- 1State Key Laboratory of Food Science and Technology, China-Canada Joint Laboratory of Food Science and Technology (Nanchang), Key Laboratory of Bioactive Polysaccharides of Jiangxi Province, Nanchang University, Nanchang, Jiangxi, China
- 2Jiangzhong Dietary Therapy Technology Co. Ltd, Jiujiang, Jiangxi, China
- 3Jiangzhong Cancer Research, Jiangxi University of Chinese Medicine, Nanchang, Jiangxi, China
- 4College of Biotechnology and Pharmaceutical Engineering, Nanjing Tech University, Nanjing, Jiangsu, China
Helicobacter pylori (H. pylori) is a gastric-persistent pathogen that can cause peptic ulcer disease, gastric cancer, and mucosal-associated lymphoid tissue lymphoma. This pathogen is commonly treated with antibiotic-based triple or quadruple therapy. However, antibiotic therapy could result in the bacterial resistance, imbalance of gut microbiota, and damage to the liver and kidneys, etc. Therefore, there is an urgent need for alternative therapeutic strategies. Interestingly, natural food resources, like vegetables, fruits, spices, and edible herbs, have potent inhibitory effects on H. pylori. In this review, we systematically summarized these foods with supporting evidence from both animal and clinical studies. The results have indicated that natural foods may possess temporary inhibition effect on H. pylori rather than durable eradication, and may help to reduce H. pylori colonization, enhance the effect of antibiotics and modulate the host’s immune response.
Introduction
Helicobacter pylori (H. pylori) is a gram-negative, microaerophilic spiral-shaped bacterium, which is classified as a Group I carcinogen by the World Health Organization. It can persistently inhabit in the stomach, which can lead to chronic gastritis, gastric ulcer, gastric cancer, and mucosa-associated lymphoid tissue lymphoma (MALT). About 70–80% of the world’s population in developing countries are infected with H. pylori, whereas,in developed countries, the infection rate is 13–50% (1). After infection and colonization with H. pylori, gastric epithelial cells trigger a series of immune response at both the celluar and molecular levels, such as NF-κB pathway (Figure 1), eventually causing gastric diseases (3). Patients infected with H. pylori are at higher risk for gastric ulcer and cancers (2). The infection of H. pylori may increase the risk of developing gastric MALT about 50 to 73% (4). In about 1% of gastric cancer patients infected with H. pylori, there co-exists increasing inflammation of the gastric corpus, the atrophic mucous membrane in the prepyloric stomach, and reduced gastric acid secretion (5). Furthermore, H. pylori infection has also been reported to be associated with insulin resistance, nonalcoholic steatohepatitis, type 2 diabetes mellitus, etc. (6). The main routes of H. pylori transmission are mouth-to-mouth, fecal-to-mouth and spread between family members (7).
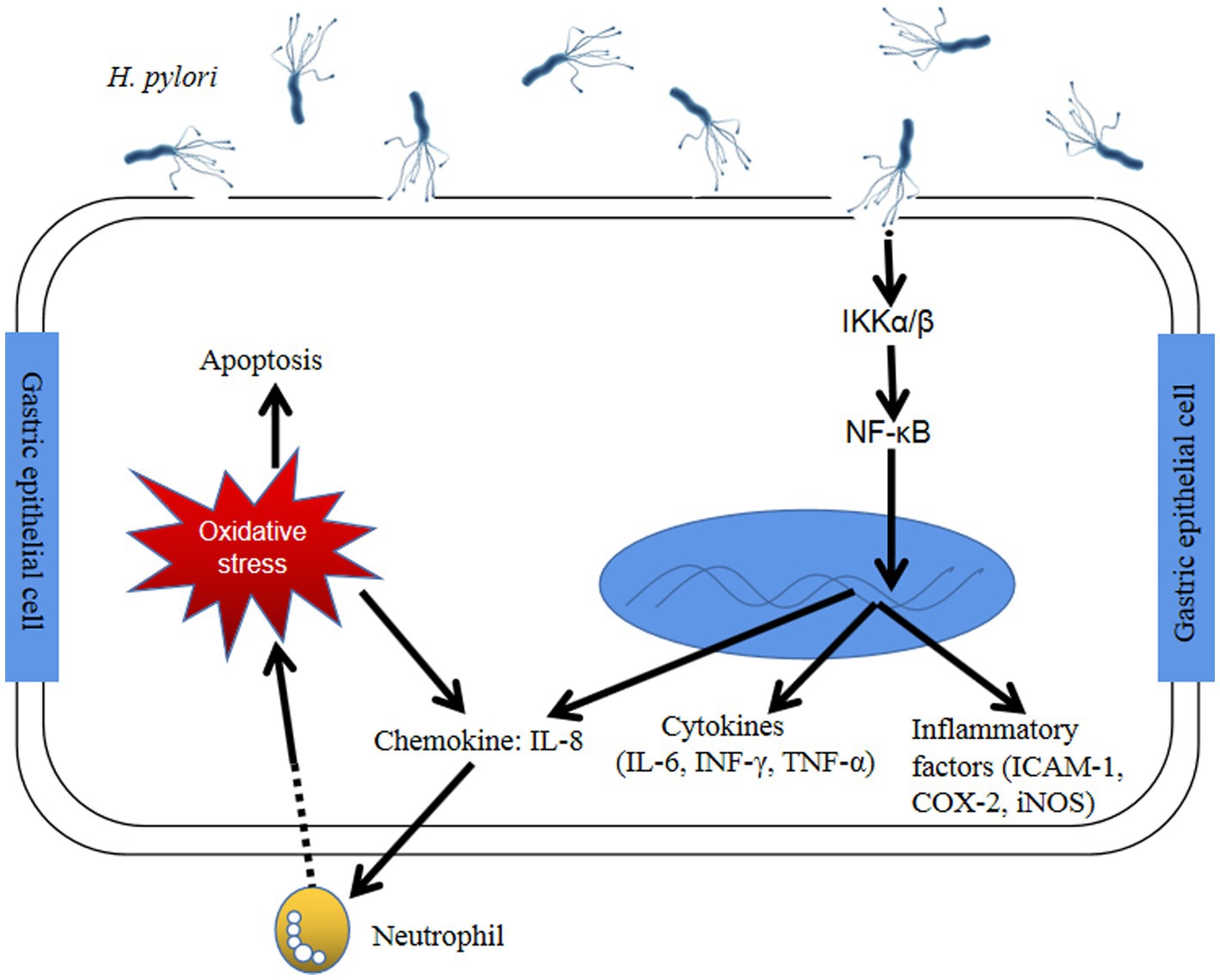
Figure 1. The immune response of gastric epithelial cells after Helicobacter pylori infection through NF-κB pathway (2) H. pylori infection activates NF-κB-related kinase, like IκB α/β, resulting in IκBα of NF-κB phosphorylation and degradation. Active NF-κB translocates into the nucleus and trigger the translation of inflammatory cytokines (COX-2, ICAM-1 and iNOS) and proinflammatory factors (NF-κB, IL-1, −6, −8, TNF-α). Furthermore, H. pylori could cause the generation of intracellular reactive oxygen species by host gastric epithelial cells and eventually led to cell apoptosis. Abbreviation: NF-κB: Nuclear factor-kappaB; IKKα/β: IκB kinase α/β; INF-γ: Interferon-γ; TNF-α: Tumor necrosis factor-α; IL: Interleukin; ICAM-1: Intracellular adhesion molecule-1; COX-2: Cyclooxygenase-2; iNOS: Inducible nitric oxide synthase.
Until now, H. pylori eradication in infected individuals remains a good choice and the most direct approach to prevent the development of H. pylori-related gastric disorders and gastric cancer (8). In general, first-line treatment for eradication of H. pylori consists of standard triple therapy (consisting of a proton pump inhibitor (PPI) and two of three antibiotics: clarithromycin and either amoxicillin or metronidazole) and bismuth-based quadruple therapy (consisting of bismuth with PPI and two antibiotics) (9, 10). The estimated efficacy of triple therapy is 82% and that of sequential therapy is 92% (11). However, various studies have reported that there are two limiting factors impairing the eradication of H. pylori, which involve development of bacterial resistance to antibiotics and the persistence of low levels of H. pylori bacteria in gastric epithelial cells (12). As we known, standard antibiotic therapy is high cost and requires at least fourteen days of drug administration and may be associated with some side effects such as diarrhea, nausea, and taste disturbances, leading to poor patient compliance (11). In addition, there are significant concerns about the issue of bacterial resistance, unaffordable treatment expenses, treatment tolerability, and cultural acceptability of antibiotic treatment (13). Apparently, long-term antibiotic therapy could lead to alarming imbalances of microbiota in the gut, promoting the growth of resistant strains of H. pylori or the emerging of other harmful micro-organisms such as Candida fungi and Clostridium difficile (14).
