Abstract
Purpose:
The accumulation of advanced glycation end products (AGEs) is associated with various diseases and age-related impairments, including loss of muscle mass and function. We investigated the association between plasma pentosidine, which is one of the AGEs, and sarcopenia, low gait speed, and mortality in patients with cirrhosis.
Methods:
This retrospective study divided 128 patients with cirrhosis into three groups by 25th and 75th quartiles of baseline plasma pentosidine levels: low (L)-, intermediate (I)-, and high (H)-pentosidine (Pen) groups. Sarcopenia was diagnosed following the Japan Society of Hepatology criteria. Low gait speed was defined as <0.8 m/s. The cumulative survival rates were compared between the three groups. Cox proportional hazards regression analysis was performed to identify independent factors associated with mortality.
Results:
Of the 128 patients, 40 (31.3%) and 34 (26.6%) had sarcopenia and low gait speed, respectively. The prevalence of sarcopenia and low gait speed significantly increased stepwise with increasing plasma pentosidine levels, with the highest in the H-Pen group (59.4% [19/32] and 56.3% [18/32], respectively) and lowest in the L-Pen group (18.8% [6/32] and 6.3% [2/32], respectively). Multivariate analysis identified plasma pentosidine levels as a significant and independent factor associated with sarcopenia (odds ratio [OR], 1.07; p = 0.036) and low gait speed (OR, 1.06; p = 0.036), with the cutoff levels of 0.0792 μg/mL (sensitivity/specificity, 0.600/0.773) and 0.0745 μg/mL (sensitivity/specificity, 0.735/0.691), respectively. The cumulative survival rates were significantly lower in the H-Pen group than in the L-Pen (hazard ratio [HR], 11.7; p = 0.001) and I-Pen (HR, 4.03; p < 0.001) groups. Plasma pentosidine levels were identified as a significant and independent prognostic factor (HR, 1.07; p < 0.001).
Conclusion:
Plasma pentosidine levels are associated with sarcopenia, low gait speed, and mortality and may serve as a useful surrogate biomarker for these clinical events in patients with cirrhosis.
Introduction
Cirrhosis is caused by persistent hepatic inflammation and progressive liver fibrosis. It in turn causes various complications and is associated with high mortality (1). Cirrhosis progresses naturally in two stages: a compensated phase followed by a decompensated phase (with ascites, encephalopathy, jaundice, and variceal hemorrhage), eventually leading to liver failure (2). The 1-year mortality rate was four times higher in patients with decompensated cirrhosis than in those with compensated cirrhosis (3). Therefore, appropriate monitoring for disease progression and cirrhosis-related events and prognosis prediction are crucial for managing patients with cirrhosis.
Sarcopenia, which is a progressive and generalized loss of skeletal muscle mass and function, is one of the most common and critical complications in patients with cirrhosis (4). A meta-analysis of 36 studies reported that the pooled prevalence of sarcopenia was 33% in patients with cirrhosis (5). Recent studies revealed the existence of sarcopenia as a high-risk factor for cirrhosis-related events, such as infections, ascites, hepatic encephalopathy, and death (6–10). The pathophysiological mechanisms of sarcopenia in chronic liver disease are multifactorial and complex (11), i.e., aging, malnutrition, low insulin-like growth factor 1 (IGF-1, an anabolic hormone) and branched-chain amino acid levels, and elevated inflammatory cytokine levels cause an imbalance between protein synthesis and breakdown. In the aging population, low levels of vitamin D, folate, and albumin, high levels of C-reactive protein, older age, lower body mass index (BMI), and the existence of chronic diseases (including diabetes and renal dysfunction with albuminuria), which reflect malnutrition and inflammatory conditions, are closely associated with sarcopenia (12–14). Furthermore, sarcopenia, combined with cognitive impairment, increases 1-year mortality in older patients discharged from acute care hospitals (15). Therefore, the development and progression of sarcopenia should be evaluated from various viewpoints.
Advanced glycation end products (AGEs) are heterogeneous molecules of irreversible products formed by non-enzymatic reactions between reducing sugars and proteins/lipids/nucleic acids (known as the Maillard reaction) (16). Various factors, such as aging, hyperglycemia, smoking, excessive alcohol consumption, inflammation, and oxidative stress, promote the accumulation of AGEs, thereby promoting reactive oxygen species production and inflammatory cascades and changes in protein structure and function, cellular apoptosis and dysfunction, and various organ damage (17). Numerous studies have revealed that AGEs play a crucial role in the pathological conditions of various diseases, such as diabetes and its complications, poor bone quality and fractures, chronic kidney disease (CKD), neurodegenerative diseases, cardiovascular disease, and chronic liver disease (16, 18–23).
Pentosidine is one of the most widely studied AGEs. Its blood and urinary content correlate with the total amount of AGEs in tissues (such as bone and skin) and thus could be a representative marker for the total AGE content in the tissues (19, 24). Several studies have revealed that high serum or urinary pentosidine levels are associated with low muscle mass, low muscle strength, low gait speed, and sarcopenia in patients with diabetes and community-dwelling adults (25–27). Furthermore, high blood pentosidine levels are associated with mortality in patients with CKD or heart failure (28, 29). Our previous study of patients with chronic liver disease revealed increased plasma pentosidine levels stepwise as the liver disease progressed, and they were correlated with hepatic fibrosis marker and Child–Pugh scores (30). Conversely, liver functional reserve-related factors, such as total bilirubin, albumin, and prothrombin time, were associated with plasma pentosidine levels. Thus, plasma pentosidine levels may be useful in predicting sarcopenia, impaired liver functional reserve, and mortality in patients with cirrhosis.
This study aimed to investigate the relationship between plasma pentosidine levels, sarcopenia, and prognosis in patients with cirrhosis.
Materials and methods
Study participants
This retrospective study included 128 patients with cirrhosis who presented to the Jikei University School of Medicine (Tokyo, Japan) and Fuji City General Hospital (Shizuoka, Japan) between 2017 and 2020. The inclusion criteria were as follows: (1) the presence of cirrhosis and (2) available measurements of skeletal muscle mass index (SMI) using bioelectrical impedance analysis (InBody S10; InBody, Tokyo, Japan) and handgrip strength using a dynamometer (T.K.K5401 GRIP-D; Takei Scientific Instruments, Niigata, Japan). The exclusion criteria were as follows: (1) alcoholic cirrhosis with different characteristics from cirrhosis caused by other etiologies (e.g., low prevalence of sarcopenia in non-elderly patients despite advanced disease stage) (31); (2) pre-existence of uncontrollable malignancies, including hepatocellular carcinoma (HCC); and (3) pacemakers, implants, or refractory ascites due to the decreased reliability of bioelectrical impedance analysis. Cirrhosis was diagnosed based on laboratory tests and imaging/endoscopic findings, such as liver deformity with atrophy of the right hepatic lobe, nodular liver surface, ascites, and esophageal/gastric varices. The model for end-stage liver disease (MELD) score and the Child–Pugh classification were used to assess the liver functional reserve (32, 33). HCC diagnosis was made based on the HCC guidelines of the American Association for the Study of Liver Diseases (34). Patients with HCC were defined as those with viable HCC at study entry. Patients who underwent liver transplantation during the observation period were considered as death and censored cases. CKD was defined as an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2 for at least 3 months (35). This study was conducted in accordance with the 2013 Declaration of Helsinki and was approved by the ethics committees of the Jikei University School of Medicine (approval number: 34-021) and Fuji City General Hospital (approval number: 279).
