- 1Hunan Key Laboratory of Skin Cancer and Psoriasis, Department of Dermatology, Hunan Engineering Research Center of Skin Health and Disease, Xiangya Hospital, Central South University, Changsha, China
- 2National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
- 3Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 4Department of Colorectal Surgical Oncology, Harbin Medical University Cancer Hospital, Harbin, China
- 5Department of Oncology, Xiangya Hospital, Central South University, Changsha, China
It is not clear whether D-dimer can be an independent predictor of coronavirus disease 2019 (COVID-19) mortality, and the cut-off of D-dimer for clinical use remains to be determined. Therefore, a comprehensive analysis is still necessary to illuminate the clinical significance of plasma D-dimer in COVID-19 mortality. We searched PubMed, Embase, Cochrane Library, and Scopus databases until November 2020. STATA software was used for all the statistical analyses. The identifier of systematic review registration was PROSPERO CRD42020220927. A total of 66 studies involving 40,614 COVID-19 patients were included in our meta-analysis. Pooled data showed that patients in high D-dimer group had poor prognosis than those in low D-dimer group [OR = 4.52, 95% CI = (3.61, 5.67), P < 0.001; HR = 2.81, 95% CI = (1.85, 4.27), P < 0.001]. Sensitivity analysis, pooled data based on different effect models and the Duval and Tweedie trim-and-fill method did not change the conclusions. Subgroup analyses stratified by different countries, cutoffs, sample size, study design, and analysis of OR/HR still keep consistent conclusions. D-dimer was identified as an independent predictor for COVID-19 mortality. A series of values including 0.5 μg/ml, 1 μg/ml, and 2 μg/ml could be determined as cutoff of D-dimer for clinic use. Measurement and monitoring of D-dimer might assist clinicians to take immediate medical actions and predict the prognosis of COVID-19.
Introduction
The outbreak and spread of Coronavirus Disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), had caused a pandemic around the world (1). Though most of patients had mild symptoms, a small minority of cases suffered from acute respiratory distress syndrome (ARDS) and even death (2). As of November 28, 2020, about 60 million cases have been reported by world health organization (WHO) and included around 1.5 million deaths globally (1). Worse still, the numbers of death are persistently increasing especially in the United States, the epicenter of COVID-19 (3). Therefore, identification of the independent predictors for COVID-19 mortality is still urgent and necessary to reduce the poor outcomes.
D-dimer, a fibrinogen degradation product, consists of two covalently bound fibrin D domains, which reflect the high coagulation and enhancement of secondary fibrinolytic activity in vivo (4, 5). Previous studies demonstrated that D-dimer was associated with the severity of COVID-19 (6–8). Hyperinflammation and hypoxia-induced injury caused by SARS-CoV-2 infection could cause the dysfunction of endothelial cells and stimulate thrombosis and elevation of D-dimer (9). Elevated D-dimer could cause the formation of pulmonary microthrombus, deep venous thrombosis, and disseminated intravascular coagulopathy, which were associated with the poor prognosis (10–12). Nowadays, increasing studies showed that D-dimer could be used as a predictor for COVID-19 mortality (9, 13). Moreover, numerous review and meta-analyses highlighted the prognostic value of D-dimer in COVID-19 mortality (14–16). However, one of the drawbacks of these analyses was that more attention was paid to D-dimer levels between survivors and non-survivors (17, 18). Actually, the abnormal elevation of D-dimer was more valuable to reflect hemodynamic changes in clinic. In addition, these meta-analyses were based primarily on the studies using univariate analysis, and it was not clear whether D-dimers play an independent role in predicting COVID-19 mortality on admission (14, 19). Another challenge is that the cutoff of D-dimer for clinical use remains to be determined (8). From the above, a comprehensive analysis of all the published studies is still necessary to illuminate the clinical significance of plasma D-dimer in COVID-19 mortality.
To our knowledge, this is the largest meta-analysis about the association between D-dimer with COVID-19 mortality. Our study will determine its cutoff and highlight the independent prognostic value of D-dimer in COVID-19 mortality to assist clinicians to take immediate medical actions and evaluate the prognosis of COVID-19.
Materials and Methods
Search Strategy
Our meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The following databases were searched: PubMed, Embase, Cochrane Library, and Scopus databases, from their inception to November 2020. No language restrictions were applied. The search terms were as follows: (“Coronavirus disease 2019” OR “Coronavirus 2019” OR “COVID-19” OR “COVID19” OR “Severe acute respiratory syndrome coronavirus 2” OR “SARS-CoV-2” OR “nCoV-2019” OR “2019-nCoV” OR “Novel coronavirus”) AND (“Mortality” OR “Death” OR “Dead” OR “Fatality” OR “Non-survival” OR “Non-survivors” OR “Non-survivor” OR “Prognosis” OR “Deceased”) AND (“D-dimer” OR “Laboratory”). Three of the authors (GD, FZ, and YL) independently screened initial records, titles, abstracts, and full text articles. Disagreements were resolved by discussion. In order to avoid missing relevant articles, we also manually reviewed the reference lists of selected retrieved papers as well as the major reviews and meta-analyses. The identifier of systematic review registration was PROSPERO CRD42020220927.
Inclusion and Exclusion Criteria
Any study reporting the relationship between D-dimer and COVID-19 mortality should be included if they met the following criteria: (1) patients were diagnosed as COVID-19; (2) dichotomous D-dimer was available to evaluate the risk of COVID-19 mortality; or (3) odds ratio (OR) or hazard ratio (HR) of the D-dimer was accessible or estimated by the provided data or Kaplan-Meier curves based on the method previously described (20, 21). Exclusion criteria were as follows: (1) patients were asymptomatic carriers of SARS-CoV-2; (2) studies with smaller sample size from the same authors or institutions; and (3) patients or studies did not fulfill the inclusion criteria.
Data Extraction and Quality Assessment
We used Endnote X9 to exclude any duplicate and irrelevant studies in our initial search. We extracted the following basic information: first authors, publication date, country of origin, study design, cases, age, sex, cutoff of D-dimer, OR, HR, and its associated 95% confidence intervals (CI). OR and HR were extracted preferentially from multivariable analysis based on lower cutoff of D-dimer. Stratified data or interquartile range such as age were converted to mean (standard deviation) based on the mathematical formulas for meta-analysis (22, 23). We used Newcastle-Ottawa Scale (NOS) for quality assessments. Two authors (GD and FZ) independently selected and evaluated the included articles. When a consensus was lacking, a third reviewer (LY) was consulted to solve the disagreements.
Statistical Analysis
STATA (Version 12.0; STATA Corporation, College Station, TX, USA) and TSA (Copenhagen trial unit) software were used for all the statistical analyses. OR with 95% CI was calculated for binary outcomes, and HR for time-to-event outcomes (24). Random-effect and fixed-effect models were both adopted in all analyses to assess the stability of results. Additionally, sensitivity analyses were performed by omitting one study each time; meta-regression and subgroup analyses were conducted based on different countries, cutoffs, sample size, study design, and analysis of OR/HR to further evaluate the consistency of our conclusions. The funnel plot and Egger test was used to evaluate publication bias, and the Duval and Tweedie trim-and-fill method was performed to adjust for this bias (25). Trial sequential analysis was used to eliminate early false positive findings. P < 0.05 was considered statistically significant.
