- 1Shanghai Clinical Center for Diabetes, Shanghai Diabetes Institute, Shanghai Key Laboratory of Diabetes Mellitus, Department of Endocrinology and Metabolism, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China
- 2Department of Radiology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China
- 3Department of General Surgery, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, China
Background: Sleeve gastrectomy is an effective bariatric procedure; however, sleeve gastrectomy-related adverse skeletal outcomes have been increasingly reported. High levels of sex hormone-binding globulin (SHBG) have been documented to be a risk factor of bone mineral density (BMD) loss with different effects observed between sexes. The aim of this study was to identify sex-specific changes in BMD following sleeve gastrectomy and to evaluate the role of SHBG in this process.
Methods: This retrospective study included 19 middle-aged men and 30 non-menopausal women with obesity who underwent sleeve gastrectomy in China. Anthropometrics, bone turnover markers, calciotropic hormones, BMD, SHBG, and gonadal steroids were measured preoperatively and at 6 and 12 months postoperatively. Longitudinal changes in BMD, bone turnover markers and SHBG were compared between sexes by linear mixed models. Multiple stepwise regression analysis was used to identify the predictors of BMD loss at the investigated bone sites.
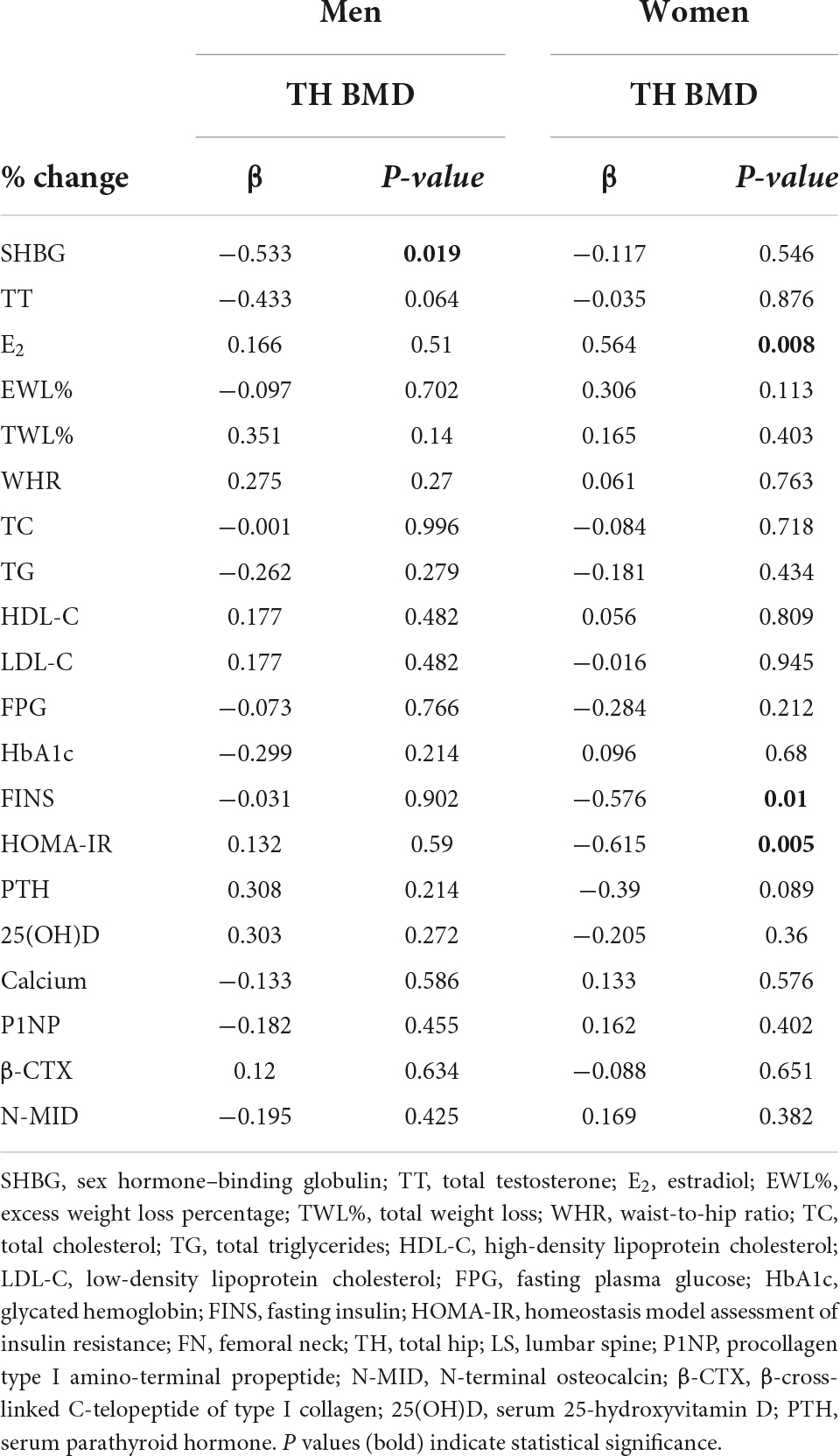
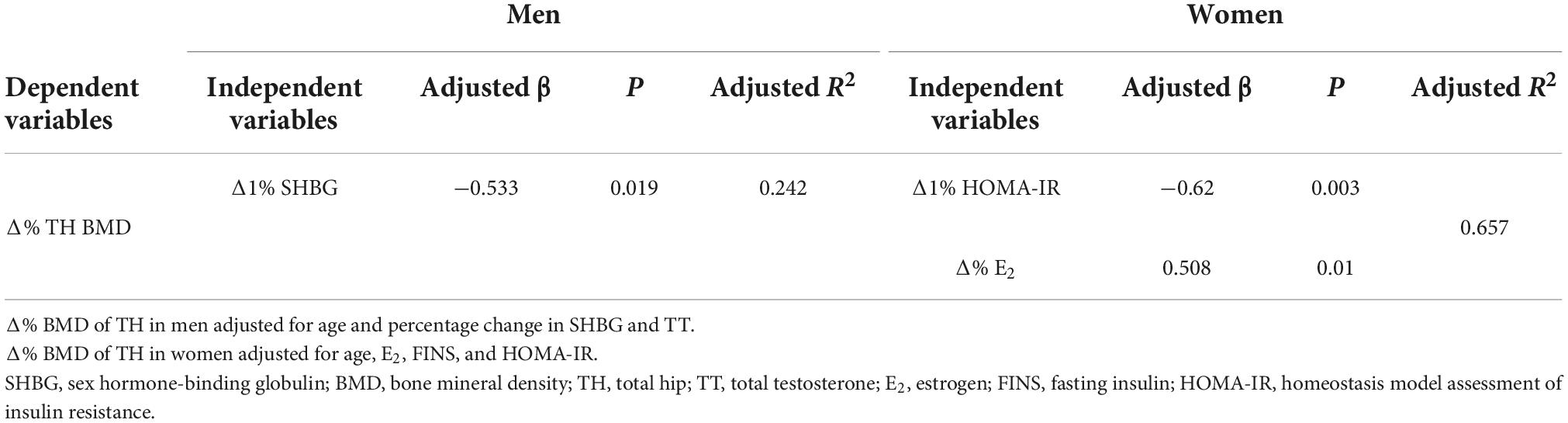
Results: Over the 12-month study period, total hip and femoral neck BMD decreased, while lumbar spine BMD remained largely unchanged in both sexes. Linear mixed models revealed significant sex × time interaction effects in total hip BMD and SHBG, showing that men had a significantly greater reduction in total hip BMD and less increase in SHBG after sleeve gastrectomy than women. In the multivariate model, SHBG was significantly associated with total hip BMD loss in men (adjusted β = −0.533, P = 0.019) but not women while total estrogen was significantly associated with total hip BMD loss in women (adjusted β = 0.508, P = 0.01) but not men.
