- 1School of Basic and Medical Biosciences, St. John’s Institute of Dermatology, King’s College London, London, United Kingdom
- 2Department of Orthopedics, University of Pittsburgh Medical Center of Central PA, Pittsburgh, PA, United States
- 3Institute of Pathology, Heidelberg University Hospital, Heidelberg, Germany
- 4Department of Surgery, Penn State College of Medicine, Hershey, PA, United States
- 5Department of Dermatology, Chelsea and Westminster Hospital NHS Foundation Trust (West Middlesex University Hospital), London, United Kingdom
- 6Institute of Immunity and Transplantation, Royal Free Hospital and University College London, London, United Kingdom
- 7Nawaloka Hospital Research and Education Foundation, Nawaloka Hospitals, Colombo, Sri Lanka
- 8Department of Genetic Medicine, The Royal Melbourne Hospital, Melbourne, VIC, Australia
- 9Department of Medicine, The University of Melbourne, Melbourne, VIC, Australia
- 10Department of Dermatology, Cambridge University Hospitals NHS Foundation Trust, Cambridge, United Kingdom
Introduction: The Ehlers-Danlos syndromes (EDS) comprise a group of inherited connective tissue disorders presenting with variable fragility to skin, soft tissue, and certain internal organs, which can cause significant complications, particularly arterial rupture, bowel perforation and joint difficulties. Currently, there are 14 proposed subtypes of EDS, with all except one subtype (hypermobile EDS) having an identified genetic etiology. An understanding of the extracutaneous features and complications within each subtype is key to maximizing clinical care and reducing the risk of further complications.
Methods: A systematic review of EDS-related extracutaneous features and complications was undertaken.
Results: We identified 839 EDS cases that met the inclusion criteria. We noted a high prevalence of joint hypermobility amongst kyphoscoliotic (39/39, 100%), spondylodysplastic (24/25, 96.0%), and hypermobile (153/160, 95.6%) EDS subtypes. The most common musculoskeletal complications were decreased bone density (39/43, 90.7%), joint pain (217/270, 80.4%), and hypotonia/weakness (79/140, 56.4%). Vascular EDS presented with cerebrovascular events (25/153, 16.3%), aneurysm (77/245, 31.4%), arterial dissection/rupture (89/250, 35.5%), and pneumothorax/hemothorax. Chronic pain was the most common miscellaneous complication, disproportionately affecting hypermobile EDS patients (139/157, 88.5%). Hypermobile EDS cases also presented with chronic fatigue (61/63, 96.8%) and gastrointestinal complications (57/63, 90.5%). Neuropsychiatric complications were noted in almost all subtypes.
Discussion: Understanding the extracutaneous features and complications of each EDS subtype may help diagnose and treat EDS prior to the development of substantial comorbidities and/or additional complications.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022308151, identifier CRD42022308151.
Introduction
The Ehlers-Danlos syndromes (EDS) are a group of rare, inherited connective tissue disorders caused by pathogenic variants in genes responsible for matrix organization including those for collagen synthesis and processing, and glycosaminoglycans metabolism (1). Presently, there are 13 distinct clinical EDS subtypes, as outlined in the 2017 revised EDS international classification system from the International EDS Consortium (see Supplementary Table 1 for subtypes and the minor/major criteria for diagnosis) (2). Since this classification, a further gene variant for EDS has been identified (AE-binding protein 1; AEBP1), leading to a 14th subtype (classical-like type 2 EDS; clEDS2) and expanding the list of EDS-associated genes (3).
EDS varies according to specific subtype, but most presentations include some degree of skin hyperextensibility, joint hypermobility and/or fragility of skin, soft tissues and certain internal organs (bowel perforation, hernias, and arterial rupture) (4). The true prevalence and incidence of EDS in the general population is difficult to ascertain, as people with milder forms of joint and skin manifestations may not seek medical care. Amongst those presenting with EDS, hypermobile EDS (hEDS) is the most frequent, with classical (cEDS), vascular (vEDS), and hEDS collectively accounting for > 90% of patients, with some subtypes [dermatosparaxis EDS (dEDS) and clEDS2] limited to a handful of family pedigrees globally (5).
Variants in the genes responsible for normal matrix organization with collagen and extracellular matrix (ECM) function have an immense impact on both the skin and internal organs, as well as the integrity of the vasculature (4). Genetic defects affecting the biosynthesis and/or structure of fibrillar procollagens cause classical (defect in type V/I collagens, OMIM 130000), vascular (defect in type III/I collagens, OMIM 130050), arthrochalasia (aEDS; defect in type I collagen, OMIM 130060), and cardiac valvular (cvEDS; defect in type I collagen, OMIM 225320) EDS subtypes (1). Dermatosparaxis EDS (dEDS; enzyme defect in procollagen N-peptidase, OMIM 225410) results in poorly structured and loosely arranged collagen fibrils in the ECM. Similarly, classical-like EDS variants (clEDS; defect in tenascin-X ECM glycoprotein, OMIM 606408) result in a loss of anti-adhesive interactions with collagen in the ECM (1). Defects in the ECM bridging molecules have been implicated in clEDS2 [deficiency in aortic carboxypeptidase-like protein, OMIM 618000] and myopathic EDS (mEDS; defect in type 12 collagen, OMIM 616471) subtypes (4).
In kyphoscoliotic EDS (kEDS; OMIM 225400), deficiency in lysyl hydroxylase (PLOD1) or FKBP22 protein (FKBP14) cause the destabilization of intermolecular collagen crosslinking and folding (6). Subtypes such as musculocontractural EDS [mcEDS; deficiency in dermatan-4-O-sulfotransferase (CHST14) or dermatan sulfate epimerase (DSE), OMIM 601776] and spondylodysplastic EDS [spEDS; deficiencies in galactosyl-transferase I (B4GALT7) or galactosyl-transferase II (B3GALT6), OMIM 130070/615349] cause specific disorders of glycosaminoglycan biosynthesis (2), whilst subtypes with more localised phenotypic features such as brittle cornea syndrome (BCS, deficiency in ZNF469 or PRDM5 proteins, OMIM 229200) and periodontal EDS (pEDS, deficiency in C1r/C1s components, OMIM 130080/617174) have been associated in dysregulation of the intracellular processes within the ECM and the complement pathway respectively (7, 8).