Diagnosis of sarcopenia and low gait speed
The diagnosis of sarcopenia was made according to the revised sarcopenia criteria proposed by the Japan Society of Hepatology (36). In brief, sarcopenia was defined as low SMI (<7.0 kg/m2 for men and <5.7 kg/m2 for women) and low handgrip strength (<28 kg for men and < 18 kg for women). Gait speed was measured over a distance of 6 m, and low gait speed was defined as <0.8 m/s (37).
Laboratory assessments
Serum total bilirubin, albumin, and creatinine were measured using standard laboratory methods. Mac-2 binding protein glycosylation isomer (M2BPGi, a hepatic fibrosis marker) was measured using a sandwich enzyme-linked immunosorbent assay (ELISA) with Wisteria floribunda lectin-recognizing carbohydrate chains (HISCL-2000i; Sysmex, Hyogo, Japan) and presented as a cut-off index (C.O.I.) calculated according to the manufacturer’s specified formula. Prothrombin time–international normalized ratio (PT–INR) was measured using a thromboplastin reagent (Coagpia PT-N; Sekisui Medical, Tokyo, Japan). The eGFR was calculated using the following formula: eGFR (mL/min/1.73 m2) = 194 × creatinine −1.094 × age −0.287 (×0.739 for women). Plasma pentosidine levels were measured using an ELISA (FSK pentosidine ELISA kit; Fushimi Pharmaceutical, Kagawa, Japan), as described elsewhere (30). Briefly, 50 μL of plasma samples were added to pronase and immediately incubated at 55°C for 1.5 h. After the enzyme reaction, they were heated in boiling water for 15 min to inactivate the enzyme. The pretreated plasma samples were incubated with pentosidine-specific rabbit antibody at 37°C for 1 h. After washing, peroxidase-labeled goat anti-rabbit IgG polyclonal antibody was added and re-incubated at room temperature for 1 h. A color-developing reagent was added to each well and the reaction was stopped after 10 min. Finally, the absorbance was measured at 450 nm (main wavelength) and 630 nm (reference wavelength).
Classification based on plasma pentosidine levels
The median plasma pentosidine level of the study cohort was 0.0665 μg/mL (interquartile range, 0.0508–0.0954 μg/mL). Patients were classified into three groups based on the first and third quartiles: low-pentosidine (L-Pen) (pentosidine level: ≤0.0508 μg/mL); (2) intermediate-pentosidine (I-Pen) (pentosidine level: 0.0508–0.0954 μg/mL); and (3) high-pentosidine (H-Pen) (pentosidine level: ≥0.0954 μg/mL).
Statistical analysis
Categorical variables and continuous variables are expressed as number (percentage) and median (interquartile range), respectively. The Shapiro–Wilk test was used to evaluate continuous variables for their normality of distribution. As a result, no continuous variables analyzed in this study were normally distributed; therefore, nonparametric tests were applied for all continuous variables. The chi-squared test for categorical variables and the Kruskal–Wallis test for continuous variables were used to assess between-group differences. The Cochran–Armitage trend test was used to evaluate trends between variables with two categories and variables with multiple categories. Univariate and multiple logistic regression analyses were performed to identify significant and independent factors associated with sarcopenia and low gait speed. The area under the receiver operating characteristic curve of pentosidine was constructed to determine the optimal cutoff values for predicting sarcopenia and low gait speed. The Kaplan–Meier method was used to estimate cumulative survival rates, and the log-rank test was used to compare between-group differences. Univariate and multiple Cox proportional hazards models were used to identify significant and independent factors associated with mortality. All statistical analyses were performed using SPSS Statistics version 27 (IBM Japan, Tokyo, Japan). A p value of <0.05 was considered statistically significant.
Results
Patient characteristics
Table 1 summarizes the baseline characteristics of the 128 study participants. This study cohort included 71 (55.5%) men, with a median age of all patients at 72.0 (63.0–78.0) years. The median plasma pentosidine level was 0.0665 (0.0508–0.0954) μg/mL. The median MELD score was 8.0 (7.0–10.0). The rates of Child–Pugh class B or C (B/C), sarcopenia, low gait speed, and HCC were 25.8% (33/128), 31.3% (40/128), 26.6% (34/128), and 21.1% (27/128), respectively.
Table 1
| Variable | All patients | L-Pen | I-Pen | H-Pen | p value |