Conclusion: Significant sex-specific BMD changes were observed after sleeve gastrectomy in the current study. Sleeve gastrectomy-related increase in SHBG may be a specific risk factor for total hip BMD loss in men. Our results indicate that sex-specific screening may be warranted to facilitate personalized postoperative bone care in this population.
Introduction
Bariatric surgery (BS) is a common and effective method for treating morbid obesity (1), of which Roux-en-Y gastric bypass and sleeve gastrectomy (SG) (2) are the most commonly performed procedures worldwide. Sleeve gastrectomy (SG) has gradually become the predominant bariatric procedure (2). However, these procedures can adversely affect bone turnover, leading to loss of bone mineral density (BMD) and skeletal fractures (3–5). Many studies have collectively confirmed the detrimental effects of Roux-en-Y gastric bypass on bone mass (6, 7) while the bone remodeling effects of SG are controversial with marked differences between sexes. At 6 months post SG, Wojciech et al. (8) reported significantly decreased BMD at the femoral neck (FN), total hip (TH), and lumbar spine (LS) in 29 obese women, while Adamczyk et al. (9) observed unchanged total body BMD, decreased FN BMD, and increased LS BMD in 25 obese men. Most of the current literature exploring the features of BS-related bone metabolism involved either men or women, with mixed studies including men and women, regardless of menstrual status. Thus, reports on sex-based differences in bone outcomes are scarce.
The cause of sex-based differences of BMD is thought to be multifactorial, including the particular sex hormones and related factors, other hormones regulating the hypothalamic-pituitary-gonadal axis, as well as genetic and mechanical factors. Sex hormones play a crucial role in skeletal integrity. Following SG, previous studies have indicated markable changes in sex hormones leading to the improvement of polycystic ovary syndrome and infertility in women (10, 11) and hypogonadism in men (12, 13) with a general trend toward sex hormones becoming more balanced. In addition, studies have shown that SG causes significant sex-specific changes in sex hormones. In men, an increase in total testosterone (TT) and sex hormone-binding globulin (SHBG) levels, and conversely, unchanged estradiol (E2) levels have been observed (13). In women, BS has been shown to significantly increase SHBG levels and decrease total E2 and TT levels (14).
SHBG is a liver-derived glycoprotein that binds tightly to testosterone and E2 and regulates their bioavailability in the serum (15). Levels of SHBG were reported to be stable during the menstrual cycle (16, 17). Clinically, it has been indicated as a potential biomarker for insulin resistance (18), polycystic ovary syndrome (19) and liver disease (20). Some studies have shown that a higher level of SHBG is a risk factor for osteoporosis and fractures, independent of sex steroid levels (21–24). As SHBG levels have been shown to increase after SG, it is worthwhile to assess whether high level of SHBG can lead to detrimental effects, such as BMD loss, postoperatively.
Few studies have described the role of SHBG in BS-related BMD loss and whether there is a sex discrepancy in the association. Therefore, the main aim of this study was to investigate sex differences in BMD and bone turnover markers (BTMs) at three different bone sites 12 months post SG. The secondary aim was to preliminarily propose the potential association between SHBG levels and SG-related BMD loss.
Materials and methods
Study design and participants
In this retrospective study, we recruited a total of 19 men and 30 women with obesity at Shanghai Jiao Tong University Affiliated Sixth People’s Hospital between March 2015 and July 2020. The inclusion criteria were as follows: (1) men aged < 50 years and women aged < 45 years with regular menstrual cycles, and (2) fulfillment of the Chinese SG guideline criteria (25). The exclusion criteria were as follows: (1) usage of medications affecting bone metabolism, including bisphosphonates, teriparatide, and oral glucocorticoids; (2) bone diseases of different etiologies (primary hyperparathyroidism, Paget disease); (3) usage of sex steroid products; (4) missing data on age, sex, body mass index (BMI), BMD, or laboratory investigations; and (5) clinical or laboratory evidence of hepatic or renal disease, gestation or lactation, or malignant disease.
All clinical measurements were recorded at baseline, 6 and 12 months postoperatively. The patients were administered with 50 μg/day of vitamin D3 and 600 mg/day of calcium postoperatively. This study was approved by the Ethics Committee for Human Investigation of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. Informed consent was obtained from all participants prior to their participation in the study. All methods were performed in accordance with the Declaration of Helsinki of the World Medical Association.
Laparoscopic sleeve gastrectomy technique
For laparoscopic SG, a 27-French oral gastric tube was placed through the pylorus, near the lesser curvature. Gastric tubulization was performed starting 2–6 cm from the pylorus by resecting straight toward the angle of His. Most of the greater curvature including the complete fundus was removed, with the antrum partially retained, leaving a small gastric tubular pouch of approximately 60–80 mL in volume. Two general surgery specialists performed the SG operations (26) for all patients using standardized laparoscopic techniques.
Bone mineral density measurement
Dual-energy X-ray absorptiometry [Hologic QDR-2000 (Hologic Corporation, Waltham, MA, United States)] was used to measure areal BMD (g/cm2) of the TH, FN, and LS (LS1–4) preoperatively and at 6 and 12 months postoperatively. The coefficient of variation was 0.8–1.0% for areal BMD. Prodigy Encore software (version 6.70, standard-array mode; GE Healthcare) was used for image analysis, as previously described (27). Two medically trained technicians performed the dual-energy X-ray absorptiometry scans and analyzed the images.
