- Department of Anesthesiology, Shengjing Hospital, China Medical University, Shenyang, China
Objectives: Erector spinae plane block (ESPB) has been used for many thoracic and abdominal surgeries. However, evidence of its analgesic efficacy following abdominal surgery, compared with that of thoracic analgesia, is insufficient. Our study explored the analgesic effect of ESPB after abdominal surgery.
Methods: We searched PubMed, Embase, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov. Primary outcomes were pain scores at 6, 12 and 24 h and 24-h opioid consumption. Secondary outcomes included time to first rescue analgesia, length of hospital stay, and incidence of postoperative nausea and vomiting (PONV). We calculated standardized mean differences (SMDs) with 95% confidence intervals (CIs) for primary outcomes and mean differences (MDs) and risk ratios (RRs) with 95% CIs for secondary outcomes.
Results: We systematically included 1,502 cases in 24 trials. Compared with placebo, ESPB significantly reduced pain scores at 6 h (SMD −1.25; 95% CI −1.79 to −0.71), 12 h (SMD −0.85; 95% CI −1.33 to −0.37) and 24 h (SMD −0.84; 95% CI −1.30 to −0.37) and 24-h opioid consumption (SMD −0.62; 95% CI −1.19 to −0.06) post-surgery. ESPB prolonged the time to first rescue analgesia and decreased the incidence of PONV. Compared with transversus abdominal plane block (TAPB), ESPB significantly reduced pain scores at 6, 12, and 24 h and 24-h opioid consumption and prolonged the time to first rescue analgesia postsurgically. Furthermore, subgroup analysis showed that ESPB significantly reduced pain scores at various time points and opioid consumption within 24 h after laparoscopic cholecystectomy, percutaneous nephrolithotomy and bariatric surgery.
Conclusion: Compared with placebo, ESPB improves the postoperative analgesic efficacy after abdominal surgery. Furthermore, our meta-analysis confirmed that ESPB provides more beneficial analgesic efficacy than TAPB.
Systematic review registration: [https://www.crd.york.ac.uk/PROSPEROFILES/301491_STRATEGY_20220104.pdf], identifier [CRD42022301491].
Introduction
Abdominal surgery is one of the most common surgical procedures clinically, and postoperative pain is a foreseeable problem. Although epidural analgesia yields good analgesic effects in major open abdominal surgery (1–4), its application is limited by the use of coagulants (5), which have unforeseeable effects on blood coagulation and compromise the safety of neuraxial techniques (6). In recent years, clinical guidelines have proven that nerve block has a better benefit/risk ratio (RR) than central neuraxial blocks and have recommended that nerve block should be performed to relieve pain after primary thoracoabdominal surgeries (7, 8). However, transversus abdominal plane block (TAPB) has several drawbacks as the nerve block is currently mainly used for abdominal surgery. For example, the needle tip may pierce the transversus abdominis (and peritoneum), injuring the internal organs and peritoneum while inducing TAPB (9).
Erector spinae plane block (ESPB) was first reported in 2016 by Forero et al. (10) and has gained much attention due to its safety and ease of application. In this technique, the local anesthetic (LA) is injected into the fascia between the erector spinae and the transverse process and diffuses in this fascia, which can block the nearby spinal nerve. ESPB not only affects the dorsal and ventral rami of spinal nerves and causes temporary loss of sensation in the corresponding body surface sensory areas innervated by them but also affects the rami communicants that transmit sympathetic fibers. It has been proven that ESPB provides both somatic and visceral sensory blocks of the abdomen (10, 11), which makes it an ideal nerve block for abdominal surgery.
There has been an increasing amount of new evidence regarding ESPB’s effectiveness in preventing pain during abdominal surgery. However, thus far, most meta-analyses have focused mainly on validating the effects of ESPB in thoracic or breast surgery and comparing them with thoracic paravertebral blocks, there is a lack of studies exploring their effectiveness in abdominal surgery or comparing them with other trunk blocks such as TAPB. The current meta-analyses (12–15) only included a small number of studies involving abdominal surgery and Daghmouri et al. (16) only researched the effect of ESPB in laparoscopic cholecystectomy (LC).
Therefore, our systematic review and meta-analysis aimed to determine the analgesic effect of ESPB after abdominal surgery. We identified randomized controlled trials (RCTs) comparing ESPB with either placebo or TAPB.
Methods
This systematic review and meta-analysis were performed and reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (17). Our meta-analysis was registered prospectively with PROSPERO (CRD42022301491).
Search strategy
Literature searches were performed using PubMed, Embase, the Cochrane Central Register of Controlled Trials, and the ClinicalTrials.gov register from 2016 until 24 September 2021 for English RCTs meeting the listed inclusion criteria, as ESPB is a new regional nerve block first introduced in 2016. The search used the MeSH keywords “Paraspinal Muscles,” “Cardiac Surgical Procedures,” “Nerve Block,” and “Anesthesia, Local.” The detailed search strategy is provided in Supplementary Appendix A.
Study selection criteria
The two authors (GZ and LL) independently screened the search results and included trials that met the following criteria: (i) adult patients (age ≥18 years) treated with abdominal surgery, including LC, percutaneous nephrolithotomy (PCNL) and bariatric surgery (BS), etc., under general anesthesia; (ii) interventions: treatment with a single-injection ESPB with LA before or after surgery; and (iii) controls: placebo (no block and sham block) and TAPB. (iv) One or more of the following outcomes were assessed: postoperative pain scores at 6, 12 and 24 h, 24-h postoperative cumulative opioid consumption (mg), the incidence of postoperative nausea and vomiting (PONV) within 24 h postoperatively; length of hospital stay (days); time to first rescue analgesia (hours). (v) Only studies published in English were included. (vi) Only studies that were RCTs were included.
In our meta-analysis, trials were excluded that met the following criteria: (i) studies which did not provide available data (ii) studies which were withdrawn;
Data extraction
Two investigators (GZ and LL) independently reviewed the full manuscripts of eligible studies and conducted data extraction using a standardized form. Extracted data included the author names, publication year; sample size; type of surgery; unilateral or bilateral; comparator(s); LA type, concentration, and volume; timing of block (before or after surgery); guidance of ESPB (ultrasound-guided or fluoroscopy-guided); postoperative outcomes including postoperative pain scores, 24-h cumulative opioid consumption, time to first rescue analgesia, length of hospital stay, and incidence of PONV. Any discrepancies regarding the extraction of data were resolved by an additional investigator (XW). When the pain score data were absent, they were replaced by the pain score data during movement, and if they were still absent, the pain score data were replaced by the pain score data at rest. If patients in the intervention and control groups received the same nerve block, this nerve block was not considered in this analysis.
To facilitate meta-analysis, medians, interquartile ranges (IQRs), and range values were approximated into standardized mean differences (SMDs) and mean differences (MDs) with their corresponding SDs. If data values were represented in a graphical format, numerical data were extracted from graphs by Web Plot Digitizer (18). The risk of bias assessment was independently assessed by two investigators, with any disagreements judged by a third investigator (XW), according to the Cochrane Collaboration tool for assessing the risk of bias (19). Studies were assessed on randomization, allocation concealment, participant and personnel blinding, outcome assessment blinding, incomplete data and selective reporting; each category of the study was assigned “low risk,” “high risk,” or “unclear risk.”
Outcome measurement
Our primary outcomes were postoperative pain scores at 6, 12, and 24 h, as well as 24-h cumulative opioid consumption. Pain scores were measured by a visual or numerical scale (0–10 scale, where 0 = no pain and 10 = worst pain imaginable). Any visual analog scale (VAS) scores reported on a 0–100 scale were converted to a 0–10 scale for analysis. All reported perioperative opioid consumption was converted to intravenous morphine equivalents (20). Our secondary outcomes were the time to first rescue analgesia measured by hours after surgery, days of hospital stay after surgery, and incidence of PONV within 24 h postoperatively.
Data analysis
All meta-analyses were conducted using Review Manager V5.4.1. (Cochrane Collaboration, Copenhagen) and Stata 16.0 software. For continuous data of primary outcomes, including postoperative pain scores and 24-h cumulative opioid consumption, SMDs with a 95% confidence interval (95% CI) were calculated, but for continuous data of secondary outcomes, including length of hospital stay and time to first rescue analgesia, MDs with 95% CIs were calculated. Dichotomous data are presented by using RRs with 95% CIs.
If I2 > 50%, differences would be regard as significant (21). The random-effects model was used for all outcomes, and forest plots were used to represent and evaluate treatment effects. Subgroups were created to explore and resolve potential heterogeneity within the intervention and control groups based on the type of surgery, the timing of the block (before surgery or after surgery), and ESPB techniques (bilateral or unilateral). Subgroup analysis was performed if the number of studies included was not less than two. For outcomes with the data of ten or more studies, Egger’s regression (DerSimonian–Laird approach) was used to assess potential publication bias of the small-study effect. Moreover, a sensitivity analysis was performed by removing each study in turn to evaluate the stability of pooled estimate. Sensitivity analyses were performed for those studies with a high degree of heterogeneity (I2 ≥ 50% or P < 0.1). Finally, pooled analyses were visualized with forest plots and tables and P < 0.05 was considered statistically significant.
Results
A total of 1,409 studies were identified by our search criteria, and 375 duplicates were removed. Of the 1,034 remaining studies screened, 24 studies (22–45) were included in this review (Figure 1), with a total of 1,502 patients (701 who received ESPB, 801 who did not). The risk of bias assessment is summarized in Figure 2. The main sources of bias from the included studies were the lack of a description of participant and personnel blinding.
