- 1Cardiology unit, Groupement des Hôpitaux de L'université Catholique de Lille, Université Catholique de Lille, Lille, France
- 2Internal Medicine Unit, Hôpital Européen Georges Pompidou, Assistance Publique des Hôpitaux de Paris and UMR_S970, Universite Paris Descartes, Inserm, Paris, France
- 3Cardiology Unit, CHU de Fann, Dakar, Senegal
- 4Cardiology Unit, Hôpital Général, Yaoundé, Cameroon
- 5Institut de Cardiologie, Abidjan, Ivory Coast
- 6Cardiology Unit, Centre Gynéco-obstétrique, Bamako, Mali
- 7Centre de Recherche et Lutte contre la Drépanocytose, Bamako, Mali
- 8Centre National de Transfusion Sanguine, Dakar, Senegal
- 9Pediatrics Unit, Centre Hospitalier National d'Enfants Albert Royer de Dakar, Université Cheikh Anta Diop, Dakar, Senegal
- 10Hematology Unit, CHU de Yopougon, Abidjan, Ivory Coast
- 11Cardiology Unit, Fondation Mère Enfant Chantal Biya, Yaoundé, Cameroon
- 12Pediatrics Unit, Centre Hospitalier d'Essos, Yaoundé, Cameroon
- 13Cardiology Unit, Libreville, Gabon
- 14Centre de Recherche et Lutte contre la Drépanocytose, Bamako, Mali
- 15UMR_S970, Université Paris Descartes, Inserm, Paris, France
- 16Cardiology Department, Hôpital Européen Georges Pompidou, Assistance Publique des Hôpitaux de Paris and UMR_S970, Université Paris Descartes, Inserm, Paris, France
Background: Several studies conducted in America or Europe have described major cardiac remodeling and diastolic dysfunction in patients with sickle cell disease (SCD). We aimed at assessing cardiac involvement in SCD in sub-Saharan Africa where SCD is the most prevalent.
Methods: In Cameroon, Mali and Senegal, SCD patients and healthy controls of the CADRE study underwent transthoracic echocardiography if aged ≥10 years. The comparison of clinical and echocardiographic features between patients and controls, and the associations between echocardiographic features and the vascular complications of SCD were assessed.
Results: 612 SCD patients (483 SS or Sβ0, 99 SC, and 19 Sβ+) and 149 controls were included. The prevalence of dyspnea and congestive heart failure was low and did not differ significantly between patients and controls. While left ventricular ejection fraction did not differ between controls and patients, left and right cardiac chambers were homogeneously more dilated and hypertrophic in patients compared to controls and systemic vascular resistances were lower (p < 0.001 for all comparisons). Three hundred and forty nine SCD patients had extra-cardiac organ damages (stroke, leg ulcer, priapism, microalbuminuria or osteonecrosis). Increased left ventricular mass index, cardiac dilatation, cardiac output, and decreased systemic vascular resistances were associated with a history of at least one SCD-related organ damage after adjustment for confounders.
Conclusions: Cardiac dilatation, cardiac output, left ventricular hypertrophy, and systemic vascular resistance are associated with extracardiac SCD complications in patients from sub-Saharan Africa despite a low prevalence of clinical heart failure. The prognostic value of cardiac subclinical involvement in SCD patients deserves further studies.
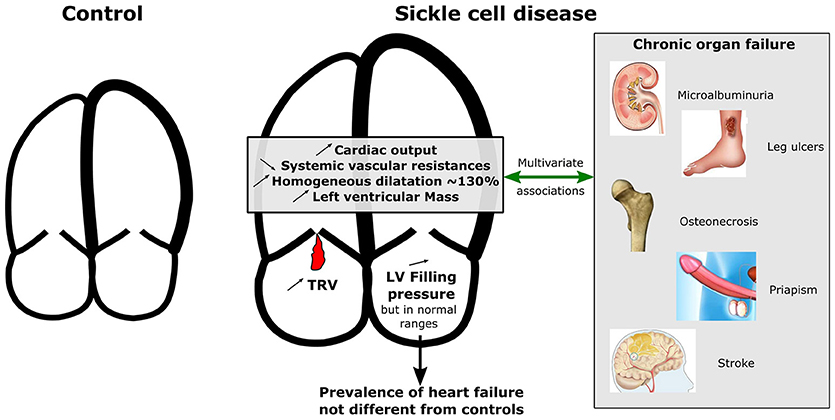
Graphical Abstract. Graphical comparison for cardiac characteristics between SCD patients and controls and multivariate associations with chronic organ failure. While left ventricular ejection fraction did not differ between controls and patients, left and right cardiac chambers were homogeneously more dilated and hypertrophic in patients compared to controls and systemic vascular resistances were lower. Increased left ventricularmass index, cardiac dilatation, cardiac output, and decreased systemic vascular resistances were associated with a history of at least one SCD-related organ damage after adjustment for confounders. TRV, Tricuspid regurgitant velocity; LV, Left ventricle.
Introduction
Sickle cell disease (SCD) is a hemoglobin genetic disorder responsible for deep chronic hemolytic anemia, recurrent acute vaso-occlusive crises, and chronic vasculopathy leading to multiple organ failure such as glomerulopathy, osteonecrosis, leg ulcers, stroke, pulmonary hypertension, and priapism. Both chronic anemia and myocardial ischemia are known to lead to heart failure in the general population (1). Major cardiac remodeling (2, 3), diastolic dysfunction and myocardial ischemia (4–6) have been described in SCD. The overwhelming majority of the data are based on reports from North America and Europe. Thus, very little is known about the prevalence of cardiac abnormalities in African SCD patients. Moreover, it is unknown whether these abnormalities are associated with other SCD-related organ damage. The CADRE (Coeur Artères et DREpanocytose, i.e., Heart Arteries and Sickle cell disease) study is a large multinational cohort of SCD patients, taking place in 5 sub-Saharan African countries. We analyzed the clinical and echocardiographic data of the patients included in the CADRE study to assess the frequency of SCD-related cardiac abnormalities in sub-Saharan Africa and looked for associations between cardiac involvement and other SCD-related organ damage.
