- 1Mashhad University of Medical Sciences, Mashhad, Iran
- 2Department of Neurology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany
- 3Department of Clinical Biochemistry, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
- 4Student Research Committee, School of Medicine, North Khorasan University of Medical Sciences, Bojnurd, Iran
- 5Student Research Committee, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
Background and aims: Previous studies have indicated a potential association between low vitamin D levels in early pregnancy and an increased risk of hypertensive disorders, including preeclampsia. Given the substantial maternal and fetal morbidity associated with preeclampsia, identifying preventive strategies is crucial. This meta-analysis aimed to evaluate the relationship between vitamin D status in early and middle pregnancy and the development of preeclampsia.
Methods: A comprehensive literature search of PubMed, Scopus, Web of Science, and Cochrane Library databases was conducted up to June 23, 2023, to identify relevant observational studies. Included studies were assessed for methodological quality, and data on maternal vitamin D concentration and the risk of preeclampsia were extracted.
Results: Twenty-nine observational studies with 74,061 participants were included. Women with preeclampsia had significantly lower vitamin D levels than those without (SMD −0.28, 95% CI −0.39 to −0.17, p < 0.001). Although lower vitamin D levels showed a trend toward higher preeclampsia risk, pooled odds ratios for insufficiency (OR 1.05, 95% CI 0.78–1.42) and deficiency (OR 1.25, 95% CI 0.89–1.76) were not statistically significant. Subgroup analyses suggested a possible dose–response relationship, especially when vitamin D was measured in early or mid-pregnancy. Additional analyses by assay method, cut-off definitions, region, and study design also supported an association between lower vitamin D and preeclampsia risk.
Conclusion: The findings of this meta-analysis suggest a potential association between low maternal vitamin D levels and an increased risk of preeclampsia, particularly when measured prior to the late pregnancy. However, the precise timing of this association requires further investigation. To definitively establish the role of vitamin D supplementation in preventing preeclampsia, well-designed randomized controlled trials are needed to determine optimal dosage and timing of intervention, as well as to assess cost-effectiveness.
Systematic review registration: Registered in OSF: https://osf.io/qwh6a (Unique ID: qwh6a).
Background
Preeclampsia is a severe pregnancy complication characterized by new-onset high blood pressure and proteinuria, often accompanied by damage to other organs. This condition typically develops after 20 weeks of gestation and is a leading cause of maternal and fetal morbidity and mortality worldwide (1). While its prevalence varies globally, it significantly impacts approximately 4.6% of pregnancies, with higher rates reported in developing countries (2). The course of preeclampsia can be dreadful if neglected, leading to adverse outcomes such as placental abruption, preterm delivery, and HELLP syndrome for the mother and preterm birth, stillbirth, low birth weight, and small for gestational age for the fetus (3). The precise etiology of preeclampsia remains elusive. However, it is widely accepted that abnormal placental development plays a central role, triggering a cascade of events including oxidative stress and systemic inflammation (4, 5). Currently, the only definitive cure for preeclampsia is delivery. Pharmacological interventions are primarily aimed at managing symptoms and preventing complications until delivery can be safely achieved (6). Recent research has focused on the potential link between maternal vitamin D status and the development of preeclampsia. Vitamin D is known to play a critical role in regulating genes involved in placental function, implantation, blood vessel development, and immune response. During pregnancy, the placenta is a key site for vitamin D activation, expressing enzymes like CYP27B1 and CYP24A1, as well as the vitamin D receptor (VDR), indicating its role in local vitamin D homeostasis (7). Gene-based studies show that vitamin D supplementation or status is linked to changes in placental gene expression. These include increased activity in amino acid transporter genes (8), reduced levels of an antiangiogenic factor tied to preeclampsia (9), and better regulation of inflammatory responses during immune challenges (10).
Moreover, low vitamin D levels during early pregnancy have been linked to an increased risk of hypertensive disorders and can also influence cholesterol levels throughout gestation (11). Vitamin D deficiency is prevalent among pregnant women, with estimates suggesting that up to 40% of this population is affected (12). Given the multiple factors influencing vitamin D levels, including diet, fortification, skin pigmentation, sun exposure, and genetics, it is considered a potential modifiable risk factor for preeclampsia prevention (13). Despite the potential association between vitamin D status and preeclampsia, clear guidelines for vitamin D supplementation during pregnancy are lacking. Observational studies have yielded inconsistent results, likely due to factors such as small sample sizes, varying definitions of vitamin D deficiency, and the often late detection of low vitamin D levels. These challenges hinder the establishment of a definitive causal relationship between vitamin D and preeclampsia prevention. The most recent meta-analysis, conducted by Hu et al. (14) in 2021, examined the association between vitamin D levels and preeclampsia before the late stage of pregnancy. Previous meta-analyses have reported an inverse relationship between vitamin D status and preeclampsia risk (15–17). However, the evolving body of evidence necessitates an updated assessment. Given the inconsistencies in the literature and the emergence of new studies, we conducted a comprehensive systematic review and meta-analysis to examine the association between vitamin D status in early and middle pregnancy and the development of preeclampsia.
Methods
Our methodology adheres to the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-analyses (18). The research protocol of this review was registered on the Open Science Framework.1
Eligibility criteria, information sources, search strategy
An advanced literature search was performed up to June 23, 2023 to retrieve relevant articles from following databases: PubMed, Scopus, Web of Science, and Cochrane Library. A comprehensive search strategy was developed using keywords and Medical Subject Headings (MeSH) terms related to vitamin D, pregnancy, and preeclampsia. These terms were combined using the Boolean operator “AND” without imposing restrictions on publication date, type, or language. Database-specific search syntax was employed for each database. To enhance the search yield, reference lists of relevant systematic reviews were manually screened. Two independent reviewers conducted the search process, with discrepancies resolved through consensus.
Early pregnancy was classified as gestational age less than 14 weeks, middle pregnancy as gestational age between 14 and 27 weeks, and late pregnancy as gestational age 28 weeks or greater (19). For studies to be considered in this meta-analysis, the following criteria should be met:
1. Observational methodology (in order to exclude the confounding effect of any intervention).
2. The main interest was to assess the link between vitamin D levels and risk of preeclampsia.
3. Study population consisted of pregnant women before the 28 weeks of gestational age.
4. Preeclampsia was defined by the new-onset of gestational hypertension and proteinuria.
5. Threshold levels for vitamin D sufficiency and insufficiency were outlined.
Studies employing methodologies other than observational designs, those conducted on animal models, or those involving pregnant women with pre-existing conditions or outcomes other than preeclampsia were excluded from the analysis.
Study selection, data extraction and study quality assessment
Two independent reviewers screened titles and abstracts of all retrieved studies to assess eligibility for the meta-analysis. Studies not meeting the inclusion criteria were excluded. Subsequently, full texts of potentially eligible studies were reviewed for final selection. Next, the following items were obtained for extraction in four sets: (1) Study characteristics (i.e., authors, location, year, and type of study), (2) patient-specific factors (i.e., gestational age), (3) Study Design (i.e., number of participants, method and period of sampling, confounding factors), (4). Outcomes (i.e., risk of preeclampsia and vitamin D concentration). Serum vitamin D concentrations reported in ng/mL were converted to nmol/L using the standard conversion factor (1 ng/mL = 2.5 nmol/L). Critical appraisal of included studies was performed by two reviewers using the Joanna Briggs Institute (JBI) checklists designed specifically for cohort, case–control, and analytical cross-sectional studies.2 A third reviewer was involved to resolve any discrepancies in selection or data extraction.
Data synthesis
We used STATA 13.1 software, developed by StataCorp LP in College Station, TX, USA, for our data analysis. Results were reported as pooled odds ratios (ORs) with a 95% confidence interval, visualized in a forest plot. We evaluated heterogeneity among the eligible studies using the I2 statistic (20). For the primary meta-analyses and meta-regression models, between-study variance (τ2) was estimated using the restricted maximum likelihood (REML) method, the DerSimonian–Laird random-effects model was additionally applied as a sensitivity estimator when substantial heterogeneity was present (I2 > 50%) (21). To explore potential sources of heterogeneity, we performed subgroup and meta-regression analyses across parameters including assay method, vitamin D threshold, geographic region, and study design. Furthermore, we conducted a sensitivity analysis, excluding one study at a time and repeating the meta-analysis. This enabled us to ensure the stability of our findings. Finally, to investigate the potential for publication bias, we adopted visual inspection of funnel plot symmetry and Egger’s regression analysis (22).