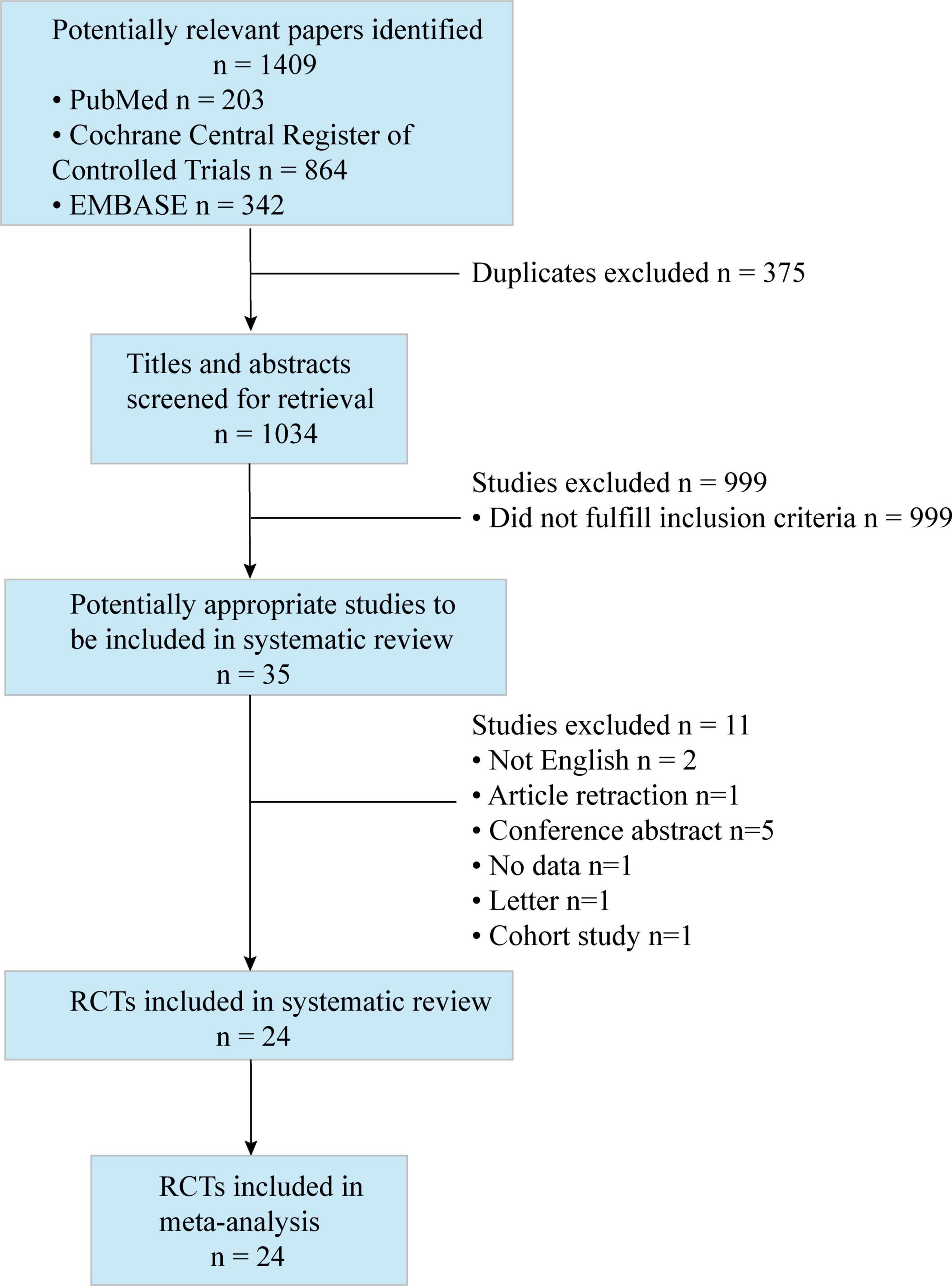
Figure 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram. The diagram shows the process and the reason for excluding studies.
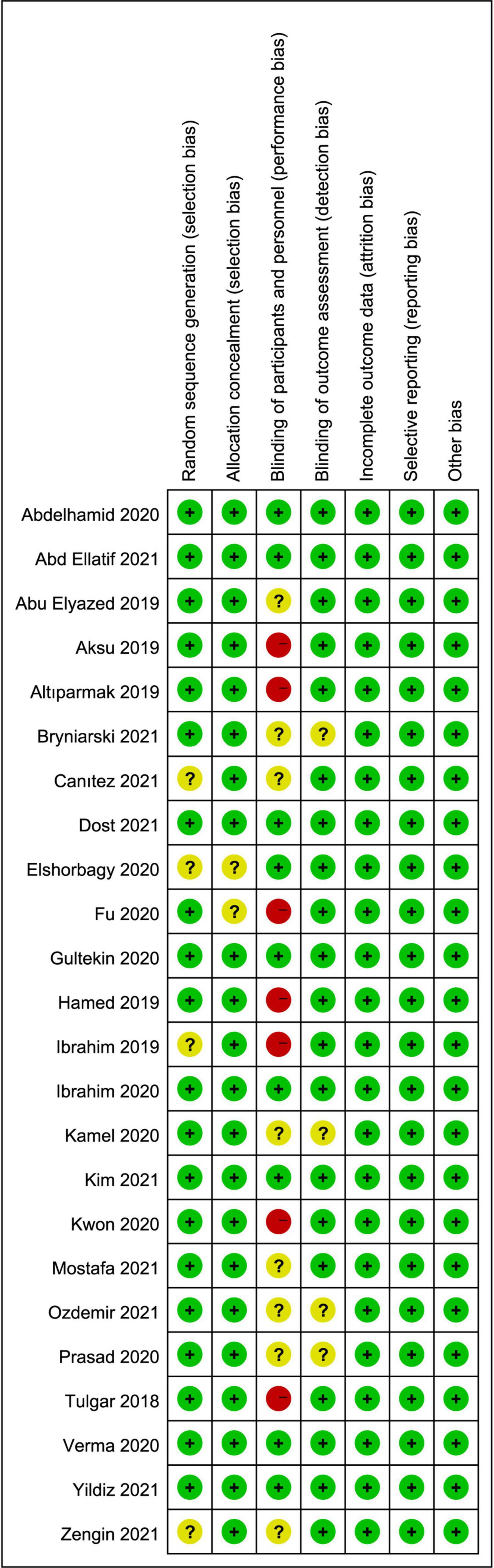
Figure 2. Risk of bias summary: review authors’ judgments about each risk of bias item for each included study. Green circle, low risk; red circle, high risk; yellow circle, unclear risk of bias.
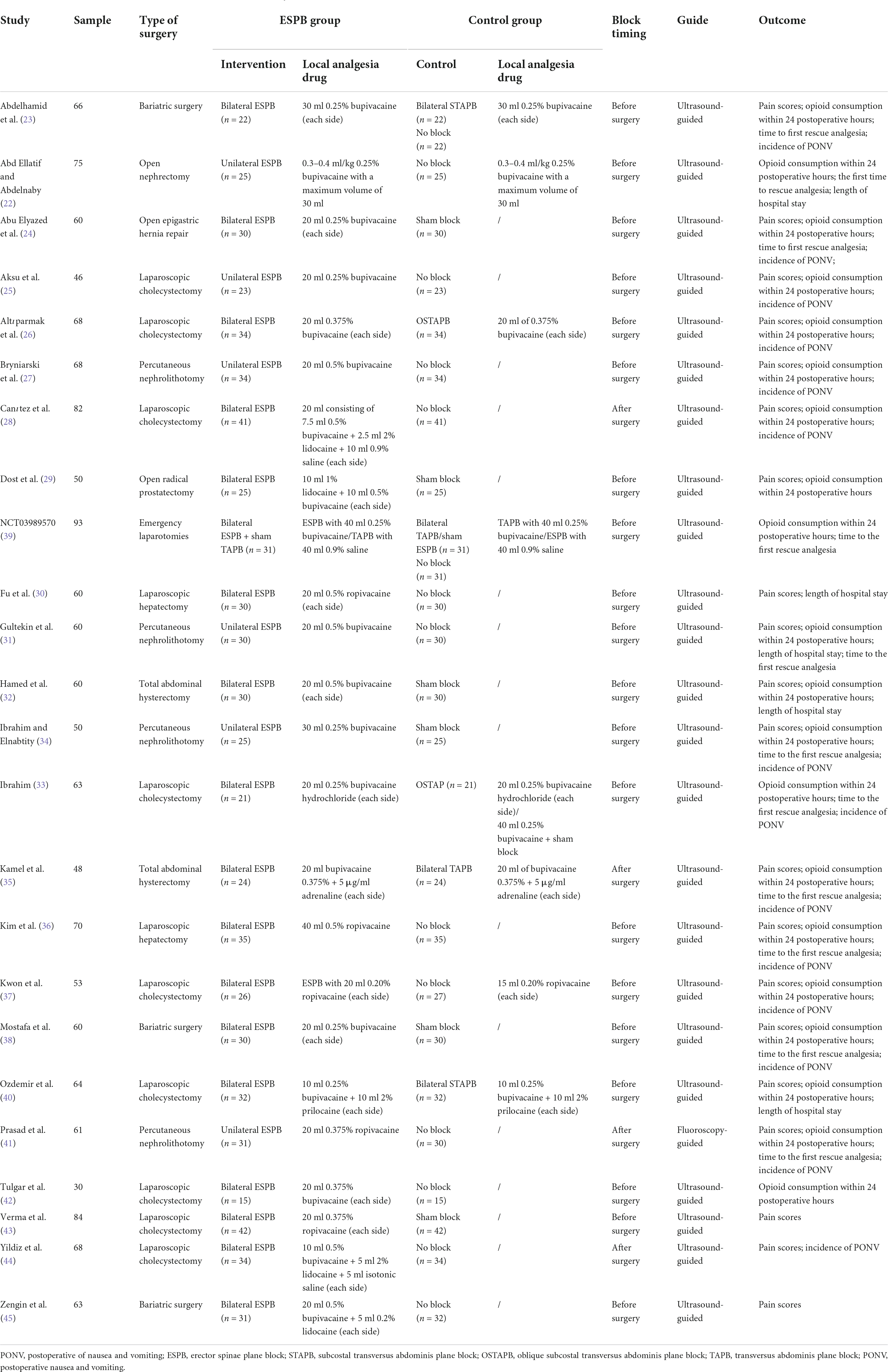
The characteristics of the included studies are summarized in Table 1. There were twenty RCTs (22–25, 27–32, 34, 36–39, 41–45) that compared ESPB with placebo, six studies (23, 26, 33, 35, 39, 40) that compared ESPB with TAPB. Abdominal surgeries were performed under general anesthesia in all studies: in nine studies (25, 26, 28, 33, 37, 40, 42–44) for LC, in four studies (27, 31, 34, 41) for PCNL, in three studies (23, 38, 45) for BS, in two studies (30, 36) for laparoscopic hepatectomy (LH), in two studies (32, 35) for total abdominal hysterectomy, in one study (22, 24) for hernia repair, in one study (22) for open nephrectomy, in one study (29) for open radical prostatectomy and in one study (39) for emergency laparotomy. Moreover, in the majority (20 of the 24) of the studies (22–27, 29–34, 36–40, 42, 43, 45), ESPB was performed before the surgery. Bilateral ESPB was conducted in 18 of the 24 studies (23, 24, 26, 28–30, 32, 33, 35–40, 42–45), while unilateral ESPB was used in the remaining studies (6 of the 24) (22, 25, 27, 31, 34, 41).
