- 1Infectious Disease Department, Hôpital de la Croix Rousse, Hospices Civils de Lyon, Lyon, France
- 2Université Claude Bernard Lyon 1, Lyon, France
- 3International Center for Research in Infectiology, INSERM U1111, Université Claude Bernard Lyon 1, CNRS, UMR5308, Ecole Normale Supérieure de Lyon, Univ Lyon, Lyon, France
- 4Centre Interrégional de Référence pour la Prise en Charge des Infections Ostéo-Articulaires Complexes (CRIOAc Lyon), Hospices Civils de Lyon, Lyon, France
- 5Plastic and Reconstructive Surgery Department, Hôpital de la Croix Rousse, Hospices Civils de Lyon, Lyon, France
- 6Orthopaedic and Traumatology Surgery Departement, Hôpital de la Croix Rousse, Hospices Civils de Lyon, Lyon, France
- 7Institut des Agents Infectieux, Laboratoire de Bactériologie, Centre National de Référence des Staphylocoques, Hôpital de la Croix-Rousse, Hospices Civils de Lyon, Lyon, France
Among carbapenem-resistant Enterobacterales, metallo-beta-lactamase producing strains represent a growing therapeutic challenge. While the association of aztreonam and ceftazidime-avibactam has been investigated in recent years for the treatment of infections involving these strains, little to no clinical data support the use of this association for the treatment of bone and joint infections. We report two cases of complex bone and joint infections involving metallo-beta-lactamase-producing Enterobacterales, successfully treated at our referral center with aztreonam and ceftazidime-avibactam for 12 weeks in continuous infusions through elastomeric infusors.
Introduction
Antimicrobial resistance is a major and growing cause of mortality in the 21st century. While it was responsible for 0.7 million deaths in 2014, this estimate is expected to grow up to 10 million deaths annually by 2050 (1). Extended-spectrum beta-lactamase (ESBL)-producing and carbapenem-resistant Enterobacterales are recognized as priority targets for the development of new antibiotic strategies. The development of new antibiotic molecules has been increasing in the last 10 years, after being at its lowest around 2010, but the introduction of new antibiotics is bound to select new resistance mechanisms among bacteria (2).
The main mechanism associated with β-lactam resistance in Enterobacterales is the production of β-lactamases, which are enzymes capable of hydrolyzing the β-lactam cycle. They are traditionally divided into four groups according to Ambler's classification. Carbapenem-resistant Enterobacterales strains most frequently produce one or several β-lactamases from either Ambler class A, B, and/or D (3). Class B β-lactamases are metallo-β-lactamases (MBL), which are all carbapenemases (e.g., VIM, IMP, and NDM). Of note, as these strains disseminate and are more and more responsible for severe infections, the use of novel antimicrobial strategies is required (4), especially to treat MBL-producing Enterobacterales.
In recent years, several innovative strategies have emerged to treat carbapenem-resistant Enterobacterales (5). On the one hand, antimicrobial combinations associating “old” β-lactams with “new” β-lactamase-inhibitors, including avibactam (AVI), have been developed—as traditional ones, such as clavulanic acid and tazobactam, cannot inhibit the hydrolytic properties of carbapenemases. These new β-lactamase-inhibitors have been associated with broad-spectrum cephalosporins or carbapenems with proven in vitro and in vivo restored activity against carbapenem-resistant Enterobacterales strains. However, these combinations are not active against MBL. On the other hand, new β-lactam molecules, such as cefiderocol, with intrinsic activity against carbapenemase-producing strains have been developed (6). However, these new strategies have inconsistent efficacy for MBL-producing Enterobacterales eradication (7, 8). Such infections remain particularly difficult to treat, with no active β-lactam/β-lactamase-inhibitor combination approved for clinical use to date and an inconsistent susceptibility to siderophore cephalosporins such as cefiderocol.
