Abstract
Background:
Body temperature (BT) has been used to evaluate the outcomes of patients with various diseases. In this study, patients with diastolic heart failure (DHF) in the intensive care unit (ICU) were examined for a correlation between BT and mortality.
Methods:
This was a retrospective cohort study of the Medical Information Mart for Intensive Care (MIMIC)-IV dataset. A total of 4,153 patients with DHF were included. The primary outcomes were 28-day ICU and higher in-hospital mortality rates. BT was used in the analyses both as a continuous variable and as a categorical variable. According to the distribution of BT, the patients were categorized into three groups (hypothermia BT <36.5°C, normal 36.5°C ≤ BT <37.5°C, and hyperthermia BT ≥37.5°C). Multivariate logistic regression analysis was performed to explore the association between BT and patient outcomes.
Results:
The proportions of the groups were 23.6, 69.2, and 7.2%, respectively. As a continuous variable, every 1°C increase in BT was associated with a 21% decrease in 28-day ICU mortality (OR: 0.79, 95% CI: 0.66–0.96, and p = 0.019) and a 23% decrease in in-hospital mortality (OR: 0.77, 95% CI: 0.66–0.91; and p = 0.002). When BT was used as a categorical variable, hypothermia was significantly associated with both 28-day ICU mortality (OR: 1.3, 95% CI: 1.03–1.65; and p = 0.026) and in-hospital mortality (OR: 1.31, 95% CI: 1.07–1.59; and p = 0.008). No statistical differences were observed between 28-day ICU mortality and in-hospital mortality with hyperthermia after adjustment.
Conclusion:
The first 24-h mean BT after ICU admission was associated with 28-day ICU and in-hospital mortality in patients with DHF. Hypothermia significantly increased mortality, whereas hyperthermia did not.
Introduction
Body temperature (BT) is a routinely measured vital sign that has been used to evaluate outcomes in patients with various diseases, such as sepsis (1), trauma (2), and multiorgan failure (3). BT abnormalities, including hypothermia and hyperthermia, are the most common symptoms in critically ill patients and the rationale for patient assessment and management (4, 5). Several previous reports claim that, under various circumstances, hypothermia can be used to predict adverse outcomes independently (6, 7). Similarly, hyperthermia has been demonstrated to increase mortality in critically ill patients (8, 9).
Diastolic heart failure (DHF) is defined as a clinical syndrome of heart failure (HF) with a preserved left ventricular ejection fraction (EF) (0.50 or more) without major valve diseases (10). It can be found in approximately one-third of patients with heart failure seen by clinicians. Patients with DHF are more likely to be women, elderly, and have increased blood pressure (11). Unfortunately, some patients with DHF may be underestimated, which hinders timely treatment because of their seemingly normal EF.
A previous study demonstrated that hypothermia is an independent sign of all-cause mortality and cardiovascular (CV) death in patients admitted with worsening HF and reduced EF (12). However, the relationship between BT and mortality among patients with DHF in the intensive care unit (ICU) remains unclear, and BT at a single point in time inevitably increases the risk of deviation (13). Hence, this study aimed to investigate the association between the mean BT of the first 24-h and 28-day ICU mortality and in-hospital mortality of patients with DHF in the ICU from the data collected in a large public clinical database.
Methods
Study cohort and data source
This study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (14). This retrospective study used data collected from the MIMIC-IV (https://mimic.mit.edu/) database (15). In an update to MIMIC-III (16), the database contains comprehensive information (demographics, laboratory results, nursing progress notes, intravenous medications, fluid balance, and other clinical variables) of patients in a Tertiary Academic Medical Center in Boston, MA, USA between 2008 and 2019. Specific diseases were documented according to The International Classification of Diseases, the ninth version (ICD-9) and the tenth version (ICD-10), by hospital staff upon patient discharge. The database is supposed to help a wide variety of healthcare research, and all researchers who have successfully completed the “protecting human subjects” training are authorized to use it. One author of this study, HX, completed the Collaborative Institutional Training Initiative examination (certification number 40287955) and is entitled to access the database for data extraction. None of the patients with MIMIC-IV were identified, and written authorization was not required.
Study population
A total of 257,366 individuals were included in the MIMIC-IV database from 2008 to 2019, of whom 50,048 were admitted to the ICU. A total of 7,397 patients with DHF in the MIMIC-IV database were identified using ICD-9 codes 42830, 42831, 42832, and 42833 and ICD-10 codes 1503, 15030, 15031, 15032, and 15033. The study included individuals above the age of 18 years. Because the same patient might have been admitted to the ICU several times, only the first ICU record was analyzed. Patients without ICU data and BT data within 24 h of ICU admission were excluded. Patients who spent <24 h in the ICU were also excluded. Ultimately, only 4,153 patients remained in this study (Figure 1).
Figure 1

A flowchart of patient selection.
Data extraction
Data extraction was performed using PostgreSQL (version 9.6) and Navicat Premium (version 12.0) software. We obtained the BT of each individual within 24 h of ICU admission by following these steps. First, we searched “temperature” from the table named “d_items,” and then obtained item “223762.” Second, we used this item to obtain the BT from the chart called “chartevents.” Each individual had a different measurement time. After removing the duplicates, we calculated the average BT of each individual using the Excel tool. We divided the patients into three groups based on the temperature performance and the existing research (17–20): hypothermia (BT <36.5°C), normal (36.5°C ≤ BT <37.5°C), and hyperthermia (BT ≥37.5°C). We obtained the survival information for the last outcome variable from the table named “patients.” From the table named “admissions,” we obtained the data regarding the length of hospital and ICU stays (21).
Taking demographics, comorbidities, scoring systems, laboratory tests, and vital signs as variables, we observed the first 24-h ICU records of patients to evaluate the average of vital signs and laboratory tests. Potential bias was prevented by the exclusiveness of data analysis in cases where variables with missing values exceeded 30%.
The following information was gathered: sex, age, ethnicity, marital status, weight, platelets, white blood cells (WBC), albumin, blood urea nitrogen (BUN), international normalized ratio (INR), partial thromboplastin time (PTT), anion gap, bicarbonate, calcium, chloride, sodium, potassium, glucose, heart rate (HR), systolic blood pressure (SBP), respiratory rate (RR), saturation of peripheral oxygen (SPO2), sequential organ failure assessment (SOFA) score, acute physiology score (APS) III, Charlson comorbidity index (CCI), urine output (UO) on the 1st day, and fluid input (FI) on the 1st day. The CCI is a scoring system used to quantify comorbidities (including myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, liver disease, diabetes, paraplegia, renal disease, malignant cancer, metastatic solid tumor, and acquired immunodeficiency syndrome).
