Abstract
Background:
Gastric polyp is an abnormally proliferative or neoplastic growth of the gastric mucosa, with a degree of tendency to transform into gastric cancer. Lack of physical activity that is significantly related to low muscle mass (LMM) and muscle strength has been identified to be associated with gastric polyps. In this study, we examine the association of LMM and different histological types of gastric polyps among Chinese asymptomatic adult males.
Methods:
In total, 1,742 male adults undergoing bioelectrical impedance analysis and upper gastrointestinal endoscopies were included. Univariate and multivariate logistic regression models were used to analyze the effect of LMM on the risk of gastric polyps and different histological types.
Results:
By univariate and multivariate analyses, LMM (OR: 1.689, 95%CI: 1.046–2.726, p = 0.032) kept independent effect on risk of gastric polyps. When ratio of appendicular skeletal muscle mass to body mass index(ASM/BMI) was brought into the analyses, it was identified to be negatively correlated with occurrence of gastric polyps (OR: 0.744, 95%CI: 0.566–0.977, p = 0.033). For different pathological types, LMM showed different effect on occurrence of gastric polyps. LMM was an independent indicator for hyperplastic and inflammatory polyps (OR: 2.378, 95%CI: 1.288–4.389, p = 0.006), rather than fundic gland polyps (OR: 1.013, 95%CI: 0.473–2.173, p = 0.973).
Conclusion:
In general, LMM was an independent indicator for hyperplastic and inflammatory polyps occurrence in Chinese asymptomatic adult males.
Introduction
Gastric polyp is an abnormally proliferative or neoplastic growth of the gastric mucosa, protruding into the lumen of stomach (1), with a degree of tendency to transform into gastric cancer (2). The incidence of gastric polyps varies between 1 and 6% in individuals who undergo upper gastrointestinal endoscopies (3–6). The most common subtypes of gastric polyps are fundic gland polyp, hyperplastic or inflammatory polyp, and adenomatous polyp (7).
A number of variables have been identified to have an impact on the risk of gastric polyps, such as age, gender, the incidence of Helicobacter pylori infection, use of proton pump inhibitors (PPIs), and physical activity (8–10). Females have a higher risk of gastric polyps (11, 12), so males with gastric polyps have received little attention. Additionally, research by Wang et al. (10) proposed that lack of regular exercise was significantly associated with gastric polyps. Reduction in physical activity was significantly related to low muscle mass (LMM), namely sarcopenia (13). It was speculated that LMM might be associated with gastric polyps. However, there were no such research about the connection between LMM and gastric polyps. To address this issue, the aim of this study was to determine whether LMM was related to gastric polyps and different histological types in Chinese adult males.
Methods
We conducted a cross-sectional study of Chinese adult males having a health-check in the First Affiliated Hospital of Wenzhou Medical University between January 2018 and June 2018.
Study Population
Inclusion criteria included: (1) males aged 18 years or older and (2) individuals who underwent bioelectrical impedance analysis (BIA) and upper gastrointestinal endoscopies from January to June 2018.
Exclusion criteria included: (1) patients with histories of PPIs administration for more than 1 year (17 individuals were eliminated from the study), (2) patients with histories of stomach surgeries (10 individuals), and (3) patients who were diagnosed with gastric cancers after upper gastrointestinal endoscopies (6 individuals).
Data Collection
Baseline characteristic were collected by predesigned questionnaires, including age, gender, alcohol and smoking consumption, comorbidities, and histories of medication and surgery.
Smoking status was classified into 3 types: non-smoker (has never smoked before), past smoker (has quit for at least 2 yeas), and current smoker (is still smoking until the day of health-check or quit < 2 years). Males with alcohol consumption >140 g per week were regarded as heavy drinkers. Comorbidities were obtained by questionnaire and measurement, including hypertension (HTN), diabetes mellitus (DM), dyslipidemia, and metabolic syndrome (MS). The blood pressure measurement, blood detection, and 13C-urea breath test were performed on the morning of the health-check. The following blood measurements were tested: serum lipid profile, fasting blood glucose, and glycated hemoglobin.