However, H. pylori has co-ecolved with humans for a long time. The human body is a complex or multiorganism ecosystem in which any chang may effect human health (15, 16). The interactions between microbiota and host are complicated and poorly understood (17). There are a few reports in literature suggesting that human beings benefit from H. pylori (10). For instance, H. pylori could protect children against gastrointestinal infection (18), reducing the prevalence of atopic diseases such as celiac disease, irritable bowel syndrome (IBS) and gastroesophageal reflux disease (GORD), etc. (19). Therefore, research into alternative or novel strategies to prevent and treat H. pylori infection has recently attracted the attention of scientists.
Notably, many natural food resources have been consumed by people since ancient times to treat gastrointestinal diseases (20) and anti-cancer (21), through which recent studies have been proven to be effective and have few side effects. These natural resources are an invaluable treasure for developing or mining drugs to treat human diseases (22). Numerous studies recently have shown that natural food resources including vegetables, fruits, spices and edible herbs contain powerful and valuable anti-H. pylori activities (12).
Herein, we have systematically reviewed published literature with supporting evidence of animal and clinical studies from the PubMed, ClinicalTrials.gov and Scopus databases. The following terms of Helicobacter pylori, food, vegetable, fruit, spices, plants, and / or herbs in all possible combination have been used for retrieval.
We expected sincerely that the appropriate combination of dietary ingredients from natural foods could be utilized to prevent, manage or treat H. pylori infection or / and related diseases such as gastric cancer. In this review, we have summarized the animal and clinical studies on the use of these natural food resources to relieve H. pylori-related infection (Figure 2).
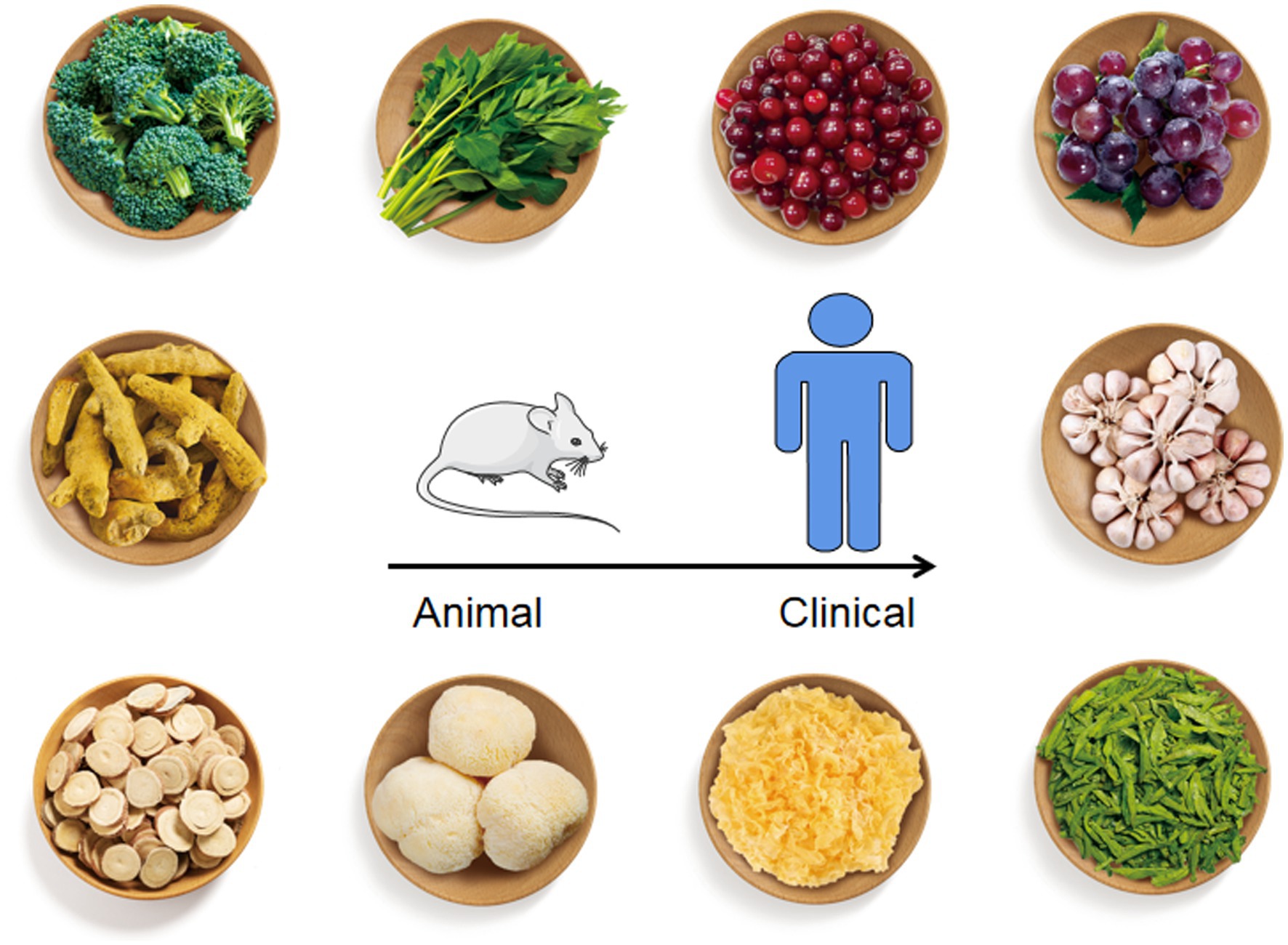
Figure 2. Natural foods listed in this review with anti-H. pylori effect. From top to bottom, left to right, the foods figures referred to broccoli, Angelica keiskei, cranberry, grape, curcumin, garlic, liquorice, Hericium erinaceus, Tremella mesenterica, and green tea.
In addition, a majority of individuals with H. pylori infection remain asymptomatic, but they are still at risk of developing the illness associated with this bacterium. However, the eradication of H. pylori in the case of asymptomatic patients in developing countries is not practical. Alternative therapies have proved to be useful in anti-inflammation, anti-oxidative, chemoprevention and gastroprotection. Based on these results, the inclusion of foods from natural products and dietary ingredients in the diet of asymptomatic patients could reduce the risk and development of H. pylori infection. Herein, the benefits from these alternative therapies are summarized in Figure 3. The summary of vegetables, fruits, spices, and edible herbs possessing anti-H. pylori activities is presented below.
