- Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
Objective: The objective of this study was to compare the efficacy differences between Chinese patent medicines combined with hormone replacement therapy (HRT) in the treatment of premature ovarian failure (POF) by the Bayesian network meta-analysis (NMA) method.
Methods: Randomized controlled trials (RCTs) reporting Chinese patent medicine combined with HRT for POF included Medline (via PubMed), Embase, Cochrane Library, China National Knowledge Infrastructure Database (CNKI), Wanfang Database (Wanfang), VIP Database (VIP), and China Biology Medicine Database (CBM) from the inception of the databases to July 2022. Two researchers independently screened the articles, extracted data, and evaluated the quality. The literature that met the inclusion criteria was screened out, the quality and risk of bias of the included studies were assessed according to the Cochrane 5.1 manual and RevMan 5.4, and NMA was performed using Stata 15.0 and R software.
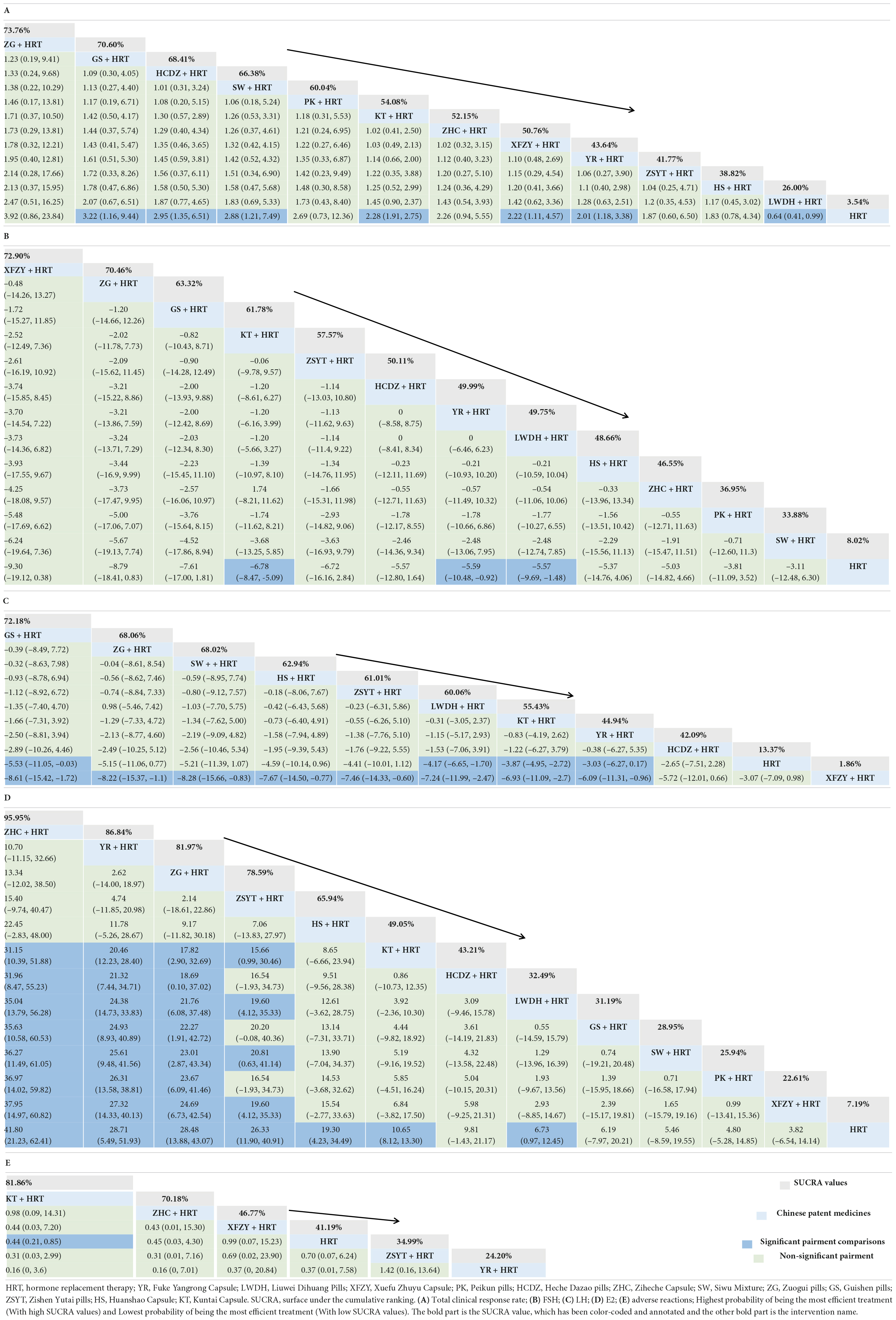
Results: Sixty-four RCTs involving 5,675 individuals containing 12 oral Chinese patent medicines combined with HRT were enrolled into the current NMA. The results showed that when compared with patients using only HRT, the total clinical response rate is greater in patients using HRT combined with one of these 12 oral Chinese patent medicines. Among them, Zuogui pills + HRT [odds ratio (OR) = 3.92; 95% credible interval (CrI) = 0.86, 23.84; SUCRA = 73.76%] is most likely to be the best intervention, and the suboptimal intervention is Guishen pills + HRT (OR = 3.22, 95% CrI = 1.16, 9.44, SUCRA = 70.60%).
Conclusion: Chinese patent medicines combined with HRT were more effective than HRT alone in the treatment of POF. Zuogui pills are good at decreasing follicle-stimulating hormone (FSH) and luteinizing hormone (LH) and more effective in the improvement of total clinical response rate; Xuefu Zhuyu capsule is also good at decreasing FSH. Ziheche capsule is an expert in improving estradiol level; Kuntai capsule shows the lowest incidence of adverse reactions. However, the quality of the literature included in this study is relatively low, so it may affect the results of the study. Therefore, higher quality and multi-center trial would be necessary for supporting these results.
Systematic review registration: [www.crd.york.ac.uk/prospero], identifier [CRD42022350587].
Introduction
Premature ovarian failure (POF) refers to the cessation of ovarian function before the age of 40 and is one of the prominent problems and diseases of female reproductive health. In recent years, the incidence of POF has gradually increased and shows a younger trend. The incidence rate before the age of 40 is about 1%, and the incidence of POF is 1 in 100 women before 40 years of age and 1 in 1,000 women before 30 years of age (1, 2). The main pathogenesis of POF is related to iatrogenic factors, immune factors, and genetic factors. The clinical features of POF are hypoestrogenism or estrogen deficiency, elevated gonadotropin levels, and lack of mature follicles. Estrogen deficiency can cause menopausal symptoms, such as sweating, hot flashes, vaginal and urinary symptoms, and vaginal dryness. However, decreased fertility and even infertility are the top POF-related concerns for women of every reproductive age. In addition, the negative effects of POF include an increased risk of cardiovascular disease, osteoporosis, and sexual dysfunction (3, 4).