Results
Literature Search and Studies Characteristics
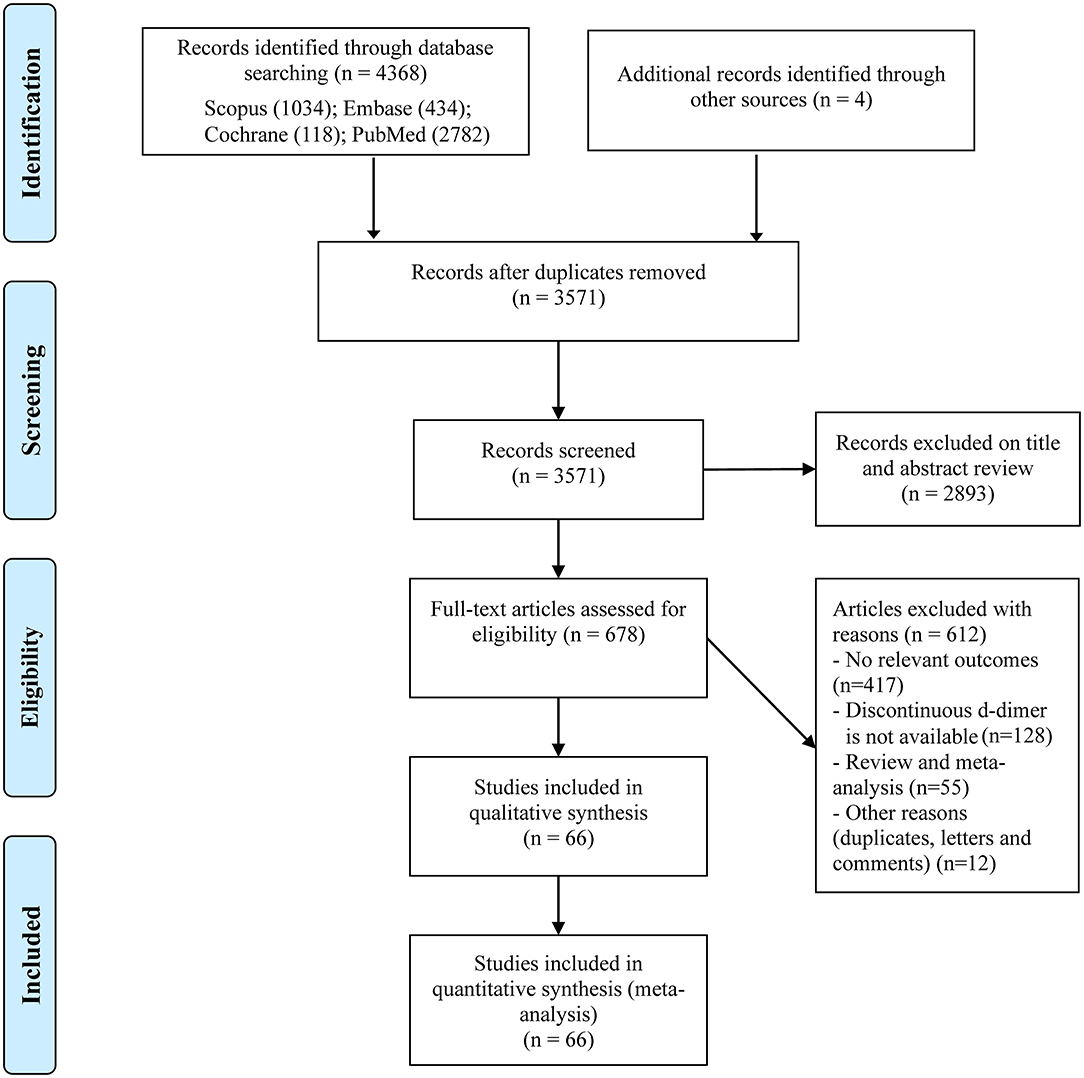
We initially identified 4,372 records through our search strategy and scanning the reference lists of related meta-analyses (Figure 1); 3,571 studies remained after excluding duplicates. Then we reviewed the titles and abstracts and obtained 678 studies for full-text scanning. We further excluded 612 studies due to studies without our concerned outcomes (n = 371), studies without dichotomous D-dimer (n = 128), review and meta-analyses (n = 55) and other reasons including duplicates, letters, and comments (n = 12). Finally, a total of 66 studies involving 40,614 COVID-19 patients were included in our meta-analysis (9, 13, 26–89).
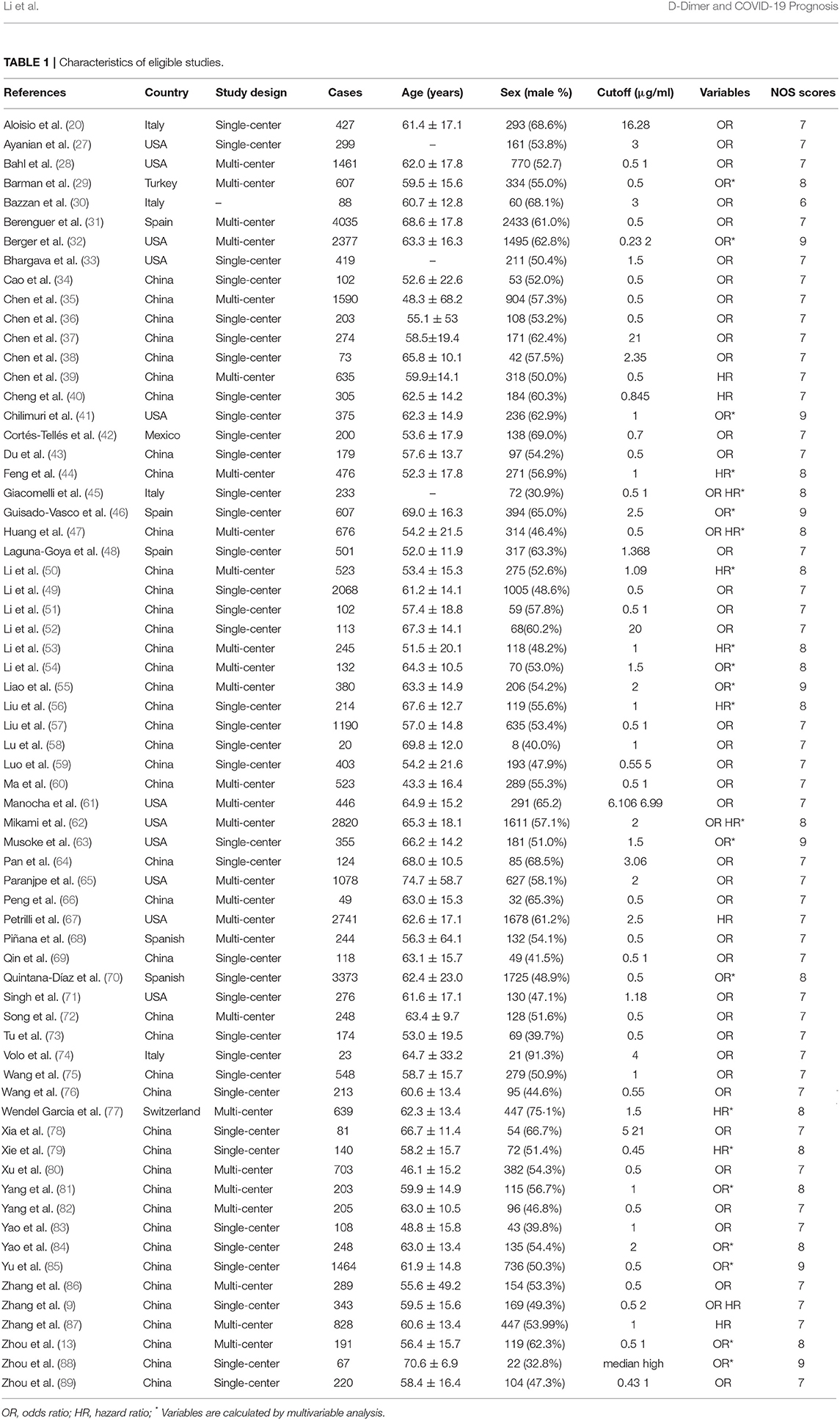
The main characteristics of eligible studies are shown in Table 1. All these 66 studies were published in 2020 and from different countries including China, the United States, Italy, Turkey, Spain, Mexico, and Switzerland. In these studies, 65 studies were written in English, and one in Chinese, and 22 studies had sample size above 500 patients. What's more, 56 studies reported OR and 15 reported HR of D-dimer. Except one study, all studies of high quality had seven or more NOS scores, and details are shown in Supplementary Table 1.
