- Drug Sector, Saudi Food and Drug Authority, Riyadh, Saudi Arabia
Background: Preterm birth (PTB) is a leading cause of neonatal morbidity and mortality.
Objective: To estimate the effect of 17-alpha-hydroxyprogesterone caproate (17-OHPC) compared to placebo in singleton gestations for reducing the risk of recurrent PTB and neonatal morbidity and mortality.
Work Design: Systematic review and meta-analysis.
Search Strategy: Searching MEDLINE, Embase, Web of Science, SCOPUS, Cochrane Library, and clinical trial registries.
Selection Criteria: Randomized controlled trials of singleton gestations with a history of PTB and treated with a weekly intramuscular injection of 17-OHPC or placebo.
Data Collection and Analysis: A random meta-analysis model was performed for the PTB outcomes (<32, <35, and <37 weeks) and neonatal outcomes (neonatal death, grade 3 or 4 intraventricular hemorrhage, respiratory distress syndrome, bronchopulmonary dysplasia, necrotizing enterocolitis, and sepsis). Effect estimates were measured by relative risk ratio (RR) with a 95% confidence interval (CI).
Main Results: Six works were included. There were no statistically significant reductions in the PTB risk following the use of 17-OHPC at <32 weeks (RR = 0.61, 95% CI: 0.13–2.77, and I2 = 39%), <35weeks (RR = 0.60, 95% CI: 0.10–3.67, and I2 = 51%), and <37 weeks (RR = 0.68, 95% CI: 0.46–1, and I2 = 75%). Furthermore, all the neonatal outcomes were statistically similar between the two groups.
Conclusion: Treatment with 17-OHPC is not associated with reducing the risk of PTB or neonatal outcomes compared to placebo.
Synopsis
Systematic review and meta-analyses of randomized trials showed that 17-OHPC is not associated with reducing the risk of PTB or neonatal outcomes compared to placebo.
Introduction
The World Health Organization defined preterm birth (PTB) as delivery before 37 weeks of gestational age. The estimated global incidence of PTB was 15 million per year (1). Several risk factors were recognized for the recurrent PTB, including women with a history of prior PTB in any pregnancy and interpregnancy interval of fewer than 12 months (2).
The use of vaginal progesterone demonstrated a significant reduction in the risk of PTB (3), and also the use of 17-alpha-hydroxyprogesterone caproate (17-OHPC), which showed promising results (4). In 2011, the Food and Drug Administration (FDA) approved 17-OHPC for reducing the risk of PTB among pregnant women with a singleton pregnancy who have a history of singleton spontaneous PTB, and it was the only approved medication for this indication (5). However, in 2020, 17-OHPC (Makena) was recommended to be withdrawn from the market after failing the PROLONG trial to confirm the clinical benefit in reducing the risk of PTB and the benefit to neonates (6, 7).
Due to the inconsistence in the evidence between approval trial and PROLONG trial, this systematic review and meta-analysis aimed to determine the effect of 17-alpha-hydroxyprogesterone compared to placebo in reducing the risk of recurrent PTB and neonatal morbidity and mortality in women with singleton pregnancy and a history of at least one previous spontaneous PTB (SPTB).
Methods
Data Source and Searches
The research proposal for this work was registered in the International Prospective Register of Systematic Reviews (PROSPERO: CRD42021228756). The manuscript was prepared based on Preferred Reporting Items for Systematic Reviews (8). We conducted a comprehensive systematic search for the following databases: MEDLINE, Embase, Web of Science, SCOPUS, and The Cochrane Library, from inception to December 31, 2020 then updated till June 28, 2021. We also searched the following clinical trial registries: International Clinical Trials Registry Platform (ICTRP) https://apps.who.int/trialsearch/, The United States Clinical Trials Registry https://www.clinicaltrials.gov/, Australian New Zealand Clinical Trials Registry (ANZCTR) http://www.anzctr.org.au/TrialSearch.aspx, Clinical Trial Registry in Japan https://www.umin.ac.jp/ctr/, International Standard Randomized Controlled Trial Number Registry (ISRCTN registry) http://www.isrctn.com/, European Union Clinical Trials Register https://www.clinicaltrialsregister.eu/ctr-search/search, and Nederland's Trial Register https://www.trialregister.nl/. Moreover, we manually searched for any potential eligible works through the references of published reviews and selected works. The search strategies are available in the Supplementary Table 1.
