- 1State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2National Clinical Research Center for Infectious Diseases, Hangzhou, China
- 3Department of Ultrasound, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 4Zhejiang Provincial Key Laboratory for Diagnosis and Treatment of Aging and Physic-Chemical Injury Diseases, Hangzhou, China
Background and Aims: Viral hepatitis are one of the main causes of liver cirrhosis. The treatment of portal hypertension caused by liver cirrhosis is difficult and diverse, and the therapeutic effect is unknown. Bayesian network meta-analysis was performed to compare the efficacy and safety of treatments for patients with portal hypertension and cirrhosis, including a transjugular intrahepatic portosystemic shunt (TIPS), endoscopic therapy, surgical therapy and medications.
Methods: Eligible articles were searched for in PubMed, Embase, Cochrane Library and Web of Science databases from their inception until June 2020. Using the “gemtc-0.8.4” package in R v.3.6.3 software and the Just Another Gibbs Sampler v.4.2.0 program, network meta-analysis was performed using a random effects model within a Bayesian framework. The odds ratios for all-cause rebleeding, bleeding-related mortality, overall survival (OS), treatment failure and hepatic encephalopathy were determined within the Bayesian framework.
Results: Forty randomized controlled trials were identified, including 4,006 adult patients and nine treatment strategies. Our results showed that distal splenorenal shunt and TIPS provided the best control of hemorrhage. Endoscopic variceal ligation with medication resulted in the highest OS rate. Medication alone resulted in poor OS and treatment failure.
Conclusions: We performed a systematic comparison of diverse treatments for cirrhotic patients with portal hypertension. Our meta-analysis indicated that a TIPS and distal splenorenal shunt resulted in lower rates of rebleeding than did other therapies. Furthermore, drugs are more suitable for combination therapy than monotherapy.
Introduction
Esophageal and gastric variceal hemorrhage is a common and life-threatening complication for patients with cirrhosis and portal hypertension (1). In approximately one-third of patients with cirrhosis, hemorrhage may cause early mortality (2). After the first variceal bleeding is addressed, the incidence of rebleeding within 1–2 years is 60–70%, and the mortality rate can be as high as ~20–33% (3, 4). Therefore, monitoring or choosing additional appropriate treatment of hemostasis for the first variceal bleeding may help improve quality of life and prognosis.
Over the past 20 years, various effective treatments for portal hypertension have been developed. Non-selective beta-blockers (β-blockers) remain the cornerstone of bleeding and have been used for more than 30 years (5). In addition, somatostatin and terlipressin are potent splanchnic vasoconstrictors (6). These agents significantly decrease both the hepatic venous pressure gradient and portal-collateral (azygos) blood flow and are used to reduce the risk of bleeding (7). Endoscopic therapy (ET), interventional therapy and surgery are also often used to control hemorrhage. ET involves mainly endoscopic injection sclerotherapy (EIS) and endoscopic variceal ligation (EVL). However, EVL has been shown to result in lower rebleeding, mortality and complication rates compared with EIS (8–10). Transjugular intrahepatic portosystemic shunt (TIPS) is a metal stent that connects the hepatic vein and intrahepatic portal vein to effectively decrease portal pressure and prevent ascites aggravation and rebleeding (11). It is ideal to maintain the portal pressure gradient of the portal and inferior vena cava between 10 and 12 mm Hg (5). Some studies have indicated that the primary unassisted patency rates of polytetrafluorethylene-covered stents are similar to those of surgical shunting (10).
Additionally, several randomized controlled trials (RCTs) have shown that combination therapy may be superior to monotherapy in terms of rebleeding, survival and complication rates (12). Argonz et al. reported increased recurrence of bleeding in the EVL group compared with the EVL plus EIS group (31.7 vs. 23%) (13). Similarly, a meta-analysis found that EVL plus nadolol or sucralfate decreased the risk of rebleeding compared with EVL alone. However, Puente et al. noted that a reduction in rebleeding did not improve survival (14).
To use existing study-level data to assess the relative effectiveness of active interventions in cirrhotic patients with a history of hemorrhage, we performed a network meta-analysis of RCTs that included rebleeding and mortality as outcomes. The purpose of this meta-analysis is to provide guidance for clinical policymakers regarding the safety and efficacy of TIPS, EVL, EIS, medication and combinations of these treatments in terms of the 1-, 2-, and 3-year rebleeding and overall survival (OS) rates, treatment failure, bleeding-related mortality, and HE.
Materials and Methods
Search Strategy
Two researchers independently screened the titles and abstracts of the articles in terms of the selection criteria. The literature search was performed in various electronic databases (PubMed, Web of Science, MEDLINE, Embase and Cochrane Library) from their inception to June 2020. A combination of free-text terms and medical subject heading terms were used for the subject search, as follows: “liver cirrhosis,” “variceal hemorrhage,” “variceal rebleeding,” “transjugular intrahepatic portosystemic shunt,” “balloon-occluded retrograde transvenous obliteration,” “endoscopic therapy,” “beta-blocker,” and “surgery.” The article type was restricted to randomized controlled trials.
Study Selection
Studies were considered eligible for inclusion if they fulfilled the following criteria: (1) focused on treatments for patients with cirrhosis and portal hypertension; (2) compared at least two factors among TIPS, EVL, EIS, medication, or combination therapies; and (3) included rebleeding as a primary endpoint. The exclusion criteria were as follows: (1) not written in English; (2) non-clinical article, such as a case report, letter, basic research study or systematic review; (3) lack of sufficient or qualified data; (4) published before 2,000 or included <20 participants per group.
Data Extraction and Quality Assessment
Two researchers independently extracted the data, and a third researcher was consulted to reach a majority decision when needed. The following information was summarized. (1) The authors' names, year of publication, treatment group, country of study, number of patients and follow-up time. (2) Clinical outcomes including all-cause rebleeding; 1-, 2-, and 3-year rebleeding rates; treatment failure; bleeding-related mortality; 1-, 2-, and 3-year OS rates; and HE. Otherwise, treatment failure was defined as the occurrence of two or more episodes of recurrent bleeding or switching to an alternative treatment. This meta-analysis was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols 2015 statement (15). The methodological quality of the RCTs was evaluated using the Cochrane Collaboration tool (16).
Statistical Analysis
Using the “gemtc” package in R 3.6.3 software, the Markov chain Monte Carlo method was applied to perform Bayesian network meta-analysis (17). This method combines both direct and indirect evidence for any given pair of management strategies and a particular endpoint. The mtc.run function was applied to generate samples, and we set 5,000 simulations for each chain as the “burn-in” period, yielding 20,000 iterations to obtain the odds ratios (ORs) for the model parameters based on three Markov chains. Rank probabilities were calculated to obtain the hierarchical position of each treatment, and a plot of rank probabilities was created using the “gemtc” package (18). Brooks–Gelman–Rubin plots, trace plots and density plots were used to assess model convergence (19).
The mtc.anohe command of the “gemtc” package was used to evaluate global heterogeneity. To ensure reliability, a sensitivity analysis was performed by removal of each trial. Begg's test and Egger's test were applied using a P < 0.1 threshold of significance for testing publication bias.
