- 1Department of Rheumatology and Immunology, Singapore General Hospital, Singapore, Singapore
- 2Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
- 3Duke-NUS Medical School, Singapore, Singapore
Aim: To evaluate the prevalence of fatigue and the factors associated with fatigue among patients with axial spondyloarthritis (axSpA) within an Asian population.
Method: We used the baseline data from a clinic registry in a tertiary referral center. All patients fulfilled the 2009 Assessment of SpondyloArthritis international Society (ASAS) classification criteria for axSpA. Severe fatigue was defined as Bath Ankylosing Spondylitis Disease Activity Index-fatigue (BASDAI-fatigue) ≥5/10 and vitality domain of Short Form-36 Health Survey (SF-36 VT) ≤10th percentile of the general population.
Results: We included 262 consecutive patients with axSpA (79% men, 82.4% Chinese). The mean (standard deviation, SD) age and duration of disease were 41.7 (13.7) and 10.1 (8.3) years, respectively. 145 (55.3%) and 52 (31.1%) patients reported severe fatigue by the BASDAI-fatigue and SF-36 VT criteria, respectively. Patients with severe fatigue had worse scores across all disease activity assessments and disease impact measures compared to those without severe fatigue. Using principal component analyses, disease activity and impact were associated with BASDAI-fatigue, while disease activity and impact, and disease chronicity were associated with SF-36 VT. In the univariable analyses, all disease activity assessments and disease impact measures correlated with both BASDAI-fatigue and SF-36 VT. In the multivariable analyses, BASDAI-axial pain, BASFI, BAS-G, and ethnicity were associated with BASDAI-fatigue, while ASQoL and BASDAI-morning stiffness were associated with SF-36 VT.
Conclusion: Fatigue is prevalent amongst patients with axSpA in Asia and is associated with disease activity, disease impact as well as patient related factors.
Introduction
Axial spondyloarthritis (axSpA) is a chronic inflammatory rheumatic disease characterized by predominant involvement of the axial skeleton, with peak disease onset in late adolescence and early adulthood (1). Associated hallmark features of axSpA include enthesitis, peripheral arthritis, and extra-articular involvement such as anterior uveitis (2). Often striking patients in their prime of life, axSpA has wide-ranging and long-lasting impacts on different aspects of life, including functional, psychological and social domains (3).
Fatigue is widely regarded as a cardinal manifestation of axSpA and is one of the three most frequently reported symptoms alongside pain and stiffness (4), with a high prevalence ranging from 49 to 66.4% in different cohorts (5–10). The importance of fatigue has been recognized as evidenced by its inclusion in the Assessment of SpondyloArthritis international Society (ASAS) core set for clinical practice (11). Fatigue is generally described as a subjective sensation of tiredness that is relentless, pervasive, unpredictable, and unresolving (12, 13). It also has adverse impacts on how axSpA patients function in daily activities (7–9, 14–16), work performance (9, 16), and quality of life (6, 9, 14, 15). However, the mechanisms driving fatigue in axSpA remains unclear. Despite the advent of biologics, fatigue remains poorly treated in many (6), thus highlighting its complexity and the management challenge it poses. Current understanding holds that fatigue in spondyloarthritis is multi-factorial and associated with disease-specific parameters (5–10, 14, 15, 17), poor psychological health (7, 8, 10, 14, 15), sleep disturbance (10, 17), and women gender (5, 8), with disease activity being the single most important predictor.
Existing literature on fatigue in axSpA is largely derived from Western populations and data on Asians with axSpA is limited. The pathogenesis of axSpA is underpinned by genetic and environmental factors (18), and there is evidence that ethnicity and geographic differences (18–22) may shape the disease manifestations, severity and comorbidities in SpA. Moreover, it has been well-known that socio-cultural differences influence perceptions on health (23). Singapore is a multi-ethnic Asian country with a unique ethnic profile of Chinese, Malay, Indians, and others in Asia. In this study, we aim to evaluate the prevalence of fatigue and the factors associated with fatigue among patients with axSpA within an Asian population.
Methods
Study Population
We used the baseline data from the PRESPOND (PREcision medicine in SPONdyloarthritis for Better Outcomes and Disease Remission) registry (3, 24) in a tertiary referral center in Singapore. The PRESPOND registry recruited consecutive and all patients who attended a designated SpA clinic from 2012 to 2015 and fulfilled the 2009 ASAS classification criteria for axSpA (11). The study protocol was approved by the SingHealth Centralized Institutional Review Board (CIRB Ref: 2012/498/E) and written informed consent was obtained from each patient prior to their participation.
Data Collection
Data was collected during clinic visit using a standardized protocol. Patients self-administered questionnaires in paper and pencil format, which captured socio-demographic information including age, gender, ethnicity, highest education level, housing type, and other patient reported outcomes (PROs). We collected data on highest education level and housing type on an ordinal scale of 1–7, adapted from the method used in the Singapore Census of Population 2010 (25, 26). Housing type has been used as a surrogate of socio-economic status in Singapore (27). Information on disease duration was retrieved from clinical records.
Patients were examined by designated rheumatologists (WF and YYL) for tender, swollen and damaged joints on a 66/68/68 diarthrodial joints diagram, as well as enthesitis using the Spondyloarthritis Research Consortium of Canada (SPARCC) enthesitis index (28). Spinal mobility parameters, including cervical rotation, tragus-to-wall distance, lateral lumbar flexion, lumbar flexion, and inter-malleolar distance, were measured twice and the better attempts were recorded. The Bath Ankylosing Spondylitis Metrology Index (BASMI10) score was then computed using the 10-step definition by Jones et al. (29). Body weight and height were measured in the clinic. Erythrocyte sedimentation rate (ESR) was measured as part of standard care.
