- Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, China
Background: The association between a diverse array of environmental risk factors and the risk of endometriosis is contradictory.
Objective: To summarize the evidence of associations between environmental risk factors and the risk of endometriosis.
Methods: Databases such as PubMed, EMBASE, Web of Science, and ClinicalTrial.gov were systematically searched in June 2020. Meta-analyses of observational studies investigated any environmental exposure (non-genetic) and endometriosis risk. For each article, we estimated the summary effect size, 95% CIs, and the 95% prediction interval (PI). We also estimated the between-study heterogeneity expressed by I2, evidence for small-study effects, and evidence of excess significance bias.
Results: About 12 eligible articles (featuring 143,422 cases and 5,112,967 participants) yielded data on 40 unique environmental risk factors, including life styles (n = 16), reproductive factors (n = 3), early life factors (n = 4), and a range of other risk factors [e.g., phthalate metabolites, endocrine-disrupting chemicals, and body mass index (BMI)]. About 25 of these 40 associations (62.5%) were statistically significant (p < 0.05) under random-effects models. Evidence for an association was indicated for alcohol intake [relative risk (RR): 1.25; 95% CI: 1.11–1.41] and the exposure to endocrine disruptor chemicals (EDCs) (RR: 1.41; 95% CI: 1.23–1.60) while 15 associations presented only weak evidence.
Conclusions: Our analyses showed that alcohol intake and exposure to endocrine-disrupting chemicals may be potential risk factors for endometriosis and supported by suggestive epidemiological evidence. However, it was evident that there was substantial heterogeneity and/or bias between the different studies featured in various meta-analyses included in this review; therefore, the outcomes of our analysis should be interpreted cautiously.
Introduction
Endometriosis is a chronic condition associated with pelvic pain, dyspareunia, and infertility and is thought to affect 6–10% of women of reproductive age (1). A recent report have demonstrated that all burden estimates of endometriosis have decreased on a global basis, however, the incidence, prevalence, and the number of years of life lived with disability, associated with this disease, exhibited an increasing trend in countries with a high sociodemographic index between 1990 and 2017 (2). These data indicate that countries with a high sociodemographic index should continue to focus on reducing the disease burden associated with endometriosis as a matter of priority.
Biologically, endometriosis is an estrogen-dependent, chronic, and inflammatory gynecological condition that is characterized by the proliferation of a functional endometrial tissue that develops outside the uterine cavity (3). In addition, it is suggested that the development of endometriosis is determined by the complex interplay and composite effects of both genetic and environmental risk factors. Families of genes associated with the immune system and inflammatory pathways, cell adhesion, and extracellular matrix remodeling have been reported to be differentially expressed when comparing between women with and without endometriosis (4, 5). As a common environmental risk factor, endocrine disruptor chemicals (EDCs) are widely present in the environment and food chains. EDCs could affect the dynamic balance of sex hormones and mediate the innate immune cell dysregulation, which may play an important role in the pathogenesis of endometriosis (6–8). In addition, dietary intake may also influence the development of endometriosis. Alcohol intake could increase the level of estrogen in blood circulation and induce a variety of cells to produce proinflammatory cytokines, which may be related to the pathogenesis of endometriosis (9). However, although previous epidemiological studies have suggested that several risk factors (e.g., diethylstilbestrol exposure, low birth weight, and early age at menarche) were associated with the risk of endometriosis (10), there is a clear lack of well-established and modifiable risk factors for this disease. Notably, there is still no conclusive evidence for these potential risk factors with respect to either the association itself or its direction. This is because several existing publications have yielded contradictory findings.
To the best of our knowledge, there has been no systematic effort to summarize and critically appraise this body of existing evidence. Therefore, we conducted the first umbrella review of the evidence arising from existing systematic reviews and meta-analyses of observational studies to provide an overview of the breadth, strength, and validity of the reported associations between a diverse array of risk factors and the risk of endometriosis. We summarize the risk factors that have been associated with endometriosis in previous meta-analyses, assess the quality of the methodology used, evaluate the evidence for diverse bias, and determine which of the associations are supported through convincing epidemiological evidence.
Methods
We followed a standardized method and reported our findings in accordance with the recommendations put forward by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (Supplementary Table 1) and Meta-analyses of Observational Studies in Epidemiology recommendations (Supplementary Table 2) (11, 12). Our study protocol was registered with PROSPERO (No: CRD42020200094).
Search Strategy
We performed an umbrella review (i.e., a systematic collection and assessment of multiple systematic reviews and meta-analyses published on a specific Research Topic) focused on the risk factors for endometriosis. We systematically searched the PubMed, EMBASE, Web of Science, and ClinicalTrial.gov databases from inception to June 30, 2020, to identify systematic reviews or meta-analyses of observational studies that were performed to examine the associations of environmental (non-genetic) factors and biomarkers with the risk of endometriosis with no restrictions. We used the following search strategy: (endometriosis) and (meta-analysis or systematic review) (as shown in Supplementary Table 3). In addition, we performed a manual search of the reference lists of all eligible retrieved publications. Two authors independently screened the titles, abstracts, and full-text articles for eligibility, and discrepancies were resolved through a consensus.
Selection and Exclusion Criteria
Publications were initially screened on the basis of the title and by reading the abstract. The full texts of potentially eligible publications were then scrutinized by the two independent investigators (YZ and N-YM). Any disagreement was solved through a discussion. We considered the publications that were meta-analyses of epidemiological studies (case-control, cohort, cross-sectional, and ecological studies) that were conducted to investigate any environmental exposure (non-genetic) and the risk of endometriosis. If an article described a separate meta-analysis of more than one environmental risk factor, we included each of these factors separately. Furthermore, if there was more than one meta-analysis on the same association, we kept the one with the largest number of primary studies included. If meta-analyses on the same association included the same number of primary studies, we kept one with the largest amount of prospective data.
The publications that investigated pure genetic markers of endometriosis were excluded because they did not fall into the remit of this study. Trials were not available for our specific research question. We excluded the systematic reviews that did not feature quantitative analysis, meta-analyses based on individual data without a systematic review, or the articles that included animal trials or laboratory studies. We also excluded the systematic reviews or meta-analyses that lacked study-specific data (risk estimates, the number of cases and controls, or the number of the total study population).
