- 1Department of Ophthalmology, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 2Department of Ophthalmology, Chang Gung Memorial Hospital, Keelung, Taiwan
- 3Department of Cardiology, Chang Gung Memorial Hospital, Keelung, Taiwan
- 4School of Traditional Chinese Medicine, Chang Gung University, Taoyuan, Taiwan
- 5Biostatistical Consultation Center of Chang Gung Memorial Hospital, Keelung, Taiwan
- 6Department of Medicine, College of Medicine, Chang Gung University, Taoyuan, Taiwan
Purpose: To investigate the risk and protective factors of dry eye disease (DED) in patients with type II diabetes mellitus (DM).
Design: A retrospective cohort study using Chang- Gung research database collecting data from 2005 to 2020.
Methods: Patients with type II DM were included, and those with previous ocular diseases were excluded. Ten thousand twenty nine developed DED (DED group), and 142,491 didn't (non-DED group). The possible risk and protective factors were compared and analyzed using the logistic regression model.
Results: A majority of the DED group were female with significantly higher initial and average glycated hemoglobin levels, and higher incidence of diabetic neuropathy and retinopathy. In conditional logistic regression model, advanced age was a risk factor. After adjusting for sex, age, and DM duration; average glycated hemoglobin level, diabetic neuropathy, retinopathy, and nephropathy with eGFR 30 ~ 59 and intravitreal injection, vitrectomy, pan-retinal photocoagulation, and cataract surgery were contributing factors of DED. Considering antihyperglycemic agents, DPP4 inhibitor, SGLT2 inhibitor, GLP-1 agonist, and insulin monotherapy and dual medications combining any two of the aforementioned agents were protective factors against DED compared with metformin alone. In the monotherapy group, SLGT2 inhibitor had the lowest odds ratio, followed by GLP1 agonist, DPP4 inhibitor, and insulin.
Conclusions: DED in patients with DM is associated with female sex, advanced age, poor diabetic control, microvascular complications and receiving ocular procedures. GLP-1 agonist, SGLT-2 inhibitor, DPP4 inhibitor, and insulin are superior to metformin alone in preventing DM-related DED. A prospective randomized control trial is warranted to clarify our results.
Introduction
Diabetes mellitus (DM) is becoming a global health issue, and the epidemic has continuously occurred over the past decades (1). According to the International Diabetes Federation, in 2021, 747,000 individuals died due to DM in southeast Asia alone, and >11% of the total population in southeast Asia will develop DM in 2045 (2). There are numerous DM-related microvascular and macrovascular comorbidities, including ocular complications. The occurrence of retinopathy, papillopathy, cataract, glaucoma, and ocular surface disease in patients with DM were well-investigated in previous studies (3–5). Given its major impact on vision, retinal disease, cataract, and glaucoma have been the major concerns of ophthalmologists (6, 7), whereas minor ocular surface diseases, such as dry eye disease (DED), were often overlooked, with a previous study showing that 51.3% of DM-related dry eye syndrome cases were underdiagnosed (8).
According to the definition given by the Tear Film & Ocular Surface Society, DED is a multifactorial disease caused by loss of tear film homeostasis and inflammation with neurosensory abnormalities potentially involved in the pathogenesis (9). In previous studies, abnormal tear dynamics was noted both in vitro and in vivo in individuals with DM with osmolarity changes (10–12). Altered enzyme metabolism and decrease mucin secretion may also contribute to DM-related DED (13). Moreover, lacrimal gland and lacrimal functional unit dysfunction (14, 15) caused by diabetic neuropathy plays an important role in DM-related DED; furthermore; the aforementioned pathology along with Meibomian gland dysfunction (8, 16) often leads to tear film instability (12) due to decreased quantity and quality of the tear lipid, resulting in DED.
In patients with DM, ocular sequelae can frequently develop and sometimes require surgical intervention. For instance, long-term hyperglycemia was associated with cataract formation (17), and cataract surgery will be indicated for visual improvement; moreover, the development of diabetic retinopathy might also require interventions at different stages, including pan-retinal photocoagulation (PRP) (18, 19), intravitreal injection (IVI) of anti-vascular endothelial growth factor or steroid (20), and even trans-pars plana vitrectomy (TPPV) (19). These ocular surgeries are associated with DED in previous studies, with the incidence of post-cataract DED of 9–32% (21, 22). Accumulating evidence also demonstrated that IVI was associated with deterioration of ocular surface health (20, 23); TPPV contributed to the development of signs and symptoms of DED, and PRP induced the decrease in tear break-up time and Schirmer test value (24).
Previous studies had investigated the prevalence of DED and relevant risk factors in patients with DM. The results regarding the prevalence of DED in patients with DM varied from studies, ranging from 15 to 54.3% (5, 25–27). Reported risk factors included but were not limited to advanced age, female sex, smoking, higher glycated hemoglobin (HbA1c) level, and DM retinopathy (3, 4, 16, 28, 29). Conflicting results regarding the role of diabetic neuropathy and nephropathy were reported among studies (26, 30, 31). However, the effects of antihyperglycemic medications on ocular surface disease are unclear. Therefore, this study aimed to investigate the associated factors of DED in a DM patient cohort, with specific focus on the effects of antihyperglycemic agents.
Methods
Data source
The study protocol was approved by the Institutional Review Board of Chang Gung Memorial Hospital (approval no.: 202001925B0C601) and followed the tenets of the Declaration of Helsinki. The Chang Gung Research Database (CGRD) contains multi-institutional standardized electronic medical records (EMR) from seven Chang Gung Memorial Hospital (CGMH) across the nation, including two medical centers, two regional hospitals, and three district hospitals. The EMR contains the patient-level data derived from electronic medical charts of patients established for administrative and healthcare purposes for CGMH. The Department of Information Systems Management of CGMH integrated and standardized all EMR from CGMH without selection criteria and established CGRD for research purposes. All data from CGRD are encrypted and de-identified, and the database contains laboratory data, inpatient data, outpatient data, emergency patient data, pathological data, nursing data, charge data, disease category data, and surgical data.
