Abstract
Legionella pneumonia (LP) is a relatively uncommon yet well-known type of atypical community-acquired pneumonia (CAP). It is characterized by a rapid progression to severe pneumonia and can be easily misdiagnosed. In most patients, chest computed tomography (CT) showed patchy infiltration, which may progress to lobar infiltration or even lobar consolidation. While pulmonary cavities are commonly observed in immunocompromised patients with LP, they are considered rare in immunocompetent individuals. Herein, we present a case of LP in an immunocompetent patient with multiple cavities in both lungs. Pathogen detection was performed using metagenomic next-generation sequencing (mNGS). This case highlights the unusual radiographic presentation of LP in an immunocompetent patient and emphasizes the importance of considering LP as a possible diagnosis in patients with pulmonary cavities, regardless of their immune status. Furthermore, the timely utilization of mNGS is crucial for early pathogen identification, as it provides multiple benefits in enhancing the diagnosis and prognosis of LP patients.
1 Introduction
Legionella pneumonia (LP) is a severe form of bacterial pneumonia caused by Legionella species. Without appropriate and timely treatment, LP can be life-threatening (1). The mortality rate for LP varies globally, ranging from 5% to 33% among the general population. However, in immunocompromised patients, the mortality rate can exceed 50% (2).
Although the imaging findings of LP are nonspecific, they are closely related to the clinical presentations and outcomes of the disease. Computed tomography (CT) is essential in detecting lung abnormalities, monitoring disease progression, and assessing therapy response. The most common CT pattern observed in LP patients is the presence of well-circumscribed infiltrates with ground-glass opacities, which can involve multiple lobes or segments (3, 4). In immunocompromised patients, it is occasionally observed that abscesses and cavitation may develop during the course of the disease. However, these findings are relatively uncommon in individuals with a healthy immune system (3, 4).