Data Extraction
For each eligible meta-analysis, we extracted the first author's name, journal name, publication year, the number of studies included, study population (general, mixed, or not report), environmental risk factors, type of effect metric in meta-analyses, and level of comparison. Also, we extracted information from each primary study used in meta-analyses, including the first author, publication year, study design (cross-sectional, case-control, or cohort), the number of cases and controls (for case-control studies), total participants or person-years (for cohort studies), risk estimates, and 95% CIs. We extracted the most fully adjusted risk estimates (odds ratio [OR], relative risk [RR], incident risk ratio [IRR], or standardized mean difference [SMD]) and 95% CIs. We also extracted information related to dose-response relationships from all meta-analyses. In practice, the measures of effect yield similar estimates because endometriosis is a rare occurrence. Two independent investigators (YZ and N-YM) extracted the data from eligible publications. In the case of discrepancies, the final decision was made through a discussion.
Statistical Analysis
Estimation of Summary Effects and Heterogeneity
For each meta-analysis, we calculated the summary effect size, along with 95% CIs and the values of p, using both fixed- and random-effects models (13). Between-study heterogeneity was assessed with the I2 statistic (14). We also assessed the uncertainty surrounding heterogeneity estimates by calculating 95% CIs and the values of p (15). I2 values of 50% or more were considered to represent high levels of heterogeneity, whereas values exceeding 75% were considered to represent very high levels of heterogeneity.
Estimation of Prediction Intervals (PIs)
We calculated 95% PIs for the random-effects estimates to account for between-study heterogeneity and to represent the possible range in which the risk estimates of new studies might lie (16).
Assessment of Small-Study Effects
We calculated the SE of the effects associated with the largest data set (with the lowest SE) for each of the included meta-analyses. If the SE was <0.10, then the 95% CI would be <0.20 (which is less than the magnitude of small effect size). Egger's regression asymmetry test was also used to determine small-study effects. The value of p < 0.10 arising from Egger's test and a summary effect size larger than the effect size of the largest study were considered to represent evidence for small-study effects (17).
Evaluation of the Excess Significance
We calculated excess significance bias by investigating whether the observed (O) number of nominally significant findings was significantly different from the expected (E) number of statistically significant studies. To do this, we performed a chi-squared test to compare the difference between O and E (18). The effect size of the largest study in each meta-analysis was used to determine the power estimates for each component of a particular study using a non-central t distribution (19). Excess statistical significance for a single meta-analysis was determined with the value of p < 0.10 and if O > E (18). Statistical analyses were conducted using the Stata version 12.0 software (Stata Corp, College Station, TX, USA) and all values of p were two-tailed.
Assessment of Methodological Quality
Two independent investigators (YZ and N-YM) assessed the methodological quality for each included systematic review and meta-analysis using the A Measurement Tool to Assess systematic Reviews (AMSTAR) 2 checklist (20–22). This is a standardized checklist including 16 criteria that refer to a corresponding methodological aspect of the study. We categorized the overall AMSTAR 2 grade as high, moderate, low, or extremely low quality.
Evidence Grading
Using the methodology described earlier and according to the grading scheme applied in previously published studies, we classified the associations that presented nominally statistically significant summary results (p < 0.05) into convincing, highly suggestive, suggestive, weak evidence, or non-significant associations (23–26). Evidence was defined as convincing when the value of p of the random-effects model was smaller than 10−6, the meta-analysis included more than 1,000 cases or more than 20,000 participants for continuous outcomes if the largest component study in the meta-analysis reported a significant result (p < 0.05), if the 95% PIs excluded the null hypothesis if the I2 statistic for heterogeneity was <50% if there was no evidence of small-study effects (p > 0.10), and if excess significance bias (p > 0.10) was indicated.
Evidence was defined as highly suggestive if the value of p for the random-effects model was <10−6, if the meta-analysis included more than 1,000 cases or more than 20,000 participants for continuous outcomes, or if the largest component study reported a significant result. Evidence was defined as suggestive if the value of p for random effects was <10−3 or if there were more than 1,000 cases or more than 20,000 participants for continuous outcomes. Evidence was defined as weak if the value of P for significant associations was <0.05. We used the “non-significant associations” classification if all associations yielded the value of p > 0.05.
Results
Literature Review
As shown in Figure 1, our initial searches identified 2,685 potentially eligible articles from PubMed, EMBASE, and Web of Science. After applying the inclusion and exclusion criteria, 40 full texts were identified for analysis; of these, we selected 12 articles (9, 27–37) including 40 meta-analyses for the current umbrella review.