Type II DM cohort
We included patients with type II DM from January 1, 2005, to December 31, 2020, using the International Classification of Disease, ninth version, Clinical Modification code (ICD-9-CM) 250.xy (x = 0, 1, 2, 3, 4, 5, 6, 7, 8, 9; y = 0, 2), and tenth version (ICD-10-CM) E11.xyza (x = 1, 2, 3, 4, 5, 6, 8, 9; y = 0, 1, 2, 3, 4, 5, 6, 7, 9; z = 0, 1, 2, 3, 4, 5, 8, 9; a = 1, 2, 3, 9). To ensure the diagnostic accuracy and for analysis of purpose, we only enrolled those who had HbA1c data, which we will brief as DM cohort in the study. The DM duration in our study is defined as the time from the day the patient was diagnosed to the last day of follow-up.
DED and non-DED groups
We further identified patients with DED using ICD-9-CM 370.33, 372.53, 375.15, 710.2, and ICD-10-CM H16.22x (x = 1, 2, 3, 9), H04.12x (x = 1, 2, 3, 9), and for diagnostic accuracy, we excluded those who did not receive either lubricant and/or topical anti-inflammation agent after the diagnosis was established. Those with DED were classified as the DED group, and those without DED were classified as the non-DED group. We excluded those who had died during the study period to exclude serious health condition. Moreover, we excluded those who are aged <18 years and not using antihyperglycemic agents and those who had pre-existing DED before the development of DM, legal blindness, previous ocular evisceration surgery, glaucoma, uveitis, and congenital eye abnormality and had undergone cornea transplantation before the diagnosis of DED.
DM comorbidity
Patients with comorbidities, including diabetic retinopathy, diabetic nephropathy, and diabetic neuropathy, which developed before the diagnosis of DED in the DED group and before the end of the follow-up in the non-DED group, were identified using ICD-9-CM and ICD-10-CM codes (Supplementary Table 1). For diabetic nephropathy, we further divided the patient into three groups based on the estimated glomerular filtration rate (eGFR) (≥60; 30–59; <30).
Diabetic antihyperglycemic agents
In the subgroup analysis for diabetic medications, the medication group was defined using medication possession ratio (MPR). Those who were not receiving either metformin, dipeptidyl peptidase 4 (DPP-4) inhibitor, glucagon-like peptide-1 receptor (GLP-1) agonist, sodium-glucose cotransporter-2 (SGLT-2) inhibitor, and insulin or in those who are using these medications but did not reach our target MPR were categorized into group “other/non-routine medications.” In the monotherapy group, patients had received either one of the aforementioned medications, with MPR >50% throughout the whole course. We defined the dual therapy group as patients who had received any two medications of metformin, DPP4 inhibitor, GLP-1 agonist, SGLT-2 inhibitor, and insulin with the MPR >60% combined and >30% each throughout the follow-up period.
Ocular procedures
In the subgroup analysis for the procedure received, we identified those who underwent IVI, TPPV, cataract surgery, and PRP before the diagnosis of DED in the DED group and before the end of the follow-up in the non-DED group using surgical coding in CGRD (Supplementary Table 2).
Outcome measurements
Risk and protective factors for DED in the DM cohort were investigated, including basic demographic data, HbA1c levels (initial HbA1c level while the diagnosis was made, and average data throughout the follow-up course), microvascular and macrovascular comorbidities (including diabetic retinopathy, diabetic nephropathy, and diabetic neuropathy), antihyperglycemic agents (including metformin, DPP-4 inhibitor, GLP-1 agonist, SGLT-2 inhibitor, insulin, and dual therapy), and ocular procedures received (including IVI, cataract surgery, TPPV, and PRP). Comorbidities were collected according to the ICD-9-CM and ICD-10-CM code, procedure using operative codes in the CGMH group, and drugs using anatomical therapeutic chemical codes.
Statistical analysis
All statistics were calculated using SPSS software version 23.0 for Windows (SPSS, Inc., Chicago, IL, USA). Continuous variables are presented as mean and standard deviation. Pearson's chi-square tests were used to compare sex, DM duration, comorbidity (nephropathy, neuropathy and retinopathy), antihyperglycemic agents, and procedures received between the DED and non-DED groups. Student's t-test was used to compare age, initial HbA1c level, and average HbA1c level between the two groups. The conditional logistic regression model was conducted to analyze the risk and protective factors for DED. A P-value <0.05 was deemed to be statistically significant. Moreover, adjusted odds ratio (OR) was used to determine the characteristic of each factor as either contributing (OR > 1) or protective factor (OR < 1).