Eligibility Criteria
We included all randomized controlled trials that compared the weekly intramuscular injection of 17-OHPC (intervention group) to weekly intramuscular injection of placebo (control group) in singleton pregnant women with a prior history of SPTB. We excluded trials that involve twin or multiple pregnancies or those used exclusively in patients with preterm rupture of membrane history, HIV history, assisted reproductive technology, arrested PTB, successful tocolysis, and placenta praevia.
Study Selection
After removing the duplicated and unrelated works, three investigators (AA, NF, and HA) independently reviewed the title and abstract, followed by a full-text review for works that met the inclusion criteria using the Rayyan software (9). Disagreement during the work selection was resolved by consensus.
Data Extraction and Quality Assessment
All the three authors (AA, NF, and HA) independently extracted the basic characteristics of the included works then cross-checked them. Disagreement was resolved by consensus. The extracted data were recorded in an Excel spreadsheet and included primary author name, year of publication, work location, work period; key inclusion criteria; sample size used for both 17-OHPC group and placebo group; and the treatment duration. The outcome measures were recorded in an Excel spreadsheet and included birth outcomes at (<32, <35, and <37 weeks) of gestation and the neonatal outcomes: neonatal death, grade 3 or 4 intraventricular hemorrhage, respiratory distress syndrome, bronchopulmonary dysplasia, necrotizing enterocolitis, and sepsis. AA, NF, and HA extracted data independently, cross-checked, and utilized consensus to resolve any disagreement. The revised Cochrane risk-of-bias tool for randomized trials was used to assess the quality of the included works for any potential biases (10). The risk of bias was assessed independently by AA, NF, and HA, then cross-checked, and agreed consensually.