Statistical analysis
Continuous variables were divided into normally and non-normally distributed variables. Normally distributed continuous variables are presented as mean ± standard deviation, and non-normally distributed variables are described as median ± interquartile range (IQR). Continuous variables were compared among the groups using a Student's t-test or one-way analysis of variance (ANOVA). Categorical variables were described as frequencies or percentages, and the chi-square test was used for comparisons. The Kruskal–Wallis test was applied to assess the significance of differences among different BT groups (the clinical cutoff point). Univariate and multivariable logistic regression analyses were employed to study the relationship between the first 24-h mean BT and 28-day ICU mortality and in-hospital mortality. Variables were chosen based on a relationship in the univariate analysis (p < 0.05), clinical judgment, or a change in effect estimate of more than 10%. In the multivariate models, we adjusted only for sex, age, marital status, and ethnicity in Model 1. In Model 2, we adjusted for Model 1 by adding laboratory data such as weight, WBC, albumin, BUN, creatinine, INR, PTT, anion gap, and bicarbonate, calcium, chloride, and sodium levels. In Model 3, we further adjusted for HR, SBP, RR, SpO2, and CCI.
Multivariate regression models were used to conduct trend tests with the median value of each BT group as a continuous variable. Tests were performed to check the results and observe the possibility of non-linearity. To address the non-linearity of the first 24-h mean BT with 28-day ICU and in-hospital mortalities, a smooth curve fitting was conducted. Subgroup analyses were performed using stratified logistic regression. Continuous variables were first changed to categorical variables in accordance with the clinical cutoff points, and then, an interaction test was performed. The adjustments and interactions of the subgroups were examined using the likelihood ratio tests. The subgroups included sex (men or women), age (<75 or ≥75 years), marital status (married, single, divorced, or widowed), ethnicity (Black, White, or other), HR (<80 or ≥80 bpm), weight (<80 or ≥80 kg), the presence of diabetes (yes or on), the presence of MI (yes or on), and the presence of renal disease (yes or no).
Based on five replications and a chained equation approach method of the MI procedure in R, multiple imputations were used to explain the missing data of <30%. The details of the missing values are shown in Supplementary Table S1.
The statistical software package R 3.3.2 (http://www.R-project.org, The R Foundation) and Free Statistics version 1.6.1 were used for all the analyses. A two-tailed test was conducted, and a p-value of <0.05 was considered significantly important.
Results
Baseline characteristics of patients
This study included 4,153 patients with DHF. The baseline characteristics of these selected patients are presented in Table 1 based on the clinical cutoff point of BT. In total, 980 patients (23.6%) had hypothermia, 298 (7.2%) had hyperthermia, and 2,875 (69.2%) were normal. Overall, the age of the patients was 76.1 ± 12.8 years on average and included approximately 1,857 (44.7%) men. Patients with hypothermia were older and had lower weight, higher CCI scores, higher BUN levels, lower white blood cell count, slower heart rate and respiratory rate, and higher 28-day ICU and in-hospital mortalities. In addition, the SOFA and ApsIII scores were higher with a higher BT. Meanwhile, patients with hyperthermia had longer lengths of stay in the ICU and hospital.
Table 1
| Variables | Over all | BT <36.5°C | 36.5 ≤ BT <37.5°C | BT ≥37.5°C | p-value |
|---|---|---|---|---|---|
| (n = 4,153) | (n = 980) | (n = 2,875) | (n=298) | ||
| Gender (%) | 0.105 | ||||
| Men | 1,857 (44.7) | 417 (42.6) | 1,293 (45) | 147 (49.3) | |
| Women | 2,296 (55.3) | 563 (57.4) | 1,582 (55) | 151 (50.7) | |
| Age (years) | 76.1 ± 12.8 | 78.2 ± 12.2 | 75.8 ± 12.8 | 71.8 ± 13.3 | <0.001 |
| Ethnicity (%) | 0.005 | ||||
| Black | 585 (14.1) | 133 (13.6) | 404 (14.1) | 48 (16.1) | |
| White | 2,964 (71.4) | 734 (74.9) | 2,036 (70.8) | 194 (65.1) | |
| Other | 604 (14.5) | 113 (11.5) | 435 (15.1) | 56 (18.8) | |
| Marital status (%) | <0.001 | ||||
| Married | 1,875 (45.1) | 409 (41.7) | 1,315 (45.7) | 151 (50.7) | |
| Single | 899 (21.6) | 199 (20.3) | 621 (21.6) | 79 (26.5) | |
| Divorced | 360 (8.7) | 74 (7.6) | 256 (8.9) | 30 (10.1) | |
| Widowed | 1,019 (24.5) | 298 (30.4) | 683 (23.8) | 38 (12.8) | |
| Weight (kg) | 83.2 ± 27.1 | 79.3 ± 26.2 | 83.9 ± 26.7 | 89.5 ± 31.8 | <0.001 |
| UO first 24 h (L) | 2.1 (1.1, 3.7) | 2.0 (0.9, 3.7) | 2.1 (1.1, 3.8) | 1.8 (1.0, 3.4) | 0.019 |