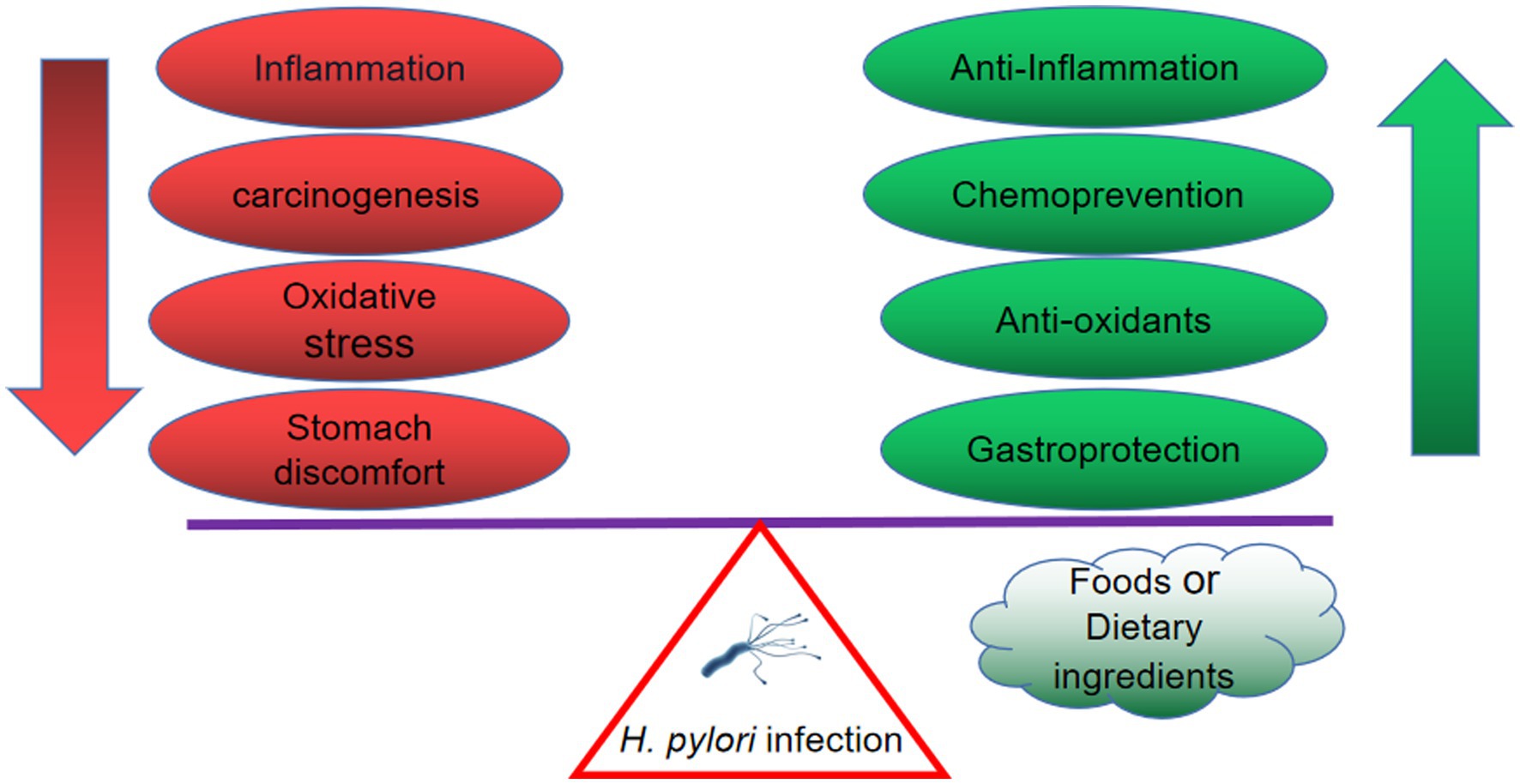
Figure 3. The H. pylori-infected individual benefits from natural products, including anti-inflammation, anti-oxidative, chemoprevention and gastroprotection.
Suppressive effects of vegetables on Helicobacter pylori infection in vivo
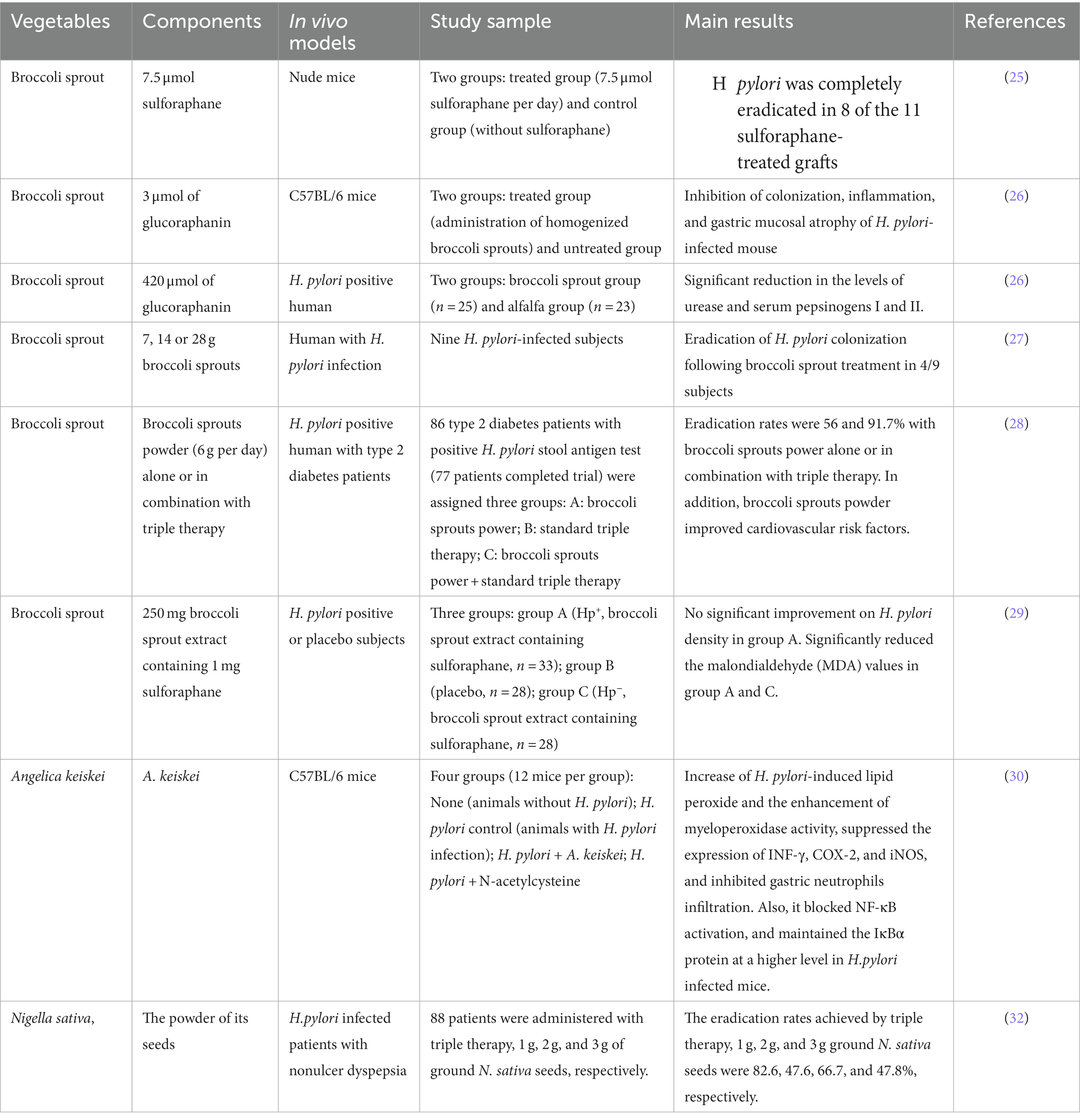
Study showed that people with a lower dietary intake of vegetables were at higher risk of H. pylori infection (23). Recently, the effects of various vegetables on H. pylori infectionwere studied. For instance, broccoli is a common vegetable that can be consumed as food and for medicinal purposes, it showed that broccoli has both anti-cancer and anti-bacterial activity (24). Broccoli sprouts, in particular, are rich in isothiocyanate sulforaphane (SF) in the form of a precursor called glucoraphanin, which has shown potent bacteriostatic activity against H. pylori (12). Lozniewski and colleagues revealed that H. pylori infections could be eradicated in 8/11 transplants treated with 7.5 μmol sulforaphane by using human gastric xenografts model in nude mice, which also suggested individuals with H. pylori infection might benefit from sulforaphane (25).
Similarly, Yanaka et al. (26) investigated the protective effect of broccoli sprouts against high salt-induced gastritis in a mouse model of H. pylori infection. The results showed that the sulforaphane contained in broccoli sprouts was effective in inhibiting colonization, inflammation and gastric mucosal atrophy. Furthermore, the asymptomatic patients with H. pylori infection consumed broccoli sprouts (70 g/d containing 420 μmol glucoraphanin), resulted in a significant decrease in H. pylori colonization and gastric inflammation, but these effects disappeared 2 months later without following intake (26). Meanwhile, a preliminary study reported temporary eradication of H. pylori in four out of nine H. pylori infected subjects who were treated with broccoli sprouts (27). Broccoli sprouts (6 g/d sulforaphane-rich) plus triple therapy treatment could significantly decrease infected patients’ systolic and diastolic blood pressure in 86 patients with type 2 diabetes (28). H. pylori density in infected patients was not significantly improved by treatment with broccoli sprouts, but it reduced malondialdehyde (MDA) values, indicating that broccoli sprout could inhibit the lipid peroxidation and exert cytoprotection effects on H. pylori related gastritis (29).
