- Department of Anesthesiology, Center for Brain Science, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
Pain relief is a major concern for patients who have undergone surgery, and it is an eternal pursuit for anesthesiologists. However, postoperative pain management is far from satisfactory, though the past decades have witnessed great progress in the development of novel analgesics and analgesic techniques. A Cochrane systematic review showed that patient-controlled analgesia (PCA) achieved better pain relief and greater patient satisfaction than traditional “on-demand” parenteral analgesia, suggesting that it might be the manner of analgesia implementation that matters for effective postoperative pain management. A wireless intelligent PCA (Wi-PCA) system that incorporated remote monitoring, an intelligent alarm, intelligent analysis and assessment of the PCA equipment, as well as automatically recording and reserving key information functions under a wireless environment was introduced in our department in 2018. The practice showed that the Wi-PCA system significantly reduced the incidence of moderate to severe postoperative pain and relevant adverse effects, shortened hospital stays, and improved patient satisfaction with postoperative pain relief. Nevertheless, for both traditional and Wi-PCA, analgesics are only administered when pain occurs, leaving behind a realm of possibilities for better postoperative pain management. With the rapid development of machinery and deep learning algorithms, artificial intelligence (AI) is changing the mode of clinical decision making. Integrating the big data collected by state-of-the-art monitoring sensors, the Internet of Things and AI algorithms, an AI-assisted PCA (Ai-PCA) may be a promising future direction for postoperative pain management.
Introduction
Pain is a physical response to harmful stimuli and is currently perceived as a disease rather than a symptom. Acute pain after surgery is common, and severe unrelieved pain leads to not only mental stresses, such as anxiety and depression, but also physical changes in respiratory, circulatory, and immune systems, affecting the patients' life quality and recovery (1). A positive attitude toward pain management by both patients and anesthesiologists has evolved enormously over the past decades. However, the scenario of pain relief remains unchanged despite the rapid progress in the development of analgesics and the emergence of novel analgesia techniques (2). Therefore, more innovative thoughts and perspectives need to be considered to bridge the gap between the ever-growing demand for better pain control and the unsatisfactory practice of pain management.
Unsatisfactory Progress in Postoperative Pain Relief Over the Past Decades
Ever since the publication of the acute pain management of operative or medical procedures and trauma guidelines by the Agency for Health Care Policy and Research in 1992 (3, 4), several national surveys on the epidemiology of post-surgical pain have been conducted in the United States, the United Kingdom, Canada, Germany, and France (5–11). All the surveys reported a pessimistically high incidence of moderate to severe pain in patients. The situation in China is also not optimistic. A multicenter investigation including 5,245 patients in 12 hospitals in Guangdong province showed that 56.19 and 29.73% of patients suffered from moderate/extreme pain in the first and second day after surgery (12). Another survey that included 2,293 patients from 17 hospitals in Southwest China suggested that the incidence of acute postoperative moderate to severe pain was 28.8% at rest and 45.1% in motion (13). Data from our hospital between November and December 2017 demonstrated that the overall moderate and severe pain (Numerical Rating Scale(NRS)≥4) incidence was 16.02% among 1,612 surgical patients (14).
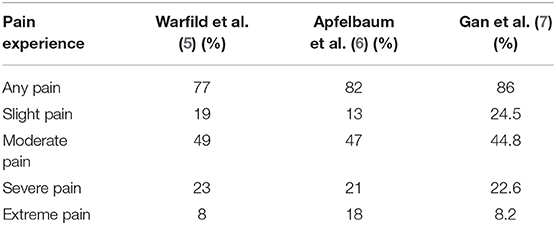
What is more disappointing is that three national surveys conducted in the United States at ~10-years intervals showed no improvement—perhaps even worsening—in the management of postoperative pain (Table 1). In 1995, Warfield et al. surveyed 500 U.S. adults, among which 27% received surgical procedures during the past 5 years. A total of 57% of patients that underwent surgery considered postoperative pain as their major concern before surgery. More than three quarters (77%) of patients experienced different levels of pain after surgery, and the percentage of patients suffering from slight, moderate, severe, and extreme pain were 19, 49, 23, and 8%, respectively. A total of 71% of patients complained of insufficient pain relief after administration of their first pain medication (5). Apfelbaum et al. conducted the second national postoperative pain survey 8 years later in 2003. The survey included 250 randomly selected participants from the National Family Opinion-World Group, which maintains a panel of more than 550,000 U.S. households. Postoperative pain was also most concerned (59%) by the participants before surgery. The postoperative pain lasted 2 weeks after discharge for 82% of patients. Among these patients, 13% experienced slight pain, 47% experienced moderate pain, 21% experienced severe pain, and 18% experienced extreme pain. Among all the patients, ~82% received pain medication in the hospital (6). Gan et al. performed the third U.S. national survey on postoperative pain prevalence in 2014 in 300 patients. A much higher percentage (80%) of patients reported post-surgical pain as their major concern before surgery. A slightly increased percentage of postoperative pain was also observed in the survey, reaching as high as 86%. The percentage of patients reporting an intensity of pain of slight, moderate, severe and extreme was 24.5, 44.8, 22.6, and 8.2%, respectively (7).