Results
Study selection
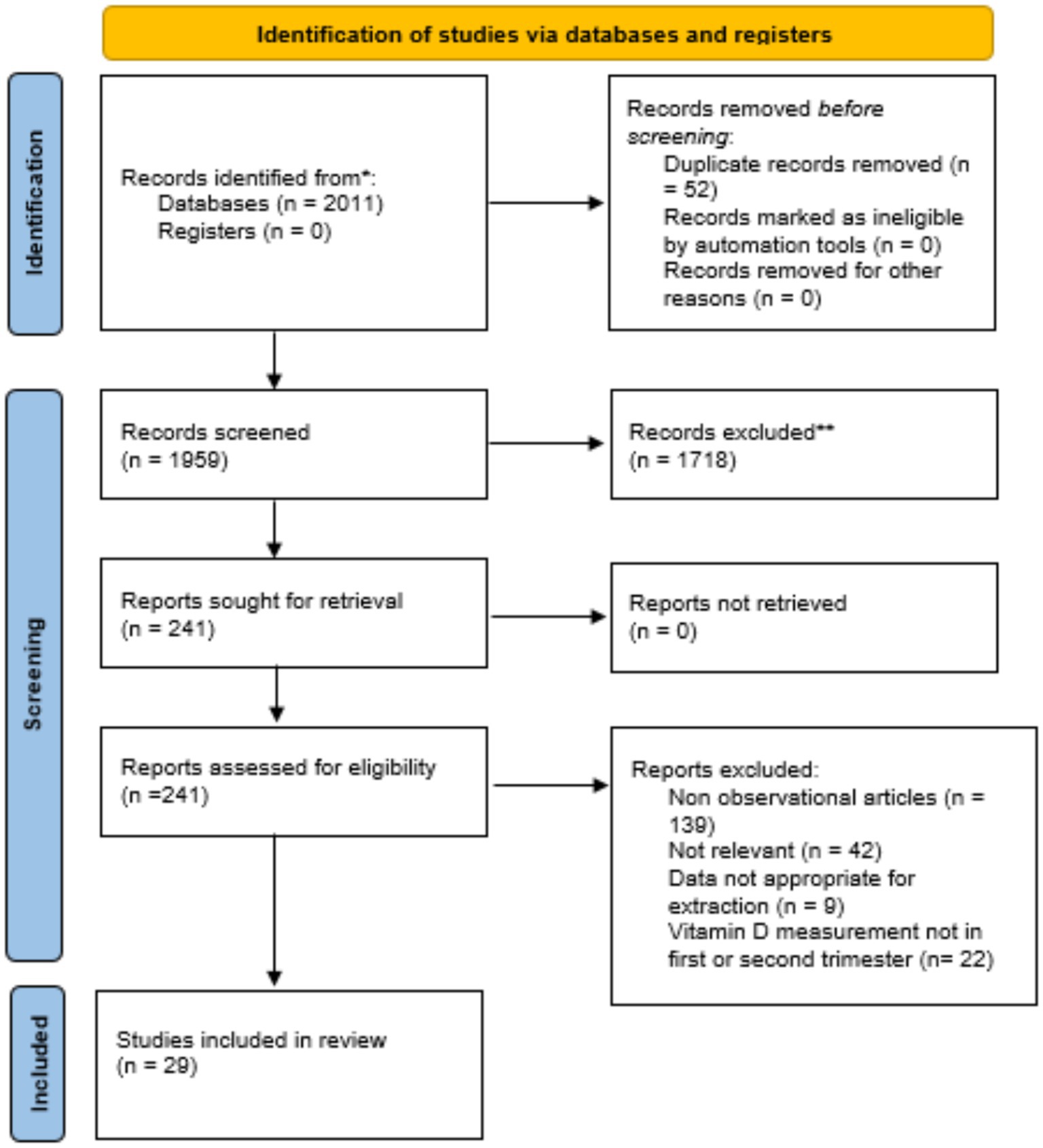
An initial search as described in Table 1 yielded 2,011 studies, of which 52 duplicates were removed, leaving 1,959 records. Abstract screening excluded 1,718 studies, resulting in 241 articles for full-text review. After this stage, 212 studies were eliminated, leaving 29 eligible for meta-analysis (Figure 1). These studies, published between 2007 and 2023, included a total of 74,061 pregnant women with vitamin D measurements obtained during the early or middle pregnancy.
Most studies used cutoff values of 75 nmol/L and 50 nmol/L to define vitamin D sufficiency and insufficiency, respectively, in accordance with Endocrine Society recommendations (23). However, four studies defined vitamin D deficiency as levels below 37.5 nmol/L based on national or local guidelines (24–27). Due to the bias of cofounders, most of the studies included in this analysis have reported data in an adjusted format. However, there is notable heterogeneity regarding the number and types of controlled variables.
Study characteristics
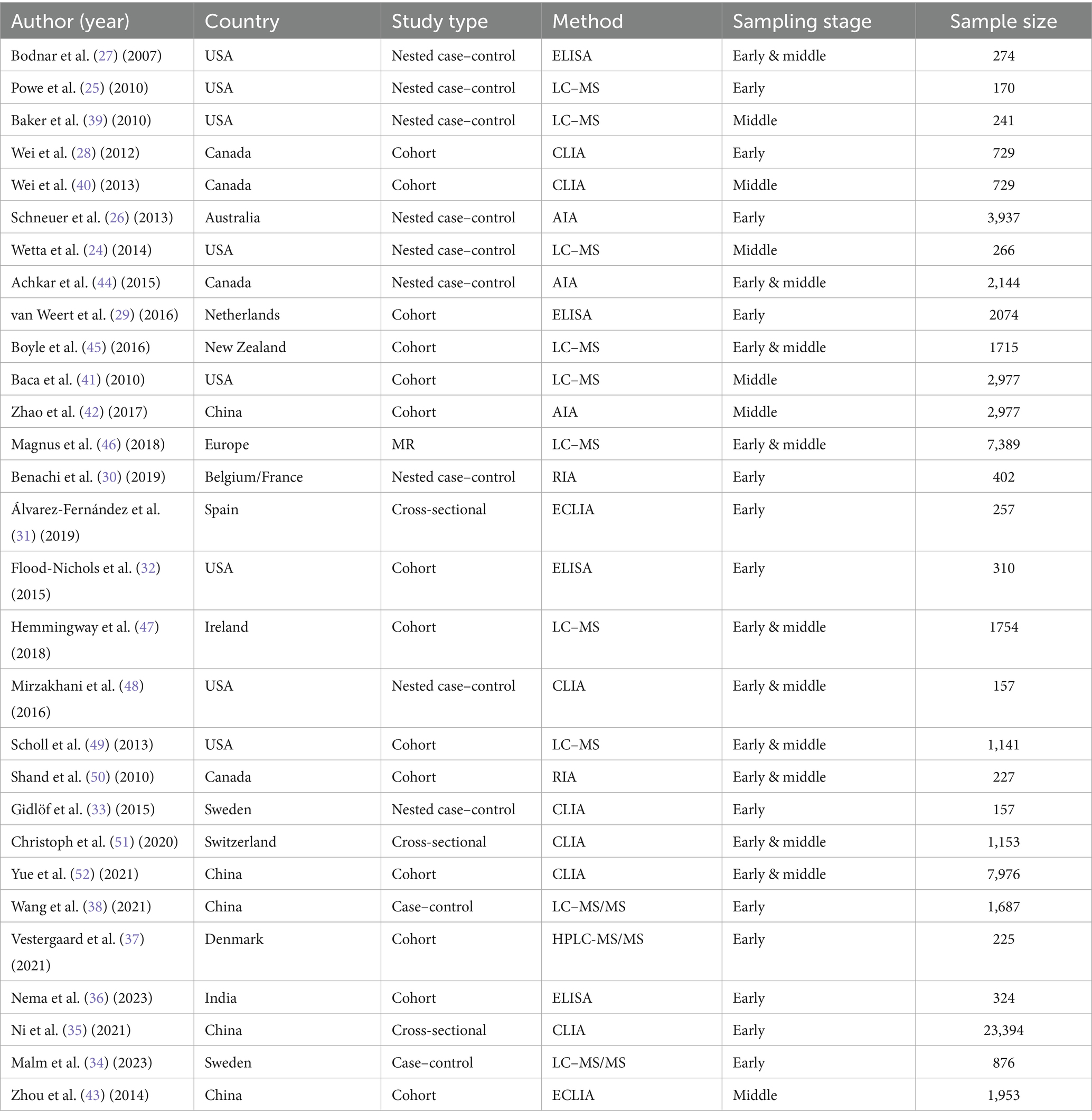
Thirteen studies quantified maternal serum concentrations of vitamin D at early pregnancy (25, 26, 28–38), six studies at middle pregnancy (24, 39–43), and 10 other studies measured maternal vitamin D concentrations during a time period overlapping between the two (<22 weeks) (27, 44–52). Out of articles included, eight were carried out in USA (24, 25, 27, 32, 39, 41, 48, 49), five were from China (35, 38, 42, 43, 52), four from Canada (28, 40, 44, 50), two from Australia and New Zealand (26, 45), one from India (36), and the rest were in Europe (29–31, 33, 34, 37, 46, 47, 51). A summary of the included studies is presented in Table 2, with detailed characteristics available in Supplementary Table S1.