Assessment of Fatigue
Fatigue was assessed using two different measures—the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) fatigue item and the Short Form-36 Health Survey (SF-36) vitality (VT) domain.
The BASDAI-fatigue item is one of the five core symptoms evaluated in the BASDAI which has been validated for axSpA patients in Singapore (30). Patients rated the severity of their fatigue over the past week on a 0-10 numeric rating scale (NRS) (0: none, 10: very severe). We defined severe fatigue as BASDAI-fatigue ≥5/10 as with previous studies on fatigue and axSpA (5, 6, 9, 10, 31, 32). The SF-36 VT is one of the eight domains of the SF-36, a generic measure of health-related quality of life (HRQoL) validated for axSpA patients in Singapore (33). The SF-36 VT domain comprises of four items on the themes of fatigue and energy levels (feeling full of life, having lots of energy, feeling worn out, and feeling tired). Each item has five response options on a Likert scale and the total raw score is converted to a 0–100 point scale (0: lowest vitality, 100: highest vitality) (34). The SF-36 VT represents a reverse concept of fatigue (35), in which a lower score is indicative of greater fatigue. We defined severe fatigue as SF-36 VT ≤10th percentile of the general Singaporean population (8). The 10th percentile SF-36 VT score of the general population was calculated to be 47.6, by applying the formula (10th percentile = mean − 1.28 × standard deviation, SD) to the data obtained from the general Singaporean population (36).
Other PROs
We collected data on BASDAI, including BASDAI-axial pain, BASDAI-peripheral joint pain, and BASDAI-morning stiffness (mean score of the questions on severity and duration), scored on a 0–10 NRS (0: none, 10: very severe). We assessed the effect of axSpA on patient's global well-being through the Bath Ankylosing Spondylitis Patient Global Score (BAS-G), which ranged from 0–100 (0: very good, 100: very bad) (37). Physical function was assessed through the Bath Ankylosing Spondylitis Functional Index (BASFI) (38). HRQoL was assessed using the SF-36 as well as the Ankylosing Spondylitis Quality of Life (ASQoL) (39), which has been validated in Singapore (40).
Statistical Analysis
We described and compared the baseline characteristics between patients with and without severe fatigue. Differences in continuous variables were analyzed using the independent samples t-test or the Mann-Whitney U test as appropriate, while that of categorical variables were analyzed using the Chi-squared test. We conducted a principal component analysis (PCA) without rotation to evaluate for clusters of variables that represent clinically distinct concepts. We selected variables that were either theoretically linked to fatigue or described in literature to be associated with fatigue (5–10, 14, 15, 17, 19, 41). Only variables with pre-defined correlations of <0.6 were included in the model. The study rheumatologists discussed to reach a consensus on the inclusion or exclusion of variables with high collinearity (Spearman's rho > 0.6). Principal components with eigenvalues > 1 were entered into linear regression models for BASDAI-fatigue and SF-36 VT, respectively, to determine component(s) significantly associated with each measure of fatigue.
We performed univariable analysis using Spearman's rank correlation to determine the relationship between the study variables and measures of fatigue. In our study, we considered correlations of magnitude ≥ 0.5 as strong, 0.3–0.49 as moderate and 0.1–0.29 as weak (42). In the multivariable analysis, we evaluated for variables associated with each measure of fatigue using stepwise linear regression. Variables with p < 0.2 in the univariate analysis for association with fatigue were included. We also considered variables that either have a strong theoretical basis for association with fatigue or were found to be predictors of fatigue in previous studies.
All statistical analyses were carried out using IBM SPSS Statistic Package, version 25 (IBM, Armonk) and statistical significance was set at p < 0.05.
