- 1Department of Emergency, Guang'anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Department of Critical Care Medicine, School of Clinical Medicine, Beijing Tsinghua Changgung Hospital, Tsinghua University, Beijing, China
Background: Targeted temperature management (TTM) is recommended in adult patients following cardiac arrest (CA) with any rhythm. However, as to non-shockable (NSR) CA, high-quality evidence of TTM supporting its practices remains uncertain. Thus, we aimed to conduct a systematic review and meta-analysis with randomized controlled trials (RCTs) to explore the efficacy and safety of TTM in this population.
Methods: We searched PubMed, Embase, and Cochrane library databases for potential trials from inception through Aug 25, 2021. RCTs evaluating TTM for CA adults due to NSR were included, regardless of the timing of cooling initiation. Outcome measurements were mortality and good neurological function. We used the Cochrane bias tools to evaluate the quality of the included studies. Heterogeneity, subgroup analyses, and sensitivity analysis were investigated to test the robustness of the primary outcomes.
Results: A total of 14 RCTs with 4,009 adults were eligible for the final analysis. All trials had a low to moderate risk of bias. Of the included trials, six compared NSR patients with or without TTM, while eight compared pre-hospital to in-hospital TTM. Pooled data showed that TTM was not associated with improved mortality (Risk ratio [RR] 1.00; 95% CI, 0.944–1.05; P = 0.89, I2 = 0%) and good neurological outcome (RR 1.18; 95% CI 0.90–1.55; P = 0.22, I2 = 8%). Similarly, use of pre-hospital TTM resulted in neither an improved mortality (RR 0.99, 95% CI 0.97–1.03; I2 = 0%, P = 0.32) nor favorable neurological outcome (RR 1.13, 95% CI 0.93–1.38; I2 = 0%, P = 0.22). These results were further confirmed in the sensitivity analyses and subgroup analyses.
Conclusions: Our results showed that using the TTM strategy did not significantly affect the mortality and neurologic outcomes in CA survival presenting initial NSR.
Introduction
Cardiac arrest (CA) is a common public health problem with an estimated annual incidence rate of 28–55 per 100,000 person-years (1). Despite the advances in cardiopulmonary resuscitation (CPR) technology, the overall mortality rate is still high, up to 90% (2). Target temperature management (TTM) has been considered as an effective therapy to improve the neurological prognosis of comatose CA survivors after the return of spontaneous circulation (ROSC) (3). The mechanism may be related to the decrease in core body temperature, which reduces inflammation and cell damage after ischemia-reperfusion injury, and promotes the brain neurons healing by reducing cerebral oxygen demand and intracranial pressure (4). Thus, the use of TTM in CA survivors had been recommended consistently by published CPR guidelines (3, 5). However, the guidelines were challenged by the most recent trial conducted by Dankiewicz et al. (6), which concluded that in patients with coma after out-of-hospital cardiac arrest, targeted hypothermia did not improve survival or neurologic good outcome rates.
CA is a highly heterogeneous entity. Many factors will affect the effect of sub-hypothermia. Among them are the two types of presenting ECG rhythm in CA patients: a shockable rhythm (SR, ventricular fibrillation, or ventricular tachycardia) or a non-shockable rhythm (NSR, asystole, or pulseless electrical activity) during CA have received the most attention (7). Currently, the guidelines recommend TTM for CA survivors with SR or NSR (3). However, compared with CA survivors with SR having conclusive evidence of TTM to support their use, studies focusing on TTM for NSR survivors have reported conflicting results (7–9). Most current clinical recommendations are based on the consensus of expert opinions and extrapolate the potential benefits of TTM in NSR patients from the evidence of SR survivors (3, 4).
Recently, several high-quality RCTs evaluating the effect of TTM in CA patients have been published (6, 8, 10–13), and most of them focus on the subgroup of NSR survivors. Therefore, with the aid of the increased power of meta-analytic techniques, we aimed to review the relevant and available RCTs to describe the effectiveness of TTM in CA survivors with initial NSR.
Methods
We conducted the current systematic review following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement (Appendix 1), and the review protocol had been published in the journal of Medicine (14).
Eligibility Criteria
Studies were considered eligible if they investigated the efficacy and safety of TTM strategy in CA survivors presenting an initial NSR were included, regardless of the methods (evaporative cooling, infusion of cold saline, and surface or systemic cooling), timing (in-hospital or pre-hospital cooling), duration of TTM, or targeted temperature (32–36°C). We excluded studies conducted in neonates, children, pregnant, and studies that did not report data on survival. In addition, articles in abstract form without predefined data available or reviews or case series were also excluded.
Search Strategy
We conducted a comprehensive systematic electronic search through PubMed, Cochrane library, and Embase databases from inception to Aug 15, 2021 (the last search) for potential RCTs, without language restriction. Boolean terms (OR and AND), Medical Subject Headings (MeSH), Emtree, and keywords were used in the search strategy. The search terms included “targeted temperature management,” “Therapeutic hypothermia,” “advanced cardiac life-support,” “cardiac arrest,” “cardiopulmonary resuscitation,” and “heart arrest.” The details of the research strategy was summarized in Appendix 2. Reference lists of relative articles were also manually checked to ensure the inclusion of all possible publications on this topic.
