- 1Department of Anesthesiology, Center for Brain Science, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
- 2Department of Anesthesiology, Second Hospital of Shanxi Medical University, Taiyuan, China
- 3Department of Anesthesiology, The First Affiliated Hospital, College of Clinical Medicine of Henan University of Science and Technology, Luoyang, China
- 4Department of Anesthesiology, Hunan Provincial People's Hospital, Changsha, China
- 5Department of Anesthesiology, The First Affiliated Hospital of Henan University of Chinese Medicine, Zhengzhou, China
- 6Department of Anesthesiology, Affiliated Hospital of Shaanxi University of Chinese Medicine, Xianyang, China
- 7Department of Anesthesiology, Henan Provincial People's Hospital, Zhengzhou, China
- 8Department of Anesthesiology, The Third Central Hospital of Tianjin, Tianjin, China
- 9Department of Anesthesiology, The First Hospital of Hunan University of Chinese Medicine, Changsha, China
- 10Department of Anesthesiology, Shanxi Traditional Chinese Medicine Hospital, Taiyuan, China
- 11Department of Anesthesiology, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 12Department of Anesthesiology, General Hospital of Ningxia Medical University, Yinchuan, China
Importance: Postoperative nausea and vomiting (PONV) gives patients a bad experience and negates their good recovery from surgery.
Objective: This trial aims to assess the preventive effectiveness of transcutaneous electrical acupoint stimulation (TEAS) on the incidence of PONV in high-risk surgical patients.
Design: The large sample size, multicenter, evaluator-blinded, and randomized controlled study was conducted between September 3, 2019 to February 6, 2021.
Setting: The 12 hospitals were from different Chinese provinces.
Participants: After obtaining ethics approval and written informed consent, 1,655 patients with Apfel score ≥ 3 points were enrolled for selective laparoscopic non-gastrointestinal surgery under general anesthesia.
Interventions: Patients were randomly allocated into the TEAS and Sham group with a 1:1 ratio. The TEAS group was stimulated on bilateral Neiguan and Zusanli acupoints after recovery from anesthesia on the surgical day and the next morning for 30 min, while the Sham group received an identical setting as TEAS but without currents delivered. Electronic patient self-reported scale was used to evaluate and record the occurrence of PONV.
Main Outcomes and Measures: Primary clinical end point is the incidence of PONV which was defined as at least one incidence of nausea, retching, or vomiting after operation within postoperative 24 h.
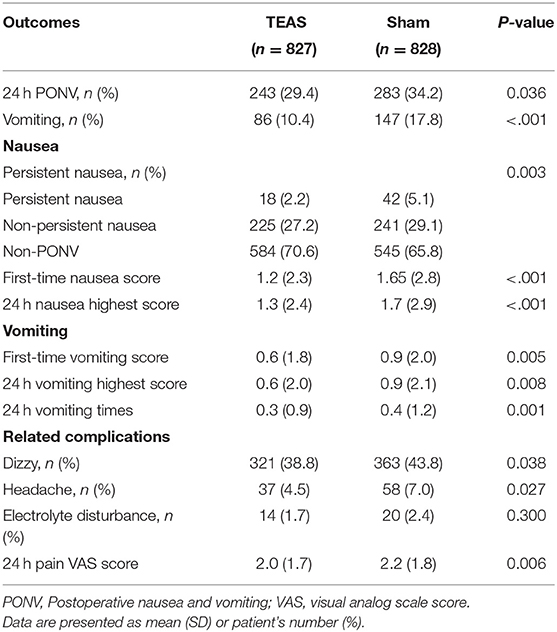
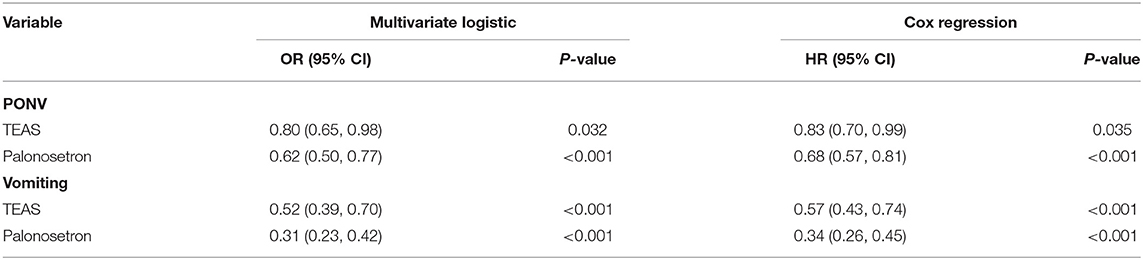
Results: Compared with the Sham treatment, the TEAS lowered the PONV incidence by 4.8% (29.4 vs. 34.2%, P = 0.036) and vomiting incidence by 7.4% (10.4 vs. 17.8%, P < 0.001). TEAS also lowered persistent nausea incidence and PONV scores and decreased PONV related complications and Quality of Recovery−40 scores (P < 0.05). TEAS lowered the 24 h PONV risk by 20% (OR, 0.80, 95% CI, 0.65 −0.98; P = 0.032), and lowered hazard ratio by 17% (HR, 0.83, 95% CI, 0.70–0.99; P = 0.035). Both TEAS and palonosetron were the independent PONV risk protective factors for 24 h PONV incidence and cumulative PONV incidence. The combination of TEAS and palonosetron was the most effective strategy to reduce the PONV incidence (P < 0.001).
Conclusions and Relevance: TEAS attenuated the PONV incidence and severity in high-risk surgical patients and may be applied clinically as a complement therapy to prevent PONV.
Clinical Trial Registration: https://clinicaltrials.gov/ct2/show/NCT04043247, identifier: NCT04043247.
Key Points
- Question: Dose transcutaneous electrical stimulation prevent postoperative nausea and vomiting (PONV) in high-risk surgical patients?
- Findings: The electrical stimulation significantly reduced the PONV incidence and its severity. Both TEAS and palonosetron were independent PONV risk protective factors for 24 h PONV incidence and cumulative PONV incidence.
- Meaning: TEAS may enhance recovery of PONV high-risk surgical patients and should be routinely used clinically as a complementary strategy.
