- 1Marine Biotechnology Fish Nutrition and Health Division, Indian Council of Agricultural Research-Central Marine Fisheries Research Institute, Kochi, India
- 2Marine Biodiversity and Environment Management Division, Indian Council of Agricultural Research-Central Marine Fisheries Research Institute, Kochi, India
- 3Faculty of Biosciences and Aquaculture, Nord University, Bodø, Norway
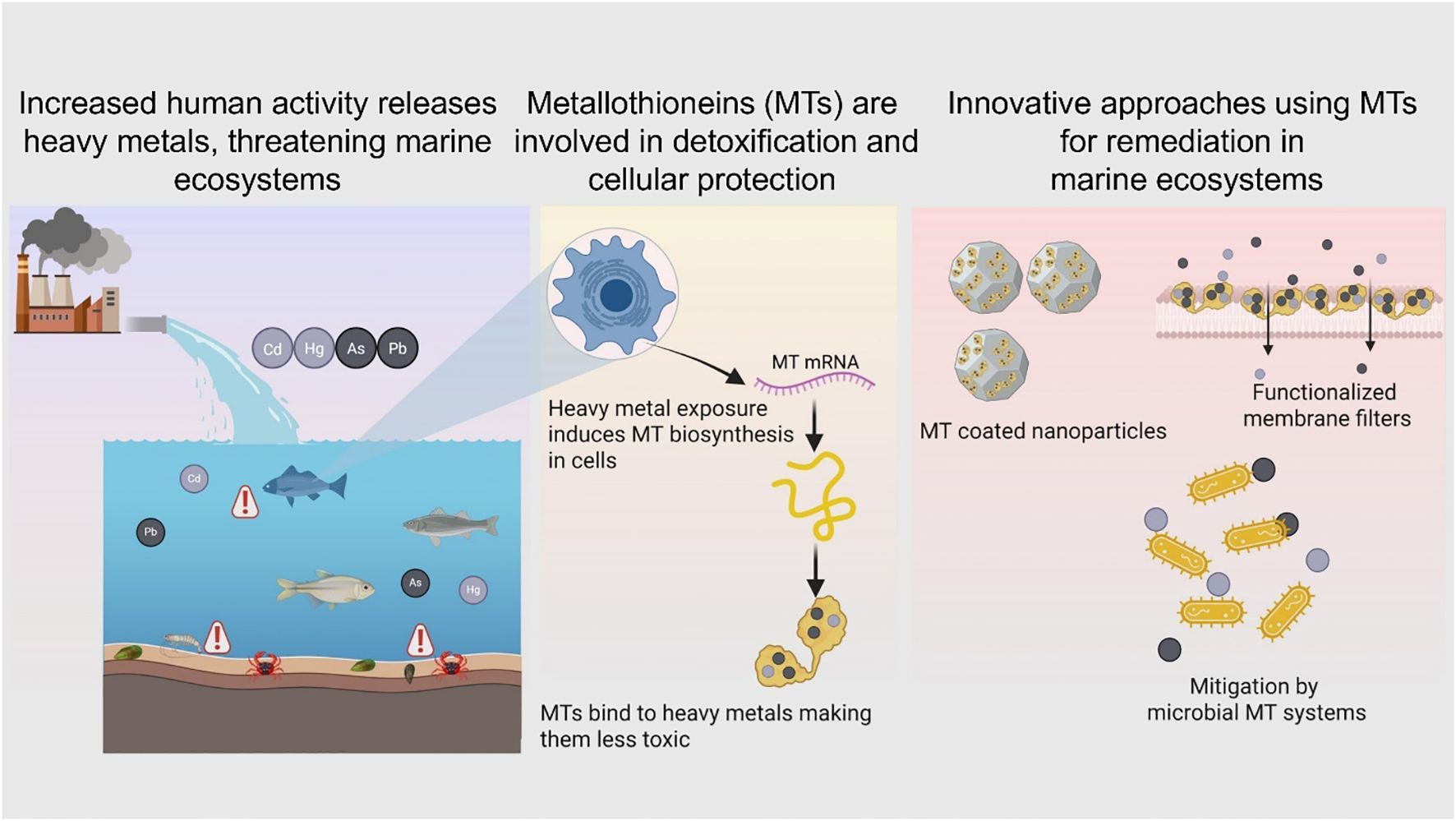
Oceans and coastal waters, vital for human survival and climate regulation, are increasingly threatened by heavy metal pollution due to human activities including industrialization and urbanization. Heavy metals including tin, zinc, mercury, copper, nickel, cadmium, cobalt, vanadium, strontium, titanium, arsenic, lead, molybdenum, and chromium are persistent pollutants that can accumulate in marine organisms, posing significant risks to ecosystems and human health. While some heavy metals are essential in trace amounts, they can exert undesirable biological effects at higher concentrations and even become toxic. In response to such toxic exposure, terrestrial and aquatic plants and animals are known to have evolved inherent mechanisms to subdue heavy metal toxicity. These responses often involve the activation of various stress-related proteins, including heat shock proteins, antioxidant enzymes, and metal-binding molecules that work collectively to restore cellular homeostasis. One of the mechanisms to mitigate metal toxicity is through the activity of metallothionein proteins. Metallothioneins, low molecular weight, cysteine-rich proteins, play a crucial role in mitigating the toxic effects of heavy metals. These proteins bind heavy metals, aiding in detoxification, protecting the cells from their undesirable effects, and maintaining metal homeostasis. Thus, metallothionein expression and activity also serve as valuable biomarkers for assessing heavy metal pollution, providing insights into the biological impact of these contaminants. The present review explores the role of bacterial metallothioneins in detoxification and their potential in environmental risk assessment, focusing on their importance in marine species exposed to heavy metal pollution. We explore the studies that report heavy metal contamination in the coastal waters, followed by elucidating the effects of heavy metal exposure on metallothionein activity and expression in marine fish, crustaceans and mollusks. Finally, we provide possible future perspectives of how bacterial metallothioneins can be employed for mitigating ecological damage caused by heavy metals. By understanding the interactions between heavy metals and metallothioneins, we can develop more effective strategies for monitoring and mitigating the effects of heavy metal contamination in marine environments.
1 Introduction
Oceans and coastal waters are essential to human survival and prosperity, supporting numerous livelihoods and economies. The size of the ocean economy was estimated to be around US$1.5 trillion in 2010, equivalent to some 3% of global GDP at the time. By 2030, its contribution is projected to double to US$3trn, providing full-time employment for around 40m people (World Ocean Initiative, 2020). Some of the major ocean-based sectors include fisheries, aquaculture, tourism, and healthcare, while renewable energies and deep-sea mining are emerging sectors with potential to improve the quality of human welfare (Dankel et al., 2023). Since the 1990s, the world oceans have consistently supplied more 80 million tonnes of aquatic animals every year for direct human consumption or feed use (FAO, 2024). Furthermore, marine renewable energy sources like tidal wave energy, ocean current energy and ocean thermal energy conversion are promising solutions for augmenting energy security for direct socioeconomic benefits even in the most remote coastal communities (Kazimierczuk et al., 2023). Therefore, world oceans play a critical role in alleviating poverty, ensuring food and energy security. Healthy oceans also contribute significantly to climate regulation (Abraham et al., 2022) and resilience (DeVries, 2022). According to the United Nations, the oceans contribute almost half of the global oxygen supply and absorb 30 percent of all carbon dioxide emissions while at the same time absorbing almost 90% of the excess heat generated by carbon emission https://www.un.org/en/climatechange/science/climate-issues/ocean. However, over the past century, coastal and oceanic waters have come under tremendous pressure from human activities (Priya et al., 2023). Industrialization, urbanization, and the improper disposal of chemical and domestic wastewater have contributed to rising levels of aquatic pollution. Moreover, the limited effectiveness of global and national governance systems has contributed to the intensification of this pollution to unprecedented levels. As a result, heavy metal concentrations in coastal and marine waters have reached alarming levels worldwide (Ramasamy et al., 2022; Zhang et al., 2025; Chidugu-Ogborigbo et al., 2025).
Heavy metals are a group of elements characterized by their high atomic weight (ranging from 63.5 to 200.6) and a density exceeding 4000 kg/m³ (Duffus, 2002). More than 50 elements on the periodic table fall into this category, including transition metals, metalloids, lanthanides, and actinides. Examples of these elements include tin (Sn), zinc (Zn), mercury (Hg), copper (Cu), nickel (Ni), cadmium (Cd), cobalt (Co), vanadium (Va), strontium (St), titanium (Ti), arsenic (As), lead (Pb), molybdenum (Mo), and chromium (Cr) (Dagdag et al., 2023). While certain heavy metals, such as iron (Fe), copper (Cu), Ni, and Zn, are essential for marine life in trace amounts, they become toxic at higher concentrations (Jomova et al., 2022). Others like As, Cd, Cr, Pb, and Hg are classified as highly lethal (Tchounwou et al., 2012). Unlike organic pollutants, heavy metals are non-biodegradable, leading to their accumulation in living organisms once released into the environment. This accumulation can have detrimental effects on the health of all living beings, including plants, animals and humans, with far-reaching consequences for marine ecosystems, mariculture activities and fisheries (Phaenark et al., 2024). Coastal sediments often serve as the depository for contaminants from various sources, such as riverine inputs, atmospheric deposition, and direct industrial discharges. These sediments act as significant sinks for trace elements through adsorption and subsequent sedimentation (Al-Mur, 2021). Heavy metals present in sediments and seawater are absorbed by aquatic organisms and bioaccumulate through the food chain, posing serious threats to both marine ecosystems and human health (Zhou et al., 2022). Numerous strategies like photocatalysis, flotation, flocculation and ion exchange etc. have been developed to mitigate heavy metal pollution in marine and coastal waters (Aziz et al., 2023). However, precise estimation of the heavy metal load in the aquatic fauna is a prerequisite for devising strategies to mitigate the adverse effects of heavy metal pollution in aquatic habitats. The extent of heavy metal contamination in coastal and marine waters can be evaluated through water quality assessments, sediment analysis for heavy metals, or by studying the accumulation of these pollutants in marine organisms (Zhang et al., 2024). Mollusks and other invertebrates, for instance, often show significantly higher levels of heavy metals compared to other marine life due to their habitat and feeding behaviors (Primost et al., 2017; Shefer et al., 2015). In addition to these direct approaches, biomarkers of exposure are powerful tools for assessing heavy metal pollution. Being highly sensitive to metal exposure, biomarkers can enable the early detection of such pollutants, allowing for timely intervention and reducing their harmful effects (Kadim and Risjani, 2022). Metallothioneins (MTs) are particularly reliable indicators of metal exposure in aquatic environments (Yang et al., 2024). MTs provide valuable insights into the biological impact of heavy metals by regulating their interference with crucial processes such as enzyme activity, gene expression, and protein functions, leading to a more accurate biological risk assessment of environmental contamination (Ruttkay-Nedecky et al., 2013; Raudenska et al., 2014; Saad et al., 2016). Furthermore, MTs have proven useful for evaluating the effectiveness of mitigation strategies against heavy metal pollution by tracking changes in biological responses as metal levels decrease in aquatic habitats over time (Tom et al., 1998; Chesman et al., 2007). This review focusses on research on metallothioneins and how they interact with heavy metals to mitigate their toxic effects in biological systems. We cover the literature about the assessment of metallothionein activity in aquatic animals in response to heavy metal exposure. Finally, an overview is provided on the metal binding properties of metallothioneins that can be leveraged to develop mitigation strategies against heavy metal pollution in aquatic habitats.
