- 1Department of Nuclear Medicine, Peking University First Hospital, Beijing, China
- 2Department of Traditional Chinese Medicine, The Seventh People's Hospital of Chongqing, Chongqing, China
- 3Department of Pathology, Peking University First Hospital, Beijing, China
Background: One of the exceptionally rare forms of non-Hodgkin’s lymphoma (NHL) is primary cardiac lymphoma (PCL). The principal clinical manifestation in patients with PCL involves cardiac symptoms resulting from myocardial infiltration by lymphoma, including arrhythmias, heart failure, and chest pain. 18F-FDG PET/CT serves as a reliable and indispensable imaging modality for assessing clinically staging NHL.
Case report: We present a rare case involving a 72-year-old woman diagnosed with primary intracardiac diffuse large B-cell lymphoma. For further staging, the patient underwent 18F-FDG PET/CT, revealing multiple nodular soft tissue density lesions in the heart and pericardium exhibiting increased FDG metabolism (SUVmax = 12.1). The supradiaphragmatic and infradiaphragmatic segments of the inferior vena cava exhibited irregular morphology with localized nodular changes and increased FDG metabolism in the surrounding area (SUVmax = 9.7). Additionally, multiple enlarged lymph nodes were identified in the left axilla, mediastinum, and adjacent to the abdominal aorta, displaying heterogeneous FDG uptake with an SUVmax of 9.3, indicating lymphoma involvement. The above imaging findings suggested that the mass was a PCL. Hence, the patient underwent a combination of chemotherapy and immunotherapy using R-CDOP (rituximab, cyclophosphamide, liposomal doxorubicin, vincristine, and prednisone). Following two courses of treatment within a span of 2 months, there was a partial remission observed in the cardiac lymphoma and the enlarged lymph nodes.
Conclusion: The case elucidated in this report contributes to an enhanced understanding of the disease for clinicians, with 18F-FDG PET/CT providing comprehensive insights into the extent of cardiac involvement, as well as the engagement of extracardiac organs and pathologic lymph nodes. The 18F-FDG PET/CT examination not only visually delineates the lesion’s location and extent but also serves as a cornerstone for clinical tumor staging, offering valuable support for treatment monitoring and subsequent follow-up.
Introduction
Cardiac and pericardial involvement by malignant lymphoma constitutes approximately 1% of cardiac tumors and 0.5% of extra-nodal non-Hodgkin’s lymphoma (NHL), representing a rare phenotype. Diffuse large B-cell lymphoma (DLBCL) is the most common type, but other cell types are also observed, such as T-cell lymphomas (1, 2). Epidemiological characteristics of primary cardiac lymphoma (PCL), according to a recent systematic review conducted in 2020, revealed a mean age of 62, a male preponderance, and a higher prevalence of cases in the Asian region followed by the European region (48% vs. 27%) (3).
One of the exceptionally rare forms of NHL is PCL. Defined by the WHO in 2015, PCL presents as a substantial lymphoma mass involving the heart and/or pericardium, with or without secondary lesions in other areas of the body. The incidence of PCL is a mere 0.056%, with only 10% identified as malignant, accounting for just 1% (4–6). While the right atrium and right ventricle are the most common sites of involvement, occurrences in the left ventricle have also been reported (4, 7). PCL exhibits a broad age range, primarily affecting the elderly and displaying a higher prevalence in men (8); it is more commonly observed in immunocompromised patients (9). The predominant symptoms are cardiac-related, contingent on the anatomical location within the heart. These include arrhythmias resulting from compressed cardiac conducting systems and manifestations of heart failure due to intra-cardiac blood flow obstruction. In some instances, PCL may even mimic myocardial ischemia or myocardial infarction (10). Petrich et al. (4) report constitutional symptoms, heart failure, and pericardial effusion as the most prevalent presenting symptoms and signs of PCL. Lymphoma-related symptoms, such as fever, night sweats, progressive weight loss, and other general systemic manifestations, are uncommon in PCL patients. Third-degree AV block constitutes an infrequent presentation of PCL. Complications may arise from mass effect, local invasion, or embolization (11).
The heart lacks lymphoid nodes, and PCL is believed to originate from the drainage of the epicardial lymph nodes (12). Although the pathogenesis of PCL remains elusive, it is potentially linked to recurrent infections and immune dysfunction, encompassing conditions like human immunodeficiency virus (HIV) infection, Epstein–Barr virus infection, congenital immunodeficiency, and allogeneic bone marrow and solid organ transplantation (2). Diagnosis hinges solely on histopathology and immunohistochemistry for precise identification, classification, and evaluation of proliferative activity (3, 5). Vogl et al. reported a successful CT-guided puncture of the cardiac tumor, enabling a prompt diagnosis of PCL and initiation of therapy without complications (13). Liquid cytology of cardiac or pleural effusion proves highly valuable. It’s essential to note that pericardial effusion cannot be directly attributed to pericardial involvement due to a lack of specific details regarding effusion detection, as cardiac insufficiency may also contribute (3). While DLBCL is the most prevalent histopathology, documented cases include Burkitt lymphoma, T-cell lymphoma, and plasmablastic lymphoma (14). In our case, cytologic analysis of drained pericardial fluid revealed DLBCL, with immunohistochemical staining confirming positivity for CD5. Given the patient’s comorbidities, including coronary artery disease and diabetes, no open chest biopsy was performed.
PCL is an extremely rare malignancy, constituting an oncologic emergency that proves fatal within a few months unless diagnosed and treated promptly (15). The principal clinical manifestation in patients with PCL involves cardiac symptoms resulting from myocardial infiltration by lymphoma, including arrhythmias, heart failure, and chest pain (4, 16). As of now, there is no specific biomarker for the early diagnosis of PCL. 2-Deoxy-2-[fluorine-18]-fluoro-D-glucose (18F-FDG) positron emission tomography combined with computed tomography (PET/CT) serves as a reliable and indispensable imaging modality for assessing clinically staging of DLBCL. Despite the rarity of primary cardiac lymphoma, some reports detailing the findings of 18F-FDG PET/CT imaging have been presented. Here, we present a rare case involving a 72-year-old woman diagnosed with primary intracardiac DLBCL, referred for 18F-FDG PET/CT imaging for staging. According to the World Health Organization (WHO), PCL can be diagnosed if it meets one of the following criteria: (i) primary lymphoma of the heart or pericardium; (ii) lymphoma with the initial cardiac-related symptoms; and (iii) lymphoma dominated by a cardiac mass (17). However, in the early stages, its clinical manifestations do not significantly differ from ordinary chest and heart diseases, often leading to limited attention being paid to the disease when patients present with symptoms such as chest pain or dyspnea (18).
