- Department of Nuclear Medicine, Affiliated Hospital of Zunyi Medical University, Zunyi, China
Background: The human epidermal growth factor receptor 2 (HER2) affibodies are multifunctional tools that, when labeled with radioactive isotopes, hold significant potential for the diagnosis and treatment of tumors exhibiting HER2 overexpression. This research focuses on the development of 131I-labeled HER2 affibodies as targeted radionuclide therapy agents (TRNT) for HER2-positive Ovarian carcinoma.
Methods: The YZHER2: V2 affibody targeting HER2 was synthesized through genetic recombination. It was labeled with 131I by the chloramine T method, and its radiochemical purity and stability were evaluated in vitro. The normal mice were subjected to a study on the pharmacokinetic characteristics of 131I-YZHER2: V2. An assessment was conducted on the uptake in tumors, biological distribution, and potential for therapeutic use of 131I-YZHER2: V2 using a HER2-positive SKOV-3 nude mouse model. The HER2-negative ID-8 mouse model was used as a negative control.
Results: 131I-YZHER2: V2 was easily prepared, and the non-decayed corrected yield of 131I-YZHER2: V2 affibody molecular probe was 96.06% ± 1.26%, showing good stability within 6 h in both normal saline (NS) and fetal bovine serum (FBS). The affinity of 131I-YZHER2: V2 was 32.9 nmol/L by cell binding assay. Scintigraphy revealed rapid uptake of the tracer in HER2-positive tumors. The retention of radioactive metabolites in the stomach, kidney, and bladder indicates that radioactive metabolites are mainly excreted through the gastrointestinal tract and urinary system. No substantial radioactive accumulation was observed in the heart, liver, lungs, or muscle tissue. Notably, significant renal retention was also evident based on in vitro biological distribution analysis. Tumor accumulation, extended retention, and advantageous distribution were observed in mice with HER2-positive tumors. Mice treated with 131I-YZHER2: V2 showed reduced tumor growth and prolonged survival. In the negative control group, there was no obvious aggregation and inhibition of tumors, and radioactive uptake in the kidney and gastrointestinal tract was also observed.
Conclusion: 131I-YZHER2: V2 has the potential to be explored as a new method for TRNT in HER2-positive ovarian cancer.
Introduction
Ovarian cancer, with a mortality rate second only to cervical cancer among malignant tumors of the female reproductive tract, is categorized into various subtypes depending on the levels of molecular biomarkers expressed. These biological indicators include the receptors for estrogen and progesterone, as well as the human epidermal growth factor receptor 2 (HER2) (1–3). The presence of HER2 gene amplification or high levels of HER2 protein can be observed in approximately 25 to 30% of ovarian malignancies (4). Overexpression of HER2 can inhibit cell apoptosis, induce angiogenesis and lymphatic angiogenesis, improve cell motility and enhance tumor invasion and metastasis, thus promoting cell growth and proliferation and tumorigenesis. Most studies have consistently shown a significant association between the existence of HER2 protein and an adverse prognosis in ovarian cancer, along with its influence on tumor sensitivity to chemotherapy and biological therapy. As a result, HER2 emerges as a crucial molecular target for further exploration into immunotherapeutic strategies (5, 6). Over the past few years, HER2-targeted therapy has become the favored strategy for the management of patients exhibiting HER2-positive status (7). While targeted therapy for HER2 is effective, the majority of patients in certain subgroups still experience disease progression due to acquired drug resistance. Many researchers are now exploring radioimmunotherapy (RIT) and other immunotherapeutic approaches, aiming to discover innovative strategies and methodologies to comprehensively address ovarian carcinoma (8).
Targeted radionuclide therapy (TRNT) is gaining popularity as a promising approach for treating cancer, particularly in individuals with advanced stages of the disease (9). Radionuclide 131I with carrier labeling is used for imaging or treatment of thyroid cancer nodules and accumulates in lesions through physiological uptake or binding to tumor cells with high specificity and affinity. These vectors include antibodies, fragments of antibodies, peptides, as well as other small molecules like affibodies (10). Consequently, TRNT delivers targeted irradiation to tumors without harming surrounding healthy tissues. When the significance of the toxicity associated with external beam radiotherapy is taken into account, TRNT emerges as the favored method for managing disseminated malignancies. Based on the dosimetric verification of Monte Carlo simulation and preclinical data, TRNT is significantly superior to traditional EBRT in terms of accuracy, safety and treatment potential for metastatic diseases through the dual mechanism of molecular targeting and local radiotherapy, especially for MCC and other solid tumors with rich blood vessels and easy to metastasis (11). In the past few years, scientists have utilized various radioactive substances to develop specialized medications for addressing ovarian carcinoma in patients with HER2-positive status. These include emitters of β-particles such as lutetium-177 (177Lu), rhenium-188 (188Re), and iodine-131 (131I), as well as emitters of Auger electrons like indium-111 (111In). Representative radiopharmaceuticals from this development include 177Lu-Dota-Trastuzumab, 177Lu-Pertuzumab, 177Lu-CHX-A”-DTPA- ABD-(ZHER2:342)2, 188Re-ZHER2: V2, and 111In-DTPA-trastuzumab (12–15). The physical characteristics of 177Lu γ-photons (208 keV, 11% abundancy) are ideal for imaging and also from a radiation safety point of view. Because of the range of β-particles, 177Lu may have an advantage in treating small lesions (16). When released from the antibody, the metallic radionuclides 177Lu are partly incorporated into the mineral bone, which may lead to excessive radiation to the bone marrow (17). Compared with 177Lu, 131I is the most extensively used radionuclide in TRNT because of its availability, its ease for chemical conjugation, and its ability to perform γ imaging and β therapeutic studies with the same biological vector (18, 19).
The affibody molecular protein framework is obtained from the Z-binding domain found in Staphylococcus A protein’s IgG binding domain. This particular domain consists of 58 amino termini and exhibits a triple helix structure, often referred to as an “engineered antibody” (20). Compared to monoclonal antibodies and their fragments, affibody molecules stand out due to their small molecular weight (7 KDa), exceptional specificity, strong affinity for antigens, and remarkable ability to penetrate tumors and is well below the glomerular filtration threshold, thus rapidly passing through the glomerular filtration to the urine. These attributes position them as prime candidates for the development of TRNT drugs (12, 15, 21–24). To achieve this objective, our aim is to synthesize a significant amount of 131I-labeled YZHER2: V2 and assess its efficacy as a TRNT agent for ovarian carcinoma with HER2-positive expression.
Materials and methods
Materials
131I was acquired from Beijing Atomic High-tech Co., LTD. (Beijing, China), Chloramine T was obtained from Tianjin Kemiou Chemical Reagent Co., LTD. (Tianjin, China), citric acid was purchased from Sigma-Aldrich Company of the United States, phosphate buffer (PBS;pH 7.4) was purchased from Guiyang Yunyan Jumei Laboratory Reagent (Guiyang, China), Ni Agarose 6FF and Q-Agarose FF were procured from Beijing Solarbio Technology Co., LTD. (Beijing, China). Chongqing Bo Maddison Biocell Center supplied ID-8 (HER2-negative) and SKOV-3 cells (HER2-positive). Agilent Technologies supplied Instant Thin Layer chromatography silica gel (iTLC-SG) paper while GE Healthcare supplied NAP-5 size exclusion column. Isoflurane (1–3% [inhaled]) was purchased from Shenzhen RWD Life Science Co., Ltd. Balb/c nude mice (6 weeks of age, 18-20 g, female), healthy Balb/c mice (4–6 weeks of age, 18–22 g, female) were obtained from Enschville Laboratory Animal Technologies, Inc. Animals were housed in standard conditions: In the absence of specific pathogens (SPF) environment, use ventilated cages, with environmental enrichment, 12 h of light/dark cycle, a controlled temperature and to access to food and water. The GC-2010 gamma radiation counter was purchased from ZONKIA at the University of Science and Technology of China in Hefei, China. Additionally, the Infinia V Hawkeye 4 SPECT/CT imaging system was acquired from GE Healthcare located in Chicago, Illinois, USA.
