- 1Department of Pediatrics, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
- 2Department of Family Medicine, Faculty of Medicine, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
This systematic review aimed to provide an overview of the clinical profile and outcome of COVID-19 infection in patients with hemoglobinopathy. The rate of COVID-19 mortality and its predictors were also identified. A systematic search was conducted in accordance with PRISMA guidelines in five electronic databases (PubMed, Scopus, Web of Science, Embase, WHO COVID-19 database) for articles published between 1st December 2019 to 31st October 2020. All articles with laboratory-confirmed COVID-19 cases with underlying hemoglobinopathy were included. Methodological quality was assessed using the Joanna Briggs Institute (JBI) critical appraisal checklists. Thirty-one articles with data on 246 patients with hemoglobinopathy were included in this review. In general, clinical manifestations of COVID-19 infection among patients with hemoglobinopathy were similar to the general population. Vaso-occlusive crisis occurred in 55.6% of sickle cell disease patients with COVID-19 infection. Mortality from COVID-19 infection among patients with hemoglobinopathy was 6.9%. After adjusting for age, gender, types of hemoglobinopathy and oxygen supplementation, respiratory (adj OR = 89.63, 95% CI 2.514–3195.537, p = 0.014) and cardiovascular (adj OR = 35.20, 95% CI 1.291–959.526, p = 0.035) comorbidities were significant predictors of mortality. Patients with hemoglobinopathy had a higher mortality rate from COVID-19 infection compared to the general population. Those with coexisting cardiovascular or respiratory comorbidities require closer monitoring during the course of illness. More data are needed to allow a better understanding on the clinical impact of COVID-19 infections among patients with hemoglobinopathy.
Clinical Trial Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020218200.
Introduction
The unprecedented coronavirus disease-2019 (COVID-19) pandemic has not abated since the first-ever reported case in Wuhan, China. The World Health Organization (WHO) declared the outbreak of Public Health Emergency of International Concern on 30th January 2020, and subsequent pandemic on 11th March 2020. According to WHO COVID Dashboard, as of 9th August 2021, COVID-19 has impacted more than 200 million patients globally, with incidence and mortality rates of 2.58 and 2.12%, respectively. The emergence of new variants that are associated with higher severity and mortality has added more burden to the already exhausted health care system.
COVID-19 is known to spread through respiratory droplets, with recent evidence of airborne transmission. Published studies suggested 17.9–30.8% of infected patients may be asymptomatic (1, 2). Symptoms of COVID-19 infection vary from the common presentation of fever, cough, and shortness of breath, to the less common ones such as anosmia, ageusia, and diarrhea (3, 4). Several risk factors were identified to be associated with higher mortality including age more than 65 years old and the presence of chronic diseases such as diabetes mellitus and cardiovascular disease.
Haemoglobinopathy itself is a chronic disease. Patients with hemoglobinopathy are a specific population with special health needs. Cardiopulmonary comorbidities that arise as complications of the disease are one of the main causes of mortality and morbidity in this population (5). Concerns arise whether this group of patients is more susceptible to COVID-19 infection with a more severe course of illness given their immunocompromised state and its many comorbidities. Clinicians need to be aware of the potential differences in how COVID-19 infection manifests in patients with hemoglobinopathies, along with the possible risk factors associated with poorer outcomes. As most evidence is being published as case series or case reports, there is a need to synthesize these findings to guide clinicians in managing COVID-19 infection in patients with hemoglobinopathy.
Hence, this systematic review aims to provide an overview of the clinical profile, including the clinical presentations, laboratory, and radiological findings, as well as the outcome of COVID-19 infection among patients with hemoglobinopathy.
Materials and Methods
Search Strategy and Selection Criteria
The study methods were in adherence to the guidelines established by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (Protocol number #CRD42020218200).
A systematic search was conducted in the following databases: PubMed, Scopus, Web of Science, Embase, and WHO COVID-19 database. The search terms included were “COVID-19” OR “severe acute respiratory syndrome coronavirus 2” OR “ncov” OR “2019-nCoV” OR “COVID-19” OR “SARS-CoV-2” AND “Hemoglobinopathies” OR “Thalassemia” OR “Anemia, Sickle Cell” OR Hemoglobin C Disease. The last search was performed on 30th October 2020.
Articles that were eligible for review included the following study designs: systematic review, cohort, case-control, cross-sectional, case report, and case series. Only articles published from 1st December 2019 to 31st October 2020, in English which reported laboratory-confirmed COVID-19 cases with underlying hemoglobinopathy were included in this review. In this review, hemoglobinopathies were defined as a heterogeneous group of inherited disorders characterized by structural alterations within the hemoglobin molecule, specifically sickle cell disease and thalassemia. This review included both the adult and pediatric populations. Articles that reported suspected COVID-19 cases without laboratory evidence, in vitro studies, animal experiments, or patients without hemoglobinopathy were excluded from this review. Papers that consisted of only an abstract were also excluded.
Outcomes of interest for this study were clinical presentation, laboratory, and radiological findings, and outcomes of COVID-19 infection among patients with hemoglobinopathies.
Data Collection and Risk of Bias Assessment
The studies extracted from the searches were identified by two independent reviewers. Citation records were managed with EndNote(R). Duplicate citations were deleted and the records were exported to an Excel sheet. Each citation was screened based on titles, abstracts, and keywords. Reasons for excluding citations were recorded. The full articles fulfilling the inclusion criteria were retrieved for review. A third investigator was consulted to resolve differences of opinion at any phase. Data retrieved from each article was cross-checked by at least two independent investigators.
The Excel data extraction form recorded the following information: author/s, study title, study design, country of study, year of publication, digital object identifier (DOI), sample size, comorbidities, clinical signs and symptoms, laboratory findings, imaging findings, outcomes.
The quality of studies included was appraised using the Joanna Briggs Institute (JBI) critical appraisal tools for the respective study designs (6). The studies were further classified into poor, moderate, or high quality based on selected criteria that would provide sufficient information for the purpose of this study (Supplementary Material). The risk of bias was assessed independently by two investigators and any discrepancies in opinions were resolved by a third investigator.
