- 1Faculty of Health, Education, Medicine and Social Care, School of Medicine, Vision and Eye Research Institute (VERI), Anglia Ruskin University, Cambridge, United Kingdom
- 2Anglia Ruskin University, Cambridge, United Kingdom
Background: Several underlying diseases have been associated with unfavorable COVID-19 related outcomes including asthma and Chronic Obstructive Pulmonary Disease (COPD), however few studies have reported risks that are adjusted for confounding variables. This study aimed to examine the adjusted risk of COVID-19 related hospitalsation, intensive care unit (ICU) admission, and mortality in patients with vs. without asthma or COPD.
Methods: A systematic review of major databases was undertaken for studies published between 1/12/2019 and 19/4/2021. Studies reporting the adjusted (for one or more confounder) risks of either hospitalsation, ICU admission, or mortality in asthmatics or COPD patients (control group = no asthma or no COPD) were identified. Risk of bias was determined via the QUIPS tool. A random effect meta-analysis was undertaken.
Findings: 37 studies were eligible for analysis, with a total of 1,678,992 participants. The pooled ORs of COVID-19 hospitalsation in subjects with asthma and COPD was 0.91 (95% CI 0.76–1.09) and 1.37 (95% CI 1.29–1.46), respectively. For ICU admission, OR in subjects with asthma and COPD was 0.89 (95% CI 0.74–1.07) and 1.22 (95% CI 1.04–1.42), respectively. For mortality, ORs were 0.88 (95% CI 0.77–1.01) and 1.25 (95% CI 1.08–1.34) for asthma and COPD, respectively. Further, the pooled risk of mortality as measured via Cox regression was 0.93 (95% CI 0.87–1.00) for asthma and 1.30 (95% CI 1.17–1.44) for COPD. All of these findings were of a moderate level of certainty.
Interpretation: COPD was significantly associated with COVID-19 related hospital admission, ICU admission, and mortality. Asthma was not associated with negative COVID-19 related health outcomes. Individuals with COPD should take precautions to limit the risk of COVID-19 exposure to negate these potential outcomes. Limitations include differing population types and adjustment for differing cofounding variables. Practitioners should note these findings when dealing with patients with these comorbidities.
Review Protocol Registration: https://www.crd.york.ac.uk/prospero/.
Introduction
In March 2020, the World Health Organization (WHO) declared the COVID-19 outbreak a global pandemic, and as of 3rd February 2021, over 103,000,000 confirmed cases have been diagnosed in more than 130 countries and areas, resulting in ~2,238,000 deaths to date (1). Several risk factors associated with increasing severity of the disease have been reported, including age (2), obesity (3), and underlying conditions such as hypertension (4), and diabetes (5).
An important risk factor for unfavorable COVID-19 outcomes is Chronic Obstructive Pulmonary Disease (COPD); a group of lung conditions including emphysema and chronic bronchitis (6), primarily caused by tobacco smoking, with air pollution, genetic factors, diet and tuberculosis also contributing to the disease (7).
COPD has been associated with increased risks of unfavorable outcomes in non-COVID-19 related pneumonia (8). For COVID-19, some primary studies have questioned whether COPD is associated with worse outcomes (9), whilst the majority of reviews conclude that COPD patients yield significantly worse outcomes than those without (10–13) and others report no effects (14).
An additional risk factor for COVID-19 related complications is the presence of asthma, a common allergy that can cause breathing difficulties including coughing, wheezing, breathlessness and a tight chest (15). Asthma exacerbations have been shown to be strongly associated with other respiratory viral infections, including previous coronaviruses (16, 17). Although some primary studies have reported associations between asthma and negative COVID-19 outcomes, the majority of reviews that have examined associations of COVID-19 outcomes and asthma have concluded a lack of association between asthma and negative COVID-19 outcomes (18, 19).
One limitation of all of the systematic reviews, to date, on COVID-19 outcomes and asthma or COPD is that they report on risk that has not been adjusted for any potential confounding factors, making the true risks of these comorbidities, and subsequent clinical implications, difficult (20)—indeed, of the 16 similar meta-analyses that were published in 2021 (as of April 2021), none of them reported exclusively on adjusted risks; they either report unadjusted risks or the inclusion of adjusted or unadjusted risks is unclear. Several primary studies report on adjusted risks that are lower than the unadjusted risks in several COVID-19 related outcomes, including in asthma (21) and COPD (22). Furthermore, several studies advocate the use of pooling adjusted effect sizes (23, 24), especially in the case of determining COVID-19 related risks (20, 25).
The aim of this review was to examine the risks of negative COVID-19 outcomes in subjects with asthma or COPD, that have been adjusted for one or more COVID-19 related risk factor, including age, sex, smoking status (20, 25), or comorbid disease. Specifically our aims were to assess:
1. Adjusted risk of COVID-19 related hospitalsation in subjects with vs. without asthma or COPD.
2. Adjusted risk of COVID-19 related intensive care unit (ICU) admission in subjects with vs. without asthma or COPD.
3. Adjusted risk of COVID-19 related overall mortality in subjects with vs. without asthma or COPD.
This review has the potential to inform clinicians regarding the true risks of unfavorable COVID-19 outcomes in patients with asthma and COPD, increase awareness in people of the potential risks should they contract COVID-19 and to inform healthcare and public health policies.
Methods
Study Registration
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (26), and was registered on 29th June 2020 with the international prospective register of systematic reviews (PROSPERO: protocol ID CRD42020194155)—note that the full PRIMSA checklist can be found in Supplementary Table 1 and justifications of any deviations from the registered protocol can be found in Supplementary Table 2.
Search Strategy
Databases were searched from 1/12/2019 to 19/4/2021 including Embase, MEDLINE, Pubmed, Scopus, Web of Science, CINAHL, The Cochrane library UK clinical Research Network: Portfolio database, and the International Standard Registered Clinical/soCial sTudy Number (ISRCTN) registry, using the following search terms:
(SARSCoV-2 OR 2019-nCoV OR COVID-19 OR coronavirus OR “Wuhan Coronavirus”)
AND
(2019 or 2020)
AND
(asthma* OR COPD OR “chronic obstructive pulmonary disease”)
No other limiters were applied.
Study Selection
Two researchers (MV,SW) independently screened titles and abstracts of all identified studies after duplicates were removed. Discrepancies between reviewers were resolved by discussion before screening full texts independently against the inclusion criteria. If it was not possible to determine whether a study met the inclusion criteria from the title and/or abstract, it was marked for a full paper review. Where necessary, the reviewers contacted corresponding authors to request missing information or clarification. All references were imported to Mendeley.
Study Inclusion and Exclusion
Two reviewers (MV & SW) independently screened all titles and abstracts. The relevance of each study was assessed according to the inclusion and exclusion criteria. Studies were included if they met the following criteria.
Population
Studies including humans with COPD and/or asthma and a confirmed case (via polymerase chain reaction or antibody test) of COVID-19 were included in this review. Children <18 yrs and animal studies were excluded from this review. We also excluded studies on previous human coronaviruses: 229E, NL63, OC43, HKU1, MERS-CoV, and SARS-CoV.
Intervention
Observational studies, including case-control and cohort studies were included. Randomized studies that reported the prognostic role of asthma/COPD in post-hoc analyses (e.g., Cox regression models) were also included.