Data Extraction and Quality Assessment
Two reviewers (Y-BZ and YY) extracted data independently from included studies on the first author' last name, publication year, study design (blinding or open-label; single or multi-centers), country where the study was conducted, study period, sample size, therapeutic regimens, follow-up duration, patient characteristics as well as all predefined outcomes. We appraised the risk of bias of the included RCTs using the Cochrane Collaboration tool for assessing the risk of bias (15). Discrepancies were identified and resolved through discussion.
Outcome Measures
The primary outcome measure was mortality (considering the longest follow-up reported by the authors). The secondary outcome was good neurological function defined as a Cerebral Performance Category (CPC) score of 1 or 2. If trials only reported good neurological recovery, we considered this outcome to be CPC 1 or CPC 2 (16).
Statistical Analysis
We pooled categorical data with risk ratios (RR) and continuous data with the mean difference (MD) using the Mantel-Haenszel, Inverse Variance fixed-effect, or Der Simonian and Laird random-effects model if needed. Some studies reported median as the measure of treatment effect, with accompanying interquartile range (IQR). Before data analysis, we estimated mean from median and standard deviations (SD) from IQR using the methods described in previous studies (17). Heterogeneity was quantified using the I2 statistic and its P-value. Studies with an I2 > 50% indicate significant heterogeneity (18).
To obtain more robust results, we estimated the pooled effect with their 95% CI if at least three studies with sufficient data available in each predefined outcome. Sensitivity analyses were performed by excluding trials that potentially biased the results. We further conducted subgroup analyses to test the robustness of the primary outcomes basing on the important clinical features (i.e., follow-up [short-term or long-term mortality; short-term mortality was defined as 28 days, ICU or hospital mortality, or mortality within 90 days of randomization, while long-term mortality was defined as a mortality rate of more than 180 days], by-stander CPR% [<50% or ≥50%], sample size [≥200 or <200], design [single-center or multi-centers], and OHCA% [100% or <100%]). Publication bias was evaluated by visually inspecting funnel plots. A two-sided P < 0.05 was considered statistically significant. All statistical analyses were performed using Review Manager, Version 5.3.
Results
Trial Identification
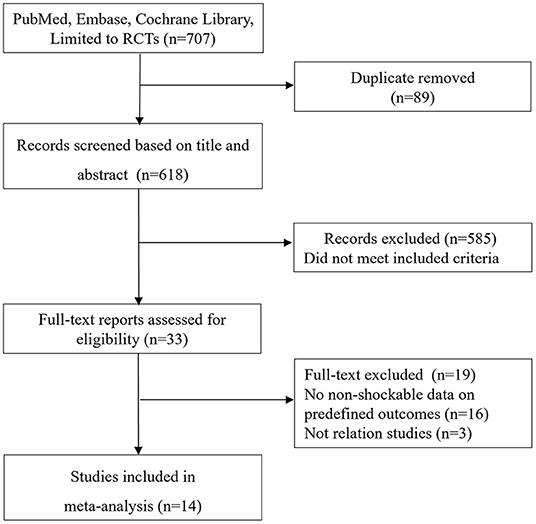
The de-duplicated results yielded 707 abstracts. The screen of abstracts and titles identified a total of 34 relevant studies for the following full-text review. Figure 1 shows a flowchart for the selection of studies. The excluded studies based on the full-text evaluation with exclusion reasons were summarized in Appendix 2. Finally, 14 RCTs (6, 10–13, 19–27) met the study inclusion criteria, of which six (6, 10, 11, 19–21) were comparing NSR patients with or without TTM while eight (12, 13, 22–27) were comparing NSR patients receiving pre-hospital or in-hospital TTM.
Quality of the Studies
The quality of the included RCTs was low to moderate risk of bias (Appendix 3). However, funnel plots did not show skewed distributions, suggesting no publication bias was involved (Appendix 4).