In addition, a leafy green vegetable, Angelica keiskei, was found to exert inhibitory effects on H. pylori-induced gastric inflammation in mice, and the possible mechanism of action could be based on the inhibition of inflammatory mediators (IFN-γ, COX-2, and iNOS) mediated by NF-κB signaling pathways (30). Another vegetable, Nigella sativa, have many active ingredients with potent medical effects such as antimicrobial, anti-inflammatory, and anti-cancer activities (31). The result of N. sativa seeds against H. pylori infection in 88 patients with nonulcer dyspepsia showed that, the eradication rates achieved by triple therapy, 1 g, 2 g, and 3 g ground N. sativa seeds were 82.6, 47.6, 66.7, and 47.8%, respectively. Notely, there was no statistically significant difference of the eradication rate between triple therapy and 2 g of seeds powder (32).
Above all, it is evident that dietary ingredients from vegetables such as broccoli sprout, A. keiskei and N. sativa have effects on inhibition of H. pylori colonization, enhancement of antibiotic sensitiveness and suppression of H. pylori-induced inflammation or oxidative stress in vivo. All results are sorted out in Table 1.
Suppressive effects of fruits on Helicobacter pylori infection in vivo
Epidemiological studies have shown that the risk of gastric cancer was lower in people with more fruit consumption (33). Fruits raw extract or active ingredients had inhibition effects on the growth of H. pylori growth (13), and anti-cancer (34). Based on the current notion that H. pylori is a key factor in the development of gastric cancer, it is easily deduced that inhibition of H. pylori activity may play an important role in reducing gastric cancer risk.
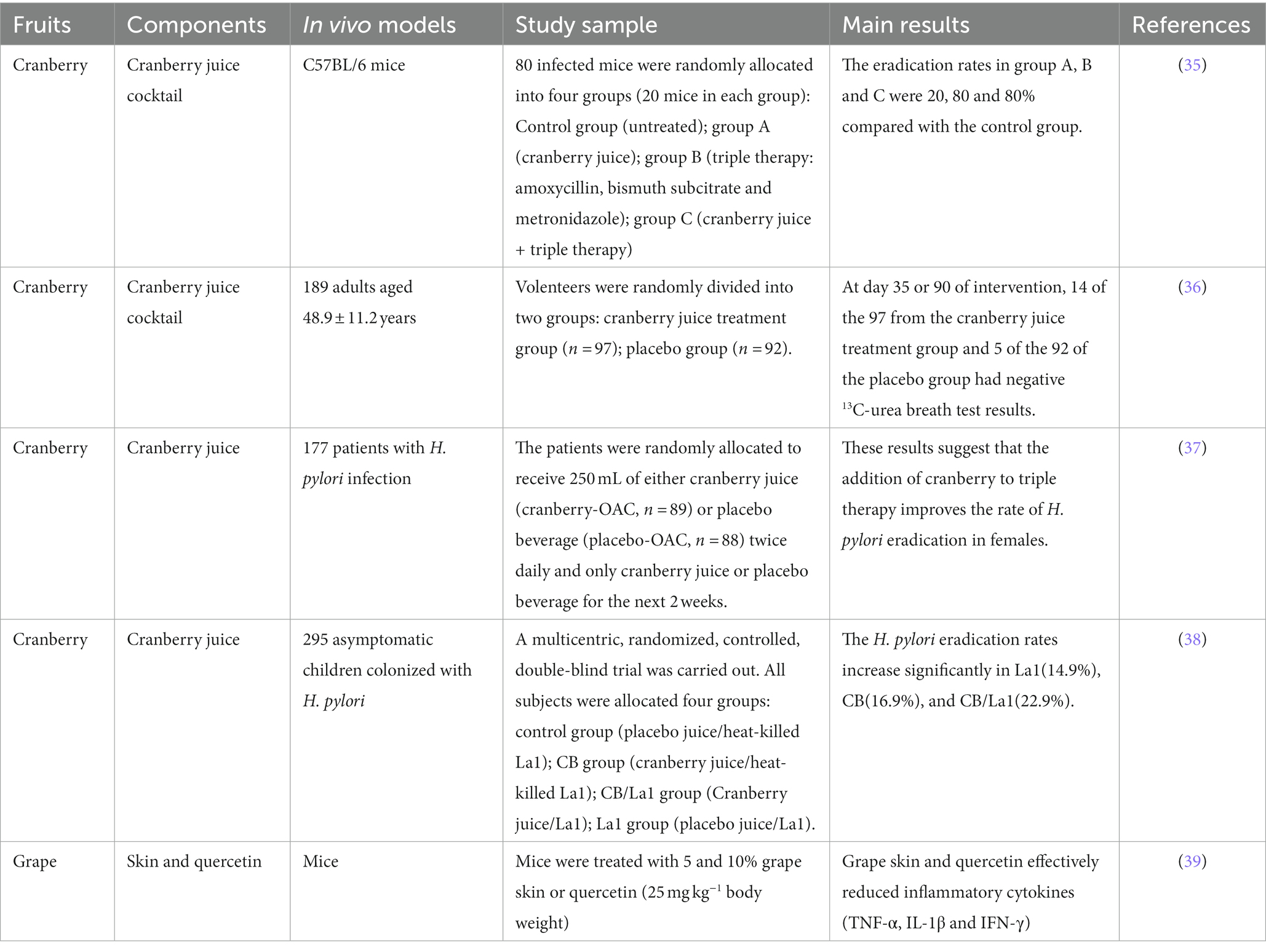
Among numerous fruits of the berries, such as cranberry, bilberry, raspberry, elderberry and strawberry, it has been widely focused their effect on inhibiting H. pylori and enhancing antibiotics sensitive to H. pylori (40). Cranberries are a great source of dietary ingredients, such as anthocyanins and proanthocyanins with high value of health benefits (41). There are a few studies that have examined the efficacy of cranberry juice on mice or human infected with H. pylori.
Cranberry juice could eliminate 80% of H. pylori colonization in infected mice after 24 h of intervention, however, the eradication rate reached only 20% after 4 weeks of treatment (35). A double-blind, randomized, placebo-controlled study of regular consumption of cranberry juice in infected Chinese subjects had yielded significantly negative 13C-urea breath test results (14/97 in the treatment group versus 5/92 in the placebo group) after 90 days intervention (36). The other study showed that the negative rate of H. pylori in female and male patients, who were administered with triple therapy during the first week and followed by cranberry supplements for two weeks, was 95.2 and 73.9%. The results indicated that the female patients may obtain more benefit form the addition of cranberry (37). Gotteland et al. (38) had shown that the H. pylori eradication rate reached 16.9% in asymptomatic infected children by daily intake of cranberry juice for three weeks, although the clearance effect disappears after cessation of cranberry juice consumption.
Grape may be the other fruit that has reported the positive effects on human infected with H. pylori. Brown et al. (39) tested the activity of grape skin together with quercetin did not significantly inhibit the growth of H. pylori, but was effective in reducing inflammatory cytokines including TNF-α, IL-1 and IFN-γ.
From the results mentioned above and summarized in Table 2, it is clear that fruits, especially like cranberry, could suppress the growth of H. pylori, and help infected subjects to attenuate the levels of inflammatory factors. The inhibitory effects of fruits may be sourced from polyphenols (34). Cranberry components showed synergistic effects with antibiotics. The potential mechanisms might be that components firstly damage the cell membranes of H. pylori, and then make cells more sensitive to antibiotics (34).
Suppressive effects of spices on Helicobacter pylori infection in vivo
Spices have been widely used as food flavors and therapeutic ingredients since ancient times. Until now, spices have been reported to process anti-cancer effects, particularly in gastric and colon cancer (42). H. pylori is one of the risk factors for gastric cancer, the anti-H. pylori effect of spices may play a crucial role in preventing the development of gastric cancer. Moreover, Liu et al. (34) have concluded that spices have anti-H. pylori activity.