In, ESHRE published a guideline for premature ovarian insufficiency, and this review presented the hormone replacement therapy (HRT) options for women with ovarian failure until natural menopause (5). Studies showed that HRT can compensate for estrogen deficiency, resulting in relief of menopausal symptoms (6). Also, it reduces the risk of cardiovascular disease (7, 8) and the impact on bone health in the long run (9–11). Since HRT has been used for a long time and cannot restore ovarian function, some researchers began to find out whether only using traditional Chinese medicine or combined with HRT can enhance the efficacy and gradually restore ovarian function without increasing adverse reactions. A meta-analysis study showed that Kuntai capsule alone had no significant difference compared with HRT in improving clinical efficacy, follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol (E2) (12). With the publication of studies on Chinese patent medicine combined with HRT in the treatment of POF, a published systematic review has confirmed the advantages of Chinese patent medicine combined with HRT in the treatment of this disease (13). At present, there are a variety of proprietary Chinese medicines for the treatment of POF, but there are few related clinical studies, and there is a lack of objective evidence-based medical evidence to confirm their safety and effectiveness. At the same time, there is currently a lack of comparison between the efficacy of different Chinese patent medicines. So, it is difficult to evaluate the efficacy and safety of various Chinese patent medicines combined with HRT in the treatment of POF. Therefore, the aim of our study was to rank the effects of various interventions using directly or indirectly available evidence through a Bayesian network meta-analysis (NMA) to gain insight into the strengths and weaknesses of these interventions to provide evidence for clinical treatment.
Methods
Registration
This systematic review and NMA are reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-NMA) statement. The study is registered with the International Prospective Register of Systematic Reviews (registration number CRD42022350587). Ethics approval or patient consent was not required, as all analyses were based on previously published studies.
Search strategies
Randomized controlled trials (RCTs) reporting Chinese patent medicine combined with HRT for POF included Medline (via PubMed), Embase, Cochrane Library, China National Knowledge Infrastructure Database (CNKI), Wanfang Database (Wanfang), VIP Database (VIP), and China Biology Medicine Database (CBM) from the inception of the databases to July 2022. The following Medical Subject Headings [MeSH] and keywords incorporating Boolean operators were applied: “premature ovarian insufficiency,” “primary ovarian insufficiency,” “POF,” “Chinese herbal,” “Traditional Chinese Medicine,” “Chinese and Western Medicine,” “capsule,” “grain,” “Oral liquid,” “pill,” “Dan,” “Gao.” We also implemented a recursive manual search to search full-text studies from obtained tracking bibliographies or similar systematic reviews to check for potentially qualified studies that we missed first.
Inclusion criteria
The inclusion criteria were constructed around the PICOS standard:
1)participants: The included subjects are all patients with POF [at least 4 months of oligomenorrhea or amenorrhea; two random measurements (> 4 weeks) of FSH > 40 IU/L] (14);
2)interventions: Chinese patent medicine combined with HRT;
3)comparison: HRT alone;
4)outcomes: main outcomes: total clinical response rate [(recovery number of cases + efficiency number of cases)/total number of cases]; secondary outcomes: serum FSH, LH, estradiol (E2) levels, and adverse reactions;
5)study design: randomized clinical trial (RCT).
Exclusion criteria
The exclusion criteria are as follows:
1)observation group or control group combined with other treatment methods;
2)literature that cannot extract complete outcome indicators or the full text cannot be obtained;
3)self-control studies, non-randomized controlled trial, experimental studies, experience summary, reviews, and case reports;
4)excluded studies in which the full text could not be obtained, the same data were repeatedly published, the use of HRT courses were unreasonable, and there were no diagnostic criteria for the disease.
Literature screening and data extraction
Selecting the studies will be accomplished by importing them into Endnote 20 (version 20.1.0, Clarivate Analytics) to manage and remove duplicate entries. Having independently screened the literature to determine whether it meets inclusion criteria, two researchers then read the abstracts and full texts to determine whether they meet the criteria. Data extraction content includes publication, patient information, intervention and control measures, treatment course, and outcome indicators.
Quality evaluation
The quality of assessment was according to Cochrane Handbook for Systematic Reviews of Interventions (15), including the following seven domains. Each of these options was evaluated as high, low, or unclear. For selection bias, it was defined as low risk of bias if the study described the method of sequence generation and allocation concealment, otherwise it was considered high risk of bias. For performance bias, the study was considered low risk of bias if it describes the method used to blind subjects, otherwise it was considered high risk of bias. For measurement bias, studies were considered low risk of bias if they described all methods of blinding outcome assessors, otherwise high risk of bias. For follow-up bias, studies were considered low risk of bias if the study described completeness of outcome data for each primary outcome (including loss to follow-up, data excluded from analysis), and high risk of bias otherwise. For reporting bias, studies were considered low risk of bias if they described how systematic reviewers examined selective outcome reporting that may have occurred, and high risk of bias otherwise. For other biases, studies were considered to be at high risk of bias if the study design was imprecise, or reporting was significantly inconsistent with previous studies. Seven items were rated as “unclear risk” when the study did not mention relevant items. Any disagreement will be resolved through discussion with the superior researcher.
Certainty of the evidence
The grading of recommendations assessment, development, and evaluation (GRADE) approach (16–18) for NMA was used to rate the certainty of the evidence of NMA estimates. Comparisons were initially rated as high-quality evidence and were downgraded accordingly, based on study limitations, imprecision, inconsistency, indirectness, and publication bias. We downgraded the study quality by one level in the study limitation, including concerns about selection bias, performance bias, detection bias, attrition bias, reporting bias, or other bias; as for imprecision item, if the sample size is insufficient or an imprecise estimate of the wide confidence interval is produced in this comparison, we will downgrade. For inconsistencies, we downgraded it by one level if study heterogeneity was found in the comparison, particularly local inconsistencies between direct and indirect evidence. If heterogeneity is observed according to four areas, namely demographic disparities, interventions, outcome measurement, and indirect comparisons, indirect projects will be downgraded. For publication bias item, we judge by asymmetric funnel diagrams. After the above assessment, the quality of evidence will be classified into one of four levels, including high, moderate, low, and very low quality. Two investigators rated the certainty of consulting with a third party.
Statistical analyses
The direct pairwise meta-analysis and bias evaluated were performed with RevMan 5.4. Variables with continuous and categorical effects were measured using the odds ratio (OR) and mean difference (MD). OR and MD were calculated using 95% credible interval [CrI]. In terms of heterogeneity, I2 represents the statistical value of 25, 50, and 75% of mild, moderate, and high heterogeneity, respectively, and was used to measure the presence or absence of substantial heterogeneity (19).
Network transitivity was considered a crucially important assumption in NMA, and its evaluation will further directly influence our analysis (20). Therefore, to ensure that multiple treatment comparisons were sufficiently similar, we estimated transmissibility by comparing clinical and methodological characteristics (e.g., patients and experimental design) across all included studies (15). The STATA/SE version 15.0 (StataCorp, College Station, TX) was used to drawn network plots and comparison-adjusted funnel plot. We employed R Version 5.0 (Mac OS X 12_5_1) with the GeMTC package to conduct NMA. The parameters in GeMTC were set as follows: initial value, 2.5; number of simulation iterations, 50,000; number of annealing times, 20,000; thinning factor, 1; and number of chains, 4. The convergence of iterations can be monitored in terms of potential scale reduction factors (PSRFs). To rank the effects of the intervention, we used the cumulative ranking probability curve (SUCRA, surface under the cumulative ranking area) and found that higher SUCRA values indicate greater efficacy (21). We did not implement the hypothesis of consistency because of non-close loops. Finally, identifying evidence of small-sample effects in networks by plotting comparative corrected funnel plots.