Herein, we present a case of community-acquired LP in an immunocompetent patient who exhibited multiple cavities in the lungs. This case highlights the importance of considering LP as a potential diagnosis in patients with pulmonary cavities, even among those who do not have compromised immune systems.
2 Case description
A 67 years-old Chinese female patient was admitted to our hospital presenting with a 5 days history of fever, cough, and exertional dyspnea. She had a productive cough with scant mucus-like sputum. She had no history of smoking and no evidence of Mycobacterium tuberculosis infection. On admission, her vital signs were recorded as follows: a body temperature of 39.6°C, a pulse rate of 79 beats per minute, a respiratory rate of 21 breaths per minute, and a blood pressure of 150/84 mmHg. Pulmonary auscultation revealed coarse breath sounds and bilateral rales.
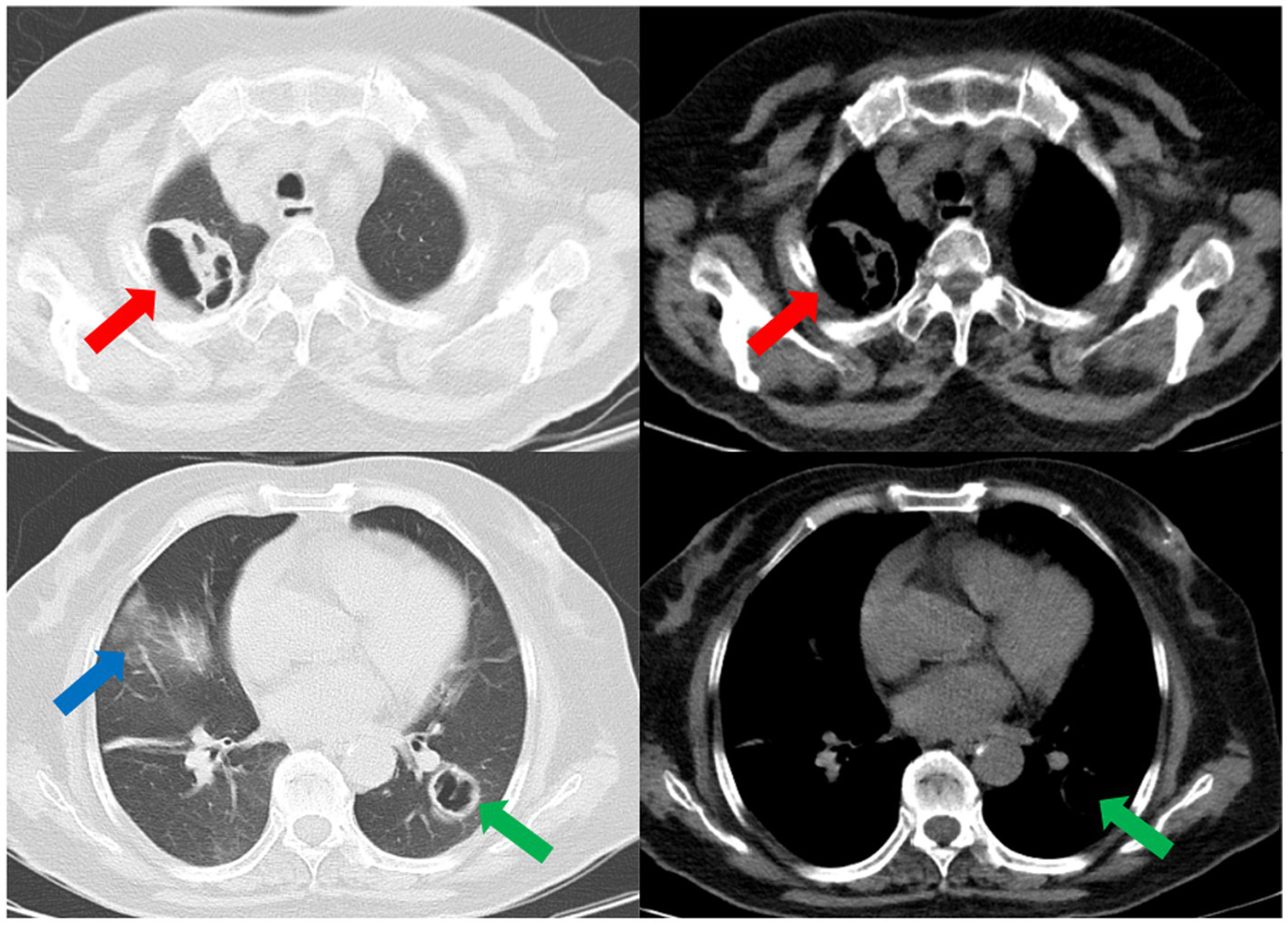
Laboratory results upon admission showed a white blood cell count of 14.29 × 109 cells/L (reference range: 3.5–9.5 × 109 cells/L) with an elevated neutrophil ratio of 88%. C-reactive protein and procalcitonin concentrations were markedly elevated at 81.40 mg/L (0–3.48 mg/L) and 1.20 ng/mL (0–0.05 ng/mL), respectively. Serum 1-3-beta-D-glucan and galactomannan tests yielded negative results. CT scans of the patient’s lungs on admission revealed multiple lung nodules and patchy infiltrations (Figure 1). The sequence of relevant events after the patient was admitted has been shown in the timeline (Figure 2).
Figure 1

CT scans of the thorax on the day of admission demonstrates multiple nodular and patchy infiltrates in the lungs. Nodular infiltrate in the right lung (red arrows). Nodular infiltrate in the left lung (green arrows). Patchy infiltrates in both lungs (blue arrows).
Figure 2
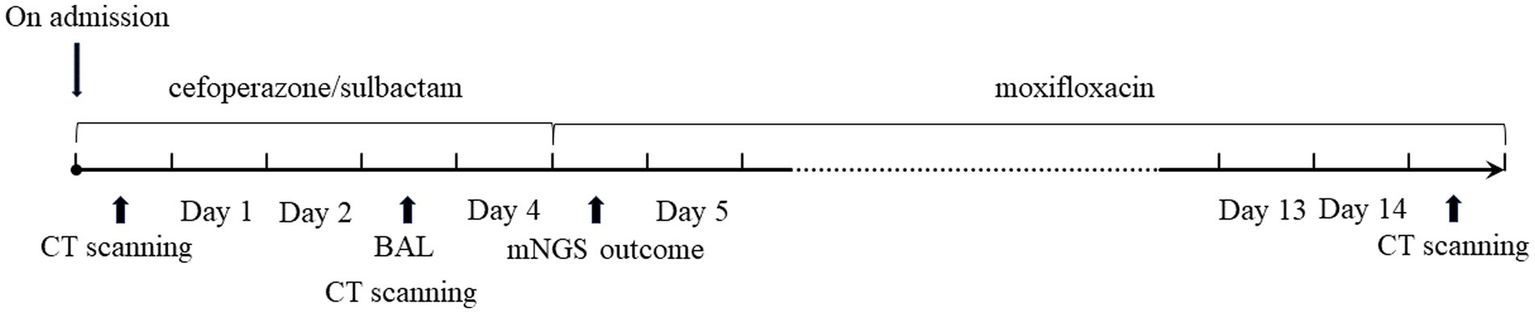
Timeline of treatment process.
The patient received empiric treatment with cefoperazone/sulbactam for suspected community-acquired pneumonia (CAP) after blood and sputum samples were obtained. Clinical response assessment after 3 days showed inadequate improvement in the patient’s clinical condition. Microbiological cultures from samples were negative for general bacteria, acid-fast bacilli and fungal elements.
