- 1Department of Pharmacology, Faculty of Medicine, Masaryk University, Brno, Czechia
- 2Department of Pharmacy, University Hospital Brno, Brno, Czechia
- 3Diagnostic and Therapeutic Centre, Emergency Services Department, University Hospital Brno, Masaryk University, Brno, Czechia
- 4Department of Laboratory Medicine, University Hospital Brno, Masaryk University, Brno, Czechia
- 5Department of Laboratory Methods, Faculty of Medicine, Masaryk University, Brno, Czechia
- 6Department of Pharmacy, Clinical Pharmacy Services Unit, University Hospital Brno, Brno, Czechia
- 7Department of Pharmacology and Toxicology, Faculty of Pharmacy, Masaryk University, Brno, Czechia
- 8Department of Laboratory Medicine, University Hospital Brno, Brno, Czechia
- 9Department of Mathematics and Statistics, Faculty of Science, Masaryk University, Brno, Czechia
- 10Department of Pediatric Hematology and Biochemistry, University Hospital Brno, Brno, Czechia
- 11Department of Pediatrics, Pediatric Nephrology Unit, University Hospital Brno, Brno, Czechia
- 12Faculty of Medicine, Masaryk University, Brno, Czechia
- 13Research Unit for Rare Diseases, Department of Pediatrics and Inherited Metabolic Disorders, First Faculty of Medicine, Charles University, Prague, Czechia
- 14General Teaching Hospital, Prague, Czechia
- 15Spadia Laboratories, Central Reference Lab, Division of Clinical Biochemistry, Ostrava, Czechia
- 16Department of Pediatric Oncology, University Hospital Brno, Brno, Czechia
Background: Assessment of kidney function in emergency settings is essential across all medical subspecialties. Daily assessment of patient creatinine results from emergency medical services showed that some deviated from expected values, implying drug-related interference.
Methods: Real-time clinical evaluation of an enzyme method (Roche CREP2) in comparison with the Jaffé gen. 2 method (Roche CREJ2) was performed. During the period of December 2022 and January 2023, we analyzed 8,498 patient samples, where 5,524 were heavily medicated STAT patient specimens, 500 were pediatric specimens, and 2,474 were from a distant general population in a different region using the same methods.
Results: In 109 out of 5,524 hospital specimens (1.97%, p < 0.001), the CREP2 value was apparently (25% or more) lower than CREJ2. Suspect interfering medication was found in a sample of 43 out of 46 reviewed patients where medication data were available. This phenomenon was not observed in the general population.
Conclusion: In a polymedicated urgent care hospital population, a creatinine enzyme method produces unreliable results, apparently due to multiple drug-related interferences.
Introduction
Determination of blood creatinine is essential to assess kidney functional status. The first method used for creatinine measurement in clinical specimens was introduced by Jaffé (1). During the past two decades, Jaffé methods have been widely replaced by enzyme-based colorimetric methods employing sarcosine oxidase/peroxidase reaction chemistry (2). It has been shown repeatedly that enzymatic methods are susceptible to negative interference caused by several drugs, such as etamsylate (3), metamizole (4, 5), paracetamol, N-acetyl-p-benzoquinone imine (NAPQUI) (5), acetylcysteine (6), derivatives of salicylic acid (5, 7), high-dose acidum ascorbicum (8), and dobesilate (9). Negative interference by catecholamines at clinically relevant concentrations was also shown to affect these methods (10), and recently, monoclonal immunoglobulins have also been reported to interfere (11, 12). In addition, Roche SmPC for enzymatic creatinine determination (CREP2) states that methyldopa and levodopa cause artificially low results. Etamsylate, acetaminophen (paracetamol), acetylcysteine, and metamizole are also mentioned as potentially interfering substances (13).
Reviewing patient result sign-outs in a large tertiary care hospital performing up to 600 STAT creatinine determinations a day, we observed that approximately 2% of creatinine results deviated from preceding or expected creatinine values in the context of patient history and diagnosis, mode of hospital admission, reference ranges, and/or delta checks. The falls in measured creatinine levels exhibited unpredictable behavior independent of diagnosis—from trauma emergency services through general surgery, urology, and obstetrics to neurology, general internal medicine, and subspecialties such as cardiology, nephrology, and oncology. Of the drugs frequently used in these emergency settings, metamizole, paracetamol, etamsylate, and acetylcysteine represent common medications known to interfere with enzymatic creatinine determination. Observing these inconsistencies, we realized an imminent occurrence of a diagnostic and/or medication error problem, the basis of which may be uncontrollable drug-induced effects leading to falsely low creatinine values by the enzyme method followed by spuriously high eGFR calculated values.
Here, we report on retrospective evaluation of the affected method (CREP2), comparing its performance to a Jaffé generation 2 method (CREJ2) in the context of real-life emergency medicine pharmacotherapy and immediate implementation of corrective measures restoring the accuracy of eGFR determinations. To the best of our knowledge, this is the first report addressing inaccuracies of enzyme-based creatinine methods relevant to emergency medical services pharmacotherapy and providing immediate, clinically feasible corrective and preventive actions applicable worldwide for hospital emergency laboratory services.
