- Ward 13 (Respiratory Digestive Geriatrics), Huzhou Third Municipal Hospital, The Affiliated Hospital of Huzhou University, Huzhou, Zhejiang, China
Background: Early detection, systematic prevention, and personalized therapy are crucial to reduce mortality in patients with gastrointestinal (GI) cancers. This systematic review and meta-analysis aimed to clarify the predictive value of osteopenia and osteosarcopenia as prognostic markers of survival and recurrence in patients with GI cancers.
Methods: Medline, Google Scholar, and Science Direct databases were searched for English-language studies that included patients who underwent surgical resection following a pathologically diagnosed GI cancer and reported the association between osteopenia and osteosarcopenia on the overall survival (OS) and recurrence-free survival (RFS). Meta-analysis was done using STATA 14.2, and the results were reported as pooled hazard ratios (HR) with 95% confidence intervals (CI). Heterogeneity was assessed using the I2 statistic and the Chi-square test. Study quality was evaluated using the Newcastle Ottawa Scale (NOS).
Results: A comprehensive literature search yielded 23 eligible studies, primarily from Japan. Osteopenia emerged as a significant risk factor for both OS (pooled HR 2.20, 95% CI: 1.74–2.79) and RFS (pooled HR 2.15, 95% CI: 1.60–2.89). Patients with osteosarcopenia exhibited threefold higher mortality rates (pooled HR 2.96, 95% CI: 1.99–4.40) and heightened risk of recurrence (pooled HR 2.75, 95% CI: 1.79–4.24). Subgroup analyses underscored the consistency of these associations across diverse contexts.
Conclusion: This meta-analysis establishes osteopenia and osteosarcopenia as robust prognostic indicators for survival and recurrence in GI cancers. Integrating musculoskeletal assessments into routine oncological care is imperative for timely interventions and optimized patient outcomes.
Introduction
Gastrointestinal (GI) cancers include malignancies of the esophagus, stomach, pancreas and biliary apparatus, liver and colon (1). GI cancers represent a formidable global health challenge, contributing significantly to morbidity and mortality (2). The prognosis of GI cancers may be influenced by many factors, such as tumor size, extent of metastases, and musculoskeletal status of patients that emerges as a critical determinant of overall well-being (3, 4).
Numerous studies have focused on the relationship between body composition and cancer prognosis. Recent reports have shown that osteopenia, characterized by low bone mineral density [BMD], sarcopenia, marked by loss of skeletal muscle mass, and osteosarcopenia, defined as the coexistence of osteopenia along with sarcopenia in cancer patients, are conditions that may potentially impact GI cancer outcomes (5, 6). Low BMD is often linked with an increased risk of falls, fractures, hospitalization, and even death, thereby negatively impacting the health-related quality of life (5). Additionally, bone loss in cancer patients may reflect osteopenia, malnutrition, and systemic inflammation (7). Recent studies demonstrated that in cancer patients, sarcopenia may be viewed not just as a malnutritional alteration but also as a systemic inflammatory change (8, 9). Furthermore, cancer-induced changes in metabolism, inflammatory status, and hormonal regulation may in turn contribute to the development and progression of osteopenia and sarcopenia (9).
The intricate relationship between osteopenia, sarcopenia, and cancer outcomes is still unclear. Existing studies often focus on individual components—tumor characteristics, treatment modalities, and patient demographics—neglecting the combined impact of bone and muscle health on patient outcomes (10, 11). GI cancers often impair nutrient absorption, leading to deficiencies that contribute to bone loss and worse clinical outcomes. Osteopenia is linked to increased chemotherapy toxicity, poor surgical recovery, and higher recurrence rates, making it a valuable early predictor of prognosis (7, 12). Therefore, due to the strong association of osteopenia with malnutrition, sarcopenia, and cancer cachexia, all of which are prevalent in GI cancer patients, it is crucial to further assess its value as a potential prognostic marker in this type of cancer. This comprehensive systematic review and meta-analysis aim to evaluate the predictive value of osteopenia and osteosarcopenia as prognostic markers of survival and recurrence in patients with GI cancers. Our results may contribute to developing tailored interventions and improving the prognostic accuracy of GI cancer outcomes.
Materials and methods
Research questions
Is there an association between osteopenia and osteosarcopenia with outcomes such as overall survival (OS) and recurrence-free survival (RFS) among patients with gastrointestinal cancers?
Objective
To evaluate the predictive value of osteopenia and osteosarcopenia as prognostic markers of survival and recurrence in patients with GI cancers.
Inclusion and exclusion criteria (PECO)
Population
Cancer patients who underwent surgical resection following a pathologically diagnosed digestive tract cancer (gastric, colorectal, esophageal, liver, biliary tract, pancreatic, and gallbladder) were chosen as study participants.
Exposure
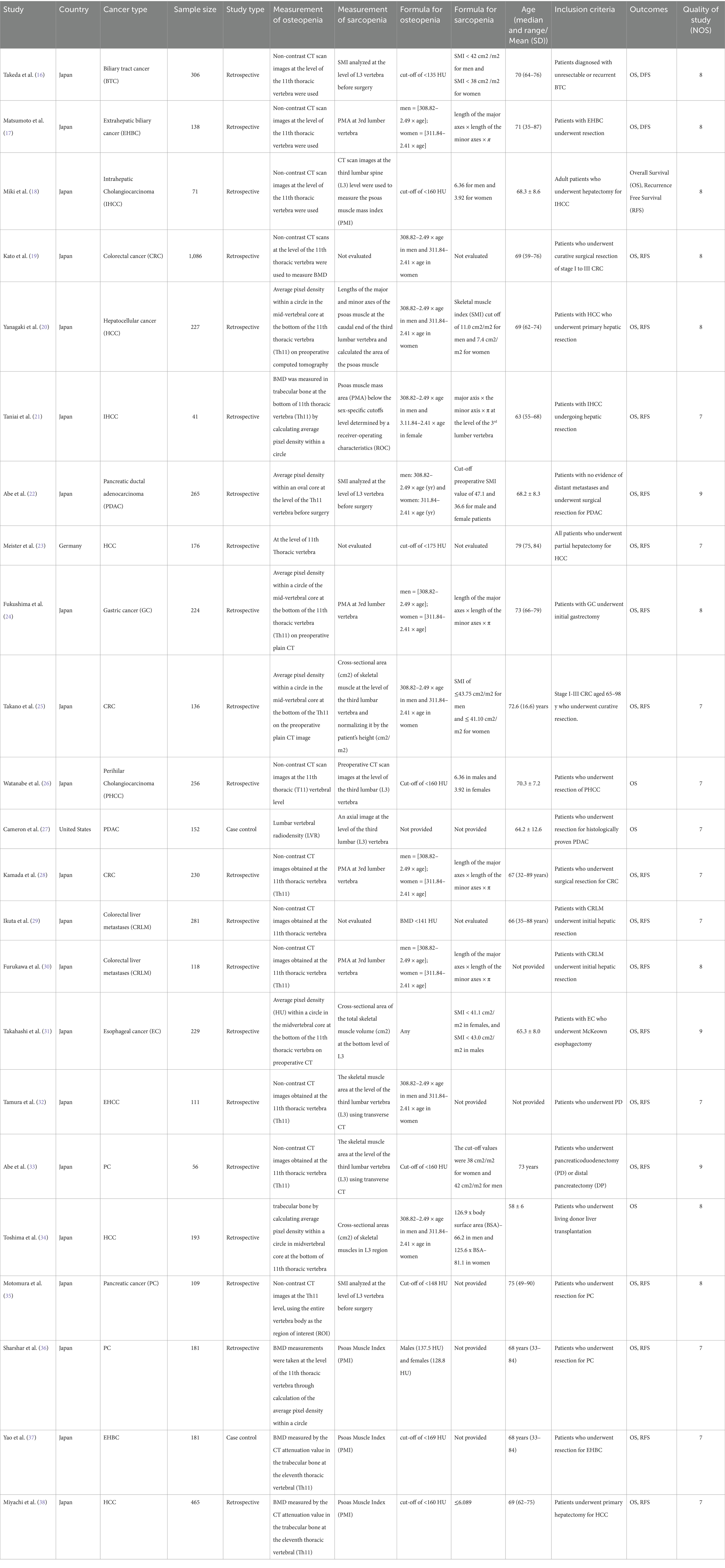
Preoperative osteopenia was the main exposure of interest. Osteopenia was defined using the BMD, in accordance with the individual studies (The individual author’s cut-offs for BMD were considered to categorize osteopenia). This study also included osteosarcopenia (coexistence of osteopenia and sarcopenia together). The definitions used for osteopenia and sarcopenia are elaborated in Table 1.