| FI first 24 h (L) | 3.9 (2.2, 6.7) | 3.9 (2.1, 6.6) | 3.9 (2.2, 6.8) | 3.9 (2.3, 6.4) | 0.681 |
| Laboratory tests | |||||
| PLT (10∧9/L) | 201.5 (150.5, 264.5) | 204.2 (147.5, 271.1) | 201.5 (152.2, 263.0) | 196.2 (146.6, 253.8) | 0.422 |
| WBC (10∧9/L) | 10.1 (7.5, 13.7) | 9.7 (7.0, 13.5) | 10.1 (7.5, 13.6) | 11.3 (8.1, 16.3) | <0.001 |
| Albumin (g/dl) | 3.0 ± 0.7 | 3.0 ± 0.7 | 3.1 ± 0.7 | 2.9 ± 0.7 | <0.001 |
| BNU (mmol/L) | 28.5 (19.0, 45.5) | 32.0 (20.5, 51.0) | 27.5 (18.5, 44.0) | 27.2 (17.0, 43.2) | <0.001 |
| INR | 1.3 (1.1, 1.7) | 1.3 (1.1, 1.9) | 1.3 (1.1, 1.6) | 1.3 (1.1, 1.7) | <0.001 |
| PTT (s) | 32.5 (28.1, 41.4) | 33.2 (28.3, 43.2) | 32.3 (28.1, 40.7) | 31.6 (27.6, 40.1) | 0.013 |
| Anion gap (mmol/L) | 13.7 ± 4.0 | 13.9 ± 4.8 | 13.6 ± 3.8 | 13.6 ± 3.9 | 0.279 |
| Bicarbonate (mmol/L) | 26.1 ± 5.6 | 25.8 ± 6.1 | 26.2 ± 5.4 | 25.9 ± 5.6 | 0.113 |
| Calcium (mmol/L) | 8.4 ± 0.9 | 8.4 ± 0.9 | 8.5 ± 0.9 | 8.4 ± 0.9 | 0.009 |
| Chloride (mmol/L) | 102.5 ± 6.4 | 102.8 ± 6.7 | 102.3 ± 6.2 | 103.7 ± 6.8 | <0.001 |
| Sodium (mmol/L) | 138.4 ± 4.9 | 138.3 ± 4.9 | 138.3 ± 4.8 | 139.4 ± 4.8 | 0.002 |
| Potassium (mmol/L) | 4.2 ± 0.7 | 4.2 ± 0.7 | 4.2 ± 0.7 | 4.1 ± 0.7 | 0.019 |
| Glucose (mmol/L) | 7.4 (6.2, 9.3) | 7.3 (6.2, 8.9) | 7.4 (6.2, 9.3) | 7.6 (6.4, 9.6) | 0.043 |
| Vital sign | |||||
| HR (bpm) | 83.1 ± 15.7 | 80.4 ± 15.6 | 83.1 ± 15.4 | 91.5 ± 16.3 | <0.001 |
| SBP (mmHg) | 120.0 ± 18.0 | 117.0 ± 18.2 | 121.1 ± 17.9 | 119.7 ± 16.8 | <0.001 |
| RR (bmp) | 19.8 ± 3.8 | 19.0 ± 3.6 | 19.8 ± 3.7 | 21.6 ± 4.4 | <0.001 |
| SpO2(%) | 96.5 ± 2.5 | 96.5 ± 3.0 | 96.5 ± 2.3 | 96.4 ± 2.4 | 0.91 |
| Score system | |||||
| SOFA | 5.0 (3.0, 7.0) | 5.0 (3.0, 7.0) | 4.0 (2.0, 6.0) | 6.0 (4.0, 9.0) | <0.001 |
| Aps III | 47.0 (37.0, 61.0) | 52.0 (40.0, 69.0) | 46.0 (36.0, 58.0) | 54.0 (40.2, 72.8) | <0.001 |
| CCI | 6.0 ± 2.2 | 6.3 ± 2.2 | 5.9 ± 2.2 | 5.8 ± 2.3 | <0.001 |
| Comorbidity | |||||
| Myocardial infarct, n (%) | 285 (6.9) | 77 (7.9) | 188 (6.5) | 20 (6.7) | 0.368 |
| Cerebrovascular disease, n (%) | 262 (6.3) | 56 (5.7) | 190 (6.6) | 16 (5.4) | 0.48 |
| Peripheral vascular disease, n (%) | 321 (7.7) | 87 (8.9) | 212 (7.4) | 22 (7.4) | 0.306 |
| Chronic pulmonary disease, n (%) | 1,185 (28.5) | 288 (29.4) | 833 (29) | 64 (21.5) | 0.019 |
| Diabetes, n (%) | 518 (12.5) | 117 (11.9) | 347 (12.1) | 54 (18.1) | 0.009 |
| Renal disease, n (%) | 1,264 (30.4) | 341 (34.8) | 833 (29) | 90 (30.2) | 0.003 |
| Severe liver disease, n (%) | 52 (1.3) | 18 (1.8) | 34 (1.2) | 0 (0) | 0.02 |
| Malignant cancer, n (%) | 304 (7.3) | 70 (7.1) | 206 (7.2) | 28 (9.4) | 0.361 |
| Outcome | |||||
| LOS hospital (day) | 3.4 ± 4.6 | 3.4 ± 4.9 | 3.2 ± 4.3 | 4.8 ± 5.7 | <0.001 |
| LOS ICU (day) | 9.3 ± 7.9 | 9.0 ± 7.9 | 9.3 ± 7.9 | 10.9 ± 7.4 | 0.002 |
| 28-day ICU mortality, n (%) | 517 (12.4) | 173 (17.7) | 302 (10.5) | 42 (14.1) | <0.001 |
| In-hospital mortality, n (%) | 795 (19.1) | 247 (25.2) | 487 (16.9) | 61 (20.5) | <0.001 |
Baseline characteristics of the patients with diastolic heart failure.
Data are denoted as mean ± SD or median (IQR) for skewed variables or numbers (proportions) for categorical variables. BT, body temperature; UO, urine output; FI, fluid intake; PLT, Platelets; WBC, white blood cells; BNU, blood urea nitrogen; INR, international normalized ratio; PTT, partial thromboplastin time; HR, heart rate; SBP, systolic blood pressure; RR, respiratory rate; SpO2, saturation of peripheral oxygen; SOFA, sequential organ failure assessment score; Aps III, acute physiology score III; CCI, Charlson comorbidity index; LOS, length of stay; ICU, intensive care unit.
Univariate and multivariate analyses
In the unadjusted analysis, the first 24-h mean BT as a continuous variable was significantly related to 28-day ICU mortality (OR 0.6, 95% CI 0.51–0.71, and p < 0.001) and in-hospital mortality (OR: 0.66, 95% CI: 0.57–0.76, and p < 0.001) (Table 2). Age, length of stay in the ICU and hospital, weight, WBC, INP, PTT, anion gap, renal disease, severe liver disease, malignant cancer, the SOFA score, CCI, HR, SBP, RR, and albumin, BUN, creatinine, bicarbonate, calcium, potassium, SpO2 levels were all associated with 28-day ICU mortality and in-hospital mortality. We categorized BT into three groups to calculate, with the normal group as reference: the ORs for 28-day ICU mortality of the hypothermia and hyperthermia groups were 1.83 (95% CI: 1.49–2.24, and p < 0.001) and 1.4 (95% CI: 0.99–1.98, and p = 0.059), respectively. The ORs for in-hospital mortality of the hypothermia and hyperthermia groups were 1.65 (95% CI: 1.39–1.97, and p < 0.001) and 1.26 (95% CI: 0.94–1.7, and p = 0.126), respectively.