Data Analysis
We performed a random effect meta-analysis model weighted by the Mantel–Haenszel method using Hartung and Knapp model for the outcomes reported in more than five works and DerSimonian and Laird random-effects model for outcomes reported in five works or less with a large ratio between the sizes of the works (11, 12). We reported the effect sizes of all meta-analyses as risk ratios (RR) with the corresponding 95% CI and graphed using forest plot figures. In addition, we quantified the between-work heterogeneity using the I2 statistics where I2 ≥ 75 percentage is indicative of high heterogeneity. We assessed the publication bias using Egger's test for the outcomes from four or more works. Subgroup analysis based on the location of the works [the United States (US vs. non-US)] was conducted for the outcome PTB at <32, <35, and <37 weeks. We also conducted sensitivity analyses for outcomes with substantial heterogeneity (≥75%) by removing one work at a time and analyzing the rest of the works to evaluate the impact of each work on the point of estimate and the heterogeneity. The statistical analysis was performed using R version 4.0.4.
Results
Studies' Characteristics
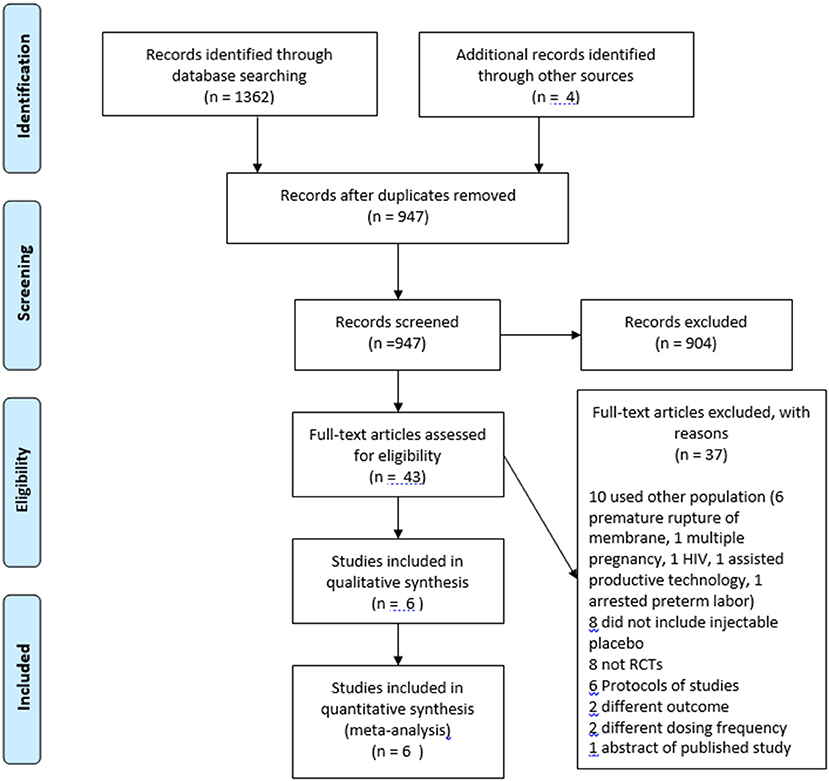
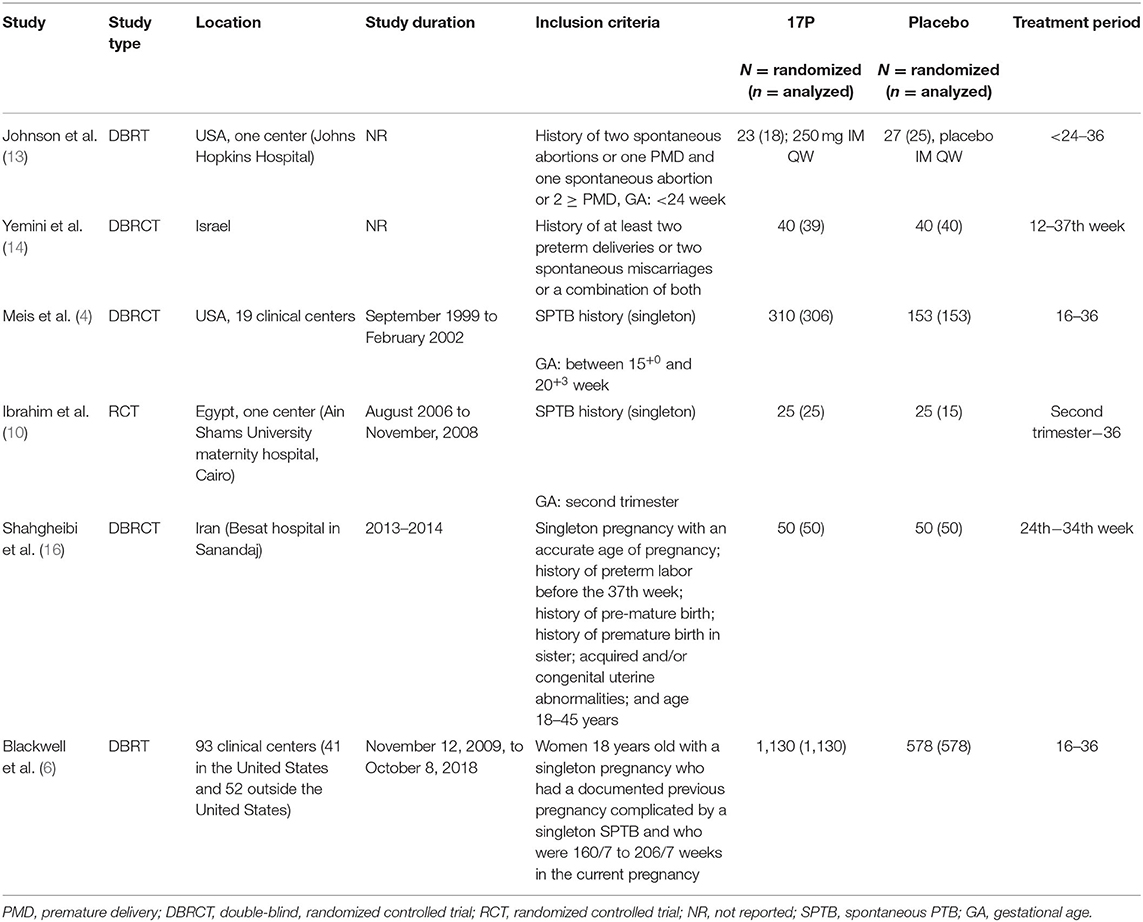
The databases search yielded 1,366 publications, and 43 publications were assessed using full-test for eligibility. Six randomized control trials met the inclusion and exclusion criteria (Figure 1) (4, 6, 13–16). The excluded works were summarized in Supplementary Table 2. The characteristics of the included works were summarized in Table 1. Four trials included 50 participants or less per arm (13–16). Three trials allowed cervical cerclage during the work period (6, 13, 14).