Introduction
Postoperative nausea and vomiting (PONV), characterized as nausea, retching, or vomiting or any these symptoms in combination after surgery, is one of the most common complaints after surgery with an overall incidence of 30%, but its incidence can be as high as to be 60–80% in high-risk patients who have three or four PONV risk factors (1). Nausea gives patients an extremely afflictive medical experience, induces stomach discomfort including the feeling of keck and poor appetite, and even causes dizziness and headache. Vomiting increases the potential risks of electrolyte imbalances, pain, incision dehiscence or hernia, dehydration, esophageal damage, or airway aspiration (2). PONV prevention has been included in enhanced recovery after surgery (ERAS) management strategy. Even though the incidence of PONV can be decreased by using less opioids and inhalational anesthetics and even using antiemetic drugs, its occurrence is still high, with up to 20% of high-risk patients treated with three antiemetic prophylaxis (1, 3).
Owning to multiple mechanisms triggering PONV, several antiemetic drugs are often used clinically in high-risk PONV patients (4), but their effectiveness is still limited and include unwanted side effects such as headaches, xerostomia, abnormal liver function, and extrapyramidal reactions (5–8). Compared to antiemetic drugs, non-pharmaceutical therapy including acupuncture has considerable advantages, for example, non-toxic effects, which has been widely used in postoperative pain analgesia and gastrointestinal function rehabilitation (9, 10). Indeed, acupuncture, a safe and effective non-pharmacotherapy, can directly or indirectly inhibit the emesis center or regulate gastrointestinal function through multiple regulatory mechanisms, such as modulating gastric motility through somatovisceral reflex and influencing the endogenous opioid system and serotonin transmission (11–14). Acupoint Neiguan (P6) stimulation was reported to be comparable to antiemetic drugs in reducing PONV incidence but its effectiveness in combination with antiemetic drugs is inconclusive (1). Transcutaneous electrical acupoint stimulation (TEAS) with transcutaneous electrical nerve stimulation applied to acupuncture points has been used to prevent PONV occurrence (15, 16). However, its effectiveness in preventing PONV has not been established yet due to small sampling size and being not well controlled in the previous studies (1). Therefore, the large sample size, multicenter, evaluator-blinded, and randomized controlled study was carried out to verify the effectiveness of TEAS on P6 and Zusanli (ST36) in reducing the incidence of PONV.
Methods
Study Design and Participants
This multicenter randomized controlled trial was approved by the Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University (XJTU1AF2019LSK-084) and then registered at ClinicalTrials.gov (NCT04043247). The detailed trial protocol is available online as Supplementary Material 1. The study was carried out in accordance with clinical research CONSORT and acupuncture clinical trial STRICTA to provide reliable concrete evidence for clinical application of TEAS in PONV prophylaxis (Supplementary Material 2). The following inclusion criteria were included: (1) selective laparoscopic non-gastrointestinal surgery under general anesthesia; (2) age 18–50 years, BMI 15–40 kg/m2, ASA class I–III; (3) Apfel score ≥ 3 points; and (4) ability to understand, sign informed consent, and coordinate intervention and evaluation. The following exclusion criteria were included: (1) pregnant and breast-feeding patients; (2) TEAS contraindications such as skin allergy, breakage, infection or itching of test points, allergic to adhesive tape, pacemaker implanted; (3) history of alcohol, opioids, or other drug abuse; (4) likely admission to ICU after surgery; and (5) participation in other clinical studies within 3 months before admission to this study. All investigators were trained according to the standardized acupoints selection and TEAS manipulation by a senior licensed acupuncturist with 20 years of practicing experience.
Enrolment, Randomization, and Blinding
After written informed consent was obtained, patients scheduled for selective laparoscopic non-gastrointestinal surgery under general anesthesia were screened for potential enrolments from 12 hospitals (listed in the authorship) in China from September 3, 2019 to February 6, 2021. The eligible patients were randomly divided into the Sham and TEAS groups in a 1:1 ratio (with a block size of 4) by Crabyter scientific research system (Xinyu Information Technology Co., Ltd.). All patients, anesthesiologists, surgeons, and evaluators were blinded for group allocation, screening, intervention treatment, and statistical analysis. Designated evaluators who were totally blinded took charge for follow-up data collections.
Clinical End Points
Primary clinical end point is the incidence of PONV, which was defined as nausea, retching, and/or vomiting after operation within postoperative 24 h. Secondary clinical end points were the postoperative 24 h PONV severity and the 40-item Quality of Recovery (QoR-40) and PONV-related complications. The PONV severity was assessed with the time and visual analog scale (VAS) score of first PONV, the cumulative numbers, and VAS score in postoperative 24 h. The specific contents of QoR-40 score include five aspects: physical comfort (12 items, 60 scores), emotional state (9 items, 45 scores), physical independence (5 items, 25 scores), psychological support (7 items, 35 scores), and pain (7 items, 35 scores). PONV-related complications are dizziness and headache, electrolyte imbalances, and pain.
Anesthesia and Surgery
After receiving 0.5 mg pre-medication of anticholinergics drug penehyclidine, atropine, or scopolamine, patients were inducted with propofol (2 mg/kg), sufentanil (0.3 μg/kg), and cisatracurium (0.15 mg/kg), and maintained with remifentanil (0.1 μg/kg/h), cisatracurium (0.1 mg/kg/h), dexmedetomidine (initial dose 1 μg/kg for 10 min, maintenance dose 0.4 μg/kg/h), and sevoflurane (1% in 2 L/min enriched oxygen). Propofol (4–8 mg/kg/h) as necessary was used for anesthesia maintenance to keep BIS 40–60 throughout surgery. Patients were treated with 50 mg non-steroidal anti-inflammatory drug flurbiprofen axeril or 40 mg parecoxib at the end of surgery, and were intravenous injection pumped with sufentanil individualized postoperative analgesia pump to keep the perioperative pain numerical score at <4 points.