Materials and Methods
Settings and Patients' Recruitment
The CADRE study is a multinational observational cohort that includes 3,747 SCD patients aged 3 years and older and 950 healthy controls matched by age and country, recruited through outpatient clinics in Yaoundé and Douala (Cameroon), Dakar (Senegal), Abidjan (Côte d'Ivoire), Franceville (Gabon) and Bamako (Mali). The overall aim of CADRE is to provide descriptive and prognostic data in SCD patients living in sub-Saharan Africa, especially regarding the cardiovascular complications of SCD. The objective of the present study is to determine the prevalence of cardiac involvement in African SCD patients and attempt to identify factors associated with adverse outcomes. The CADRE protocol was approved by the relevant national ethics committee in each of the participating countries: Côte d'Ivoire (N043/MSLS/CNER-dkn), Mali (N18 MS-SG-CNESS/2011), Senegal (N12/40 MSAS/DS/CNERS), Cameroon (N016-CNE-SE-2011), and Gabon (N023-CNER-GB_2011). The patients were recruited from February 2011 to December 2013. A detailed protocol of the study is available elsewhere (7).
Inclusion Criteria
Three centers of the CADRE study (Yaoundé, Dakar and Bamako) participated to the echocardiographic study. These centers were selected based on the possibility to collaborate with a senior cardiologist who met the requested expertise in cardio-echography practice. In these centers, all SCD patients aged 10 or more attending the participating outpatient clinics were invited to participate in the present study. Patients (or their parents) were required to sign an informed consent form. Controls were either healthy voluntary parents or siblings of SCD patients, or healthy voluntary members of the center's medical or nursing staff.
Data Collection
The medical visits were performed at a steady state defined as the absence of any vaso-occlusive crisis (VOC) for the last 15 days, absence of fever or infectious disease for the last 8 days and absence of transfusion for the last 2 months. We recorded medical history, including the main complications of SCD and physical examination findings on standardized forms. Dyspnea was assessed by the New York Heart Association (NYHA) classification. Congestive heart failure was defined by the combination of NHYA class ≥ II and congestion or a history of congestion. Physical activity was considered if there was more than 30 min of walking 5 times per week or 20 min of physical activity reaching the threshold of breathlessness 3 times a week. Laboratory tests included alkaline hemoglobin electrophoresis and/or high-performance liquid chromatography to confirm the SCD phenotype (if not already available), complete blood counts, reticulocyte counts, bilirubin and lactate dehydrogenase (LDH) serum levels, serum creatinine, urine creatinine and quantitative albuminuria. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI formula without adjusting for African ethnicity for adults (8) and the Schwartz formula for children younger than 15 years old (9).
Echocardiography
Echocardiographies were performed by a single experienced cardiologist in each center. Each cardiologist has been trained in Europe and on site by the first author to ensure good quality and reproducibility of the measures. Patients and controls were scanned by the same machine in each country. Respectively, the Sonosite Micromaxx (Sonosite, Bothell, USA), the Philips HP Sonos 5500 (Philips, Andover, Massachusetts) and the Vivid E7 commercial (GE Medical Systems, Horten, Norway) ultrasound scanner was used. We therefore adjusted for the country to limit the measurement bias. The following anatomic measurements were recorded according to the recommendation of the American Society of Echocardiography (10): septal wall, posterior wall thickness, and left ventricular (LV) minor axis dimension at end-diastole and end-systole were obtained with the use of M-mode recordings obtained at a sweep speed of 100 mm/s or with parasternal long axis view 2D imaging. LV mass was calculated using the Devereux's formula (0.8 (1.04 [septal + posterior wall + LV telediastolic diameter]3—[LV telediastolic diameter]3) + 0.6) and indexed for the body surface area. Hypertrophy was considered if indexed left ventricular mass was higher than 95 g/m2 in women and 105 g/m2 in men. Relative wall thickness was the ratio of the septum thickness plus posterior wall thickness divided by the left ventricular end diastolic diameter. Concentric hypertrophy was considered if relative wall thickness was strictly higher than 0.42. LV volume was measured by surfacing the LV from the apical four-chamber view. LV systolic ejection fraction was calculated by the Simpson's method from the apical four-chamber view. Cardiac output index was calculated as the product of the forward stroke volume (time-velocity integral x D2/4 x π, D being the diameter of the LV outflow tract) and heart rate divided by body surface area. Cardiac work index was calculated as the product of mean arterial pressure and cardiac output index. Systemic vascular resistances were calculated as the subtraction of estimated right atrial pressure to mean arterial pressure divided by cardiac output. Atrial surfaces were measured at ventricular end-systole in the apical four-chamber view and were indexed to body surface area. Pulsed-wave Doppler was performed in the apical 4-chamber view at end-expiration with a 3-mm sample volume to obtain mitral inflow velocities (E and A waves).(11) Pulsed-wave tissue Doppler imaging was sequentially performed to acquire lateral and septal mitral annular velocities (E'l and E's waves), and both velocities were averaged to calculate the E/E' ratio (which correlates reasonably well with LV end-diastolic pressure) as previously described (12). E/E' ratio <8 and E/E' ≥13 were considered as low and high LV filling pressures, respectively. Diastolic dysfunction was considered if E'l <10 cm/s or E's <8 cm/s (11) and was classified in three grades using E/A ratio, E wave deceleration time and E/E' ratio as previously described (11). The tricuspid regurgitation pressure gradient and the trans-pulmonary pressure gradient was recorded from any view with continuous-wave Doppler imaging with the modified Bernouilli equation (4 × V2), where V is the peak tricuspid regurgitation velocity or the end diastole pulmonary regurgitation. Estimated pulmonary pressures were obtained when tricuspid regurgitation and/or pulmonary regurgitation jets were available, as recommended. Pulmonary vascular resistances were calculated as the subtraction of estimated right atrial pressure to mean pulmonary arterial pressure divided by cardiac output as left and right atrium pressure are very similar. Because right heart catheterization was not an option, a tricuspid regurgitant jet velocity (TRV) strictly above 3 m/s was used as a surrogate marker of pulmonary hypertension (13, 14).