Materials and methods
Survey setting and patient population
The study was performed as an exploratory survey based on pharmacovigilance signals on the potential influence of medications on laboratory tests. The aim was to identify and minimize potential risks from patient medications. The survey was approved by the multicentric IRB of University Hospital Brno under the number 03-091122/EK. Creatinine measurements were performed as a single additional analysis of all accessible clinical specimens covered by legally required informed consent. All methods were applied as described by the manufacturer. All participating laboratories were accredited under the ISO15189 standard. Central and pediatric laboratories of the University Hospital Brno and a Central reference laboratory Spadia lab, a.s. were involved. Altogether, 8,498 paired, real-time patient creatinine measurements were performed. Of those, 5,524 were STAT determinations at the Department of Laboratory Medicine, University Hospital Brno (A), 500 measurements at the Children’s Hospital laboratories (B), and 2,474 at Spadia lab a.s. (C).
(A) Hospital adult urgent care population: STAT creatinine determinations were analyzed by both CREP2 and CREJ2 methods summing up to 5,524 measurements from 21 December 2022 to 19 January 2023. This population consisted of 3,449 patients, 45.21% male patients and 54.79% female patients, with an age range of 18–99 years and a median age of 62 years.
(B) Hospital pediatric population: Five hundred STAT creatinine determinations were analyzed using both methods from 15 January 2023 to 9 February 2023. The population at the Children’s Hospital is mostly constituted of five intensive care units (neonatal, general pediatric, resuscitation care, infectious diseases, and pediatric oncology/transplantation). This population consisted of 317 patients, 54.9% male patients and 45.1% female patients, with an age range of 0–24 years and a median age of 8 years.
(C) Common adult population: From 5 January 2023 to 31 January 2023, we performed 2,474 measurements in a different geographic region (North Moravia, Czechia) using identical equipment and methods (Roche c702). This dataset served as a “field verification set” performed at the same time in an approximately 200 km distant population assumingly not taking medications used in urgent care hospital settings. This patient population was a general regional outpatient population unrelated to a University Hospital Brno referral population. This population consisted of 2,474 patients, 42.3% male patients and 57.7% female patients, with an age range of 1–99 years and a median of 60 years.
Laboratory methods
Creatinine and cystatin C measurements were performed using Roche platforms Cobas 8,000, Pure, or Integra. The CREP2 (enzyme-based) and CREJ2 (rate-blanked and compensated Jaffé gen. 2) methods were installed on the Cobas 8,000 c702 serving as the main measurement device in our hospital. All methods were conducted as described by the manufacturer. Creatinine methods were calibrated using C.f.a.s. Roche IDMS-traceable calibration. Pediatric STAT creatinine determinations were performed at the Children’s Hospital using Roche Cobas Integra 400+ instruments as recommended by the manufacturer.
Statistical analysis
The Bland–Altman plot was used to define the limits of agreement between the values of creatinine concentration with a 95% confidence interval. Scatterplots with identity lines were used to visualize log-transformed values of paired measurements. The chi-square test for equality of proportions was used to assess the significance of differences among the populations analyzed. Statistical significance was defined as a value of p of <0.05. Statistical analyses were performed using R Statistical Software (v4.2.2; R Core Team 2021) (14–16). The datasets generated and analyzed in the current study are not publicly available as they are based on individual patient measurements. The ISO15189-accredited laboratories store the primary data as per the requirement of this document.
Results
Data analysis
We used logarithmic transformation of the rank-ordered creatinine concentrations (x-axis) determined by the CREJ2 method (the lowest value first) and CREP2 methods to present all datapoints for population (A) as shown in Figure 1, Panel A. A substantial portion of CREP2 values deviates from a central tendency set by a blue sigmoid line of increasing CREJ2 concentrations. Panel 1b presents both creatinine methods as log-transformed data. The Panel 1d shows Bland–Altman plots of the full dataset as from Panel A1 visualizing a dispersion of creatinine concentrations. Panel 1e shows the identical dataset but with 25% of outlying values eliminated. Panels 1c and 1f describe the irrelevant general population (C) showing log-transformed values and a Bland–Altman plot showing very good agreement between the two methods. The results for the pediatric population are presented in Figure 2 as full data Bland–Altman plot (2a left panel) and after elimination of outliers greater than 25% (2b right panel). Figure 3 shows ratios of paired creatinine measurements as extracted from the table ranked from the first/s/third event (hospital admission > next morning sampling > next day sampling, etc.) through the tenth event (i.e., last measurements) as available. Interquartile ranges and medians are shown.