The BIA and upper gastrointestinal endoscopy were performed in each individual. The appendicular skeletal muscle mass (ASM, kg) was measured by BIA (InBody770; InBody Korean Inc.). The ASM (kg)/BMI (kg/m2) was calculated to evaluate the muscle mass. According to the Foundation for the National Institutes of Health (FNIH) (14, 15), males with ASM/BMI <0.789 m2 were regarded as having low skeletal muscle mass. The endoscopic characteristics of polyps were recorded and analyzed, including existence, location, number, and size of pathological diagnosis. Helicobacter Pylori infection was detected by histopathological result of biopsied stomach specimen or 13C-urea breath test.
Statistical Analysis
Category variables were compared by Pearson χ2 tests and displayed as frequencies (percentages). The normal distribution of continuous variables was tested by Shapiro–Wilk test. Continuous variables were compared by Wilcox test and displayed as median (range). Univariate and multivariate logistic regression analyses were performed to determine the effect of LMM on gastric polyp occurrence, containing different pathological types. Variables, including age, smoking status, heavy drinking status, diabetes mellitus, hypertension, dyslipidemia, metabolic syndrome, Helicobacter Pylori infection, and LMM, were analyzed by univariate analysis. Variables with p < 0.1 in univariate analysis were incorporated into the subsequent multivariate analysis. In addition, we compared ASM/BMI between individuals with and without gastric polyps by Wilcox test.
All statistical analyses were carried out using R version 3.6.1 (https://www.r-project.org/). All analyses were two-tailed, with p < 0.05 considered significant.
Results
Baseline Characteristics
We included 1,742 males, 1,487 of whom were in the group without LMM and 255 of them in the group with LMM (Table 1). The median of ASM/BMI was 0.903, range from 0.555 to 1.934. One hundred and ten males were diagnosed with gastric polyps (Table 2), with an incidence rate of 6.31%. There were three pathological types: fundic gland polyps (N = 54), hyperplastic and inflammatory polyps (N = 53), and adenomatous polyps (N = 1). The histology was not available on two individuals because of unsuitable biopsy specimens.
Table 1
| Variables | Total (N = 1,742) | Individuals without LMM (N = 1,487) | Individuals with LMM (N = 255) | P-value |
|---|---|---|---|---|
| Age (years) | 46 (18–84) | 45 (18–78) | 50 (21–84) | <0.001 |
| Smoking (%) | <0.001 | |||
| Never | 1172 (67.28) | 1020 (68.59) | 152 (59.61) | |
| Past | 20 (1.15) | 12 (0.81) | 8 (3.14) | |
| Current | 550 (31.57) | 455 (30.60) | 95 (37.25) | |
| Heavy drinker (%) | 0.429 | |||
| Yes | 316 (18.14) | 265 (17.82) | 51 (20.00) | |
| No | 1426 (81.86) | 1222 (82.18) | 204 (80.00) | |
| Diabetes mellitus (%) | <0.001 | |||
| Yes | 179 (10.28) | 134 (9.01) | 45 (17.65) | |
| No | 1563 (89.72) | 1353 (90.99) | 210 (82.35) | |
| Hypertension (%) | <0.001 | |||
| Yes | 594 (34.10) | 470 (31.61) | 124 (48.63) | |
| No | 1148 (65.90) | 1017 (68.39) | 131 (51.37) | |
| Dyslipidemia (%) | 0.014 | |||
| Yes | 1147 (65.84) | 959 (64.49) | 188 (73.73) | |
| No | 591 (33.93) | 524 (35.24) | 67 (26.27) | |
| Missing | 4 (0.23) | 4 (0.27) | 0 (0.00) | |
| Metabolic syndrome (%) | <0.001 | |||
| Yes | 312 (17.91) | 230 (15.47) | 82 (32.16) | |
| No | 1430 (82.09) | 1257 (84.53) | 173 (67.84) | |
| Helicobacter Pylori infection (%) | 0.868 | |||
| Yes | 784 (45.01) | 666 (44.79) | 118 (46.27) | |
| No | 939 (53.90) | 804 (54.07) | 135 (52.94) | |
| Missing | 19 (1.09) | 17 (1.14) | 2 (0.78) |
Baseline characteristics between individuals with and without low skeletal muscle mass [median (range) or n (%)].