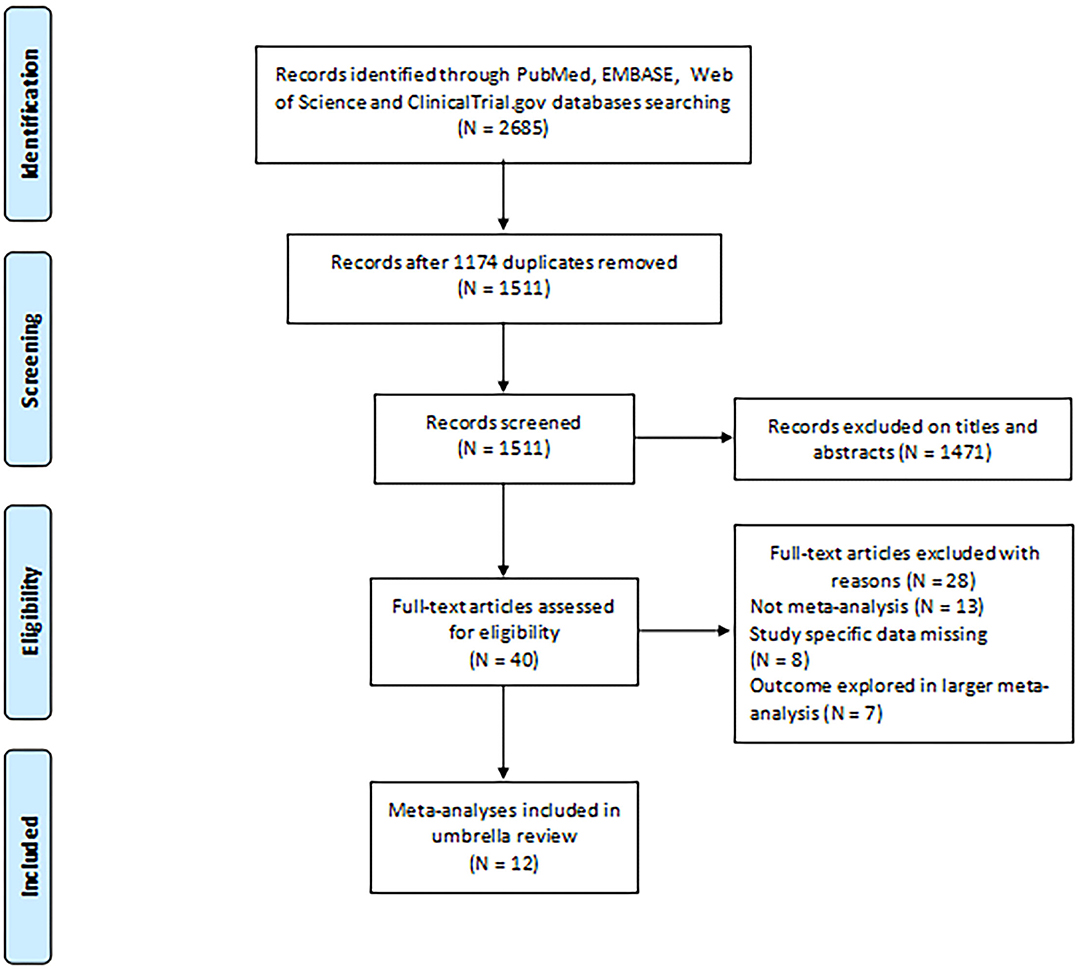
Figure 1. Flowchart depicting the selection of studies for inclusion in our umbrella review relating to the potential association between environmental risk factors and endometriosis.
Characteristics of the Meta-Analyses
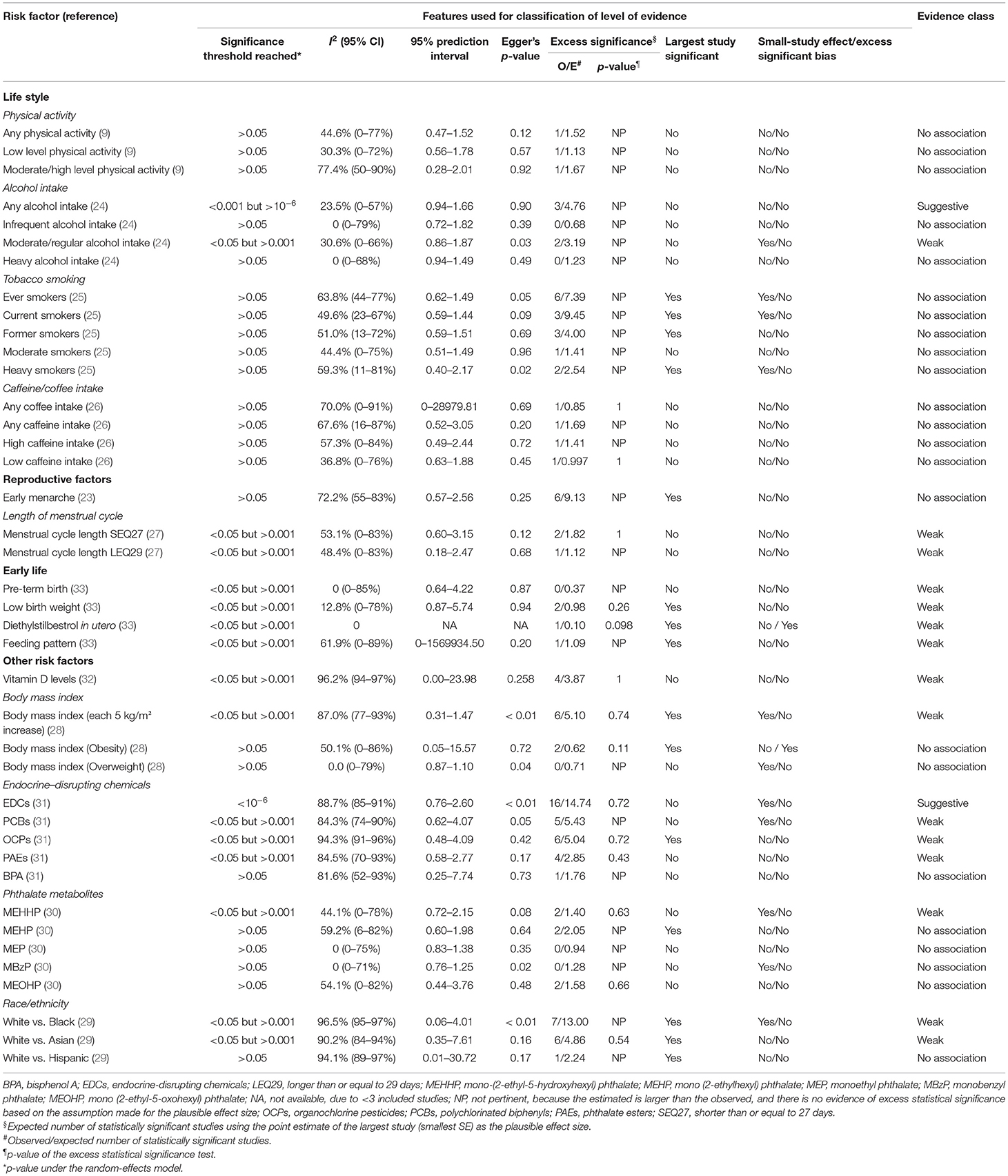
As reported in Table 1 and Supplementary Table 4, the eligible meta-analyses were published between 2012 and 2020, and the median number of articles included for each risk factor was nine (range: 2–30). Of the 354 unique studies, 81 (22.9%) adopted cohort designs, 258 (72.9%) adopted a case-control design, and 15 (4.2%) were cross-sectional studies. About 40 associations between environmental risk factors and endometriosis were identified and based on data from 143,422 cases and a population of 5,112,967. A total of 26 associations included at least 1,000 cases of endometriosis. The meta-analyses reported a wide range of environmental risk factors related to lifestyles (n = 16), reproductive factors (n = 3), early life factors (n = 4), and a range of other risk factors, including vitamin D levels (n = 1), body mass index (BMI) (n = 3), an exposure to EDCs (n = 5), phthalate metabolites (n = 5), and race/ethnicity (n = 3).