Risk of Bias Assessment
The overall risk of bias assessment of included works showed a low risk of bias in five works, but one work had some concern due to the open-label nature of the work and the lack of information about the baseline characteristics of included patients (Supplementary Table 3) (15). One work did not provide enough information about the adherence to the interventions which may raise a concern of the effect of adherence to the treatment (13). Another work had some concern due to the imbalance in the mean of previous preterm deliveries between the treatment arm and control arm at the baseline characteristics (4).
Risk of PTB Before 32, 35, and 37 Weeks
Three works provided data about the risk of PTB before 32 and 35 weeks (4, 6, 13). The pooled estimate showed no significant difference in reducing the risk of PTB prior 32 weeks (RR = 0.61, 95% CI: 0.13–2.77, and I2 = 39%, Figure 2A) and prior 35 weeks (RR = 0.60, 95% CI: 0.10–3.67, and I2 = 51%, Figure 2B). All six works provided data about the risk PTB before 37 weeks. The pooled estimate did not show a significant difference in the reduction in PTB risk before 37 weeks following the use of 17-OHPC compared to placebo (RR = 0.68, 95% CI: 0.46–1, and I2 = 75%, Figure 2C).
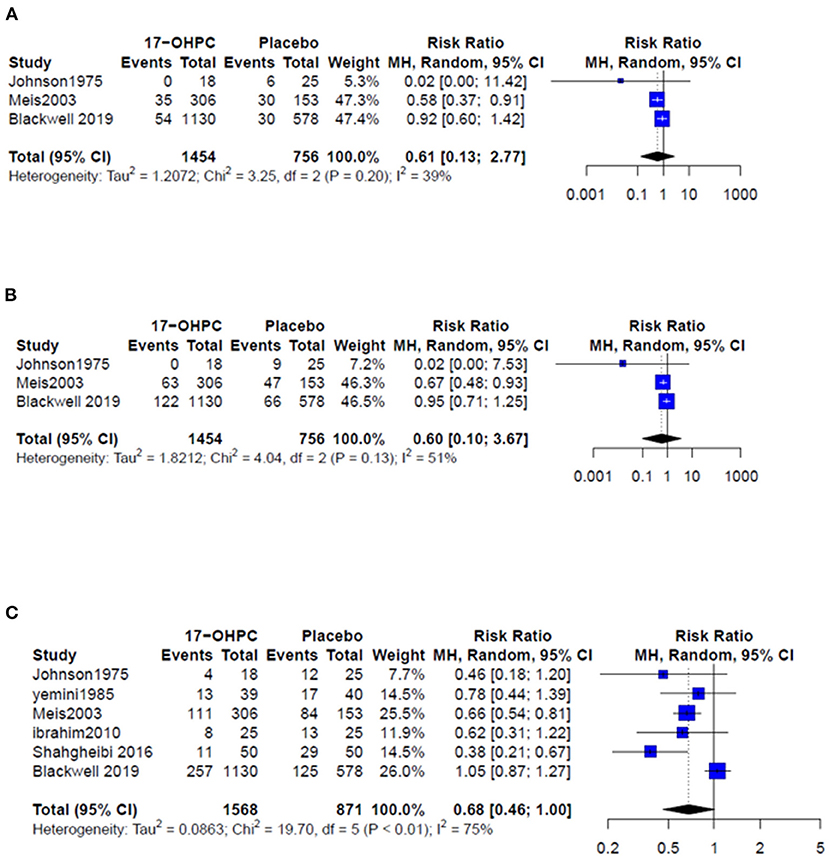
Figure 2. Forest plots of the risk of recurrent PTB below (A) 32 weeks, (B) 35 weeks, and (C) 37 weeks.
