- 1Department of Gastroenterology, Guanghua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Shanghai Guanghua Hospital of Integrative Medicine, Shanghai, China
- 3The Research Institute for Joint Diseases, Shanghai Academy of Traditional Chinese Medicine, Shanghai, China
Objective: To investigate the factors associated with the recurrence of colorectal polyps.
Methods: Data on polyp recurrence and related factors, including gender, age, BMI, family history, smoking history, alcohol consumption history, gallbladder disease history, food allergy, polyp size, number, and pathological classification, Helicobacter pylori (Hp) infection, parathyroid hormone, gastrin, and blood lipid levels, were collected as exposure factors. Polyp recurrence was used as the outcome measure. Logistic regression analysis was used to evaluate risk and protective factors for colorectal polyp recurrence. The diagnostic performance of the identified risk factor model was assessed using ROC curve analysis.
Results: Among the 318 patients, 170 experienced polyp recurrence, while 148 did not. Logistic regression analysis revealed that gender (OR = 1.927, 95% CI = 1.134–3.276, P = 0.015), age60–80 years (OR = 3.228, 95% CI = 1.846–5.647, P < 0.001), history of gallbladder disease (OR = 2.011, 95% CI = 1.147–3.523, P = 0.015), food allergy (OR = 2.246, 95% CI = 1.211–4.545, P = 0.012), pathological classification (OR = 5.023, 95% CI = 2.932–8.606, P < 0.001), and Hp infection (OR = 1.970, 95% CI = 1.171–3.312, P = 0.011) were positively associated with polyp recurrence. Conversely, polyp size (OR = 0.324, 95% CI = 0.127–0.827, P = 0.018) was negatively associated with recurrence. Logit(p) = −2.459 + 0.656 × Gender + 1.172 × Age 61–80 years + 0.698 × Gallbladder Disease + 0.853 × Food Allergy−1.127 × Polyp Size + 1.164 × Pathological Classification + 0.678 × Hp Infection. The risk prediction model can be used to predict post-surgical recurrence of colorectal polyps with a sensitivity of 0.88 and specificity of 0.56. The cutoff value for this odds prediction model is 0.44.
Conclusion: Elderly (61–80 years old) male patients with adenomatous colorectal cancer and the history of Helicobacter pylori (Hp) infection, gallbladder disease and food allergy have higher odds to experience recurrence after surgical resection. On the contrary, those patients with a larger polyp size (≥2 cm) are less odds to experience recurrence. Patients with a risk prediction model value greater than or equal to 0.44 have increased odds to experience postoperative recurrence.
1 Introduction
Colorectal polyps are growths on the colonic mucosa that can cause symptoms such as abdominal pain, diarrhea, constipation, rectal bleeding, and mucus in the stool. In some cases, however, there may be no obvious symptoms (1). The most common type of colorectal polyp is the adenomatous polyp, which is also the most significant precancerous lesion for colorectal cancer (CRC). Research shows that 85%–90% of colorectal cancers develop from adenomas, and the polyp-adenoma-cancer sequence is now widely recognized as the primary pathway for colorectal cancer progression (2, 3). Colorectal cancer ranks as the third most common cancer worldwide and is the fourth leading cause of cancer-related deaths, posing a significant threat to human health (4). In China, the incidence and mortality of colorectal cancer are rising annually, with a growing trend among younger populations. Studies indicate that even after polyp removal, patients still face a relatively high risk of recurrence or malignant transformation (5), underscoring the importance of early prevention and treatment to reduce the risk of colorectal cancer. Even after endoscopic polyp removal, the recurrence rate remains as high as 30%–50% (6–8). The Delphi Initiative for the Early-Onset Colorectal Cancer (DIRECt) International Management Guidelines (9) highlights relevant risk factors, including hereditary CRC syndromes, long-term inflammatory bowel disease (10, 11), ethnicity (12), sugar intake (13, 14), obesity (15, 16), gender, hyperlipidemia, smoking, alcohol consumption (17), dietary habits and lifestyle (18), among others (19, 20). However, the evidence for these associations remains controversial. Consequently, many experts recommend combining risk factors into a comprehensive risk score, but no standardized framework for clinical application has yet been established.