Comparison
Comparator groups include humans with confirmed COVID-19 and no evidence of COPD and/or asthma.
Outcomes
Studies had to report one or more of the following:
1. Number of COVID-19 cases hospitalised vs. COVID-19 cases non-hospitalised cases.
2. Number of hospitalised COVID-19 cases treated in intensive care unit (ICU) vs. hospitalsation but not admitted for ICU care.
3. Number of COVID-19 related deaths vs. survival.
Furthermore, studies were excluded if they were:
1. Not written in English.
2. Not peer reviewed (e.g., preprints).
3. Studies in a non-adult (<18 years) population.
4. Had insufficient data to calculate an adjusted odds ratio (aOR; adjusted for more than one COVID-19 related covariate) related to the stated outcomes.
Data Extraction
Data was extracted by two reviewers (MT & MV) and included: first author, study title, date of study, dates in which study data were collected, country, aim/objective, study type, number of participants, disease investigated, method of disease diagnoses, method of COVID-19 diagnosis, outcome type, sample size, participant characteristics, adjusted OR and 95% confidence intervals (CIs) (or raw data in which an adjusted odds ratio could be calculated), details of confounding variables the OR was adjusted for. Where data was missing, required clarification or particular variables of interest were not reported in the paper, corresponding authors were contacted to enable inclusion in the meta-analysis, and given 2 weeks to respond. If no response was received within 2 weeks, or the data was unavailable, these studies were excluded.
Quality Assessment
Risk of bias was assessed by two independent researchers (MT & MV) using the Quality In Prognosis Studies (QUIPS) tool (27). The QUIPS is a non-scoring appraisal tool for assessing the scientific validity of articles, which requires the identification of whether or not relevant information is present in each article using a yes, no or not applicable rating, with an overall verdict of “low,” “medium,” or “high” risk of bias. Any discrepancies over the final risk of bias verdict was made by consensus, with involvement of a third review author (SP) where necessary.
Statistical Analysis
Due to anticipated heterogeneity, a random-effects model was conducted using the DerSimonian and Laird method, with studies weighted according the inverse variance, using Comprehensive Meta-Analysis (28). The meta-analysis was conducted using the following steps:
(1) Adjusted odds ratios (aORs), or adjusted Hazard Ratios (aHRs) and 95% CIs were inputted (with significance set as p = 0.05). Note that if the raw data were available, a binary logistic regression was conducted.
(2) Heterogeneity between studies was assessed using the I2 statistic (29). If high (>50%) heterogeneity was found, sub-group analyses were conducted based on total participants (>10 vs. <10k participants).
(3) Publication bias was assessed with a visual inspection of funnel plots and with the Egger bias test (30). As per the recommendations by Fu et al. (31) and Sterne et al. (32), these tests were only conducted if the number of studies in each analysis exceeded ten.
(4) Sensitivity analyses were conducted to assess the robustness of the pooled effect sizes through the one study removed method.
Certainty of Evidence
To ascertain the certainty of the evidence, the Grading of Recommendations, Assessment, Development and Evaluations (33) (GRADE) framework was used.
Results
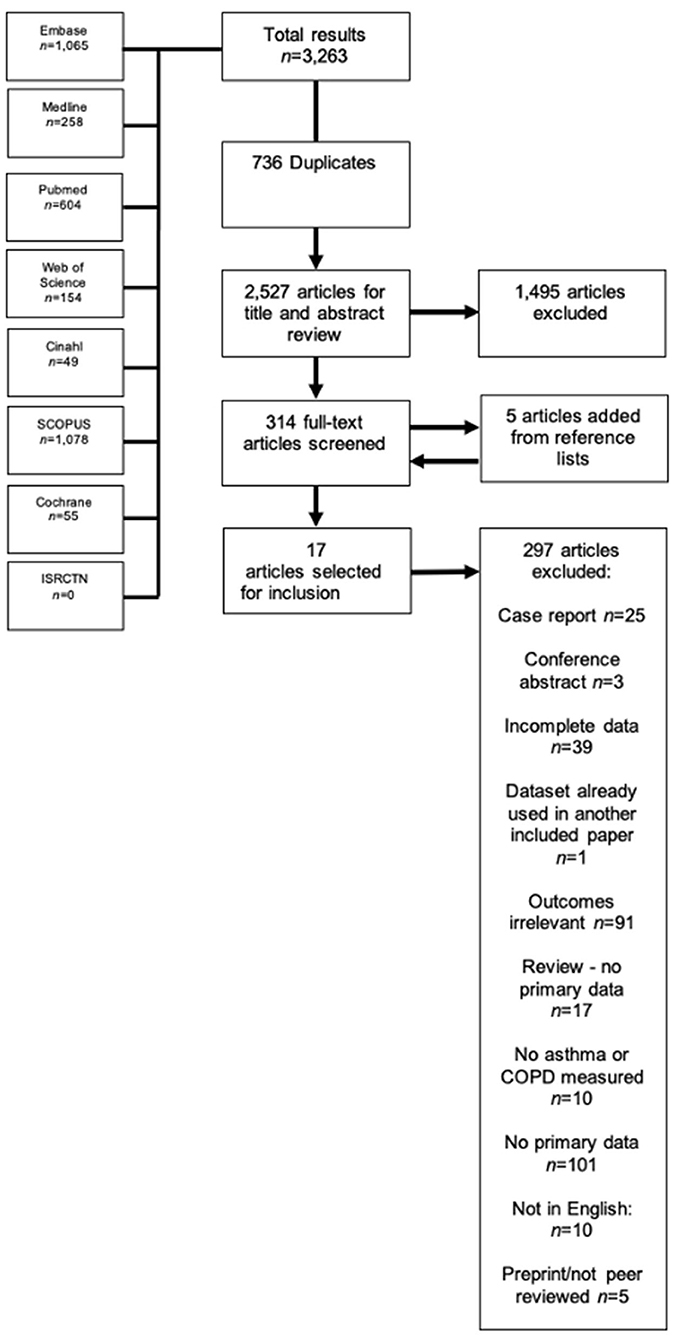
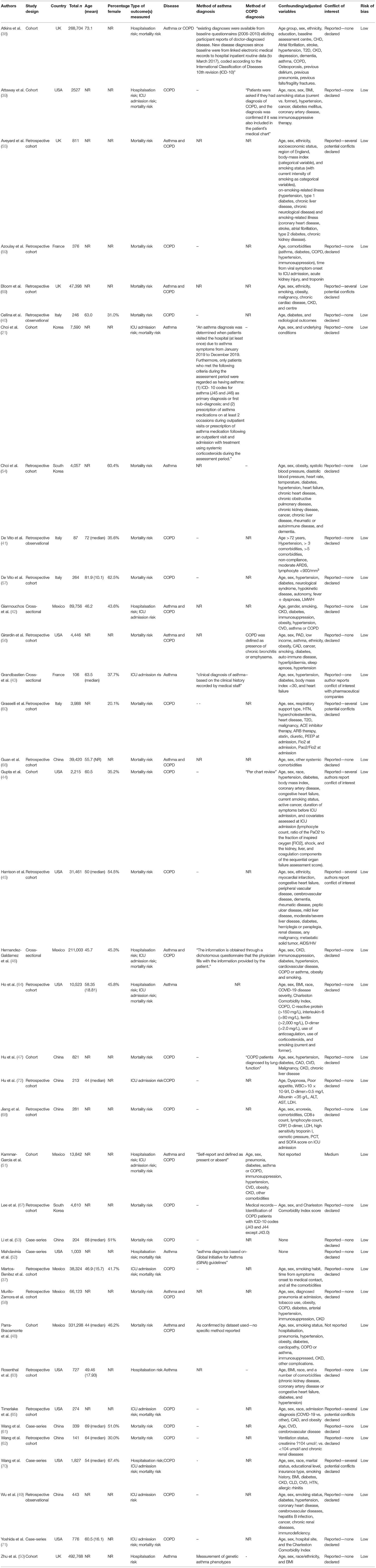
The literature search yielded 3,701 results, of which 780 were duplicates and were automatically removed, leaving 2,921 studies to be screened using the title and abstract. Of these studies, 416 full-texts were screened, where five extra studies were obtained by way of reference lists, resulting in 421 full texts that were finally screened. Thirty-eight studies appeared to be eligible for inclusion, however one (34) was excluded because the reported 95% CIs were not symmetrical, and therefore could not be pooled, leaving 37 finally eligible for inclusion (21, 35–69). The full PRISMA flowchart is shown in Figure 1, and a full list of excluded studies with reasons for exclusion can be found in Supplementary Table 2. There were a total of 1,678,992 participants across the included studies, with a mean age range of 45.7–81.9 years. Of the included studies, 10 (38, 42, 46, 48, 51, 55, 56, 58, 66, 69) examined outcomes in both asthma and COPD, seven (21, 43, 50, 52, 63, 64) examined outcomes exclusively in asthma, and the remaining 20 studies (37, 39–41, 44, 45, 47, 49, 53, 57, 59–62, 65, 67, 68, 70, 71) reported on outcomes exclusively regarding COPD. All but one study was classified as having low risk of bias (see Supplementary Table 4 for full QUIPS scoring). Full descriptive characteristics of included studies are shown in Table 1.