Risk of bias of included studies
The methodological quality of included studies was assessed using the JBI tool. All case–control studies achieved a high quality score of 9 out of 10. Cohort studies generally demonstrated excellent quality, with a score of 11 out of 11, except for Nema et al. (36) (score of 6/11) and Vestergaard et al. (37) (score of 9/11). Among the cross-sectional studies, Ni et al. (35) obtained a score of 8 out of 8, while the remaining two studies scored 6 out of 8. To assess publication bias, Egger’s regression, Begg’s test, and funnel plot analyses were conducted for all comparisons. Evidence of publication bias was detected in the comparison of sufficient versus insufficient vitamin D levels based on Begg’s test (p < 0.1) and in the comparison of sufficient versus insufficient or deficient levels of vitamin D based on Egger’s test and funnel plot asymmetry (Figure 2). These findings suggest that publication bias may have influenced the results. Furthermore, the trim-and-fill analysis for the mean difference outcome showed no imputed studies, indicating a low risk of publication bias; the pooled effect size remained stable after adjustment (mean difference: –4.009; 95% CI: −4.743 to −3.276). For the odds ratio outcomes, some imputed studies were identified. In the Sufficient vs. Insufficient category, two studies were imputed, and the pooled OR shifted slightly from 1.37 (95% CI: 1.13–1.61) to 1.33 (95% CI: 1.10–1.57). In the Sufficient vs. Deficient category, three studies were imputed, with the pooled OR changing from 1.43 (95% CI: 1.15–1.71) to 1.40 (95% CI: 1.13–1.68). In the Sufficient/Insufficient vs. Deficient category, three studies were imputed, and the pooled OR decreased marginally from 1.40 (95% CI: 1.16–1.65) to 1.38 (95% CI: 1.13–1.62). Finally, in the Sufficient vs. Insufficient/Deficient category, no studies were imputed, indicating stable results. Overall, these findings suggest that although minor adjustments occurred after imputing potentially missing studies, the effect sizes remained consistent, indicating that publication bias is unlikely to have influenced the conclusions of this meta-analysis in a meaningful way.
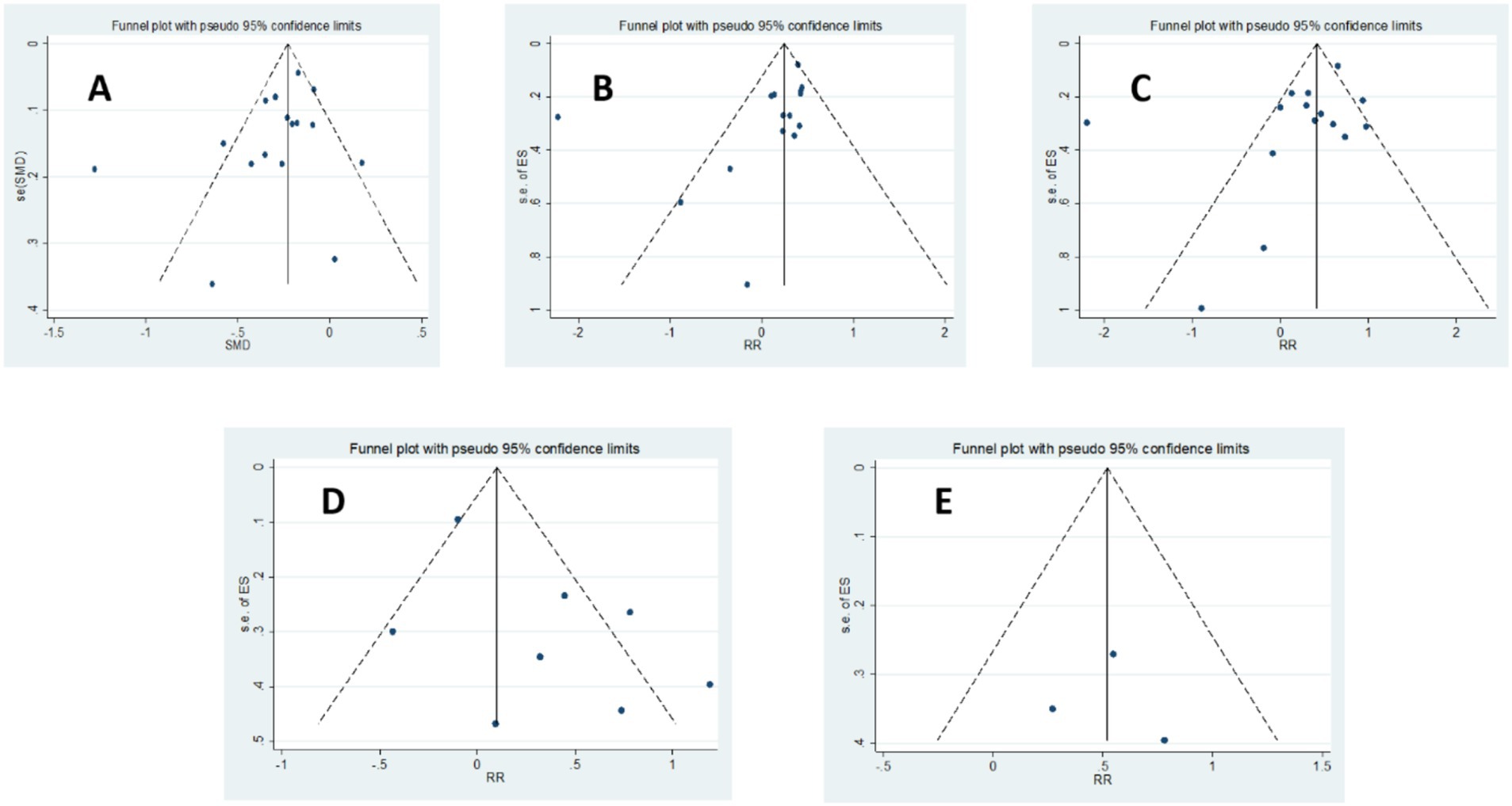
Figure 2. Funnel plot of the studies represented in the meta-analysis; (A) Vitamin D concentration in preeclampsia versus normal pregnancy, (B) Sufficient levels of Vitamin D (≥75 nmol/L) versus Insufficient levels of Vitamin D (50–75 nmol/L or 37.5–75 nmol/L), (C) Sufficient Levels of Vitamin D (≥75 nmol/L) versus Deficient Levels of Vitamin D (<50 nmol/L or <37.5 nmol/L), (D) Sufficient or Insufficient Levels of Vitamin D (≥50 nmol/L) versus Deficient Levels of Vitamin D (<50 nmol/L or < 37.5 nmol/L), (E) Sufficient Levels of Vitamin D (≥75 nmol/L) versus Insufficient or Deficient Levels of Vitamin D (<75 nmol/L).
Sensitivity studies
Sensitivity analysis indicated that the removal of any individual study or subgroup of studies had minimal impact on the pooled risk ratio and its confidence interval (Supplementary Figures S1, S2), suggesting the robustness of our findings.
Principal findings
Vitamin D concentration in preeclampsia versus normal pregnancy
A total of 20 studies reported the mean concentration of vitamin D among participants, out of which four (27, 31, 32, 41), did not provide the standard deviation of data. Finally, 16 studies (2, 24–26, 28, 30, 33, 36–38, 40, 42, 44, 45, 47, 48) comprising 24,247 pregnant women (including 1,133 with pre-eclampsia) were eligible for the meta-analysis comparing vitamin D status in pregnant women with and without pre-eclampsia. The analysis revealed that women with pre-eclampsia had significantly lower vitamin D levels compared to those without it (SMD, −0.28; 95% CI, −0.39, −0.17; p-value<0.001). A subgroup analysis showed that this observation persisted regardless of whether serum vitamin D was measured during the early and middle pregnancy (as shown in Figure 3). However, there was significant heterogeneity among these studies (I2 = 73.0%). Therefore, a random effects model was applied. Subgroup analyses confirmed this association across various methodological and clinical parameters, including assay type, vitamin D cut-off definitions, geographic region, and study design. Although effect sizes varied slightly among subgroups, the direction of association remained consistent. No heterogeneity was detected among subgroups (see Supplementary Table S2 for details).