Statistical Methods
Continuous data are presented as median (25–75th percentiles). Qualitative data are presented as absolute values and percentages. Comparison between patients and controls were performed using multilevel logistic regression with country as a nested variable (to control for potential inter-center variability) adjusted for age and sex.
To investigate the association of cardiac measurements with the SCD complications we performed separated regressions in the SCD population, including clinical complications as the explained variable and cardiac measurements as explanatory variables. SCD-related organ damage was defined by the history of at least one of the following complications: microalbuminuria, osteonecrosis, leg ulcer, stroke, or priapism. We used multilevel logistic regression adjusted for age, mean BP, heart rate, hemoglobin, SCD phenotypic group, and country. Continuous variables were scaled in order to make the Odds Ratios comparable. A 2-tailed P value of < 0.05 was considered to be statistically significant. All analyses were performed using R software (version 3.1.0).
Results
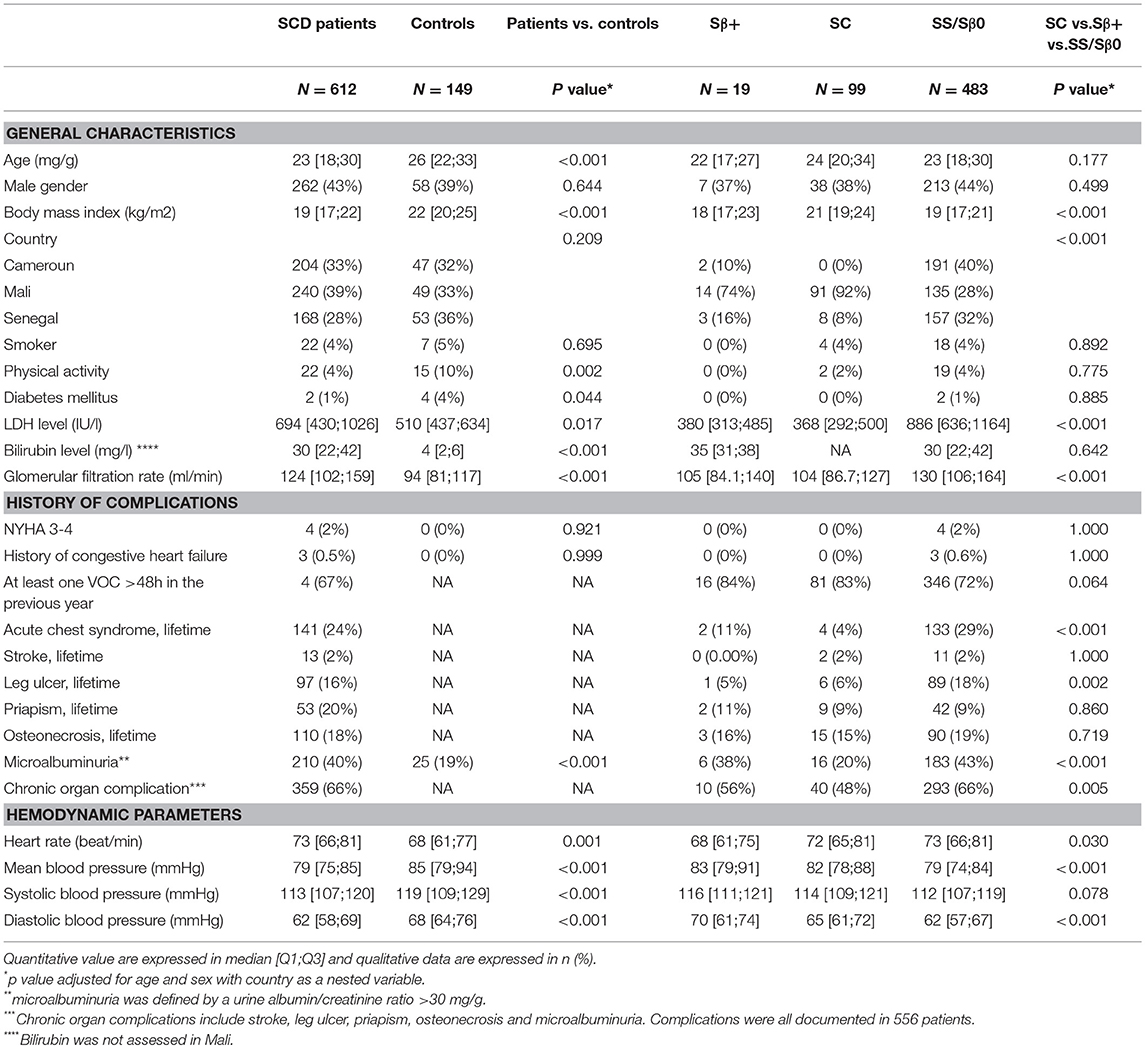
Patients' and Controls' Baseline Characteristics (Table 1)
Six hundred and twelve SCD patients (483 SS or Sβ0, 99 SC and 19 Sβ+) and 149 healthy controls underwent echocardiography. Their median (Q1;Q3) age was respectively 23 [18;30] and 26 [22;33] years old. Clinical and biological SCD features are described in Table 1 and were significantly different between the SCD phenotypic groups: SS and Sβ0 patients had a lower hemoglobin level, higher hemolysis markers, leucocyte and platelet counts and glomerular filtration rate, more frequent history of leg ulcers, more frequent micro-albuminuria (p < 0.05 for all comparisons). A history of at least one organ damage was present in 66% of SS and Sβ0 patients, 48% of SC, and 56% of Sβ+ patients (p = 0.001). Noteworthy, <1% of patients were treated with hydroxyurea or iterative blood transfusions. The prevalence of participants with NYHA class III or IV dyspnea was 2 % in patients and 0 % in controls (p = 0.921). Likewise, the prevalence of congestive heart failure was similar in patients and in controls (0.5 vs. 0% p = 0.999). There was no difference between SS/Sβ0, SC or Sβ+ patients for the prevalence of NYHA III/IV or congestive heart failure. Heart rate was significantly higher in patients than in controls and higher in SS-Sβ0 than in SC or Sβ+ patients (p = 0.001). Mean blood pressure (BP) was significantly lower in patients than in controls (p < 0.001) and lower in SS-Sβ0 than in SC or Sβ+ patients, even after adjustment for age (p < 0.001). Noteworthy, in the entire CADRE cohort including 3,627 patients with SCD (median age: 15 years old) and 943 healthy controls (median age: 18 years old), the prevalence of NYHA class III/IV and the prevalence of clinical heart failure did not differ in patients and in controls (respectively 1.6 vs. 0.7 %, p = 0.127 and 0.1 vs. 0% p = 0.998).