Meta-Analysis
Risk of COVID-19 Related Hospitalsation
When adjusted for one or more comorbidity, the pooled aOR was 0.87 (95% CI 0.73–1.05; p = 0.15; I2 = 85.36) for asthma and 1.39 (95% CI 1.31–1.48; p = < 0.001; I2 = 4.24) for COPD (see Table 2 and Figure 2). The sensitivity analysis found that the removal of any one study did not significantly change the direction of results for either asthma or COPD (see Supplementary Figures 1, 2 for full details).
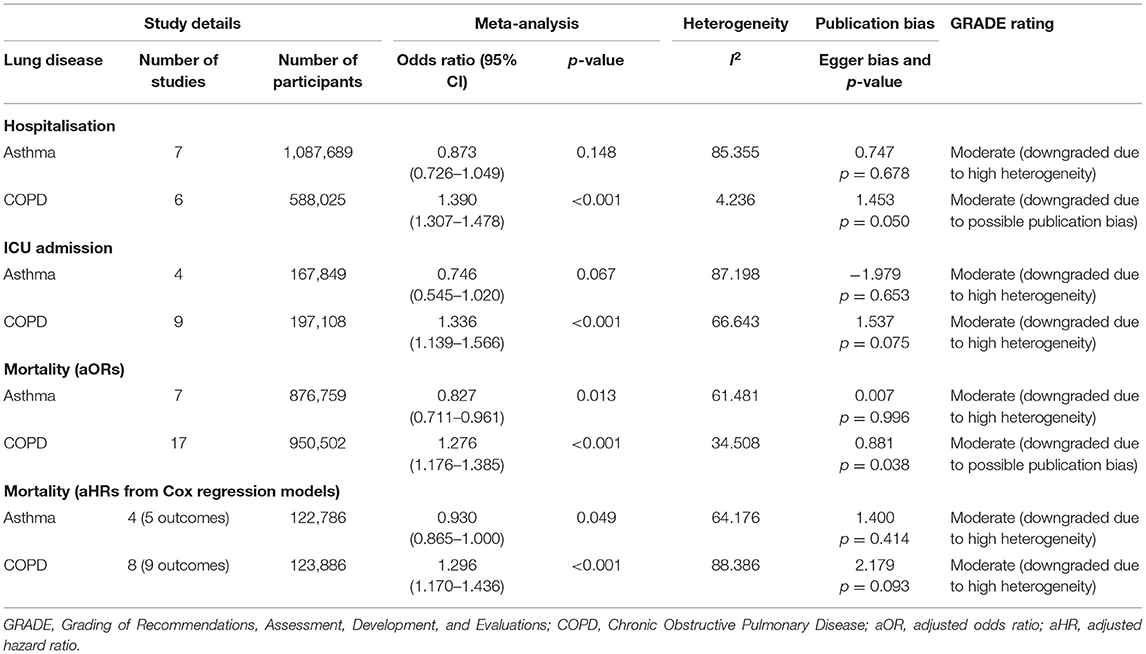
Table 2. Meta-analysis showing the pooled adjusted risk of unfavorable COVID-19 outcomes in subjects with asthma or COPD.
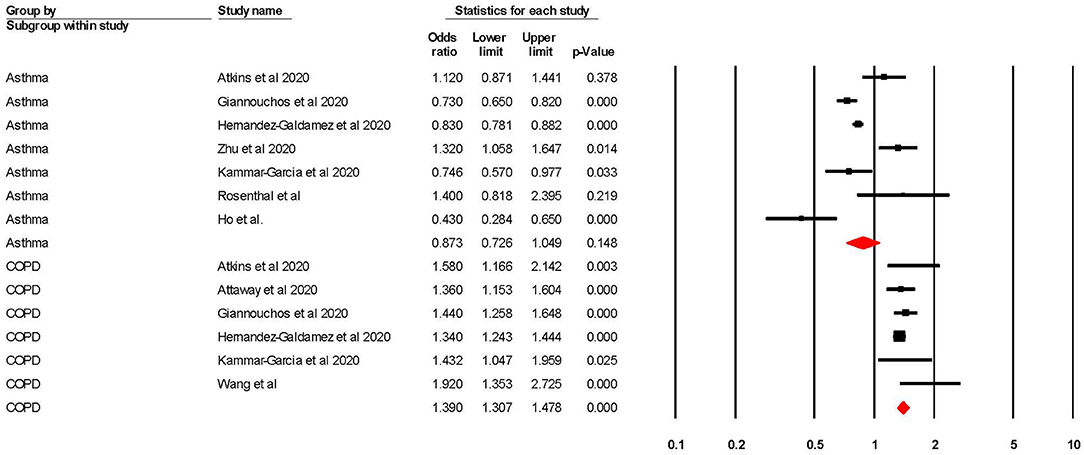
Figure 2. Forest plot showing odds ratios (adjusted for at least one confounder) for COVID-19 related hospitalisation in subjects with asthma or Chronic Obstructive Pulmonary Disease (COPD).
Risk of COVID-19 Related ICU Admission
When adjusted for one or more comorbidity, the pooled aOR was 0.75 (95% CI 0.55–1.02; p = 0.07; I2 = 87.20) for asthma and 1.34 (95% CI 1.14–1.57; p = < 0.001; I2 = 66.64) for COPD (see Table 2 and Figure 3). The sensitivity analysis found that for asthma the aOR became significant with the removal of one study (46) (OR = 0.65 95% CI 0.44–0.97 p = 0.04). The removal of any one study did not significantly change the direction of results for COPD (see Supplementary Figures 3, 4 for full details).