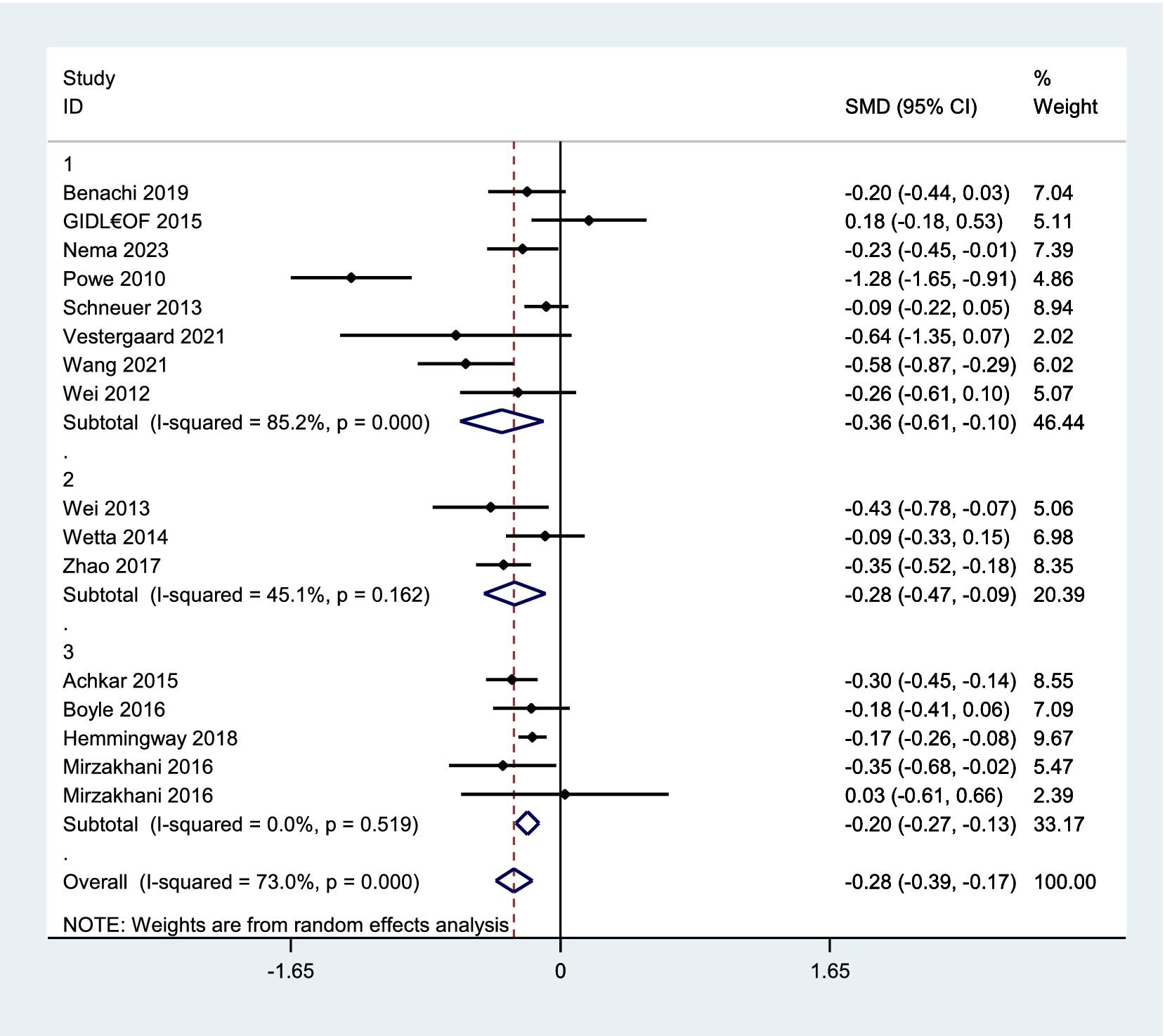
Figure 3. Forest plot for vitamin D concentration in preeclampsia versus normal pregnancy. 1. Early pregnancy, 2. Middle pregnancy, 3. Early or middle pregnancy.
Sufficient levels of vitamin D (≥75 nmol/L) versus insufficient levels of vitamin D (50–75 nmol/L or 37.5–75 nmol/L)
A total of 27,448 pregnant women from 15 studies were included in this analysis, with 8,756 having sufficient levels of vitamin D and 18,692 having insufficient levels. There was a trend for an increased risk of preeclampsia in women with vitamin D insufficiency compared to women with sufficient levels, but it was not statistically significant (OR 1.05, 95% CI 0.78–1.42, I2 = 85.1%; p-value<0.001, random-effect). However, the subgroup analysis revealed a significant increase in the risk of preeclampsia in studies of women with insufficient vitamin D levels during early pregnancy or overlapping between early and middle pregnancy, but not in middle pregnancy alone (early pregnancy: OR 1.37, 95% CI 1.07–1.76, I2 = 0%; p-value = 0.584, random-effect; middle pregnancy: OR 0.72, 95% CI 0.26–1.99, I2 = 96.4%; p-value < 0.001, random-effect; early and middle pregnancy: OR 1.34, 95% CI 1.10–1.64, I2 = 15.3%; p-value = 0.316, random-effect), detailed in Figure 4A. Further subgroup analyses based on assay method, vitamin D cut-off definitions, region, and study design consistently supported the association between vitamin D insufficiency and increased risk of preeclampsia. No significant between-group heterogeneity was found (see Supplementary Table S3 for full subgroup results).
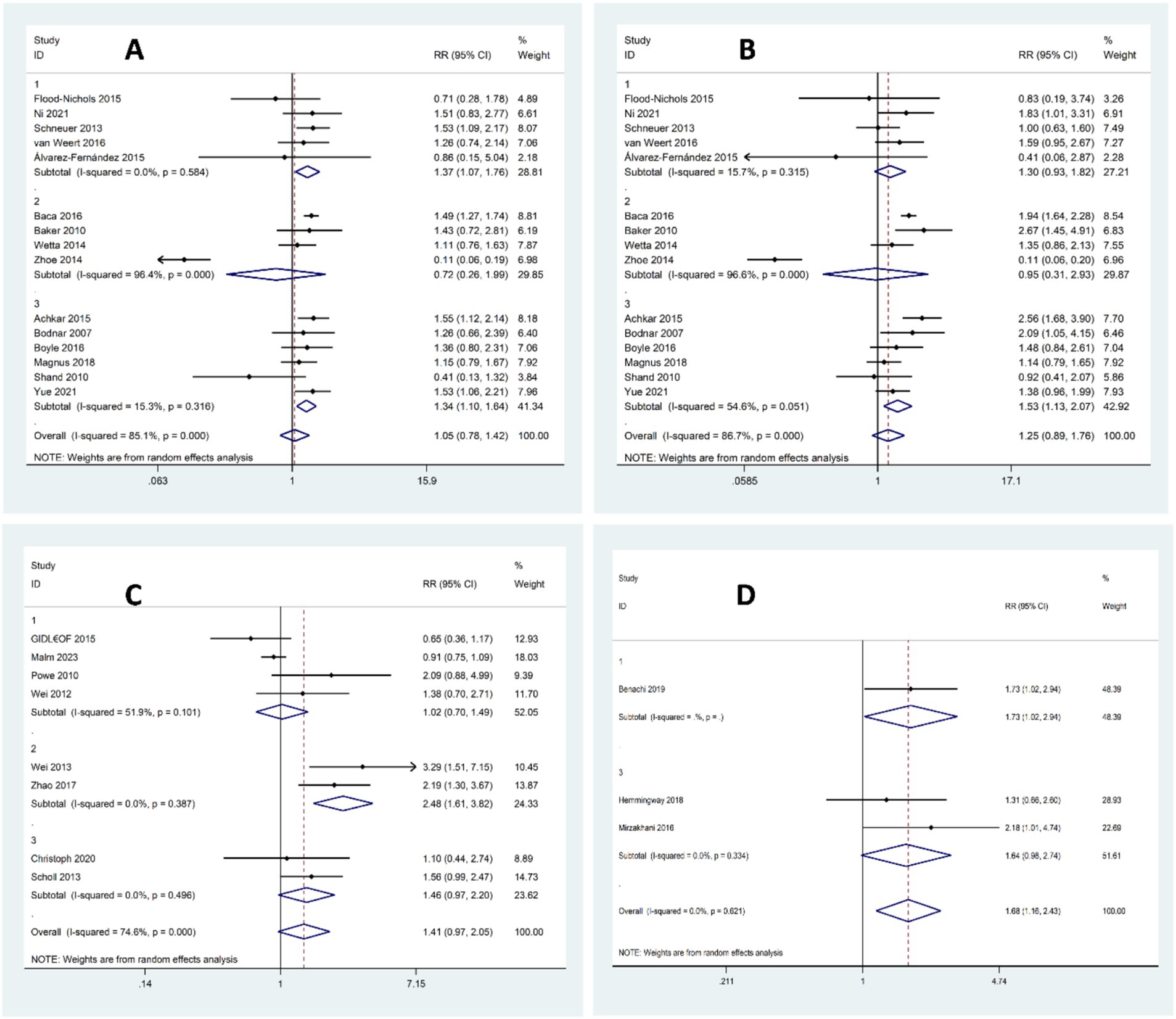
Figure 4. Forest plot for the risk of preeclampsia according to vitamin D status; (A) Sufficient Levels of Vitamin D (≥75 nmol/L) versus Insufficient levels of Vitamin D (50–75 nmol/L or 37.5–75 nmol/L), (B) Sufficient Levels of Vitamin D (≥75 nmol/L) versus Deficient Levels of Vitamin D (<50 nmol/L or <37.5 nmol/L), (C) Sufficient or Insufficient Levels of Vitamin D (≥50 nmol/L) versus Deficient Levels of Vitamin D (<50 nmol/L or < 37.5 nmol/L), (D) Sufficient Levels of Vitamin D (≥75 nmol/L) versus Insufficient or Deficient Levels of Vitamin D (<75 nmol/L).