Outcome
The primary outcomes of interest were OS and RFS. OS was defined as the patient’s death between the date of resection and the last point of contact with the patient. RFS was calculated from the date of the tumor’s resection to the first recurrence at any site.
Study design
The review included all analytical designs, including cross-sectional, prospective, and retrospective studies.
Exclusion criteria
Studies not reported in English, studies that were not retrievable, case reports, case series, and grey literature were excluded. The search was not restricted to a specific region or publication year.
Our literature search encompassed three databases: Medline, Google Scholar, and Science Direct, from inception until December 2023.
Primary and secondary data screenings were independently conducted by both authors. Any conflicts that arose between them were resolved through mutual consensus. The reporting of our review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) framework (13). During the primary screening, both authors screened titles and abstracts of the studies, removing any duplicates. In the subsequent secondary screening, full texts of the selected studies were reviewed using the inclusion criteria, and relevant information was extracted.
Both authors created and meticulously checked a data extraction template to ensure completeness and accuracy. Information such as author details, region, study design, inclusion criteria, type of cancer, sample size, definition of OS, SP, OSP, and the cut-offs used were extracted from individual studies and entered into the template.
The databases and PROSPERO were examined to ascertain the absence of prior systematic reviews on the same topic, confirming the novelty of our review (CRD42023493216).
Search strategy
The following Medical subject heading (MeSH) terms were used: “Digestive tract cancer” OR “Digestive tract tumours” OR “Gastrointestinal neoplasms” AND “Osteopenia” OR “Low BMD” AND “Osteosarcopenia” AND “Survival” OR “Death” AND “Outcome” AND “Recurrence free survival” AND “Disease free survival” AND “Observational studies” OR “Cohort studies” OR “Prospective studies.” Reference list of included articles were screened for any potentially relevant studies. The detailed search strategy is provided as Supplementary material.
Statistical analysis
All statistical analyses were performed using STATA 14.2. Binary outcomes (OS, DFS & RFS) were analyzed using the inverse variance method to combine effects across various studies, expressing outcomes as pooled hazards ratios (HR) with 95% confidence intervals (CIs). The Freeman-Tukey double arcsine transformation was applied to mitigate the potential influences of both large and small studies on pooled estimates. Diligent attempts were made to contact the authors for missing data. Results, presented as pooled effect sizes, were visually depicted through forest plots. Publication bias was assessed using funnel plots, and statistical tests were conducted using Egger’s test (14). Heterogeneity was assessed by I2 statistic and the Chi-square heterogeneity test. Heterogeneity levels were categorized as mild (I2 < 25%), moderate (I2 between 25 and 75%), and substantial (I2 > 75%). Due to expected heterogeneity in study definition and population, a random-effects model was used to account for the variation in effect sizes among the included studies. The between-study variance (τ2) was estimated using the Der Simonian and Laird technique, and the pooled hazard ratios (HRs) for survival outcomes were calculated using the inverse variance approach. p < 0.05 was statistically significant.
Quality assessment of included studies
The Newcastle Ottawa Scale (NOS) (15) was used to evaluate study quality. This scale assesses studies based on outcomes, selection of study groups, and comparability, with a maximum score of nine for each study.
Results
Study selection
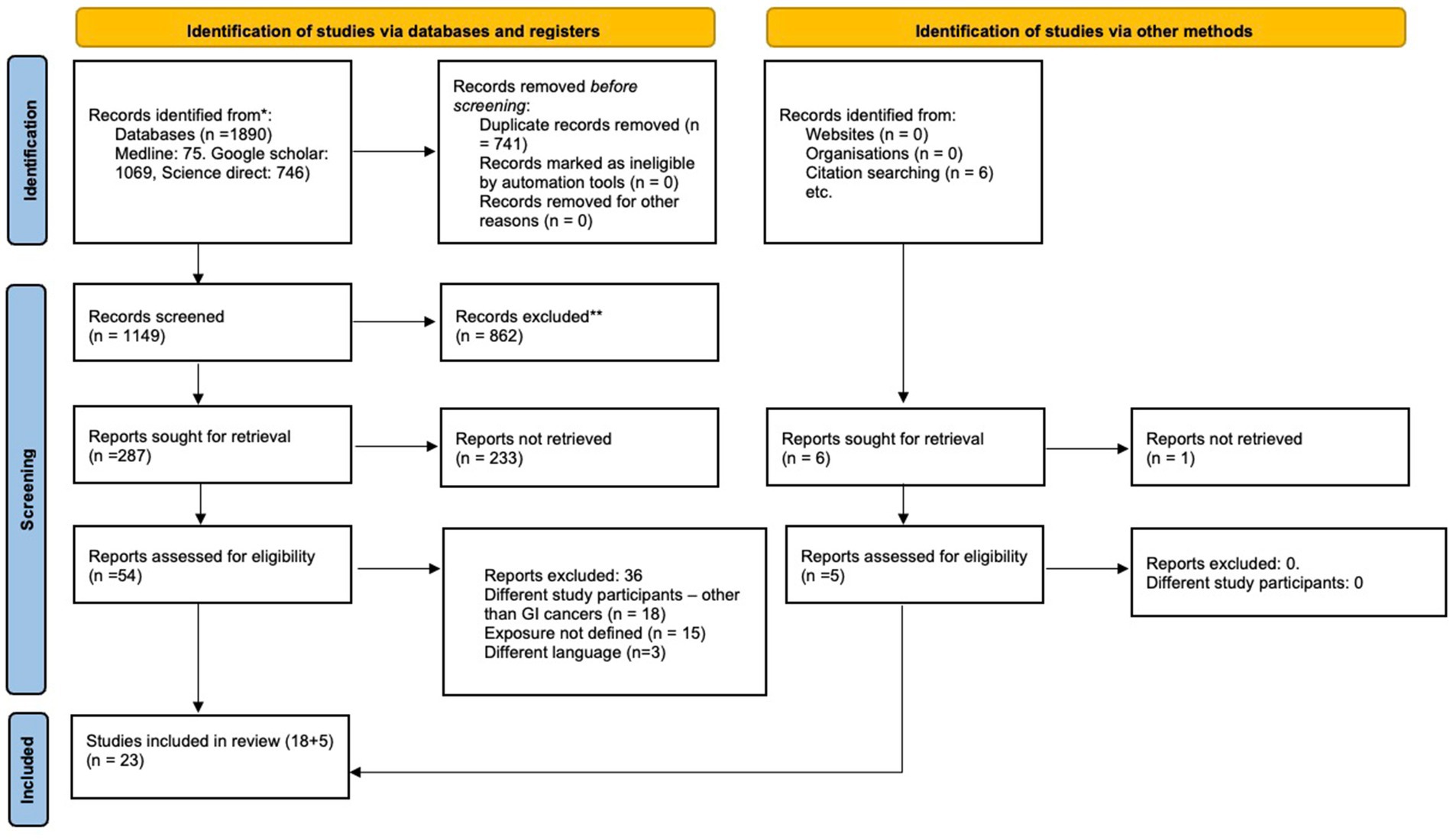
The initial search identified 1890 articles. After primary screening, 741 studies were removed as duplicates, and an additional 862 studies were removed at the stage of titles and abstracts evaluation. Of the remaining 287 articles, 54 free full-texts were retrieved for secondary screening, and 23 articles were ultimately selected for this systematic review and meta-analysis (16–38).