In our comprehensive literature search on the PubMed database spanning from 2009 to 2023, utilizing keywords related to diffuse large B-cell lymphoma and 18F-FDG PET/CT, we identified a total of 20 available case reports. The summarized case reports are presented in Table 1.
Case presentation
A 72-year-old woman presented with chest tightness and breath-holding symptoms a month before seeking medical attention, aggravated by physical activity and hindering her ability to recline at night. The patient had a history of coronary artery disease and diabetes mellitus; however, she has no family history of genetic disorders or tumors. Acute coronary syndrome was initially ruled out. Laboratory tests revealed CK-MB level at 1.5 ng/mL (normal range: 0–5 ng/mL), high-sensitivity troponin I at 19.3 ng/L (normal range: 0–0.04 ng/mL), and natriuretic peptide at 127.00 pg./mL (normal range: 0–100 pg./mL). Blood gas analysis indicated hypoxemia. The patient initiated diuretic treatment with oral furosemide. However, her breath-holding symptoms intensified after 2 weeks, leading to her hospital admission. An electrophysiology study showed third-degree atrioventricular block. A chest CT scan disclosed an enlarged and dysmorphic heart, displaying multiple nodular and clumped slightly hypodense foci in the heart and pericardium, along with effusions in the pericardium and pleural cavity (Figures 1A,B). The diagnostic imaging physician suspected a malignant tumor of interlobar origin in the heart. Subsequent tumor marker panel analysis revealed elevated CA125 at 203 U/mL (normal range: 0–35 U/mL), CYFRA21-1 at 5.40 ng/mL (normal range: 0–3.3 ng/mL), and NSE at 17.75 ng/mL (normal range: 0–16.3 ng/mL). Cytologic examination of pericardial and pleural effusions exhibited microscopic cells characterized by large, deeply stained nuclei, scanty cytoplasm, moderate cellular anisotropy, visible small nucleoli, and distinct nuclear fission images and apoptosis (Figure 2A). Immunohistochemical staining demonstrated positive expression of CD5, CD10, CD20, MUM1, and Bcl2 (Figures 2B–F). In addition, Ki-67 was observed to be positive in 40% of the tumor cells. In situ hybridization remained negative for Epstein–Barr virus (EBV)-encoded small RNAs (EBERs). The pathological diagnosis of cell smears and cell wax blocks in pericardial and pleural effusion confirms DLBCL.
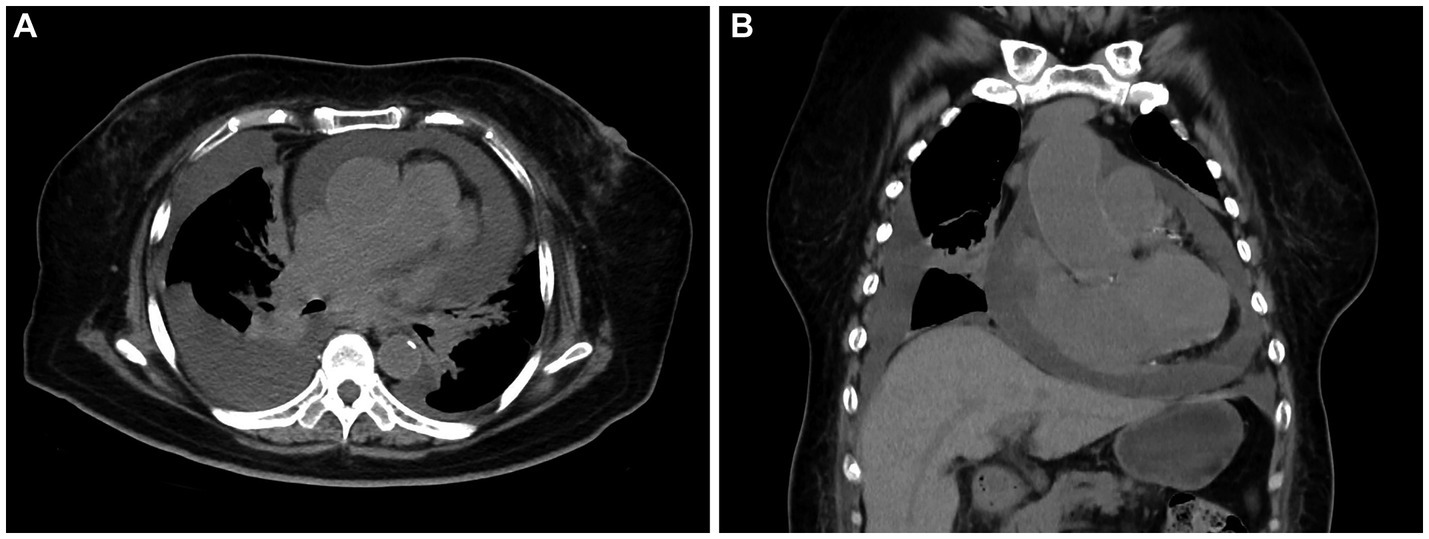
Figure 1. Computed tomography (CT) images of primary cardiac lymphoma (PCL). Transverse (A) and coronal (B) images revealed multiple nodular and clumped slightly hypodense foci in the heart and pericardium, along with effusions in the pericardium and pleural cavity.