This study utilized a cross-sectional retrospective cohort design to investigate patients who underwent colorectal polyp resection, with a follow-up period of 1 year. Exposure factors included patient characteristics at the time of surgery, such as gender, age, BMI, family history, smoking history, alcohol consumption history, history of gallbladder disease, food allergy, polyp size, number, pathological classification, Helicobacter pylori infection, parathyroid hormone, gastrin, and blood lipid levels. The outcome measure was defined as polyp recurrence within one year postoperatively. This study aims to explore the risk factors for colorectal polyp recurrence in depth, providing new perspectives for the prevention and treatment of patients with colorectal polyps.
2 Materials and methods
2.1 Patients
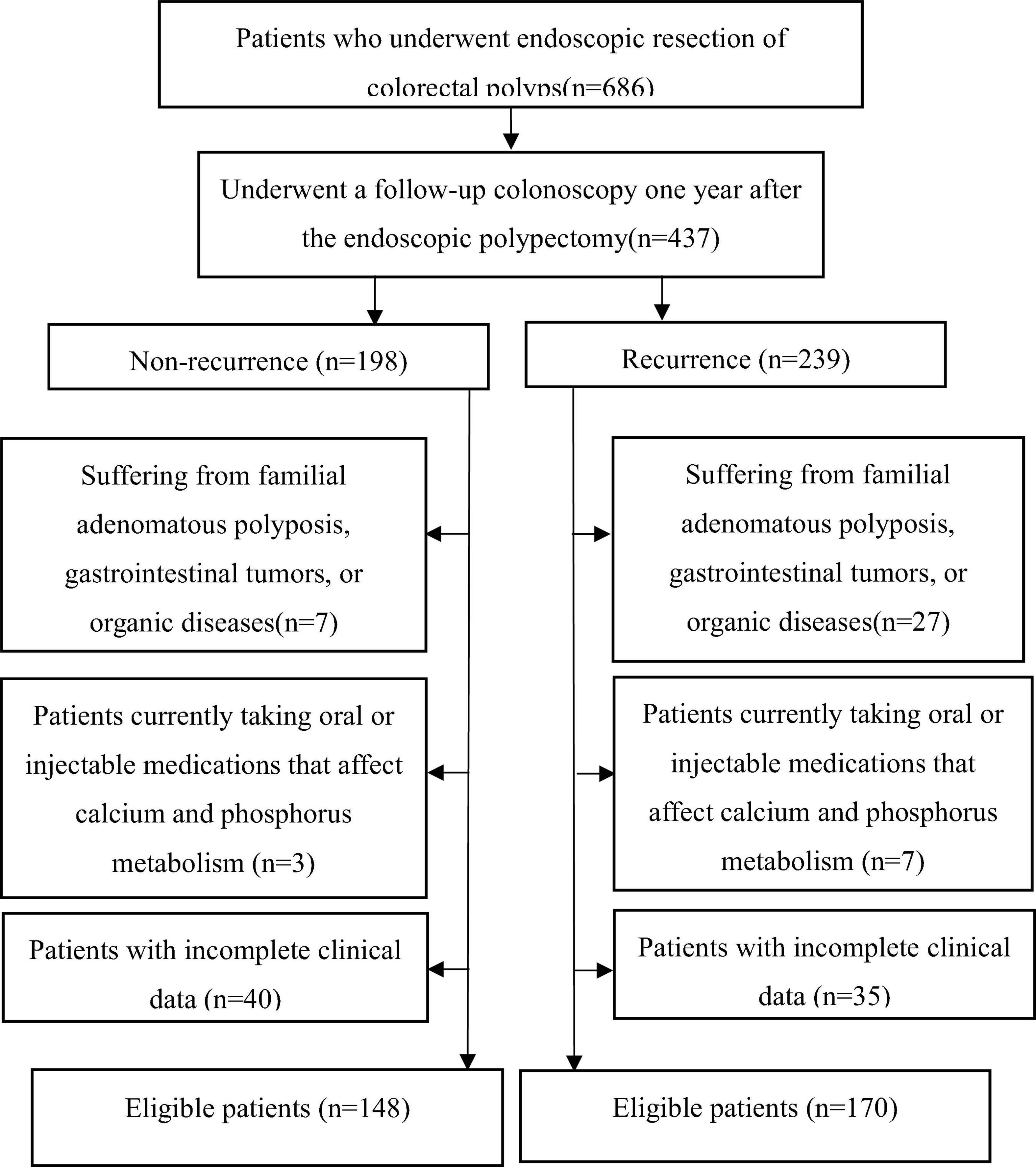
We retrospectively screened 686 patients who underwent endoscopic colorectal polyp resection between 1 January 2022, and 1 September 2024. Among these, 437 patients underwent follow-up colonoscopy one year postoperatively. According to the with recurrence or not, all subjects were divided into two groups, recurrence group (n = 239), Non-recurrence group (n = 198). After applying exclusion criteria, 318 patients (148 non-recurrence and 170 recurrence) met the inclusion criteria and completed the study. The study flowchart is shown in Figure 1. All patients were diagnosed with colorectal polyps and underwent endoscopic resection of colorectal polyps. This study was approved by the Ethics Committee of Guanghua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine (2021-K-37). The inclusion requirements were as follows: (1) Individuals who satisfied the diagnostic criteria for colorectal polyps in 2024 ESGE Guideline (21) and the 2021 Colorectal Cancer Screening Guidelines (22) and had undergone endoscopic resection; (2) No restrictions on gender or age; (3) Patients with clear consciousness who were able to cooperate in the collection of clinical data and related laboratory tests; (4) Patients who provided written informed consent to participate in the study. The exclusion criteria were as follows: (1) Patients with other significant intestinal diseases, familial adenomatous polyposis, or confirmed malignancies; (2) those with pathological findings of high-grade intraepithelial neoplasia, suspected malignancies, or confirmed malignant changes; (3) those with severe dysfunction of the heart, lungs, liver, or kidneys; compromised immune function; or malignant tumors; (4) those who had previously undergone gastrectomy; (5) those currently participating in other clinical trials; (6) female patients planning to conceive, currently pregnant, breastfeeding, or those with mental disorders or unable to complete the study. As shown in Figure 1, the process of including or excluding patients is summarized.