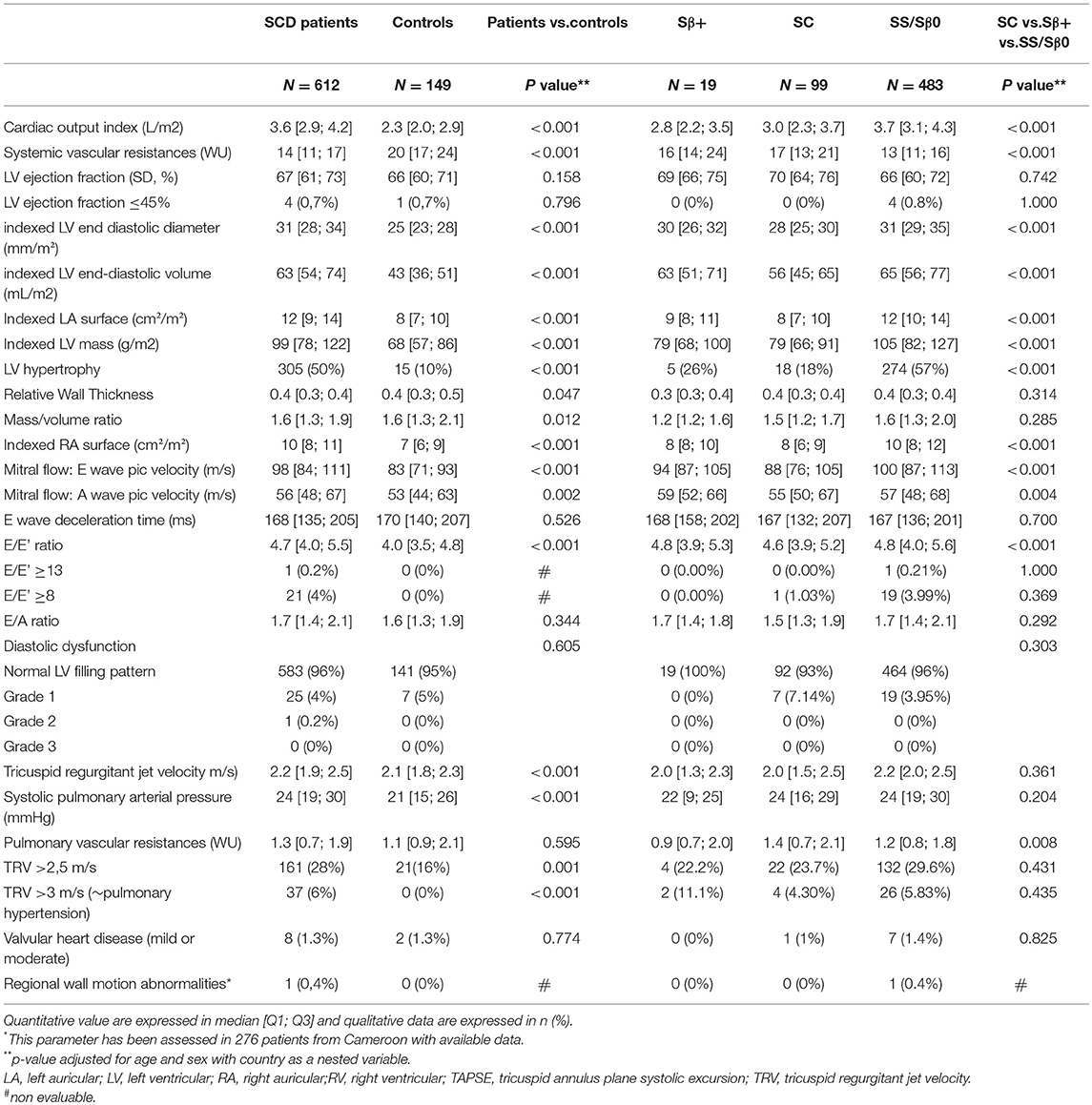
Echocardiographic Parameters (Table 2)
Cardiac Morphology
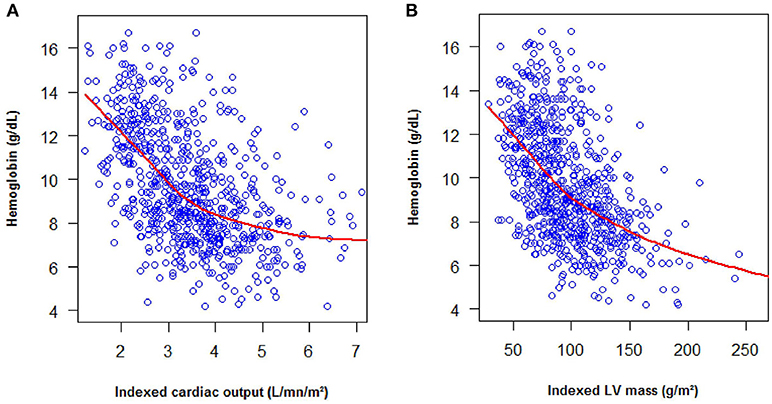
The patients and controls were young with a low body mass index, therefore no patient was excluded for technical issue. The four cardiac chambers were homogeneously dilated in SCD patients (Table 2). Left ventricular mass index (99 [78;122] vs. 68 [57;86] g/m2, p < 0.001), cardiac output index (3.6 [2.9;4.2] vs. 2.3 [2.0;2.9] L/min/m2, p < 0.001) and cardiac work index (277 [229;337] vs. 209 [165;254] L.mmHg/min/m2, p < 0.001) were higher in SCD patients than in controls. Fifty percent of the patients (57% of SS/ Sβ0 patients and 18% of SC patients) had cardiac hypertrophy vs. 10% in the control group p < 0.001. The hypertrophy was eccentric in 62% and concentric in 38% of the SCD patients. Left ventricular mass index and cardiac output were negatively correlated with hemoglobin level and the correlation was particularly strong for patients with a hemoglobin level inferior to of 9 g/dL (Figure 1).