Neonatal Outcomes
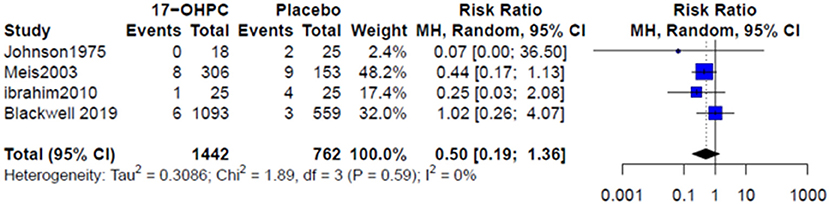
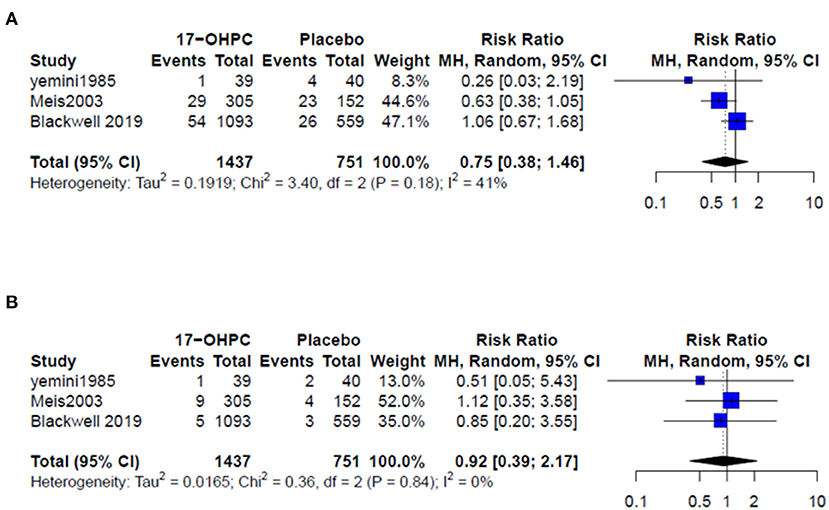
Four works reported information about the risk of neonatal death (4, 6, 13, 15). There was no significant difference in reducing the risk of neonatal death between 17-OHPC and placebo (RR = 0.50, 95% CI: 0.19–1.36, and I2 = 0%, Figure 3). Three works had data about the risks of respiratory distress and sepsis (4, 6, 14). The pooled estimates did not show a significant difference in reducing the risk of both outcomes between 17-OHPC and placebo with (RR = 0.75, 95% CI: 0.38–1.46, and I2 = 41%, Figure 4A) for respiratory distress and (RR = 0.92, 95% CI: 0.39–2.17, and I2 = 0%, Figure 4B) for sepsis. Two works provided data about the risks of grade 3 or 4 intravascular hemorrhage, bronchopulmonary dysplasia, and necrotizing enterocolitis (4, 6). Results from the meta-analysis for these outcomes did not show a significant difference between 17-OHPC and placebo (Figures 5A–C).
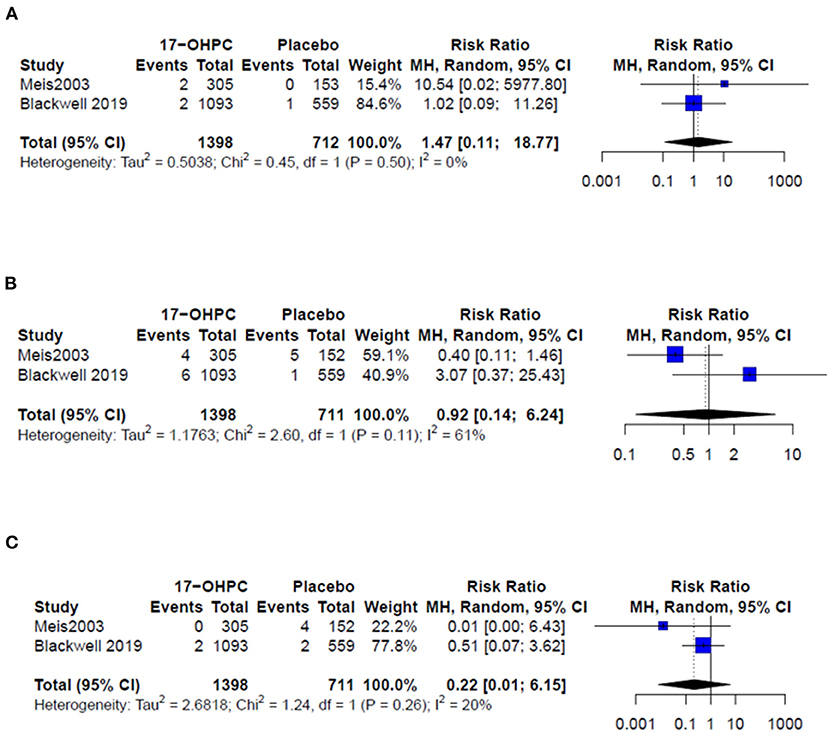
Figure 5. Forest plots of the risks of recurrent PTB below (A) grade 3 or 4 intravascular hemorrhage, (B) bronchopulmonary dysplasia, and (C) necrotizing enterocolitis.