2.2 Exposure factors
The exposure factors analyzed in this study include: (1) Patient Information and Medical History: gender, age, BMI, family history, smoking history, alcohol consumption history, and history of gallbladder disease. (2) Laboratory Test Results: food allergen analysis, polyp size, number and pathological classification, 13C urea breath test, parathyroid hormone levels, gastrin levels, and blood lipid profiles. These exposure factors were included as independent variables to explore their association with polyp recurrence.
2.3 Outcome measures
After one year of follow-up, patients underwent a repeat colonoscopy to evaluate their postoperative recovery status. Following the recommendations from the Asia-Pacific Consensus on Colorectal Cancer Screening (23), recurrence was determined based on polyp presence, recording their number, size, and pathological nature.
2.4 Establishment of the predictive model
A logistic regression analysis was conducted to identify factors associated with polyp recurrence. A total of 14 factors, including: gender, age, BMI, smoking history, drinking history, history of gallbladder disease (based on the abdominal ultrasound report), Helicobacter pylori (Hp) status, polyp size, polyp number, pathological classification, abnormal blood lipid levels, gastrin levels, parathyroid hormone levels and food allergy were considered as independent variables (X), and whether polyp recurrence occurred as dependent variable (Y). The backward LR method with the entry probability at 0.10 and the removal probability at 0.15 was used to construct a multivariable logistic regression model, with details of risk factors and their coding presented in Supplementary Table 1. Then the multivariable logistic regression model was used to estimate the probability of postoperative polyp recurrence by ROC curve (AUC) analysis.
2.5 Statistical analysis
Statistical analysis was conducted using SPSS 26.0 software, following a stepwise approach. Continuous variables were expressed as mean±standard deviation (x±s). Categorical variables were expressed as numbers and percentages and analyzed using the χ2 test. All tests were two-tailed unless otherwise specified, with P < 0.05 considered statistically significant. For normally distributed variables with homogeneity of variance, independent samples t-tests were used. For normally distributed variables with heterogeneity of variance, approximate t’ tests were applied. For non-normally distributed variables, the Wilcoxon rank-sum test was used.