2 Heavy metal pollution in coastal and marine habitats
Heavy metals are naturally occurring elements dispersed throughout the Earth’s crust, typically present in trace amounts—ranging from mere parts per trillion for noble metals to as much as 5% for iron. During the Earth’s early formation, around 4 to 5 billion years ago, when the mantle was still molten, these heavy metals largely settled into the planet’s core (Engwa et al., 2019). Natural processes such as biogenic activity, the release of marine salts, volcanic eruptions, and weathering are recognized as contributors to the release of heavy metals into the environment (Aziz et al., 2023). Once these metals enter the soil, often carried by rain, they can either accumulate within the soil or be further mobilized by irrigation and additional rainfall. A portion of the leached heavy metals infiltrates groundwater (Wang and Lei, 2018), while the remainder is carried to coastal waters via runoff. However, anthropogenic activities—such as industrial operations, mining, and modern agricultural practices—play a far more significant role in contributing to heavy metal pollution in aquatic habitats (Li et al., 2022). As a result of these activities, millions of tons of heavy metals—such as Cr, Cu, Zn, As, Cd, Pb, Sn—are introduced into natural waters each year (Tchounwou et al., 2012). These heavy metals bioaccumulate in aquatic organisms, posing significant risks to human health and threatening the delicate balance of aquatic ecosystems. Heavy metal accumulation in aquatic environments often exhibits differential partitioning among water, sediment, and biota (Phaenark et al., 2024). In Pullicat Lake, located on the Coromandel Coast straddling the border of Andhra Pradesh and Tamil Nadu, and the second-largest brackish water lagoon in India, the prevalence of heavy metals followed the order Pb > Cr > Zn > Cu in water and Cu > Cr > Pb > Zn in sediments (Akila et al., 2022). In contrast, fish species like Lutjanus fulviflamma, Chanos chanos, Arius sp., and Terapon jarbua, that are found commonly in the lake, exhibited a distinct accumulation pattern, with the order Cu > Cr > Zn > Pb. The variation in heavy metal accumulation across water, sediment, and fish in this case helps to understand the differences in metal chemistry, environmental partitioning, and biological uptake. While Pb and Cr tend to bind to particulates and hence may explain their higher accumulation in sediments. On the other hand, essential metals like Cu and Zn are more bioavailable and actively taken up by fish, leading to higher concentrations in their tissues. Notably, the concentrations of Cu and Cr (3.3 to 39.1 µg/g and 0.22-23.1 µg/g, respectively) in fish muscle tissues exceeded the permissible limits (30 µg/g and 0.05 µg/g) established by the World Health Organization (WHO, 1989), underscoring potential health risks associated with the regular consumption of fish from this ecosystem and the urgent need for regulatory measures (Akila et al., 2022). Assessment of heavy metal contamination in coastal sediments from the southwestern Bay of Bengal, India, indicated notable ecological risk levels. The geo-accumulation index showed Cd and As concentrations ranging from 0.20 to 21.76 μg/g and 2.09 to 28.18 μg/g, respectively—values consistent with contamination thresholds (WHO, 1989). Further analysis using the ecological risk index classified the area as being subject to moderate to high ecological risk, emphasizing the vulnerability of these coastal sediments to heavy metal pollution (Naik et al., 2023). In an elaborate study in North-West Bay of Bengal was conducted by Hasan et al. (2022) six different indices were measured to portray the degree of pollution (heavy metal pollution index, heavy metal evaluation index and Nemerow pollution index) and risk assessment (potential ecological risk index, hazard quotient, and hazard index). The pollution indices suggested that the north-west region of Bay of Bengal is under moderate to high degree of Pb, Cu and Cd pollution. Though human health hazards are comparatively low according to hazard quotient and hazard index, the study also showed that the heavy metals Fe, Cu and Pb (466.57, 45.05, 90.16 μg/l, repsectively) exceeded the lowest biological chronic safety limit recommended by the National Oceanographic and Atmospheric Administration, USA (>50, >3.1 and >8.1 μg/l, for Fe, Cu and Pb respectively). On the west coast of India, an investigation into heavy metal dynamics within the Kadalundi-Vallikkunnu Community Reserve—a coastal habitat comprising mudflats, mangroves, and sand beaches at the mouth of the Kadalundi River—revealed a clear pattern of trophic transfer (Aarif et al., 2023). Although relatively low concentrations of Zn, Cu, Co, Cr, Pb, and Cd were detected in sediments, significantly higher levels were observed in invertebrate prey species. Metal concentrations in prey ranged from 84.72–224.74 mg/kg for Zn, 26.63–170.36 mg/kg for Cu, 13.98–14.42 mg/kg for Co, 14.78–98.16 mg/kg for Cr, 18.95–157.29 mg/kg for Pb, and 9.33–60.56 mg/kg for Cd. Corresponding increases were also recorded in shorebird droppings, with Zn, Cu, Co, Cr, Pb, and Cd concentrations ranging from 41.33–58.8, 31.42–52.11, 36.34–55.68, 52.3–68.21, 25.94–43.13, and 5.53–16.4 mg/kg, respectively. This progressive increase in metal concentrations from sediment to prey to avian excreta highlights the potential for biomagnification through dietary exposure. Additionally, biofilm samples exhibited high concentrations of Cr (22.64 mg/kg), Pb (28.09 mg/kg), and Cd (18.46 mg/kg), suggesting that biofilm-feeding shorebirds may be accumulating heavy metals through this alternative trophic pathway. In another study, an assessment of heavy metal accumulation in Indian Mackerel (Rastrelliger kanagurta) harvested from nine coastal sites revealed substantial contamination linked to local anthropogenic activities (Mangalagiri et al., 2020). These sites were selected based on their proximity to industrial operations—including aquaculture hatcheries, commercial fishing zones, steel and thermal power plants, ports, and various factories (pharmaceutical, petrochemical, and textile)—as well as domestic pollution sources such as sewage canals. The concentrations of aluminum (Al: 34.66–58.55 μg/g), chromium (Cr: 2.62–3.24 μg/g), manganese (Mn: 0.86–1.36 μg/g), arsenic (As: 0.67–1.47 μg/g), and lead (Pb: 0.06–0.37 μg/g) in fish muscle tissues exceeded established normal levels, indicating significant bioaccumulation and raising concerns about the safety of fish consumption from these regions.
These studies underscore the pervasive and multifaceted bioaccumulation of heavy metals in marine ecosystems, with far-reaching implications for both environmental and human health. The accumulation of heavy metals in organisms across multiple trophic levels—invertebrates, fish, biofilms, and even bird droppings—highlights significant risks, particularly for coastal communities that rely heavily on seafood. These findings reinforce the need for integrated monitoring strategies employing bioindicators, stricter regulatory enforcement, and targeted risk management efforts to address the impacts of heavy metal pollution. By deepening our understanding of how heavy metals affect aquatic organisms, we can better predict ecological consequences and develop effective mitigation strategies to protect both ecosystems and human health.
3 Impact of heavy metal exposure, metallothioneins and detoxification
Heavy metals in their elemental forms are only sparingly soluble in water. However, their solubility depends strongly on the speciation and presence of complexing agents (like organic matter or chloride ions) tending to bind with suspended particulate matter and sediments. The degree to which these metals accumulate in the tissues and organs of marine organisms varies significantly by species, driven by species-specific detoxification and metabolic processes (Giri and Singh, 2014; Ray and Vashishth, 2024). As a result, different organisms in the same polluted environment may exhibit vastly different levels of metal accumulation, underscoring the importance of selecting appropriate sentinel species for assessing heavy metal burdens in aquatic ecosystems. Numerous species—including fish, benthic macroinvertebrates, shrimp, crustaceans, bacteria, periphyton, and diatoms—have been utilized to study the effects of heavy metal contamination in marine environments (Kadim and Risjani, 2022). However, bioaccumulation varies significantly due to these species-specific mechanisms. For example, in the Subarnarekha River in Ranchi, India, the muscles of Penaeus indicus were found to accumulate As (0.61 ug g-1) and Cd (0.0.28 ug g-1) at levels 2 and 23 times higher, respectively, than the dorsal muscles of Mugil gulio (As: 0.32 and Cu 0.03 ug g-1) (Giri and Singh, 2014). Furthermore, considerable variability in heavy metal accumulation can be observed even within a single taxonomic class, across different species (Ray and Vashishth, 2024) and across various tissues within the same species (Malik and Maurya, 2014). For instance, in the Ennore Creek in Southeast India the Hg concentration in Perna viridis (0.85 µg/g) was about double that of Crassostrea madrasensis (0.43 µg/g hepatopancreas) (Kumar et al., 2013). In two fish species collected from the same region of the Kali river, India, the concentration of Cd in the muscle during summer months was 22.40 µg/g in Puntius ticto whereas in Heteropneustis fossilis, muscle Cd concentration was more than double (45.4 µg/g) In Mastacembelus armatus obtained from, the Cu concentration in the muscle was 41.3 µg/g and 271.6 µg/g in the liver indicating the differential deposition of heavy metals in the tissues of the same animal (Javed and Usmani, 2013). This variability highlights the complexity of bioaccumulation processes and emphasizes the importance of careful species selection and analysis to accurately assess heavy metal contamination in aquatic environments.
For a metal to exert its biological effect—whether beneficial or toxic—it must first cross the cell membrane, enter the cell, and bind, either reversibly or irreversibly, to a cellular target, altering specific biochemical processes (Hollenberg, 2010). Heavy metals can enter organisms through various physiological routes, primarily via ingestion through the digestive tract from water and food, or through non-dietary absorption across membranes such as the skin and gills. In fish, heavy metals are often absorbed through the gill surfaces and then distributed to tissues like muscle, liver, intestines, gills, and kidneys (Ray and Vashishth, 2024). Once inside biological systems, metals may either participate in physiological processes or cause harm, depending on their concentration. Essential elements like Mn, Fe, Co, Cu, Zn, and Mo are vital for growth and normal physiological functions, but they become toxic at elevated levels (Jomova et al., 2022). On the other hand, elements like Pb, Hg, and Cd have no physiological role and are toxic even at low concentrations (Kortei et al., 2020; Lall, 2022). Toxic metals exert their harmful effects through several common mechanisms, such as enzyme inhibition, disruption of subcellular organelles, and interaction with DNA, which can result in mutagenesis, apoptosis, or carcinogenesis (Beyersmann and Hartwig, 2008) (Figure 1). These metals may also covalently modify proteins, displace essential metals in metal-dependent proteins (Thompson, 2022). Some metals generate free radicals, leading to damage of vital cellular proteins, membranes, and organelles, potentially causing apoptosis (Engwa et al., 2019). The cumulative outcome of these toxic mechanisms is damage to various organs, including the nervous, respiratory, endocrine, and reproductive systems (Balali-Mood et al., 2021). As a defense, many animals have evolved mechanisms to mitigate heavy metal toxicity by forming non-toxic metal-protein complexes with proteins like MTs, which play a crucial role in detoxification.
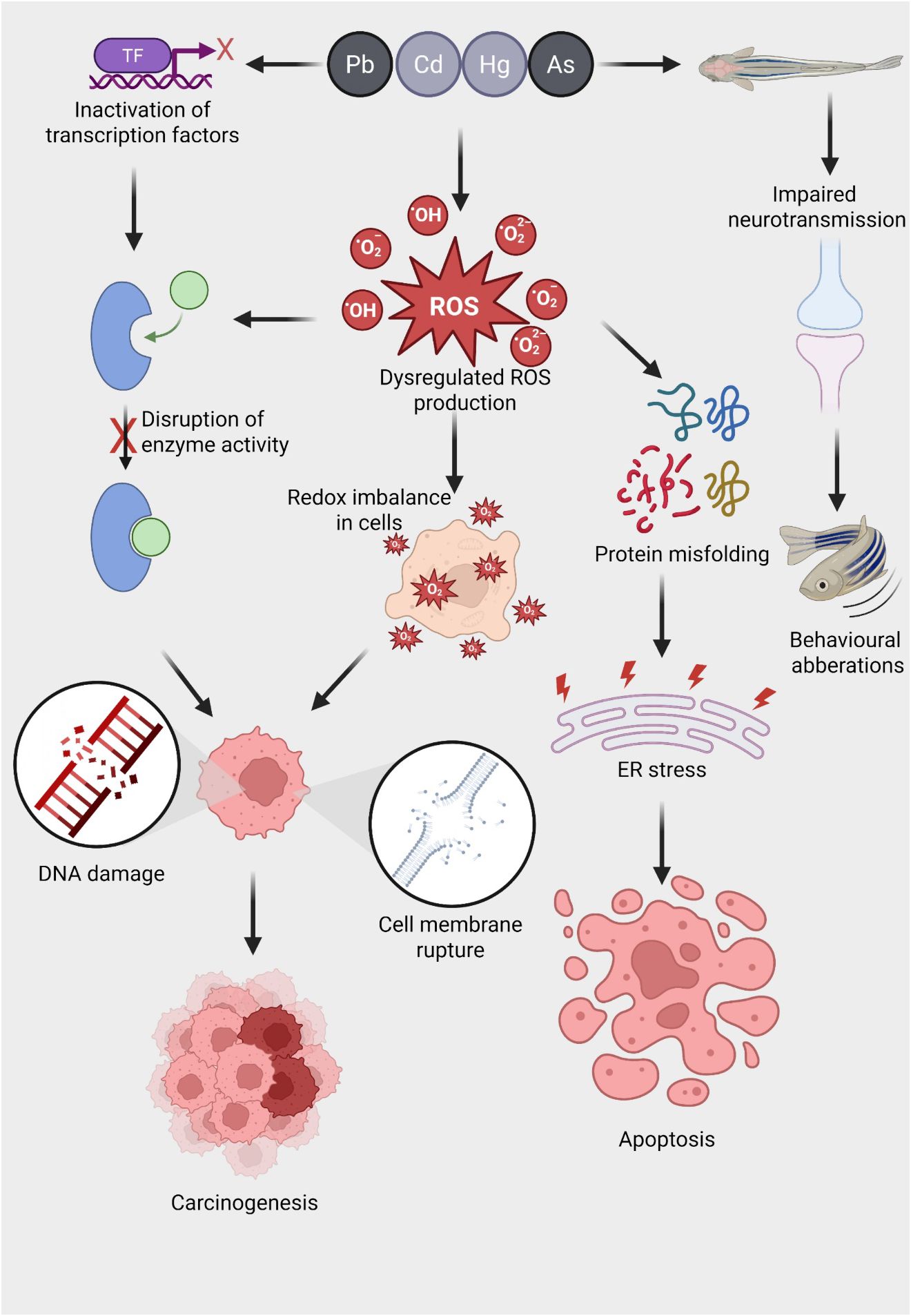
Figure 1. Mechanisms of heavy metal-induced oxidative stress and its downstream toxicological effects in aquatic organisms. Exposure to heavy metals such as lead (Pb), cadmium (Cd), mercury (Hg), and arsenic (As) triggers a cascade of toxicological responses in aquatic animals. These metals inactivate transcription factors (TFs), disrupt normal gene regulation, and interfere with enzyme function by binding to active sites or altering protein structure. A key consequence of metal exposure is the dysregulation of reactive oxygen species (ROS) production, including superoxide anions (O₂⁻), hydroxyl radicals (·OH), and hydrogen peroxide (H₂O₂), leading to a redox imbalance within cells. This oxidative stress results in widespread cellular damage, including (i) DNA strand breaks and mutations that can drive carcinogenesis, (ii) disruption of membrane integrity causing cell lysis, and (iii) protein misfolding that triggers endoplasmic reticulum (ER) stress. Persistent ER stress promotes apoptotic cell death. In neural tissues, heavy metal exposure impairs neurotransmission, potentially through ROS-mediated synaptic damage, resulting in behavioral aberrations such as altered locomotion and reduced responsiveness. Collectively, these molecular and cellular disruptions underlie the chronic toxicity and disease pathology associated with metal pollution in aquatic ecosystems.