Association of D-Dimer and COVID-19 Mortality
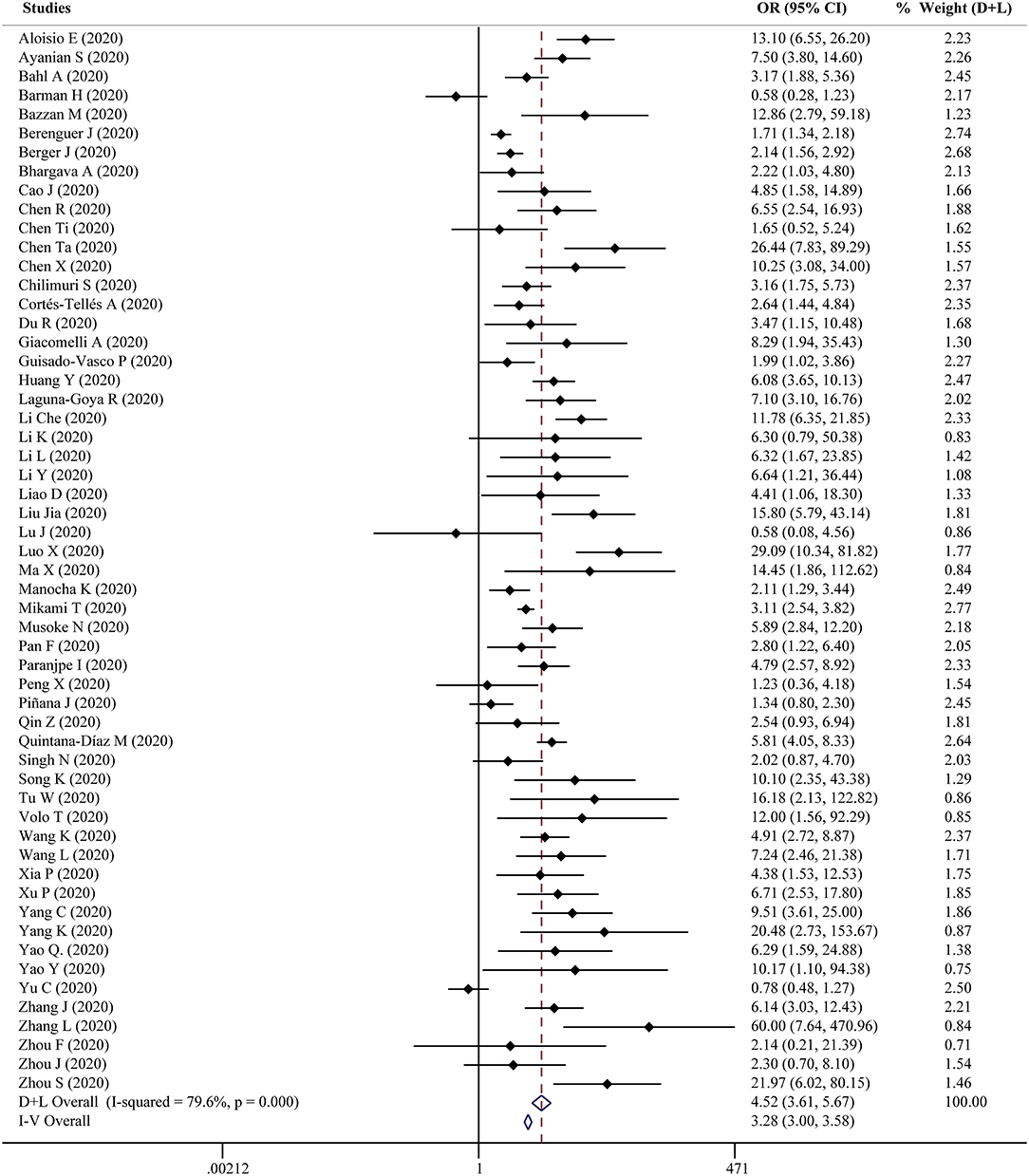
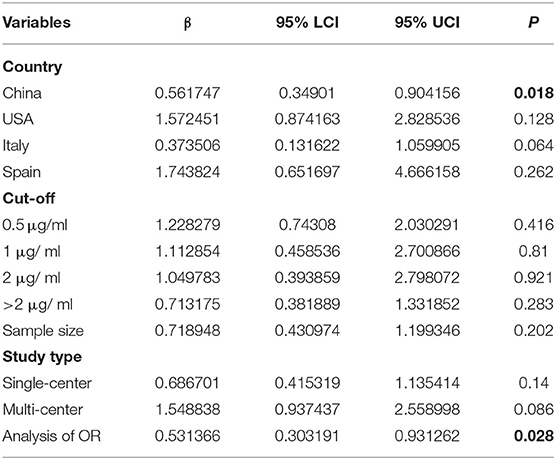
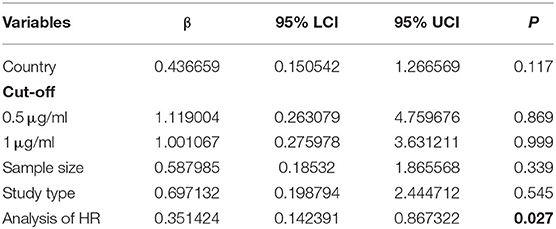
Fifty-six studies reported the proportion of non-survivors between high and low D-dimer groups. With heterogeneity (I2 = 79.6%, P < 0.001), the random-effect model was performed and suggested that patients in the high D-dimer group had higher proportion of mortality than those in the low D-dimer group [OR = 4.52, 95% CI = (3.61, 5.67), P < 0.001]. The conclusion did not change when using the fixed-effect model for meta-analysis [OR = 3.28, 95% CI = (3.00, 3.58), P < 0.001] (Figure 2). Sensitivity analysis did not change the conclusion (Supplementary Figure 1). The funnel plot was not in a form of symmetry, indicating the existence of potential publication bias (Supplementary Figure 2A). Then we used Egger test to detect the presence of publication bias (P < 0.001) (Supplementary Figure 2B). However, the conclusion did not change in fixed-effect model [OR = 2.92, 95% CI = (2.68, 3.18), P < 0.001] or random-effect model [OR = 3.33, 95% CI = (2.66, 4.16), P < 0.001] after filling 15 studies in the comparison. To examine whether the observed heterogeneity could be contributed by possible moderators, univariate meta-regression was performed and suggested that country and analysis of OR were possible significant moderators (Table 2). To further assess the stability of the conclusion, we conducted the subgroup analysis stratified by different countries, cutoffs, sample size, study design, and analysis of OR. The conclusion did not change, highlighting the independent prognostic value of D-dimer and that the cutoff of D-dimer could be determined as a series of values including 0.5 μg/ml, 1 μg/ ml, and 2 μg/ ml (Figure 3).