Operational Definitions
Symptoms were considered present if they occurred at any time from presentation to discharge. Duration of symptoms was presented in days.
All laboratory data results were categorized into high, normal, or low, according to the local laboratory reference values in the respective articles. This was done taking into consideration that different laboratories would have different reference ranges. For articles that reported mean values for multiple samples, the researcher attempted to email the original authors for their raw data for further analysis. In the event that raw data was not available, they were considered as missing data.
Radiological findings were categorized based on the descriptive changes reported by the authors: normal, ground glass opacity, consolidation, combined ground glass opacity/consolidation and others.
Clinical staging for COVID severity was based on the National Institute of Health guidelines category 1 for asymptomatic presentation, category 2 for mild illness (mild systemic and respiratory symptoms with no clinical evidence of lower respiratory involvement), category 3 for moderate illness (clinical signs and symptoms of lower respiratory involvement with oxygen saturation of or more than 95% on room air at sea level), category 4 for severe illness (lower respiratory involvement with oxygen saturation of <95% on room air at sea level) and category 5 for critical illness (presence of acute respiratory distress syndrome, septic shock or multiorgan involvement) (7).
Statistical Analysis
Descriptive statistics were reported using frequencies, percentages, and ranges. The proportion ratios and prevalence rates were also determined. Simple logistic regression was done to determine the crude odds ratio for various comorbidities and COVID-19 mortality. Comorbidities with p-value of <0.25 were included in the model for binary logistic regression. Binary logistic regression was conducted to determine the independent associations between selected predictors with COVID-19 mortality, with adjustment for age, gender, and need for oxygen supplementation. All statistical analyses were done using Statistical Package for the Social Sciences (SPSS) version 26. The α for statistical significance was set at 0.05.
Results
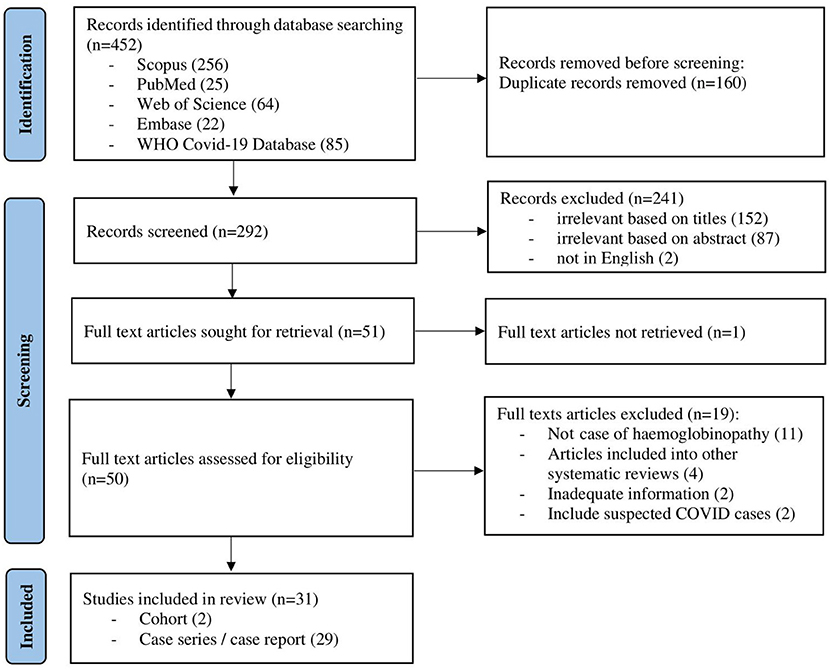
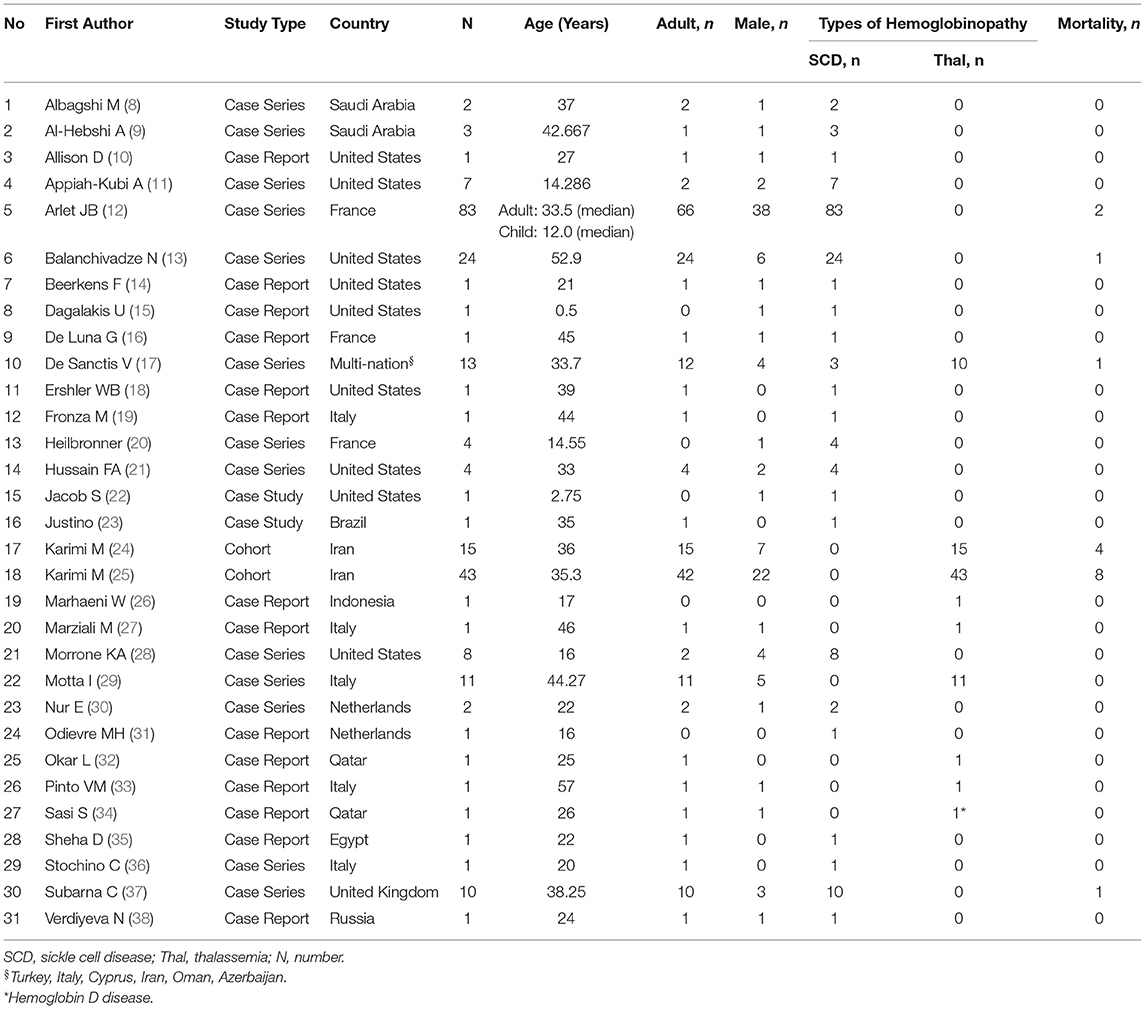