MTs are a group of low molecular mass (∼7 kDa), cysteine-rich and evolutionarily conserved metal-binding proteins (Lynes et al., 2014). MTs are ubiquitous proteins, found not only throughout the animal kingdom, but also in higher plants, and some prokaryotes. They are a family of proteins characterized by sulfur-based metal clusters. The MTs were initially discovered in equine renal cortex (Figure 2) (Margoshes and Vallee, 1957) and the first amino acid sequences of mammalian MTs revealed that these proteins contained 61–62 amino acids, of which 20 were highly conserved cysteines residues. In fact, over the years, four isoforms of MTs were unraveled in mammals: the MT-1, MT-2, and MT-4 isoforms consist of 61–62 amino acids and the MT-3 isoform comprises 68 amino acids (Vašák and Meloni, 2011). With respect to aquatic species, the cadmium-binding MTs was first studied in the marine fish Sebastes seboides (Olafson and Thompson, 1974). As in mammals, fish MTs are composed of two globular domains and each domain contains a ‘mineral core’ enclosed by two large helical turns of the polypeptidic chains (Vergani et al., 2005). The N-terminal right-handed β domain binds three bivalent metal ions. The C-terminal α domain is left-handed and binds four bivalent ions. Despite the similarity in the basic features, fish MTs have a higher flexible character with respect to mammalian metallothionein, that can facilitate functionality at the low temperatures that fish may experience in marine or freshwater environments (Capasso et al., 2003). However, it was revealed that the higher flexibility in the fish MTs is not a function of thermal regime but rather, it displays a significant phylogenetic dependence (Scudiero et al., 2005). In contrast to mammalian and teleost MTs, the invertebrates appear to have a more diverse structure of MTs. The main difference lies in the ability of the invertebrate MTs to bind the bivalent ions. For instance, in crabs the two domains bind three bivalent metals each (Otvos et al., 1982; Overnell et al., 1988). In comparison with mammalians, molluscan MTs usually have higher glycine content (15% in mussels), randomly distributed throughout the sequence (Roesijadi et al., 1988). Additionally, research on the oyster Crassostrea virginica has shown that its MTs are both cysteine- and glycine-rich, lacking methionine, histidine, arginine, and aromatic amino acids, further highlighting the distinctive amino acid profile of molluscan MTs (Bakiu et al., 2013).
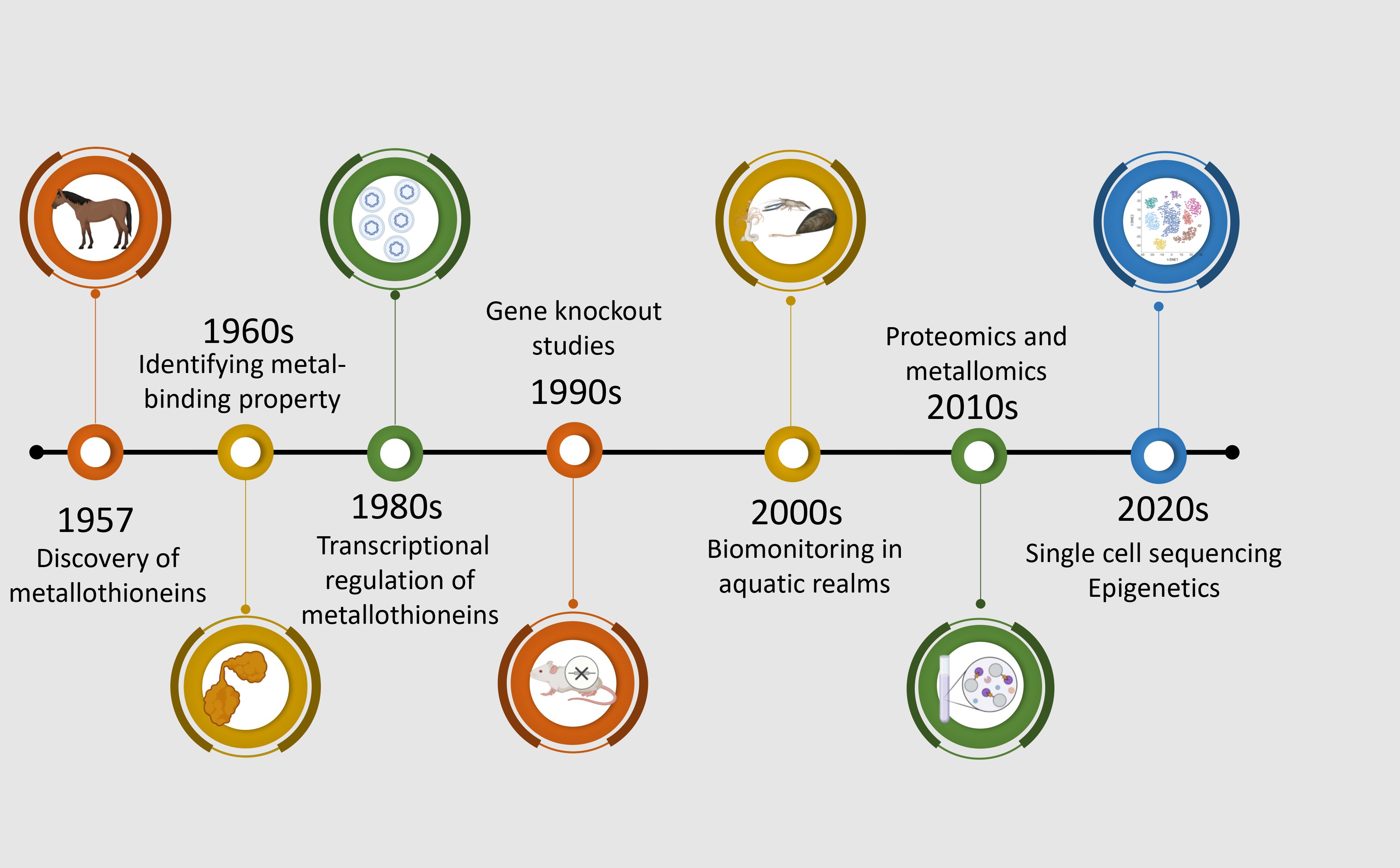
Figure 2. Chronological progression of significant discoveries and methodological advancements in metallothionein research from 1957 to the 2020s. The timeline begins with the discovery of metallothioneins in horse kidney tissues (Margoshes and Vallee, 1957), followed by the identification of their two-domain structure in the 1960s (Kagi and Valee, 1960). The 1980s saw the use of northern blotting and radiolabeled probes in MT research (Karin et al., 1980). In 1990, gene knockout models were employed to investigate metallothionein function (Masters et al., 1994). The 2000s marked a shift toward ecotoxicological applications using metallothioneins in aquatic indicator species (Amiard et al., 2006). Proteomics and metallomics approaches were integrated in the 2010s to explore metallothionein interactions and metal-binding profiles (Shabb et al., 2017). The most recent advances in the 2020s involve single-cell sequencing and epigenetic studies, providing insights into cell-specific regulation and environmental responsiveness of metallothioneins (Lu et al., 2018; Maleckaite et al., 2019).
MTs bind heavy metals and play essential roles in metal ion homeostasis, detoxification, and cellular protection against oxidative stress induced by heavy metal exposure (Rajizadeh and Pourbabaki, 2024). They are particularly important in regulating the levels of essential metals like Zn and Cu, as well as detoxifying harmful metals such as Cd and Hg (Roesijadi, 2000; Isani and Carpenè, 2014). Their role in preventing metal toxicity was established in early experiments in which mammalian cell lines were found to be highly sensitive to metal ion toxicity and cells that express excess levels of MTs were found to be extraordinarily resistant to heavy metal toxicity (Palmiter, 1998; Smith et al., 2008). There are several mechanisms that are put forth to explain how cells use the MTs to evade metal toxicity. Primarily, they bind to toxic heavy metals, preventing them from interacting with cellular components and causing oxidative (Barrera-Escorcia and Wong-Chang, 2010) and DNA damage (Qu et al., 2013). MTs also facilitating the excretion of heavy metals. Since these proteins do not present secondary structures and adopt their specific three-dimensional structure after the binding of metal ions (Si and Lang, 2018). The metal-MT complex can then be transported to the kidney, where the metal is excreted due to degradation of MT or changing pH (Samuel et al., 2021). However, in some organisms like fishes, metals can have a long biological half-life that may extend to more than a year in the liver and kidney, reflecting the poor efficiency of excretion pathways (Haux and Larsson, 1984). Nevertheless, MTs also protect cells from oxidative stress by scavenging free radicals and modulating redox states (Ruttkay-Nedecky et al., 2013). MTs also regulate the functioning of the enzymes which need metal ions as cofactors (Thompson, 2022). The binding of MTs with metal ions also regulates the intracellular concentrations of certain essential metals, particularly Zn and Cu, which are vital for numerous enzymatic reactions. Certain MTs can also shape the heavy metal repertoire in immune cells and promote metal uptake by an invading intracellular pathogen (Vignesh and Deepe, 2017). Field studies have shown significant correlations among accumulated metal concentrations and both MT protein and mRNA levels in fish (Knapen et al., 2007; M’kandawire et al., 2017). However, some studies have shown that MT protein levels may not bear significant correlation with the mRNA levels in rats (Misra et al., 1997; Vasconcelos et al., 2002), emphasizing the need to measure both changes in MT levels and transcriptional as well as translation levels. Nevertheless, an important feature of the MTs that has relevance for environmental studies is the induction of MT expression reported in several invertebrate (Tom and Auslander, 2005) species like Perna perna (Baraj et al., 2011), Crasosstrea spp (Hertika et al., 2019), Meretrix meretrix (Sun et al., 2024) and Peneaus vannamei (Moksnes et al. (1995) and vertebrates like fishes (Thanomsit et al., 2013; Bouraoui et al., 2008) in response to metal exposure making them important species to understand the biological effects of heavy metal pollution (Table 1).
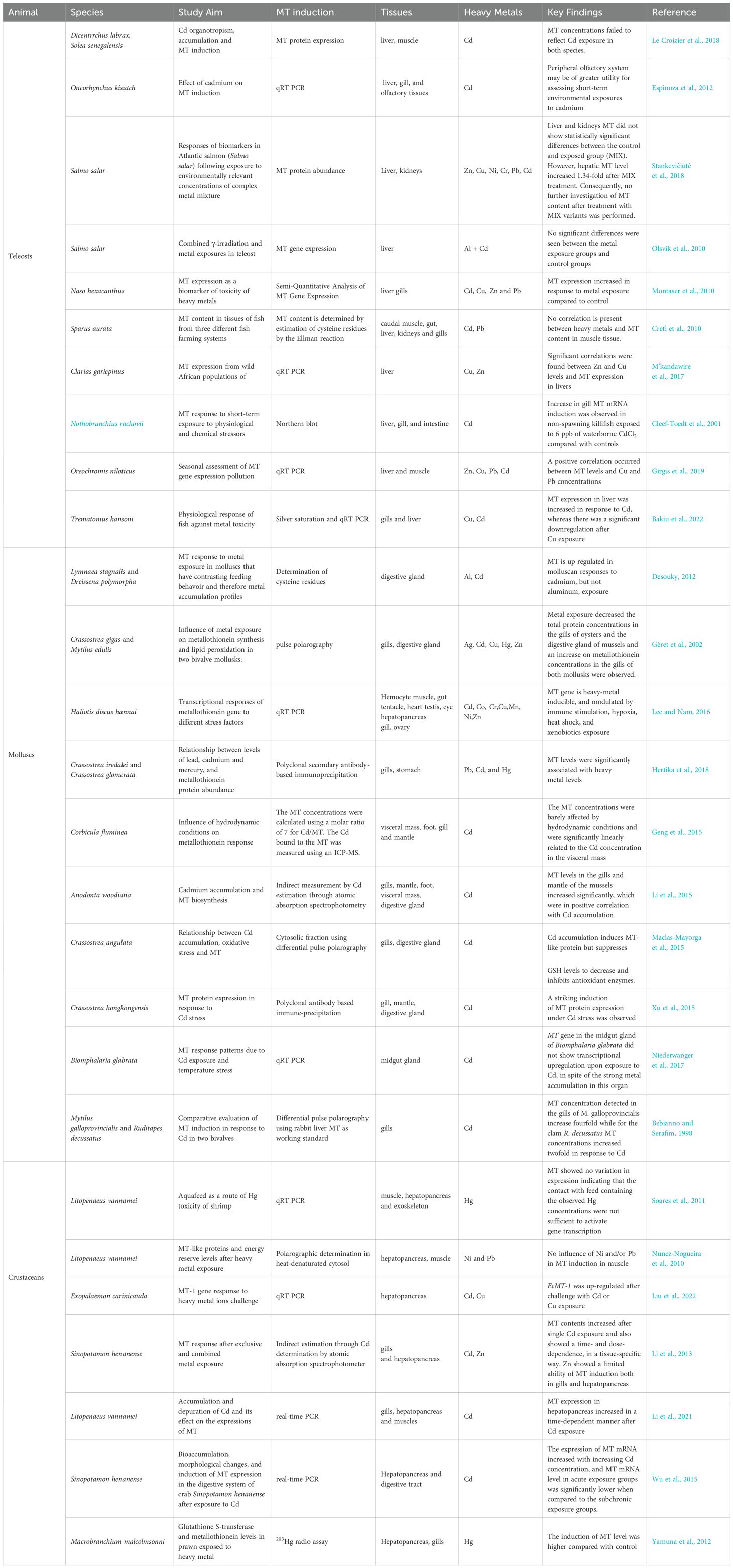
Table 1. Studies that evaluated MT protein or gene expression in response to heavy metal exposure in aquatic animals.