Results
Literature retrieval process and results
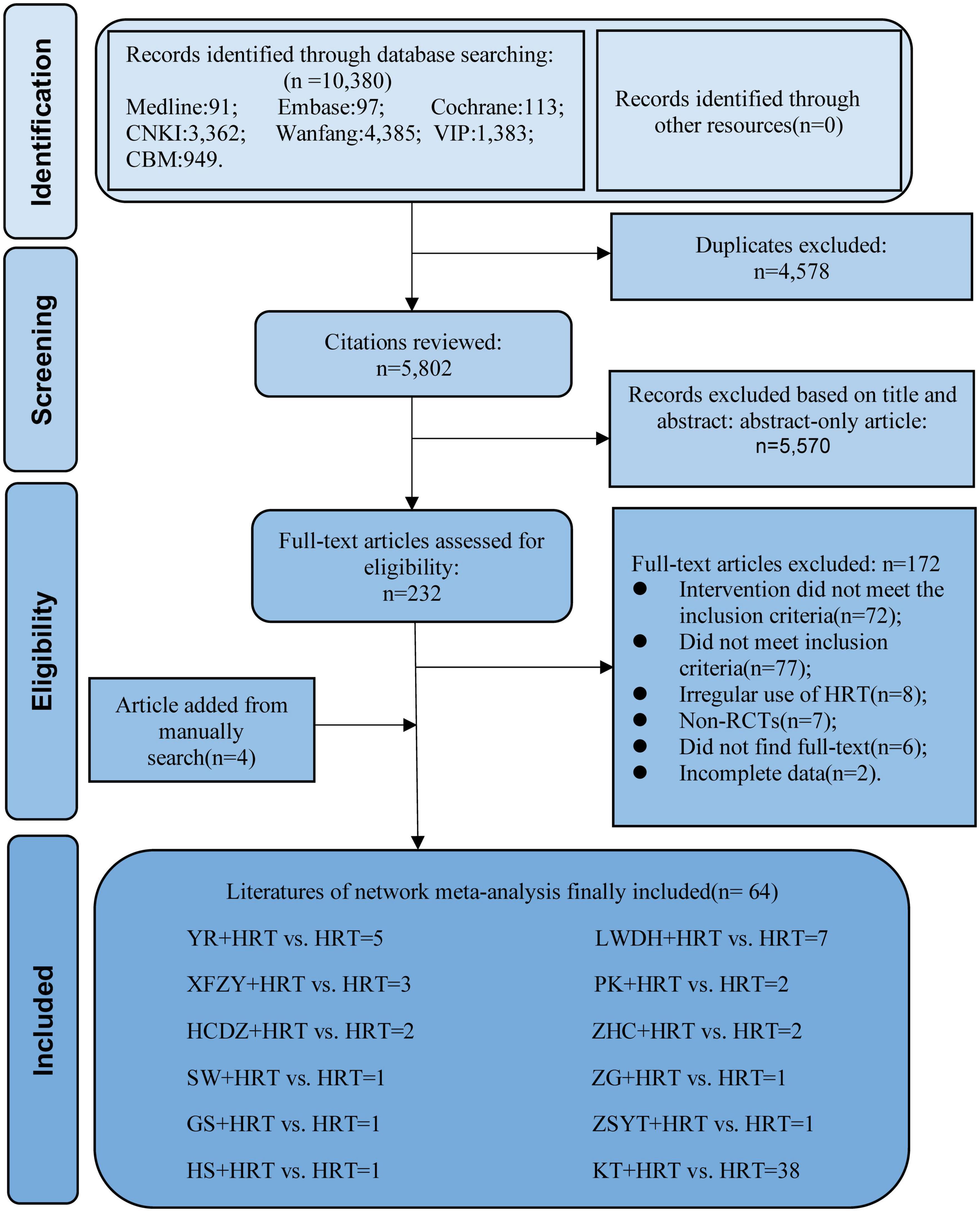
A total of 10,380 literature from seven databases were included in this study. First, we have removed duplicate 4,578 studies and, by reading the title and abstract, removed incompatible 5,570 studies. Second, by reading the full text, 127 incompatible studies were removed. The reasons for exclusion are as follows: (1) intervention did not meet the inclusion criteria (n = 72); (2) did not meet inclusion criteria (n = 77); (3) irregular use of HRT (n = 8); (4) non-RCTs (n = 7); (5) did not find full text (n = 6); (6) incomplete data (n = 2). Finally, 64 articles (22–85) were included for research. The selection process is illustrated in Figure 1.
Quality of the included studies
All 64 RCTs were mentioned randomization, of which 30 were random number table method, one was simple random method, and one was the envelope lottery method, rated as low risk. The rest were only described as “random” and rated as unclear risk; in terms of allocation concealment, blinding of participants or doctors, and blinding of outcome evaluator, all articles were not described and rated as unclear risk; all articles with complete data and no selective reporting were rated as unclear risk, unable to judge other sources of bias, rated as unclear risk. The assessment results of the risk of bias test are shown in Supplementary Figure 1. The GRADE level of evidence for the primary outcome was rated very low to low quality, shown in Supplementary Table 1.
Network meta-analysis
Figure 2 shows all outcome measures, with all included Chinese patent medicines combined with HRT being compared at least once, while there is a lack of closed loop between various types of Chinese patent medicines combined with HRT.
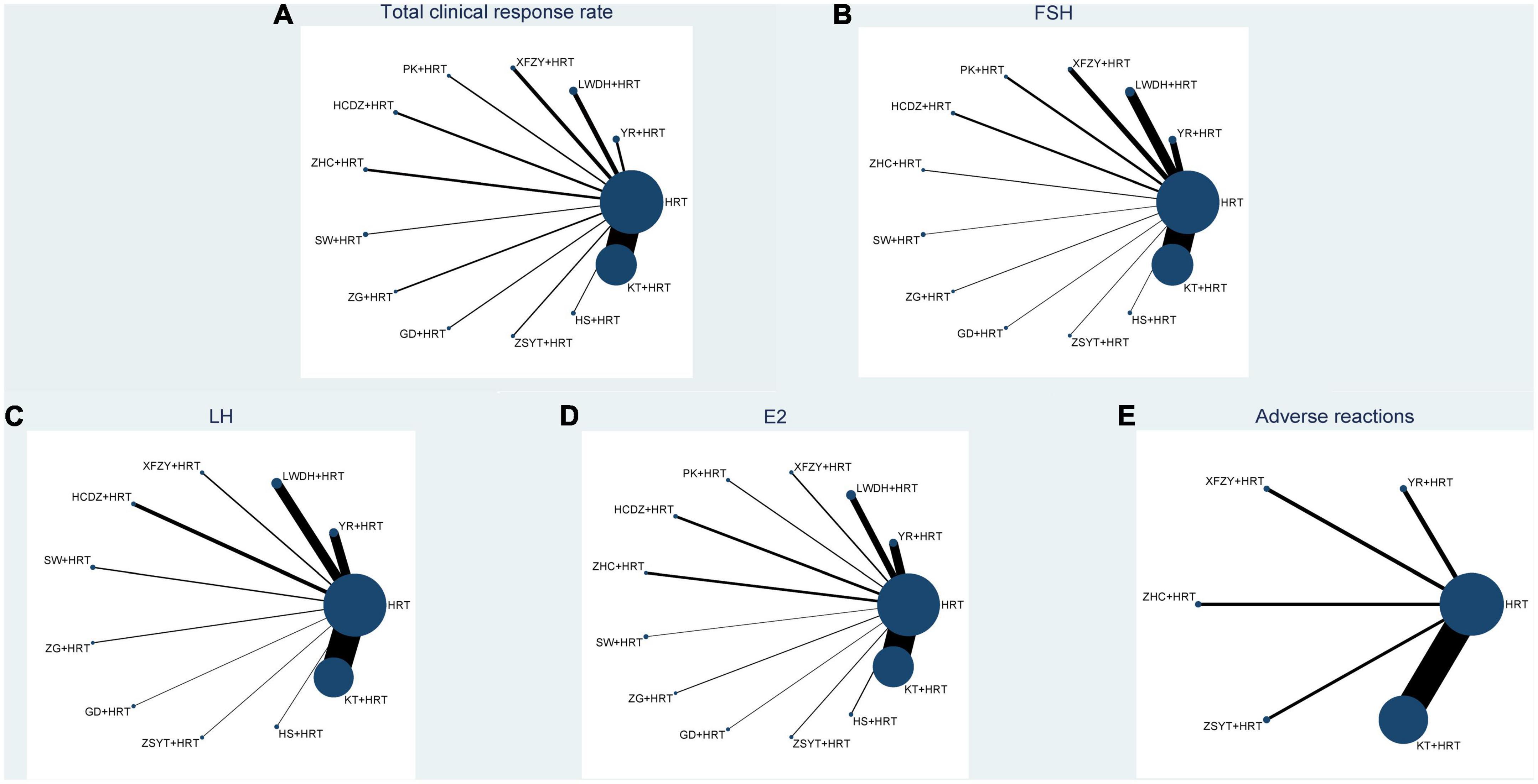
Figure 2. Network plot of all the trials based on the outcomes. P.S: (A) total clinical response rate; (B) FSH; (C) LH; (D) E2; (E) adverse reactions; HRT, hormone replacement therapy; YR, Fuke Yangrong Capsule; LWDH, Liuwei Dihuang Pills; XFZY, Xuefu Zhuyu Capsule; PK, Peikun pills; HCDZ, Heche Dazao pills; ZHC, Ziheche Capsule; SW, Siwu Mixture; ZG, Zuogui pills; GS, Guishen pills; ZSYT, Zishen Yutai pills; HS, Huanshao Capsule; KT, Kuntai Capsule.