Results
Eligible Studies and Characteristics
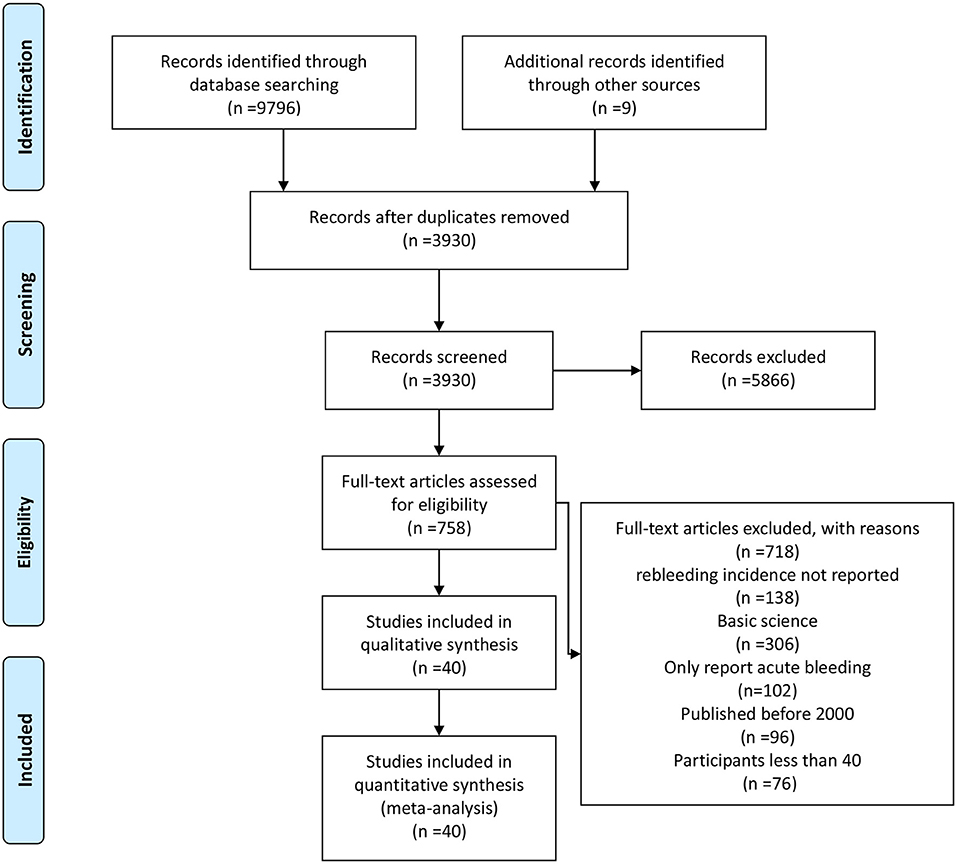
The literature search generated 9,805 relevant clinical records. After screening titles and abstracts, removing duplicates and assessing eligibility, 5,866 articles were excluded; the remaining articles were subjected to full text review. Finally, 40 RCTs including a total of 4,006 patients met the inclusion criteria and were selected for the meta-analysis. A flow chart of the detailed screening process can be found in Figure 1.
Study Characteristics and Quality
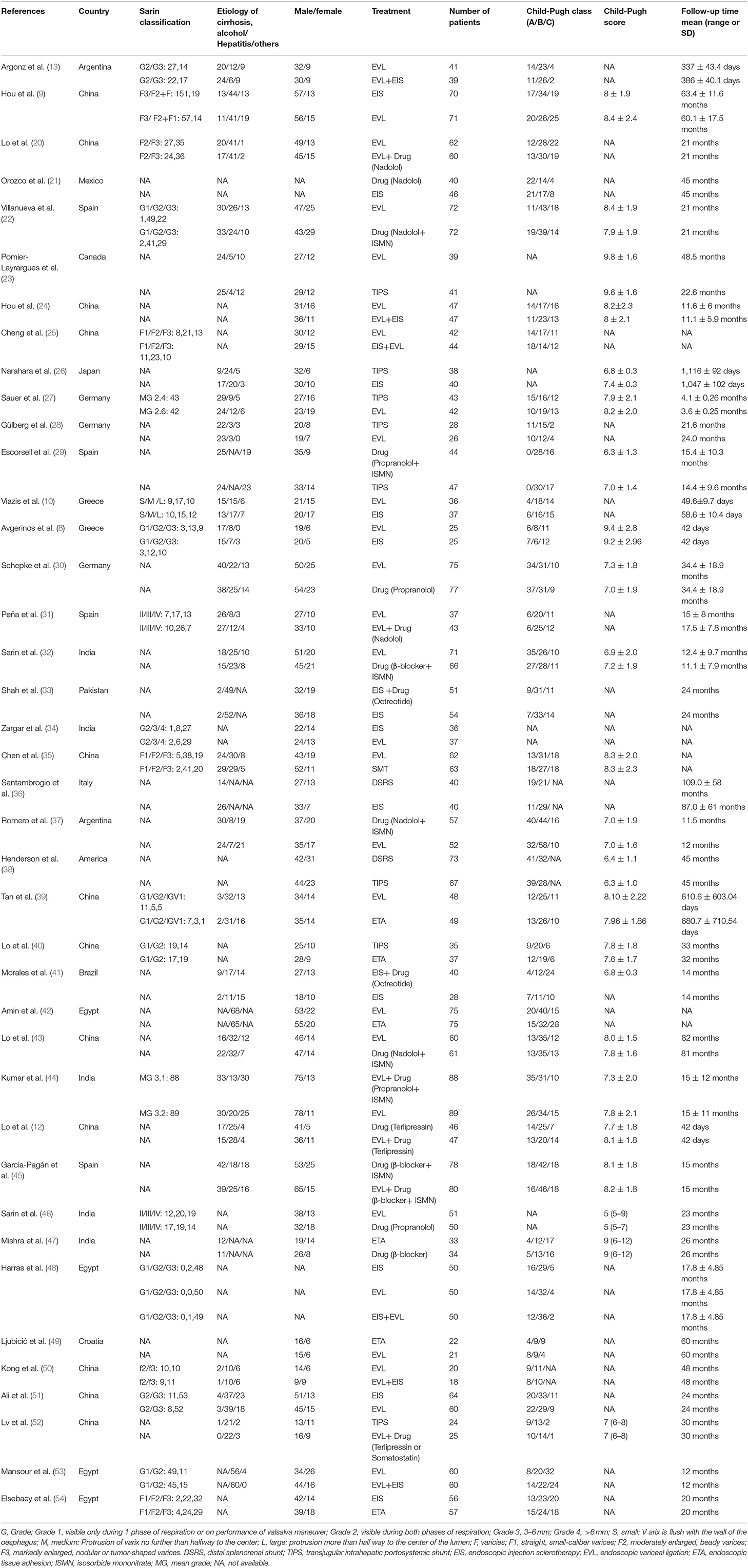
The basic characteristics of the selected studies are summarized in Table 1. In total, 4,006 patients were enrolled in nine different treatment strategies. The 40 studies included 12 from China, five from India, one from Japan, one from Pakistan, one from Mexico, two from Argentina, one from Canada, one from the USA, one Croatia, one Brazil, one from Italy, four from Egypt, three from Germany, four from Spain, and two from Greece. Thus, 19 studies were performed in Asia, 11 in Europe, six in America, and four in Africa. Seventeen of the studies had a mean follow up time of >2 years. Medications included propranolol, nadolol, octreotide, terlipressin, and isosorbide-5-mononitrate; ET included EIS, EVL, and endoscopic tissue adhesive (ETA) injection. There were two major types of surgical shunts: portacaval and distal splenorenal shunts. All studies were two-arm trials, except for Harras consisting of three arms: EIS, EVL, and EIS + EVL. Detailed results of the bias assessment are shown in Supplementary Table 1.
Network Structure Diagrams
Nine different therapeutic strategies were included among the trials: EVL, TIPS, distal splenorenal shunt (DSRS), medication, EVL + EIS, EIS, EVL + medication, ETA, and EIS + medication. Network structure diagrams were applied to depict the direct associations among the treatment strategies. The thickness of the lines is proportional to the number of comparisons, and the diameter of the circles is proportional to the number of treatments included in the meta-analysis. All diagrams are presented in Figure 2.