Subsequently, a bronchoscopy with bronchoalveolar lavage was performed on the fourth day after admission. Bronchoalveolar lavage fluid (BALF) was sent to Jiangsu Simcere Medical Diagnostics Co., Ltd., and then metagenomic next-generation sequencing (mNGS) was performed on the Illumina next-generation high-throughput sequencing platform. For detailed methodological description, please refer to State Key Laboratory of Translational Medicine and Innovative Drug Development & Jiangsu Simcere Diagnostics Co., Ltd. previously published paper (5). The mNGS results on the 5th day after admission showed that 17,667 original sequences of Legionella pneumophila were identified, with a relative abundance of 98.3% and a coverage of 7.22% (Figure 3). Meanwhile, a number of other pathogens have been identified, including certain fungi and bacteria (Table 1). After our analysis, we are more inclined to classify them as respiratory custom flora or background flora derived from the environment or samples, regardless of considering them as pathogenic bacteria. On the same day, a repeated CT scan revealed the presence of bilateral lung cavities (Figure 4). These findings were consistent with LP. Immediate treatment with moxifloxacin injection at a dosage of 0.4 g daily was initiated. After 3 days, the patient’s fever resolved, and there was improvement in cough symptoms. A follow-up CT scan 2 weeks later showed that the cavity in the right lung was smaller. Although the cavity in the left lung was slightly enlarged, the walls of the cavity were thinner, and the infiltration from both lungs had been resolved (Figure 5).
Figure 3
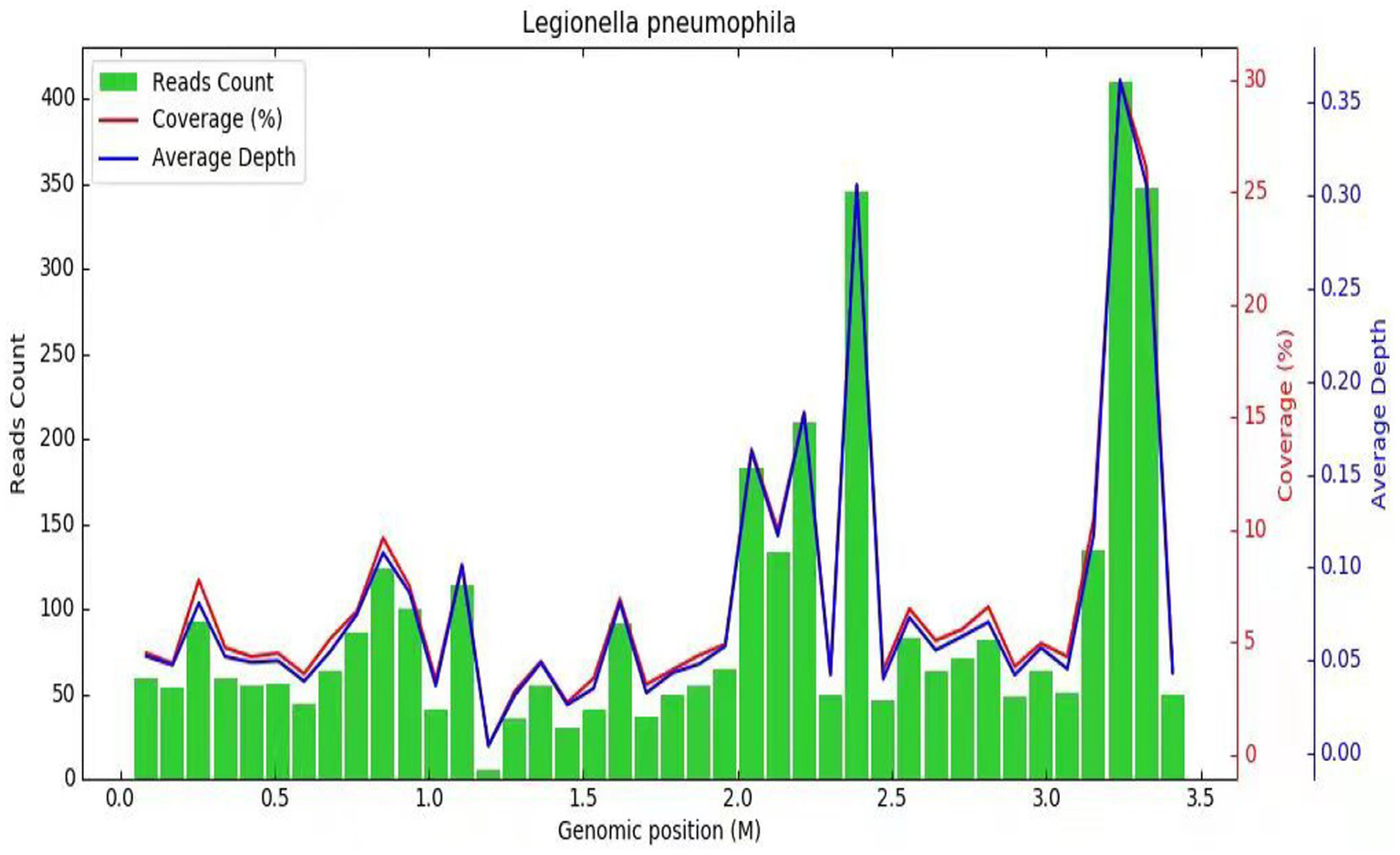
Diagnosis of Legionella pneumophila infection using mNGS. The horizontal axis (abscissa) represents the genome of the Legionella bacterium and the vertical axis (ordinate) represents the number of reads detected. The red line indicates the coverage, which represents the ratio of the detected nucleic acid sequence of Legionella to the entire gene sequence of Legionella. The blue line represents the sequencing depth, which is a measure of how many times a specific segment of the Legionella genome has been detected during the sequencing process.