Table 2
| Variables | Total (N = 107) | Fundic gland polyps (N = 54) | Hyperplastic and inflammatory polyps (N = 53) | P-value |
|---|---|---|---|---|
| Age (years) | 49.0 (25.0–84.0) | 48.5 (25.0–84.0) | 49.0 (29.0–78.0) | 0.945 |
| Smoking (%) | 0.558 | |||
| Never | 73 (68.22) | 38 (70.37) | 35 (66.04) | |
| Past | 2 (1.86) | 0 (0.00) | 2 (3.77) | |
| Current | 32 (29.91) | 16 (29.63) | 16 (30.19) | |
| Heavy drinker (%) | 0.797 | |||
| Yes | 17 (15.89) | 8 (14.81) | 9 (16.98) | |
| No | 90 (84.11) | 46 (85.19) | 44 (83.02) | |
| Diabetes mellitus (%) | 1.000 | |||
| Yes | 11 (10.28) | 6 (11.11) | 5 (9.43) | |
| No | 96 (89.72) | 48 (88.89) | 48 (90.57) | |
| Hypertension (%) | 0.173 | |||
| Yes | 45 (42.06) | 19 (35.19) | 26 (49.06) | |
| No | 62 (57.94) | 35 (64.81) | 27 (50.94) | |
| Dyslipidemia (%) | 0.605 | |||
| Yes | 72 (67.29) | 38 (70.37) | 34 (64.15) | |
| No | 34 (31.78) | 16 (29.63) | 18 (33.96) | |
| Missing | 1 (0.93) | 0 (0.00) | 1 (1.89) | |
| Metabolic syndrome (%) | 0.639 | |||
| Yes | 22 (20.56) | 10 (18.52) | 12 (22.64) | |
| No | 85 (79.44) | 44 (81.48) | 41 (77.36) | |
| Helicobacter Pylori infection (%) | 0.012 | |||
| Yes | 25 (23.36) | 7 (12.96) | 18 (33.96) | |
| No | 82 (76.64) | 47 (87.04) | 35 (66.04) | |
| Low muscle mass (%) | 0.067 | |||
| Yes | 24 (22.43) | 8 (14.81) | 16 (30.19) | |
| No | 83 (77.57) | 46 (85.19) | 37 (69.81) | |
| Polyp location (%) | <0.001 | |||
| Antrum | 10 (9.35) | 0 (0.00) | 10 (18.87) | |
| Body | 60 (56.07) | 37 (68.52) | 23 (43.40) | |
| Fundus | 18 (16.82) | 13 (24.07) | 5 (9.43) | |
| Polysite | 9 (8.41) | 3 (5.56) | 6 (11.32) | |
| Other sites | 10 (9.35) | 1 (1.85) | 9 (16.98) | |
| Number of polyps | ||||
| Single | 77 (71.96) | 38 (70.37) | 39 (73.58) | 0.830 |
| Multiple* | 30 (28.04) | 16 (29.63) | 14 (26.42) | |
| Polyp size (cm) | ||||
| <0.5 | 89 (83.18) | 46 (85.19) | 43 (81.13) | 0.028 |
| ≥0.5 | 14 (13.08) | 4 (7.41) | 10 (18.87) | |
| undefined | 4 (3.74) | 4 (7.41) | 0 (0.00) |
Baseline characteristics according to different pathological type of gastric polyps [median (range) or n (%)].