Results
Overall, 333,803 patients with type II DM were identified from January 1, 2005, to December 31, 2020, in the CGRD, and 255,490 of them had HbA1c data. We further excluded those who are aged <18 years (n = 1,239), without any antihyperglycemic agent use (n = 85,953), and those who had pre-existing DED before DM occurred (n = 16,916), legal blindness (n = 296), previous ocular evisceration surgery (n = 4), glaucoma (n = 8,290), uveitis (n = 1,193), and congenital eye abnormality (n = 89) and had underwent cornea transplantation (n = 92) before the diagnosis of DED or before the end of follow-up for the non-DED group. Moreover, those who did not have eGFR data were also excluded (n = 13,241). A total of 152,520 remained, with 10,029 of them having DED (DED group) and 142,291 did not develop DED throughout the follow-up course (non-DED group) (Figure 1). In the demographic data before age and sex matching, female predominance was noted in the DED group (p < 0.001) with younger age at the diagnosis of type II DM (p = 0.012). The HbA1c level was higher in the DED group in both initial data (p < 0.001) and average data (p < 0.001) (Table 1). After age and sex matching, the incidence of both retinopathy and neuropathy was higher in the DED group (p < 0.001, p < 0.001, respectively); however, the incidence of nephropathy was lower in the DED group but was not statistically significant (p = 0.835). However, when we further examined the distribution of different stages of nephropathy using eGFRs, those who had lower eGFR (30–59 group and <30 group), which indicated more severe forms of diabetic nephropathy, was significantly observed in the DED group (p < 0.001) (Table 2). In ocular procedures received, the percentage of all procedures, including IVI, TPPV, PRP, and cataract surgery, was significantly higher in the DED group compared with the non-DED group (p < 0.001, p < 0.001, p < 0.001, p < 0.001, respectively) (Table 3). In conditional logistic regression model, female sex was a contributing factor without statistical significance, and advanced age was a risk factor for DED (OR, 1.18, p < 0.001); however, longer DM duration (5–10 years, >10 years) seems to be protective against DED in our cohort (OR, 0.70, p < 0.001; OR, 0.33, p < 0.001) (Table 4). After adjusting for age, sex, and DM duration, we performed three different models regarding IVI (referred to model 1), TPPV (referred to model 2), and PRP (referred to model 3) as their population was highly overlapping, which caused interference if we analyzed them in the same model. As a result, IVI, PRP, TPPV, and cataract surgery were all demonstrated as risk factors for the development of DM-related DED (OR, 1.86; p < 0.001; OR, 2.41, p < 0.001; OR, 3.72, p < 0.001; OR, 3.81–4.16, p < 0.001, respectively). Higher average HbA1c level was a contributing factor in all three models (OR, 2.14, p < 0.001; OR, 2.14, p < 0.001; OR, 2.09, p < 0.001, respectively). For diabetic comorbidity, retinopathy (OR, 2.40, p < 0.001; OR, 2.22, p < 0.001; OR, 2.15, p < 0.001, respectively) and neuropathy (OR, 1.69, p < 0.001; OR, 1.71, p < 0.001; OR, 1.66, p < 0.001, respectively) were contributing factors in all three models. As for nephropathy, compared with those with eGFR ≥60, an eGFR between 30 and 59 was a significant risk factor in all three models (OR, 1.17, p < 0.001; OR, 1.17, p < 0.001; OR, 1.16, p < 0.001, respectively), although eGFR <30 was a risk factor without statistical significance. Taking anti-glycemic medications into consideration, non-routine/other medications appeared to be a protective factor against DED in all three models (OR, 0.54, p < 0.001, in all three models). Compared with metformin monotherapy, DPP4 inhibitor, GLP-1 agonist, SGLT-2 inhibitor, and insulin monotherapy demonstrated as protective factors for DED and dual therapy. When adopting OR as a predictor for the efficacy of preventing DED, SLGT-2 inhibitor had lower OR in all three models (OR, 0.09, p < 0.001; OR, 0.09, p < 0.001; OR, 0.09, p < 0.001, respectively), followed by GLP-1 agonist (OR, 0.14, p < 0.001; OR, 0.13, p < 0.001; OR, 0.14, p < 0.001, respectively), DPP-4 inhibitor (OR, 0.54, p < 0.001; OR, 0.54, p < 0.001; OR, 0.54, p < 0.001, respectively), and insulin alone (OR, 0.71, p = 0.004; OR, 0.72, p = 0.006; OR, 0.72, p = 0.005, respectively). For dual therapy group, the OR between DPP4 inhibitor and GLP-1 agonist (OR, 0.33, p < 0.001; OR, 0.34, p < 0.001; OR, 0.34, p < 0.001, respectively) (Table 5). Given the retrospective nature of our study, without active interventions, there were no safety concern and any adverse events.
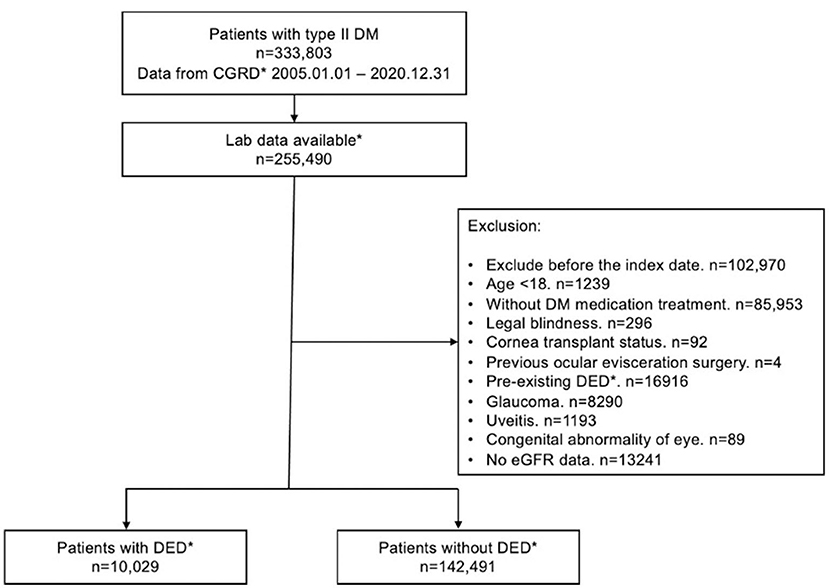
Figure 1. Flow chart for inclusion and exclusion of patients from the CGRD. CGRD, Chang Gung Research Database. DED, dry eye disease; Laboratory data available: with available HbA1c data.