Systolic Function, Diastolic Function, and Cardiac Filling Pressure
LV ejection fraction was similar in patients and in controls (67 [61;73] vs. 66 [60;71]%, p = 0.158). E wave deceleration time and diastolic dysfunction were not different between SCD patients and controls. E and A waves were higher in patients than in controls (respectively, 98 [84;111] vs. 83 [71;93] cm/s, p < 0.001 and 56 [48;67] vs. 53 [44;63], p = 0.002), but the E/A ratio was similar in both groups (p = 0.344). E/E' ratio was higher in patients than in controls (4.7 [4.0;5.5] vs. 4.0 [3.5;4.8], p < 0.001) but rarely reached a pathological level: E/E' was ≥8 in 21 (3.5%) patients vs. 0 controls (p = 1) and E/E' was ≥13 in 1 (0.2%) patient and 0 control, p = 1). Right ventricular function assessed by TAPSE (17 [15;20] vs. 14 [13;16], p < 0.001) and tricuspid annular Sdti (15 [13;17] vs. 13[12;15], p < 0.001) was higher in patients than in controls. Systemic resistances were lower in SCD patients (14 [11;17] vs. 20 [17;24] WU, p < 0.001].
Pulmonary Pressure
TRV (2.2 [1.9;2.5] vs. 2.1 [1.8;2.3], p < 0.001) and sPAP (24 [19;30] vs. 21 [15;26], p < 0.001) were higher in patients than in controls. Conversely, systolic pulmonary resistances were not different between patients and controls (1.3 [0.7;1.9] vs. 1.1 [0.9;2.1] WU, p = 0.595). A TRV of more than 3 m/s was observed in 37 (6%) patients and 0 (0%) controls (p < 0.001).
Other Echocardiographic Features
Echocardiographic wall motion disorder was only evaluated in Cameroun and was observed in one (0.4%) patient vs. 0 control (p = 0.624). Valvular heart disease were equally prevalent in patients compared to controls [8 (1.3%) vs. 2 (1.3%), p = 0.774], all of them were of mild or moderate severity.
Relationship Between SCD-Related Organ Complications and Cardiac Variables (Table 3)
In SCD patients, after adjustment for age, heart rate, mean arterial pressure, serum hemoglobin, phenotype of hemoglobin and country, cardiac chamber dilatation (LV, left and right atrium), LV mass (aOR = 1.7 [1.3;2.3]) and cardiac output (aOR = 1.3 [1;1.6]) were positively associated with SCD-related organ damages. After adjustment for the same variables, systemic vascular resistance (aOR = 0.7 [0.5;0.9]) was negatively associated with SCD-related organ damages. A sensibility analysis was conducted, which did not include osteonecrosis (that is typically associated with hyper-viscosity) among the other organ damages (that are typically associated with hyper-hemolysis), which displayed similar results (data not shown). A sensibility analysis was performed in the subgroup of SS/Sβ0 patients, showing similar results (Table S1).
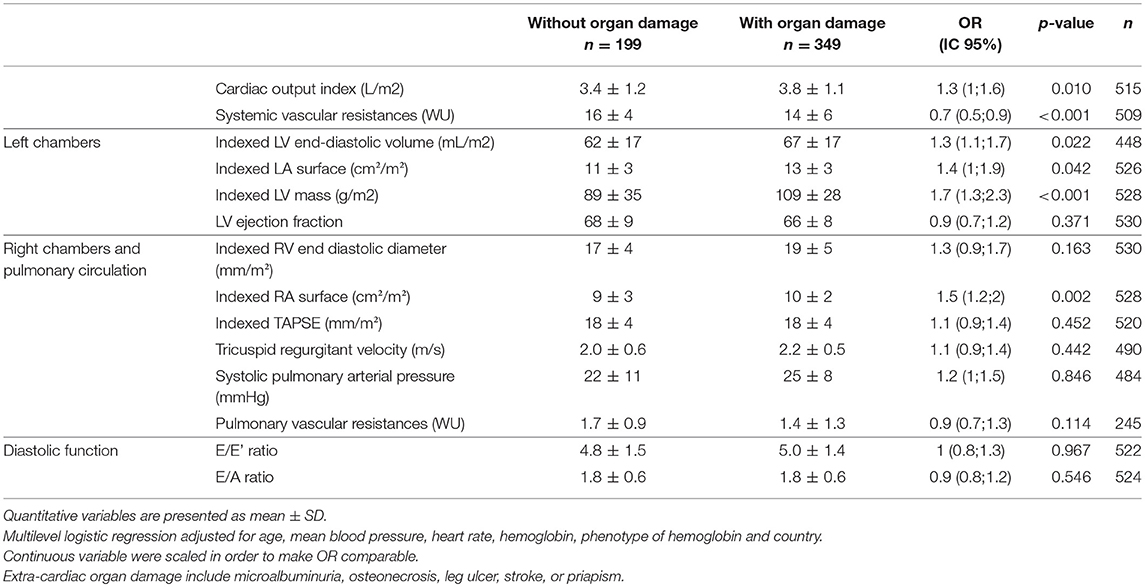
Table 3. Multivariate associations between SCD-related extra-cardiac organ damage and echocardiographic measurements, adjusted for age, mean blood pressure, heart rate, hemoglobin, phenotype of hemoglobin and country.
Discussion
In our sub-Saharan African study of teenagers and young adults, congestive heart failure and cardiac functional symptoms were extremely rare. We confirmed that SCD patients display a harmonious dilatation of cardiac atria and ventricles, as well as a high cardiac output, high cardiac work index with normal LVEF. However, unlike previous studies, we observed a very low frequency of pathological LV filling pressure in our population.
