- 1Center of Medicine Clinical Research, Department of Pharmacy, Medical Supplies Center, People's Liberation Army of Chinese General Hospital, Beijing, China
- 2Department of Pharmacy, Medical Supplies Center, People's Liberation Army of Chinese General Hospital, Beijing, China
Treatment of multidrug-resistant (MDR) Gram-negative bacteria (GNB) infections has led to a global public health challenging due to the bacterial resistance and limited choices of antibiotics. Cefiderocol (CFDC), a novel siderophore cephalosporin possessed unique drug delivery systems and stability to β-lactamases, has the potential to become first-line therapy for most aggressive MDR Gram-negative pathogens infection. However, there have been reports of drug resistance in the course of using CFDC. This study provides an overview of the in-vitro and in-vivo activity of CFDC and potential resistance mechanism was also summarized. In general, CFDC shows excellent activity against a broad range of MDR GNB pathogens including Enterobacteriaceae, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia. The expressions of metallo-β-lactamases such as inosine 5'-monophosphate (IMP), Verona integron-mediated metallo-β-lactamase (VIM), and New Delhi metallo-β-lactamase (NDM) are associated with a higher resistance rate of CFDC. Carbapenem-resistant phenotype has little effect on the resistance rate, although the acquisition of a particular carbapenemase may affect the susceptibility of the pathogens to CFDC. For potential resistance mechanism, mutations in β-lactamases and TonB-dependent receptors, which assist CFDC entering bacteria, would increase a minimum inhibitory concentration (MIC)90 value of CFDC against MDR pathogens. Since the development of CFDC, resistance during its utilization has been reported thus, prudent clinical applications are still necessary to preserve the activity of CFDC.
Introduction
As an ongoing challenge to global health, the emergence of antibiotic-resistant infections results in substantial morbidity and mortality (1). Gram-negative bacteria (GNB) are increasingly associated with high rates of antimicrobial resistance, especially for the carbapenem-resistant Enterobacteriaceae (CRE), multidrug-resistant (MDR) Acinetobacter baumannii (A. baumannii), Klebsiella pneumoniae (K. pneumoniae), and Pseudomonas aeruginosa (P. aeruginosa) (2). Due to the current limited options of MDR pathogen-caused infections and the resistance for “cunning bacterias” to drugs, new therapeutic options are of particular concern and urgently necessary (3–5).
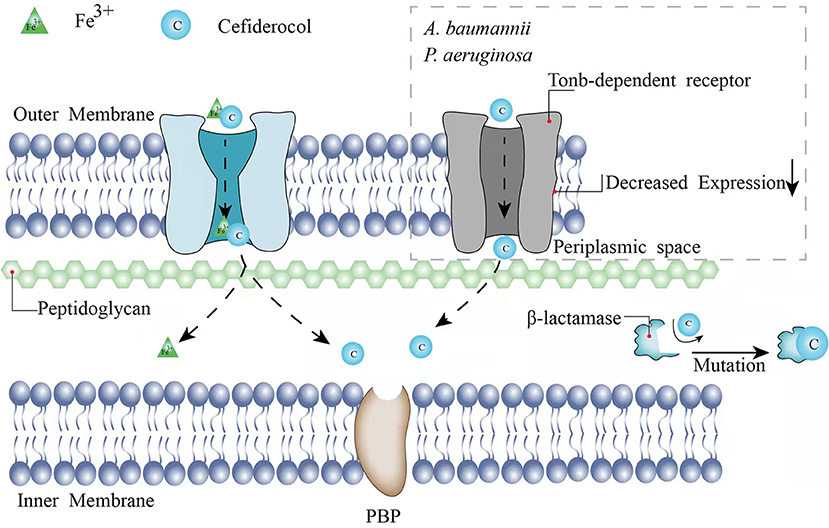
As a novel injectable siderophore cephalosporin, cefiderocol (CFDC) has been approved by the United States Food and Drug Administration (FDA) for the treatment of complicated urinary tract infections (cUTIs) in 2019, hospital-acquired bacterial pneumonia (HABP), and ventilator-associated bacterial pneumonia (VABP) caused by GNB in 2020. In a study consisting of 377 patients with GNB-induced cUTI, 73% of 252 patients in the CFDC group was cured according to clinical response and microbiological response compared with the imipenem-cilastatin group (55% of 119 patients), indicating the good activity of CFDC (6). Same as other cephalosporins, the principal mechanism of CFDC is the inhibition of the cell wall by combining with penicillin-binding protein-3 (PBP-3), which the affinities [50% inhibitory concentrations (IC50s)] of cefiderocol for PBP-3 of Escherichia coli (E. coli) NIHJ JC-2, K. pneumoniae SR22291, P. aeruginosa ATCC 27853, and A. baumannii ATCC 17978 were 0.04 to 0.67 μg/ml (7). However, CFDC is more stable to β-lactamases because of its “Trojan horse” strategy (7). CFDC combines a cephalosporin core and a catechol-type siderophore, which are highly effective to acquire bacterial iron (Fe3+). Through binding to bacterial iron transporter outer membrane protein, CFDC can enter the bacterial periplasmic space to avoid the degradation of β-lactamase produced by the pathogen (7, 8) (Figure 1). Therefore, it shows activity against GNB pathogens including extended-spectrum β-lactamases (ESBL)-producing GNB, CRE, P. aeruginosa, A. baumannii, K. pneumoniae, Klebsiella oxytoca, and Stenotrophomonas maltophilia (S. maltophilia) (9–19). Serratia marcescens, Citrobacter koseri, Burkholderia cepacia (B. cepacia), and Citrobacter freundii (C. freundii) are also sensitive to CFDC with a minimum inhibitory concentration (MIC)90 value of under 1 mg/l (9, 11, 14–16, 19–21). The breakpoints of CFDC have been interpreted by different standards