In individuals with multiple polyps, size was based on the largest polyp.
Univariate and Multivariate Analyses for Gastric Polyps
As Table 3 showed, variables including HTN (odds ratio [OR]: 1.523, 95% confidence interval [CI]: 1.021–2.272, p = 0.039), Helicobacter Pylori infection (OR: 0.339, 95%CI: 0.216–0.534, p < 0.001), and LMM (OR:1.689, 95%CI: 1.046–2.726, p = 0.032) had an independent effect on the risk of gastric polyps.
Table 3
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Crode OR (95% CI) | P-value | Adjust OR (95% CI) | P-value | |
| Age, ≥50 vs. <50 | 1.511 (1.025–2.227) | 0.037 | 1.309 (0.875–1.959) | 0.191 |
| Smoking (%) | ||||
| Past vs. never | 1.649 (0.375–7.240) | 0.508 | ||
| Current vs. never | 0.978 (0.643–1.487) | 0.916 | ||
| Heavy drinker, yes vs. no | 0.876 (0.520–1.474) | 0.618 | ||
| Diabetes mellitus, yes vs. no | 1.184 (0.649–2.158) | 0.582 | ||
| Hypertension, yes vs. no | 1.540 (1.042–2.276) | 0.030 | 1.523 (1.021–2.272) | 0.039 |
| Dyslipidemia, yes vs. no | 1.146 (0.755–1.741) | 0.522 | ||
| Metabolic syndrome, yes vs. no | 1.378 (0.867–2.192) | 0.175 | ||
| Helicobacter Pylori infection, yes vs. no | 0.349 (0.222–0.548) | <0.001 | 0.339 (0.216–0.534) | <0.001 |
| Low muscle mass, yes vs. no | 1.792 (1.124–2.861) | 0.014 | 1.689 (1.046–2.726) | 0.032 |
Univariate and multivariate analyses for gastric polyps.
When ASM/BMI was brought into the analyses as a continuous variable (Supplementary Table 1), it was identified to be negatively correlated with the occurrence of gastric polyps in univariate (OR: 0.727, 95%CI: 0.557–0.948, p = 0.019) and multivariate analyses (OR: 0.744, 95%CI: 0.566–0.977, p = 0.033).
Univariate and Multivariate Analyses for Different Histological Types of Gastric Polyps
In univariate analysis (Supplementary Table 2), only Helicobacter Pylori infection (OR: 0.171, 95%CI: 0.077–0.380, p < 0.001; Supplementary Table 2) showed an association with risk of fundic gland polyps. It was inferred that LMM (OR: 1.013, 95%CI: 0.473–2.173, p = 0.973; Table 4) had no significant effect on the occurrence of fundic gland polyps.
Table 4
| LMM | Effect of LMM for fundic gland polyps | Effect of LMM for hyperplastic and inflammatory polyps | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Univariate analysis | 1.013 (0.473–2.173) | 0.973 | 2.620 (1.435–4.784) | 0.002 |
| Multivariate analysis | 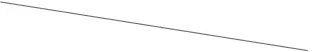 |  | 2.378 (1.288–4.389) | 0.006 |
Logistic Regression Analyses for the different histologic types in gastric polyps.
In univariate and multivariate analyses (Supplementary Table 3), compared with the group without LMM, there was an 137.8% rise in the risk of hyperplastic and inflammatory polyps in individuals with LMM (OR: 2.378, 95%CI: 1.288–4.389, p = 0.006; Table 4).
Different ASM/BMI in Individuals With and Without Gastric Polyps
As Figure 1 showed, individuals with and without gastric polyps displayed significantly different ASM/BMI (p = 0.032). Individuals with hyperplastic and inflammatory polyps showed relatively lower ASM/BMI (p = 0.010) compared to individuals without hyperplastic and inflammatory polyps. However, this association was not observed in fundic gland polyps (p = 0.959).