The initial search of the electronic databases yielded 452 articles. Manual searching through the references of these articles yielded no additional eligible articles. After removing 160 duplicates, the titles and abstracts for 292 articles were screened. Following this, 50 full-text articles were retrieved with the full text of 1 article being unable to be retrieved. Finally, out of the 50 eligible full-text articles, 31 articles were included in this review (Figure 1). Of these, 2 were retrospective cohort studies, 13 were case series and 16 were case reports (Table 1). As the majority of the articles were case reports and case series, the level of evidence was low. The methodological quality for all of the studies is described in the supplementary data (Supplementary Material).
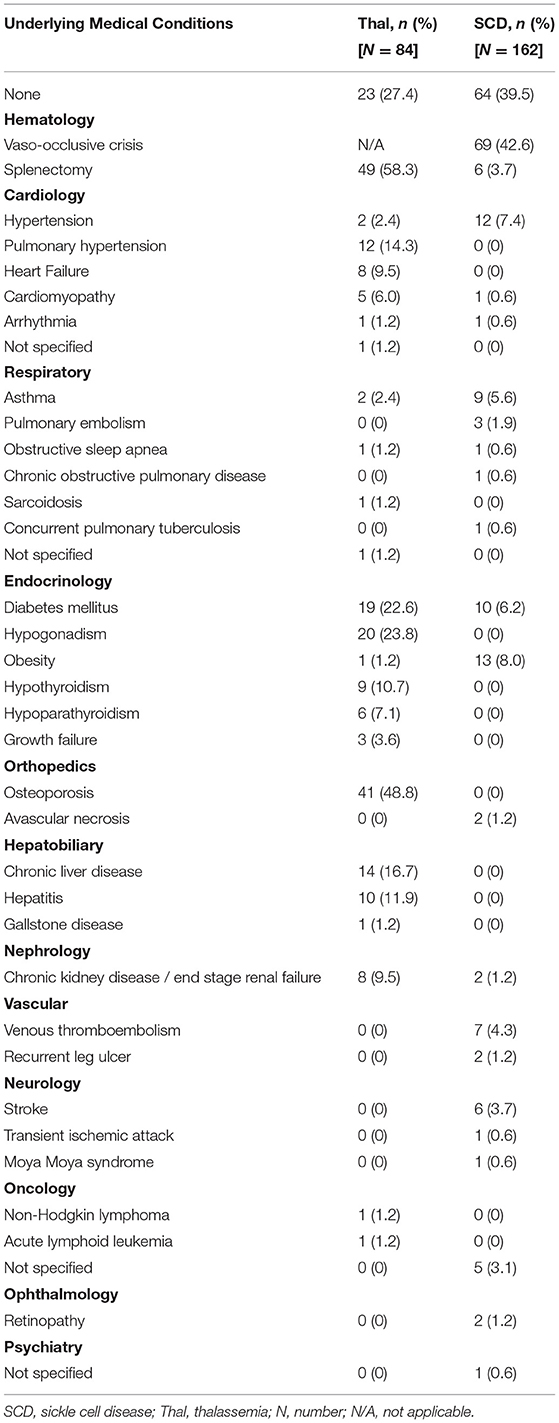
Overall, a total of 246 patients with hemoglobinopathies were reported to have COVID-19 infection. The patients' age ranged from 0.5 to 61 years old, with the majority (83.7%) being adults (age above 18 years old). Out of this, 140 (56.9%) had sickle cell anemia, 22 (8.9%) had sickle cell trait, 68 (27.6%) transfusion-dependent thalassemia and 16 (6.5%) non-transfusion-dependent thalassemia. Two-third (64.6%) of the patients had at least one underlying comorbidity and 22.4% had undergone splenectomy (Table 2). Among the sickle cell anemia patients, 42.6% had a history of vaso-occlusive crisis.
Table 3 summarized the reported clinical symptoms, laboratory markers, and radiological findings for COVID-19 infection in these patients. Twenty-nine (35.8%) patients had severe COVID-19 (Stage 4 or 5). The three most common presenting symptoms were fever (69.2%), vaso-occlusive crises (55.6%), and cough (54.2%). A small number of patients presented with mild non-respiratory symptoms such as gastrointestinal symptoms, conjunctivitis, and anorexia. Eight (6.7%) adult COVID-19 patients were detected through mass screening and were asymptomatic. Half (55.8%) of the patients had normal SpO2 during admission. Complete blood counts and C-reactive protein (CRP) were the most commonly reported laboratory results (Table 3). Overall, 79.5% (n = 105) of patients were anemic at presentation and 50.7% (n = 34) had leukocytosis. Radiographic findings were only reported in a small number of patients (n = 45) with 64.1% and 76.4% having radiological features of COVID-19 in chest x-ray and computed tomography, respectively.