4 Metallothioneins as biomarkers of heavy metal pollution
MTs are considered central in the intracellular regulation of metals such as Cu, Zn, Hg and Cd. Increased MT synthesis is associated with increased capacity for binding these metals and protection against metal toxicity (Bakiu et al., 2022). Though MT induction at the level of transcription is most extensively studied, the induction of MTs in response to heavy metals is known to occur at transcriptional, translational and post-translational levels. After entering the cytoplasm, the ion interacts with a complex of metal-regulatory transcription factor-1 (MTF-1) to bind to a regulatory sequence of DNA called metal-responsive element (MRE) (Günther et al., 2012). MREs contain a 7-bp core sequence (TGCRCNC) and are present in multiple copies in the promoter/enhancer regions of almost all metal-inducible MT genes (Searle, 1987). There are two possible models how the MTF-1 can bind to MREs in the genome (Figure 3). The first view is that MTF-1 acts as a metal sensor that cannot bind to DNA in the absence of metals (Günther et al., 2012). As cells accumulate metals, the metal binds to the α and β domains, causing a conformational change in the protein and subsequent binding to the MRE. When MT levels are insufficient, it chelates the metal from the domains forcing the MTF-1 to relinquish the MREs and thus ceasing the transcription. An alternative model proposes that the existence of a metal-sensitive inhibitor, generally known as the metal synthesis inhibitor (MTI) which complexes with MTF-1 rendering it inactive (Palmiter, 1994). As cellular metal levels increase, the metal binds to the MTI releasing it from MTF-1, allowing the transcription factor to bind to the MRE to activate transcription. When MT levels are sufficient, the metal is removed from the MTI, allowing it once again to bind to MTF-1, thereby inhibiting transcription (Morby et al., 1993).
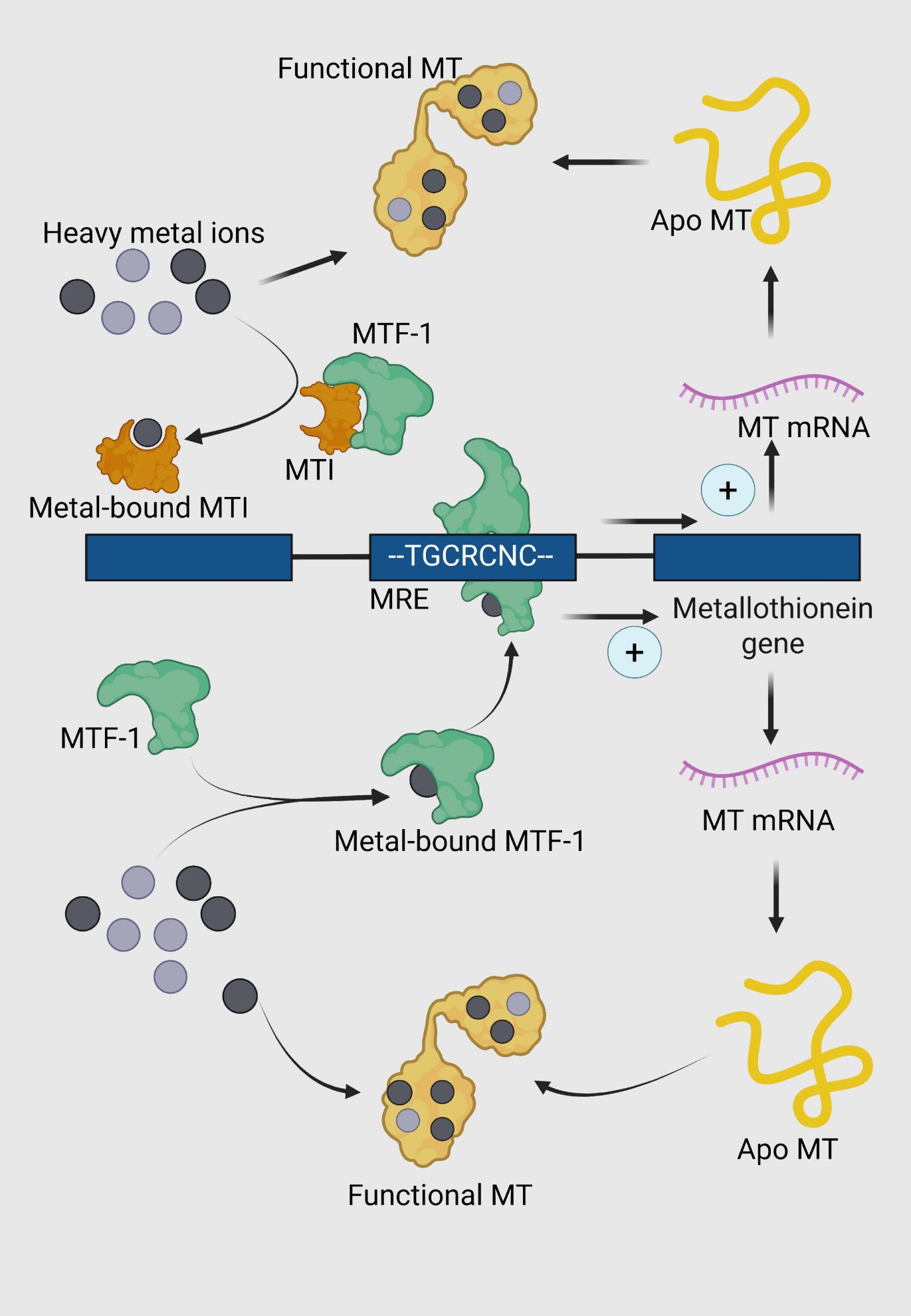
Figure 3. Regulation of metallothionein (MT) expression and function in response to heavy metal stress. Metal-responsive transcription factor 1 (MTF-1, green) is activated through the intracellular accumulation of heavy metals. The activation is mediated either through direct binding of heavy metals or through the detachment from MTI protein. Upon activation, MTF-1 undergoes a conformational change and translocates to the nucleus. Metal-bound MTF-1 binds to specific metal response elements (MREs) in the promoter region of the metallothionein gene, represented by the consensus sequence TGCRCNC. This binding upregulates the transcription of MT genes, leading to the synthesis of MT mRNA. The transcribed mRNA is translated into apo-metallothionein (apo MT), a cysteine-rich protein (yellow) devoid of bound metals. Apo MT binds to intracellular heavy metal ions, forming functional MT (yellow with metal ions).
4.1 Teleosts
In Asian seabass, MT expression in response to cadmium nitrate (CdNO₃) exposure demonstrated a biphasic dose-response pattern, with the highest MT mRNA levels observed at a dose of 4 mg/kg, but no significant induction at 6 mg/kg (Thanomsit et al., 2013). Studies in other fish species further reinforce the utility of MTs for assessing metal pollution. For instance, in Oreochromis mossambicus (tilapia), both laboratory and field studies in the Chicamba reservoir of Revue river system, Mozambique, showed that elevated Hg levels induced MT overexpression in hepatic tissues, establishing a strong correlation between Hg exposure and MT expression (Monjane-Mabuie et al., 2022). This supports the potential of MTs as reliable biomarkers for monitoring metal contamination in aquatic environments. However, the effectiveness of MTs as biomarkers must take into account several factors, including tissue-specific, species-specific, sex-specific, and time-dependent variations. In Nile tilapia, for example, Cheung et al. (2004) demonstrated that while hepatic MT mRNA levels were significantly induced by exposure to bivalent metals like Cd2+and Zn2+, the renal MT mRNA levels were not similarly affected. In contrast, the gills showed a higher sensitivity to metal ion exposure, emphasizing the importance of selecting the appropriate tissue for MT assessment. Time-dependent responses have also been observed in juvenile seabream, where significant MT induction occurred 12, 24, and 48 hours after exposure to CdCl2, indicating that the timing of sampling is crucial for accurately capturing MT expression patterns following heavy metal challenge (Bouraoui et al., 2008). Additionally, sex-specific differences have been reported, with male Nile tilapia showing significantly higher hepatic MT gene expression compared to females in metal-polluted environments (Awad et al., 2024), suggesting that sex may influence MT regulation in response to heavy metal exposure. Beyond pollution monitoring, MTs also play a role in mitigating heavy metal toxicity. In carp myocytes, for instance, arsenic exposure led to a significant reduction in the expression of zinc transporters (znt1, znt5, and znt7), Zn-regulated iron-regulated transporter-like proteins (zip8 and zip10), and MTs. However, zinc supplementation was found to alleviate this suppression, restoring MT expression and highlighting the protective role of MTs in maintaining metal homeostasis under toxic conditions (Dong et al., 2024). In conclusion, while MTs are valuable biomarkers for assessing heavy metal contamination, their application must consider various biological and environmental factors, including species, tissue specificity, sex, and timing. These factors contribute to the complexity of MT regulation but also offer insights into their broader role in heavy metal detoxification and protection in aquatic species.
4.2 Molluscs
Compared to teleosts, the process of biomineralization in invertebrates complicates the relationship between MTs and metal exposure (Mason and Jenkins, 1995). However, a positive aspect of studying invertebrate MT responses to assess aquatic pollution is that invertebrates tend to be more accurate representatives of the local environment where they are found (Tom and Auslander, 2005). Consequently, research has focused on understanding metal uptake in invertebrates as a reflection of pollution in aquatic habitats (Viarengo et al., 1999). Invertebrate gills and digestive glands are the primary organs where heavy metals accumulate (Gomes et al., 2012). For example, studies on the gills of mussels, such as the brown mussel (Perna perna), have shown that Cd bioaccumulation leads to significant MT induction (Baraj et al., 2011). Similarly, exposure to metals like Ag, Cd, Hg, and Zn for 21 days resulted in increased MT synthesis in the gills of Mytilus edulis and the digestive gland of Crassostrea gigas (Géret et al., 2002). In other bivalves, such as Crassostrea cuculata and Crassostrea glomerata, MT concentrations were consistently higher in the stomach compared to the gills, and these levels correlated with the metal concentrations in their respective habitats (Hertika et al., 2019). In Meretrix casta, an economically significant shellfish species, a metallothionein gene was recently characterized, showing predominant expression in the hemocytes and hepatopancreas. Yang et al. (2023) cloned and characterized a MT gene from the hard clam Meretrix meretrix, (designated MmMT) identifying a 629−bp ORF encoding a 76−amino−acid, cysteine-rich protein typical of MTs, with highest expression observed in hepatopancreas and hemocytes. They also demonstrated that the MT transcripts were significantly upregulated in response to Vibrio splendidus stimulation, and that Escherichia coli expressing MT exhibited enhanced tolerance to Cd2+and Cu2+. This study indicated the role of MT in both metal detoxification and immune response. Another study showed that MT expression in response to Cd exposure was significantly upregulated only at a water salinity of around 20 ppt, with peak expression at 7 days post-exposure in Meretrix meretrix. Interestingly, this increased expression diminished by 35 days, highlighting the importance of salinity in regulating the metal-metallothionein interaction (Sun et al., 2024). Similarly, Mytillus coruscus exhibited time-dependent MT mRNA expression in the hepatopancreas following exposure to Cu2+and Pb2+, peaking at 7 days and returning to normal levels by day 15 (Ge et al., 2020). These findings underscore the complex relationship between metal accumulation, environmental factors such as salinity, and MT expression in invertebrates, which must be considered when using MTs as biomarkers for aquatic pollution.