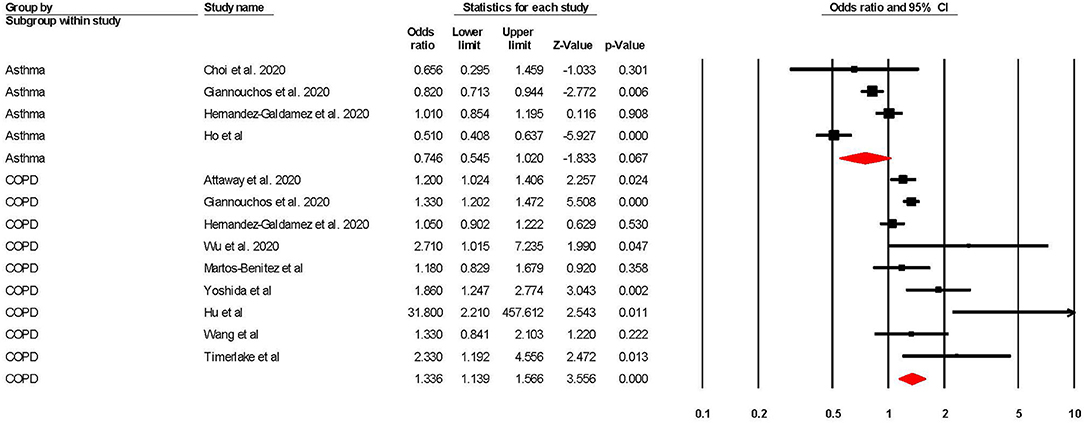
Figure 3. Forest plot showing odds ratios (adjusted for at least one comorbidity) for COVID-19 related intensive care admission in subjects with asthma or Chronic Obstructive Pulmonary Disease (COPD).
Risk of COVID-19 Related Mortality
When adjusted for one or more comorbidity, the pooled aOR was 0.83 (95% CI 0.71–0.96; p = 0.01; I2 = 61.48) for asthma and 1.28 (95% CI 1.18–1.39; p = < 0.001; I2 = 34.51) for COPD (see Table 2 and Figure 4). The sensitivity analysis found that for asthma the aOR became non-significant with the removal of one study (46) (OR = 0.83 95% CI 0.66–1.05 p = 0.118), and the results did not significantly change for COPD when any one study was removed (see Supplementary Figures 5, 6 for full details).
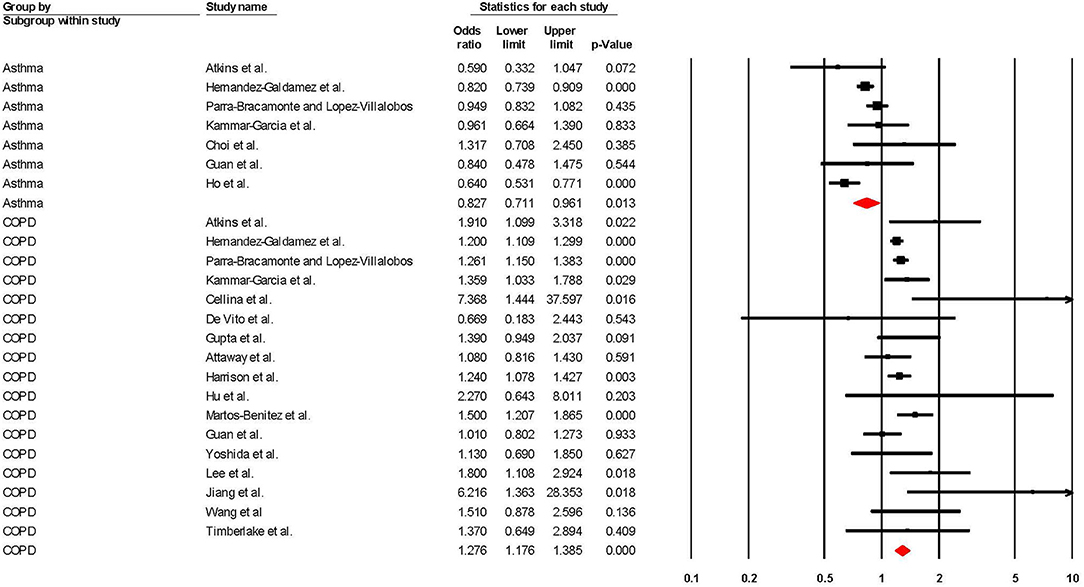
Figure 4. Forest plot showing odds ratios (adjusted for at least one comorbidity) for COVID-19 related overall mortality in subjects with asthma or Chronic Obstructive Pulmonary Disease (COPD).
Regarding studies that reported aHRs in the form of Cox regression models, the pooled risk of mortality was 0.93 (95% CI 0.87–1.00; p = 0.049; I2 = 64.18) for asthma and 1.30 (95% CI 1.17–1.44; p = < 0.001; I2 = 88.39) for COPD (see Table 2 and Figure 5). The sensitivity analysis found that the removal of any one study did not significantly change the direction of results for COPD, and the removal of any one of three studies (56, 58, 69) changed the significance of results in asthma (see Supplementary Figures 7, 8 for full details).
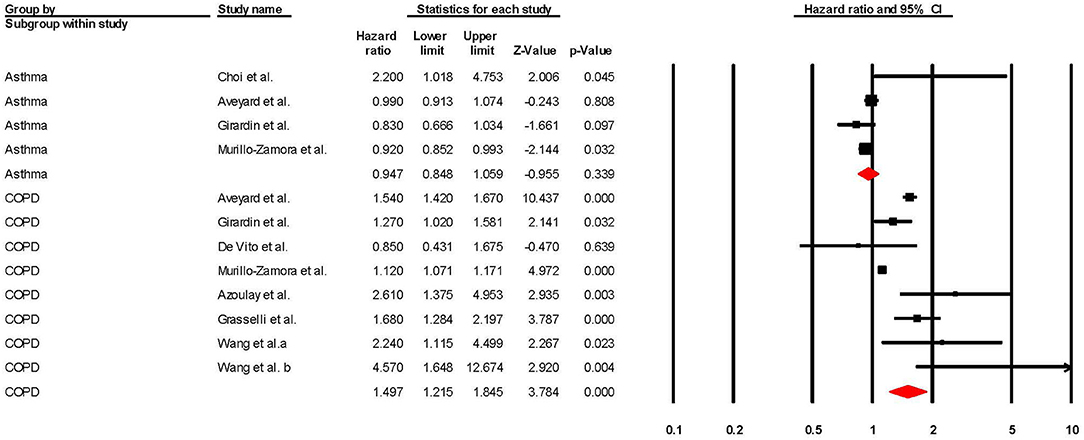
Figure 5. Forest plot showing Cox regression hazard ratios (adjusted for at least one comorbidity) for COVID-19 related overall mortality in subjects with asthma or Chronic Obstructive Pulmonary Disease (COPD).
Certainty of Evidence Using the GRADE Approach
Using the GRADE (33) approach, all of the results were rated as being a “moderate” level of certainty. The two reasons why the level of evidence was not rated as “high” was because of either (1) high heterogeneity, or (2) the presence of publication bias.