Study Characteristics
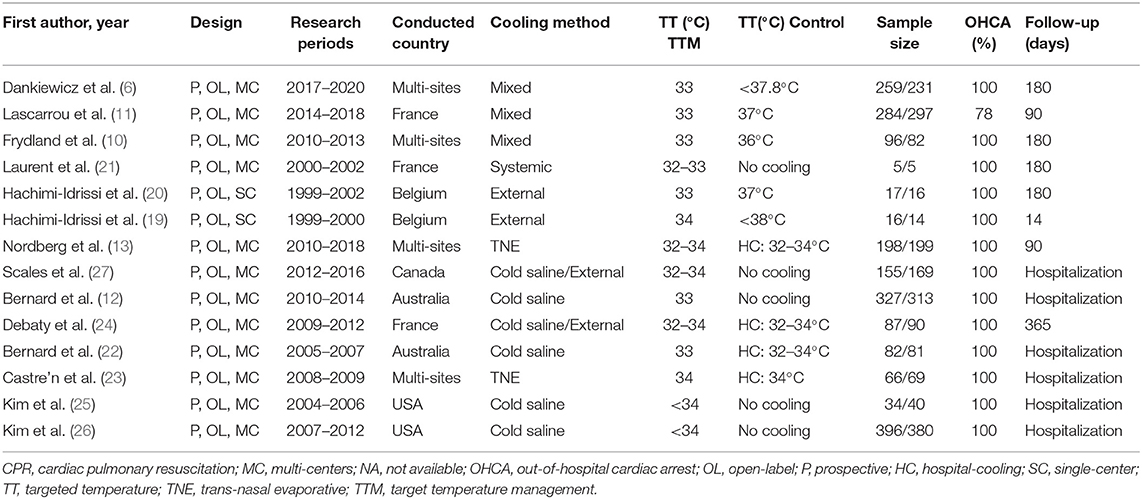
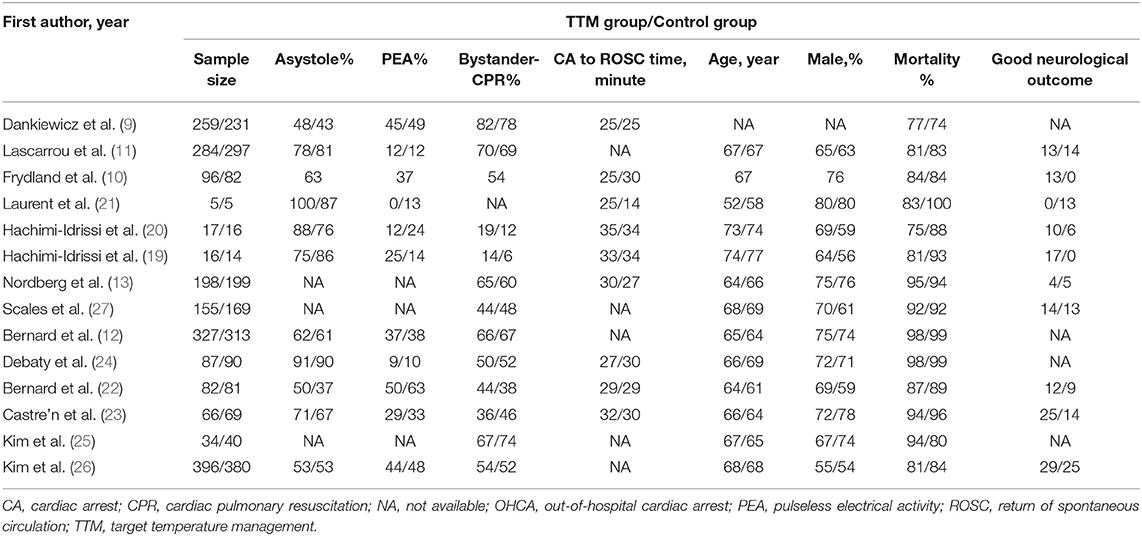
Tables 1, 2 shows the characteristics of the 14 included RCTs. Of these studies, 2 were single-center trials (19, 20), and 12 were multi-center trials (6, 10–13, 21–27). These RCTs were published between 2007 and 2021 from the France (n = 3), USA (n = 2), Australia (n = 2), Belgium (n = 2), Canada (n = 1), and multi-site (n = 4). A total of 4,009 NSR survivors were included in the final analysis (sample size ranging from 10 to 776 patients), with 2,022 patients in the TTM group and 1,987 patients in the non-TTM group. As to the initial rhythm, 11 RCTs included patients with SR or NSR (6, 12, 13, 19–21, 23–27), while the remaining three included only patients with NSR (10, 11, 22). Cooling methods varied among the RCTs, such as trans-nasal evaporative cooling, infusion of cold saline, and surface or systemic cooling.
