marsh H. (Springer International Publishing, Cham, Switzerland), 155–231. doi: 10.1007/978-3-030-90742-6_5
Edwards H., Hostetler J., Stith B., and Martin J. (2021). Monitoring abundance of aggregated animals (Florida manatees) using an unmanned aerial system (UAS). Sci. Rep. 11, 12920. doi: 10.1038/s41598-021-92437-z
Erftemeijer P. and Shuail D. (2012). Seagrass habitats in the Arabian Gulf: Distribution, tolerance thresholds and threats. Aquat. Ecosystem Health Manage. 15, 73–83. doi: 10.1080/14634988.2012.668479
Garrigue C., Patenaude N., and Marsh H. (2008). Distribution and abundance of the dugong in New Caledonia, southwest Pacific. Mar. Mammal Sci. 24, 81–90. doi: 10.1111/j.1748-7692.2007.00173.x
Hagihara R., Jones R. E., Sobtzick S., Cleguer C., Garrigue C., and Marsh H. (2018). Compensating for geographic variation in detection probability with water depth improves abundance estimates of coastal marine megafauna. PloS One 13, e0191476. doi: 10.1371/journal.pone.0191476
Hammond P. S., Francis T. B., Heinemann D., Long K. J., Moore J. E., Punt A. E., et al. (2021). Estimating the abundance of marine mammal populations. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.735770
Hanafy M., Abu El-Regal M., Ali S., and Fouda M. (2006). The dugong, Dugong dugon, in Egyptian waters: Distribution, relative abundance and threats. Zoology Middle East 39, 17–24. doi: 10.1080/09397140.2006.10638178
Heinsohn G. E. (1972). A study of dugongs (Dugong dugon) in northern Queensland, Australia. Biol. Conserv. 4, 205–213. doi: 10.1016/0006-3207(72)90170-X
Heinsohn G. E., Wake J., Marsh H., and Spain A. V. (1977). The dugong (Dugong dugon (Müller)) in the seagrass system. Aquaculture 12, 235–248. doi: 10.1016/0044-8486(77)90064-3
Hensel E., Wenclawski S., and Layman C. (2018). Using a small, consumer grade drone to identify and count marine megafauna in shallow habitats. Latin Am. J. Aquat. Res. 46, 1025–1033. doi: 10.3856/vol46-issue5-fulltext-15
Hodgson A. (2007). The distribution, abundance and conservation of dugongs and other marine megafauna in Shark Bay Marine Park, Ningaloo Reef Marine Park and Exmouth Gulf (Report to the Western Australia Department of Environment and Conservation). Western Aust. Department Environ. Conserv.
Hodgson A. J. (2009). “Marine mammals,” in Marine Atlas of Bahrain. Eds. Loughland R. A. and Zainal A. J. M. (GEOMATEC Bahrain Centre for Studies and Research, Manama, Bahrain), 232–261.
Hodgson A., Kelly N., and Peel D. (2013). Unmanned aerial vehicles (UAVs) for surveying marine fauna: A dugong case study. PloS One 8, e79556. doi: 10.1371/journal.pone.0079556
Hodgson A., Peel D., and Kelly N. (2017). Unmanned aerial vehicles for surveying marine fauna: Assessing detection probability. Ecological Applications 27 (4), 1253–1267. doi: 10.1002/eap.1519
Kamranzad B., EtemadShahidi A., and Chegini V. (2013). Assessment of wave energy variation in the Persian Gulf. Ocean Eng. 70, 72–80. doi: 10.1016/j.oceaneng.2013.05.027
Khamis A., Abdulla A., D’Souza E., Kelkar N., Arthur R., Al Khalifa E., et al. (2023). Long-term persistence of large dugong groups in a conservation hotspot around Hawar Island, Kingdom of Bahrain. Aquat. Conservation: Mar. Freshw. Ecosyst. 33, 592–605. doi: 10.1002/aqc.3936
Lanyon J. M. (2003). Distribution and abundance of dugongs in Moreton Bay, Queensland, Australia. Wildlife Res. 30, 397–409. doi: 10.1071/WR98082
Lefebvre J.-P. (2023). How can dugongs (Dugong dugon) travel along the water column at low energetic cost? A novel hypothesis. Ecol. Model. 485, 110505. doi: 10.1016/j.ecolmodel.2023.110505
Lin M., Turvey S. T., Han C., Huang X., Mazaris A. D., Liu M., et al. (2022). Functional extinction of dugongs in China. R. Soc. Open Sci. 9, 211994. doi: 10.1098/rsos.211994
Marsh H. (2018). “Dugong: dugong dugon,” in Encyclopedia of marine mammals, 3rd ed. Eds. Würsig B., Thewissen J. G. M., and Kovacs K. M. (London, UK: Academic Press), 274–277. doi: 10.1016/B978-0-12-804327-1.00110-2
Marsh H., Eros C., Corkeron P., and Breen B. (1999). A conservation strategy for dugongs: Implications of Australian research. Mar. Freshw. Res. 50, 979–990. doi: 10.1071/MF99080
Marsh H., Eros C., Hugues J., and Penrose H. (2002). Dugong: Status reports and action plans for countries and territories (Early Warning and Assessment Report Series) (UNEP). Available online at: https://portals.iucn.org/library/node/8013 (Accessed June 29, 2025).