Results
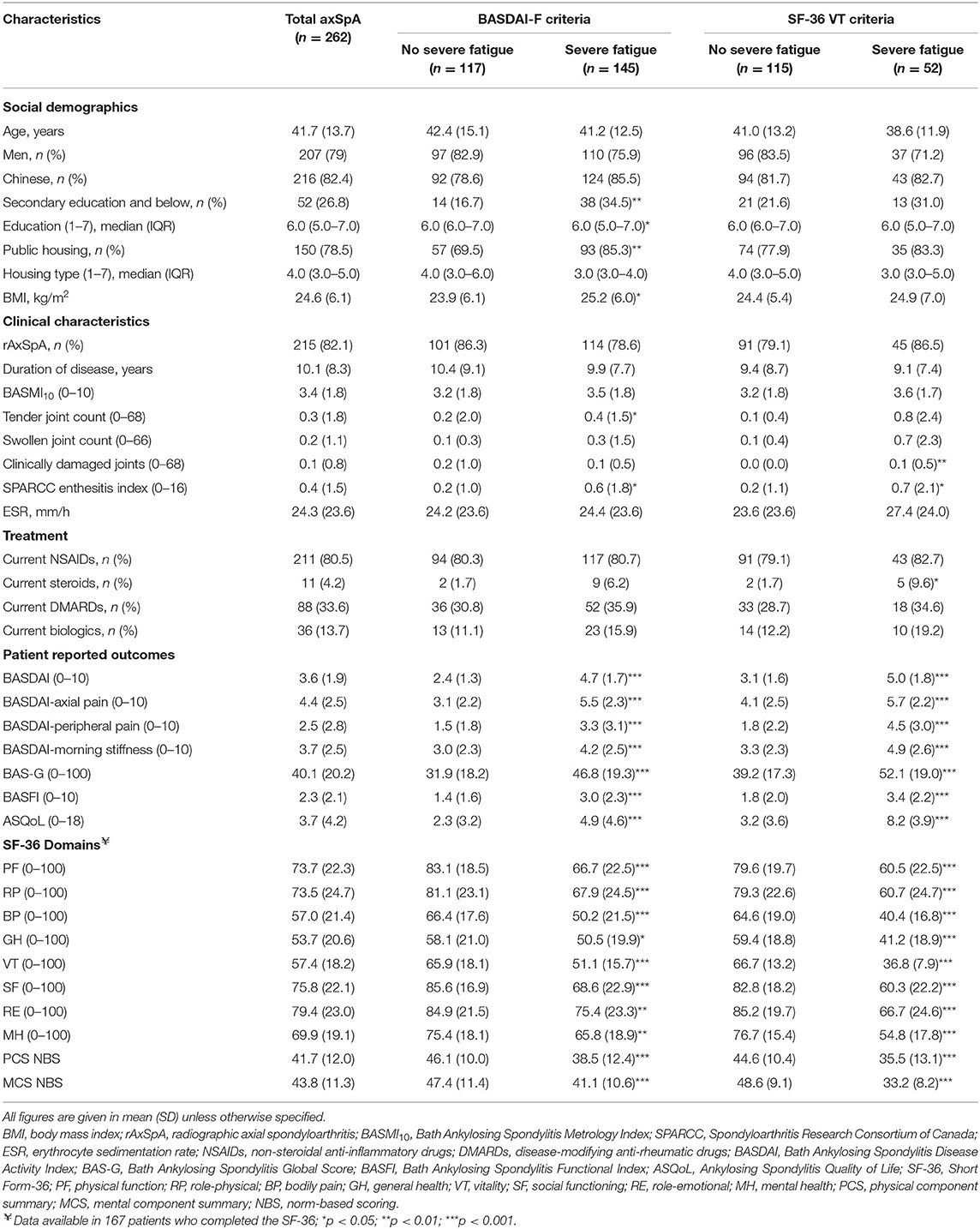
A total of 262 consecutive patients with axSpA were included in this study, of which 262 had complete data for BASDAI-fatigue, and only 167 had available data for SF-36 and SF-36 VT. The baseline characteristics of the patients are detailed in Table 1. 207 (79%) were men and 216 (82.4%) were of Chinese ethnicity. The mean (standard deviation, SD) age was 41.7 (13.7) years, while that of duration of disease was 10.1 (8.3) years (Table 1).
Among the 262 patients, 145 (55.3%) reported severe fatigue by the BASDAI-fatigue criteria (BASDAI-fatigue ≥ 5/10). Among the 167 patients with data for SF-36, 52 (31.1%) had severe fatigue by the SF-36 VT criteria (VT ≤ 47.6/100). Patients who had severe fatigue by the BASDAI-fatigue and SF-36 VT criteria had worse scores across all disease-activity assessments (BASDAI, axial pain, peripheral joint pain, morning stiffness, SPARCC enthesitis index) as well as PROs measuring disease impact (BAS-G, BASFI, ASQoL, and all SF-36 domains), compared to those without severe fatigue. Additionally, patients with severe fatigue by the BASDAI-fatigue criteria had higher BMI and lower education levels, with a higher proportion of them reporting an education level of secondary or below and living in public housing, compared to those without severe fatigue. Patients who had severe fatigue by the SF-36 VT criteria had a higher damaged joint count compared to those without severe fatigue.
In the PCA, we included gender, ethnicity, age, education, housing type, body mass index (BMI), duration of disease, swollen joint count, SPARCC enthesitis index, BASMI10, clinically damaged joints, BASDAI-axial pain, BASDAI-peripheral joint pain, BASDAI-morning stiffness, BAS-G, BASFI, and ASQoL. BASDAI-axial pain and BASDAI-peripheral joint pain were included separately as they represent distinctive domains of disease activity. Tender joint count was excluded due to collinearity with swollen joint count. Due to a sizable amount of missing data for SF-36, we chose ASQoL over the SF-36 domains into the model. Six principal components explaining 64.4% of the total variance (Figure 1 and Supplementary Table 1) were derived. From components 1 to 6, the concepts represented are disease activity and impact, disease chronicity, socio-economic status, enthesitis and socio-demographics, peripheral joint damage, and BMI/ethnicity, respectively. Component 1, explaining 21.7% of the variance, reflected disease activity, and impact. The variables included are BASFI, BASDAI-morning stiffness, BAS-G, BASDAI-axial pain, ASQoL, and BASDAI-peripheral joint pain. Component 2, accounting for 12.5% of the variance, represented the concept of disease chronicity and comprised of age, duration of disease and BASMI10. In the regression model analysis, only component 1 was significantly associated with BASDAI-fatigue (p < 0.001), with the model accounting for 30.1% of variance in BASDAI-fatigue. In the SF-36 VT model, both components 1 and 2 were significantly associated with fatigue (p < 0.001), with the model explaining 44.2% of variance in VT.