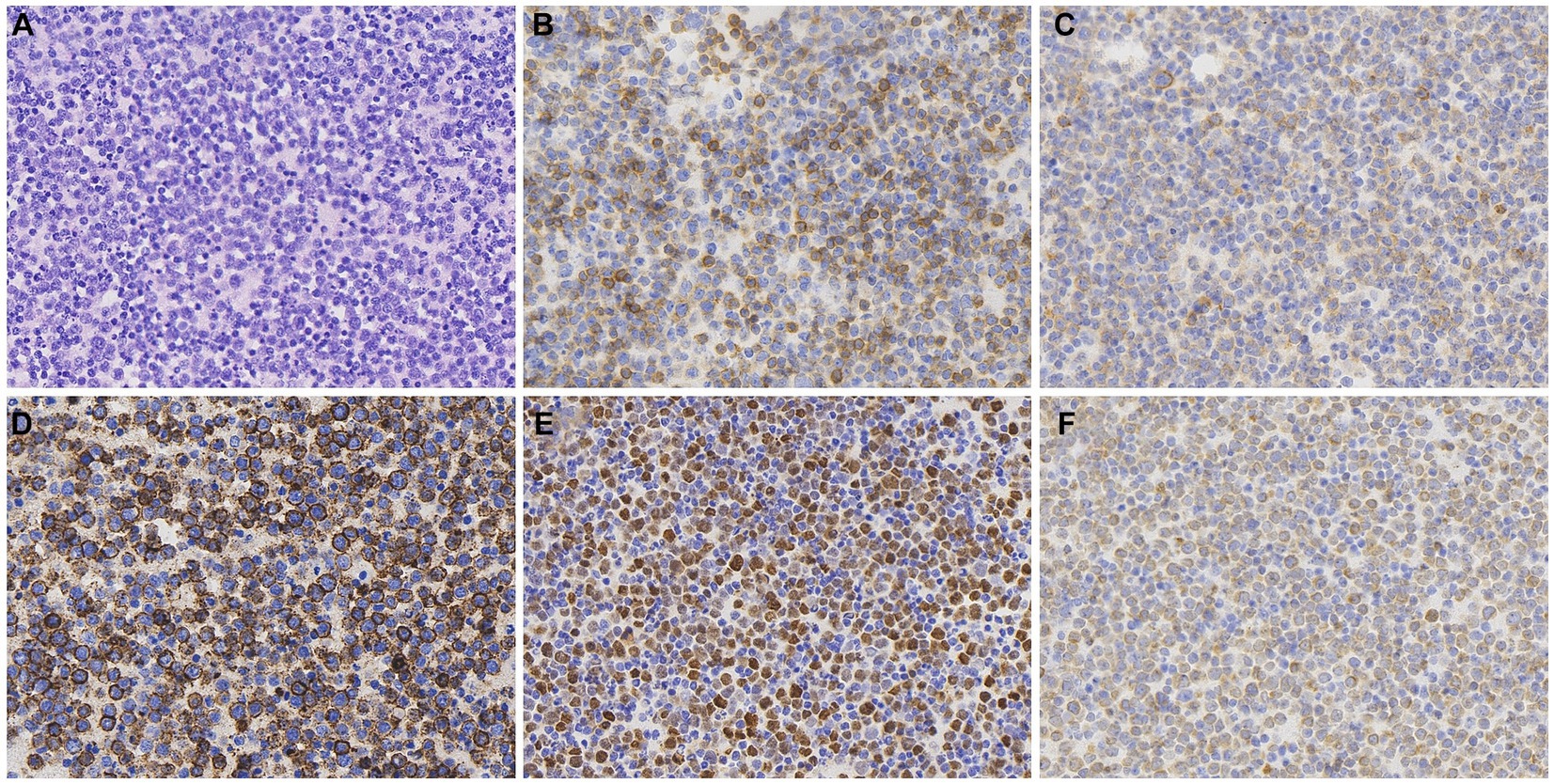
Figure 2. Histopathological and immunohistochemical images of PCL. Hematoxylin–eosin (HE) staining (magnification ×400) of the lesion (A) revealed microscopic cells characterized by large, deeply stained nuclei, scanty cytoplasm, moderate cellular anisotropy, visible small nucleoli, and distinct nuclear fission images and apoptosis. Immunohistochemical staining (400×) showed tumor cells were positive for the expression of CD5 (B), CD10 (C), CD20 (D), MUM1 (E), and Bcl-2 (F).
For further staging, the patient underwent an 18F-FDG PET/CT scan. The patient was instructed to follow a high-fat low-carbohydrate diet for 24 h prior to the 18F-FDG PET/CT study and to avoid strenuous exercise (34, 35). Additionally, a low carbohydrate meal was advised before starting the 6-h fasting period to maintain blood glucose levels below 11.1 mmol/L. In this examination, 3,000 units of unfractionated heparin was intravenously administered 15 min before FDG administration. The 18F-FDG PET/CT scan, performed utilizing Philips GXL-16 PET/CT machines, was conducted 60 min after the intravenous administration of 18F-FDG. To ensure optimal hydration, patients were instructed to consume 1,000 mL of water and empty their bladders after 60 min of quiet rest. Routine scans were conducted from the head to mid-thigh, with separate acquisitions of the head and torso. In alignment with the European Association of Nuclear Medicine Research Limited (EARL) standards, we reconstructed the SUVmax for improved reproducibility and comparability (36, 37). This revealed multiple nodular soft tissue density lesions in the heart and pericardium, exhibiting increased FDG metabolism (SUVmax = 12.1). The supradiaphragmatic and infradiaphragmatic segments of the inferior vena cava exhibited irregular morphology with localized nodular changes and increased FDG metabolism in the surrounding area (SUVmax = 9.7). Additionally, multiple enlarged lymph nodes were identified in the mediastinum, left axilla, and near the left kidney, displaying heterogeneous FDG uptake with an SUVmax of 9.3, indicating lymphoma involvement (Figure 3). The above imaging findings suggested that the mass was a PCL.
