- Division of Gastroenterology and Hepatology, Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Republic of Korea
Background/aims: Colonic diverticular bleeding (CDB) is a common cause of acute lower gastrointestinal bleeding. Patients with CDB are at increased risk for recurrence. Here, we aimed to evaluate the clinical course of patients with CDB and identify risk factors for recurrent CDB (rCDB).
Methods: We included patients who were hospitalized at a single tertiary center for management of CDB between January 2005 and March 2020. A Cox proportional hazards regression analysis was performed to evaluate the risk factors of patients with rCDB as follows: model 1 adjusted by age, Charlson comorbidity index (CCI), and presence of bilateral colon diverticula; model 2 adjusted by age, CCI, and presence of left side colon diverticula; model 3 adjusted by age, CCI, and presence of sigmoid colon diverticula.
Results: Among 219 patients (mean age, 68.0 years; 55 females), 56 and 163 had definite and presumptive CDB, respectively. During the median period of 506 days, 62 patients (28.3%) experienced rCDB. CCI score ≥ 4 was independently associated with rCDB in models 1, 2 and 3 (all p < 0.05). Age ≥ 75 years was independently associated with rCDB in models 1 and 2 (both p < 0.05). The presence of bilateral colon and sigmoid colon diverticula were independently associated with rCDB in models 1 and 3, respectively (both p < 0.05).
Conclusion: rCDB frequently occurred at any time in patients with previous CDB. High CCI scores and distribution of colon diverticula were associated with rCDB. Clinicians should consider a possible rCDB for a patient considering age, comorbidity, and distribution of colon diverticula.
1. Introduction
Despite the decreasing incidence of upper gastrointestinal bleeding, the incidence of lower gastrointestinal bleeding (LGIB) is gradually increasing (1). Colonic diverticular bleeding (CDB) is a common cause of LGIB (2), and the risk factors include old age, male sex, obesity, non-steroidal anti-inflammatory drug (NSAID) or antithrombic agent (AT) use, and underlying comorbidities, including cardiovascular diseases, hypertension, or diabetes (3). Recurrent CDB (rCDB) occurs within 1 year at rates of approximately 4–35% (4–7). Despite several efforts, including avoiding or decreasing the number of probable triggers, rCDB may occur at various times and in clinical situations, leading to increased morbidities, rehospitalizations, and medical costs. The risk factors associated with rCDB seem to be similar but inconclusive, as previous studies included a small number of patients and a lack of long-term follow-up data, and related clinical situations at each period may differ. Therefore, studies with a larger number of completed long-term follow-up patients considering the overall clinical factors, including age, comorbidities, and prescribed medications, for patients with rCDB are needed. Therefore, we aimed to evaluate the clinical course of patients with CDB and identify risk factors for rCDB.
2. Methods
2.1. Study population
This study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki and was approved by the institutional review board of Chonnam National University Hospital (IRB No.: CNUH-2020-115).
From January 2005 to March 2020, a total of 3,539 consecutive patients with gastrointestinal bleeding were admitted to our center, of which 1,572 had obscure GIB or LGIB. We excluded 1,351 patients with bleeding of small bowel origin, colon cancer, ischemic colitis, inflammatory bowel disease, hemorrhoids, angiodysplasia, colonic ulcers, or other conditions based on a medical chart review. We also excluded two patients who died on the first admission. Finally, 219 patients with CDB were included in this study (Supplementary Figure S1).
We retrospectively reviewed electronic medical records and extracted information on demographic factors and clinical characteristics, including comorbidities, medication history or laboratory findings, diagnostic or therapeutic procedural outcomes, and the presence and relevance of rCDB with mortality.
2.2. Assessment of comorbidity and Charlson comorbidity index score
The components of the Charlson comorbidity index (CCI) include the following: acquired immunodeficiency syndrome status, cerebrovascular accident, chronic obstructive pulmonary disease, congestive cardiac failure, connective tissue disease, dementia, diabetes, hemiplegia, leukemia, liver disease, lymphoma, peptic ulcer disease, peripheral vascular disease, previous myocardial infarction, renal disease, and solid tumor with or without metastasis. We calculated the CCI score by adding the weights of all comorbid parameters.
2.3. Definition of CDB
All patients underwent colonoscopy and abdominal computed tomography (CT) to exclude other diseases, such as colonic inflammatory bowel disease or cancer. Definite CDB was diagnosed using colonoscopic findings of stigmata of recent diverticular hemorrhage (active bleeding, visible vessel, or adherent clot). Presumptive CDB was diagnosed using endoscopic features of a diverticulum with fresh blood clots in the colon or abdominal CT findings of a colonic extravasation, and colonoscopic findings of a diverticulum without other bleeding foci. Additional examinations, including esophagogastroduodenoscopy, abdominal CT, and capsule endoscopy, cannot confirm other bleeding foci (4, 6).
2.4. Location of diverticula
The anatomical distribution of diverticula was divided into two groups for comparison in three ways: the presence or absence of right-sided colonic diverticula in the cecum, ascending colon, or transverse colon; the presence or absence of left-sided diverticula in the descending or sigmoid colon; bilateral or unilateral colonic diverticulosis.
2.5. Treatment method
Endoscopic hemostasis, endoscopic clipping (EC), epinephrine injection therapy, and argon plasma coagulation were used. Trans arterial embolization was also performed when extravasation was prominent on CT. If the bleeding was not controlled by endoscopic treatment or embolization, surgical treatment was performed.