Marsh H., Heinsohn G. E., and Marsh L. M. (1984). Breeding cycle, life history, and population dynamics of the dugong, Dugong dugon (Sirenia: Dugongidae). Aust. J. Zoology 32, 767–788. doi: 10.1071/ZO9840767
Marsh H., O’Shea T. J., and Reynolds J. E. (2011). Ecology and conservation of Sirenia: Dugongs and manatees (Cambridge, UK: Cambridge University Press). doi: 10.1017/CBO9781139013277
Marsh H., Prince R. I. T., Saafeld W. K., and Shepherd R. (1994). The distribution and abundance of the dugong in Shark Bay, Western Australia. Wildlife Res. 21, 149–161. doi: 10.1071/WR9940149
Marsh H. and Saalfeld W. (1989). Distribution and abundance of dugongs in the northern Great Barrier Reef Marine Park. Wildlife Res. 16, 429–440. doi: 10.1071/WR9890429
Marsh H. and Saalfeld W. (1990). The distribution and abundance of dugongs in the Great Barrier Reef Marine Park south of Cape Bedford. Wildlife Res. 17, 511–524. doi: 10.1071/WR9900511
Marsh H. and Saalfeld W. (1991). The status of the dugong in Torres Strait (Research Publication No. 11) (Great Barrier Reef Marine Park Authority). Available online at: https://elibrary.gbrmpa.gov.au/jspui/handle/11017/309 (Accessed June 28, 2025).
Marsh H. and Sobtzick S. (2019). “Dugong dugon (amended version of 2015 assessment),” in International Union for Conservation of Nature (IUCN) Red List of Threatened Species (IUCN). Available online at: https://www.iucnredlist.org/species/6909/160756767 (Accessed June 28, 2025).
Marshall C. D., Al Ansi M., Dupont J., Warren C., Al Shaikh I., and Cullen J. (2018). Large dugong (Dugong dugon) aggregations persist in coastal Qatar. Mar. Mammal Sci. 34, 1154–1163. doi: 10.1111/mms.12497
Ng S. Z. H., Ow Y. X., and Jaafar Z. (2022). Dugongs (Dugong dugon) along hyper-urbanized coastlines. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.947700
Nishiwaki M., Kasuya T., Miyazaki N., Tobayama N., and Kataoka T. (1979). Present distribution of the dugong in the world. Sci. Rep. Whales Res. Institute 31, 133–141. Available at: https://www.icrwhale.org/pdf/SC031133-141.pdf?utm_source=chatgpt.com (Accessed June 28, 2025).
Owlad E., Stoffelen A., Ghafarian P., and Gholami S. (2022). Wind field and gust climatology of the Persian Gulf during 1988–2010 using in-situ, reanalysis and satellite sea surface winds. Regional Stud. Mar. Sci. 52, 102255. doi: 10.1016/j.rsma.2022.102255
Ponnampalam L. S., Fairul Izmal J. H., Adulyanukosol K., Ooi J. L. S., and Reynolds J. E. (2015). Aligning conservation and research priorities for proactive species and habitat management: The case of dugongs Dugong dugon in Johor, Malaysia. Oryx 49, 743–749. doi: 10.1017/S0030605313001580
Pollock K. H., Marsh H., and Lawler I. R. (2006). Estimating animal abundance in heterogeneous environments: An application to aerial surveys for dugongs. Journal of Wildlife Management 70 (1), 255–262. doi: 10.2193/0022-541X(2006)70[255:EAAIHE]2.0.CO;2
Preen A. R. (1992). Interactions between dugongs and seagrasses in a subtropical environment (Doctoral dissertation, James Cook University of North Queensland). Townsville, QLD, Australia: James Cook University. Available online at: https://researchonline.jcu.edu.au/1086/ (accessed December 5, 2023).