Clinical and laboratory assessments
Anthropometric and laboratory parameters, including sex hormone levels, bone metabolic indexes, and glucose-lipid profiles, were assessed preoperatively and 6 and 12 months postoperatively. Anthropometric measurements, including height (cm), weight (kg), and waist circumference (cm), were recorded using standardized methods (27). BMI (kg/m2) was calculated as weight (kg)/[height (m)]2. The excess weight loss percentage was calculated as (preoperative weight - current weight)/(preoperative weight - ideal weight) where ideal weight was based on a BMI of 24 kg/m2 (28). The percentage of total weight loss was calculated as 100 × (initial weight–postoperative weight)/initial weight. Laboratory parameters were determined using overnight fasting venous blood samples. Sex hormones, including total serum SHBG, TT, and total E2, were measured using an automated Roche electrochemiluminescence system (Roche Diagnostics GmbH, Germany). The bone metabolism variables of parathyroid hormone, 25-hydroxy vitamin D, procollagen type I amino-terminal propeptide (P1NP), N-terminal osteocalcin (N-MID), and β-cross-linked C-telopeptide of type I collagen (β-CTX), were determined by Roche electrochemiluminescence system (Roche Diagnostics GmbH, Germany). Plasma glucose levels were determined using the glucose oxidase method and glycated hemoglobin levels were measured using high-performance liquid chromatography (Bio-Rad Inc., Hercules, CA, United States). Serum insulin was assayed using radioimmunoassay with specific insulin detection kits (Roche Diagnostics [Shanghai] Limited Company, Shanghai, China). Homeostatic model assessment for insulin resistance was calculated as fasting insulin (mIU/L) × fasting glucose (mM)/22.5. The homeostasis model assessment of β-cell function was assessed as [20 × insulin (mIU/mL)]/[glucose (mmol/L)– 3.5] (29). Lipid profiles including serum total cholesterol, total triglycerides, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol levels were analyzed using standard enzymatic methods with a 7600–120 Hitachi automatic analyzer (Hitachi, Tokyo, Japan).
Statistical analyses
Data distribution was evaluated using the Shapiro–Wilk test. Normally distributed data were reported as mean ± standard deviation, while skewed data were reported as median (interquartile range). The skewed variables were logarithmically transformed (log-transformed) before the analysis. The clinical characteristics at 6 or 12 months after SG were compared with baseline using a paired-samples test or Wilcoxon signed-rank test within each sex group. A linear mixed effects model was used to assess the time and sex effects of bone metabolic variables and SHBG. The estimated marginal means of BTMs and SHBG were derived from the log-transformed data. After adjusting for baseline values, the percent change from baseline to 6 or 12 months postoperatively was calculated as 100 × (initial measurement–postoperative measurement)/initial measurement. Correlations between TH BMD and clinical variables including SHBG and sex steroids were assessed using single linear regression analysis, while potential variables accounting for BMD were entered into multiple stepwise regression analysis. These analyses were performed as subgroup analyses by sex to determine between-group differences. Significance was set at a two-sided test with a P value < 0.05. All statistical analyses were performed using the IBM Statistical Product and Service Solutions Statistics for Windows (version 26.0; IBM Corp., Armonk, NY, United States).
Results
Sleeve gastrectomy-related changes in anthropometrics and metabolic characteristics
This retrospective study included 49 participants with a mean age of 32.9 ± 7.80 years with no significant difference between sexes. The comparable mean BMI at baseline was 35.01 ± 4.71 kg/m2 (P > 0.05). The mean waist circumference and waist-to-hip ratio at baseline were 110.88 ± 9.82 cm and 0.97 ± 0.06, respectively, with a lower waist-to-hip ratio observed in women. Anthropometric values decreased postoperatively over 12 months in both men and women. TT levels significantly increased in men (11.77 ± 4.31 nM vs. 19.86 ± 6.38 nM, baseline vs. 12 months follow-up, P < 0.001), but decreased in women (1.13 ± 0.52 nM vs. 0.73 ± 0.34 nM, baseline vs. 12 months follow-up, P < 0.027). Levels of E2 significantly increased in women over the 12 months (221.01 ± 155.29 nM vs. 418.052 ± 369.23 nM, P < 0.016), but no significant change were observed in men (P = 0.298). Except for the total cholesterol and low-density lipoprotein cholesterol levels, the glucose-lipid metabolic variables improved significantly in both sexes, as expected (P < 0.05). Serum calcium levels remained stable (P > 0.05). 25-hydroxy vitamin D levels significantly increased in women (13.73 ± 5.54 mM vs. 18.92 ± 9.59 mM) but remained largely unchanged in men. Phosphorus levels increased (P < 0.05) while parathyroid hormone levels did not change significantly over 12 months (P > 0.05) in both sexes. Most of the anthropometric and clinical characteristics of participants varied as a function of SG, and the mean values were shown within each sex group in Table 1 with the distribution of data in Supplementary Table 1.
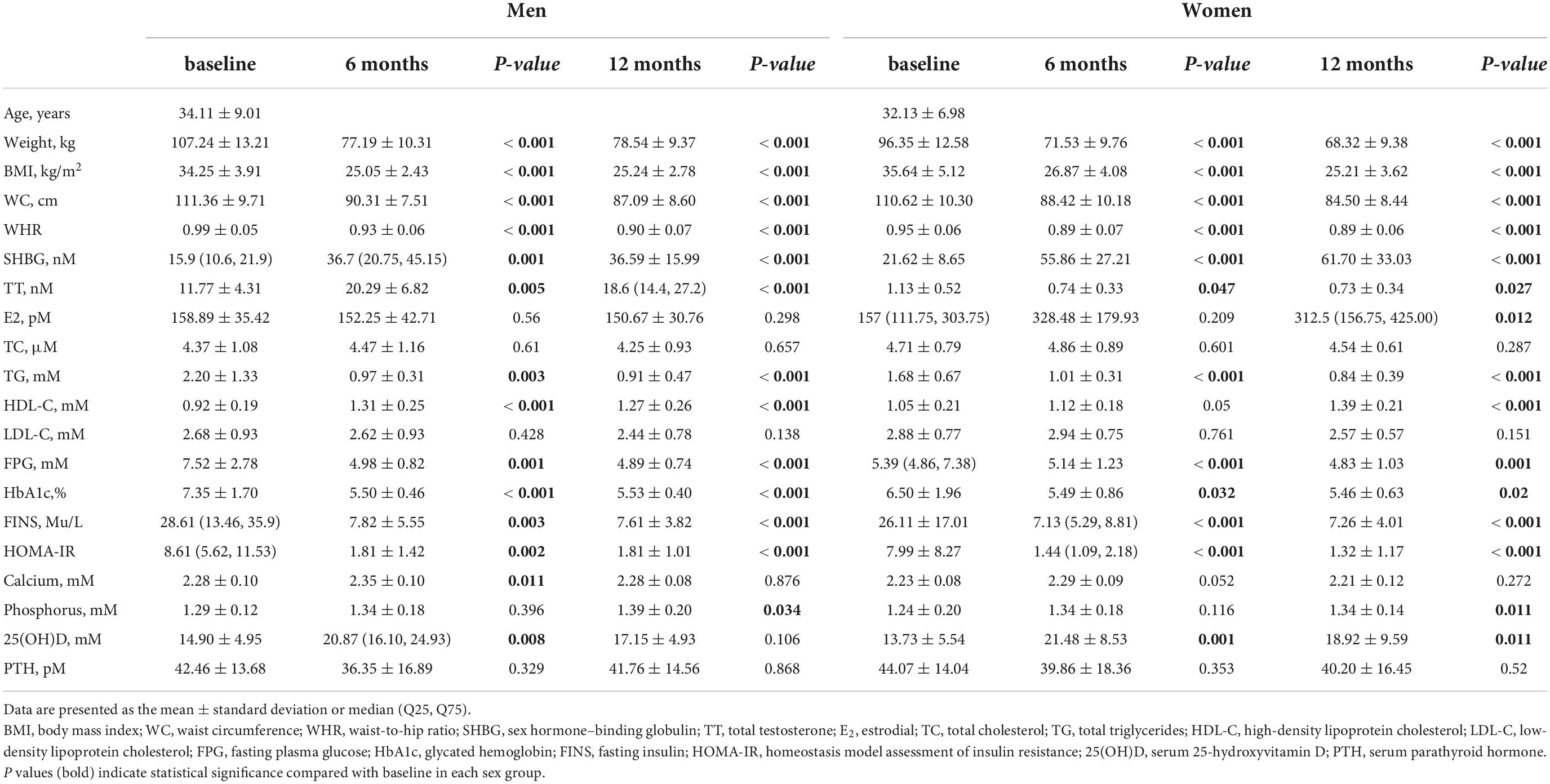
Table 1. Clinical characteristics of the participants at baseline and at 6 and 12 months after sleeve gastrectomy.