Subgroup Analyses
The results from subgroup analyses based on the region from three works for PTB <32 weeks and 35 weeks indicated that no significant difference between 17-OHPC and placebo in the US-based works with RR = 0.51, 95% CI: 0.07–3.90, and I2 = 0%, Supplementary Figure 1, and RR = 0.58, 95% CI: 0.03–12.92, and I2 = 18%, Supplementary Figure 2, respectively. However, the risk of PTB <32 weeks based on one work showed a 76% increase in the risk of PTB in 17-OHPC users vs. placebo users (RR = 1.76, CI: 1.02–3.02, Supplementary Figure 1). Data about the risk of PTB <37 weeks were obtained from three US-based works (4, 6, 13), and four non-US-based works (6, 14–16). The was no significant difference between 17-OHPC and placebo in the US-based works (RR = 0.78, 95% CI: 0.27–2.28, I2 = 80%, Supplementary Figure 3) and non-US-based works (RR = 0.69, 95% CI: 0.35–1.37, I2 = 72%, Supplementary Figure 3).
Sensitivity Analyses
Exploring the effect of removing one work at a time for the risk of PTB <37 weeks (I2 = 75%) showed that removing PROLONG work resulted in reducing the heterogeneity (I2) to 0.04 % and changing the overall estimate to RR = 0.60, 95% CI: 0.43–0.83, which represent a significant reduction in the risk of PTB birth <37weeks following the use of 17-OHPC compared to placebo (Supplementary Figure 4).
Publication Bias
The publication bias was assessed for two outcomes: PTB <37 weeks and neonatal death. Egger's test results for both outcomes did not suggest a significant publication bias with a p-value of 0.24 and 0.56, respectively.
Discussion
Overall, the meta-analysis of the included works showed no significant association between 17-OHPC use and reduced risk for PTB in singleton gestations. They also found no significant differences in neonatal outcomes between 17-OHPC and placebo arms despite the low clinical and methodological heterogeneity across the works.
On the contrary, a recent meta-analysis (17) of four clinical trials comparing 17-OHPC vs. placebo or no treatment found that 17-OHPC can reduce PTB before 37 weeks of gestation by 29% (RR = 0.71; 95% CI = 0.53–0.96; P = 0.001). Regarding neonatal outcomes, a reduction in neonatal death by 68% (RR = 0.32; 95% CI = 0.15–0.66; P = 0.002) and a reduction in birthweights under 2,500 g by 35% (RR = 0.65; 95% CI = 0.50–0.84; P < 0.001) were reported. However, this meta-analysis did not include the data from the PROLONG trial. Compared to our review, which included only works that compared 17-OHPC and placebo treatments, Fernandez-Macias et al. meta-analysis compared 17-OHPC with placebo and no treatment groups (17).
Methodologically, both Meis et al. and PROLONG trials had almost similar inclusion and exclusion criteria (4, 6). However, there was an imbalance in baseline characteristics and risk factors in the treatment groups of PROLONG and Meis et al. trials. The PROLONG trial had a higher mean of gestational age, women's age, body mass index (BMI), smoking, alcohol, and illicit drug usage during pregnancy compared to the Meis et al. trial and a higher number of black women. The protocol of the PROLONG trial was also modified to allow the participation of women if they received any progesterone and stopped at least 4 weeks before the first dose of the work medication. However, PROLONG trial did not provide information about evaluating the results in patients who received any progesterone before the first dose of the work medication and also in patients who did not receive any progesterone. This would help to rule out any residual effect of using progesterone product before the randomization. The Meis et al. trial was conducted in the USA, while the PROLONG trial was a multinational, multicenter work, and the background rate of PTBs may not be similar in the included countries. In addition, the Meis et al. trial included patients from academic centers, which may not be a good representative of the general population.