Table 2
| Variable | 28-day ICU mortality | in-hospital mortality | ||
|---|---|---|---|---|
| OR (95%CI) | P -value | OR (95%CI) | P -value | |
| BT (°C) | 0.6 (0.51–0.71) | <0.001 | 0.66 (0.57–0.76) | <0.001 |
| 36.5 ≤ BT <37.5°C | ref | ref | ||
| BT <36.5°C | 1.83 (1.49–2.24) | <0.001 | 1.65 (1.39–1.97) | <0.001 |
| BT ≥37.5°C | 1.4 (0.99–1.98) | 0.059 | 1.26 (0.94–1.7) | 0.126 |
| Age (years) | 1.03 (1.02–1.04) | <0.001 | 1.02 (1.01–1.03) | <0.001 |
| LOS hospital | 0.98 (0.97–0.99) | 0.007 | 1 (0.99–1.01) | 0.795 |
| LOS ICU | 1.04 (1.03–1.06) | <0.001 | 1.05 (1.03–1.06) | <0.001 |
| Weight (kg) | 0.99 (0.98–0.99) | <0.001 | 0.99 (0.99–0.99) | <0.001 |
| WBC (10∧9/L) | 1.04 (1.02–1.05) | <0.001 | 1.02 (1.01–1.03) | <0.001 |
| Albumin (g/dl) | 0.54 (0.47–0.62) | <0.001 | 0.43 (0.38–0.49) | <0.001 |
| BNU (mmol/L) | 1.02 (1.01–1.02) | <0.001 | 1.01 (1.01–1.02) | <0.001 |
| Creatinine (mmol/L) | 1.07 (1.03–1.12) | 0.002 | 1.12 (1.08–1.16) | <0.001 |
| INR | 1.23 (1.16–1.3) | <0.001 | 1.19 (1.13–1.25) | <0.001 |
| PTT (s) | 1.01 (1.01–1.02) | <0.001 | 1.01 (1.01–1.01) | <0.001 |
| Anion gap (mmol/L) | 1.07 (1.05–1.09) | <0.001 | 1.07 (1.05–1.09) | <0.001 |
| Bicarbonate (mmol/L) | 0.95 (0.94–0.97) | <0.001 | 0.95 (0.94–0.97) | <0.001 |
| Calcium (mmol/L) | 0.86 (0.77–0.95) | 0.003 | 0.77 (0.71–0.85) | <0.001 |
| Potassium (mmol/L) | 1.21 (1.07–1.36) | 0.003 | 1.15 (1.04–1.28) | 0.008 |
| HR, bpm | 1.02 (1.02–1.03) | <0.001 | 1.02 (1.01–1.02) | <0.001 |
| SBP (mmHg) | 0.96 (0.95–0.97) | <0.001 | 0.98 (0.97–0.98) | <0.001 |
| RR, bpm | 1.09 (1.07–1.12) | <0.001 | 1.07 (1.05–1.09) | <0.001 |
| Renal disease, n (%) | 1.4 (1.15–1.7) | 0.001 | 1.46 (1.25–1.72) | <0.001 |
| Severe liver disease, n (%) | 2.38 (1.26–4.49) | 0.007 | 1.9 (1.05–3.43) | 0.035 |
| Malignant cancer, n (%) | 2.14 (1.6–2.85) | <0.001 | 1.81 (1.39–2.35) | <0.001 |
| Cerebrovascular disease, n (%) | 1.42 (1.01–1.99) | 0.046 | 1.25 (0.92–1.68) | 0.152 |
| Peripheral vascular disease, n (%) | 1.47 (1.08–2) | 0.014 | 1.15 (0.87–1.52) | 0.334 |
| SpO2 (%) | 0.87 (0.84–0.9) | <0.001 | 0.92 (0.89–0.95) | <0.001 |
| SOFA | 1.33 (1.29–1.36) | <0.001 | 1.23 (1.2–1.26) | <0.001 |
| CCI | 1.18 (1.14–1.23) | <0.001 | 1.11 (1.07–1.15) | <0.001 |
Results of univariate analysis of 28-day ICU mortality and in-hospital mortality.
Data presented are ORs and 95% CIs. BT, body temperature; LOS, length of stay; WBC, white blood cells; BNU, blood urea nitrogen; INR, international normalized ratio; PTT, partial thromboplastin time; HR, heart rate; SBP, systolic blood pressure; RR, respiratory rate; SpO2, saturation of peripheral oxygen; SOFA, sequential organ failure assessment score; APS III, acute physiology score III; CCI, Charlson comorbidity index.
Multivariable logistic analysis was used to explore the adjusted association among the first 24-h mean BT, 28-day ICU mortality, and in-hospital mortality. BT as a continuous variable was correlated with 28-day in-ICU mortality and in-hospital mortality in all three models after adjusting for confounders. In the full variables adjusted model, every 1°C increase in BT was associated with a 21% decrease in 28-day ICU mortality and a 23% decrease in in-hospital mortality; the adjusted ORs were 0.79 (95% CI: 0.66–0.96, and p = 0.019) and 0.77 (95% CI: 0.66–0.91, and p = 0.002), respectively. When analyzed as a categorical variable, the hypothermia group was both relevant to an increased risk of 28-day ICU mortality and in-hospital mortality after adjustment in Model 3; the reference was the normal group, and the adjusted ORs for 28-day ICU mortality and in-hospital mortality were 1.3 (95% CI: 1.03–1.65, and p = 0.026) and 1.31 (95% CI: 1.07–1.59, and p = 0.008), respectively. Moreover, the adjusted ORs were significant in all the models (Table 3). However, there was no sign of independent correlation in the hyperthermia group with 28-day ICU mortality and in-hospital mortality after multivariable adjustment; the ORs for 28-day ICU mortality and in-hospital mortality in Model 3 were 1.3 (95% CI: 0.88–1.93, and p = 0.187) and 1.13 (95% CI: 0.81–1.57, and p = 0.463), respectively.