Interventions
Both groups received 5 mg dexamethasone (before induction) combined with 0.075 mg palonosetron (before induction) as the first choice. When palonosetron was not available at some hospitals, patients received 5 mg dexamethasone (before induction) combined with 2 mg tropisetron (at the end of surgery) instead. According to the criteria of WHO acupuncture points, P6 and ST36 were selected in this study (Supplementary Figure 1). These acupoints are far from the operation site. P6 is located at 2 inches above palm wrist transverse striation, between the palm long tendon and the temporal flexor tendon. ST36 is located at 3 inches below EX-LE5 (the lateral pit of the patella and patellar ligament), and one transverse finger outward lateral tibia. The true or sham TEAS was given to the TEAS or sham group patients, respectively, in the PACU when recovered from anesthesia (to be awake and calm, Richmond Agitation-Sedation Scale = 0) on the same surgical day and on the next morning of surgical ward for 30 min. The bilateral P6 and ST36 were pressed to achieve a tingling sensation called “de qi”, and electrode slices were stuck and fixed on those acupoints. Interveners connected electrode slices to electrical stimulator (TEAS stimulator, SDZ-V; Hwato, China) and selected the distant-dense wave with the frequency of 2/10 Hz. After adjusting the intensity to the patient's maximum tolerance, the treatment lasted for 30 min. Patients in the sham group received identical settings and manipulation to the TEAS group, except that the wire between electrode pads and TEAS stimulator was cut so that no current could be delivered. The 5-HT3 receptor antagonists or propofol or haloperidol were used for PONV rescue when necessarily.
Data Collection
Electronic patient self-reported scale (Jiangsu Rehn Medtech Technology Co., Ltd.) was used to evaluate and record the occurrence of PONV, including time and corresponding severity. The severity was evaluated with VAS score which is a psychometric response scale to measure the intensity of subjective characteristics or attitudes and has been widely used for pain quantification. In our study, the severity of nausea and vomiting was rated on the VAS score from 0 to 10 (0 = no nausea or vomiting, 10 = maximum severity). Once patients suffered PONV, patients or their caregivers pressed the buttons on an electronic scale and reported the related information (Supplementary Figure 2). Postoperative 24 h cumulative PONV incidence refers to the cumulative number of PONV episodes in the postoperative 24 h period. Persistent nausea was defined as continuous nausea lasting over 5 min. To avoid the leakage of allocation, evaluators went to the wards after 12 a.m. at the 1st and 2nd postoperative day to check the electronic patient self-reported system running condition and obtain the information of QoR-40 and complications. The postoperative 30-day complications and adverse events were obtained through telephone follow-ups and were stored in the electronic medical record system.
Statistical Analysis
Power Calculation