Table 1
| Genus | Number of original sequences | Relative abundance | Species | Number of original sequences |
|---|---|---|---|---|
| Legionella | 17,667 | 98.30% | Legionella pneumophila | 17,667 |
| Candida | 4 | 26.67% | Candida albicans | 4 |
| Pneumocystis | 2 | 13.33% | Pneumocystis jirovecii | 2 |
| Neisseria | 97 | 0.54% | Neisseria subflava | 55 |
| Streptococcus | 71 | 0.40% | Streptococcus australis | 25 |
| Tannerella | 29 | 0.16% | Tannerella forsythia | 27 |
| Rothia | 21 | 0.12% | Rothia mucilaginosa | 21 |
| Fusobacterium | 19 | 0.11% | Fusobacterium nucleatum | 18 |
| Parvimonas | 15 | 0.08% | Parvimonas micra | 15 |
Reported cases of Legionella pneumonia.
Number of original sequences: refers to the number of sequences matching the microbial pathogen at the genus/species level. Relative abundance: the proportion of microorganisms of the same type detected in the entire specimen.
Figure 4
The repeated CT scan conducted on the fourth day after admission showed cavitary lesions in both lungs (red arrows and green arrows). Remaining patchy infiltrate in the right lung (blue arrow).
Figure 5
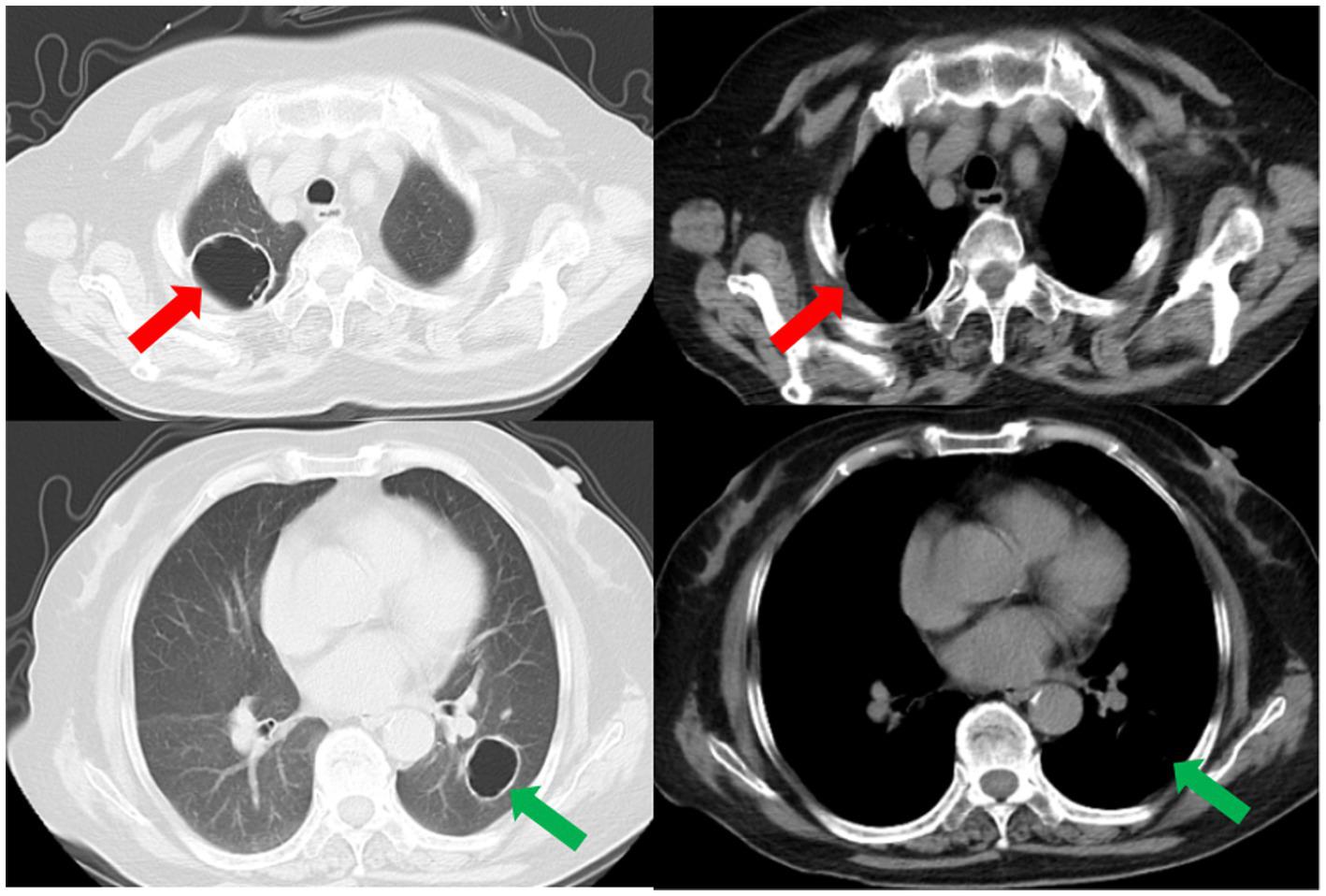
A subsequent CT scan 2 weeks later showed that the cavity in the right lung had decreased in size (red arrow), while the cavity in the left lung had slightly enlarged (green arrow). However, the thickness of the walls of both cavities had diminished, and the bilateral pulmonary infiltration indicated by the blue arrows had been resolved.