|---|---|---|---|---|---|
| Patients, n (%) | 128 | 32 (25.0) | 64 (50.0) | 32 (25.0) | |
| Men/Women, n (%) | 71(55.5)/57(44.5) | 18 (56.3)/14 (43.8) | 37(57.8)/27(42.2) | 16 (50.0)/16 (50.0) | 0.764 |
| Age (years) | 72.0 (63.0–78.0) | 68.5 (59.3–73.5) | 73.5 (64.0–80.0) | 75.5 (63.5–78.0) | 0.024 |
| BMI (kg/m2) | 23.8 (21.3–26.3) | 25.4 (22.6–30.1) | 24.1 (21.5–26.1) | 22.3 (19.1–24.6) | 0.004 |
| Diabetes mellitus, n (%) | 56 (43.8) | 19 (59.4) | 18 (28.1) | 19 (59.4) | 0.002 |
| Chronic kidney disease, n (%) | 66 (51.6) | 16 (50.0) | 30 (46.9) | 21 (65.6) | 0.212 |
| Etiology | |||||
| HBV/HCV/other, n | 17/61/50 | 4/9/19 | 9/35/20 | 4/17/11 | 0.090 |
| Child-Pugh B/C, n (%) | 33 (25.8) | 3 (9.4) | 10 (15.6) | 20 (62.5) | < 0.001 |
| MELD score | 8.0 (7.0–10.0) | 7.5 (7.0–9.0) | 8.0 (7.0–9.0) | 10.0 (8.0–13.8) | < 0.001 |
| Total bilirubin (mg/dL) | 0.8 (0.6–1.1) | 0.9 (0.6–1.1) | 0.8 (0.6–1.1) | 0.8 (0.4–1.4) | 0.870 |
| Albumin (g/dL) | 3.9 (3.5–4.3) | 4.1 (3.6–4.4) | 4.0 (3.6–4.3) | 3.4 (2.9–3.7) | < 0.001 |
| Prothrombin time INR | 1.08 (1.00–1.18) | 1.01 (1.00–1.13) | 1.10 (1.00–1.16) | 1.12 (1.05–1.26) | 0.036 |
| Creatinine (mg/dL) | 0.89 (0.69–1.10) | 0.80 (0.62–1.12) | 0.90 (0.70–1.06) | 1.05 (0.70–1.20) | 0.217 |
| eGFR (mL/min/1.73m2) | 59 (47–72) | 61 (50–87) | 60 (51–69) | 49 (38–72) | 0.049 |
| M2BPGi (C.O.I.) | 2.94 (1.41–5.40) | 1.56 (1.16–2.71) | 2.86 (1.54–4.20) | 6.22 (3.36–7.87) | < 0.001 |
| Pentosidine (μg/mL) | 0.0665 (0.0508–0.0954) | 0.0421 (0.0377–0.0447) | 0.0665 (0.0560–0.0768) | 0.1299 (0.1087–0.1841) | < 0.001 |
| SMI (kg/m2) | |||||
| All patients | 6.55 (5.69–7.75) | 7.72 (5.85–8.47) | 6.64 (5.92–7.54) | 6.01 (5.21–6.98) | 0.007 |
| Men | 7.19 (6.48–8.24) | 8.28 (7.57–8.99) | 7.13 (6.51–8.02) | 6.93 (6.25–7.18) | 0.002 |
| Women | 5.81 (5.07–6.35) | 5.54 (4.78–7.21) | 5.96 (5.64–6.32) | 5.31 (4.93–5.80) | 0.154 |
| Handgrip strength (kg) | |||||
| All patients | 22.7 (16.8–32.2) | 29.7 (16.0–36.9) | 23.6 (18.9–34.9) | 17.6 (13.3–24.5) | 0.001 |
| Men | 30.5 (24.0–38.1) | 35.2 (31.3–42.9) | 31.6 (23.9–39.0) | 23.8 (14.4–28.0) | < 0.001 |
| Women | 17.0 (14.2–21.3) | 15.6 (14.5–21.1) | 19.0 (14.2–21.9) | 15.8 (13.1–17.7) | 0.351 |
| Sarcopenia, n (%) | 40 (31.3) | 6 (18.8) | 15 (23.4) | 19 (59.4) | < 0.001 |
| Gait speed (m/s) | 1.06 (0.79–1.21) | 1.12 (0.99–1.25) | 1.10 (0.87–1.24) | 0.73 (0.55–1.04) | < 0.001 |
| Low gait speed, n (%) | 34 (26.6) | 2 (6.3) | 14 (21.9) | 18 (56.3) | < 0.001 |
| HCC, n (%) | 27 (21.1) | 3 (9.4) | 13 (20.3) | 11 (34.4) | 0.096 |
Baseline characteristics of the three groups classified according to the plasma pentosidine levels.
Continuous variables are shown as median (interquartile range). Statistical analysis was performed using the chi-squared test or the Kruskal-Wallis test, as appropriate. BMI, body mass index; C.O.I., cut-off index; eGFR, estimated glomerular filtration rate; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; INR, international normalized ratio; M2BPGi, Mac-2 binding protein glycosylation isomer; MELD, model for end-stage liver disease; Pen, pentosidine; SMI, skeletal muscle mass index.
Clinical characteristics of patients according to pentosidine levels
The distributions of the L-Pen, I-Pen, and H-Pen groups were 25.0% (32/128), 50.0% (64/128), and 25.0% (32/128), respectively (Table 1). Age, BMI, diabetes mellitus prevalence, eGFR, M2BPGi, liver functional reserve-related factors (Child–Pugh class B/C, MELD score, albumin, and PT–INR), and sarcopenia-related factors (SMI, hand grip strength, and gait speed) significantly differed between the three groups. Of note, the prevalence of sarcopenia and low gait speed was highest in the H-Pen group (59.4% [19/32] and 56.3% [18/32], respectively) and lowest in the L-Pen group (18.8% [6/32] and 6.3% [2/32], respectively) (Figures 1A,B). The prevalence of these comorbidities significantly increased stepwise with increasing plasma pentosidine levels.
Figure 1
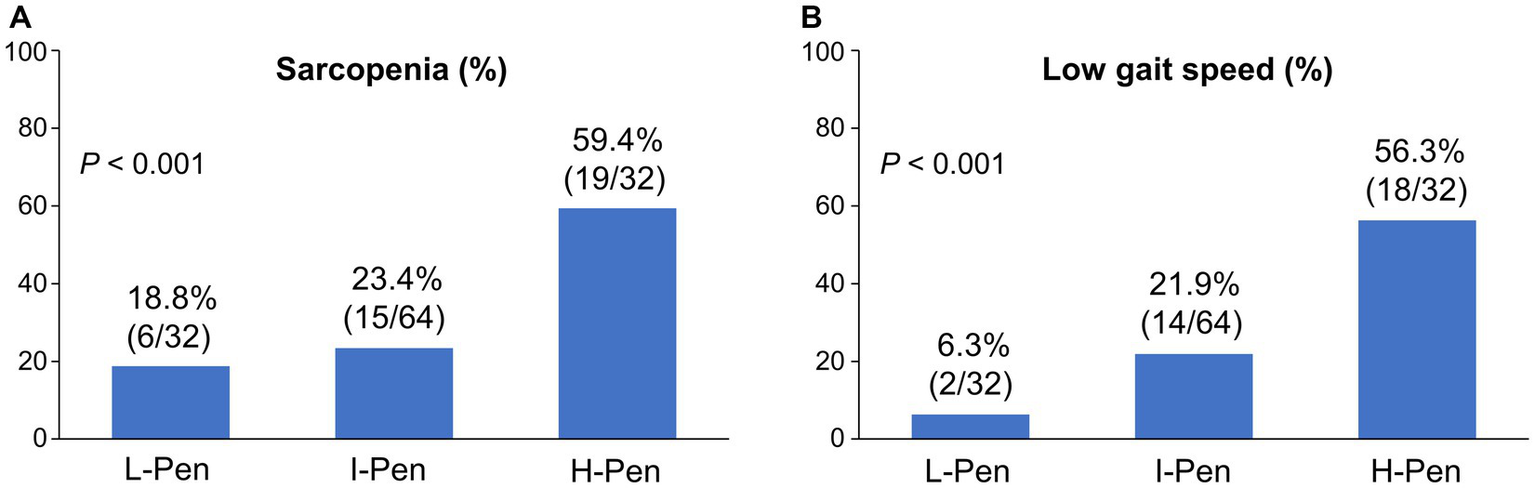
Comparison of the prevalence of sarcopenia and low gait speed between the three groups stratified according to baseline plasma pentosidine levels. (A,B) The prevalence of sarcopenia and low gait speed significantly increased stepwise with increasing plasma pentosidine levels (p < 0.001 for both). L-Pen, low-pentosidine group; I-Pen, intermediate-pentosidine group; H-Pen, high-pentosidine group.