Aztreonam is a β-lactam of the monobactam family. Its antimicrobial spectrum includes a wide range of gram-negative bacteria. Usually, this antibiotic is mainly used for the treatment of gram-negative bacteria infections in patients allergic to penicillin or cephalosporins as allergic cross-reactivity with aztreonam almost never occurs (9). Interestingly, aztreonam is not hydrolyzed by MBL, but MBL-producing strains are usually resistant to aztreonam due to the frequent co-production of other β-lactamases (e.g., ESBLs, serine-carbapenemases, or OXA-like carbapenemases), which hydrolyze aztreonam (10).
Hereby, the combination of aztreonam (ATM) with a broad-spectrum β-lactamase-inhibitor (e.g., AVI, only available for clinical use through the ceftazidime-avibactam combination) has recently emerged as a last resort treatment option for MBL-producing Enterobacterales and has been investigated in recent in vitro studies. In vitro susceptibility rate of MBL-producing Enterobacterales strains to ATM and CAZ-AVI in association ranges from 80 to 100% of strains in most reported series (10–12). Nevertheless, there is still little clinical evidence supporting the use of the association of ATM with AVI (or CAZ-AVI) in a clinical setting, notably for infections with issues concerning the poor diffusion of antibiotics and the length of treatment such as bone and joint infections (BJI) (12–14). Moreover, ATM and CAZ-AVI are antibiotics that are frequently prescribed and are administered at the dose of 2 g and 2 g/0.5 g, respectively, every 8 h as discontinuous therapy, but as they are time-dependent antibiotics (as other beta-lactam antibiotics), it would be relevant to perform continuous infusions of these antibiotics to optimize the time above the minimum inhibitory concentration (MIC) of the targeted pathogen. Regarding the stability of these drugs, each of them was considered to be stable in elastomeric infusors during 12 h (15). Elastomeric infusors are useful to treat patients in the outpatient setting, and their use should be considered in the setting of complex infections such as BJI.
The aim of this case series is to illustrate the combined use of ATM and CAZ-AVI in two patients with BJI involving MBL-producing Enterobacterales who were treated recently at our referral center.
Methods
The CRIOAc Lyon (https://www.crioac-lyon.fr/en) is a French referral center for the management of complex BJI. Since the setup of the ongoing prospective cohort study called the Lyon BJI cohort study in 2017 (NCT02817711) that includes all patients managed in our center, two patients infected with MBL-producing Enterobacterales strains were treated with ATM and CAZ-AVI. They were identified through a keyword screening of the database that included more than 5,000 patients.
This study was reviewed and approved by Hospices Civils de Lyon Ethics Committee under the MR004 regulation (N°22-5034). All patients were informed of this study, and their consent for the use of their medical data was sought in an opt-out design.
Susceptibility testing was performed using Vitek 2 (BioMérieux, Marcy l'Etoile, France), and the minimal inhibitor concentrations for some antibiotics were determined using UMIC tests (Biocentric, Bandol, France) or E-test strips (Biomérieux) according to CASFM/EUCAST guidelines. The MIC of the combination of ATM/CAZ-AVI was measured using a CAZ-AVI strip placed onto the agar for 10 min after inoculation and then replaced by an ATM strip (strip superposition method) (16). A representative figure showing the results of MIC testing is shown in Supplementary Figure 1. Carbapenemase production was assessed using the multiplex PCR (Xpert Carba-R®, Cepheid, Sunnyvale, USA) or immunochromatographic test (Resist, Coris BioConcept, Gembloux, Belgium).
Case 1
A 70-year-old woman who had a history of breast cancer was in complete remission for 8 years and had essential hypertension. She was a victim of a traffic accident during a leisure vacation in Uzbekistan and suffered multiple traumas, including a left humeral diaphyseal fracture. She was first cared for in Turkey, where she underwent humeral plate osteosynthesis. She required a stay of several days in intensive care, during which she developed ventilation-acquired pneumonia, seemingly with a carbapenem-resistant strain (although we were not able to retrospectively collect the microbiological results obtained in the Turkish lab), requiring treatment with colistin and tigecycline. Upon sanitary repatriation to our referral center 32 days later, her surgical wound was found to be inflammatory, with a purulent discharge highly evocative of surgical site infection. Therefore, she underwent surgical site debridement and implant removal 35 days after the first surgery (see post-operative X-ray in Figure 1). Pre-operative findings were an abscess around the osteosynthesis and showed no sign of fracture healing. Multiple microbiological samples were collected during surgery. Immediately after surgery, the patient was started on empiric antibiotic therapy associated with vancomycin, cefepime, and metronidazole, and quickly switched on day 1 to meropenem, colistin, and daptomycin.