Primary outcome
Total clinical response rate
A total of 52 studies involving 4,702 patents reported the total clinical response rate. Among them, GS + HRT, HCDZ + HRT, SW + HRT, KT + HRT, XFZY + HRT, YR + HRT, and LWDH + HRT compared with HRT have statistical significance (see Table 1). ZG + HRT [odds ratio (OR) = 3.92; 95% credible interval (CrI) 0.86, 23.84; SUCRA = 73.76%] had the highest rate of impact on POF patients in terms of the other 11 Chinese patent medicines combined with HRT. Next, the second was GS + HRT (OR = 3.22, 95% CrI = 1.16, 9.44, SUCRA = 70.60%) and HCDZ + HRT (OR = 2.95, 95% CrI = 1.35, 6.51, SUCRA = 68.41%) was third. The comparison-correction funnel plot showed that not all studies were symmetrically distributed around the X = 0 line, and two studies were located outside the funnel chart, which provides evidence for small-sample effects in the study network (see in Supplementary Figure 2). None of the five outcome indicators in this study exhibited a closed loop, so there is no necessary to do a consistency test.
Secondary outcome
A total of 59 studies involving 5,415 patents reported FSH. Only KT + HRT (MD = –6.78, 95% CrI = –8.47, –5.09), YR + HRT (MD = –5.59, 95% CrI = –10.48, –0.92), and LWDH + HRT (MD = –5.57, 95% CrI = –9.69, –1.48) had a significant benefit compared with HRT, and other Chinese patent medicines combined with HRT had no statistical differences. According to the SUCRA value, XFZY + HRT was ranked first (SUCRA = 72.90%) (see Table 1).
There are 51 studies involving 4,629 patents reported LH. Only GS + HRT (MD = –5.53, 95% CrI = –11.05, –0.03), LWDH + HRT (MD = –4.17, 95% CrI = –6.65, –1.7), KT + HRT (MD = –3.87, 95% CrI = –4.95, –2.72), and YR + HRT (MD = –3.03, 95% CrI = –6.27, –0.17) had a significant benefit compared with HRT, and other Chinese patent medicines combined with HRT had no statistical differences. According to the SUCRA value, GS + HRT was ranked first (SUCRA = 72.18%) (see Table 1).
A total of 58 studies involving 5,315 patents reported E2. ZHC + HRT (MD = 41.8; 95% CrI = 21.23, 62.41; SUCRA = 95.95%) had the highest rate of impact on POF patients in terms of the other 11 Chinese patent medicines combined with HRT. Next, the second was YR + HRT (MD = 28.71, 95% CrI = 5.49, 51.93, SUCRA = 86.84%), and ZG + HRT (MD = 28.48, 95% CrI = 13.88, 43.07, SUCRA = 81.97%) was third (see Table 1).
A total of 13 studies involving 1,168 patents reported the adverse reactions. Only KT + HRT had a significant benefit compared with HRT. KT + HRT (OR = 0.44, 95% CrI = 0.21, 0.85, SUCRA = 81.86%) shows the best, and ZHC + HRT (OR = 0.45, 95% CrI = 0.03, 4.3, SUCRA = 70.18%) was as follows. The comparison-correction funnel plot of all secondary outcome is shown in Supplementary Figure 2.
Discussion
Our study has adopted a Bayesian NMA which involves 64 RCTs to evaluate the effectiveness of 12 Chinese patent medicines combined with HRT in POF patients. In terms of primary outcome, ZG + HRT appears to be the most promising way to help patients with POF improve their clinically integrated outcomes.
In recent years, with the development of social economy, women’s increasing mental stress and poor living habits have led to the gradual rejuvenation of patients with POF, which is a devastating diagnosis for women of childbearing age, because it indicates a decline in fertility (1, 86). As a first-line treatment widely used in patients with POF, HRT can improve the clinical symptoms caused by estrogen deficiency in patients, but it is not insisted on by patients because it cannot restore ovarian function and the treatment time is too long (recommended until natural menopause) (5). At present, in addition to hormone therapy, there are still complementary therapies, such as inositol, vitamin D, and traditional Chinese medicine. Inositol has been found to be a natural endogenous compound, which includes D-Chiro-Inositol (DCI) and Myo-inositol (MI), of which MI is the second messenger of insulin and FSH, which can improve insulin resistance (87), correct the FSH/LH ratio, and promote ovulation (88); DCI can improve the quality of oocytes and blastocysts, which has a potential role in improving fertility (89). Similarly, the fact that vitamin D receptors are present in women’s central and peripheral reproductive organs, tissues, and cells suggests that vitamin D plays a key role in fertility. Vitamin D supplementation promotes oocyte development, improves embryo quality, and increases endometrial tolerance (90); in addition, inositol can reduce fasting insulin, blood total cholesterol, blood triglycerol levels, thereby reducing diabetes, dyslipidemia, and cardiovascular disease risk, vitamin D supplementation can also reduce the risk of osteoporosis, and no adverse reactions have been shown, which is undoubtedly a significant advantage for perimenopausal women (91).
In addition, for patients of reproductive age with POF, how to protect fertility at a young age is a key concern. Vitrification of oocytes is an effective technique for fertility protection that allows women to preserve gametes for future fertility in advance. One prospective study found no statistically significant difference in pregnancy and clinical pregnancy rates per cycle between vitrified oocytes and sibling fresh oocytes in closed systems (92). Another prospective study found that open and closed vitrification protocols were equally effective for sibling oocyte cycles when performing blastocyst embryo transfers (93). Thus, vitrification of oocytes offers women the possibility of delaying fertility until the end of treatment or finding a suitable time for fertility. However, the psychological support given during the treatment of infertile patients is also a point that cannot be ignored. POF can cause depression and anxiety disorders in most women. In terms of fertility, studies have found that due to gender differences, infertile couples, although they are seriously affected by infertility diagnosis, in terms of behavior, relationship, social, emotional, and cognitive aspects, regardless of the way they are conceived, women are more likely than men to “seek social support” (94, 95). Therefore, understanding the sources and changes of psychological stress in female patients and providing patients with specific psychotherapy can benefit them in terms of interventions and outcomes of infertility treatment.