While “heart failure” has traditionally been considered common in adult SCD patients, there is no large screening study that describes the prevalence of congestive heart failure or cardiac functional symptoms. Although Sadchev and coll. showed that high E/E' ratio and TRV were associated to poor exercise capacity (15), recent studies have focused on echocardiographic parameters at a resting state. Systolic function is preserved in the majority of SCD patients (2, 13, 14, 16–19). Congestive heart failure has mainly been observed during acute complications of SCD such as vaso-occlusive crisis or acute chest syndrome. We could hypothesize that an acute drop of hemoglobin level leads to a cardiac work overload that may not be supported by the heart's contractile reserve (3, 4). However, pathological analysis of 52 hearts from deceased patients with sickle cell anemia found cardiac dilatation and hypertrophy with signs of congestive heart failure in 17/52 cases with neither coronary atherosclerosis, nor infarction (20). By contrast, in our population of teenagers and young adults at steady state, the prevalence of congestive heart failure was extremely low, not statistically different from null. It is possible that some cardiac failure-related symptoms were erroneously attributed to SCD (for instance, dyspnea attributed to anemia) but clinical acute congestive heart failure is unlikely to have been underdiagnosed. Nevertheless, some characteristics of our study may have led to underestimate the prevalence of heart failure. First, we did not assess the incidence of acute heart failure during vaso-occlusive crisis, acute chest syndrome or other causes of acute anemia. Second, the SCD population is younger in Africa than in the USA or Europe, and we cannot rule out an increased incidence of heart failure in case of prolonged survival, although the prevalence in the oldest patients (older than 40 years old) was not significantly higher; Third, the cross-sectional design of the study cannot rule out a survivor bias, that may be higher than in Europe and USA studies due to earlier death. Nonetheless, our study suggests that cardiac failure is rare feature in patients living with SCD in Africa.
In contrast, echocardiographic measures revealed a major cardiac remodeling in these patients with homogeneous dilatation and LV hypertrophy, in line with echocardiographic studies performed in SCD patients from other regions, as well as in patients with other causes of chronic anemia (1). We found no difference of prevalence of LV systolic or diastolic dysfunction between SCD patients and healthy controls using the usual echocardiographic definitions [LV ejection fraction <45%; E'l <10 cm/s or E's <8 cm/s (11)]. However, although they did not reach a pathological level, LV filling pressure (defined by E/E' ratio) were higher in patients than in controls which argues in favor of a subclinical cardiac dysfunction. Noteworthy, those findings are subject to criticism because echocardiographic diastolic dysfunction criteria are poorly validated in the case of high cardiac output (21). As other authors, we did not find any echocardiographic LV systolic dysfunction. As the LV ejection fraction is not the best measure to evaluate early LV systolic dysfunction, several authors have used longitudinal strain which assesses more accurately myocardial deformation. Most studies did not show any significant abnormality in LV strain (22–26) and only one study reported right ventricular lower strain in children with SCD compared to healthy children (24).
In theory, different mechanisms could lead to heart failure in SCD patients, including volume overload or myocardial ischemia. Volume overload is correlated with the degree of chronic anemia, especially when the hemoglobin is under 9 g/dL (Figure 1). This overload is probably responsible for heart failure in case of acute anemia. Noteworthy, Bahl et al. found that chronic severe anemia alone did not lead to cardiac disease (27). A few studies support the role of ischemia in SCD heart failure as the myocardial flow reserve is abnormal in SCD patients (4–6) but, as mentioned above, the occurrence of myocardial infarction is rare(5, 20). These findings are in accordance with the very low rate of regional wall motion abnormalities observed in our study. Rare myocardial infarctions are likely due to capillary obstruction whilst coronary arteries are usually normal (5, 28). Noteworthy, despite the African location of our study, the rate of valvular heart disease was lower than expected (29), possibly due to the penicillin prophylaxis that is recommended during childhood in SCD, thereby preventing the occurrence of rheumatic heart disease.
Regarding the pulmonary circulation, we found higher TRV in patients than in controls, which corroborates other large scale studies (13, 14). As demonstrated by Parent et al. (12) in an invasive hemodynamic study, the estimated high sPAP by echocardiography in SCD patients is mostly due to high cardiac output, and only rarely reflects a true pulmonary arterial hypertension (high pulmonary arterial resistances) or a cardiac dysfunction (post capillary pulmonary hypertension due to the rise of LV filling pressure). In our study, pulmonary vascular resistances assessed by echocardiography were similar in patients and controls while there was a clear decrease in systemic vascular resistances (30), suggesting a lack of pulmonary resistances adaptation.
Although we observed that SCD cardiac remodeling rarely went to pathological filling pressure and clinical symptoms of congestive heart failure, cardiac dilatation (LA, L,V, and RA surfaces) and LV mass were independently associated with the presence of SCD-related organ damages, including glomerulopathy, osteonecrosis, leg ulcers, priapism, and stroke. This association persisted after adjustment for major confounders (age, mean BP, heart rate, hemoglobin, sickle cell phenotype and country). Lower systemic vascular resistances were also independently associated with SCD-related organ damages. Surprisingly, TRV ratio was not associated with extra-cardiac organ damages in our cohort, whereas high TRV has been reported to be more frequent in patients with hyper-hemolysis phenotype, together with more frequent leg ulcers and priapism (31). However in another report of the CADRE study, we showed that this association between high TRV, leg ulcers, priapism and increased hemolysis markers was not clinically relevant in our African population (32). Nevertheless, high TRV has been consistently reported as a strong risk factor of mortality in SCD and is certainly worth monitoring as a global prognostic factor (14, 18).
The main strength of this study is to be the largest and the first multicentric cohort of SCD patients with cardiac evaluation in sub-Saharan Africa to date. Furthermore, patients were prospectively recruited irrespective of their symptoms, to avoid any referral bias, and both children (over 10 years) and adults of all SCD phenotypes were represented. However, we acknowledge some notable methodological limitations, especially the absence of Echo Core lab, which was impossible to set up in this multicentric African context and the absence of inter- and intra-reader variability calculation. Moreover, the cross-sectional design of the study does not allow inferring any temporality or causal link between cardiac abnormalities and extra-cardiac organ damage.