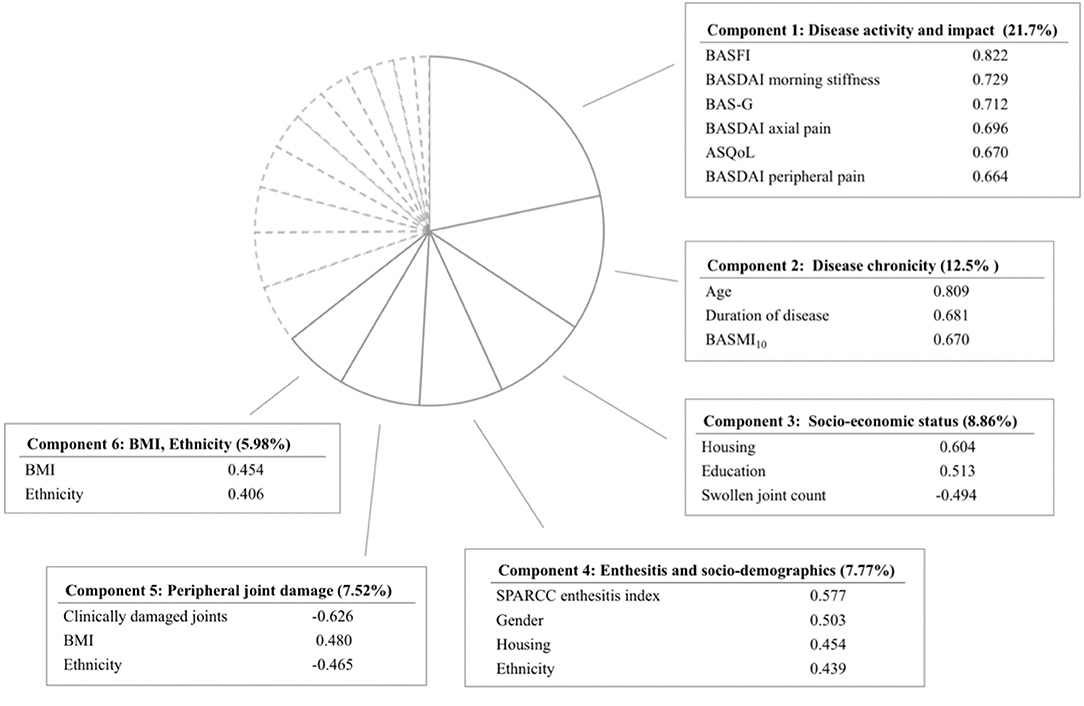
Figure 1. Principal component analysis yielding 6 components with eigenvalue greater than 1. Only variables with high factor loading are shown. BASFI, Bath Ankylosing Spondylitis Functional Index; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BAS-G, Bath Ankylosing Spondylitis Global Score; ASQoL, Ankylosing Spondylitis Quality of Life; BASMI10, Bath Ankylosing Spondylitis Metrology Index; SPARCC, Spondyloarthritis Research Consortium of Canada; BMI, Body Mass Index.
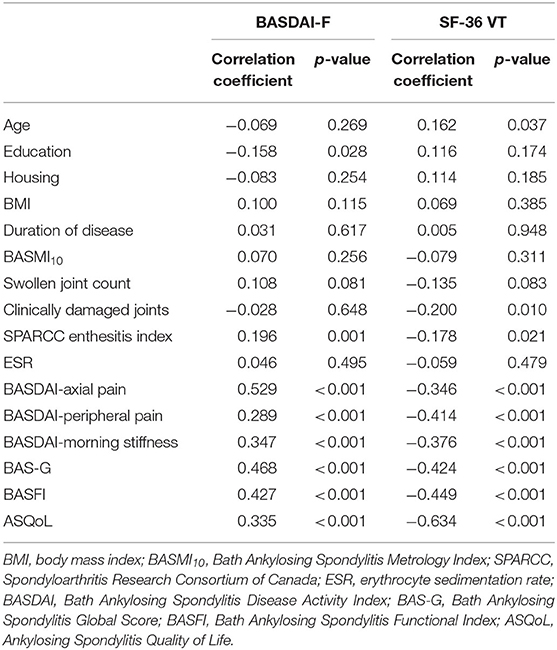
In the univariable analysis (Table 2), BASDAI-fatigue correlated strongly with BASDAI-axial pain; moderately with BAS-G, BASFI, BASDAI-morning stiffness, and ASQoL; and weakly with BASDAI-peripheral joint pain, SPARCC enthesitis index and education. In contrast, SF-36 VT correlated strongly with ASQoL; moderately with BASFI, BAS-G, BASDAI-peripheral joint pain, BASDAI-morning stiffness, and BASDAI-axial pain; and weakly with clinically damaged joints, SPARCC enthesitis index and age.
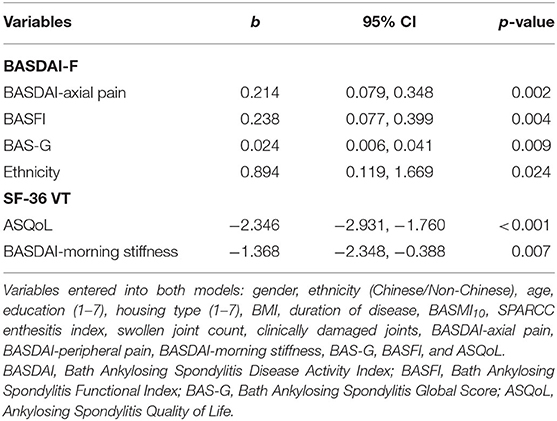
In the multivariable analysis, we selected variables with p < 0.2 in the univariable analysis, which included education, swollen joint count, SPARCC enthesitis index, BASDAI-axial pain, BASDAI-peripheral joint pain, BASDAI-morning stiffness, BAS-G, BASFI, and ASQoL. We also included gender (5, 8), age (14), and BMI (41) as these were previously reported associations with fatigue. Ethnicity and housing type as a surrogate of socio-economic status (27) were included for theoretical exploration. In addition, we included duration of disease, clinically damaged joints and BASMI10 to explore possible associations between fatigue and the concepts of disease chronicity and damage (peripheral and axial). The results of the multivariate analysis are presented in Table 3. In the BASDAI-fatigue model, BASDAI-axial pain, BASFI, BAS-G, and ethnicity were significantly associated with fatigue. In the SF-36 VT model, ASQoL, and BASDAI-morning stiffness were significantly associated with fatigue.