Preparation of HER2 affibody protein
E. coli BL21 cells were subjected to a transformation procedure using the plasmid pET22b (+), which contained a gene fragment encoding HER2-targeted biophiles. The amino terminal has tyrosine and the amino acid sequence is MAYHEHEHEA ENKFNKEMRN AYWEIALLPN LTNQQKRAFI RSLYDDPSQS ANLLAEAKKL NDAQ. The bacterial cells were grown in LB broth supplemented with kanamycin (50 μg/mL) at 37°C and stimulated for protein expression using IPTG (1 mmol/L). After the completion of harvest, ultrasonic treatment was employed to lyse the cells, followed by centrifugation for the removal of cellular debris. Subsequently, the resulting clarified cell lysate underwent heat treatment at a temperature of 60°C for a duration of 10 min in order to induce precipitation of a fraction of the inherent E. coli protein and filtration using a 0.22 μm filter. The affibody was isolated using immobilized metal affinity chromatography (IMAC, Ni Agarose 6FF) and subsequently purified through anion exchange chromatography (Q-Agarose FF).
The affibody underwent analysis utilizing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and matrix-assisted laser desorption/ionization tandem time-of-flight mass spectrometry (Maldi-TOF/TOF).
Prepared and characterized 131I-YZHER2: V2
Add 200 μL of YZHER2: V2 solution (1 mg/mL in PBS, pH 7.4) into an EP tube, followed by gentle mixing with 100 μL of Na131I (3.7 GBq/mL). Subsequently, introduce 80 μL of chloramine T solution (1 g/L). Incubate the mixture at room temperature for 10 min, occasionally shaking, and then quench the reaction by adding 40 μL of Na2S2O3 (0.25 mol/L). Thereafter, purify the reaction mixture using a NAP-5 column and employing PBS as the eluent, through size-exclusion chromatography.
The determination of the labeling efficiency of the molecular probe 131I-YZHER2: V2 was performed utilizing reverse-phase high-performance liquid chromatography (RP-HPLC). In essence, the analysis was conducted utilizing an LC-16 HPLC system from Shimadzu (Suzhou, China) equipped with a UV–visible detector (λ = 260 nm) and a radioactivity flow detector from ECKERT & ZIEGLER Radiopharma (Berlin, Germany). A WondaSil C18-WR column (5 μm, 100 Å, 4.6 I. D × 150 mm, Cat. No.5020–39,031, Suzhou, China) was employed for the analysis. The identification conditions were as follows: (1) Application of a C18 reverse-phase column. (2) Mobile phase A consists of acetonitrile with 0.1% trifluoroacetic acid, gradually increasing from 5 to 80%. Mobile phase B, on the other hand, is composed of water with 0.1% trifluoroacetic acid and gradually decreasing from 95 to 20%. (3) The rate of liquid flow is 1 mL/min. (4) UV detector wavelength set at 260 nm. (5) Sample run time set to 20 min. The radioactive chemical purity (RCP) of 131I-YZHER2: V2 was determined using HPLC, and rapid analysis was conducted using instant thin-layer chromatography (ITLC). The ITLC technique utilized Agilent’s ITLC-paper along with a mobile phase consisting of citrate buffer (pH = 4.5). 131I-YZHER2: V2 was incubated at room temperature (approximately 37°C) in both normal saline (NS) and fetal bovine serum (FBS) to assess its in vitro stability. The RCP was evaluated using the aforementioned ITLC method within 8 h.
Cell lines
The SKOV-3 and ID-8 cells were incubated in a 5% CO2 incubator at 37°C for 2 to 3 days, using DMEM medium supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. Once the cell fission rate reached approximately 90%, the initial culture medium was discarded, followed by two washes with PBS. Subsequently, a suitable solution of trypsin (0.25%) containing EDTA (0.02%) was added to detach cells from the flasks. When the cells shrank back and became round, supplemented DMEM was added into the complete culture medium to stop digestion, the wall of the bottle was gently blown with a sterile pipette, the cells attached to the wall were blown off and transferred to the centrifuge tube. After being subjected to centrifugation at 217 × g for a duration of 5 min, the supernatant was removed. Subsequently, DMEM was introduced into the complete culture medium and thoroughly blended through blowing and mixing processes. Subsequently, cells were introduced into the culture bottle at a concentration ratio of 1:3 for passage.
Specific binding and cellular uptake
SKOV-3 cell line was selected the positive control and ID-8 cell line as negative control. They were inoculated into 24-well plates (2 × 105/well, 1 mL) and cultured at 37°C overnight. When the cells had attached as a monolayer, the original medium was discarded, the cells rinsed twice with PBS, and fresh serum-free medium (DMEM) was added. Freshly prepared 131I-YZHER2: V2 at different concentrations (0.52, 2.59, 12.97, 64.86, 162.16, 405.43 nM, n = 3) were added to each well. After subjecting the cells to incubation at a temperature of 4°C for a duration of 2 h, the medium was extracted and the cells were rinsed thrice with cold PBS. Subsequently, lysis was initiated by exposing them to 1 M NaOH. The resulting lysates obtained from the cells were gathered and quantified using a gamma counter. Using GraphPad Prism 9 software through nonlinear fitting (total number of unit points and non-specific binding) calculate equilibrium dissociation constant (equilibrium dissociation constant, Kd).
SKOV-3 cells were inoculated in culture dishes (106/dish, 2 mL) and cultured overnight at 37°C to form a monolayer. Eliminate the medium, wash twice with PBS, and introduce a fresh serum-free medium. 131I-YZHER2: V2 was added to the solution at a concentration of 32.9 nM and incubated at a temperature of 37°C. At a predetermined time point (1, 2, 4, 8, 12, and 24 h after the start of incubation), a set of petri dishes (n = 3) were extracted. The liquid portion was gathered while the cells underwent three rounds of cold PBS rinsing. The washing medium was mixed with the supernatant previously collected, which represented unbinding. Then, the cells were exposed to a chilled urea buffer (4 M urea, 0.2 M glycine, pH 2.5) for a period of 5 min, and the cells were washed 3 times to collect the urea buffer, which represents membrane binding. Finally, cell lysis was accomplished using a solution containing 1 mol/L NaOH, and the culture dish was cleaned three times, and the cell lysate and cleaning solution were collected, which represented the cell internalization. The gamma counter quantifies the amount of gamma-emitting radionuclides in a sample and determines the proportion of radioactivity bound to the membrane and internalized over time.
Animal models
All experiments involving animals have received ethical approval from the Ethics Committee of the Affiliated Hospital of Zunyi Medical University (Approval number, KLLYA-2021-019). Euthanasia by cervical dislocation was performed 4 weeks after the end of the protocol and had been planned in case of poor tolerance but was not required. The mice were kept in adequately ventilated cages with filter tops and given unrestricted availability to a standard diet and water. SKOV-3 and ID-8 cells were cultured to the exponential growth phase and were then re-suspended in PBS buffers after trypsin digestion. SKOV-3 and ID-8 cells (5 × 106, 100 μL) were implanted into the right axillary region of 21 female Balb/C mice aged 6–8 weeks, respectively, and tumors were formed within about 2 weeks. The size of the tumor was assessed using a vernier caliper. Once the tumor volume reached approximately 1 cm3, it was utilized for conducting a biological distribution analysis and scintigraphy.
Pharmacokinetics of 131I-YZHER2: V2
A pharmacokinetic study of 131I-YZHER2: V2 was conducted on 33 Balb/c mice, aged between 6 and 8 weeks, with an approximate average weight of 18.0 ± 1.6 g, procured from Ensville Co., Ltd., Chongqing, China. Each mouse received an intravenous injection in the tail vein of 0.74 MBq of 131I-YZHER2: V2, equivalent to 5 μg of YZHER2: V2 in 100 μL of PBS. Blood samples were drawn at specific time points post-injection: 0, 10, 15, 30 min, and 1, 2, 4, 8, 24, 48, and 72 h, with three mice sampled at each interval. At these respective times, a blood sample of 0.2 mL was obtained post-enucleation of the eyeball, placed into test tubes, and the radioactivity of each sample was assessed using a γ-counter. The level of radioactivity in the bloodstream was quantified as the ratio of the administered dose to tissue weight (%ID/g). A time-radioactivity curve of blood was generated. Pharmacokinetic parameters were calculated using DAS 2.0 software from Shanghai, China, and a two-compartment model was utilized to assess the half-life of 131I-YZHER2: V2 in the bloodstream.