Sub-Group Analyses
When sub-grouped between studies with >10 vs. <10k participants, no significant changes were found, except for in risk of mortality (as measured by Cox regression) in participants with COPD. It was found that studies with >10k participants yielded significantly lower (p = 0.001) risk of mortality (aHR = 1.13 95% CI 1.10–1.17) when compared to studies that had <10k participants (aHR = 1.59 95% CI 1.31–1.94), and also yielded lower heterogeneity in this subgroup (>10k = 36.19%; <10k = 58.32%). Although the differences between sub-groups were significant, both pooled aHRs were still, respectively, statistically significant. Full information can be found in Table 3 and in Supplementary Figures 9–16.
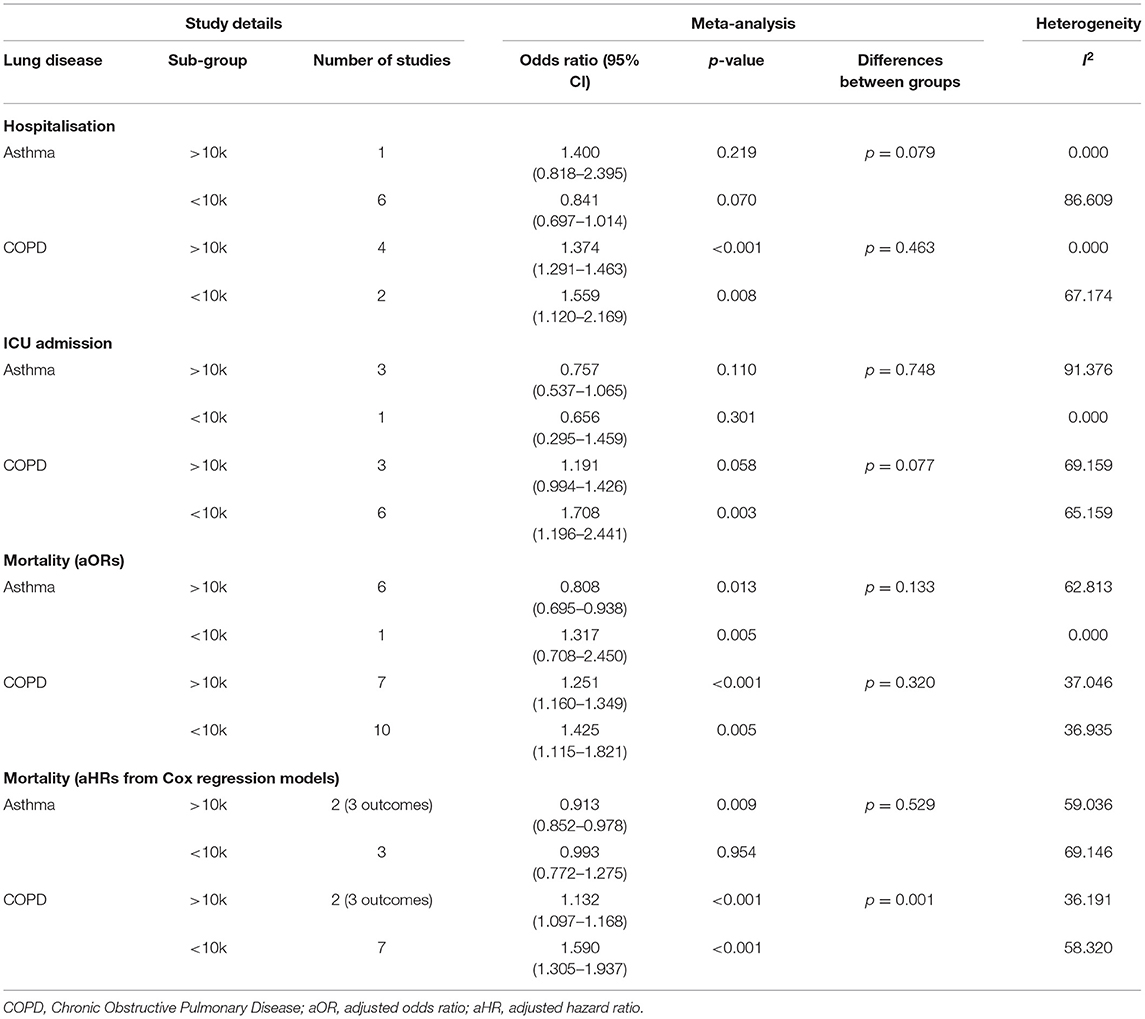
Table 3. Sub-group analyses showing the pooled adjusted risk of unfavorable COVID-19 outcomes in participants with asthma or COPD stratified >10 vs. <10k participants.
Discussion
This meta-analysis included 37 studies examined the adjusted risks of COVID-19 related hospitalsation, ICU-admission, and mortality in populations with and without either asthma or COPD. The analysis suggests with a moderate level of certainty that COPD is a significant risk factor for COVID-19 related hospitalsation, ICU admission, or mortality when the risks were adjusted for at least one comorbidity. Furthermore, with a moderate level of certainty, asthma was not shown to be a significant risk factor for COVID-19 related hospitalsation, ICU admission, or mortality when adjusted for at least one comorbidity.
COPD was shown to be a significant risk factor in all three outcomes, with the sensitivity analysis reporting robustness in all outcomes. These results broadly agree with previous meta-analyses exploring similar outcomes in this population (10–14). When directly comparing reported risks, this study shows a marked decrease in mortality risk (5.69 vs. 1.25) when compared to Lippi and Henry (10), which would be expected. Although the mechanisms that underpin this risk are not clear, several hypotheses, including the increased expression of the angiotensin-converting enzyme 2 (ACE-2) in COPD patients, have been reported as COVID-19's route of entry into susceptible cells (73). Furthermore, it has been reported that morbidity and mortality in COPD patients are frequently related to acute exacerbation (12), and severe respiratory failure (67) which may add to already compromised respiratory capacity among COVID-19 patients (12, 74, 75). Moreover, the effect of smoking could be a reason why people with COPD appear to have increased COVID-19 risks; indeed, a recent systematic review and meta-analysis (76) reported that both current and former smokers have increased risks of COVID-19 related deaths, although these risks do not appear to have been adjusted for any co-variates. Further exploration into adjusted smoking risk, in particular adjusted for COPD and/or asthma presence, would be beneficial.
Other comorbidities have also been shown to be significant risk factors for unfavorable COVID-19 related outcomes including (but not limited to), hypertension (4), diabetes (5), and obesity (3). It is difficult to directly compare our results with previous data as these previous estimates report unadjusted data making true risks of each comorbidity hard to compare. We agree with Jordan et al. (20) and recommend that future studies aim to report risks based on adjustments for, at the very least, age, sex, and smoking status so that true risks can be determined. It is recommended that clinicians continue to consider COPD patients to be at greater risk of COVID-19 related morbid outcomes. Individuals with COPD should take extra precautions to ensure that exposure to COVID-19 is minimal.