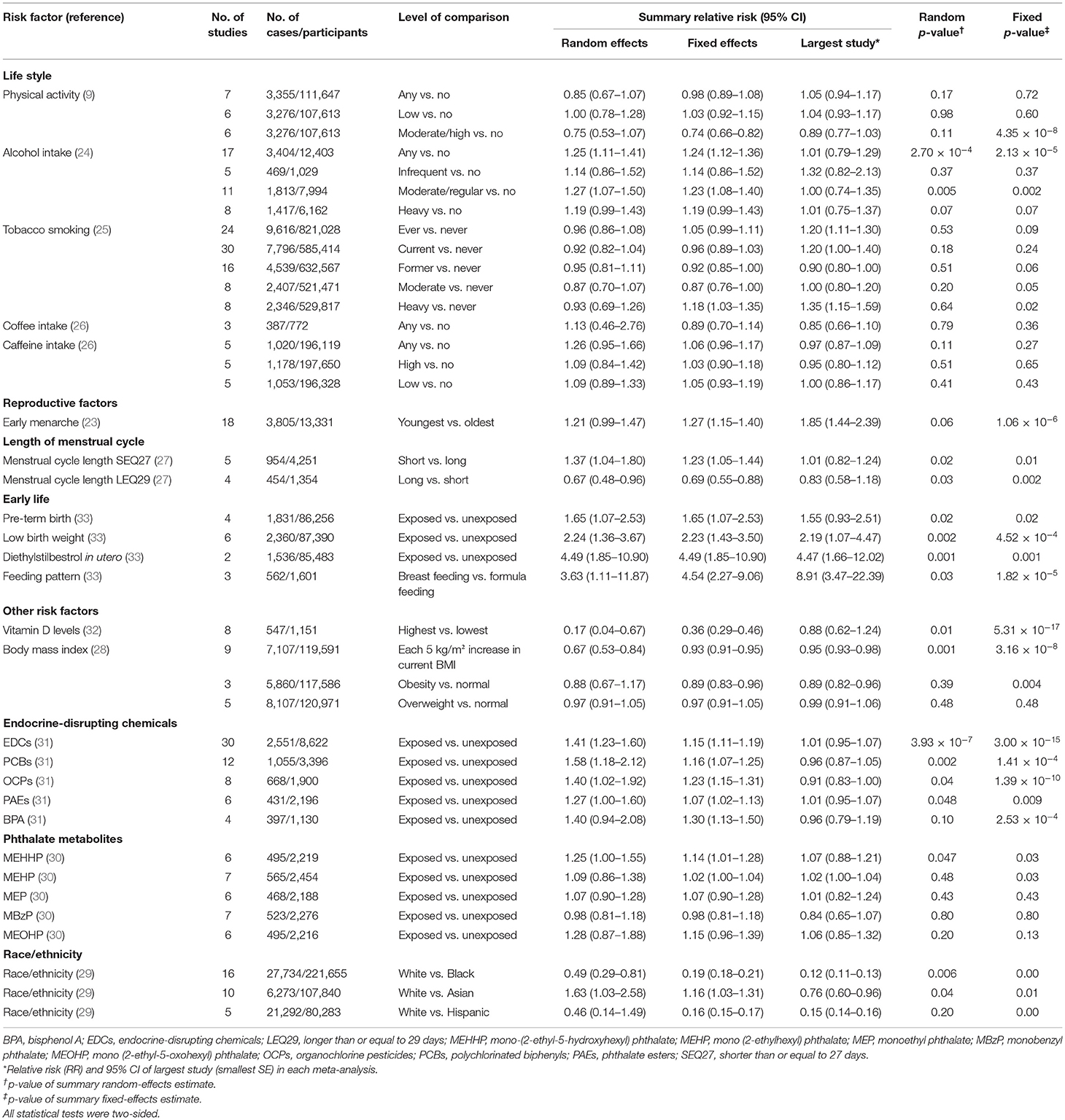
Table 1. Characteristics and quantitative synthesis of the eligible meta-analyses of multiple risk factors for endometriosis.