Preen A. (2004). Distribution, abundance and conservation status of dugongs and dolphins in the southern and western Arabian Gulf. Biol. Conserv. 118, 205–218. doi: 10.1016/j.biocon.2003.08.014
Preen A. and Marsh H. (1995). Response of dugongs to large-scale loss of seagrass from Hervey Bay, Queensland, Australia. Wildlife Res. 22, 507–519. doi: 10.1071/WR9950507
Price A. R. G. and Coles S. L. (1992). Aspects of seagrass ecology along the western Arabian Gulf coast. Hydrobiologia 234, 129–141. doi: 10.1007/BF00014245
Rausch R. and Dirks H. (2024). A hydrogeological overview of the Upper Mega Aquifer System on the Arabian Platform. Hydrogeology J. 32, 621–634. doi: 10.1007/s10040-023-02760-0
Said N. E., Cleguer C., Lavery P., Hodgson A. J., Gorham C., Tyne J. A., et al. (2025). Sparse seagrass meadows are critical dugong habitat: A novel rapid assessment of habitat–wildlife associations using paired drone and in-water surveys. Ecol. Indic. 171, 113135. doi: 10.1016/j.ecolind.2025.113135
Seddon J. M., Ovenden J. R., Sneath H. L., Broderick D., Dudgeon C. L., and Lanyon J. M. (2014). Fine scale population structure of dugongs (Dugong dugon) implies low gene flow along the southern Queensland coastline. Conserv. Genet. 15, 1381–1392. doi: 10.1007/s10592-014-0624-x
Shawky A. M. (2019). Evidence of the occurrence of a large dugong in the Red Sea, Egypt. Egyptian J. Aquat. Res. 45, 247–250. doi: 10.1016/j.ejar.2019.08.001
Sheppard J. K., Preen A. R., Marsh H., Lawler I. R., Whiting S. D., and Jones R. E. (2006). Movement heterogeneity of dugongs, Dugong dugon (Müller), over large spatial scales. J. Exp. Mar. Biol. Ecol. 334, 64–83. doi: 10.1016/j.jembe.2006.01.011
Smith R., Purnama A., and Al-Barwani H. H. (2007). Sensitivity of hypersaline Arabian Gulf to seawater desalination plants. Appl. Math. Model. 31, 2347–2354. doi: 10.1016/j.apm.2006.09.010
State of Qatar (2004). Law No. 19 of 2004 on the protection of marine species. (Doha, Qatar: Al Meezan Qatar Legal Portal, Government of Qatar). Available online at: https://www.almeezan.qa/LawPage.aspx?id=139&language=en (Accessed date June 29, 2025).
Thoppil P. G. and Hogan P. J. (2010). Persian Gulf response to a wintertime shamal wind event. Deep Sea Res. Part I: Oceanographic Res. Papers 57, 946–955. doi: 10.1016/j.dsr.2010.03.002
Tol S., Coles R., and Congdon B. (2016). Dugong dugon feeding in tropical Australian seagrass meadows: Implications for conservation planning. PeerJ 4, e2194. doi: 10.7717/peerj.2194
Vasou P., Krokos G., Langodan S., Sofianos S., and Hoteit I. (2024). Contribution of surface and lateral forcing to the Arabian Gulf warming trend. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1260058
Weather Underground Philadelphia, PA weather history. Available online at: https://www.wunderground.com/history/daily/us/pa/philadelphia (Accessed July 31, 2025).
Westlake E. L., Keesing J. K., Hardiman L. K., Tonks M., and Olsen Y. (2022). Growth, biomass and productivity of the seagrass Thalassia hemprichii at Ashmore Reef, Australia. Aquat. Bot. 183, 103557. doi: 10.1016/j.aquabot.2022.103557
Whitehead S. (2015). Seasonality of a short-lived seagrass relative to environmental factors and the development of an adaptable, functional-structured plant model (Master’s thesis, The University of Western Australia). Perth, WA, Australia: The University of Western Australia. Available online at: https://api.research-repository.uwa.edu.au/ws/portalfiles/portal/4725969/Whitehead_Sam_2015.pdf (Accessed June 27, 2025).
Williams P. J., Hooten M. B., Womble J. N., and Bower M. R. (2017). Estimating occupancy and abundance using aerial images with imperfect detection. Methods Ecol. Evol. 8, 1679–1689. doi: 10.1111/2041-210X.12815
Yamamuro M., Aketa K., and Uchida S. (2004). Carbon and nitrogen stable isotope ratios of the tissues and gut contents of a dugong from the temperate coast of Japan. Mammal Study 29, 179–183. doi: 10.3106/mammalstudy.29.179
Keywords: dugongs, largest herd, group size estimation, UAV survey, Arabian Gulf
Citation: Alagha DI, Soliman Y, Marshall C and Alansi M (2025) Detection of the largest herd of dugongs (Dugong dugon) in the Central Arabian Gulf using unmanned aerial vehicles. Front. Mar. Sci. 12:1620194. doi: 10.3389/fmars.2025.1620194
Received: 29 April 2025; Accepted: 22 September 2025;
Published: 14 October 2025.
Edited by:
Xuelei Zhang, Ministry of Natural Resources, ChinaReviewed by:
John Man Kon Wong, Ministry of Environment and Climate Change, QatarCharles Deutsch, Florida Fish & Wildlife Conservation Commission, United States
Copyright © 2025 Alagha, Soliman, Marshall and Alansi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yousria Soliman, eW91c3JhLnNvbGltYW5AZ21haWwuY29t
 Danah I. Alagha1
Danah I. Alagha1 Yousria Soliman
Yousria Soliman Christopher Marshall
Christopher Marshall