Conclusion
Our results suggest that the major cardiac remodeling observed in the SCD exceptionally leads to clinical or echocardiographic heart failure in West and Central African patients with SCD but is associated with other organ damages. The prognostic value of cardiac measurements in sub-Saharan Africa will be better assessed during the follow-up of the patients. It will help identify non-invasive predictors of SCD-related organ damages and better tailor the monitoring and treatment of SCD patients in this resource constrained setting.
Author Contributions
XJ and IBD conceived the presented idea. AM and XJ designed the study. XJ encouraged AM to investigate and XJ supervised the findings of this work. AM wrote the manuscript with support from BR. AM and LO performed the analytic calculations. BR, XJ, SM, and MM contributed to the final version of the manuscript. BR and XJ supervised the project. AM, IBD, SK, RN, MD, DD, SD, ID-L, AT, IS, DC, GW, ID, BF, MS, AT, KB, GK, EA, CD, YT, GL, IK, and LO contributed to the implementation of the research, and collected the data. IBD, SK, RN, MD, DD, SD, ID-L, AT, IS, and DC supervised the study in the different countries. All authors discussed the results and contributed to the final manuscript.
Funding
Sorbonne Paris Cité University, laboratory of excellence GrEX (http://www.labex-grex.com).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aknowledgments
We thank all the investigators for enrolling patients in the CADRE study: Cameroon (Yaoundé and Douala): SK, Dr Marcel MONNY LOBE (deceased), Dr Suzanne BELINGA, DC, Dr Odette GUIFO, GW, Dr Françoise NGO SACKS, Dr Angèle PONDY; Côte d'Ivoire (Abidjan): Pr Jean Baptiste ANZOUAN, Pr Kouadio Euloge KRAMOH, RN, IS, Pr Diebkilé Aïssata TOLO, KB, IK, GK; Gabon (Franceville): Pr Simon ATEGBO, Dr Paul BIE, Lucas SICA, Estelle Sonya ZANG EDOU, EA; Mali (Bamako): DD, MD, Dr Karim DEMBELE, CD, YT; Senegal (Dakar): IBD, SD, ID, Dr Daniella BAMBARA, ID-L, BF, Dr Gaelle LEGUEUN, Dr Mbacké SARR, MS.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2018.00323/full#supplementary-material
References
1. Varat MA, Adolph RJ, Fowler NO. Cardiovascular effects of anemia. Am Heart J. (1972) 83:415–26. doi: 10.1016/0002-8703(72)90445-0
2. Lester LA, Sodt PC, Hutcheon N, Arcilla RA. Cardiac abnormalities in children with sickle cell anemia. Chest (1990) 98:1169–74. doi: 10.1378/chest.98.5.1169
3. Gladwin MT, Sachdev V. Cardiovascular abnormalities in sickle cell disease. J Am Coll Cardiol. (2012) 59:1123–33. doi: 10.1016/j.jacc.2011.10.900
4. Almeida AG, Araújo F, Rego F, David C, Lopes MG, Ducla-Soares J. Abnormal myocardial flow reserve in sickle cell disease: a myocardial contrast echocardiography study. Echocardiogr Mt Kisco N. (2008) 25:591–9. doi: 10.1111/j.1540-8175.2008.00666.x
5. James TN. Homage to James B. Herrick: a contemporary look at myocardial infarction and at sickle-cell heart disease: the 32nd Annual Herrick Lecture of the Council on Clinical Cardiology of the American Heart Association. Circulation (2000) 101:1874–87. doi: 10.1161/01.CIR.101.15.1874
6. Chacko P, Kraut EH, Zweier J, Hitchcock C, Raman SV. Myocardial infarction in sickle cell disease: use of translational imaging to diagnose an under-recognized problem. J Cardiovasc Transl Res. (2013) 6:752–61. doi: 10.1007/s12265-012-9426-z
7. Ranque B, Menet A, Diop IB, Thiam MM, Diallo D, Diop S, et al. Early renal damage in patients with sickle cell disease in sub-Saharan Africa: a multinational, prospective, cross-sectional study. Lancet Haematol. (2014) 1:e64–73. doi: 10.1016/S2352-3026(14)00007-6
8. Arlet J-B, Ribeil J-A, Chatellier G, Eladari D, De Seigneux S, Souberbielle JC, et al. Determination of the best method to estimate glomerular filtration rate from serum creatinine in adult patients with sickle cell disease: a prospective observational cohort study. BMC Nephrol. (2012) 13:83. doi: 10.1186/1471-2369-13-83
9. Abraham AG, Schwartz GJ, Furth S, Warady BA, Muñoz A. Longitudinal formulas to estimate GFR in children with CKD. Clin J Am Soc Nephrol. (2009) 4:1724–30. doi: 10.2215/CJN.01860309
10. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification. Eur J Echocardiogr J Work Group Echocardiogr Eur Soc Cardiol. (2006) 7:79–108. doi: 10.1016/j.euje.2005.12.014
11. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr J Work Group Echocardiogr Eur Soc Cardiol. (2009) 10:165–93. doi: 10.1016/j.echo.2008.11.023
12. Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation (2000) 102:1788–94. doi: 10.1161/01.CIR.102.15.1788
13. Parent F, Bachir D, Inamo J, Lionnet F, Driss F, Loko G, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. (2011) 365:44–53. doi: 10.1056/NEJMoa1005565
14. Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, et al. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. (2004) 350:886–95. doi: 10.1056/NEJMoa035477
15. Sachdev V, Kato GJ, Gibbs JSR, Barst RJ, Machado RF, Nouraie M, et al. Echocardiographic markers of elevated pulmonary pressure and left ventricular diastolic dysfunction are associated with exercise intolerance in adults and adolescents with homozygous sickle cell anemia in the United States and United Kingdom. Circulation (2011) 124:1452–60. doi: 10.1161/CIRCULATIONAHA.111.032920