Summary Effect Size
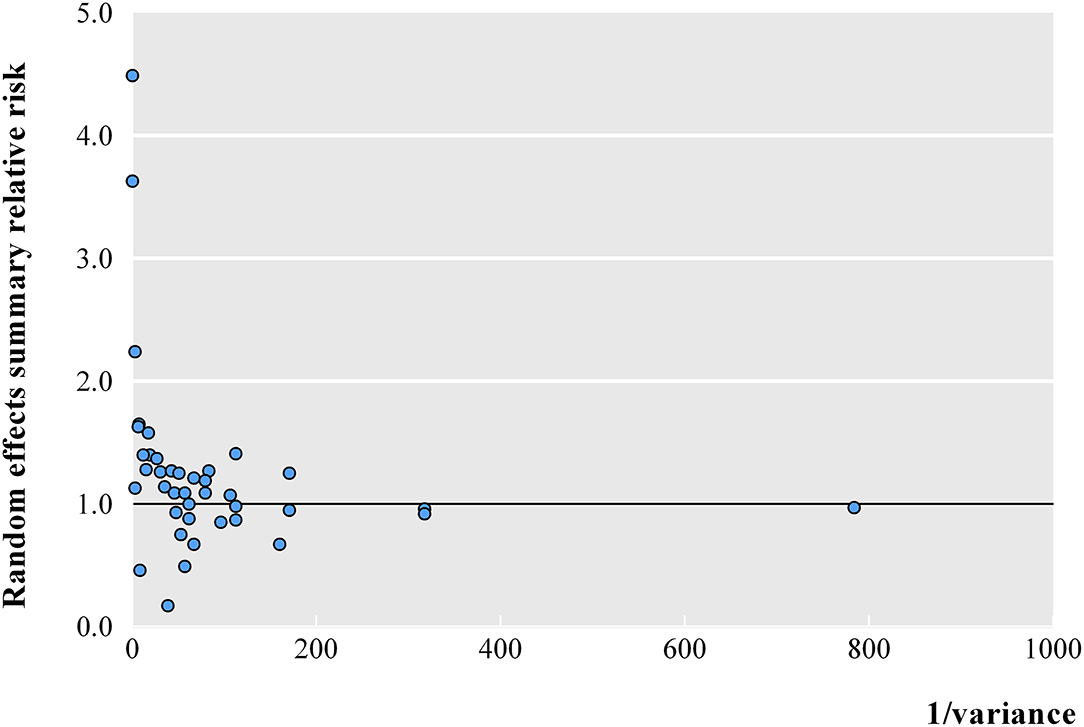
At a threshold of p < 0.05, the summary effect sizes were significant for 25 (62.5%) and 17 (42.5%) association estimates in fixed- and random-effects models, respectively. At a more conservative threshold of p < 10−6, 7 (17.5%) and 1 (2.5%) associations were statistically significant in fixed- and random-effects models, respectively. The magnitude of the observed summary random effect estimates was in the range from 0.17 and 4.49, and 62.5% of the observed estimates were between 0.70 and 1.30 (Figure 2). In a meta-analysis with small variances, it was observed that the summary effect size tended to be 1. However, three associations exhibited evident outliers (feeding pattern in early life, diethylstilbestrol in utero, and BMI of overweight subjects vs. normal subjects).
Heterogeneity and Bias Tests for Meta-Analyses
Overall, the largest study described statistically significant results in 15 meta-analyses (37.5%). About 12 meta-analyses (30.0%) exhibited large heterogeneity (I2 ≥ 50% and I2 ≤ 75%), and 11 (27.5%) showed very large heterogeneity (I2 > 75%) (Table 2). We further assessed the uncertainty of the summary random effects by calculating their 95% PIs; however, the 95% PI excluded the null hypothesis in none of the associations.
Evidence for significant small-study effects was observed in 11 meta-analyses, and evidence for statistically significant excess significance bias was noted for the one risk factors (diethylstilbestrol in utero).
The Methodological Quality of the Meta-Analyses
After evaluating the risk of bias using the AMSTAR 2 tool, the conduct of the articles was rated as low quality for 33.3% (n = 4) of the published articles, and critically low for 66.7% (n = 8) (Supplementary Figure 1). The most frequently deficient critical domains were the lack of a registered protocol (9 of 12 articles) and the lack of a list of excluded studies or justification of the exclusions (9 of 12 articles).
Grading of the Evidence
By applying the predefined methodological criteria, we further investigated whether the nominally significant associations between environmental risk factors and endometriosis were supported by convincing evidence, highly suggestive evidence, suggestive evidence, weak evidence, or no association (Table 2). Overall, no association was supported through convincing and highly suggestive evidence, whereas the associations of the two risk factors (any alcohol intake and exposure to EDCs) for endometriosis were supported by suggestive evidence by virtue of the fact that these two risk factors involved >1,000 cases and the value of p for random effects <10−3. Furthermore, 15 associations (moderate/regular alcohol intake, the length of menstrual cycle [shorter than or equal to 27 days (SEQ27) and longer than or equal to 29 days (LEQ29)], pre-term birth, low birth weight, diethylstilbestrol in utero, feeding pattern, vitamin D levels, BMI, polychlorinated biphenyls (PCBs), organochlorine pesticides (OCPs), phthalate esters (PAEs), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), and race/ethnicity [white vs. black or Asian]) presented weak evidence with the value of p for random effects <0.05. Finally, 23 associations did not present even a nominally statistically significant result.
Discussion
This umbrella review involves previous meta-analyses of observational studies and provides a comprehensive overview and critical assessment of the environmental risk factors associated with endometriosis. About 40 risk factors were investigated for their association with endometriosis, including lifestyle, reproductive factors, early life factors, race/ethnicity, and other risk factors. However, among these factors, only the two factors (any alcohol intake and EDCs) presented suggestive evidence to indicate a strong, significant, and positive association with endometriosis. Several other putative risk factors (e.g., moderate/regular alcohol intake, the length of menstrual cycle, and early life factors such as, pre-term birth, low birth weight, and feeding pattern) presented weak evidence for their association with endometriosis.
Four of the included meta-analyses investigated the relationship between alcohol intake and the risk of endometriosis (9). However, only the “any alcohol intake” criterion was supported by evidence with suggestive epidemiological credibility. Furthermore, despite a significant positive relationship between any alcohol intake and the risk of endometriosis, the 95% PI of the effect size included the null hypothesis, thus showing that in some settings the effect of any alcohol intake on endometriosis might be absent. Consistent with our findings, a review conducted by Agarwal et al. (38) reported that alcohol consumption may catalyze the production of oxidative stress and reactive oxygen species; these factors may increase the risk of endometriosis (38). Endometriosis is an estrogen-dependent disease (39), and ovarian sex steroid receptors have been identified in ectopic endometrial tissue (40). Women suffering from alcohol dependence or abuse are often anovulatory and exhibit ovarian pathology, luteal phase defects, recurrent abortion, and infertility (41). Occasionally, alcoholics have premenstrual symptoms, dysmenorrhea, and a heavy menstrual flow (41). All of these factors are known to be related to endometriosis (41). It is plausible that alcohol could increase the activity of aromatase, an enzyme that converts testosterone to estrogen, thus leading to a reduction in testosterone levels and an increase in estrogen levels (42). Alcohols may also interact with a luteinizing hormone derived from the pituitary, thus causing the ovaries to release more amount of estradiol (43). The summary RR was significant and showed a relatively strong effect between the exposure to EDCs and the risk of endometriosis with suggestive epidemiological evidence. However, this particular meta-analysis (35) exhibited very large levels of heterogeneity, small-study effects; moreover, the 95% PI included the null hypothesis, thus showing that the effect size of the relationship might vary in different settings.