Biodistribution study
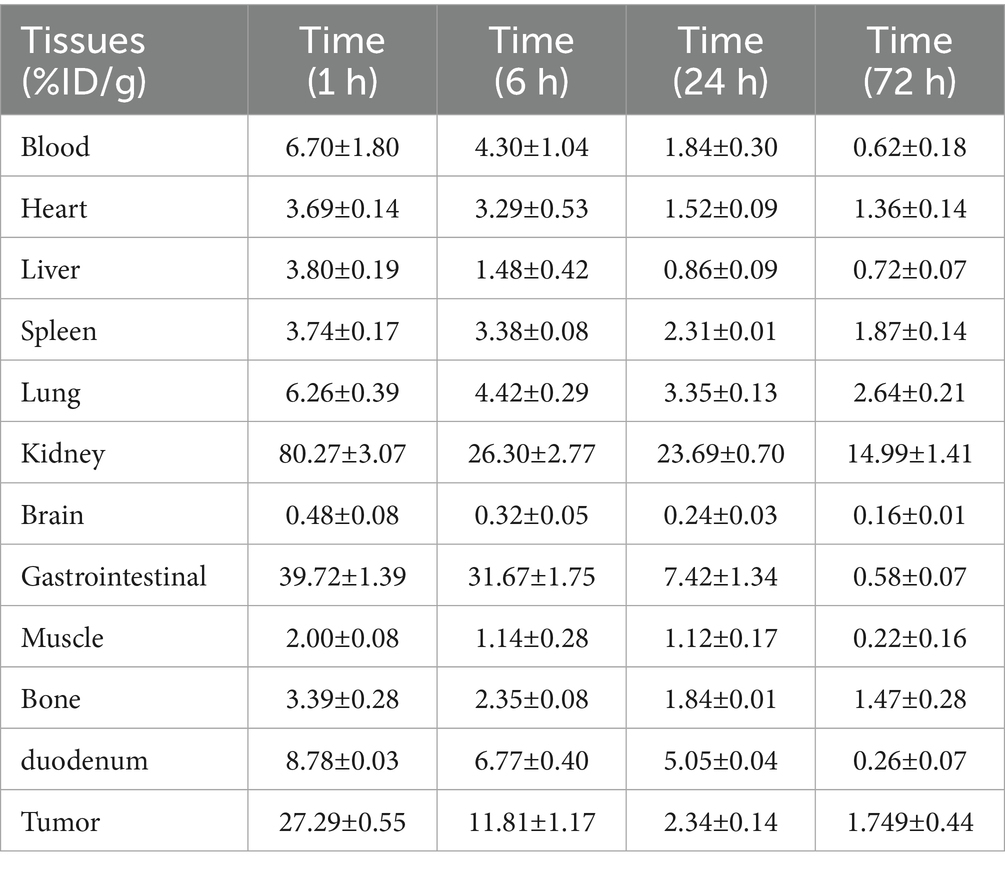
The injection of approximately 1.85 MBq (10 μg)of 131I-YZHER2: V2 was administered into the lateral tail vein of mice bearing SKOV3 tumors. Animals were euthanized at 1, 6, 24, and 72 h after injection. To minimize animal suffering, humane euthanasia was performed promptly via CO2 inhalation. Following euthanasia, animals were placed in lead-lined containers for 3 days and subsequently cremated after surface contamination measurements confirmed absence of residual radioactivity. Samples including blood, tumor, cardiac tissue, hepatic tissue, splenic tissue, pulmonary tissue, renal tissue, cerebral tissue, muscular tissue, femoral bone marrow, gastric mucosa and duodenal mucosa were gathered. A γ-counter measures the radioactive count of a sample. The quantification of biological distribution was represented as the ratio of injected dose to tissue weight (%ID/g).
Scintigraphy and immunohistochemistry analyses
SKOV-3 ovarian cancer-containing nude mice were divided into two groups, one group of 3 days in advance take feed a large excess of non-radioactive Iodine (1% NaI) was used to block uptake of radioactive I into the thyroid gland, with isoflurane anesthesia (4–5% induction, maintain at 1–3%). Each group was injected with about 1.85 MBq of 131I-YZHER2: V2 (YZHER2: V2 content was about 10 μg) through different administration routes: caudal vein, subcutaneous and abdominal. Scintigraphy was conducted at intervals of 1, 3, 6, 24, 48, and 72 h post-injection. Image acquisition parameters were 128 × 128, magnification was 2.5 times, and radiation count was 200.
Therapeutic efficacy
The established SKOV3 tumor-bearing mice were randomly assigned to three groups, each comprising seven mice. PBS (100 μL), YZHER2: V2 (100 μL, equivalent to 20 μg) dissolved in sterile water, and 131I-YZHER2: V2 (3.7 MBq, 100 μL), corresponding to 20 μg of YZHER2: V2, were administered in each group. Treatment consisted of every 5 days, a total of 5 times. Tumor volume in the mice was measured after each treatment. The nude mice were euthanized when the tumor volume was greater than 2000 mm3, weight loss was 20–25%, tumor ulceration or necrosis occurred, or other reasons related to animal ethical violations occurred.
Statistical analysis
SPSS 18.0 software was used. Data that followed a normal distribution were expressed as mean ± standard deviation (−x ± s). The independent samples t-test was utilized to compare two groups, while One-Way ANOVA was used for comparing multiple groups. A significance threshold of p < 0.05 was chosen, with levels of significance indicated by *p < 0.05; **p < 0.01; ***p < 0.001.
Results
Quality control and stability of 131I-YZHER2: V2
The successful radiolabeling of YZHER2: V2 with 131I was accomplished using the chloramine-T technique. Characterization of 131I-YZHER2: V2 was conducted through HPLC and ITLC. As depicted in (Figures 1A,B), the HPLC analysis of 131I-YZHER2: V2 exhibited a solitary radioactive peak with a retention time of 13.77 min, whereas the HPLC outcome for 131I demonstrated a solitary radioactive peak with a retention time of 1.66 min. The labeling yield of 131I-YZHER2: V2 was found to be high at (96.06% ± 1.26%) (n = 5), eliminating the requirement for further purification in biological experiments. The bacterial endotoxin assay demonstrated a minimal presence of less than 1 EU/mL. 131I-YZHER2: V2 exhibited a retention factor ranging from 0 to 0.2 (Figure 1C), while iodine ions had a retention factor ranging from 0.5 to 0.7 (Figure 1D). Furthermore, the stability of 131I-YZHER2: V2 was analyzed in both normal saline (NS) and fetal bovine serum (FBS) at room temperature (approximately 37°C) via the ITLC method. The stability of 131I-YZHER2: V2 was maintained in both NS and FBS, with a RCP of over 90% achieved within 8 h (Figure 2).
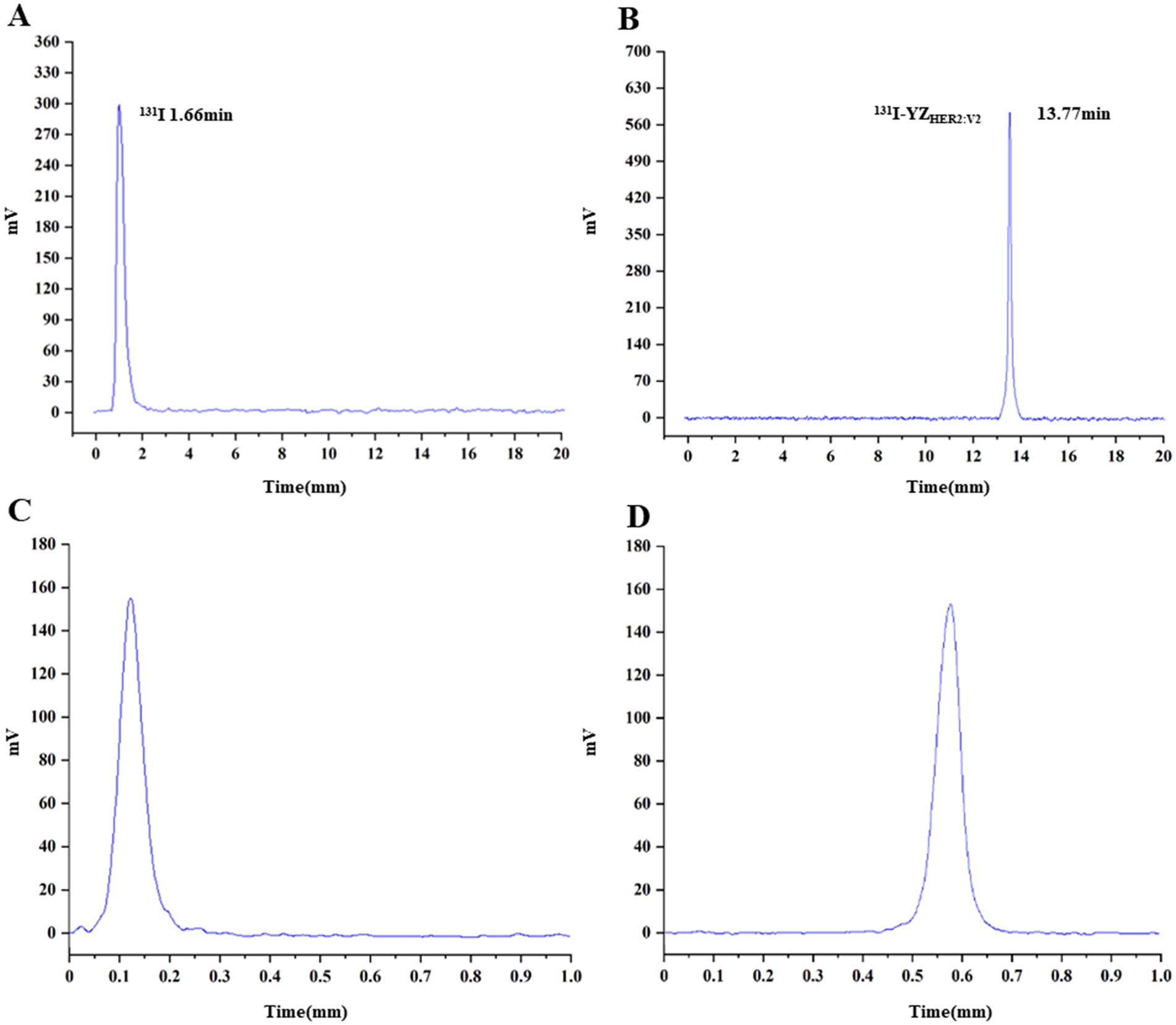
Figure 1. Characterization and stability assessment of 131I-YZHER2: V2 were conducted. HPLC analysis was performed on 131I-YZHER2: V2 (A) and 131I (B). The results of instant thin-layer chromatography (ITLC) showed the presence of 131I-YZHER2: V2 (C) and 131I (D).