2.6. Adjustment of antithrombotic agents
We classified antithrombotic agents (ATs) into antiplatelet agents and anticoagulants. Antiplatelet agents included aspirin, thienopyridine, and cilostazol. Anticoagulants included low-molecular-weight heparin (LMWH; enoxaparin and dalteparin), warfarin, and direct oral anticoagulants (DOAC; apixaban, edoxaban, and rivaroxaban). Decisions to use ATs during hospitalization and after discharge were made by the attending physician based on a medical judgment of necessity. We defined ‘adjustment of ATs’ as the discontinuation of any AT during admission and after discharge.
2.7. Confirmation of death outside the hospital
The patient’s date of death and related disease codes were confirmed by request from the Korea National Statistical Agency. Disease codes were classified by the Korean Standard Classification of Disease and Cause of Death (KCD7) (8, 9).
2.8. Outcomes
The primary outcomes were the risk factors of patients with rCDB. rCDB was defined as a significant amount of fresh bloody or wine-colored stool with hemodynamic instability, a need for transfusion, identification of stigmata of recent hemorrhage on repeated colonoscopy, or identification of extravasation in the colonic diverticula on repeated abdomen CT after discharge. The secondary outcomes were the rebleeding rates and the cause of death in patients with CDB.
2.9. Statistical analysis
Continuous and categorical data are expressed as means ± standard deviations or medians (ranges) and absolute or relative frequencies, respectively. Continuous variables were analyzed using a Student’s t-test. Categorical data were examined using the Fisher exact test or χ2 test. Rebleeding-free days were calculated from the date of the first bleeding to the date of recurrence or death using the Kaplan–Meier method. Patients without evidence of recurrence were classified as alive and event-free at the date of the last follow-up. We performed a univariate analysis using the log-rank test. Variables with a value of p < 0.05 were analyzed by multivariate analysis using a Cox proportional hazard model to evaluate the risk factors associated with rCDB. In all statistical tests, a two-sided value of p of <0.05 was considered statistically significant. The Kaplan–Meier analysis was performed using GraphPad Prism (version 5.0; GraphPad Software Inc., San Diego, CA), and all other analyses were performed using SPSS (version 25.0; SPSS Inc., Chicago, IL).