4.3 Crustaceans
Early studies on Artemia demonstrated the presence of MTs and their induction in response to Cd exposure (Bing-liang and Chien, 1994). A dose-response and time-course study by Moksnes et al. (1995) further confirmed a strong correlation between MT induction and cadmium accumulation in the hepatopancreas of the tropical shrimp Penaeus vannamei, as well as between their concurrent depuration during the recovery phase. After 16 days post-exposure, over 80% of the induced MT had been lost, and depuration correlated significantly with time (R=0.94, p < 0.01) and cadmium levels in the hepatopancreas, indicating that MTs can serve as reliable biomarkers for Cd pollution in shrimp farming. In 2005, MTs in Litopenaeus vannamei were characterized, showing a positive correlation between MT expression and Cd content in the gills and hepatopancreas, although zinc exposure did not induce MT expression (Wu and Chen, 2005). Interestingly, MT expression in L. vannamei can also be induced by both hypersaline and hyposaline conditions, suggesting that salinity plays a role in MT dynamics (Wang et al., 2014). In the caridean shrimp Exopalaemon carinicauda, a complex pattern of MT expression has been observed in response to different metal ions. The hepatopancreas was identified as the major site of MT production, with expression levels hundreds of times higher than in other tissues such as muscle, stomach, intestine, and hemocytes (Zhang et al., 2014). Notably, MT expression in the hepatopancreas was upregulated after exposure to CuSO₄ but downregulated after exposure to CdCl₂ (Zhang et al., 2013, 2014). A more recent study, however, reported that MT mRNA expression in E. carinicauda increases in a dose-dependent manner when exposed to both CuSO₄ and CdCl₂, and identified three distinct MT isoforms: a copper-inducible isoform, a cadmium-inducible isoform, and a third isoform (MT-1) that responds to both Cu and Cd exposure (Liu et al., 2022). Similarly, MT genes were recently identified in the transcriptome data of Gammarus fossarum, a sentinel amphipod species, revealing two MT-coding transcripts, mt1 and mt2, with organ-specific expression patterns in the gills and caeca. In silico analysis and experimental exposures to Cd, Zn and Ag showed that mt1 is strongly induced by Cd exposure and is more significantly expressed in the caeca, while mt2 is more inducible in the gills (Esposti et al., 2024). These studies emphasize the central role of MTs as biomarkers for assessing heavy metal-induced damage in aquatic organisms.
However, recent findings reveal that the metal-MT interaction is more complex than was initially thought (Florianczyk, 2007; Chen et al., 2014). An ideal biomarker should reliably reflect exposure to pollutants and accurately assess the effectiveness of mitigation strategies. The specificity of MTs as indicators of heavy metal pollution is complicated by external factors such as bacterial infections and salinity changes, which can also influence MT expression. Moreover, MTs participate in immune signaling pathways (Crowthers et al., 2000), and the presence of multiple isoforms with metal-specific affinities complicates their use as universal biomarkers. These complexities hinder the development of broad-spectrum MT biomarkers capable of detecting a wide range of metals across diverse aquatic environments. Establishing a reliable MT biomarker requires considering the physicochemical properties of the habitat and the species under study. Additionally, interspecific variations in MT structure-function relationships and differences in tissue distribution within the same species must be addressed to develop effective assays for detecting heavy metal pollution in coastal and marine ecosystems. Despite these challenges, the metal-binding capabilities of MTs can still be harnessed to design effective strategies for mitigating heavy metal pollution in aquatic environments. Understanding the intricacies of MT dynamics across different species and environmental conditions will be crucial for advancing both biomonitoring and remediation efforts in marine and coastal waters.
5 Leveraging bacterial metallothioneins in mitigating heavy metal induced ecological damage
The use of MTs for mitigating heavy metal pollution in aquatic ecosystems has garnered growing interest due to their capacity to bind and sequester metal ions. Bioremediation using bacterial MTs is particularly effective, as it offers a reliable, efficient, and cost-effective approach to heavy metal removal. Numerous microbes, including microalgae and bacteria, harbor metal-binding MTs, and their capacity to bind metal ions has been extensively studied. For instance, the unicellular cyanobacterium Synechococcus sp. PCC 7942 was shown to bind significant amounts of heavy metals: 11.3 mg of Cu (II), 30.4 mg of Pb (II), 3.2 mg of Ni (II), 7.2 mg of Cd (II), and 5.4 mg of Cr (III) per gram of biomass under repeated flow conditions (Gardea-Torresdey et al., 1998). While this study did not directly correlate MT expression with metal uptake, PCR identified genomic loci coding for MTs, suggesting potential future avenues for enhancing heavy metal sequestration.
Genetic modification to enhance metal uptake has also been explored. For example, MTs from Synechococcus were expressed in E. coli using the pGEX3X expression vector. In vivo studies of E. coli expressing the smtA gene showed increased accumulation of Zn and Cd ions (Shi et al., 1992). Recently, E. coli overexpressing human metallothionein 3 were immobilized in biobeads and used for detoxifying Cu- and Cd-contaminated water, achieving reductions of 87.2% for copper, 32.8% for zinc, and 27.3% for Cd, highlighting the potential for this approach in treating toxic wastewater (Gupta et al., 2019). These findings indicate that microbe-based biofilters, particularly those utilizing genetically modified organisms with enhanced MT expression, hold promise as bioremediation tools. However, significant challenges persist, such as ecological risks, uncontrolled spread and horizontal gene transfer, high development and maintenance costs, and potential human health risks.
To address these concerns, the development of metallothionein-functionalized materials present a more sustainable and eco-friendly alternative for mitigating heavy metal pollution in coastal and marine habitats. Although these materials also rely on genetically modified organisms, the risk of environmental release and gene spread is considerably lower. For example, a novel genetically engineered fusion protein, combining metallothionein from Cancer pagurus, a cellulose-binding module from Cellulomonas fimi, and superfolder GFP, was expressed in E. coli. The resulting biosorbent, created by mixing E. coli lysates with cellulose, efficiently removed Pb from water (Xiao et al., 2020). Another study constructed a biosorbent using E. coli that featured metallothionein and a carbohydrate-binding module, with biosorption capacities for Pb and Zn ions reaching 39.02 mg/g and 29.28 mg/g, respectively (Mwandira et al., 2020).
Nanotechnology has further enhanced the potential of these biofunctionalized materials. A recent example includes a nanobiohybrid magnetic adsorbent (Zarei et al., 2016) developed by immobilizing a recombinant form of rice metallothionein onto synthesized amine-functionalized magnetic nanocomposite particles, achieving Cd retention of 476 mg/g. In another case, MTs extracted from soybean debris were immobilized on nano-silica extracted from rice straw, resulting in a maximum adsorption capacity of 325 mg/g for lead removal from battery industry wastewater, with a removal efficiency of 98%.
Despite these promising developments, the widespread adoption of biofunctionalized materials faces obstacles such as high production costs, scalability for industrial applications, and challenges in disposing of biosorbents. These issues need to be resolved before these technologies can be widely implemented in large-scale water treatment and remediation efforts, particularly in coastal and marine environments.
6 Conclusion
With the rise of anthropogenic activities worldwide, the load of heavy metal pollution in aquatic environments has surged, posing a serious threat to aquatic biodiversity and the ecological balance of coastal and marine waters. Heavy metal pollution not only endangers marine life but also impacts human health due to the bioaccumulation of heavy metals in seafood. As a result, there is an urgent need to understand the dynamics of heavy metal contamination in coastal ecosystems and to develop efficient strategies for their removal. MTs, present in aquatic vertebrates, invertebrates, and microbes, can bind heavy metals, making them valuable tools for assessing the responses of ecologically significant species to metal pollution and for designing mitigation strategies to prevent heavy metal pollution. However, a closer examination of metallothionein expression or activity in response to heavy metal exposure reveals a more complex relationship than previously understood. While many studies report altered metallothionein levels in various tissues following heavy metal exposure, the magnitude, and sometimes the direction, of this induction is influenced by external factors such as salinity, oxygen levels, and the immune status of the organisms. In some cases, there is no clear correlation between heavy metal exposure and metallothionein induction, suggesting that the response is more nuanced and context dependent. Nevertheless, the metal binding ability of the MTs can be harnessed to develop efficient mitigation strategies to subdue the heavy metal load in aquatic habitats.
7 Future perspectives
The non-specific nature of MTs calls for the development of multi-biomarker approaches, integrating them with other established biomarkers such as superoxide dismutase and heat shock proteins (An et al., 2008). This combined strategy could lead to a more robust biomonitoring platform, improving the accuracy and reliability of assessments of heavy metal pollution in marine environments. By expanding the range of biological responses being monitored, researchers can gain deeper insights into how heavy metals affect marine ecosystems. Moreover, MTs hold potential beyond traditional pollution monitoring, offering new avenues for research in the context of climate change. Recent studies have shown that metallothionein expression is sensitive to environmental factors such as temperature changes, dissolved oxygen levels, and salinity fluctuations—key stressors linked to climate change (Martins and Bianchini, 2009; Abdel-Tawwab and Wafeek, 2014; Giannetto et al., 2017). Future studies could explore the use of MTs as biomarkers for these climate-related stressors in model organisms, helping to elucidate the complex interactions between pollution and shifting environmental conditions.
In addition to studying heavy metals, it is important to investigate how metallothionein expression interacts with other pollutants, such as organic contaminants. This could offer a more comprehensive approach to assessing the multifaceted environmental stressors that often coexist in aquatic ecosystems. Understanding these interactions would significantly contribute to the development of integrated environmental risk assessment frameworks. Furthermore, emerging pollutants, such as 6PPD and its degradation product 6PPD-quinone, are increasingly concerning due to their global impact on marine life (Tian et al., 2021; Varshney et al., 2022). These pollutants, derived from tire wear particles, present new challenges to aquatic ecosystems. While the effects of these contaminants on metallothionein expression are still unclear, investigating their impact on aquatic species could be essential in developing relevant animal models. Such models would facilitate the assessment of the biological effects of tire wear particles and inform mitigation strategies for these emerging threats to marine biodiversity.
In the present decade, there has been significant research on omics approaches in MTs especially in the domains of DNA methylation patterns of MT genes and single-cell RNA sequencing in human cell lines. These studies have revealed unprecedented insights into how MTs can regulate diseases (Lu et al., 2018; Maleckaite et al., 2019). Although such deeper molecular studies in aquatic animal metallothioneins are missing, in the future such studies have the potential to improve the usage of bacterial and marine aquatic animal metallothion for better biomarkers and screening. The integration of artificial intelligence and machine learning in MT research is a growing area with promising potential. Although in infancy, these platforms can significantly enhance our understanding of mechanisms that regulate MT response under the conditions of metal toxicity. In 2023, a machine learning classifier was built with heavy metal signature biomarker genes as features to distinguish between heavy metal exposure from non-heavy metal exposure gene expression samples. While this platform developed by Mukherjee (2023) effectively classifies gene expression profiles based on heavy metal exposure using a biomarker gene panel, it remains unclear to what extent MT genes contribute to the model’s decision-making. Clarifying the individual feature importance of MTs would enhance our understanding of their diagnostic value and mechanistic role in metal-specific toxicity classification. Another example is the platform by Receptor.ai that provides a systematic, AI-enhanced framework for identifying and prioritizing binding pockets on metallothionein 1L (MT1L) gene and screening small molecules against it. This opens avenues for chemical biology probes, therapeutic lead discovery, and deeper mechanistic characterization of MT1L and related isoforms. These future research directions could strengthen the use of MTs as key biomarkers in monitoring the health of coastal and marine environments, enhancing our ability to address complex environmental challenges.
Author contributions
AG: Writing – review & editing, Writing – original draft, Data curation, Conceptualization, Methodology. MS: Data curation, Writing – review & editing, Conceptualization, Resources, Formal Analysis. SR: Data curation, Writing – review & editing, Software, Investigation. QA: Data curation, Formal analysis, Validation, Visualization, Writing – review & editing. KC: Methodology, Supervision, Conceptualization, Writing – review & editing. DP: Conceptualization, Writing – review & editing, Investigation, Data curation. RL: Writing – review & editing, Data curation. PS: Conceptualization, Validation, Formal analysis, Software, Writing – review & editing, Data curation. PA: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work is performed under the project “Impact of coastal marine pollution on the ecosystems health, biodiversity, and mitigation measures (MBEMD/MPC/28)” funded by ICAR-Central Marine Fisheries Research Institute.
Acknowledgments
Authors from CMFRI are thankful to Director, CMFRI for his support. The figures in the article are created with biorender.com. The authors also extend their gratitude to the Norwegian Agency for Shared Services in Education and Research for supporting the OA publication fee through Nord University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aarif K. M., Rubeena K. A., Nefla A., Musilova Z., Musil P., Shaju S. S., et al. (2023). Heavy metals in wetlands of southwestern India: from sediments through invertebrates to migratory shorebirds. Chemosphere 345, 140445. doi: 10.1016/j.chemosphere.2023.140445
Abdel-Tawwab M. and Wafeek M. (2014). Influence of water temperature and waterborne cadmium toxicity on growth performance and metallothionein–cadmium distribution in different organs of Nile tilapia, Oreochromis niloticus (L.). J. Thermal Biol. 45, 157–162. doi: 10.1016/j.jtherbio.2014.09.002
Abraham J., Cheng L., Mann M. E., Trenberth K., and von Schuckmann K. (2022). The ocean response to climate change guides both adaptation and mitigation efforts. Atmospheric Oceanic Sci. Lett. 15, 100221. doi: 10.1016/j.aosl.2022.100221
Akila M., Anbalagan S., Lakshmisri N. M., Janaki V., Ramesh T., Merlin R. J., et al. (2022). Heavy metal accumulation in selected fish species from Pulicat Lake, India, and health risk assessment. Environ. Technol. Innovation 27, 102744. doi: 10.1016/j.eti.2022.102744
Al-Mur B. A. (2021). Assessment of heavy metal contamination in water, sediments, and Mangrove plant of Al-Budhai region, Red Sea Coast, Kingdom of Saudi Arabia. J. Taibah Univ. Sci. 15, 423–441. doi: 10.1080/16583655.2021.1985871
Amiard J. C., Amiard-Triquet C., Barka S., Pellerin J., and Rainbow P. S. (2006). Metallothioneins in aquatic invertebrates: their role in metal detoxification and their use as biomarkers. Aquat. Toxicol. 76, 160–202. doi: 10.1016/j.aquatox.2005.08.015
An K. W., Shin H. S., and Choi C. Y. (2008). Physiological responses and expression of metallothionein (MT) and superoxide dismutase (SOD) mRNAs in olive flounder, Paralichthys olivaceus exposed to benzo [a] pyrene. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 149, 534–539. doi: 10.1016/j.cbpb.2007.12.004
Awad S. T., Hemeda S. A., El Nahas A. F., Abbas E. M., Abdel-Razek M. A., Ismail M., et al. (2024). Gender-specific responses in gene expression of Nile tilapia (Oreochromis niloticus) to heavy metal pollution in different aquatic habitats. Sci. Rep. 14, 14671. doi: 10.1038/s41598-024-64300-4
Aziz K. H. H., Mustafa F. S., Omer K. M., Hama S., Hamarawf R. F., and Rahman K. O. (2023). Heavy metal pollution in the aquatic environment: efficient and low-cost removal approaches to eliminate their toxicity: a review. RSC Adv. 13, 17595–17610. doi: 10.1039/D3RA00723E
Bakiu R., Pacchini S., Piva E., Schumann S., Tolomeo A. M., Ferro D., et al. (2022). Metallothionein expression as a physiological response against metal toxicity in the striped rockcod Trematomus hansoni. Int. J. Mol. Sci. 23, 12799. doi: 10.3390/ijms232112799
Bakiu R., Santovito G., Hoda A., Shehu J., Durmishaj S., Irato P., et al. (2013). Metallothionein (MT): A good biomarker in marine sentinel species like sea bream (sparus aurata). Albanian J. Agric. Sci. 12, 247–253.