Proprietary Chinese medicine has always been a hot topic in Chinese medical research and is widely used in the clinical frontline, especially by gynecologists and fertility center doctors in general hospitals. Based on this purpose, we conducted a study of proprietary Chinese medicine combined with HRT in the treatment of POF, trying to obtain relatively objective evidence, hoping to give full play to the advantages of the three combination of the two, gradually restore ovarian function, to shorten the treatment time, and look for the best efficacy of proprietary Chinese medicine combined with HRT to provide an evidence base. The essence of POF is considered to be the depletion of follicles, and experimental studies have found that Zuogui pills may improve the therapeutic effect of chemotherapy-induced POF by inhibiting the pathway of mitochondrial-dependent apoptosis, laying an experimental foundation for Zuogui pills as a reasonable treatment choice for POF (96). A meta-analysis study also shows that ZG + HRT is more therapeutic and safer than HRT alone (97). Combined with the findings of this study, in the primary outcome, total clinical response rate, ZG + HRT (OR = 3.92; 95% CrI = 0.86, 23.84) is the best in all treatments. Therefore, we can cautiously think that ZG + HRT seems to be the most effective proprietary Chinese medicine in terms of improving total clinical response rate.
This study showed that XFZY + HRT (SUCRA = 72.90%) worked best at reducing FSH levels, but it has no statistical differences. Some scholars found that the ovarian artery peak systolic velocity in POF patients was negatively correlated with FSH (98). Modern pharmacological studies have found that the extract of Honghai–Taoren drug pair can promote blood circulation by affecting hemodynamics, plasma coagulation, and platelet aggregation (99, 100). XFTZ Capsule is composed of a variety of traditional Chinese medicines for promoting blood circulation and removing blood stasis, including Honghai and Taoren. So we infer that XFZY Capsule may reduce FSH levels by improving hemodynamics and improving ovarian blood supply, but this inference lacks experimental research. For the outcome of E2, ZHC + HRT (MD = 41.8; 95% CrI = 21.23, 62.41; SUCRA = 95.95%) was best in all treatments. The composition of Ziheche Capsule is Ziheche. Modern pharmacological studies have found that Ziheche contains a large number of hormones, including gonadotropin, corticotropin-releasing hormone, thyrotropin, prolactin, a variety of LHs, and erythropoietin, which can directly stimulate ovarian tissue and promote endometrial hyperplasia (101). Therefore, we can cautiously conclude that ZHC + HRT has the best effect in improving the E2 level. However, outcome measures for many interventions were not statistically significant, so more long-term and high-quality, large-sample, multi-center RCTs are needed in future to further confirm this.
Innovation and limitations of this study
In this study, for the first time, a NMA was used to classify and analyze the relevant efficacy indicators of proprietary Chinese patient medicines combined with HRT in the treatment of POF. Giving full play to the advantages of proprietary Chinese patient medicines, it provides evidence-based evidence for reasonable and targeted drugs in clinical practice. In addition, this study searched seven databases and finally included 64 RCTs involving 5,675 POF patients, with a large sample size and many sources of evidence.
At the same time, this study has certain limitations: (1) There are differences in the number of studies included in different interventions. For example, there are 42 studies involving Kuntai capsule, and only one study involving Zuogui pills, Guishen pills, Zishen Yutai pills, Huanshao capsule, and Siwu mixture. The difference in number may affect the results of this study make an impact; (2) in this study, 12 proprietary Chinese patient medicines were indirectly compared with HRT, and there was no RCTs that directly compared the efficacy of proprietary Chinese patient medicines, and the evidence network graphs of the five outcome indicators did not form a closed loop, which affected the credibility and stability of the results to a certain extent. (3) In terms of literature quality evaluation, all studies were single-center, Chinese literature, and most of the random methods used the random number table method, and none of them mentioned the allocation concealment, the blinding method of subjects and outcome evaluators, and other sources of bias could not be judged. Therefore, we look forward to conducting more RCTs with large samples, multicenter, and high methodological quality in future to provide more robust and reliable evidence support for clinical drug use.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
H-ZZ served as principal author, had full access to all the data in the study, took responsibility for the accuracy of the data analysis and the integrity of the data, and contributed to the draft of the manuscript. C-LB and M-YL contributed to the conception and design. X-LY, C-LB, and S-YZ contributed to data acquisition and interpretation. X-LY, M-YL, and S-BW contributed to revising of the article and final approval. All authors contributed to the article and approved the submitted version.
Acknowledgments
We affirm that the work submitted for publication is original and has not been published other than as an abstract or preprint in any language or format and has not been submitted elsewhere for print or electronic publication consideration. We affirm that each person listed as authors participated in the work in a substantive manner, in accordance with ICMJE authorship guidelines, and is prepared to take public responsibility for it. All authors consent to the investigation of any improprieties that may be alleged regarding the work. Each author further releases and holds harmless the Endocrine Society from any claim or liability that may arise therefrom.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1043390/full#supplementary-material
References
1. Tinjić S, Abazović D, Ljubić D, Vojvodić D, Božanović T, Ibrišimović M, et al. Influence of autologous in vitro activation of ovaries by stem cells and growth factors on endocrine and reproductive function of patients with ovarian insufficiency-a clinical trial study. Int J Fertil Steril. (2021) 15:178–88. doi: 10.22074/IJFS.2020.134678
2. Jankowska K. Premature ovarian failure. Prz Menopauzalny. (2017) 16:51–6. doi: 10.5114/pm.2017.68592
3. Honigberg MC, Zekavat SM, Niroula A, Griffin GK, Bick AG, Pirruccello JP, et al. Premature menopause, clonal hematopoiesis, and coronary artery disease in postmenopausal women. Circulation. (2021) 143:410–23. doi: 10.1161/CIRCULATIONAHA.120.051775
4. Beck-Peccoz P, Persani L. Premature ovarian failure. Orphanet J Rare Dis. (2006) 1:9. doi: 10.1186/1750-1172-1-9
5. European Society for Human Reproduction and Embryology [ESHRE] Guideline Group on POI, Webber L, Davies M, Anderson R, Bartlett J, Braat D, et al. ESHRE guideline: management of women with premature ovarian insufficiency. Hum Reprod. (2016) 31:926–37. doi: 10.1093/humrep/dew027
6. Webber L, Anderson RA, Davies M, Janse F, Vermeulen N. HRT for women with premature ovarian insufficiency: a comprehensive review. Hum Reprod Open. (2017) 2017:hox007. doi: 10.1093/hropen/hox007
7. Løkkegaard E, Jovanovic Z, Heitmann BL, Keiding N, Ottesen B, Pedersen AT. The association between early menopause and risk of ischaemic heart disease: influence of hormone therapy. Maturitas. (2006) 53:226–33. doi: 10.1016/j.maturitas.2005.04.009
8. Longcope C. Adrenal and gonadal androgen secretion in normal females. Clin Endocrinol Metab. (1986) 15:213–28. doi: 10.1016/s0300-595x(86)80021-4
9. Cartwright B, Robinson J, Seed PT, Fogelman I, Rymer J. Hormone replacement therapy versus the combined oral contraceptive pill in premature ovarian failure: a randomized controlled trial of the effects on bone mineral density. TJ Clin Endocrinol Metab. (2016) 101:3497–505. doi: 10.1210/jc.2015-4063
10. Kodama M, Komura H, Kodama T, Nishio Y, Kimura T. Estrogen therapy initiated at an early age increases bone mineral density in turner syndrome patients. Endocr J. (2012) 59:153–9. doi: 10.1507/endocrj.ej11-0267
11. Popat VB, Calis KA, Kalantaridou SN, Vanderhoof VH, Koziol D, Troendle JF, et al. Bone mineral density in young women with primary ovarian insufficiency: results of a three-year randomized controlled trial of physiological transdermal estradiol and testosterone replacement. J Clin Endocrinol Metab. (2014) 99:3418–26. doi: 10.1210/jc.2013-4145
12. Jiao C, Lu XJ, Yin JY, Zhang MH, Gu WL. Meta-analysis of Kuntai Capsules in the treatment of premature ovarian failure. World Chin Med. (2021) 16:1552–6.