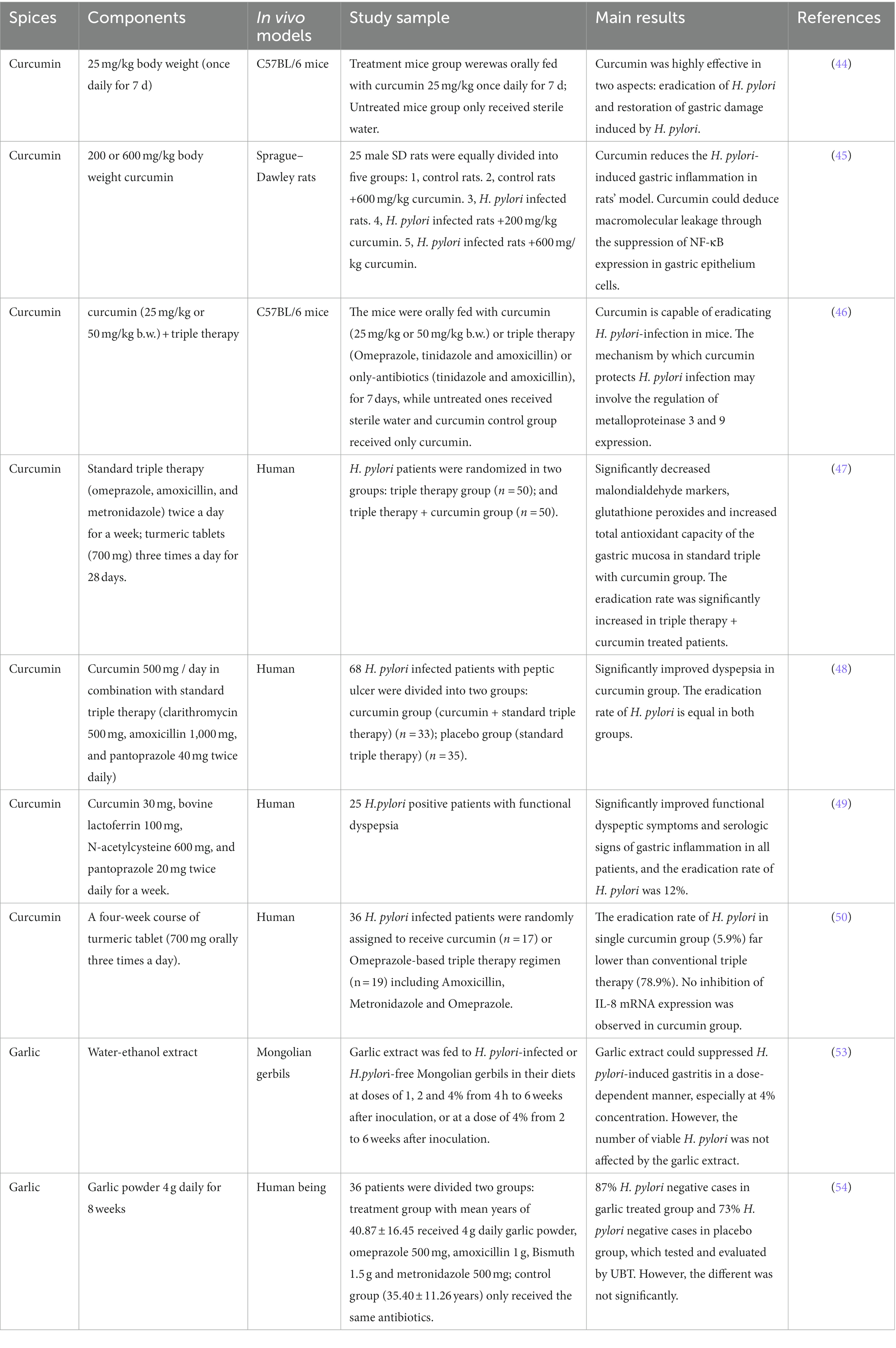
Curcumin is a polyphenolic yellow pigment which is rich in turmeric root. Turmeric is widely used as spices, food coloring and medicines in India and Southeast Asia (43). Curcumin presented strong therapeutic effect against H. pylori infection for it not only could eradicate H. pylori, but also completely repair the gastric damage induced by H. pylori (44). 200 or 600 mg/kg of curcumin had anti-inflammatory effect by suppressing expression of NF-κB p65 (45). Similarly, Kundu et al. (46) revealed that curcumin had healing-promoting effect on H. pylori infected mice and could restrict the expression of metalloproteinases-3 and 9 (46).
Furthermore, clinical studies have been conducted for exploring the inhibition effects of curcumin on H. pylori. Judaki et al. (47) investigated the clinical effect of curcumin (700 mg / three times a day for 28 days) combined with triple therapy regimens for patients with chronic gastritis-associated H. pylori infection. The result suggested that the additive curcumin could significantly decrease MDA level and glutathione peroxides, improve the total antioxidant capacity of the gastric mucosa, increase the H. pylori eradication rate compared to triple therapy alone (86.4% vs. 74.5%, p < 0.05) (47).
The other clinical study showed that standard triple therapy with curcumin significantly improves dyspeptic symptoms, despite no obvious effect on H. pylori eradication (48). Similar results have been reported by di Mario et al. (49), they found that curcumin-based therapy could relieve functional dyspeptic symptoms as well as serological signs of gastric inflammation, and increase 12% level of eradication rate (49). In another case, the H. pylori eradication rate was only 5.9% when patients were treated with curcumin alone, however, extra finding was that curcumin could modulate the production of inflammatory cytokines (50).
Garlic (Allium sativum), the other food flavoring, shares high scores in therapeutic properties (51). It has been reported that garlic consumption has therapeutic benefits in precancerous gastric lesions (52). Limuro et al. showed that the water-ethanol extract of garlic was able to inhibit H. pylori-induced early gastritis in a dose-dependent manner in Mongolian gerbils (53). Moreover, the administration of 4 g/day garlic powder increased the eradication rate of H. pylori in the treatment group (87%) compared to the placebo group (73%), although the difference was not statistically significant (54).
In general, these results pointed toward that spices have potent healing effect on gastric damage caused by H. pylori (Table 3). All above observations not only suggest the therapeutic effect of curcumin against H.pylori infections but underline the anti-inflamatory effect of curcumin. Curcumin may be as a potential therapeutic candidate for H.pylori associated disease.
Suppressive effects of edible herbs on Helicobacter pylori infection in vivo
China have rich experience in using medicinal herbs to manage stomach diseases over the long history, which is gaining popularity recently. Parts of medicinal herbs were developed as food because of their safe and functional properties.
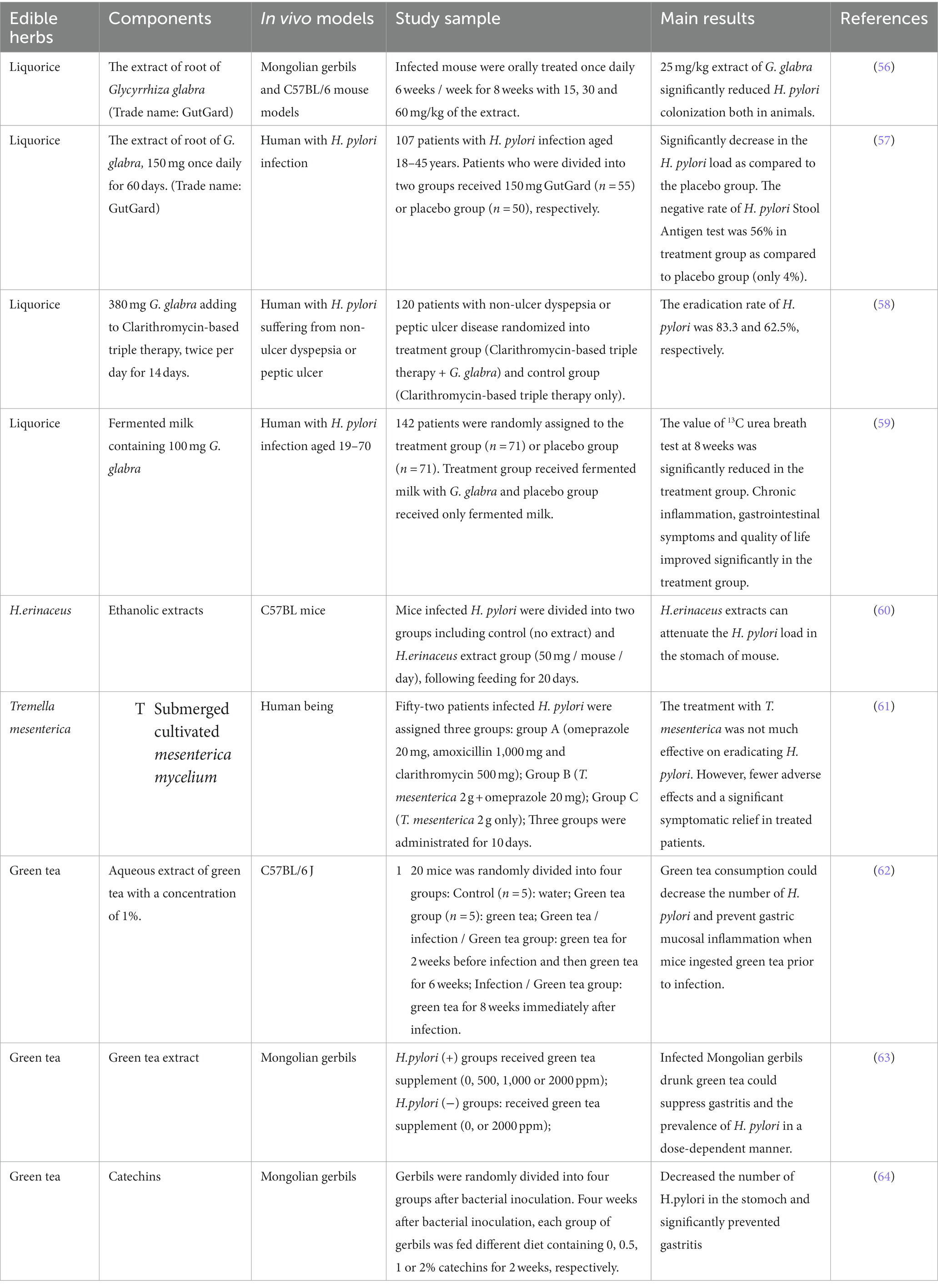
Licorice root (Glycyrrhiza glabra L.), which could be found in almost all Traditional Chinese Medicine (TCM) regimens. It is a common practice to use G. glabra to treat gastric ulcers in TCM. Aqueous licorice root extract had shown anti-adhesion effect on H. pylori by interfering with the binding action between the bacterial adhesins and human gastric tissue (55). Administration of G. glabra extract had anti-H. pylori effect in both C57B/L mice and Mongolian gerbils (56).