Other works have also demonstrated the superiority of vaginal progesterone to intramuscular progesterone in reducing the risk for PTB. In a previous meta-analysis that included only randomized works comparing the efficacy of vaginal progesterone with that of 17-OHPC in preventing SPTB, lower rates of SPTB below 34 weeks were observed with vaginal progesterone (17.5 vs. 25.0%; RR = 0.71; 95% CI = 0.53–0.95) compared to 17-OHPC (18). The pooled analysis also found vaginal progesterone associated with lower rates of adverse maternal side effects and admission to NICU (18). Consistent with these findings, Maher et al., showed a beneficial effect of the vaginal progesterone over the intramuscular 17-OHPC in reducing the number of deliveries before 34 weeks of gestation (OR = 0.58; 95% CI = 0.37–0.89; P = 0.02), with lower adverse events in a Saudi-based cohort (19).
There are several strengths of our work. First, our analysis involved data from randomized controlled works of 2,451 women treated with either 17-OHPC or injectable placebo. Second, we included in our analysis the risk of preterm at three different time points (<32, <35, and <37 weeks) and also key neonatal outcomes. Third, we conducted a sensitivity analysis to assess the influence of removing one work at a time on the heterogeneity for the risk of recurrent PTB <37 weeks. The PROLONG work had a huge impact on the heterogeneity and the point estimate. It was the only multicenter, international trial with larger sample size. Finally, we were able to conduct a subgroup analysis to assess the difference in the risk of PTB (<32, <35, and <37 weeks) based on the work location to assess whether the effect of 17-OHPC differs in US-based works vs. non-US-based works. In general, the work location seems not to affect the overall conclusion even though one non-US-based work showed an increased risk of PTB <32 weeks among the 17-OHPC group, which may need more works to evaluate the effect of 17-OHPC on reducing the risk of PTB <32weeks.
We also acknowledge that our work has some limitations. First, not all outcomes were reported in the included works except in two works (4, 6). However, the Mesi et al. trial and PROLONG work were the largest trials which may allow us to perform an adequate estimate of the effect of 17-OHPC vs. placebo for all outcomes. Second, the included works did not provide enough information about the outcomes based on the cervical length at the starting of the work, smoking status, ethnicity, and the number of prior PTBs. Therefore, we were not able to run a meta-analysis to estimate the effect of 17-OHPC in these groups. Third, one of the works used an open-label method which may introduce some potential biases in managing and observing patients treated with 17-OHPC differed from those treated with placebo (15). However, in the sensitivity analysis, removing this work did not alter the conclusion (15). Finally, the lack of adequate information about the distribution of risk factors of PTB among treatment groups in all works may result in individual differences between the works. These differences may explain the heterogeneity between the works, especially for PTB outcomes <37 weeks.
In conclusion, our meta-analysis of the available randomized trials showed that the use of 17-OHPC might not be useful in reducing the risk of PTB or neonatal outcomes compared to placebo in women with singleton pregnancy and a history of SPTB. Further research discoveries are needed to provide effective treatment for PTB.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author Contributions
AA: analysis and interpretation of results. All authors: work conception, design and data collection, draft manuscript preparation, reviewed the results, and approved the final version of the manuscript.