13. Shen, QX, Zhang LL, Lin XY, Liang YJ, Liu GT, Lu RL. Meta-analysis of the efficacy of Kuntai Capsule combined with climmon in the treatment of premature ovarian failure. Chin Modern Appl Pharm. (2020) 1:78–84. doi: 10.13748/j.cnki.issn1007-7693.2020.01.015.
14. Chen ZJ, Tian QJ, Qiao J, Liu JY, Yang DZ, Huang HF, et al.. Chinese expert consensus on clinical diagnosis and treatment of premature ovarian insufficiency. Chin J Obstet Gynecol. (2017) 52:577–81. doi: 10.3760/cma.j.issn.0253-3766.2019.07.002
15. Higgins J, Thomas J, Chandler J, Cumpston M, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester: John Wiley & Sons (2019).
16. Brignardello-Petersen R, Izcovich A, Rochwerg B, Florez ID, Hazlewood G, Alhazanni W, et al. GRADE approach to drawing conclusions from a network meta-analysis using a partially contextualised framework. BMJ. (2020) 371:m3907. doi: 10.1136/bmj.m3907
17. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6.
18. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1.Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64:383–94.
19. Melsen WG, Bootsma MC, Rovers MM, Bonten MJ. The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin Microbiol Infect. (2014) 20:123–9. doi: 10.1111/1469-0691.12494
20. Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. (2012) 3:80–97. doi: 10.1002/jrsm.1037
21. Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2. Med Decis Making. (2012) 33:607–17. doi: 10.1177/0272989X12458724
22. Li J. Clinical effect of gynecology Yangrong capsule combined with estrogen and progesterone artificial cycle sequential therapy in patients with premature ovarian failure. Chin Foreign Med Res. (2018) 9:153–4. doi: 10.14033/j.cnki.cfmr.2018.9.078
23. Liu HY. Effect of gynecology Yangrong capsule combined with estrogen and progesterone artificial cycle sequential treatment on patients with premature ovarian failure. Med J Chin Peoples Health. (2021) 16:73–5.
24. Dai LH, Chen M, Xu SJ. Clinical curative effect of fuke yangrong capsule combining estrogen and progesterone artificial cycle sequential treatment on patients with premature ovarian failure. Chin J Woman Child Health Res. (2017) 5:567–9.
25. Zhang LP. Study on the effect of gynecology Yangrong capsule combined with estrogen and progesterone artificial cycle sequential therapy on premature ovarian failure. Contemp Med Symp. (2019) 13:128–9.
26. Huang SH, Zhang XJ. Clinical study of combined therapy of traditional Chinese medicine and western medicine on primary ovarian insufficiency. CJTCMP. (2017) 4:1707–11.
27. Du D, Wei JL, Chen SY. Clinical research on six taste glutinous Rehmannia pill combined with artificial cycle therapy in treatment of premature ovarian failure. Chin Arch Tradit Chin Med. (2013) 12:2738–40. doi: 10.13193/j.issn.1673-7717.2013.12.047
28. Du JM. Clinical observation of hormone combined with Liuwei Dihuang Pill in the treatment of premature ovarian failure. Shenzhen J Integr Tradit Chin Western Med. (2016) 4:51–3. doi: 10.16458/j.cnki.1007-0893.2016.04.025
29. Liang QF, Chen Q, Mao HF, Cao YG. Value of estrogen and progesterone replacement therapy combined with Liuwei Dihuang Pills in the treatment of kidney yin deficiency type premature ovarian failure. J Bengbu Med Coll. (2020) 45:588–92. doi: 10.13898/j.cnki.issn.1000-2200.2020.05.008
30. Sun JJ. Significance of estrogen and progesterone replacement therapy combined with Liuwei Dihuang Pill in the treatment of premature ovarian failure of kidney yin deficiency type. Health Must Read Mag. (2021) 5:39.
31. Qin LH. Clinical effect of hormone combined with Liuwei Dihuang Pill on premature ovarian failure. J Clin Med. (2017) 80:15775. doi: 10.16281/j.cnki.jocml.2017.80.108
32. Li, Q, Tang JS, Chou S, et al. The clinical effect of drug combination by Liuwei Dihuang Pills and climen in treatment of POF. Hebei Med. (2016) 2:339–41.
33. Zuo L, Cai F. Experimental study on the treatment of premature ovarian failure with Liuwei Dihuang Pill. World Chin Med. (2015) 10:501–2.
34. Li Q, Tang JS, Chou S, Zhang LH, Luo HY, Qiu M, et al. Clinical effect analysis of combined Chinese and western medicine on premature ovarian failure of qi stagnation and blood stasis type. Chin J Trauma Disabil Med. (2016) 24:11–2. doi: 10.13214/j.cnki.cjotadm.2016.15.007
35. Wang YF, Yan L. Clinical observation on the treatment of premature ovarian failure with qi stagnation and blood stasis by combination of traditional Chinese and Western medicine. J Pract Tradit Chin Med. (2015) 4:301.
36. Liu J. Clinical efficacy of combined Chinese and western medicine treatment of premature ovarian failure of blood stasis due to qi stagnation. Sichuan Med J. (2012) 8:1404–6. doi: 10.16252/j.cnki.issn1004-0501-2012.08.008
37. Feng P, Li QK. Clinical observation on 33 cases of premature ovarian failure treated with artificial cycle and peikun pill. J New Chin Med. (2013) 5:82–4. doi: 10.13457/j.cnki.jncm.2013.05.089
38. Li P, Fan YB, Leng W. Clinical observation of peikun pill in treating 24 cases with impaired ovarian function. Cardiovasc Dis J Integr Tradit Chin Western Med. (2017) 4:16–7. doi: 10.16282/j.cnki.cn11-9336/r.2017.04.009
39. Chu JJ, Li WL, Xiong CQ. Clinical efficacy of Heche Dazao capsule combined with climen in treatment of premature ovarian failure. J Anhui Univ Chin Med. (2015) 3:26–8.
40. Zhou WQ. Clinical effect of Heche Dazao pills combined with hormone replacement therapy in the treatment of premature ovarian failure. Clin Res Pract. (2019) 11:122–4. doi: 10.19347/j.cnki.2096-1413.201911049
41. Jiang WF. Effect of Zihe Che on serum FSH and E2 in patients with premature ovarian failure levels and menstrual effects. Guide China Med. (2009) 18:36–7.