3. Results
3.1. Baseline demographic and clinical characteristics
A total of 219 patients (mean age, 68.0 ± 12.4 years; 55 females) were enrolled in the study. The baseline characteristics of the 219 patients are described in Supplementary Table S1. The most common comorbidities were hypertension (62.4%) and diabetes (33.3%). ATs and NSAIDs were administered to 109 (49.8%) patients and 22 (10.0%) patients, respectively. There were 86 (39.3%) patients with a CCI score of 0 and 133 (60.7%) with a CCI score ≥ 1. Comorbidities contributing to CCI are shown in Supplementary Table S2.
Definite and presumptive CDB were diagnosed in 56 (25.6%) and 163 (74.4%) patients, respectively. Among the 219 patients, right-sided colon diverticula were observed in 189 (86.3%), left-sided colon diverticula were observed in 87 (39.7%), and bilateral colon diverticula were observed in 57 (26.0%).
Fifty-three (24.2%) patients experienced shock at admission, and 164 (74.9%) underwent urgent colonoscopy within 24 h of admission. Cecal intubation was successfully performed in 208 (95.0%) patients. While bleeding stopped spontaneously in 152 (69.4%) patients, urgent endoscopic, radiologic, and surgical interventions were performed in 55 (25.1%), 10 (4.6%), and 2 (0.9%) patients, respectively. The median duration of hospitalization was 6 days.
3.2. Risk factors of rCDB
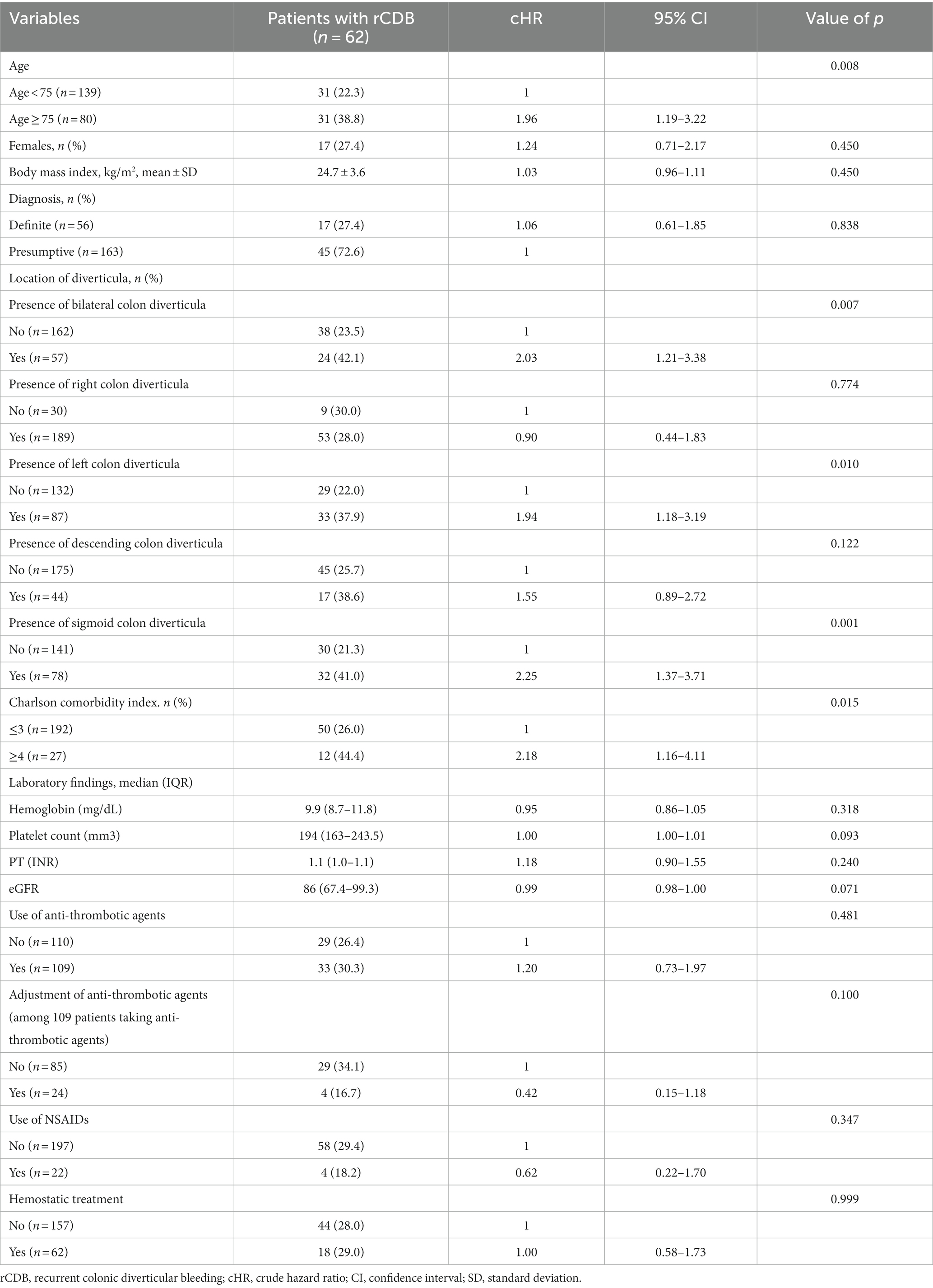
During the median follow-up of 60 months (range, 0.1–182.4), rebleeding occurred in 28.3% (62/219) of patients, and third rebleeding occurred in 11.0% (24/219) of patients (Figure 1). The cumulative incidence rates of rCDB at 1, 6, 12, and 24 months were 5.0% (11/219), 9.1% (20/219), 12.8% (28/219), and 17.4% (38/219), respectively.
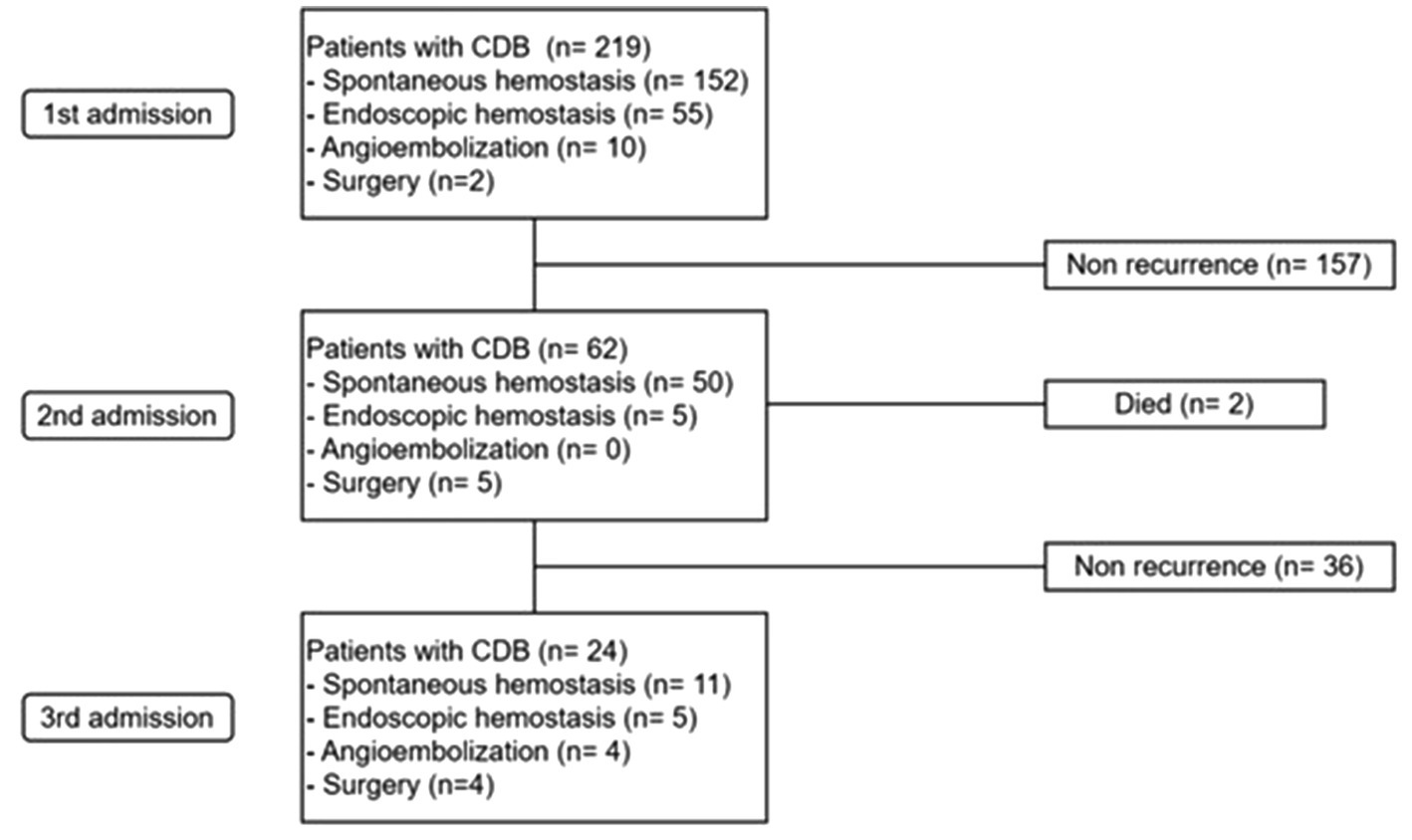
Figure 1. Long-term outcomes of re-admission and therapeutic modalities in study population. CDB, colonic diverticular bleeding.