Although asthma has been related to worse outcomes in other viral infections, including other forms of coronavirus (16, 17), our analysis did not suggest asthma as a significant risk factor for any of the outcomes measured in this review, apart from mortality (measured as a non-time dependent OR), however sensitivity analysis suggested that the significance of this outcome was subject to the influence of one large study. These results broadly agree with previous meta-analyses that concluded that asthma was not a significant risk factor for either mortality or “severe” health outcomes (14, 18, 35, 77). When directly comparing reported risks across these meta-analyses, this study's mortality risk is lower (0.83 and 0.93 vs. 0.96 and 1.03) (35, 77), which is an expected result given we pooled adjusted ORs and the other meta-analyses were not adjusted for any other covariates. These results, however, need to be interpreted with caution as the included studies have used asthma as an umbrella term and did not differentiate between different types or severities of the disease. The National Health Service (NHS) in the UK has severe asthma listed “high risk of severe outcomes,” and other severities at “moderate risk” of COVID-19 (78), and although this study does not support this, more data is required to differentiate between different severity of asthma, and, as such, individuals with asthma should still aim to minimize their risk of COVID-19 exposure.
Although this is the first review to systematically examine risks of unfavorable COVID-19 outcomes in populations with asthma or COPD with effect sizes adjusted for at least one covariate, our results should be considered within its limitations. Firstly, although the majority were deemed as low risk of bias, the effect of methodological bias cannot be ruled out. Secondly, the pooling of adjusted ORs (with different studies adjusting for different covariates) inherently creates a degree of inconsistency, meaning that the results should be treated only as indicative. Thirdly, there was considerable heterogeneity in some of the reported analyses, especially in the asthmatic populations, which could not be explained by the presence of large studies vs. smaller ones. One probable reason for this is the different asthma diagnosis methods, in particular regarding the type and severity of asthma. Furthermore, there was some evidence of publication bias, which could not be explained. Lastly, meta-analyses have inherent limitations: their findings are dependent on estimates selected from each primary study and thus are dependent on the accuracy of primary studies (79).
Conclusions
COPD is significantly associated with worse COVID-19 related, hospital admission, ICU admission and mortality, even when adjusted for at least one comorbidity. Asthma, when pooling risks were adjusted for other comorbidities, was not associated with a higher risk of COVID-19 related hospitalsation, ICU admission and mortality. Clinicians should note these findings when dealing with patients with these comorbidities. Furthermore, individuals with COPD should take special precautions to limit the risk of COVID-19 exposure to negate these potential outcomes.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
MT and MV acquisition and analysis. MT, MV, and SP drafted the work. MT and MV verified the underlying data. All authors made substantial contributions to the conception, design of the work, interpretation of data for the work, revising it critically for important intellectual content and final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.668808/full#supplementary-material
References
1. World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. (2021). Available online at: https://covid19.who.int (accessed February 3, 2021).
2. Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: a comparison with young and middle-aged patients. J Infect. (2020) 80:e14–8. doi: 10.1016/j.jinf.2020.03.005
3. Tamara A, Tahapary DL. Obesity as a predictor for a poor prognosis of COVID-19: a systematic review. Diabetes Metab Syndr Clin Res Rev. (2020) 14:655–9. doi: 10.1016/j.dsx.2020.05.020
4. Pranata R, Lim MA, Huang I, Raharjo SB, Lukito AA. Hypertension is associated with increased mortality and severity of disease in COVID-19 pneumonia: a systematic review, meta-analysis and meta-regression. J Renin-Angiotensin-Aldosterone Syst JRAAS. (2020) 21:1470320320926899. doi: 10.1177/1470320320926899
5. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr Clin Res Rev. (2020) 14:395–403. doi: 10.1016/j.dsx.2020.04.018
6. National Health Service. Overview: Chronic Obstructive Pulmonary Disease (COPD). (2019). Available online at: https://www.nhs.uk/conditions/chronic-obstructive-pulmonary-disease-copd/ (accessed January 26, 2021).
7. Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, et al. An official American thoracic society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2010) 182:693–718. doi: 10.1164/rccm.200811-1757ST
8. Restrepo MI, Mortensen EM, Pugh JA, Anzueto A. COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J. (2006) 28:346–51. doi: 10.1183/09031936.06.00131905
9. Schultze A, Walker AJ, MacKenna B, Morton CE, Bhaskaran K, Brown JP, et al. Risk of COVID-19-related death among patients with chronic obstructive pulmonary disease or asthma prescribed inhaled corticosteroids: an observational cohort study using the OpenSAFELY platform. Lancet Respir Med. (2020) 8:1106–20. doi: 10.1016/S2213-2600(20)30415-X
10. Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir Med. (2020) 167:105941. doi: 10.1016/j.rmed.2020.105941
11. Leung JM, Niikura M, Yang CWT, Sin DD. COVID-19 and COPD. Eur Respir J. (2020) 56:2002108. doi: 10.1183/13993003.02108-2020
12. Pranata R, Soeroto A, Huang I, Lim M, Santoso P, Permana H, et al. Effect of chronic obstructive pulmonary disease and smoking on the outcome of COVID-19. Int J Tuberc Lung Dis. (2020) 24:838–43. doi: 10.5588/ijtld.20.0278
13. Alqahtani JS, Oyelade T, Aldhahir AM, Alghamdi SM, Almehmadi M, Alqahtani AS, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS ONE. (2020) 15:e0233147. doi: 10.1371/journal.pone.0233147
14. Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PLoS ONE. (2020) 15:e0238215. doi: 10.1371/journal.pone.0238215
15. National Health Service. Overview: Asthma. (2018). Available online at: https://www.nhs.uk/conditions/asthma/ (accessed January 26, 2021).
16. Papadopoulos NG, Christodoulou I, Rohde G, Agache I, Almqvist C, Bruno A, et al. Viruses and bacteria in acute asthma exacerbations - A GA 2LEN-DARE* systematic review. Allergy Eur J Allergy Clin Immunol. (2011) 66:458–68. doi: 10.1111/j.1398-9995.2010.02505.x
17. Busse WW, Lemanske RF, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. (2010) 376:826–34. doi: 10.1016/S0140-6736(10)61380-3
18. Soeroto AY, Purwiga A, Roesli R. Asthma does not increase COVID-19 mortality and poor outcomes: a systematic review and meta-analysis. Asian Pac J Allergy Immunol. (2021). doi: 10.12932/AP-110920-0955. [Epub ahead of print].
19. Sunjaya AP, Allida SM, Di Tanna GL, Jenkins C. Asthma and risk of infection, hospitalisation, ICU admission and mortality from COVID-19: Systematic review and meta-analysis. J Asthma. (2021) 1:1–22. doi: 10.1080/02770903.2021.1888116
20. Jordan RE, Adab P, Cheng KK. Covid-19: risk factors for severe disease and death. BMJ. (2020) 368:m1198. doi: 10.1136/bmj.m1198
21. Choi YJ, Park J-Y, Lee HS, Suh J, Song JY, Byun MK, et al. Effect of asthma and asthma medication on the prognosis of patients with COVID-19. Eur Respir J. (2020) 57:2002226. doi: 10.1183/13993003.02226-2020
22. Cen Y, Chen X, Shen Y, Zhang X-H, Lei Y, Xu C, et al. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019—a multi-centre observational study. Clin Microbiol Infect. (2020) 26:1242–7. doi: 10.1016/j.cmi.2020.05.041
23. Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. (2005) 143:199–211. doi: 10.7326/0003-4819-143-3-200508020-00006
24. Schuch FB, Vancampfort D, Firth J, Rosenbaum S, Ward PB, Silva ES, et al. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am J Psychiatry. (2018) 175:631–48. doi: 10.1176/appi.ajp.2018.17111194
25. Kobayashi T, Jung S, Linton NM, Kinoshita R, Hayashi K, Miyama T, et al. Communicating the risk of death from novel coronavirus disease (COVID-19). J Clin Med. (2020) 9:580. doi: 10.3390/jcm9020580
26. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
27. Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. (2013) 158:280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
28. Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta Analysis. Englewood, NJ: Biostat (2013).
29. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
30. Egger M, Smith GD, Schneider M, Minder C. Bias in meta - analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
31. Fu R, Gartlehner G, Grant M, Shamliyan T, Sedrakyan A, Wilt TJ, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the effective health care program. J Clin Epidemiol. (2011) 64:1187–97. doi: 10.1016/j.jclinepi.2010.08.010
32. Sterne JA, Egger M, Moher D. Addressing Reporting Biases. Cochrane Handb Syst Rev Interv Cochrane Book Ser. Chichester: John Wiley & Sons, Ltd (2008). doi: 10.1002/9780470712184.ch10
33. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
34. Islam M, Riaz B, Islam A, Khanam F, Akhter J, Choudhury R, et al. Risk factors associated with morbidity and mortality outcomes of COVID-19 patients on the 28th day of the disease course: a retrospective cohort study in Bangladesh. Epidemiol Infect. (2020) 148:e263. doi: 10.1017/S0950268820002630
35. Wang Y, Chen J, Chen W, Liu L, Dong M, Ji J, et al. Does asthma increase the mortality of patients with COVID-19?: A systematic review and meta-analysis. Int Arch Allergy Immunol. (2020) 22:1–7. doi: 10.1159/000510953
36. Nogueira PJ, de Araújo Nobre M, Costa A, Ribeiro RM, Furtado C, Bacelar Nicolau L, et al. The role of health preconditions on COVID-19 deaths in portugal: evidence from surveillance data of the first 20293 infection cases. J Clin Med. (2020) 9:2368. doi: 10.3390/jcm9082368
37. Martos-Benítez FD, Soler-Morejón CD, García-del Barco D. Chronic comorbidities and clinical outcomes in patients with and without COVID-19: a large population-based study using national administrative healthcare open data of Mexico. Intern Emerg Med. (2021) 7:1–11. doi: 10.1007/s11739-020-02597-5
38. Atkins JL, Masoli JAH, Delgado J, Pilling LC, Kuo CL, Kuchel GA, et al. Pre-existing comorbidities predicting COVID-19 and mortality in the UK Biobank community cohort. J Gerontol A Biol Sci Med Sci. (2020) 75:2224–30. doi: 10.1093/gerona/glaa183
39. Attaway AA, Zein J, Hatipoglu US. SARS-CoV-2 infection in the COPD population is associated with increased healthcare utilization: an analysis of Cleveland clinic's COVID-19 registry. EClinicalMedicine. (2020) 26:100515. doi: 10.1016/j.eclinm.2020.100515
40. Cellina M, Gibelli D, Valenti Pittino C, Toluian T, Marino P, Oliva G. Risk factors of fatal outcome in patients with COVID-19 pneumonia. Disaster Med Public Health Prep. (2020). doi: 10.1017/dmp.2020.346. [Epub ahead of print].
41. De Vito A, Geremia N, Fiore V, Princic E, Babudieri S, Madeddu G, et al. Clinical features, laboratory findings and predictors of death in hospitalized patients with COVID-19 in Sardinia, Italy. Eur Rev Med Pharmacol Sci. (2020) 24:7861–8. doi: 10.26355/eurrev_202007_22291
42. Giannouchos TV, Sussman RA, Mier JM, Poulas K, Farsalinos K. Characteristics and risk factors for COVID-19 diagnosis and adverse outcomes in Mexico: an analysis of 89,756 laboratory–confirmed COVID-19 cases. Eur Respir J. (2020). doi: 10.1101/2020.06.04.20122481. [Epub ahead of print].
43. Grandbastien M, Piotin A, Godet J, Abessolo-Amougou I, Ederlé C, Enache I, et al. SARS-CoV-2 pneumonia in hospitalized asthmatic patients did not induce severe exacerbation. J Allergy Clin Immunol Pract. (2020) 8:2600–7. doi: 10.1016/j.jaip.2020.06.032
44. Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, et al. Factors associated with death in critically Ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. (2020) 180:1436–47. doi: 10.1001/jamainternmed.2020.3596
45. Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GYH. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: A federated electronic medical record analysis. PLoS Med. (2020) 17:e3321. doi: 10.1371/journal.pmed.1003321
46. Hernández-Galdamez DR, González-Block MÁ, Romo-Dueñas DK, Lima-Morales R, Hernández-Vicente IA, Lumbreras-Guzmán M, et al. Increased risk of hospitalization and death in patients with COVID-19 and pre-existing non-communicable diseases and modifiable risk factors in Mexico. Arch Med Res. (2020) 51:683–9. doi: 10.1016/j.arcmed.2020.07.003
47. Hu W, Dong M, Xiong M, Zhao D, Zhao Y, Wang M, et al. Clinical courses and outcomes of patients with chronic obstructive pulmonary disease during the covid-19 epidemic in hubei, china. Int J COPD. (2020) 15:2237–48. doi: 10.2147/COPD.S265004
48. Parra-Bracamonte GM, Lopez-Villalobos N, Parra-Bracamonte FE. Clinical characteristics and risk factors for mortality of patients with COVID-19 in a large data set from Mexico. Ann Epidemiol. (2020) 52:93–8.e2. doi: 10.1016/j.annepidem.2020.08.005
49. Wu F, Zhou Y, Wang Z, Xie M, Shi Z, Tang Z, et al. Clinical characteristics of COVID-19 infection in chronic obstructive pulmonary disease: a multicenter, retrospective, observational study. J Thorac Dis. (2020) 12:1811–23. doi: 10.21037/jtd-20-1914
50. Zhu Z, Hasegawa K, Ma B, Fujiogi M, Camargo CA, Liang L. Association of asthma and its genetic predisposition with the risk of severe COVID-19. J Allergy Clin Immunol. (2020) 146:327–9. doi: 10.1016/j.jaci.2020.06.001
51. García AK, Mayo J de JV, Zertuche JMV, Hernández ML, López OV, Badilla OS, et al. Impact of comorbidities in Mexican SARS-CoV-2-positive patients: a retrospective analysis in a national cohort. Rev Invest Clin. (2020) 72:151–8. doi: 10.24875/RIC.20000207
52. Mahdavinia M, Foster KJ, Jauregui E, Moore D, Adnan D, Andy-Nweye AB, et al. Asthma prolongs intubation in COVID-19. J Allergy Clin Immunol Pract. (2020) 8:2388–91. doi: 10.1016/j.jaip.2020.05.006
53. Li P, Chen L, Liu Z, Pan J, Zhou D, Wang H, et al. Clinical features and short-term outcomes of elderly patients with COVID-19. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. (2020) 97:245–50. doi: 10.1016/j.ijid.2020.05.107
54. Choi H, Wee JH, Kim SY, Kim J, Kim HI, Park J, et al. Association between asthma and clinical mortality/morbidity in COVID-19 patients using clinical epidemiologic data from Korean disease control and prevention. Allergy. (2020) 76:921–4. doi: 10.22541/au.160279752.20065578/v1
55. Aveyard P, Gao M, Lindson N, Hartmann-Boyce J, Watkinson P, Young D, et al. Association between pre-existing respiratory disease and its treatment, and severe COVID-19: a population cohort study. Lancet Respir Med. (2021) 76:921–24. doi: 10.1016/S2213-2600(21)00095-3