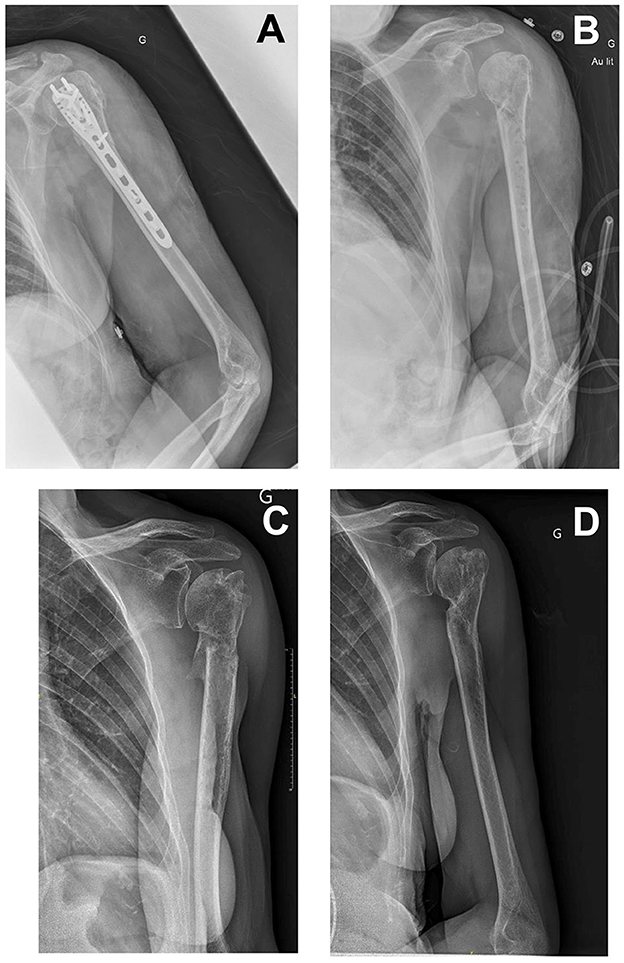
Figure 1. (A) An X-ray image of 2 days before debridement and implant removal surgery. (B) An X-ray image immediately after debridement and implant removal surgery. (C) An X-ray image of 6 months after surgery. (D) An X-ray image of 18 months after surgery.
An NDM-producing Klebsiella pneumoniae strain was cultured from all pre-operative samples. Antibiotic susceptibility testing notably found it to be resistant to meropenem (MIC > 32 mg/L), colistin (MIC:64 mg/L), levofloxacin, fosfomycin, and co-trimoxazole but susceptible to tigecycline (MIC: 0.25 mg/L). No other bacteria were found in the culture. Upon these findings, meropenem and colistin were stopped on day 3, and she was started on tigecycline. Further tests showed that the MIC of ATM combined with CAZ-AVI was 0.19 mg/L, while ATM and CAZ-AVI individual MICs were ≥ 256 mg/L. Therefore, tigecycline was discontinued, and she was started on a triple antibiotic therapy including ATM (continuous infusion of 3 g in 150 ml of NaCl 0.09% per 12 h, twice a day), CAZ-AVI (continuous infusion of 3 g/0.75 g in 150 ml of NaCl 0.09% per 12 h, twice a day), and delivered as a continuous infusion using elastomeric infusor and fosfomycin (3 g/8 h, discontinuous infusion).
The postoperative outcome was favorable: there was no clinical relapse, and inflammatory biomarkers decreased over the weeks following surgery. As a result, fosfomycin was discontinued 4 weeks after surgery, allowing the patient to be discharged from the hospital. ATM and CAZ-AVI were administered for a total of 12 weeks after surgery. There was no adverse effect of this antibiotherapy.