Postoperative pain scores
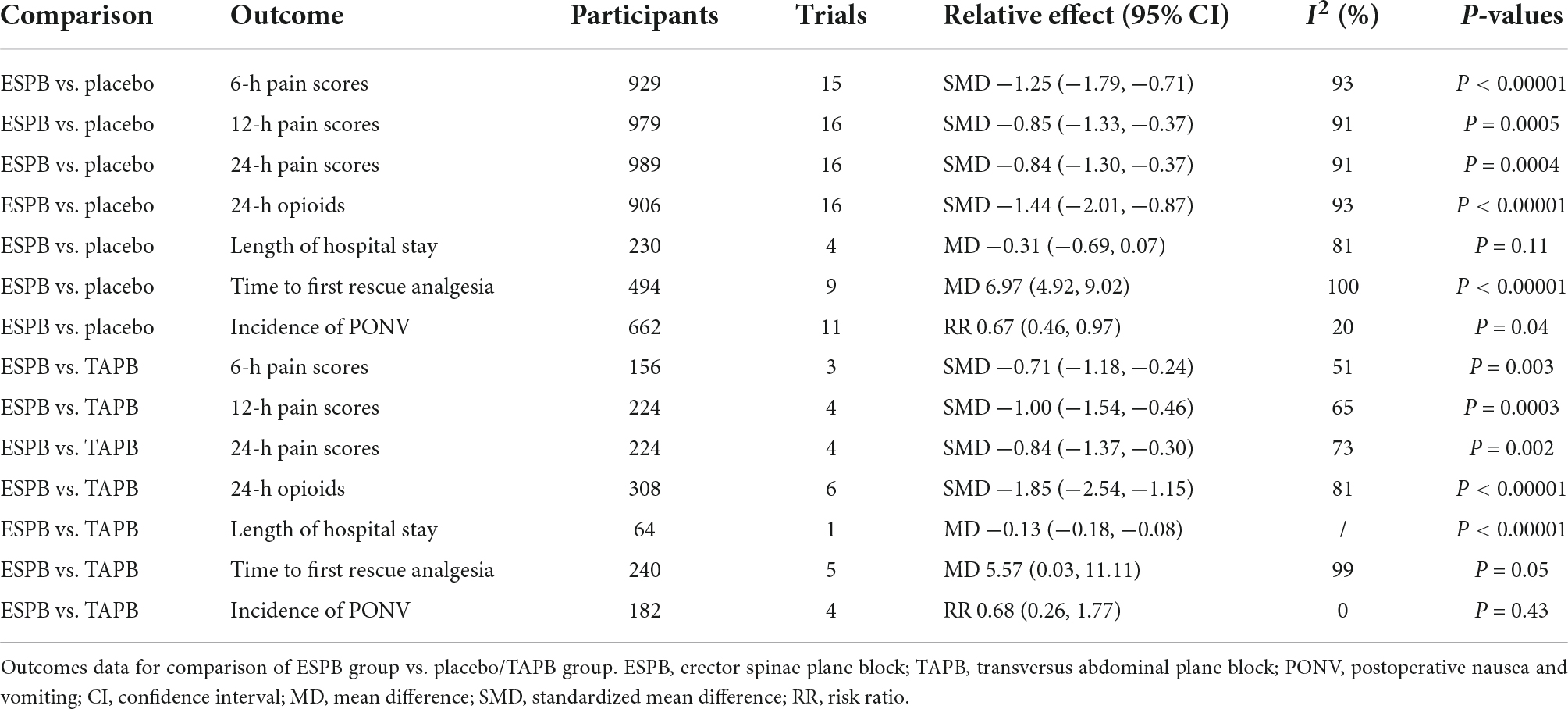
Compared with the placebo group, there was a significant reduction in postoperative pain scores in the ESPB group at various time points (Table 2): fifteen studies (23–25, 27–29, 31, 32, 36–38, 41, 43–46) reported significantly lower pain scores at 6 h (−1.25 cm; 95% CI −1.79 to −0.71; P < 0.00001; I2 = 93%) (Figure 3A). However, 16 studies (23–25, 27–29, 31, 32, 34, 36–38, 41, 43–45) reported significantly lower pain scores at 12 h (−0.85 cm; 95% CI −1.33 to −0.37; P = 0.0005; I2 = 91%) (Figure 3B) and 24 h (−0.84 cm; 95% CI −1.30 to −0.37; P = 0.0004; I2 = 91%) (Figure 3C). In our meta-analysis, compared with TAPB, ESPB significantly reduced pain scores at time points after abdominal surgery (Table 2): three trials (23, 35, 40) reported significantly lower pain scores at 6 h (−0.71 cm; 95% CI −1.18 to −0.24; P = 0.003; I2 = 51%) (Figure 4A) after abdominal surgery and four studies (23, 26, 35, 40) revealed significantly lower postoperative pain scores at 12 h (−1.00 cm; 95% CI −1.54 to −0.46 P = 0.0003; I2 = 65%) (Figure 4B) and 24 h (−0.84 cm; 95% CI −1.37 to −0.30; P = 0.002; I2 = 73%) (Figure 4C). Moreover, we conducted subgroup analyses to determine the postoperative analgesia conferred by ESPB compared with placebo in different types of surgery. The subgroup analysis of primary outcomes was performed as follows.
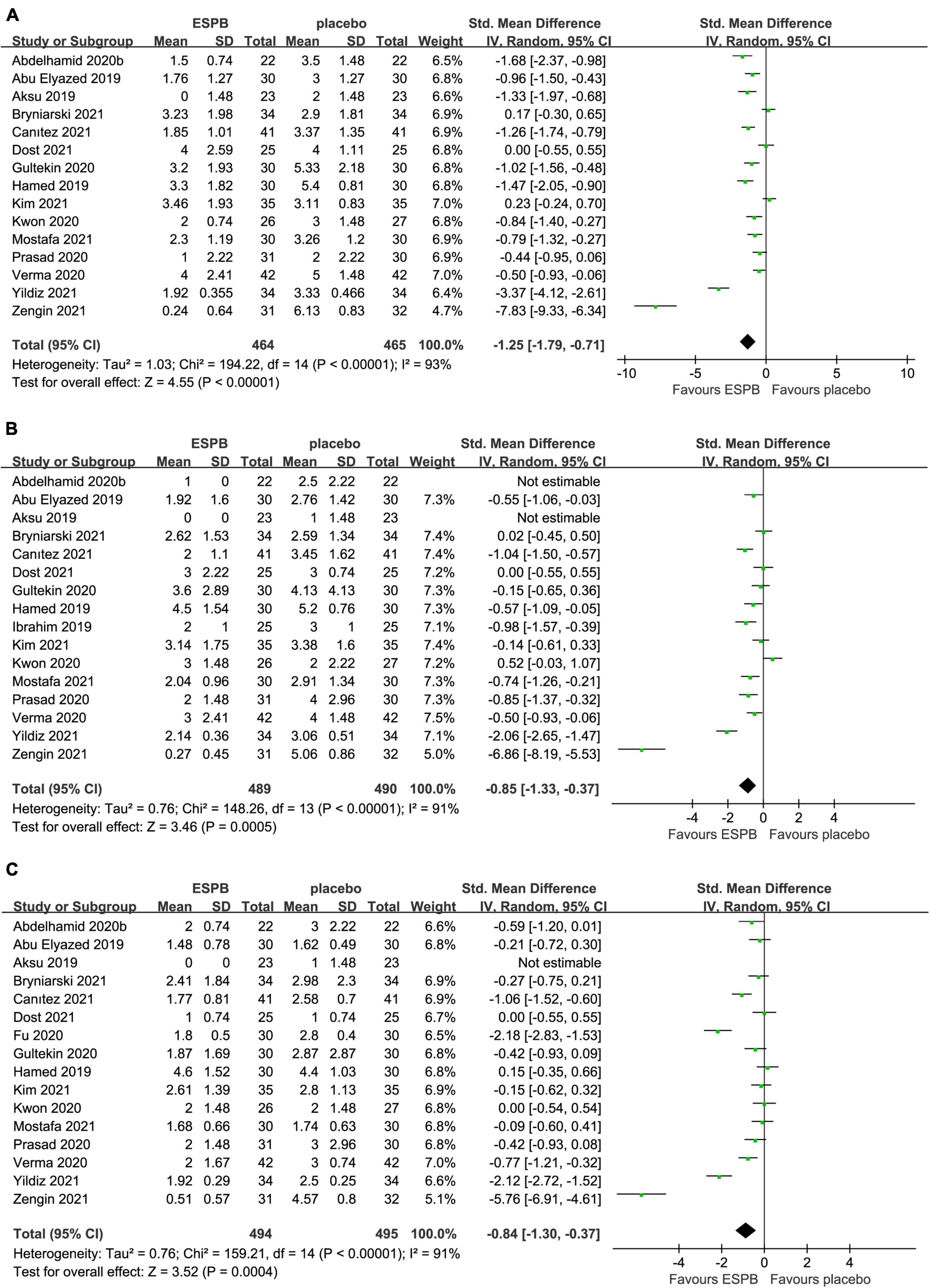
Figure 3. Forest plot of pain scores for the ESPB vs. placebo in the first 24 h after surgery. (A) Pain scores at 6 h after surgery. (B) Pain scores at 12 h after surgery. (C) Pain scores at 24 h after surgery.