3 Discussion and literature review
To begin, a literature search was conducted using relevant databases such as Embase and Medline, spanning up to October 2023. The process carried out during this literature search is further detailed in Supplementary Table S3. After identifying the target papers through this search, the titles and abstracts of the database records were reviewed. Based on this initial screening, the full text of studies that were considered suitable for the evaluation were retrieved. Following the retrieval of the full text, the relevant case data was extracted from these studies.
Among the 14 previously reported cases of LP that were reviewed, 8 patients developed cavitation or abscesses, accounting for 57.1% of the cases (Table 2). Among these 8 patients, 7 were accompanied by immunosuppressive factors, accounting for 87.5%. This suggests that LP patients with immunodeficiency factors are more likely to develop cavities or abscesses, phenomena that are less likely to occur in LP patients with normal immune function. Within the reviewed LP cases, there were 4 deaths (Table 2). In 75% of the fatal cases, the correct pathogen was not identified before the patients’ deaths. Among the 11 surviving cases, 36.4% had Next-Generation Sequencing (NGS) used in diagnosis, and only 27.3% had metagenomic Next-Generation Sequencing (mNGS) used (Table 2). While the application of NGS or mNGS is not yet widespread, all patients who used NGS for LP diagnosis survived and achieved satisfactory results. As evident from the cases reviewed in Table 2, the vast majority of patients received appropriate anti-infective treatment following a correct diagnosis (see Table 3).
Table 2
| Case No. | Gender | Age | Underlying condition/immunosuppression factors | Imaging findings | Diagnosis mode | Therapeutic drugs | Serogroup | Treatment effect |
|---|---|---|---|---|---|---|---|---|
| 1 (6) | M | 52 | Good’s syndrome; methylprednisolone 60 mg daily for 2 weeks | Bilateral pleural effusion with patchy shadows and consolidation; pulmonary abscess and cavity formation | Blood mNGS; BALF and soft tissue specimens PCR | FQ, MA | N/D | Good response |
| 2 (7) | F | 34 | N/D | Interstitial inflammation with multiple lymphadenopathies | tNGS on thoracic lymph nodes specimen | FQ | N/D | Good response |
| 3 (8) | M | 31 | Smoking history | Consolidation of the right middle and lower lobe | Blood mNGS; culture with BALF | GA, CP, FQ, AG | N/D | Good response |
| 4 (9) | F | 34 | Liver transplantation | Infiltration progressive cavitation | Urine serotype 1 antigen assay, tissue specimens culture | FQ | I | Good response |
| 5 (10) | F | 65 | Breast cancer | Patchy consolidation | Blood, sputum, and pleural effusion NGS | FQ | N/D | Good response |
| 6 (11) | F | 39 | SLE | Cavity, diffuse infiltration & abscess | Serotype 1 antigen assay | MA, LS | I | Good response |
| 7 (12) | M | 44 | N/D | Alveolar infiltrates, cavitation | Postmortem Pus antigen analysis | BA, MA, AG | I | Bad response |
| 8 (12) | M | 31 | N/D | Infiltrates | Postmortem tissue antigen analysis | BA, MA, AG, LS | N/D | Bad response |
| 9 (13) | F | 39 | Breast cancer | Infiltration, cavitation | Culture with BALF, urine serotype 1 antigen assay | MA | I | Good response |
| 10 (14) | M | 10 | Idiopathic thrombocytopaenia | Round-shaped infiltrates, segmental both-sided pneumonia, a central abcedation inside the infiltrate | BALF PCR& urine by a serotype 1 antigen assay | FQ | I | Good response |
| 11 (15) | F | 20 | SLE | Patchy infiltrate | Lung tissue autopsy | AG, LS, BA | N/D | Bad response |
| 12 (16) | M | 63 | N/D | Extensive consolidation | BALF mNGS | FQ | N/D | Good response |
| 13 (17) | F | 30 | Idiopathic thrombocytopaenia, SLE | Cavitation appeared in consolidation | tracheal aspirates DFA | MA, AG, BA | VI | Bad response |
| 14 (18) | M | 45 | Chronic myelogenous leukemia | Consolidation, with a abscess formation | Pus biopsy, urine serotype 1 antigen assay | CP, MA, LS | I | Good response |
Reported cases of Legionella pneumonia.