Factors associated with sarcopenia and low gait speed
Six variables (age, BMI, diabetes mellitus, Child–Pugh class B/C, albumin, and pentosidine) were revealed as significant factors associated with sarcopenia in univariate analysis (Supplementary Table S1). Multivariate analysis identified advanced age (odds ratio [OR], 1.08; 95% confidence interval [CI], 1.02–1.14; p = 0.012), low BMI (OR, 0.73; 95% CI, 0.62–0.86; p < 0.001), and high pentosidine level (OR, 1.07; 95%CI, 1.01–1.15; p = 0.036) as significant and independent factors associated with sarcopenia in patients with cirrhosis (Table 2).
Table 2
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95%CI) | p value | OR (95%CI) | p value | |
| Age (years) | 1.08 (1.03–1.13) | 0.001 | 1.08 (1.02–1.14) | 0.012 |
| BMI (kg/m2) | 0.69 (0.59–0.81) | < 0.001 | 0.73 (0.62–0.86) | < 0.001 |
| Diabetes mellitus | 2.63 (1.22–5.65) | 0.014 | ||
| Child-Pugh B/C | 2.33 (1.02–5.32) | 0.044 | ||
| Albumin (g/dL) | 0.53 (0.28–1.01) | 0.054 | ||
| Pentosidine (x102) (μg/mL) | 1.10 (1.02–1.19) | 0.012 | 1.07 (1.01–1.15) | 0.036 |
Significant factors associated with sarcopenia in patients with cirrhosis.
BMI, body mass index; CI, confidence interval; OR, odds ratio.
Meanwhile, six variables (age, diabetes mellitus, Child–Pugh class B/C, MELD score, albumin, and pentosidine) were revealed as significant factors associated with low gait speed in univariate analysis (Supplementary Table S2). Multivariate analysis identified advanced age (OR, 1.10; 95%CI, 1.04–1.17; p = 0.001), Child–Pugh class B/C (OR, 4.31; 95%CI, 1.49–12.5; p = 0.007), and high plasma pentosidine level (OR, 1.06; 95%CI, 1.00–1.13; p = 0.036) as significant and independent factors associated with low gait speed (Table 3).
Table 3
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95%CI) | p value | OR (95%CI) | p value | |
| Age (years) | 1.07 (1.02–1.12) | 0.007 | 1.10 (1.04–1.17) | 0.001 |
| Diabetes mellitus | 1.95 (0.88–4.31) | 0.099 | ||
| Child-Pugh B/C | 4.03 (1.71–9.46) | 0.001 | 4.31 (1.49–12.5) | 0.007 |
| MELD score | 1.13 (0.98–1.30) | 0.083 | ||
| Albumin (g/dL) | 0.50 (0.26–0.99) | 0.047 | ||
| Pentosidine (x102) (μg/mL) | 1.10 (1.02–1.18) | 0.012 | 1.06 (1.00–1.13) | 0.036 |
Significant factors associated with low gait speed in patients with cirrhosis.
CI, confidence interval; OR, odds ratio.
Optimal cutoff plasma pentosidine levels for predicting sarcopenia and low gait speed
Figure 2 shows the cutoff values and diagnostic performance of plasma pentosidine levels for predicting sarcopenia and low gait speed. The cutoff plasma pentosidine levels for predicting sarcopenia and low gait speed were 0.0792 μg/mL (area under the curve [AUC], 0.70; sensitivity/specificity, 0.600/0.773) and 0.0745 μg/mL (AUC, 0.74; sensitivity/specificity, 0.735/0.691), respectively.
Figure 2
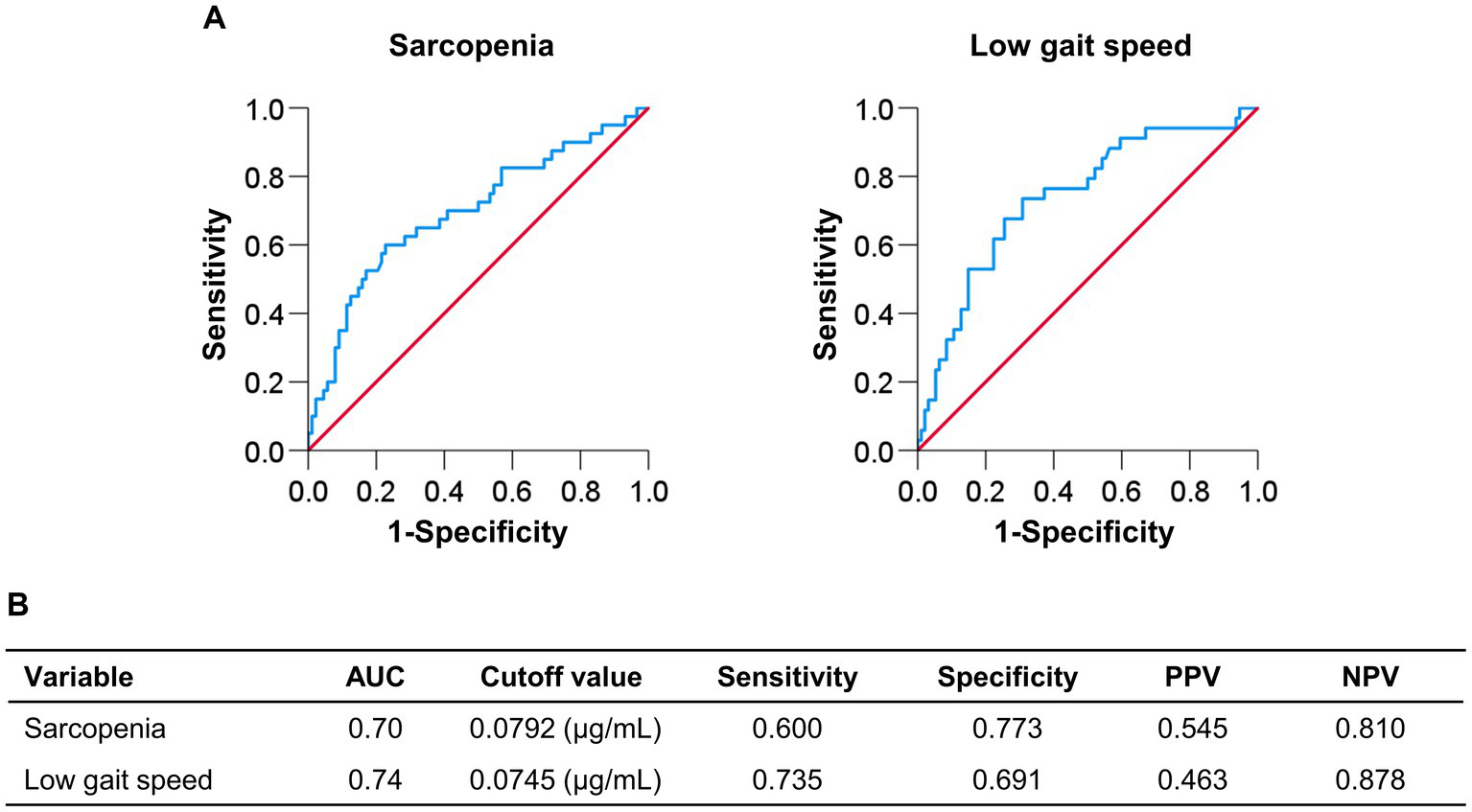
Receiver operating characteristic curve analysis of plasma pentosidine levels for the prediction of sarcopenia and low gait speed. (A,B) The cutoff value for predicting sarcopenia was 0.0792 μg/mL, with an area under the curve (AUC), sensitivity, and specificity of 0.70, 0.600, and 0.773, respectively. The cutoff value for predicting low gait speed was 0.0745 μg/mL, with an AUC, sensitivity, and specificity of 0.74, 0.735, and 0.691, respectively. PPV, positive predictive value; NPV, negative predictive value.