Follow-ups at 3, 6, 9, 18, and 24 months showed favorable outcomes. The humeral fracture was healed at 6 months. There was a slight limitation of abduction angle, not associated with any significant loss of functional ability.
Case 2
A 71-year-old man had a history of obesity (100 kg), with essential hypertension, hypercholesterolemia, right knee total arthroplasty, and bilateral total hip arthroplasty. He underwent total left knee arthroplasty, with early post-operative infection caused by methicillin-susceptible Staphylococcus aureus (MSSA), methicillin-resistant Staphylococcus epidermidis (MRSE), Streptococcus agalactiae, and Finegoldia magna. Despite two iterative debridement surgeries and adequate antibiotic therapy for 6 months, the outcome was not favorable, and he showed clinical symptoms of relapsing left knee infection 1 month after antibiotic discontinuation. He had left knee dermohypodermitis, and there was a purulent discharge through a fistula in direct continuity with the prosthetic articulation. He received 10 days of ceftobiprole, which successfully cured dermohypodermitis. He then underwent the first surgery of a two-stage left knee arthroplasty replacement strategy (see pre-operative photography and post-operative X-ray in Figure 2). The implant was removed, soft tissues were debrided, and a non-articulated spacer was molded with gentamicin- and vancomycin-loaded polymethylmethacrylate cement (COPAL® G+V). An anterolateral thigh flap was performed. Empiric post-operative antibiotherapy was daptomycin, cefepime, and metronidazole. Definitive antibiotic therapy with co-trimoxazole and rifampicin was started upon finding only MSSA in all pre-operative sample cultures at day 15.
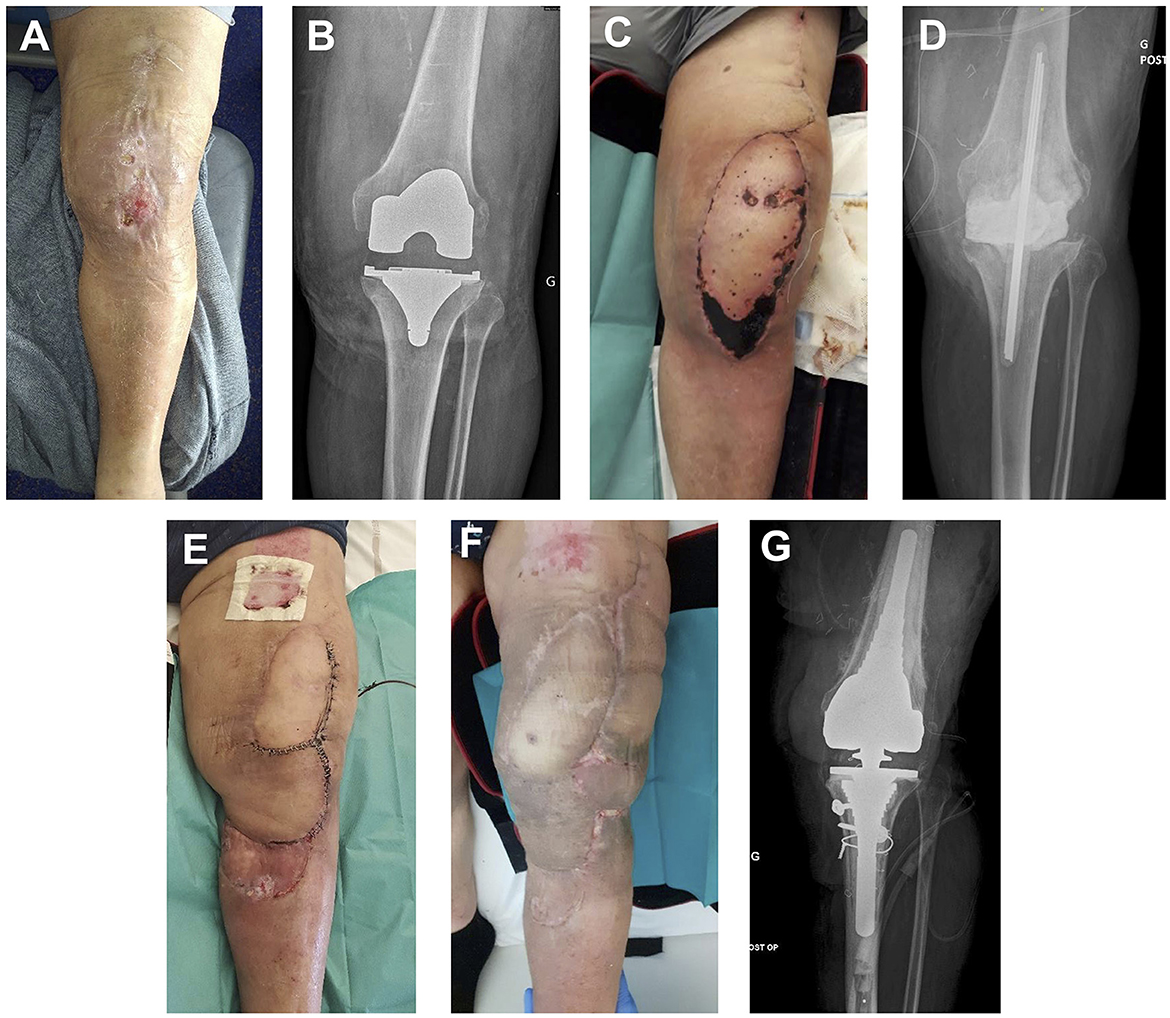
Figure 2. (A) Initial presentation after 10 days of ceftobiprole for left knee cellulitis. (B) X-ray at initial presentation. (C) Thigh flap partial necrosis 10 days after implant removal surgery. (D) X-ray after implant removal surgery. (E) Second surgery: spacer replacement, rotation flap, and STSG. (F) Clinical presentation 2 months after last surgery (total knee arthroplasty reimplanted). (G) X-ray at 12 months follow-up after total knee arthroplasty reimplantation.