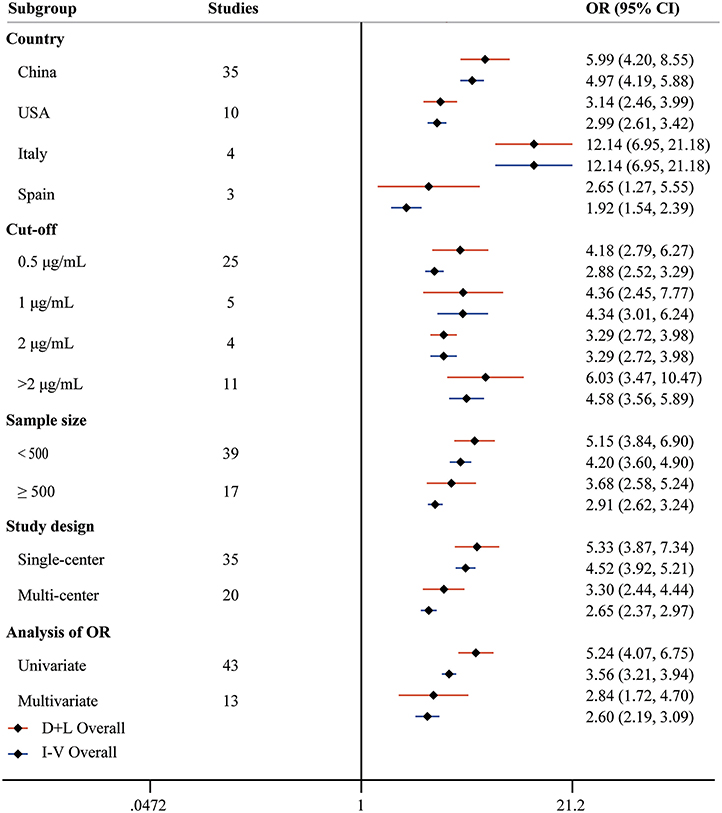
Figure 3. Forest plot to assess OR of COVID-19 mortality for D-dimer stratified by different countries, cutoffs, sample size, study design, and analysis of OR.
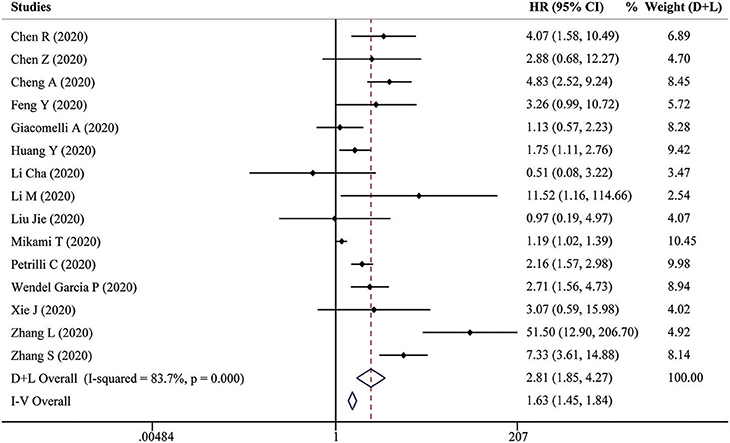
Fifteen studies reported HRs of high D-dimer vs. low D-dimer. Due to the heterogeneity among studies (I2 = 83.7%, P < 0.001), the random-effect model was used, and pooled data showed that patients in the high D-dimer group were significantly associated with poor overall survival [HR = 2.81, 95% CI = (1.85, 4.27), P < 0.001]. This result was consistent when using the fixed-effect model to analyze the pooled data [HR = 1.63, 95% CI = (1.45, 1.84), P < 0.001] (Figure 4). We further performed a sensitivity analysis through excluding any one specific study each time. We did not observe obvious decline of heterogeneity, and the conclusion was consistent (Supplementary Figure 3A). The funnel plot identified four studies over the pseudo 95% CI (Supplementary Figure 3B), and the Egger test detected the presence of publication bias (P = 0.013) (Supplementary Figure 3C). Then the Duval and Tweedie trim-and-fill method was adopted, but no studies were trimmed and filled. To explore the origin of heterogeneity, we performed the univariate meta-regression and found that analysis of HR was possible significant moderator (Table 3). Subgroup analysis based on different countries, cutoffs, sample size, study design, and analysis of HR did not change the conclusion, which means D-dimer is an independent indicator for COVID-19 mortality, and the cutoff of D-dimer (0.5μg/ ml or 1μg/ ml) could be used clinically (Figure 5).
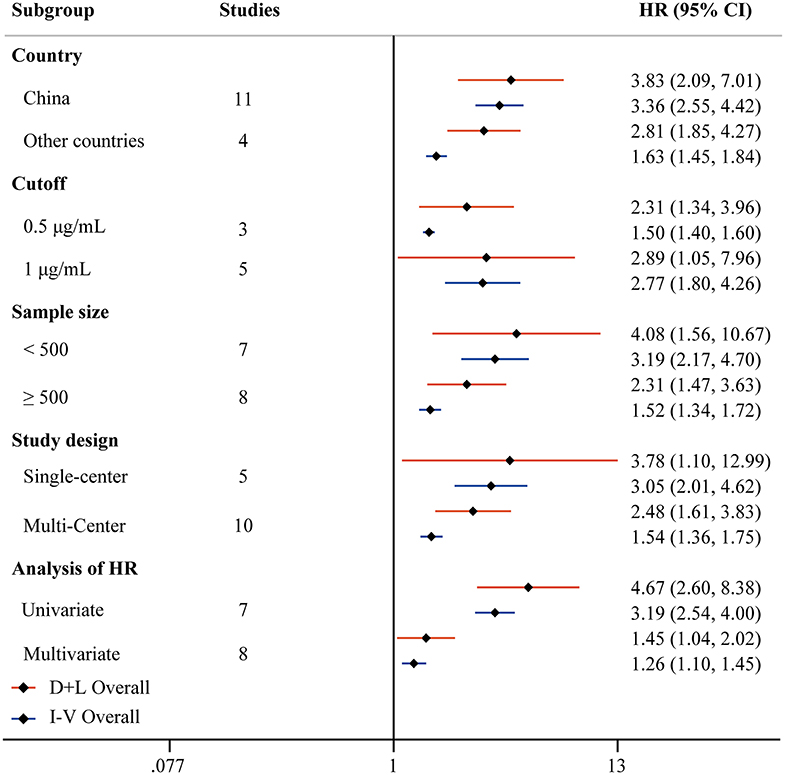
Figure 5. Forest plot to assess HR of COVID-19 mortality for D-dimer stratified by different countries, cutoffs, sample size, study design, and analysis of HR.
Trial Sequential Analysis
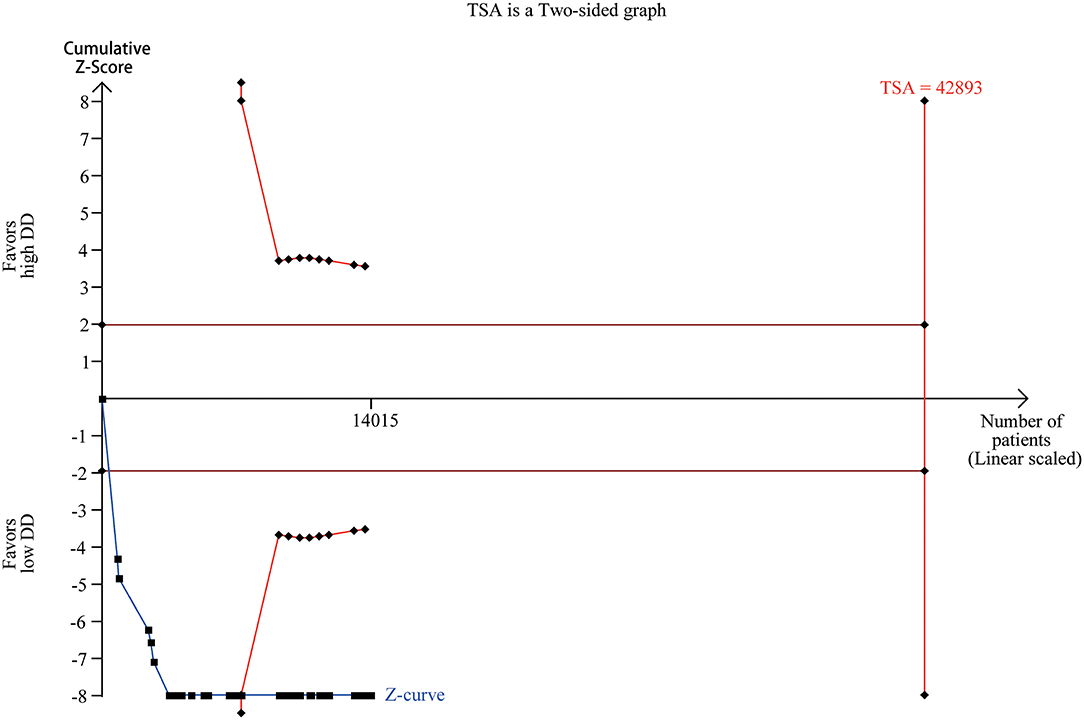
Trial sequential analysis has been widely used to improve the reliability of conclusion and eliminate early false positive findings due to imprecision and repeated significance testing in meta-analyses (90). We collected the numbers of death and total numbers of patients in the high and low D-dimer group from 37 studies (Supplementary Table 2). Trial sequential analysis on data for death supported a 20% risk ratio reduction in the low D-dimer group compared with high D-dimer group. The required information size of 42,893 was calculated based on a control event proportion of 11.5% (based on data in our meta-analysis), a risk of type I error of 5%, a power of 80%, and a diversity of 87.16%. Although the actual information size did not reach the required information size, the cumulated Z-curve (blue curve) crossed the traditional boundary of 5% significance (horizontal red line) and the trial sequential monitoring boundary (red curve), implying that firm evidence was reached (Figure 6).