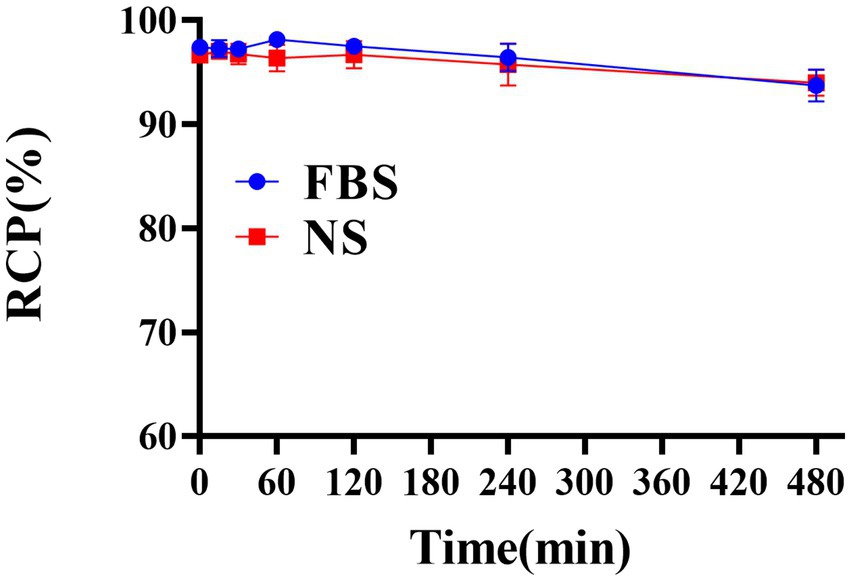
Figure 2. Assessing the stability of 131I-YZHER2: V2 in a solution of normal saline (NS) at room temperature and fetal bovine serum (FBS) at 37°C for up to 8 h.
Pharmacokinetics of 131I-YZHER2: V2
The drug-time curve and pharmacokinetic parameters of 131I-YZHER2: V2 are depicted in Figure 3. The administration of 131I-YZHER2: V2 intravenously into the mouse tail resulted in an immediate peak observed at zero Tmax value (Tmax = 0). The distribution half-life (T1/2α) was found to be approximately 0.015 ± 0.016 h, while the elimination half-life (T1/2β) was estimated to be around 1.198 ± 0.128 h. A maximum concentration achieved (Cmax) of roughly (74.27 ± 6.53)% ID/g was noted, along with an average residence time of about 14.9 ± 0.337 h and a clearance rate of approximately (0.326 ± 0.02)% ID/g/h.
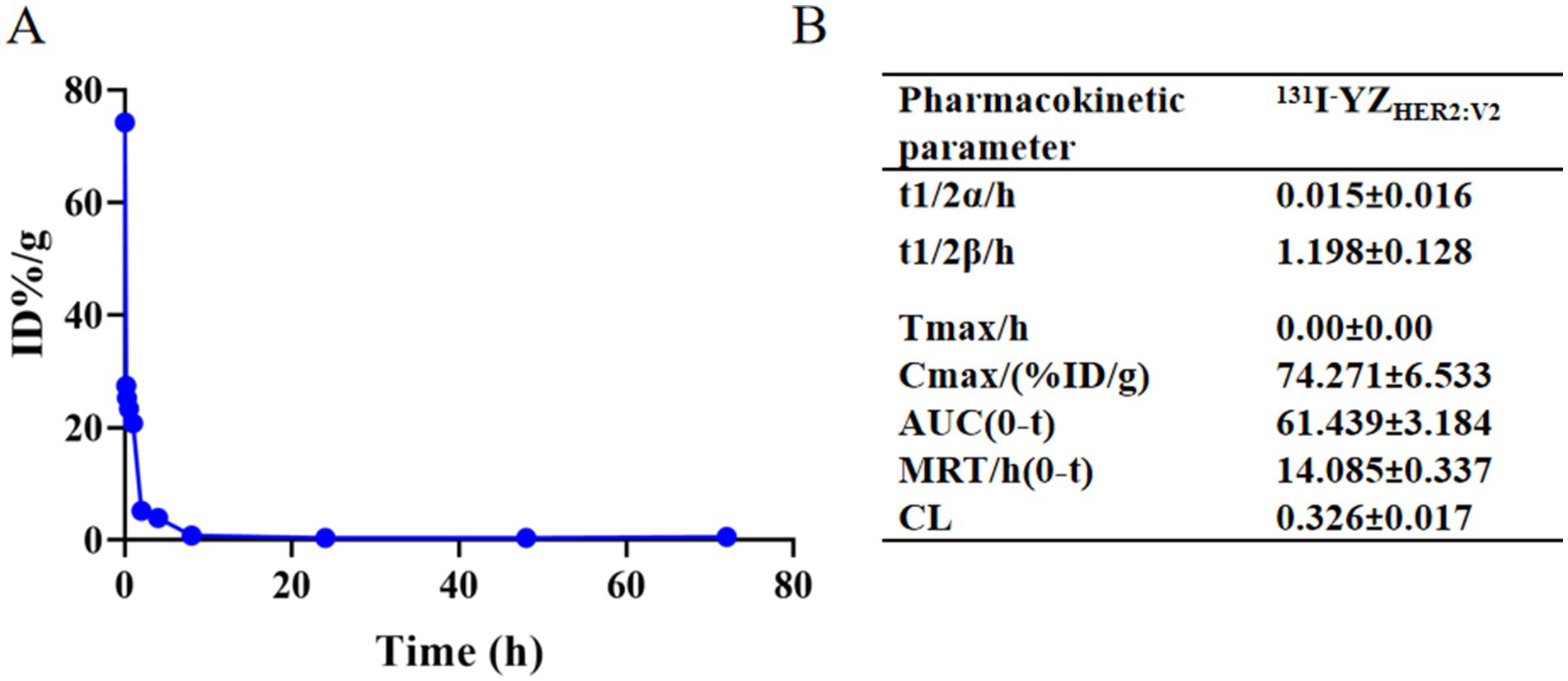
Figure 3. (A) Time-concentration profile of the drug 131I-YZHER2: V2; (B) Pharmacokinetic characteristics of 131I-YZHER2: V2.
Binding specificity and cellular uptake
At all concentration points, the radioactivity of 131I-YZHER2: V2 in SKOV-3 cells was found to be significantly greater than that observed in ID-8 cells (p < 0.05) (Figures 4A,B). 131I-YZHER2: V2 has an affinity for SKOV-3 cells with KD values of about (32.9 ± 0.69) nM. Cell uptake studies showed that SKOV-3 cells rapidly took up 131I-YZHER2: V2, and the uptake rate was (1.51 ± 0.22)% at 1 h, and then slowly increased, and the uptake rate was (1.78 ± 0.35)% at 6 h. The internalization of 131I-YZHER2: V2 by SKOV3 cells increased over time, with (3.78 ± 0.25)% of total cell-binding radioactivity internalized after 14 h of incubation (Figure 4C).