Sufficient levels of vitamin D (≥75 nmol/L) versus deficient levels of vitamin D (<50 nmol/L or <37.5 nmol/L)
In the next analysis that included a total of 33,321 pregnant women from 15 studies, 8,756 had sufficient levels of vitamin D while 24,565 had deficient levels. There was an overall trend indicating an increased risk of preeclampsia in women with vitamin D deficiency compared to those with sufficient levels, but the findings did not reach statistical significance p-value<0.001 (OR 1.25, 95% CI 0.89–1.76, I2 = 86.7%;, random-effect). The next subgroup analysis revealed a significant increase in the risk of preeclampsia in women with deficient Vitamin D levels during the overlapping period between early and middle pregnancy, but not in early or middle pregnancy alone (early pregnancy: OR 1.30, 95% CI 0.93–1.82, I2 = 15.7%; p-value = 0.315, random-effect; middle pregnancy: OR 0.95, 95% CI 0.31–2.93, I2 = 96.6%; p-value < 0.001, random-effect; early and middle pregnancy: OR 1.53, 95% CI 1.13–2.07, I2 = 54.6%; p-value = 0.061, random-effect), detailed in Figure 4B. Further subgroup analyses by assay method, cut-off definitions, region, and study design generally supported the association between vitamin D deficiency and elevated preeclampsia risk. However, no significant between-group heterogeneity was detected (see Supplementary Table S4 for detailed subgroup results).
Sufficient or insufficient levels of vitamin D (≥50 nmol/L) versus deficient levels of vitamin D (<50 nmol/L or < 37.5 nmol/L)
In the next analysis that included a total of 15,751 pregnant women from 8 studies, 4,952 had sufficient or insufficient levels of Vitamin D while 10,799 had deficient levels. While there was an overall trend for higher risk of preeclampsia in women with vitamin D deficiency compared to those with sufficient or insufficient levels, the results were not statistically significant (OR 1.41, 95% CI 0.97–2.05, I2 = 74.6%; p-value<0.001, random-effect). The next subgroup analysis showed a significant increase in preeclampsia risk only among women with deficient vitamin D levels in their middle pregnancy (early pregnancy: OR 1.02, 95% CI 0.70–1.49, I2 = 51.9%, p-value = 0.101, random-effect; middle pregnancy: OR 2.48, 95% CI 1.61–3.82, I2 = 0%; p-value = 0.387, random-effect; early and middle pregnancy: OR 1.41, 95% CI 0.97–2.05, I2 = 0%; p-value = 0.496, random-effect), detailed in Figure 4C. Further subgroup analyses by assay method, cut-off definitions, region, and study design generally supported the association between vitamin D deficiency and elevated preeclampsia risk. However, no significant between-group heterogeneity was detected (see Supplementary Table S5 for detailed subgroup results).
Sufficient levels of vitamin D (≥75 nmol/L) versus insufficient or deficient levels of vitamin D (<75 nmol/L)
In the next analysis that included a total of 2,313 pregnant women from three studies, 576 had sufficient levels of vitamin D and 1,737 had insufficient or deficient levels of vitamin D. The risk of preeclampsia was significantly higher in women with vitamin D status of insufficiency or deficiency compared to those with sufficient levels (OR 1.68, 95% CI 1.16–2.43, I2 = 0%; p-value = 0.621, fixed-effect), detailed in Figure 4D. Subgroup analyses revealed some variation based on assay method, threshold definitions, region, and study design. However, it should be noted that most subgroups included only one or two studies, which limits the robustness and generalizability of these subgroup findings (see Supplementary Table S6 for full subgroup results).
Meta-regression analysis
Meta-regression analyses were conducted to examine whether assay method, deficiency cut-off, sufficiency cut-off, study region, or study design contributed to the observed heterogeneity (Supplementary Table S7). In most comparisons, none of these factors had a statistically significant effect on the results (all p > 0.05). An exception was found for the comparison between sufficient vitamin D levels (≥75 nmol/L) and insufficient or deficient levels (<75 nmol/L), where assay method (p = 0.037) and study region (p < 0.001) were significant predictors. These findings should be interpreted with caution, as most subgroups for these moderators included only one or two studies, which limits the strength and generalizability of the conclusions.
Moreover, an ordinal meta-regression across commonly applied thresholds (37.5, 50, and 75 nmol/L; REML) did not indicate a significant trend (β = 0.17, 95% CI − 0.27 to 0.61; Wald χ2 = 0.56; p = 0.455), with heterogeneity increasing slightly (τ2 from 0.0165 at baseline to 0.0214; residual I2 = 27.9%; adjusted R2 = 0%). In a sensitivity analysis restricted to uniform thresholds, studies using 50 nmol/L as the cut-off produced an overall OR of 1.23 (95% CI 0.51–1.95; τ2 = 0.3617; I2 = 61.4%; REML), while those applying 75 nmol/L yielded OR = 1.12 (95% CI 0.99–1.26; τ2 = 0.0120; I2 = 20.8%). These results were directionally consistent with the main model, suggesting that the findings are not dependent on the specific threshold applied.
The relationship between vitamin D thresholds and preeclampsia risk was further examined using bubble and Galbraith plots (Supplementary Figures S3, S4). In the bubble plot the linear prediction line was nearly flat, and confidence intervals remained wide, indicating no significant trend across increasing thresholds. In contrast, the Galbraith plot, suggested that studies with higher precision tended to report somewhat stronger associations. Although this pattern should be interpreted with caution given the variability among studies.
Comparison with existing literature
This meta-analysis of 29 observational studies investigated the association between vitamin D status during the early and middle pregnancy and the risk of preeclampsia using two approaches: (1) comparing mean vitamin D levels between pregnant women with and without preeclampsia, and (2) assessing the risk of preeclampsia based on predefined vitamin D deficiency, insufficiency, and normal ranges.
Our findings demonstrate significantly lower vitamin D levels in pregnant women who developed preeclampsia compared to those without. However, due to the observational nature of this study, a causal relationship between low vitamin D and preeclampsia cannot be definitively established. Nevertheless, our results align with previous meta-analyses linking vitamin D deficiency (below 50 nmol/L) to an increased risk of preeclampsia (15–17). These findings support the potential benefit of vitamin D supplementation in reducing this risk (53–55). One recent meta-analysis showed that when vitamin D levels falls below 40 nmol/L, the risk of preeclampsia dramatically increases (56). Consistent with previous research, a meta-analysis by (53) demonstrated a reduced risk of preeclampsia with vitamin D supplementation of up to 2,000 IU/day. His finding is supported by multiple meta-analyses of randomized controlled trials (RCTs) showing a similar association, despite variations in supplementation doses (54, 55, 57, 58). However, one meta-analysis of three RCTs reported no association between vitamin D supplementation and preeclampsia risk. The authors attributed this inconsistency to potential confounding effects of a single study and the limited sample size (59).