including the Clinical and Laboratory Standards Institute (CLSI), the FDA, and the European Committee on Antimicrobial Susceptibility Testing (EUCAST). The breakpoints of CLSI are commonly used and available for Enterobacteriaceae, A. baumannii, P. aeruginosa, and S. maltophilia (susceptible ≤ 4 mg/l, intermediate 8 mg/l, and resistant ≥16 mg/l). The breakpoints of the EUCAST for Enterobacteriaceae and P. aeruginosa are susceptible ≤ 2 mg/l and resistant >2 mg/l (22). The FDA breakpoints for Enterobacteriaceae have been changed from (susceptible ≤ 2 mg/l, intermediate 4 mg/l, and resistant ≥8 mg/l) in 2019 to (susceptible ≤ 4 mg/l, intermediate 8 mg/l, and resistant ≥16 mg/l) in 2020 and the standard for A. baumannii (susceptible ≤ 1 mg/l and resistant ≥4 mg/l) has been added. The breakpoints for P. aeruginosa remain as (susceptible ≤ 1 mg/l and resistant ≥4 mg/l). Broth microdilution and disk diffusion methods are both available for different standards.
Although it has not been long since CFDC appears in the market and its indications are limited, CFDC is highly anticipated and acts as a new option for the treatment of various MDR pathogens (23). Recently, a randomized controlled phase-3 trial study has reported that CFDC has similar clinical efficacy to the best available therapy in infections caused by carbapenem-resistant GNB (24). Another study has reported that CFDC is non-inferior to high-dose and extended-infusion meropenem in the treatment of MDR GNB infections (25).
In this study, we aim to review the in-vitro and in-vivo activity of CFDC to evaluate its global effectiveness so far (Supplementary Table 1) and to discuss the potential mechanism of CFDC resistance.
Reports of in-vitro Resistance to CFDC
Resistance Rate in Enterobacterales
Generally, CFDC has a high activity against Enterobacterales with most of the MIC90 values ≤ 8 mg/l (10, 11, 13–16, 18–21, 26, 27). Mariana et al. have reported a resistance rate of 5% for 335 Enterobacterales isolates according to the CLSI breakpoints, which are originated from the United States, Canada, and Singapore, with an MIC50 value ranging from 0.015 to >64 mg/l and an MIC90 value of 8 mg/l (21). However, the resistance rate shows obvious differences according to different phenotypes of β-lactamases. Based on the Ambler Classification system, β-lactamases are divided into four classes as follows: classes A, C, and D of serine β-lactamases and class B known as metallo-β-lactamases (MBLs). The β-lactamases that confer the reduction of drug sensitivity in Enterobacterales belong to the abovementioned types: two serine-β-lactamase including ESBL and K. pneumoniae carbapenemases (KPCs) and class B [MBL, especially New Delhi metallo-β-lactamases (NDM)]. Several reports have shown that the resistance rate of MBL-producing Enterobacterales is higher compared with non-MBL-producing pathogens (10, 28, 29). Two studies from Europe and the United Kingdom in 2020 have reported that the resistance rate of NDM-positive strains against CFDC is up to 59 and 48.6% (the EUCAST breakpoints), respectively, with an MIC90 value reaching 32 mg/l (10, 28). Two other studies have also reported the widest MIC90 range of CFDC against NDM-positive Enterobacteriaceae up to >64 mg/l (3) or 8 mg/l (30) compared with other β-lactamase phenotypes. Following NDM, another type of Verona integron-mediated MBL (VIM)-positive strain exhibits a high resistance of 19.1 and 21%, respectively (10, 28). The resistance rate of CFDC against class A β-lactamase-positive strains and carbapenemases, such as GES, IMI, SME, and TEM enzyme, is relatively lower compared with class B β-lactamase-positive strains (3, 10, 28). An MIC90 value of CFDC against ESBL + porin loss Enterobacterales ranges from 0.125 to 32 mg/l with a resistance rate of 38.5% (28). Studies from Europe have shown that the resistance rate of KPC-producing pathogens to CFDC is 8.9 and 16.4%, respectively, based on the EUCAST breakpoints (10, 28). Similarly, class D β-lactamase OXA-48-positive Enterobacterales show a resistance rate of 7.1 and 11.8%, respectively, with an MIC90 value reaching 8 mg/l (10, 28). However, another study from North America and Europe has shown that all the 75 KPC-positive and 32 OXA-48-positive strains are sensitive to CFDC (30). The class C β-lactamase AmpC-positive strains are susceptible to CFDC with the resistance rate of 0% (28). Meredith et al. have shown that the resistance rates of meropenem non-susceptible (MIC90 ≥2 mg/l) strains are all susceptible according to the CLSI breakpoints (19).