In previous studies, the PONV incidence was reported to be ~40%, and the PONV incidence was reduced by 7% with the TEAS treatment (15). With significance set at 0.05 and power set at 80%, the sample size required to detect differences was 742 patients in each group calculated with the Pass 11 software (NCSS, LLC. Kaysville, Utah, USA). Taking into account a loss-to-follow-up rate of about 10%, 1,634 patients in total were enrolled to meet the minimum sample size requirement.
Intention-to-treat analysis was used for all enrolled patients. Continuous variables were presented as mean ± SD if data are normally distributed; otherwise, they were presented as median (interquartile range, IQR). Categorical values were presented as numbers (percentages). The differences between two groups were compared using the Mann-Whitney or Chi-square tests. The Kaplan-Meier log-rank test was used to illustrate the cumulative incidence of nausea and vomiting. Variables with significant differences (P < 0.10) in univariate analysis were included in multivariate analysis, which was used to identify the associated risk factors of the PONV occurrence. The comparison of independent risk factors related to PONV was further analyzed by chi-square tests or Kaplan-Meier log-rank test. A 2-sided P-value < 0.05 was considered to be of statistical significance. All these statistical analyses were performed using the SPSS software (version 25.0, IBM Corporation, NY, USA). All authors had access to the study data and reviewed and approved the final manuscript.
Results
Characteristics of Study Population
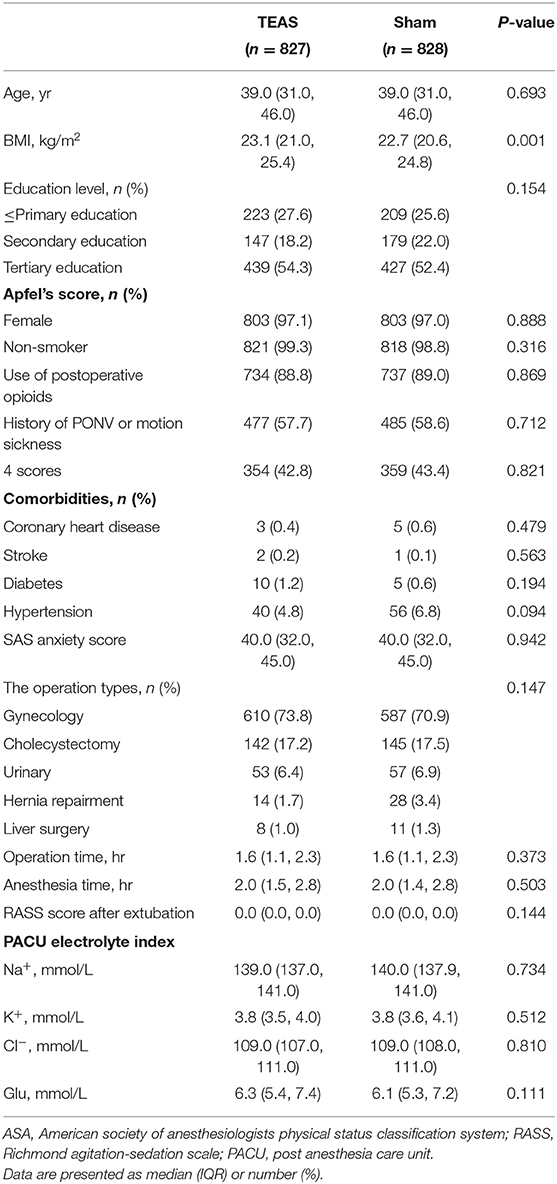
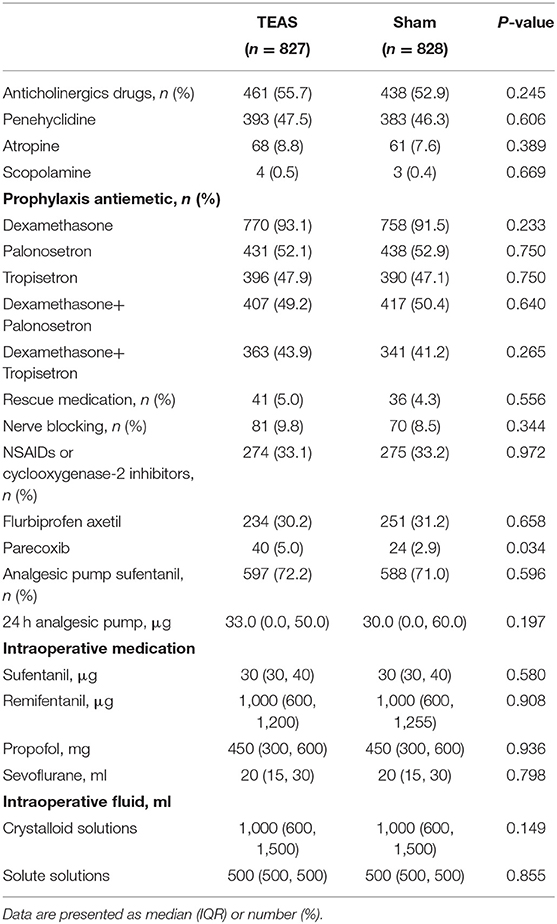
A total of 1,734 patients were scheduled for laparoscopic non-gastrointestinal surgery at 12 hospitals in China between September 3, 2019 and February 6, 2021. At two-step recruitment screenings, 48 patients were excluded at the first screening and of those, 31 patients were excluded at the second screening. A total of 1,655 patients were enrolled and randomly divided into two groups: 828 patients allocated to the Sham group and 827 patients to the TEAS group. All 1,655 patients were included in the intention-to-treat analysis (Figure 1). No TEAS-related adverse effects occurred in the study. The demographics, comorbidities, preoperative SAS anxiety score, surgical and anesthesia data, RASS scores after extubation, and PACU electrolyte index between the TEAS and Sham group were presented in Table 1. The selective laparoscopic non-gastrointestinal surgery under general anesthesia included gynecology (>70%), cholecystectomy, urinary, hernia repairment, and liver surgery. The anticholinergics, antiemetics, and multimode analgesics used during the perioperative period showed no significant differences between the two groups, except the parecoxib usage was more often higher in the TEAS group than in the Sham group (Table 2). The patients with or without parecoxib had no significant difference of PONV incidences.
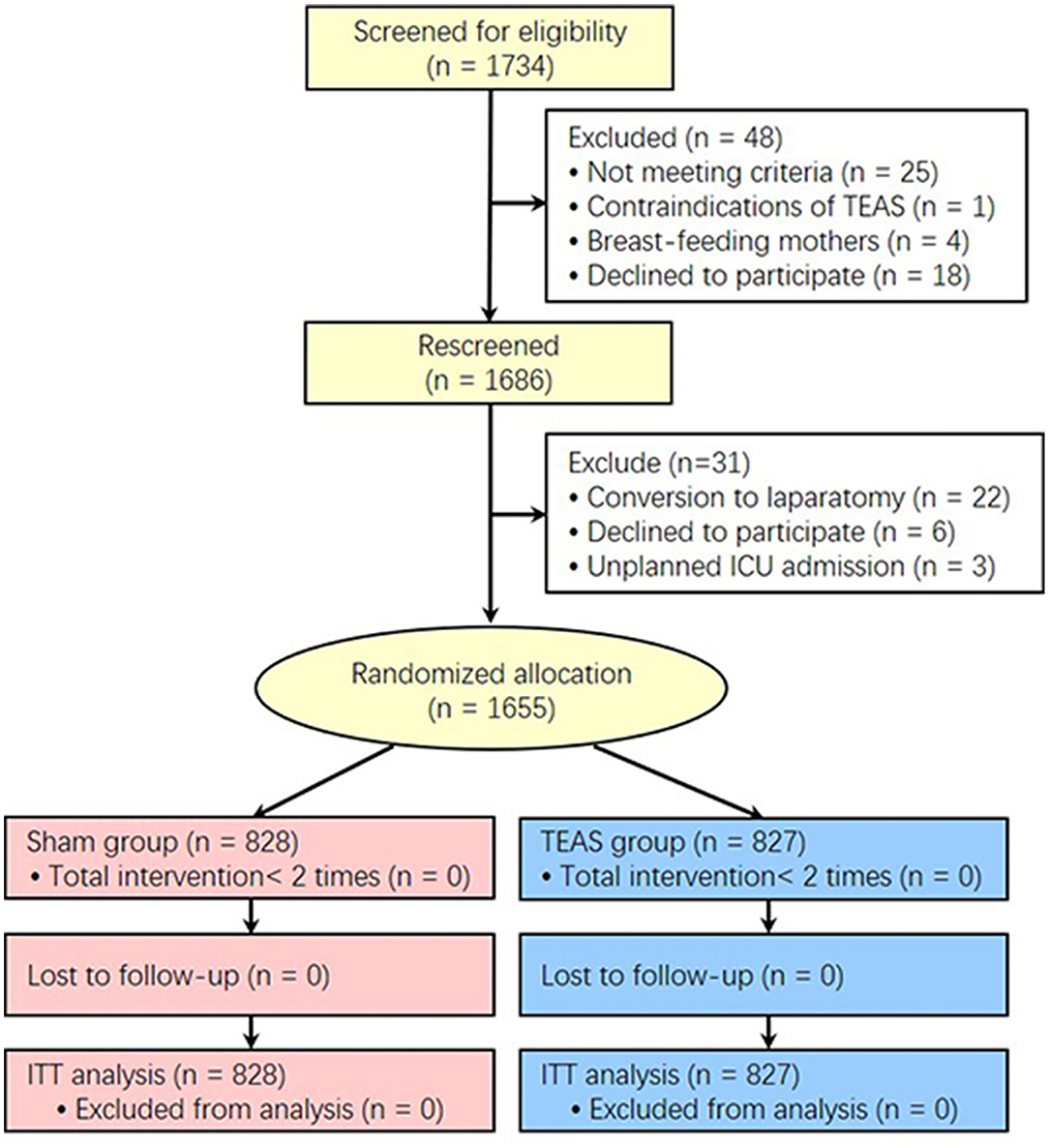
Figure 1. CONSORT diagram for patients enrolled in the study. TEAS, transcutaneous electrical acupoint stimulation; ITT, Intention-To-Treat.