Licorice root extract showed a significant reduction of H. pylori load compared to the placebo group. The results also showed that the eradication of H. pylori by stool antigen test in GutGuard treatment group was 56% compared to 4% in the placebo group (57). Adding licorice to the clarithromycin-based triple therapy could significantly increase the H. pylori eradication rate reached 83.3% over the control group (62.5%) (58). Moreover, fermented milk in combination with licorice could significantly reduce H. pylori density and relieve gastrointestinal symptoms and histological inflammation (59).
Hericium erinaceus referred to as the lion’s mane mushroom or monkey’s head mushroom (in Chinese), can be used as both edible and medicinal fungus. H. erinaceus has been widely used in TCM to treat chronic superficial gastritis and gastric ulcers (60). Wang et al. (60) showed that the ethanolic extracts of H. erinaceus significantly reduced the H. pylori colonization in the stomach of mice (60). Despite the fact that monkey head mushrooms have been used in Chinese medicine for thousands of years, however, few clinical studies have examined the suppressive effects of H. erinaceus on H. pylori infection. In addition, Tremella mesenterica is another fungus found to have anti-H. pylori activity. Clinical study showed that T. mesenterica had immunomodulatory effect on H. pylori-infected patients when administered 2 g/day for 10 days (61).
Green tea is one of the most popular beverages in China and Japan. Green tea has been found to have suppressive effects on H. pylori growth (62). Intake of green tea in advance could attenuate this microbe colonization and gastric mucosal inflammation before infection (62). Matsubara et al. had found that green tea aqueous extract exerted a dose-dependent suppressive effect under three different concentration (500, 1,000 and 2000 ppm) on gastritis and the prevalence of H. pylori in infected Mongolian gerbils (63). One of main antioxidant compounds in green tea was catechins, which showed antibacterial activity against H. pylori in vivo (51). In infected Mongolian gerbils, H. pylori was eradicated in 36% animals with the dietary intake of 2% catechins for 14 days, and with significant decreases mucosal hemorrhage and erosion (64). The authors indicated that tea catechins have an anti-H. pylori effect and may have a therapeutic effect against gastic mucosa injury induced by H. pylori.
Taken together, edible herbs share in nature the characteristics of food and medicine. Some common but important edible herbs with anti-H. pylori effect including licorice, fungus and green tea were selected for this review (Table 4). All results have shown that edible herbs inhibit H. pylori colonization to some extend and improve H. pylori-related symptoms, likely by attenuating the development of inflammation. It is worth noting that numerous edible herbs are an invaluable treasure for the Chinese and people of East Asia. The potential of edible herbs to combat gastric disorders, particularly Helicobacter pylori infection, is worthy to be explored in the future.
Conclusion
Taken together, the results in this review are not completely satisfactory because in most cases H. pylori eradication is not obtained. However, this review showed that H. pylori colonization, severity of gastrointestinal inflammation and antibiotic therapy results would be improved by dietary ingredients from natural food resources. From the human beings perspective, the alternative treatment by single or combination of dietary ingredients from natural food resources to reduce H. pylori colonization is a promising and valuable strategy. The underlined mechanism of action has not yet been well explored about these natural food resources. Meanwhile, the safety and efficacy of the active compounds isolated from these natural foods are also lack. In future, it is necessary to further formulate active ingredients and make them available for clinical use.
Given the fact that complete eradication with natural foods or dietary ingredients may be impractical. The supplement of these foodstuff may provide a useful adjunct or alternative to conventional drug treatments, and should be tested in infected human for clinical trials. The natural products and dietary ingredients might even shift the consumers’ life patterns due to their low cost and relative safety after consuming for a long time. Moreover, the decline of excessive antibiotic use would have profound effects on human health and environment, including reduction of antibiotic resistance and protection of human microbiota diversity.
Author contributions
CW: Writing – original draft. MY: Writing – review & editing. HZ: Writing – review & editing. SM: Writing – review & editing. FS: Writing – review & editing. SN: Writing – review & editing. MX: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The financial supported by the Key Technological Project of Jiangxi Province (20212AAF01005).
Acknowledgments
The authors would like to thank the Key Technological Project of Jiangxi Province.
Conflict of interest
MY and HZ is employed by Jiangzhong dietary therapy technology Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Peleteiro, B, Bastos, A, Ferro, A, and Lunet, N. Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with National Coverage. Dig Dis Sci (2014) 59:1698–709. doi: 10.1007/s10620-014-3063-0
2. Wang, YC. Medicinal plant activity on Helicobacter pylori related diseases. World J Gastroenterol (2018) 20:10368–82. doi: 10.3748/wjg.v20.i30.10368
3. Naito, Y, and Yoshikawa, T. Molecular and cellular mechanisms involved in Helicobacter pylori-induced inflammation and oxidative stress. Free Radic Biol Med (2002) 33:323–36. doi: 10.1016/S0891-5849(02)00868-7
4. Parkin, DM, Pisani, P, and Ferlay, J. Global cancer statistics. Ca A Cancer Journal for Clinicians [J] (2009) 49:33–64. doi: 10.3322/canjclin.49.1.33
5. Bartnik, W. Clinical aspects of Helicobacter pylori infection. Pol Arch Med Wewn (2008) 118:426–30. doi: 10.20452/pamw.440
6. Antonietta Gerarda Gracina, RMZ, De Musis, C, Romano, L, Loguercio, C, and Romano, M. Helicobacter pylori and extragastric diseases. Helicobacter (2014) 19:52–8. doi: 10.3748/wjg.v24.i29.3204
7. Bastos, J, Carreira, H, La Vecchia, C, and Lunet, N. Childcare attendance and Helicobacter pylori infection. European J cancer prevention (2013) 22:311–9. doi: 10.1097/CEJ.0b013e32835b69aa
8. Pimanov, SI, Makarenko, EV, Voropaeva, AV, Matveenko, ME, and Voropaev, EV. Helicobacter pylori eradication improves gastric histology and decreases serum gastrin, pepsinogen I and pepsinogen II levels in patients with duodenal ulcer. J Gastroenterol Hepatol (2008) 23:1666–71. doi: 10.1111/j.1440-1746.2007.04983.x
9. Chey, WD, and Wong, BC. American College of Gastroenterology guideline on the Management of Helicobacter pylori infection. Am J Gastroenterol (2007) 102:1808–25. doi: 10.1111/j.1572-0241.2007.01393.x
10. Malfertheiner, P, Megraud, F, O'Morain, C, Bazzoli, F, el-Omar, E, Graham, D, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III consensus report. Australian & New Zealand J Ophthalmol (2007) 56:772–81. doi: 10.1136/gut.2006.101634
11. Lukasz Holubiuk, JI. Diet and Helicobacter pylori infection. Gastroenterology Rev (2016) 3:150–4. doi: 10.5114/pg.2016.61487
12. Fahey, JW, Haristoy, X, Dolan, PM, Kensler, TW, Scholtus, I, Stephenson, KK, et al. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci U S A (2002) 99:7610–5. doi: 10.1073/pnas.112203099
13. Fahey, JW, Stephenson, KK, and Wallace, AJ. Dietary amelioration of helicobacter infection. Nutr Res (2015) 35:461–73. doi: 10.1016/j.nutres.2015.03.001
14. Inger Addamsson, CEN, Lundquist, P, Sjostedt, S, and Edlund, C. Comparative effects of omeprazole, amoxycillin plus metronidazole versus omeprazole, clarithromycin plus metronidazole on the oral, gastric and intestinal microflora in Helicobacter pylori-infected patients. J Antimicrob Chemother (1999) 44:629–40. doi: 10.1093/jac/44.5.629
15. Dethlefsen, L, Eckburg, PB, Bik, EM, and Relman, DA. Assembly of the human intestinal microbiota. Trends in ecology & evolution (2006) 21:517–23.