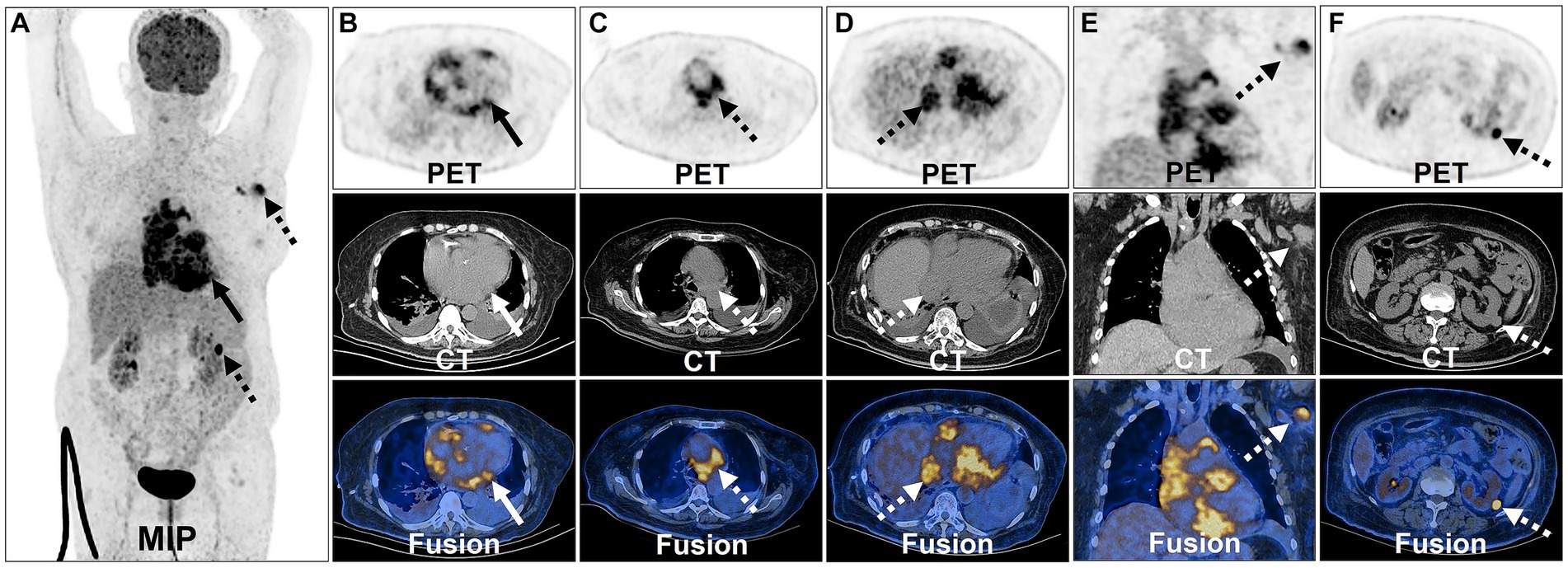
Figure 3. 18F-FDG PET/CT images of PCL. The anteroposterior 3-dimensional maximum intensity projection image (MIP) demonstrated FDG-avid lesions on the heart, pericardium (long arrows) and multiple lymph nodes (dash arrows) (A). Transverse and coronal images showed multiple nodular soft tissue density lesions in the heart and pericardium exhibiting increased FDG metabolism (B). Enlarged lymph nodes were identified in the mediastinum displaying heterogeneous FDG uptake (C). The supradiaphragmatic segments of the inferior vena cava exhibited irregular morphology with localized nodular changes and increased FDG metabolism in the surrounding area (D). Enlarged lymph node was identified in the left axilla displaying heterogeneous FDG uptake (E). Enlarged lymph node was identified near the left kidney displaying heterogeneous FDG uptake (F).
Hence, the patient underwent a combination of chemotherapy and immunotherapy using R-CDOP (rituximab, cyclophosphamide, liposomal doxorubicin, vincristine, and prednisone). Following two courses of treatment within a span of 2 months, there was a partial remission observed in the cardiac lymphoma and the enlarged lymph nodes. She was closely monitored throughout the chemo-immunotherapy, with an established emergency plan in place to address potential complications, including arrhythmia and cardiac rupture.
Discussion
Clinical imaging examinations frequently provide crucial insights for the differential diagnosis of a cardiac mass. On CT, PCL typically manifests as multiple iso-attenuating to hypo-attenuating masses infiltrating the myocardium (15, 38). Noteworthy, CT reveals specific anatomical findings of unobstructed coronary arteries. In the case presented in this study, while CT served as the initial diagnostic modality for PCL, it fell short in revealing the details of myocardial and pericardial infiltration. Nevertheless, it did contribute additional information regarding extra-cardiac involvement. Presently, magnetic resonance imaging (MRI) stands as the preferred tomographic modality for investigating PCL, effectively distinguishing the mass from normal myocardial tissue through tissue characterization sequences (39). Beyond its radiation-free nature, MRI boasts advantages over CT, including high contrast and spatial resolution, making it the most useful modality for precisely defining the anatomy of the lesion. Kaida et al. (19) reported PCL to exhibit low signal on T1WI and high signal on both T2WI and fat suppression T2WI. Regrettably, our patient did not undergo a cardiac MRI examination.
18F-FDG PET/CT stands out as a noninvasive tool for discerning the metabolic activity of tumors, proving exceptionally beneficial in delineating the staging of malignant lymphoma, tracking relapse, and evaluating therapeutic response (40, 41). When a patient is referred for evaluation of a heart lesion or an area very close to the myocardium, additional dietary recommendations can be helpful. While numerous options exist to reduce normal glucose uptake by the myocardium, common recommendations may include advising the patient to follow a low-carbohydrate diet for 24 h prior to the 18F-FDG PET/CT study, or at the very least, consume a low-carbohydrate meal before the 6-h fasting period preceding the study (34, 35). A low-carbohydrate diet helps transition the myocardium from using glucose as its primary energy source to utilizing fatty acids, thereby reducing glucose uptake by the myocardium. Physiological FDG accumulation in the cardiac wall often interferes with the evaluation of cardiac lesions. Ishimaru et al. (42) underscored the significant role of heparin administration before FDG injection in detecting cardiac sarcoidosis. Heparin induces the release of free fatty acids into the circulation and serves to reduce the physiological FDG uptake by the myocardium (43, 44). Plasma glucose levels must be measured before administering FDG. If the plasma glucose level is 11 mmol/L (about 200 mg/dL) or higher, the 18F-FDG PET/CT study should be rescheduled or the patient excluded based on individual circumstances and the nature of the study being conducted. For patients with Hodgkin and non-Hodgkin lymphoma, 18F-FDG PET/CT has emerged as the standard procedure for staging, monitoring, and restaging the disease. During therapy assessment at mid-treatment and post-completion of chemotherapy, the Deauville score (DS) is recommended for distinguishing between responders and non-responders (45, 46). The DS is a clinical tool used to categorize patients with lymphoma based on the comparison between lesion and reference organ uptake of 18F-FDG, thus reflecting their disease status.