Endocrine disruptor chemicals are exogenous chemical entities or mixtures of compounds, which exert their toxicity by interfering with the normal hormonal homeostatic mechanisms that promote the growth and development of tissues (44). Our results are consistent with several previous experimental studies, which reported that the exposure of prenatal mice to bisphenol A (BPA) can cause endometriosis-like symptoms in offspring (45, 46). The relationship between EDCs and the risk of endometriosis is credible because EDCs exhibit a variety of biological effects, including the ability to alter hormone synthesis, regulate receptors, or act as agonists or antagonists (47). Estrogen is necessary for the proliferation and survival of endometriotic tissues (48). A study conducted by Lemaire et al. (49) indicated that many EDCs bind and activate estrogen receptor- (ER-) alpha and exhibit a dose-dependent agonist/antagonist effect on ER signaling; this effect is essential for angiogenesis and inflammatory signaling during the development of endometriotic lesions.
Additional potential risk factors showed weak evidence for endometriosis, including moderate/regular alcohol intake, the length of the menstrual cycle, early life factors (e.g., pre-term birth, low birth weight, and feeding pattern) vitamin D levels, BMI, certain types of EDCs (e.g., PCBs and OCPs), MEHHP, and race/ethnicity factors. This might be due to the fact that these meta-analyses had large or very large levels of heterogeneity and small-study effect/excess significant bias. We also found that breastfeeding was positively associated with the risk of endometriosis although this may be affected by a certain amount of bias (37). A previous experimental study involving Wistar rats described the evolution of endometriotic implants from pregnancy to lactation and showed a marked tendency for regression in the grade of growth (50). Histologically, during lactation, the endometriotic implants showed signs of reduced cellular activity, such as the presence of tiny cysts in the epithelium-lining that were devoid of vesicular nuclei or prominent nucleoli with only a small extent of apical cytoplasmic secretion (50). To some extent, this suggests that breastfeeding may reduce the risk of endometriosis. This might be due to the fact that breastfeeding can prolong post-partum amenorrhea, influence retrograde menstruation, increase concentrations of circulating oxytocin, and inhibit circulating concentrations of estrogen, gonadotrophin-releasing hormone, luteinizing hormone, and follicle-stimulating hormone (51).
With regard to race/ethnicity, the results related to the risk of endometriosis have been inconsistent thus far. For example, a meta-analysis conducted by Bougie indicated that the risk of endometriosis is higher in Asian women and lower in black women (33); this was contradictory to our present findings. This might be related to socioeconomic factors. When the unit of analyses was a group, race/ethnicity was kept constant, the socioeconomic level of the entire study population was regarded as homogeneous, the prevalence of endometriosis was positively associated with socioeconomic status (52). In another paper, Ridley stated that the frequency of endometriosis increases in any racial group as socioeconomic status improves. It would be expected that women with a higher socioeconomic status in more affluent countries may have better access to care and therefore would be more likely to be diagnosed.
To the best of our knowledge, this is the first systematic and comprehensive assessment of the potential association between environmental factors and the risk of endometriosis using a robust analysis and by evaluating biases and methodological limitations. The classification of evidence was based on extensive statistical analysis and a large number of previous meta-analyses and aims to assess the strength and validity of the published evidence. The criteria chosen to classify each meta-analysis by evidence level (i.e., convincing, highly suggestive, suggestive, or weak) is a transparent and systematic means of assessing the strength of evidence in the literature. In addition, we used AMSTAR-2 to assess the methodological quality of the mate-analyses included; this strategy had helped to identify the most common reasons for reduced quality and will help to improve the quality of future articles in this field.
Nevertheless, there are some possible limitations and caveats associated with this study. First, the present analysis relies upon the articles cited by the original authors and the results that have already been published in systematic reviews and meta-analyses. Although some studies may have been missed in the initial searches, this is unlikely to have affected our results, as repeat meta-analysis resulted in similar results. Second, the statistical tests we used to explore the existence of bias can only provide clues for the presence of potential bias, these tests cannot prove the existence of bias or its exact source. However, our estimates may be conservative because a negative result for bias does not rule out its potential existence. Third, we did not assess the quality of the preliminary studies for individual components as this was beyond the scope of this umbrella review; rather, the initial systematic review and meta-analysis were responsible for this aspect. However, we classified epidemiological evidence on the basis of well-recognized and pre-specified criteria. Fourth, some of the included meta-analyses exhibited large or very large levels of heterogeneity, along with signs of small-study effects or excess significance. In cases of particularly extensive heterogeneity, it is possible that Egger's test may lead to false signals with small-study effects (53, 54). Heterogeneity may often be an expression of bias in some studies involving meta-analysis although this can also arise from real discrepancies between different studies. To our knowledge, the incidence and prevalence of endometriosis show obvious geographical heterogeneity; this may manifest as risk factors showing differential relationships in different geographical regions (2). In addition, there are several other factors that could contribute to heterogeneity, including the integration of cohort studies and case-control studies, discrepancies in the estimation of exposure, discrepancies in the definition and diagnosis of endometriosis, the frequency of exposure in control groups, the types of exposure and the source of controls, and differential response rates among cases and controls. Therefore, we should consider the association between environmental factors and risk factors for endometriosis with caution, particularly for meta-analyses exhibiting extensive heterogeneity. Finally, while we are concerned about bias and other issues that may lead to false-positive relationships, it is also that false negatives may also exist, particularly for associations with limited evidence. Despite these potential limitations, we describe the state of association between environmental factors and the risk of endometriosis. The potential clinical significance of identifying strong correlations between these parameters is to identify individuals at a higher risk of endometriosis. This may allow us to organize appropriate screening programs to detect the preclinical phases of endometriosis.