42. Shan XL. Clinical observation on 35 cases of premature ovarian failure treated with Ziheche capsule. Zhejiang J TCM. (2010) 7:508.
43. Liu J. Clinical study on Siwu mixture for premature ovarian failure in adjuvant treatment. J New Chin Med. (2019) 2:175–7. doi: 10.13457/j.cnki.jncm.2019.02.053
44. Han Y, Cao YH, Luo Y, Gong HP, Chen XY, Yuan L. Effect of zuogui pill combined with bemeric and medroxyprogesterone acetate on anti mullerian hormone in patients with premature ovarian failure. Mod J Integr Tradit Chin Western Med. (2018) 2: 168–71.
45. Lu Y. Clinical observation on the treatment of premature ovarian failure with deficiency of kidney yin by combination of Chinese and western medicine. J Pract Tradit Chin Med. (2019) 2:203–4.
46. Xie J, Chen MF. Clinical effect of Zishen Yutai pill combined with climen on premature ovarian failure. J Hunan Univ Chin Med. (2017) 12:1396–9.
47. Shen L, Zhang Y. Clinical study on Huanshao capsules combined with complex packing estradiol valerate tablets, estradiol valerate and cyproterone acetate tablets in treatment of premature ovarian failure. Drugs Clin. (2018) 4:916–20.
48. Zhou XH. Clinical observation of Kuntai capsules combined with hormone for premature ovarian failure. J New Chin Med. (2017) 3:79–81. doi: 10.13457/j.cnki.jncm.2017.03.027
49. Yang XQ. Clinical effect of Kuntai capsule combined with estrogen and progesterone on premature ovarian failure. Henan Med Res. (2018) 12:2236–7.
50. Li YF. Effect of sequential combination of estrogen and progesterone combined with kuntai capsule on premature ovarian failure and their influence on ovarian hemodynamic indexes of patients. Chin J Pract Med. (2020) 18:110–4.
51. Yang YJ. Effect of Kuntai capsule combined with hormone replacement therapy on sex hormone and blood lipid levels in patients with premature ovarian failure. Henan Med Res. (2019) 15:2804–5.
52. Liu YP, Wang C, Jin QF. Effect of Kuntai capsule combined with sequential therapy of estrogen and progesterone on premature ovarian failure and its effect on Treg and Th17 balance and cytokine level. Matern Child Health Care China. (2019) 22:5308–11.
53. Wang Y, Guo YY. Clinical effect of Kuntai capsule combined with estradiol valerate tablets in the treatment of premature ovarian failure and its influences on ovarian stromal hemodynamic indexes, TGF-βRII and VEGF levels. Clin Res Pract. (2022) 18:139–42. doi: 10.19347/j.cnki.2096-1413.202218039
54. Liang ML, Chen LY, Wang YT. Clinical observation on 46 cases of premature ovarian failure treated by hormone replacement therapy combined with Kuntai Capsule. Chin J Ethnomed Ethnopharmacy. (2019) 19:93–5.
55. Xiao PM, Xu YY, Shi YH. Clinical efficacy of climen combined with Kuntai capsule for premature ovarian failure. Chin J Gen Pract. (2015) 5:774–5. doi: 10.16766/j.cnki.issn.1674-4152.2015.05.036
56. Liu Q. Clinical observation on the combination of climen and Kuntai capsules in the treatment of premature ovarian failure. Shenzhen J Integr Tradit Chin Western Med. (2015) 9:81–2. doi: 10.16458/j.cnki.1007-0893.2015.09.044
57. Wu SJ. Study on the value of Kuntai capsule combined hormone replacement therapy in the treatment of premature ovarian failure. Electr J Pract Gynecol Endocrinol. (2019) 14:114–9. doi: 10.16484/j.cnki.issn2095-8803.2019.14.084
58. Pan SR, Wang XL, Lv JY. Treatment of 53 cases of premature ovarian failure with Kuntai capsule Combined with artificial cycle therapy. China Pharma. (2015) 4:77–8.
59. Liang W. Effect analysis of climen tablet combined with Kuntai capsule in treating premature ovarian failure. Henan Med Res. (2017) 8:1426–7.
60. Li AF. Effect of Kun Tai capsule combined with artificial periodic hormone therapy on patients with premature ovarian failure. China Contin Med Educ. (2018) 9:104–6.
61. Yuan HF, Hu YJ. Effect of Kuntai capsule combined with hormone replacement therapy on premature ovarian failure. Hubei J TCM. (2019) 1:16–8.
62. Li HP, Xu GZ, Mai JX, Mou W, Li XM. Effect of Kuntai capsule combined with estrogen and progesterone on ovarian blood flow and sex hormone level in patients with premature ovarian failure. Med Pract. (2018) 8:38–41.
63. Li HY. Clinical analysis of Keling Meng combined with Kuntai Capsule in treating premature ovarian failure. Super Baby. (2021) 12:115.
64. Wang HY. Effect of Keling Meng combined with Kuntai Capsule on premature ovarian failure and related hormones. Womans Health Res. (2022) 4:33–4.
65. Li HZ. Effect of Kuntai Capsule Combined with hormone replacement therapy on patients with premature ovarian failure. Med J Chin Peoples Health. (2020) 19:87–9.
66. Cai J, Li X. Effect of Kuntai capsule combined with hormone replacement therapy on clinical symptoms and sex hormone level in patients with premature ovarian failure. Mod J Integr Tradit Chin Western Med. (2019) 35:3933–6.
67. Guo J. The effect of hormone replacement therapy combined with kuntai capsule on serum lipid and sex hormone levels in premature ovarian failure. J Heze Med Coll. (2017) 4:20–2.
68. Chen JQ. Effect of estradiol valerate tablets and estradiol cycloproprogesterone tablets combined with Kuntai capsules on patients with premature ovarian failure. Med Equip. (2017) 8:92–3.
69. Chen Q. Effect of Kuntai capsule Combined with hormone replacement therapy on premature ovarian failure. Henan Med Res. (2019) 13:2426–8.
70. Wang R. Effect of Kuntai capsule Combined with hormone replacement therapy on premature ovarian failure. Harbin Med J. (2021) 6:129–30.
71. Dai SM. Clinical observation of Keling Meng combined with Kuntai capsule in the treatment of premature ovarian failure. Electr J Pract Gynecol Endocrinol. (2016) 12:151–2. doi: 10.16484/j.cnki.issn2095-8803.2016.12.030
72. Gui SM. Clinical observation on treatment of premature ovarian failure with Kuntai capsule combined with hormone replacement. J Pract Tradit Chin Med. (2021) 9:1570–2.
73. Kang SQ. Application effect of hormone replacement therapy combined with Kuntai capsule in the treatment of premature ovarian failure. China Mod Med. (2018) 28:136–8.
74. Luo YH, Chen MF, Huang X, Fan ZJ. Clinical analysis of Keling Meng combined with Kuntai capsule in the treatment of premature ovarian failure. Jiangxi Med J. (2019) 12:1610–33.
75. Xing YR. Clinical observation on treatment of premature ovarian failure with Kuntai capsule combined with climen. China Foreign Med Treat. (2016) 18:111–2. doi: 10.16662/j.cnki.1674-0742.2016.18.111
76. Lu YX. Clinical effect of Kuntai capsule combined with sequential therapy of estrogen and progesterone on premature ovarian failure. Chin J Clin Ration Drug Use. (2019) 36:89–90. doi: 10.15887/j.cnki.13-1389/r.2019.36.044
77. Zhao YY. Clinical observation of Kuntai capsule combined with climen on patients with premature ovarian failure. Health Must Read Maga. (2020) 7:110–1.