Balali-Mood M., Naseri K., Tahergorabi Z., Khazdair M. R., and Sadeghi M. (2021). Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 12, 643972. doi: 10.3389/fphar.2021.643972
Baraj B., Niencheski F., Fillmann G., and De Martinez Gaspar Martins C. (2011). Assessing the effects of Cu, Cd, and exposure period on metallothionein production in gills of the Brazilian brown mussel Perna perna by using factorial design. Environ. Monit. Assess. 179, 155–162. doi: 10.1007/s10661-010-1725-8
Barrera-Escorcia G. and Wong-Chang I. (2010). Lipid peroxidation and metallothionein induction by chromium and cadmium in Oyster Crassostrea virginica (Gmelin) from Mandinga Lagoon, Veracruz. Hidrobiológica 20, 31–40.
Bebianno M. J. and Serafim M. A. (1998). Comparison of MT induction in response to cadmium in the gills of the bivalve molluscs Mytilus galloprovincialis and Ruditapes decussatus. Sci. Total Environ. 214, 123–131. doi: 10.1016/S0048-9697(98)00059-X
Beyersmann D. and Hartwig A. (2008). Carcinogenic metal compounds: recent insight into molecular and cellular mechanisms. Arch. Toxicol. 82, 493–512. doi: 10.1007/s00204-008-0313-y
Bing-liang C. and Chien P. K. (1994). The induction and extraction of metallothioneins in Artemia. Chin. J. Oceanology Limnology 12, 175–179. doi: 10.1007/BF02850516
Bouraoui Z., Banni M., Ghedira J., Clerandeau C., Guerbej H., Narbonne J. F., et al. (2008). Acute effects of cadmium on liver phase I and phase II enzymes and metallothionein accumulation on sea bream Sparus aurata. Fish Physiol. Biochem. 34, 201–207. doi: 10.1007/s10695-007-9177-y
Capasso C., Carginale V., Scudiero R., Crescenzi O., Spadaccini R., Temussi P. A., et al. (2003). Phylogenetic divergence of fish and mammalian metallothionein: relationships with structural diversification and organismal temperature. J. Mol. Evol. 57, S250–S257. doi: 10.1007/s00239-003-0034-z
Chen L., Ma L., Bai Q., Zhu X., Zhang J., Wei Q., et al. (2014). Heavy metal-induced metallothionein expression is regulated by specific protein phosphatase 2A complexes. J. Biol. Chem. 289, 22413–22426. doi: 10.1074/jbc.M114.548677
Chesman B. S., O’hara S., Burt G. R., and Langston W. J. (2007). Hepatic metallothionein and total oxyradical scavenging capacity in Atlantic cod Gadus morhua caged in open sea contamination gradients. Aquat. Toxicol. 84, 310–320. doi: 10.1016/j.aquatox.2007.06.008
Chidugu-Ogborigbo R. U., Nkopuyo U. S., Nikolas J. H., and Barker J. (2025). Bioaccumulation and genotoxic effect of heavy metal pollution in marine sponges from the Niger Delta. Mar. pollut. Bull. 211, 117386. doi: 10.1016/j.marpolbul.2024.117386
Cheung A. P., Lam T. H. J., and Chan K. M. (2004). Regulation of Tilapia metallothionein gene expression by heavy metal ions. Mar. Environ. Res. 58, 389–94. doi: 10.1016/j.marenvres.2004.03.084
Cleef-Toedt K. A. V., Kaplan L. A., and Crivello J. F. (2001). Killifish MT messenger RNA expression following temperature perturbation and cadmium exposure. Cell Stress Chaperones 6, 351. doi: 10.1379/1466-1268(2001)006<0351:kmmref>2.0.co;2
Cretì P., Trinchella F., and Scudiero R. (2010). Heavy metal bioaccumulation and MT content in tissues of the sea bream Sparus aurata from three different fish farming systems. Environ. Monit. Assess. 165, 321–329. doi: 10.1007/s10661-009-0948-z
Crowthers K. C., Kline V., Giardina C., and Lynes M. A. (2000). Augmented humoral immune function in metallothionein-null mice. Toxicol. Appl. Pharmacol. 166, 161–172. doi: 10.1006/taap.2000.8961
Dagdag O., Quadri T. W., Haldhar R., Kim S. C., Daoudi W., Berdimurodov E., et al. (2023). “An overview of heavy metal pollution and control,” in Heavy metals in the environment: management strategies for global pollution, (Heidelberg: Springer Nature), 3–24.
Dankel D. J., Aasly K., Carić H., Ellefmo S. L., Gaspers A., Hixson R., et al. (2023). “Ocean sectors: Case studies of human activity in the Ocean-based economy,” in Oceans and human health (Academic Press, Cambridge, USA), 531–546).
Desouky M. M. (2012). MT is up-regulated in molluscan responses to cadmium, but not aluminum, exposure. J. Basic Appl. Zoology 65, 139–143. doi: 10.1016/j.jobaz.2012.07.008
DeVries T. (2022). The ocean carbon cycle. Annu. Rev. Environ. Resour. 47, 317–341. doi: 10.1146/annurev-environ-120920-111307
Dong H., Song H., Liu Y., and Zou H. (2024). Zinc-mediated endoplasmic reticulum stress and metallothionein alleviate arsenic-induced cardiotoxicity in Cyprinus carpio. Biol. Trace Element Res. 202, 4203–4215. doi: 10.1007/s12011-023-03975-8
Duffus J. H. (2002). Heavy metals” a meaningless term? (IUPAC Technical Report). Pure Appl. Chem. 74, 793–807. doi: 10.1351/pac200274050793
Engwa G. A., Ferdinand P. U., Nwalo F. N., and Unachukwu M. N. (2019). “Mechanism and health effects of heavy metal toxicity in humans,” in Poisoning in the modern world-new tricks for an old dog (London: IntechOpen), 70–90. doi: 10.5772/intechopen.82511
Espinoza H. M., Williams C. R., and Gallagher E. P. (2012). Effect of cadmium on glutathione S-transferase and MT gene expression in coho salmon liver, gill and olfactory tissues. Aquat. Toxicol. 110, 37–44. doi: 10.1016/j.aquatox.2011.12.012
Esposti D. D., Lalouette A., Gaget K., Lepeule L., Chaabi Z., Leprêtre M., et al. (2024). Identification and organ-specific patterns of expression of two metallothioneins in the sentinel species Gammarus fossarum. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 269, 110907. doi: 10.1016/j.cbpb.2023.110907
FAO (2024). The state of world fisheries and aquaculture 2024 – blue transformation in action (Rome: Food and Agriculture Organization of the United Nations). doi: 10.4060/cd0683en
Florianczyk B. (2007). Metallothioneins and its role in metal regulation, binding of reactive oxygen species, apoptosis and cell differentiation. J. Pre-clinical Clin. Res. 1, 16–18.
Gardea-Torresdey J. L., Arenas J. L., Francisco N. M. C., Tiemann K. J., and Webb R. (1998). Ability of immobilized cyanobacteria to remove metal ions from solution and demonstration of the presence of metallothionein genes in various strains. J. Hazardous Subst. Res. 1, 2. doi: 10.4148/1090-7025.1001
Ge D., Zhang L., Long Z., Chi C., and Liu H. (2020). A novel biomarker for marine environmental pollution: a metallothionein from Mytilus coruscus. Aquaculture Rep. 17, 100364. doi: 10.1016/j.aqrep.2020.100364
Geng N., Wang C., Wang P., Qi N., and Ren L. (2015). Cadmium accumulation and metallothionein response in the freshwater bivalve Corbicula fluminea under hydrodynamic conditions. Biol. Trace Element Res. 165, 222–232. doi: 10.1007/s12011-015-0266-y
Géret F., Jouan A., Turpin V., Bebianno M. J., and Cosson R. P. (2002). Influence of metal exposure on metallothionein synthesis and lipid peroxidation in two bivalve mollusks: the oyster (Crassostrea gigas) and the mussel (Mytilus edulis). Aquat. Living Resour. 15, 61–66. doi: 10.1016/S0990-7440(01)01147-0
Giannetto A., Maisano M., Cappello T., Oliva S., Parrino V., Natalotto A., et al. (2017). Effects of oxygen availability on oxidative stress biomarkers in the Mediterranean mussel Mytilus galloprovincialis. Mar. Biotechnol. 19, 614–626. doi: 10.1007/s10126-017-9780-6
Giri S. and Singh A. K. (2014). Assessment of human health risk for heavy metals in fish and shrimp collected from Subarnarekha River, India. Int. J. Environ. Health Res. 24, 429–449. doi: 10.1080/09603123.2013.857391
Girgis S. M., Mabrouk D. M., Hanna M. I., and Abd ElRaouf A. (2019). Seasonal assessment of some heavy metal pollution and Metallothionein gene expression in cultured Oreochromis niloticus. Bull. Natl. Res. Cent. 43, 131. doi: 10.1186/s42269-019-0167-x
Gomes T., Pereira C. G., Cardoso C., Pinheiro J. P., Cancio I., and Bebianno M. J. (2012). Accumulation and toxicity of copper oxide nanoparticles in the digestive gland of Mytilus galloprovincialis. Aquatic Toxicology 118, 72–79. doi: 10.1016/j.aquatox.2012.03.017
Günther V., Lindert U., and Schaffner W. (2012). The taste of heavy metals: gene regulation by MTF-1. Biochim. Biophys. Acta (BBA)-Molecular Cell Res. 1823, 1416–1425. doi: 10.1016/j.bbamcr.2012.01.005
Gupta D., Satpati S., Dixit A., and Ranjan R. (2019). Fabrication of biobeads expressing heavy metal-binding protein for removal of heavy metal from wastewater. Appl. Microbiol. Biotechnol. 103, 5411–5420. doi: 10.1007/s00253-019-09852-6
Hasan M., Rahman M., al Ahmed A., Islam M. A., and Rahman M. (2022). Heavy metal pollution and ecological risk assessment in the surface water from a marine protected area, Swatch of No Ground, north-western part of the Bay of Bengal. Reg. Stud. Mar. Sci. 52, 102278. doi: 10.1016/j.rsma.2022.102278
Haux C. and Larsson Å. (1984). Long-term sublethal physiological effects on rainbow trout, Salmo gairdneri, during exposure to cadmium and after subsequent recovery. Aquat. Toxicol. 5, 129–142. doi: 10.1016/0166-445X(84)90004-3
Hertika A. M. S., Kusriani K., Indrayani E., Nurdiani R., and Putra R. B. (2018). Relationship between levels of the heavy metals lead, cadmium and mercury, and metallothionein in the gills and stomach of Crassostrea iredalei and Crassostrea glomerata. F1000Research 7. doi: 10.1186/s40850-023-00183-8
Hertika A. M. S., Kusriani K., Indrayani E., Yona D., and Putra R. B. D. S. (2019). Metallothionein expression on oysters (Crassostrea cuculata and Crassostrea glomerata) from the southern coastal region of East Java. F1000Research 8. doi: 10.12688/f1000research.17381.2
Hollenberg P. F. (2010). Introduction: mechanisms of metal toxicity special issue. Chem. Res. Toxicol. 23, 292–93. doi: 10.1021/tx900456p
Isani G. and Carpenè E. (2014). Metallothioneins, unconventional proteins from unconventional animals: a long journey from nematodes to mammals. Biomolecules 4, 435–457. doi: 10.3390/biom4020435
Javed M. and Usmani N. (2013). Assessment of heavy metal (Cu, Ni, Fe, Co, Mn, Cr, Zn) pollution in effluent dominated rivulet water and their effect on glycogen metabolism and histology of Mastacembelus armatus. SpringerPlus 2, 1–13. doi: 10.1186/2193-1801-2-390
Jomova K., Makova M., Alomar S. Y., Alwasel S. H., Nepovimova E., Kuca K., et al (2022). Essential metals in health and disease. Chem. Biol. Interact. 367, 110173. doi: 10.1016/j.cbi.2022.110173
Kadim M. K. and Risjani Y. (2022). Biomarker for monitoring heavy metal pollution in aquatic environment: An overview toward molecular perspectives. Emerging Contaminants 8, 195–205. doi: 10.1016/j.emcon.2022.02.003
Kagi J. H. and Vallee B. L. (1960). Metallothionein: a cadmium-and zinc-containing protein from equine renal cortex. J. Biol. Chem. 235, 3460–65. doi: 10.1016/S0021-9258(18)64490-4
Karin M., Andersen R. D., Slater E., Smith K., and Herschman H. R. (1980). Metallothionein mRNA induction in HeLa cells in response to zinc or dexamethasone is a primary induction response. Nature 286, 295–297. doi: 10.1038/286295a0
Kazimierczuk K., Henderson C., Duffy K., Hanif S., Bhattacharya S., Biswas S., et al. (2023). A socio-technical assessment of marine renewable energy potential in coastal communities. Energy Res. Soc. Sci. 100, 103098. doi: 10.1016/j.erss.2023.103098
Knapen D., Reynders H., Bervoets L., Verheyen E., and Blust R. (2007). Metallothionein gene and protein expression as a biomarker for metal pollution in natural gudgeon populations. Aquat. Toxicol. 82, 163–172. doi: 10.1016/j.aquatox.2007.02.008
Kortei N. K., Heymann M. E., Essuman E. K., Kpodo F. M., Akonor P. T., Lokpo S. Y., et al. (2020). Health risk assessment and levels of toxic metals in fishes (Oreochromis noliticus and Clarias Anguillaris) from Ankobrah and Pra basins: Impact of illegal mining activities on food safety. Toxicol. Rep 7, 360–369. doi: 10.1016/j.toxrep.2020.02.011
Kumar C. S., Jaikumar M., Robin R. S., Karthikeyan P., and Kumar C. S. (2013). Heavy metal concentration of sea water and marine organisms in Ennore Creek, Southeast Coast of India. J. Toxicol. Health 103, 192–201.