For E. coli, most of the reports have shown that an MIC90 value of CFDC is ≤ 4 mg/l ranging from 0.25 to 4 mg/l (9, 11, 13, 14, 16, 18–21, 29). Two studies have compared the MIC90 values between isolates from Europe and North America, showing that an MIC90 value of CFDC to the strains from Europe is twice higher compared with those from North America (16, 19), although all the stains are susceptible. Class B β-lactamase-positive E. coli has the highest resistance to CFDC (27, 29). Naoki et al. have reported that 26.3% of 19 isolates from NDM-1-producing E. coli are resistant to CFDC according to the CLSI breakpoints (29). The MIC90 values of CFDC against NDM (-1/4/5/6/7), VIM (-1/2/4/19), or inosine 5'-monophosphate (IMP) (-1/8) E. coli are significantly higher compared with other β-lactamase phenotypes isolates such as KPC (-2/3) or OXA-48 type (16 vs. 1 or 0.5 mg/l) (27).
For K. pneumoniae, an MIC90 value of CFDC is mostly ≤ 8 mg/L (9, 11, 13–16, 18, 21, 27, 29). In 2018, 689 carbapenem non-susceptible strains from North America and Europe are mostly susceptible, with an MIC90 value of 4 mg/l (18). However, C Paul et al. have reported that the resistance rate of 15 carbapenem-resistant K. pneumoniae is up to 20% based on the CLSI breakpoints, with an MIC90 value of 32 mg/l (20). Kenneth et al. have also reported the widest MIC90 range >64 mg/l and a resistance rate of 4.3% of CFDC against carbapenem-resistant K. pneumoniae according to the CLSI breakpoints (9). Different phenotypes also affect the sensitivity of K. pneumoniae. An MIC90 (4 mg/l) value of CFDC against MBL-positive strains is twice or four times higher than that of KPC- or OXA-48-positive strains (27). For class A β-lactamases, the resistance rate of CFDC against 23 isolates from ESBL-producing K. pneumoniae is 2.7%, with an MIC90 value ranging from 0.125 to >64 mg/l (9). However, the other two studies both found that ESBL-positive isolates were sensitive to CFDC, with the highest MIC90 value of 4 mg/l (14, 29). The KPC (−2/3/11) and OXA (-48/162/163/181/204/232) isolates are also susceptible to CFDC with an MIC90 value <4 mg/l (15, 27, 29). Besides, the same genus of bacteria may have different MICs when large samples of detection conducted by different regions. James et al. have reported that an MIC90 value of CFDC to the strains from Europe (2 mg/l) is four times higher compared with those from North America (0.5 mg/l) (16).
For other Enterobacterales, the decreased sensitivity is mainly attributed to the production of OXA or MBLs (NDM, VIM, or IMP), with an MIC90 value (4 mg/l) four times higher compared with KPC-positive strains (27). An MIC90 value of CFDC against carbapenem-resistant pathogens is also increased up to 8 mg/l, ranging from 0.06 to 32 mg/l (18). Enterobacter cloacae (E. cloacae) is susceptible to CFDC with an MIC90 value ≤ 1 mg/l (11, 14, 16, 29). According to the CLSI breakpoints, C Paul et al. have reported that the resistance rate of carbapenem-resistant E. cloacae complex is 13%, with an MIC90 value of 16 mg/l (20). The resistance rate of CFDC against 38 ESBL-positive strains is 5.3% using the CLSI breakpoints, with an MIC90 value ranging from < 0.03 to >64 mg/l (9). CFDC shows excellent activity against nonenzyme-producing Klebsiella spp., Serratia spp., Citrobacter spp., and Proteus mirabilis with a resistance rate of 0% (9, 11, 14, 16, 18–20, 29). Carbapenem non-susceptibility is the main factor for the decreased MIC90 value of Klebsiella spp., Serratia spp., and Citrobacter spp. and will result in a two- or four-time increase of CFDCs MIC90 (18, 20). Although an MIC90 value of C. freundii ranges from ≤ 0.063 to >64 mg/l and the resistance rate is not provided by Naoki et al., an MIC90 value is as low as 0.125 mg/l (29).
Pseudomonas aeruginosa
Generally, CFDC shows an excellent bactericidal effect against P. aeruginosa with an MIC90 value of ≤ 2 mg/l (3, 13–19, 27, 30). MBLs are still correlated to their CFDC resistance. A study from the United Kingdom has reported that the resistance rate of isolates from 11 NDM-positive and 30 IMP-positive P. aeruginosa is 54.5 and 20%, respectively, according to the EUCAST breakpoints, with the upper range of an MIC90 value of ≥ 128 mg/l (28). The resistance rate of the class A β-lactamase (GES, PER, and VEB)-producing isolates is relatively high at 10–33.3% (28). However, two European studies have reported that the resistance rates of VIM-, IMP-, NDM-, and GES-positive P. aeruginosa are all 0% (10, 30). Although Dobias et al. did not provide the resistance rate against CFDC, an MIC90 value of 2 mg/l reflects the high activity of CFDC against VIM-, IMP-, KPC-, SPM-, or GIM-producing P. aeruginosa (27). An MIC90 value of CFDC against carbapenemase-producing P. aeruginosa is a little bit higher compared with non-carbapenemase ones (2 vs. 0.5 mg/l) in a German study, with a resistance rate of 9.1 and 0%, respectively (11). A study from the USA has also reported a high MIC90 value of 8 mg/l in carbapenem-resistant strains (20). The activity of CFDC against carbapenem non-susceptible or MDR P. aeruginosa remains well, with an MIC90 value of ≤ 2 mg/l (14, 17, 19, 30).