Outcomes
With or Without TTM
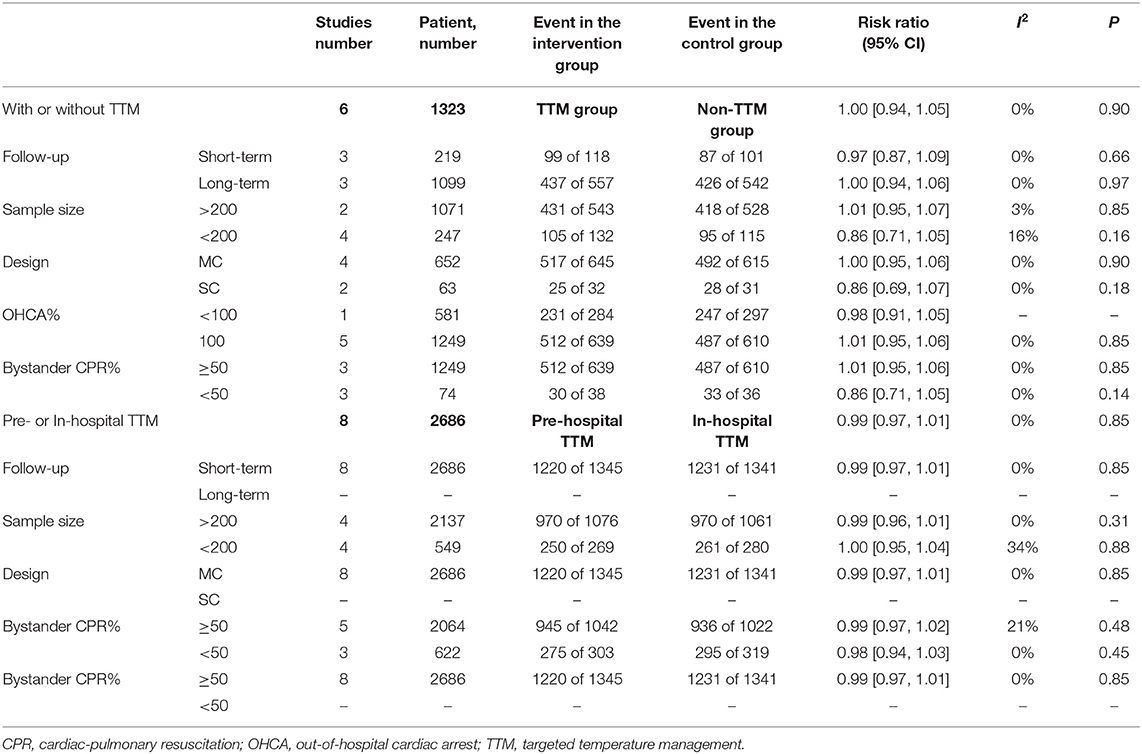
Six studies compared NSR survivors with or without TTM, and all reported outcomes of mortality (6, 10, 11, 19–21). Of the 677 patients in the TTM group, 542 died, compared to 520 of 646 patients in the non-TTM group. The pooled analysis suggested TTM did not affect the mortality (RR = 1.00; 95% CI, 0.944–1.05; P = 0.89, I2 = 0%) (Figure 2A). Five studies focused on neurological outcomes as interests (10, 11, 19–21). Pooled analysis showed the good neurological outcome was comparable between the TTM group and non-TTM groups (n = 1,232; RR 1.39; 95% CI 0.92–2.11; P = 0.11, I2 = 0%) (Figure 2B).
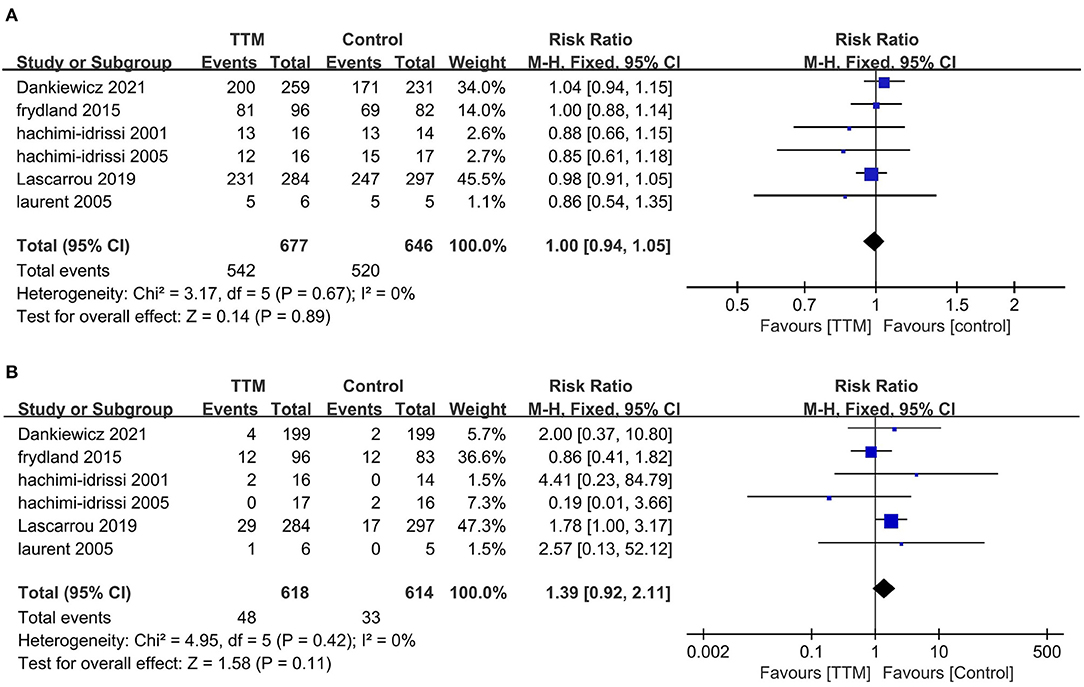
Figure 2. Forest plot of the non-shockable rhythm survivors with or without targeted temperature management. (A) Mortality; (B) Good neurological outcome.
In the sensitivity analysis, excluding any single test did not significantly change the overall combined OR of the survival (P-value ranged from 0.21 to 0.87) and neurological outcomes (P-value ranged from 0.19 to 0.98). Similarly, subgroup analyses based on study design, sample size, country, or initial rhythm showed no differences in the survival outcomes and good neurological outcomes (Table 2) between the TTM and non-TTM groups (Table 3).