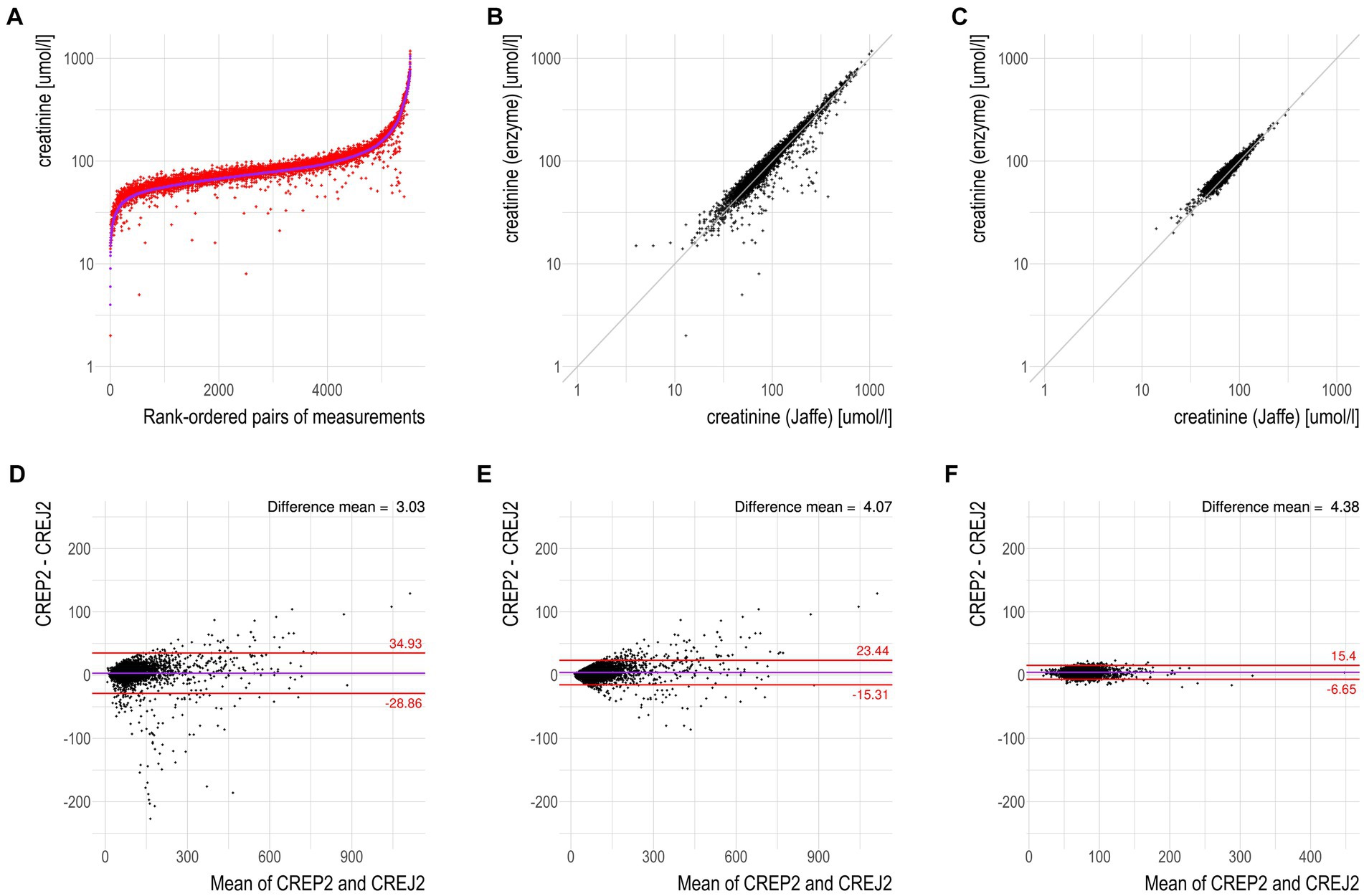
Figure 1. CREP2 exhibits a strong susceptibility to underestimate creatinine concentrations in the “hospital adult urgent care population” in contrast to the “common adult population.” (A) Logarithmic-scale scatterplot with paired data for the dataset “hospital adult urgent care population” with the samples being rank-ordered by creatinine concentrations determined by the CREJ2 method. The data show that CREP2 values were much lower than CREJ2 values in approximately 2% of the samples. (B) Logarithmic-scale scatterplot with identity line for the same dataset as in panel (A). The identity line represents perfect agreement between the two methods; no negative interferences in the urgent care cohort with the CREJ2 method were observed. (C) Logarithmic-scale scatterplot with identity line for the dataset “common adult population” showing agreement between the two methods. (D) Bland–Altman plot for dataset “hospital adult urgent care population” from panel (A); the horizontal solid line represents the mean difference between the two values, whereas the red (dotted) lines represent the limits of agreement (1.96 SD) of individual differences. (E) Bland–Altman plot for the same dataset as in panel (D) where outlying observations greater than 25% were removed. (F) Bland–Altman plot for dataset “common adult population” where no deviations between the two methods in the general community patient cohort were observed.