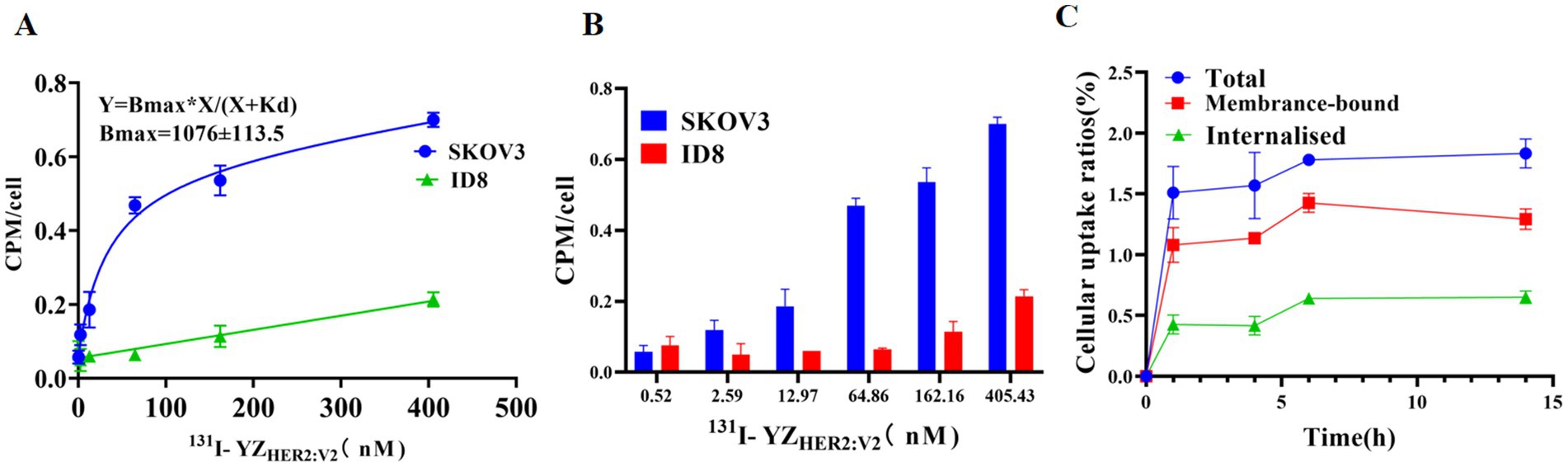
Figure 4. Binding specificity and cellular uptake. (A) Detection of 131I-YZHER2: V2 using Affibody and its application in the identification of HER2-positive cells. (B) Examining the impact of different concentrations of 131I-YZHER2: V2 on SKOV3 and ID8 cell lines (n = 3). (C) Internalization and absorption of 131I-YZHER2: V2 in SKOV3 cells.
Biodistribution in SKOV-3 nude mice
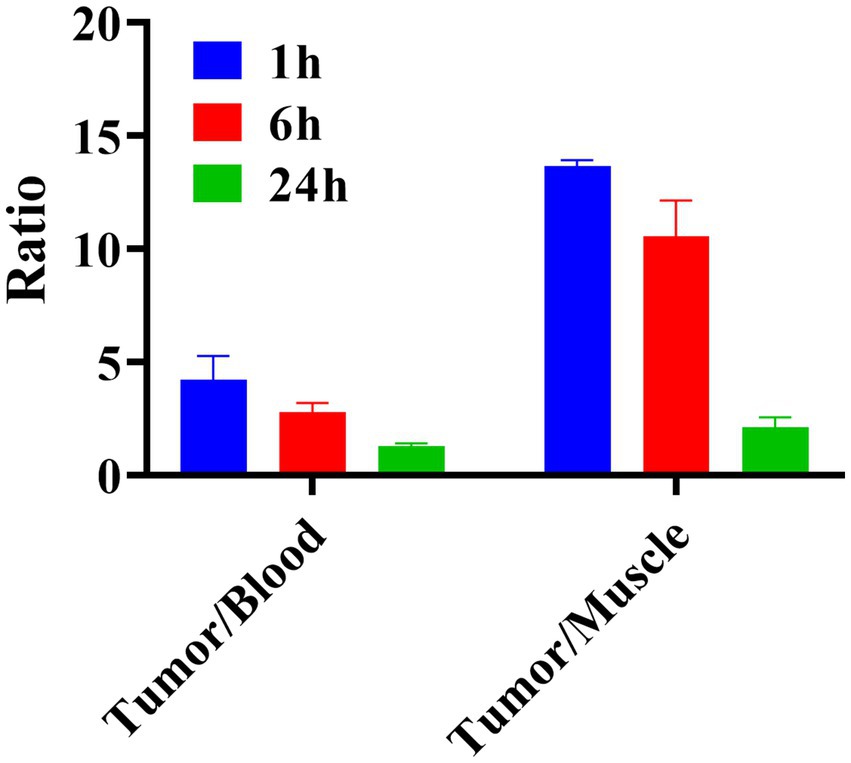
The tumor uptake was (27.29 ± 0.55) %ID/g at 1 h and (1.75 ± 0.44) %ID/g at 24 h after injection of 131I-YZHER2: V2. The biological distribution data for 131I-YZHER2: V2 in SKOV-3 nude mice are shown. An hour post-injection, variations in the ratio between tumor and blood were detected. The tumor-to-muscle ratio at 1 h was significantly higher than at 6 and 24 h, as depicted in Figure 5.
Scintigraphy of SKOV-3 nude mice
The schematic diagram for scintigraphy is presented in Figure 6. After injection of 131I-YZHER2: V2 into the tail vein of SKOV-3 ovarian cancer bearing nude mice, the tumor radionuclide concentration was clearly shown. Over time, it becomes evident that intraperitoneal injection offers a more distinct visualization of the tumor. This enhanced clarity is possibly attributed to the peritoneal absorption, rendering it a promising administration route for our subsequent treatment experiments. One hour following the injection of 131I-YZHER2: V2, a significant accumulation of radioactive isotopes within the tumor is observed. The detection of radioactivity buildup in the kidneys, bladder, and gastrointestinal tract suggests that 131I-YZHER2: V2 primarily exits the body through the urinary and digestive systems, aligning with its natural distribution. In the group where the thyroid uptake of radioactive I is blocked by a superabundance of non-radioactive I, no radioactive isotopic accumulation was detected in the thyroid. This minimizes the risk of thyroid damage and further enhances labeling efficiency. By manually delineating the tumor and lower limb (depicting the level of radioactivity in muscles), we obtained the average radiation count per unit volume. The results show a decreasing ratio with time, in line with the biological distribution pattern. However, no radionuclide concentration was observed in ID8 tumors, as shown in Supplementary Figure S4.