Establishing a clear cut-off point for vitamin D status is crucial for developing effective nutritional interventions to prevent preeclampsia. A significant challenge in this field has been the inconsistent use of vitamin D level thresholds across studies. Most studies included in this meta-analysis aligned with the Endocrine Society guidelines, categorizing vitamin D status as sufficient (>30 ng/mL or >75 nmol/L), insufficient (20–30 ng/mL or 50–75 nmol/L), or deficient (<20 ng/mL or <50 nmol/L) (23). Previous meta-analyses encompassing vitamin D measurements across the entire pregnancy period have consistently linked vitamin D levels below 50 nmol/L to an increased risk of preeclampsia (15–17). Narrowing down the time of measurement to the early and middle pregnancy allows for the use of preventive methods before the disease progresses. Interestingly, a meta-analysis conducted by Hu et al. (14), found that pregnant women with early or mid-pregnancy vitamin D levels below 50 nmol/L exhibited an increased risk of preeclampsia. Nevertheless, two studies reported no association between vitamin D levels and preeclampsia risk during pregnancy (60, 61). Our analysis revealed a potential trend suggesting that vitamin D levels exceeding 75 nmol/L during early or middle pregnancy might be associated with a reduced preeclampsia risk compared to lower levels. This association was most pronounced when comparing sufficient versus insufficient or deficient vitamin D status across three studies. While subgroup analysis by pregnancy stage strengthened these findings, the inconsistent reporting of pregnancy stages across studies limited definitive conclusions regarding stage-specific effects.
The discrepancies observed among meta-analyses can be attributed to several factors, primarily heterogeneity in study design and methodology. Variations in study type, timing of vitamin D measurement, assay methods, and definitions of vitamin D deficiency can significantly influence pooled results. Our subgroup analyses addressed some of these sources of heterogeneity, showing that the association between vitamin D status and preeclampsia was generally consistent despite variations in assay techniques (e.g., ELISA, LC–MS/MS), cut-off thresholds (e.g., 50 vs. 37.5 nmol/L), and study design (e.g., cohort, case–control). Results from the meta-regression further supported this consistency, as none of these factors showed statistically significant contributions to heterogeneity. Although adjusted odds ratios account for potential confounders, overadjustment may obscure true associations. Additionally, the meta-analysis methodology, including inclusion criteria and statistical models, can impact the overall findings.
The pathogenesis of preeclampsia is not yet fully understood. It is proposed that insufficient trophoblast cell invasion and defective remodeling of the uterine spiral arteries causes placental ischemia. This reduction in placental blood flow triggers an immune system imbalance, where there is an increase in proinflammatory CD4+ T cells and cytokines, and a decrease in regulatory T cells and anti-inflammatory cytokines. As a result, chronic inflammation develops, leading to oxidative stress, elevated proinflammatory cytokines, and the production of autoantibodies (62). Interestingly, low levels of vitamin D could further sensitize the endothelium to inflammation and induce oxidative stress (63). Conversely, adequate vitamin D has been found to affect the endovascular system directly by suppressing vascular wall cell proliferation and having immunomodulatory effects (64), and indirectly through the regulation of calcium levels and blood pressure (65). Therefore, inadequate amounts of vitamin D may contribute to the development of preeclampsia.
Strengths and limitations
Our meta-analysis has several strengths. By focusing on vitamin D measurements from early and middle pregnancy, we targeted a critical period for potential intervention. Additionally, incorporating both mean vitamin D levels and odds ratios enhanced the comprehensiveness of our analysis, given the diverse reporting of these data in the literature. Our use of three vitamin D status categories provided a more nuanced assessment compared to dichotomizing into deficient and non-deficient groups. Subgroup analyses and meta-regression across multiple parameters, including assay methods, vitamin D thresholds, region, and study design, further validated the consistency of findings.
This meta-analysis has also several limitations that should be addressed. Firstly, there was significant heterogeneity among studies for Preeclampsia risk regarding gestational weeks, study design, methods of vitamin D assessment, threshold for vitamin D status, and participants’ characteristics. Additionally, few studies specified or adjusted for the season of sampling, which is known to influence vitamin D concentrations. Secondly, to To minimize the influence of confounding factors, only adjusted RRs were entered into the analysis, but unidentified confounders could still skew the results. Some evidence of publication bias was detected in specific comparisons; however, trim-and-fill analysis showed few imputed studies and only minor shifts in pooled estimates. These small changes suggest that publication bias is unlikely to have significantly affected the overall conclusions. Thirdly, the ambiguous reporting of the pregnancy stage during which vitamin D was measured in some studies made it difficult to compare the association between vitamin D levels and preeclampsia during early versus middle pregnancy. Furthermore, none of the included studies distinguished between early-onset and late-onset preeclampsia, therefore we could not extract or analyze this data, which limited our ability to explore potential differences in the relationship between vitamin D deficiency and preeclampsia subtypes. Finally, although threshold modelling was attempted through ordinal and sensitivity approaches, continuous meta-regression remained limited by the small number of distinct and overlapping cut-offs, reducing statistical power.
Conclusion and implications
In summary, this systematic review and meta-analysis investigated the role of vitamin D status prior to the late pregnancy in the development of preeclampsia. Based on the evidence presented in this study, it is suggested that low concentrations of maternal vitamin D prior to the late stage of pregnancy could be associated with an increased risk of preeclampsia, but the significance of this association depends on the time of measurement including the season of the year. To fully understand the role of vitamin D in preventing and managing preeclampsia, there is a need for well-designed trials on vitamin D supplementation. These trials should focus on identifying the optimal time for vitamin D enrichment during pregnancy, as well as evaluating the cost-effectiveness of this approach. Future research should explore more specific areas, such as investigating vitamin D status at the preconception stage and its potential impact on the risk of preeclampsia. Additionally, studies should consider subtypes of preeclampsia (e.g., early- or late-onset) to better understand how vitamin D deficiency may influence the development of these subtypes. Multi-center, multinational studies are recommended to further examine the impact of geographic, ethnic, and lifestyle factors.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
FZ: Conceptualization, Writing – original draft. QB: Writing – original draft. HD: Writing – original draft. ArA: Data curation, Formal analysis, Software, Visualization, Writing – original draft, Writing – review & editing. ReZ: Data curation, Investigation, Writing – original draft. RaZ: Data curation, Investigation, Writing – review & editing. AmA: Data curation, Investigation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We appreciate all authors of included studies.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1535359/full#supplementary-material
Abbreviations
PRISMA, preferred reporting items for systematic reviews and meta-analyses; MESH, medical subject headings; JBI, Joanna Briggs Institute; OR, odds ratio.