Sex discrepancy of sleeve gastrectomy-related changes in bone mineral density
During the 12-month follow-up period, none of the patients developed osteoporosis. The mean BMD values at three sites at baseline and 6 and 12 months after SG were shown in Supplementary Table 2. TH BMD and FN BMD was comparable between sexes at baseline, respectively. The mean LS BMD level was higher in women than in men at baseline (1.253 ± 0.135 g/cm2 vs. 1.166 ± 0.103 g/cm2, P < 0.01). Longitudinal data analysis, according to linear mixed effects models, showed a significant SG-related impact of BMD loss at TH (P < 0.001) and FN (P < 0.001) over 12 months, but not at LS (P = 0.234) in both sexes. The dynamic changes of BMDs across different time points in men and women were present in Figure 1, with estimated marginal means and corresponding standard errors. When investigating the interaction effect of sex and SG across time points, men demonstrated greater TH BMD loss compared to women at 12 months (β = 0.025, SE = 0.009, P = 0.008) (Table 2). Overall, men with obesity might be more vulnerable to SG-related BMD loss than women with obesity, especially for TH BMD.
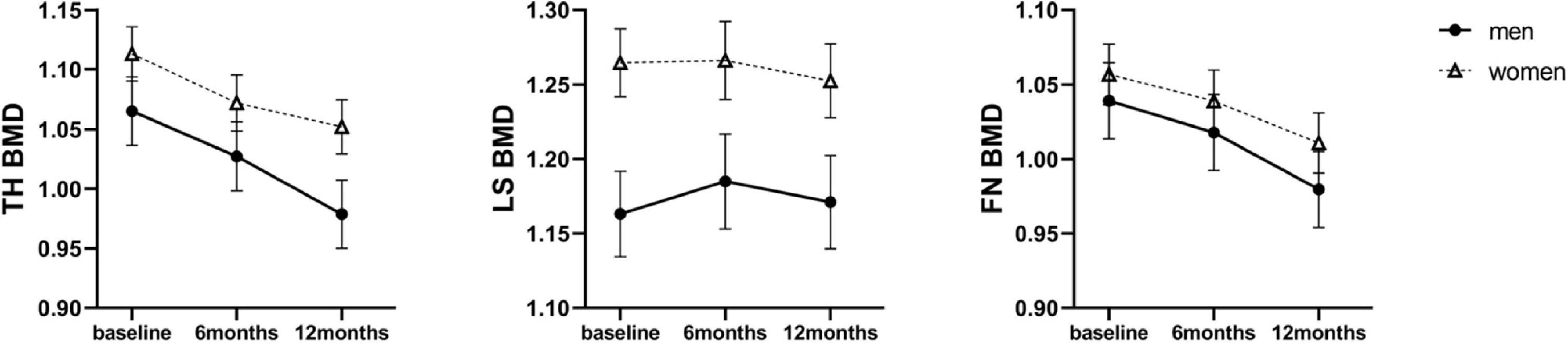
Figure 1. Estimated marginal means (y-axis) of BMD for men and women group over time points (x-axis). Error bars indicate standard error. BMD, bone mineral density; TH, total hip; LS, lumbar spine; FN, femoral neck.
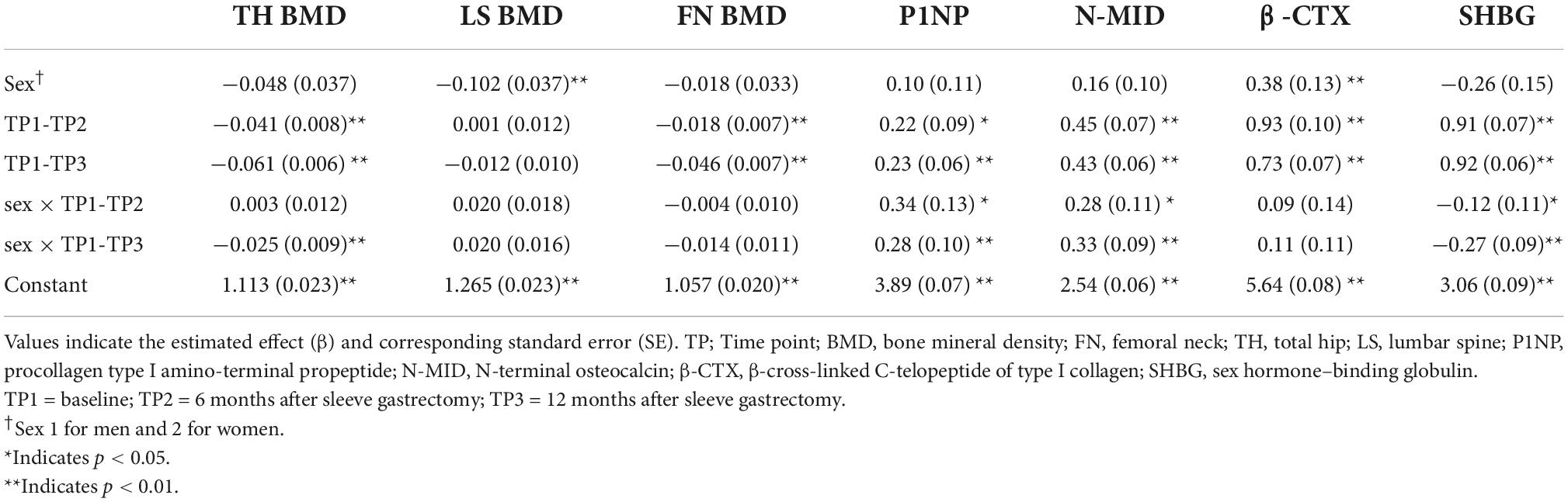
Table 2. Linear mixed effects in sex- and sleeve gastrectomy -related changes of bone metabolic variables and SHBG at 6 and 12 months of follow-up.