Acinetobacter spp.
Most studies have demonstrated that an MIC90 value of CFDC against non-enzymes-producing A. baumannii is <4 mg/l (3, 10, 13, 15, 16, 19, 27, 30, 31). Ceftazidime resistant had little effect on an MIC90 value of CFDC to A. baumannii (13). However, one study has reported that an MIC90 value of CFDC to 97 A. baumannii isolates is 32 mg/l according to the CLSI breakpoints, with a resistance rate of 33%. The pathogens collected from the United States, Canada, and Singapore from 1996 to 2015 possess one or multiple types of gene expression including blaCMY, blaCTX−M, blaFOX, blaIMI, blaIMP, blaKPC, blaNDM, blaOXA−48−like, blaSHV, blaSME, and blaTEM (21). A. baumannii resistance is attributed to the production of OXA- or NDM-type enzymes. Moreover, the resistance rate varies according to different phenotypes of OXA enzymes. The resistance rate of CFDC against OXA-23-positive A. baumannii is 14.6%, while it is 11.1, 10, and 5.3% for OXA-24/40-positive strains, OXA-58-positive strains, and OXA-51-positive strains, respectively, using non-species special pharmacokinetic-pharmacodynamic (PK-PD) breakpoints (22, 28). Akinobu et al. have demonstrated that the resistance rate of CFDC against OXA-23-positive strains (16.7%) is relatively higher compared with other phenotypes of OXA-positive A. baumannii (0%), with the maximum range of an MIC90 value of >32 mg/l (31). Iregui et al. have also reported that the resistance rate of blaOXA−23 A. baumannii is 8.8% according to the CLSI breakpoints, with an MIC90 value of 8 mg/l (13). However, Delgado et al. from Spain and Christopher et al. from Europe have reported that the resistance rate of OXA-24/40-positive strains is 12 and 6.8%, respectively, which is higher compared with other phenotypes (10, 15). A study from the United Kingdom has demonstrated that 20 NDM-producing pathogens show the highest resistance of 50% based on non-species special PK-PD breakpoints, with an MIC90 value ranging from 1 to ≥128 mg/l (22, 28) (since no MIC90 criteria have been provided for CFDC to A. baumannii). A Chinese study has reported that the resistance rate of imipenem-resistant pathogens is 7% in 2020 using the CLSI breakpoints, with an MIC90 value ranging from 0.06 to >64 mg/l and an MIC90 value of 8 mg/l (17). Other studies have also shown that carbapenem-resistant strains are more resistant to CFDC compared with the susceptible strains, with a slightly higher MIC90 value or MIC90 range (3, 19, 31). MDR A. baumannii exhibits the highest resistance with an MIC90 value of 8 mg/l and an MIC90 range reaching >256 mg/l (18). Two studies have compared an MIC90 value between the isolates from Europe and North America and no significant difference has been found (16, 19).
According to the CLSI breakpoints, Kenneth et al. have shown that the resistance rate of Acinetobacter spp. is 10%, with an MIC90 value of 4 mg/l (9). However, CFDC has high activity against Acinetobacter pittii from North America and Europe, reported by James et al., with the resistance rate of 0% and an MIC90 value of 0.5 mg/l (16).
Other Strains
For S. maltophilia, B. cepacia complex, Morganellaceae, Achromobacter xylosoxidans, and Proteus mirabilis, they show excellent susceptibility to CFDC, with a resistance rate of 0% and an MIC90 value of <1 mg/l, as reported by worldwide studies (11, 14–21, 26).
Reports of in-vivo Resistance to CFDC
Several animal studies demonstrated that strains carring KPC or NDM may reduce the suscepitbility to CFDC. An in-vivo study using neutropenic murine thigh and lung infection models has shown that an MIC90 of NDM-producing GNB including E. coli, K. pneumoniae, and P. aeruginosa was 8- to 64-fold higher than non-producing strains (32). The MIC90 of NDM-1-producing K. pneumoniae sequence type 14 (ST14) reach to 16 mg/l, which is resistant to CFDC (32). The MIC90 of KPC-producing pathogens is 16 times higher than non-producing K. pneumoniae (4 vs. 0.25 mg/l) (32). In a immunocompetent rat respiratory tract infection model, an MIC90 value at 8 mg/l of NDM-1-positive K. pneumoniae is two times higher than KPC-2-positive K. pneumoniae (33). In in-vivo models, the amount of inoculation will also affect the MIC90 of CFDC. An MIC90 value at 16–64 mg/l was observed in a K. pneumoniae infected neutropenic murine thigh model, which was infected with 107 CFU/ml bacterial suspension (34). According to the EUCAST breakpoints, Hobson et al. have also reported that high inocula (107 CFU/ml) with KPC-producing Enterobacteriaceae will lead the resistance to CFDC compared to usual inocula (105 CFU/ml) in 2021 (35).