Discussion
Fatigue is prevalent in our study cohort, underscoring its importance as a major symptom in axSpA. In our study, 55.3% of all patients experienced severe fatigue by the BASDAI-fatigue criteria (BASDAI-fatigue ≥ 5/10) while 31.1% experienced severe fatigue by the SF-36 VT criteria (VT ≤ 10 percentile of the general population). Patients who experienced severe fatigue generally reported higher disease activity and greater perceived disease impact. In addition, patients with severe fatigue by the BASDAI-fatigue criteria had higher BMI and lower socio-economic status. In the PCA, disease activity and impact variables were associated with both measures of fatigue, and disease chronicity was additionally associated with SF-36 VT. In the multivariable analyses, BASDAI-axial pain, BASFI, BAS-G, and ethnicity (non-Chinese vs. Chinese) were associated with BASDAI-fatigue; while ASQoL and BASDAI-morning stiffness were associated with SF-36 VT.
The prevalence of severe fatigue in our axSpA cohort was largely concordant with that reported in previous studies. In several Western cohorts using the same definition (BASDAI-fatigue ≥ 5/10), the prevalence of fatigue ranged from 49 to 66.4% (5–10). In a Norwegian study, which defined severe fatigue as SF-36 VT ≤ 10th percentile of the general population, 32% of their patients with axSpA were found to have severe fatigue (8). In our study, disease activity assessments stood out consistently as key predictors of fatigue. Disease activity is a well-recognized driver of fatigue in axSpA (5–10, 14, 15, 17). This suggests a component of fatigue that is amenable to disease modifying treatment. In fact, studies have demonstrated that biologics therapy reduced fatigue levels, albeit suboptimally (6), highlighting the multi-factorial nature of fatigue in axSpA. ESR, which may not be a good reflection of disease activity (43), was not associated with fatigue in our study. This is consistent with findings from other studies (8, 17). C-reactive protein may be a better reflection of disease activity but was not consistently captured in our study. Besides disease activity, we also found significant associations between disease impact and fatigue. In the multi-variable analysis, physical function (measured by BASFI), and BAS-G were associated with BASDAI-fatigue, while ASQoL was associated with SF-36 VT. Our findings are consistent with other studies that reported BASFI (7–9, 14, 15, 17), BAS-G (5, 9, 14), and ASQoL (6, 9, 14, 15) as predictors of fatigue. This highlights patients' perception of fatigue as a disabling symptom and accentuates the importance of recognizing and addressing fatigue in clinical practice. Moreover, poor mental health including anxiety and depression has been found to be associated with fatigue (7, 8, 10, 14, 15, 32). In a study on patients with ankylosing spondylitis using brain magnetic resonance imaging, the left thalamus volume of patients with severe fatigue was significantly larger than that of healthy controls and patients without fatigue (32), suggesting a role of the central nervous system in the subjective symptoms of fatigue. This emphasizes the need for a holistic bio-psycho-cognitive approach in the clinical management of fatigue in axSpA. In our study, we also found an inverse correlation between damaged joint count and SF-36 VT. We postulate that joint damage could possibly increase the effort required in performing daily activities, resulting in greater fatigue, as hypothesized in studies in rheumatoid arthritis (44), where peripheral joint damage features prominently. Spinal damage, which was measured by BASMI10 and clustered together with age and disease duration in the PCA, was associated with SF-36 VT. However, it was not associated with fatigue measures in the multivariable analysis, which is in keeping with other studies (6–9, 15, 17).
Several patient-related factors were associated with fatigue in our study. We found that Chinese ethnicity is significantly associated with BASDAI-fatigue in the multivariable analysis after adjusting for other variables. Some recent studies have found that Blacks, African Americans, and African Brazilians have higher disease activity compared to Whites (19–21), suggesting that ethnicity may influence disease activity in axSpA. We also found that patients with severe BASDAI-fatigue had higher BMI, though statistical significance was lost in the multivariable analyses. This is consistent with other studies (17, 41), and could possibly be explained by a heightened level of pro-inflammatory cytokines resulting from increased adiposity (45), which is increasingly recognized for its immunological properties. Obesity has been associated with various disease outcomes in SpA (24, 46). Clinicians should therefore consider weight loss in the management of fatigue in patients with high BMI. Moreover, we found that patients with severe BASDAI-fatigue had lower socio-economic status, as measured by education and housing type. Housing type is a unique surrogate of socio-economic status in Singapore (47). Socio-economic status is a well-known determinant of health (48), and may influence disease activity as well as the progression of spinal damage in axSpA (49, 50). Lower education levels were similarly reported to be associated with higher fatigue levels in a French cohort (5). Age positively correlated with SF-36 VT in our study, but not with BASDAI-fatigue. This could possibly be due to the greater discrepancy in vitality perceived by younger patients when they compare themselves to their healthy peers. Except for a Dutch study (14), previous studies have largely reported no association between age and fatigue (15, 17). Some studies have demonstrated that women have higher fatigue levels compared to men (5, 8). In our study, gender was not significantly associated with fatigue. This could possibly be ascribed to the small sample size of women in our cohort.