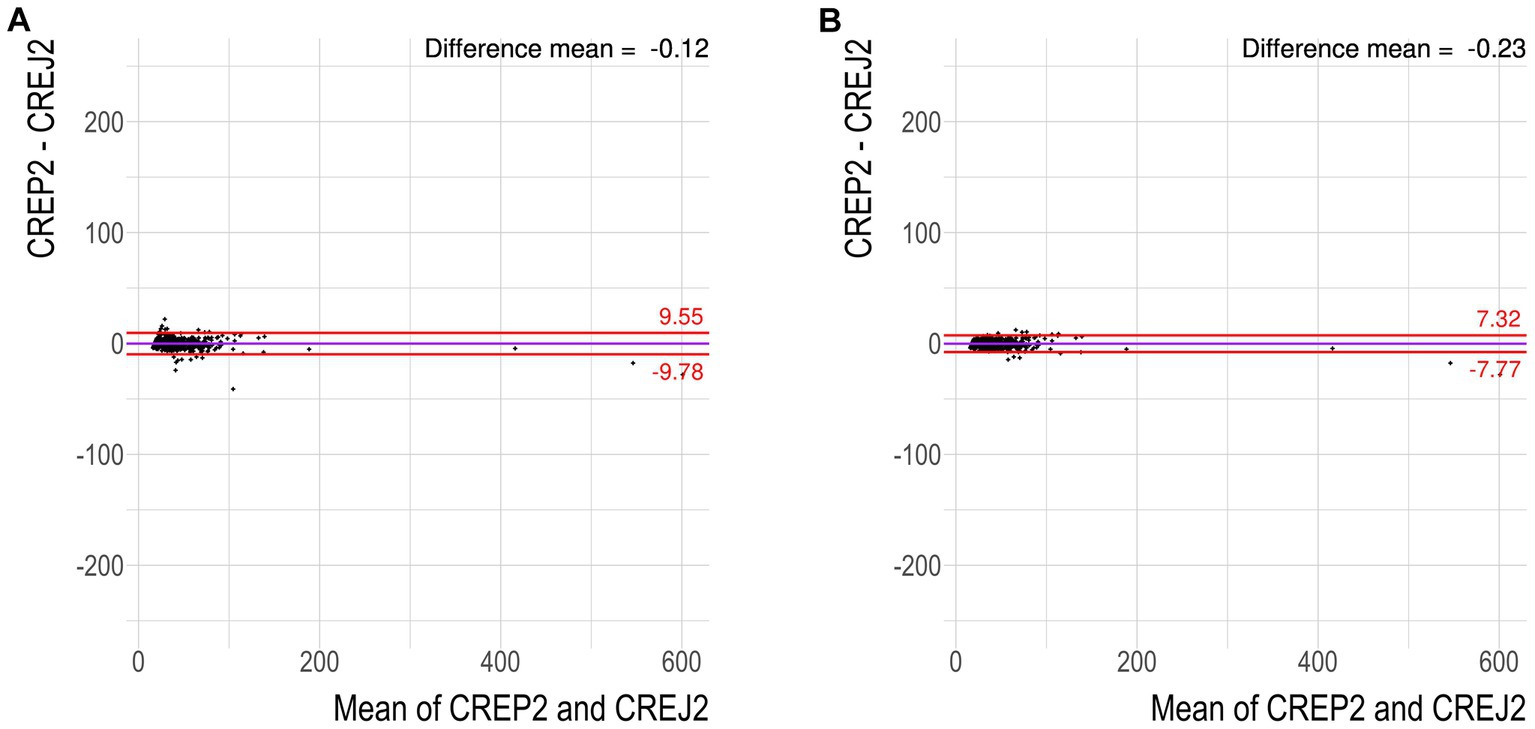
Figure 2. Pediatric hospital population does not exhibit susceptibility to underestimate creatinine concentrations by CREP2. (A) Bland–Altman plot for dataset “pediatric hospital population” showing 25 of 500 values greater than 1.96 SD. These cases were then individually screened by a pediatric nephrologist (JS) and a specialist in pediatric laboratory medicine (DV) for suspect medication. (B) Bland–Altman plot for the same dataset with any samples exhibiting the difference between the two methods greater than 25% removed.