Primary and Secondary Outcomes
Compared to the Sham group, patients in the TEAS group had significantly lower PONV incidence (29.4 vs. 34.2%, P = 0.036) and lower vomiting incidence (10.4 vs. 17.8%, P < 0.001). For nausea, the TEAS group showed lower persistent nausea incidence (2.2 vs. 5.1%, P = 0.003), lower first-time nausea score, and 24 h nausea highest scores (P < 0.001). Specifically, in 827 patients of the TEAS group, 29.4% (243) patients suffered nausea, including 0.7% (6) patients who suffered both persistent nausea and vomiting, 1.5% (12) patients who suffered persistent nausea without vomiting, 9.7% (80) patients who suffered vomiting without persistent nausea, and 17.5% (145) patients who suffered nausea without persistent nausea or vomiting. Correspondingly, in 828 patients of the Sham group, these incidences were, respectively, 2.1% (17), 3.0% (25), 15.7% (130), and 13.4% (111) (Supplementary Figure 3).
In the vomiting patients, the TEAS group showed lower first-time vomiting score (P = 0.005) and lower highest vomiting scores (P = 0.008) together with vomiting times at the postoperative 24 h (P = 0.001). TEAS also significantly decreased the PONV-related postoperative 30 days complications including dizziness (P = 0.038), headache (P = 0.027), and postoperative 24 h Pain (P = 0.006) (Table 3). Compared to the non-PONV patients, the PONV patients had worse QoR-40 scores in all the five items (all P < 0.001). Compared to the 24 h QoR-40 scores in the Sham group, patients in the TEAS group had better physical comfort (P = 0.003), emotional state (P = 0.001), psychological support (P = 0.002), and pain (P = 0.012). Compared to the vomiting patients, the non-vomiting patients had significantly better scores in the same items (all P < 0.001) (Supplementary Table 1).
Multivariate Binary Logistic and COX Regression Analysis for PONV
Both TEAS and palonosetron were independently protective factors of PONV (Table 4). Compared with the Sham group, the TEAS group had 20%lower risk of PONV (OR, 0.80, 95% CI, 0.65–0.98; P = 0.032) and 48%lower risk of vomiting occurrence (OR, 0.52, 95% CI, 0.39–0.70; P < 0.001) by multivariate logistic regression analysis; the TEAS group delayed PONV occurrence time by 17% (HR, 0.83, 95% CI, 0.70–0.99; P = 0.035) and delayed vomiting occurrence time by 43% (HR, 0.57, 95% CI, 0.43–0.74; P < 0.001) by COX regression analysis (Supplementary Figure 4). Compared with the tropisetron antiemetic, palonosetron reduced risk of PONV by 38% (OR, 0.62, 95% CI, 0.50–0.77; P < 0.001) by multivariate logistic regression analysis; the palonosetron delayed postoperative 24 h PONV occurrence time by 32% (OR, 0.68, 95% CI, 0.57–0.81; P < 0.001) by COX regression analysis.
The combined TEAS and palonosetron group had the lowest PONV incidence (25.1%), followed with combined Sham and palonosetron (28.8%) and combined TEAS and tropisetron (34.1%), and the combined Sham and tropisetron group had the highest PONV incidence (40.3%) (P < 0.001). The similar distribution of cumulative PONV incidences and vomiting among the four groups was noted (Figure 2).
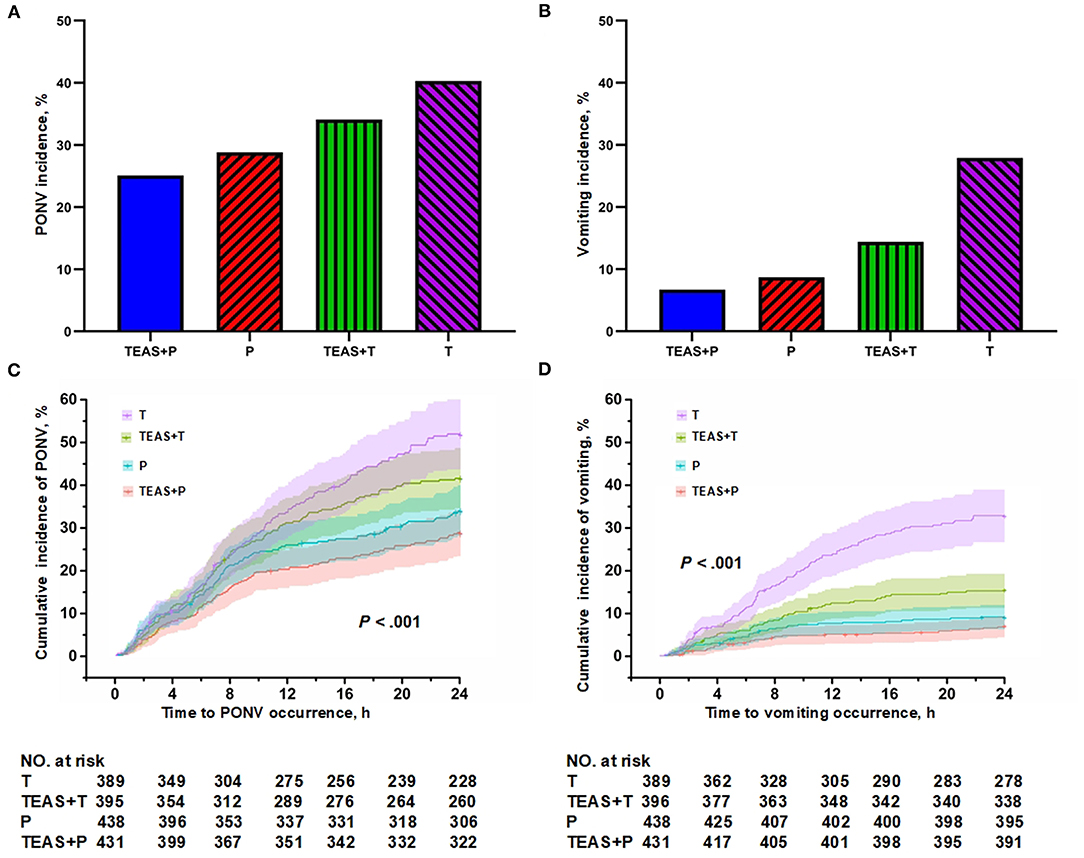
Figure 2. The postoperative 24 h (A) PONV incidences, (B) vomiting incidences, postoperative 24 h cumulative (C) PONV and (D) vomiting incidences among combined TEAS and palonosetron (TEAS + P), combined Sham and palonosetron (Sham + P), combined TEAS and tropisetron (TEAS + T), and combined Sham and tropisetron (Sham + T).