The candidate genes that are responsible for the various EDS subtypes also cause development of extracutaneous manifestations. Understanding these specific changes is crucial, as it allows for accurate clinical subclassification of these manifestations, can help guide a clinician to a clinical diagnosis of EDS and can provide additional evidence to support the use of next generation sequencing technologies for a molecular diagnosis. Chronic deficits in these downstream pathways may also give rise to substantial long-term complications, particularly within the musculoskeletal, vascular, ocular, gastrointestinal, respiratory, and psychiatric systems. Some of these complications are potentially fatal (i.e., arterial rupture, bowel perforation, and pneumothorax) whilst other complications can cause a significant health burden for affected individuals. It must also be stressed that each EDS subtype is unique and is associated with an entirely separate clinical presentation and set of possible complications.
Thus, an assessment of the extracutaneous manifestations of EDS, as well as the complications associated with each EDS subtype is required to improve the clinical diagnosis of EDS and to prevent EDS-associated complications. Owing to the rarity of this complex group of genetic disorders, the paucity of evidence outlining the extracutaneous features and complications of EDS and the introduction of an additional subtype since the 2017 re-classification of EDS, a systematic review was undertaken.
Materials and methods
A systematic search of the literature was conducted to identify published data pertaining to EDS. The databases included PubMed and Web of Science. Database searches were performed from the date of publication of the 2017 International Classification of EDS (March 2017) until 15 January 2022. Search strings were used to examine electronic databases for relevant references (see Supplementary Table 2). Search results were screened initially by title and abstract. Reference lists of included studies were screened for additional studies. Clinical findings for each EDS subtype were defined as per the 2017 International Classification of EDS (2). Articles were excluded if they did not contain a formal diagnosis of EDS as per the subtype classification. Only original, published cases in humans were included. Animal models were not included as they did not accurately predict human responses and complications. This study followed PRISMA guidelines (9).
Data extraction and aggregation were performed by a single investigator (BD), with uncertainties resolved through discussion with the principal investigator (NB). After identifying potential articles, the EDS Skin Working Group reviewed the papers to establish a consensus on inclusion. For each case, demographics, variant, extracutaneous features, Beighton scores, and complications were included. Joint pain was defined as pain associated with one or more joints, whilst chronic pain was defined as pain in one or more anatomic regions that persists or recurs for longer than 3 months and is associated with significant emotional distress or significant functional disability and that cannot be better explained by another chronic pain condition (10). For complications involving body systems (i.e., cardiovascular, gastrointestinal, urological, ocular, and neuropsychiatric) a broad group of findings were collected, some of which could be limited to, or have no significance in their association to EDS (refer to the footnote in Table 2 for a comprehensive list of findings for each system). Complications such as aneurysms, arterial dissection, and rupture events were excluded from the overall cardiovascular events and collected individually due to their clinical significance.
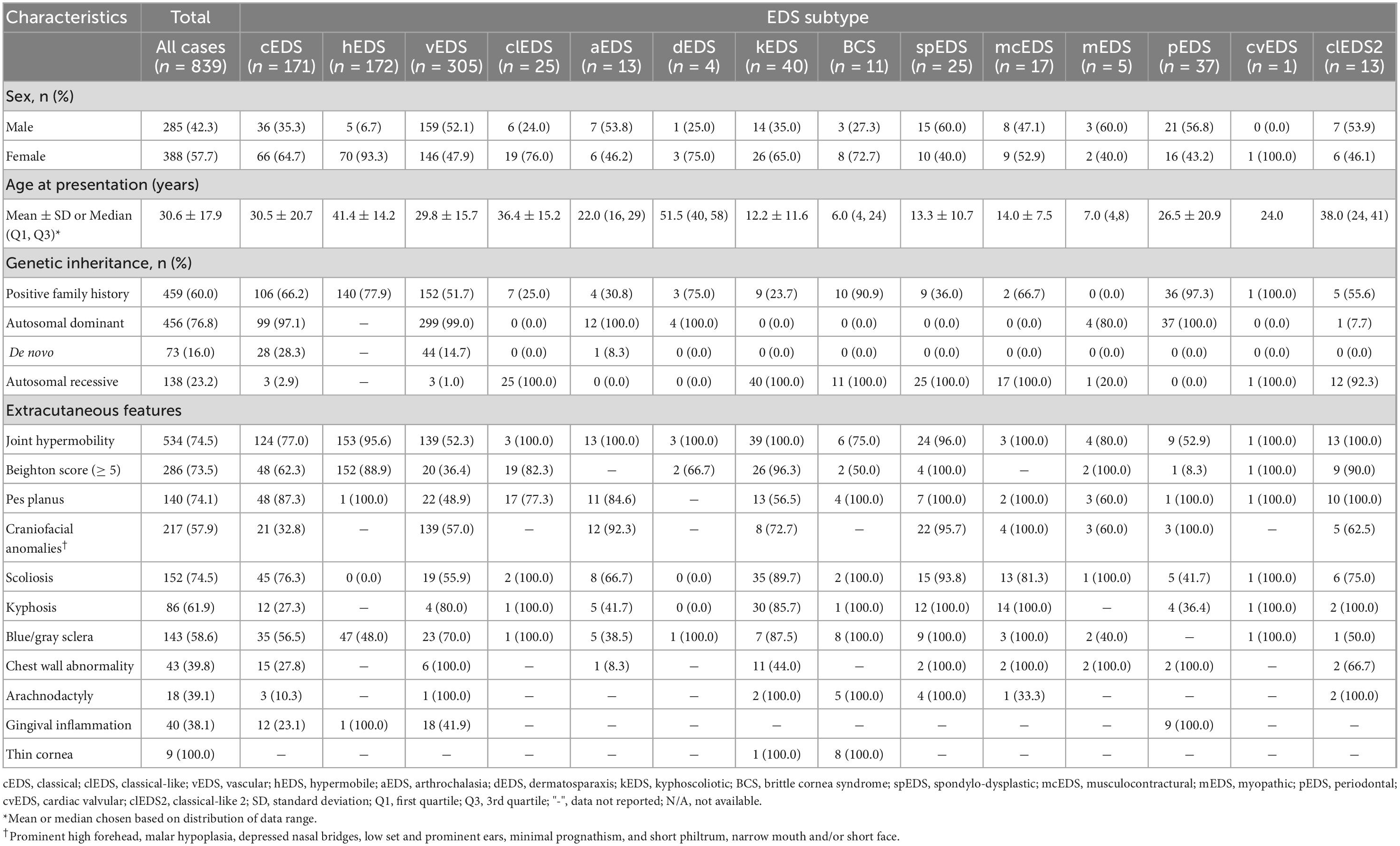
Table 1. Demographics, inheritance, and extracutaneous features of patients with Ehlers-Danlos syndrome.