The reasons for exclusion were as follows: 18 studies reported on patients with other cancers, 15 did not define the exposure clearly, and 3 were not in English.
Characteristics of the included studies
The general characteristics of the included studies are outlined in Table 1. Of 23 studies, 21 were from Japan, and one study each was from Germany and the United States. Sample sizes of included studies ranged from 41 to 1,086. A majority (21/23) were retrospective. Figure 1 explains the study selection process. Twenty-one articles reported on the association between osteopenia and OS (18–38), 18 reported on the association between osteopenia and RFS (18–25, 28–33, 35–38). The association between OS and osteosarcopenia was reported by six studies (16, 17, 20–22, 25), RFS and osteosarcopenia were reported by five studies (17, 20–22, 25) and thus were pooled for the meta-analysis.
Association between osteopenia (low BMD) with OS and DFS
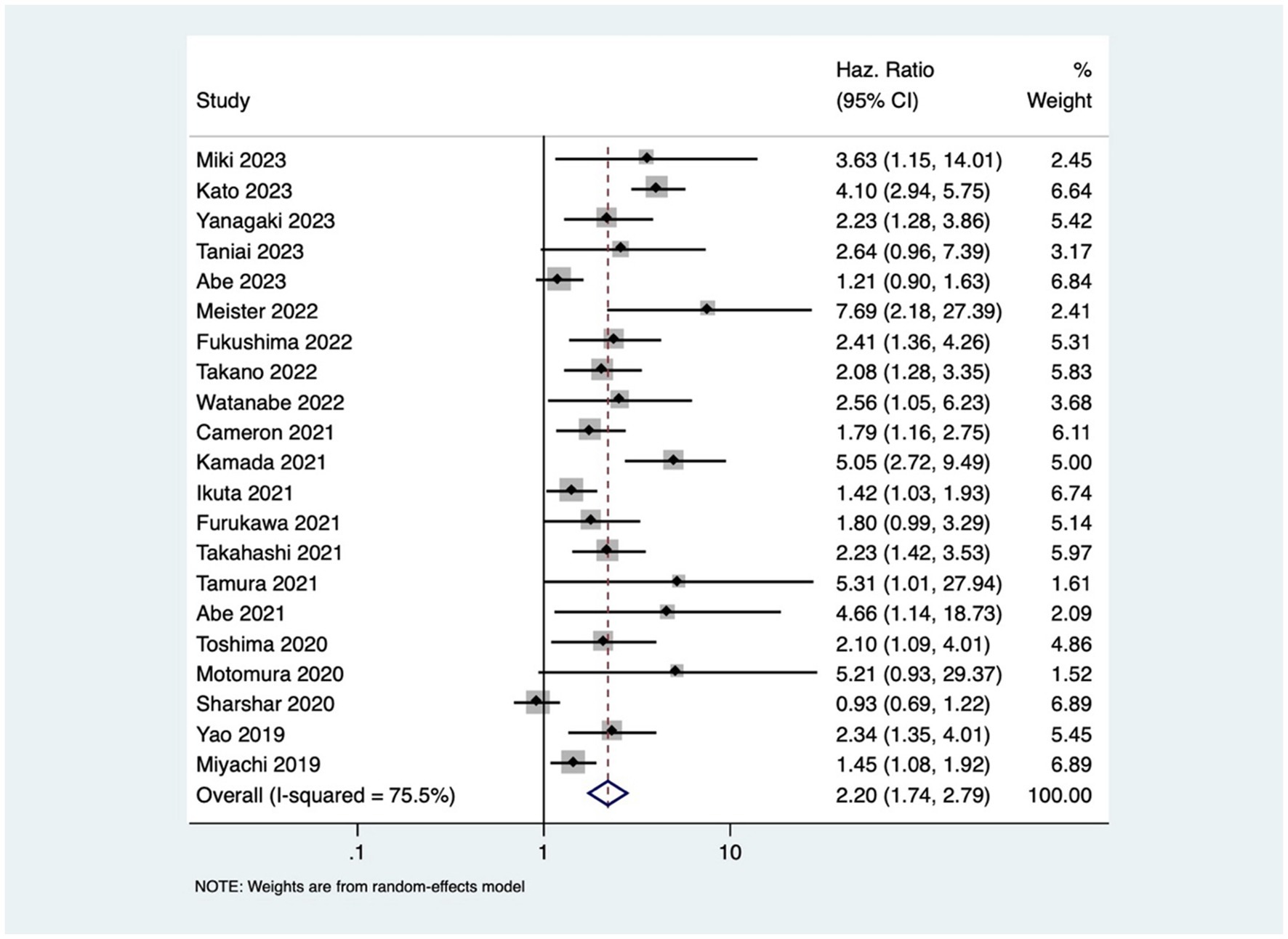
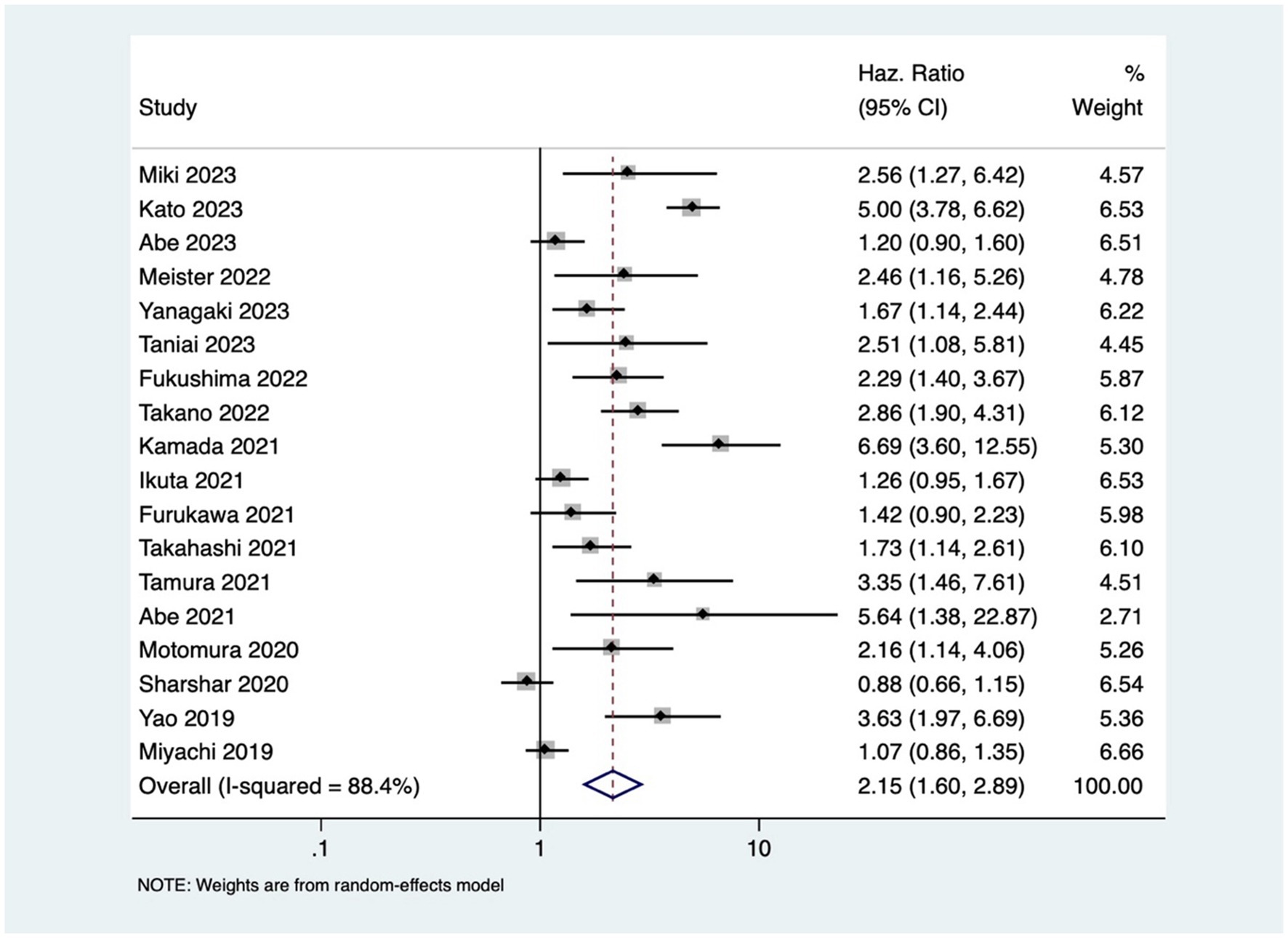
Patients with osteopenia or low BMD had significantly poorer OS (pooled HR of 2.20, 95% CI: 1.74–2.79, with high heterogeneity I2=75.5, p-value <0.001) (Figure 2). The osteopenia was associated with lower RFS (pooled HR of 2.15, 95% CI: 1.60–2.89, with high heterogeneity I2=88.4, p-value <0.001) (Figure 3). Due to the high heterogeneity observed across the studies, subgroup analysis was done to investigate the reasons for clinical heterogeneity. The type of GI cancer, geographical region of included studies, and sample size showed a significant association between incidences of osteopenia and survival outcomes (except for the association between low BMD with OS and low BMD with RFS among pancreatic cancer patients) (Supplementary Figures 1–6).