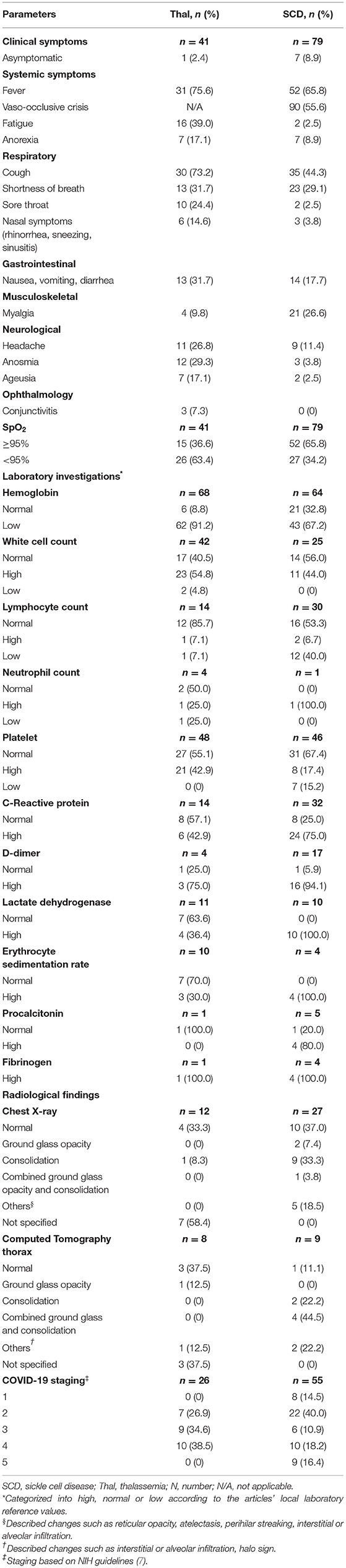
Table 3. Summary of clinical presentations, laboratory investigations and radiological imaging of the study population.
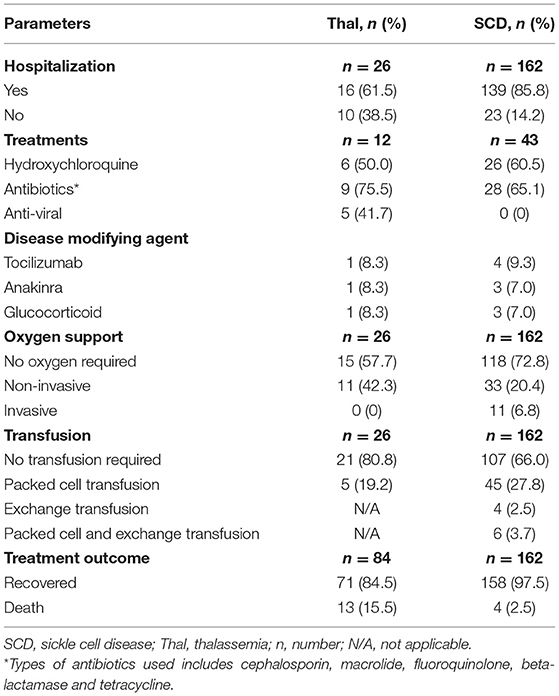
Twenty-eight articles reported treatment given to the patients (n = 93) (Table 4). Antibiotics (67.3%) and hydroxychloroquine (58.2%) were the more commonly administered drugs. The majority (82.4%) of patients required hospital admission with about 29.3% of them requiring supplemental oxygen (either non-invasive or invasive), and 31.9% required blood or exchange transfusion. There were 17 (6.9%) deaths reported; out of those, 13 (76.5%) were thalassemia patients while the remaining were patients with SCD.
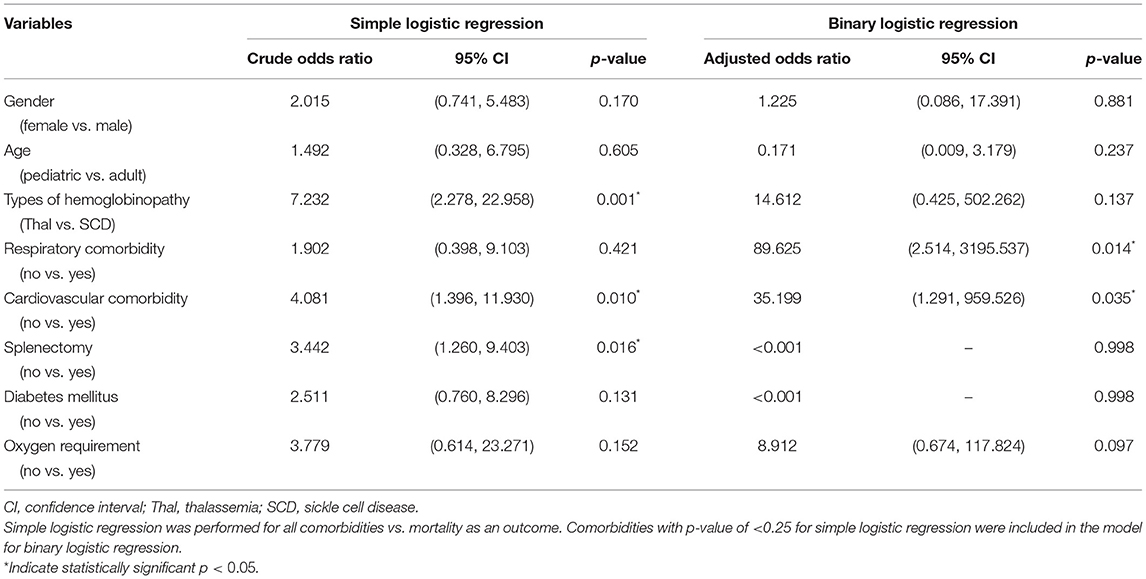
Binary logistic regression analysis was done to determine the independent association between comorbidities and mortality, adjusting for age, gender, types of hemoglobinopathy, and oxygen supplementation. The model predicted between 8.0 and 36.5% variance in the outcome and was able to correctly predict 97.3% of mortality outcomes. The presence of respiratory and cardiovascular comorbidities was independently associated with mortality in patients with underlying hemoglobinopathies who were infected with COVID-19 (Table 5). Respiratory comorbidities were associated with 89.63 times risk of death compared to those without respiratory comorbidities (adj OR = 89.63, 95% CI 2.514–3195.537, p = 0.014). Cardiovascular comorbidities were associated with 35.20 times risk of death compared to those without (adj OR = 35.20, 95% CI 1.291–959.526, p = 0.035).