Sex discrepancy of sleeve gastrectomy-related changes in bone turnover markers
Bone tissue undergoes continuous remodeling to maintain a dynamic balance between bone formation and resorption, which contributes to BMD. The following serum markers of bone remodeling were measured: P1NP and N-MID, representing bone formation, and β-CTX representing bone resorption. The raw mean values of BTMs at baseline and 6 and 12 months after SG are shown in Supplementary Table 2. The level of β-CTX was higher in men than in women at baseline (463.09 ± 181.59 mg/mL vs. 322.57 ± 182.10 mg/mL, P < 0.05). All values of BTMs increased in both sexes across 12 months (Figure 2). When investigating the different effects of SG between sexes across time points, linear mixed effects models revealed higher levels of P1NP and N-MID in men than women at both 6 and 12 months (Table 2). Therefore, the bone activity was greater in men than that in women 12 months after SG.
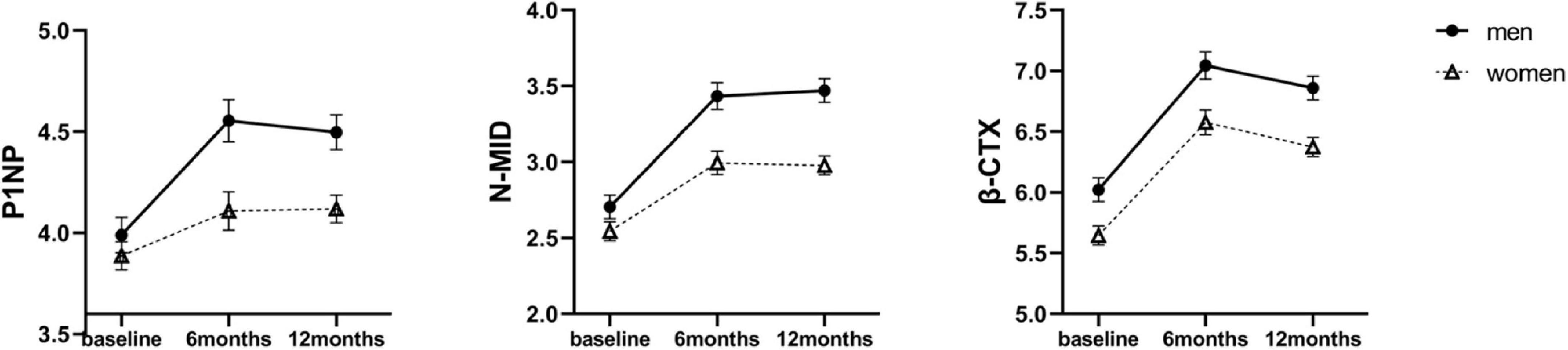
Figure 2. Estimated marginal means (y-axis) of bone turnover markers for men and women group over time points (x-axis). Error bars indicate standard error. P1NP, procollagen type I amino-terminal propeptide; β-CTX, β-cross-linked C-telopeptide of type I collagen; N-MID, N-terminal osteocalcin.
Sex discrepancy of sleeve gastrectomy-related changes in serum sex hormone-binding globulin
There was no significant sex difference in SHBG levels at baseline [18.42 ± 7.93 nM (men) vs. 21.62 ± 8.65 nM (women), P = 0.144]. Compared with baseline, SHBG levels increased to 38.59 ± 17.24 nM at 6 months and decreased slightly to 36.59 ± 15.99 nM at 12 months in men (P < 0.001). In women, SHBG levels also increased progressively over the 12 months to 61.70 ± 33.03 nM (P < 0.001). The linear mixed model shown significant main interaction effects of sex × time for SHBG at 6 months (β = −0.12, SE = 0.11, P = 0.019) and 12 months (β = −0.27, SE = 0.09, P = 0.001) after SG. This indicated that the increase in SHBG levels in men was far less than that in women over the 12 months (Table 2 and Figure 3).
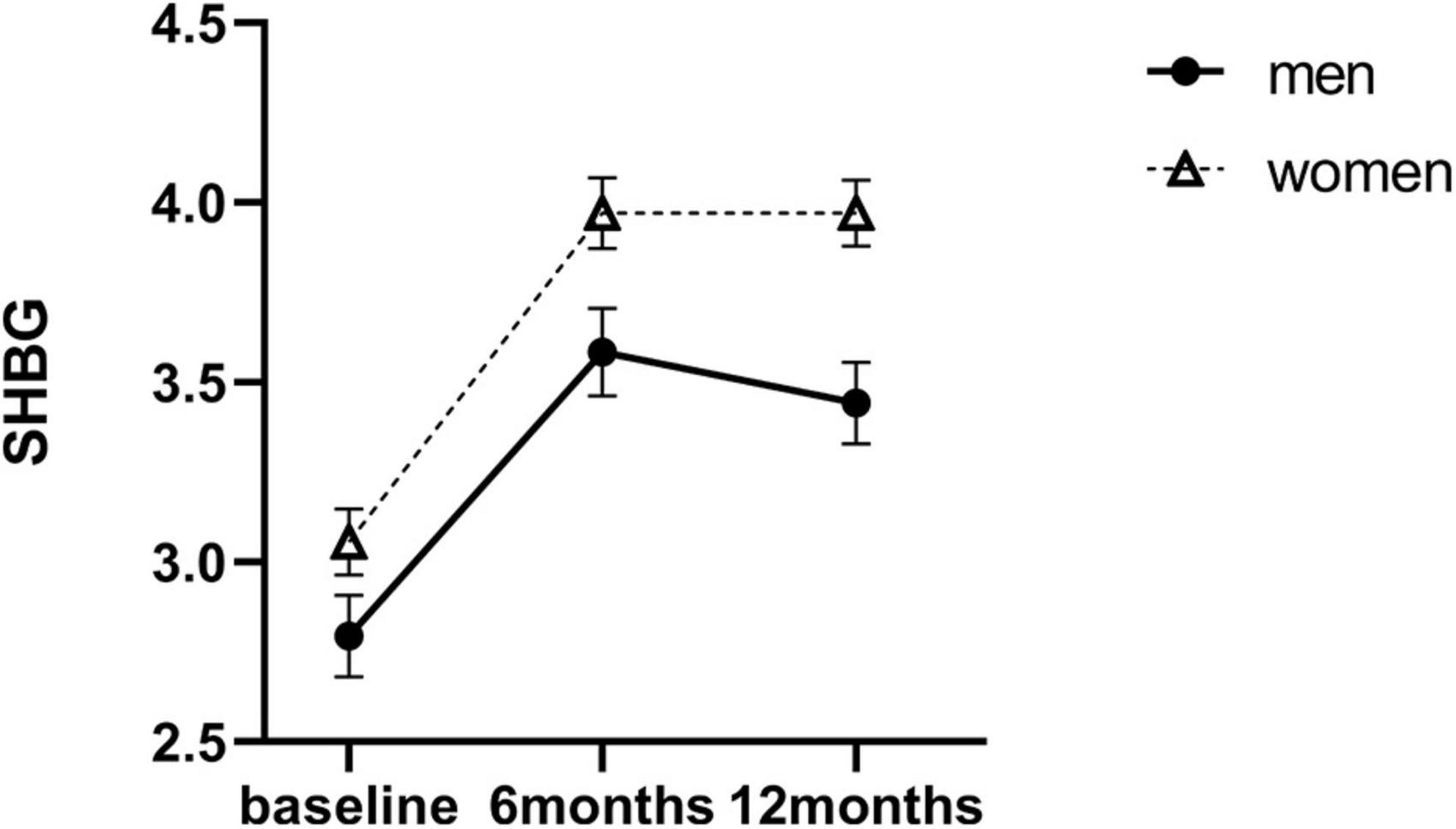
Figure 3. Estimated marginal means (y-axis) of SHBG for men and women group over time points (x-axis). Error bars indicate standard error. SHBG, sex hormone–binding globulin.