Association between osteosarcopenia with OS and DFS
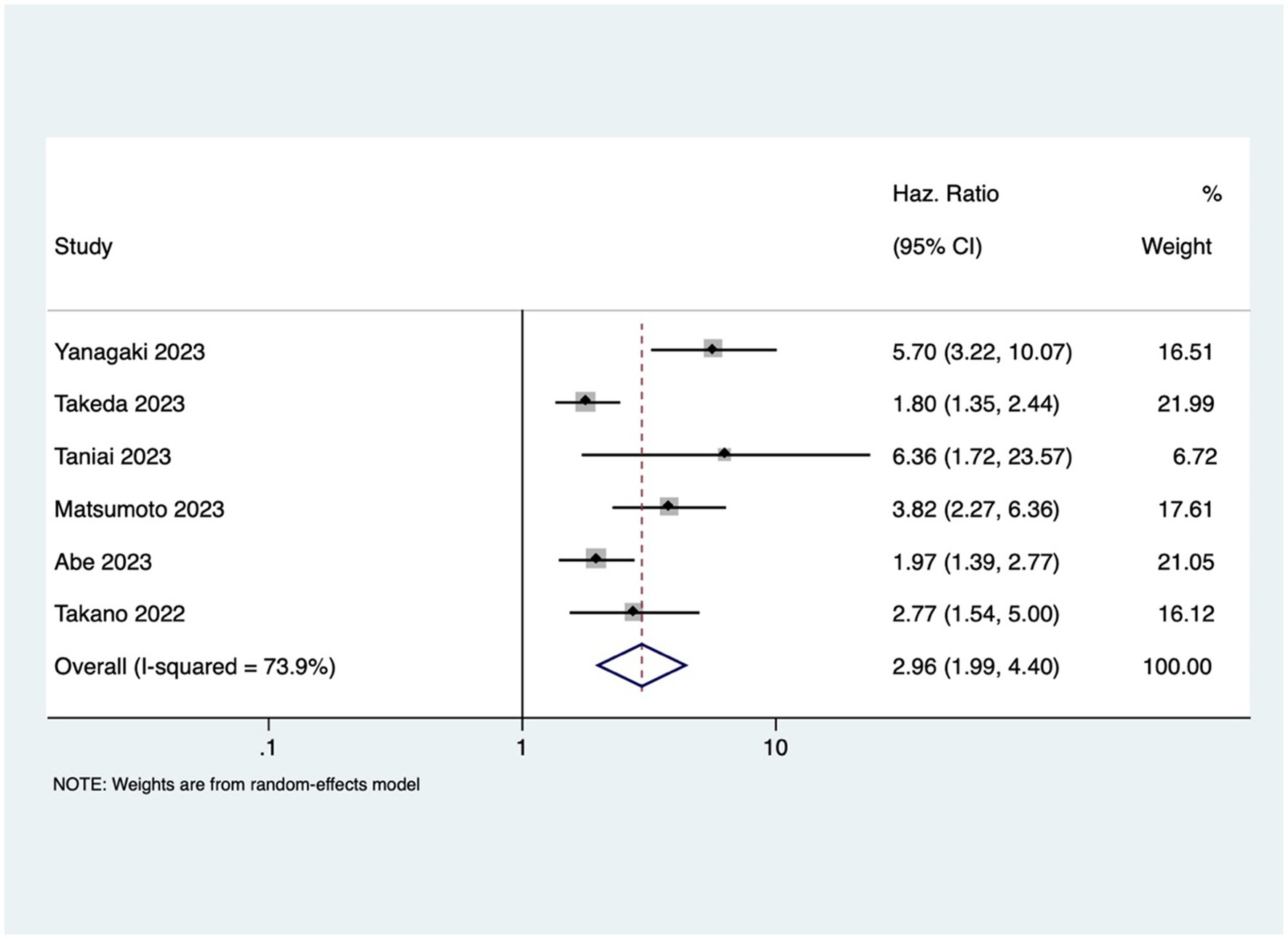
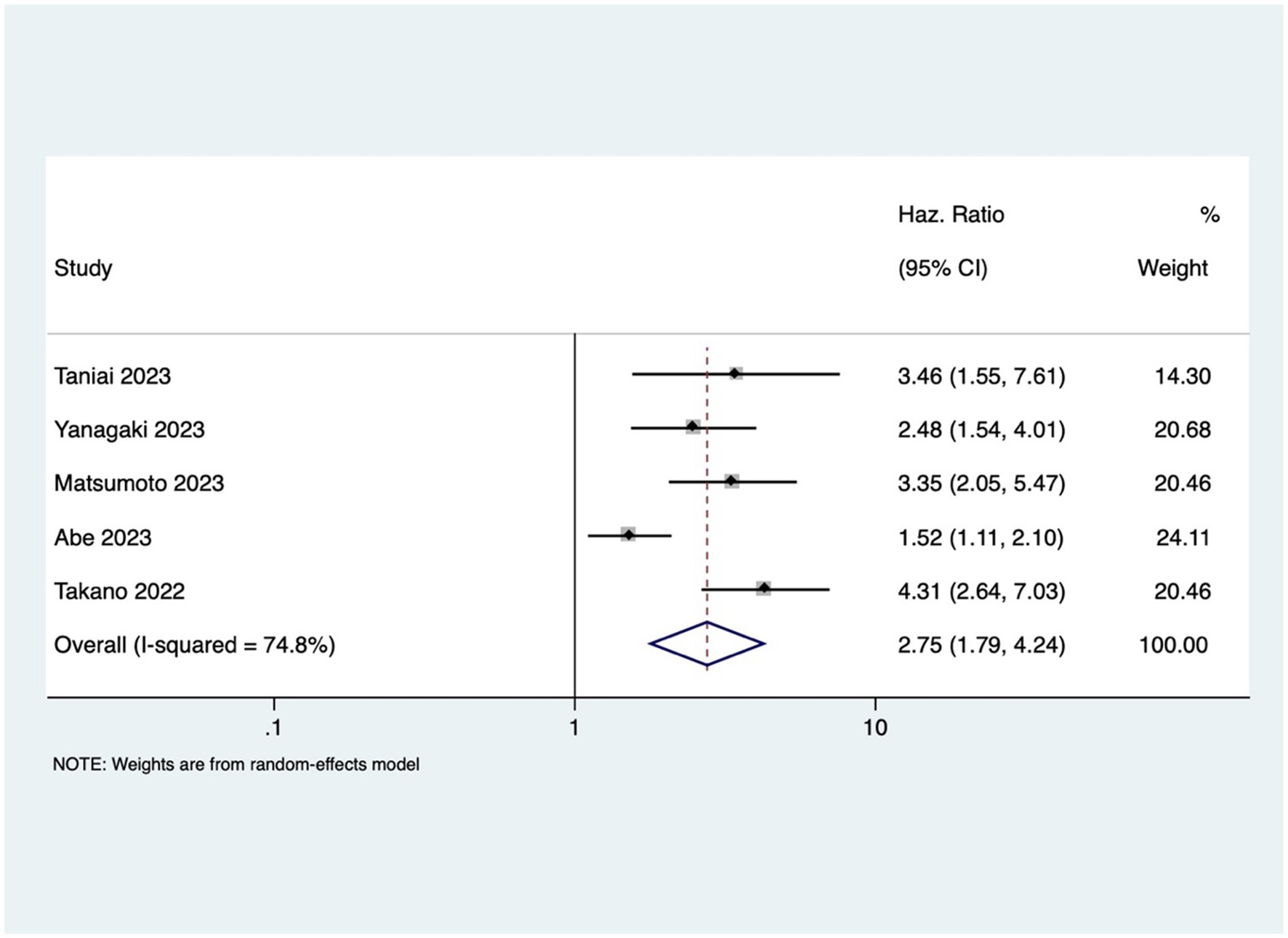
GI cancer patients with osteosarcopenia had three 3 times higher mortality risk compared to patients without osteosarcopenia (pooled HR 2.96, 95% CI: 1.99–4.40, with high heterogeneity I2=73.9, p-value <0.001) (Figure 4). Osteosarcopenia was a significant risk factor for poor RFS (pooled HR of 2.75, 95% CI: 1.79–4.24, with high heterogeneity I2=74.8, p-value <0.001) (Figure 5).
Risk of bias
The asymmetric funnel plot showed evidence of publication bias for the association between osteopenia with OS and RFS (Supplementary Figures 7, 8).
Table 1 summarizes the risk of bias in the included studies, as assessed by NOS. Supplementary Figures 9, 10 show the effect of the risk of bias on the association between OS and RFS with osteopenia.
Discussion
This meta-analysis showed that low BMD, osteopenia, and osteosarcopenia are potentially significant risk factors for poor OS and RFS among GI cancer patients. These findings highlight the need for preoperative assessment of GI cancer patients for timely interventions that may improve patient outcomes.
Together with genetics and ethnicity, BMD is a composite indicator reflecting exposure to multiple factors over the course of a patient’s life (19). BMD positively correlates with patient’s levels of estrogens, calcium and vitamin D intake, weight, and physical activity (39). Low BMD, therefore, is closely associated with factors that influence GI cancers either positively (calcium, vitamin D, oral contraceptives, physical activity) or negatively (age, BMI, smoking, alcohol) (40).