Unfortunately, he developed necrosis on the distal portion of his flap, prompting him to perform a new surgery 1 month after the previous one. Soft tissues were debrided, and an intra-articular collection was evacuated; multiple samples were collected. The spacer was removed, and a new one was implanted (COPAL® G+V with manual addition at the time of spacer preparation of 2 g of fosfomycin). A new flap and a split-thickness skin graft were performed. Post-operative empiric antibiotherapy was piperacillin-tazobactam and daptomycin. An NDM and OXA-48-producing Enterobacter cloacae strain was cultured in all samples. It was resistant to meropenem, levofloxacin, and co-trimoxazole. It had intermediate susceptibility to tigecycline (MIC: 1.5 mg/L). It was susceptible to colistin (MIC: 1 mg/L), fosfomycin (MIC:12 mg/L), and amikacin. Moreover, it was resistant to ATM and CAZ-AVI (MIC > 256 mg/L) but susceptible to ATM + CAZ-AVI (CMI: 0.19 mg/L). The final diagnosis was a carbapenem-resistant E. cloacae-associated spacer super-infection. Definitive antibiotherapy was ATM (continuous infusion of 3 g in 150 ml of NaCl 0.09% per 12 h, twice a day), CAZ-AVI (continuous infusion of 3 g/0.75 g in 150 ml of NaCl 0.09% per 12 h, twice a day) as a continuous infusion using elastomeric infusor, colistin (4 MUI per 8 h), and daptomycin (850 mg per day).
After 2 weeks of colistin, the patient developed acute kidney injury with elevated eosinophils on blood test analyses. Immunoallergic interstitial nephritis associated with colistin was evocated: colistin was discontinued, and the patient received systemic corticosteroids. Renal function and eosinophil count improved shortly thereafter. ATM and CAZ plasmatic concentrations were monitored, and dosages were adapted accordingly to reach a steady-state concentration of at least four times the minimal inhibitor concentration (steady-state concentrations were 13.7 mg/ml and 85 mg/ml, respectively). No adverse event associated with ATM and CAZ was reported.