Potential variables accounting for total hip bone mineral density loss between sexes
To evaluate potential variables related to the sex-specific TH BMD loss, single linear regression analysis was performed in both sexes (Table 3). Subsequently, stepwise multiple logistic regression analysis was performed to identify potential independent variables of TH BMD loss. The results of these analyses are presented for men and women separately in Table 4.
For men, SHBG (adjusted β = −0.533, P = 0.019) was the best predictor of TH BMD loss (R2 = 0.242). For women, the best predictors for TH BMD loss were homeostatic model assessment for insulin resistance (adjusted β = −0.62, P = 0.003) and E2 (adjusted β = 0.508, P = 0.01) (R2 = 0.657). This indicated that SHBG might play a key role in TH BMD loss in men, but not in women. In conclusion, predictors for TH BMD loss are different between men and women.
Discussion
In this longitudinal study, we observed significant sex-related differences in SG-related bone remodeling and in the association between SHBG and BMD. Although BMD was reduced postoperatively in both men and women, men had significantly more TH BMD loss and relatively higher levels of bone formation markers (P1NP and N-MID) than women. Changes in SHBG level was negatively associated with TH BMD loss in men, whereas in women, SHBG levels was not associated with BMD loss at any of the measured sites.
Sex-related differences in BMD changes after SG have not been clearly or extensively described in the literature. A previous meta-analysis (30) reported that TH and FN BMD decreased in both sexes 12 months after SG, but LS BMD remained unchanged. In this regard, our data are largely in agreement with the results. Furthermore, our linear mixed effects models indicated that TH BMD reduction was more profound in men than women, and no significant sex difference of FN BMD levels was observed. In contrast to our results, a study in Romania reported significant BMD loss in men at all investigated sites, including LS and FN, compared with women (31) and a Chinese study conducted by Wang et al. (32) found that BMD was significantly decreased in women but unchanged in men after SG. Variations in study parameters such as ethnicity, age of participants, and study period may explain these differences.
Studies have proved that bone remodeling is activated to a lesser extent after SG than after Roux-en-Y gastric bypass (33, 34). However, sex discrepancies in BTM changes after BS have rarely been reported. In the Chinese population, Wang et al. (32) found that type I collagen and osteocalcin increased in both men and women after SG. In line with this study, our data shown that all BTMs including P1NP, N-MID and β-CTX increased postoperatively. These results indicated that in both men and women, SG enhanced the bone remodeling. Furthermore, we displayed the effects of sex and time in BTM changes after SG in linear mixed effects model, revealing a more significant increase in P1NP and N-MID in men than women. Our results indicated that men were more susceptible to BMD loss, especially TH BMD loss, which may be due to the greater activation of bone turnover in men.
A possible explanation for sex-specific SG-related BMD loss could be the postoperative endocrine-disrupting chemicals. They affect estrogenic or androgenic signaling pathways or act independently of the sex steroid axis. Higher levels of E2, as observed in menstruating women, is a widely accepted factor for the sex disparity in BMD. This might explain the positive relationship of percentile change between E2 and TH BMD observed in women but not in men in our study. Correspondingly, levels of SHBG, which modulate sex hormones, might also be relevant to the sex-specific BMD changes after SG. A higher SHBG level has been associated with BMD loss (21, 35). Moreover, previous studies have reported that SG causes a significant increase in SHBG levels in men (13) and women (14) separately. Our study found that, over 12 months, SHBG levels increased in both sexes, with a greater effect in women. However, increase in SHBG levels were inversely related to TH BMD loss in men but were not associated with BMD loss at any site in women. Yang et al. (36) conducted a cross-sectional study of 6,434 participants to examine the association between sex hormone levels and BMD and found a differential effect of SHBG on BMD by sex, which was similar to our findings.
The mechanism by which SHBG influences BMD in different sexes remains unclear. One possible explanation for the sex disparity may be the “free hormone hypothesis” (37), whereby SHBG acts as a biologically inert buffer to modulate and transport androgens or estrogens from circulation to bone cells with various affinities to sex steroids: dihydrotestosterone > testosterone > androstenediol > E2 > estrone (19). Grasa et al. (38) also reported that the binding capacity of SHBG was affected by sex due to the differences in structure and affinities of gonadal steroids (binding sites are mostly occupied by testosterone due to a lower estrogen-binding capacity in men). In addition to the indirect effect of SHBG on BMD, SHBG can directly participate in signal transduction in bone cells with an SHBG membrane receptor (39) or by endocytic facilitation of SHBG-bound androgens and estrogens (40). Moreover, genetic polymorphisms in SHBG might also contribute to the difference in BMD loss between men and women. Haplotype CCGGT (consisting of rs11078701C, rs1017163C, rs9898876G, rs62059836G, and rs2541012T) and haplotype CGGT (consisting of rs858521C, rs858518G, rs6259G, and rs727428T) in SHBG and neighboring genes have been associated with a significant risk effect for osteoporosis in Chinese men (41). In addition, polymorphism in the 5’UTR (G to A base change) of rs1799941 has been associated with lower TH BMD in postmenopausal women (42). However, further studies are needed to elucidate the sex-specific genetic variants of SHBG in BMD loss following SG.
To the best of our knowledge, this is the first study to show sex differences in post-SG BMD loss between middle-aged Chinese men and non-menopausal Chinese women by using linear mixed effects model. Furthermore, the detrimental effects of increased SHBG levels on BMD in men who underwent SG were demonstrated for the first time. Our results indicate the need for personalized bone care. However, this study has some limitations. First, the sample size was not large enough to draw definitive conclusions. Second, its retrospective nature may have led to a bias, including the lack of data pertaining to other factors which may affect bone health such as patients’ activity levels and nutritional intake. Third, causality cannot be assumed by this observational study, and this pilot study can only propose the possible link between SHBG levels and SG-related BMD loss. Further studies with larger sample sizes, longer follow-up periods, and polymorphisms in SHBG testing may be needed to evaluate the utility of these results in providing personalized care for patients post SG. In addition, animal or cell experiments are needed to elucidate the exact mechanisms for SHBG on bone metabolism.
In conclusion, men were more susceptible than women to the detrimental effects of SG on BMD, particularly TH BMD. This may be related to increased levels of SHBG after SG. Therefore, early targeted screening and intervention may be necessary for patients undergoing this surgical procedure. Furthermore, the determination of serum SHBG levels may be a useful screening tool for a personalized bone care postoperatively.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee for Human Investigation of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
DY, JH, and HY designed the study. DY, YY, and YT analyzed the data, prepared the figures, and drafted the manuscript. RX and YX recruited participants and collected the data. HZ, WL, and PZ were responsible for bariatric surgery. YB, JH, and HY contributed to the discussion and revised the manuscript. All authors have read and approved the final study.