Grande et al. have reported that a 63-year-old male patient with septic shock is presented at the intensive care unit (ICU) due to the initial infection of ESBL K. pneumoniae, oxacillin-sensitive Staphylococcus aureus, and multi-sensitive P. aeruginosa. Then, a VIM-producing XDR P. aeruginosa grows from his sputum on day 26 (36). On day 54, the regimen of colistin and meropenem is switched to CFDC 2 g q8h infused over 3 h plus metronidazole 500 mg TID as P. aeruginosa is susceptible to CFDC. The treatment regimen is discontinued after 6 weeks (36). On day 128, GES- and VIM-producing XDR P. aeruginosa are isolated from ischial eschar with an MIC90 value of CFDC increased to 8 mg/l (36). It indicates that P. aeruginosa develops resistance during CFDC treatment.
Resistance Mechanisms
Previous studies have shown that the presence of single-type MBLs may increase an MIC90 value of CFDC against part of the isolates. However, one study has demonstrated that the co-expression of MBLs and serine-type β-lactamases is related to the non-susceptibility of CFDC (37). An MIC90 value to CFDC presents an 8- to 64-fold and 8-fold reduction against CFDC-resistant Enterobacterales (including E. coli and K. pneumoniae) and A. baumannii, respectively, when both the dipicolinic acid (an MBL inhibitor) and avibactam (a serine-β-lactamase inhibitor) are added to the susceptible level (≤0.5 μg/ml) (37). However, an MIC90 value of ≤ 2-fold for CFDC has not been observed by the addition of dipicolinic acid or avibactam alone (37). Mutations in β-lactamases may also lead to CFDC resistance (Figure 1). A 4- to 32-fold increase of an MIC90 was observed in D179Y-H274Y mutations of KPC-31 compared to the wild-type alleles reported by Hobson et al. in 2021 (35). Shields et al. have reported that the deletion of positions 292 and 293, which are located in the R2 loop of AmpC, causes the decreased susceptibility of Enterobacterales (38). The mutations lead to the disappearance of the H10 helix in the R2 loop and the expansion of the substrate-binding site, resulting in a more stable binding to the bulkier side chain possessed by CFDC (38). Akito et al. have demonstrated the alanine-proline deletion at positions 294 and 295 located in the R2 loop, which is also associated with the reduced susceptibility to CFDC in E. coli and E. cloacae (39). Especially for E. cloacae, the depletion of A294_L295 results in an increase of >32-fold in an MIC90 value of CFDC (39).
TonB-dependent receptors commonly exist in GNB outer membrane and assist CFDC to enter the bacterial periplasmic space via cooperation with the TonB-ExbB-ExbD complex located in the cytoplasmic membrane (40). The energy required for the transport of CFDC is provided by TonB-ExbB-ExbD complex (40). The main TonB-dependent receptors of A. baumannii are termed as PiuA and PirA (41). Malik et al. have reported that the change from a hydrophobic amino acid to an aromatic amino acid at location 275 of PirA and the downregulation of pirA, possibly in combination with loss of piuA, cause the decreased expressions of TonB-dependent receptors, which interpret the increased resistance to CFDC in A. baumannii (42) (Figure 1). Decreased sensitivity to CFDC has also been observed in P. aeruginosa when the loss of piuA and downregulation of TonB-dependent receptors occur (41) (Figure 1). Alexandre et al. have shown that the decreased expression of PiuA ortholog, termed as PiuD, which is encoded by piuD, is more important than the loss of piuA (43). They had tested an MIC90 value of P. aeruginosa when piuA or piuD is depleted and found that the deletion of piuA increases the CFDC MIC90 value by 2-fold, while such elevation for the deletion of piuD is 32-fold (43). Moynié et al. have considered that TonB3-ExbB3-ExbD3 complex not only provides energy for the siderophore transport, but also is associated with siderophore acquiring Fe3+42. Mutation of TonB3-ExbB3-ExbD3 by insertion of A at position 9 in the exbD3 gene, deletion of A at position 319, and insertion of A at position 243 in the tonB3 gene would impede energy acquisition for transport and iron availability. Therefore, the transmission of CFDC to bacteria would be inhibited (41).
Conclusion
In conclusion, CFDC has demonstrated excellent activity against GNB including ESBL Enterobacterales, CRE, MDR A. baumannii, and carbapenem-resistant P. aeruginosa. The expressions of MBLs are associated with the decreased sensitivity of pathogens to CFDC. However, the acquisition of a particular β-lactamase does not ensure resistance and additional mechanisms such as mutations in β-lactamases are necessary for overt resistance to develop. Moreover, since the CFDC resistance has already been reported during its anti-infective therapy, the clinical application needs to be cautious to preserve the activity of CFDC.