Comparison of cumulative survival rates according to plasma pentosidine levels
The median observation period was 54.3 (33.6–61.1) months. During the observation period, 29 (22.7%) patients died of liver disease-related events (liver failure, n = 12; HCC, n = 11; liver transplantation, n = 2; rupture of esophageal varices, n = 4). The 1-, 3-, and 5-year cumulative survival rates were 87.5, 66.3, and 47.6% in the H-Pen group; 100, 91.7, and 82.7% in the I-Pen group; and 100, 93.3, and 93.3% in the L-Pen group, respectively. The cumulative survival rates were significantly lower in the H-Pen group than in the L-Pen (hazard ratio [HR], 11.7; 95%CI, 2.66–51.4; p = 0.001) and I-Pen (HR, 4.03; 95%CI, 1.83–8.85; p < 0.001) groups (Figure 3).
Figure 3
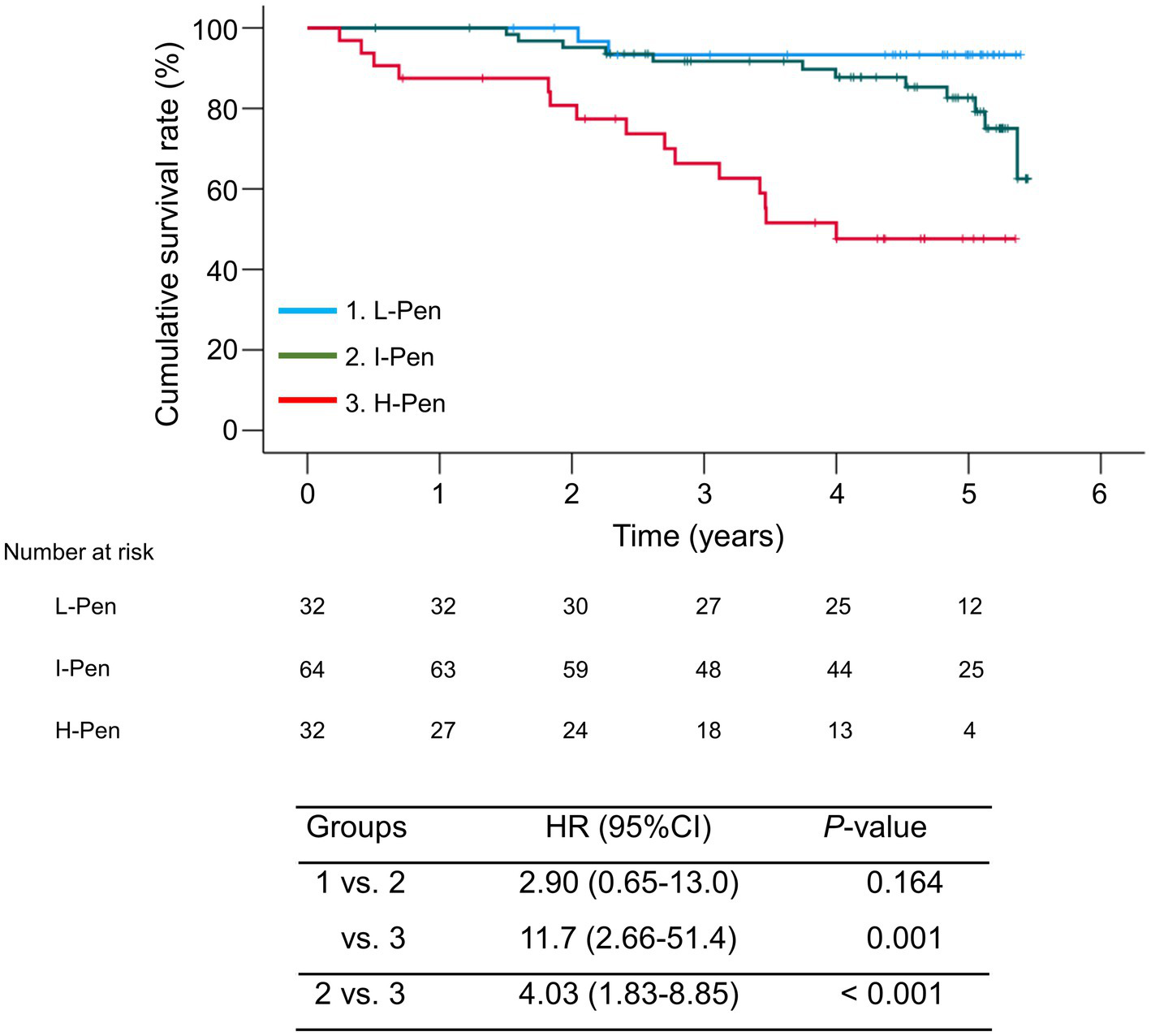
Comparison of the cumulative survival rates between the three groups stratified according to baseline plasma pentosidine levels. The cumulative survival rates were significantly lower in the high-pentosidine (H-Pen) group than in the low-pentosidine (L-Pen) and intermediate-pentosidine (I-Pen) groups (p = 0.001 and <0.001, respectively). Bands above and below polygonal lines denote 95% confidence interval.
Factors associated with mortality
Child–Pugh class B/C, MELD score, M2BPGi, pentosidine, sarcopenia, low gait speed, and HCC were significant factors associated with mortality in univariate analysis (Supplementary Table S3). Cox proportional hazards regression analysis identified high pentosidine level (HR, 1.07; 95% CI, 1.04–1.10; p < 0.001), low gait speed (HR, 3.58; 95% CI, 1.66–7.72; p < 0.001), and HCC (HR, 4.50; 95% CI, 2.05–9.86; p < 0.001) as significant and independent prognostic factors in patients with cirrhosis (Table 4).