Funding
This work was funded by the National Key Research and Development Project of China (2016YFA0502003), Clinical Research Plan of SHDC (No. SHDC2020CR1017B), National Natural Sciences Foundation of China (82170867) to Junfeng Han, Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (No. 20191920), Shanghai “Science and Technology Innovation Action Plan” Science and Technology support project in biomedical field (20S31903200), and Shanghai Key Clinical Center for Metabolic Disease (No. 2017ZZ01013).
Acknowledgments
We would like to thank all patients, clinicians, nurses, and technicians who participated in this work. We would also like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.957478/full#supplementary-material
Abbreviations
BS, bariatric surgery; SG, sleeve gastrectomy; BMD, bone mineral density; FN, femoral neck; TH, total hip; LS, lumbar spine; TT, total testosterone; E2, estradiol; SHBG, sex hormone-binding globulin; BTM, bone turnover marker; P1NP, procollagen type I amino-terminal propeptide; N-MID, N-terminal osteocalcin; β -CTX, β -cross-linked C-telopeptide of type I collagen.
References
1. Arterburn DE, Olsen MK, Smith VA, Livingston EH, Van Scoyoc L, Yancy WS Jr., et al. Association between bariatric surgery and long-term survival. JAMA. (2015) 313:62–70. doi: 10.1001/jama.2014.16968
2. Paccou J, Tsourdi E, Meier C, Palermo A, Pepe J, Body JJ, et al. Bariatric surgery and skeletal health: A narrative review and position statement for management by the European Calcified Tissue Society (ECTS). Bone. (2022) 154:116236. doi: 10.1016/j.bone.2021.116236
3. Alsaed OS, Al-Allaf AW, Elgenaied I, Jebril RA, Sasi S, Ahmed AO, et al. Increased fracture risk after bariatric surgery: a case-controlled study with a long-term follow-up. Obes Surg. (2021) 31:4853–60. doi: 10.1007/s11695-021-05655-9
4. Ieong K, Ardila-Gatas J, Yang J, Zhang X, Tsui ST, Spaniolas K, et al. Bone mineral density changes after bariatric surgery. Surg Endosc. (2021) 35:4763–70. doi: 10.1007/s00464-020-07953-2
5. Kim J, Nimeri A, Khorgami Z, El Chaar M, Lima AG, Vosburg RW. Metabolic bone changes after bariatric surgery: 2020 update, american society for metabolic and bariatric surgery clinical issues committee position statement. Surg Obes Relat Dis. (2021) 17:1–8. doi: 10.1016/j.soard.2020.09.031
6. Lindeman KG, Greenblatt LB, Rourke C, Bouxsein ML, Finkelstein JS, Yu EW. Longitudinal 5-Year Evaluation of Bone Density and Microarchitecture After Roux-en-Y Gastric Bypass Surgery. J Clin Endocrinol Metab. (2018) 103:4104–12. doi: 10.1210/jc.2018-01496
7. Krez A, Agarwal S, Bucovsky M, McMahon DJ, Hu Y, Bessler M, et al. Long-term bone loss and deterioration of microarchitecture after gastric bypass in african american and latina women. J Clin Endocrinol Metab. (2021) 106:e1868–79. doi: 10.1210/clinem/dgaa654
8. Pluskiewicz W, Bužga M, Holéczy P, Bortlík L, Šmajstrla V, Adamczyk P. Bone mineral changes in spine and proximal femur in individual obese women after laparoscopic sleeve gastrectomy: a short-term study. Obes Surg. (2012) 22:1068–76. doi: 10.1007/s11695-012-0654-8
9. Adamczyk P, Bužga M, Holéczy P, Švagera Z, Šmajstrla V, Zonèa P, et al. Bone mineral density and body composition after laparoscopic sleeve gastrectomy in men: A short-term longitudinal study. Int J Surg. (2015) 23:101–7. doi: 10.1016/j.ijsu.2015.09.048
10. Machado Júnior AS, Ribeiro C, Santa-Cruz F, Sena BF, Figueiredo JL, Áab F, et al. The effect of sleeve gastrectomy on the hormonal profile of patients with polycystic ovary syndrome. Obes Surg. (2019) 29:2415–9. doi: 10.1007/s11695-019-03854-z
11. Ezzat RS, Abdallah W, Elsayed M, Saleh HS, Abdalla W. Impact of bariatric surgery on androgen profile and ovarian volume in obese polycystic ovary syndrome patients with infertility. Saudi J Biol Sci. (2021) 28:5048–52. doi: 10.1016/j.sjbs.2021.05.022
12. Zhu C, Zhang Y, Wang X, Gao J, Lu L, Zhou D, et al. [Effect of laparoscopic sleeve gastrectomy on sex hormone in male severe obesity]. Zhonghua Wei Chang Wai Ke Za Zhi. (2017) 20:405–10.
13. Zhu C, Zhang Y, Zhang L, Gao J, Mei F, Zhu B, et al. Changes in sex hormones after laparoscopic sleeve gastrectomy in chinese obese men: a 12-month Follow-Up. Obes Surg. (2019) 29:869–77. doi: 10.1007/s11695-018-3611-3
14. Emami MR, Safabakhsh M, Khorshidi M, Moradi Moghaddam O, Mohammed SH, Zarezadeh M, et al. Effect of bariatric surgery on endogenous sex hormones and sex hormone-binding globulin levels: a systematic review and meta-analysis. Surg Obes Relat Dis. (2021) 17:1621–36. doi: 10.1016/j.soard.2021.05.003
15. Siiteri PK, Murai JT, Hammond GL, Nisker JA, Raymoure WJ, Kuhn RW. The serum transport of steroid hormones. Recent Prog Horm Res. (1982) 38:457–510. doi: 10.1016/b978-0-12-571138-8.50016-0
16. Jia MC, Zhou LY, Ren S, Dong L, Xiao B. Serum SHBG levels during normal menstrual cycle and after insertion of levonorgestrel-releasing IUD. Adv Contracept. (1992) 8:33–40. doi: 10.1007/BF01849346
17. Rezaii T, Gustafsson TP, Axelson M, Zamani L, Ernberg M, Hirschberg AL, et al. Circulating androgens and SHBG during the normal menstrual cycle in two ethnic populations. Scand J Clin Lab Invest. (2017) 77:184–9. doi: 10.1080/00365513.2017.1286685
18. Gascón F, Valle M, Martos R, Ruz FJ, Ríos R, Montilla P, et al. Sex hormone-binding globulin as a marker for hyperinsulinemia and/or insulin resistance in obese children. Eur J Endocrinol. (2000) 143:85–9. doi: 10.1530/eje.0.1430085
19. Qu X, Donnelly R. Sex Hormone-Binding Globulin (SHBG) as an Early Biomarker and Therapeutic Target in Polycystic Ovary Syndrome. Int J Mol Sci. (2020) 21:8191. doi: 10.3390/ijms21218191