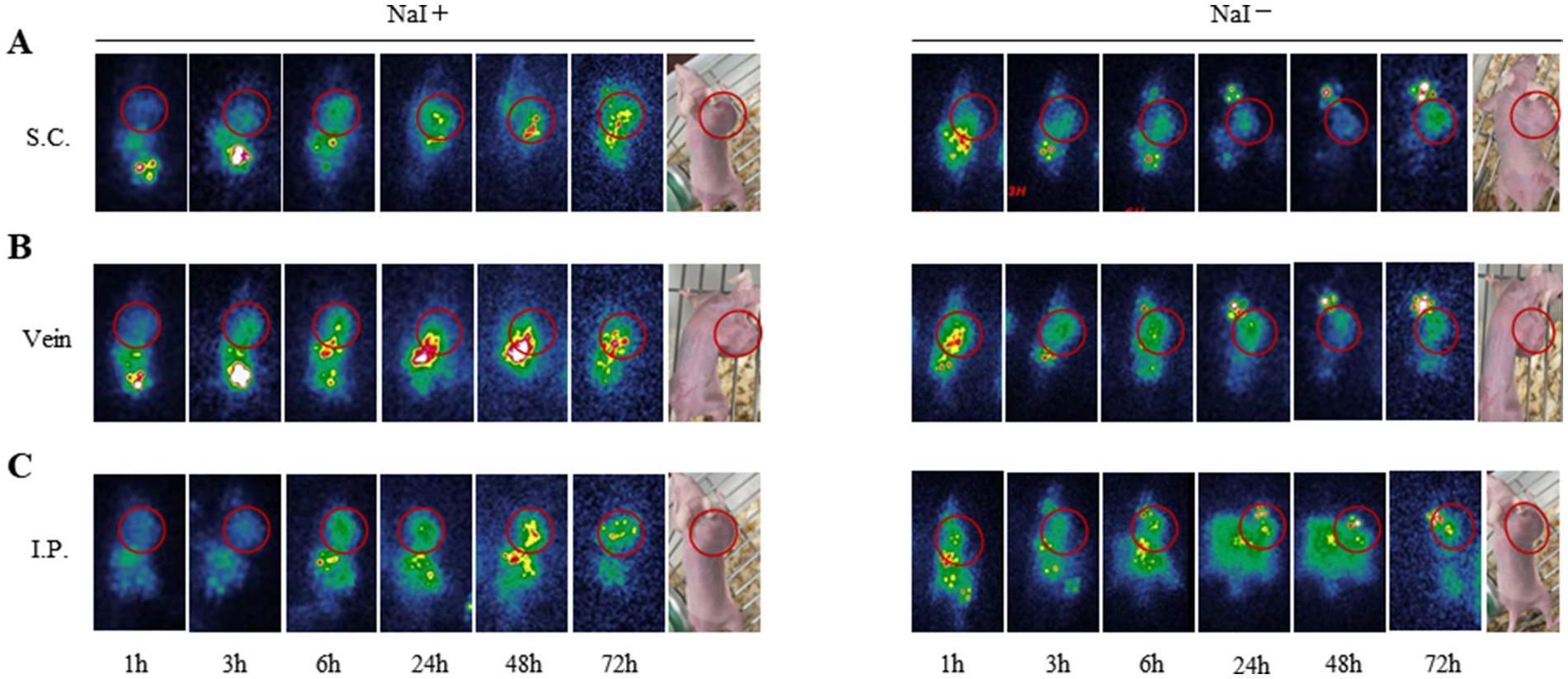
Figure 6. Scintigraphy of SKOV3 tumor bearing mice in different administration routes and imaging time. (A) subcutaneous injection (B) intravenous injection (C) intraperitoneal injection of 1 h, 3 h, 6 h, 24 h, 48 h, 72 h imaging. (NaI-/NaI+).
Therapeutic results
After 53 days of treatment, we observed similar growth patterns in the relative tumor volume of the YZHER2: V2 group (24.80 ± 5.36) times and the PBS group (26.62 ± 4.33) times, with no significant differences in their relative tumor volumes, both of which exceeded that of the 131I-YZHER2: V2 group (17.00 ± 4.32) times. Tumors in mice subjected to YZHER2: V2 and PBS treatments exhibited exponential growth, while those receiving 131I-YZHER2: V2 treatment experienced a delay in tumor growth, as illustrated in Figure 7A. In addition, as shown in Figure 7B, the survival time of 131I-YZHER2: V2 group was 3 days longer than that of YZHER2: V2 group and PBS group, with a statistically significant difference (p < 0.05), indicating that 131I-YZHER2: V2 related tumor shrinkage can affect survival.
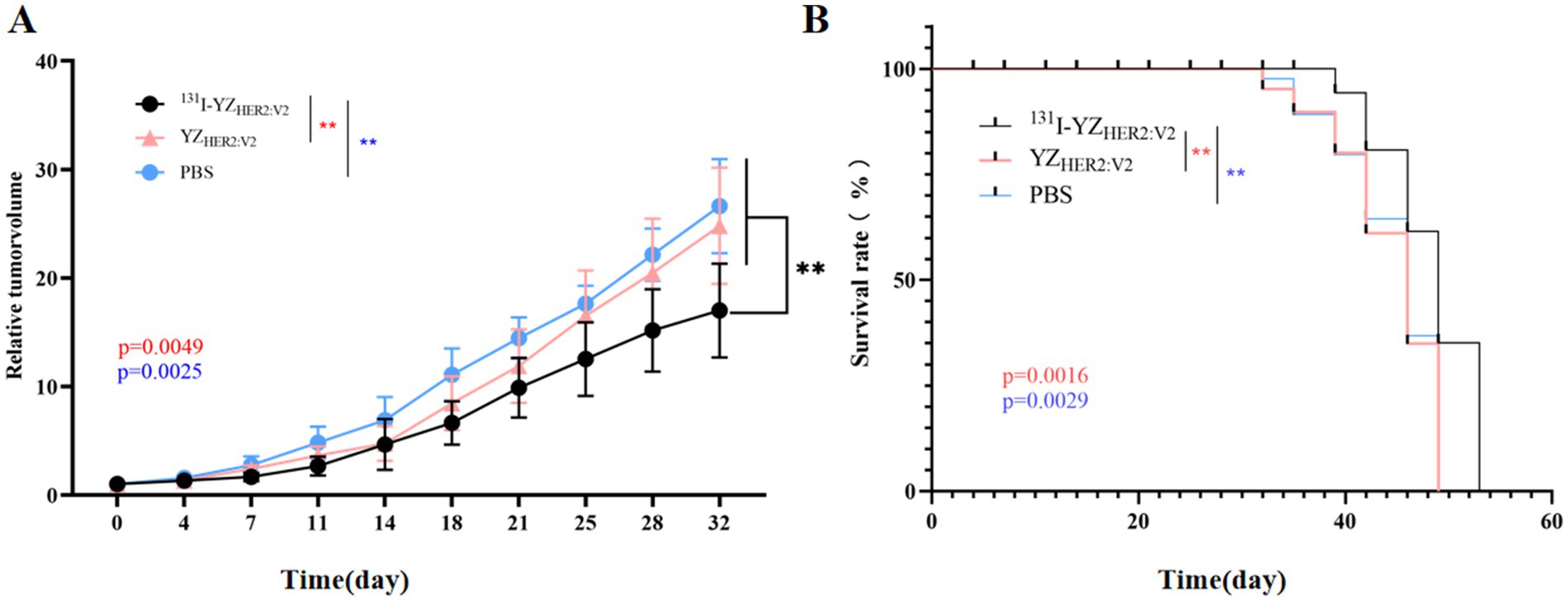
Figure 7. In vivo therapeutic efficacy of 131I-YZHER2: V2 in HER2-positive tumors. (A) Relative tumor volume. (B) survival rate. The red asterisk (*) indicates a comparison between the experimental group 131I-YZHER2: V2 and the control group YZHER2: V2, while the blue asterisk (*) represents a comparison between 131I-YZHER2: V2 and the blank group PBS.
Discussion
In the clinical trials that were conducted, HER2 affibody was labeled using diagnostic radionuclides including fluorine-18 (18F), gallium-68 (68Ga), technetium-99 (99mTc), and 111In to evaluate tumor targeting, biological distribution, blood clearance, dosimetry, and safety (25–28). The successful clinical transformation of affibody targeting HER2-positive tumors has prompted efforts to create additional TRNT drugs targeting HER2 for the treatment of cancer. In 2007, Tolmachev et al. (15). were the first to introduce the application of CHX-A”-DTPA as a chelating agent in order to attach 177Lu to the affibody ZHER2:342 for labeling purposes. The findings indicated that the administration of 177Lu-CHX-A”-DTPA-ABD-(ZHER2:342)2 effectively inhibited the growth of SKOV-3 cells characterized by high HER2 receptor expression. The survival of nude mice treated with 177Lu-CHX-A “-DTPA-ABD -(ZHER2:342)2 was significantly longer than that of the control group injected with PBS and the blocking group injected with 177Lu-CHX-A” -DTPA-ABD -(ZHER2:342)2. In the control group, 70% of mice developed tumors between 36 and 62 days (median 43 days) and died due to tumor growth between 67 and 104 days (median 67 days). None of the mice in the 21.6 MBq treatment group developed tumors, and the survival rate was 100%, which was significantly different from that in the control group (p < 0.001). However, its clinical translation is limited due to its elevated radioactive retention in blood and bone marrow. In this research, we assessed the efficacy of YZHER2: V2 labeled with 131I as a TRNT agent for managing transplanted tumors of HER2-positive breast cancer in mice. 131I is an ideal choice for radionuclide therapy and SPECT imaging due to its emission of both β-particles and γ-rays. To date, HER2-positive ovarian cancer has not been treated with 131I-labeled HER2 affibody. Altai et al. (22) attached different amino acid sequences to the carboxyl terminus, labeled these modifiers with 188Re, and compared them in vitro and in vivo. The results showed that the tumor uptake of 188Re-ZHER2: V2, a molecular probe with GGGC sequence at the carboxyl terminus, was significantly higher than that of any organ tissue (including liver and kidney) at 4 h after injection. This molecular probe has a high yield and maintains a high affinity for HER2. 188Re-ZHER2: V2 provides highly efficient targeting of HER2-expressing xenografts, rapid blood clearance, and low renal and bone uptake. Preclinical studies have shown that 188Re-labeled YZHER2: V2 specifically binds to SKOV-3 cells and is rapidly cleared from the bloodstream. Four hours after injection, radioactive uptake in the tumor exceeded that in the kidneys, with scintigraphy further emphasizing the tumor as the primary site of radioactive accumulation. Yang et al. (29) and Hu et al. (30) used a rapid and simple method to directly label HER2: V2 with 99mTc. This method is simple, rapid, and has a high labeling rate and high radiochemical purity. The radiolabelled probe showed good HER2 tumor targeting, rapid blood clearance, low gastric and salivary gland radioactivity, low renal radioactivity, and low hepatobiliary excretion. HER2 affibody YZHER2: V2 labeled with 131I was used in current study. Additionally, 131I can be efficiently coupled with YZHER2: V2, which has tyrosine residues, using the chloramine-T method. At present, different methods are commonly used to label iodine with radioisotopes, including chloramine-T, iodogen, and lactoperoxidase techniques (31–33). Furthermore, SGMIB has emerged as a novel technique for radioiodination, offering notable benefits compared to conventional iodination methods. These advantages stem from the gentle reaction conditions, excellent reproducibility, and minimal compromise of biological activity, resulting in enhanced biodistribution and superior tumor uptake of radioiodinated biomolecules (34) (see Table 1).