The mean and standard deviation (SD) is presented for parametric variables, and the median and interquartile range is presented for non-parametric variables. Categorical variables are presented as frequencies together with proportions. Categorical data were compared with Chi-square tests. Statistical significance was defined as p < 0.05.
Results
Our search strategy identified 139 papers containing 839 EDS cases, which met the inclusion criteria (Supplementary Figure 1). There was a female-to-male ratio of 1.36:1 (p < 0.01), though higher ratios were noted for hEDS (11.8:1, p < 0.001) and clEDS (2.6:1, p < 0.001) subtypes. Genetic inheritance revealed predominantly autosomal dominant transmission, with some de novo involvement, reflecting the large cohort of cEDS and vEDS cases. A positive family history was present in 60.0% (459/795) of cases, with a higher prevalence noted in rarer autosomal recessive EDS subtypes, capturing family pedigree case studies. Demographics, genetic analysis, and extra-cutaneous features were collected (Table 1), as well as EDS-associated complications (Table 2).
Facial features
Craniofacial abnormalities (i.e., prominent high forehead, malar hypoplasia, depressed nasal bridges, low-set and prominent ears, prognathism, short philtrum, and/or short face) were variably observed in spEDS (22/23, 95.7%), aEDS (12/13, 92.3%), and to a lesser extent in vEDS (139/244, 57.0%) subtypes. An expected high prevalence of blue/gray sclera (8/8, 100%) and thin cornea (8/8, 100%) were noted in BCS. Similarly, gingival inflammation was a prominent feature amongst pEDS cases (9/9, 100%).
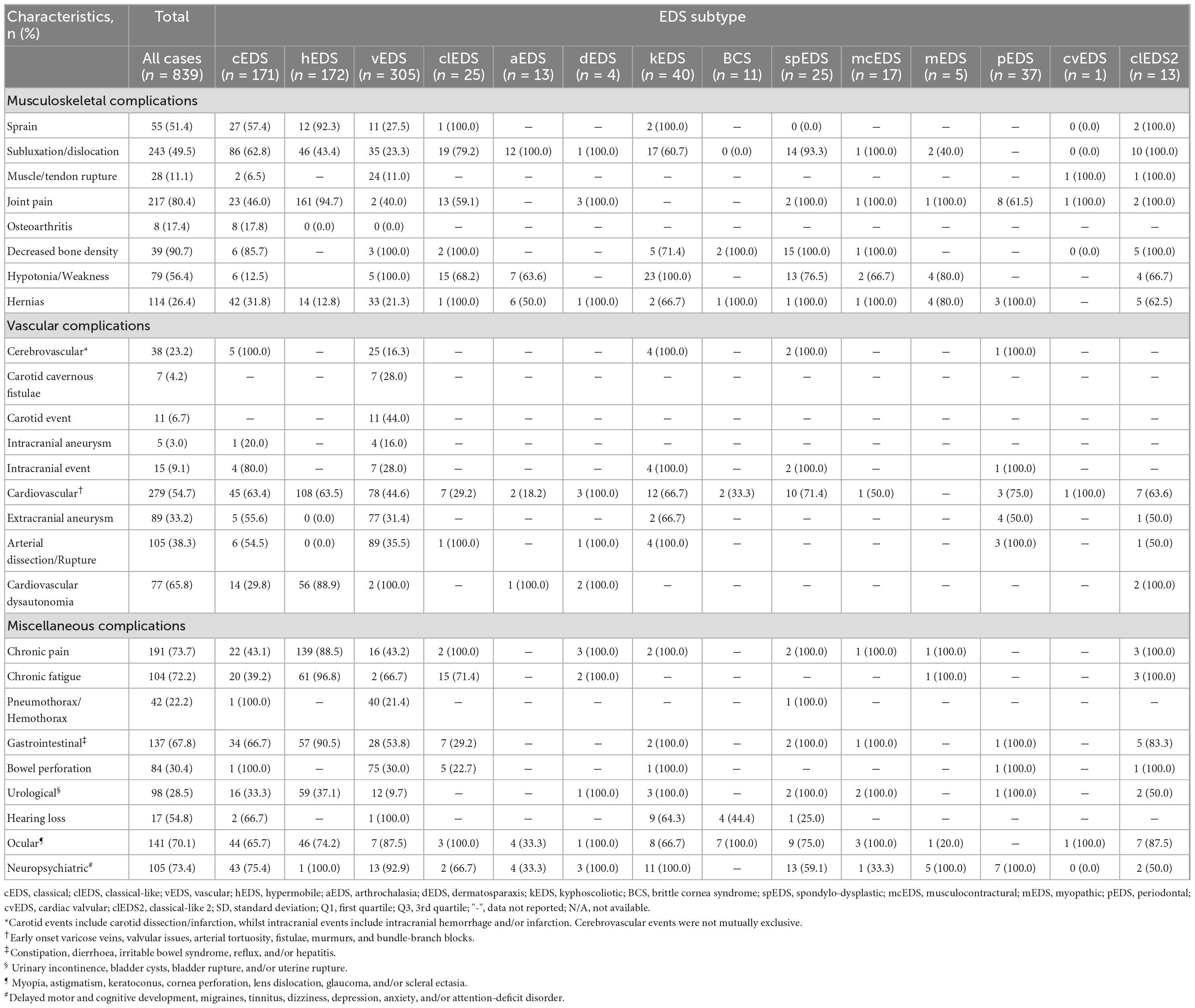
Musculoskeletal complications
Overall, we noted a high prevalence of joint hypermobility and clinically significant Beighton scores amongst EDS cases, particularly amongst kEDS (39/39, 100%), spEDS (24/25, 96.0%), and hEDS (153/160, 95.6%) subtypes. The most common musculoskeletal complications were decreased bone density (39/43, 90.7), joint pain (217/270, 80.4%), and hypotonia/weakness (79/140, 56.4%). Notably, joint pain was a significant feature observed in hEDS cases (161/170, 94.7%). Muscle/tendon rupture was almost entirely exclusive to vEDS cases, whilst sprains and subluxations/dislocations had a higher prevalence amongst hEDS, aEDS, spEDS, and cEDS cases. Abnormalities in the curvature of the spine were most notable in kEDS, spEDS, with scoliosis a prevalent feature amongst cEDS cases (45/59, 76.3%).