Author Disclaimer
The contents of this manuscript are solely the authors' views and may not be understood or quoted as being made on behalf of or reflecting the position of the Saudi Food and Drug Authority.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.764855/full#supplementary-material
References
2. Yamashita M, Hayashi S, Endo M, Okuno K, Fukui O, Mimura K, et al. Incidence and risk factors for recurrent spontaneous preterm birth: a retrospective cohort study in Japan. J Obstet Gynaecol Res. (2015) 41:1708–14. doi: 10.1111/jog.12786
3. Romero R, Conde-Agudelo A, Da Fonseca E, O'Brien JM, Cetingoz E, Creasy GW, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. (2018) 218:161–80. doi: 10.1016/j.ajog.2017.11.576
4. Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. (2003) 348:2379–85. doi: 10.1056/NEJMoa035140
5. Gandell DL, Randell MD, Gudeman JL. FDA approved vs. Pharmacy compounded 17-OHPC-current issues for obstetricians to consider in reducing recurrent preterm birth. Curr Med Res Opin. (2020) 36:1393–401. doi: 10.1080/03007995.2020.1783220
6. Blackwell SC, Gyamfi-Bannerman C, Biggio JR Jr, Chauhan SP, Hughes BL, Louis JM, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG Study): a multicenter, international, randomized double-blind trial. Am J Perinatol. (2020) 37:127–36. doi: 10.1055/s-0039-3400227
7. Chang CY, Nguyen CP, Wesley B, Guo J, Johnson LL, Joffe HV. Withdrawing approval of Makena – a proposal from the FDA Center for Drug Evaluation and Research. N Engl J Med. (2020) 383:e131. doi: 10.1056/NEJMp2031055
8. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021) 372:n160. doi: 10.1136/bmj.n160
9. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
10. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
11. Hartung J, Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med. (2001) 20:3875–89. doi: 10.1002/sim.1009
12. Inthout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. (2014) 14:25. doi: 10.1186/1471-2288-14-25
13. Johnson JW, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17α-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med. (1975) 293:675–80. doi: 10.1056/NEJM197510022931401
14. Yemini M, Borenstein R, Dreazen E, Apelman Z, Mogilner BM, Kessler I, et al. Prevention of premature labor by 17 alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. (1985) 151:574–7. doi: 10.1016/0002-9378(85)90141-3
15. Ibrahim M, Arm ARM, Younis MA-F. Progesterone supplementation for prevention of preterm labor: a randomized controlled trial. Middle East Fertil Soc J. (2010) 15:39–41. doi: 10.1016/j.mefs.2010.03.007
16. Shahgheibi S, Soofizadeh N, Mojtahedzadeh A, Rezaei M, et al. The effect of 17α-Hydroxyprogestrone caproate on prevention of preterm labor in High-Risk pregnant women: a clinical trial study. Int J Med Res Health Sci. (2016) 5:261–6. doi: 10.5812/ircmj.26150
17. Fernandez-Macias R, Martinez-Portilla RJ, Cerrillos L, Figueras F, Palacio M. A systematic review and meta-analysis of randomized controlled trials comparing 17-alpha-hydroxyprogesterone caproate versus placebo for the prevention of recurrent preterm birth. Int J Gynaecol Obstet. (2019) 147:156–64. doi: 10.1002/ijgo.12940
18. Saccone G, Khalifeh A, Elimian A, Bahrami E, Chaman-Ara K, Bahrami MA, et al. Vaginal progesterone vs intramuscular 1α-hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth in singleton gestations: systematic review and meta-analysis of randomized controlled trials. Ultrasound Obstet Gynecol. (2017) 49:315–21. doi: 10.1002/uog.17245
Keywords: 17-alpha-hydroxyprogesterone caproate, 17-OHPC, preterm birth, recurrent preterm birth, systematic review and meta-analysis
Citation: Almutairi AR, Aljohani HI and Al-fadel NS (2021) 17-Alpha-Hydroxyprogesterone vs. Placebo for Preventing of Recurrent Preterm Birth: A Systematic Review and Meta-Analysis of Randomized Trials. Front. Med. 8:764855. doi: 10.3389/fmed.2021.764855
Received: 26 August 2021; Accepted: 29 October 2021;
Published: 01 December 2021.
Edited by:
Ola Andersson, Lund University, SwedenReviewed by:
Nazan Yurtcu, Sivas Cumhuriyet University Faculty of Medicine, TurkeyAyman Oraif, King Abdulaziz University, Saudi Arabia
Abdullah Abdulaziz Alalwan, Qassim University, Saudi Arabia
Copyright © 2021 Almutairi, Aljohani and Al-fadel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdulaali R. Almutairi, YXJtdXRhaXJpQHNmZGEuZ292LnNh
 Abdulaali R. Almutairi
Abdulaali R. Almutairi Hadir I. Aljohani
Hadir I. Aljohani Nouf S. Al-fadel
Nouf S. Al-fadel