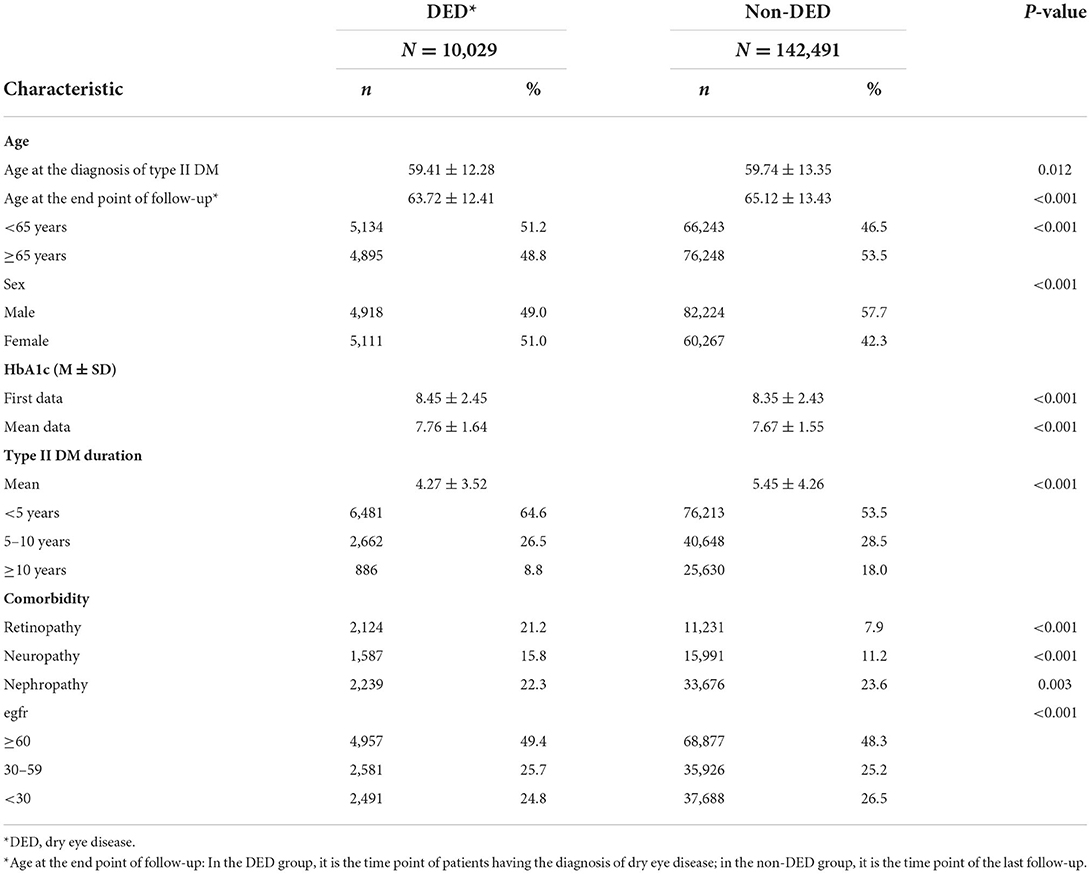
Table 1. Demographics and characteristics of dry eye disease (DED) and non-dry eye disease (non-DED) groups in patients with type II diabetes mellitus.
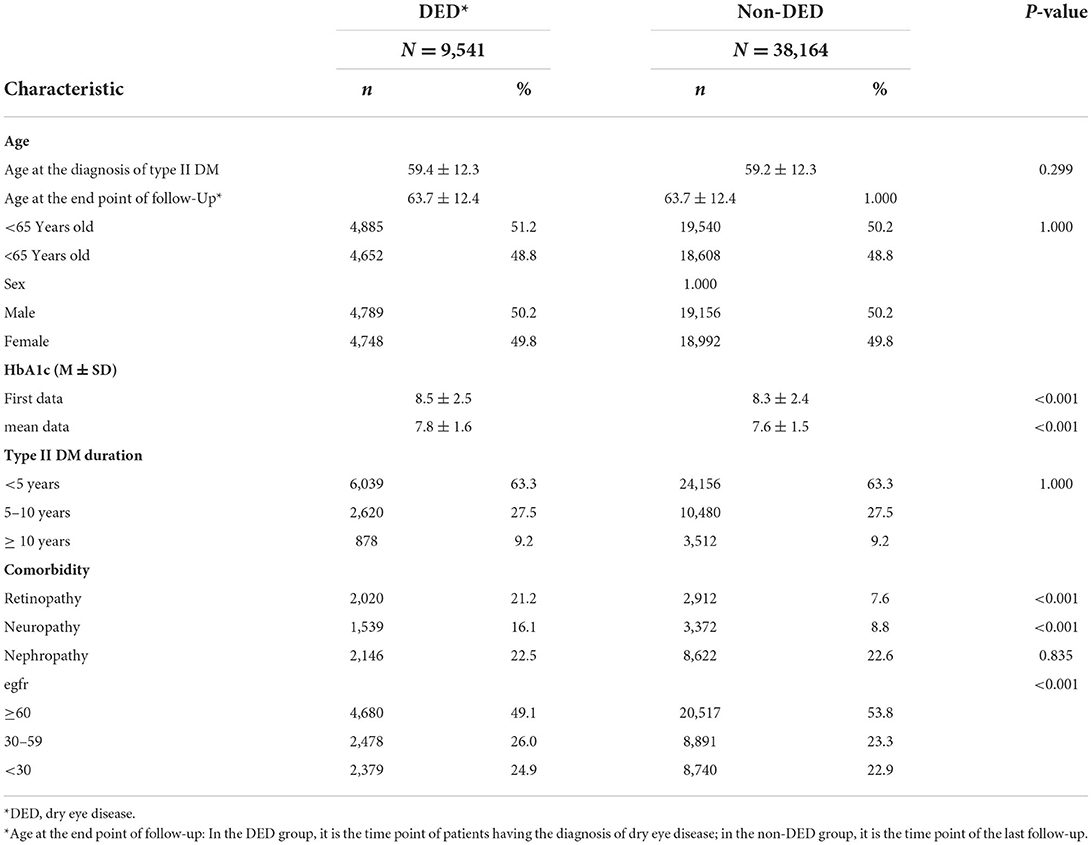
Table 2. Demographics and characteristics of the dry eye disease and non-dry eye disease groups in patients with type II diabetes mellitus after age and sex match.