Notably, the existing literature on PCL predominantly comprises case reports, emphasizing its increased metabolic state with substantial FDG uptake (20). PCL may present as either multiple nodular lesions or extensive soft tissue masses (19, 21). The reported SUVmax range for cardiac lymphoma spans from 9.24 to 37 (5, 7, 10, 11, 14, 19–33). Kikuchi et al. (47) underscored a significantly elevated SUV in PCL patients compared to those with other cardiac malignant tumors (e.g., metastases, sarcoma) and benign tumors, demonstrating a lack of overlap. When imaging reveals increased 18F-FDG uptake in multiple cardiac tumors alongside significant pericardial effusion, PCL should be a primary consideration. In the case under scrutiny, 18F-FDG PET/CT provided comprehensive insights into both the extent of cardiac involvement and the absence of extracardiac organ participation. Another pivotal role of 18F-FDG PET/CT in PCL management lies in its ability to monitor treatment response, a critical aspect in restaging numerous common lymphoma types (48). Adequate repeatability and reproducibility are essential for the clinical management of patients and the use of FDG PET/CT within multicenter trials. The new developments in PET/CT have been shown to affect the SUVmax in lesions (49). Consequently, it is conceivable that patients examined on various PET/CT scanners, utilizing different hardware, software, and acquisition parameters, may yield varied SUVmax. In future studies, we recommend that researchers adopt scan acquisition and image reconstruction protocols that are consistent with the EARL Coordination Program (36, 37). This approach will not only enhance the reproducibility and comparability of our findings but also facilitate the meaningful comparison of results across different PET/CT scanners.
In contrast, during the restaging of lymphoma, MRI may persist in exhibiting contrast enhancement, potentially linked to residual inflammation or scar, even when the tumor is no longer metabolically active. Consequently, PET/CT emerged as the preferred modality for assessing treatment response in this scenario. To ensure unequivocal findings, the post-chemotherapy scan was conducted after a 12-h abstinence from a high-fat low-carbohydrate diet, aiming to achieve a favorable tumor-to-background contrast and mitigate potential confounding physiological myocardial uptake in the 18F-FDG PET/CT image. Differential diagnoses encompass benign myxoma, the most prevalent type of cardiac tumor, and angiosarcoma, the predominant malignant heart tumor typically located in the left cavities (50–52). While imaging examinations are valuable for detecting and characterizing cardiac masses, arriving at a definitive diagnosis remains challenging. Patient treatment and prognosis hinge significantly on the tissue type and biological behavior of the tumor.
The primary cause of mortality in PCL was heart failure, succeeded by sepsis and the progression of lymphoma. Less frequently observed causes included arrhythmia, embolism, cerebrovascular accidents, and sudden cardiac death (4). Existing strategies for PCL treatment encompass surgery, chemotherapy, radiotherapy, and immunotherapy. Complete resection was deemed unfeasible in our report due to the tumor’s extensive involvement of the pericardium and diffuse infiltration of the myocardium. Early diagnosis and the precise selection of chemotherapy and immunotherapy, guided by cardiac imaging and pathological examination, may significantly improve the prognosis of PCL in atypical locations. Prognostic risk factors include extracardiac involvement, immune deficiency, arrhythmias such as complete atrioventricular block, and left heart involvement (3). The impact of surgical interventions proved limited, necessitating careful consideration of surgical decisions, especially in patients diagnosed with cardiac lymphoma (53). In cases where clinical and radiological suspicions of PCL exist, aggressive diagnostic procedures should be implemented, and therapy initiated before irreversible cardiac damage ensues.
The CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) regimen remains the conventional choice for treating DLBCL. However, the prognosis of PCL remains unfavorable; Rolla et al. (54) reported on 66 PCL patients, among whom 31 underwent a CHOP-based chemotherapy regimen, resulting in a median survival mean of 7 months. With the widespread use of rituximab (R), the integration of immunotherapy with chemotherapy (R-CHOP regimen) has become the preferred treatment for DLBCL, displacing surgical approaches, regardless of the tumor stage (55). Side effects, observed in approximately 10% of cases, include tumor lysis syndrome and sepsis. Additionally, chemotherapy carries the risk of significant thromboembolism, cardiac wall perforation, ventricular septal rupture, life-threatening arrhythmias, and pericardial effusion. While surgical management does not constitute the primary treatment, prompt surgical debulking is indicated in patients with acute and severe presentations, especially those experiencing rapidly progressing heart failure. Therefore, clinicians must maintain a heightened vigilance index and provide timely interventions to optimize patient prognosis.