Table 4
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95%CI) | p value | HR (95%CI) | p value | |
| Child-Pugh B/C | 5.47 (2.56–11.7) | < 0.001 | ||
| MELD score | 1.22 (1.11–1.35) | < 0.001 | ||
| M2BPGi (C.O.I.) | 1.06 (0.10–1.12) | 0.073 | ||
| Pentosidine (x102) (μg/mL) | 1.09 (1.06–1.12) | < 0.001 | 1.07 (1.04–1.10) | < 0.001 |
| Sarcopenia | 2.49 (1.20–5.17) | 0.015 | ||
| Low gait speed | 3.62 (1.72–7.61) | < 0.001 | 3.58 (1.66–7.72) | < 0.001 |
| HCC | 4.69 (2.22–9.90) | < 0.001 | 4.50 (2.05–9.86) | < 0.001 |
Significant factors associated with mortality in patients with cirrhosis.
CI, confidence interval; C.O.I., cut-off index; HCC, hepatocellular carcinoma; HR, hazard ratio; Mac-2 binding protein glycosylation isomer; MELD, model for end-stage liver disease.
Discussion
AGEs, which are harmful compounds of irreversible non-enzymatic glycation products, play a crucial role in various diseases pathogenesis, including musculoskeletal diseases (16, 18–23). Several studies suggested that the accumulation of AGEs in the muscle induces loss of muscle mass and strength, and increased levels of blood AGEs are associated with sarcopenia and low gait speed (25–27, 38). Furthermore, high levels of plasma/serum AGEs and their precursors were associated with impaired liver functional reserve, leading to poor prognosis (30, 39–41). The present study investigated the relationship between plasma pentosidine levels (a representative marker of total AGEs), sarcopenia or low gait speed, and mortality in patients with cirrhosis. We found the highest prevalence of sarcopenia and low gait speed in the H-Pen group and the lowest in the L-Pen group. These comorbidities had significantly increased prevalence in a stepwise manner with increasing plasma pentosidine levels. Additionally, multivariate analysis identified plasma pentosidine levels as an independent factor associated with sarcopenia and low gait speed. One study of community-dwelling elderly adults revealed significant and negative associations between the urinary pentosidine level and handgrip strength and gait speed (26). Another study of middle-aged and elderly men with diabetes mellitus revealed serum pentosidine level as an independent risk factor of sarcopenia, and the prevalence of sarcopenia increased stepwise with an increased quartile of serum pentosidine levels (27). The other study of postmenopausal women with diabetes mellitus revealed increased serum pentosidine and decreased serum IGF-1 levels to be independently associated with loss of muscle mass (25). Further, older women with high serum carboxymethyl-lysine (CML) levels, which is one of the AGEs, had an increased risk of severe walking disability (i.e., inability to walk or ability to walk only at a speed of <0.4 m/s) (42). An in vivo study of mice with streptozotocin-induced diabetes revealed that AGE accumulation in hind limb muscles resulted in a decrease in muscle mass, muscle endurance, and regenerative capacity (38). In contrast, the administration of alagebrium chloride, which is an inhibitor of AGEs, ameliorated muscle atrophy and muscle regenerative capacity. An in vitro revealed that IGF-1 promoted the differentiation of myoblastic C2C12 cells, whereas AGEs decreased endogenous expression levels of IGF-1 mRNA and inhibited IGF-1-induced Akt activation, thereby increasing apoptosis and suppressing myogenic differentiation in C2C12 cells (43). IGF-1 is primarily synthesized in hepatocytes, and its levels are reduced in patients with advanced liver disease (11). Our previous study revealed an association between low serum IGF-1 levels and sarcopenia and low gait speed in patients with cirrhosis (44). Collectively, these reports indicate that increased plasma/serum AGE levels and decreased IGF-1 levels due to impaired liver functional reserve and accumulated AGEs in the muscle tissues are associated with sarcopenia and impaired physical performance and may be a predictor of the development and progression of these comorbidities in clinical settings. To our best knowledge, the present study is the first to focus on and clarify the relationship between plasma pentosidine (representative of AGEs) levels and sarcopenia or low gait speed in patients with cirrhosis, because of the few related literature.
This study revealed decreased survival rates stepwise with increasing plasma pentosidine levels, which were a significant and independent prognostic factor. One study of patients with CKD revealed a positive association between plasma pentosidine levels and oxidative stress and inflammation markers and plasma pentosidine levels as an independent predictor of mortality (28). Further, a study of patients with heart failure reported an independent association between high serum pentosidine levels and cardiac events (defined as cardiac death and rehospitalization due to heart failure exacerbation) (29). These findings indicate that plasma/serum pentosidine levels may be useful for predicting prognosis in disease conditions with increased AGEs.
Circulating AGEs are eliminated via the scavenger receptors of sinusoidal Kupffer and endothelial cells in the liver (45). Accordingly, liver disease progression causes a decline in the clearance of circulating molecules due to incomplete subendothelial basal membrane formation (40). Indeed, plasma/serum AGE levels correlate with liver fibrosis markers and Child-Pugh scores (30, 39–41). Intriguingly, liver transplantation decreases by approximately 50% in the CML level within 3 months after transplantation, despite persistent renal impairment, thereby highlighting the crucial role of the liver in AGE elimination (39). Thus, more advanced liver function impairment may facilitate the accumulation of AGEs in patients with cirrhosis. In turn, the accumulated AGEs will increase oxidative stress and inflammation, thereby further deteriorating liver injury and fibrosis (17). A clinical study reported significantly higher serum AGE levels in patients with HCC than in those with non-alcoholic steatohepatitis and control subjects (46). Furthermore, high serum AGE levels were independently associated with an increased risk of HCC, particularly in patients with hepatitis C-related cirrhosis (47). In vitro studies revealed that AGEs and related-receptors were associate with angiogenesis and tumor differentiation of HCC (48, 49). Hence, high AGE levels may be involved in liver fibrosis progression, liver function impairment, and HCC tumorigenesis, and consequently, worsen prognosis in patients with chronic liver disease.
This study has some limitations. First, we were unable to assess the accumulation of AGEs in muscle tissues. Second, we did not investigate the patient’s lifestyle, such as dietary intake or physical exercise, which may impact the development and progression of sarcopenia. Finally, this was a retrospective, small-group study conducted at two medical centers; therefore, prospective, large-scale, multicenter studies are required to confirm the obtained findings.
Conclusion
In conclusion, the plasma pentosidine level was significantly and independently associated with sarcopenia, low gait speed, and mortality. Therefore, it may serve as a surrogate biomarker for these clinical events in patients with cirrhosis.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Jikei University School of Medicine (approval number: 34-021) and Fuji City General Hospital (approval number: 279). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because of the retrospective nature of the study.
Author contributions
CS and MS participated in the conception and design of the study. CS and AT acquired, analyzed and interpreted the data, and drafted the manuscript. MS and AT interpreted the data and revised the manuscript. AT substantively revised and completed the manuscript. All authors read and approved the final version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1212899/full#supplementary-material
References
1.
Ginès P Krag A Abraldes JG Solà E Fabrellas N Kamath PS . Liver cirrhosis. Lancet. (2021) 398:1359–76. doi: 10.1016/S0140-6736(21)01374-X
2.