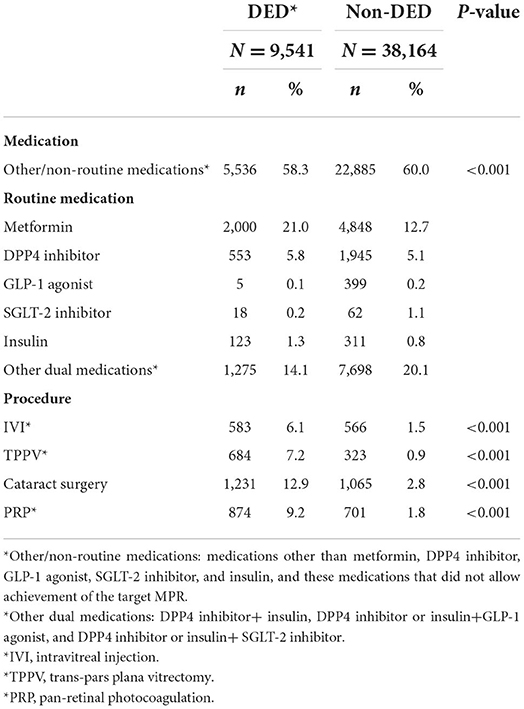
Table 3. Hypoglycemic medications used and procedures received in both dry eye disease and non-dry eye disease groups after age and sex match.
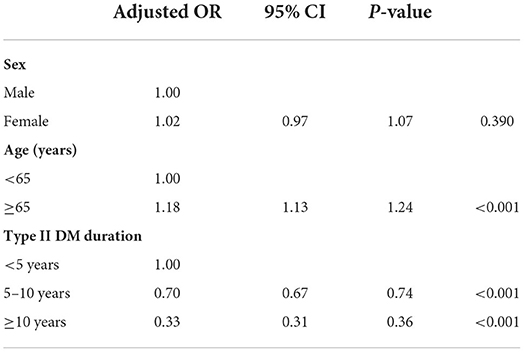
Table 4. Risk and protective factor analysis using conditional logistic regression model assessing the influence of age, sex, and DM duration on DED.
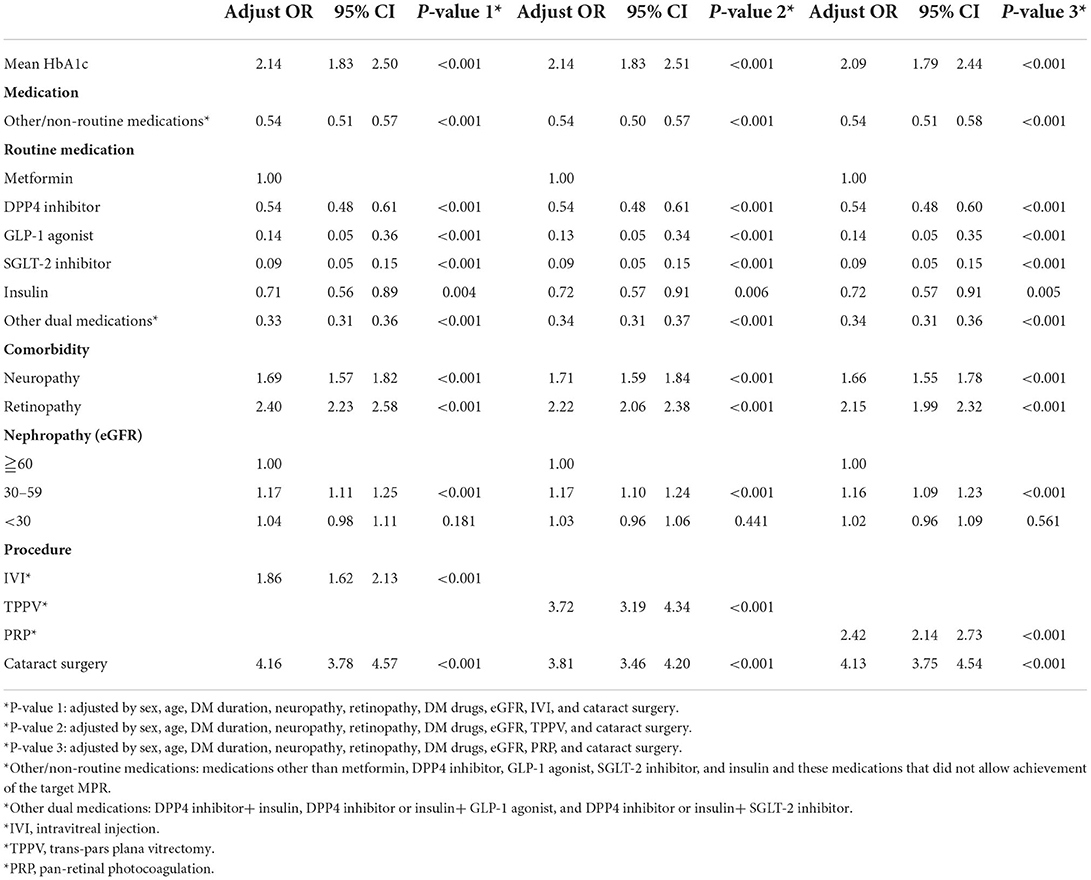
Table 5. Risk and protective factor analysis for dry eye disease using the conditional logistic regression model with control of age, sex, and DM.