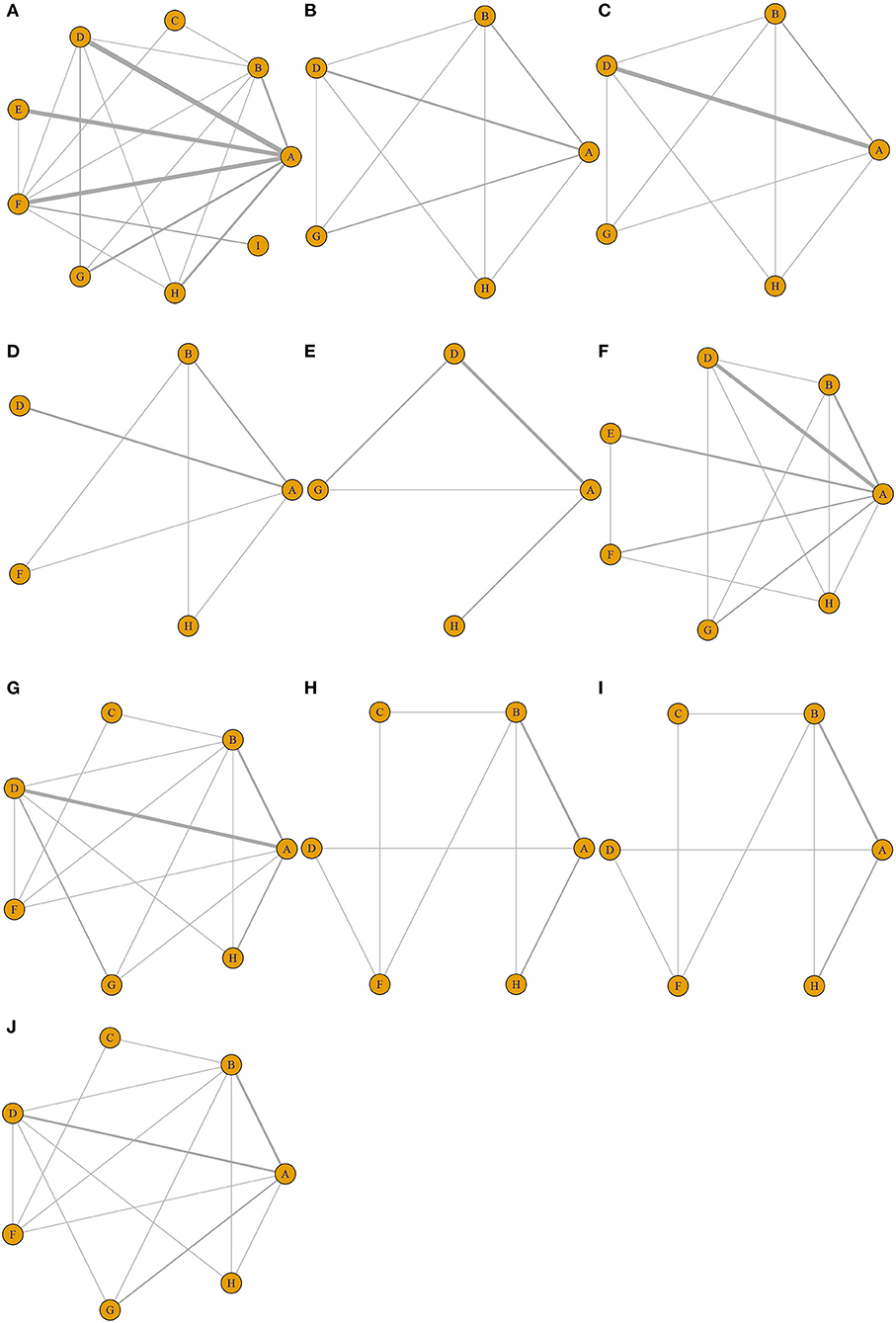
Figure 2. Network structure diagrams. As shown in the figure, the thickness of the lines is proportional to the number of comparisons, and the diameter of the circles is proportional to the number of treatments included in the meta-analysis. (A) All-cause rebleeding. Rebleeding at (B) 1 year, (C) 2 years, and (D) 3 years. (E) Treatment failure. (F) Bleeding-related mortality. OS at (G) 1 year, (H) 2 years, and (I) 3 years. (J) Hepatic encephalopathy.
Brooks–Gelman–Rubin Diagnostic Plot, Density Plot, and Trace Plot
Brooks–Gelman–Rubin diagnostic plots, trace plots and density plots were obtained to assess the convergence of our model. As suggested by Brooks and Gelman (55), the model was considered to be well-fitted if the curves of the plots were consistent and stable, and if the potential scale reduction factor was close to 1.0. For trace plots, each Markov Chain Monte Carlo chain achieved stable fusion from the beginning, and the overlapping area accounted for the majority of chain fluctuation in the subsequent calculations. The fluctuation of single chains could not be recognized by the naked eye, and therefore the degree of convergence was considered satisfactory, as shown in Supplementary Figure 1. In the density diagram, the bandwidth tended to be zero and stable, and a smooth curve that conformed to the normal distribution indicated that the model had good convergence (Supplementary Figure 1). Furthermore, the potential scale reduction factor for each analysis was close to 1.0 in the Brooks–Gelman–Rubin diagnostic plot, as shown in Supplementary Figure 2 and Supplementary Table 2.
All-Cause Rebleeding
Forty studies, including 4,006 patients with nine therapeutic schedules, reported all-cause rebleeding. There were many significant differences among the therapeutic schedules, such as TIPS vs. EVL (OR 0.31, 95% CI 0.15–0.66), medication vs. TIPS (OR 5.4, 95% CI 2.3–13.0), EIS vs. TIPS (OR 4.1, 95% CI 1.7–10.), medication vs. DSRS (OR 1.3, 95% CI 0.30–5.6), EIS vs. DSRS (OR 5.3, 95% CI 1.3–23.), medication vs. EVL + EIS (OR 2.9, 95% CI 1.1–8), EVL + medication vs. medication alone (OR 0.36, 95% CI 0.15–0.85), ETA vs. medication (OR 0.26, 95% CI 0.10–0.66), and ETA vs. EIS (OR 0.34, 95% CI 0.13–0.88) (Figure 3). Subgroup analyses of rebleeding occurring at 1, 2, and 3 years are summarized in Supplementary Figures 3–5. Compared with TIPS, EVL (OR 6.1, 95% CI 2.2–18.0; OR 6.6, 95% CI 2.5–18.0; and OR 5.0, 95% CI 0.9–28.0 at 1, 2, and 3 years, respectively) and medication (OR 4.9, 95% CI 1.7–17.0; OR 7.9, 95% CI 2.9–23.0; and OR 11, 95% CI 1.0–120.0 at 1, 2, and 3 years, respectively) had higher rates of rebleeding in all years. In descending order, all-cause rebleeding was best controlled by DSRS, TIPS, EIS + medication, ETA, EVL + medication, EVL + EIS, EVL, EIS, and medication alone (Figure 4). In order of decreasing efficacy, rebleeding at 1, 2, and 3 years were best controlled by TIPS, ETA, EVL + medication/EIS, EVL, and medication alone (Supplementary Figure 6).
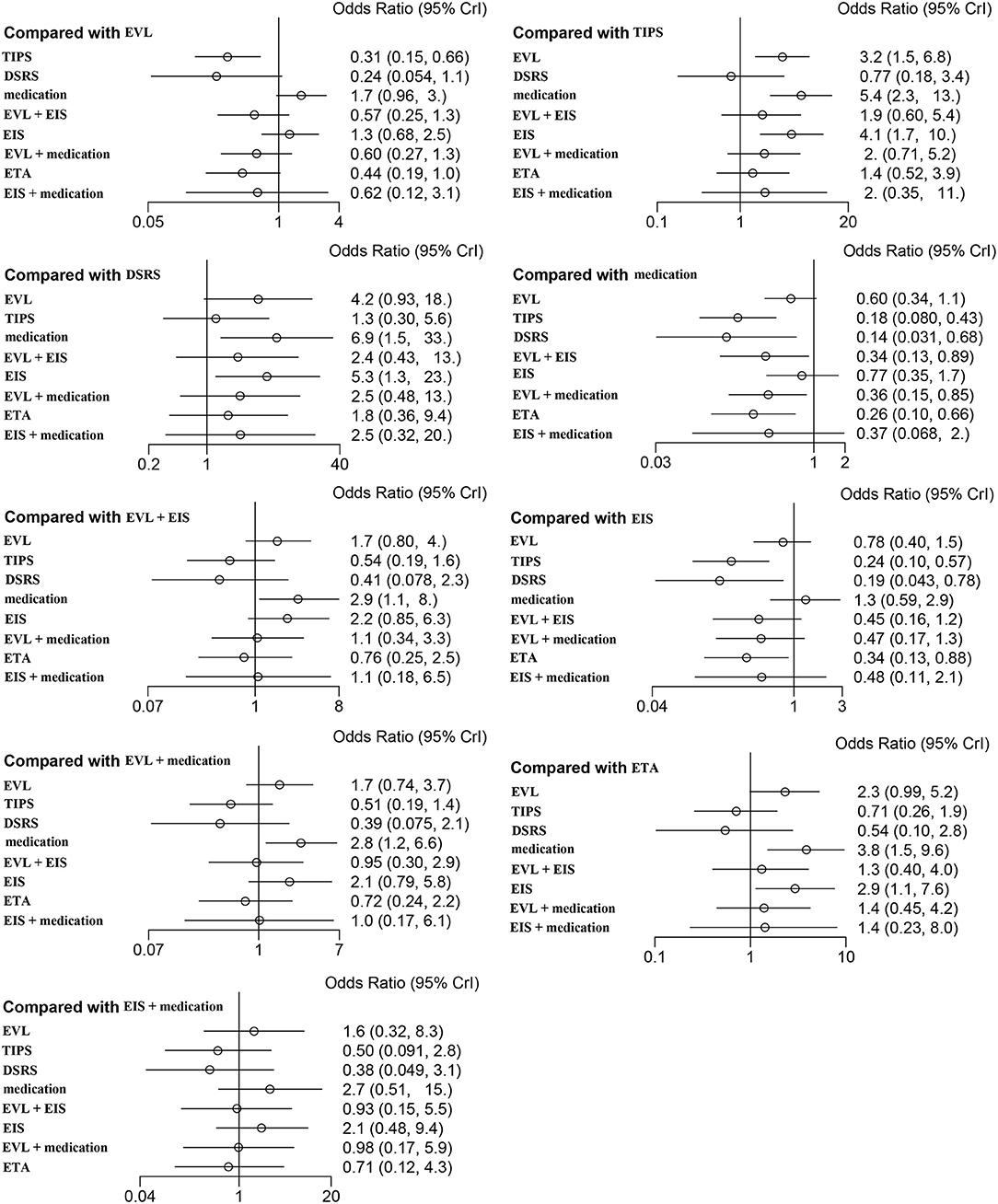
Figure 3. Forest plot of the odds ratios for all-cause rebleeding based on different pairwise comparisons. EVL, endoscopic variceal ligation; TIPS, transjugular intrahepatic portosystemic shunt; DSRS, distal splenorenal shunt; EIS, endoscopic injection sclerotherapy; ETA, endoscopic tissue.