Lall S. P. (2022). “The minerals,” in Fish nutrition. Eds. Hardy R. W. and Kaushik S. J. (Academic Press, London, United Kingdom), 469–554.
Le Croizier G., Lacroix C., Artigaud S., Le Floch S., Raffray J., Penicaud V., et al. (2018). Significance of MTs in differential cadmium accumulation kinetics between two marine fish species. Environ. pollut. 236, 462–476. doi: 10.1016/j.envpol.2018.01.002
Lee S. Y. and Nam Y. K. (2016). Transcriptional responses of metallothionein gene to different stress factors in Pacific abalone (Haliotis discus hannai). Fish Shellfish Immunol. 58, 530–541. doi: 10.1016/j.fsi.2016.09.030
Li L., Shen Y. C., Liang J. R., Liu H., Chen T. C., and Guo H. (2021). Accumulation and Depuration of Cd and its Effect on the Expressions of Metallothionein and Apoptotic Genes in Litopenaeus vannamei. Bull Environ Contam Toxicol 106 (3), 501–506. doi: 10.1007/s00128-021-03115-9
Li Y., Chai X., Wu H., Jing W., and Wang L. (2013). The response of metallothionein and malondialdehyde after exclusive and combined Cd/Zn exposure in the crab Sinopotamon henanense. PloS One 8, e80475. doi: 10.1371/journal.pone.0080475
Li C., Wang H., Liao X., Xiao R., Liu K., Bai J., et al. (2022). Heavy metal pollution in coastal wetlands: A systematic review of studies globally over the past three decades. J. Hazardous Materials 424, 127312. doi: 10.1016/j.jhazmat.2021.127312
Li Y., Yang H., Liu N., Luo J., Wang Q., and Wang L. (2015). Cadmium accumulation and metallothionein biosynthesis in cadmium-treated freshwater mussel Anodonta woodiana. PloS One 10, e0117037. doi: 10.1371/journal.pone.0117037
Liu Y., Wu Z., Guo K., Zhou Y., Xing K., Zheng J., et al. (2022). Metallothionein-1 gene from Exopalaemon carinicauda and its response to heavy metal ions challenge. Mar. pollut. Bull. 175, 113324. doi: 10.1016/j.marpolbul.2022.113324
Lu J., Baccei A., da Rocha E. L., Guillermier C., McManus S., Finney L. A., et al. (2018). Single-cell RNA sequencing reveals metallothionein heterogeneity during hESC differentiation to definitive endoderm. Stem Cell Res. 28, 48–55. doi: 10.1016/j.scr.2018.01.015
Lynes M. A., Hidalgo J., Manso Y., Devisscher L., Laukens D., and Lawrence D. A. (2014). Metallothionein and stress combine to affect multiple organ systems. Cell Stress Chaperones 19, 605–611. doi: 10.1007/s12192-014-0501-z
M’kandawire E., Mierek-Adamska A., Stürzenbaum S. R., Choongo K., Yabe J., Mwase M., et al. (2017). MT from wild populations of the African Catfish Clarias gariepinus: from sequence, protein expression and metal binding properties to transcriptional biomarker of metal pollution. Int. J. Mol. Sci. 18, 1548. doi: 10.3390/ijms18071548
Macías-Mayorga D., Laiz I., Moreno-Garrido I., and Blasco J. (2015). Is oxidative stress related to cadmium accumulation in the Mollusc Crassostrea angulata? Aquat. Toxicol. 161, 231–241. doi: 10.1016/j.aquatox.2015.02.007
Maleckaite R., Zalimas A., Bakavicius A., Jankevicius F., Jarmalaite S., and Daniunaite K. (2019). DNA methylation of metallothionein genes is associated with the clinical features of renal cell carcinoma. Oncol. Rep. 41, 3535–3544. doi: 10.3892/or.2019.7109
Malik D. S. and Maurya P. K. (2014). Heavy metal concentration in water, sediment, and tissues of fish species (Heteropneustis fossilis and Puntius ticto) from Kali River, India. Toxicological Environ. Chem. 96, 1195–1206. doi: 10.1080/02772248.2015.1015296
Mangalagiri P., Bikkina A., Sundarraj D. K., and Thatiparthi B. R. (2020). Bioaccumulation of heavy metals in Rastrelliger kanagurta along the coastal waters of Visakhapatnam, India. Mar. pollut. Bull. 160, 111658. doi: 10.1016/j.marpolbul.2020.111658
Margoshes M. and Vallee B. L. (1957). A cadmium protein from equine kidney cortex. J. Am. Chem. Soc. 79, 4813–4814. doi: 10.1021/ja01574a064
Martins C. D. M. G. and Bianchini A. (2009). Metallothionein-like proteins in the blue crab Callinectes sapidus: effect of water salinity and ions. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 152, 366–371.
Mason A. Z. and Jenkins K. D. (1995). “Metal detoxification in aquatic organisms,” in Metal speciation and bioavailability in aquatic systems (Wiley: Chichester), 479–608.
Masters B. A., Kelly E. J., Quaife C. J., Brinster R. L., and Palmiter R. D. (1994). Targeted disruption of metallothionein I and II genes increases sensitivity to cadmium. Proc. Natl. Acad. Sci. 91, 584–588. doi: 10.1073/pnas.91.2.584
Misra R. R., Crance K. A., Bare R. M., and Waalkes M. P. (1997). Lack of correlation between the inducibility of metallothionein mRNA and metallothionein protein in cadmium-exposed rodents. Toxicology 117, 99–109. doi: 10.1016/S0300-483X(96)03557-3
Moksnes P. O., Lindahl U., and Haux C. (1995). Metallothionein as a bioindicator of heavy metal exposure in the tropical shrimp, Penaeus vannamei: a study of dose-dependent induction. Mar. Environ. Res. 39, 143–146. doi: 10.1016/0141-1136(94)00057-V
Monjane-Mabuie A., Mondlane-Milisse A., Pedro O., Leão-Buchir J., and Correia D. (2022). Mercury pollution assessment and metallothionein gene expression in tilapia (Oreochromis mossambicus): a case study of Revuè River in Manica, Mozambique. Rendiconti Lincei. Sci. Fisiche e Naturali 33, 513–526. doi: 10.1007/s12210-022-01092-7
Montaser M., Mahfouz M. E., El-Shazly S. A., Abdel-Rahman G. H., and Bakry S. (2010). Toxicity of heavy metals on fish at Jeddah coast KSA: MT expression as a biomarker and histopathological study on liver and gills. World J. Fish Mar. Sci. 2, 174–185. Available online at: https://idosi.org/wjfms/wjfms2(3)10/2.pdf.
Morby A. P., Turner J. S., Huckle J. W., and Robinson N. J. (1993). SmtB is a metal-dependent repressor of the cyanobacterial metallothionein gene smtA: identification of a Zn inhibited DNA-protein complex. Nucleic Acids Res. 21, 921–925. doi: 10.1093/nar/21.4.921
Mukherjee S. (2023). Machine Learning classifier built with heavy metal signature biomarker genes as features to distinguish between heavy metal exposure from non-heavy metal exposure gene expression samples. HAL open science, hal-04084188 , version 1 (27-04-2023).
Mwandira W., Nakashima K., Togo Y., Sato T., and Kawasaki S. (2020). Cellulose-metallothionein biosorbent for removal of Pb (II) and Zn (II) from polluted water. Chemosphere 246, 125733. doi: 10.1016/j.chemosphere.2019.125733
Naik S., Pradhan U., Karthikeyan P., Bandyopadhyay D., Sahoo R. K., Panda U. S., et al. (2023). Ecological risk assessment of heavy metals in the coastal sediment in the South-western Bay of Bengal. Front. Mar. Sci. 10, 1255466. doi: 10.3389/fmars.2023.1255466
Niederwanger M., Dvorak M., Schnegg R., Pedrini-Martha V., Bacher K., Bidoli M., et al. (2017). Challenging the metallothionein (MT) gene of Biomphalaria glabrata: unexpected response patterns due to cadmium exposure and temperature stress. Int. J. Mol. Sci. 18, 1747. doi: 10.3390/ijms18081747
Nunez-Nogueira G., Mouneyrac C., Muntz A., and Fernandez-Bringas L. (2010). Metallothionein-Like Proteins and Energy Reserve Levels after Ni and Pb Exposure in the Pacific White Prawn Penaeus vannamei. J. Toxicol. 2010, 407360. doi: 10.1155/2010/407360
Olafson K. W. and Thompson J. A. J. (1974). Isolation of heavy metal binding proteins from marine vertebrates. Mar. Biol. 28, 83–86. doi: 10.1007/BF00396298
Olsvik P. A., Heier L. S., Rosseland B. O., Teien H. C., and Salbu B. (2010). Effects of combined γ-irradiation and metal (Al+Cd) exposures in Atlantic salmon (Salmo salar L.). J. Environ. Radioactivity 101, 230–236. doi: 10.1016/j.jenvrad.2009.11.004
Otvos J. D., Olafson R. W., and Armitage I. M. (1982). Structure of an invertebrate metallothionein from Scylla serrata. J. Biol. Chem. 257, 2427–2431. doi: 10.1016/S0021-9258(18)34941-X
Overnell J., Good M., and Vašak M. (1988). Spectroscopic studies on cadmium (II)-and cobalt (II)-substituted metallothionein from the crab Cancer pagurus: Evidence for one additional low-affinity metal-binding site. Eur. J. Biochem. 172, 171–177. doi: 10.1111/j.1432-1033.1988.tb13869.x
Palmiter R. D. (1994). Regulation of metallothionein genes by heavy metals appears to be mediated by a zinc-sensitive inhibitor that interacts with a constitutively active transcription factor, MTF-1. Proc. Natl. Acad. Sci. 91, 1219–1223. doi: 10.1073/pnas.91.4.1219
Palmiter R. D. (1998). The elusive function of metallothioneins. Proc. Natl. Acad. Sci. 95, 8428–8430. doi: 10.1073/pnas.95.15.8428
Phaenark C., Phankamolsil Y., and Sawangproh W. (2024). Ecological and health implications of heavy metal bioaccumulation in Thai Fauna: A systematic review. Ecotoxicology Environ. Saf. 285, 117086. doi: 10.1016/j.ecoenv.2024.117086
Primost M. A., Gil M. N., and Bigatti G. (2017). High bioaccumulation of cadmium and other metals in Patagonian edible gastropods. Mar. Biol. Res. 13, 774–781. doi: 10.1080/17451000.2017.1296163
Priya A. K., Muruganandam M., Rajamanickam S., Sivarethinamohan S., Reddy M. K., Velusamy P., Gomathi R., et al. (2023). Impact of climate change and anthropogenic activities on aquatic ecosystem–A review. Environ. Res. 238, 117233. doi: 10.1016/j.envres.2023.117233
Qu W., Pi J., and Waalkes M. P. (2013). Metallothionein blocks oxidative DNA damage in vitro. Arch. Toxicol. 87, 311–321. doi: 10.1007/s00204-012-0927-y
Rajizadeh M. A. and Pourbabaki R. (2024). Oxidative stress and exposure to metals. InTechOpen. doi: 10.5772/intechopen.1006077
Ramasamy V., Senthil S., Paramasivam K., and Suresh G. (2022). Potential toxicity of heavy metals in beach and intertidal sediments: A comparative study. Acta Ecologica Sin. 42, 57–67. doi: 10.1016/j.chnaes.2021.03.006
Raudenska M., Gumulec J., Podlaha O., Sztalmachova M., Babula P., Eckschlager T., et al. (2014). Metallothionein polymorphisms in pathological processes. Metallomics 6, 55–68. doi: 10.1039/C3MT00132F
Ray S. and Vashishth R. (2024). From water to plate: reviewing the bioaccumulation of heavy metals in fish and unraveling human health risks in the food chain. Emerging Contaminants 10, 100358. doi: 10.1016/j.emcon.2024.100358
Roesijadi G. (2000). Metal transfer as a mechanism for metallothionein-mediated metal detoxification. Cell. Mol. Biol. (Noisy-le-Grand France) 46, 393–405.