D'Amico G Garcia-Tsao G Pagliaro L . Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. (2006) 44:217–31. doi: 10.1016/j.jhep.2005.10.013
3.
Zipprich A Garcia-Tsao G Rogowski S Fleig WE Seufferlein T Dollinger MM . Prognostic indicators of survival in patients with compensated and decompensated cirrhosis. Liver Int. (2012) 32:1407–14. doi: 10.1111/j.1478-3231.2012.02830.x
4.
Saeki C Takano K Oikawa T Aoki Y Kanai T Takakura K et al . Comparative assessment of sarcopenia using the JSH, AWGS, and EWGSOP2 criteria and the relationship between sarcopenia, osteoporosis, and osteosarcopenia in patients with liver cirrhosis. BMC Musculoskelet Disord. (2019) 20:615. doi: 10.1186/s12891-019-2983-4
5.
Mazeaud S Zupo R Couret A Panza F Sardone R Castellana F . Prevalence of sarcopenia in liver cirrhosis: a systematic review and meta-analysis. Clin Transl Gastroenterol. (2023) 14:e00584. doi: 10.14309/ctg.0000000000000584. Online ahead of print
6.
Kim G Kang SH Kim MY Baik SK . Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PLoS One. (2017) 12:e0186990. doi: 10.1371/journal.pone.0186990
7.
Wijarnpreecha K Werlang M Panjawatanan P Kroner PT Cheungpasitporn W Lukens FJ et al . Association between sarcopenia and hepatic encephalopathy: a systematic review and meta-analysis. Ann Hepatol. (2020) 19:245–50. doi: 10.1016/j.aohep.2019.06.007
8.
Kikuchi N Uojima H Hidaka H Iwasaki S Wada N Kubota K et al . Prospective study for an independent predictor of prognosis in liver cirrhosis based on the new sarcopenia criteria produced by the Japan Society of Hepatology. Hepatol Res. (2021) 51:968–78. doi: 10.1111/hepr.13698
9.
Zeng X Shi ZW Yu JJ Wang LF Luo YY Jin SM et al . Sarcopenia as a prognostic predictor of liver cirrhosis: a multicentre study in China. J Cachexia Sarcopenia Muscle. (2021) 12:1948–58. doi: 10.1002/jcsm.12797
10.
Tantai X Liu Y Yeo YH Praktiknjo M Mauro E Hamaguchi Y et al . Effect of sarcopenia on survival in patients with cirrhosis: a meta-analysis. J Hepatol. (2022) 76:588–99. doi: 10.1016/j.jhep.2021.11.006
11.
Saeki C Tsubota A . Influencing factors and molecular pathogenesis of sarcopenia and osteosarcopenia in chronic liver disease. Life. (2021) 11:899. doi: 10.3390/life11090899
12.
Zupo R Moroni A Castellana F Gasparri C Catino F Lampignano L et al . A machine-learning approach to target clinical and biological features associated with sarcopenia: findings from northern and southern Italian aging populations. Meta. (2023) 13:565. doi: 10.3390/metabo13040565
13.
Moreno-Gonzalez R Corbella X Mattace-Raso F Tap L Sieber C Freiberger E et al . Prevalence of sarcopenia in community-dwelling older adults using the updated EWGSOP2 definition according to kidney function and albuminuria: the screening for CKD among older people across Europe (SCOPE) study. BMC Geriatr. (2020) 20:327. doi: 10.1186/s12877-020-01700-x
14.
Formiga F Moreno-González R Corsonello A Carlsson A Ärnlöv J Mattace-Raso F et al . Diabetes, sarcopenia and chronic kidney disease; the screening for CKD among older people across Europe (SCOPE) study. BMC Geriatr. (2022) 22:254. doi: 10.1186/s12877-022-02916-9
15.
Zengarini E Giacconi R Mancinelli L Riccardi GR Castellani D Vetrano DL et al . Prognosis and interplay of cognitive impairment and sarcopenia in older adults discharged from acute care hospitals. J Clin Med. (2019) 8:1693. doi: 10.3390/jcm8101693
16.
Fernando DH Forbes JM Angus PW Herath CB . Development and progression of non-alcoholic fatty liver disease: the role of advanced glycation end products. Int J Mol Sci. (2019) 20:5037. doi: 10.3390/ijms20205037
17.
Rungratanawanich W Qu Y Wang X Essa MM Song BJ . Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp Mol Med. (2021) 53:168–88. doi: 10.1038/s12276-021-00561-7
18.
Khalid M Petroianu G Adem A . Advanced glycation end products and diabetes mellitus: mechanisms and perspectives. Biomol Ther. (2022) 12:542. doi: 10.3390/biom12040542
19.
Saito M Marumo K . Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int. (2010) 21:195–214. doi: 10.1007/s00198-009-1066-z
20.
Rabbani N Thornalley PJ . Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. (2018) 93:803–13. doi: 10.1016/j.kint.2017.11.034
21.
Li J Liu D Sun L Lu Y Zhang Z . Advanced glycation end products and neurodegenerative diseases: mechanisms and perspective. J Neurol Sci. (2012) 317:1–5. doi: 10.1016/j.jns.2012.02.018
22.
Pinto RS Minanni CA de Araújo Lira AL Passarelli M . Advanced glycation end products: a sweet flavor that embitters cardiovascular disease. Int J Mol Sci. (2022) 23:2404. doi: 10.3390/ijms23052404
23.
Semba RD Nicklett EJ Ferrucci L . Does accumulation of advanced glycation end products contribute to the aging phenotype?J Gerontol A Biol Sci Med Sci. (2010) 65:963–75. doi: 10.1093/gerona/glq074
24.
Kida Y Saito M Shinohara A Soshi S Marumo K . Non-invasive skin autofluorescence, blood and urine assays of the advanced glycation end product (AGE) pentosidine as an indirect indicator of AGE content in human bone. BMC Musculoskelet Disord. (2019) 20:627. doi: 10.1186/s12891-019-3011-4
25.
Tanaka K Kanazawa I Sugimoto T . Elevated serum pentosidine and decreased serum IGF-I levels are associated with loss of muscle mass in postmenopausal women with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. (2016) 124:163–6. doi: 10.1055/s-0035-1565103
26.
Moriwaki K Matsumoto H Tanimura C Osaki M Nagashima H Hagino H . Urinary pentosidine level is associated with grip strength and gait speed in community-dwelling adults: a cross-sectional study. BMC Musculoskelet Disord. (2021) 22:392. doi: 10.1186/s12891-021-04279-5
27.
Zhang X Liu J Zhang Q Lu A Du Y Ye X . Elevated serum pentosidine is independently associated with the high prevalence of sarcopenia in Chinese middle-aged and elderly men with type 2 diabetes mellitus. J Diabetes Investig. (2021) 12:2054–61. doi: 10.1111/jdi.13581
28.