Table 3
| Outcomes | n.event_% | Univariate | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Crude OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value | ||
| 28-day ICU mortality | |||||||||
| BT (°C) | 517 (12.4) | 0.6 (0.51–0.71) | <0.001 | 0.65 (0.54–0.77) | <0.001 | 0.78 (0.65–0.94) | 0.009 | 0.79 (0.66–0.96) | 0.019 |
| 36.5 ≤ BT <37.5°C | 302 (10.5) | Ref | Ref | Ref | Ref | ||||
| BT <36.5°C | 173 (17.7) | 1.83 (1.49–2.24) | <0.001 | 1.72 (1.4–2.11) | <0.001 | 1.39 (1.11–1.73) | 0.004 | 1.3 (1.03–1.65) | 0.026 |
| BT ≥37.5°C | 42 (14.1) | 1.4 (0.99–1.98) | 0.059 | 1.58 (1.11–2.24) | 0.011 | 1.48 (1.02–2.14) | 0.039 | 1.3 (0.88–1.93) | 0.187 |
| p for trend | <0.001 | 0.001 | 0.161 | 0.233 | |||||
| In-hospital mortality | |||||||||
| BT (°C) | 795 (19.1) | 0.66 (0.57–0.76) | <0.001 | 0.69 (0.59–0.8) | <0.001 | 0.8 (0.68–0.93) | 0.005 | 0.77 (0.66–0.91) | 0.002 |
| 36.5 ≤ BT <37.5°C | 487 (16.9) | Ref | Ref | Ref | Ref | ||||
| BT <36.5°C | 247 (25.2) | 1.65 (1.39–1.97) | <0.001 | 1.59 (1.34–1.9) | <0.001 | 1.33 (1.1–1.6) | 0.003 | 1.31 (1.07–1.59) | 0.008 |
| BT ≥37.5°C | 61 (20.5) | 1.26 (0.94–1.7) | 0.126 | 1.36 (1–1.83) | 0.047 | 1.26 (0.92–1.74) | 0.149 | 1.13 (0.81–1.57) | 0.463 |
| p for trend | <0.001 | 0.001 | 0.083 | 0.057 | |||||
Multivariable logistic model analysis of BT with 28-day ICU mortality and in-hospital mortality.
Data presented are ORs and 95% CIs. BT, body temperature. Model 1 adjusted for gender, age, marital status, and ethnicity; model 2 adjusted for model 1 + weight, WBC, albumin, BUN, creatinine, INR, PTT, anion gap, bicarbonate, calcium, chloride, and sodium; model 3 adjusted for model 2+HR, SBP, RR, SpO2, renal disease, severe liver disease, malignant cancer, myocardial infarction, cerebrovascular disease, peripheral vascular disease, diabetes, chronic pulmonary disease, and CCI.
Results of non-linearity of BT and mortality
In the current study, we described the relationship between BT and 28-day ICU mortality and in-hospital mortality in a non-linear model (Figure 2). The smooth curve and the results of the logistic proportional hazards regression model with cubic spline functions demonstrated a non-linear relationship between BT and mortality after adjusting for sex, age, marital status, ethnicity, weight, WBC, albumin, BUN, creatinine, INR, PTT, anion gap, bicarbonate, calcium, chloride, sodium, HR, SBP, RR, SpO2 levels, and CCI. The P-value for non-linearity is <0.001.
Figure 2

Non-linear relationship between BT and 28-day ICU mortality (A) and in-hospital mortality (B). Adjustment factors included gender, age, marital status, ethnicity, weight, WBC, albumin, BUN, INR, PTT, anion gap, bicarbonate, calcium, chloride, sodium, HR, SBP, DBP, MBP, RR, SpO2, the presence of renal disease, the presence of severe liver disease, the presence of malignant cancer, the presence of myocardial infarction, the presence of cerebrovascular disease, the presence of peripheral vascular disease, the presence of diabetes, the presence of chronic pulmonary disease, and CCI.
Subgroup analyses
We used sex, age, ethnicity, marital status, HR, weight, and the presence of diabetes, MI, and renal disease as stratification variables to observe the trend of effect sizes between BT with 28-day ICU mortality and in-hospital mortality in Models 1 and 3 (Figures 3, 4). Consistent results were observed in both Models 1 and 3 (Supplementary Tables S2–S5). We noted that only a few variables showed an interaction between BT and 28-day ICU mortality, including marital status (P-values for interaction = 0.041) and absence of renal disease (P-values for interaction = 0.045). However, no stratification variables showed an interaction between BT and in-hospital mortality in the two models.
Figure 3

Subgroup analyses of BT and 28-day ICU mortality and in-hospital mortality. Adjustment factors included gender, age, marital status, ethnicity, weight, WBC, albumin, BUN, INR, PTT, anion gap, bicarbonate, calcium, chloride, sodium, HR, SBP, DBP, MBP, RR, SpO2, the presence of renal disease, the presence of severe liver disease, the presence of malignant cancer, the presence of myocardial infarction, the presence of cerebrovascular disease, the presence of peripheral vascular disease, the presence of diabetes, the presence of chronic pulmonary disease, and CCI. BT, body temperature; HR, heart rate; MI, myocardial infarct.