N/D, not determined; MA, macrolides antibiotics; FQ, fluoroquinolone; GA, glycopeptide antibiotic; CP, carbapenem; AG, aminoglycoside; LS, lincosamides; BA, β-lactam antibiotics; F, female; M, male.
Table 3
| Medline | (Legionnaires pneumonia) AND (abscess); (legionnaires pneumonia) AND (cavity); (legionnaires pneumonia) AND (Next-Generation Sequencing) |
| Embase | (“Legionnaires pneumonia” OR (legionnaires AND (“pneumonia”/exp OR pneumonia))) AND (“abscess”/exp OR abscess); (“legionnaires pneumonia” OR (legionnaires AND (“pneumonia”/exp OR pneumonia))) AND cavity; (“legionnaires pneumonia” OR (legionnaires AND (“pneumonia”/exp OR pneumonia))) AND (“high throughput sequencing”/exp OR “high throughput sequencing”) |
Keywords for searching case reports on abscesses or cavities caused by Legionella and its diagnostic methods.
Based on the reviewed literature and the presented case, there are several noteworthy points that deserve attention and discussion. Firstly, the presence of cavities in the lungs, which is more commonly seen in immunosuppressed patients with LP, was found in an immunocompetent individual. Generally, immunocompetent individuals are more efficient in clearing Legionella bacteria, leading to fewer abscesses and cavities compared to immunocompromised patients (19, 20). In cases where immune function is compromised due to factors such as immune deficiency, organ transplantation, chemotherapy, or prolonged use of immunosuppressants, the immune response may become inadequate, potentially allowing bacterial growth and resulting in abscesses and cavities in the lungs (6, 9, 10, 15, 17, 18). In our patient, investigations for underlying causes of immunosuppression did not reveal any significant findings. When the immune system recognizes the presence of Legionella bacteria, it mounts a strong inflammatory response to eliminate the infection (20). However, this intense immune response can also cause collateral damage to the surrounding lung tissue, leading to the formation of abscesses or cavities (21).
Secondly, mNGS plays an important role in detecting Legionella pneumophila based on the analysis of BALF. Since LP lacks specific radiographic features, pathogen evidence seems to be more convincing in diagnosis compared with radiographic findings. In our case, the routine microbiological tests yield negative results and it is difficult to diagnose the causative agent Legionella pneumophila using traditional methods. Legionella culture and urine antigen testing are commonly used clinical methods for Legionella detection. However, they have limitations such as time-consuming culture results, sensitivity issues, and potential false positives (3, 22–24). In contrast, mNGS has proven to be effective in identifying challenging-to-culture pathogens like Legionella (25). It utilizes nucleic acid detection and molecular techniques, which offer enhanced sensitivity, specificity, and rapid pathogen detection throughput (26). This technology can be particularly useful in clinical settings for precise diagnosis and timely treatment (27).
Finally, this patient responded well to treatment for LP, indicating the effectiveness of the selected antibiotics in combating the infection. According to the etiological characteristics of Legionella, most macrolides, tetracyclines, ketolides, and quinolones are effective, with good efficacy and relatively few side effects, while Beta-lactams and aminoglycosides are ineffective (3). For severe or life-threatening LP, the British Thoracic Society recommends the use of fluoroquinolones (28). Studies have shown that moxifloxacin, in comparison to levofloxacin, exhibits at least an equivalent anti-Legionella effect and superior pharmacological parameters in vitro (29–31), as well as in clinical treatment (32). Notably, moxifloxacin does not require dosage adjustment in patients with liver and kidney dysfunction, making it a preferred choice due to its convenient dosing frequency and feasible administration method. The optimal duration of LP treatment varies based on clinical presentation and individual factors, however, a 2 weeks course of highly active anti-Legionella antibiotics is typically deemed sufficient (24). In severe cases of LP, particularly in patients who are immunocompromised or have failed initial treatment, the treatment duration should be extended to 4–6 weeks (24, 33). While there is still uncertainty about the ideal treatment for cavitary LP, studies have suggested that both macrolides and quinolones have shown efficacy against cavitary disease (34). In general, anti-infective treatment for cavitary LP is recommended for at least 4 weeks or until the lung cavity disappears (35).
4 Conclusion
In summary, this case illustrates an unusual form of multiple cavities in both lungs in an immunocompetent patient with LP. mNGS is an advanced diagnostic tool that can be valuable in confirming the diagnosis of Legionella infection and guiding treatment decisions. Either a fluoroquinolone or a macrolide is recommended as a first-line antibiotic treatment for LP, including cavitary LP. Although rare, LP should be considered in the differential diagnosis of cavitary lung diseases, even in immunocompetent patients.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of Shandong First Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZG: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing, Software, Visualization. AZ: Conceptualization, Investigation, Writing – original draft. XL: Investigation, Writing – original draft. YJ: Software, Writing – original draft. SY: Investigation, Writing – original draft. DL: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The present study was supported by the Collaborative Innovation Center for Intelligent Molecules with Multi-effects and Nanomedicine (Grant No. 2019-01), Shandong Province, China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1329381/full#supplementary-material
References
1.