Vascular complications
A high prevalence was noted of vascular complications such as cerebrovascular events (25/153, 16.3%), extracranial aneurysm (77/245, 31.4%), and arterial dissection/rupture (89/250, 35.5%) amongst vEDS cases. In particular, carotid cavernous fistulae were exclusively noted within vEDS patients. Cardiovascular complications such as early onset varicose veins, valvular incompetence, arterial tortuosity, fistulae, murmurs, and bundle-branch blocks were noted as prominent features amongst almost all subtypes, particularly cEDS, hEDS, and kEDS. Interestingly, cardiovascular dysautonomia was a major feature amongst most hEDS cases (56/63, 88.9%).
Miscellaneous complications
Overall, chronic pain disproportionately affected the hEDS subtype (139/157, 88.5%) compared to other subtypes. Hypermobile EDS cases commonly exhibited chronic fatigue (61/63, 96.8%), gastrointestinal complications (57/63, 90.5%), and urological complications (59/159, 37.1%). Additional complications such as pneumothorax/hemothorax and bowel perforation were almost entirely exclusive to the vEDS subtype. Within this group, the mean age for first pneumothorax/hemothorax was 25.6 ± 9.0 years, whilst for bowel perforation it was 26.6 ± 10.9 years. Neuropsychiatric complications were noted in all kEDS, mEDS, and pEDS cases (100.0%), however, a substantial number of cases were also noted amongst vEDS and cEDS subtypes.
Discussion
Facial features
Craniofacial abnormalities were noted in almost all spEDS and aEDS cases. The gene variants noted in spEDS (B4GALT7/B3GALT6) cause significant impairment to glycosaminoglycan biosynthesis, producing a perturbed deposition of type I collagen, a disorganized ECM, and a misshapen and partly absent cartilage structure within connective tissue (11). Furthermore, during early development these variants can result in absent ossified bone structure, resulting in the clinical appearance of a short, round face with midface hypoplasia ± frontal bossing and proptosis (11). For aEDS specific variants in EXON6 COL1A1 or COL1A2 prevent cleavage of the collagen type I N-propeptide and disrupt the normal interhelical cross-linking of collagen fibers, which can cause significant bone and soft tissue abnormalities (11). Interestingly, similar craniofacial abnormalities are seen in osteogenesis imperfecta, which is also caused by variants in COL1A1 or COL1A2 and can show clinical overlap (12).
We noted that all BCS cases, and the majority of kEDS cases presented with blue/gray sclera and a thin cornea. BCS is due to biallelic variants in ZNF469 or PRDM5. These genes encode proteins that are crucial role in the normal development of the anterior chamber segment and cornea, and maintenance of the ECM (13). Thus, pathogenic variants result in downstream thinning of the sclera, producing a bluish appearance. Both sensorineural and conductive deafness are seen in BCS, in addition to hypermobile tympanic membranes which can result in devastating polysensory loss when coupled with ophthalmic complications (7, 13).
Musculoskeletal complications
Joint hypermobility and elevated Beighton scores were associated with most EDS subtypes, with many EDS subtypes showing some degree of joint laxity in their clinical presentation (2). It was also prevalent in rarer subtypes such as kEDS and spEDS, as well as hEDS. In PLOD1-related kEDS, the deficiency in collagen lysyl hydroxylase 1 causes under hydroxylation of lysyl residues in collagens and impairs the cross-link formation with consequent mechanical instability of joint ligaments (14). Similarly, as previously mentioned, the gene variants noted in spEDS (B4GALT7/B3GALT6) cause perturbed deposition of type I collagen, a disorganized ECM within connective tissues, contributing to joint hypermobility (11).
Though a consistent genetic association has not yet been discovered for hEDS, transcriptome profiling of hEDS fibroblasts has shown a differential expression of many adhesion molecule-encoding genes including members of cadherins, protocadherins and desmosomes, which are involved in the formation of specialized cell-cell junction complexes essential for maintaining epithelial integrity, morphogenesis, and tissue architecture (15). It is hypothesized that these transcriptional changes that suggest a fibroblast-to-myofibroblast transition, may cause deficiencies in the cytoskeletal architecture needed for joint stability (16). This could explain the high prevalence of sprains and subluxation/dislocations observed amongst hEDS, aEDS, spEDS, and cEDS cases. Furthermore, it is also these ECM/cytoskeletal deficiencies that contribute to weak spinal muscles, resulting in a lack of spinal column support and manifesting with spinal deformities as noted in kEDS, spEDS and cEDS cases (17).