Footnotes
References
1. Tranquilli, A, Dekker, G, Magee, L, Roberts, J, Sibai, B, Steyn, W, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertension. (2014):97–104. doi: 10.1016/j.preghy.2014.02.001
2. Abalos, E, Cuesta, C, Grosso, AL, Chou, D, and Say, L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. (2013) 170:1–7. doi: 10.1016/j.ejogrb.2013.05.005
3. Magee, LA, Nicolaides, KH, and Von Dadelszen, P. Preeclampsia. N Engl J Med. (2022) 386:1817–32. doi: 10.1056/NEJMra2109523
4. Michalczyk, M, Celewicz, A, Celewicz, M, Woźniakowska-Gondek, P, and Rzepka, R. The role of inflammation in the pathogenesis of preeclampsia. Mediat Inflamm. (2020) 2020:1–9. doi: 10.1155/2020/3864941
5. Aouache, R, Biquard, L, Vaiman, D, and Miralles, F. Oxidative stress in preeclampsia and placental diseases. Int J Mol Sci. (2018) 19:1496. doi: 10.3390/ijms19051496
6. Amaral, LM, Wallace, K, Owens, M, and LaMarca, B. Pathophysiology and current clinical management of preeclampsia. Curr Hypertens Rep. (2017) 19:1–6. doi: 10.1007/s11906-017-0757-7
7. Ashley, B, Simner, C, Manousopoulou, A, Jenkinson, C, Hey, F, Frost, JM, et al. Placental uptake and metabolism of 25 (OH) vitamin D determine its activity within the fetoplacental unit. eLife. (2022) 11:e71094. doi: 10.7554/eLife.71094
8. Cleal, J, Day, P, Simner, C, Barton, SJ, Mahon, PA, Inskip, H, et al. Placental amino acid transport may be regulated by maternal vitamin D and vitamin D-binding protein: results from the Southampton women's survey. Br J Nutr. (2015) 113:1903–10. doi: 10.1017/S0007114515001178
9. Schulz, EV, Cruze, L, Wei, W, Gehris, J, and Wagner, CL. Maternal vitamin D sufficiency and reduced placental gene expression in angiogenic biomarkers related to comorbidities of pregnancy. J Steroid Biochem Mol Biol. (2017) 173:273–9. doi: 10.1016/j.jsbmb.2017.02.003
10. Liu, NQ, Larner, DP, Yao, Q, Chun, RF, Ouyang, Y, Zhou, R, et al. Vitamin D-deficiency and sex-specific dysregulation of placental inflammation. J Steroid Biochem Mol Biol. (2018) 177:223–30. doi: 10.1016/j.jsbmb.2017.06.012
11. Lepsch, J, Eshriqui, I, Farias, DR, Vaz, JS, Figueiredo, ACC, Adegboye, ARA, et al. Association between early pregnancy vitamin D status and changes in serum lipid profiles throughout pregnancy. Metabolism. (2017) 70:85–97. doi: 10.1016/j.metabol.2017.02.004
12. Wheeler, BJ, Taylor, BJ, De Lange, M, Harper, MJ, Jones, S, Mekhail, A, et al. A longitudinal study of 25-hydroxy vitamin D and parathyroid hormone status throughout pregnancy and exclusive lactation in New Zealand mothers and their infants at 45° S. Nutrients. (2018) 10:86. doi: 10.3390/nu10010086
13. Suárez-Varela, MM, Uçar, N, Peraita-Costa, I, Huertas, MF, Soriano, JM, Llopis-Morales, A, et al. Vitamin D-related risk factors for maternal morbidity during pregnancy: a systematic review. Nutrients. (2022) 14:3166. doi: 10.3390/nu14153166
14. Hu, K-L, Zhang, C-X, Chen, P, Zhang, D, and Hunt, S. Vitamin D levels in early and middle pregnancy and preeclampsia, a systematic review and meta-analysis. Nutrients. (2022) 14:999. doi: 10.3390/nu14050999
15. Akbari, S, Khodadadi, B, Ahmadi, SAY, Abbaszadeh, S, and Shahsavar, F. Association of vitamin D level and vitamin D deficiency with risk of preeclampsia: a systematic review and updated meta-analysis. Taiwanese J Obstetrics Gynecol. (2018) 57:241–7. doi: 10.1016/j.tjog.2018.02.013
16. Tabesh, M, Salehi-Abargouei, A, Tabesh, M, and Esmaillzadeh, A. Maternal vitamin D status and risk of pre-eclampsia: a systematic review and meta-analysis. J Clin Endocrinol Metabol. (2013) 98:3165–73. doi: 10.1210/jc.2013-1257
17. Wei, S-Q, Qi, H-P, Luo, Z-C, and Fraser, WD. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J. Matern Fetal Neonatal Med. (2013) 26:889–99. doi: 10.3109/14767058.2013.765849
18. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DGPRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
19. Zeng, Y, and He, G. Association of blood parameters in early pregnancy with anemia during late pregnancy: a multicenter cohort study in China. J Matern Fetal Neonatal Med. (2024) 37:2299110. doi: 10.1080/14767058.2023.2299110
20. Higgins, JP, and Thompson, SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
21. DerSimonian, R, and Kacker, R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. (2007) 28:105–14. doi: 10.1016/j.cct.2006.04.004
22. Sterne, JA, and Harbord, RM. Funnel plots in meta-analysis. Stata J. (2004) 4:127–41. doi: 10.1177/1536867X0400400204
23. Holick, MF, Binkley, NC, Bischoff-Ferrari, HA, Gordon, CM, Hanley, DA, Heaney, RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metabol. (2011) 96:1911–30. doi: 10.1210/jc.2011-0385
24. Wetta, LA, Biggio, JR, Cliver, S, Abramovici, A, Barnes, S, and Tita, AT. Is midtrimester vitamin D status associated with spontaneous preterm birth and preeclampsia? Am J Perinatol. (2013) 31:541–6. doi: 10.1055/s-0033-1356483
25. Powe, CE, Seely, EW, Rana, S, Bhan, I, Ecker, J, Karumanchi, SA, et al. First trimester vitamin D, vitamin D binding protein, and subsequent preeclampsia. Hypertension. (2010) 56:758–63. doi: 10.1161/HYPERTENSIONAHA.110.158238
26. Schneuer, FJ, Roberts, CL, Guilbert, C, Simpson, JM, Algert, CS, Khambalia, AZ, et al. Effects of maternal serum 25-hydroxyvitamin D concentrations in the first trimester on subsequent pregnancy outcomes in an Australian population. Am J Clin Nutr. (2014) 99:287–95. doi: 10.3945/ajcn.113.065672
27. Bodnar, LM, Catov, JM, Simhan, HN, Holick, MF, Powers, RW, and Roberts, JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metabol. (2007) 92:3517–22. doi: 10.1210/jc.2007-0718
28. Wei, S, Audibert, F, Hidiroglou, N, Sarafin, K, Julien, P, Wu, Y, et al. Longitudinal vitamin D status in pregnancy and the risk of pre-eclampsia. BJOG Int J Obstet Gynaecol. (2012) 119:832–9. doi: 10.1111/j.1471-0528.2012.03307.x
29. van Weert, B, van den Berg, D, Hrudey, EJ, Oostvogels, AJ, de Miranda, E, and Vrijkotte, TG. Is first trimester vitamin D status in nulliparous women associated with pregnancy related hypertensive disorders? Midwifery. (2016) 34:117–22. doi: 10.1016/j.midw.2015.12.007
30. Benachi, A, Baptiste, A, Taieb, J, Tsatsaris, V, Guibourdenche, J, Senat, M-V, et al. Relationship between vitamin D status in pregnancy and the risk for preeclampsia: a nested case-control study. Clin Nutr. (2020) 39:440–6. doi: 10.1016/j.clnu.2019.02.015
31. Álvarez-Fernández, I, Prieto, B, Rodríguez, V, Ruano, Y, Escudero, AI, and Álvarez, FV. Role of vitamin D and sFlt-1/PlGF ratio in the development of early-and late-onset preeclampsia. Clin Chem Lab Med. (2015) 53:1033–40. doi: 10.1515/cclm-2014-1039
32. Flood-Nichols, SK, Tinnemore, D, Huang, RR, Napolitano, PG, and Ippolito, DL. Vitamin D deficiency in early pregnancy. PLoS One. (2015) 10:e0123763. doi: 10.1371/journal.pone.0123763
33. Gidlöf, S, Silva, AT, Gustafsson, S, and Lindqvist, PG. Vitamin D and the risk of preeclampsia–a nested case–control study. Acta Obstet Gynecol Scand. (2015) 94:904–8. doi: 10.1111/aogs.12658
34. Malm, G, Lindh, CH, Hansson, SR, Källén, K, Malm, J, and Rylander, L. Maternal serum vitamin D level in early pregnancy and risk for preeclampsia: a case-control study in southern Sweden. PLoS One. (2023) 18:e0281234. doi: 10.1371/journal.pone.0281234
35. Ni, M, Zhang, Q, Zhao, J, Shen, Q, Yao, D, Wang, T, et al. Relationship between maternal vitamin D status in the first trimester of pregnancy and maternal and neonatal outcomes: a retrospective single center study. BMC Pediatr. (2021) 21:330. doi: 10.1186/s12887-021-02730-z