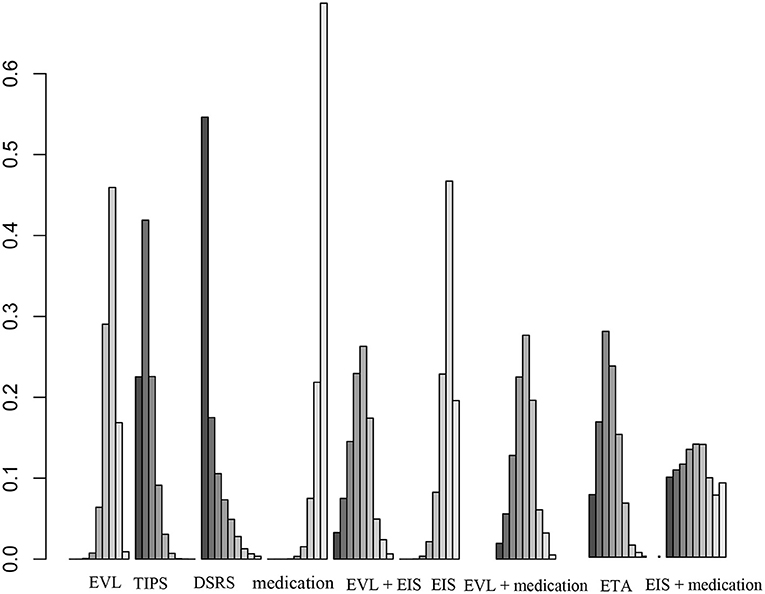
Figure 4. Ranking of therapies based on all-cause rebleeding. EVL, endoscopic variceal ligation; TIPS, transjugular intrahepatic portosystemic shunt; DSRS, distal splenorenal shunt; EIS, endoscopic injection sclerotherapy; ETA, endoscopic tissue.
Bleeding-Related Mortality
Twenty-one articles including seven different treatments (EVL, TIPS, medication, EVL + EIS, EIS, EVL + medication, and ETA) were used in the analysis of bleeding-related mortality. No significant results were identified regarding the previously discussed treatments. Our results showed that EVL (OR 5.0, 95% CI 0.6–100.0), TIPS (OR 2.6, 95% CI 0.2–51.0), medication alone (OR 5.0, 95% CI 0.5–100.0), EIS (OR 5.4, 95% CI 0.5–130.0), EVL + medication (OR 2.4, 95% CI 0.2–54.0), and ETA (OR 2.3, 95% CI 0.2–50.0) were associated with a relatively high rate of rebleeding compared with EVL + EIS (Supplementary Figure 7). As indicated in the cumulative ranking, EVL + EIS ranked most favorably among the treatments (Supplementary Figure 8).
Overall Survival (OS)
Twenty-one of the selected studies assessed the 1-year OS rate in a total of 2,163 patients with cirrhosis. Eleven of the studies assessed 2- and 3-year OS rates in a total of 967 cirrhosis patients. Our results showed that TIPS (OR 1.1, 95% CI 0.5–2.7), DSRS (OR, 0.9 95% CI 0.2–5.9), medication (OR 0.6, 95% CI 0.3–1.3), EIS (OR 0.7, 95% CI 0.2–2.5), EVL + medication (OR 1.5, 95% CI 0.5–5.1), and ETA (OR 1.2, 95% CI 0.4–3.9) did not differ significantly from EVL in terms of 1-year OS (Figure 5). There was also no significant difference among the other treatment methods (Figure 5). EVL, TIPS, DSRS, medication, EIS and ETA offered no significant benefit in terms of the 2- or 3-year OS rate (Supplementary Figures 9, 10). Rank probability analysis showed that medication, EIS, DSRS, TIPS, EVL, ETA, and EVL + medication ranked from worst to best in terms of 1-year OS, and that medication, TIPS, EVL, ETA, DSRS, and EIS ranked from worst to best in terms of 3-year OS (Figure 6 and Supplementary Figures 11, 12).
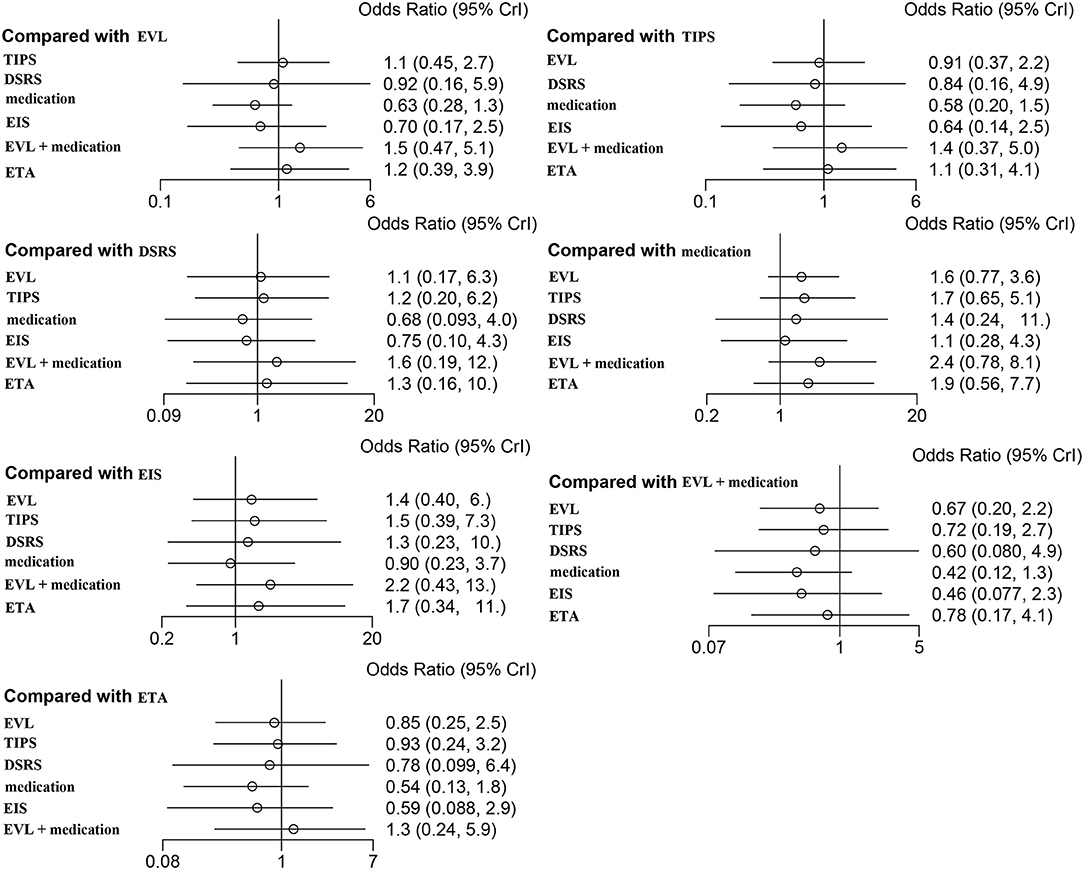
Figure 5. Forest plot of the odds ratios for 1-year OS based on different pairwise comparisons. EVL, endoscopic variceal ligation; TIPS, transjugular intrahepatic portosystemic shunt; DSRS, distal splenorenal shunt; EIS, endoscopic injection sclerotherapy; ETA, endoscopic tissue.