Author Contributions
JY wrote the first draft of the manuscript. JW, MC, and YC contributed to manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (81770004 and 82073894) and the Cultivation Project of PLA General Hospital for Distinguished Young Scientists (2020-JQPY-004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.741940/full#supplementary-material
References
1. Bart S, Rubin D, Kim P, Farley J, Nambiar S. Trends in hospital-acquired and ventilator-associated bacterial pneumonia trials. Clin Infect Dis. (2020) 2020:ciaa1712. doi: 10.1093/cid/ciaa1712
2. Karakonstantis S, Kritsotakis EI, Gikas A. Treatment options for pneumoniae K, P. aeruginosa and A. baumannii co-resistant to carbapenems, aminoglycosides, polymyxins and tigecycline: an approach based on the mechanisms of resistance to carbapenems. Infection. (2020) 48:835–51. doi: 10.1007/s15010-020-01520-6
3. Jacobs MR, Abdelhamed AM, Good CE, Rhoads DD, Hujer KM, Hujer AM, et al. ARGONAUT-I: activity of cefiderocol (S-649266), a siderophore cephalosporin, against gram-negative bacteria, including carbapenem-resistant nonfermenters and enterobacteriaceae with defined extended-spectrum β-lactamases and carbapenemases. Antimicrob Agents Chemother. (2019) 63:18. doi: 10.1128/AAC.01801-18
4. Wang Y, Wang J, Wang R, Cai Y. Resistance to ceftazidime-avibactam and underlying mechanisms. J Glob Antimicrob Resist. (2020) 22:18–27. doi: 10.1016/j.jgar.2019.12.009
5. Chen J, Zeng Y, Zhang R, Cai J. In vivo emergence of colistin and tigecycline resistance in carbapenem-resistant hypervirulent Klebsiella pneumoniae during antibiotics treatment. Front Microbiol. (2021) 12:702956. doi: 10.3389/fmicb.2021.702956
6. Portsmouth S, van Veenhuyzen D, Echols R, Machida M, Ferreira JCA, Ariyasu M, et al. Cefiderocol vs. imipenem-cilastatin for the treatment of complicated urinary tract infections caused by Gram-negative uropathogens: a phase 2, randomised, double-blind, non-inferiority trial. Lancet Infect Dis. (2018) 18:1319–28. doi: 10.1016/S1473-3099(18)30554-1
7. Ito A, Sato aT, Ota aM, Takemura aM, Nishikawa aT. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against Gram-negative bacteria. Antimicrob Agents Chemother. (2018) 62:e01454–17. doi: 10.1128/AAC.01454-17
8. Aoki T, Yoshizawa H, Yamawaki K, Yokoo K, Sato J, Hisakawa S, et al. Cefiderocol (S-649266), a new siderophore cephalosporin exhibiting potent activities against Pseudomonas aeruginosa and other gram-negative pathogens including multi-drug resistant bacteria: structure activity relationship. Eur J Med Chem. (2018) 155:847–68. doi: 10.1016/j.ejmech.2018.06.014
9. Rolston KVI, Gerges B, Shelburne S, Aitken SL, Raad I, Prince RA. Activity of cefiderocol and comparators against isolates from cancer patients. Antimicrob Agents Chemother. (2020) 64:19. doi: 10.1128/AAC.01955-19
10. Longshaw C, Manissero D, Tsuji M, Echols R, Yamano Y. In vitro activity of the siderophore cephalosporin, cefiderocol, against molecularly characterized, carbapenem-non-susceptible Gram-negative bacteria from Europe. JAC-Antimicrobial Resistance. (2020) 2:dlaa060. doi: 10.1093/jacamr/dlaa060
11. Kresken M, Korte-Berwanger M, Gatermann SG, Pfeifer Y, Pfennigwerth N, Seifert H, et al. In vitro activity of cefiderocol against aerobic Gram-negative bacterial pathogens from Germany. Int J Antimicrob Agents. (2020) 56:106128. doi: 10.1016/j.ijantimicag.2020.106128
12. Johnston BD, Thuras P, Porter SB, Anacker M, VonBank B, Snippes Vagnone P, et al. Activity of cefiderocol, ceftazidime-avibactam, and eravacycline against carbapenem-resistant Escherichia coli isolates from the United States and International Sites in Relation to Clonal Background, Resistance Genes, Coresistance, and Region. Antimicrob Agents Chemother. (2020) 64:20. doi: 10.1128/AAC.00797-20
13. Iregui A, Khan Z, Landman D, Quale J. Activity of cefiderocol against enterobacterales, Pseudomonas aeruginosa, and Acinetobacter baumannii endemic to medical centers in New York City. Microbial Drug Resistance. (2020) 26:722–6. doi: 10.1089/mdr.2019.0298
14. Golden AR, Adam HJ, Baxter M, Walkty A, Lagacé-Wiens P, Karlowsky JA, Zhanel GG. In vitro activity of cefiderocol, a novel siderophore cephalosporin, against gram-negative bacilli isolated from patients in Canadian Intensive Care Units. Diagn Microbiol Infect Dis. (2020) 97:115012. doi: 10.1016/j.diagmicrobio.2020.115012