Discussion
Our study found that the TEAS significantly decreased the 24 h PONV incidence and also lowered persistent nausea incidence, PONV scores, and postoperative 24 h cumulative PONV incidences. Further, the TEAS also significantly decreased PONV-related complications, and improved postoperative 24 h physical comfort and emotional state, psychological support scores, and pain score in 24 h QoR-40 Scores. We also found that both TEAS and palonosetron were the independent PONV protective factors for 24 h PONV incidence and cumulative PONV incidence.
PONV is triggered by the nerve projected to the vomiting center, which is influenced by the cerebral cortex, vestibular and cerebellar nuclei, and chemoreceptor trigger band (4). The surface of the chemoreceptor trigger band covers various receptors, such as 5-HT3, 5-HT4, opioid, cholinergic, cannabis, and dopamine receptor (17). It has been reported that the incidence of PONV can be decreased by 26% for each additional type of antiemetic drug. The incidence of PONV was 52, 37, 28, and 22% in the absence or presence of 1, 2, and 3 types of antiemetic drugs, respectively (3). The Enhanced Recovery after Surgery Society recommended that more than two types of antiemetics should be used for patients undergoing gynecologic procedures (18), while three types of antiemetics did not show improved efficacy over the two agents (19). Thus, two types antiemetics in combination (dexamethasone with tropisetron/palonosetron) were used in our study. Compared to the first-generation 5-HT3 receptor antagonists of tropisetron, the second-generation 5-HT3 receptor antagonist palonosetron has a higher binding affinity and longer half-life and duration of action, and a single injection of a 0.075 mg dose of palonosetron for PONV prevention was used for up to 24 h after surgery (20).
However, the combination of two types of antiemetic drugs only inhibited part of the PONV-related receptors, and the prophylaxis effect reached the maximum effect and increasing their doses did not increase antiemetic effects due to receptor occupancy saturation. However, the side effects were increased (7). Correspondingly, the TEAS can modulate comprehensive PONV triggering receptors and reflex pathways to prevent PONV. For example, TEAS stimulates neuronal pathways from the soma splanchnic neurons to the paraventricular nucleus of the hypothalamus (21); TEAS also reduces secretion of 5-HT in the duodenum and suppresses the activation of the nucleus tractus solitarii in the brain-stem (22). TEAS inhibits sympathetic nerve and stimulates parasympathetic nerve and hence increases the activity of acetylcholinesterase (23); TEAS also stimulates receptors such as NO, CCK-A, cannabinoid, opioid receptor, and others and regulates neurotransmitters such as serotonin, GABA, and catecholamines (14). All these result in a decreased incidence of PONV.
Furthermore, the PONV prophylaxis outcome of TEAS might be due to stimulating acupoints of P6 and ST3. P6 is currently recognized as the standard acupoint for the prevention of PONV (24), and its effect was comparable to that of antiemetic drugs (25). Stimulation of P6 and ST36 in laparoscopic radical gastrectomy for gastric cancer significantly reduced the incidence of PONV, decreased early postoperative pain intensity and analgesic dosage, shortened the time of exhaust and excretion, promoted the recovery of gastrointestinal function, and improved patient satisfaction (26). Stimulation of P6 alone or combined with ST36 for 30 min before the lumbar anesthesia of cesarean section decreased PONV, and the mechanism is related to the reduction of plasma 5-HT5 concentration (27). Electroacupuncture at P6 and St36 together was reported to decrease period-dominant frequency in the electrogastrograph, and this effect was abolished by naloxone, indicating a central opioid pathway involvement (12).
It is worth noting that we enrolled the PONV high risk patients who had Apfel ≥ 3. The Apfel score includes female gender, non-smoker, history of motion sickness or PONV, and postoperative opioid use. In our study, 97.0% of patients were female. Besides accounting for a quarter of Apfel score, females were more likely to be non-smoking (28) and have motion sickness than males (29). This agreed with the previous epidemiological study, in which females accounted for 93.3% in Apfel score 3 population and 100% in Apfel score 4 population (30). Without any PONV prophylaxis, the estimated probability of PONV in Apfel ≥ 2 patients were between 39 and 78%, whereas if Apfel ≥ 3 is present, it may rise up to 78% (1). In addition, we enrolled high PONV risk surgical types. The laparoscopic gynecological operation and the laparoscopic cholecystectomy accounts for about 70 and 18% in our study, respectively (Table 1). Without any PONV prophylaxis, the PONV incidence of gynecological surgery and cholecystectomy surgery were 69 and 59.6% (31). When PONV prophylaxis with dexamethasone and tropisetron, the PONV incidence of laparoscopic gynecological surgery had been reported even up to 77.4% (32). In contrast, we found that the PONV incidence was much lower. What factors caused these discrepancies between previous studies and the current study remain unknown. However, the study heterogeneity was avoided in our study which may be one of reasons; for example, almost all patients were female and non-smokers and the SAS anxiety score were comparable among patients.
Limitations
The strengths of our trial are its large sample size, multiple centers, high PONV risk patients and surgeries, and the effectiveness of TEAS group in reducing PONV was clearly demonstrated. However, the unavoidable limitation in our study was that patients cannot be blinded to the intervention totally as they can sense the acupoint stimuli in the TEAS group whilst others in the sham group did not. As such, any “placebo” effects of TEAS are unknown. In addition, the balance anesthesia as a potential factor to reduce PONV, the non-standardization of anesthesia management, and the use of different regimens to prevent PONV during surgery may cause potential bias on the results. Antiemetic prophylactic medication used during our study remained similar but not identical due to different medical supplies in various centers. We have narrowed 5-HT3 receptor antagonists down to two specific drugs (palonosetron or tropisetron). Meanwhile, only 92.3% patients received dexamethasone, although there is no significant difference regarding dexamethasone usage between the TEAS group and Sham group (Table 2). Lastly, the sample size calculation was based on the previous publications without any antiemetic prophylaxis medication (15) which might induce sample size bias. However, our study is a large sample size and a multi-centers trial and, therefore, all those limitations were unlikely to have affected our conclusions.