A logistic regression analysis was conducted to identify factors associated with polyp recurrence. The Backward LR method with the entry probability at 0.10 and the removal probability at 0.15 was used to construct a multivariable logistic regression model. p-value of LR test < 0.0001 indicates that the model is statistically significant overall.
3 Results
3.1 Patient characteristics
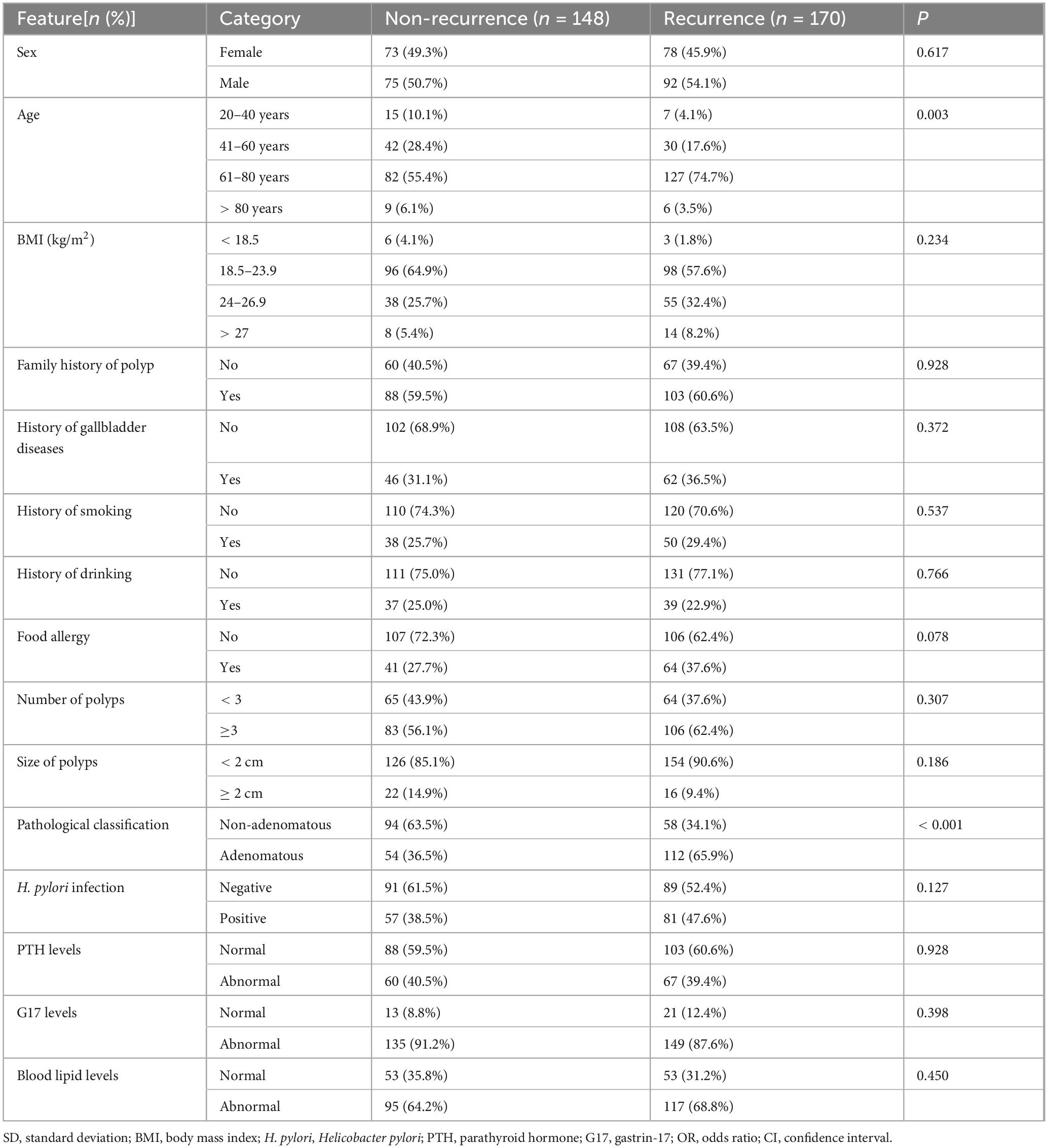
The study included a total of 318 patients. Among them, 148 cases were categorized as non-recurrence, and 170 cases as recurrence. Detailed patient information is presented in Table 1. The risk factors and corresponding variable assignments are listed in Supplementary Table 1. Except for age and pathological classification, there were no statistically significant differences in the baseline comparisons of other exposure factors between the recurrence and non-recurrence groups.