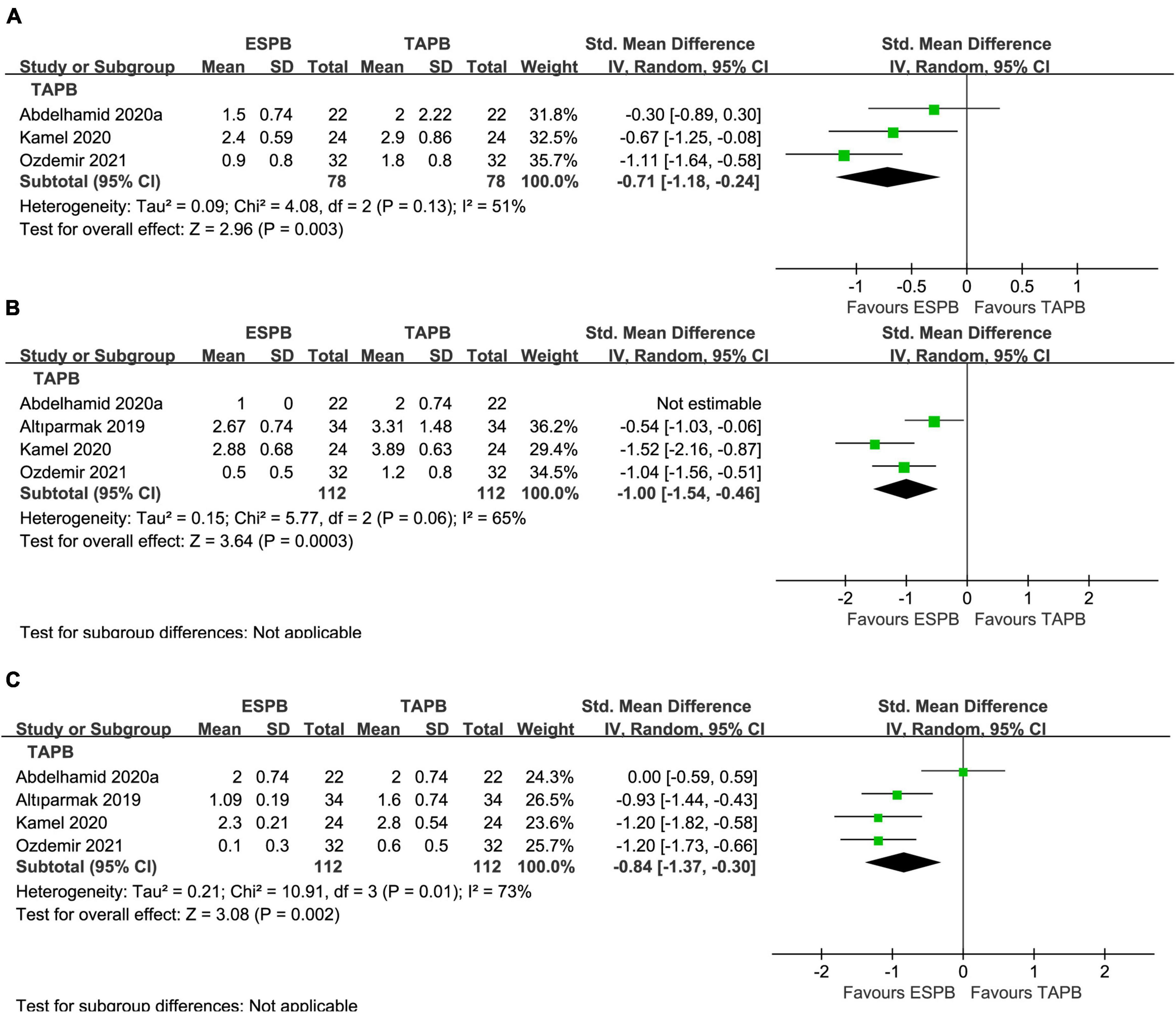
Figure 4. Forest plot of pain scores for the ESPB vs. TAPB in the first 24 h after surgery. (A) Pain scores at 6 h after surgery. (B) Pain scores at 12 h after surgery. (C) Pain scores at 24 h after surgery.
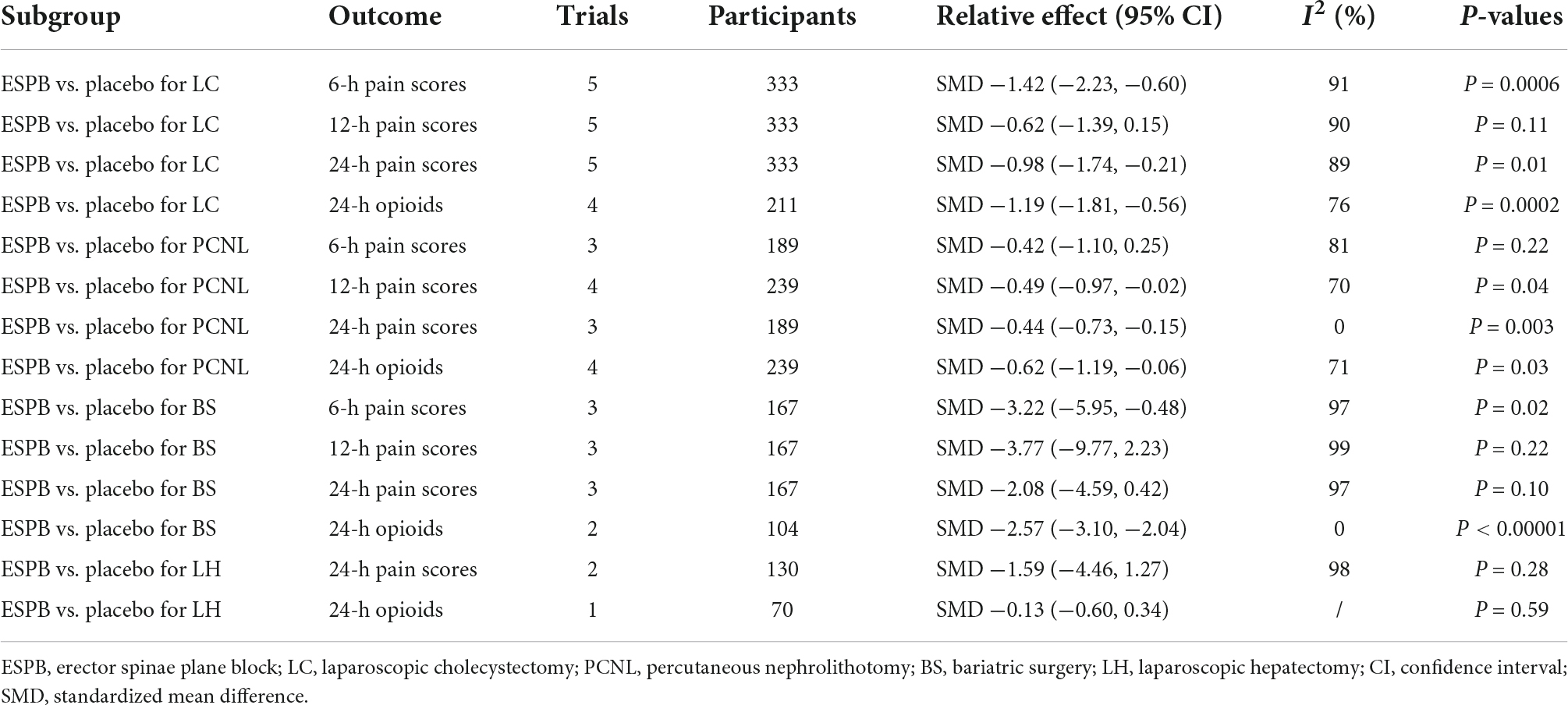
In the subgroup analysis of LC, five trials (25, 28, 37, 43, 44) reported that, compared with placebo, ESPB significantly reduced pain scores at 6 h (−1.42 cm; 95% CI −2.23 to −0.60; P = 0.0006; I2 = 91%) and 24 h (−0.98 cm; 95% CI −1.74 to −0.21; P = 0.01; I2 = 89%) (Table 3). Interestingly, five trials (25, 28, 37, 43, 44) showed that no significant difference was detected in postoperative pain scores at 12 h (−0.62 cm; 95% CI −1.39 to 0.15; P = 0.11; I2 = 90%) between the groups (Table 3).
In the subgroup analysis of PCNL, three studies (27, 31, 41) reported that, compared with placebo, ESPB provided comparable pain scores at 6 h (−0.42 cm; 95% CI −1.10 to 0.25; P = 0.22; I2 = 81%) and significantly lower postoperative pain scores at 24 h (−0.44 cm; 95% CI −0.73 to −0.15; P = 0.003; I2 = 0%) (Table 3). Meanwhile, four studies (27, 31, 34, 41) reported a significant reduction in postoperative pain scores at 12 h (−0.49 cm; 95% CI −0.97 to −0.02; P = 0.04; I2 = 70%) (Table 3) in the ESPB group after PCNL, compared with placebo.
In the subgroup analysis of BS, three trials (23, 38, 45) revealed that, compared with placebo, ESPB significantly reduced pain scores at 6 h (−3.22 cm; 95% CI −5.95 to −0.48; P = 0.02; I2 = 97%) (Table 3). However, no significant difference was found in postoperative pain scores at 12 h (−3.77 cm; 95% CI −9.77 to 2.23; P = 0.22; I2 = 99%) and 24 h (−2.08 cm; 95% CI −4.59 to 0.42; P = 0.10; I2 = 97%) after BS between the groups (Table 3).
In the subgroup analysis of LH, two studies (30, 36) found no significant difference in postoperative pain scores at 24 h (−1.59 cm; 95% CI −4.46 to 1.27; P = 0.28; I2 = 98%) between ESPB and placebo groups (Table 3). However, subgroup analysis was not performed due to the limited number of studies involving pain scores at 6 and 12 h after LH.
Postoperative 24-h cumulative opioid consumption
The 24-h cumulative opioid consumption after abdominal surgery was investigated in 16 studies (22–25, 27–29, 31, 32, 34, 36–39, 41, 42), with a significant reduction (−1.44 mg; 95% CI −2.01 to −0.87; P < 0.00001; I2 = 93%) in the ESPB group compared with the placebo group (Table 2 and Figure 5A).