Conclusion
In conclusion, PCL is a rare entity in clinical practice, often associated with a poor prognosis. The advent of 18F-FDG PET/CT has marked a significant stride, expanding the potential evaluation capabilities of conventional imaging (MRI and CT). When high 18F-FDG uptake is evident in multiple cardiac tumors, accompanied by a considerable pericardial effusion, PCL should be considered a primary diagnostic consideration. The case elucidated in this report contributes to an understanding of the disease for clinicians, with 18F-FDG PET/CT providing comprehensive insights into the extent of cardiac involvement, as well as the engagement of extracardiac organs and pathologic lymph nodes. The 18F-FDG PET/CT examination not only visually delineates the lesion’s location and extent but also serves as a cornerstone for clinical tumor staging, offering valuable support for treatment monitoring and subsequent follow-up.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The patients provided their written informed consent to participate in this study. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author contributions
WH: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. ZZ: Investigation, Writing – original draft. YZ: Data curation, Writing – original draft. YQ: Conceptualization, Data curation, Writing – original draft. YP: Data curation, Writing – original draft. QY: Conceptualization, Writing – review & editing. WW: Conceptualization, Funding acquisition, Software, Validation, Writing – original draft. LK: Conceptualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82171970), the Beijing Science Foundation for Distinguished Young Scholars (JQ21025), the Beijing Municipal Science & Technology Commission (Z221100007422027), and National High Level Hospital Clinical Research Funding (Interdisciplinary Research Project of Peking University First Hospital, 2023IR17).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Johri, A, Baetz, T, Isotalo, PA, Nolan, RL, Sanfilippo, AJ, and Ropchan, G. Primary cardiac diffuse large B cell lymphoma presenting with superior vena cava syndrome. Can J Cardiol. (2009) 25:e210–2. doi: 10.1016/s0828-282x(09)70110-2
2. Hsueh, S-C, Chung, M-T, Fang, R, Hsiung, M-C, Young, M-S, and Lu, H-F. Primary cardiac lymphoma. J Chin Med Assoc. (2006) 69:169–74. doi: 10.1016/S1726-4901(09)70200-X
3. Chen, H, Qian, S, Shi, P, Liu, L, and Yang, F. A presentation, treatment, and survival analysis of primary cardiac lymphoma cases reported from 2009 to 2019. Int J Hematol. (2020) 112:65–73. doi: 10.1007/s12185-020-02881-2
4. Petrich, A, Cho, SI, and Billett, H. Primary cardiac lymphoma: an analysis of presentation, treatment, and outcome patterns. Cancer. (2011) 117:581–9. doi: 10.1002/cncr.25444
5. Venugopala, D, Dsouza, NV, Acharya, V, Rai, M, Venkataramana, CG, and Boussios, S. Constrictive pericarditis-a cloak camouflaging lymphoma. Hematol Rep. (2023) 15:166–71. doi: 10.3390/hematolrep15010017
6. Gowda, RM, and Khan, IA. Clinical perspectives of primary cardiac lymphoma. Angiology. (2003) 54:599–604. doi: 10.1177/000331970305400510
7. Thiagaraj, A, Kalamkar, P, Rahman, R, Farah, V, and Poornima, I. An unprecedented case report of primary cardiac lymphoma exclusive to left ventricle: a diagnostic and therapeutic challenge. Eur Heart J Case Rep. (2018) 2:yty029. doi: 10.1093/ehjcr/yty029
8. Yin, K, Brydges, H, Lawrence, KW, Wei, Y, Karlson, KJ, McAneny, DB, et al. Primary cardiac lymphoma. J Thorac Cardiovasc Surg. (2022) 164:573–580.e1. doi: 10.1016/j.jtcvs.2020.09.102
9. Bulum, J, Banfić, L, Strozzi, M, Aurer, I, and Jelasić, D. Primary cardiac lymphoma presenting as atrial flutter and total heart block. Heart Vessel. (2007) 22:52–4. doi: 10.1007/s00380-006-0924-2
10. Alansari, YE, Yudhistiara, B, Michel, RP, and Elstein, E. Case report: multimodality imaging to diagnose cardiac diffuse large B-cell lymphoma. Eur Heart J Case Rep. (2021) 5:ytab172. doi: 10.1093/ehjcr/ytab172
11. Lee, JC, Platts, DG, Huang, Y-TT, and Slaughter, RE. Positron emission tomography combined with computed tomography as an integral component in evaluation of primary cardiac lymphoma. Clin Cardiol. (2010) 33:E106–8. doi: 10.1002/clc.20725
12. Economopoulos, T, Asprou, N, Stathakis, N, Papageorgiou, E, Dervenoulas, J, Xanthaki, K, et al. Primary extranodal non-Hodgkin’s lymphoma in adults: clinicopathological and survival characteristics. Leuk Lymphoma. (1996) 21:131–6. doi: 10.3109/10428199609067590
13. Vogl, TJ, Martin, SS, Koch, V, Scholtz, J-E, Booz, C, Leistner, DM, et al. Letter to the editor: CT guided biopsy of a right ventricle primary cardiac lymphoma-a case report. Cardiovasc Intervent Radiol. (2023) 46:970–2. doi: 10.1007/s00270-023-03482-2
14. Imataki, O, Kubo, H, Fujita, H, and Uemura, M. Metabolic steal of the myocardium by primary cardiac lymphoma. Case Rep Oncol. (2023) 16:7–12. doi: 10.1159/000527638
15. Coulier, B, Colin, GC, Tourmous, H, Floris, N, Van Eeckhout, P, and Scavée, C. Imaging features of primary cardiac lymphoma. Diagn Interv Imaging. (2018) 99:115–7. doi: 10.1016/j.diii.2017.05.013