Figure 4
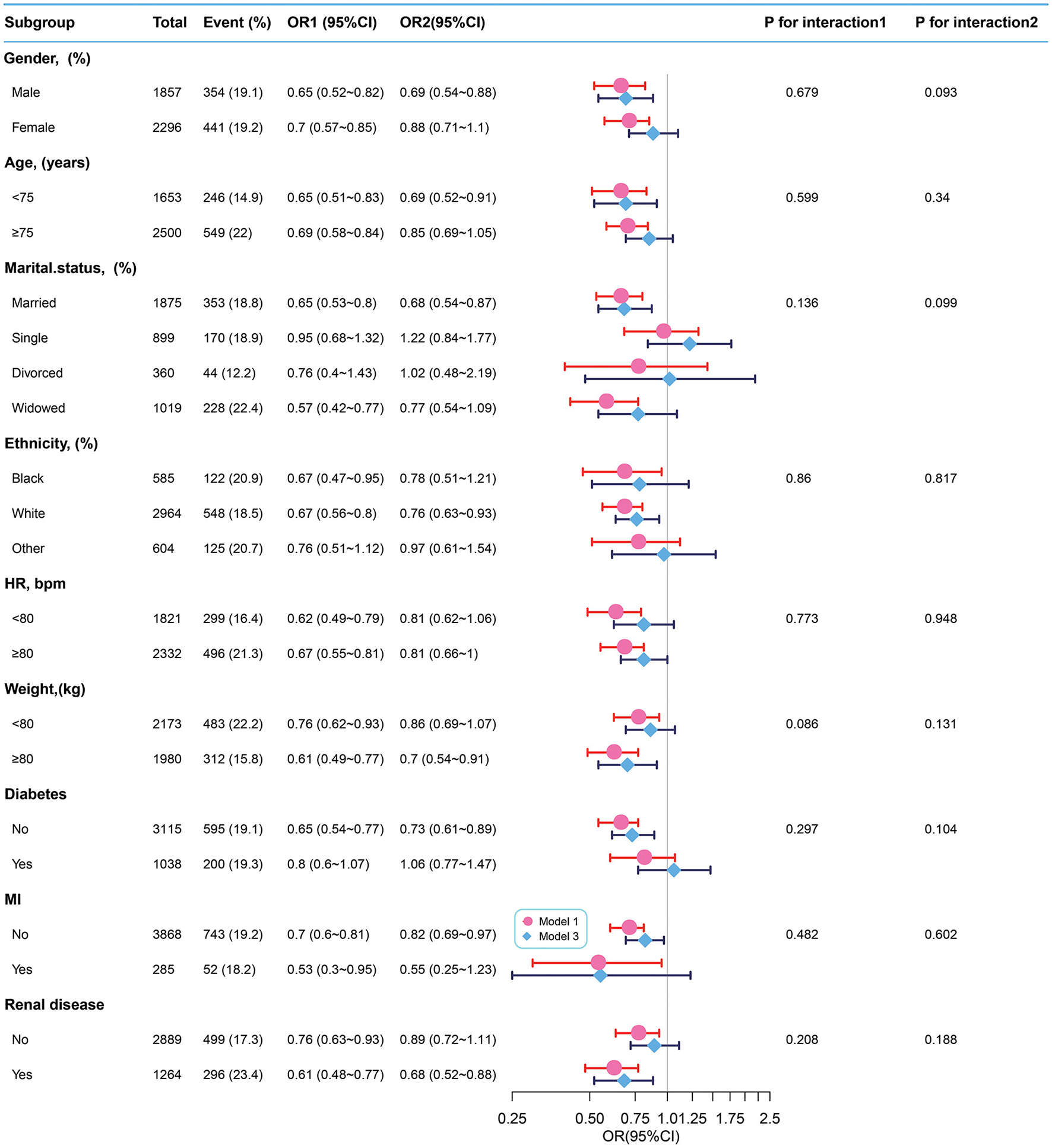
Subgroup analyses of BT and in-hospital mortality. Adjustment factors included gender, age, marital status, ethnicity, weight, WBC, albumin, BUN, INR, PTT, anion gap, bicarbonate, calcium, chloride, sodium, HR, SBP, DBP, MBP, RR, SpO2, the presence of renal disease, the presence of severe liver disease, the presence of malignant cancer, the presence of myocardial infarction, the presence of cerebrovascular disease, the presence of peripheral vascular disease, the presence of diabetes, the presence of chronic pulmonary disease, and CCI. BT, body temperature; HR, heart rate; MI, myocardial infarct.
Discussion
In this retrospective study, we demonstrated the association between the first 24-h mean BT after ICU admission and 28-day ICU mortality and in-hospital mortality among patients with DHF in the ICU. Hypothermia (BT <36.5°C) had significantly increased mortality; however, no statistical associations were observed between hyperthermia (BT ≥37.5°C) and mortality after adjusting the variables.
As a vital sign value, BT can be used to independently assess prognostic risk (5). A previous study found that hypothermia was seemingly more harmful than hyperthermia because it increased 1-year mortality (22) and hypothermia was associated with a significantly higher mortality in patients with worsening HF (12), sepsis (23), frailty in older adults in the ED (7) and after CABG (24). These results are consistent with our results when BT was analyzed as a categorical variable. The reason might be that hypothermia produces significant physiological changes (such as a left shift of the oxygenation curve, a decline in coagulation function, and arrhythmia), which could aggravate tissue hypoxia and multiple organ functions (25).
However, the conclusions of some analyses were inconsistent with our findings. Wade et al. revealed that hypothermia and hyperthermia during hospital admission are associated with increased in-hospital mortality (26). Furthermore, a retrospective study reported that, in patients with infection, an elevated BT peak in the first 24-h ICU admission was associated with a reduction in in-hospital mortality (27). Hyperthermia was thought to be an adaptive physiological response, whereas hypothermia was thought to be a maladaptive response (28). Romanovsky et al. (29) suggested that fever in the mild-to-moderate stage of the disease was a measure to clean the pathogen. In the late stage, the disease has already developed, so hypothermia might be the outcome of a more severe condition with multiorgan failure, inflammatory processes, and hormonal factors involving thermoregulation, or low body temperature might directly result in adverse consequences (12, 30, 31).
Heart failure (HF) is a common but complex syndrome caused by a wide variety of etiologies, initially called systolic heart failure (SHF) and DHF. More recently, based on the calculated ejection fraction (EF) of 50%, HF was described as HFrEF and HFpEF (32). One report showed that patients with DHF might have a similar EF to those with HFpEF (33). In March 2021, the European Journal of Heart Failure published the first version of the Universal Definition and Classification of Heart Failure, led by the Heart Failure Society of America (HFSA) (34), ending the history of inconsistent definitions and classifications of heart failure. The data for our study were extracted from the MIMIC-IV, in which patient information was recorded from 2008 to 2019. The database uses a previous standard to diagnose DHF. Patients with DHF were documented according to The International Classification of Diseases ninth version (ICD-9) codes 42830, 42831, 42832, and 42833 and tenth version (ICD-10) codes 1503, 15030, 15031, 15032, and 15033. In a large clinical trial, Payvar et al. revealed the relationship between low BT and adverse consequences in hospitalized patients with worsening HF. In an average of 9.9 months following the study of 4,108 patients after discharge, lower body temperature at randomization showed a linear correlation with an increased possibility of CV death or HF rehospitalization (12). Casscells and colleagues identified that hypothermia at hospital arrival was significantly related to higher in-hospital mortality, observed in another retrospective study of 291 HF hospitalized patients for HF exacerbation (35). The patients in these previous studies were all patients with HFrEF, and few studies have explored HFpEF. The patients in our study were diagnosed with DHF, whose EF was ≥0.50 and similar to HFpEF.