ATM, CAZ-AVI, and daptomycin were continued for 12 weeks. The patient underwent another surgery after 6 weeks of antibiotic treatment: the spacer was changed again (COPAL® G+V). Further outcomes were favorable, and all antibiotics were discontinued at 12 weeks.
Unfortunately, upon the total knee arthroplasty reimplantation surgery performed after 3 months, pre-operative findings were evocative of persistent chronic infection, and microbiologic samples were positive for Staphylococcus epidermidis. Iterative surgery was required 1 month later because of unfavorable local outcomes and found evidence of Candida parapsilosis super-infection. There was no other occurrence of carbapenem-resistant E. cloacae-associated infection. Infection control was obtained after adequate antibiotic treatment, and a suspensive antimicrobial treatment with doxycycline and fluconazole was decided. Further outcomes were favorable at 24 months after the last surgery: no pain was reported, the patient could walk although there was a limitation in flexion angle, and there was no radiologic evidence of persistent chronic infection.
Conclusion
The two patients described in this case series were both treated with ATM and CAZ-AVI for an MBL-producing Enterobacterales-associated BJI, with a favorable outcome despite the very high surgical and microbiological complexity of their cases.
Earlier cases of infections involving MBL-producing Enterobacterales treated with ATM and CAZ-AVI have already been reported (12) although most of them were bloodstream infections (including central line-associated bloodstream infections), UTIs, and LRTIs. In a multicentric prospective cohort in Italy and Greece (17) which compared ATM and CAZ-AVI to other active antibiotics in MBL-producing Enterobacterales-associated BSIs, the association of ATM and CAZ-AVI was associated with greater survival rates. Regarding BJI, few cases have been reported (13, 14), but favorable outcomes were reached with this association. Moreover, a case of VIM-producing Pseudomonas aeruginosa associated osteomyelitis successfully treated with ATM, CAZ-AVI, and amikacin was reported (18).
Although only two cases of BJIs treated favorably with ATM and CAZ-AVI are reported, our study provides further data supporting the use of this association in MBL-producing Enterobacterales involving BJIs. There are still challenges limiting the use of this association. First, ATM-AVI is not currently available without combining ATM and CAZ-AVI. Thus, treating patients with ATM and CAZ-AVI currently requires exposition to an unnecessary and potentially toxic CAZ treatment. Of note, the association ATM-AVI is currently under phase 3 investigation and might be commercially available in the future. Second, the administration of ATM and CAZ-AVI requires repeated intravenous infusions, which can be a hindrance to patient rehabilitation and hospital discharge. Nevertheless, our study provides data supporting the safety of at-home administration of ATM and CAZ-AVI with the use of elastomeric infusors.
In conclusion, 12 weeks treatment with the combination of ATM and CAZ-AVI through elastomeric infusors was associated with favorable outcomes in these two complex MBL-producing Enterobacterales-associated BJI patients. These results support further investigation of this association for routine treatment of such infections.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Hospices Civils de Lyon Ethics Committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
YM, AC, CK, and TF were involved in drafting the manuscript and revising it critically for important intellectual content. YM, AC, SB, AS, CH, SL, CB, and TF were involved in patient care and contributed to acquisition and interpretation of the data. FL, CK, and TR-G were responsible for microbiological analyses and contributed to acquisition and interpretation of the data. All authors read and approved the final manuscript.