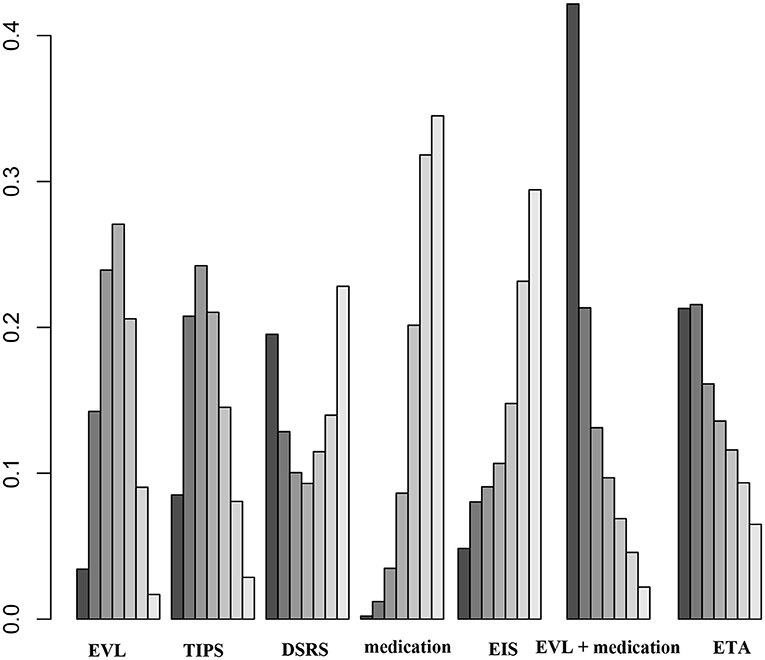
Figure 6. Ranking of 1-year OS among the different therapies. EVL, endoscopic variceal ligation; TIPS, transjugular intrahepatic portosystemic shunt; DSRS, distal splenorenal shunt; EIS, endoscopic injection sclerotherapy; ETA, endoscopic tissue.
Treatment Failure
The incidence of treatment failure was examined in nine direct comparisons and just four different treatments among 1,099 patients. The incidence of treatment failure is shown in Supplementary Figure 13. Compared with EVL, medication (OR 1.3, 95% CI 0.4–5.7), EVL + medication (OR 0.3, 95% CI 0.0–2.4), and ETA (OR 1.2, 95% CI 0.1–13.0) were not significantly associated with treatment failure. When other treatments were compared, the results were similar. We created a rank probability plot, which showed that EVL + medication had the lowest rate of treatment failure (Supplementary Figure 14).
Hepatic Encephalopathy
A total of 1,956 patients experienced hepatic encephalopathy in trials that included 19 direct comparisons and seven different treatments. As shown in Figure 7, DSRS and TIPS were associated with a significantly higher incidence of hepatic encephalopathy compared with EVL, medication, EIS, EVL + medication, and ETA. The rank probability analysis confirmed this finding (Supplementary Figure 15).
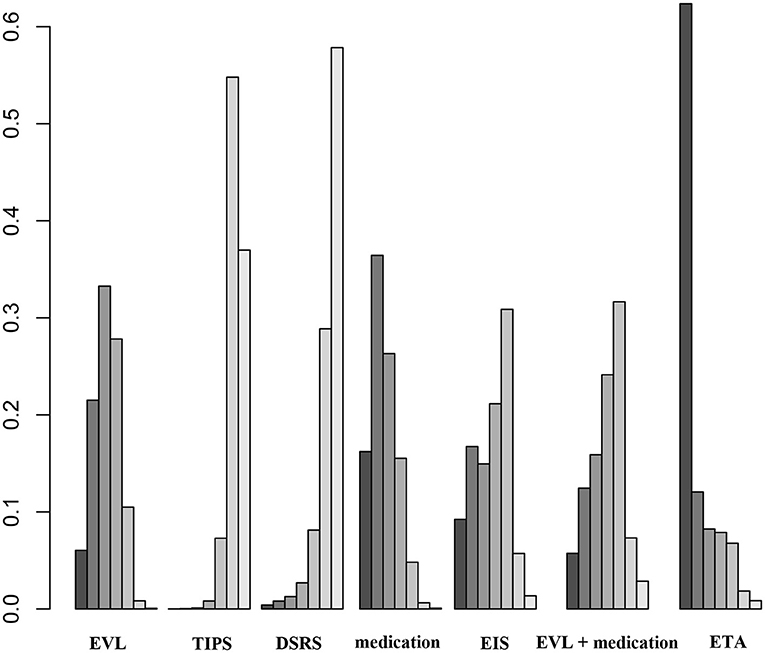
Figure 7. Ranking of the odds ratios for HE based on different pairwise comparisons. EVL, endoscopic variceal ligation; TIPS, transjugular intrahepatic portosystemic shunt; DSRS, distal splenorenal shunt; EIS, endoscopic injection sclerotherapy; ETA, endoscopic tissue.
Sensitivity Analysis and Publication Bias
Sensitivity analysis was performed by excluding several studies. The results were consistent with those of the primary meta-analysis. Begg's and Egger's tests showed that no clear publication bias existed (P > 0.1).
Discussion
Abundant research has shown that variceal bleeding and rebleeding are among the most serious complications of portal hypertension in patients with cirrhosis because of a severe impact on prognosis (11, 56). Treating patients based on their individual risk of portal hypertension-related bleeding undoubtedly affects the prognosis. ET, a classic treatment that has been routinely used for 30–40 years, plays a pivotal role in the management of variceal bleeding and rebleeding (57). However, ET is effective for only a short time because portal pressure and blood flow remain unchanged, and varices frequently recur (in ~50% of cases within 2 years). β-blockers, such as propranolol, timolol, nadolol and carvedilol, decrease cardiac output by β1 adrenergic receptors and reduce splanchnic blood flow by β2 receptors. None of these medications are clearly more effective than others; their usage is driven by doctors' recommendations and patient compliance (58, 59). It has been reported that combination therapy with β-blockers and EVL is significantly more effective than either EVL or medication alone in preventing recurrent hemorrhage (46, 60). In addition, adding low-dose isosorbide-5-mononitrate to β-blockers has been shown to provide a greater portal pressure-reducing effect than β-blockers alone (37, 61). As shown in our network diagram, many studies have directly compared the above treatments, but several treatments have not been compared directly (e.g., EIS + medication vs. DSRS, EIS + medication vs. TIPS, etc.). We conducted our network meta-analysis to address these gaps and provide further guidance for clinical practice.
Our network meta-analysis included 40 RCTs that were conducted within the past 20 years and compared rates of rebleeding, treatment failure, OS and HE due to variceal hemorrhage. A total of 4,006 patients were treated with nine therapeutic methods, including vasoactive medications, DSRS, EVL, EIS, ETA, and combination therapies.
We found that TIPS and DSRS were associated with a lower likelihood of variceal rebleeding compared with ET or medication, either alone or combined. However, TIPS and DSRS were also associated with a higher rate of HE, which is consistent with the American Association for the Study of Liver Diseases practice guidelines (62). Patients experienced a peak incidence of ascites early after DSRS placement (~10% within the 1st month) (38, 63). Conversely, there was a high rate of ascites in the TIPS group at later follow-up time points. TIPS is preferred over DSRS for patients with poor liver function after ineffective conservative therapy and ET. Otherwise, both DSRS and TIPS appear to offer equivalent outcomes (59). According to the AALSD and Baveno guidelines, although TIPS and DSRS are effective in controlling rebleeding, we need to discuss treatment indications the issue of critical liver reserve. TIPS is only recommended for early intervention (72 h) and is not suitable for serious decompensated cirrhosis patients, such as multi-organ failure, abnormal coagulation function, etc. Besides, it should be mentioned that our study did not distinguish between bare and covered stents, which may underestimate the effectiveness of TIPS.