Compared with previous studies, our study has several advantages. First, the focus of the study was on the influence of overall body temperature changes in the patients on the first 24 h in the ICU instead of the adverse outcomes associated with a single time point. Therefore, our study might be particularly valuable for the temperature management of patients in the ICU. Second, this was an observational study and, therefore, vulnerable to potential confounding factors. A statistical adjustment was performed to reduce the residual confounders as much as possible. Third, the target independent variables were classified as both continuous and categorical variables. Such clarification could decrease contingency in data analysis and increase the robustness of the results. Fourth, the subgroup analysis suggested that the consistent relationships between BT and 28-day ICU mortality and in-hospital mortality were significant in the different subgroups in this study.
To the best of our knowledge, this is the first study to observe an independent relationship between the first 24-h mean BT and 28-day ICU mortality and in-hospital mortality in patients with DHF in the ICU. This would help highlight the significance of abnormal BT and might help reduce the adverse consequences of hypothermia in these patients. Although BT can be obtained easily, lower LOS values are often neglected by clinical staff, and this study would inspire further concern regarding this simple and easily available finding.
However, the present study has some limitations. First, the sample size of the hyperthermia group was relatively small, which needs to be enlarged, and a multicenter database is necessary for verification in the future. Second, the BT varied according to the measurement site. Clarification of the method and site in the MIMIC-IV database is not clear, which might lead to deviations in the results. Further research is needed to clarify these details. Third, as a retrospective study, this study used cases of DHF diagnosed according to administrative diagnosis codes. Although this study used the first sequence of diagnoses, it is still possible that there are false associations caused by misclassifications. Fourth, in the MIMIC-IV database, there was no mention of varying degrees of diastolic dysfunction, and this study may not have compared the differences for varying degrees of diastolic dysfunction. Finally, the study was conducted at a single institution and might have selection bias, and further studies are necessary to investigate the external generalizability.
Conclusion
This retrospective observational study proved that the mean BT of patients in the first 24 h in ICU was related to 28-day ICU mortality and in-hospital mortality in patients with DHF. Hypothermia significantly increased mortality, whereas hyperthermia did not. The significance of BT monitoring is worthy of attention for improving risk stratification and the treatment selection process for patients with DHF undergoing clinical treatment.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
HX conceived the idea, conducted data analysis, and drafted the manuscript. YX interpreted the results and helped to revise the manuscript. XS conducted the data collection. NF conducted data analysis and reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgments
We thank Jie Liu (People's Liberation Army of China General Hospital, Beijing, China) and Yang Qilin (The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China) for their contribution to the statistical support, study design consultations, and comments regarding the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1028122/full#supplementary-material
Abbreviations
BT, body temperature; DHF, diastolic heart failure; ICU, intensive care unit; MIMIC-IV, medical information mart for intensive care-IV; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; ICD, international classification of diseases; SOFA, sequential organ failure assessment; APS III, acute physiology score III; CCI, Charlson comorbidity index; UO, urine output; FI, fluid intake; HR, heart rate; SBP, systolic blood pressure; RR, respiratory rate; SpO2, saturation of peripheral oxygen; LOS, length of stay.
References
1.
Baek MS Kim JH Kwon YS . Cluster analysis integrating age and body temperature for mortality in patients with sepsis: a multicenter retrospective study. Sci Rep. (2022) 12:1090. 10.1038/s41598-022-05088-z
2.
Okada A Okada Y Narumiya H Ishii W Kitamura T Iiduka R . Body temperature and in-hospital mortality in trauma patients: analysis of a nationwide trauma database in Japan. Eur J Trauma Emerg Surg. (2022) 48:163–71. 10.1007/s00068-020-01489-9
3.
Whittle JL Bates JH . Thermoregulatory failure secondary to acute illness: complications and treatment. Arch Intern Med. (1979) 139(4):418-21. 10.1001/archinte.139.4.418
4.
Laupland KB . Fever in the critically ill medical patient. Crit Care Med. (2009) 37:S273–8. 10.1097/CCM.0b013e3181aa6117
5.
O'Grady NP Barie PS Bartlett JG Bleck T Carroll K Kalil AC et al . Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American college of critical care medicine and the infectious diseases society of America. Crit Care Med. (2008) 36:1330–49. 10.1097/CCM.0b013e318169eda9
6.
Xu F Zhang C Liu C Bi S Gu J . Relationship between first 24-h mean body temperature and clinical outcomes of post-cardiac surgery patients. Front Cardiovasc Med. (2021) 8:746228. 10.3389/fcvm.2021.746228
7.
Alakare J Kemp K Strandberg T Castren M Tolonen J Harjola VP . Low body temperature and mortality in older patients with frailty in the emergency department. Aging Clin Exp Res. (2022) 34:1453–7. 10.1007/s40520-022-02098-9
8.
Niven DJ Laupland KB . Pyrexia: aetiology in the ICU. Crit Care. (2016) 20:247. 10.1186/s13054-016-1406-2
9.
Laupland KB Shahpori R Kirkpatrick AW Ross T Gregson DB Stelfox HT . Occurrence and outcome of fever in critically ill adults. Crit Care Med. (2008) 36:1531–5. 10.1097/CCM.0b013e318170efd3
10.
Vasan RS Levy D . Defining diastolic heart failure: a call for standardized diagnostic criteria. Circulation. (2000) 101:2118–21. 10.1161/01.CIR.101.17.2118
11.
Vasan RS Benjamin EJ Levy D . Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective. J Am Coll Cardiol. (1995) 26:1565–74. 10.1016/0735-1097(95)00381-9
12.
Payvar S Spertus JA Miller AB Casscells SW Pang PS Zannad F et al . Association of low body temperature and poor outcomes in patients admitted with worsening heart failure: a substudy of the efficacy of vasopressin antagonism in heart failure outcome study with tolvaptan (EVEREST) trial. Eur J Heart Fail. (2013) 15:1382–9. 10.1093/eurjhf/hft113
13.
Shen Y Lou Y Zhu S . Hyperthermia is a predictor of high mortality in patients with sepsis. Crit Care. (2020) 24:543. 10.1186/s13054-020-03263-0
14.
von Elm E Altman DG Egger M Pocock SJ Gøtzsche PC Vandenbroucke JP . The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. (2014) 12:1495–9. 10.1016/j.ijsu.2014.07.013
15.