Discussions
Our review found that in terms of severity, more patients with hemoglobinopathy (35.8%) had severe COVID-19 infection compared to the general population (11.1–19.1%) (39, 40). This could explain the higher mortality due to COVID-19 in this review (6.9%) compared to the general population (2.2–5.0%) (3, 39, 40). The mortality of 6.9% in patients with hemoglobinopathy was higher than reported COVID-19 mortality in patients with chronic kidney disease (1.54%) (41). However, it was lower compared to other immunocompromised conditions such as HIV infection (12.65%) (42) and malignancy (25.6%) (43). Majority of the published cases in this review was from high-income countries (25 articles; 17 thalassemia and 159 SCD patients) with four (2.5%) deaths involving SCD patients reported. In contrast, most of the thalassemia cases reported were from the low-middle-income countries (5 articles; 63 thalassemia patients) of which, there were 13 (20.6%) deaths. As the type of hemoglobinopathy is not a significant predictor of mortality in our logistic regression, we postulate that the higher mortality rate among the thalassemia patients could be attributed to the low health care system capacity in the low-middle-income countries.
This review also found that the presence of respiratory and cardiovascular comorbidities were independent predictors of mortality in COVID-19 infection. COVID-19 infection can lead to multi-system inflammation, resulting in myocardial injuries such as myocarditis, arrhythmia, acute coronary syndrome, and venous thromboembolism (44). COVID-19 related myocardial injury may further aggravate the burden of patients with underlying cardiovascular comorbidities, thereby putting them at higher risk of death (44). COVID-19 infection is primarily a viral respiratory illness. In chronic pulmonary disease, alteration of the pulmonary structures may result in a more severe COVID-19 infection. Up-regulation of ACE-2 expression in COPD, and delayed innate antiviral immune response in asthma (45) are mechanisms that possibly explain the higher risk of severe COVID-19 infection and mortality among those with respiratory comorbidities (40).
Several studies have found that SCD patients with heart and lung comorbidities were at higher risk of severe COVID-19 infections (46, 47). Similarly, the mortality rate among thalassemia patients with cardiovascular comorbidities was significantly higher when compared to its counterpart without underlying cardiovascular disease (48). Thalassemia patients are expected to be at higher risk of cardiovascular comorbidities compared to SCD patients. This is due to the higher risk of cardiomyopathy associated with iron overload compared to patients with sickle cell disease (49). Cardiomyopathy is also the leading cause of mortality and morbidity in thalassemia patients (50, 51). However, this excess risk of COVID-19 mortality due to the presence of cardiovascular comorbidity remained statistically significant despite statistically controlling for the type of hemoglobinopathy. Due to the relatively small number of samples, the precision of the adjusted OR was affected, resulting in an extremely wide confidence interval. As more evidence is being generated, a more precise estimate of the true adjusted odds ratio can be determined.
In this review, the clinical presentations of COVID-19 infection among patients with hemoglobinopathy were almost similar to the general population, with fever and cough being reported as the most common symptoms (52, 53). COVID-19 infection may also lead to hypoxia, triggering vaso-occlusive crisis, making this one of the common manifestations seen in patients with SCD. Anemia, leukocytosis, lymphopenia, thrombocytosis, and raised CRP were among the abnormal laboratory changes reported (54, 55). These are among the common non-specific findings in the presence of infections. The treatment and management of COVID-19 in these patients were similar to that of COVID-19 patients in the general population (56–58). Exchange transfusion was only required in a small number of patients with sickle cell disease who presented with vaso-occlusive crises and severe COVID-19 infections (n=10, 6.2%). Meanwhile, blood transfusion was generally required when patients with hemoglobinopathy had anemia during presentation (mean hemoglobin 8.2 ± 1.6 g/dL). As the data available was limited, we were unable to determine whether the requirement for transfusion was influenced by the severity of COVID-19 infection and oxygen requirement. None of the articles in this review reported on the impact of COVID-19 pandemic on blood transfusion services at their center, and whether this indirectly affect the management as well as the outcome of patients with hemoglobinopathy when they were admitted for COVID-19 infection. Two studies conducted in Eastern Mediterranean reported no interruption on blood transfusion services for patients with hemoglobinopathy despite the pandemic (59, 60). However, more data is needed especially from the low- and middle-income countries with regards to this matter.
This systematic review summarized the clinical manifestations, investigations, and outcomes of COVID-19 in the hemoglobinopathy population. One of the limitations was that studies included in this review were published during the early phase of COVID-19 pandemic, thus most of them were case reports or case series with low levels of evidence. The small sample size also discouraged further analysis based on types of hemoglobinopathy. Furthermore, not all of the studies reported their laboratory and radiological findings, hence a meta-analysis was not feasible to conclusively determine the association between laboratory markers and disease outcomes. Another limitation in our review was that the radiological findings were categorized based on the descriptive changes reported by the authors. As both COVID-19 pneumonia and acute chest syndrome in SCD patients may have similar findings (consolidation, ground glass opacity, and atelectasis), we were unable to differentiate what causes these radiological changes. The outcome of the study population reported in this systematic review was limited to mortality rate as no follow-up data were available with regards to long term effects of COVID-19 infection to patients with hemoglobinopathy. Finally, the actual number of COVID-19 cases or deaths among patients with hemoglobinopathy may be underreported especially in developing or under-developed nations, given limitations of resources for testing. The development of an authoritative international registry to capture the data on COVID-19 infections among patients with hemoglobinopathy will allow a more accurate and impactful analysis.