15. Delgado-Valverde M, Conejo MDC, Serrano L, Fernández-Cuenca F, Pascual Á. Activity of cefiderocol against high-risk clones of multidrug-resistant Enterobacterales, Acinetobacter baumannii, Pseudomonas aeruginosa and Stenotrophomonas maltophilia. J Antimicrob Chemother. (2020) 75:1840–9. doi: 10.1093/jac/dkaa117
16. Karlowsky JA, Hackel MA, Tsuji M, Yamano Y, Echols R, Sahm DF. In vitro activity of cefiderocol, a siderophore cephalosporin, against gram-negative bacilli isolated by clinical laboratories in North America and Europe in 2015-2016: SIDERO-WT-2015. Int J Antimicrob Agents. (2019) 53:456–66. doi: 10.1016/j.ijantimicag.2018.11.007
17. Hsueh SC, Lee YJ, Huang YT, Liao CH, Tsuji M, Hsueh PR. In vitro activities of cefiderocol, ceftolozane/tazobactam, ceftazidime/avibactam and other comparative drugs against imipenem-resistant Pseudomonas aeruginosa and Acinetobacter baumannii, and Stenotrophomonas maltophilia, all associated with bloodstream infections in Taiwan. J Antimicrob Chemother. (2019) 74:380–6. doi: 10.1093/jac/dky425
18. Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of gram-negative bacilli collected worldwide in 2014 to 2016. Antimicrob Agents Chemother. (2018) 62:17. doi: 10.1128/AAC.01968-17
19. Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. In vitro activity of the siderophore cephalosporin, cefiderocol, against a recent collection of clinically relevant gram-negative bacilli from North America and Europe, including carbapenem-nonsusceptible isolates (SIDERO-WT-2014 study). Antimicrob Agents Chemother. (2017) 61:17. doi: 10.1128/AAC.00093-17
20. Morris CP, Bergman Y, Tekle T, Fissel JA, Tamma PD, Simner PJ. Cefiderocol antimicrobial susceptibility testing against multidrug-resistant gram-negative bacilli: a comparison of disk diffusion to broth microdilution. J Clin Microbiol. (2020) 59:20. doi: 10.1128/JCM.01649-20
21. Albano M, Karau MJ, Schuetz AN, Patel R. Comparison of agar dilution to broth microdilution for testing in vitro activity of cefiderocol against gram-negative bacilli. J Clin Microbiol. (2020) 2020:20. doi: 10.1128/JCM.00966-20
22. EUCAST. Breakpoints for Cefiderocol From EUCAST. Addendum (May 20202) to EUCAST Breakpoint Tables V.10.0. Breakpoints to be Included in EUCAST Breakpoint Tables v 11.0, January 2021. (2020). Available online at: https://yc.mlpla.mil.cn/s/org/eucast/www/G.https/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/Addenda/Cefiderocol_addendum_20200501.pdf
23. Wu JY, Srinivas P, Pogue JM. Cefiderocol: a novel agent for the management of multidrug-resistant gram-negative organisms. Infect Dis Ther. (2020) 9:17–40. doi: 10.1007/s40121-020-00286-6
24. Bassetti M, Echols R, Matsunaga Y, Ariyasu M, Doi Y, Ferrer R, et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect Dis. (2020) 2020:9. doi: 10.1016/S1473-3099(20)30796-9
25. Wunderink RG, Matsunaga Y, Ariyasu M, Clevenbergh P, Echols R, Kaye KS, et al. Cefiderocol vs. high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. (2020) 2020:3. doi: 10.1016/S1473-3099(20)30731-3
26. Jacobs MR, Abdelhamed AM, Good CE, Rhoads DD, Hujer KM, Hujer AM, et al. In vitro activity of cefiderocol (s-649266), a siderophore cephalosporin, against enterobacteriaceae with defined extended-spectrum b-lactamases and carbapenemases. Open ForInfect Dis. (2018) 5:S413–4. doi: 10.1093/ofid/ofy210.1182
27. Dobias J, Dénervaud-Tendon V, Poirel L, Nordmann Nordmann P, Activity of the novel siderophore cephalosporin cefiderocol against multidrug-resistant Gram-negative pathogens. Eur J Clin Microbiol Infect Dis. (2017) 36:2319–27. doi: 10.1007/s10096-017-3063-z
28. Mushtaq S, Sadouki Z, Vickers A, Livermore DM, Woodford N. In vitro activity of cefiderocol, a siderophore cephalosporin, against multidrug-resistant gram-negative bacteria. Antimicrobial Agents Chemotherapy. (2020) 64:20. doi: 10.1128/AAC.01582-20
29. Kohira N, West J, Ito A, Ito-Horiyama T, Nakamura R, Sato T, et al. In vitro antimicrobial activity of a siderophore cephalosporin, s-649266, against enterobacteriaceae clinical isolates, including carbapenem-resistant strains. Antimicrob Agents Chemother. (2016) 60:729–34. doi: 10.1128/AAC.01695-15