Conclusion
In conclusion, the intake of any alcohol and exposure to EDCs may represent potential risk factors for endometriosis; these associations were linked through suggestive epidemiological evidence. However, further research is still needed. Such research should incorporate data from a larger number of studies and investigate the specific sources of heterogeneity so that we can better understand the relationship between these factors and the risk of endometriosis.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. Requests to access these datasets should be directed to bWFuaW5neWVoZWFkQDE2My5jb20=.
Author Contributions
N-YM: conceived and designed the study. YZ: literature search, data curation, formal analysis, and writing the original draft. All authors: writing, reviewing, and editing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.680833/full#supplementary-material
References
1. Kuznetsov L, Dworzynski K, Davies M, Overton C. Diagnosis and management of endometriosis: summary of NICE guidance. BMJ. (2017) 358:j3935. doi: 10.1136/bmj.j3935
2. Zhang S, Gong TT, Wang HY, Zhao YH, Wu QJ. Global, regional, and national endometriosis trends from 1990 to 2017. Ann N Y Acad Sci. (2021) 1484:90–101. doi: 10.1111/nyas.14468
3. Donnez J, Van Langendonckt A, Casanas-Roux F, Van Gossum JP, Pirard C, Jadoul P, et al. Current thinking on the pathogenesis of endometriosis. Gynecol Obstet Invest. (2002) 54(Suppl. 1):52–8. doi: 10.1159/000066295
4. Eyster KM, Klinkova O, Kennedy V, Hansen KA. Whole genome deoxyribonucleic acid microarray analysis of gene expression in ectopic versus eutopic endometrium. Fertil Steril. (2007) 88:1505–33. doi: 10.1016/j.fertnstert.2007.01.056
5. Wren JD, Wu Y, Guo SW. A system-wide analysis of differentially expressed genes in ectopic and eutopic endometrium. Hum Reprod. (2007) 22:2093–102. doi: 10.1093/humrep/dem129
6. Caserta D, Maranghi L, Mantovani A, Marci R, Maranghi F, Moscarini M. Impact of endocrine disruptor chemicals in gynaecology. Hum Reprod Update. (2008) 14:59–72. doi: 10.1093/humupd/dmm025
7. Sharma P, Tseng HH, Lee JL, Tsai EM, Suen JL. A prominent environmental endocrine disruptor, 4-nonylphenol, promotes endometriosis development via plasmacytoid dendritic cells. Mol Hum Reprod. (2020) 26:601–14. doi: 10.1093/molehr/gaaa039
8. Soave I, Caserta D, Wenger JM, Dessole S, Perino A, Marci R. Environment and Endometriosis: a toxic relationship. Eur Rev Med Pharmacol Sci. (2015) 19:1964–72.
9. Parazzini F, Cipriani S, Bravi F, Pelucchi C, Chiaffarino F, Ricci E, et al. A metaanalysis on alcohol consumption and risk of endometriosis. Am J Obstet Gynecol. (2013) 209:101–6. doi: 10.1016/j.ajog.2013.05.039
10. Zondervan KT, Becker CM, Missmer SA. Endometriosis. N Engl J Med. (2020) 382:1244–56. doi: 10.1056/NEJMra1810764
11. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
12. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
13. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
14. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
15. Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. (2007) 335:914–6. doi: 10.1136/bmj.39343.408449.80
16. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. (2011) 342:d549. doi: 10.1136/bmj.d549
17. Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
18. Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials. (2007) 4:245–53. doi: 10.1177/1740774507079441
19. Tsilidis KK, Panagiotou OA, Sena ES, Aretouli E, Evangelou E, Howells DW, et al. Evaluation of excess significance bias in animal studies of neurological diseases. PLoS Biol. (2013) 11:e1001609. doi: 10.1371/journal.pbio.1001609
20. Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. (2007) 7:10. doi: 10.1186/1471-2288-7-10
21. Shea BJ, Bouter LM, Peterson J, Boers M, Andersson N, Ortiz Z, et al. External validation of a measurement tool to assess systematic reviews (AMSTAR). PLoS ONE. (2007) 2:e1350. doi: 10.1371/journal.pone.0001350
22. Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. (2009) 62:1013–20. doi: 10.1016/j.jclinepi.2008.10.009
23. Kalliala I, Markozannes G, Gunter MJ, Paraskevaidis E, Gabra H, Mitra A, et al. Obesity and gynaecological and obstetric conditions: umbrella review of the literature. BMJ. (2017) 359:j4511. doi: 10.1136/bmj.j4511
24. Tsilidis KK, Papatheodorou SI, Evangelou E, Ioannidis JP. Evaluation of excess statistical significance in meta-analyses of 98 biomarker associations with cancer risk. J Natl Cancer Inst. (2012) 104:1867–78. doi: 10.1093/jnci/djs437
25. Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. (2015) 350:g7607. doi: 10.1136/bmj.g7607
26. Kim JY, Son MJ, Son CY, Radua J, Eisenhut M, Gressier F, et al. Environmental risk factors and biomarkers for autism spectrum disorder: an umbrella review of the evidence. Lancet Psychiat. (2019) 6:590–600. doi: 10.1016/S2215-0366(19)30181-6
27. Nnoaham KE, Webster P, Kumbang J, Kennedy SH, Zondervan KT. Is early age at menarche a risk factor for endometriosis? A systematic review and meta-analysis of case-control studies. Fertil Steril. (2012) 98:702–12. doi: 10.1016/j.fertnstert.2012.05.035
28. Bravi F, Parazzini F, Cipriani S, Chiaffarino F, Ricci E, Chiantera V, et al. Tobacco smoking and risk of endometriosis: a systematic review and meta-analysis. BMJ Open. (2014) 4:e6325. doi: 10.1136/bmjopen-2014-006325