Discussion
The ongoing spread of COVID-19 is posing a huge threat to global public health. Nowadays, the numbers of deaths caused by COVID-19 are still increasing while there is still no effective medication (64). Thus, it's imperative to identify the predictors for COVID-19 mortality. With regard to the role of plasma D-dimer in COVID-19 mortality, studies have reported associations that vary in strength and direction. Therefore, a comprehensive meta-analysis is necessary to illuminate the clinical significance of plasma D-dimer in COVID-19 mortality.
In this meta-analysis, a total of 66 studies involving 40,614 COVID-19 patients were enrolled. We found that patients in high D-dimer group had a poorer prognosis than those in low D-dimer group, independent of countries, cutoffs, sample size, study design, and analysis of OR/HR. Sensitivity analysis and pooled data based on different effect models were used to explore the consistency of our conclusions, and the conclusions were still consistent. Additionally, even though there exist publication bias in the combined outcomes of high D-dimer vs. low D-dimer, the conclusion still did not change after the Duval and Tweedie trim-and-fill method. Trial sequential analysis further confirmed our conclusions. Based on the above findings, we could conclude that D-dimer was an independent predictor for COVID-19 mortality. Furthermore, subgroup analyses based on cutoffs of dimer highlighted that a series of values including 0.5 μg/ ml, 1 μg/ ml, and 2 μg/ ml could be determined as the cutoff of D-dimer for clinic use.
D-dimer is one of the commonest laboratory findings for COVID-19 patients. As early as February 2019, Guan et al. reported that severe patients had a significantly higher level of D-dimer than non-severe patients through analyzing 1,099 patients with laboratory-confirmed COVID-19 from over 550 hospitals in China (91). Moreover, Zhou and his colleague conducted a retrospective study involving 191 COVID-19 patients and found that elevated D-dimer at admission was a risk factor for death of adult patients (13). However, this conclusion was not consistent in other studies. Xie et al. found that D-dimer is not a risk factor after adjustment of age and gender through analyzing 140 COVID-19 patients (79). Besides, Liu and his team even did not find the difference in the unadjusted association between D-dimer and all-cause death in COVID-19 patients (35). Therefore, our findings are necessary to solve the problem and highlight the clinical significance of plasma D-dimer in COVID-19 mortality.
The mechanism is still unknown about the association between elevated D-dimer with COVID-19 mortality. Wang et al. previously showed that the significantly increased D-dimer and corresponding hypoxemia could induce the formation of pulmonary microthrombus in the 2009 novel influenza A(H1N1) (10). A recent study conducted by Klok and his colleague demonstrated that approximately 31% COVID-19 patients in intensive care unit had the thrombotic complications (11). Moreover, D-dimer could be used to indicate deep venous thrombosis in COVID-19 patients with cardiovascular diseases (92). That means elevated level of D-dimer, the indicator of thrombotic complications, might be the cause of COVID-19 mortality. However, other studies held different opinions that COVID-19 progress is the cause of the increase of D-dimer level. One possible mechanism is that SARS-CoV-2 infections are usually accompanied by an aggressive inflammatory response and even cytokine storm. The hyperinflammation could induce the dysfunction and damage of endothelial cells, resulting in the elevation of D-dimer and excess thrombin generation (93). Additionally, organ damage and corresponding hypoxemia caused by SARS-CoV-2 infection could stimulate thrombosis through increasing blood viscosity and activating hypoxia-inducible transcription factor-dependent signaling pathways (94, 95). Recently, Turagam et al. found that mortality is mostly associated with pulseless electrical activity. Whether D-dimer-associated thrombosis could cause pulseless electrical activity and ultimately mortality needs to be clarified (96). Overall, The underlying mechanism is unsolved about the relationship between elevated D-dimer and COVID-19 mortality. Our finding highlights the association, and more studies are needed to dig out the detailed mechanism.
To our knowledge, this is the largest meta-analysis to evaluate the clinical significance of plasma D-dimer in COVID-19 mortality. However, several limitations must be acknowledged. First, noticeable heterogeneity exists in all of the analyses. Sensitivity analysis pooled the data based on different effect models, yet the heterogeneity could not be eliminated completely. Second, publication bias exists in all the comparisons, though the conclusion did not change through the Duval and Tweedie trim-and-fill method. Finally, our study could not clarify the underlying mechanism between D-dimer with COVID-19 mortality.
In conclusion, D-dimer was identified as an independent predictor for COVID-19 mortality. A series of values including 0.5 μg/ ml, 1 μg/ ml, and 2 μg/ ml could be determined as cutoff of D-dimer for clinic use. Measurement and monitoring of D-dimer might assist clinicians to take immediate medical actions and predict the prognosis of COVID-19.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
YL and YD: data curation, formal analysis, methodology, roles/writing-original draft. LY: conceptualization, data curation, formal analysis, methodology, roles/writing-original draft. HS: formal analysis, methodology, roles/writing-revised draft. SD and HH: methodology, software, validation. FZ, GD, and XC: conceptualization, data curation, formal analysis, software, supervision, validation, visualization, roles/writing—original draft, writing—review & editing. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the National Natural Science Foundation of China (62041208), the fellowship of China postdoctoral Science Foundation (No. 2020M682594), and The Youth Science Foundation of Xiangya Hospital (2020Q10).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.638097/full#supplementary-material
Supplementary Figure 1. Sensitivity analyses of OR of COVID-19 mortality for D-dimer.
Supplementary Figure 2. Funnel plot (A) and Egger test (B) of OR of COVID-19 mortality for D-dimer.
Supplementary Figure 3. Sensitivity analyses (A), funnel plot (B) and Egger test (C) of HR of COVID-19 mortality for D-dimer.
Supplementary Table 1. Methodological quality of studies included in the meta-analysis.
Supplementary Table 2. Raw data for all the analyses in the study.