Conclusion
This review has shown that there is no difference in terms of clinical manifestations, laboratory and imaging findings for COVID-19 infections among patients with hemoglobinopathy compared to the general population. There is higher COVID-19 mortality among patients with hemoglobinopathy compared to the general population. Clinicians who manage COVID-19 infections in patients with underlying hemoglobinopathy should therefore exercise greater caution, especially in the presence of coexisting cardiovascular or respiratory comorbidities. Further large-scale, longitudinal studies are needed to evaluate the impact and long-term morbidity of COVID-19 infection on patients with hemoglobinopathy.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
JL, WC, CT, and SL contributed to the conception and design of the study. JL and WC conducted the systematic search of the study. JL, WC, and CT contributed to the data analysis. All authors contributed to the drafting and revising the article and gave final approval of the version for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.757510/full#supplementary-material
References
1. Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance. (2020) 25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180
2. Nishiura H, Kobayashi T, Miyama T, Suzuki A, Jung S-m, Hayashi K, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). Int J Infect Dis. (2020) 94:154–5. doi: 10.1016/j.ijid.2020.03.020
3. Yang BY, Barnard LM, Emert JM, Drucker C, Schwarcz L, Counts CR, et al. Clinical characteristics of patients With Coronavirus Disease 2019 (COVID-19) receiving emergency medical services in King County, Washington. JAMA Netw Open. (2020) 3:e2014549. doi: 10.1001/jamanetworkopen.2020.14549
4. Li J, Huang DQ, Zou B, Yang H, Hui WZ, Rui F, et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. (2021) 93:1449–58. doi: 10.1002/jmv.26424
5. Vij R, Machado RF. Pulmonary complications of hemoglobinopathies. Chest. (2010) 138:973–83. doi: 10.1378/chest.10-0317
6. Aromataris E, Munn Z. JBI Manual for Evidence Synthesis. JBI (2020). Available online at: https://synthesismanual.jbi.global/ (accessed April 21, 2021).
7. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines: National Institutes of Health. (2021). Available online at: https://www.covid19treatmentguidelines.nih.gov/ (accessed April 21, 2021).
8. Albagshi M, Albagshi M. Sickle cell disease: high risk or no risk for coronavirus disease 2019 infection. J Appl Hematol. (2020) 11:89–90. doi: 10.4103/joah.joah_95_20
9. Al-Hebshi A, Zolaly M, Alshengeti A, Al Qurainees G, Yamani S, Hamdan N, et al. A Saudi family with sickle cell disease presented with acute crises and COVID-19 infection. Pediatr Blood Cancer. (2020) 67:e28547. doi: 10.1002/pbc.28547
10. Allison D, Campbell-Lee S, Crane J, Vidanovic V, Webb S, Fraidenburg D, et al. Red blood cell exchange to avoid intubating a COVID-19 positive patient with sickle cell disease? J Clin Apher. (2020) 35:378–81. doi: 10.1002/jca.21809
11. Appiah-Kubi A, Acharya S, Fein Levy C, Vlachos A, Ostovar G, Murphy K, et al. Varying presentations and favourable outcomes of COVID-19 infection in children and young adults with sickle cell disease: an additional case series with comparisons to published cases. Br J Haematol. (2020) 190:e221–4. doi: 10.1111/bjh.17013
12. Arlet JB, de Luna G, Khimoud D, Odievre MH, de Montalembert M, Joseph L, et al. Prognosis of patients with sickle cell disease and COVID-19: a French experience. Lancet Haematol. (2020) 7:e632–4. doi: 10.1016/S2352-3026(20)30204-0
13. Balanchivadze N, Kudirka AA, Askar S, Almadhoun K, Kuriakose P, Fadel R, et al. Impact of COVID-19 infection on 24 patients with sickle cell disease. One Center Urban Experience, Detroit, MI, USA. Hemoglobin. (2020) 44:284–9. doi: 10.1080/03630269.2020.1797775
14. Beerkens F, John M, Puliafito B, Corbett V, Edwards C, Tremblay D. COVID-19 pneumonia as a cause of acute chest syndrome in an adult sickle cell patient. Am J Hematol. (2020) 95:E154–6. doi: 10.1002/ajh.25809
15. Dagalakis U, Hammershaimb E, McArthur MA, Macatangay RA. SARS-CoV-2 infection in pediatric patient with hemoglobin SC disease. Pediatr Blood Cancer. (2020) 67:e28430. doi: 10.1002/pbc.28430
16. De Luna G, Habibi A, Deux JF, Colard M, Pham Hung d'Alexandry d'Orengiani AL, Schlemmer F, et al. Rapid and severe COVID-19 pneumonia with severe acute chest syndrome in a sickle cell patient successfully treated with tocilizumab. Am J Hematol. (2020) 95:876–8. doi: 10.1002/ajh.25833
17. de Sanctis V, Canatan D, Corrons JLV, Karimi M, Daar S, Kattamis C, et al. Preliminary Data on COVID-19 in Patients with Hemoglobinopathies: A Multicentre ICET-A Study. Mediterr J Hematol Infect Dis. (2020) 12:e2020046. doi: 10.4084/mjhid.2020.046
18. Ershler WB, Holbrook ME. Sickle cell anemia and COVID-19: use of voxelotor to avoid transfusion. Transfusion. (2020) 60:3066–7. doi: 10.1111/trf.16068