Discussion
To the best of our knowledge, this is the first study to investigate the association between DED and antihyperglycemic agent, and we found that DPP4 inhibitor, GLP-1 agonist, SGLT-2 inhibitor, and insulin monotherapy are all superior to metformin alone. In terms of predicting the protective effect using OR, SGLT-2 inhibitor is the highest, followed by GLP-1 agonist, DPP4 inhibitor, and insulin. As for the protective effect of dual medications, due to insufficient case number, all individuals using dual medications are pooled together for analysis, and thus it is difficult for us to identify the effect of different combinations. However, the combined protective effect between DPP4 inhibitor and GLP-1 agonist was observed. In our study, we also verified different possible risk factors for DM-related DED and found consistent results compared with previous studies (3, 4, 16, 28, 29), including female sex, advanced age, poor glycemic control evidenced by higher average HbA1c level, and presence of diabetic retinopathy. For diabetic neuropathy and nephropathy, controversial results were shown among different studies (26, 30, 31). In our cohort, neuropathy was demonstrated as a risk factor for DED in the DM population, and the risk increased with the deterioration of renal function (eGFR) in DM nephropathy, which was consistent with the results of a previous study (31).
An intriguing finding is that longer DM duration appeared to be a protective factor for the development of DED in our study. In previous studies, DM duration either did not have a significant effect or is a contributing factor for DED (32, 33). However, studies had discovered that, in patients with longer DM duration, self-reported symptoms and decreased corneal sensitivity along with inferior whorl length destruction were noted with high underdiagnosis rate for DED (8, 34). Therefore, in a retrospective database study, a proportion of underdiagnosed individuals were anticipated as patients would not seek for medical help due to minimal symptom, especially in those with long-term DM. In our study, when using CGRD rather than the national health insurance database, retrieving laboratory data became possible. However, if the patients visited ophthalmologists elsewhere and had been diagnosed with DED, these patients will be categorized as non-DED group in CGRD database. According to the criteria we set to define the DM duration of the non-DED group, the DM duration of these patients would not meet its endpoint until the last follow-up date in our hospital system rather than the day they had been diagnosed with DED; thus, the duration in the non-DED group might be prolonged. To deal with this bizarre scenario, we had adjusted the influence of DM duration in our regression model for other risk factor analyses.
DED is a multifactorial disease, in which inflammation and neurosensory abnormalities are involved in its pathogenesis (9); moreover, lacrimal functional unit dysfunction is known to be related to diabetic neuropathy, which leads to the development of DM-related DED (14, 15). The neuroprotective and anti-inflammatory effect of DPP4 inhibitor, SGLT-2 inhibitor, and GLP-1 agonist had been investigated in previous studies (35). For instance, SGLT-2 inhibitors have a dose-dependent effect on diabetic neuropathy in mice (36), and its ability to reduce oxidative stress and inflammation had also been reported (37). As for GLP-1 agonist, a preclinical trial had shown beneficial effects on diabetic polyneuropathy and peripheral nerve degeneration in human and neuroprotective effect in animal models (38). In DPP-4 inhibitors, different studies on diabetic rats had pointed out its ability to reduce the decrease in nerve density, restoring mechanical sensitivity thresholds, and improving nerve conduction velocity and slowing nerve fiber atrophy (39–41). In humans, DPP-4 inhibitor was found to be superior to sulfonylurea drugs in preventing diabetic neuropathy (42). In contrast, metformin was found to be associated with more severe diabetic peripheral neuropathy compared with non-metformin treatment (43). From the results of our study, all GLP-1 agonists, SGLT-2 inhibitors, and DPP-4 inhibitors are superior to metformin alone in preventing DM-related DED. We presume that the possible mechanisms contributing to the results are the neuroprotective effect against DM neuropathy as demonstrated by these medications, which further prevents the occurrence of lacrimal function unit dysfunction, resulting in lower incidence of DM-related DED in patients using antihyperglycemic agents.
There are several limitations in this study. First, when using CGRD database, we could not investigate the integral follow-up course for some patients as a few of them might have visited other institutions, which might cause bias regarding our grouping. Second, the CGRD does not contain diagnostic data for DED including Schirmer test and ocular surface disease index questionnaire; therefore, we can only define DED using ICD diagnostic code combined with medications used. Moreover, most patients are Asian; thus, the result of our study may need further validation to be applicable to other ethnicities. Lastly, due to the retrospective nature of our study, despite the strict inclusion and exclusion criteria, there might still be some bias regarding grouping and accuracy of diagnosis.
Conclusion
DED in patients with DM are associated with female sex, advanced age, poor glycemic control, and development of comorbidities. Ocular procedures, including IVI, PRP, TPPV, and cataract surgery, also increase the risk of developing DED. However, in our cohort, longer DM duration appeared to be protective against DED, which might be masked due to underdiagnosis caused by peripheral neuropathy. As for antihyperglycemic agent, GLP-1 agonist, SGLT-2 inhibitor, DPP4 inhibitor, and insulin monotherapy are all superior to metformin alone, with SGLT2 inhibitor and GLP-1 agonist having significantly lower OR, followed by DPP-4 inhibitor and insulin. However, a prospective randomized control trial will be needed to further consolidate our results.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Review Board of Chang Gung Memorial Hospital (approval no.: 202001925B0C601). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
L-YP and C-CS contributed to statistical analysis and manuscript composition. Y-KK, L-YP, C-CS, and T-HC contributed to study design and data collection. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by a grant from the Alcon Services AG, Taiwan Branch (Investigator-Initiated Research IIR # 57241343).