References
2. Xu B, Fan CY, Wang AL, Zou YL, Yu YH, He C, et al. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J Infect. (2020) 81:e51–60. doi: 10.1016/j.jinf.2020.04.012
3. Gross SA, Robbins DH, Greenwald DA, Schnoll-Sussman FH, Pochapin MB. Preparation in the big apple: New York City, a new epicenter of the COVID-19 pandemic. Am J Gastroenterol. (2020) 115:801–4. doi: 10.14309/ajg.0000000000000636
4. Fang P, Du L, Cai D. Evaluation of plasma D-dimer for the diagnosis in Chinese patients with hepatocellular carcinoma: a meta-analysis. Medicine. (2020) 99:e19461. doi: 10.1097/MD.0000000000019461
5. Favaloro EJ, Thachil J. Reporting of D-dimer data in COVID-19: some confusion and potential for misinformation. Clin Chem Lab Med. (2020) 58:1191–9. doi: 10.1515/cclm-2020-0573
6. Lippi G, Favaloro EJ. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. (2020) 120:876–8. doi: 10.1055/s-0040-1709650
7. Wicki J, Perneger TV, Junod AF, Bounameaux H, Perrier A. Assessing clinical probability of pulmonary embolism in the emergency ward: a simple score. Arch Intern Med. (2001) 161:92–7. doi: 10.1001/archinte.161.1.92
8. Gungor B, Atici A, Baycan OF, Alici G, Ozturk F, Tugrul S. Elevated D-dimer levels on admission are associated with severity and increased risk of mortality in COVID-19: a systematic review and meta-analysis. Am J Emerg Med. (2020) 39:173–9. doi: 10.1016/j.ajem.2020.09.018
9. Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. (2020) 18:1324–9. doi: 10.1111/jth.14859
10. Wang ZF, Su F, Lin XJ, Dai B, Kong LF, Zhao HW, et al. Serum D-dimer changes and prognostic implication in 2009 novel influenza A(H1N1). Thromb Res. (2011) 127:198–201. doi: 10.1016/j.thromres.2010.11.032
11. Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. (2020) 191:145–7. doi: 10.1016/j.thromres.2020.04.013
12. Townsend L, Fogarty H, Dyer A, Martin-Loeches I, Bannan C, Nadarajan P, et al. Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. J Thromb Haemost. (2021) 19:1064–70. doi: 10.1111/jth.15267
13. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
14. Shah S, Shah K, Patel SB, Patel FS, Osman M, Velagapudi P, et al. Elevated D-dimer levels are associated with increased risk of mortality in coronavirus disease 2019: a systematic review and meta-analysis. Cardiol Rev. (2020) 28:295–302. doi: 10.1097/CRD.0000000000000330
15. Zhou X, Cheng Z, Shu D, Lin W, Ming Z, Chen W, et al. Characteristics of mortal COVID-19 cases compared to the survivors. Aging. (2020) 12:24579–95. doi: 10.18632/aging.202216
16. Lima WG, Barra A, Brito JCM, Nizer WSC. D-Dimer serum levels as a biomarker associated for the lethality in patients with coronavirus disease 2019: a meta-analysis. Blood Coagul Fibrinolysis. (2020) 31:335–8. doi: 10.1097/MBC.0000000000000927
17. Shi L, Wang Y, Wang YD, Duan GC, Yang HY. D-dimer is associated with the risk of mortality in Coronavirus Disease 2019 patients. Eur Rev Med Pharmacol Sci. (2020) 24:8576–9. doi: 10.26355/eurrev_202008_22655
18. Nugroho J, Wardhana A, Maghfirah I, Mulia EPB, Rachmi DA, A'Yun M Q, et al. Relationship of D-dimer with severity and mortality in SARS-CoV-2 patients: A meta-analysis. Int J Lab Hematol. (2020) 43:110–5. doi: 10.1111/ijlh.13336
19. Simadibrata DM, Lubis AM. D-dimer levels on admission and all-cause mortality risk in COVID-19 patients: a meta-analysis. Epidemiol Infect. (2020) 148:e202. doi: 10.1017/S0950268820002022
20. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. (1998) 17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8
21. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
22. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
23. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. (2014) 14:135. doi: 10.1186/1471-2288-14-135
24. Zeng F, Chen L, Liao M, Chen B, Long J, Wu W, et al. Laparoscopic versus open gastrectomy for gastric cancer. World J Surg Oncol. (2020) 18:20. doi: 10.1186/s12957-020-1795-1
25. Zeng F, Li L, Zeng J, Deng Y, Huang H, Chen B, et al. Can we predict the severity of coronavirus disease 2019 with a routine blood test? Pol Arch Intern Med. (2020) 130:400–6. doi: 10.20452/pamw.15331
26. Aloisio E, Chibireva M, Serafini L, Pasqualetti S, Falvella FS, Dolci A, et al. A comprehensive appraisal of laboratory biochemistry tests as major predictors of COVID-19 severity. Arch Pathol Lab Med. (2020) 144:1457–64. doi: 10.5858/arpa.2020-0389-SA
27. Ayanian S, Reyes J, Lynn L, Teufel K. The association between biomarkers and clinical outcomes in novel coronavirus pneumonia in a US cohort. Biomark Med. (2020) 14:1091–7. doi: 10.2217/bmm-2020-0309
28. Bahl A, Van Baalen MN, Ortiz L, Chen NW, Todd C, Milad M, et al. Early predictors of in-hospital mortality in patients with COVID-19 in a large American cohort. Intern Emerg Med. (2020) 15:1485–99. doi: 10.1007/s11739-020-02509-7
29. Barman HA, Atici A, Sahin I, Alici G, Aktas Tekin E, Baycan ÖF, et al. Prognostic significance of cardiac injury in COVID-19 patients with and without coronary artery disease. Coron Artery Dis. (2020). doi: 10.1097/mca.0000000000000914. [Epub ahead of print].
30. Bazzan M, Montaruli B, Sciascia S, Cosseddu D, Norbiato C, Roccatello D. Low ADAMTS 13 plasma levels are predictors of mortality in COVID-19 patients. Intern Emerg Med. (2020) 15:861–3. doi: 10.1007/s11739-020-02394-0
31. Berenguer J, Ryan P, Rodríguez-Baño J, Jarrín I, Carratalà J, Pachón J, et al. Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain. Clin Microbiol Infect. (2020) 26:1525–36. doi: 10.1016/j.cmi.2020.07.024
32. Berger JS, Kunichoff D, Adhikari S, Ahuja T, Amoroso N, Aphinyanaphongs Y, et al. Prevalence and outcomes of d-dimer elevation in hospitalized patients with COVID-19. Arterioscler Thromb Vasc Biol. (2020):2539–47. doi: 10.1161/ATVBAHA.120.314872
33. Bhargava A, Sharma M, Riederer K, Fukushima EA, Szpunar SM, Saravolatz L. Risk factors for in-hospital mortality from COVID-19 infection among black patients—an urban center experience. Clin Infect Dis. (2020). doi: 10.1093/cid/ciaa1468. [Epub ahead of print].