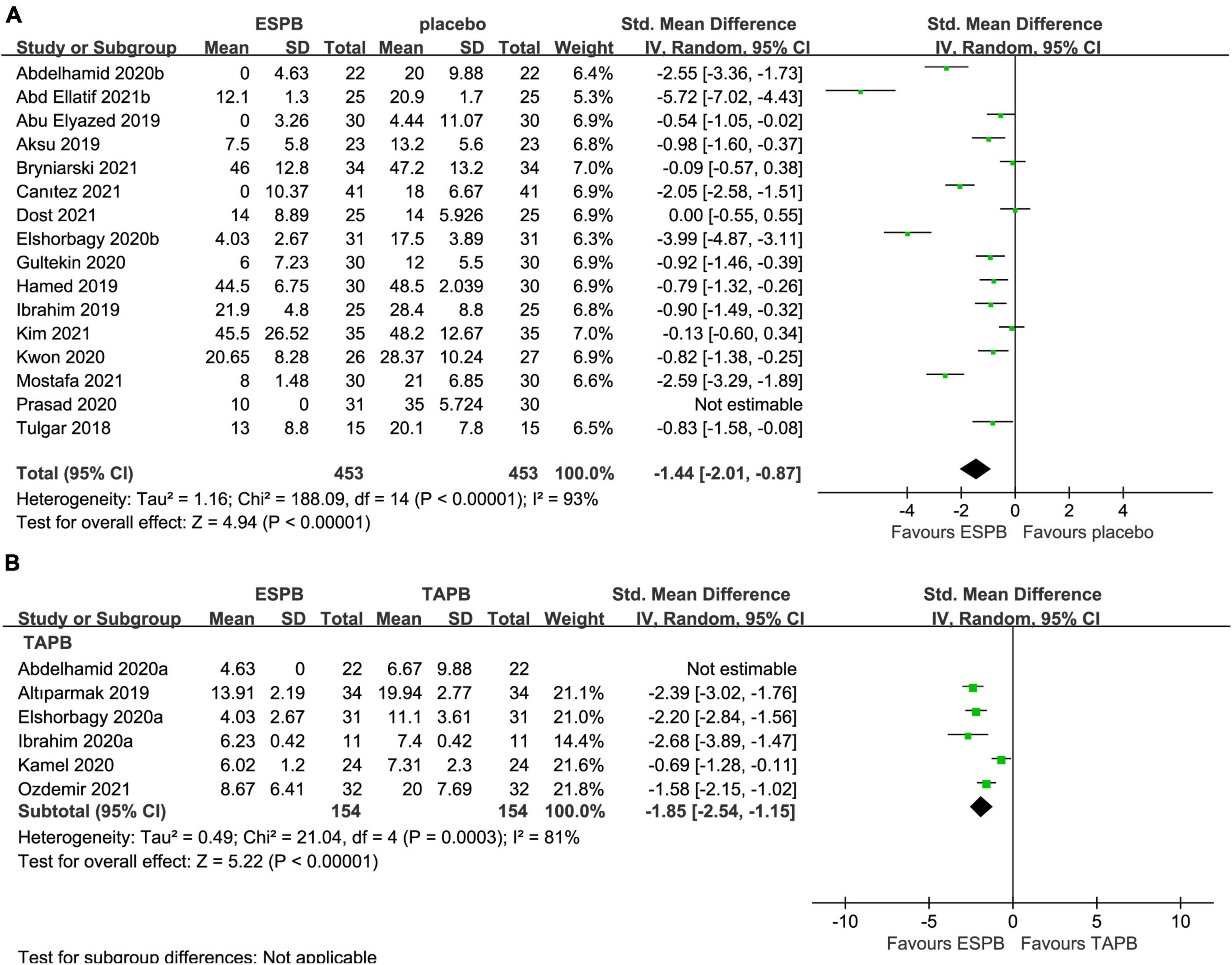
Figure 5. Forest plot for the comparison of intravenous morphine equivalents (mg) in the first 24 h after surgery. (A) Twenty-four hours cumulative opioid consumption for the ESPB vs. placebo studies. (B) Twenty-four hours cumulative opioid consumption for the ESPB vs. TAPB studies.
The 24-h cumulative opioid consumption after abdominal surgery was investigated by six studies (23, 26, 33, 35, 39, 40), with a significant reduction in opioid intake (−1.85 mg; 95% CI −2.54 to −1.15; I2 = 81%; P < 0.00001) in the ESPB group compared with TAPB group (Table 2 and Figure 5B).
In the subgroup analysis of LC, four studies (25, 28, 37, 42) reported that, compared with placebo, ESPB significantly reduced the 24-h cumulative opioid consumption (−1.19 mg; 95% CI −1.81 to −0.56; P = 0.0002; I2 = 76%) after LC (Table 3).
In the subgroup analysis of PCNL, compared with placebo group, four studies (27, 31, 34, 41) reported that ESPB significantly reduced 24-h cumulative opioid consumption (−0.62 mg; 95% CI −1.19 to −0.06; P = 0.03; I2 = 71%) (Table 3).
In the subgroup analysis of BS, two studies (23, 38) revealed that, compared with placebo group, ESPB significantly reduced 24-h cumulative opioid consumption (−2.57 mg; 95% CI −3.10 to −2.04; P < 0.00001; I2 = 0%) (Table 3). However, due to the limitation of the number of studies involving 24-h cumulative opioid consumption after LH, subgroup analysis was not performed (Table 3).
Secondary outcome measures
Time to first rescue analgesia
The time to first rescue analgesia after abdominal surgery was reported in nine trials (22–24, 31, 34, 36, 38, 39, 41), and compared with the placebo group, ESPB significantly prolonged the time to first rescue analgesia (6.97 h; 95% CI 4.92 to 9.02; P < 0.0001; I2 = 100%) (Table 2). Five studies (23, 33, 35, 39, 40) including 240 patients undergoing abdominal surgery reported that, compared with the TAPB group, ESPB significantly extended the time to first rescue analgesia (5.57 h; 95% CI 0.03 to 11.11; P = 0.05; I2 = 99%) (Table 2).
Length of hospital stay
Four trials (22, 30–32) compared the length of hospital stay of 230 patients undergoing abdominal surgery between the ESPB group and placebo group. However, the length of hospital stay was not significantly different (−0.31 days; 95% CI −0.69 to 0.07; P = 0.11; I2 = 81%) between the groups (Table 2). In one study by Ozdemir et al., which compared ESPB with TAPB for LC, there was a significantly shorter hospital stay in the ESPB group (40) (Table 2).
Incidence of postoperative nausea and vomiting
Eleven trials (23–25, 27, 28, 34, 36–38, 41, 44) reported the impact of ESPB on the incidence of PONV in 662 patients undergoing abdominal surgery. ESPB significantly reduced the incidence of PONV (RR 0.67; 95% CI 0.46 to 0.97; P = 0.04; I2 = 20%) compared with that in the placebo group (Table 2 and Figure 6A). In addition, four studies (23, 26, 33, 35) analyzed the incidence of PONV in patients receiving ESPB vs. TAPB. However, there was no significant difference in the incidence of PONV (RR 0.68; 95% CI 0.26 to 1.77; P = 0.43; I2 = 0%) between the groups (Table 2 and Figure 6B).
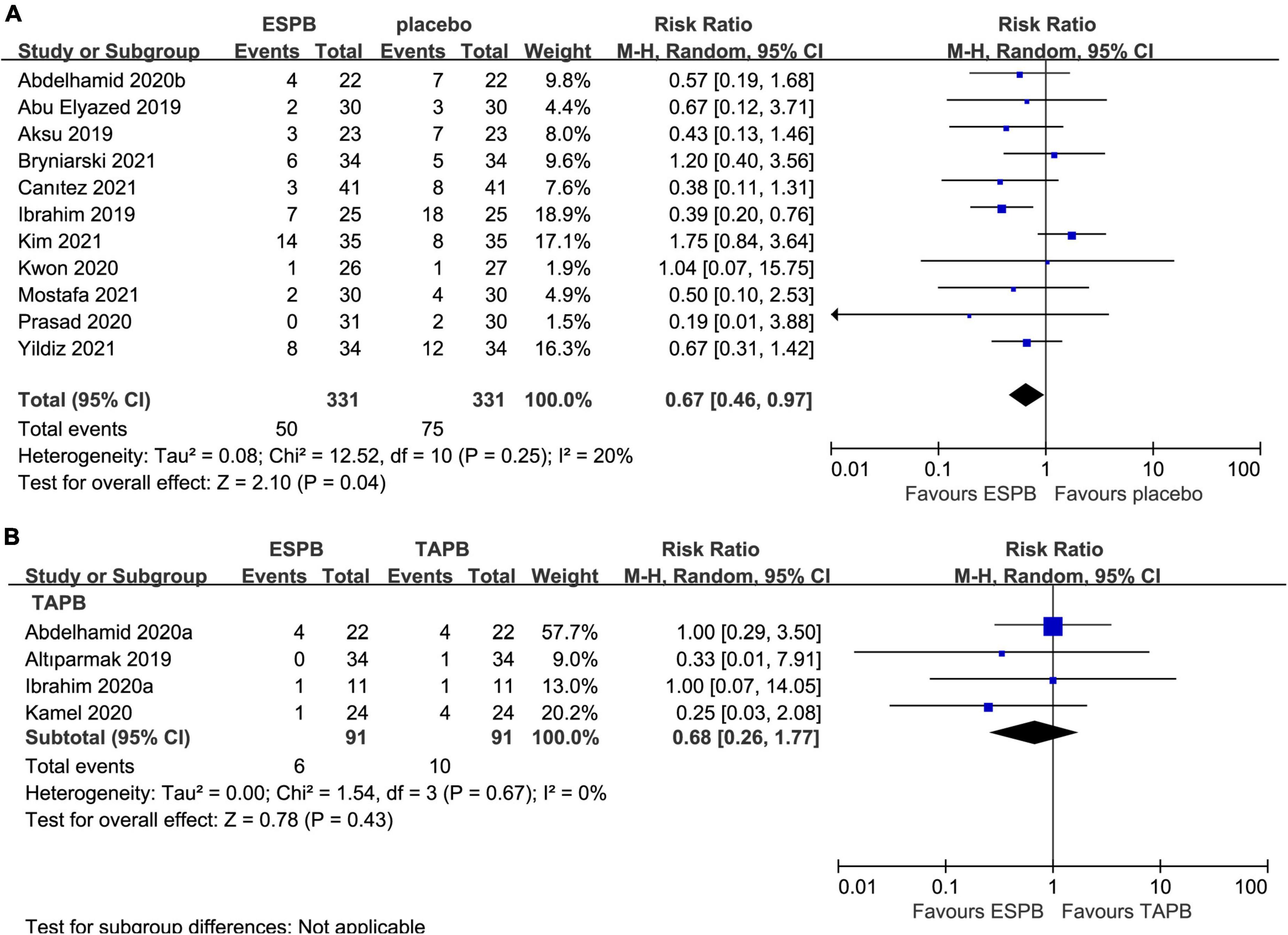
Figure 6. Forest plot for the comparison of the incidence of postoperative PONV. (A) Postoperative incidence of PONV for the ESPB vs. placebo studies. (B) Postoperative incidence of PONV for the ESPB vs. TAPB studies.