16. Simmons BE, Santhanam V, Castaner A, Rao KR, Sachdev N, Cooper R. Sickle cell heart disease. Two-dimensional echo and Doppler ultrasonographic findings in the hearts of adult patients with sickle cell anemia. Arch Intern Med. (1988) 148:1526–8. doi: 10.1001/archinte.1988.00380070044011
17. Covitz W, Espeland M, Gallagher D, Hellenbrand W, Leff S, Talner N. The heart in sickle cell anemia. The Cooperative Study of Sickle Cell Disease (CSSCD). Chest (1995) 108:1214–9. doi: 10.1378/chest.108.5.1214
18. Sachdev V, Machado RF, Shizukuda Y, Rao YN, Sidenko S, Ernst I, et al. Diastolic dysfunction is an independent risk factor for death in patients with sickle cell disease. J Am Coll Cardiol. (2007) 49:472–9. doi: 10.1016/j.jacc.2006.09.038
19. Lewis JF, Maron BJ, Castro O, Moosa YA. Left ventricular diastolic filling abnormalities identified by Doppler echocardiography in asymptomatic patients with sickle cell anemia. J Am Coll Cardiol. (1991) 17:1473–8. doi: 10.1016/0735-1097(91)90634-L
20. Gerry JL, Bulkley BH, Hutchins GM. Clinicopathologic analysis of cardiac dysfunction in 52 patients with sickle cell anemia. Am J Cardiol. (1978) 42:211–6. doi: 10.1016/0002-9149(78)90902-5
21. Hammoudi N, Charbonnier M, Levy P, Djebbar M, Stankovic Stojanovic K, Ederhy S, et al. Left atrial volume is not an index of left ventricular diastolic dysfunction in patients with sickle cell anaemia. Arch Cardiovasc Dis. (2015) 108:156–62. doi: 10.1016/j.acvd.2014.09.010
22. Braga JCMS, Assef JE, Waib PH, de Sousa AG de MR, de Mattos Barretto RB, Guimarães Filho FV, et al. Altered left ventricular twist is associated with clinical severity in adults and adolescents with homozygous sickle cell anemia. J Am Soc Echocardiogr. (2015) 28:692–9. doi: 10.1016/j.echo.2015.01.019
23. Barbosa MM, Vasconcelos MCM, Ferrari TCA, Fernandes BM, Passaglia LG, Silva CM, et al. Assessment of ventricular function in adults with sickle cell disease: role of two-dimensional speckle-tracking strain. J Am Soc Echocardiogr. (2014) 27:1216–22. doi: 10.1016/j.echo.2014.07.014
24. Blanc J, Stos B, de Montalembert M, Bonnet D, Boudjemline Y. Right ventricular systolic strain is altered in children with sickle cell disease. J Am Soc Echocardiogr. (2012) 25:511–7. doi: 10.1016/j.echo.2012.01.011
25. Ahmad H, Gayat E, Yodwut C, Abduch MC, Patel AR, Weinert L, et al. Evaluation of myocardial deformation in patients with sickle cell disease and preserved ejection fraction using three-dimensional speckle tracking echocardiography. Echocardiogr Mt Kisco N. (2012) 29:962–9. doi: 10.1111/j.1540-8175.2012.01710.x
26. Hammoudi N, Arangalage D, Djebbar M, Stojanovic KS, Charbonnier M, Isnard R, et al. Subclinical left ventricular systolic impairment in steady state young adult patients with sickle-cell anemia. Int J Cardiovasc Imaging (2014) 30:1297–304. doi: 10.1007/s10554-014-0473-1
27. Bahl VK, Malhotra OP, Kumar D, Agarwal R, Goswami KC, Bajaj R, et al. Noninvasive assessment of systolic and diastolic left ventricular function in patients with chronic severe anemia: a combined M-mode, two-dimensional, and Doppler echocardiographic study. Am Heart J. (1992) 124:1516–23. doi: 10.1016/0002-8703(92)90066-5
28. Nicholson GT, Hsu DT, Colan SD, Manwani D, Burton WB, Fountain D, et al. Coronary artery dilation in sickle cell disease. J Pediatr. (2011) 159:789–94.e1-2.
29. Marijon E, Ou P, Celermajer DS, Ferreira B, Mocumbi AO, Jani D, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. (2007) 357:470–6. doi: 10.1056/NEJMoa065085
30. Ranque B, Menet A, Boutouyrie P, Diop IB, Kingue S, Diarra M, et al. Arterial stiffness impairment in sickle cell disease associated with chronic vascular complications: the multinational African CADRE study. Circulation. (2016) 31:923–33. doi: 10.1161/CIRCULATIONAHA.115.021015
31. Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. (2007) 21:37-47. doi: 10.1016/j.blre.2006.07.001
Keywords: sickle cell disease, heart failure, hemolytic anemia, cardiac remodeling, and global health
Citation: Menet A, Ranque B, Diop IB, Kingue S, N'guetta R, Diarra M, Diallo D, Diop S, Diagne I, Sanogo I, Chelo D, Wamba G, Deme-Ly I, Faye BF, Seck M, Tolo A, Boidy K, Koffi G, Abough EC, Diakite CO, Traore Y, Legueun G, Kamara I, Offredo L, Marechaux S, Mirabel M and Jouven X (2018) Subclinical Cardiac Dysfunction Is Associated With Extracardiac Organ Damages. Front. Med. 5:323. doi: 10.3389/fmed.2018.00323
Received: 15 April 2018; Accepted: 31 October 2018;
Published: 20 November 2018.
Edited by:
John Joseph Strouse, Duke University, United StatesReviewed by:
Payal Chandarana Desai, The Ohio State University, United StatesLili Barouch, Johns Hopkins University, United States
Copyright © 2018 Menet, Ranque, Diop, Kingue, N'guetta, Diarra, Diallo, Diop, Diagne, Sanogo, Chelo, Wamba, Deme-Ly, Faye, Seck, Tolo, Boidy, Koffi, Abough, Diakite, Traore, Legueun, Kamara, Offredo, Marechaux, Mirabel and Jouven. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aymeric Menet, YXltZXJpY21lbmV0QGdtYWlsLmNvbQ==
†These authors contributed equally to this work
 Aymeric Menet
Aymeric Menet Brigitte Ranque2†
Brigitte Ranque2†