Our study is one of the few (17) to evaluate factors associated with axSpA-fatigue in an Asian cohort, thereby supplementing current limited understanding on fatigue amongst patients with axSpA in Asia. Moreover, our preliminary data allowed for the study of fatigue among different Asian ethnicities. However, ethnicity was eventually analyzed as a dichotomous variable (Chinese vs. non-Chinese) due to limited representation from other ethnicities, thus limiting generalizability of the finding to other Asian ethnicities. We assessed fatigue using two different measures, which allowed for comparison and identification of variables consistently associated with both measures of fatigue. However, we recognize that our study has several limitations. Firstly, the sample size of our cohort is small. Secondly, this is a cross-sectional study and hence it is not possible to establish the causal relationship between fatigue and the variables. In addition, a third of our patients did not have data captured for SF-36. This resulted from a non-differential omission in distributing the SF-36 questionnaires on some clinic days where research assistant support was not available. However, the baseline characteristics of patients who completed the SF-36 were not different from those who did not. Moreover, a sensitivity analyses for BASDAI-fatigue limited to the 167 patients with SF-36 data yielded consistent results (data not shown). Lastly, variables which could potentially be linked to fatigue such as work productivity, sleep disturbance, anxiety, depression, fibromyalgia, and previous use of medication including traditional Chinese medicine were not evaluated in this study. These could have accounted for some of the unexplained variance in the PCA.
In conclusion, fatigue is prevalent amongst patients with axSpA in Singapore. Disease activity is a key predictor of fatigue in our study, suggesting that disease modifying treatment should continue to be a cardinal pillar in the management of fatigue. We also found that fatigue has significant associations with patients' perception of well-being, physical function, and quality of life. In addition, patient-related factors such as ethnicity, BMI, and socio-economic status were linked to fatigue. This study underscores the multi-factorial nature of fatigue amongst Asian patients with axSpA and emphasizes the need for a holistic approach in the management of fatigue.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: All data are available upon reasonable request to the corresponding author. Requests to access these datasets should be directed to a2F0eS5sZXVuZy55LnlAc2luZ2hlYWx0aC5jb20uc2c=.
Ethics Statement
The studies involving human participants were reviewed and approved by SingHealth Centralized Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YYL and WF conceptualized the study design and performed the data collection. WZL and YYL performed the statistical analysis. All authors authored and reviewed the manuscript and approved the version of this article to be published.
Funding
YYL was supported by the National Medical Research Council, Singapore (NMRC/CSA-INV/0022/2017).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all patients who participated in the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.603941/full#supplementary-material
References
1. Strand V, Singh JA. Evaluation and management of the patient with suspected inflammatory spine disease. Mayo Clin Proc. (2017) 92:555–64. doi: 10.1016/j.mayocp.2016.12.008
2. Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. (2016) 375:1303. doi: 10.1056/NEJMra1406182
3. Hong C, Kwan YH, Leung YY, Lui NL, Fong W. Comparison of ankylosing spondylitis and non-radiographic axial spondyloarthritis in a multi-ethnic Asian population of Singapore. Int J Rheum Dis. (2019) 22:1506–11. doi: 10.1111/1756-185X.13603
4. Calin A, Edmunds L, Kennedy LG. Fatigue in ankylosing spondylitis–why is it ignored? J Rheumatol. (1993) 20:991–5.
5. Gossec L, Dougados M, D'Agostino MA, Fautrel B. Fatigue in early axial spondyloarthritis. Results from the French DESIR cohort. Joint Bone Spine. (2016) 83:427–31. doi: 10.1016/j.jbspin.2015.07.012
6. Bedaiwi M, Sari I, Thavaneswaran A, Ayearst R, Haroon N, Inman RD. Fatigue in ankylosing spondylitis and nonradiographic axial spondyloarthritis: analysis from a longitudinal observation cohort. J Rheumatol. (2015) 42:2354–60. doi: 10.3899/jrheum.150463
7. Da Costa D, Dritsa M, Ring A, Fitzcharles MA. Mental health status and leisure-time physical activity contribute to fatigue intensity in patients with spondylarthropathy. Arthritis Rheum. (2004) 51:1004–8. doi: 10.1002/art.20841
8. Dagfinrud H, Vollestad NK, Loge JH, Kvien TK, Mengshoel AM. Fatigue in patients with ankylosing spondylitis: a comparison with the general population and associations with clinical and self-reported measures. Arthritis Rheum. (2005) 53:5–11. doi: 10.1002/art.20910
9. Connolly D, Fitzpatrick C, O'Shea F. Disease activity, occupational participation, and quality of life for individuals with and without severe fatigue in ankylosing spondylitis. Occup Ther Int. (2019) 2019:3027280. doi: 10.1155/2019/3027280
10. Aissaoui N, Rostom S, Hakkou J, Berrada Ghziouel K, Bahiri R, Abouqal R, et al. Fatigue in patients with ankylosing spondylitis: prevalence and relationships with disease-specific variables, psychological status, and sleep disturbance. Rheumatol Int. (2012) 32:2117–24. doi: 10.1007/s00296-011-1928-5
11. Sieper J, Rudwaleit M, Baraliakos X, Brandt J, Braun J, Burgos-Vargas R, et al. The Assessment of Spondylo Arthritis International Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann Rheum Dis. (2009) 68(Suppl. 2):ii1–44. doi: 10.1136/ard.2008.104018
12. Davies H, Brophy S, Dennis M, Cooksey R, Irvine E, Siebert S. Patient perspectives of managing fatigue in ankylosing spondylitis, and views on potential interventions: a qualitative study. BMC Musculoskelet Disord. (2013) 14:163. doi: 10.1186/1471-2474-14-163
13. Farren W, Goodacre L, Stigant M. Fatigue in ankylosing spondylitis: causes, consequences and self-management. Musculoskeletal Care. (2013) 11:39–50. doi: 10.1002/msc.1029
14. van Tubergen A, Coenen J, Landewe R, Spoorenberg A, Chorus A, Boonen A, et al. Assessment of fatigue in patients with ankylosing spondylitis: a psychometric analysis. Arthritis Rheum. (2002) 47:8–16. doi: 10.1002/art1.10179
15. Schneeberger EE, Marengo MF, Dal Pra F, Maldonado Cocco JA, Citera G. Fatigue assessment and its impact in the quality of life of patients with ankylosing spondylitis. Clin Rheumatol. (2015) 34:497–501. doi: 10.1007/s10067-014-2682-3
16. Espahbodi S, Bassett P, Cavill C, Freeth M, Hole J, Sengupta R. Fatigue contributes to work productivity impairment in patients with axial spondyloarthritis: a cross-sectional UK study. Clin Exp Rheumatol. (2017) 35:571–8.