Table 1 shows the univariate analysis for risk factors of rCDB, in which age ≥ 75 years (p = 0.008), CCI score ≥ 4 (p = 0.015), and location of diverticula [the presence of bilateral colon diverticula (p = 0.007), left colon diverticula (p = 0.010), and sigmoid colon diverticula (p = 0.001)] were associated with rCDB. There were no significant differences between the two groups in terms of sex, underlying comorbidities, laboratory findings, use of ATs or NSAIDs, and hemostatic treatments (all p > 0.05) (Table 1). The Kaplan–Meier analysis showed that rCDB occurred more in patients with CCI scores ≥4 and in those with a presence of sigmoid colon, left colonic, and bilateral diverticulosis (All p ≤ 0.01) (Figure 2).
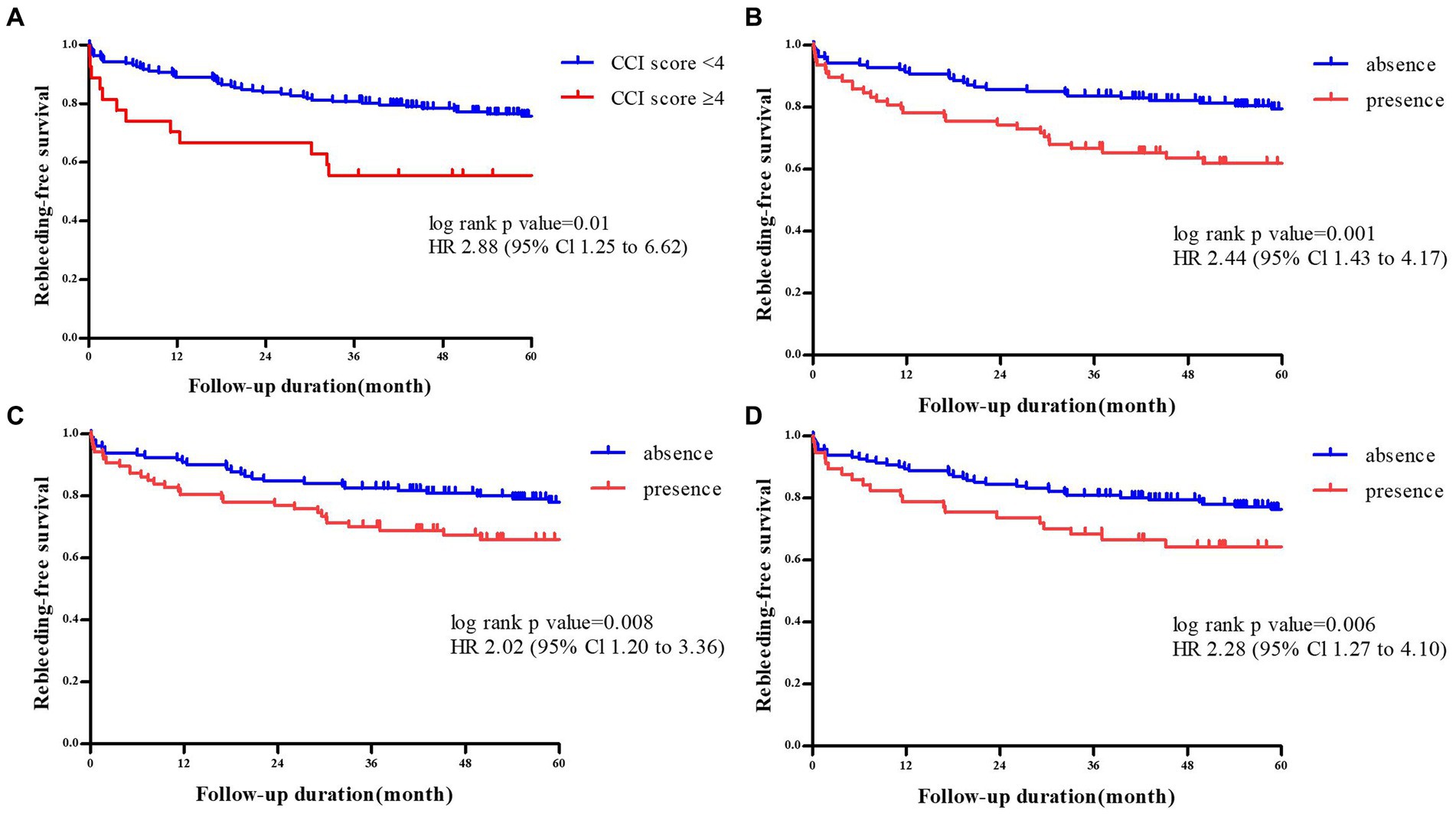
Figure 2. Comparison of rebleeding -free survival rate according to the Charlson comorbidity index scores of≥ 4 or <4 (A), the presence of sigmoid colon diverticuli (B), the presenceof left colonic diverticuli (C), and bilatral diverticuli (D).
We performed a multivariate analysis including variables with a value of p <0.05 (age ≥ 75 years, CCI score ≥ 4), considering the location of colon diverticula (the presence of bilateral colon, left side colon, and sigmoid colon diverticula): model 1 adjusted by age, CCI, and the presence of bilateral colon diverticula; model 2 adjusted by age, CCI, and the presence of left side colon diverticula; model 3 adjusted by age, CCI, and the presence of sigmoid colon diverticula.