Each treatment group received five doses at a dose frequency of 0.1 mCi for the cumulative dose of the treatment described herein. We observed that the YZHER2: V2 labeled with 131I exhibited a significantly high RCP and demonstrated satisfactory stability for up to 6 h in an in vitro setting. After the administration of 131I-YZHER2: V2 via intravenous injection in mice, rapid elimination from the bloodstream is observed, facilitating the acquisition of early diagnostic images characterized by excellent contrast. As expected, HER2-positive tumors became detectable within one hour of administration, whereas minimal accumulation in HER2-negative tumors was observed during the corresponding time period. The T/M ratio of HER2-positive SKOV-3 xenografts was consistently higher compared to the negative controls at all time points. The imaging results of intravenous, subcutaneous and intraperitoneal injection routes showed that the tumors were visualized by all three injection methods, but the animal model injected intraperitoneally had better imaging effect over time. In vivo tumor targeting specificity of 131I-YZHER2: V2 against HER2-positive tumors was further confirmed by observing a significant increase in tumor uptake during biological distribution experiments conducted 1 h post-injection. Swift elimination from the bloodstream may help reduce radiation exposure to non-target tissues, thereby minimizing potential side effects or toxicity. However, it might lead to insufficient drug concentration in the target tissues, compromising the therapeutic efficacy. As a result, to achieve the desired therapeutic outcome, more frequent dosing or higher infusional doses might be necessary.
The efficacy of 131I-YZHER2: V2 in treating HER2-positive tumors was proven through the administration of 5 treatment sessions. The tumor suppressive effect of 131I-YZHER2: V2 was attributed to HER2-mediated targeting of ovarian cancer cells for tumor uptake, which was also associated with longer survival. After treatment with 131I-YZHER2: V2, the survival time in this group was extended to 53 days, while all mice in the PBS group and the YZHER2: V2 group were euthanized at this time point due to ethical reasons, such as tumor volumes exceeding 2,000 mm3, a weight loss of 20–25%, tumor ulceration, or necrosis. The two groups were observed for 53 days, and the 131I-YZHER2: V2 group had prolonged cell survival compared with the YZHER2: V2 group and PBS group. Further preclinical studies need longer observation periods to evaluate the duration of delayed tumor growth.
Taken together, the in vivo results indicate that 131I-YZHER2: V2 exhibits promising efficacy and safety profiles, suggesting its potential as a TRNT agent for ovarian cancer with HER2 overexpression.
The results show that the affinity labeling method is feasible, rapid and highly radiochemically pure. The rapid blood clearance, the good tumor targeting, and the high renal and gastrointestinal radioactivity suggest renal and gastrointestinal excretion of unbound avidin. 131I-YZHER2: V2 is a promising probe for the diagnosis and treatment of HER2-overexpressing tumors. In our research, due to the strong binding affinity and effective tumor targeting capabilities of YZHER2: V2, we selected it as a promising candidate for the development of molecular imaging agents and targeted therapy drugs in order to diagnose and treat HRE2-positive cancers. The dosimetric safety of 131I-YZHER2: V2 has not been evaluated before treatment, which needs to be further improved in future studies.
Conclusion
131I-YZHER2: V2, synthesized utilizing the Chloramine-T technique, demonstrates a remarkable efficiency in radiolabeling and exceptional stability when tested in vitro. The produced 131I-YZHER2: V2 demonstrates tumor accumulation, prolonged tumor retention, a high tumor-to-background ratio, favorable biodistribution and therapeutic efficacy in mice bearing HER2-positive tumors. Our findings indicate that 131I-YZHER2: V2 exhibits significant potential as a highly promising therapeutic radiopharmaceutical for future clinical applications.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used. The animal study was approved by Ethics Committee of the Affiliated Hospital at Zunyi Medical University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
HH: Conceptualization, Data curation, Formal analysis, Resources, Writing – original draft. XH: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – original draft. FL: Investigation, Methodology, Validation, Writing – original draft. GW: Project administration, Software, Supervision, Writing – original draft. JC: Conceptualization, Formal analysis, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was financially supported by the National Natural Science Foundation of China (NSFC, grant number: 82260353), Guizhou Province Science and Technology Plan Project (grant number: Qiankehe-ZK[2024]-329), Guizhou Provincial Health Commission Science and Technology Fund (grant number: gzwkj2025-480) and Zunyi Science and Technology Joint Fund (grant number: HZ-2023-284).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1507596/full#supplementary-material
Abbreviations
CT, computed tomography; HPLC, high-performance liquid chromatography; HER2, human epidermal growth factor receptor 2; ITLC, instant thin-layer chromatography; RCP, radiochemical purity; TRNT, targeted radionuclide therapy; T/M, tumor-to-muscle; IPTG, isopropyl beta-d-thiogalactoside.
References
1. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Xia, C, Dong, X, Li, H, Cao, M, Sun, D, He, S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. (2022) 135:584–90. doi: 10.1097/CM9.0000000000002108
3. Prat, A, Pineda, E, Adamo, B, Galván, P, Fernández, A, Gaba, L, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. (2015) 24:S26–35. doi: 10.1016/j.breast.2015.07.008
4. Lin, C-K, Lin, W-L, Chen, F-L, Lee, M-Y, Kuo, J-F, Ruan, A, et al. Assessing the impact of polysomy-17 on HER2 status and the correlations of HER2 status with prognostic variables (ER, PR, p53, Ki-67) in epithelial ovarian cancer: a tissue microarray study using immunohistochemistry and fluorescent in situ hybridization. Int J Gynecol Pathol. (2011) 30:372–9. doi: 10.1097/PGP.0b013e31820c9ff3
5. Mendelsohn, J, and Baselga, J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. (2003) 21:2787–99. doi: 10.1200/JCO.2003.01.504
6. Chavez-Blanco, A, Perez-Sanchez, V, Gonzalez-Fierro, A, Vela-Chavez, T, Candelaria, M, Cetina, L, et al. HER2 expression in cervical cancer as a potential therapeutic target. BMC Cancer. (2004) 4:59. doi: 10.1186/1471-2407-4-59
7. Oh, D-Y, and Bang, Y-J. HER2-targeted therapies - a role beyond breast cancer. Nat Rev Clin Oncol. (2020) 17:33–48. doi: 10.1038/s41571-019-0268-3
8. Puglisi, F, Minisini, AM, De Angelis, C, and Arpino, G. Overcoming treatment resistance in HER2-positive breast cancer: potential strategies. Drugs. (2012) 72:1175–93. doi: 10.2165/11634000-000000000-00000
9. Kleinendorst, SC, Oosterwijk, E, Bussink, J, Westdorp, H, Konijnenberg, MW, and Heskamp, S. Combining targeted radionuclide therapy and immune checkpoint inhibition for Cancer treatment. Clin Cancer Res. (2022) 28:3652–7. doi: 10.1158/1078-0432.CCR-21-4332
10. Goldsmith, SJ. Targeted radionuclide therapy: a historical and personal review. Semin Nucl Med. (2020) 50:87–97. doi: 10.1053/j.semnuclmed.2019.07.006
11. Ramirez-Fort, MK, Meier-Schiesser, B, Lachance, K, Mahase, SS, Church, CD, Niaz, MJ, et al. Folate hydrolase-1 (FOLH1) is a novel target for antibody-based brachytherapy in Merkel cell carcinoma. Skin Health Dis. (2021) 1:e9. doi: 10.1002/ski2.9
12. Orlova, A, Tran, TA, Ekblad, T, Karlström, AE, and Tolmachev, V. Re-ma SGS-Z (HER2:342), a potential Affibody conjugate for systemic therapy of HER2-expressing tumours. Eur J Nucl Med Mol Imaging. (2010) 37:260–9. doi: 10.1007/s00259-009-1268-9
13. Persson, M, Tolmachev, V, Andersson, K, Gedda, L, Sandström, M, and Carlsson, J. [(177) Lu]pertuzumab: experimental studies on targeting of HER-2 positive tumour cells. Eur J Nucl Med Mol Imaging. (2005) 32:1457–62. doi: 10.1007/s00259-005-1902-0
14. Miranda, ACC, Dos Santos, SN, Fuscaldi, LL, Balieiro, LM, Bellini, MH, Guimarães, MICC, et al. Radioimmunotheranostic pair based on the anti-HER2 monoclonal antibody: influence of chelating agents and radionuclides on biological properties. Pharmaceutics. (2021) 13:971. doi: 10.3390/pharmaceutics13070971
15. Tolmachev, V, Orlova, A, Pehrson, R, Galli, J, Baastrup, B, Andersson, K, et al. Radionuclide therapy of HER2-positive microxenografts using a 177Lu-labeled HER2-specific Affibody molecule. Cancer Res. (2007) 67:2773–82. doi: 10.1158/0008-5472.CAN-06-1630
16. de Jong, M, Breeman, WA, Bernard, BF, Bakker, WH, Visser, TJ, Kooij, PP, et al. Tumor response after [(90)Y-DOTA(0), Tyr(3)]octreotide radionuclide therapy in a transplantable rat tumor model is dependent on tumor size. J Nucl Med. (2001) 42:1841–6.