Advantages and Flaws of Traditional Patient-Controlled Analgesia (PCA)
The innovative concept of PCA was first proposed by Sechzer et al. (15), and it was put into practice in the mid-1970s following the emergence of microprocessors. The first generation PCA employed mechanical analgesia pumps, while electronic pumps are currently playing dominant roles in clinical practice (16, 17). Several routes of PCA administration, such as intravenous, epidural, and nerve block, have also been developed, allowing for the self-administration of small doses of analgesics by patients. PCA brought pain management into a new era, shifting postoperative analgesia from “scheduled” and “requested” modes to “self-administration” modes, rendering pain control patient autonomous.
PCA has gained popularity among both patients and anesthesiologists. However, contrary to the expected high analgesia efficiency, the latest Cochrane systemic review showed that PCA only displayed marginal superiority (8%) over traditional methods of pain management (18).
One of the non-neglectable defects of the traditional PCA is that it is decentralized. PCA equipment is scattered in patient wards without direct or instant connection with medical personnel. Patients have to master the operation of the usually pre-programmed PCA equipment following a short tutorial by medical staff. There might be no problem when the equipment works properly. However, if no immediate response is made by medical staff when mechanical problems occur or when a patient's analgesia requires adjustments, the analgesia efficiency will be compromised. Moreover, the unresolved alarm sound might even trigger undesired nervous or panic emotions in patients.
Better Performance of Wi-PCA Than Traditional PCA
The rapid development of communication technologies, especially wireless communication techniques, spawned the Internet of Things, making instant gathering and sharing information possible. The Wi-PCA that connects electronic PCA pumps and other mobile terminals with a central computer sever installed with an information control system under a wireless environment has enabled remote monitoring, intelligent alarms, intelligent analysis and assessment of the PCA equipment, and automatically recording and reserving key information (19). The Wi-PCA significantly enhanced the dynamic management of postoperative pain, and practice showed that Wi-PCA significantly reduced the incidence of moderate to severe postoperative pain as well as relevant adverse reactions, shortened the length of hospital stays, and improved patient satisfaction with postoperative pain relief compared with the traditional PCA.
Data from Cao et al. indicated that the incidence of rest (NRS ≥ 4) and motion (NRS ≥ 4) pain was significantly lower in patients using Wi-PCA in the Tumor Hospital Affiliated to Nantong University than patients using traditional PCA in 12 hospitals in Guangdong province (20), and the incidence of nausea and vomiting was significantly lower while satisfaction was higher among patients using Wi-PCA (Tables S1, S2). They also performed a historical comparison between Wi-PCA (from 2016 to 2017) and traditional PCA (in 2015) in the Tumor Hospital Affiliated to Nantong University. The data suggested that the incidence of rest pain and motion pain in the first three days after operation was significantly reduced in the Wi-PCA group, while the incidence of nausea and vomiting increased slightly (Table S3).
The Wi-PCA system was introduced to our department in the beginning of 2018. Comparison of traditional analgesic data from 1,612 patients between November and December in 2017 and Wi-PCA data from 6,191 patients between January and August in 2018 in the First Affiliated Hospital of Xi'an Jiaotong University suggested that the incidence of moderate or severe pain in surgical patients with traditional PCA was 16.02%, while the introduction of the Wi-PCA system significantly reduced the incidence to as low as 1% (Figure S1A). The patient satisfaction with pain relief was 15% higher in the Wi-PCA group (Figure S1B). Moreover, a declining trend for postoperative nausea and vomiting (PONV) was observed in contrast to Cao's observation (Figure S1C).