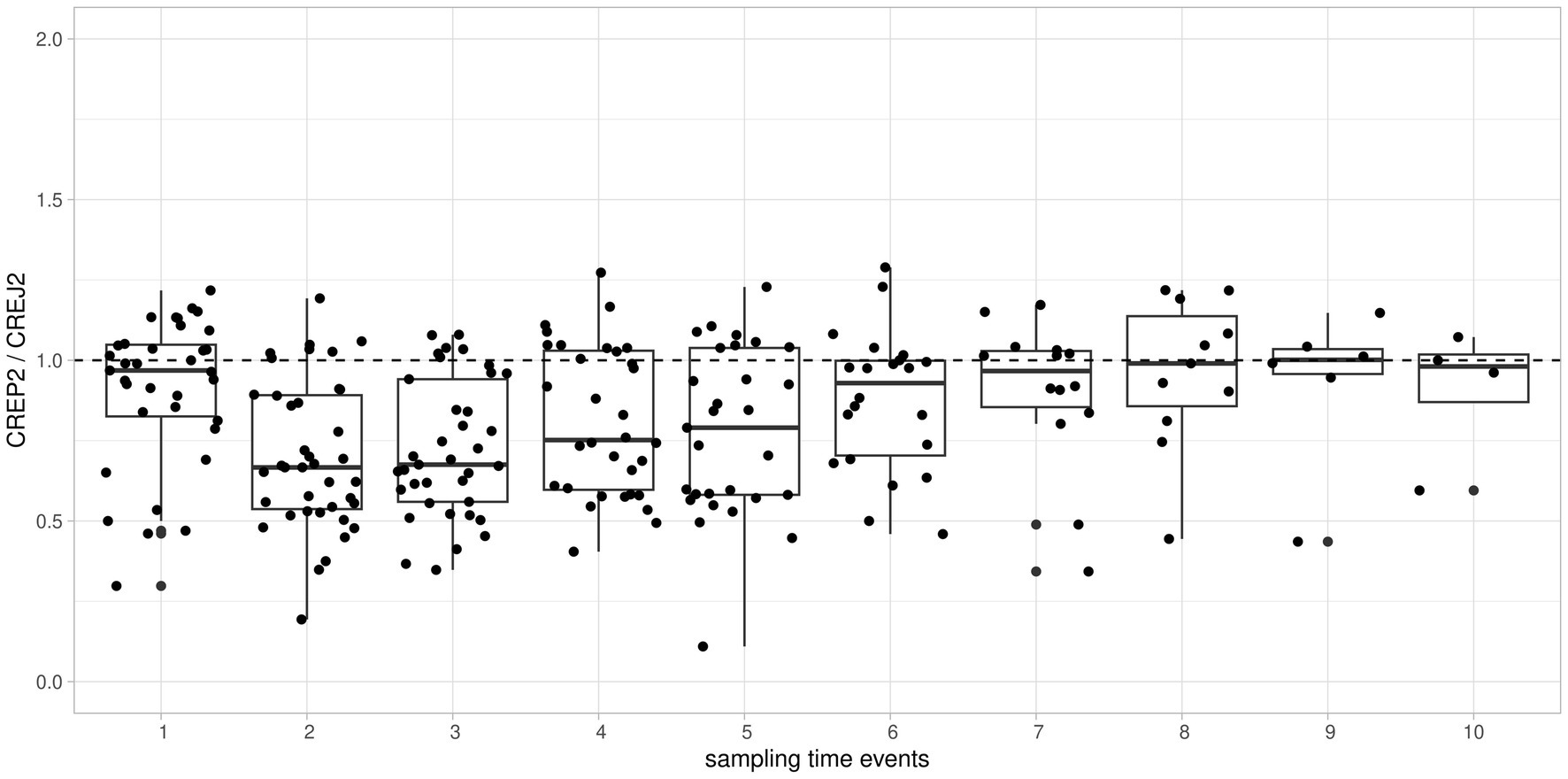
Figure 3. Time-dependent individual patient trends of consecutive creatinine measurements expressed as CREP2/CREJ2 ratio and aggregated and ranked to “sampling time events” from the first event through the tenth event. To visualize individual time-dependent trends of ratios of creatinine concentrations, we used relative timescale aggregating measurements as ordinal data. Boxplots of all patients/all measurements showed as “sampling time events 1 through 10” on the x-axis against the ratio of creatinine concentrations by CREP2/CREJ2 are shown. Major deviations from the median line observed among the 2nd through 7th measurement events implying a drug effect present but then returning to result concordance showed as a ratio approximately 1 implying a drug effect being “washed out.”
Hospital patient record review
The clinical signal for this observational study was derived retrospectively from daily sign-out reviews. Values of CREP2 discrepant to CREJ2 with preceding measurements higher than 25% by CREP2 were first screened by two clinicians and two clinical pharmacists (MR and DB; KH and SK). We examined the use of etamsylate, metamizole (dipyrone), acetylcysteine, paracetamol, catecholamines, high-dose acidum ascorbicum, and dobesilate during the 72-h period before the time of collection of a sample with a suspiciously low CREP2 value. In urgent care patients, we reviewed drug administration timing in the context of creatinine concentrations that were extracted from daily LIMS results. The criteria were either delta checks and/or inconsistent patient result history. We identified the concerned medication in a sample of 43 of 46 reviewed patients where medication data were available for post-hoc access. All cases identified on the basis of 25% differences between creatinine measurements and selected for review are presented in Table 1. Figure 3 shows the evolution of discrepancies during the hospital stay between the two methods. Creatinine values usually were similar by both methods in the first sample collected after patient entry. With subsequent samples, the enzyme method tended to give low values, presumably because intensive drug treatment had started. With longer treatment, the difference between the methods became smaller, presumably because drug treatment had ended.

Table 1. Clinical cases showing blood creatinine concentrations against patient emergency pharmacotherapy timeframe.
Result summary
In the hospital adult urgent care population (A), we found 109 samples (1.97%) where the CREP2 value was 25% or more below the CREJ2 value. There were no such samples in the common adult population (C). The proportions were significantly different (p < 0.001) between the population (A) and population (C). We conclude that in a polymedicated urgent care hospital population, the creatinine enzyme method produces unreliable results due to multiple drug-related interferences.