Pre-hospital or In-hospital TTM
All eight trials reported outcome of mortality (1,345 in pre-hospital group and 1,341 in in-hospital group) (12, 13, 22–27). The pooled mortality rate was similar when we compared the pre-hospital TTM group with the in-hospital TTM group (8 trials, N = 2,682; RR 0.99, 95% CI 0.97–1.01, I2 = 0) (Figure 3A). Five RCTs focused on neurological outcomes as interests. When pooled, the result showed no difference in favorable neurological outcome (6 trials, N = 1,955; RR 1.13, 95% CI 0.93–1.18, I2 = 0) (Figure 3B).
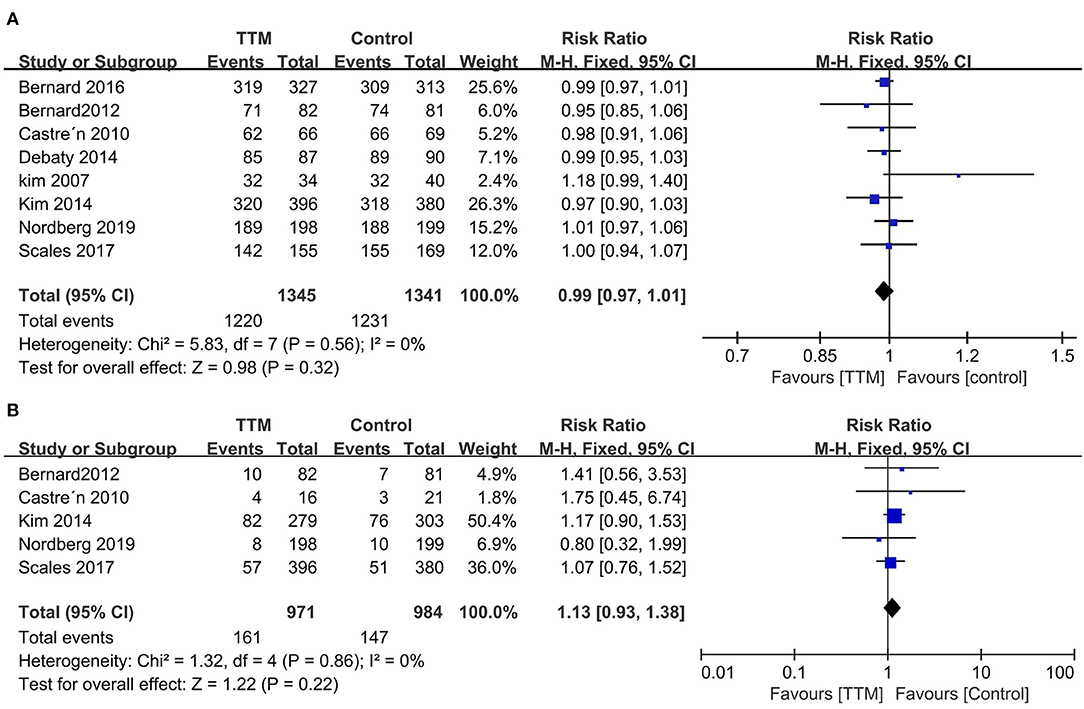
Figure 3. Forest plot of the targeted temperature management used in pre-hospital or in-hospital survivors with non-shockable rhythm. (A) Mortality; (B) Good neurological outcome.
Discussion
In the current meta-analysis, we included 14 RCTs focusing on CA survivors with NSR to evaluate the prognosis of TTM for these patients. Most included studies were low to medium risk of bias in quality. Our results showed that TTM did not significantly improve survival or neurological outcomes in CA patients with NSR than those who did not. Meanwhile, early starting cooling strategies such as prehospital TTM showed no more benefits in such a patient population. These results were further supported by subgroup analysis and sensitivity analysis.
Comparison With Previous Studies
Our results showed that many CA survivors with NSR received TTM despite no high-quality evidence to support its use. This may be due to the recommendations of the CPR guidelines (3, 5), which had consistently recommended TTM for CA survivors with both SR and NSR. However, the recommendation for the NSR patients is mainly based on an extrapolation of SR patients but no clear evidence. The latest 2021 guideline has been modified to suggest TTM for adult CA patients with initial NSR who remain unresponsive after ROSC (3). However, it is still a weak recommendation and is from very low-quality evidence.
So far, there have been 3 meta-analyses published on this topic with inconsistent results (7–9). However, the main problem of these articles was the inclusion of most observational studies but only a small number of RCTs, leading to a high risk of bias and random error in these studies. To address the limitations of the previous meta-analysis, we only included RCTs and added several recent published trials, with a total sample size of 4,009 patients in the current study (6, 10–13, 19–27). Thus, our sample size allowed for better statistical power and other sensitivities and subgroup analyses. The results of subgroup and sensitivity analyses for ICHA initial rhythm, study start date, study design, OHCA%, and recent and long-term prognosis corroborated the robustness of our findings. Thus, our results provide conclusive evidence regarding the impact of TTM on mortality and neurologic outcomes of CA survivors with initial NSR.