Ai-PCA: a Promising Future Analgesia Direction
The Wi-PCA is still not intelligent enough, as this kind of system is not equipped with a “brain” that thinks and makes decisions independently. Indeed, to some extent, no substantial progress has been made in traditional “scheduled or requested” analgesia, traditional PCA, and Wi-PCA, as they all provide salvage analgesia instead of preventive analgesia. Preventive analgesia, a broader perioperative pain management strategy, aimed at blocking the induction of central sensitization has led to less intense pain and reduced analgesic consumption (21–23). However, in regard to postoperative PCA, no reliable parameters exist for determining the optimal time to trigger the small bolus dose of analgesic before pain occurs.
Artificial intelligence (AI) algorithms give machines the ability to reason and make decisions in a supervised or unsupervised manner. AI-powered technologies are thriving, and they are currently changing medical practice. AI has surpassed humans in several medical areas, such as disease diagnosis based on medical or pathological images and disease activity monitoring for atrial fibrillation and epilepsy relapse (24–29). Pioneering work of AI applications in anesthesiology has been carried out in several aspects, including anesthesia depth monitoring, control of anesthesia, risk prediction, and logistics management (30). In pain management, AI has been used to select patients who may benefit from preoperative pain consultation (31).
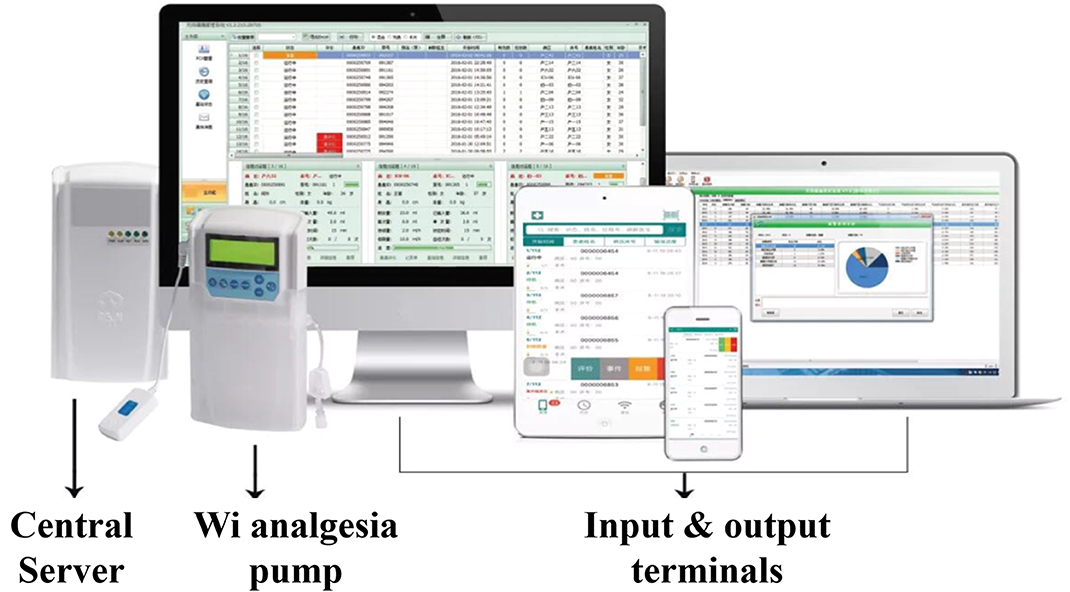
Like the biological neural circuit, an integrated AI application requires both input and output terminals apart from the central model built with specific AI algorithms. Indeed, the Wi-PCA provides the hardware structure of Ai-PCA, that is the computer server and the terminals that send signals to and receive signals from the server (Figure 1). Ai-PCA requires more specific detectors that gather and send more specific signals to the server and equips the server with an AI model.