56. Girardin J-L, Seixas A, Ramos Cejudo J, Osorio RS, Avirappattu G, Reid M, et al. Contribution of pulmonary diseases to COVID-19 mortality in a diverse urban community of New York. Chron Respir Dis. (2021) 18:1479973120986806. doi: 10.1177/1479973120986806
57. De Vito A, Fiore V, Princic E, Geremia N, Panu Napodano CM, Muredda AA, et al. Predictors of infection, symptoms development, and mortality in people with SARS-CoV-2 living in retirement nursing homes. PLoS ONE. (2021) 16:e0248009. doi: 10.1371/journal.pone.0248009
58. Murillo-Zamora E, Hernandez-Suarez CM. Survival in adult inpatients with COVID-19. Public Health. (2021) 190:1–3. doi: 10.1016/j.puhe.2020.10.029
59. Azoulay E, Fartoukh M, Darmon M, Géri G, Voiriot G, Dupont T, et al. Increased mortality in patients with severe SARS-CoV-2 infection admitted within seven days of disease onset. Intensive Care Med. (2020) 46:1714–22. doi: 10.1007/s00134-020-06202-3
60. Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. (2020) 180:1345–55. doi: 10.1001/jamainternmed.2020.3539
61. Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. (2020) 80:639–45. doi: 10.1016/j.jinf.2020.03.019
62. Wang T, Tang C, Chen R, Ruan H, Liang W, Guan W, et al. Clinical features of coronavirus disease 2019 patients with mechanical ventilation: a nationwide study in China. Crit Care Med. (2020) 48:e809–12. doi: 10.1097/CCM.0000000000004473
63. Rosenthal JA, Awan SF, Fintzi J, Keswani A, Ein D. Asthma is associated with increased risk of intubation but not hospitalization or death in coronavirus disease 2019. Ann Allergy Asthma Immunol. (2021) 126:93–5. doi: 10.1016/j.anai.2020.10.002
64. Ho KS, Howell D, Rogers L, Narasimhan B, Verma H, Steiger D. The relationship between asthma, eosinophilia, and outcomes in coronavirus disease 2019 infection. Ann Allergy Asthma Immunol. (2021) 1–7. doi: 10.1016/j.anai.2021.02.021
65. Timberlake DT, Narayanan D, Ogbogu PU, Raveendran R, Porter K, Scherzer R, et al. Severity of COVID-19 in hospitalized patients with and without atopic disease. World Allergy Organ J. (2021) 14:100508. doi: 10.1016/j.waojou.2021.100508
66. Guan W, Liang W, Shi Y, Gan L, Wang H, He J, et al. Chronic respiratory diseases and the outcomes of COVID-19: A nationwide retrospective cohort study of 39,420 cases. J Allergy Clin Immunol Pract. (2021). doi: 10.1016/j.jaip.2021.02.041. [Epub ahead of print].
67. Lee SC, Son KJ, Han CH, Park SC, Jung JY. Impact of COPD on COVID-19 prognosis: a nationwide population-based study in South Korea. Sci Rep. (2021) 11:1–8. doi: 10.1038/s41598-021-83226-9
68. Jiang Y, Abudurexiti S, An M-M, Cao D, Wei J, Gong P. Risk factors associated with 28-day all-cause mortality in older severe COVID-19 patients in Wuhan, China: a retrospective observational study. Sci Rep. (2020) 10:1–13. doi: 10.1038/s41598-020-79508-3
69. Bloom CI, Drake TM, Docherty AB, Lipworth BJ, Johnston SL, Nguyen-Van-Tam JS, et al. Risk of adverse outcomes in patients with underlying respiratory conditions admitted to hospital with COVID-19: a national, multicentre prospective cohort study using the ISARIC WHO Clinical Characterisation Protocol UK. Lancet Respir Med. (2021). doi: 10.1016/S2213-2600(21)00013-8. [Epub ahead of print].
70. Wang L, Foer D, Bates DW, Boyce JA, Zhou L. Risk factors for hospitalization, intensive care, and mortality among patients with asthma and COVID-19. J Allergy Clin Immunol. (2020) 146:808–12. doi: 10.1016/j.jaci.2020.07.018
71. Yoshida Y, Gillet SA, Brown MI, Zu Y, Wilson SM, Ahmed SJ, et al. Clinical characteristics and outcomes in women and men hospitalized for coronavirus disease 2019 in New Orleans. Biol Sex Differ. (2021) 12:1–11. doi: 10.1186/s13293-021-00359-2
72. Hu X, Hu C, Yang Y, Chen J, Zhong P, Wen Y, et al. Clinical characteristics and risk factors for severity of COVID-19 outside Wuhan: a double-center retrospective cohort study of 213 cases in Hunan, China. Ther Adv Respir Dis. (2020) 14:1753466620963035. doi: 10.1177/1753466620963035
73. Leung JM, Yang CX, Tam A, Shaipanich T, Hackett T-L, Singhera GK, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. (2020) 55:2000688. doi: 10.1183/13993003.00688-2020
74. Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. (2006) 173:1114–21. doi: 10.1164/rccm.200506-859OC
75. Rohde G, Wiethege A, Borg I, Kauth M, Bauer T, Gillissen A, et al. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. (2003) 58:37–42. doi: 10.1136/thorax.58.1.37
76. Umnuaypornlert A, Kanchanasurakit S, Lucero-Prisno DEI, Saokaew S. Smoking and risk of negative outcomes among COVID-19 patients: a systematic review and meta-analysis. Tob Induc Dis. (2021) 19:9. doi: 10.18332/tid/132411
77. Wang Y, Ao G, Qi X, Xie B. The association between COVID-19 and asthma: a systematic review and meta-analysis. Clin Exp Allergy J Br Soc Allergy Clin Immunol. (2020) 50:1274–7. doi: 10.1111/cea.13733
78. National Health Service. Who's at Higher Risk From Coronavirus. (2021). Available online at: https://www.nhs.uk/conditions/coronavirus-covid-19/people-at-higher-risk/whos-at-higher-risk-from-coronavirus/ (accessed January 28, 2021).
Keywords: COVID-19, COPD, asthma, mortality, hospitalsation, meta-analysis, ICU, intensive care
Citation: Pardhan S, Wood S, Vaughan M and Trott M (2021) The Risk of COVID-19 Related Hospitalsation, Intensive Care Unit Admission and Mortality in People With Underlying Asthma or COPD: A Systematic Review and Meta-Analysis. Front. Med. 8:668808. doi: 10.3389/fmed.2021.668808
Received: 17 February 2021; Accepted: 17 May 2021;
Published: 16 June 2021.
Edited by:
Longxiang Su, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Citra Fragrantia Theodorea, University of Indonesia, IndonesiaZsolt Szakács, University of Pécs, Hungary
Michael Anthonius Lim, University of Pelita Harapan, Indonesia
Copyright © 2021 Pardhan, Wood, Vaughan and Trott. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mike Trott, bWlrZS50cm90dEBhcnUuYWMudWs=
 Shahina Pardhan
Shahina Pardhan Samantha Wood
Samantha Wood Megan Vaughan1
Megan Vaughan1 Mike Trott
Mike Trott