16. Jeudy, J, Burke, AP, and Frazier, AA. Cardiac lymphoma. Radiol Clin North Am. (2016) 54:689–710. doi: 10.1016/j.rcl.2016.03.006
17. Burke, A, and Tavora, F. The 2015 WHO classification of tumors of the heart and pericardium. J Thorac Oncol. (2016) 11:441–52. doi: 10.1016/j.jtho.2015.11.009
18. Carras, S, Berger, F, Chalabreysse, L, Callet-Bauchut, E, Cordier, J-F, Salles, G, et al. Primary cardiac lymphoma: diagnosis, treatment and outcome in a modern series. Hematol Oncol. (2017) 35:510–9. doi: 10.1002/hon.2301
19. Kaida, H, Kumode, T, Kimura, M, and Ishii, K. 18F-FDG PET/CT finding of primary cardiac lymphoma. Clin Nucl Med. (2020) 45:319–21. doi: 10.1097/RLU.0000000000002957
20. Qiang, Y, Zeng, K, Zhang, B, Guan, R, Liu, Y, Liu, Z, et al. Atypical location of primary cardiac lymphoma in the left heart with atypical clinical presentation: a case report and literature review. Front Surg. (2022) 9:1036519. doi: 10.3389/fsurg.2022.1036519
21. Erhamamcı, S, and Aslan, N. A rare case of primary cardiac diffuse large B-cell lymphoma imaged with 18F-FDG PET/CT. Mol Imaging Radionucl Ther. (2022) 31:148–50. doi: 10.4274/mirt.galenos.2021.60590
22. Tong, AK, Neo, SH, and Kok, TY. Disseminated lymphoma evolving into neurolymphomatosis during mid-cycle of chemotherapy detected by (18)F-FDG PET/CT. Ann Acad Med Singap. (2015) 44:545–7. doi: 10.47102/annals-acadmedsg.V44N11p545
23. Chang, L, Gong, C, Lu, H, Liu, Y, Kang, L, Chen, J, et al. Percutaneous intravenous catheter forceps biopsy in right atrial mass: two case reports and literature review. BMC Cardiovasc Disord. (2022) 22:63. doi: 10.1186/s12872-022-02507-x
24. Higgins, AY, Taylor, EP, Baldassarre, LA, Miller, EJ, and Rosenfeld, LE. Diagnosis of extensive myocardial infiltration by diffuse large B-cell lymphoma using 18F-fluorodeoxyglucose positron emission tomography (18-FDG PET). J Nucl Cardiol. (2018) 25:1869–71. doi: 10.1007/s12350-017-1099-1
25. Su, H-Y, Huang, H-L, Sun, C-M, Hou, S-M, and Chen, M-L. Primary cardiac lymphoma evaluated with integration of PET/CT and contrast-enhanced CT. Clin Nucl Med. (2009) 34:298–301. doi: 10.1097/RLU.0b013e31819e527c
26. Goldman, M, Matthews, R, Meng, H, Bilfinger, T, and Kort, S. Evaluation of cardiac involvement with mediastinal lymphoma: the role of innovative integrated cardiovascular imaging. Echocardiography. (2012) 29:E189–92. doi: 10.1111/j.1540-8175.2012.01746.x
27. Takaya, T, Takeuchi, Y, Nakajima, H, Nishiki-Kosaka, S, Hata, K, Kijima, Y, et al. Usefulness of transesophageal echocardiographic observation during chemotherapy for cardiac metastasis of non-Hodgkin lymphoma complicated with left ventricular diastolic collapse. J Cardiol. (2009) 53:447–52. doi: 10.1016/j.jjcc.2008.08.009
28. Franc, B, Yoshida, E, Herfkens, R, and Goris, M. Pericardial lymph node involvement in lymphoma as identified on PET. Clin Nucl Med. (2004) 29:741–2. doi: 10.1097/00003072-200411000-00021
29. Yang, Y, Chen, P, Liu, L, Lin, J, and Huang, S. The value of 18F-FDG PET/CT in evaluating the efficacy of chemotherapy for diffuse large B-cell lymphoma with cardiac involvement. J Nucl Cardiol. (2022) 29:3548–53. doi: 10.1007/s12350-021-02551-8
30. Tsugu, T, Nagatomo, Y, Matsuyama, E, Lancellotti, P, and Mitamura, H. Very delayed sinus arrest during complete remission of diffuse large B-cell lymphoma invading right atrium. Turk Kardiyol Dern Ars. (2021) 49:414–8. doi: 10.5543/tkda.2021.57474
31. Panareo, S, Urso, L, Santi, I, Rigolin, GM, Cuneo, A, Cittanti, C, et al. Right atrium mass assessed with 18F-FDG PET/CT scan turns out to be an uncommon relapse of testicular diffuse large B-cell lymphoma: a case report. Diagnostics. (2020) 10:987. doi: 10.3390/diagnostics10110987
32. Tagami, K, Tanda, S, Kato, H, Tashiro, A, Saji, K, Komaru, T, et al. Detection of asymptomatic cardiac metastasis and successful salvage chemotherapy comprising a prednisone, etoposide, procarbazine, and cyclophosphamide regimen in an elderly Japanese patient suffering from a delayed recurrence of diffuse large B-cell lymphoma. Case Rep Oncol. (2012) 5:62–8. doi: 10.1159/000336447
33. Kaderli, AA, Baran, I, Aydin, O, Bicer, M, Akpinar, T, Ozkalemkas, F, et al. Diffuse involvement of the heart and great vessels in primary cardiac lymphoma. Eur J Echocardiogr. (2010) 11:74–6. doi: 10.1093/ejechocard/jep111
34. Lum, DP, Wandell, S, Ko, J, and Coel, MN. Reduction of myocardial 2-deoxy-2-[18F]fluoro-D-glucose uptake artifacts in positron emission tomography using dietary carbohydrate restriction. Mol Imaging Biol. (2002) 4:232–7. doi: 10.1016/s1095-0397(01)00062-0
35. Coulden, R, Chung, P, Sonnex, E, Ibrahim, Q, Maguire, C, and Abele, J. Suppression of myocardial 18F-FDG uptake with a preparatory “Atkins-style” low-carbohydrate diet. Eur Radiol. (2012) 22:2221–8. doi: 10.1007/s00330-012-2478-2
36. Kaalep, A, Sera, T, Oyen, W, Krause, BJ, Chiti, A, Liu, Y, et al. EANM/EARL FDG-PET/CT accreditation - summary results from the first 200 accredited imaging systems. Eur J Nucl Med Mol Imaging. (2018) 45:412–22. doi: 10.1007/s00259-017-3853-7