36. Nema, J, Wadhwani, N, Randhir, K, Dangat, K, Pisal, H, Kadam, V, et al. Association of maternal vitamin D status with the risk of preeclampsia. Food Funct. (2023) 14:4859–65. doi: 10.1039/D3FO00007A
37. Vestergaard, AL, Justesen, S, Volqvartz, T, Aagaard, SK, Andreasen, MF, Lesnikova, I, et al. Vitamin D insufficiency among Danish pregnant women-prevalence and association with adverse obstetric outcomes and placental vitamin D metabolism. Acta Obstet Gynecol Scand. (2021) 100:480–8. doi: 10.1111/aogs.14019
38. Wang, W, Du, T, and Jiang, X. Correlation between 25-Hydroxyvitamin D, sFlt-1, PLGF, and hypertension in pregnancy. J Healthcare Eng. (2021) 2021:1–11. doi: 10.1155/2021/9371953
39. Baker, AM, Haeri, S, Camargo, CA Jr, Espinola, JA, and Stuebe, AM. A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia. J Clin Endocrinol Metabol. (2010) 95:5105–9. doi: 10.1210/jc.2010-0996
40. Wei, SQ, Audibert, F, Luo, ZC, Nuyt, AM, Masse, B, Julien, P, et al. Maternal plasma 25-hydroxyvitamin D levels, angiogenic factors, and preeclampsia. Am J Obstet Gynecol. (2013) 208:390.e1–6. doi: 10.1016/j.ajog.2013.03.025
41. Baca, KM, Simhan, HN, Platt, RW, and Bodnar, LM. Low maternal 25-hydroxyvitamin D concentration increases the risk of severe and mild preeclampsia. Ann Epidemiol. (2016) 26:853–857.e1. e1. doi: 10.1016/j.annepidem.2016.09.015
42. Zhao, X, Fang, R, Yu, R, Chen, D, Zhao, J, and Xiao, J. Maternal vitamin D status in the late second trimester and the risk of severe preeclampsia in southeastern China. Nutrients. (2017) 9:138. doi: 10.3390/nu9020138
43. Zhou, J, Su, L, Liu, M, Liu, Y, Cao, X, Wang, Z, et al. Associations between 25-hydroxyvitamin D levels and pregnancy outcomes: a prospective observational study in southern China. Eur J Clin Nutr. (2014) 68:925–30. doi: 10.1038/ejcn.2014.99
44. Achkar, M, Dodds, L, Giguère, Y, Forest, J-C, Armson, BA, Woolcott, C, et al. Vitamin D status in early pregnancy and risk of preeclampsia. Am J Obstet Gynecol. (2015) 212:511.e1. e7–7. doi: 10.1016/j.ajog.2014.11.009
45. Boyle, VT, Thorstensen, EB, Mourath, D, Jones, MB, McCowan, LM, Kenny, LC, et al. The relationship between 25-hydroxyvitamin D concentration in early pregnancy and pregnancy outcomes in a large, prospective cohort. Br J Nutr. (2016) 116:1409–15. doi: 10.1017/S0007114516003202
46. Magnus, MC, Miliku, K, Bauer, A, Engel, SM, Felix, JF, Jaddoe, VW, et al. Vitamin D and risk of pregnancy related hypertensive disorders: mendelian randomisation study. BMJ. (2018) 361:k2167. doi: 10.1136/bmj.k2167
47. Hemmingway, A, Kenny, LC, Malvisi, L, and Kiely, ME. Exploring the concept of functional vitamin D deficiency in pregnancy: impact of the interaction between 25-hydroxyvitamin D and parathyroid hormone on perinatal outcomes. Am J Clin Nutr. (2018) 108:821–9. doi: 10.1093/ajcn/nqy150
48. Mirzakhani, H, Litonjua, AA, McElrath, TF, O’Connor, G, Lee-Parritz, A, Iverson, R, et al. Early pregnancy vitamin D status and risk of preeclampsia. J Clin Invest. (2016) 126:4702–15. doi: 10.1172/JCI89031
49. Scholl, TO, Chen, X, and Stein, TP. Vitamin D, secondary hyperparathyroidism, and preeclampsia. Am J Clin Nutr. (2013) 98:787–93. doi: 10.3945/ajcn.112.055871
50. Shand, A, Nassar, N, Von Dadelszen, P, Innis, S, and Green, T. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. BJOG Int J Obstet Gynaecol. (2010) 117:1593–8. doi: 10.1111/j.1471-0528.2010.02742.x
51. Christoph, P, Challande, P, Raio, L, and Surbek, D. High prevalence of severe vitamin D deficiency during the first trimester in pregnant women in Switzerland and its potential contributions to adverse outcomes in the pregnancy. Swiss Med Wkly. (2020) 150:w20238. doi: 10.4414/smw.2020.20238
52. Yue, C-Y, Gao, J-p, Zhang, C-Y, and Ying, C-M. Is serum vitamin D deficiency before gestational 20 weeks a risk factor for preeclampsia? Clin Nutr. (2021) 40:4430–5. doi: 10.1016/j.clnu.2020.12.040
53. Irwinda, R, Hiksas, R, Lokeswara, AW, and Wibowo, N. Vitamin D supplementation higher than 2000 IU/day compared to lower dose on maternal–fetal outcome: systematic review and meta-analysis. Womens Health. (2022) 18:17455057221111066. doi: 10.1177/17455057221111066
54. Khaing, W, Vallibhakara, SA-O, Tantrakul, V, Vallibhakara, O, Rattanasiri, S, McEvoy, M, et al. Calcium and vitamin D supplementation for prevention of preeclampsia: a systematic review and network meta-analysis. Nutrients. (2017) 9:1141. doi: 10.3390/nu9101141
55. Kinshella, M-LW, Omar, S, Scherbinsky, K, Vidler, M, Magee, LA, von Dadelszen, P, et al. Effects of maternal nutritional supplements and dietary interventions on placental complications: an umbrella review, meta-analysis and evidence map. Nutrients. (2021) 13:472. doi: 10.3390/nu13020472
56. Zhao, R, Zhou, L, Wang, S, Xiong, G, and Hao, L. Association between maternal vitamin D levels and risk of adverse pregnancy outcomes: a systematic review and dose–response meta-analysis. Food Funct. (2022) 13:14–37. doi: 10.1039/D1FO03033G
57. Fogacci, S, Fogacci, F, Banach, M, Michos, ED, Hernandez, AV, Lip, GY, et al. Vitamin D supplementation and incident preeclampsia: a systematic review and meta-analysis of randomized clinical trials. Clin Nutr. (2020) 39:1742–52. doi: 10.1016/j.clnu.2019.08.015
58. Palacios, C, Kostiuk, LK, and Peña-Rosas, JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. (2019) 7:Cd008873. doi: 10.1002/14651858.CD008873.pub4
59. Pérez-López, FR, Pasupuleti, V, Mezones-Holguin, E, Benites-Zapata, VA, Thota, P, Deshpande, A, et al. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. (2015) 103:1278–1288.e4. e4. doi: 10.1016/j.fertnstert.2015.02.019
60. Aguilar-Cordero, M, Lasserrot-Cuadrado, A, Mur-Villar, N, León-Ríos, X, Rivero-Blanco, T, and Pérez-Castillo, I. Vitamin D, preeclampsia and prematurity: a systematic review and meta-analysis of observational and interventional studies. Midwifery. (2020) 87:102707. doi: 10.1016/j.midw.2020.102707
61. Martínez-Domínguez, SJ, Tajada, M, Chedraui, P, and Pérez-López, FR. Systematic review and meta-analysis of Spanish studies regarding the association between maternal 25-hydroxyvitamin D levels and perinatal outcomes. Gynecol Endocrinol. (2018) 34:987–94. doi: 10.1080/09513590.2018.1472761
62. Cornelius, DC. Preeclampsia: from inflammation to immunoregulation. Clin Med Insights Blood Disord. (2018) 11:1179545X17752325. doi: 10.1177/1179545X17752325
63. Kim, D-H, Meza, CA, Clarke, H, Kim, J-S, and Hickner, RC. Vitamin D and endothelial function. Nutrients. (2020) 12:575. doi: 10.3390/nu12020575
64. Pál, É, Ungvári, Z, Benyó, Z, and Várbíró, S. Role of vitamin D deficiency in the pathogenesis of cardiovascular and cerebrovascular diseases. Nutrients. (2023) 15:334. doi: 10.3390/nu15020334
Keywords: vitamin D, preeclampsia, pregnancy, systematic review, meta-analysis
Citation: Zakerinasab F, Behfar Q, Daghistani H, Ansari A, Zadeh RH, Zadeh RH and Amirbeik A (2025) Vitamin D levels in early and middle pregnancy and preeclampsia: a systematic review and meta-analysis. Front. Med. 12:1535359. doi: 10.3389/fmed.2025.1535359
Edited by:
Javier Diaz-Castro, University of Granada, SpainReviewed by:
Gulsym Serikbaivna Manasova, Odessa National Medical University, UkraineHan Zo Choi, Kyung Hee University Hospital, Republic of Korea
Copyright © 2025 Zakerinasab, Behfar, Daghistani, Ansari, Zadeh, Zadeh and Amirbeik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arina Ansari, YXJpbmFhbnNhcmk4MEBnbWFpbC5jb20=
 Faezeh Zakerinasab1
Faezeh Zakerinasab1 Hussam Daghistani
Hussam Daghistani Arina Ansari
Arina Ansari