78. Su AF, Nan Y. Clinical observation of Kuntai capsule for the treatment of idiopathic premature ovarian failure. SH J TCM. (2014) 5:79–80. doi: 10.16305/j.1007-1334.2014.05.036
79. Wu HX. Premature ovarian failure using Kuntai capsule combined with hormone replacement therapy. Contin Med Educ. (2020) 1:154–6.
80. Wang J, He ZY, Sun GL. Clinical effect of Kuntai capsule combined with climen in the treatment of premature ovarian failure. China Pract Med. (2021) 17:167–70. doi: 10.14163/j.cnki.11-5547/r.2021.17.062
81. Xie JR, Cheng Y, Lv QL. Clinical observation on treatment of premature ovarian failure with Kuntai capsule combined with estrogen and progesterone. J New Chin Med. (2016) 9:94–6. doi: 10.13457/j.cnki.jncm.2016.09.043
82. Xu J, Zhang FL, Yao F, Liu N, Ren Y, Fan MH, et al. Analysis of curative effect of two methods in treating premature ovarian failure. J Med Theory Pract. (2016) 13:1764–6. doi: 10.19381/j.issn.1001-7585.2016.13.050
83. Wu YY, Wu ZX, Lu YX. Effect of Kuntai capsule combined with hormone replacement therapy on premature ovarian failure. Matern Child Health Care China. (2016) 21:4425–7.
84. Guo XL. Effect of Kuntai capsule combined with artificial cycle hormone therapy on clinical symptoms and estrogen level in patients with premature ovarian failure. China Health Care Nutr. (2017) 27:105–6. doi: 10.3969/j.issn.1004-7484.2017.07.143
85. Zeng SY, Xiao SJ. Clinical observation of Kuntai capsule combined with hormone in treating premature ovarian failure. New Chin Med. (2014) 11:129–30. doi: 10.13457/j.cnki.jncm.2014.11.049
86. Di Prospero F, Luzi S, Iacopini Z. Cigarette smoking damages women’s reproductive life. Reprod Biomed Online. (2004) 8:246–7. doi: 10.1016/s1472-6483(10)60525-1
87. D’Anna R, Corrado F, Loddo S, Gullo G, Giunta L, Di Benedetto A. Myoinositol plus α-lactalbumin supplementation, insulin resistance and birth outcomes in women with gestational diabetes mellitus: a randomized, controlled study. Sci Rep. (2021) 11:8866. doi: 10.1038/s41598-021-88329-x
88. Gullo G, Carlomagno G, Unfer V, D’Anna R. Myo-inositol: from induction of ovulation to menopausal disorder management. Minerva Ginecol. (2015) 67: 485–6.
89. Pericuesta E, Laguna-Barraza R, Ramos-Ibeas P, Gutierrez-Arroyo JL, Navarro JA, Vera K, et al. D-chiro-inositol treatment affects oocyte and embryo quality and improves glucose intolerance in both aged mice and mouse models of polycystic ovarian syndrome. Int J Mol Sci. (2020) 21:6049. doi: 10.3390/ijms21176049
90. Menichini D, Forte G, Orrù B, Gullo G, Unfer V, Facchinetti F. The role of vitamin D in metabolic and reproductive disturbances of polycystic ovary syndrome: a narrative mini-review. Int J Vitam Nutr Res. (2022) 92:126–33. doi: 10.1024/0300-9831/a000691
91. Huang YZ, Zhang LL, Xue SY, Yang GY. A network meta-analysis of the efficacy of various hypoglycemic drugs and inositol in the treatment of polycystic ovary syndrome. Chin J Endocrinol Metab. (2021) 37:1096–105. doi: 10.3760/cma.j.cn311282-20210204-00078.
92. Papatheodorou A, Vanderzwalmen P, Panagiotidis Y, Petousis S, Gullo G, Kasapi E, et al. How does closed system vitrification of human oocytes affect the clinical outcome? A prospective, observational, cohort, noninferiority trial in an oocyte donation program. Fertil Steril. (2016) 106:1348–55. doi: 10.1016/j.fertnstert.2016.07.1066
93. Gullo G, Petousis S, Papatheodorou A, Panagiotidis Y, Margioula-Siarkou C, Prapas N, et al. Closed vs. Open oocyte vitrification methods are equally effective for blastocyst embryo transfers: prospective study from a sibling oocyte donation program. Gynecol Obstet Invest. (2020) 85:206–12. doi: 10.1159/000506803
94. Burgio S, Polizzi C, Buzzaccarini G, Laganà AS, Gullo G, Perricone G, et al. Psychological variables in medically assisted reproduction: a systematic review. Prz Menopauzalny. (2022) 21:47–63. doi: 10.5114/pm.2022.114404
95. Gullo G, Cucinella G, Perino A, Gullo D, Segreto D, Laganà AS, et al. The gender gap in the diagnostic-therapeutic journey of the infertile couple. Int J Environ Res Public Health. (2021) 18:6184. doi: 10.3390/ijerph18126184
96. Peng H, Zeng L, Zhu L, Luo S, Xu L, Zeng L, et al. Zuogui pills inhibit mitochondria-dependent apoptosis of follicles in a rat model of premature ovarian failure. J Ethnopharmacol. (2019) 238:111855. doi: 10.1016/j.jep.2019.111855
97. Jiao C, Lu XJ. Meta analysis of the combination of hormonal combination of left gui pill plus and minus prescription for the treatment of premature ovarian failure. J Chin Physician. (2019) 7:1002–6.
98. Wang J. Transvaginal color doppler ultrasound to detect changes in blood flow in premature ovarian failure and its relationship with sex hormone levels. Med Innov China. (2021) 28:158–61.
99. Liu L, Duan JA, Tang Y, Guo J, Yang N, Ma H, et al. Taoren-Honghua herb pair and its main components promoting blood circulation through influencing on hemorheology, plasma coagulation and platelet aggregation. J Ethnopharmacol. (2012) 139:381–7. doi: 10.1016/j.jep.2011.11.016
100. Yang KL, Zeng LT, Ge AQ, Ge JW, Long ZY, Bao TT, et al. Based on network pharmacology, the molecular mechanism of peach kernel-safflower on blood activation and stasis was discussed. World Sci Technol. (2018) 2208–16.
Keywords: Chinese patent medicine, hormone replacement therapy (HRT), premature ovarian failure (POF), network meta-analysis (NMA), validity
Citation: Zhong H-Z, Li M-Y, Yin X-L, Bin C-L, Zhou S-Y and Wei S-B (2022) Chinese patent medicines combined with hormone replacement therapy for premature ovarian failure: A Bayesian network meta-analysis. Front. Med. 9:1043390. doi: 10.3389/fmed.2022.1043390
Received: 13 September 2022; Accepted: 24 October 2022;
Published: 17 November 2022.
Edited by:
Mingsan Miao, Henan University of Traditional Chinese Medicine, ChinaReviewed by:
Giuseppe Gullo, Azienda Ospedaliera Ospedali Riuniti Villa Sofia Cervello, ItalyJinsong Geng, Nantong University, China
Copyright © 2022 Zhong, Li, Yin, Bin, Zhou and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shao-Bin Wei, d2Vpc2hhb2JpbjU2MjBAMTYzLmNvbQ==
 Han-Zhi Zhong
Han-Zhi Zhong Mao-Ya Li
Mao-Ya Li Cheng-Li Bin
Cheng-Li Bin Shao-Bin Wei
Shao-Bin Wei