This study showed that GI cancer patients with osteopenia have 2-fold higher risk of death [pooled HR of 2.20, 95% CI: 1.74–2.79] and cancer recurrence [pooled HR of 2.15, 95% CI: 1.60–2.89]. These findings are comparable to the previous study done by Watanabe et al. that reported pooled HR of 2.02 and 1.96 for OS and RFS, respectively (41) The observed high heterogeneity in the association between osteopenia/osteosarcopenia and the outcomes might be attributed to variations in cancer types and stages, reflecting the heterogeneous nature of GI cancers. Despite the increased risk, the mechanism underlying osteopenia’s negative impact on prognosis is still unclear. One possible mechanism of this effect may be osteoclast stimulation brought on by cancer cachexia (severe, unintentional loss of weight, muscle mass, and strength due to chronic inflammation and metabolic dysfunction), resulting in bone loss (42). The compromised structural integrity of bones in patients with osteopenia may render them more susceptible to the skeletal complications of cancer (such as osteoporosis, fracture, and bone loss), contributing to the observed increased rates of mortality and cancer recurrence. Additionally, cytokines produced from cancer cells, such as PTHrP, interleukin (IL)-1, IL-6, and IL-8, create and activate osteoclasts through activating the RANK/RANKL receptors, and subsequently, NF-κB (43), which leads to muscle loss and sarcopenia (5, 44, 45). This study revealed that osteosarcopenia that encompasses both bone and muscle deficits was associated with 3-times higher mortality in GI cancer patients.