30. Kazmierczak KM, Tsuji M, Wise MG, Hackel M, Yamano Y, Echols R, et al. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-β-lactamase-producing isolates (SIDERO-WT-2014 Study). Int J Antimicrob Agents. (2019) 53:177–84. doi: 10.1016/j.ijantimicag.2018.10.007
31. Ito A, Kohira N, Bouchillon SK, West J, Rittenhouse S, Sader HS, et al. In vitro antimicrobial activity of S-649266, a catechol-substituted siderophore cephalosporin, when tested against non-fermenting Gram-negative bacteria. J Antimicrob Chemother. (2016) 71:670–7. doi: 10.1093/jac/dkv402
32. Nakamura R, Ito-Horiyama T, Takemura M, Toba S, Matsumoto S, Ikehara T, et al. In vitro pharmacodynamic study of cefiderocol, a novel parenteral siderophore cephalosporin, in murine thigh and lung infection models. Antimicrobial Agents Chemother. (2019) 63:e02031–18. doi: 10.1128/AAC.02031-18
33. Matsumoto S, Singley CM, Hoover J, Nakamura R, Echols R, Rittenhouse S, et al. Efficacy of cefiderocol against carbapenem-resistant gram-negative bacilli in immunocompetent-rat respiratory tract infection models recreating human plasma pharmacokinetics. Antimicrobial Agents Chemother. (2017) 61:e00700–17. doi: 10.1128/AAC.00700-17
34. Monogue ML, Tsuji M, Yamano Y, Echols R, Nicolau DP. Efficacy of humanized exposures of cefiderocol (S-649266) against a diverse population of gram-negative bacteria in a murine thigh infection model. Antimicrobial Agents Chemother. (2017) 61:e01022–17. doi: 10.1128/AAC.01022-17
35. Hobson CA, Cointe A, Jacquier H, Choudhury A, Magnan M, Courroux C, et al. Cross resistance to cefiderocol and ceftazidime-avibactam in KPC beta-lactamase mutants and inoculum effect. Clin Microbiol Infect. (2021) 2021:16. doi: 10.1016/j.cmi.2021.04.016
36. Grande Perez C, Maillart E, Miendje Deyi VY, Huang TD, Kamgang P, Dernier Y, et al. Compassionate use of cefiderocol in a pancreatic abscess and emergence of resistance. Médecine et Maladies Infectieuses. (2020) 2020:22. doi: 10.1016/j.medmal.2020.10.022
37. Kohira N, Hackel MA, Ishioka Y, Kuroiwa M, Sahm DF, Sato T, et al. Reduced susceptibility mechanism to cefiderocol, a siderophore cephalosporin, among clinical isolates from a global surveillance programme (SIDERO-WT-2014). J Glob Antimicrob Resist. (2020) 22:738–41. doi: 10.1016/j.jgar.2020.07.009
38. Shields RK, Iovleva A, Kline EG, Kawai A, McElheny CL, Doi Y. Clinical evolution of AmpC-mediated ceftazidime-avibactam and cefiderocol resistance in Enterobacter cloacae complex following exposure to cefepime. Clin Infect Dis. (2020) 71:2713–6. doi: 10.1093/cid/ciaa355
39. Kawai A, McElheny CL, Iovleva A, Kline EG, Sluis-Cremer N, Shields RK, et al. Structural basis of reduced susceptibility to ceftazidime-avibactam and cefiderocol in Enterobacter cloacae due to AmpC R2 loop deletion. Antimicrob Agents Chemother. (2020) 64:20. doi: 10.1128/AAC.00198-20
40. Hartney SL, Mazurier S, Girard MK, Mehnaz S, Davis EW 2nd, Gross H, et al. Ferric-pyoverdine recognition by Fpv outer membrane proteins of Pseudomonas protegens Pf-5. J Bacteriol. (2013) 195:765–76. doi: 10.1128/JB.01639-12
41. Moynié L, Luscher A, Rolo D, Pletzer D, Tortajada A, Weingart H, et al. Structure and function of the PiuA and PirA siderophore-drug receptors from Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob Agents Chemother. (2017) 61:16. doi: 10.1128/AAC.02531-16
42. Malik S, Kaminski M, Landman D, Quale J. Cefiderocol resistance in Acinetobacter baumannii: roles of β-lactamases, siderophore receptors, and penicillin binding protein 3. Antimicrob Agents Chemother. (2020) 64:20. doi: 10.1128/AAC.01221-20
Keywords: Gram-negative bacteria, activity, cefiderocol, resistance mechanisms, cephalosporins (therapeutic use)
Citation: Yao J, Wang J, Chen M and Cai Y (2021) Cefiderocol: An Overview of Its in-vitro and in-vivo Activity and Underlying Resistant Mechanisms. Front. Med. 8:741940. doi: 10.3389/fmed.2021.741940
Received: 15 July 2021; Accepted: 02 November 2021;
Published: 07 December 2021.
Edited by:
Leonardo Neves Andrade, University of São Paulo, BrazilReviewed by:
Naveen Kumar Devanga Ragupathi, The University of Sheffield, United KingdomCinara Feliciano, University of São Paulo, Brazil
Copyright © 2021 Yao, Wang, Chen and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Cai, Y2FpY2FpX2hoQDEyNi5jb20=; Mengli Chen, aGVsbG9saWx5MzAxY25AMTI2LmNvbQ==
 Jiahui Yao1
Jiahui Yao1 Yun Cai
Yun Cai