Interestingly, we noted osteopenia/osteoporosis as a common complication amongst all EDS subtypes. Recent research has shown the various roles of collagens in osteoblastogenesis within the ECM (18, 19). An estimated 90% of the ECM in bone is comprised of collagen [primarily type I collagen, with lesser amounts of type V (∼10%)], which serves as a tissue scaffold but also provides a substrate for cell anchorage and regulates bioavailability of growth factors and cytokines (19). It is the collagen fibrils, containing type I and V collagen molecules, which are cross-linked in bone osteogenesis. Therefore, as expected variants causing altered or decreased collagen I and V (cEDS, vEDS, aEDS, and cvEDS), cause impaired bone mineralization (20). In addition, type III collagen is expressed at high levels during embryonic skeletal development and is expressed by osteoblasts for trabecular bone development. Thus, loss of function variants in COL3A1 (vEDS) can impair osteoblast differentiation (19). Consequently, bone mineralization was substantiated in the clinical setting, where EDS patients (cEDS/hEDS) had reduced bone mineral density and bone quality, with an increased prevalence of vertebral fractures, compared to matched control subjects (21).
Joint pain is common in EDS patients (80.4%), particularly within hEDS cases (94.7%). Consequently, joint pain, particularly hypermobility-related pain is one of the leading disability determinants in joint hypermobility related disorders (22). The natural history of pain in joint hypermobility and related disorders indicates that pain is usually nociceptive (physical pain caused by structural dysfunction) at the beginning of the disease (23). Furthermore, studies have suggested that patients with EDS may experience neuropathic pain, whilst others suggest joint pain spreads beyond the joints, causing fatigue, and resembling fibromyalgia (24, 25). In contrast, a pilot study of 24 EDS patients (cEDS, vEDS, hEDS) demonstrated the presence of small fiber neuropathy, supporting the additional features of paresthesias, dysesthesias, neuropathic pain, and autonomic symptoms, which are often observed in EDS patients (26).
Vascular complications
These are especially common in vEDS (variants in COL3A1/COL1A1) and are the most significant due to the risk of life-threatening features of tissue fragility that can cause cerebrovascular events, aneurysm, and arterial dissection/rupture (27). Vascular EDS patients with pathogenic variants in COL3A1, have structural defects of the C-propeptide chain of collagen type III, defects that are characterized by decreased thermal stability and abnormalities in proteolytic processing that can produce procollagen suicide (28). Substitutions of triple helical glycine residues and splice donor site variants, leading to exon skipping, are generally associated with a shorter life expectancy, whereas variants leading to COL3A1 haploinsufficiency are usually associated with a milder phenotype, a delay in the onset of complications, and a longer life expectancy (29, 30). Interestingly, we noted 3 cases of vEDS autosomal recessive inheritance, with one case being compound heterozygous for two COL3A1 variants, and two cases being homozygous for a COL3A1 missense variant, with all parents unaffected (31). For vEDS patients, there are also significant medical challenges in early adulthood, with American and European clinical data suggesting ∼20–25% of vEDS patients will have a major vascular complication by age 20, and ∼70–80% having at least one major complication by age 40 (29, 32). Furthermore, early vascular complications or bowel rupture are well-recognized even before adolescence (31). Given these alarming statistics, early diagnosis is therefore vital. The most common presentation in childhood is easy bruising that may be accompanied by skin lucency and vascular visibility, as well as a possible family history of vascular complications (33).
Amongst all subtypes, we noted a prevalence of cardiovascular complications. This is an important finding, as non-vascular EDS subtypes can still present with vascular complications. It has been noted that vascular complications are most frequently reported in mcEDS (CHST14/DSE) and in clEDS (TNXB), occur in two thirds of the patients, respectively (30). Furthermore, cEDS, cvEDS, dEDS, and kEDS subtypes also show a lower, but nonetheless important risk of vascular complications, such as intracranial hemorrhages, arterial aneurysms, and arterial dissections, compared to vEDS (30).
We also observed a high prevalence of cardiovascular dysautonomia amongst hEDS cases. There is growing recognition of the association between hEDS and cardiovascular autonomic dysfunction, which can present as orthostatic intolerance, orthostatic hypotension, postural orthostatic tachycardia syndrome, or neurally mediated hypotension (34). It was noted in a cohort study that ∼50% of adults with hEDS or hypermobility spectrum disorder had diagnostic features of cardiovascular dysautonomia (35). So far, no convincing plausible mechanism has been identified, although there are multiple hypotheses such as abnormal connective tissue in dependent blood vessels causing abnormal vein distention (36), neuropathy with tissue laxity and vasoactive mediation (37), and pathogenic adrenergic and muscarinic autoantibody development (34).
Miscellaneous complications
The most notable group presenting with additional complications were from hEDS cases, with high rates of chronic pain, chronic fatigue, gastrointestinal complications (constipation, diarrhea, irritable bowel syndrome, reflux and/or hepatitis), and urological complications (urinary incontinence, bladder cysts/rupture and/or uterine rupture). As previously mentioned, multiple hypotheses have been proposed to try and explain the multi-system involvement of hEDS patients. For hEDS patients, a reduced expression of specific fibroblast miRNA has been demonstrated, which is implicated in the Wnt pathway for normal ECM regulation. Decreased levels of this miRNA were also found in cerebrospinal fluid and serum of patients with fibromyalgia (38), implying a possible involvement of specific miRNA signatures or a common disease pattern in both conditions (16). In addition, clinical studies have shown that hEDS patients suffer from widespread pain and manifest signs compatible with central sensitization, causing fibromyalgia-like, widespread pain and hyperalgesia to painful stimuli (39). This suggests that the chronic pain and fatigue experienced may reflect abnormal central processing of the nociceptive input in the dorsal horn neurons, possibly involving the descending modulatory system (40, 41). These pathways most likely input into the gastrointestinal (42) and urological systems (43), further explaining the multi-system presentation.