The interplay between chronic inflammation (increased IL-6 and TNF-α leading to osteoclast activation and muscle protein breakdown, increased NF-κB and RANKL expression), muscle-bone crosstalk dysregulation (myostatin overexpression, irisin and osteocyte dysfunction), metabolic dysfunction, and tumor microenvironment alterations (IGF-1 suppression and adipokines and endocrine dysfunction) underlies the association between osteopenia/osteosarcopenia and poor survival in GI cancers (44, 45).
The results of this study further corroborate other reports highlighting the compounded impact of this complex condition (46). While our findings were comparable with previous reports (16, 17, 22), the observed mortality rates associated with osteosarcopenia were slightly lower compared to other studies [HR >5] (20, 21). It is plausible that variations in study design, sample size, patient demographics, and follow-up period could cause this disparity. It’s possible that selection bias was more likely to affect earlier research with smaller sample numbers, which resulted in inflated hazard ratios. Inconsistencies between studies may have also been caused by differences in diagnostic thresholds, imaging modalities, and definitions of osteosarcopenia (47).
Additionally, this study showed that osteosarcopenia was associated with poorer RFS (pooled HR of 2.75; p < 0.001). This observation further emphasizes the need for a comprehensive assessment that includes both musculoskeletal aspects.
The subgroup analysis showed that osteopenia was associated with poor OS in patients with colorectal cancer (HR of 2.5) and lower RFS in patients with bile duct and colorectal cancer (HR of 3 and 2.75, respectively). These results are in agreement with the previous meta-analysis by Watanabe et al. that showed the highest mortality rates in patients with colorectal cancer in combination with osteopenia and a maximum risk for recurrence in patients with osteopenia and colorectal or bile duct cancer (41).
However, no association was detected in pancreatic cancer patients. This discrepancy may be due to the aggressive tumor biology and early metastatic spread of pancreatic cancer, which may overshadow the impact of osteopenia on survival. Additionally, treatment-related malabsorption (Whipple surgery leading to malabsorption, etc), cachexia, and vitamin D deficiencies might have confounded the relationship between survival and low bone mineral density. Variations in assessment methods, such as computer tomography (CT) vs. dual-energy X-ray absorptiometry (DXA) and heterogeneity in patient cohorts could also contribute to the inconsistency (48, 49).
It is also important to consider that cancer chemotherapies, including alkylating agents, FOLFIRI, antimetabolites, glucocorticoids, and platinum-derived cisplatin, cause direct dysregulation of bone turnover and nephrotoxicity, which expedite bone loss (46, 50). Additionally, low BMD-specific outcomes, especially frailty fractures, could significantly impair functional status and physical activity. This, in turn, could result in non-cancer mortality or non-adherence to cancer treatment, which triggers recurrence (51).
Strengths and limitations
The main strengths of this review and meta-analysis are the inclusion of a substantial number of studies, rigorous screening processes, and comprehensive subgroup analyses that enhance the robustness of our findings.
However, this study has certain limitations. The high heterogeneity between the studies might impact the precision of our estimates.
One major limitation is the lack of standardized definitions for osteopenia and sarcopenia, which varied across the included studies. This might have introduced heterogeneity in the findings, affecting the comparability of results. Additionally, while DXA is considered a gold standard for diagnosing osteopenia, all studies included in this review diagnosed osteopenia using preoperative CT. Thus, over-reliance on CT-based measurements instead of DXA to assess BMD presents another challenge. Moreover, different studies used different threshold values for defining osteopenia. Most included studies were from Japan, thus limiting the generalisability of the findings. The predominance of Japanese studies in this meta-analysis raises concerns about the generalizability of our findings due to cultural, genetic, dietary, and healthcare system differences. Traditional Japanese diets, lower obesity rates, and distinct genetic factors influencing bone and muscle metabolism may affect the prevalence and impact of osteopenia differently than in Western populations. Finally, this study was unable to rule out the potential publication and language biases (since the review included only studies published in English).
Conclusion and recommendations
In conclusion, this systematic review and meta-analysis show that osteopenia and osteosarcopenia are associated with significantly worse outcomes in patients with GI cancers. These results shed light on the intricate interplay between musculoskeletal health and outcomes in this population of patients. This study provides a robust foundation for integrating musculoskeletal assessments such as routine sarcopenia and osteopenia screening using tools like CT-based body composition analysis or DXA into the prognostic considerations for these cancers, and further strengthens the need of a holistic approach to GI cancer management that considers not only tumor characteristics but also patient’s bone and muscle health. Future research should also explore interventional strategies aimed at mitigating the negative impact of osteopenia and sarcopenia in GI cancer patients. Trials investigating the use of exercise therapies (resistance training and muscle mass training) nutritional supplementation, and pharmacological interventions (such as anti-resorptive agents like bisphosphonates or denosumab) among cancer patients with osteopenia and osteosarcopenia with standardized diagnostic criteria are necessary.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: Medline, Google Scholar, and Science Direct, from inception until December 2023.
Author contributions
XZ: Conceptualization, Data curation, Formal analysis, Writing – original draft, Methodology. YW: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing, Project administration, Validation, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1527829/full#supplementary-material
SUPPLEMENTARY FIGURE 1 | Forest plot of overall survival for osteopenia grouped by cancer type.
SUPPLEMENTARY FIGURE 2 | Forest plot of overall survival for osteopenia grouped by country.
SUPPLEMENTARY FIGURE 3 | Forest plot of overall survival for osteopenia grouped by sample size.
SUPPLEMENTARY FIGURE 4 | Forest plot of recurrence free survival for osteopenia grouped by cancer type.
SUPPLEMENTARY FIGURE 5 | Forest plot of recurrence free survival for osteopenia grouped by country.
SUPPLEMENTARY FIGURE 6 | Forest plot of recurrence free survival for osteopenia grouped by sample size.
SUPPLEMENTARY FIGURE 7 | Funnel plot of overall survival for osteopenia.
SUPPLEMENTARY FIGURE 8 | Funnel plot of recurrence free survival for osteopenia.
SUPPLEMENTARY FIGURE 9 | Funnel plot of overall survival for osteopenia by quality of included studies.
SUPPLEMENTARY FIGURE 10 | Funnel plot of recurrence free survival for osteopenia by quality of included studies.