16. Salminen, S, Benno, Y, and De Vos, W. Intestinal colonisation, microbiota and future probiotics? Asia Pacific journal of clinical nutrition (2006) 15:558–62.
17. O’Hara, AM, and Shanahan, F. Gut microbiota: mining for therapeutic potential. Clinical Gastroenterology and Hepatology (2007) 5:274–84. doi: 10.1016/j.cgh.2006.12.009
18. Dietrich Rothenbacher, GB, Brenner, H, and Blaser, MJ. Inverse relationship between gastric colonization of helicobacter pylori and diarrheal illnesses in children: results of a population-based cross-sectonal study. J Infect Dis (2000) 182:1446–9. doi: 10.1086/315887
19. Lin, D, and Koskella, B. Friend and foe: factors influencing the movement of the bacterium Helicobacter pylori along the parasitism-mutualism continuum. Evol Appl (2015) 8:9–22. doi: 10.1111/eva.12231
20. Kim, KJ, Liu, X, Komabayashi, T, Jeong, SI, and Selli, S. Natural products for infectious diseases. Evid Based Complement Alternat Med (2016) 2016:9459047. doi: 10.1155/2016/9459047
21. Zhou, Y, Li, Y, Zhou, T, Zheng, J, Li, S, and Li, HB. Dietary natural products for prevention and treatment of liver cancer. Nutrients (2016) 8:156. doi: 10.3390/nu8030156
22. Newman, DJ, and Cragg, GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod (2016) 79:629–61. doi: 10.1021/acs.jnatprod.5b01055
23. Amaral, O, Fernandes, I, Veiga, N, Pereira, C, Chaves, C, Nelas, P, et al. Living conditions and Helicobacter pylori in adults. Biomed Res Int (2017) 2017:9082716. doi: 10.1155/2017/9082716
24. Herr, I, and Buchler, MW. Dietary constituents of broccoli and other cruciferous vegetables: implications for prevention and therapy of cancer. Cancer Treat Rev (2010) 36:377–83. doi: 10.1016/j.ctrv.2010.01.002
25. Haristoy, X, Angioi-Duprez, K, Duprez, A, and Lozniewski, A. Efficacy of sulforaphane in eradicating Helicobacter pylori in human gastric xenografts implanted in nude mice. Antimicrob Agents Chemother (2003) 47:3982–4. doi: 10.1128/AAC.47.12.3982-3984.2003
26. Yanaka, A, Fahey, JW, Fukumoto, A, Nakayama, M, Inoue, S, Zhang, S, et al. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res (Phila) (2009) 2:353–60. doi: 10.1158/1940-6207.CAPR-08-0192
27. Galan, MV, Kishan, AA, and Silverman, AL. Oral broccoli sprouts for the treatment of Helicobacter pylori infection: a preliminary report. Dig Dis Sci (2004) 49:1088–90. doi: 10.1023/B:DDAS.0000037792.04787.8a
28. Mirmiran, P, Bahadoran, Z, Golzarand, M, Zojaji, H, and Azizi, F. A comparative study of broccoli sprouts powder and standard triple therapy on cardiovascular risk factors following H. pylori eradication: a randomized clinical trial in patients with type 2 diabetes. J Diabetes Metab Disord (2014) 13:64. doi: 10.1186/2251-6581-13-64
29. Chang, YW, Jang, JY, Kim, YH, Kim, JW, and Shim, JJ. The effects of broccoli sprout extract containing Sulforaphane on lipid peroxidation and Helicobacter pylori infection in the gastric mucosa. Gut Liver (2015) 9:486–93. doi: 10.5009/gnl14040
30. Kim, A, Lim, JW, Kim, H, and Kim, H. Supplementation with Angelica keiskei inhibits expression of inflammatory mediators in the gastric mucosa of Helicobacter pylori-infected mice. Nutr Res (2016) 36:488–97. doi: 10.1016/j.nutres.2015.12.017
31. Murali, MR, Naveen, SV, Son, CG, and Raghavendran, HRB. Current knowledge on alleviating Helicobacter pylori infections through the use of some commonly known natural products: bech to beside. Integrative Medicine Research (2014) 3:111–8. doi: 10.1016/j.imr.2014.04.001
32. Salem, EM, Yar, T, Bamosa, AO, al-Quorain, A, Yasawy, MI, Alsulaiman, RM, et al. Comparative study of nigella Sativa and triple therapy in eradication of Helicobacter Pylori in patients with non-ulcer dyspepsia. Saudi J Gastroenterol (2010) 16:207–14. doi: 10.4103/1319-3767.65201
33. Wang, T, Cai, H, Sasazuki, S, Tsugane, S, Zheng, W, Cho, ER, et al. Fruit and vegetable consumption, Helicobacter pylori antibodies, and gastric cancer risk: a pooled analysis of prospective studies in China, Japan, and Korea. Int J Cancer (2017) 140:591–9. doi: 10.1002/ijc.30477
34. Liu, Q, Meng, X, Li, Y, Zhao, CN, Tang, GY, Li, S, et al. Natural products for the prevention and Management of Helicobacter pylori infection. Compr Rev Food Sci Food Saf (2018) 17:937–52. doi: 10.1111/1541-4337.12355
35. Shi, T, and XIao, S. Cranberry juice cocktail for prevention and treatment of Helicobater pylori infection in mice model (in Chinese). Chin. J Gastroenterol (2003) 8:265–8.