EIS has been supplanted by EVL as the main therapeutic strategy because of the growing evidence that EVL has lower complication rates (51, 64). EIS is associated with severe complications such as transient dysphagia, retrosternal chest discomfort, low-grade fever and esophageal ulceration. Complications occur in up to 40% of patients. ETA employs N-butyl-cyanoacrylate, a strong tissue adhesive used for hemostasis that causes endothelial fibrosis and venous obturation. ETA is associated with a rebleeding risk of 20–25% when endoscopic tissue adhesion achieves hemostasis (65). Our results showed that rebleeding was more frequent after ET than after TIPS or DSRS. However, patients had significantly lower rates of HE after ETA. According to the 2015 United Kingdom guidelines and the 2017 American Association for the Study of Liver Diseases guidelines, combined therapies are favored over EIS, EVL or ETA alone (66, 67). Similarly, based on our network meta-analysis, EVL + medication resulted in a higher 1-year OS rate compared with EVL, EIS or ETA alone and a lower treatment failure rate compared with EVL or ETA alone. EVL + EIS was superior to EVL or EIS alone in terms of bleeding-related mortality.
β-blockers such as propranolol and nadolol have been used for more than 30 years (5). Terlipressin and somatostatin are potent splanchnic vasoconstrictors that also have systemic circulatory effects; they increase arterial pressure and systemic vascular resistance, inhibit glucagon and other vasoactive peptides, and facilitate adrenergic vasoconstriction (68, 69). Our meta-analysis showed that simple conservative treatment offers little benefit. β-blockers, terlipressin and somatostatin drugs do not offer greater benefits compared with endoscopy or interventional therapy (29). However, Patch et al. (70) and Saran et al. (46) reported that propranolol is as effective as EVL in preventing variceal rebleeding within a median follow-up period of 1–2 years. This discrepancy may be attributed to the relatively small sample size in these studies; there is a clear difference in efficacy between medications alone and ET + medication (12, 45).
To the best of our knowledge, this is the first study to comprehensively analyze the safety and efficacy of various treatments for patients with portal hypertension and cirrhosis in terms of bleeding-related complications. Our study has several advantages. First, the data were extracted from 40 high-quality randomized controlled trials that involved over 4,000 patients in 13 countries. Second, multiple endpoints were observed, including 1-, 2-, and 3-year rebleeding rates, treatment failure, bleeding-related mortality, 1-, 2-, and 3-year OS rates, and HE.
Our study also had several limitations. First, some of the included subgroups were too small to evaluate effectively. Thus, several subgroup analyses (e.g., balloon-occluded retrograde transvenous obliteration, EVL + EIS + β-blocker, bare stent, covered stents etc.) were not performed. Second, patient characteristics that may have resulted in unavoidable methodological heterogeneity, such as Child–Pugh class, age and sex, varied among individual studies and could not be further addressed by subgroup or sensitivity analyses. Third, many different medications were used, including isosorbide mononitrate, somatostatin, octreotide, terlipressin and β-blockers, and there may have been differences in dosage among the studies.
According to our meta-analysis, TIPS and DSRS were superior to other therapies in terms of short-term and long-term bleeding control. However, these therapies may increase the risk of HE. There was no significant difference among the groups in the 1- or 3-year OS rate. Based on the complexity of the network meta-analysis model, the results of the meta-analysis are closely related to the model parameters, including the initial values and number of iterations. Therefore, the results of this meta-analysis should be interpreted with caution. Prospective RCTs are required to provide more data on TIPS, balloon-occluded retrograde transvenous obliteration and combination therapy.
Conclusions
In conclusion, TIPS and DSRS should be given priority in patients with portal hypertension and cirrhosis to control rebleeding, which may not improve survival. ET together with medication may improve survival. Furthermore, medications should be used in combination with ET or other treatments rather than as the sole therapeutic intervention.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
QY: conception and design and data collection. QY, WC, CY, and JY: data analysis, interpretation, and drafting the manuscript. TJ and HC: reviewed data analysis, interpretation, writing the manuscript, read, and approved the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Key Research and Development Program of China (No. 2020YFA0113003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.712918/full#supplementary-material
Abbreviations
JAGS, Just Another Gibbs Sampler; MCMC, Markov Chain Monte Carlo chain; PSRF, Potential Scale Reduction Factor; CI, Confidence interval; OS, Overall survival; RR, Odds ratio; RCT, Randomized controlled trial; PRISMA-P, Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols; EIS, endoscopic sclerotherapy; EVL, endoscopic variceal ligation; ETA, endoscopic tissue adhesion; ISMN, isosorbide mononitrate; TIPS, transjugular intrahepatic portosystemic shunt; DSRS, distal splenorenal shunt.
References
1. Mauro E, Gadano A. What's new in portal hypertension? Liver Int. (2020) 2020:122–7. doi: 10.1111/liv.14366
2. Thalheimer U, Mela M, Patch D, Burroughs A. Prevention of variceal rebleeding. Lancet. (2003) 361:2244–5. doi: 10.1016/S0140-6736(03)12778-X
3. Wu K, Song Q, Gou Y, He S. Sandwich method with or without lauromacrogol in the treatment of gastric variceal bleeding with liver cirrhosis: a meta-analysis. Medicine. (2019) 98:e16201. doi: 10.1097/MD.0000000000016201
4. de Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis. (2001) 5:645–63. doi: 10.1016/S1089-3261(05)70186-0
5. Brunner F, Berzigotti A, Bosch J. Prevention and treatment of variceal haemorrhage in 2017. Liver Int. (2017) 2017:104–15. doi: 10.1016/s1089-3261(05)70186-0
6. Bunchorntavakul A, Rajender Reddy K. Pharmacologic management of portal hypertension. Clin Liver Dis. (2014) 18:303–17. doi: 10.1016/j.cld.2013.12.003
7. Ivan Troisi R, Vanlander A, Cesare Giglio M, Van Limmen J, Scudeller L, Heyse B, et al. Somatostatin as inflow modulator in liver-transplant recipients with severe portal hypertension: a randomized trial. Ann Surg. (2019) 269:1025–33. doi: 10.1097/SLA.0000000000003062
8. Avgerinos A, Armonis A, Stefanidis G, Mathou N, Vlachogiannakos J, Kougioumtzian A, et al. Sustained rise of portal pressure after sclerotherapy, but not band ligation, in acute variceal bleeding in cirrhosis. Hepatology. (2004) 39:1623–30. doi: 10.1002/hep.20236
9. Hou MC, Lin HC, Lee FY, Chang FY, Lee SD. Recurrence of esophageal varices following endoscopic treatment and its impact on rebleeding: comparison of sclerotherapy and ligation. J Hepatol. (2000) 32:202–8. doi: 10.1016/S0168-8278(00)80064-1
10. Viazis N, Armonis A, Vlachogiannakos J, Rekoumis G, Stefanidis G, Papadimitriou N, et al. Effects of endoscopic variceal treatment on oesophageal function: a prospective, randomized study. Eur J Gastroenterol Hepatol. (2002) 14:263–9. doi: 10.1097/00042737-200203000-00010
11. Magaz M, Baiges A, Hernández-Gea V. Precision medicine in variceal bleeding: are we there yet? J Hepatol. (2020) 72:774–84. doi: 10.1016/j.jhep.2020.01.008
12. Lo G, Chen W, Wang H, Lin C, Chan H, Tsai W, et al. Low-dose terlipressin plus banding ligation versus low-dose terlipressin alone in the prevention of very early rebleeding of oesophageal varices. Gut. (2009) 58:1275–80. doi: 10.1136/gut.2008.165910