References
1. Siegel, RL, Miller, KD, Fuchs, HE, and Jemal, A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
2. Peery, AF, Crockett, SD, Murphy, CC, Lund, JL, Dellon, ES, Williams, JL, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. (2019) 156:254–272.e11. doi: 10.1053/j.gastro.2018.08.063
3. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyère, O, Cederholm, T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:601. doi: 10.1093/ageing/afz046
4. Su, H, Ruan, J, Chen, T, Lin, E, and Shi, L. CT-assessed sarcopenia is a predictive factor for both long-term and short-term outcomes in gastrointestinal oncology patients: a systematic review and meta-analysis. Cancer Imaging. (2019) 19:82. doi: 10.1186/s40644-019-0270-0
5. Verschueren, S, Gielen, E, O’Neill, TW, Pye, SR, Adams, JE, Ward, KA, et al. Sarcopenia and its relationship with bone mineral density in middle-aged and elderly European men. Osteoporos Int. (2013) 24:87–98. doi: 10.1007/s00198-012-2057-z
6. Kirk, B, Zanker, J, and Duque, G. Osteosarcopenia: epidemiology, diagnosis, and treatment-facts and numbers. J Cachexia Sarcopenia Muscle. (2020) 11:609–18. doi: 10.1002/jcsm.12567
7. Pin, F, Bonewald, LF, and Bonetto, A. Role of myokines and osteokines in cancer cachexia. Exp Biol Med. (2021) 246:2118–27. doi: 10.1177/15353702211009213
8. Lavalle, S, Valerio, MR, Masiello, E, Gebbia, V, and Scandurra, G. Unveiling the intricate dance: how Cancer orchestrates muscle wasting and sarcopenia. In Vivo. (2024) 38:1520–9. doi: 10.21873/invivo.13602
9. Looijaard, SMLM, Te Lintel Hekkert, ML, Wüst, RCI, Otten, RHJ, Meskers, CGM, and Maier, AB. Pathophysiological mechanisms explaining poor clinical outcome of older cancer patients with low skeletal muscle mass. Acta Physiol. (2021) 231:e13516. doi: 10.1111/apha.13516
10. Clemente-Suárez, VJ, Redondo-Flórez, L, Rubio-Zarapuz, A, Martínez-Guardado, I, Navarro-Jiménez, E, and Tornero-Aguilera, JF. Nutritional and exercise interventions in Cancer-related Cachexia: an extensive narrative review. Int J Environ Res Public Health. (2022) 19:4604. doi: 10.3390/ijerph19084604
11. Prado, CM, Purcell, SA, and Laviano, A. Nutrition interventions to treat low muscle mass in cancer. J Cachexia Sarcopenia Muscle. (2020) 11:366–80. doi: 10.1002/jcsm.12525
12. Hirase, Y, Arigami, T, Matsushita, D, Shimonosono, M, Tsuruda, Y, Sasaki, K, et al. Prognostic significance of osteosarcopenia in patients with stage IV gastric cancer undergoing conversion surgery. Langenbeck's Arch Surg. (2024) 410:7. doi: 10.1007/s00423-024-03574-8
13. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
14. Egger, M, Davey Smith, G, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
15. Lo, CK-L, Mertz, D, and Loeb, M. Newcastle-Ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
16. Takeda, T, Okamoto, T, Sasaki, T, Hirai, T, Ishitsuka, T, Yamada, M, et al. The impact of osteosarcopenia in patients with unresectable or recurrent biliary tract cancer receiving palliative chemotherapy. Jpn J Clin Oncol. (2023) 53:1051–7. doi: 10.1093/jjco/hyad097
17. Matsumoto, M, Onda, S, Igarashi, Y, Hamura, R, Uwagawa, T, Furukawa, K, et al. Osteosarcopenia is a significant predictor of recurrence and the prognosis after resection for extrahepatic bile duct cancer. Surg Today. (2023) 54:407–18. doi: 10.1007/s00595-023-02747-0
18. Miki, A, Sakuma, Y, Watanabe, J, Endo, K, Sasanuma, H, Teratani, T, et al. Osteopenia is associated with shorter survival in patients with intrahepatic cholangiocarcinoma. Curr Oncol. (2023) 30:1860–8. doi: 10.3390/curroncol30020144
19. Kato, H, Seishima, R, Mizuno, S, Matsui, S, Shigeta, K, Okabayashi, K, et al. The prognostic impact of preoperative osteopenia in patients with colorectal Cancer. Dis Colon Rectum. (2023) 66:e1225–33. doi: 10.1097/DCR.0000000000002961
20. Yanagaki, M, Haruki, K, Taniai, T, Igarashi, Y, Yasuda, J, Furukawa, K, et al. The significance of osteosarcopenia as a predictor of the long-term outcomes in hepatocellular carcinoma after hepatic resection. J Hepatobiliary Pancreat Sci. (2023) 30:453–61. doi: 10.1002/jhbp.1246
21. Taniai, T, Haruki, K, Yanagaki, M, Igarashi, Y, Furukawa, K, Onda, S, et al. Osteosarcopenia predicts poor prognosis for patients with intrahepatic cholangiocarcinoma after hepatic resection. Surg Today. (2023) 53:82–9. doi: 10.1007/s00595-022-02550-3
22. Abe, T, Nakata, K, Nakamura, S, Ideno, N, Ikenaga, N, Fujita, N, et al. Prognostic impact of preoperative Osteosarcopenia for patients with pancreatic ductal adenocarcinoma after curative resection. Ann Surg Oncol. (2023) 30:6673–9. doi: 10.1245/s10434-023-13936-z
23. Meister, FA, Verhoeven, S, Mantas, A, Liu, W-J, Jiang, D, Heij, L, et al. Osteopenia is associated with inferior survival in patients undergoing partial hepatectomy for hepatocellular carcinoma. Sci Rep. (2022) 12:18316. doi: 10.1038/s41598-022-21652-z
24. Fukushima, N, Tsuboi, K, Nyumura, Y, Hoshino, M, Masuda, T, Suzuki, T, et al. Prognostic significance of preoperative osteopenia on outcomes after gastrectomy for gastric cancer. Ann Gastroenterol Surg. (2023) 7:255–64. doi: 10.1002/ags3.12635
25. Takano, Y, Kodera, K, Tsukihara, S, Takahashi, S, Kobayashi, Y, Koyama, M, et al. Prognostic significance of osteosarcopenia in older adults with colorectal cancer. Ann Gastroenterol Surg. (2023) 7:637–44. doi: 10.1002/ags3.12663
26. Watanabe, J, Miki, A, Sakuma, Y, Shimodaira, K, Aoki, Y, Meguro, Y, et al. Preoperative osteopenia is associated with significantly shorter survival in patients with Perihilar cholangiocarcinoma. Cancers. (2022) 14:2213. doi: 10.3390/cancers14092213
27. Cameron, ME, Underwood, PW, Williams, IE, George, TJ, Judge, SM, Yarrow, JF, et al. Osteopenia is associated with wasting in pancreatic adenocarcinoma and predicts survival after surgery. Cancer Med. (2022) 11:50–60. doi: 10.1002/cam4.4416
28. Kamada, T, Furukawa, K, Takahashi, J, Nakashima, K, Nakaseko, Y, Suzuki, N, et al. Prognostic significance of osteopenia in patients with colorectal cancer: a retrospective cohort study. Ann Gastroenterol Surg. (2021) 5:832–43. doi: 10.1002/ags3.12491