Roesijadi G., Unger M. E., and Morris J. E. (1988). Immunochemical quantification of metallothioneins of a marine mollusc. Can. J. Fish. Aquat. Sci. 45, 1257–1263. doi: 10.1139/f88-148
Ruttkay-Nedecky B., Nejdl L., Gumulec J., Zitka O., Masarik M., Eckschlager T., et al. (2013). The role of metallothionein in oxidative stress. Int. J. Mol. Sci. 14, 6044–6066. doi: 10.3390/ijms14036044
Saad A. A., El-Sikaily A., and Kassem H. (2016). Metallothionein and glutathione content as biomarkers of metal pollution in mussels and local fishermen in Abu Qir Bay, Egypt. J. Health pollut. 6, 50–60. doi: 10.5696/2156-9614-6-12.50
Samuel M. S., Datta S., Khandge R. S., and Selvarajan E. (2021). A state of the art review on characterization of heavy metal binding metallothioneins proteins and their widespread applications. Sci. Total Environ. 775, 145829. doi: 10.1016/j.scitotenv.2021.145829
Scudiero R., Temussi P. A., and Parisi E. (2005). Fish and mammalian metallothioneins: a comparative study. Gene 345, 21–26. doi: 10.1016/j.gene.2004.11.024
Searle P. F. (1987). Metallothionein gene regulation. Biochem. Soc. Trans. 15, 584–6. doi: 10.1042/bst0150584
Shabb J. B., Muhonen W. W., and Mehus A. A. (2017). “Quantitation of human metallothionein isoforms in cells, tissues, and cerebrospinal fluid by mass spectrometry,” in Methods in enzymology, proteomics in biology part B, vol. 586 . Ed. Shukla A. (Academic Press, Cambridge, USA), 413–431.
Shefer E., Silverman J., and Herut B. (2015). Trace metal bioaccumulation in Israeli Mediterranean coastal marine mollusks. Quaternary Int. 390, 44–55. doi: 10.1016/j.quaint.2015.10.030
Shi J., Lindsay W. P., Huckle J. W., Morby A. P., and Robinson N. J. (1992). Cyanobacterial metallothionein gene expressed in Escherichia coli Metal-binding properties of the expressed protein. FEBS Lett. 303, 159–163.
Si M. and Lang J. (2018). The roles of metallothioneins in carcinogenesis. J. Hematol. Oncol. 11, 1–20. doi: 10.1186/s13045-018-0645-x
Smith P. J., Wiltshire M., Furon E., Beattie J. H., and Errington R. J. (2008). Impact of overexpression of metallothionein-1 on cell cycle progression and zinc toxicity. Am. J. Physiology-Cell Physiol. 295, C1399–C1408. doi: 10.1152/ajpcell.00342.2008
Soares T. M., Coutinho D. A., Lacerda L. D. D., Moraes M. O., and Rebelo M. F. (2011). Mercury accumulation and metallothionein expression from aquafeeds by Litopenaeus vannamei Boone 1931 under intensive aquaculture conditions. Braz. J. Biol. 71, 131–137. doi: 10.1590/S1519-69842011000100019
Stankevičiūtė M., Sauliutė G., Makaras T., Markuckas A., Virbickas T., and Baršienė J. (2018). Responses of biomarkers in Atlantic salmon (Salmo salar) following exposure to environmentally relevant concentrations of complex metal mixture (Zn, Cu, Ni, Cr, Pb, Cd). Part II. Ecotoxicology 27, 1069–1086. doi: 10.1007/s10646-018-1960-2
Sun M., Jing Y., Zhang T., Hu F., Chen Q., and Liu G. (2024). Effect of salinity on the toxicokinetics, oxidative stress, and metallothionein gene expression in Meretrix meretrix exposed to cadmium. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 279, 109863.
Tchounwou P. B., Yedjou C. G., Patlolla A. K., and Sutton D. J. (2012). “Heavy metal toxicity and the environment,” in Molecular, clinical and environmental toxicology: Volume 3: Environmental toxicology. Ed. Luch A. (Springer Science & Business Media, Secaucus, USA), 133–164.
Thanomsit C., Nantanawat P., Wassmur B., Gräns J., Celander M. C., and Kanchanopas-Barnette P. (2013). Characterization of metallothionein from Asian sea bass (Lates calcarifer, Bloch) and application as a biomarker for heavy metal exposure in Thailand. Asian J. Water Environ. pollut. 10, 53–64. doi: 10.3233/AJW-2013-10_4_07
Thompson M. W. (2022). Regulation of zinc-dependent enzymes by metal carrier proteins. Biometals 35, 187–213. doi: 10.1007/s10534-022-00373-w
Tian Z., Zhao H., Peter K. T., Gonzalez M., Wetzel J., Wu C., et al. (2021). A ubiquitous tire rubber–derived chemical induces acute mortality in coho salmon. Science 371, 185–189. doi: 10.1126/science.abd6951
Tom M., Moran O., Jakubov E., Cavari B., and Rinkevich B. (1998). Molecular characterization of metallothionein-cDNA of Sparus aurata used for detecting heavy metal pollution along the Mediterranean coast of Israel. Mar. pollut. Bull. 36, 131–137. doi: 10.1016/S0025-326X(97)87421-8
Tom M. and Auslander M. (2005). Transcript and protein environmental biomarkers in fish—a review. Chemosphere 59, 155–162. doi: 10.1016/j.chemosphere.2004.10.063
Varshney S., Gora A. H., Siriyappagouder P., Kiron V., and Olsvik P. A. (2022). Toxicological effects of 6PPD and 6PPD quinone in zebrafish larvae. J. Hazardous Materials 424, 127623. doi: 10.1016/j.jhazmat.2021.127623
Vašák M. and Meloni G. (2011). Chemistry and biology of mammalian metallothioneins. JBIC J. Biol. Inorganic Chem. 16, 1067–1078. doi: 10.1007/s00775-011-0799-2
Vasconcelos M. H., Tam S. C., Hesketh J. E., Reid M., and Beattie J. H. (2002). Metal-and tissue-dependent relationship between metallothionein mRNA and protein. Toxicol. Appl. Pharmacol. 182, 91–97. doi: 10.1006/taap.2002.9428
Viarengo A., Burlando B., Dondero F., Marro A., and Fabbri R. (1999). Metallothionein as a tool in biomonitoring programmes. Biomarkers 4, 455–66. doi: 10.1080/135475099230615
Vergani L., Grattarola M., Borghi C., Dondero F., and Viarengo A. (2005). Fish and molluscan metallothioneins: a structural and functional comparison. FEBS J. 272, 6014–23. doi: 10.1111/j.1742-4658.2005.04993.x
Vignesh S. K. and Deepe G. S. Jr. (2017). Metallothioneins: emerging modulators in immunity and infection. Int. J. Mol. Sci. 18, 2197. doi: 10.3390/ijms18102197
Wang Z. and Lei G. (2018). “Study on penetration effect of heavy metal migration in different soil types,” in IOP conference series: materials science and engineering, vol. 394. (IOP Publishing), 052033. doi: 10.1088/1757-899X/394/5/052033
Wang Y., Zhao Z., Zhang L., Hu C., Ren C., and Yuan L. (2014). Molecular characterization of metallothionein from white shrimp, Litopenaeus vannamei and its expression response to salinity stress. Mar. Biol. Res. 10, 731–737. doi: 10.1080/17451000.2013.850513
WHO (1989). Heavy metals-environmental aspects (World Health Organization). Environmental Health Criteria. No. 85.
World Ocean Initiative (2020). A sustainable ocean economy in 2030: opportunities and challenges (London: The Economist Group Limited). Available online at: https://bvearmb.do/handle/123456789/5669 (Accessed June 25, 2025).
Wu J. P. and Chen H. C. (2005). Metallothionein induction and heavy metal accumulation in white shrimp Litopenaeus vannamei exposed to cadmium and zinc. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 140, 383–394.
Wu H., Li Y., Lang X., and Wang L. (2015). Bioaccumulation, morphological changes, and induction of metallothionein gene expression in the digestive system of the freshwater crab Sinopotamon henanense after exposure to cadmium. Environ. Sci. pollut. Res. 22, 11585–11594. doi: 10.1007/s11356-015-4419-5
Xiao Q., Han J., Jiang C., Luo M., Zhang Q., He Z., et al. (2020). Novel fusion protein consisting of metallothionein, cellulose binding module, and superfolder GFP for lead removal from the water decoction of traditional Chinese medicine. ACS omega 5, 2893–2898. doi: 10.1021/acsomega.9b03739
Xu D., Yu B., Zhang Y., Cui M., and Zhang Q. (2015). Metallothionein protein expression of Crassostrea hongkongensis in response to cadmium stress. J. Shellfish Res. 34, 311–318. doi: 10.2983/035.034.0213
Yamuna A., Bhavan P. S., and Geraldine P. (2012). Glutathione S-transferase and metallothionein levels in the freshwater prawn Macrobrachium malcolmsonii exposed to mercury. J. Environ. Biol. 33, 133.
Yang J., Guo Y., Hu J., Bao Z., and Wang M. (2023). A metallothionein gene from hard clam Meretrix meretrix: Sequence features, expression patterns, and metal tolerance activities. Dev. Comp. Immunol. 149, 105057. doi: 10.1016/j.dci.2023.105057
Yang R., Roshani D., Gao B., Li P., and Shang N. (2024). Metallothionein: A comprehensive review of its classification, structure, biological functions, and applications. Antioxidants 13, 825. doi: 10.3390/antiox13070825
Zarei M., Shahpiri A., Esmaeilnejad-Ahranjani P., and Arpanaei A. (2016). Metallothionein-immobilized silica-coated magnetic particles as a novel nanobiohybrid adsorbent for highly efficient removal of cadmium from aqueous solutions. RSC Adv. 6, 46785–46793. doi: 10.1039/C6RA05210J
Zhang H., Tang D., He J., Yang X., Feng Z., Fu Y., et al. (2024). Assessment of heavy metal contamination in the surface sediments, seawater and organisms of the Pearl River Estuary, South China Sea. Sci. Total Environ. 950, 175266. doi: 10.1016/j.scitotenv.2024.175266
Zhang J., Wang J., Gui T., Sun Z., and Xiang J. (2014). A copper-induced metallothionein gene from Exopalaemon carinicauda and its response to heavy metal ions. Int. J. Biol. Macromolecules 70, 246–250. doi: 10.1016/j.ijbiomac.2014.06.020
Zhang J., Wang J., and Xiang J. (2013). A cadmium metallothionein gene of ridgetail white prawn Exopalaemon carinicauda (Holthuis 1950) and its expression. Chin. J. Oceanology Limnology 31, 1204–1209. doi: 10.1007/s00343-013-3089-8
Zhang P., Ye Z., Huang L., Wang X., and Zhang W. (2025). Heavy metal alarm of marine fish consumption surrounding Qiongzhou Strait, the South China Sea. Front. Mar. Sci. 12, 1557963. doi: 10.3389/fmars.2025.1557963
Keywords: detoxification, heavy metals, pollution, aquatic habitat, remediation
Citation: Gora AH, Sreeram MP, Rehman S, Ain QU, Chakraborty K, Prema D, Lavanya R, Siriyappagouder P and Asha PS (2025) A review on metallothionein research in marine and estuarine realms: past paradigms and future vistas. Front. Mar. Sci. 12:1636760. doi: 10.3389/fmars.2025.1636760
Received: 29 May 2025; Accepted: 07 July 2025;
Published: 31 July 2025.
Edited by:
Rachel Ann Hauser-Davis, Oswaldo Cruz Foundation (Fiocruz), BrazilReviewed by:
Verdiana Vellani, University of Trieste, ItalyKamalesh Sen, University of Burdwan, India
Copyright © 2025 Gora, Sreeram, Rehman, Ain, Chakraborty, Prema, Lavanya, Siriyappagouder and Asha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. P. Sreeram, bXBzY21mcmlAZ21haWwuY29t; Prabhugouda Siriyappagouder, cHJhYmh1Z291ZGEuc2lyaXlhcHBhZ291ZGVyQG5vcmQubm8=
†Present address: Kajal Chakraborty, ICAR-National Bureau of Fish Genetic Resources, Lucknow, India
 Adnan H. Gora
Adnan H. Gora M. P. Sreeram2*
M. P. Sreeram2* Saima Rehman
Saima Rehman Prabhugouda Siriyappagouder
Prabhugouda Siriyappagouder