19. Fronza M, Bardaro F, Stirpe E. Acute lung failure due to COVID-19 in a patient with sickle cell anemia. Minerva Pneumol. (2020) 59:44–6. doi: 10.23736/S0026-4954.20.01880-5
20. Heilbronner C, Berteloot L, Tremolieres P, Dupic L, de Saint Blanquat L, Lesage F, et al. Patients with sickle cell disease and suspected COVID-19 in a paediatric intensive care unit. Br J Haematol. (2020) 190:e21–4. doi: 10.1111/bjh.16802
21. Hussain FA, Njoku FU, Saraf SL, Molokie RE, Gordeuk VR, Han J. COVID-19 infection in patients with sickle cell disease. Br J Haematol. (2020) 189:851–2. doi: 10.1111/bjh.16734
22. Jacob S, Dworkin A, Romanos-Sirakis E. A pediatric patient with sickle cell disease presenting with severe anemia and splenic sequestration in the setting of COVID-19. Pediatr Blood Cancer. (2020) 67:e28511. doi: 10.1002/pbc.28511
23. Justino CC, Campanharo FF, Augusto MN, Morais SC, Figueiredo MS. COVID-19 as a trigger of acute chest syndrome in a pregnant woman with sickle cell anemia. Hematol Transfus Cell Ther. (2020) 42:212–4. doi: 10.1016/j.htct.2020.06.003
24. Karimi M, Haghpanah S, Azarkeivan A, Zahedi Z, Zarei T, Akhavan Tavakoli M, et al. Prevalence and mortality in β-thalassaemias due to outbreak of novel coronavirus disease (COVID-19): the nationwide Iranian experience. Br J Haematol. (2020) 190:e137–40. doi: 10.1111/bjh.16911
25. Karimi M, Haghpanah S, Zarei T, Azarkeivan A, Shirkavand A, Matin S, et al. Prevalence and severity of Coronavirus disease 2019 (COVID-19) in Transfusion Dependent and Non-Transfusion Dependent beta-thalassemia patients and effects of associated comorbidities: an Iranian nationwide study. Acta Bio Med. (2020) 91:e2020007. doi: 10.23750/abm.v91i3.10155
26. Marhaeni W, Wijaya AB, Kusumaningtyas P, Mapianto RS. Thalassemic child presenting with anosmia due to COVID-19. Indian J Pediatr. (2020) 87:750. doi: 10.1007/s12098-020-03370-4
27. Marziali M, Ribersani M, Losardo AA, Taglietti F, Pugliese P, Micozzi A, et al. COVID-19 pneumonia and pulmonary microembolism in a patient with B-thalassemia major. Clin Case Rep. (2020) 8:3139–42. doi: 10.1002/ccr3.3275
28. Morrone KA, Strumph K, Liszewski MJ, Jackson J, Rinke ML, Silver EJ, et al. Acute chest syndrome in the setting of SARS-COV-2 infections-A case series at an urban medical center in the Bronx. Pediatr Blood Cancer. (2020) 67:e28579. doi: 10.1002/pbc.28579
29. Motta I, Migone De Amicis M, Pinto VM, Balocco M, Longo F, Bonetti F, et al. SARS-CoV-2 infection in beta thalassemia: preliminary data from the Italian experience. Am J Hematol. (2020) 95:E198–9. doi: 10.1002/ajh.25840
30. Nur E, Gaartman AE, van Tuijn CFJ, Tang MW, Biemond BJ. Vaso-occlusive crisis and acute chest syndrome in sickle cell disease due to 2019 novel coronavirus disease (COVID-19). Am J Hematol. (2020) 95:725–6. doi: 10.1002/ajh.25821
31. Odièvre MH, de Marcellus C, Ducou Le Pointe H, Allali S, Romain AS, Youn J, et al. Dramatic improvement after tocilizumab of severe COVID-19 in a child with sickle cell disease and acute chest syndrome. Am J Hematol. (2020) 95:E192–4. doi: 10.1002/ajh.25855
32. Okar L, Ali M, Parengal J, Yassin MA. COVID-19 and thalassemia beta major in splenectomized patient: clinical case progression and literature review. Clin Case Rep. (2020) 8:2918–22. doi: 10.1002/ccr3.3345
33. Pinto VM, Derchi GE, Bacigalupo L, Pontali E, Forni GL. COVID-19 in a patient with ß-thalassemia major and severe pulmonary arterial hypertension. Hemoglobin. (2020) 44:218–20. doi: 10.1080/03630269.2020.1779082
34. Sasi S, Yassin MA, Nair AP, Al Maslamani MS. A case of COVID-19 in a patient with asymptomatic hemoglobin D thalassemia and glucose-6-phosphate dehydrogenase deficiency. Am J Case Rep. (2020) 21:e925788. doi: 10.12659/AJCR.925788
35. Sheha D, El-Shayeb M, Eid Y, Amin M, Saeed A, Abdou D, et al. Unfolding of sickle cell trait by coronavirus disease 2019 (COVID-19) infection. Br J Haematol. (2020) 191:e38–40. doi: 10.1111/bjh.17089
36. Stochino C, Villa S, Zucchi P, Parravicini P, Gori A, Raviglione MC. Clinical characteristics of COVID-19 and active tuberculosis co-infection in an Italian reference hospital. Eur Respir J. (2020) 56:2001708. doi: 10.1183/13993003.01708-2020
37. Subarna C, Giselle P-P, Fester I, Virginia T, Charlotte G, David R, et al. COVID-19 in patients with sickle cell disease - a case series from a UK Tertiary Hospital. Haematologica. (2020) 105:2691–3. doi: 10.3324/haematol.2020.254250
38. Verdiyeva N, Ibrahimova T, Nasibova A, Huseynov V. How to treat and manage COVID-19 in SCD patients. Hematol Transfusion Cell Ther. (2020) 42:76. doi: 10.1016/j.htct.2020.09.137
39. Epidemiology Working Group for NCIP Epidemic Response Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Chin J Epidemiol. (2020) 41:145–51. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003
40. Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, et al. Coronavirus disease 2019 case surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:759–65. doi: 10.15585/mmwr.mm6924e2