Spontaneous pneumothoraces/hemothoraces was noted amongst vEDS cases. Type III collagen in the lung is expressed in vessels, parenchymal fibroblasts and is particularly rich in the pleural linings (44). Thus, pulmonary complications of vEDS are proposed to result from poor tissue integrity (45). In addition, there may be longstanding damage from inefficient repair of the lung parenchyma and pulmonary vessels after injury, with type I collagen favored over the absent type III collagen (46). We also noted that for vEDS cases, age of first pneumothorax/hemothorax was 25.6 ± 9.0 years. Thus, spontaneous pneumothorax/hemothorax is an early manifestation of vEDS and frequently precedes an arterial complication or intestinal perforation (first presentation at 26.6 ± 10.9 years) (47).
Neuropsychiatric complications were prevalent amongst many of the EDS subtypes, possibly indicating the significant impact that joint hypermobility and EDS complications have on the quality of life of EDS patients. Anxiety symptoms and disorders have a well-established link with hypermobility, yet growing evidence also now points toward associations across psychiatric diagnoses, notably with affective illnesses, increasingly with neurodevelopmental disorders and with stress-sensitive medical conditions, including fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome (48). However, future research is needed to investigate the genetic, neural and psychophysiological mechanisms for such mind-body interactions.
Limitations
Due to the retrospective nature of this study, severity of clinical features could not be assessed. Furthermore, due to a lack of a standardized assessment of craniofacial characteristics and cerebrovascular, cardiovascular, and neuropsychiatric complications, a sub-analysis of individual features could not be undertaken. It must also be noted that in many cases (particularly vEDS cases) that it was not possible to gather information on the scans that were used to identify aneurysms and/or dissection and whether the patient presented symptomatically, or if this was an incidental finding. Given that assessments were performed by physicians and EDS clinicians there is an inherent subjectivity when performing Beighton score assessments. The systematic and predefined search strategy aimed to identify all potentially relevant studies, but the risk of selection bias is not negligible. Due to the rarity of some EDS subtypes, only small case numbers (predominantly from family pedigrees) were reported, which may impact the generalizability of the findings.
In conclusion, the extracutaneous features of the EDS subtypes, as well as the associated complications of each EDS subtype have been presented. We observed significant features that were specific to EDS subtypes, which can help the clinician in an accurate individual assessment of EDS and assist in the decision to undertaken next generation sequencing technologies for a molecular diagnosis. Furthermore, having a comprehensive understanding of the possible complications (particularly for vEDS) may help diagnose and treat EDS prior to the development of substantial comorbidities and/or additional complications. To expand our understanding of EDS and to help optimize management and care guidelines, future research should be focused on further systematic documentation of clinical features and complications, as well as the natural history of each EDS subtype.
Data availability statement
Datasets from this systematic review can be provided, upon reasonable request by contacting the corresponding author.
Author contributions
BD collected preliminary data, contributed to the analysis, and development of the original and revised manuscripts. ML, IH, FM, SS, and IW contributed to the analysis of included manuscript for this review and significant contributions to the revision of the manuscript. JS contributed to the statistical analysis of the raw data, collation of tables, and revision of the manuscript. NB was supervisor of this project who contributed to the design and analysis of this project, review of all manuscript included in the review, and significant contribution to the original and revised manuscripts. All authors contributed to the article and approved the submitted version.
Funding
This research was in part funded by a microgrant from the Ehlers-Danlos Society.
Acknowledgments
We wish to recognize the Ehlers-Danlos Society and the Ehlers-Danlos International Consortium for facilitation of the EDS Skin working group. We would like to thank the authors of published manuscript for providing individual patient data from their studies, with special thanks to Assistant Professor Jennifer Andrews (University of Arizona, USA), Associate Professor Christina Laukaitis (University of Arizona, USA), Professor Benaim Charles (Lausanne University Hospital, Switzerland), Dr. Jessica Eccles (Brighton and Sussex University Hospitals NHS Trust, UK), and Professor Sherene Shalhub (University of Washington, USA).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1053466/full#supplementary-material
References
1. Malfait F, Castori M, Francomano CA, Giunta C, Kosho T, Byers PH. The ehlers-danlos syndromes. Nat Rev Dis Primers. (2020) 6:64. doi: 10.1038/s41572-020-0194-9
2. Malfait F, Francomano C, Byers P, Belmont J, Berglund B, Black J, et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. (2017) 175:8–26.
3. Blackburn PR, Xu Z, Tumelty KE, Zhao RW, Monis WJ, Harris KG, et al. Bi-allelic Alterations in AEBP1 Lead to defective collagen assembly and connective tissue structure resulting in a variant of ehlers-danlos syndrome. Am J Hum Genet. (2018) 102:696–705. doi: 10.1016/j.ajhg.2018.02.018
5. Ritelli M, Colombi M. Molecular genetics and pathogenesis of ehlers-danlos syndrome and related connective tissue disorders. Genes. (2020) 11:547. doi: 10.3390/genes11050547
6. Lim PJ, Lindert U, Opitz L, Hausser I, Rohrbach M, Giunta C. Transcriptome profiling of primary skin fibroblasts reveal distinct molecular features between PLOD1- and FKBP14-kyphoscoliotic ehlers-danlos syndrome. Genes. (2019) 10:517. doi: 10.3390/genes10070517
7. Walkden A, Burkitt-Wright E, Au L. Brittle cornea syndrome: current perspectives. Clin Ophthalmol. (2019) 13:1511–6. doi: 10.2147/OPTH.S185287
8. Kapferer-Seebacher I, van Dijk FS, Zschocke J. Periodontal ehlers-danlos syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Gripp KW, et al. editors. GeneReviews(®). Seattle, WA: University of Washington (2021).
9. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
10. Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. A classification of chronic pain for ICD-11. Pain. (2015) 156:1003–7. doi: 10.1097/j.pain.0000000000000160
11. Syx D, Delbaere S, Bui C, De Clercq A, Larson G, Mizumoto S, et al. Alterations in glycosaminoglycan biosynthesis associated with the Ehlers-Danlos Syndromes. Am J Physiol Cell Physiol. (2022) 323:C1843–59. doi: 10.1152/ajpcell.00127.2022
12. Lu Y, Wang Y, Rauch F, Li H, Zhang Y, Zhai N, et al. Osteogenesis imperfecta type III/Ehlers-Danlos overlap syndrome in a Chinese man. Intractable Rare Dis Res. (2018) 7:37–41. doi: 10.5582/irdr.2018.01010
13. Burkitt Wright EM, Porter LF, Spencer HL, Clayton-Smith J, Au L, Munier FL, et al. Brittle cornea syndrome: recognition, molecular diagnosis and management. Orphanet J Rare Dis. (2013) 8:68. doi: 10.1186/1750-1172-8-68
14. Rohrbach M, Vandersteen A, Yis U, Serdaroglu G, Ataman E, Chopra M, et al. Phenotypic variability of the kyphoscoliotic type of Ehlers-Danlos syndrome (EDS VIA): clinical, molecular and biochemical delineation. Orphanet J Rare Dis. (2011) 6:46. doi: 10.1186/1750-1172-6-46
15. McCrea PD, Maher MT, Gottardi CJ. Nuclear signaling from cadherin adhesion complexes. Curr Top Dev Biol. (2015) 112:129–96. doi: 10.1016/bs.ctdb.2014.11.018
16. Chiarelli N, Ritelli M, Zoppi N, Colombi M. Cellular and molecular mechanisms in the pathogenesis of classical, vascular, and hypermobile ehlersdanlos syndromes. Genes. (2019) 10:609. doi: 10.3390/genes10080609
17. Marathe N, Lohkamp LN, Fehlings MG. Spinal manifestations of Ehlers-Danlos syndrome: a scoping review. J Neurosurg Spine. (2022) 37:783–93. doi: 10.3171/2022.6.SPINE211011
18. Yano H, Hamanaka R, Nakamura-Ota M, Adachi S, Zhang JJ, Matsuo N, et al. Sp7/Osterix induces the mouse pro-alpha2(I) collagen gene (Col1a2) expression via the proximal promoter in osteoblastic cells. Biochem Biophys Res Commun. (2014) 452:531–6. doi: 10.1016/j.bbrc.2014.08.100
19. Volk SW, Shah SR, Cohen AJ, Wang Y, Brisson BK, Vogel LK, et al. Type III collagen regulates osteoblastogenesis and the quantity of trabecular bone. Calcif Tissue Int. (2014) 94:621–31. doi: 10.1007/s00223-014-9843-x
20. Eyre DR, Weis MA. Bone collagen: new clues to its mineralization mechanism from recessive osteogenesis imperfecta. Calcif Tissue Int. (2013) 93:338–47. doi: 10.1007/s00223-013-9723-9
21. Eller-Vainicher C, Bassotti A, Imeraj A, Cairoli E, Ulivieri FM, Cortini F, et al. Bone involvement in adult patients affected with Ehlers-Danlos syndrome. Osteoporos Int. (2016) 27:2525–31. doi: 10.1007/s00198-016-3562-2
22. Scheper MC, Juul-Kristensen B, Rombaut L, Rameckers EA, Verbunt J, Engelbert RH. Disability in adolescents and adults diagnosed with hypermobility-related disorders: a meta-analysis. Arch Phys Med Rehabil. (2016) 97:2174–87. doi: 10.1016/j.apmr.2016.02.015
23. Guarnieri V, Castori M. Clinical relevance of joint hypermobility and its impact on musculoskeletal pain and bone mass. Curr Osteoporos Rep. (2018) 16:333–43. doi: 10.1007/s11914-018-0460-x
24. Rombaut L, Scheper M, De Wandele I, De Vries J, Meeus M, Malfait F, et al. Chronic pain in patients with the hypermobility type of Ehlers-Danlos syndrome: evidence for generalized hyperalgesia. Clin Rheumatol. (2015) 34:1121–9. doi: 10.1007/s10067-014-2499-0
25. Voermans NC, Knoop H. Both pain and fatigue are important possible determinants of disability in patients with the Ehlers-Danlos syndrome hypermobility type. Disabil Rehabil. (2011) 33:706–7. doi: 10.3109/09638288.2010.531373
26. Cazzato D, Castori M, Lombardi R, Caravello F, Bella ED, Petrucci A, et al. Small fiber neuropathy is a common feature of Ehlers-Danlos syndromes. Neurology. (2016) 87:155–9. doi: 10.1212/WNL.0000000000002847
27. Byers PH, Belmont J, Black J, De Backer J, Frank M, Jeunemaitre X, et al. Diagnosis, natural history, and management in vascular Ehlers-Danlos syndrome. Am J Med Genet C Semin Med Genet. (2017) 175:40–7. doi: 10.1002/ajmg.c.31553
28. Beridze N, Frishman WH. Vascular Ehlers-Danlos syndrome: pathophysiology, diagnosis, and prevention and treatment of its complications. Cardiol Rev. (2012) 20:4–7. doi: 10.1097/CRD.0b013e3182342316
29. Frank M, Albuisson J, Ranque B, Golmard L, Mazzella JM, Bal-Theoleyre L, et al. The type of variants at the COL3A1 gene associates with the phenotype and severity of vascular Ehlers-Danlos syndrome. Eur J Hum Genet. (2015) 23:1657–64. doi: 10.1038/ejhg.2015.32
30. D’Hondt S, Van Damme T, Malfait F. Vascular phenotypes in nonvascular subtypes of the Ehlers-Danlos syndrome: a systematic review. Genet Med. (2018) 20:562–73. doi: 10.1038/gim.2017.138
31. Horn D, Siebert E, Seidel U, Rost I, Mayer K, Jamra RA, et al. Biallelic COL3A1 mutations result in a clinical spectrum of specific structural brain anomalies and connective tissue abnormalities. Am J Med Genet A. (2017) 173:2534–8. doi: 10.1002/ajmg.a.38345