Subgroup analysis of block techniques and timing of block
Subgroup analyses of block techniques (unilateral or bilateral ESPB) and the timing of block (before or after surgery) are presented in Table 4. Our meta-analysis revealed that performing ESPB after surgery significantly prolonged the time to first request for analgesia after abdominal surgery compared with that in the before-surgery subgroup (P = 0.002, DerSimonian–Laird approach). However, for other outcomes, the block technique and the timing of the block showed no statistical subgroup differences (P > 0.05).
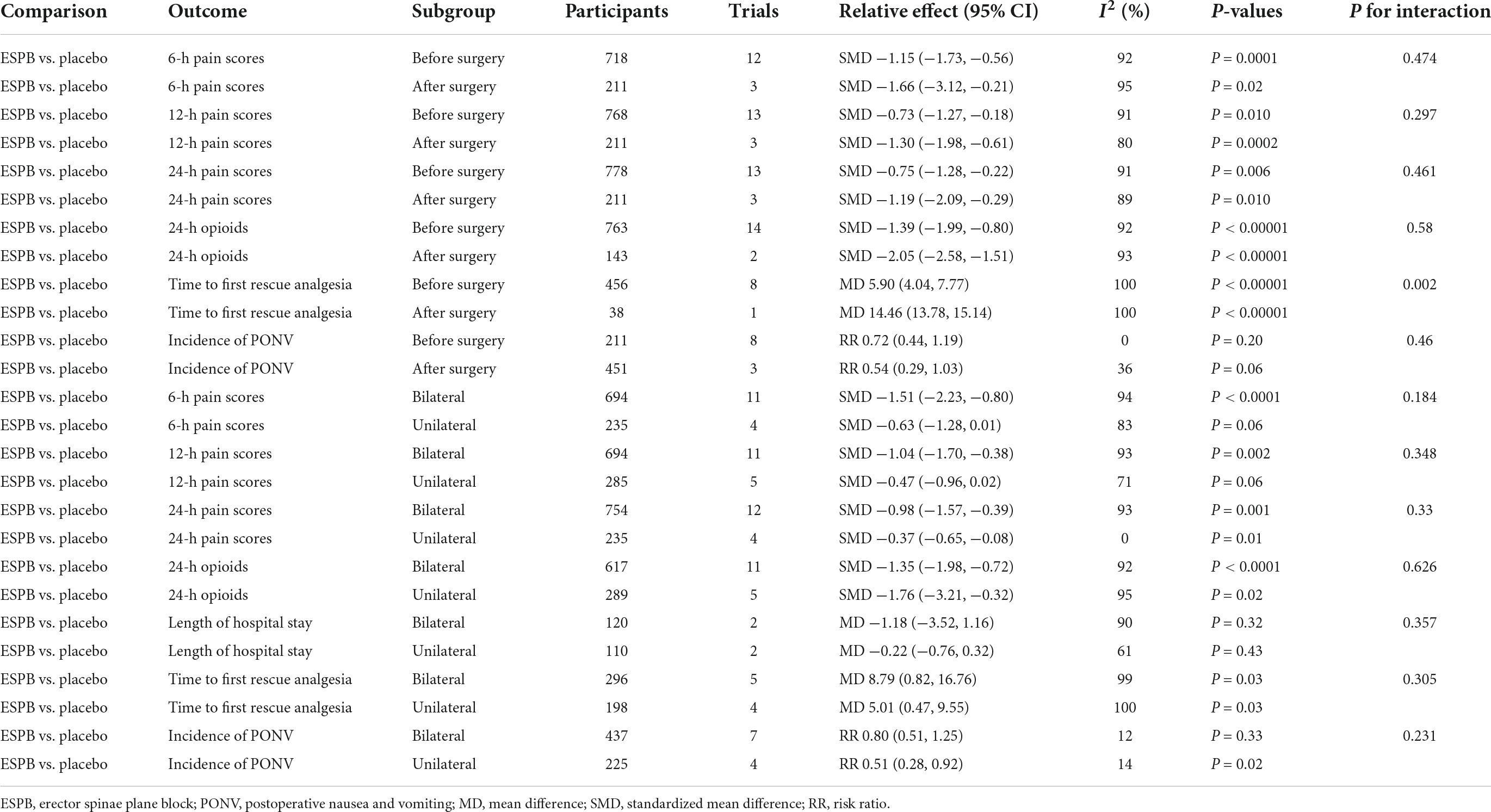
Table 4. Subgroup analysis of timing of block (before/after surgery) and ESPB techniques (unilateral/bilateral).
Publication bias and sensitivity analysis
Egger’s test showed that the P-value for postoperative pain scores at 6, 12, and 24 h and for 24-h cumulative opioid consumption was less than 0.0001, which was less than 0.05 (Table 5). Therefore, some publication bias existed in the primary outcome and may have influenced the final result, but publication bias did not exist for the incidence of PONV.
According to the sensitivity analysis, most of overall outcomes did not change after the exclusion of a single study except postoperative pain scores at 6 h and time to first rescue analgesia between ESPB and TAPB (Supplementary Figures 2, 4).
Discussion
This systematic review and meta-analysis showed the postoperative analgesic efficacy of ESPB in adults undergoing abdominal surgery under general anesthesia. When compared with placebo (e.g., no block and sham block), ESPB provided better postoperative analgesia at various time points and reduced opioid consumption within 24 h after surgery. Furthermore, ESPB was associated with a longer time to first rescue analgesia and a lower incidence of PONV postoperatively after abdominal surgery. However, it was not beneficial in shortening the length of hospital stay.
Compared with TAPB, ESPB also provided significantly lower pain scores at the various time points and lower opioid consumption within 24 h after surgery. Meanwhile, ESPB significantly prolonged the time to first rescue analgesia after abdominal surgery. However, we found no significant differences in the incidence of PONV between the groups.
Moreover, we tried to perform a subgroup analysis to explore the effect of ESPB on different types of surgery. Our meta-analysis showed that ESPB seems to be most beneficial in terms of reduction not only in pain scores but also in opioid consumption for patients undergoing LC, PCNL, and BS. Based on our meta-analysis, the best indication for performing ESPB for postoperative analgesia is LC (e.g., reduced postoperative pain at 6 and 24 h and 24-h cumulative opioid consumption) and PCNL (e.g., reduced pain at 12 and 24 h and 24-h cumulative opioid consumption). Similarly, ESPB could be recommended for BS (e.g., reduced pain at 6 h and 24-h postoperative cumulative opioid consumption). However, due to a limited number of studies, there is no effective recommendation for the effect of ESPB in reducing pain or opioid consumption in other types of surgery, such as LH, hernia repair, open nephrectomy, open radical prostatectomy or emergency laparotomy.
Due to the high heterogeneity of the outcomes, we also tried to perform subgroup analyses of the timing of block (before/after the surgery) and ESPB technique (unilateral/bilateral). The time to first rescue analgesia was significantly prolonged by ESPB in both the before-surgery and after-surgery subgroups, and the effect on the after-surgery subgroup was significantly more powerful than that in the before-surgery subgroup. However, the heterogeneity was still high (I2 = 100%) for both subgroups, and the number of studies for the after-surgery subgroup was limited (only one). Consequently, the results need to be confirmed by more research.
A cadaver study (10) reported the use of ESPB to inject LA into the fascia between the erector spinae and the transverse process; the LA was able to pass through the fascia to infiltrate and paralyze the spinal nerves. ESPB can act on dorsal and ventral branches of the spinal nerves and rami communicants that transmit sympathetic fibers. Due to the erector spinae and erector spinae plane extending down to the lumbar spine, ESPB can provide analgesia for abdominal surgery if the injection is performed at the lower levels of the thoracic spine. Recently, few cadaveric and radiological studies have described the LA diffusion range of ESPB. The results showed that ESPB seemed to work by spreading the LA to the epidural and paravertebral space. In this way, ESPB would be able to implement somatic and visceral analgesic effects such as epidural anesthesia (47–50). Moreover, the transverse process of the spine can now act as a puncture needle support point and anatomic landmark on ultrasound, which means ESPB is easy to perform (22, 26, 51).
A previous meta-analysis (16) that included 5 RCTs with 250 patients undergoing LC concluded that, compared with placebo, ESPB significantly decreased postoperative pain scores and 24-h cumulative opioid consumption as well as significantly prolonged the time to first rescue analgesia. In our analysis, we obtained similar results. We believe these findings suggest that ESPB plays an important role in postoperative analgesia for LC. Moreover, similar to our meta-analysis, a few previous meta-analyses (12–15) demonstrated that the ESPB group had significantly lower pain scores, lower 24-h cumulative opioid consumption, longer time to first rescue analgesia and lower incidence of PONV among patients undergoing surgery. However, these meta-analyses analyzed various surgeries, and as they only contained a small number of trials of abdominal surgery, they could not demonstrate the analgesic effect of ESPB in abdominal surgery. Our meta-analysis included 24 RCTs and performed a meta-analysis to compare ESPB with placebo or TAPB for postoperative analgesia in abdominal surgery patients based on a larger sample size. Moreover, the quality of trials in these meta-analyses should also be considered, as two of them (12, 15) included trials (52) that have been retracted.