Discussion
Inaccurate clinical inference on renal functions may severely affect diagnostic workup and threaten the safety and effectiveness of treatment. Among a number of putative filtration biomarkers, only cystatin C has been partially adopted in clinical practice, mostly in nephrology specialties, but not in emergency medical services (17). Despite common criticism, blood creatinine remains the only biomarker accessible worldwide. Therefore, the accuracy of its determinations is of paramount importance to reliably calculate eGFR that is predominantly recommended for clinical use (18). Although mentioned in SmPCs of the respective drugs (19–21) and a procedure manual of the affected method (22) as well, real-life urgent care practice often dictates an immediate clinical need for medication and, at the same time, renal function assessment. As we show here, enzyme methods applied on patient STAT specimens with multiple medications do not provide a reliable platform for creatinine determination. These findings led us to reintroduce the CREJ2 method to clinical practice as the main tool for creatinine determination. The Roche CREJ2 rate-blanked and compensated method has an IDMS-traceable calibration and is therefore suitable for eGFR calculations. Significant improvements in the original Jaffé method have been made, with this generation showing reduced interferences. We kept the CREP2 methods on our instrumentation available as needed, that is, double-checks, patients with muscular wasting, sarcopenia, pediatric population with creatinine less than 20 μmoL/L, and/or clinical trials with new drugs whose behavior toward creatinine determination is not known.
Clinical practice in our hospitals set new laboratory standards for the evaluation of renal functions. The screening tier in the adult population consists of (i) CREJ2 determination resulting in CKD-EPI eGFR calculation always available as STAT test. The second tier consists of (ii) CREJ2 + cystatin C determinations available STAT on a physician’s specific request resulting in a composite CKD-EPI (“DUO”) eGFR calculation—this procedure is intended as a quantitative measure of eGFR covering clinical situations where creatinine may be biologically unreliable, such as muscle wasting processes and extremity amputations. For pediatric populations, the screening tier is (iii) CREJ2 plus patient height resulting in Schwartz-bedside eGFR calculation, and (iv) the quantitative tier is urea+CREJ2 + cystatin C determination and eGFR calculation using the “full” Schwartz equation (23). This practice integrated our findings and general recommendations as well (24, 25).
Conclusion
From a clinical point of view, treatment is always a priority, especially in the emergency setting. Diagnostic laboratories shall use methods suitable for the intended purpose such as in specific patient populations. However, it is the IVD manufacturer’s responsibility to provide such technologies declaring suitability for a given patient setting such as emergency medicine.
Regarding kidney function biomarkers, we think that combining both biological markers, creatinine and cystatin C, reduces the disadvantages associated with each of them separately. This may advance cystatin C usage to urgent care testing in large referral centers as they deal with polymedicated specimens from severely sick patients often with no medical history available. Our new practice greatly improved the reliability of renal function evaluation in our hospitals and the safety of diagnostic procedures and eGFR-based drug dosage as well. Although the nature of these drug-related interferences is not fully clear (26), our hypothesis is that drugs and/or drug-related substances with reducing chemical properties may interfere with the hydrogen peroxide/peroxidase step, thus severely affecting the reporter reaction.
Limitations
Our report has several limitations. First, we did not mention which of the two methods produces “true” results. From a laboratory point of view, the personalized and often temporary nature of these abnormalities does not enable their detection by external means such as proficiency testing surveys, where both methods perform satisfactorily, nor by internal means such as delta checks in patients with no medical record history. Nevertheless, under our survey design, the CREJ2 method did not produce any false-negative results. Second, the lower limit of quantitation is higher for CREJ2 methods than enzyme methods, which is a limitation in pediatric medicine. Therefore, our pediatric algorithm for specimens <20 μmoL/L by CREJ2 implies that both methods are used; notably, in interference-free specimens, the CREP2 method showed excellent analytical performance, stability, and linearity down to 7 μmoL/L (data not shown). Third, it was not technically possible to timely screen all patient records for all possible medications, in part due to the legal inaccessibility of some records.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, upon reasonable request.