34. Cao J, Tu WJ, Cheng W, Yu L, Liu YK, Hu X, et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. (2020) 71:748–55. doi: 10.1093/cid/ciaa243
35. Chen R, Liang W, Jiang M, Guan W, Zhan C, Wang T, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest. (2020) 158:97–105. doi: 10.1016/j.chest.2020.04.010
36. Chen T, Dai Z, Mo P, Li X, Ma Z, Song S, et al. Clinical Characteristics and outcomes of older patients with coronavirus disease 2019 (Covid-19) in Wuhan, China: a single-centered, retrospective study. J Gerontol A Biol Sci Med Sci. (2020) 75:1788–95. doi: 10.1093/gerona/glaa089
37. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. (2020) 368:m1295. doi: 10.1136/bmj.m1091
38. Chen X, Yan L, Fei Y, Zhang C. Laboratory abnormalities and risk factors associated with in-hospital death in patients with severe COVID-19. J Clin Lab Anal. (2020) 34:e23467. doi: 10.1002/jcla.23467
39. Chen Z, Zhang F, Hu W, Chen Q, Li C, Wu L, et al. Laboratory markers associated with COVID-19 progression in patients with or without comorbidity: a retrospective study. J Clin Lab Anal. (2020) 35:e23644. doi: 10.1002/jcla.23644
40. Cheng A, Hu L, Wang Y, Huang L, Zhao L, Zhang C, et al. Diagnostic performance of initial blood urea nitrogen combined with D-dimer levels for predicting in-hospital mortality in COVID-19 patients. Int J Antimicrob Agents. (2020) 56:106110. doi: 10.1016/j.ijantimicag.2020.106110
41. Chilimuri S, Sun H, Alemam A, Mantri N, Shehi E, Tejada J, et al. Predictors of mortality in adults admitted with COVID-19: Retrospective cohort study from New York City. West J Emerg Med. (2020) 21:779–84. doi: 10.5811/westjem.2020.6.47919
42. Cortés-Tellés A, López-Romero S, Mancilla-Ceballos R, Ortíz-Farías DL, Núñez-Caamal N, Figueroa-Hurtado E. Risk factors for mortality among hospitalized patients with COVID-19. An overview in Mexican population. Tuberc Respir Dis. (2020) 83(Suppl 1):S46–S54. doi: 10.4046/trd.2020.0095
43. Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. (2020) 55:2000524. doi: 10.1183/13993003.00524-2020
44. Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. (2020) 201:1380–8. doi: 10.1164/rccm.202002-0445OC
45. Giacomelli A, Ridolfo AL, Milazzo L, Oreni L, Bernacchia D, Siano M, et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: A prospective cohort study. Pharmacol Res. (2020) 158:104931. doi: 10.1016/j.phrs.2020.104931
46. Guisado-Vasco P, Valderas-Ortega S, Carralón-González MM, Roda-Santacruz A, González-Cortijo L, Sotres-Fernández G, et al. Clinical characteristics and outcomes among hospitalized adults with severe COVID-19 admitted to a tertiary medical center and receiving antiviral, antimalarials, glucocorticoids, or immunomodulation with tocilizumab or cyclosporine: a retrospective observational study (COQUIMA cohort). EClinMed. (2020) 28:100591. doi: 10.1016/j.eclinm.2020.100591
47. Huang Y, Lyu X, Li D, Wang L, Wang Y, Zou W, et al. A cohort study of 676 patients indicates D-dimer is a critical risk factor for the mortality of COVID-19. PLoS ONE.(2020) 15:e0242045. doi: 10.1371/journal.pone.0242045
48. Laguna-Goya R, Utrero-Rico A, Talayero P, Lasa-Lazaro M, Ramirez-Fernandez A, Naranjo L, et al. IL-6-based mortality risk model for hospitalized patients with COVID-19. J Allergy Clin Immunol. (2020) 146:799–807.e9. doi: 10.1016/j.jaci.2020.07.009
49. Li C, Jiang J, Wang F, Zhou N, Veronese G, Moslehi JJ, et al. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J Mol Cell Cardiol. (2020) 147:74–87. doi: 10.1016/j.yjmcc.2020.08.008
50. Li C, Ye J, Chen Q, Hu W, Wang L, Fan Y, et al. Elevated Lactate Dehydrogenase (LDH) level as an independent risk factor for the severity and mortality of COVID-19. Aging. (2020) 12:15670–81. doi: 10.18632/aging.103770
51. Li K, Li K, Chen D, Chen D, Chen S, Chen S, et al. Predictors of fatality including radiographic findings in adults with COVID-19. Respiratory Res. (2020) 21:146. doi: 10.1186/s12931-020-01411-2
52. Li L, Zhang S, He B, Chen X, Wang S, Zhao Q. Risk factors and electrocardiogram characteristics for mortality in critical inpatients with COVID-19. Clin Cardiol. (2020) 43:1624–30. doi: 10.1002/clc.23492
53. Li M, Cheng B, Zeng W, Chen S, Tu M, Wu M, et al. Analysis of the risk factors for mortality in adult covid-19 patients in Wuhan: a multicenter study. Front Med. (2020) 7:545. doi: 10.3389/fmed.2020.00545
54. Li Y, Han X, Alwalid O, Cui Y, Cao Y, Liu J, et al. Baseline characteristics and risk factors for short-term outcomes in 132 COVID-19 patients with diabetes in Wuhan China: A retrospective study. Diabetes Res Clin Pract. (2020) 166:108299. doi: 10.1016/j.diabres.2020.108299
55. Liao D, Zhou F, Luo L, Xu M, Wang H, Xia J, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. (2020) 7:e671–8. doi: 10.1016/S2352-3026(20)30217-9
56. Liu J, Liu Z, Jiang W, Wang J, Zhu M, Song J, et al. Clinical predictors of COVID-19 disease progression and death: analysis of 214 hospitalized patients from Wuhan, China. Clin Respir J. (2020) 15:293–309. doi: 10.1111/crj.13296
57. Liu J, Zhang S, Wu Z, Shang Y, Dong X, Li G, et al. Clinical outcomes of COVID-19 in Wuhan, China: a large cohort study. Ann Intensive Care. (2020) 10:99. doi: 10.1186/s13613-020-00706-3
58. Lu J, Zhang Y, Cheng G, He J, Wu F, Hu H, et al. [Clinical characteristics and outcomes of adult critically ill patients with COVID-19 in Honghu, Hubei Province]. Nan Fang Yi Ke Da Xue Xue Bao. (2020) 40:778–85. doi: 10.12122/j.issn.1673-4254.2020.06.02
59. Luo X, Xia H, Yang W, Wang B, Guo T, Xiong J, et al. Characteristics of patients with COVID-19 during epidemic ongoing outbreak in Wuhan, China. medRxiv [Preprint]. (2020). doi: 10.1101/2020.03.19.20033175
60. Ma X, Li A, Jiao M, Shi Q, An X, Feng Y, et al. Characteristic of 523 COVID-19 in Henan Province and a Death Prediction Model. Front Public Health. (2020) 8:475. doi: 10.3389/fpubh.2020.00475
61. Manocha KK, Kirzner J, Ying X, Yeo I, Peltzer B, Ang B, et al. Troponin and other biomarker levels and outcomes among patients hospitalized with COVID-19: derivation and validation of the HA2T2 COVID-19 mortality risk score. J Am Heart Assoc. (2020) 10:e018477. doi: 10.1161/JAHA.120.018477
62. Mikami T, Miyashita H, Yamada T, Harrington M, Steinberg D, Dunn A, et al. Risk factors for mortality in patients with COVID-19 in New York City. J Gen Intern Med. (2020) 36:1–10. doi: 10.1007/s11606-020-05983-z
63. Musoke N, Lo KB, Albano J, Peterson E, Bhargav R, Gul F, et al. Anticoagulation and bleeding risk in patients with COVID-19. Thromb Res. (2020) 196:227–30. doi: 10.1016/j.thromres.2020.08.035
64. Pan F, Yang L, Li Y, Liang B, Li L, Ye T, et al. Factors associated with death outcome in patients with severe coronavirus disease-19 (Covid-19): a case-control study. Int J Med Sci. (2020) 17:1281–92. doi: 10.7150/ijms.46614
65. Paranjpe I, Russak A, De Freitas JK, Lala A, Miotto R, Vaid A, et al. Clinical Characteristics of Hospitalized Covid-19 Patients in New York City. medRxiv [Preprint]. (2020). doi: 10.1101/2020.04.19.20062117