Figure 1
In individuals with age ≥ 50 (p = 0.028) and individuals without HP infection (p = 0.016), ASM/BMI was still significantly different between individuals with and without gastric polyps (Figure 2). Similarly, in individuals with age ≥ 50 (p = 0.028) and individuals without HP infection (p = 0.015), ASM/BMI was significantly higher in individuals without hyperplastic and inflammatory polyps (Figure 3).
Figure 2
Figure 3
Discussion
In this study, we found an association between LMM and increased occurrence of hyperplastic and inflammatory polyps in asymptomatic males. Up to now, there has been no research concerning the relation between reduced muscle mass and occurrence of gastric polyps. However, the inverse associations between physical activity and the presence of colorectal polyps, adenomas, and even cancer have been previously reported (16–21). A study by Wang et al. (10) mentioned that lack of physical exercise was significantly associated with gastric polyps. Physical inactivity was a major cause of sarcopenia, which manifested as reduced muscle mass and strength (22). There might be an underlying connection between low muscle mass, gastric polyps, and physical inactivity. A relevant mechanism study about the relationship is needed in the future.
The population enrolled in this study was Chinese asymptomatic adult males. Moreover, we included 1,100 females and discovered that there was no connection between LMM and gastric polyps with p-value of 0.168 (Supplementary Table 4). Sarcopenia and gastric polyps are more common in females (23, 24). But the correlation between low muscle mass and gastric polyps was found to exist in males in this study. It was inferred that sex hormones were responsible for the gender-specific difference. A further study about the underlying mechanism of gender-specific correlation between low muscle mass and gastric polyps is needed in the future.
The pathological types of gastric polyps in our study found to be associated with low muscle mass was hyperplastic or inflammatory polyps. It was caused by hyperplasia of foveolar cells accompanied by their increased exfoliation (25, 26). Gastric hyperplastic or inflammatory polyps commonly reside in gastric mucosa with longstanding Helicobacter Pylori infection-associated atrophy or metaplasia (25). In a study by Baeg et al. (27) it was discovered that Helicobacter Pylori eradication treatment increased ghrelin secretion and reduced the risk of low muscle mass. Thus, the correlation between gastric hyperplastic or inflammatory polyps and LMM might be Helicobacter Pylori infection-associated.
Muscle mass, which could be easily quantitatively detected by BIA or dual X-ray absorptiometry (DXA), is cost-effective and of great importance for the diagnosis of sarcopenia (14). It was necessary to detect the muscle mass before gastrointestinal endoscopies, which is predictive for hyperplastic and inflammatory polyps. In addition, enhanced physical exercise might be a preventive action to avoid possible gastric polyps to some extent. However, further prospective studies are required to determine the effect of physical activity on risk of gastric polyps.
Study Strengths and Limitations
It was cross-sectional study with large sample size, and there was a new-found association between low muscle mass and hyperplastic or inflammatory polyps. Detection of muscle mass prior to gastrointestinal endoscopies could help doctors with psychological preparation for corresponding removal of polyps.
Yet, there were some limitations to our study: (1) cross-sectional nature of the research, which could not prove causality; (2) a single-center analysis with ineluctable selective bias; (3) a lack of data of muscle strengthening, which caused failure to diagnosis individuals with sarcopenia; and (4) a lack of data on physical activity/exercise, which mediated the process of low muscle mass and gastric polyps to some extent.
Conclusion
In general, low muscle mass was a potential indicator for predicting occurrence of hyperplastic or inflammatory polyps, rather than fundic gland polyps. The ASM/BMI was negatively correlated with the occurrence of hyperplastic or inflammatory polyps.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The study was approved by the Institutional Review Board (IRB) of The First Affiliated Hospital of Wenzhou Medical University. Due to its cross-sectional nature, consent was waived by IRB and the data was anonymous. All data was extracted from electronic medical records and analyzed in accordance with the principles of the Declaration of Helsinki.