41. Cai R, Zhang J, Zhu Y, Liu L, Liu Y, He Q. Mortality in chronic kidney disease patients with COVID-19: a systematic review and meta-analysis. Int Urol Nephrol. (2021) 53:1623–9. doi: 10.1007/s11255-020-02740-3
42. Ssentongo P, Heilbrunn ES, Ssentongo AE, Advani S, Chinchilli VM, Nunez JJ, et al. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: a systematic review and meta-analysis. Sci Rep. (2021) 11:6283. doi: 10.1038/s41598-021-85359-3
43. Saini KS, Tagliamento M, Lambertini M, McNally R, Romano M, Leone M, et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer. (2020) 139:43–50. doi: 10.1016/j.ejca.2020.08.011
44. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. (2020) 17:543–58. doi: 10.1038/s41569-020-0413-9
45. Ejaz H, Alsrhani A, Zafar A, Javed H, Junaid K, Abdalla AE, et al. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health. (2020) 13:1833–9. doi: 10.1016/j.jiph.2020.07.014
46. Mucalo L, Brandow AM, Dasgupta M, Mason SF, Simpson PM, Singh A, et al. Comorbidities are risk factors for hospitalization and serious COVID-19 illness in children and adults with sickle cell disease. Blood Adv. (2021) 5:2717–24. doi: 10.1182/bloodadvances.2021004288
47. Minniti CP, Zaidi AU, Nouraie M, Manwani D, Crouch GD, Crouch AS, et al. Clinical predictors of poor outcomes in patients with sickle cell disease and COVID-19 infection. Blood advances. (2021) 5:207–15. doi: 10.1182/bloodadvances.2020003456
48. Zafari M, Rad MT, Mohseni F, Nikbakht N. β-Thalassemia major and coronavirus-19, mortality and morbidity: a systematic review study. Hemoglobin. (2021) 45:1–4. doi: 10.1080/03630269.2020.1857266
49. Meloni A, Puliyel M, Pepe A, Berdoukas V, Coates TD, Wood JC. Cardiac iron overload in sickle-cell disease. Am J Hematol. (2014) 89:678–83. doi: 10.1002/ajh.23721
50. Tantiworawit A, Tapanya S, Phrommitikul A, Norasetthada L, Chai-Adisaksopha C, Rattarittamrong E, et al. Cardiovascular complications of thalassemia patients in the iron chelation therapy era. Blood. (2013) 122:5602. doi: 10.1182/blood.V122.21.5602.5602
51. Pennell DJ, Udelson JE, Arai AE, Bozkurt B, Cohen AR, Galanello R, et al. Cardiovascular function and treatment in β-thalassemia major: a consensus statement from the American Heart Association. Circulation. (2013) 128:281–308. doi: 10.1161/CIR.0b013e31829b2be6
52. Mehta OP, Bhandari P, Raut A, Kacimi SEO, Huy NT. Coronavirus Disease (COVID-19): comprehensive review of clinical presentation. Front Public Health. (2021) 8:582932. doi: 10.3389/fpubh.2020.582932
53. Wong CKH, Wong JYH, Tang EHM, Au CH, Wai AKC. Clinical presentations, laboratory and radiological findings, and treatments for 11,028 COVID-19 patients: a systematic review and meta-analysis. Sci Rep. (2020) 10:19765. doi: 10.1038/s41598-020-74988-9
54. Goudouris ES. Laboratory diagnosis of COVID-19. J Pediatr. (2021) 97:7–12. doi: 10.1016/j.jped.2020.08.001
55. Zhang Z-L, Hou Y-L, Li D-T, Li F-Z. Laboratory findings of COVID-19: a systematic review and meta-analysis. Scand J Clin Lab Investig. (2020) 80:441–7. doi: 10.1080/00365513.2020.1768587
56. Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug treatments for COVID-19: living systematic review and network meta-analysis. BMJ. (2020) 370:m2980. doi: 10.1136/bmj.m2980
57. Tahvildari A, Arbabi M, Farsi Y, Jamshidi P, Hasanzadeh S, Calcagno TM, et al. Clinical features, diagnosis, and treatment of COVID-19 in hospitalized patients: a systematic review of case reports and case series. Front Med. (2020) 7:231. doi: 10.3389/fmed.2020.00231
58. Juul S, Nielsen EE, Feinberg J, Siddiqui F, Jørgensen CK, Barot E, et al. Interventions for treatment of COVID-19: Second edition of a living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). PLoS ONE. (2021) 16:e0248132. doi: 10.1371/journal.pone.0248132
59. Al-Riyami AZ, Abdella YE, Badawi MA, Panchatcharam SM, Ghaleb Y, Maghsudlu M, et al. The impact of COVID-19 pandemic on blood supplies and transfusion services in Eastern Mediterranean Region. Transfus Clin Biol. (2021) 28:16–24. doi: 10.1016/j.tracli.2020.11.002
Keywords: COVID-19, hemoglobinopathies, sickle cell disease, thalassemia, severe acute respiratory syndrome coronavirus 2, systematic review
Citation: Lee JX, Chieng WK, Lau SCD and Tan CE (2021) COVID-19 and Hemoglobinopathies: A Systematic Review of Clinical Presentations, Investigations, and Outcomes. Front. Med. 8:757510. doi: 10.3389/fmed.2021.757510
Received: 12 August 2021; Accepted: 20 September 2021;
Published: 13 October 2021.
Edited by:
Eleni Gavriilaki, G. Papanikolaou General Hospital, GreeceReviewed by:
Mohamed A. Yassin, Hamad Medical Corporation, QatarEfthymia Vlachaki, Aristotle University of Thessaloniki, Greece
Copyright © 2021 Lee, Chieng, Lau and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sie Chong Doris Lau, ZG9yaXNAcHB1a20udWttLmVkdS5teQ==
†These authors have contributed equally to this work and share first authorship
 Jun Xin Lee
Jun Xin Lee Wei Keong Chieng
Wei Keong Chieng Sie Chong Doris Lau
Sie Chong Doris Lau Chai Eng Tan
Chai Eng Tan