Acknowledgments
The authors are grateful to the Lyon Bone and Joint Infection Study Group. Coordinator: TF; Infectious Diseases Specialists: TF, Florent Valour, Thomas Perpoint, Florence Ader, Sandrine Roux, Agathe Becker, Claire Triffault-Fillit, AC, Pierre Chauvelot, Clément Javaux, Marie Wan, Etienne Garneret, Sarah Soueges, Sophie Landre, Olivier Bahuaud, Johanna Lippman, and Evelyne Braun; Surgeons: SL, Elvire Servien, CB, Stanislas Gunst, Etienne Deroche, Léopold Joseph, Contant Foissy, William Barnoud, Michel-Henry Fessy, Anthony Viste, Jean-Luc Besse, Philippe Chaudier, Lucie Louboutin, Aram Gazarian, Christophe Gaillard, Antoine Bertani, Frédéric Rongieras, Sébastien Martres, Franck Trouillet, Cédric Barrey, Ali Mojallal, Mathilde Lherm, SB, Hélène Person, Caroline Ospital, Philippe Céruse, Carine Fuchsmann, and Clémentine Daveau; Anesthesiologists: Frédéric Aubrun, Mikhail Dziadzko, Caroline Macabéo, Dana Patrascu, and Audrey Chevreau-Ciliberti; Microbiologists: FL, Laetitia Beraud, TR-G, Céline Dupieux, and CK; Imaging: Fabien Craighero, Loic Boussel, Jean-Baptiste Pialat, and Isabelle Morelec; PK/PD specialists: Sylvain Goutelle and Romain Garreau; Clinical research assistant and database manager: Johanna Boulant.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1224922/full#supplementary-material
References
1. Antimicrobial resistance. Global report on surveillance. (2023). Available online at: https://www.who.int/publications-detail-redirect/9789241564748 (accessed January 26, 2023).
2. Zheng W, Sun W, Simeonov A. Drug repurposing screens and synergistic drug-combinations for infectious diseases: drug repurposing for infectious diseases. Br J Pharmacol. 175:181–91. doi: 10.1111/bph.13895
3. Tompkins K, van Duin D. Treatment for carbapenem-resistant Enterobacterales infections: recent advances and future directions. Eur J Clin Microbiol Infect Dis. 40:2053–68. doi: 10.1007/s10096-021-04296-1
4. Cattoir V, Felden B. Future antibacterial strategies: from basic concepts to clinical challenges. J Infect Dis. (2019) 220:350–60. doi: 10.1093/infdis/jiz134
5. Yahav D, Giske CG, Grāmatniece A, Abodakpi H, Tam VH, Leibovici L. New β-Lactam–β-lactamase inhibitor combinations. Clin Microbiol Rev. (2020) 34:e00115–20. doi: 10.1128/CMR.00115-20
6. Longshaw C, Manissero D, Tsuji M, Echols R, Yamano Y. In vitro activity of the siderophore cephalosporin, cefiderocol, against molecularly characterized, carbapenem-non-susceptible Gram-negative bacteria from Europe. JAC Antimicrob Resist. (2020) 2:dlaa060. doi: 10.1093/jacamr/dlaa060
7. Boattini M, Comini S, Bianco G, Iannaccone M, Casale R, Cavallo R, et al. Activity of cefiderocol and synergy of novel β-lactam-β-lactamase inhibitor-based combinations against metallo-β-lactamase-producing gram-negative bacilli: insights from a two-year study (2019–2020). J Chemother. 2022:1–7. doi: 10.1080/1120009X.2022.2090615
8. Larcher R, Laffont-Lozes P, Roger C, Doncesco R, Groul-Viaud C, Martin A, et al. Last resort beta-lactam antibiotics for treatment of New-Delhi Metallo-Beta-Lactamase producing Enterobacterales and other Difficult-to-Treat Resistance in Gram-negative bacteria: A real-life study. Front Cell Infect Microbiol. (2022) 12:1048633. doi: 10.3389/fcimb.2022.1048633
9. Caruso C, Valluzzi RL, Colantuono S, Gaeta F, Romano A. β-lactam allergy and cross-reactivity: a clinician's guide to selecting an alternative antibiotic. J Asthma Allergy. (2021). 14:31-46. doi: 10.2147/JAA.S242061