3.2 Elderly male patients with adenomatous colorectal cancer are higher odds to experience recurrence after surgical resection
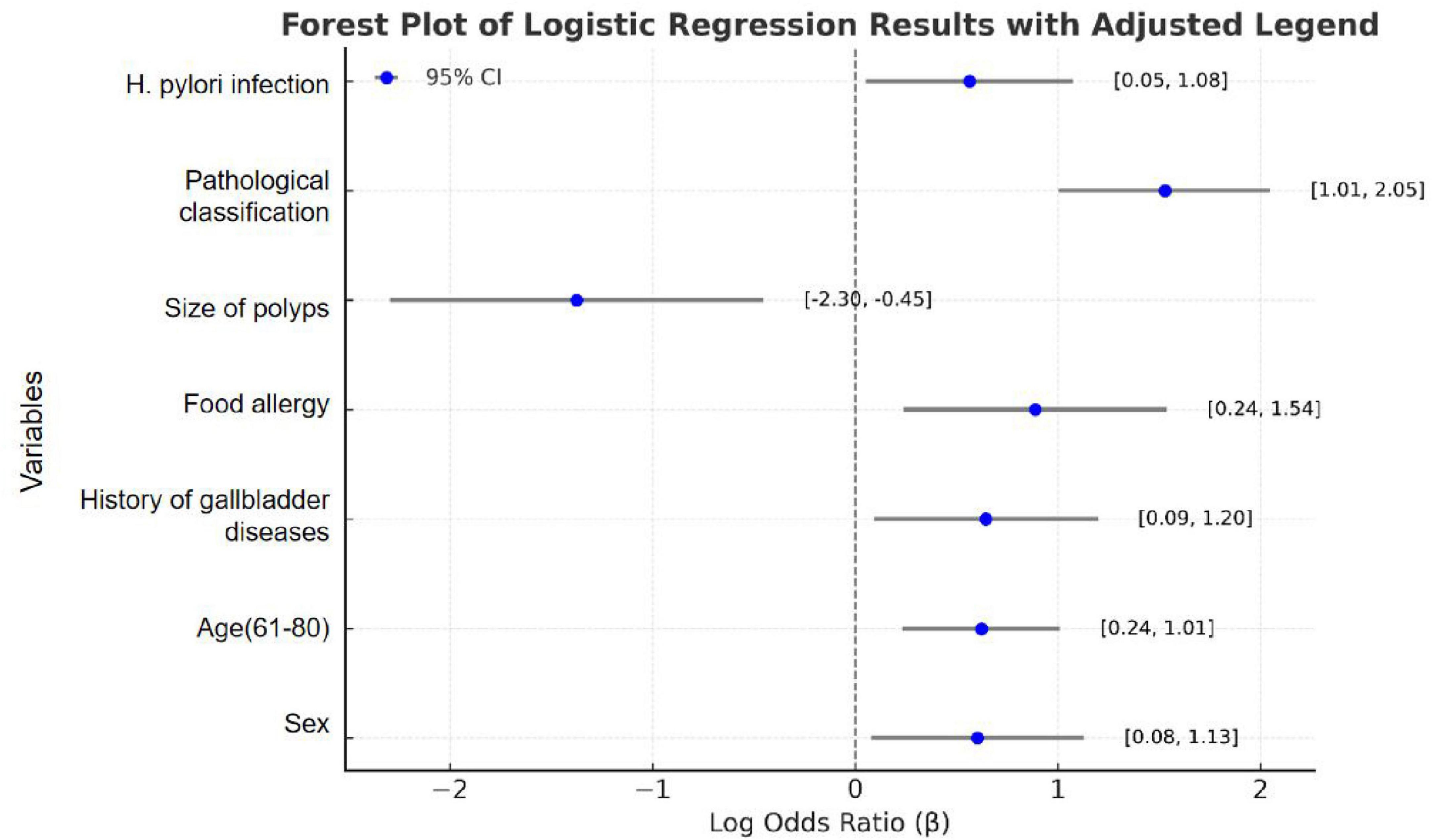
To identify significant risk factors for recurrence following colorectal cancer resection, we employed the Logistic stepwise regression method (Backward LR). The analysis used the following parameters: Constant Term, −3.2043; Pseudo R2, 0.1399; Log-Likelihood, −188.93. Logistic regression analysis identified several significant factors associated with colorectal polyp recurrence. As shown in Table 2 and Figure 2, male patients (OR = 1.927, 95% CI = 1.134–3.276, P = 0.015) had higher odds to experience recurrence compared to female patients, corresponding to an 92.7% increased odds. The odds of recurrence in patients aged 61–80 years is 3.228 times higher than that in the 20–40-year-old group (OR = 3.228, 95% CI = 1.846–5.647, P < 0.001). Patients with a history of gallbladder disease have 2.011 times higher odds of recurrence compared to those without such a history, indicating a 101.1% increased odds (OR = 2.011, 95% CI = 1.147–3.523, P = 0.015). Patients with