Model 1 showed that an age ≥ 75 years, a CCI score ≥ 4, and the presence of bilateral colon diverticula were all independent risk factors for rCDB (adjusted hazard ratio [aHR] 1.70, 95% confidence interval [CI] 1.02–2.83, p = 0.04 for age ≥ 75 years; aHR 2.24, 95% CI 1.19–4.23, p = 0.01 for a CCI score ≥ 4; aHR 2.24, 95% CI 1.19–4.23, p = 0.01 for the presence of bilateral colon diverticula). Model 2 showed that age ≥ 75 years and a CCI score ≥ 4 were both independent risk factors for rCDB (aHR 1.92, 95% CI 1.17–3.16, p = 0.01 for age ≥ 75 years; aHR 2.11, 95% CI 1.12–3.98, p = 0.02 for a CCI score ≥ 4). Model 3 showed that a CCI score ≥ 4 and the presence of sigmoid colon diverticula were both independent risk factors for rCDB (aHR 2.48, 95% CI 1.31–4.68, p < 0.01 for a CCI score ≥ 4; aHR 2.41, 95% CI 1.46–3.98, p < 0.01 for the presence of sigmoid colon diverticula) (Table 2).
Table 2. Cox proportional hazards models of risk factors for recurrent colonic diverticular bleeding.
3.3. Adjustment of ATs in patients taking ATs
Among 109 (49.8%) patients that were taking ATs, 24 (22.0%) discontinued AT agents after discharge upon medical judgment by an attending physician. A Kaplan–Meier analysis showed that rCDB occurred less in patients with adjustment of ATs than in patients who continued using ATs, but this was statistically insignificant (HR 1.98, 95% CI 0.90–4.37 p = 0.08) (Supplementary Figure S2).
3.4. Causes of death
Two patients died at the first admission because of diverticular bleeding and acute myocardial infarction, respectively. During the follow-up after discharge, 31 out of the remaining 219 patients died, of which 11 (21.6%) had rCDB and 20 (11.8%) did not have rCDB (p = 0.08). One patient with rCDB had a CDB-related death. The most common cause of death during the follow-up was pneumonia (n = 5, 16.1%) (Supplementary Table S3).
4. Discussion
The incidence of CDB is increasing because of the increase in colonic diverticulosis, an aging population, and AT use (3). Approximately 70–90% of diverticular bleeding events spontaneously stop, complicating the definite diagnosis of diverticular bleeding (10–12). In our study, only 25% of patients were diagnosed with definite CDB, which was similar to proportions in previous studies (19–42%) (6, 13, 14), and CDB spontaneously stopped in 69.4% of patients. Despite this, about 25% of the patients with CDB experienced hypovolemic shock at admission, which was also similar to a previous study (25.6%) (7). Therefore, any changes in symptoms or vital signs should be closely monitored, and adequate resuscitation should be provided for all patients with CDB. This study presented the clinical course of 219 patients with CDB over 15 years in a tertiary referral center. The recurrence rate was as high as 28.3%.
The CCI was developed to determine the association between patients’ various underlying diseases and one-year mortality (15). The CCI is helpful in classifying patients by weighing their comorbidities and has been validated in various disease subgroups, such as cardiac, renal, stroke-related, and liver diseases (15–19). Recently, the CCI has been used to predict the prognosis in various patient groups (16–20), with high CCI scores becoming a risk factor for severe CDB (21). In this study, we used the CCI score to reflect the overall status of comorbidities. The incidence of rCDB was about two times higher in patients with CCI scores ≥4 after adjusting factors found to be significant in a univariate analysis, such as age and location of diverticula. Therefore, clinicians should consider the occurrence of rCDB and make active efforts, such as controlling underlying diseases and adjusting the related medication in patients with diverticular bleeding, especially in patients with a high CCI score.
While left-sided colonic diverticulosis is more common in Western countries, right-sided or bilateral colon diverticula are more common in Asian countries (13, 22–25), which was similar to our study. Over 95% of patients in our study underwent colonoscopy with a full examination, which allowed the precise detection of the anatomical distribution of diverticulum. Interestingly, our study identified that the location of the colon diverticula was associated with the occurrence of rCDB. Patients with bilateral colon diverticula had a higher occurrence of rCDB, which was similar to previous studies (24, 26, 27). We also demonstrated that patients with sigmoid colon diverticula had a higher occurrence of rCDB. Left-sided colonic diverticulosis is considered acquired rather than congenital and increases with age (28). However, even after adjustment for significant factors, such as CCI and age, sigmoid colon diverticulosis was an independent risk factor for rCDB. Colonoscopy, especially performed in an emergency situation, is not perfect due to inadequate bowel preparation and remaining bloody clots attached to the multiple diverticular sacs and colonic mucosa. This limited surface visualization may lead to blind spots, especially in the flexure, behind folds, and angulated area of the colon. Compared to other locations of the colon, it can be difficult to find the bleeding focus from sigmoid colon diverticula using a colonoscope due to anatomical characteristics, such as the many folds and angulation of the sigmoid colon. In addition, the sigmoid colon length can vary depending on the endoscopist’s colonoscopic manipulation. This length ranges from 40 to 70 cm when stretched during scope insertion and is shortened to only 30–35 cm when the scope is straightened fully (29). For these reasons, endoscopists may miss the hidden bleeding focus in the sigmoid colon, leading to rCDB. Further studies are needed to elucidate the relationship between the location of colon diverticula and rCDB.