17. Zhou W, Guo J, He M, Li J, Chen Y, Liu J, et al. Fatigue and contributing factors in Chinese patients with ankylosing spondylitis. Clin Rheumatol. (2020) 39:2337–44. doi: 10.1007/s10067-020-04976-x
18. Benegas M, Munoz-Gomariz E, Font P, Burgos-Vargas R, Chaves J, Palleiro D, et al. Comparison of the clinical expression of patients with ankylosing spondylitis from Europe and Latin America. J Rheumatol. (2012) 39:2315–20. doi: 10.3899/jrheum.110687
19. Jamalyaria F, Ward MM, Assassi S, Learch TJ, Lee M, Gensler LS, et al. Ethnicity and disease severity in ankylosing spondylitis a cross-sectional analysis of three ethnic groups. Clin Rheumatol. (2017) 36:2359–64. doi: 10.1007/s10067-017-3767-6
20. Singh DK, Magrey MN. Racial differences in clinical features and comorbidities in ankylosing spondylitis in the United States. J Rheumatol. (2019) 47:835–38. doi: 10.3899/jrheum.181019
21. Skare TL, Bortoluzzo AB, Goncalves CR, Braga da Silva JA, Ximenes AC, Bertolo MB, et al. Ethnic influence in clinical and functional measures of Brazilian patients with spondyloarthritis. J Rheumatol. (2012) 39:141–7. doi: 10.3899/jrheum.110372
22. Lau CS, Burgos-Vargas R, Louthrenoo W, Mok MY, Wordsworth P, Zeng QY. Features of spondyloarthritis around the world. Rheum Dis Clin North Am. (1998) 24:753–70. doi: 10.1016/S0889-857X(05)70040-5
24. Lee YX, Kwan YH, Png WY, Lim KK, Tan CS, Lui NL, et al. Association of obesity with patient-reported outcomes in patients with axial spondyloarthritis: a cross-sectional study in an urban Asian population. Clin Rheumatol. (2017) 36:2365–70. doi: 10.1007/s10067-017-3585-x
25. Department of Statistics, Ministry of Trade and Industry, Republic of Singapore. Singapore Census of Population 2010, Statistical Release 1: Demographic Characteristics, Education, Language and Religion. (2010). Available online at: https://www.singstat.gov.sg/publications/cop2010/census10_stat_release1 (accessed May 8, 2020).
26. Department of Statistics Ministry of Trade and Industry Republic of Singapore. Singapore Census of Population 2010, Statistical Release 2: Households and Housing. (2010). Available online at: https://www.singstat.gov.sg/publications/cop2010/census10_stat_release2 (accessed May 8, 2020).
27. Ng CW, Tan WS, Gunapal PP, Wong LY, Heng BH. Association of socioeconomic status (SES) and social support with depressive symptoms among the elderly in Singapore. Ann Acad Med Singapore. (2014) 43:576–87.
28. Maksymowych WP, Mallon C, Morrow S, Shojania K, Olszynski WP, Wong RL, et al. Development and validation of the Spondyloarthritis Research Consortium of Canada (SPARCC) enthesitis index. Ann Rheum Dis. (2009) 68:948–53. doi: 10.1136/ard.2007.084244
29. Jones SD, Porter J, Garrett SL, Kennedy LG, Whitelock H, Calin A. A new scoring system for the Bath Ankylosing Spondylitis Metrology Index (BASMI). J Rheumatol. (1995) 22:1609.