food allergies had a 2.246-fold higher odds of recurrence compared to those without allergies, equating to a 124.6% increased odds (OR = 2.246, 95% CI = 1.211–4.545, P = 0.012). Patients with adenomatous polyps had 5.023 times higher odds to experience recurrence compared to those with non-adenomatous polyps, representing a 402.3% increased odds (OR = 5.023, 95% CI = 2.932–8.606, P < 0.001). Patients with Helicobacter pylori infection had 1.970 times higher odds to experience recurrence compared to uninfected patients, equating to a 97.0% increased risk (OR = 1.970, 95% CI = 1.171–3.312, P = 0.011). Conversely, patients with larger polyps (≥2 cm) had 0.324 times higher adds to experience recurrence compared to those with smaller polyps (<2 cm), indicating a 67.6% reduction in recurrence risk (OR = 0.324, 95% CI = 0.127–0.827, P = 0.018). The results indicate that elderly male patients are higher odds to experience recurrence after surgery. A history of food allergies significantly increases the odds of recurrence, while the presence of polyps larger than 2 cm at the time of surgical resection serves as a protective factor for patient prognosis.
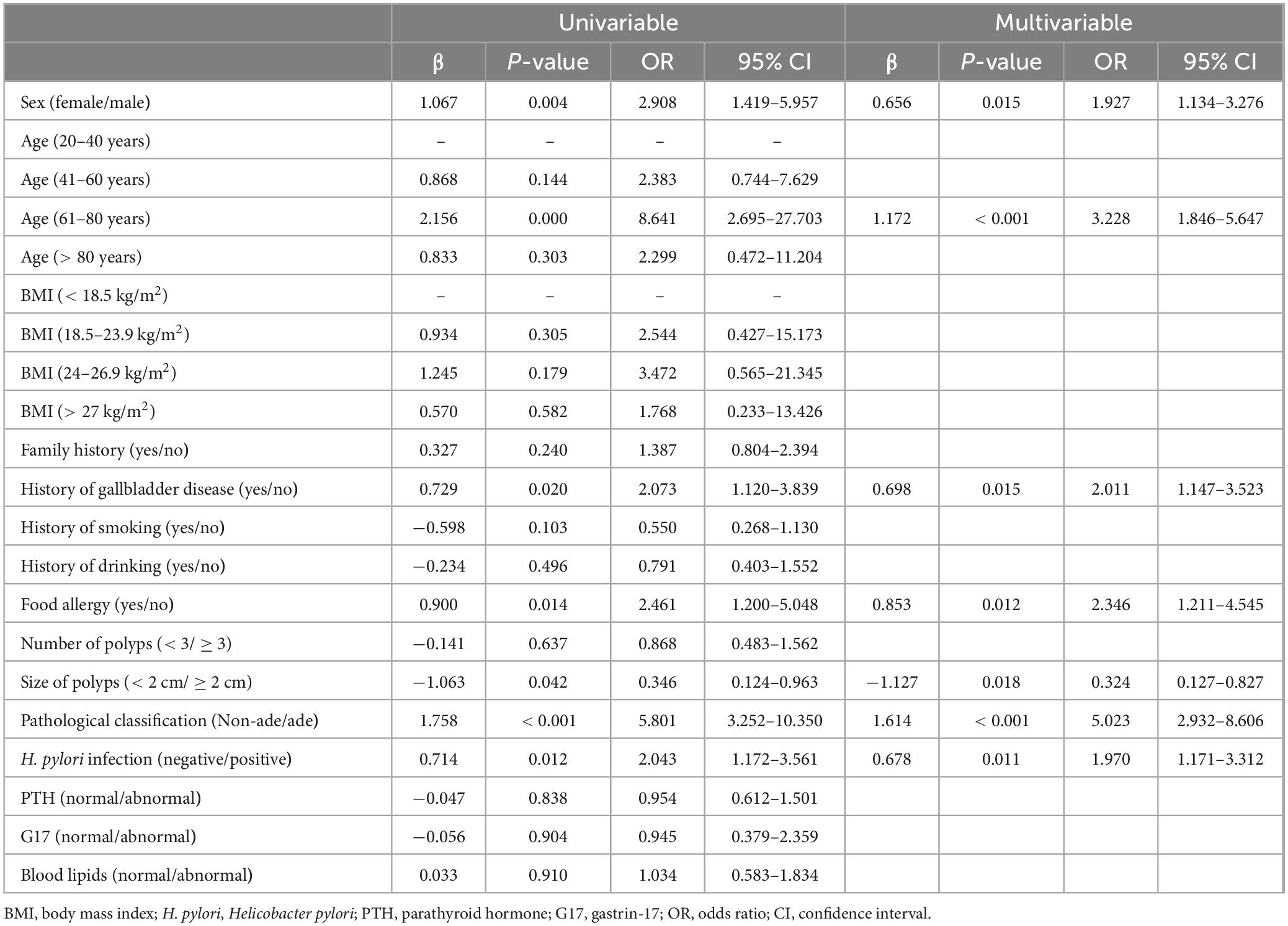
Table 2. Univariable and multivariable logistic regression analyses for predicting factors associated with polyp recurrence in the polyp patients.
In this study,
3.3 A predictive model for assessing postoperative recurrence risk of colorectal polyps
To further explore risk factors for predicting postoperative recurrence of colorectal polyps, we constructed a risk prediction model based on the identified factors and evaluated its diagnostic performance. The predictive logistic regression model for postoperative recurrence of colorectal polyps is as follows: Logit(p) = −2.459 + 0.656 × Gender + 1.172 × Age 61–80 years + 0.698 × Gallbladder Disease + 0.853 × Food Allergy−1.127 × Polyp Size + 1.164 × Pathological Classification + 0.678 × Hp Infection.
Gender: Male = 1, Female = 0
Age: Patient’s age (20–40 years = 0, 0, 0, 0; 41–60 years = 0, 1, 0, 0; 61–80 years = 0, 0, 1, 0; > 80 = 0, 0, 0, 1)
Gallbladder Disease: History of gallbladder disease (Yes = 1, No = 0)
Food Allergies: Presence of food allergies (Yes = 1, No = 0)
Polyp Size: Size of the polyp (> 2 cm = 1, < 2 cm = 0)