66. Peng X, Chen Y, Deng L, Liu Q, Li Q, Xiong J, et al. Clinical features of critically ill patients infected with SARS-CoV-2 outside Wuhan with and without diabetes. Int J Diabetes Dev Ctries. (2020) 40:1–9. doi: 10.1007/s13410-020-00888-3
67. Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. (2020) 369:m1966. doi: 10.1136/bmj.m1966
68. Piñana JL, Martino R, García-García I, Parody R, Morales MD, Benzo G, et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol. (2020) 9:21. doi: 10.1186/s40164-020-00177-z
69. Qin ZJ, Liu L, Sun Q, Li X, Luo JF, Liu JS, et al. Impaired immune and coagulation systems may be early risk factors for COVID-19 patients: a retrospective study of 118 inpatients from Wuhan, China. Medicine. (2020) 99:e21700. doi: 10.1097/md.0000000000021700
70. Quintana-Díaz M, Andrés-Esteban EM, Ramírez-Cervantes KL, Olivan-Blázquez B, Juárez-Vela R, Gea-Caballero V. Coagulation parameters: an efficient measure for predicting the prognosis and clinical management of patients with COVID-19. J Clin Med. (2020) 9:3482. doi: 10.3390/jcm9113482
71. Singh N, Anchan RK, Besser SA, Belkin MN, Dela Cruz M, Lee L, et al. High sensitivity troponin-T for prediction of adverse events in patients with COVID-19. Biomarkers. (2020) 25:1–26. doi: 10.1080/1354750x.2020.1829056
72. Song K, Gong H, Xu B, Dong X, Li L, Hu W, et al. Association between recent oncologic treatment and mortality among patients with carcinoma who are hospitalized with COVID-19: a multicenter study. Cancer. (2020) 127:437–48. doi: 10.1002/cncr.33240
73. Tu WJ, Cao J, Yu L, Hu X, Liu Q. Clinicolaboratory study of 25 fatal cases of COVID-19 in Wuhan. Intensive Care Med. (2020) 46:1117–20. doi: 10.1007/s00134-020-06023-4
74. Volo T, Stritoni P, Battel I, Zennaro B, Lazzari F, Bellin M, et al. Elective tracheostomy during COVID-19 outbreak: to whom, when, how? Early experience from Venice, Italy. Eur Arch Otorhinolaryngol. (2020) 278:1–9. doi: 10.1007/s00405-020-06190-6
75. Wang K, Zhang Z, Yu M, Tao Y, Xie M. 15-day mortality and associated risk factors for hospitalized patients with COVID-19 in Wuhan, China: an ambispective observational cohort study. Intensive Care Med. (2020) 46:1472–4. doi: 10.1007/s00134-020-06047-w
76. Wang L, He WB, Yu XM, Hu DL, Jiang H. Prolonged prothrombin time at admission predicts poor clinical outcome in COVID-19 patients. World J Clin Cases. (2020) 8:4370–9. doi: 10.12998/wjcc.v8.i19.4370
77. Wendel Garcia PD, Fumeaux T, Guerci P, Heuberger DM, Montomoli J, Roche-Campo F, et al. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort. EClinMed. (2020) 25:100449. doi: 10.1016/j.eclinm.2020.100449
78. Xia P, Wen Y, Duan Y, Su H, Cao W, Xiao M, et al. Clinicopathological features and outcomes of acute kidney injury in critically ill COVID-19 with prolonged disease course: a retrospective cohort. J Am Soc Nephrol. (2020) 31:2205–21. doi: 10.1681/asn.2020040426
79. Xie J, Covassin N, Fan Z, Singh P, Gao W, Li G, et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. (2020) 95:1138–47. doi: 10.1016/j.mayocp.2020.04.006
80. Xu PP, Tian RH, Luo S, Zu ZY, Fan B, Wang XM, et al. Risk factors for adverse clinical outcomes with COVID-19 in China: a multicenter, retrospective, observational study. Theranostics. (2020) 10:6372–83. doi: 10.7150/thno.46833
81. Yang C, Liu F, Liu W, Cao G, Liu J, Huang S, et al. Myocardial injury and risk factors for mortality in patients with COVID-19 pneumonia. Int J Cardiol. (2020) 326:230–6. doi: 10.1016/j.ijcard.2020.09.048
82. Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. (2020) 21:904–13. doi: 10.1016/S1470-2045(20)30310-7
83. Yao Q, Wang P, Wang X, Qie G, Meng M, Tong X, et al. A retrospective study of risk factors for severe acute respiratory syndrome coronavirus 2 infections in hospitalized adult patients. Polish Arch Intern Med. (2020) 130:390–9. doi: 10.20452/pamw.15312
84. Yao Y, Cao J, Wang Q, Shi Q, Liu K, Luo Z, et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J Intensive Care. (2020) 8:49. doi: 10.1186/s40560-020-00466-z
85. Yu C, Lei Q, Li W, Wang X, Liu W, Fan X, et al. Clinical characteristics, associated factors, and predicting covid-19 mortality risk: a retrospective study in Wuhan, China. Am J Prev Med. (2020) 59:168–75. doi: 10.1016/j.amepre.2020.05.002
86. Zhang JJ, Cao YY, Tan G, Dong X, Wang BC, Lin J, et al. Clinical, radiological, and laboratory characteristics and risk factors for severity and mortality of 289 hospitalized COVID-19 patients. Allergy. (2020) 76:533–50. doi: 10.1111/all.14496
87. Zhang S, Guo M, Duan L, Wu F, Hu G, Wang Z, et al. Development and validation of a risk factor-based system to predict short-term survival in adult hospitalized patients with COVID-19: a multicenter, retrospective, cohort study. Crit Care. (2020) 24:438. doi: 10.1186/s13054-020-03123-x
88. Zhou J, Huang L, Chen J, Yuan X, Shen Q, Dong S, et al. Clinical features predicting mortality risk in older patients with COVID-19. Curr Med Res Opin. (2020) 36:1753–9. doi: 10.1080/03007995.2020.1825365
89. Zhou S, Mi S, Luo S, Wang Y, Ren B, Cai L, et al. Risk factors for mortality in 220 patients with COVID-19 in Wuhan, China: a single-center, retrospective study. Ear Nose Throat J. (2020) 100(2_suppl):140S−7S. doi: 10.1177/0145561320972608
90. Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User Manual for Trial Sequential Analysis. Copenhagen: CTU (2017).
91. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
92. Li Y, Zhao K, Wei H, Chen W, Wang W, Jia L, et al. Dynamic relationship between D-dimer and COVID-19 severity. Br J Haematol. (2020) 190:e24–7. doi: 10.1111/bjh.16811
93. Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. (2017) 149:38–44. doi: 10.1016/j.thromres.2016.11.007
94. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. (2020) 18:1094–9. doi: 10.1111/jth.14817
95. Gupta N, Zhao YY, Evans CE. The stimulation of thrombosis by hypoxia. Thromb Res. (2019) 181:77–83. doi: 10.1016/j.thromres.2019.07.013
Keywords: COVID-19, SARS-CoV-2, D-dimer, independent, cutoff, meta-analysis
Citation: Li Y, Deng Y, Ye L, Sun H, Du S, Huang H, Zeng F, Chen X and Deng G (2021) Clinical Significance of Plasma D-Dimer in COVID-19 Mortality. Front. Med. 8:638097. doi: 10.3389/fmed.2021.638097
Received: 05 December 2020; Accepted: 29 March 2021;
Published: 25 May 2021.
Edited by:
Zisis Kozlakidis, International Agency For Research On Cancer (IARC), FranceReviewed by:
Mohit Turagam, Mount Sinai Hospital, United StatesRami M. Elshazli, Horus University, Egypt
Copyright © 2021 Li, Deng, Ye, Sun, Du, Huang, Zeng, Chen and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Furong Zeng, emVuZ2Zsb3JhY2huQGhvdG1haWwuY29t; Xiang Chen, Y2hlbnhpYW5nY2tAMTI2LmNvbQ==; Guangtong Deng, ZGVuZ2d1YW5ndG9uZ0BvdXRsb29rLmNvbQ==
†These authors have contributed equally to this work
 Yayun Li1,2†
Yayun Li1,2† Songtao Du
Songtao Du Furong Zeng
Furong Zeng Xiang Chen
Xiang Chen Guangtong Deng
Guangtong Deng