It is also worth noting that the International Liaison Committee on Resuscitation (ILCOR) recently published a systematic review that there was no improvement in survival or favorable neurologic outcome in TTM groups compared with normothermia groups. The prehospital cooling groups also showed no benefits in survival or favorable neurologic outcome in comparison with on prehospital cooling groups. These findings may warrant an update of international cardiac arrest guidelines (28).
Explaining Our Findings
We found TTM did not benefit CA patients with initial NSR concerning the mortality and neurological prognosis. Some might contribute to this. On the one hand, NSR has a marked impact on the CA prognosis, not only the reduced chances of obtaining ROSC but also the chances of surviving hospital discharge. The CA patients' leading cause of death is the neurological injury from anoxic brain damage, independent of initial rhythm (29, 30). The potential mechanism of TTM included anti-oxidant, anti-apoptotic and anti-inflammatory effects and a decrease in the accumulation or release of excitotoxic amino acids (4, 28, 31). The positive results from animal studies and trials led to the inclusion of TTM in the guidelines (32, 33). However, NSR survivors have more poor prognostic factors than those of SR (26, 34). As shown in our results, NSR survivors were usually older, had more comorbidities, and suffered an increased risk of multiple organ dysfunction. Thus, those risks might partly weaken the advantages of TTM application.
On the other hand, the included studies spanned an extensive range of periods, during which CPR and CA guidelines have been updated several times (3, 5). CA prognosis might benefit from several improved techniques such as bystander intervention, advanced cardiac life support, emergency cardiac catheterization, and optimal support for brain functions (3). Therefore, these techniques may limit the theoretical benefits of TTM to reduce free radical-mediated reperfusion injury in hypoxic brain injury.
Additionally, the prehospital cooling strategy also showed no more prognosis benefits in NSR patients after CA than those receiving TTM after hospital arrival. Several reasons might help explain the negative findings. First, prehospital cooling patients have more re-arrest episodes and pulmonary edema due to the infusion of a large amount of cold intravenous saline immediately after ROSC (26). Meanwhile, the rapid infusion can also cause an increase in right atrium pressure, which may reduce coronary perfusion pressure and, therefore, myocardial perfusion (35). Second, the drop in temperature by rapid infusion of cold saline might be too slight. As shown in the study by Bernard et al., the authors found no differences in outcomes when cooling was initiated before hospital arrival between the groups (12). Third, prehospital cooling did not significantly reduce the time to targeted temperature. Scales et al. (27) reported that achieving a target temperature of <34°C within 6-h of hospital arrival was not significantly different between CA survivors with or without prehospital cooling patients. In addition, studies have shown that lower myocardial temperature increases the rate of successful defibrillation. However, this does not affect patients with NSR (36).
Research Limitations
Our study has several limitations. First, all of the included RCTs are open-label designs. This might result in the selection bias of our research. Second, CA is a highly heterogeneous entity, and many factors may affect the efficacy of TTM, such as the cooling methods, sedative drugs, timing, and shivering monitoring methods. Third, although we had used subgroup analyses and sensitivity analysis to explore the possible confounding factors, our results may be affected by unmeasured factors. Fourth, prognostic assessment methods varied among the included studies. Some clinicians chose telephone interviews rather than face-to-face interviews. Fifth, the non-TTM CA survivors varied in the temperature management, such as target 36°C to <38°C or no cooling, which may affect the robustness of our conclusions. Finally, the included CA patients have different underlying diseases, demographic characteristics and use different disease severity scoring standards. However, due to the number of studies, we cannot further perform subgroup analysis to clarify this point.
Conclusions
Among patients with NSR, the use of TTM showed no more benefits than usual care in survival and favorable neurological outcomes. However, our results were based on observational findings and warranted a randomized clinical trial to assess the efficacy of TTM for such a patient population.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
Y-BZ contributed to conception and design and drafted the manuscript. YY and Y-BZ contributed to searching the scientific literature and data interpretation. J-ZF and YR helped to collect the data and performed statistical analyses. H-BH was responsible for the integrity of the work as a whole, from inception to publication of the article. All authors read and approved the manuscript.
Funding
This research was supported by the Science and Technological Innovation Project of China Academy of Chinese Medical Sciences (CACMS) Innovation Fund (CI2021A02904).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors want to thank Daxing Yu (Guang'anmen Hospital) and the CACMS Innovation Fund (CI2021A02904) for the support of this research.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.910560/full#supplementary-material
Abbreviations
CA, cardiac arrest; CI, confidence interval; ICU, intensive care unit; IHCA, in-hospital cardiac arrest; ILCOR, International Liaison Committee on Resuscitation; IQR, interquartile range; LOS, length of stay; MD, mean difference; NSR, non-shockable rhythm; OHCA, out-of-hospital cardiac arrest; ROSC, return of spontaneous circulation; RR, risk ratio; RCTs, randomized controlled trials; SD, standard deviations; SR, shockable rhythm; TTM, targeted temperature management.