Barimani MJ . Legionella: an uncommon cause of community-acquired pneumonia. JAAPA. (2022) 35:38–42. doi: 10.1097/01.Jaa.0000873792.00538.78
2.
El-Ebiary M Sarmiento X Torres A Nogué S Mesalles E Bodí M et al . Prognostic factors of severe Legionella pneumonia requiring admission to ICU. Am J Respir Crit Care Med. (1997) 156:1467–72. doi: 10.1164/ajrccm.156.5.97-04039
3.
Cunha BA Burillo A Bouza E . Legionnaires’ disease. Lancet. (2016) 387:376–85. doi: 10.1016/s0140-6736(15)60078-2
4.
Mittal S Singh AP Gold M Leung AN Haramati LB Katz DS . Thoracic imaging features of Legionnaire’s disease. Infect Dis Clin N Am. (2017) 31:43–54. doi: 10.1016/j.idc.2016.10.004
5.
Zhang J Gao L Zhu C Jin J Song C Dong H et al . Clinical value of metagenomic next-generation sequencing by Illumina and Nanopore for the detection of pathogens in bronchoalveolar lavage fluid in suspected community-acquired pneumonia patients. Front Cell Infect Microbiol. (2022) 12:1021320. doi: 10.3389/fcimb.2022.1021320
6.
Li S Jiang W Wang CY Weng L Du B Peng JM . A case of disseminated Legionnaires’ disease: the value of metagenome next-generation sequencing in the diagnosis of legionnaires. Front Med. (2022) 9:955955. doi: 10.3389/fmed.2022.955955
7.
Li S Tong J Li H Mao C Shen W Lei Y et al . L. pneumophila infection diagnosed by tNGS in a lady with lymphadenopathy. Infect Drug Resist. (2023) 16:4435–42. doi: 10.2147/idr.S417495
8.
McBee DB Mizu R Hamdi AM . A case of severe, difficult-to-diagnose Legionnaires’ disease in a young welder. Cureus. (2023) 15:e42250. doi: 10.7759/cureus.42250
9.
Fraser TG Zembower TR Lynch P Fryer J Salvalaggio PR Yeldandi AV et al . Cavitary Legionella pneumonia in a liver transplant recipient. Transpl Infect Dis. (2004) 6:77–80. doi: 10.1111/j.1399-3062.2004.00053.x
10.
Yi H Fang J Huang J Liu B Qu J Zhou M . Legionella pneumophila as cause of severe community-acquired pneumonia, China. Emerg Infect Dis. (2020) 26:160–2. doi: 10.3201/eid2601.190655
11.
Senécal JL St-Antoine P Béliveau C . Legionella pneumophila lung abscess in a patient with systemic lupus erythematosus. Am J Med Sci. (1987) 293:309–14. doi: 10.1097/00000441-198705000-00005
12.
Lewin S Brettman LR Goldstein EJ Holzman RS Devila H Taubman F et al . Legionnaires’ disease. A cause of severe abscess-forming pneumonia. Am J Med. (1979) 67:339–42. doi: 10.1016/0002-9343(79)90411-x
13.
Guy SD Worth LJ Thursky KA Francis PA Slavin MA . Legionella pneumophila lung abscess associated with immune suppression. Intern Med J. (2011) 41:715–21. doi: 10.1111/j.1445-5994.2011.02508.x
14.
Heine S Fuchs A von Müller L Krenn T Nemat S Graf N et al . Legionellosis must be kept in mind in case of pneumonia with lung abscesses in children receiving therapeutic steroids. Infection. (2011) 39:481–4. doi: 10.1007/s15010-011-0131-7
15.
Jacox RF Stuard ID . Legionnaires’ disease in a patient with systemic lupus erythematosus. Arthritis Rheum. (1978) 21:975–7. doi: 10.1002/art.1780210816
16.
Du R Feng Y Wang Y Huang J Tao Y Mao H . Metagenomic next-generation sequencing confirms the diagnosis of Legionella pneumonia with rhabdomyolysis and acute kidney injury in a limited resource area: a case report and review. Front Public Health. (2023) 11:1145733. doi: 10.3389/fpubh.2023.1145733
17.
Dowling JN Kroboth FJ Karpf M Yee RB Pasculle AW . Pneumonia and multiple lung abscesses caused by dual infection with Legionella micdadei and Legionella pneumophila. Am Rev Respir Dis. (1983) 127:121–5. doi: 10.1164/arrd.1983.127.1.121
18.
Schindel C Siepmann U Han S Ullmann AJ Mayer E Fischer T et al . Persistent Legionella infection in a patient after bone marrow transplantation. J Clin Microbiol. (2000) 38:4294–5. doi: 10.1128/jcm.38.11.4294-4295.2000
19.
Palusinska-Szysz M Janczarek M . Innate immunity to Legionella and toll-like receptors—review. Folia Microbiol. (2010) 55:508–14. doi: 10.1007/s12223-010-0084-8
20.