Machowska A Sun J Qureshi AR Isoyama N Leurs P Anderstam B et al . Plasma pentosidine and its association with mortality in patients with chronic kidney disease. PLoS One. (2016) 11:e0163826. doi: 10.1371/journal.pone.0163826
29.
Koyama Y Takeishi Y Arimoto T Niizeki T Shishido T Takahashi H et al . High serum level of pentosidine, an advanced glycation end product (AGE), is a risk factor of patients with heart failure. J Card Fail. (2007) 13:199–206. doi: 10.1016/j.cardfail.2006.11.009
30.
Saeki C Saito M Kanai T Nakano M Oikawa T Torisu Y et al . Plasma pentosidine levels are associated with prevalent fractures in patients with chronic liver disease. PLoS One. (2021) 16:e0249728. doi: 10.1371/journal.pone.0249728
31.
Saeki C Kanai T Nakano M Oikawa T Torisu Y Saruta M et al . Clinical characteristics of sarcopenia in patients with alcoholic liver cirrhosis. JGH Open. (2021) 5:763–9. doi: 10.1002/jgh3.12582
32.
Pugh RN Murray-Lyon IM Dawson JL Pietroni MC Williams R . Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. (1973) 60:646–9. doi: 10.1002/bjs.1800600817
33.
Kamath PS Wiesner RH Malinchoc M Kremers W Therneau TM Kosberg CL et al . A model to predict survival in patients with end-stage liver disease. Hepatology. (2001) 33:464–70. doi: 10.1053/jhep.2001.22172
34.
Marrero JA Kulik LM Sirlin CB Zhu AX Finn RS Abecassis MM et al . Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. (2018) 68:723–50. doi: 10.1002/hep.29913
35.
Webster AC Nagler EV Morton RL Masson P . Chronic kidney disease. Lancet. (2017) 389:1238–52. doi: 10.1016/S0140-6736(16)32064-5
36.
Nishikawa H Shiraki M Hiramatsu A Hara N Moriya K Hino K et al . Reduced handgrip strength predicts poorer survival in chronic liver diseases: a large multicenter study in Japan. Hepatol Res. (2021) 51:957–67. doi: 10.1111/hepr.13679
37.
Cruz-Jentoft AJ Bahat G Bauer J Boirie Y Bruyère O Cederholm T . Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
38.
Chiu CY Yang RS Sheu ML Chan DC Yang TH Tsai KS et al . Advanced glycation end-products induce skeletal muscle atrophy and dysfunction in diabetic mice via a RAGE-mediated, AMPK-down-regulated, Akt pathway. J Pathol. (2016) 238:470–82. doi: 10.1002/path.4674
39.
Sebeková K Kupcová V Schinzel R Heidland A . Markedly elevated levels of plasma advanced glycation end products in patients with liver cirrhosis – amelioration by liver transplantation. J Hepatol. (2002) 36:66–71. doi: 10.1016/s0168-8278(01)00232-x
40.
Yagmur E Tacke F Weiss C Lahme B Manns MP Kiefer P et al . Elevation of Nε-(carboxymethyl)lysine-modified advanced glycation end products in chronic liver disease is an indicator of liver cirrhosis. Clin Biochem. (2006) 39:39–45. doi: 10.1016/j.clinbiochem.2005.07.016
41.
Michel M Hess C Kaps L Kremer WM Hilscher M Galle PR et al . Elevated serum levels of methylglyoxal are associated with impaired liver function in patients with liver cirrhosis. Sci Rep. (2021) 11:20506. doi: 10.1038/s41598-021-00119-7
42.
Sun K Semba RD Fried LP Schaumberg DA Ferrucci L Varadhan R . Elevated serum carboxymethyl-lysine, an advanced glycation end product, predicts severe walking disability in older women: the Women's Health and Aging Study I. J Aging Res. (2012) 2012:586385. doi: 10.1155/2012/586385
43.
Adachi N Kanazawa I Tanaka KI Takeno A Notsu M Tanaka S et al . Insulin-like growth factor-I protects against the detrimental effects of advanced glycation end products and high glucose in myoblastic C2C12 cells. Calcif Tissue Int. (2019) 105:89–96. doi: 10.1007/s00223-019-00537-w
44.
Saeki C Kanai T Nakano M Oikawa T Torisu Y Saruta M et al . Low serum branched-chain amino acid and insulin-like growth factor-1 levels are associated with sarcopenia and slow gait speed in patients with liver cirrhosis. J Clin Med. (2020) 9:3239. doi: 10.3390/jcm9103239
45.
Smedsrød B Melkko J Araki N Sano H Horiuchi S . Advanced glycation end products are eliminated by scavenger-receptor-mediated endocytosis in hepatic sinusoidal Kupffer and endothelial cells. Biochem J. (1997) 322:567–73. doi: 10.1042/bj3220567
46.
Kan H Yamagishi S Ojima A Fukami K Ueda S Takeuchi M et al . Elevation of serum levels of advanced glycation end products in patients with non-B or non-C hepatocellular carcinoma. J Clin Lab Anal. (2015) 29:480–4. doi: 10.1002/jcla.21797
47.
Abdel-Razik A Shabana W El Nakib AM Abdelsalam M Abdelwahab A Hasan AS et al . De novo hepatocellular carcinoma in hepatitis C-related cirrhosis: are advanced glycation end products a key driver?Front Cell Infect Microbiol. (2021) 11:662431. doi: 10.3389/fcimb.2021.662431
48.
Takino J Yamagishi S Takeuchi M . Glycer-AGEs-RAGE signaling enhances the angiogenic potential of hepatocellular carcinoma by upregulating VEGF expression. World J Gastroenterol. (2012) 18:1781–8. doi: 10.3748/wjg.v18.i15.1781
49.
Hiwatashi K Ueno S Abeyama K Kubo F Sakoda M Maruyama I et al . A novel function of the receptor for advanced glycation end-products (RAGE) in association with tumorigenesis and tumor differentiation of HCC. Ann Surg Oncol. (2008) 15:923–33. doi: 10.1245/s10434-007-9698-8
Summary
Keywords
pentosidine, cirrhosis, sarcopenia, low gait speed, prognosis
Citation
Saeki C, Saito M and Tsubota A (2023) Plasma pentosidine as a useful biomarker of sarcopenia, low gait speed, and mortality in patients with cirrhosis. Front. Med. 10:1212899. doi: 10.3389/fmed.2023.1212899
Received
27 April 2023
Accepted
04 September 2023
Published
15 September 2023
Volume
10 - 2023
Edited by
Lei Kuang, Central South University, China
Reviewed by
Roberta Zupo, University of Bari Aldo Moro, Italy; Paolo Fabbietti, National Institute of Science and Health for Aging (IRCCS), Italy
Updates
Copyright
© 2023 Saeki, Saito and Tsubota.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chisato Saeki, chisato@jikei.ac.jpAkihito Tsubota, atsubo@jikei.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.