20. Thaler MA, Seifert-Klauss V, Luppa PB. The biomarker sex hormone-binding globulin - from established applications to emerging trends in clinical medicine. Best Pract Res Clin Endocrinol Metab. (2015) 29:749–60. doi: 10.1016/j.beem.2015.06.005
21. Zha XY, Hu Y, Pang XN, Zhu JH, Chang GL, Li L. Sex hormone-binding globulin (SHBG) as an independent determinant of bone mineral density (BMD) among Chinese middle-aged and elderly men. Endocrine. (2014) 47:590–7. doi: 10.1007/s12020-013-0155-0
22. Hsu B, Seibel MJ, Cumming RG, Blyth FM, Naganathan V, Bleicher K, et al. Progressive temporal change in serum shbg, but not in serum testosterone or estradiol, is associated with bone loss and incident fractures in older men: the concord health and ageing in men project. J Bone Miner Res. (2016) 31:2115–22. doi: 10.1002/jbmr.2904
23. Vandenput L, Mellström D, Kindmark A, Johansson H, Lorentzon M, Leung J, et al. High serum SHBG predicts incident vertebral fractures in elderly men. J Bone Miner Res. (2016) 31:683–9. doi: 10.1002/jbmr.2718
24. Aleksova J, Wong P, McLachlan R, Choy KW, Ebeling PR, Milat F, et al. Sex hormone-binding globulin is a biomarker associated with nonvertebral fracture in men on dialysis therapy. Kidney Int. (2018) 94:372–80. doi: 10.1016/j.kint.2018.02.02
25. Du Y, Zhang J, Chen G, Sun Z. Formulation and interpretation of the Chinese Guidelines for Surgical Treatment of Obesity and Type 2 Diabetes Mellitus. Biosci Trends. (2021) 15:299–304. doi: 10.5582/bst.2021.01287
26. Wang L, Shi C, Yan H, Xia M, Zhu X, Sun X, et al. Acute effects of sleeve gastrectomy on glucose variability, glucose metabolism, and ghrelin response. Obes Surg. (2021) 31:4005–14. doi: 10.1007/s11695-021-05534-3
27. Wang J, Ma J, Yu H, Zhang P, Han J, Bao Y. Unacylated ghrelin is correlated with the decline of bone mineral density after Roux-en-Y gastric bypass in obese Chinese with type 2 diabetes. Surg Obes Relat Dis. (2019) 15:1473–80. doi: 10.1016/j.soard.2019.04.011
28. Tu Y, Pan Y, Han J, Pan J, Zhang P, Jia W, et al. A total weight loss of 25% shows better predictivity in evaluating the efficiency of bariatric surgery. Int J Obes (Lond). (2021) 45:396–403. doi: 10.1038/s41366-020-00690-5
29. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. (2004) 27:1487–95. doi: 10.2337/diacare.27.6.1487
30. Jaruvongvanich V, Vantanasiri K, Upala S, Ungprasert P. Changes in bone mineral density and bone metabolism after sleeve gastrectomy: a systematic review and meta-analysis. Surg Obes Relat Dis. (2019) 15:1252–60. doi: 10.1016/j.soard.2019.06.006
31. Malinici E, Sirbu A, Popa M, Andrei M, Ioacara S, Copaescu C, et al. Bone mineral density trends during the first year after laparoscopic sleeve gastrectomy-a cohort study on 241 patients. Obes Surg. (2021) 31:4885–92. doi: 10.1007/s11695-021-05661-x
32. Wang X, Li L, Zhu C, Gao J, Qu S. Alteration of bone mineral density differs between genders in obese subjects after laparoscopic sleeve gastrectomy: bone morphogenetic protein 4 may count. Obes Surg. (2018) 28:3221–6. doi: 10.1007/s11695-018-3298-5
33. Ivaska KK, Huovinen V, Soinio M, Hannukainen JC, Saunavaara V, Salminen P, et al. Changes in bone metabolism after bariatric surgery by gastric bypass or sleeve gastrectomy. Bone. (2017) 95:47–54. doi: 10.1016/j.bone.2016.11.001
34. Hofsø D, Hillestad T, Halvorsen E, Fatima F, Johnson LK, Lindberg M, et al. Bone Mineral density and turnover after sleeve gastrectomy and gastric bypass: a randomized controlled trial (Oseberg). J Clin Endocrinol Metab. (2021) 106:501–11. doi: 10.1210/clinem/dgaa808
35. Jing Y, Wang X, Yu J, Wang X, Zhou Y, Tao B, et al. Associations of serum sex hormone binding globulin with bone mineral densities and higher 10-year probability of fractures in postmenopausal women with type 2 diabetes mellitus. Ann Transl Med. (2019) 7:457. doi: 10.21037/atm.2019.08.46
36. Yang F, Yang D, Zhou Y, Wu J. Associations of sex hormone-binding globulin with bone mineral density among us adults, NHANES 2013-2016. Int J Gen Med. (2021) 14:7707–17. doi: 10.2147/IJGM.S329992
37. Goldman AL, Bhasin S, Wu F, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone’s binding in circulation: physiological and clinical implications. Endocr Rev. (2017) 38:302–24. doi: 10.1210/er.2017-00025
38. Grasa M, Gulfo J, Camps N, Alcalá R, Monserrat L, Moreno-Navarrete JM, et al. Modulation of SHBG binding to testosterone and estradiol by sex and morbid obesity. Eur J Endocrinol. (2017) 176:393–404. doi: 10.1530/EJE-16-0834
39. Kahn SM, Hryb DJ, Nakhla AM, Romas NA, Rosner W. Sex hormone-binding globulin is synthesized in target cells. J Endocrinol. (2002) 175:113–20. doi: 10.1677/joe.0.1750113
40. Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, Schulz H, et al. Role of endocytosis in cellular uptake of sex steroids. Cell. (2005) 122:751–62. doi: 10.1016/j.cell.2005.06.032
41. Zha XY, Hu Y, Pang XN, Zhu JH, Chang GL, Li L. The association between sex hormone-binding globulin gene polymorphism with bone mineral density. Steroids. (2016) 106:9–18. doi: 10.1016/j.steroids.2015.11.011
Keywords: sex heterogeneity, bone mineral density, sleeve gastrectomy, sex hormone-binding globulin, obesity
Citation: Yang D, Ye Y, Tu Y, Xu R, Xiao Y, Zhang H, Liu W, Zhang P, Yu H, Bao Y and Han J (2022) Sex-specific differences in bone mineral density loss after sleeve gastrectomy. Front. Med. 9:957478. doi: 10.3389/fmed.2022.957478
Received: 31 May 2022; Accepted: 20 September 2022;
Published: 26 October 2022.
Edited by:
Ziyi Zhang, University of Toronto, CanadaCopyright © 2022 Yang, Ye, Tu, Xu, Xiao, Zhang, Liu, Zhang, Yu, Bao and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haoyong Yu, eXVoYW95b25nMTExQDE2My5jb20=; Junfeng Han, amZoYW5Ac2p0dS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Di Yang
Di Yang Yafen Ye
Yafen Ye Yinfang Tu1
Yinfang Tu1 Haoyong Yu
Haoyong Yu