29. Ikuta, S, Aihara, T, Nakajima, T, Kasai, M, and Yamanaka, N. Computed tomography-measured bone mineral density as a surrogate marker of survival after resection of colorectal liver metastases. Ann Transl Med. (2021) 9:21. doi: 10.21037/atm-20-3751
30. Furukawa, K, Haruki, K, Taniai, T, Hamura, R, Shirai, Y, Yasuda, J, et al. Osteosarcopenia is a potential predictor for the prognosis of patients who underwent hepatic resection for colorectal liver metastases. Ann Gastroenterol Surg. (2021) 5:390–8. doi: 10.1002/ags3.12428
31. Takahashi, K, Nishikawa, K, Furukawa, K, Tanishima, Y, Ishikawa, Y, Kurogochi, T, et al. Prognostic significance of preoperative osteopenia in patients undergoing Esophagectomy for esophageal Cancer. World J Surg. (2021) 45:3119–28. doi: 10.1007/s00268-021-06199-w
32. Tamura, S, Ashida, R, Sugiura, T, Okamura, Y, Ito, T, Yamamoto, Y, et al. The prognostic impact of skeletal muscle status and bone mineral density for resected distal cholangiocarcinoma. Clin Nutr. (2021) 40:3552–8. doi: 10.1016/j.clnu.2020.12.011
33. Abe, K, Furukawa, K, Okamoto, T, Matsumoto, M, Futagawa, Y, Haruki, K, et al. Impact of osteopenia on surgical and oncological outcomes in patients with pancreatic cancer. Int J Clin Oncol. (2021) 26:1929–37. doi: 10.1007/s10147-021-01986-w
34. Toshima, T, Yoshizumi, T, Kosai-Fujimoto, Y, Inokuchi, S, Yoshiya, S, Takeishi, K, et al. Prognostic impact of osteopenia in patients who underwent living donor liver transplantation for hepatocellular carcinoma. World J Surg. (2020) 44:258–67. doi: 10.1007/s00268-019-05206-5
35. Motomura, T, Uchiyama, H, Iguchi, T, Ninomiya, M, Yoshida, R, Honboh, T, et al. Impact of osteopenia on oncologic outcomes after curative resection for pancreatic Cancer. In Vivo. (2020) 34:3551–7. doi: 10.21873/invivo.12198
36. Sharshar, M, Kaido, T, Shirai, H, Okumura, S, Yao, S, Miyachi, Y, et al. Impact of the preoperative bone mineral density on the outcomes after resection of pancreatic cancer. Surg Today. (2020) 50:757–66. doi: 10.1007/s00595-019-01954-y
37. Yao, S, Kaido, T, Okumura, S, Iwamura, S, Miyachi, Y, Shirai, H, et al. Bone mineral density correlates with survival after resection of extrahepatic biliary malignancies. Clin Nutr. (2019) 38:2770–7. doi: 10.1016/j.clnu.2018.12.004
38. Miyachi, Y, Kaido, T, Yao, S, Shirai, H, Kobayashi, A, Hamaguchi, Y, et al. Bone mineral density as a risk factor for patients undergoing surgery for hepatocellular carcinoma. World J Surg. (2019) 43:920–8. doi: 10.1007/s00268-018-4861-x
39. Dawson-Hughes, B, and Harris, SS. Calcium intake influences the association of protein intake with rates of bone loss in elderly men and women. Am J Clin Nutr. (2002) 75:773–9. doi: 10.1093/ajcn/75.4.773
40. Ganry, O, Lapôtre-Ledoux, B, Fardellone, P, and Dubreuil, A. Bone mass density, subsequent risk of colon cancer and survival in postmenopausal women. Eur J Epidemiol. (2008) 23:467–73. doi: 10.1007/s10654-008-9256-0
41. Watanabe, J, Saitsu, A, Miki, A, Kotani, K, and Sata, N. Prognostic value of preoperative low bone mineral density in patients with digestive cancers: a systematic review and meta-analysis. Arch Osteoporos. (2022) 17:33. doi: 10.1007/s11657-022-01060-6
42. Sakuma, K, Hamada, K, Yamaguchi, A, and Aoi, W. Current nutritional and pharmacological approaches for attenuating sarcopenia. Cells. (2023) 12:2422. doi: 10.3390/cells12192422
43. Ham, SL, Nasrollahi, S, Shah, KN, Soltisz, A, Paruchuri, S, Yun, YH, et al. Phytochemicals potently inhibit migration of metastatic breast cancer cells. Integr Biol. (2015) 7:792–800. doi: 10.1039/C5IB00121H
44. Zain, NM, Seriramulu, VP, and Chelliah, KK. Bone mineral density and breast Cancer risk factors among premenopausal and postmenopausal women a systematic review. Asian Pac J Cancer Prev. (2016) 17:3229–34. doi: 10.14456/apjcp.2016.80/APJCP.2016.17.7.3229
45. Stewart, A, Kumar, V, Torgerson, DJ, Fraser, WD, Gilbert, FJ, and Reid, DM. Axial BMD, change in BMD and bone turnover do not predict breast cancer incidence in early postmenopausal women. Osteoporos Int. (2005) 16:1627–32. doi: 10.1007/s00198-005-1886-4
46. Dolly, A, Lecomte, T, Bouché, O, Borg, C, Terrebonne, E, Douillard, J-Y, et al. Concurrent losses of skeletal muscle mass, adipose tissue and bone mineral density during bevacizumab / cytotoxic chemotherapy treatment for metastatic colorectal cancer. Clin Nutr. (2020) 39:3319–30. doi: 10.1016/j.clnu.2020.02.017
47. Xu, F, Xi, H, Liao, M, Zhang, Y, Ma, H, Wu, M, et al. Repurposed antipsychotic chlorpromazine inhibits colorectal cancer and pulmonary metastasis by inducing G2/M cell cycle arrest, apoptosis, and autophagy. Cancer Chemother Pharmacol. (2022) 89:331–46. doi: 10.1007/s00280-021-04386-z
48. Cao, Y, Efetov, SK, He, M, Fu, Y, Beeraka, NM, Zhang, J, et al. Updated clinical perspectives and challenges of chimeric antigen receptor-T cell therapy in colorectal Cancer and invasive breast Cancer. Arch Immunol Ther Exp. (2023) 71:19. doi: 10.1007/s00005-023-00684-x
49. Khalilieh, S, Iyer, A, Hammelef, E, Zohar, N, Gorgov, E, Yeo, TP, et al. Major pancreatic resection increases bone mineral density loss, osteoporosis, and fractures. Ann Surg. (2024). doi: 10.1097/SLA.0000000000006326. Epub ahead of print
50. Sturgeon, KM, Mathis, KM, Rogers, CJ, Schmitz, KH, and Waning, DL. Cancer- and chemotherapy-induced musculoskeletal degradation. JBMR Plus. (2019) 3:e10187. doi: 10.1002/jbm4.10187
Keywords: osteopenia, GI cancers, overall survival, recurrence-free survival, osteosarcopenia
Citation: Zou X and Wang Y (2025) Predictive value of osteopenia as prognostic marker for survival and recurrence in patients with gastrointestinal cancers: a systematic review and meta-analysis. Front. Med. 12:1527829. doi: 10.3389/fmed.2025.1527829
Edited by:
Hua Zhong, University of Hawaii at Manoa, United StatesReviewed by:
Yiqian Zhang, Tulane University, United StatesH. -S. Chen, University of California, Irvine, United States
Chuang Li, AbbVie, United States
Copyright © 2025 Zou and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Wang, V2FuZ3lhbmc2Njk4MTFAMTYzLmNvbQ==
 Xinmei Zou
Xinmei Zou Yang Wang
Yang Wang