30. Kwan YH, Tan JJ, Phang JK, Fong W, Lim KK, Koh HL, et al. Validity and reliability of the Ankylosing Spondylitis Disease Activity Score with C-reactive protein (ASDAS-CRP) and Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) in patients with axial spondyloarthritis (axSpA) in Singapore. Int J Rheum Dis. (2019) 22:2206–12. doi: 10.1111/1756-185X.13735
31. Dernis-Labous E, Messow M, Dougados M. Assessment of fatigue in the management of patients with ankylosing spondylitis. Rheumatology. (2003) 42:1523–8. doi: 10.1093/rheumatology/keg421
32. Li T, Zhou L, Zhao H, Song J, Wang X, Liu S, et al. Fatigue in ankylosing spondylitis is associated with psychological factors and brain gray matter. Front Med. (2019) 6:271. doi: 10.3389/fmed.2019.00271
33. Kwan YH, Fong WW, Lui NL, Yong ST, Cheung YB, Malhotra R, et al. Validity and reliability of the Short Form 36 Health Surveys (SF-36) among patients with spondyloarthritis in Singapore. Rheumatol Int. (2016) 36:1759–65. doi: 10.1007/s00296-016-3567-3
35. Neuberger GB. Measures of fatigue: The fatigue questionnaire, fatigue severity scale, multidimensional assessment of fatigue scale, and Short Form-36 vitality (energy/fatigue) subscale of the short form health survey. Arthritis Care Res. (2003) 49:S175–83. doi: 10.1002/art.11405
36. Thumboo J, Wu Y, Tai ES, Gandek B, Lee J, Ma S, et al. Reliability and validity of the English (Singapore) and Chinese (Singapore) versions of the Short-Form 36 version 2 in a multi-ethnic urban Asian population in Singapore. Qual Life Res. (2013) 22:2501–8. doi: 10.1007/s11136-013-0381-1
37. Jones SD, Steiner A, Garrett SL, Calin A. The Bath Ankylosing Spondylitis Patient Global Score (BAS-G). Br J Rheumatol. (1996) 35:66–71. doi: 10.1093/rheumatology/35.1.66
38. Calin A, Garrett S, Whitelock H, Kennedy LG, O'Hea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol. (1994) 21:2281–5.
39. Doward LC, Spoorenberg A, Cook SA, Whalley D, Helliwell PS, Kay LJ, et al. Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Ann Rheum Dis. (2003) 62:20–6. doi: 10.1136/ard.62.1.20
40. Leung YY, Lee W, Lui NL, Rouse M, McKenna SP, Thumboo J. Adaptation of Chinese and English versions of the Ankylosing Spondylitis quality of life (ASQoL) scale for use in Singapore. BMC Musculoskelet Disord. (2017) 18:353. doi: 10.1186/s12891-017-1715-x
41. Zepa J, Bulina I, Lavrentjevs V, Vinkalna I, Nikitina-Zake L, Andersone D, et al. The impact of body mass index on disease progression in ankylosing spondylitis. Proc Latvian Acad Sci Sect B Nat Exact Appl Sci. (2018) 72:23–8. doi: 10.1515/prolas-2018-0002
43. Elmamoun M, Leung YY, O'Sullivan D, Steinkoenig I, Chandran V, Gladman DD, et al. Using Acute-phase reactants to inform the development of instruments for the updated psoriatic arthritis core outcome measurement set. J Rheumatol. (2019) 46:266–73. doi: 10.3899/jrheum.180195
44. Druce KL, Basu N. Predictors of fatigue in rheumatoid arthritis. Rheumatology. (2019) 58(Suppl. 5):v29–34. doi: 10.1093/rheumatology/kez346
45. Gremese E, Bernardi S, Bonazza S, Nowik M, Peluso G, Massara A, et al. Body weight, gender and response to TNF-alpha blockers in axial spondyloarthritis. Rheumatology. (2014) 53:875–81. doi: 10.1093/rheumatology/ket433
46. Fitzgerald G, Gallagher P, O'Shea FD. Multimorbidity in axial spondyloarthropathy and its association with disease outcomes: results from the ankylosing spondylitis registry of ireland cohort. J Rheumatol. (2020) 47:218–26. doi: 10.3899/jrheum.181415
47. Chan CQH, Lee KH, Low LL. A systematic review of health status, health seeking behaviour and healthcare utilisation of low socioeconomic status populations in urban Singapore. Int J Equity Health. (2018) 17:39. doi: 10.1186/s12939-018-0751-y
48. Blane D. Social determinants of health–socioeconomic status, social class, and ethnicity. Am J Public Health. (1995) 85:903–5. doi: 10.2105/AJPH.85.7.903
49. Gensler L, Haroon N, Reveille J, Learch T, Brown M, Weisman M, et al. FRI0468 socioeconomic status predicts radiographic progression in ankylosing spondylitis. Ann Rheumat Dis. (2014) 72:A533. doi: 10.1136/annrheumdis-2013-eular.1595
Keywords: fatigue, spondyloarthritis, disease activity, disease impact, ethnic
Citation: Lim WZ, Fong W, Kwan YH and Leung YY (2021) Exploring the Prevalence and Factors Associated With Fatigue in Axial Spondyloarthritis in an Asian Cohort in Singapore. Front. Med. 8:603941. doi: 10.3389/fmed.2021.603941
Received: 08 September 2020; Accepted: 14 January 2021;
Published: 04 February 2021.
Edited by:
Xenofon Baraliakos, Rheumazentrum Ruhrgebiet, GermanyReviewed by:
Jose Inciarte-Mundo, Hospital Clínic de Barcelona, SpainHuangHsi Chen, Chung Shan Medical University Hospital, Taiwan
Copyright © 2021 Lim, Fong, Kwan and Leung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Ying Leung, a2F0eWNjY0Bob3RtYWlsLmNvbQ==
 Wei Ze Lim1,2
Wei Ze Lim1,2 Warren Fong
Warren Fong Ying Ying Leung
Ying Ying Leung