References
1. Berdowski J, Berg RA, Tijssen JG, Koster RW. Global incidences of out-of-hospital cardiac arrest and survival rates: systematic review of 67 prospective studies. Resuscitation. (2010) 81:1479–87. doi: 10.1016/j.resuscitation.2010.08.006
2. Wissenberg M, Lippert FK, Folke F, Weeke P, Hansen CM, Christensen EF, et al. Association of national initiatives to improve cardiac arrest management with rates of bystander intervention and patient survival after out-of-hospital cardiac arrest. JAMA. (2013) 310:1377–84. doi: 10.1001/jama.2013.278483
3. Nolan JP, Sandroni C, Böttiger BW, Cariou A, Cronberg T, Friberg H, et al. European Resuscitation Council and European Society of intensive care medicine guidelines 2021. post-resuscitation care. Resuscitation. (2021) 161:220–69. doi: 10.1016/j.resuscitation.2021.02.012
4. Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. (2012) 13:267–78. doi: 10.1038/nrn3174
5. Donnino MW, Andersen LW, Berg KM, Reynolds JC, Nolan JP, Morley PT, et al. Temperature management after cardiac arrest: an advisory statement by the advanced life support task force of the International Liaison Committee on Resuscitation and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Circulation. (2015) 132:2448–56. doi: 10.1161/CIR.0000000000000313
6. Dankiewicz J, Cronberg T, Lilja G, Jakobsen JC, Levin H, Ullen S, et al. Hypothermia versus normothermia after out-of-hospital cardiac arrest. N Engl J Med. (2021) 384:2283–94. doi: 10.1056/NEJMoa2100591
7. Osman M, Munir MB, Regner S, Osman K, Benjamin MM, Kheiri B, et al. Induced hypothermia in patients with cardiac arrest and a non-shockable rhythm: meta-analysis and trial sequential analysis. Neurocrit Care. (2021) 34:279–86. doi: 10.1007/s12028-020-01034-x
8. Song L, Wei L, Zhang L, Lu Y, Wang K, Li Y. The role of targeted temperature management in adult patients resuscitated from nonshockable cardiac arrests: an updated systematic review and meta-analysis. Biomed Res Int. (2016) 2016:2350974. doi: 10.1155/2016/2350974
9. Barbarawi M, Alabdouh A, Barbarawi O, Lakshman H, Alkasasbeh M, Rizk F, et al. Targeted temperature management in cardiac arrest patients with an initial non-shockable rhythm: a systematic review and meta-analysis. Shock. (2020) 54:623–30. doi: 10.1097/SHK.0000000000001550
10. Frydland M, Kjaergaard J, Erlinge D, Wanscher M, Nielsen N, Pellis T, et al. Target temperature management of 33 degrees C and 36 degrees C in patients with out-of-hospital cardiac arrest with initial non-shockable rhythm - a TTM sub-study. Resuscitation. (2015) 89:142–8. doi: 10.1016/j.resuscitation.2014.12.033
11. Lascarrou J-B, Merdji H, Le Gouge A, Colin G, Grillet G, Girardie P, et al. Targeted temperature management for cardiac arrest with nonshockable rhythm. N Engl J Med. (2019) 381:2327–37. doi: 10.1056/NEJMoa1906661
12. Bernard SA, Smith K, Finn J, Hein C, Grantham H, Bray JE, et al. Induction of therapeutic hypothermia during out-of-hospital cardiac arrest using a rapid infusion of cold saline: the RINSE Trial (Rapid Infusion of Cold Normal Saline). Circulation. (2016) 134:797–805. doi: 10.1161/CIRCULATIONAHA.116.021989
13. Nordberg P, Taccone FS, Truhlar A, Forsberg S, Hollenberg J, Jonsson M, et al. Effect of trans-nasal evaporative intra-arrest cooling on functional neurologic outcome in out-of-hospital cardiac arrest: the PRINCESS Randomized Clinical Trial. JAMA. (2019) 321:1677–85. doi: 10.1001/jama.2019.4149
14. Zhu Y, Huang H, Feng J, Ren Y, Li W. Therapeutic hypothermia for cardiac arrest due to non-shockable rhythm: a protocol for systematic review and meta-analysis. Medicine. (2020) 99:e21452. doi: 10.1097/MD.0000000000021452
15. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
16. Becker LB, Aufderheide TP, Geocadin RG, Callaway CW, Lazar RM, Donnino MW, et al. Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association. Circulation. (2011) 124:2158–77. doi: 10.1161/CIR.0b013e3182340239
17. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
19. Hachimi-Idrissi S, Corne L, Ebinger G, Michotte Y, Huyghens L. Mild hypothermia induced by a helmet device: a clinical feasibility study. Resuscitation. (2001) 51:275–81. doi: 10.1016/S0300-9572(01)00412-9
20. Hachimi-Idrissi S, Zizi M, Nguyen DN, Schiettecate J, Ebinger G, Michotte Y, et al. The evolution of serum astroglial S-100 beta protein in patients with cardiac arrest treated with mild hypothermia. Resuscitation. (2005) 64:187–92. doi: 10.1016/j.resuscitation.2004.08.008