Conclusions
This multi-center randomized controlled study concluded that the use of postoperative TEAS (at anesthesia recovery arrival and in the following morning) in conjunction with antiemetic prophylaxis may significantly reduce PONV incidence and its severity. Both TEAS and palonosetron were independent PONV risk protective factors for 24 h PONV incidence and cumulative PONV incidence. The combination of TEAS and palonosetron was the most effective strategy to reduce the PONV incidence. Our work may suggest that one should consider implementing TEAS clinically for patients' better and smoother postoperative recovery.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University (XJTU1AF2019LSK-084). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WG and QW: study conception, critically revised the manuscript for important intellectual content, and obtained funding. WG, LZ, XH, LW, JF, XZ, JZ, HW, QZ, CW, WC, XN, LY, RD, GW, and BL: acquisition or interpretation of data. WG and YL: statistical analyses. WG, LY, and QW: drafted the manuscript. WG, LZ, XH, LW, JF, XZ, JZ, HW, QZ, CW, WC, XN, LY, RD, GW, BL, and QW: provided administrative, technical, or material support.
Funding
This study was supported by the Clinical Research Award of the First Affiliated Hospital of Xi'an Jiaotong University, China (No. XJTU1AF2021CRF-012), the National Nature Science Foundation of China (Nos. 81971290 and 81771485), and the Key Research and Development Program of Shaanxi Province (2020SF-136).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Prof. Daqing Ma, MD, PhD, FRCA, MAE, Imperial College London, Chelsea and Westminster Hospital, London, UK for his critical comments during manuscript preparation. The authors thank the Department of Anesthesiology of the Second Hospital of Hebei Medical University, Department of Anesthesiology of Hebei Hospital of Traditional Chinese Medicine, Department of Anesthesiology of Xiyuan Hospital of China Academy of Chinese Medical Sciences, Department of Anesthesiology of Fujian Provincial Hospital, and Department of Anesthesiology of Ningxia Chinese Medicine Research Center for their guidance in protocol.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.766244/full#supplementary-material
Abbreviations
PONV, Postoperative nausea and vomiting; TEAS, transcutaneous electrical acupoint stimulation; QoR-40, the 40-item Quality of Recovery; P6, Neiguan; ST36, Zusanli; EX-LE5, Xiyan; 5-HT3, 5-hydroxytryptamine 3; PACU, postoperative recovery unit; VAS, visual analog scale score.
References
1. Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, Habib AS, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analgesia. (2020) 131:411–48. doi: 10.1213/ANE.0000000000004833
2. Schmitt ARM, Ritto FG, de Azevedo J, Medeiros PJD, de Mesquita MCM. Efficacy of gastric aspiration in reducing postoperative nausea and vomiting after orthognathic surgery: a double-blind prospective study. J Oral Maxillofac Surg. (2017) 75:701–8. doi: 10.1016/j.joms.2016.10.002
3. Apfel C, Korttila K, Abdalla M, Kerger H, Turan A, Vedder I, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. N Engl J Med. (2004) 350:2441–51. doi: 10.1056/NEJMoa032196
4. Chandrakantan A, Glass PS. Multimodal therapies for postoperative nausea and vomiting, and pain. Br J Anaesth. (2011) 107 (Suppl. 1):i27–40. doi: 10.1093/bja/aer358
5. Cao X, White PF, Ma H. An update on the management of postoperative nausea and vomiting. J Anesth. (2017) 31:617–26. doi: 10.1007/s00540-017-2363-x
6. Kovac AL. Comparative pharmacology and guide to the use of the serotonin 5-HT3 receptor antagonists for postoperative nausea and vomiting. Drugs. (2016) 76:1719–35. doi: 10.1007/s40265-016-0663-3
7. Gan T, White P, Scuderi P, Watcha M, Kovac A. FDA “black box” warning regarding use of droperidol for postoperative nausea and vomiting: is it justified? Anesthesiology. (2002) 97:287. doi: 10.1097/00000542-200207000-00059
8. Kovac A. Prevention and treatment of postoperative nausea and vomiting. Drugs. (2000) 59:213–43. doi: 10.2165/00003495-200059020-00005
9. Chen J, Zhang Y, Li X, Wan Y, Ji X, Wang W, et al. Efficacy of transcutaneous electrical acupoint stimulation combined with general anesthesia for sedation and postoperative analgesia in minimally invasive lung cancer surgery: a randomized, double-blind, placebo-controlled trial. Thorac Cancer. (2020). doi: 10.1111/1759-7714.13343
10. Zhou D, Hu B, He S, Li X, Gong H, Li F, et al. Transcutaneous electrical acupoint stimulation accelerates the recovery of gastrointestinal function after cesarean section: a randomized controlled trial. Evid Based Complement Alternat Med. (2018) 2018:7341920. doi: 10.1155/2018/7341920
11. Wang C, Chen X, Xie PY. Electroacupuncture at PC6 or ST36 influences the effect of tacrine on the motility of esophagus. Evid Based Complement Alternat Med. (2014) 2014:263489. doi: 10.1155/2014/263489
12. Streitberger K, Ezzo J, Schneider A. Acupuncture for nausea and vomiting: an update of clinical and experimental studies. Auton Neurosci. (2006) 129:107–17. doi: 10.1016/j.autneu.2006.07.015
13. Stoicea N, Gan TJ, Joseph N, Uribe A, Pandya J, Dalal R, et al. Alternative therapies for the prevention of postoperative nausea and vomiting. Front Med. (2015) 2:87. doi: 10.3389/fmed.2015.00087
14. Wang C, Zhou D, Shuai X, Liu J, Xie P. Effects and mechanisms of electroacupuncture at PC6 on frequency of transient lower esophageal sphincter relaxation in cats. World J Gastroenterol. (2007) 13:4873–80. doi: 10.3748/wjg.v13.i36.4873
15. Chen J, Tu Q, Miao S, Zhou Z, Hu S. Transcutaneous electrical acupoint stimulation for preventing postoperative nausea and vomiting after general anesthesia: a meta-analysis of randomized controlled trials. Int J Surg. (2020) 73:57–64. doi: 10.1016/j.ijsu.2019.10.036
16. Fu C, Wu T, Shu Q, Song A, Jiao Y. Acupuncture therapy on postoperative nausea and vomiting in abdominal operation: a Bayesian network meta analysis. Medicine. (2020) 99:e20301. doi: 10.1097/MD.0000000000020301
17. Dabbous A, Jabbour-Khoury S, Nasr V, Moussa A, Zbeidy R, Khouzam N, et al. Dexamethasone with either granisetron or ondansetron for postoperative nausea and vomiting in laparoscopic surgery. Middle East J Anaesthesiol. (2010) 20:565–70.