This meta-analysis showed the beneficial effect and ease of application of postoperative analgesia compared with placebo and our sensitivity analysis also showed strong ability of the pooled analysis (Supplementary Figures 1, 3–5). ESPB has been applied in various kinds of surgeries, including lumbar spine surgery (53), LC (16), breast cancer surgery (54), and other thoracic and abdominal surgeries, and no side effects or complications related to this block have been reported. Our present meta-analysis provides novel evidence that ESPB is an effective nerve block for analgesia after abdominal surgeries.
In comparison with that of TAPB, the injection point of ESPB is remote from the peritoneum and abdominal wall and poses a lower risk of abdominal organ damage and peritoneal breach (9, 10). While ESPB provides somatic and visceral sensory block of the abdomen (10, 11), TAPB only supplies analgesia to the anterolateral abdominal wall (55). Therefore, ESPB may provide more effective analgesia after abdominal surgery. Due to the combination of its efficacy and lower risk of complications, ESPB has been regarded as an alternative to TAPB for postoperative analgesia in certain surgical operations. In addition, our review revealed that, compared with TAPB, ESPB is associated with a longer time to first rescue analgesia and a comparable incidence of PONV after abdominal surgery. However, perhaps due to the influence of different surgical types, different postoperative analgesia regimens and differences in clinician habits, time to first rescue analgesia can vary. For example, Ozdemir et al. (40) reported a longer time to first rescue analgesia in the TAPB group, while other studies report longer time in the ESPB group. It was worth mentioning that sensitivity analysis of postoperative pain scores at 6 h and time to first rescue analgesia found that the outcomes were not stable (Supplementary Figures 2, 4). Moreover, since not many studies have reported on these two outcomes, subgroup analysis was not performed to explore the effect of timing of block (before/after the surgery) and ESPB technique, so the veracity of both outcomes deserve further research. Even so, our meta-analysis still provides new evidence that ESPB may be a promising alternative to TAPB after abdominal surgery. However, the differences in analgesic effects and other postoperative anesthetic outcomes of both nerve blocks still require direct comparison in future large-volume and well-designed RCTs.
However, some limitations of our meta-analysis should be mentioned. First, the main drawback of our meta-analysis is that high heterogeneity was observed between studies. The sources of high heterogeneity also included differences in the types and doses of LAs, differences in multimodal analgesia, performer differences and patient differences (age, sex, etc.), etc. Second, Egger’s tests of primary outcomes revealed a high risk of small-study effects, which also reduced the reliability of our meta-analysis. Third, our meta-analysis only included studies involving abdominal surgeries instead of all kinds of surgical procedures. Therefore, the effect of postoperative analgesia may be exaggerated due to selection bias. Fourth, our meta-analysis only focused on the comparison of the ESPB group with the placebo or TAPB group. We did not compare the postoperative analgesic effect of ESPB with that of other postoperative analgesic methods (such as intrathecal morphine, quadratus lumborum block, or local infiltration). Therefore, more studies in this area are needed in the future. Fifth, the sample sizes of the studies included in this review were all relatively small. The largest sample size of the experimental group was only 42 patients. In the future, large-volume studies are needed in this area. Finally, relatively few studies have focused on the same surgery. Subgroup analyses by type of surgery were only applied to LC, PCNL, BS, and LH, with 6, 4, 3, and 2 RCTs, respectively.
Conclusion
In summary, ESPB is a novel, beneficial nerve block for adult patients undergoing abdominal surgery. Moreover, our meta-analysis confirms that ESPB provides more beneficial postoperative analgesic efficacy than TAPB. Therefore, our research recommends ESPB as a supplement to the multimodal analgesic regimen for abdominal surgery and a valid alternative to TAPB. Future, large-volume, well-designed RCTs with extensive follow-up are needed to confirm and update the findings in this area.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YG and XW were responsible for the conception and design of the study, and drafting and revising the manuscript. YG, LL, and XW conducted the literature retrieval, screening, and quality evaluation. YG, YC, and JZ analyzed the data and explained the results. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Natural Science Foundation of Liaoning Province (Grant No. 20180551189).
Acknowledgments
We would like to thank our friend Kun Zhao, who patiently provided many valuable suggestions to improve the quality of this article. YG wants to thank, in particular, for the support, encouragement and patience from Yang Yu in the last years.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.934866/full#supplementary-material
References
1. Nishimori M, Ballantyne JC, Low JH. Epidural pain relief versus systemic opioid-based pain relief for abdominal aortic surgery. Cochrane Database Syst Rev. (2006) CD005059. doi: 10.1002/14651858.CD005059.pub2
2. Capdevila X, Moulard S, Plasse C, Peshaud J-L, Molinari N, Dadure C, et al. Effectiveness of epidural analgesia, continuous surgical site analgesia, and patient-controlled analgesic morphine for postoperative pain management and hyperalgesia, rehabilitation, and health-related quality of life after open nephrectomy: a prospective, randomized, controlled study. Anesth Analg. (2017) 124:336–45.
3. Oh TK, Lim MC, Lee Y, Yun JY, Yeon S, Park S-Y. Improved postoperative pain control for cytoreductive surgery in women with ovarian cancer using patient-controlled epidural analgesia. Int J Gynecol Cancer. (2016) 26:588–93. doi: 10.1097/IGC.0000000000000644
4. Bardia A, Sood A, Mahmood F, Orhurhu V, Mueller A, Montealegre-Gallegos M, et al. Combined epidural-general anesthesia vs general anesthesia alone for elective abdominal aortic aneurysm repair. JAMA Surg. (2016) 151:1116–23. doi: 10.1001/jamasurg.2016.2733
5. Hemmerling TM. Pain management in abdominal surgery. Langenbecks Arch Surg. (2018) 403:791–803. doi: 10.1007/s00423-018-1705-y
6. Novak-Jankoviè V, Markoviè-Božiè J. Regional anaesthesia in thoracic and abdominal surgery. Acta Clin Croat. (2019) 58:96–100. doi: 10.20471/acc.2019.58.s1.14
7. Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of postoperative pain: a clinical practice guideline from the American pain society, the American society of regional anesthesia and pain medicine, and the American society of anesthesiologists’ committee on regional anesthesia, executive committee, and administrative council. J Pain. (2016) 17:131–57. doi: 10.1016/j.jpain.2015.12.008
8. SFAR Committees on Pain and Local Regional Anaesthesia and on Standards. Expert panel guidelines (2008). Postoperative pain management in adults and children. SFAR committees on pain and local regional anaesthesia and on standards. Ann Fr Anesth Reanim. (2009) 28:403–9. doi: 10.1016/j.annfar.2009.02.019
9. Tran DQ, Bravo D, Leurcharusmee P, Neal JM. Transversus abdominis plane block: a narrative review. Anesthesiology. (2019) 131:1166–90. doi: 10.1097/ALN.0000000000002842
10. Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. (2016) 41:621–7. doi: 10.1097/AAP.0000000000000451
11. Chin KJ, Malhas L, Perlas A. The erector spinae plane block provides visceral abdominal analgesia in bariatric surgery: a report of 3 cases. Reg Anesth Pain Med. (2017) 42:372–6. doi: 10.1097/AAP.0000000000000581
12. Fanelli A, Torrano V, Cozowicz C, Mariano ER, Balzani E. The opioid sparing effect of erector spinae plane block for various surgeries: a meta-analysis of randomized-controlled trials. Minerva Anestesiol. (2021) 87:903–14. doi: 10.23736/S0375-9393.21.15356-8
13. Huang J, Liu J-C. Ultrasound-guided erector spinae plane block for postoperative analgesia: a meta-analysis of randomized controlled trials. BMC Anesthesiol. (2020) 20:83. doi: 10.1186/s12871-020-00999-8
14. Cai Q, Liu G-Q, Huang L-S, Yang Z-X, Gao M-L, Jing R, et al. Effects of erector spinae plane block on postoperative pain and side-effects in adult patients underwent surgery: a systematic review and meta-analysis of randomized controlled trials. Int J Surg. (2020) 80:107–16. doi: 10.1016/j.ijsu.2020.05.038
15. Kendall MC, Alves L, Traill LL, De Oliveira GS. The effect of ultrasound-guided erector spinae plane block on postsurgical pain: a meta-analysis of randomized controlled trials. BMC Anesthesiol. (2020) 20:99. doi: 10.1186/s12871-020-01016-8
16. Daghmouri MA, Akremi S, Chaouch MA, Mesbahi M, Amouri N, Jaoua H, et al. Bilateral Erector spinae plane block for postoperative analgesia in laparoscopic cholecystectomy: a systematic review and meta-analysis of randomized controlled trials. Pain Pract. (2021) 21:357–65. doi: 10.1111/papr.12953
17. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. (2015) 350:g7647. doi: 10.1136/bmj.g7647
18. Drevon D, Fursa SR, Malcolm AL. Intercoder reliability and validity of webplotdigitizer in extracting graphed data. Behav Modif. (2017) 41:323–39. doi: 10.1177/0145445516673998
19. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
21. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
22. Abd Ellatif SE, Abdelnaby SM. Ultrasound guided erector spinae plane block versus quadratus lumborum block for postoperative analgesia in patient undergoing open nephrectomy: a randomized controlled study. Egypt J Anaesth. (2021) 37:123–34. doi: 10.1080/11101849.2021.1894661
23. Abdelhamid BM, Khaled D, Mansour MA, Hassan MM. Comparison between the ultrasound-guided erector spinae block and the subcostal approach to the transversus abdominis plane block in obese patients undergoing sleeve gastrectomy: a randomized controlled trial. Minerva Anestesiol. (2020) 86:816–26. doi: 10.23736/s0375-9393.20.14064-1
24. Abu Elyazed MM, Mostafa SF, Abdelghany MS, Eid GM. Ultrasound-Guided erector spinae plane block in patients undergoing open epigastric hernia repair: a prospective randomized controlled study. Anesth Analg. (2019) 129:235–40. doi: 10.1213/ane.0000000000004071
25. Aksu C, Kus A, Yörükoglu HU, Tor Kılıç C, Gürkan Y. The effect of erector spinae plane block on postoperative pain following laparoscopic cholecystectomy: a randomized controlled study. Anestezi Dergisi. (2019) 27:9–14. doi: 10.5222/jarss.2019.14632
26. Altıparmak B, Korkmaz Toker M, Uysal AI, Kuşçu Y, Gümüş Demirbilek S. Ultrasound-guided erector spinae plane block versus oblique subcostal transversus abdominis plane block for postoperative analgesia of adult patients undergoing laparoscopic cholecystectomy: randomized, controlled trial. J Clin Anesth. (2019) 57:31–6. doi: 10.1016/j.jclinane.2019.03.012
27. Bryniarski P, Bialka S, Kepinski M, Szelka-Urbanczyk A, Paradysz A, Misiolek H. Erector spinae plane block for perioperative analgesia after percutaneous nephrolithotomy. Int J Environ Res Public Health. (2021) 18:3625. doi: 10.3390/ijerph18073625
28. Canıtez A, Kozanhan B, Aksoy N, Yildiz M, Tutar MS. Effect of erector spinae plane block on the postoperative quality of recovery after laparoscopic cholecystectomy: a prospective double-blind study. Br J Anaesth. (2021) 127:629–35. doi: 10.1016/j.bja.2021.06.030
29. Dost B, Kaya C, Ozdemir E, Ustun YB, Koksal E, Bilgin S, et al. Ultrasound-guided erector spinae plane block for postoperative analgesia in patients undergoing open radical prostatectomy: a randomized, placebo-controlled trial. J Clin Anesth. (2021) 72:110277. doi: 10.1016/j.jclinane.2021.110277
30. Fu J, Zhang G, Qiu Y. Erector spinae plane block for postoperative pain and recovery in hepatectomy: a randomized controlled trial. Medicine. (2020) 99:e22251. doi: 10.1097/md.0000000000022251
31. Gultekin MH, Erdogan A, Akyol F. Evaluation of the efficacy of the erector spinae plane block for postoperative pain in patients undergoing percutaneous nephrolithotomy: a randomized controlled trial. J Endourol. (2020) 34:267–72. doi: 10.1089/end.2019.0777
32. Hamed MA, Goda AS, Basiony MM, Fargaly OS, Abdelhady MA. Erector spinae plane block for postoperative analgesia in patients undergoing total abdominal hysterectomy: a randomized controlled study original study. J Pain Res. (2019) 12:1393–8. doi: 10.2147/jpr.S196501
33. Ibrahim M. Erector spinae plane block in laparoscopic cholecystectomy, is there a difference? A randomized controlled trial. Anesth Essays Res. (2020) 14:119–26. doi: 10.4103/aer.AER_144_19
34. Ibrahim M, Elnabtity AM. Analgesic efficacy of erector spinae plane block in percutaneous nephrolithotomy: a randomized controlled trial. Der Anaesthesist. (2019) 68:755–61. doi: 10.1007/s00101-019-00673-w
35. Kamel AAF, Amin OAI, Ibrahem MAM. Bilateral ultrasound-guided erector spinae plane block versus transversus abdominis plane block on postoperative analgesia after total abdominal hysterectomy. Pain Physician. (2020) 23:375–82.
36. Kim D, Kim JM, Choi G-S, Heo G, Kim GS, Jeong JS. Ultrasound-guided erector spinae plane block for postoperative analgesia in laparoscopic liver resection: a prospective, randomised controlled, patient and observer-blinded study. Eur J Anaesthesiol. (2021) 38:S106–12. doi: 10.1097/eja.0000000000001475
37. Kwon HM, Kim DH, Jeong SM, Choi KT, Park S, Kwon HJ, et al. Does erector spinae plane block have a visceral analgesic effect?: a randomized controlled trial. Sci Rep. (2020) 10:8389. doi: 10.1038/s41598-020-65172-0
38. Mostafa SF, Abdelghany MS, Abu Elyazed MM. Ultrasound-guided erector spinae plane block in patients undergoing laparoscopic bariatric surgery: a prospective randomized controlled trial. Pain Pract. (2021) 21:445–53. doi: 10.1111/papr.12975
39. NCT03989570 Transversus Abdominis Plane Block. Versus Erector Spinae Plane Block. (2019). Available online at: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01952617/full (accessed June 30, 2020).
40. Ozdemir H, Araz C, Karaca O, Turk E. Comparison of ultrasound-guided erector spinae plane block and subcostal transversus abdominis plane block for postoperative analgesia after laparoscopic cholecystectomy: a randomized, controlled trial. J Invest Surg. (2021) 35:870–7. doi: 10.1080/08941939.2021.1931574
41. Prasad MK, Varshney RK, Jain P, Choudhary AK, Khare A, Jheetay GS. Postoperative analgesic efficacy of fluoroscopy-guided erector spinae plane block after percutaneous nephrolithotomy (PCNL): a randomized controlled study. Saudi J Anaesth. (2020) 14:480–6. doi: 10.4103/sja.SJA_26_20
42. Tulgar S, Kapakli MS, Senturk O, Selvi O, Serifsoy TE, Ozer Z. Evaluation of ultrasound-guided erector spinae plane block for postoperative analgesia in laparoscopic cholecystectomy: a prospective, randomized, controlled clinical trial. J Clin Anesth. (2018) 49:101–6. doi: 10.1016/j.jclinane.2018.06.019
43. Verma R, Srivastava D, Saxena R, Singh TK, Gupta D, Agarwal A, et al. Ultrasound-guided bilateral erector spinae plane block for postoperative analgesia in laparoscopic cholecystectomy: a randomized controlled trial. Anesth Essays Res. (2020) 14:226–32. doi: 10.4103/aer.AER_41_20
44. Yildiz M, Kozanhan B, Iyisoy MS, Canıtez A, Aksoy N, Eryigit A. The effect of erector spinae plane block on postoperative analgesia and respiratory function in patients undergoing laparoscopic cholecystectomy: a double-blind randomized controlled trial. J Clin Anesth. (2021) 74:110403. doi: 10.1016/j.jclinane.2021.110403
45. Zengin SU, Ergun MO, Gunal O. Effect of ultrasound-guided erector spinae plane block on postoperative pain and intraoperative opioid consumption in bariatric surgery. Obes Surg. (2021) 31:5176–82. doi: 10.1007/s11695-021-05681-7
46. Mostafa SF, El Mourad MB. Ultrasound guided erector spinae plane block for percutaneous radiofrequency ablation of liver tumors. Egypt J Anaesth. (2020) 36:305–11. doi: 10.1080/11101849.2020.1854156
47. Celik M, Tulgar S, Ahiskalioglu A, Alper F. Is high volume lumbar erector spinae plane block an alternative to transforaminal epidural injection? Evaluation with MRI. Reg Anesth Pain Med. (2019) Epub ahead of print. doi: 10.1136/rapm-2019-100514
48. Yang HM, Choi YJ, Kwon HJ, O J, Cho TH, Kim SH. Comparison of injectate spread and nerve involvement between retrolaminar and erector spinae plane blocks in the thoracic region: a cadaveric study. Anaesthesia. (2018) 73:1244–50. doi: 10.1111/anae.14408
49. Greenhalgh K, Womack J, Marcangelo S. Injectate spread in erector spinae plane block. Anaesthesia. (2019) 74:126–7. doi: 10.1111/anae.14523
50. Vidal E, Giménez H, Forero M, Fajardo M. Erector spinae plane block: a cadaver study to determine its mechanism of action. Rev Esp Anestesiol Reanim (Engl Ed). (2018) 65:514–9. doi: 10.1016/j.redar.2018.07.004
51. Hacibeyoglu G, Topal A, Arican S, Kilicaslan A, Tekin A, Uzun ST. USG guided bilateral erector spinae plane block is an effective and safe postoperative analgesia method for living donor liver transplantation. J Clin Anesth. (2018) 49:36–7. doi: 10.1016/j.jclinane.2018.06.003
52. Tulgar S, Kapakli MS, Kose HC, Senturk O, Selvi O, Serifsoy TE, et al. Evaluation of ultrasound-guided erector spinae plane block and oblique subcostal transversus abdominis plane block in laparoscopic cholecystectomy: randomized, controlled, prospective study. Anesth Essays Res. (2019) 13:50–6. doi: 10.4103/aer.AER_194_18
53. Oh SK, Lim BG, Won YJ, Lee DK, Kim SS. Analgesic efficacy of erector spinae plane block in lumbar spine surgery: a systematic review and meta-analysis. J Clin Anesth. (2022) 78:110647. doi: 10.1016/j.jclinane.2022.110647
54. Hussain N, Brull R, Noble J, Weaver T, Essandoh M, McCartney CJ, et al. Statistically significant but clinically unimportant: a systematic review and meta-analysis of the analgesic benefits of erector spinae plane block following breast cancer surgery. Reg Anesth Pain Med. (2021) 46:3–12. doi: 10.1136/rapm-2020-101917
Keywords: erector spinae plane block, abdominal surgery, nerve block, opioid consumption, anesthesia
Citation: Gao Y, Liu L, Cui Y, Zhang J and Wu X (2022) Postoperative analgesia efficacy of erector spinae plane block in adult abdominal surgery: A systematic review and meta-analysis of randomized trials. Front. Med. 9:934866. doi: 10.3389/fmed.2022.934866
Received: 03 May 2022; Accepted: 19 August 2022;
Published: 04 October 2022.
Edited by:
Shun Ming Chan, Tri-Service General Hospital, TaiwanReviewed by:
Ece Yamak Altinpulluk, Morphological Madrid Research Center, SpainAntonio Sarría-Santamera, Nazarbayev University School of Medicine, Kazakhstan
Copyright © 2022 Gao, Liu, Cui, Zhang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiuying Wu, V3V4aXV5aW5nMDQxNUAxNjMuY29t; d3V4eUBzai1ob3NwaXRhbC5jb20=
 Yuzheng Gao
Yuzheng Gao Xiuying Wu
Xiuying Wu