32. Shalhub S, Black JH III, Cecchi AC, Xu Z, Griswold BF, Safi HJ, et al. Molecular diagnosis in vascular Ehlers-Danlos syndrome predicts pattern of arterial involvement and outcomes. J Vasc Surg. (2014) 60:160–9. doi: 10.1016/j.jvs.2014.01.070
33. Stembridge N, Doolan BJ, Lavallee ME, Hausser I, Pope FM, Seneviratne SL, et al. The role of cutaneous manifestations in the diagnosis of the Ehlers-Danlos syndromes. Skin Health Dis. (2022) 2022:e140. doi: 10.1002/ski2.140
34. Hakim A, O’Callaghan C, De Wandele I, Stiles L, Pocinki A, Rowe P. Cardiovascular autonomic dysfunction in Ehlers-Danlos syndrome-Hypermobile type. Am J Med Genet C Semin Med Genet. (2017) 175:168–74. doi: 10.1002/ajmg.c.31543
35. Celletti C, Borsellino B, Castori M, Censi F, Calcagnini G, Camerota F, et al. A new insight on postural tachycardia syndrome in 102 adults with hypermobile Ehlers-Danlos Syndrome/hypermobility spectrum disorder. Monaldi Arch Chest Dis. (2020) 90:1286. doi: 10.4081/monaldi.2020.1286
36. Rowe PC, Barron DF, Calkins H, Maumenee IH, Tong PY, Geraghty MT. Orthostatic intolerance and chronic fatigue syndrome associated with Ehlers-Danlos syndrome. J Pediatr. (1999) 135:494–9. doi: 10.1016/S0022-3476(99)70173-3
37. De Wandele I, Rombaut L, Leybaert L, Van de Borne P, De Backer T, Malfait F, et al. Dysautonomia and its underlying mechanisms in the hypermobility type of Ehlers-Danlos syndrome. Semin Arthritis Rheum. (2014) 44:93–100. doi: 10.1016/j.semarthrit.2013.12.006
38. Bjersing JL, Lundborg C, Bokarewa MI, Mannerkorpi K. Profile of cerebrospinal microRNAs in fibromyalgia. PLoS One. (2013) 8:e78762. doi: 10.1371/journal.pone.0078762
39. Lluch E, Torres R, Nijs J, Van Oosterwijck J. Evidence for central sensitization in patients with osteoarthritis pain: a systematic literature review. Eur J Pain. (2014) 18:1367–75. doi: 10.1002/j.1532-2149.2014.499.x
40. Di Stefano G, Celletti C, Baron R, Castori M, Di Franco M, La Cesa S, et al. Central sensitization as the mechanism underlying pain in joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type. Eur J Pain. (2016) 20:1319–25. doi: 10.1002/ejp.856
41. Truini A, Garcia-Larrea L, Cruccu G. Reappraising neuropathic pain in humans–how symptoms help disclose mechanisms. Nat Rev Neurol. (2013) 9:572–82. doi: 10.1038/nrneurol.2013.180
42. Zarate N, Farmer AD, Grahame R, Mohammed SD, Knowles CH, Scott SM, et al. Unexplained gastrointestinal symptoms and joint hypermobility: is connective tissue the missing link? Neurogastroenterol Motil. (2010) 22:252–e78. doi: 10.1111/j.1365-2982.2009.01421.x
43. Gilliam E, Hoffman JD, Yeh G. Urogenital and pelvic complications in the Ehlers-Danlos syndromes and associated hypermobility spectrum disorders: a scoping review. Clin Genet. (2020) 97:168–78. doi: 10.1111/cge.13624
44. McLees BD, Schleiter G, Pinnell SR. Isolation of type III collagen from human adult parenchymal lung tissue. Biochemistry. (1977) 16:185–90. doi: 10.1021/bi00621a004
45. Boone PM, Scott RM, Marciniak SJ, Henske EP, Raby BA. The genetics of pneumothorax. Am J Respir Crit Care Med. (2019) 199:1344–57. doi: 10.1164/rccm.201807-1212CI
46. Hatake K, Morimura Y, Kudo R, Kawashima W, Kasuda S, Kuniyasu H. Respiratory complications of Ehlers-Danlos syndrome type IV. Leg Med. (2013) 15:23–7. doi: 10.1016/j.legalmed.2012.07.005
47. Shalhub S, Neptune E, Sanchez DE, Dua A, Arellano N, McDonnell NB, et al. Spontaneous pneumothorax and hemothorax frequently precede the arterial and intestinal complications of vascular Ehlers-Danlos syndrome. Am J Med Genet A. (2019) 179:797–802. doi: 10.1002/ajmg.a.61094
Keywords: Ehlers-Danlos syndrome, heritable connective tissue disorders (HCTD), extracutaneous, complications, joint hypermobility
Citation: Doolan BJ, Lavallee ME, Hausser I, Schubart JR, Michael Pope F, Seneviratne SL, Winship IM and Burrows NP (2023) Extracutaneous features and complications of the Ehlers-Danlos syndromes: A systematic review. Front. Med. 10:1053466. doi: 10.3389/fmed.2023.1053466
Received: 26 September 2022; Accepted: 03 January 2023;
Published: 23 January 2023.
Edited by:
Tomoki Kosho, Shinshu University, JapanReviewed by:
Glenda Sobey, Sheffield Children’s Hospital, United KingdomTakashi Hashimoto, Osaka City University, Japan
Copyright © 2023 Doolan, Lavallee, Hausser, Schubart, Michael Pope, Seneviratne, Winship and Burrows. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brent J. Doolan,  Yi5kb29sYW5AbmhzLm5ldA==
Yi5kb29sYW5AbmhzLm5ldA==
 Brent J. Doolan
Brent J. Doolan Mark E. Lavallee
Mark E. Lavallee Ingrid Hausser
Ingrid Hausser Jane R. Schubart
Jane R. Schubart F. Michael Pope5
F. Michael Pope5 Ingrid M. Winship
Ingrid M. Winship Nigel P. Burrows
Nigel P. Burrows