Ethics statement
The studies involving humans were approved by the University Hospital Brno, Multicentric Ethics Review Board, approval No. 03-091122/EK. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by-product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
RD conceived the topic, codesigned methods and pharmacovigilance issues, participated in the result interpretation and writing of the manuscript, was confirmed to have full access to all the data in the study, and accepted the responsibility to submit for publication. SK conceived the topic, codesigned methods, performed medication analysis, participated in result interpretation and writing of the manuscript, was confirmed to have full access to all the data in the study, and accepted the responsibility to submit for publication. MRih conceived the topic, performed interactions with clinical wards with documentation reviews and medication analyses, participated in the result interpretation and writing of the manuscript, was confirmed to have full access to all the data in the study, and accepted the responsibility to submit for publication. DB reviewed patient documentation, performed pediatric analyses with composite eGFR calculations such as Schwartz formulas, participated in the writing of the manuscript, was confirmed to have full access to all the data in the study, and accepted the responsibility to submit for publication. KH reviewed patient documentation, performed medication analyses, participated in the result interpretation and writing of the manuscript, was confirmed to have full access to all the data in the study, and accepted the responsibility to submit for publication. OW performed laboratory workups, participated in conceiving the topic and writing of the manuscript, was confirmed to have full access to all the data in the study, and accepted the responsibility to submit for publication. LB participated in the design of the survey and its conception, performed statistical analyses, participated in the result interpretation and writing of the manuscript, was confirmed to have full access to all the data in the study, and accepted the responsibility to submit for publication. IS surveyed statistical analyses, participated in the writing of the manuscript, was confirmed to have full access to all the data in the study, and accepted the responsibility to submit for publication. MP performed laboratory analyses, participated in the writing of the manuscript, was confirmed having full access to all the data in the study, and accepted the responsibility to submit for publication. AK performed clinical case analyses, participated in conceiving the manuscript and in result interpretation and writing of the manuscript, was confirmed to have full access to all the data in the study, and accepted the responsibility to submit for publication. AM performed pediatric laboratory analyses, participated in the writing of the manuscript, was confirmed to have full access to all the data in the study, and accepted the responsibility to submit for publication. MB performed laboratory analyses, participated in the writing of the manuscript, was confirmed to have full access to all the data in the study, and accepted the responsibility to submit for publication. JS coordinated laboratory practice for pediatric patients and participated in the writing of the manuscript, was confirmed to have full access to all the data in the study, and accepted the responsibility to submit for publication. MRa coordinated laboratory analyses in an outpatient population, participated in result interpretation and writing of the manuscript, was confirmed to have full access to all the data in the study, and accepted the responsibility to submit for publication. MRic performed laboratory analyses in an outpatient population, participated in the writing of the manuscript, was confirmed to have full access to all the data in the study, and accepted the responsibility to submit for publication. LZ conceived the topic, coordinated working activities, surveyed medication analyses with a focus on antimicrobial therapy, coordinated overall interpretations of findings and writing of the manuscript, was confirmed to have full access to all the data in the study, and accepted the responsibility to submit for publication. DV conceived the topic, coordinated all working activities, surveyed all analyses, coordinated final result interpretation, edited and coordinated the writing of the manuscript, was confirmed to have full access to all the data in the study, and accepted the responsibility to submit for publication. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. This study was supported by LRI CZECRIN (LM2018128), CZECRIN_4 PATIENTS (CZ.02.1.01/0.0/0.0/16_013/0001826), MHCZ-DRO (FNBr, 65269705), ERN PaedCan grant 101085543, and CREATIC grant agreement 101059788.
Acknowledgments
DV declares that the study is (i) the own work of the author’s team and that (ii) neither the DV nor any of the coauthors have a conflict of interest in regard to this manuscript to declare.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
STAT, testing performed immediately (“statim”); eGFR, estimated glomerular filtration rate; IRB, institutional review board; SmPC, summary of product characteristics; IDMS, isotope dilution mass spectrometry; LIMS, laboratory information and management system.
References
1. Jaffe, M. Ueber den Niederschlag, welchen Pikrinsäure in normalem Harn erzeugt und über eine neue Reaction des Kreatinins. Z Physiol Chem. (1886) 10:391–400.
2. Guder, WG, Hoffmann, GE, Hubbuch, A, Poppe, WA, Siedel, J, and Price, CP. Multicentre evaluation of an enzymatic method for creatinine determination using a sensitive colour reagent. J Clin Chem Clin Biochem. (1986) 24:889–902.
3. Wiewiorka, O, Dastych, M, and Čermáková, Z. Strong negative interference of ethamsylate (Dicynone®) in serum creatinine quantification via enzymatic assay using Trinder reaction. Scand J Clin Lab Invest. (2013) 73:449–51. doi: 10.3109/00365513.2013.794300
4. Bojko, L, Ripka, GP, Dionísio, LM, Borges, CL, Borato, DCK, and Moss, MF. Drug dosing using estimated glomerular filtration rate: misclassification due to metamizole interference in a creatinine assay. Ann Clin Biochem. (2021) 58:474–80. doi: 10.1177/00045632211020029
5. Luna-Záizar, H, Virgen-Montelongo, M, Cortez-Álvarez, CR, Ruiz-Quezada, SL, Escutia-Gutiérrez, R, García-Lemus, CR, et al. In vitro interference by acetaminophen, aspirin, and metamizole in serum measurements of glucose, urea, and creatinine. Clin Biochem. (2015) 48:538–41. doi: 10.1016/j.clinbiochem.2015.01.007
6. McCudden, C, Clark, EG, Akbari, A, Kong, J, Kanji, S, and Hiremath, S. N-acetylcysteine interference with creatinine measurement: an in vitro analysis. Kidney Int Rep. (2021) 6:1973–6. doi: 10.1016/j.ekir.2021.04.006
7. Orieux, A, Brunier, J, Rigothier, C, Pinson, B, Dabernat, S, and Bats, ML. Plasma creatinine below limit of quantification in a patient with acute kidney injury. Clin Chim Acta. (2022) 524:101–5. doi: 10.1016/j.cca.2021.12.001
8. Martinello, F, and Luiz da Silva, E. Mechanism of ascorbic acid interference in biochemical tests that use peroxide and peroxidase to generate chromophore. Clin Chim Acta. (2006) 373:108–16. doi: 10.1016/j.cca.2006.05.012
9. Shen, H, Chen, K, and Cao, J. A new method for anti-negative interference of calcium dobesilate in serum creatinine enzymatic analysis. J Clin Lab Anal. (2021) 35:e23928. doi: 10.1002/jcla.23928
10. Saenger, AK, Lockwood, C, Snozek, CL, Milz, CT, Karon, BS, and Scott, MG. Catecholamine interference in enzymatic creatinine assays. Clin Chem. (2009) 55:1732–6. doi: 10.1373/clinchem.2009.127373
11. Flowers, KC, Tuddenham, E, Leiva, A, Garrison, L, Morris, JE, and Cromwell, T. Negative interference from immunoglobulin M paraproteinaemia on the Roche enzymatic creatinine method. Ann Clin Biochem. (2022) 59:205–10. doi: 10.1177/00045632221074867
12. Metraiah, EH, Regan, H, Louw, J, and Kidder, D. Deceiving proteins! A case of lymphoma and high creatinine. BMJ Case Rep. (2017) 2017:bcr2016217946. doi: 10.1136/bcr-2016-217946
13. Roche Diagnostics Corporation. [Method Sheet] CREP2 "Kreatinin plus 2. verze" [CZ]. no. 0105168589190c701V13.0 [Print]. (2022).