Acknowledgments
Special thanks to the statistical team from Health Information and Epidemiology Laboratory of Chang Gung Memorial Hospital, Chiayi Branch.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.980714/full#supplementary-material
References
1. Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol. (2011) 8:228–36. doi: 10.1038/nrendo.2011.183
2. Ogurtsova K, Guariguata L, Barengo NC, Ruiz PL, Sacre JW, Karuranga S, et al. IDF Diabetes Atlas: global estimates of undiagnosed diabetes in adults for 2021. Diabetes Res Clin Pract. (2022) 183:109118. doi: 10.1016/j.diabres.2021.109118
3. Huang X, Zhang P, Zou X, Xu Y, Zhu J, He J, et al. Two-year incidence and associated factors of Dry eye among residents in Shanghai communities with type 2 diabetes mellitus. Eye Contact Lens. (2020) 46:S42–9. doi: 10.1097/ICL.0000000000000626
5. Kaiserman I, Kaiserman N, Nakar S, Vinker S. Dry eye in diabetic patients. Am J Ophthalmol. (2005) 139:498–503. doi: 10.1016/j.ajo.2004.10.022
6. Nakamura M, Kanamori A, Negi A. Diabetes mellitus as a risk factor for glaucomatous optic neuropathy. Ophthalmologica. (2005) 219:1–10. doi: 10.1159/000081775
7. Murtha T, Cavallerano J. The management of diabetic eye disease in the setting of cataract surgery. Curr Opin Ophthalmol. (2007) 18:13–8. doi: 10.1097/ICU.0b013e32801129fc
8. Schwartz S, Halleran C, Doll T, Harthan JS, Williams MT, O'Dell LE. Does diabetes make a difference in dry eye? Invest Ophthalmol Vis Sci. (2018) 59:956.
9. Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo CK, et al. TFOS DEWS II definition and classification report. Ocul Surf. (2017) 15:276–83. doi: 10.1016/j.jtos.2017.05.008
10. Zou X, Zhang P, Xu Y, Lu L, Zou H. Quantitative proteomics and weighted correlation network analysis of tear samples in Type 2 diabetes patients complicated with dry eye. Proteomics Clin Appl. (2020) 14:e1900083. doi: 10.1002/prca.201900083
11. Ramos-Remus C, Suarez-Almazor M, Russell AS. Low tear production in patients with diabetes mellitus is not due to Sjögren's syndrome. Clin Exp Rheumatol. (1994) 12:375–80.
12. Derakhshan A, Abrishami M, Khajedaluee M, Omidtabrizi A, Moghaddam SG. Comparison between tear film osmolar cocentration and other tear film function parameters in patients with diabetes mellitus. Korean J Ophthalmol. (2019) 33:326–32. doi: 10.3341/kjo.2013.0146
13. Tseng SCG, Hirst LW, Maumenee AE, Kenyon KR, Sun TT, Green WR. Possible mechanisms for the loss of goblet cells in mucin-deficient disorders. Ophthalmology. (1984) 91:545–52. doi: 10.1016/S0161-6420(84)34251-8
14. Ljubimov AV. Diabetic complications in the cornea. Vision Res. (2017) 139:138–52. doi: 10.1016/j.visres.2017.03.002
15. Gekka M, Miyata K, Nagai Y, Nemoto S, Sameshima T, Tanabe T, et al. Corneal epithelial barrier function in diabetic patients. Cornea. (2004) 23:35–7. doi: 10.1097/00003226-200401000-00006
16. Sandra Johanna GP, Antonio LA, Andrés GS. Correlation between type 2 diabetes, dry eye and Meibomian glands dysfunction. J Optom. (2019) 12:256–62. doi: 10.1016/j.optom.2019.02.003
17. Henriques J, Vaz-Pereira S, Nascimento J, Rosa PC. [Diabetic eye disease]. Acta Med Port. (2015) 28:107–13. doi: 10.20344/amp.5361
18. Reddy SV, Husain D. Panretinal photocoagulation: a review of complications. Semin Ophthalmol. (2018) 33:83–8. doi: 10.1080/08820538.2017.1353820
19. El Rami H, Barham R, Sun JK, Silva PS. Evidence-based treatment of diabetic retinopathy. Semin Ophthalmol. (2017) 32:67–74. doi: 10.1080/08820538.2016.1228397
20. Srinagesh V, Ellenberg D, Scharper PH, Etter J. Intravitreal dry eye study. Invest Ophthalmol Vis Sci. (2014) 55:3696.
21. Naderi K, Gormley J, O'Brart D. Cataract surgery and dry eye disease: a review. Eur J Ophthalmol. (2020) 30:840–55. doi: 10.1177/1120672120929958
22. Mikalauskiene L, Grzybowski A, Zemaitiene R. Ocular surface changes associated with ophthalmic surgery. J Clin Med. (2021) 10:1642. doi: 10.3390/jcm10081642
23. Laude A, Lim JW, Srinagesh V, Tong L. The effect of intravitreal injections on dry eye, and proposed management strategies. Clin Ophthalmol. (2017) 11:1491–7. doi: 10.2147/OPTH.S136500
24. Jamali H, Eslami J, Kalashipour F, Nowroozzadeh MH. Effect of panretinal photocoagulation on corneal sensation and tear function in patients with diabetic retinopathy. Retina. (2021) 41:338–44. doi: 10.1097/IAE.0000000000002821
25. Manaviat MR, Rashidi M, Afkhami-Ardekani M, Shoja MR. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol. (2008) 8:10. doi: 10.1186/1471-2415-8-10
26. Najafi L, Malek M, Valojerdi AE, Aghili R, Khamseh ME, Fallah AE, et al. Dry eye and its correlation to diabetes microvascular complications in people with type 2 diabetes mellitus. J Diabetes Complications. (2013) 27:459–62. doi: 10.1016/j.jdiacomp.2013.04.006
27. Hung N, Kang EY, Lee TW, Chen TH, Shyu YC, Sun CC. The risks of corneal surface damage in aqueous-deficient dry eye disease: a 17-year population-based study in Taiwan. Am J Ophthalmol. (2021) 227:231–9. doi: 10.1016/j.ajo.2021.03.013
28. Ward MF II, Le P, Donaldson JC, Van Buren E, Lin FC, Lefebvre C, et al. Racial and ethnic differences in the association between diabetes mellitus and dry eye disease. Ophthal Epidemiol. (2019) 26:295–300. doi: 10.1080/09286586.2019.1607882
29. Olaniyan SI, Fasina O, Bekibele CO, Ogundipe AO. Relationship between dry eye and glycosylated haemoglobin among diabetics in Ibadan, Nigeria. Pan Afr Med J. (2019) 33:14. doi: 10.11604/pamj.2019.33.14.14074
30. Zou X, Lu L, Xu Y, Zhu J, He J, Zhang B, et al. Prevalence and clinical characteristics of dry eye disease in community-based type 2 diabetic patients: the Beixinjing eye study. BMC Ophthalmol. (2018) 18:117. doi: 10.1186/s12886-018-0781-7
31. Camacho Ordoñez A, Fernandez O, Alegria ED, Del Valle CP, Velasco R, Baca O, et al. Correlation of dry eye syndrome with diabetic retinopathy, Kidney Disease Outcomes Quality Initiative and lipids panel. Invest Ophthalmol Vis Sci. (2015) 56:4450.