21. Laurent I, Adrie C, Vinsonneau C, Cariou A, Chiche JD, Ohanessian A, et al. High-volume hemofiltration after out-of-hospital cardiac arrest: a randomized study. J Am Coll Cardiol. (2005) 46:432–7. doi: 10.1016/j.jacc.2005.04.039
22. Bernard SA, Smith K, Cameron P, Masci K, Taylor DM, Cooper DJ, et al. Induction of prehospital therapeutic hypothermia after resuscitation from nonventricular fibrillation cardiac arrest*. Crit Care Med. (2012) 40:747–53. doi: 10.1097/CCM.0b013e3182377038
23. Castren M, Nordberg P, Svensson L, Taccone F, Vincent JL, Desruelles D, et al. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation. (2010) 122:729–36. doi: 10.1161/CIRCULATIONAHA.109.931691
24. Debaty G, Maignan M, Savary D, Koch FX, Ruckly S, Durand M, et al. Impact of intra-arrest therapeutic hypothermia in outcomes of prehospital cardiac arrest: a randomized controlled trial. Intensive Care Med. (2014) 40:1832–42. doi: 10.1007/s00134-014-3519-x
25. Kim F, Olsufka M, Longstreth WT, Maynard C, Carlbom D, Deem S, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4°C normal saline. Circulation. (2007) 115:3064–70. doi: 10.1161/CIRCULATIONAHA.106.655480
26. Kim F, Nichol G, Maynard C, Hallstrom A, Kudenchuk PJ, Rea T, et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. JAMA. (2014) 311:45–52. doi: 10.1001/jama.2013.282173
27. Scales DC, Cheskes S, Verbeek PR, Pinto R, Austin D, Brooks SC, et al. Prehospital cooling to improve successful targeted temperature management after cardiac arrest: a randomized controlled trial. Resuscitation. (2017) 121:187–94. doi: 10.1016/j.resuscitation.2017.10.002
28. Granfeldt A, Holmberg MJ, Nolan JP, Soar J, Andersen LW, International Liaison Committee on Resuscitation (ILCOR) Advanced Life Support Task Force. Targeted temperature management in adult cardiac arrest: systematic review and meta-analysis. Resuscitation. (2021) 167:160–72. doi: 10.1016/j.resuscitation.2021.08.040
29. Dragancea I, Rundgren M, Englund E, Friberg H, Cronberg T. The influence of induced hypothermia and delayed prognostication on the mode of death after cardiac arrest. Resuscitation. (2013) 84:337–42. doi: 10.1016/j.resuscitation.2012.09.015
30. Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. (2004) 30:2126–8. doi: 10.1007/s00134-004-2425-z
31. Sun YJ, Zhang ZY, Fan B, Li GY. Neuroprotection by therapeutic hypothermia. Frontiers Neurosci. (2019) 13:586. doi: 10.3389/fnins.2019.00586
32. American Heart Association. Part 7.5. postresuscitation support. Circulation. (2005) 112:IV-84-IV-8. doi: 10.1161/CIRCULATIONAHA.105.166560
33. Nolan JP, Deakin CD, Soar J, Bottiger BW, Smith G. European resuscitation council guidelines for resuscitation 2005. Resuscitation. (2005) 67:S39–86. doi: 10.1016/j.resuscitation.2005.10.009
34. Donnino MW, Andersen LW, Giberson T, Gaieski DF, Abella BS, Peberdy MA, et al. Initial lactate and lactate change in post-cardiac arrest: a multicenter validation study. Crit Care Med. (2014) 42:1804–11. doi: 10.1097/CCM.0000000000000332
35. Yannopoulos D, Zviman M, Castro V, Kolandaivelu A, Ranjan R, Wilson RF, et al. Intra-cardiopulmonary resuscitation hypothermia with and without volume loading in an ischemic model of cardiac arrest. Circulation. (2009) 120:1426–35. doi: 10.1161/CIRCULATIONAHA.109.848424
Keywords: non-shockable rhythm, cardiac arrest, targeted temperature management, neurological outcome, meta-analysis
Citation: Zhu Y-B, Yao Y, Ren Y, Feng J-Z and Huang H-B (2022) Targeted Temperature Management for Cardiac Arrest Due to Non-shockable Rhythm: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Med. 9:910560. doi: 10.3389/fmed.2022.910560
Received: 01 April 2022; Accepted: 16 May 2022;
Published: 03 June 2022.
Edited by:
Elisa Damiani, Università Politecnica delle Marche, ItalyReviewed by:
Roberta Domizi, Ospedali Riuniti Umberto I, ItalyArtem N. Kuzovlev, Research Institute General Resuscitation in V.A. Negovskogo, Russia
Copyright © 2022 Zhu, Yao, Ren, Feng and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-Bin Huang, aGhiYTAyOTIyQGJ0Y2guZWR1LmNu
†These authors have contributed equally to this work
 Yi-Bing Zhu
Yi-Bing Zhu Yan Yao
Yan Yao Yu Ren
Yu Ren Jing-Zhi Feng
Jing-Zhi Feng Hui-Bin Huang
Hui-Bin Huang