36. Zhang, L, Ma, J, Pan, K, Go, VLW, Chen, J, and You, WC. Efficacy of cranberry juice on Helicobacter pylori infection: a double-blind, randomized placebo-controlled trial. Helicobacter (2005) 10:139–45. doi: 10.1111/j.1523-5378.2005.00301.x
37. Shmuely, H, Yahav, J, Samra, Z, Chodick, G, Koren, R, Niv, Y, et al. Effect of cranberry juice on eradication of Helicobacter pylori in patients treated with antibiotics and a proton pump inhibitor. Molecular nutrition & food research (2007) 51:746–51. doi: 10.1002/mnfr.200600281
38. Gotteland, M, Andrews, M, Toledo, M, Muñoz, L, Caceres, P, Anziani, A, et al. Modulation of Helicobacter pylori colonization with cranberry juice and Lactobacillus johnsonii La1 in children. Nutrition (2008) 24:421–6. doi: 10.1016/j.nut.2008.01.007
39. Brown, JC, Wang, J, Kasman, L, Jiang, X, and Haley-Zitlin, V. Activities of muscadine grape skin and quercetin against Helicobacter pylori infection in mice. J Appl Microbiol (2011) 110:139–46. doi: 10.1111/j.1365-2672.2010.04870.x
40. Chatterjee, A, Yasmin, T, Bagchi, D, and Stohs, SJ. Inhibition of Helicobacter pylori in vitro by various berry extracts, with enhanced susceptibility to clarithromycin. Mol Cell Biochem (2004) 265:19–26. doi: 10.1023/B:MCBI.0000044310.92444.ec
41. Pedersen, CB, Kyle, J, Jenkinson, AME, Gardner, PT, McPhail, D, and Duthie, GG. Effects of blueberry and cranberry juice consumption on the plasma antioxidant capacity of healthy female volunteers. Eur J Clin Nutr (2000) 54:405–8. doi: 10.1038/sj.ejcn.1600972
42. Zheng, J, Zhou, Y, Li, Y, Xu, DP, Li, S, and Li, HB. Spices for prevention and treatment of cancers. Nutrients (2016) 8:nu8080495. doi: 10.3390/nu8080495
43. Sarkar, A, De, R, and Mukhopadhyay, AK. Curcumin as a potential therapeutic candidate for Helicobacter pylori associated diseases. World J Gastroenterol (2016) 22:2736–48. doi: 10.3748/wjg.v22.i9.2736
44. de, R, Kundu, P, Swarnakar, S, Ramamurthy, T, Chowdhury, A, Nair, GB, et al. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob Agents Chemother (2009) 53:1592–7. doi: 10.1128/AAC.01242-08
45. Sintara, K, Thong-Ngam, D, Patumraj, S, Klaikeaw, N, and Chatsuwan, T. Curcumin suppresses gastric NF-kappaB activation and macromolecular leakage in Helicobacter pylori-infected rats. World J Gastroenterol (2010) 16:4039–46. doi: 10.3748/wjg.v16.i32.4039
46. Kundu, P, de, R, Pal, I, Mukhopadhyay, AK, Saha, DR, and Swarnakar, S. Curcumin alleviates matrix metalloproteinase-3 and -9 activities during eradication of Helicobacter pylori infection in cultured cells and mice. PLoS One (2011) 6:e16306. doi: 10.1371/journal.pone.0016306
47. JUDAKI, A, RAHMANI, A, FEIZI, J, ASADOLLAHI, K, and HAFEZI AHMADI, MR. Curcumin in combination with triple therapy regimes ameliorates oxidative stress and histopathologic changes in chronic gastritis-associated Helicobacter Pylori infection. Arq Gastroenterol (2017) 54:177–82. doi: 10.1590/s0004-2803.201700000-18
48. Khonche, A, Biglarian, O, Panahi, Y, Valizadegan, G, Soflaei, SS, Ghamarchehreh, ME, et al. Adjunctive therapy with curcumin for peptic ulcer: a randomized controlled trial. Drug Res (Stuttg) (2016) 66:444–8. doi: 10.1055/s-0042-109394
49. di Mario, F, Cavallaro, LG, Nouvenne, A, Stefani, N, Cavestro, GM, Iori, V, et al. A curcumin-based 1-week triple therapy for eradication of Helicobacter pylori infection: something to learn from failure? Helicobacter (2007) 12:238–43. doi: 10.1111/j.1523-5378.2007.00497.x
50. Koosirirat, C, Linpisarn, S, Changsom, D, Chawansuntati, K, and Wipasa, J. Investigation of the anti-inflammatory effect of Curcuma longa in Helicobacter pylori-infected patients. Int Immunopharmacol (2010) 10:815–8. doi: 10.1016/j.intimp.2010.04.021
51. Ayala, G, Escobedo-Hinojosa, WI, de la Cruz-Herrera, CF, and Romero, I. Exploring alternative treatments for Helicobacter pylori infection. World J Gastroenterol (2014) 20:1450–69. doi: 10.3748/wjg.v20.i6.1450
52. You, WC, Brown, LM, Zhang, L, Li, JY, Jin, ML, Chang, YS, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst (2006) 98:974–83. doi: 10.1093/jnci/djj264
53. Iimuro, M, Shibata, H, Kawamori, T, Matsumoto, T, Arakawa, T, Sugimura, T, et al. Suppressive effects of garlic extract on Helicobacter pylori-induced gastritis in Mongolian gerbils. Cancer Lett (2002) 187:61–8. doi: 10.1016/S0304-3835(02)00401-9
54. Hekmatdoost, A, Ghobeh, M, Shaker-Hosseini, R, MirSattari, D, Rastmanesh, R, Rashidkhani, B, et al. The effect of garlic consumption on Helicobacter pylori treatment using urea breath test: a randomized clinical trial. J nutr Sci Diet (2015) 1:21–7.
55. Wittschier, N, Faller, G, and Hensel, A. Aqueous extracts and polysaccharides from liquorice roots (Glycyrrhiza glabra L.) inhibit adhesion of Helicobacter pylori to human gastric mucosa. J Ethnopharmacol (2009) 125:218–23. doi: 10.1016/j.jep.2009.07.009
56. Kim, JM, Zheng, HM, Lee, BY, Lee, WK, and Lee, DH. Anti-Helicobacter pylori properties of GutGard. Prev Nutr Food Sci (2013) 18:104–10. doi: 10.3746/pnf.2013.18.2.104
57. Puram, S, Suh, HC, Kim, SU, Bethapudi, B, Joseph, JA, Agarwal, A, et al. Effect of GutGard in the Management of Helicobacter pylori: a randomized double blind placebo controlled study. Evid Based Complement Alternat Med (2013, 2013) 2013:263805. doi: 10.1155/2013/263805
58. Hajiaghamohammadi, AA, Zargar, A, Oveisi, S, Samimi, R, and Reisian, S. To evaluate of the effect of adding licorice to the standard treatment regimen of Helicobacter pylori. Braz J Infect Dis (2016) 20:534–8. doi: 10.1016/j.bjid.2016.07.015
59. Yoon, JY, Cha, JM, Hong, SS, Kim, HK, Kwak, MS, Jeon, JW, et al. Fermented milk containing lactobacillus paracasei and Glycyrrhiza glabra has a beneficial effect in patients with Helicobacter pylori infection: a randomized, double-blind, placebo-controlled study. Medicine (Baltimore) (2019) 98:e16601. doi: 10.1097/MD.0000000000016601
60. Wang, G, Zhang, X, Maier, SE, Zhang, L, and Maier, RJ. In vitro and in vivo inhibition of Helicobacter pylori by Ethanolic extracts of lions mane medicinal mushroom, Hericium erinaceus (Agaricomycetes). Intern J Med Mushroom (2019) 21:1–11. doi: 10.1615/IntJMedMushrooms.2018029487
61. Lachter, J, Yampolsky, Y, Gafni-Schieber, R, and Wasser, SP. Yellow brain culinary-medicinal mushroom,Tremella mesenterica Ritz.:Fr.(higher basidiomycetes) is subjectively but not objectively effective for eradication of Helicobacter pylori: a prospective controlled trial. Intern J Med Mushroom (2012) 14:55–63. doi: 10.1615/IntJMedMushr.v14.i1.60
62. Stoicov, C, Saffari, R, and Houghton, J. Green tea inhibits helicobacter growth in vivo and in vitro. Int J Antimicrob Agents (2009) 33:473–8. doi: 10.1016/j.ijantimicag.2008.10.032
63. Matsubara, S, Shibata, H, Ishikawa, F, Yokokura, T, Takahashi, M, Sugimura, T, et al. Suppression of Helicobacter pylori-induced gastritis by green tea extract in Mongolian gerbils. Biochem Biophys Res Commun (2003) 310:715–9. doi: 10.1016/j.bbrc.2003.09.066
Keywords: Helicobacter pylori, antibiotics, natural foods, eradication, dietatary ingredients
Citation: Wang C, Yao M, Zhong H, Meena SS, Shu F, Nie S and Xie M (2023) Natural foods resources and dietary ingredients for the amelioration of Helicobacter pylori infection. Front. Med. 10:1324473. doi: 10.3389/fmed.2023.1324473
Edited by:
Pragya Tiwari, Yeungnam University, Republic of KoreaReviewed by:
Qiaomei Yang, Zhejiang Ocean University, ChinaJinxuan Cao, Beijing Technology and Business University, China
Copyright © 2023 Wang, Yao, Zhong, Meena, Shu, Nie and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaoping Nie, c3BuaWVAbmN1LmVkdS5jbg==; Mingyong Xie, bXl4aWVAbmN1LmVkdS5jbg==
 Chengyuan Wang
Chengyuan Wang Meixiang Yao2
Meixiang Yao2 Stephene S. Meena
Stephene S. Meena Fuxing Shu
Fuxing Shu Shaoping Nie
Shaoping Nie