18. Nelson G, Altman AD, Nick A, Meyer LA, Ramirez PT, Achtari C, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: enhanced recovery after surgery (ERAS(R)) society recommendations–part I. Gynecol Oncol. (2016) 140:313–22. doi: 10.1016/j.ygyno.2015.11.015
19. Benevides ML, Oliveira SS, de Aguilar-Nascimento JE. The combination of haloperidol, dexamethasone, and ondansetron for prevention of postoperative nausea and vomiting in laparoscopic sleeve gastrectomy: a randomized double-blind trial. Obes Surg. (2013) 23:1389–96. doi: 10.1007/s11695-013-0923-1
20. Li Y, Wei X, Zhang S, Zhou L, Zhang J. A meta-analysis of palonosetron for the prevention of postoperative nausea and vomiting in adults. Journal of Perianesthesia Nurs. (2015) 30:398–405. doi: 10.1016/j.jopan.2015.05.116
21. Shu C, Yong CY, Chen H, Xiao C, Chao Z, Cheng T, et al. Response of gastric-related neurons in the hypothalamic paraventricular nucleus to acupuncture at Neiguan and Zusanli in a rat model of gastric distension. Chin J Tissue Eng Res. (2014) 18:675–80.
22. Cui Y, Wang L, Shi G, Liu L, Pei P, Guo J. Electroacupuncture alleviates cisplatin-induced nausea in rats. Acupunct Med. (2016) 34:120–6. doi: 10.1136/acupmed-2015-010833
23. Li Y, Zhu B, Rong P, Ben H, Li YH. Neural mechanism of acupuncture-modulated gastric motility. World J Gastroenterol. (2007) 13:709–16. doi: 10.3748/wjg.v13.i5.709
24. Lee A, Chan S, Fan L. Stimulation of the wrist acupuncture point PC6 for preventing postoperative nausea and vomiting. Cochrane Database Syst Rev. (2015) 2015:CD003281. doi: 10.1002/14651858.CD003281.pub4
25. Coloma M, White P, Ogunnaike B, Markowitz S, Brown P, Lee A, et al. Comparison of acustimulation and ondansetron for the treatment of established postoperative nausea and vomiting. Anesthesiology. (2002) 97:1387–92. doi: 10.1097/00000542-200212000-00009
26. Gu SH, Lang HB, Gan JH, Zheng ZW, Zhao F, Q T. Effect of transcutaneous electrical acupoint stimulation on gastrointestinal function recovery after laparoscopic radical gastrectomy – A randomized controlled trial - ScienceDirect. Eur J Integr Med. (2019) 26:11–7. doi: 10.1016/j.eujim.2019.01.001
27. El-Deeb AM, Ahmady MS. Effect of acupuncture on nausea and/or vomiting during and after cesarean section in comparison with ondansetron. J Anesth. (2011) 25:698–703. doi: 10.1007/s00540-011-1198-0
28. Wang YJ, Li ZX, Gu HQ, Zhai Y, Jiang Y, Zhao XQ, et al. China stroke statistics 2019: a report from the National Center for Healthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vasc Neurol. (2020) 5:211–39. doi: 10.1136/svn-2020-000457
29. Mucci V, Canceri JM, Brown R, Dai M, Yakushin SB, Watson S, et al. Mal de Debarquement syndrome: a retrospective online questionnaire on the influences of gonadal hormones in relation to onset and symptom fluctuation. Front Neurol. (2018) 9:362. doi: 10.3389/fneur.2018.00362
30. Canakci E, Catak T, Basar HE, Cebeci Z, Coskun I, Saltali AO, et al. Prevalence study for postoperative nausea vomiting: a training hospital example. Niger J Clin Pract. (2021) 24:1633–40. doi: 10.4103/njcp.njcp_399_20
31. Apfel C, Kranke P, Eberhart LJA. Comparison of surgical site and patient's history with a simplified risk score for the prediction of postoperative nausea and vomiting. Anaesthesia. (2004) 59:1078–82. doi: 10.1111/j.1365-2044.2004.03875.x
32. Xiong Q, Min S, Wei K, Yang Y, Ma J, Liu D, et al. Transcutaneous electrical acupoint stimulation combined with dexamethasone and tropisetron prevents postoperative nausea and vomiting in female patients undergoing laparoscopic sleeve gastrectomy: a prospective, randomized controlled trial. Obes Surg. (2021) 31:1912–20. doi: 10.1007/s11695-020-05205-9
Keywords: antiemetic drugs, laparoscopic non-gastrointestinal surgery, nausea, transcutaneous electrical stimulation, vomiting
Citation: Gao W, Zhang L, Han X, Wei L, Fang J, Zhang X, Zhang J, Wang H, Zhou Q, Wang C, Chen W, Ni X, Yang L, Du R, Wang G, Liu B, Li Y, Zhang S and Wang Q (2022) Transcutaneous Electrical Acupoint Stimulation Decreases the Incidence of Postoperative Nausea and Vomiting After Laparoscopic Non-gastrointestinal Surgery: A Multi-Center Randomized Controlled Trial. Front. Med. 9:766244. doi: 10.3389/fmed.2022.766244
Received: 28 August 2021; Accepted: 09 February 2022;
Published: 14 March 2022.
Edited by:
Ata Murat Kaynar, University of Pittsburgh, United StatesReviewed by:
Lei Zhao, Capital Medical University, ChinaAlberto A. Uribe, The Ohio State University, United States
Copyright © 2022 Gao, Zhang, Han, Wei, Fang, Zhang, Zhang, Wang, Zhou, Wang, Chen, Ni, Yang, Du, Wang, Liu, Li, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Wang, ZHIud2FuZ3FpYW5nQG1haWwueGp0dS5lZHUuY24=
 Wei Gao
Wei Gao Linzhong Zhang2
Linzhong Zhang2 Jiaqiang Zhang
Jiaqiang Zhang Haiyun Wang
Haiyun Wang Wenting Chen
Wenting Chen