32. Nadeem H, Malik TG, Mazhar A, Ali A. Association of dry eye disease with diabetic retinopathy. J Coll Physicians Surg Pak. (2020) 30:493–7. doi: 10.29271/jcpsp.2020.05.493
33. Shujaat S, Jawed M, Memon S, Talpur KI. Determination of risk factors and treatment of dry eye disease in Type 1 diabetes before corneal complications at Sindh Institute of Ophthalmology and Visual Sciences. Open Ophthalmol J. (2017) 11:355–61. doi: 10.2174/1874364101711010355
34. Lyu Y, Zeng X, Li F, Zhao S. The effect of the duration of diabetes on dry eye and corneal nerves. Cont Lens Anterior Eye. (2019) 42:380–5. doi: 10.1016/j.clae.2019.02.011
35. El Mouhayyar C, Riachy R, Khalil AB, Eid A, Azar S. SGLT2 inhibitors, GLP-1 agonists, and DPP-4 inhibitors in diabetes and microvascular complications: a review. Int J Endocrinol. (2020) 2020:1762164. doi: 10.1155/2020/1762164
36. Takakura S, Toyoshi T, Hayashizaki Y, Takasu T. Effect of ipragliflozin, an SGLT2 inhibitor, on progression of diabetic microvascular complications in spontaneously diabetic Torii fatty rats. Life Sci. (2016) 147:125–31. doi: 10.1016/j.lfs.2016.01.042
37. Cersosimo E, DeFronzo RA. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diabetes Metab Res Rev. (2006) 22:423–36. doi: 10.1002/dmrr.634
38. Seufert J, Gallwitz B. The extra-pancreatic effects of GLP-1 receptor agonists: a focus on the cardiovascular, gastrointestinal and central nervous systems. Diabetes Obes Metab. (2014) 16:673–88. doi: 10.1111/dom.12251
39. Bianchi R, Cervellini I, Porretta-Serapiglia C, Oggioni N, Burkey B, Ghezzi P, et al. Beneficial effects of PKF275-055, a novel, selective, orally bioavailable, long-acting dipeptidyl peptidase IV inhibitor in streptozotocin-induced diabetic peripheral neuropathy. J Pharmacol Exp Ther. (2012) 340:64–72. doi: 10.1124/jpet.111.181529
40. Kawanami D, Matoba K, Sango K, Utsunomiya K. Incretin-based therapies for diabetic complications: basic mechanisms and clinical evidence. Int J Mol Sci. (2016) 17:1223. doi: 10.3390/ijms17081223
41. Tsuboi K, Mizukami H, Inaba W, Baba M, Yagihashi S. The dipeptidyl peptidase IV inhibitor vildagliptin suppresses development of neuropathy in diabetic rodents: effects on peripheral sensory nerve function, structure and molecular changes. J Neurochem. (2016) 136:859–70. doi: 10.1111/jnc.13439
42. Kolaczynski WM, Hankins M, Ong SH, Richter H, Clemens A, Toussi M. Microvascular outcomes in patients with type 2 diabetes treated with vildagliptin vs. sulfonylurea: A retrospective study using German electronic medical records. Diabetes Ther. (2016) 7:483–96. doi: 10.1007/s13300-016-0177-8
Keywords: hypoglycemic agent, dry eye disease, diabetes mellitus, risk and protective factors, SLGT-2 inhibitor, GLP-1 agonist, DPP4 inhibitor, insulin
Citation: Pan L-Y, Kuo Y-K, Chen T-H and Sun C-C (2022) Dry eye disease in patients with type II diabetes mellitus: A retrospective, population-based cohort study in Taiwan. Front. Med. 9:980714. doi: 10.3389/fmed.2022.980714
Received: 28 June 2022; Accepted: 29 July 2022;
Published: 23 August 2022.
Edited by:
Alejandro Navas, Instituto de Oftalmología Fundación de Asistencia Privada Conde de Valenciana, I.A.P, MexicoReviewed by:
Shohei Kaneko, Jichi Medical University Saitama Medical Center, JapanAthanasia Papazafiropoulou, Tzaneio Hospital, Greece
Copyright © 2022 Pan, Kuo, Chen and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chi-Chin Sun, YXJ2aW4uc3VuQG1zYS5oaW5ldC5uZXQ=
 Li-Yen Pan
Li-Yen Pan Yu-Kai Kuo2
Yu-Kai Kuo2 Tien-Hsing Chen
Tien-Hsing Chen Chi-Chin Sun
Chi-Chin Sun