10. Karlowsky JA, Kazmierczak KM, de Jonge BLM, Hackel MA, Sahm DF, Bradford PA. In vitro activity of aztreonam-avibactam against enterobacteriaceae and pseudomonas aeruginosa isolated by clinical laboratories in 40 countries from 2012 to 2015. Antimicrob Agents Chemother. (2017) 61:e00472–17. doi: 10.1128/AAC.00472-17
11. Marshall S, Hujer AM, Rojas LJ, Papp-Wallace KM, Humphries RM, Spellberg B, et al. Can ceftazidime-avibactam and aztreonam overcome β-lactam resistance conferred by metallo-β-lactamases in enterobacteriaceae? Antimicrob Agents Chemother. 61:e02243–16. doi: 10.1128/AAC.02243-16
12. Mauri C, Maraolo AE, Di Bella S, Luzzaro F, Principe L. The revival of aztreonam in combination with avibactam against metallo-β-lactamase-producing gram-negatives: a systematic review of in vitro studies and clinical cases. Antibiotics (Basel). (2021) 10:1012. doi: 10.3390/antibiotics10081012
13. Cairns KA, Hall V, Martin GE, Griffin DWJ, Stewart JD, Khan SF, et al. Treatment of invasive IMP-4 Enterobacter cloacae infection in transplant recipients using ceftazidime/avibactam with aztreonam: a case series and literature review. Transpl Infect Dis. 23:2. doi: 10.1111/tid.13510
14. Mittal J, Szymczak WA, Guo Y, Levi MH, Chen L, Kreiswirth BN, et al. Two for the price of one: emerging carbapenemases in a returning traveller to New York City. BMJ Case Rep. (2018) 2018:bcr2018225440. doi: 10.1136/bcr-2018-225440
15. Loeuille G, D'Huart E, Vigneron J, Nisse Y-E, Beiler B, Polo C, et al. Stability studies of 16 antibiotics for continuous infusion in intensive care units and for performing outpatient parenteral antimicrobial therapy. Antibiotics (Basel). (2022) 11:458. doi: 10.3390/antibiotics11040458
16. Emeraud C, Escaut L, Boucly A, Fortineau N, Bonnin RA, Naas T, et al. Aztreonam plus clavulanate, tazobactam, or avibactam for treatment of infections caused by metallo-β-lactamase-producing Gram-negative bacteria. Antimicrob Agents Chemother. (2019) 63(5). doi: 10.1128/AAC.00010-19
17. Falcone M, Daikos GL, Tiseo G, Bassoulis D, Giordano C, Galfo V, et al. Efficacy of ceftazidime-avibactam plus aztreonam in patients with bloodstream infections caused by metallo-β-lactamase–producing enterobacterales. Clin Infect Dis. (2021) 72:1871–1878. doi: 10.1093/cid/ciaa586
Keywords: antibiotic resistance, ceftazidime-avibactam, aztreonam, Enterobacterales, bone and joint infections, metallo-beta-lactamase, elastomeric infusor
Citation: Merad Y, Conrad A, Brosset S, Schmidt A, Hanriat C, Lustig S, Laurent F, Kolenda C, Roussel-Gaillard T, Batailler C, Ferry T and Lyon BJI Study group (2023) Case report: Continuous infusions of ceftazidime-avibactam and aztreonam in combination through elastomeric infusors for 12 weeks for the treatment of bone and joint infections due to metallo-β-lactamase producing Enterobacterales. Front. Med. 10:1224922. doi: 10.3389/fmed.2023.1224922
Received: 18 May 2023; Accepted: 05 July 2023;
Published: 03 August 2023.
Edited by:
Natividad Benito, Universitat Autònoma de Barcelona, SpainReviewed by:
Marta Ulldemolins, Bellvitge University Hospital, SpainEfthymia Giannitsioti, University General Hospital Attikon, Greece
Copyright © 2023 Merad, Conrad, Brosset, Schmidt, Hanriat, Lustig, Laurent, Kolenda, Roussel-Gaillard, Batailler, Ferry and Lyon BJI Study group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanis Merad, eWFuaXMubWVyYWRAY2h1LWx5b24uZnI=
 Yanis Merad
Yanis Merad Anne Conrad
Anne Conrad Sophie Brosset4,5
Sophie Brosset4,5 Frederic Laurent
Frederic Laurent Camille Kolenda
Camille Kolenda Tiphaine Roussel-Gaillard
Tiphaine Roussel-Gaillard Cecile Batailler
Cecile Batailler Tristan Ferry
Tristan Ferry