Endoscopic hemostasis can achieve a high rate of active bleeding control (30, 31) and is typically considered the first-line treatment for CDB management (32, 33). In this study, 75% of patients had undergone urgent colonoscopy examinations within 24 h. Although aggressive colonoscopic evaluations were performed as the first evaluation, an endoscopic intervention was performed in only a third of the patients. A literature review reported an endoscopic hemostasis rate ranging from 20.8 to 32.8% in Western countries (34, 35) and 16.8 to 34.1% in Eastern countries (36, 37), which were similar to the rates in our study. Angiographic treatment and surgical resection are other modalities used if endoscopic hemostasis fails (38, 39). In our study, radiological interventions were performed in 11 (5.0%) patients and surgical intervention in two (0.9%). While the most of diverticular bleeding episodes resolve spontaneously with conservative treatment, surgical treatment becomes necessary under specific circumstances, including persistent bleeding, recurrent bleeding episodes, and even the development of hypovolemic shock (40, 41). Urgent surgery is required in approximately 10 to 25% of patients who experience hemodynamic instability (42). Studies have reported morbidity and mortality rates of 17 and 8.3%, respectively, among patients with acute CDB who underwent urgent surgery (43). Recent research has underscored that the presence of comorbid diseases is an independent risk factor for requiring urgent colectomy for CDB, with both mortality and morbidity rates as high as 20% (40). Consequently, patients with more comorbid diseases may face increased risks when undergoing urgent colectomy.
Although NSAIDs, steroids, ATs, obesity, hypertension, and chronic kidney disease are risk factors for rCDB, each study showed inconsistent results (4–6, 44). In this study, medications, including ATs, NSAIDs, and steroids, did not increase the risk of rCDB. However, rCDB occurred less in patients with an adjustment of ATs than in patients with a continuation of ATs. Thus, the modification of ATs should be considered to prevent rebleeding. However, it is possible that the discontinuation of ATs could result in fatal thromboembolic events, particularly in elderly patients. Therefore, we believe that the discontinuation of ATs should be carefully considered along with their risks and benefits.
In this study, CDB-related death occurred in two patients, and the overall mortality rate was twice as high in patients with rCDB compared to those without rCDB. Although CDB itself was not the immediate cause of death, increased hospitalization and morbidity due to CDB may have affected the increase in mortality.
Our study had two limitations. First, this was a retrospective cohort study conducted at a single referral center. However, a larger number of cases were evaluated in this study than in previous studies. Second, this study did not reflect the effects of variable endoscopic hemostatic methods, such as endoscopic band ligation, endoscopic detachable snare ligation, use of topical hemostatic agents, and over-the-scope clip (36, 45–47). We typically performed endoscopic hemostasis using endoscopic clipping or endoscopic injection therapy. Recent studies showed that endoscopic band ligation was more helpful in decreasing early rCDB (13, 39).
5. Conclusion
rCDB frequently occurred at any time in patients with previous CDB. High CCI scores and distribution of colon diverticula were associated with rCDB. Clinicians should consider a possible rCDB for a patient considering age, comorbidity, and distribution of colon diverticula.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the institutional review board of Chonnam National University Hospital (IRB No.: CNUH-2020-115). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
S-YP and H-SK developed the concept of the study, analyzed the collected data, wrote the manuscript, revised it critically for intellectual content and supervised the study. H-SY, S-YC, and DK collected clinical data, analyzed th electronic medical records, and wrote the manuscript. CP and SC collected clinical data. All authors have read and approved the final manuscript.
Funding
This study was supported by grants (BCRI 23033 and BCRI 23016) of the Chonnam National University Hospital Biomedical Research Institute.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1195051/full#supplementary-material
Abbreviations
AT, antithrombotic agent; CCI, Charlson comorbidity index; CDB, colonic diverticular bleeding; CI, confidence interval; CT, computed tomography; HR, hazard ratio; LGIB, lower gastrointestinal bleeding; LMWH, low-molecular-weight heparin; NSAIDs, non-steroidal anti-inflammatory drugs; DOAC, direct oral anticoagulant; rCDB, recurrent CDB.
References
1. Lanas, A, García-Rodríguez, LA, Polo-Tomás, M, Ponce, M, Alonso-Abreu, I, Perez-Aisa, MA, et al. Time trends and impact of upper and lower gastrointestinal bleeding and perforation in clinical practice. Am J Gastroenterol. (2009) 104:1633–41. doi: 10.1038/ajg.2009.164
2. Longstreth, GF. Epidemiology and outcome of patients hospitalized with acute lower gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. (1997) 92:419–24.
3. Kinjo, K, Matsui, T, Hisabe, T, Ishihara, H, Maki, S, Chuman, K, et al. Increase in colonic diverticular hemorrhage and confounding factors. World J Gastrointest Pharmacol Ther. (2016) 7:440–6. doi: 10.4292/wjgpt.v7.i3.440
4. Niikura, R, Nagata, N, Yamada, A, Akiyama, J, Shimbo, T, and Uemura, N. Recurrence of colonic diverticular bleeding and associated risk factors. Color Dis. (2012) 14:302–5. doi: 10.1111/j.1463-1318.2011.02611.x
5. Nishikawa, H, Maruo, T, Tsumura, T, Sekikawa, A, Kanesaka, T, and Osaki, Y. Risk factors associated with recurrent hemorrhage after the initial improvement of colonic diverticular bleeding. Acta Gastroenterol Belg. (2013) 76:20–4.
6. Taki, M, Oshima, T, Tozawa, K, Taniguchi, Y, Tomita, T, Ohda, Y, et al. Analysis of risk factors for colonic diverticular bleeding and recurrence. Medicine (Baltimore). (2017) 96:e8090. doi: 10.1097/MD.0000000000008090
7. Poncet, G, Heluwaert, F, Voirin, D, Bonaz, B, and Faucheron, JL. Natural history of acute colonic diverticular bleeding: a prospective study in 133 consecutive patients. Aliment Pharmacol Ther. (2010) 32:466–71. doi: 10.1111/j.1365-2036.2010.04362.x
8. Organization WH. International statistical classification of diseases and related health problems: Tabular list. World Health Organization (2004).