13. Argonz J, Kravetz D, Suarez A, Romero G, Bildozola M, Passamonti M, et al. Variceal band ligation and variceal band ligation plus sclerotherapy in the prevention of recurrent variceal bleeding in cirrhotic patients: a randomized, prospective and controlled trial. Gastrointestinal Endosc. (2000) 51:157–63. doi: 10.1016/S0016-5107(00)70411-1
14. Puente A, Hernández-Gea V, Graupera I, Roque M, Colomo A, Poca M, et al. Drugs plus ligation to prevent rebleeding in cirrhosis: an updated systematic review. Liver Int. (2014) 34:823–33. doi: 10.1111/liv.12452
15. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. (2015) 350:g7647. doi: 10.1136/bmj.g7647
16. Higgins J, Altman D, Gøtzsche P, Jüni P, Moher D, Oxman A, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
17. Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. (2005) 331:897–900. doi: 10.1136/bmj.331.7521.897
18. Veroniki A, Vasiliadis H, Higgins J, Salanti G. Evaluation of inconsistency in networks of interventions. Int J Epidemiol. (2013) 42:332–45. doi: 10.1093/ije/dys222
19. Wu H, Huang J, Lin H, Liao W, Peng Y, Hung K, et al. Comparative effectiveness of renin-angiotensin system blockers and other antihypertensive drugs in patients with diabetes: systematic review and bayesian network meta-analysis. BMJ. (2013) 347:f6008. doi: 10.1136/bmj.f6008
20. Lo GH, Chen WC, Wang HM, Lee CC. Controlled trial of ligation plus nadolol versus nadolol alone for the prevention of first variceal bleeding. Hepatology. (2010) 52:230–7. doi: 10.1002/hep.23617
21. Orozco H, Mercado MA, Chan C, Guillen-Navarro E, Lopez-Martinez LM. A comparative study of the elective treatment of variceal hemorrhage with betablockers, transendoscopic sclerotherapy, and surgery: a prospective, controlled, and randomized trial during 10 years. Ann Surg. (2000) 232:216–9. doi: 10.1097/00000658-200008000-00011
22. Villanueva C, Miñana J, Ortiz J, Gallego A, Soriano G, Torras X, et al. Endoscopic ligation compared with combined treatment with nadolol and isosorbide mononitrate to prevent recurrent variceal bleeding. N Engl J Med. (2001) 345:647–55. doi: 10.1056/NEJMoa003223
23. Pomier-Layrargues G, Villeneuve JP, Deschenes M, Bui B, Perreault P, Fenyves D, et al. Transjugular intrahepatic portosystemic shunt (TIPS) versus endoscopic variceal ligation in the prevention of variceal rebleeding in patients with cirrhosis: a randomised trial. Gut. (2001) 48:390–6. doi: 10.1136/gut.48.3.390
24. Hou MC, Chen WC, Lin HC, Lee FY, Chang FY, Lee SD. A new “sandwich” method of combined endoscopic variceal ligation and sclerotherapy versus ligation alone in the treatment of esophageal variceal bleeding: a randomized trial. Gastrointest Endosc. (2001) 53:572–8. doi: 10.1067/mge.2001.114058
25. Cheng YS, Pan S, Lien GS, Suk FM, Wu MS, Chen JN, et al. Adjuvant sclerotherapy after ligation for the treatment of esophageal varices: a prospective, randomized long-term study. Gastrointest Endosc. (2001) 53:566–71. doi: 10.1067/mge.2001.114061
26. Narahara Y, Kanazawa H, Kawamata H, Tada N, Saitoh H, Matsuzaka S, et al. A randomized clinical trial comparing transjugular intrahepatic portosystemic shunt with endoscopic sclerotherapy in the long-term management of patients with cirrhosis after recent variceal hemorrhage. Hepatol Res. (2001) 21:189–198. doi: 10.1016/s1386-6346(01)00104-8
27. Sauer P, Hansmann J, Richter GM, Stremmel W, Stiehl A. Endoscopic variceal ligation plus propranolol vs. transjugular intrahepatic portosystemic stent shunt: a long-term randomized trial. Endoscopy. (2002) 34:690–7. doi: 10.1055/s-2002-33565
28. Gülberg V, Schepke M, Geigenberger G, Holl J, Brensing KA, Waggershauser T, et al. Transjugular intrahepatic portosystemic shunting is not superior to endoscopic variceal band ligation for prevention of variceal rebleeding in cirrhotic patients: a randomized, controlled trial. Scand J Gastroenterol. (2002) 37:338–43. doi: 10.1080/003655202317284255
29. Escorsell A, Bañares R, García-Pagán J, Gilabert R, Moitinho E, Piqueras B, et al. TIPS versus drug therapy in preventing variceal rebleeding in advanced cirrhosis: a randomized controlled trial. Hepatology. (2002) 35:385–92. doi: 10.1053/jhep.2002.30418
30. Schepke M, Kleber G, Nürnberg D, Willert J, Koch L, Veltzke-Schlieker W, et al. German Study Group for the Primary Prophylaxis of Variceal Bleeding. Ligation versus propranolol for the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology. (2004) 40:65–72. doi: 10.1002/hep.20284
31. de la Peña J, Brullet E, Sanchez-Hernández E, Rivero M, Vergara M, Martin-Lorente JL, et al. Variceal ligation plus nadolol compared with ligation for prophylaxis of variceal rebleeding: a multicenter trial. Hepatology. (2005) 41:572–8. doi: 10.1002/hep.20584
32. Sarin SK, Wadhawan M, Gupta R, Shahi H. Evaluation of endoscopic variceal ligation (EVL) versus propanolol plus isosorbide mononitrate/nadolol (ISMN) in the prevention of variceal rebleeding: comparison of cirrhotic and noncirrhotic patients. Dig Dis Sci. (2005) 50:1538–47. doi: 10.1007/s10620-005-2877-1
33. Shah HA, Mumtaz K, Jafri W, Abid S, Hamid S, Ahmad A, et al. Sclerotherapy plus octreotide versus sclerotherapy alone in the management of gastro-oesophageal variceal hemorrhage. J Ayub Med Coll Abbottabad. (2005) 17:10–4.
34. Zargar SA, Javid G, Khan BA, Shah OJ, Yattoo GN, Shah AH, et al. Endoscopic ligation vs. sclerotherapy in adults with extrahepatic portal venous obstruction: a prospective randomized study. Gastrointest Endosc. (2005) 61:58–66. doi: 10.1016/s0016-5107(04)02455-1
35. Chen WC, Lo GH, Tsai WL, Hsu PI, Lin CK, Lai KH. Emergency endoscopic variceal ligation versus somatostatin for acute esophageal variceal bleeding. J Chin Med Assoc. (2006) 69:60–7. doi: 10.1016/S1726-4901(09)70115-7
36. Santambrogio R, Opocher E, Costa M, Bruno S, Ceretti AP, Spina GP. Natural history of a randomized trial comparing distal spleno-renal shunt with endoscopic sclerotherapy in the prevention of variceal rebleeding: a lesson from the past. World J Gastroenterol. (2006) 12:6331–8. doi: 10.3748/wjg.v12.i39.6331
37. Romero G, Kravetz D, Argonz J, Vulcano C, Suarez A, Fassio E, et al. Comparative study between nadolol and 5-isosorbide mononitrate vs. endoscopic band ligation plus sclerotherapy in the prevention of variceal rebleeding in cirrhotic patients: a randomized controlled trial. Aliment Pharmacol Therapeut. (2006) 24:601–11. doi: 10.1111/j.1365-2036.2006.03007.x
38. Henderson J, Boyer T, Kutner M, Galloway J, Rikkers L, Jeffers L, et al. Distal splenorenal shunt versus transjugular intrahepatic portal systematic shunt for variceal bleeding: a randomized trial. Gastroenterology. (2006) 130:1643–51. doi: 10.1053/j.gastro.2006.02.008
39. Tan PC, Hou MC, Lin HC, Liu TT, Lee FY, Chang FY, et al. A randomized trial of endoscopic treatment of acute gastric variceal hemorrhage: N-butyl-2-cyanoacrylate injection versus band ligation. Hepatology. (2006) 43:690–7. doi: 10.1002/hep.21145
40. Lo GH, Liang HL, Chen WC, Chen MH, Lai KH, Hsu PI, et al. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy. (2007) 39:679–85. doi: 10.1055/s-2007-966591
41. Morales GF, Pereira Lima JC, Hornos AP, Marques DL, Costa CSD, Lima Pereira L, et al. Octreotide for esophageal variceal bleeding treated with endoscopic sclerotherapy: a randomized, placebo-controlled trial. Hepatogastroenterology. (2007) 54:195–200.
42. El Amin H, Abdel Baky L, Sayed Z, Abdel Mohsen E, Eid K, Fouad Y, et al. A randomized trial of endoscopic variceal ligation versus cyanoacrylate injection for treatment of bleeding junctional varices. Trop Gastroenterol. (2010) 31:279–84.