17. Müller, WA, Scháffer, EH, and Linzner, U Studies on incorporated short-lived beta-emitters with regard to the induction of late effects Radiat Environ Biophys (1980) 18 1–11 doi: 10.1007/BF01324368 1
18. Lauter, A, Strumpf, A, Platzbecker, U, Schetelig, J, Wermke, M, Radke, J, et al. 188Re anti-CD66 radioimmunotherapy combined with reduced-intensity conditioning and in-vivo T cell depletion in elderly patients undergoing allogeneic haematopoietic cell transplantation. Br J Haematol. (2010) 148:910–7. doi: 10.1111/j.1365-2141.2009.08025.x
19. Schneider, S, Strumpf, A, Schetelig, J, Wunderlich, G, Ehninger, G, Kotzerke, J, et al. Reduced-intensity conditioning combined with (188) rhenium radioimmunotherapy before allogeneic hematopoietic stem cell transplantation in elderly patients with acute myeloid leukemia: the role of in vivo T cell depletion. Biol Blood Marrow Transplant. (2015) 21:1754–60. doi: 10.1016/j.bbmt.2015.05.012
20. Ståhl, S, Gräslund, T, Eriksson Karlström, A, Frejd, FY, Nygren, P-Å, and Löfblom, J. Affibody molecules in biotechnological and medical applications. Trends Biotechnol. (2017) 35:691–712. doi: 10.1016/j.tibtech.2017.04.007
21. Hu, X, Hu, H, Li, D, Wang, P, and Cai, J. Affibody-based molecular probe 99mTc-(HE)3ZHER2:V2 for non-invasive HER2 detection in ovarian and breast cancer xenografts. Open Med. (2024) 19:20241027. doi: 10.1515/med-2024-1027
22. Altai, M, Wållberg, H, Honarvar, H, Strand, J, Orlova, A, Varasteh, Z, et al. 188Re-ZHER2:V2, a promising affibody-based targeting agent against HER2-expressing tumors: preclinical assessment. J Nucl Med. (2014) 55:1842–8. doi: 10.2967/jnumed.114.140194
23. Liu, Y, Vorobyeva, A, Orlova, A, Konijnenberg, MW, Xu, T, Bragina, O, et al. Experimental therapy of HER2-expressing xenografts using the second-generation HER2-targeting Affibody molecule 188Re-ZHER2:41071. Pharmaceutics. (2022) 14:1092. doi: 10.3390/pharmaceutics14051092
24. Orlova, A, Jonsson, A, Rosik, D, Lundqvist, H, Lindborg, M, Abrahmsen, L, et al. Site-specific radiometal labeling and improved biodistribution using ABY-027, a novel HER2-targeting affibody molecule-albumin-binding domain fusion protein. J Nucl Med. (2013) 54:961–8. doi: 10.2967/jnumed.112.110700
25. Bragina, O, von Witting, E, Garousi, J, Zelchan, R, Sandström, M, Orlova, A, et al. Phase I study of 99mTc-ADAPT6, a scaffold protein-based probe for visualization of HER2 expression in breast cancer. J Nucl Med. (2021) 62:493–9. doi: 10.2967/jnumed.120.248799
26. Glaser, M, Iveson, P, Hoppmann, S, Indrevoll, B, Wilson, A, Arukwe, J, et al. Three methods for 18F labeling of the HER2-binding affibody molecule Z (HER2:2891) including preclinical assessment. J Nucl Med. (2013) 54:1981–8. doi: 10.2967/jnumed.113.122465
27. Sörensen, J, Sandberg, D, Sandström, M, Wennborg, A, Feldwisch, J, Tolmachev, V, et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J Nucl Med. (2014) 55:730–5. doi: 10.2967/jnumed.113.131243
28. Sörensen, J, Velikyan, I, Sandberg, D, Wennborg, A, Feldwisch, J, Tolmachev, V, et al. Measuring HER2-receptor expression in metastatic breast Cancer using [68Ga]ABY-025 Affibody PET/CT. Theranostics. (2016) 6:262–71. doi: 10.7150/thno.13502
29. Yang, Y, Zhao, X, Xing, Y, Yu, T, Zhang, J, and Wang, J. Preclinical evaluation of 99mTc direct labeling ZHER2:V2 for HER2 positive tumors imaging. Oncol Lett. (2018) 16:5361–6. doi: 10.3892/ol.2018.9279
30. Hu, X, Liang, Z, Qi, L, Li, F, Cai, X, and Cai, J. Radiosynthesis, optimization and pharmacokinetic study of the 99mTc-labeled human epidermal growth factor receptor 2 affibody molecule probe 99mTc-(HE)3ZHER2:V2. Nucl Med Commun. (2023) 44:244–51. doi: 10.1097/MNM.0000000000001660
31. Yamada, A, Traboulsi, A, Dittert, LW, and Hussain, AA. Chloramine-T in radiolabeling techniques. III. Radioiodination of biomolecules containing thioether groups. Anal Biochem. (2000) 277:232–5. doi: 10.1006/abio.1999.4378
32. Flemmig, J, Gau, J, Schlorke, D, and Arnhold, J. Lactoperoxidase as a potential drug target. Expert Opin Ther Targets. (2016) 20:447–61. doi: 10.1517/14728222.2016.1112378
33. Chen, J, Wang, M, Joyce, A, DeFranco, D, Kavosi, M, Xu, X, et al. Comparison of succinimidyl [(125) I] iodobenzoate with iodogen iodination methods to study pharmacokinetics and ADME of biotherapeutics. Pharm Res. (2014) 31:2810–21. doi: 10.1007/s11095-014-1378-3
Keywords: human epidermal growth factor receptor 2, affibody, 131 I, radionuclide targeted therapy, HER2
Citation: Hu H, Hu X, Li F, Wang G and Cai J (2025) Affibody-based targeting agent 131I-YZHER2: V2 for HER2-positive ovarian cancer xenografts. Front. Med. 12:1507596. doi: 10.3389/fmed.2025.1507596
Edited by:
Giorgio Treglia, Ente Ospedaliero Cantonale (EOC), SwitzerlandReviewed by:
Calogero D’Alessandria, Technical University of Munich, GermanyChristopher Stephen Lange, Downstate Health Sciences University, United States
Copyright © 2025 Hu, Hu, Li, Wang and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiong Cai, amlvbmdfY2FpQDE2My5jb20=
†These authors have contributed equally to this work
 Hongyu Hu
Hongyu Hu Xianwen Hu
Xianwen Hu Fangming Li
Fangming Li Jiong Cai
Jiong Cai