Choosing the right parameter as the input signal is key in Ai-PCA development. The preventive analgesia concept is based on the “injury discharge” phenomenon, a basic science discovery that peripheral nerve injury-triggered afferent barrage consists of high-frequency bursts which are different from normal neural responses by natural stimuli (32). The “injury-discharge” might be a good candidate, but the feasibility depends on its accessibility by wearable detectors. Hence, cooperation between basic and applied scientists are necessary for successful Ai-PCA development.
Another issue in AI applications in pain management is ethical and safety concerns, although the use of AI applications in postoperative pain management is an irresistible trend. Future disputes might be focused on whether the best course of action is the use of Ai-PCA or AI-controlled analgesia.
Conclusions
The disappointing fact that almost no real progress has been made in the past two decades in postoperative management requires innovation in the development of analgesia strategy. AI is a promising approach to shift salvage analgesia to a preventive era.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
QW, RW, SW, and ND contributed conception and design of the study. SW organized the database. RW and ND performed the statistical analysis. RW wrote the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This article was funded by Natural Science Basic Research Program of Shaanxi (Program No. 2017JZ029), Natural Science Basic Research Program of Shaanxi (Program No. 2019JQ509), and National Natural Science Foundation of China (Program No. 81802443).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.00145/full#supplementary-material
References
1. Cousins MJ, Brennan F, Carr DB. Pain relief: a universal human right. Pain. (2004) 112:1–4. doi: 10.1016/j.pain.2004.09.002
2. Correll DJ, Vlassakov KV, Kissin I. No evidence of real progress in treatment of acute pain, 1993-2012: scientometric analysis. J Pain Res. (2014) 7:199–210. doi: 10.2147/JPR.S60842
3. Acute pain management: operative or medical procedures and trauma part 1. Agency for health care policy and research. Clin Pharm. (1992)11:309–31.
4. Acute pain management: operative or medical procedures and trauma part 2. Agency for health care policy and research. Clin Pharm. (1992) 11:391–414.
5. Warfield CA, Kahn CH. Acute pain management. Programs in US. hospitals and experiences and attitudes among US. adults. Anesthesiology. (1995) 83:1090–4. doi: 10.1097/00000542-199511000-00023
6. Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. (2003) 97:534–40. doi: 10.1213/01.ANE.0000068822.10113.9E
7. Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. (2014) 30:149–60. doi: 10.1185/03007995.2013.860019
8. McGrath B, Elgendy H, Chung F, Kamming D, Curti B, King S. Thirty percent of patients have moderate to severe pain 24 hr after ambulatory surgery: a survey of 5,703 patients. Can J Anaesth. (2004) 51:886–91. doi: 10.1007/BF03018885
9. Bruster S, Jarman B, Bosanquet N, Weston D, Erens R, Delbanco TL. National survey of hospital patients. BMJ. (1994) 309:1542–6. doi: 10.1136/bmj.309.6968.1542
10. Maier C, Nestler N, Richter H, Hardinghaus W, Pogatzki-Zahn E, Zenz M, Osterbrink J. The quality of pain management in German hospitals. Dtsch Arztebl Int. (2010) 107:607–14. doi: 10.3238/arztebl.2010.0607
11. Fletcher D, Fermanian C, Mardaye A, Aegerter P Pain and Regional Anesthesia Committee of the French Anesthesia and Intensive Care Society (SFAR). A patient-based national survey on postoperative pain management in France reveals significant achievements and persistent challenges. Pain. (2008) 137:441–51. doi: 10.1016/j.pain.2008.02.026
12. She S. Multi-Center Survey Report on Postoperative Analgesia in Guangdong, China. Congress of Chinese Association of Anesthesiology (2011).
13. Peng LH, Jing JY, Qin PP, Su M. A multi-centered cross-sectional study of disease burden of pain of inpatients in Southwest China. Chin Med J (Engl). (2016) 129:936–41. doi: 10.4103/0366-6999.179788
14. Duan N, Wang S, Dong M, Bu Y, Li X, Wang Q. Clinical effect observation of artificial intelligence patient controlled analgesia on acute postoperative pain. Chin Med J. (2019) 54:1011–4.
15. Sechzer PH. Objective measurements of pain. Anesthesiology. (1968) 29:209–10. doi: 10.1097/00000542-196801000-00104
16. Grass JA. Patient-controlled analgesia. Anesth Analg. (2005) 101(5 Suppl.):S44–61. doi: 10.1213/01.ANE.0000177102.11682.20
17. Xuan XC, Cao H, Hu Y. Current status and application of patient-controlled analgesia: a literature review. Int J Clin Exp Med. (2019) 12:2633–41.