14. R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing. (2022). Available at: https://www.R-project.org/.
16. Rudis, B. Hrbrthemes: additional themes, theme components and utilities for 'ggplot2'. R package version 0.8.0. (2020). Available at: https://CRAN.R-project.org/package=hrbrthemes.
17. Bongiovanni, C, Magrini, L, Salerno, G, Gori, CS, Cardelli, P, Hur, M, et al. Serum cystatin C for the diagnosis of acute kidney injury in patients admitted in the emergency department. Dis Markers. (2015) 2015:416059. doi: 10.1155/2015/416059
18. Inker, LA, Eneanya, ND, Coresh, J, Tighiouart, H, Wang, D, and Sang, Y. New creatinine-and cystatin C-based equations to estimate GFR without race. N Engl J Med. (2021) 385:1737–49. doi: 10.1056/NEJMoa2102953
19. State Institute for Drug Control (SÚKL). [SmPC] ACC-Injekt Summary of product characteristics [CZ]. sp.zn.sukls315481/2020. Available at: https://www.sukl.cz/modules/medication/download.php?file=SPC175927.pdf&type=spc&as=acc-injekt-spc.
20. State Institute for Drug Control (SÚKL). [SmPC] Dicynone-Summary of product characteristics [CZ]. sp.zn. sukls26699/2022. Available at: https://www.sukl.cz/modules/medication/download.php?file=SPC185883.pdf&type=spc&as=dicynone-spc.
21. State Institute for Drug Control (SÚKL). [SmPC] Novalgin-Summary of product characteristics [CZ]. sp. zn. sukls39194/2022. Available at: https://www.sukl.cz/modules/medication/download.php?file=SPC184586.pdf&type=spc&as=novalgin-spc.
22. Roche Diagnostics Corporation. CREP2 "Kreatinin plus 2. verze" [CZ]. no. 0105168589190c701V13.0. (2022).
23. Schwartz, GJ, Muñoz, A, Schneider, MF, Mak, RH, Kaskel, F, and Warady, BA. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. (2009) 20:629–37. doi: 10.1681/ASN.2008030287
24. Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. (2012) 120:c179–84. doi: 10.1159/000339789
25. Kellum, JA, Romagnani, P, Ashuntantang, G, Ronco, C, Zarbock, A, and Anders, HJ. Acute kidney injury. Nat Rev Dis Primers. (2021) 7:52. doi: 10.1038/s41572-021-00284-z
Keywords: creatinine, renal functions, eGFR, urgent care medication, cystatin C
Citation: Demlova R, Kozakova S, Rihacek M, Buckova D, Horska K, Wiewiorka O, Boucek L, Selingerova I, Podborska M, Korberova A, Mikuskova A, Starha J, Benovska M, Radina M, Richter M, Zdrazilova Dubska L and Valik D (2024) Emergency medicine pharmacotherapy compromises accuracy of plasma creatinine determination by enzyme-based methods: real-world clinical evidence and implications for clinical practice. Front. Med. 10:1236948. doi: 10.3389/fmed.2023.1236948
Edited by:
Guiomar Nascimento Gomes, Federal University of São Paulo, BrazilReviewed by:
Gus Schoorlemmer, Federal University of São Paulo, BrazilShuangxin Liu, Guangdong Provincial People’s Hospital, China
Copyright © 2024 Demlova, Kozakova, Rihacek, Buckova, Horska, Wiewiorka, Boucek, Selingerova, Podborska, Korberova, Mikuskova, Starha, Benovska, Radina, Richter, Zdrazilova Dubska and Valik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dalibor Valik, dmFsaWsuZGFsaWJvckBmbmJybm8uY3o=
†These authors have contributed equally to this work
 Regina Demlova1†
Regina Demlova1† Michal Rihacek
Michal Rihacek Lubos Boucek
Lubos Boucek Iveta Selingerova
Iveta Selingerova Dalibor Valik
Dalibor Valik