9. Shin, H-Y, Lee, J-Y, Kim, J-E, Lee, S-M, Youn, H-J, Kim, H-R, et al. Cause-of-death statistics in 2016 in the Republic of Korea. J Korean Med Assoc. (2018) 61:573–84. doi: 10.5124/jkma.2018.61.9.573
10. Goldberg, S, Nivatvongs, S, and Rothenberger, D. Diverticular disease with acute hemorrhage In: SI Schwartz, GT Shires, and FC Spencer, editors. Principles of Surgery. New York: McGraw-Hill (1989). 255–9.
11. McGuire, HH Jr. Bleeding colonic diverticula. A reappraisal of natural history and management. Ann Surg. (1994) 220:653–6. doi: 10.1097/00000658-199411000-00008
12. Lewis, M. Bleeding colonic diverticula. J Clin Gastroenterol. (2008) 42:1156–8. doi: 10.1097/MCG.0b013e3181862ad1
13. Honda, H, Ishii, N, Takasu, A, Shiratori, Y, and Omata, F. Risk factors of early rebleeding in the endoscopic management of colonic diverticular bleeding. J Gastroenterol Hepatol. (2019) 34:1784–92. doi: 10.1111/jgh.14669
14. Oguri, N, Ikeya, T, Kobayashi, D, Yamamoto, K, Yoshimoto, T, Takasu, A, et al. Effectiveness of risk scoring systems in predicting endoscopic treatment in colonic diverticular bleeding. J Gastroenterol Hepatol. (2020) 35:815–20. doi: 10.1111/jgh.14901
15. Charlson, ME, Pompei, P, Ales, KL, and MacKenzie, CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
16. Goldstein, LB, Samsa, GP, Matchar, DB, and Horner, RD. Charlson index comorbidity adjustment for ischemic stroke outcome studies. Stroke. (2004) 35:1941–5. doi: 10.1161/01.STR.0000135225.80898.1c
17. Hemmelgarn, BR, Manns, BJ, Quan, H, and Ghali, WA. Adapting the Charlson comorbidity index for use in patients with ESRD. Am J Kidney Dis. (2003) 42:125–32. doi: 10.1016/S0272-6386(03)00415-3
18. Lee, DS, Donovan, L, Austin, PC, Gong, Y, Liu, PP, Rouleau, JL, et al. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. (2005) 43:182–8. doi: 10.1097/00005650-200502000-00012
19. Myers, RP, Quan, H, Hubbard, JN, Shaheen, AA, and Kaplan, GG. Predicting in-hospital mortality in patients with cirrhosis: results differ across risk adjustment methods. Hepatology. (2009) 49:568–77. doi: 10.1002/hep.22676
20. Kim, S, Kim, DH, Park, SY, Park, CH, Kim, HS, Choi, SK, et al. Association between Charlson comorbidity index and complications of endoscopic resection of gastric neoplasms in elderly patients. BMC Gastroenterol. (2020) 20:213. doi: 10.1186/s12876-020-01360-6
21. Kinjo, K, Matsui, T, Hisabe, T, Ishihara, H, Kojima, T, Chuman, K, et al. Risk factors for severity of colonic diverticular hemorrhage. Intest Res. (2018) 16:458–66. doi: 10.5217/ir.2018.16.3.458
22. Stollman, N, and Raskin, JB. Diverticular disease of the colon. Lancet. (2004) 363:631–9. doi: 10.1016/S0140-6736(04)15597-9
23. Casarella, WJ, Kanter, IE, and Seaman, WB. Right-sided colonic diverticula as a cause of acute rectal hemorrhage. N Engl J Med. (1972) 286:450–3. doi: 10.1056/NEJM197203022860902
24. Suh, S, Seo, PJ, Park, H, Shin, CM, Jo, HJ, Kim, HY, et al. The risk factors for colonic diverticular bleeding. Korean J Gastroenterol. (2012) 60:349–54. doi: 10.4166/kjg.2012.60.6.349
25. Imaeda, H, and Hibi, T. The burden of diverticular disease and its complications: west versus east. Inflamm Intest Dis. (2018) 3:61–8. doi: 10.1159/000492178
26. Sato, Y, Yasuda, H, Nakamoto, Y, Kiyokawa, H, Yamashita, M, Matsuo, Y, et al. Risk factors for late Rebleeding of colonic diverticular bleeding in elderly individuals. J Anus Rectum Colon. (2021) 5:148–57. doi: 10.23922/jarc.2020-081
27. Arena, R, Lisotti, A, Mussetto, A, Merighi, A, Pezzoli, A, and Triossi, O. Right-sided diverticulosis is an independent risk factor for bleeding in patients admitted for diverticular disease. Dig Liver Dis. (2021) 53:835–40. doi: 10.1016/j.dld.2020.09.027
28. Nakaji, S, Danjo, K, Munakata, A, Sugawara, K, MacAuley, D, Kernohan, G, et al. Comparison of etiology of right-sided diverticula in Japan with that of left-sided diverticula in the west. Int J Color Dis. (2002) 17:365–73. doi: 10.1007/s00384-002-0403-x
29. Jayasekeran, V, Holt, B, and Bourke, M. Normal adult colonic anatomy in colonoscopy. Video J Encyclop GI Endos. (2013) 1:390–2. doi: 10.1016/S2212-0971(13)70173-0
30. Yen, EF, Ladabaum, U, Muthusamy, VR, Cello, JP, McQuaid, KR, and Shah, JN. Colonoscopic treatment of acute diverticular hemorrhage using endoclips. Dig Dis Sci. (2008) 53:2480–5. doi: 10.1007/s10620-007-0151-4