Friedman H Yamamoto Y Newton C Klein T . Immunologic response and pathophysiology of Legionella infection. Semin Respir Infect. (1998) 13:100–8. PMID:
21.
Cunha LD Zamboni DS . Recognition of Legionella pneumophila nucleic acids by innate immune receptors. Microbes Infect. (2014) 16:985–90. doi: 10.1016/j.micinf.2014.08.008
22.
Pierre DM Baron J Yu VL Stout JE . Diagnostic testing for Legionnaires’ disease. Ann Clin Microbiol Antimicrob. (2017) 16:59. doi: 10.1186/s12941-017-0229-6
23.
Mercante JW Winchell JM . Current and emerging Legionella diagnostics for laboratory and outbreak investigations. Clin Microbiol Rev. (2015) 28:95–133. doi: 10.1128/cmr.00029-14
24.
Cunha BA . The atypical pneumonias: clinical diagnosis and importance. Clin Microbiol Infect. (2006) 12:12–24. doi: 10.1111/j.1469-0691.2006.01393.x
25.
Zhu N Zhou D Yuan R Ruzetuoheti Y Li J Zhang X et al . Identification and comparison of Chlamydia psittaci, Legionella and Mycoplasma pneumonia infection. Clin Respir J. (2023) 17:384–93. doi: 10.1111/crj.13603
26.
Maurer FP Christner M Hentschke M Rohde H . Advances in rapid identification and susceptibility testing of bacteria in the clinical microbiology laboratory: implications for patient care and antimicrobial stewardship programs. Infect Dis Rep. (2017) 9:6839. doi: 10.4081/idr.2017.6839
27.
Gu W Miller S Chiu CY . Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol. (2019) 14:319–38. doi: 10.1146/annurev-pathmechdis-012418-012751
28.
Levy ML Le Jeune I Woodhead MA Macfarlaned JT Lim WS . Primary care summary of the British Thoracic Society guidelines for the management of community acquired pneumonia in adults: 2009 update. Endorsed by the Royal College of General Practitioners and the Primary Care espiratory Society UK. Prim Care Respir J. (2010) 19:21–7. doi: 10.4104/pcrj.2010.00014
29.
Schubert S Dalhoff A Stass H Ullmann U . Pharmacodynamics of moxifloxacin and levofloxacin simulating human serum and lung concentrations. Infection. (2005) 33:15–21. doi: 10.1007/s15010-005-8203-1
30.
Gómez-Lus R Adrián F del Campo R Gómez-Lus P Sánchez S García C et al . Comparative in vitro bacteriostatic and bactericidal activity of trovafloxacin, levofloxacin and moxifloxacin against clinical and environmental isolates of Legionella spp.Int J Antimicrob Agents. (2001) 18:49–54. doi: 10.1016/s0924-8579(01)00339-9
31.
Jonas D Engels I Friedhoff C Spitzmüller B Daschner FD Frank U . Efficacy of moxifloxacin, trovafloxacin, clinafloxacin and levofloxacin against intracellular Legionella pneumophila. J Antimicrob Chemother. (2001) 47:147–52. doi: 10.1093/jac/47.2.147
32.
Garau J Fritsch A Arvis P Read RC . Clinical efficacy of moxifloxacin versus comparator therapies for community-acquired pneumonia caused by Legionella spp.J Chemother. (2010) 22:264–6. doi: 10.1179/joc.2010.22.4.264
33.
Fields BS Benson RF Besser RE . Legionella and Legionnaires’ disease: 25 years of investigation. Clin Microbiol Rev. (2002) 15:506–26. doi: 10.1128/cmr.15.3.506-526.2002
34.
Domingo C Roig J Planas F Bechini J Tenesa M Morera J . Radiographic appearance of nosocomial Legionnaires’ disease after erythromycin treatment. Thorax. (1991) 46:663–6. doi: 10.1136/thx.46.9.663
35.
O’Reilly KM Urban MA Barriero T Betts RF Trawick DR . Persistent culture-positive Legionella infection in an immunocompromised host. Clin Infect Dis. (2005) 40:e87–9. doi: 10.1086/429826
Summary
Keywords
Legionella, Legionella pneumophila , immunocompetent patient, Legionella pneumonia, pulmonary cavity, metagenomic next-generation sequencing
Citation
Guo Z, Zuo A, Liu X, Jiang Y, Yang S and Lu D (2024) Multiple pulmonary cavities in an immunocompetent patient: a case report and literature review. Front. Med. 11:1329381. doi: 10.3389/fmed.2024.1329381
Received
28 October 2023
Accepted
16 February 2024
Published
27 February 2024
Volume
11 - 2024
Edited by
Talat Kilic, İnönü University, Türkiye
Reviewed by
Ronald Balczon, University of South Alabama, United States
Hilal Ermis, İnönü University, Türkiye
Updates
Copyright
© 2024 Guo, Zuo, Liu, Jiang, Yang and Lu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Degan Lu, deganlu@126.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.