37. Boellaard, R, Delgado-Bolton, R, Oyen, WJG, Giammarile, F, Tatsch, K, Eschner, W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. (2015) 42:328–54. doi: 10.1007/s00259-014-2961-x
38. Carter, BW, Wu, CC, Khorashadi, L, Godoy, MCB, de Groot, PM, Abbott, GF, et al. Multimodality imaging of cardiothoracic lymphoma. Eur J Radiol. (2014) 83:1470–82. doi: 10.1016/j.ejrad.2014.05.018
39. Asadian, S, Rezaeian, N, Hosseini, L, Toloueitabar, Y, and Hemmati Komasi, MM. The role of cardiac CT and MRI in the diagnosis and management of primary cardiac lymphoma: a comprehensive review. Trends Cardiovasc Med. (2022) 32:408–20. doi: 10.1016/j.tcm.2021.08.010
40. Zanoni, L, Bezzi, D, Nanni, C, Paccagnella, A, Farina, A, Broccoli, A, et al. PET/CT in non-Hodgkin lymphoma: an update. Semin Nucl Med. (2023) 53:320–51. doi: 10.1053/j.semnuclmed.2022.11.001
41. Yuan, H, Qiu, J, Chiu, KWH, Chan, LWC, Zhang, F, Wei, X, et al. PET/CT morphology and cardiac conduction disorders help discriminate primary cardiac lymphoma from primary cardiac sarcoma. J Nucl Cardiol. (2022) 29:2866–77. doi: 10.1007/s12350-022-03042-0
42. Ishimaru, S, Tsujino, I, Takei, T, Tsukamoto, E, Sakaue, S, Kamigaki, M, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J. (2005) 26:1538–43. doi: 10.1093/eurheartj/ehi180
43. Nuutila, P, Koivisto, VA, Knuuti, J, Ruotsalainen, U, Teräs, M, Haaparanta, M, et al. Glucose-free fatty acid cycle operates in human heart and skeletal muscle in vivo. J Clin Invest. (1992) 89:1767–74. doi: 10.1172/JCI115780
44. Minamimoto, R, Morooka, M, Kubota, K, Ito, K, Masuda-Miyata, Y, Mitsumoto, T, et al. Value of FDG-PET/CT using unfractionated heparin for managing primary cardiac lymphoma and several key findings. J Nucl Cardiol. (2011) 18:516–20. doi: 10.1007/s12350-011-9358-z
45. Barrington, SF, Qian, W, Somer, EJ, Franceschetto, A, Bagni, B, Brun, E, et al. Concordance between four European centres of PET reporting criteria designed for use in multicentre trials in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. (2010) 37:1824–33. doi: 10.1007/s00259-010-1490-5
46. Biggi, A, Gallamini, A, Chauvie, S, Hutchings, M, Kostakoglu, L, Gregianin, M, et al. International validation study for interim PET in ABVD-treated, advanced-stage hodgkin lymphoma: interpretation criteria and concordance rate among reviewers. J Nucl Med. (2013) 54:683–90. doi: 10.2967/jnumed.112.110890
47. Kikuchi, Y, Oyama-Manabe, N, Manabe, O, Naya, M, Ito, YM, Hatanaka, KC, et al. Imaging characteristics of cardiac dominant diffuse large B-cell lymphoma demonstrated with MDCT and PET/CT. Eur J Nucl Med Mol Imaging. (2013) 40:1337–44. doi: 10.1007/s00259-013-2436-5
48. Israel, O, Keidar, Z, and Bar-Shalom, R. Positron emission tomography in the evaluation of lymphoma. Semin Nucl Med. (2004) 34:166–79. doi: 10.1053/j.semnuclmed.2004.03.002
49. Hsu, DFC, Ilan, E, Peterson, WT, Uribe, J, Lubberink, M, and Levin, CS. Studies of a next-generation silicon-photomultiplier-based time-of-flight PET/CT system. J Nucl Med. (2017) 58:1511–8. doi: 10.2967/jnumed.117.189514
50. Hoffmeier, A, Sindermann, JR, Scheld, HH, and Martens, S. Cardiac tumors--diagnosis and surgical treatment. Dtsch Arztebl Int. (2014) 111:205–11. doi: 10.3238/arztebl.2014.0205
51. Kassab, J, Gebrael, G, Chedid El Helou, M, El Dahdah, J, Haroun, E, Kassab, R, et al. Case report: primary cardiac lymphoma manifesting as superior vena cava syndrome. Front Cardiovasc Med. (2023) 10:1257734. doi: 10.3389/fcvm.2023.1257734
52. Li, X, Chen, Y, Liu, J, Xu, L, Li, Y, Liu, D, et al. Cardiac magnetic resonance imaging of primary cardiac tumors. Quant Imaging Med Surg. (2020) 10:294–313. doi: 10.21037/qims.2019.11.13
53. Henn, MC. Commentary: Don’t mess with primary cardiac lymphoma. J Thorac Cardiovasc Surg. (2022) 164:582–3. doi: 10.1016/j.jtcvs.2020.09.049
54. Rolla, G, Bertero, MT, Pastena, G, Tartaglia, N, Corradi, F, Casabona, R, et al. Primary lymphoma of the heart. a case report and review of the literature. Leuk Res. (2002) 26:117–20. doi: 10.1016/s0145-2126(01)00092-3
Keywords: malignant lymphoma, diffuse large B-cell lymphoma, primary cardiac lymphoma, computed tomography, 18F-FDG PET/CT, case report
Citation: Huang W, Zheng Z, Zhang Y, Qiu Y, Peng Y, Yang Q, Wang W and Kang L (2024) A rare case of primary cardiac diffuse large B-cell lymphoma imaged with 18F-FDG PET/CT: a case report and literature review. Front. Med. 11:1373773. doi: 10.3389/fmed.2024.1373773
Edited by:
Carmelo Caldarella, Fondazione Policlinico Universitario A. Gemelli IRCCS, ItalyReviewed by:
Andor W. J. M. Glaudemans, University of Groningen, NetherlandsPriscilla Guglielmo, Veneto Institute of Oncology (IRCCS), Italy
Copyright © 2024 Huang, Zheng, Zhang, Qiu, Peng, Yang, Wang and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Kang, a2FuZ2xlaUBiam11LmVkdS5jbg==
†These authors have contributed equally to this work
 Wenpeng Huang
Wenpeng Huang Zuohuan Zheng2†
Zuohuan Zheng2† Yongbai Zhang
Yongbai Zhang Yongkang Qiu
Yongkang Qiu Yushuo Peng
Yushuo Peng Qi Yang
Qi Yang Lei Kang
Lei Kang