Author contributions
CL: data curation. MC: methodology. NW: writing—original draft. WL: writing—review and editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.861065/full#supplementary-material
References
1.
ParkDYLauwersGY. Gastric polyps: classification and management. Arch Pathol Lab Med. (2008) 132:633–40. 10.5858/2008-132-633-GPCAM
2.
WaldumHFossmarkR. Gastritis, gastric polyps and gastric cancer. Int J Mol Sci. (2021) 22:6548. 10.3390/ijms22126548
3.
CaoHWangBZhangZZhangHQuR. Distribution trends of gastric polyps: an endoscopy database analysis of 24 121 northern Chinese patients. J Gastroenterol Hepatol. (2012) 27:1175–80. 10.1111/j.1440-1746.2012.07116.x
4.
CarmackSWGentaRMSchulerCMSaboorianMH. The current spectrum of gastric polyps: a 1-year national study of over 120,000 patients. Am J Gastroenterol. (2009) 104:1524–32. 10.1038/ajg.2009.139
5.
Velázquez-DohornMELópez-DurandCFGamboa-DomínguezA. Changing trends in gastric polyps. Rev Invest Clin. (2018) 70:40–5. 10.24875/RIC.17002430
6.
FanNNYangJSunGLuZSLing HuEQWangXDet al. Changes in the spectrum of gastric polyps in the Chinese population. World J Gastroenterol. (2015) 21:9758–64. 10.3748/wjg.v21.i33.9758
7.
CarmackSWGentaRMGrahamDYLauwersGY. Management of gastric polyps: a pathology-based guide for gastroenterologists. Nat Rev Gastroenterol Hepatol. (2009) 6:331–41. 10.1038/nrgastro.2009.70
8.
JalvingMKoornstraJJWesselingJBoezenHMSDEJKleibeukerJH. Increased risk of fundic gland polyps during long-term proton pump inhibitor therapy. Aliment Pharmacol Ther. (2006) 24:1341–8. 10.1111/j.1365-2036.2006.03127.x
9.
ElhanafiSSaadiMLouWMallawaarachchiIDwivediA.ZuckermanMet al. Gastric polyps: Association with Helicobacter pylori status and the pathology of the surrounding mucosa, a cross sectional study. World J Gastrointest Endosc. (2015) 7:995–1002. 10.4253/wjge.v7.i10.995
10.
WangFWYoungSCChenRYLinKHChenYHHsuPIet al. The prevalence and risk factors of gastric polyp in asymptomatic patients receiving health examination. Gastroenterol Res Pract. (2018) 2018:9451905. 10.1155/2018/9451905
11.
ZhengENiSYuYWangYWengXZhengL. Impact of gender and age on the occurrence of gastric polyps: data analysis of 69575 southeastern Chinese patients. Turk J Gastroenterol. (2015) 26:474–9. 10.5152/tjg.2015.0099
12.
HuangHRongYWangMGuoZYuY.LongZet al. Analysis of gastroscopy results among healthy people undergoing a medical checkup: a retrospective study. BMC Gastroenterol. (2020) 20:412. 10.1186/s12876-020-01557-9
13.
Bowden DaviesKAPicklesSSprungVSKempGJAlamUMooreDRet al. Reduced physical activity in young and older adults: metabolic and musculoskeletal implications. Ther Adv Endocrinol Metab. (2019) 10:2042018819888824. 10.1177/2042018819888824
14.
ChenLKWooJAssantachaiPAuyeungTWChouMY.IijimaKet al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7.e2. 10.1016/j.jamda.2019.12.012
15.
ChoeYRJohJYKimYP. Clinically relevant cut-off points for the diagnosis of sarcopenia in older korean people. J Gerontol A Biol Sci Med Sci. (2017) 72:1724–31. 10.1093/gerona/glx052
16.
HeXWuKOginoSGiovannucciELChanATSongM. Association between risk factors for colorectal cancer and risk of serrated polyps and conventional adenomas. Gastroenterology. (2018) 155:355–73.e18. 10.1053/j.gastro.2018.04.019
17.