31. Kobayashi, K, Furumoto, Y, Akutsu, D, Matsuoka, M, Nozaka, T, Asano, T, et al. Endoscopic detachable snare ligation improves the treatment for colonic diverticular hemorrhage. Digestion. (2020) 101:208–16. doi: 10.1159/000498847
32. Bloomfeld, RS, Rockey, DC, and Shetzline, MA. Endoscopic therapy of acute diverticular hemorrhage. Am J Gastroenterol. (2001) 96:2367–72. doi: 10.1111/j.1572-0241.2001.04048.x
33. Kaise, M, Nagata, N, Ishii, N, Omori, J, Goto, O, and Iwakiri, K. Epidemiology of colonic diverticula and recent advances in the management of colonic diverticular bleeding. Dig Endosc. (2020) 32:240–50. doi: 10.1111/den.13547
34. Kaltenbach, T, Watson, R, Shah, J, Friedland, S, Sato, T, Shergill, A, et al. Colonoscopy with clipping is useful in the diagnosis and treatment of diverticular bleeding. Clin Gastroenterol Hepatol. (2012) 10:131–7. doi: 10.1016/j.cgh.2011.10.029
35. Jensen, DM, Machicado, GA, Jutabha, R, and Kovacs, TO. Urgent colonoscopy for the diagnosis and treatment of severe diverticular hemorrhage. N Engl J Med. (2000) 342:78–82. doi: 10.1056/NEJM200001133420202
36. Ishii, N, Setoyama, T, Deshpande, GA, Omata, F, Matsuda, M, Suzuki, S, et al. Endoscopic band ligation for colonic diverticular hemorrhage. Gastrointest Endosc. (2012) 75:382–7. doi: 10.1016/j.gie.2011.07.030
37. Ishii, N, Hirata, N, Omata, F, Itoh, T, Uemura, M, Matsuda, M, et al. Location in the ascending colon is a predictor of refractory colonic diverticular hemorrhage after endoscopic clipping. Gastrointest Endosc. (2012) 76:1175–81. doi: 10.1016/j.gie.2012.07.040
38. Browder, W, Cerise, EJ, and Litwin, MS. Impact of emergency angiography in massive lower gastrointestinal bleeding. Ann Surg. (1986) 204:530–6. doi: 10.1097/00000658-198611000-00004
39. Ishii, N, Omata, F, Nagata, N, and Kaise, M. Effectiveness of endoscopic treatments for colonic diverticular bleeding. Gastrointest Endosc. (2018) 87:58–66. doi: 10.1016/j.gie.2017.08.013
40. Chen, C-Y, Wu, C-C, Jao, S-W, Pai, L, and Hsiao, C-W. Colonic diverticular bleeding with comorbid diseases may need elective colectomy. J Gastrointest Surg. (2009) 13:516–20. doi: 10.1007/s11605-008-0731-4
41. Cirocchi, R, Grassi, V, Cavaliere, D, Renzi, C, Tabola, R, Poli, G, et al. New trends in acute management of colonic diverticular bleeding: a systematic review. Medicine. (2015) 94:e1710. doi: 10.1097/MD.0000000000001710
42. Zuccaro, G Jr. Management of the adult patient with acute lower gastrointestinal bleeding. J Am College Gastroenterol. (1998) 93:1202–8. doi: 10.1111/j.1572-0241.1998.00395.x
43. Ríos, A, Montoya, MJ, Rodríguez, JM, Serrano, A, Molina, J, Ramírez, P, et al. Severe acute lower gastrointestinal bleeding: risk factors for morbidity and mortality. Langenbeck’s Arch Surg. (2007) 392:165–71. doi: 10.1007/s00423-006-0117-6
44. Aytac, E, Stocchi, L, Gorgun, E, and Ozuner, G. Risk of recurrence and long-term outcomes after colonic diverticular bleeding. Int J Color Dis. (2014) 29:373–8. doi: 10.1007/s00384-013-1804-8
45. Hashimoto, R, Hamamoto, H, and Tanuma, T. Endoscopic hemostasis of diverticular bleeding by using detachable snares. Gastrointest Endosc. (2016) 84:379–80. doi: 10.1016/j.gie.2016.01.008
46. Barkun, AN, Moosavi, S, and Martel, M. Topical hemostatic agents: a systematic review with particular emphasis on endoscopic application in GI bleeding. Gastrointest Endosc. (2013) 77:692–700. doi: 10.1016/j.gie.2013.01.020
Keywords: colon, comorbidity, diverticular diseases, diverticulum, recurrence
Citation: You H-S, Kim DH, Cho S-Y, Park S-Y, Park CH, Kim H-S and Choi SK (2023) Risk factors for patients hospitalized with recurrent colon diverticular bleeding: a single center experience. Front. Med. 10:1195051. doi: 10.3389/fmed.2023.1195051
Edited by:
Giuseppe Losurdo, University of Bari Medical School, ItalyReviewed by:
Emad Aljahdli, King Abdulaziz University, Saudi ArabiaAlessio Vagliasindi, Oncological Center of Basilicata (IRCCS), Italy
Copyright © 2023 You, Kim, Cho, Park, Park, Kim and Choi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seon-Young Park, ZHJwc3lAbmF2ZXIuY29t; Hyun-Soo Kim, Y29sb25oc2tAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Hye-Su You
Hye-Su You Dong Hyun Kim
Dong Hyun Kim Seo-Yeon Cho
Seo-Yeon Cho Seon-Young Park
Seon-Young Park