43. Lo GH, Chen WC, Lin CK, Tsai WL, Chan HH, Chen TA, et al. Improved survival in patients receiving medical therapy as compared with banding ligation for the prevention of esophageal variceal rebleeding. Hepatology. (2008) 48:580–7. doi: 10.1002/hep.22358
44. Kumar A, Jha SK, Sharma P, Dubey S, Tyagi P, Sharma BC, et al. Addition of propranolol and isosorbide mononitrate to endoscopic variceal ligation does not reduce variceal rebleeding incidence. Gastroenterology. (2009) 137:892–901. doi: 10.1053/j.gastro.2009.05.049
45. García-Pagán JC, Villanueva C, Albillos A, Bañares R, Morillas R, Abraldes JG, et al. Spanish Variceal Bleeding Study Group. Nadolol plus isosorbide mononitrate alone or associated with band ligation in the prevention of recurrent bleeding: a multicentre randomised controlled trial. Gut. (2009) 58:1144–50. doi: 10.1136/gut.2008.171207
46. Sarin S, Gupta N, Jha S, Agrawal A, Mishra S, Sharma B, et al. Equal efficacy of endoscopic variceal ligation and propranolol in preventing variceal bleeding in patients with noncirrhotic portal hypertension. Gastroenterology. (2010) 139:1238–45. doi: 10.1053/j.gastro.2010.06.017
47. Mishra SR, Chander Sharma B, Kumar A, Sarin SK. Endoscopic cyanoacrylate injection versus beta-blocker for secondary prophylaxis of gastric variceal bleed: a randomised controlled trial. Gut. (2010) 59:729–35. doi: 10.1136/gut.2009.192039
48. Harras F, Sheta el S, Shehata M, El Saadany S, Selim M, Mansour L. Endoscopic band ligation plus argon plasma coagulation versus scleroligation for eradication of esophageal varices. J Gastroenterol Hepatol. (2010) 25:1058–65. doi: 10.1111/j.1440-1746.2010.06265.x
49. Ljubicić N, Bisćanin A, Nikolić M, Supanc V, Hrabar D, Pavić T, et al. A randomized controlled trial of endoscopic treatment of acute esophageal variceal hemorrhage: N-butyl-2-cyanoacrylate injection vs. variceal ligation. Hepatogastroenterology. (2011) 58:438–43.
50. Kong DR, Wang JG, Chen C, Yu FF, Wu Q, Xu JM. Effect of intravariceal sclerotherapy combined with esophageal mucosal sclerotherapy using small-volume sclerosant for cirrhotic patients with high variceal pressure. World J Gastroenterol. (2015) 21:2800–6. doi: 10.3748/wjg.v21.i9.2800
51. Ali S, Wu S, Xu H, Liu H, Hao J, Qin C. A prospective study of endoscopic injection sclerotherapy and endoscopic variceal ligation in the treatment of esophageal varices. J Laparoendosc Adv Surg Techniq A. (2017) 27:333–41. doi: 10.1089/lap.2016.0436
52. Lv Y, Qi X, He C, Wang Z, Yin Z, Niu J, et al. PVT-TIPS Study Group. Covered TIPS versus endoscopic band ligation plus propranolol for the prevention of variceal rebleeding in cirrhotic patients with portal vein thrombosis: a randomised controlled trial. Gut. (2018) 67:2156–68. doi: 10.1136/gutjnl-2017-314634
53. Mansour L, El-Kalla F, El-Bassat H, Abd-Elsalam S, El-Bedewy M, Kobtan A, et al. Randomized controlled trial of scleroligation versus band ligation alone for eradication of gastroesophageal varices. Gastrointest Endosc. (2017) 86:307–15. doi: 10.1016/j.gie.2016.12.026
54. Elsebaey MA, Tawfik MA, Ezzat S, Selim A, Elashry H, Abd-Elsalam S. Endoscopic injection sclerotherapy versus N-Butyl-2 Cyanoacrylate injection in the management of actively bleeding esophageal varices: a randomized controlled trial. BMC Gastroenterol. (2019) 19:23. doi: 10.1186/s12876-019-0940-1
55. Brooks PS, Gelman A. General methods for monitoring convergence of iterative simulations. J Computat Graph Statist. (1998) 7:434–55.
56. Stanley A, Laine L. Management of acute upper gastrointestinal bleeding. BMJ. (2019) 364:l536. doi: 10.1136/bmj.l536
57. Lanas A. International consensus guidelines for nonvariceal gastrointestinal bleeding: a step forward. Ann Internal Med. (2019) 171:853–4. doi: 10.7326/M19-2789
58. Reiberger T, Ulbrich G, Ferlitsch A, Payer B, Schwabl P, Pinter M, et al. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non-response to propranolol. Gut. (2013) 62:1634–41. doi: 10.1136/gutjnl-2012-304038
59. Villanueva C, Aracil C, Colomo A, Hernández-Gea V, López-Balaguer J, Alvarez-Urturi C, et al. Acute hemodynamic response to beta-blockers and prediction of long-term outcome in primary prophylaxis of variceal bleeding. Gastroenterology. (2009) 137:119–28. doi: 10.1053/j.gastro.2009.03.048
60. Villanueva C, Balanzó J, Novella M, Soriano G, Sáinz S, Torras X, et al. Nadolol plus isosorbide mononitrate compared with sclerotherapy for the prevention of variceal rebleeding. N Engl J Med. (1996) 334:1624–9. doi: 10.1056/NEJM199606203342502
61. Villanueva C, Graupera I, Aracil C, Alvarado E, Miñana J, Puente Á, et al. A randomized trial to assess whether portal pressure guided therapy to prevent variceal rebleeding improves survival in cirrhosis. Hepatology. (2017) 65:1693–707. doi: 10.1002/hep.29056
62. Boyer T, Haskal Z. The role of transjugular intrahepatic portosystemic shunt (TIPS) in the management of portal hypertension: update 2009. Hepatology. (2010) 51:306. doi: 10.1002/hep.23383
63. Elwood D, Pomposelli J, Pomfret E, Lewis W, Jenkins R. Distal splenorenal shunt: preferred treatment for recurrent variceal hemorrhage in the patient with well-compensated cirrhosis. Archiv Surg. (2006) 141:385–8. doi: 10.1001/archsurg.141.4.385
64. Dai C, Liu W, Jiang M, Sun M. Endoscopic variceal ligation compared with endoscopic injection sclerotherapy for treatment of esophageal variceal hemorrhage: a meta-analysis. World J Gastroenterol. (2015) 21:2534–41. doi: 10.3748/wjg.v21.i8.2534
65. Al-Khazraji A, Curry M. The current knowledge about the therapeutic use of endoscopic sclerotherapy and endoscopic tissue adhesives in variceal bleeding. Expert Rev Gastroenterol Hepatol. (2019) 13:893–7. doi: 10.1080/17474124.2019.1652092
66. Tripathi D, Stanley A, Hayes P, Patch D, Millson C, Mehrzad H, et al. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. (2015) 64:1680–704. doi: 10.1136/gutjnl-2015-309262
67. Garcia-Tsao G, Abraldes J, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. (2017) 65:310–35. doi: 10.1002/hep.28906
68. Wiest R, Tsai M, Groszmann R. Octreotide potentiates PKC-dependent vasoconstrictors in portal-hypertensive and control rats. Gastroenterology. (2001) 120:975–83. doi: 10.1053/gast.2001.22529
69. Zhang J, Liu J, Wu Y, Romeiro F, Levi Sandri G, Zhou X, et al. Effect of terlipressin on renal function in cirrhotic patients with acute upper gastrointestinal bleeding. Ann Transl Med. (2020) 8:340. doi: 10.21037/atm.2020.02.135
Keywords: endoscopic therapy, transjugular intrahepatic portosystemic shunt, portal hypertension, liver cirrhosis, network meta-analysis, all-cause rebleeding
Citation: Yao Q, Chen W, Yan C, Yu J, Jiang T and Cao H (2021) Efficacy and Safety of Treatments for Patients With Portal Hypertension and Cirrhosis: A Systematic Review and Bayesian Network Meta-Analysis. Front. Med. 8:712918. doi: 10.3389/fmed.2021.712918
Received: 21 May 2021; Accepted: 12 August 2021;
Published: 03 September 2021.
Edited by:
Yijin Wang, Southern University of Science and Technology, ChinaReviewed by:
Jiacheng Liu, Huazhong University of Science and Technology, ChinaCihan Yurdaydin, Koç University Hospital, Turkey
Copyright © 2021 Yao, Chen, Yan, Yu, Jiang and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongcui Cao, aGNjYW9Aemp1LmVkdS5jbg==
†These authors have contributed equally to this work
 Qigu Yao1,2†
Qigu Yao1,2† Wenyi Chen
Wenyi Chen Jiong Yu
Jiong Yu Tian'an Jiang
Tian'an Jiang Hongcui Cao
Hongcui Cao