BrennerDRShawEYannitsosDHWarkentinMTBrocktonNTMcGregorSEet al. The association between recreational physical activity, sedentary time, and colorectal polyps in a population screened for colorectal cancer. Cancer Epidemiol. (2018) 53:12–20. 10.1016/j.canep.2017.12.017
18.
ØinesMHelsingenLMBretthauerM. Emilsson epidemiology L, and risk factors of colorectal polyps. Best Pract Res Clin Gastroenterol. (2017) 31:419–24. 10.1016/j.bpg.2017.06.004
19.
RosenbergLBoggsDWiseLAPalmerJRRoltschMHMakambiKHet al. A follow-up study of physical activity and incidence of colorectal polyps in African-American women. Cancer Epidemiol Biomarkers Prev. (2006) 15:1438–42. 10.1158/1055-9965.EPI-06-0079
20.
SongJHKimYSYangSYChungSJParkMJLimSHet al. Physical activity and other lifestyle factors in relation to the prevalence of colorectal adenoma: a colonoscopy-based study in asymptomatic Koreans. Cancer Causes Control. (2013) 24:1717–26. 10.1007/s10552-013-0247-4
21.
FriedenreichCMShawENeilsonHKBrennerDR. Epidemiology and biology of physical activity and cancer recurrence. J Mol Med (Berl). (2017) 95:1029–41. 10.1007/s00109-017-1558-9
22.
BoothFWRobertsCKLayeMJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. (2012) 2:1143–211. 10.1002/cphy.c110025
23.
LaurentMRDedeyneLDupontJMellaertsBDejaegerMGielenE. Age-related bone loss and sarcopenia in men. Maturitas. (2019) 122:51–6. 10.1016/j.maturitas.2019.01.006
24.
Argüello ViúdezLCórdovaHUchimaHSánchez-MontesCGinès ÀI.Araujoet al. Gastric polyps: Retrospective analysis of. 41,253 upper endoscopies. Gastroenterol Hepatol. (2017) 40:507–14. 10.1016/j.gastre.2017.01.007
25.
MarkowskiARMarkowskaAGuzinska-UstymowiczK. Pathophysiological and clinical aspects of gastric hyperplastic polyps. World J Gastroenterol. (2016) 22:8883–91. 10.3748/wjg.v22.i40.8883
26.
ArteagaCDWadhwaR. Gastric Polyp, in StatPearls. (2021). StatPearls Publishing Copyright © Treasure Island, FL: StatPearls Publishing LLC (2021).
27.
BaegMKChoiMGKoSHLimCHKimJSChoYKet al. Elderly women who received Helicobacter pylori-eradicating therapy have reduced risk of low skeletal muscle mass. Clin Interv Aging. (2015) 10:1771–7. 10.2147/CIA.S95007
Summary
Keywords
ASM/BMI, gastric polyps, Chinese asymptomatic adult males, BIA, upper gastrointestinal endoscopy, LMM
Citation
Wang N, Chen M, Lin C and Lin W (2022) Association Between Low Muscle Mass and Gastric Hyperplastic and Inflammatory Polyps in Chinese Asymptomatic Adult Males. Front. Med. 9:861065. doi: 10.3389/fmed.2022.861065
Received
24 January 2022
Accepted
08 March 2022
Published
06 May 2022
Volume
9 - 2022
Edited by
Pedro M. Baptista, Health Research Institute of Aragon (IIS Aragon), Spain
Reviewed by
Yuji Naito, Kyoto Prefectural University of Medicine, Japan; Ricardo Aurélio Carvalho Sampaio, Federal University of Sergipe, Brazil
Updates
Copyright
© 2022 Wang, Chen, Lin and Lin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weihong Lin ylbjzxlwh@163.com
This article was submitted to Gastroenterology, a section of the journal Frontiers in Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.