Goldberger AL Amaral LA Glass L Hausdorff JM Ivanov PC Mark RG et al . PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. (2000) 101:E215–20. 10.1161/01.CIR.101.23.e215
16.
Johnson AE Pollard TJ Shen L Lehman LW Feng M Ghassemi M et al . MIMIC-III, a freely accessible critical care database. Sci Data. (2016) 3:160035. 10.1038/sdata.2016.35
17.
Nam K Jo WY Kwon SM Kang P Cho YJ Jeon Y et al . Association between postoperative body temperature and all-cause mortality after off-pump coronary artery bypass graft surgery: a retrospective observational study. Anesth Analg. (2020) 130:1381–8. 10.1213/ANE.0000000000004416
18.
Murtuza F Farrier AJ Venkatesan M Smith R Khan A Uzoigwe CE et al . Is there an association between body temperature and serum lactate levels in hip fracture patients?Ann R Coll Surg Engl. (2015) 97:513–8. 10.1308/rcsann.2015.0012
19.
Faizi M Farrier AJ Venkatesan M Thomas C Uzoigwe CE Balasubramanian S et al . Is body temperature an independent predictor of mortality in hip fracture patients?Injury. (2014) 45:1942–5. 10.1016/j.injury.2014.09.024
20.
Uzoigwe CE Khan A Smith RP Venkatesan M Balasubramanian S Isaac S et al . Hypothermia and low body temperature are common and associated with high mortality in hip fracture patients. Hip Int. (2014) 24:237–42. 10.5301/hipint.5000124
21.
Johnson AE Stone DJ Celi LA Pollard TJ . The MIMIC Code Repository: enabling reproducibility in critical care research. J Am Med Inform Assoc. (2018) 25:32–9. 10.1093/jamia/ocx084
22.
Erkens R Wernly B Masyuk M Muessig JM Franz M Schulze PC et al . Admission body temperature in critically ill patients as an independent risk predictor for overall outcome. Med Princ Pract. (2020) 29:389–95. 10.1159/000505126
23.
Drewry AM Fuller BM Skrupky LP Hotchkiss RS . The presence of hypothermia within 24 h of sepsis diagnosis predicts persistent lymphopenia. Crit Care Med. (2015) 43:1165–9. 10.1097/CCM.0000000000000940
24.
Hannan EL Samadashvili Z Wechsler A Jordan D Lahey SJ Culliford AT et al . The relationship between perioperative temperature and adverse outcomes after off-pump coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. (2010) 139:1568–75. 10.1016/j.jtcvs.2009.11.057
25.
Martini WZ . The effects of hypothermia on fibrinogen metabolism and coagulation function in swine. Metabolism. (2007) 56:214–21. 10.1016/j.metabol.2006.09.015
26.
Wade CE Salinas J Eastridge BJ McManus JG Holcomb JB . Admission hypo- or hyperthermia and survival after trauma in civilian and military environments. Int J Emerg Med. (2011) 4:35. 10.1186/1865-1380-4-35
27.
Young PJ Saxena M Beasley R Bellomo R Bailey M Pilcher D et al . Early peak temperature and mortality in critically ill patients with or without infection. Intensive Care Med (. (2012). 10.1007/s00134-012-2478-3
28.
Leon LR . Hypothermia in systemic inflammation: role of cytokines. Front Biosci. (2004) 9:1877–88. 10.2741/1381
29.
Romanovsky AA Székely M . Fever and hypothermia: two adaptive thermoregulatory responses to systemic inflammation. Med Hypotheses. (1998) 50:219–26. 10.1016/S0306-9877(98)90022-6
30.
Rumbus Z Matics R Hegyi P Zsiboras C Szabo I Illes A et al . Fever is associated with reduced, hypothermia with increased mortality in septic patients: a meta-analysis of clinical trials. PLoS ONE. (2017) 12:e0170152. 10.1371/journal.pone.0170152
31.
Singer AJ Taira BR Thode HC . Jr., McCormack JE, Shapiro M, Aydin A, et al. The association between hypothermia, prehospital cooling, and mortality in burn victims. Acad Emerg Med. (2010) 17:456–9. 10.1111/j.1553-2712.2010.00702.x
32.
Katz AM Rolett EL . Heart failure: when form fails to follow function. Eur Heart J. (2016) 37:449–54. 10.1093/eurheartj/ehv548
33.
Schockmel M Agrinier N Jourdain P Alla F Eicher JC Coulon P et al . Socioeconomic factors and mortality in diastolic heart failure. Eur J Clin Invest. (2014) 44:372–83. 10.1111/eci.12246
34.
Bozkurt B Coats AJS Tsutsui H Abdelhamid CM Adamopoulos S Albert N et al . Universal definition and classification of heart failure: a report of the heart failure society of America, heart failure association of the European society of cardiology, Japanese heart failure society and writing committee of the universal definition of heart failure: endorsed by the Canadian heart failure society, heart failure association of India, cardiac society of Australia and New Zealand, and Chinese heart failure association. Eur J Heart Fail. (2021) 23:352–80. 10.1002/ejhf.2115
35.
Casscells W Vasseghi MF Siadaty MS Madjid M Siddiqui H Lal B et al . Hypothermia is a bedside predictor of imminent death in patients with congestive heart failure. Am Heart J. (2005) 149:927–33. 10.1016/j.ahj.2004.07.038
Summary
Keywords
diastolic heart failure, body temperature, hypothermia, mortality, intensive care unit
Citation
Xu H, Xie Y, Sun X and Feng N (2022) Association between first 24-h mean body temperature and mortality in patients with diastolic heart failure in intensive care unit: A retrospective cohort study. Front. Med. 9:1028122. doi: 10.3389/fmed.2022.1028122
Received
25 August 2022
Accepted
28 November 2022
Published
20 December 2022
Volume
9 - 2022
Edited by
Ata Murat Kaynar, University of Pittsburgh, United States
Reviewed by
Stefano Carugo, IRCCS Ca 'Granda Foundation Maggiore Policlinico Hospital, Italy; Qing Zhang, Sichuan University, China
Updates
Copyright
© 2022 Xu, Xie, Sun and Feng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyu Xu ✉ xuhy009@163.com
This article was submitted to Intensive Care Medicine and Anesthesiology, a section of the journal Frontiers in Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.