29. Chiaffarino F, Bravi F, Cipriani S, Parazzini F, Ricci E, Vigano P, et al. Coffee and caffeine intake and risk of endometriosis: a meta-analysis. Eur J Nutr. (2014) 53:1573–9. doi: 10.1007/s00394-014-0662-7
30. Wei M, Cheng Y, Bu H, Zhao Y, Zhao W. Length of menstrual cycle and risk of endometriosis: a meta-analysis of 11 case-control studies. Medicine. (2016) 95:e2922. doi: 10.1097/MD.0000000000002922
31. Ricci E, Vigano P, Cipriani S, Chiaffarino F, Bianchi S, Rebonato G, et al. Physical activity and endometriosis risk in women with infertility or pain: systematic review and meta-analysis. Medicine. (2016) 95:e4957. doi: 10.1097/MD.0000000000004957
32. Liu Y, Zhang W. Association between body mass index and endometriosis risk: a meta-analysis. Oncotarget. (2017) 8:46928–36. doi: 10.18632/oncotarget.14916
33. Bougie O, Yap MI, Sikora L, Flaxman T, Singh S. Influence of race/ethnicity on prevalence and presentation of endometriosis: a systematic review and meta-analysis. BJOG. (2019) 126:1104–15. doi: 10.1111/1471-0528.15692
34. Cai W, Yang J, Liu Y, Bi Y, Wang H. Association between phthalate metabolites and risk of endometriosis: a meta-analysis. Int J Environ Res Public Health. (2019) 16:3678. doi: 10.3390/ijerph16193678
35. Wen X, Xiong Y, Qu X, Jin L, Zhou C, Zhang M, et al. The risk of endometriosis after exposure to endocrine-disrupting chemicals: a meta-analysis of 30 epidemiology studies. Gynecol Endocrinol. (2019) 35:645–50. doi: 10.1080/09513590.2019.1590546
36. Qiu Y, Yuan S, Wang H. Vitamin D status in endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. (2020) 302:141–52. doi: 10.1007/s00404-020-05576-5
37. Ottolina J, Schimberni M, Makieva S, Bartiromo L, Fazia T, Bernardinelli L, et al. Early-life factors, in-utero exposures and endometriosis risk: a meta-analysis. Reprod Biomed Online. (2020) 41:279–89. doi: 10.1016/j.rbmo.2020.04.005
38. Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. (2012) 10:49. doi: 10.1186/1477-7827-10-49
39. Barbieri RL, Gordon AM. Hormonal therapy of endometriosis: the estradiol target. Fertil Steril. (1991) 56:820–2. doi: 10.1016/S0015-0282(16)54648-2
40. Bergqvist A. Steroid receptors in endometriosis. In: Thomas EJ, Rock JA, editors. Modern Approaches to Endometriosis. Lancaster: Kluwer Academic Publishers (1991). p. 33–55. doi: 10.1007/978-94-011-3864-2_3
41. Thylan S. Endometriosis and moderate alcohol use. Am J Public Health. (1995) 85:1021–2. doi: 10.2105/AJPH.85.7.1021-a
42. Gavaler JS, Van Thiel DH. The association between moderate alcoholic beverage consumption and serum estradiol and testosterone levels in normal postmenopausal women: relationship to the literature. Alcohol Clin Exp Res. (1992) 16:87–92. doi: 10.1111/j.1530-0277.1992.tb00642.x
43. Rettori V, McCann SM. The mechanism of action of alcohol to suppress gonadotropin secretion. Mol Psychiatry. (1997) 2:350–4. doi: 10.1038/sj.mp.4000306
44. Sifakis S, Androutsopoulos VP, Tsatsakis AM, Spandidos DA. Human exposure to endocrine disrupting chemicals: effects on the male and female reproductive systems. Environ Toxicol Pharmacol. (2017) 51:56–70. doi: 10.1016/j.etap.2017.02.024
45. Signorile PG, Spugnini EP, Mita L, Mellone P, D'Avino A, Bianco M, et al. Pre-natal exposure of mice to bisphenol A elicits an endometriosis-like phenotype in female offspring. Gen Comp Endocrinol. (2010) 168:318–25. doi: 10.1016/j.ygcen.2010.03.030
46. Signorile PG, Spugnini EP, Citro G, Viceconte R, Vincenzi B, Baldi F, et al. Endocrine disruptors in utero cause ovarian damages linked to endometriosis. Front Biosci. (2012) 4:1724–30. doi: 10.2741/e493
47. Sonnenschein C, Soto AM. An updated review of environmental estrogen and androgen mimics and antagonists. J Steroid Biochem Mol Biol. (1998) 65:143–50. doi: 10.1016/S0960-0760(98)00027-2
49. Lemaire G, Mnif W, Mauvais P, Balaguer P, Rahmani R. Activation of alpha- and beta-estrogen receptors by persistent pesticides in reporter cell lines. Life Sci. (2006) 79:1160–9. doi: 10.1016/j.lfs.2006.03.023
50. Barragan JC, Brotons J, Ruiz JA, Acien P. Experimentally induced endometriosis in rats: effect on fertility and the effects of pregnancy and lactation on the ectopic endometrial tissue. Fertil Steril. (1992) 58:1215–9. doi: 10.1016/S0015-0282(16)55572-1
51. Farland LV, Eliassen AH, Tamimi RM, Spiegelman D, Michels KB, Missmer SA. History of breast feeding and risk of incident endometriosis: prospective cohort study. BMJ. (2017) 358:j3778. doi: 10.1136/bmj.j3778
52. Houston DE. Evidence for the risk of pelvic endometriosis by age, race and socioeconomic status. Epidemiol Rev. (1984) 6:167–91. doi: 10.1093/oxfordjournals.epirev.a036270
53. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. doi: 10.1136/bmj.d4002
Keywords: endometriosis, meta-analysis, observational study, risk factor, umbrella review
Citation: Zhang Y and Ma N-Y (2021) Environmental Risk Factors for Endometriosis: An Umbrella Review of a Meta-Analysis of 354 Observational Studies With Over 5 Million Populations. Front. Med. 8:680833. doi: 10.3389/fmed.2021.680833
Received: 15 March 2021; Accepted: 23 September 2021;
Published: 25 October 2021.
Edited by:
Michael David Mueller, Bern University Hospital, SwitzerlandReviewed by:
Donatella Caserta, Sapienza University of Rome, ItalySvend Lindenberg, Copenhagen Fertility Center, Denmark
Copyright © 2021 Zhang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning-Ye Ma, bWFuaW5neWVoZWFkQDE2My5jb20=
 Ye Zhang
Ye Zhang Ning-Ye Ma
Ning-Ye Ma