18. Hudcova J, McNicol E, Quah C, Lau J, Carr DB. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database Syst Rev. 18:CD003348. doi: 10.1002/14651858.CD003348.pub2
19. Wang N, Wang JG. Research on Intelligent Analgesia Pump. Procedia Comput Sci. (2019) 154:256–9. doi: 10.1016/j.procs.2019.06.038
20. Cao H, Huang W, Peng S, Xu L, Wang S, Zhang J, et al. Effect of intelligentized patient-controlled analgesia management on quality of postoperative analgesia. Chin J Anesthesiol. (2019) 38:1077–81.
21. Kissin I. A call to reassess the clinical value of preventive (preemptive) analgesia. Anesth Analg. (2011) 113:977–8. doi: 10.1213/ANE.0b013e31822d39f9
22. Katz J, Clarke H, Seltzer Z. Review article: preventive analgesia: quo vadimus? Anesth Analg. (2011) 113:1242–53. doi: 10.1213/ANE.0b013e31822c9a59
23. Katz J, McCartney CJ. Current status of preemptive analgesia. Curr Opin Anaesthesiol. (2002) 15:435–41. doi: 10.1097/00001503-200208000-00005
24. Bera K, Schalper KA, Rimm DL, Velcheti V, Madabhushi A. Artificial intelligence in digital pathology - new tools for diagnosis and precision oncology. Nat Rev Clin Oncol. (2019) 16:703–15. doi: 10.1038/s41571-019-0252-y
25. Niazi MKK, Parwani AV, Gurcan MN. Digital pathology and artificial intelligence. Lancet Oncol. (2019) 20:e253–61. doi: 10.1016/S1470-2045(19)30154-8
26. Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts H. Artificial intelligence in radiology. Nat Rev Cancer. (2018) 18:500–10. doi: 10.1038/s41568-018-0016-5
27. Attia ZI, Noseworthy PA, Lopez-Jimenez F, Asirvatham SJ, Deshmukh AJ, Gersh BJ, et al. Friedman: an artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. (2019) 394:861–7. doi: 10.1016/S0140-6736(19)31721-0
28. Kearney H, Byrne S, Cavalleri GL, Delanty N. Tackling epilepsy with high-definition precision medicine: a review. JAMA Neurol. (2019). doi: 10.1001/jamaneurol.2019.2384. [Epub ahead of print].
29. Briganti G, Le Moine O. Artificial intelligence in medicine: today and tomorrow. Front Med. 7:27. doi: 10.3389/fmed.2020.00027
30. Hashimoto DA, Witkowski E, Gao L, Meireles O, Rosman G. Artificial intelligence in anesthesiology: current techniques, clinical applications, and limitations. Anesthesiology. (2020) 132:379–94. doi: 10.1097/ALN.0000000000002960
31. Tighe PJ, Lucas SD, Edwards DA, Boezaart AP, Aytug H, Bihorac A. Use of machine-learning classifiers to predict requests for preoperative acute pain service consultation. Pain Med. (2012) 13:1347–57. doi: 10.1111/j.1526-4637.2012.01477.x
Keywords: Ai-PCA, Wi-PCA, patient-controlled analgesia, artificial intelligence, postoperative pain
Citation: Wang R, Wang S, Duan N and Wang Q (2020) From Patient-Controlled Analgesia to Artificial Intelligence-Assisted Patient-Controlled Analgesia: Practices and Perspectives. Front. Med. 7:145. doi: 10.3389/fmed.2020.00145
Received: 01 December 2019; Accepted: 03 April 2020;
Published: 22 May 2020.
Edited by:
Mert Sentürk, Istanbul University, TurkeyReviewed by:
Natalija Vukovic, University of Nis, SerbiaVesna D. Dinic, Clinical Center Niš, Serbia
Alparslan Kus, Kocaeli University, Turkey
Copyright © 2020 Wang, Wang, Duan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Wang, ZHIud2FuZ3FpYW5nQDEzOS5jb20=
 Rui Wang
Rui Wang Na Duan
Na Duan Qiang Wang
Qiang Wang