Pathological Classification: Adenomatous = 1, Non-adenomatous = 0
H. pylori Infection: Presence of H. pylori infection (Yes = 1, No = 0).
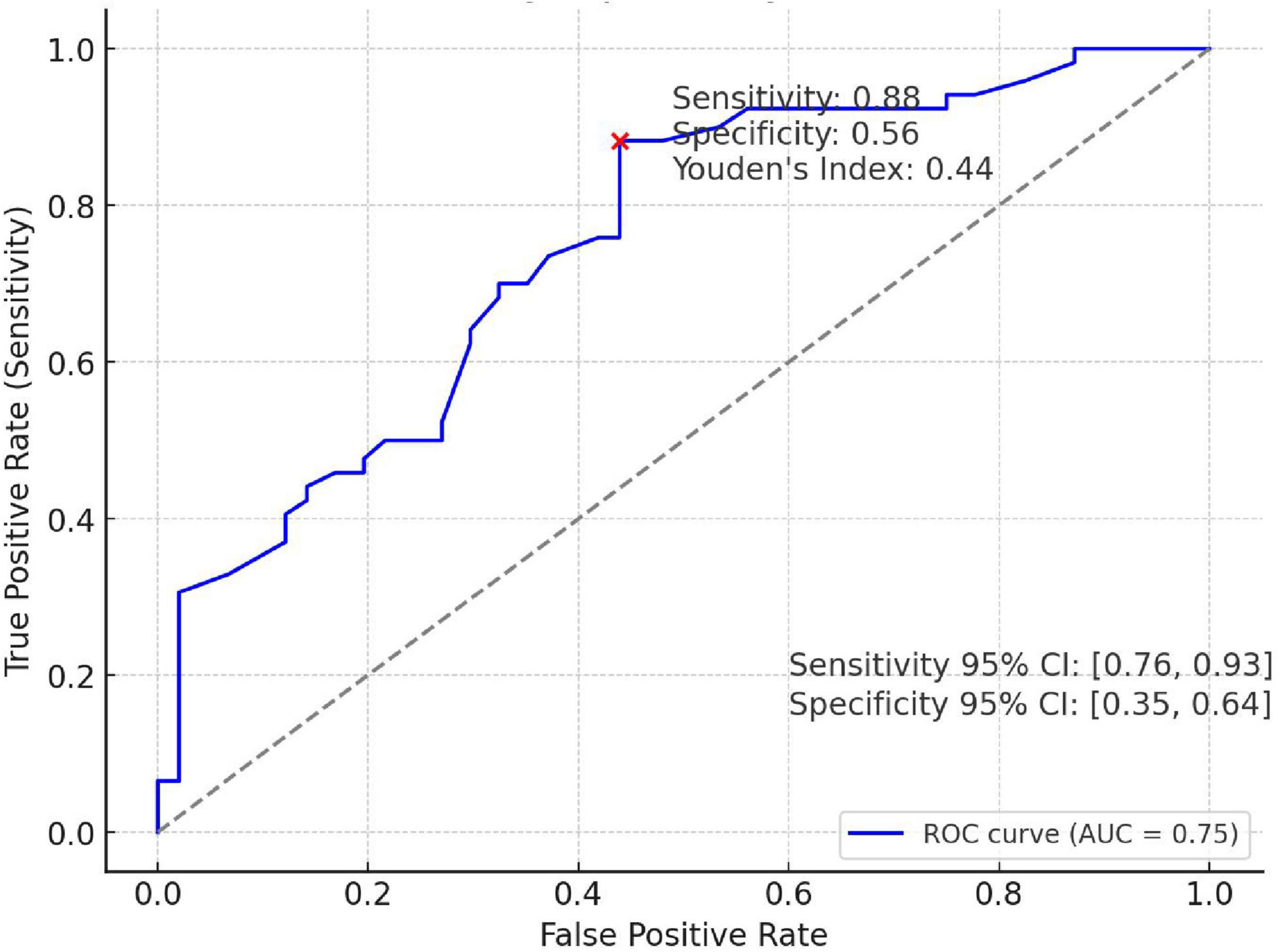
This model can be used to estimate the probability of postoperative polyp recurrence based on individual patient characteristics. As shown in Figure 3, area under the ROC curve (AUC) of the model was 0.75, with the sensitivity was 0.88, the specificity was 0.56, and Youden’s index = 0.44. The results indicate that colorectal polyp patients with predicted model values greater than or equal to 0.44 are more odds to experience postoperative recurrence.
4 Discussion
Early prevention and treatment of colorectal polyps are critical in preventing colorectal cancer. Currently, colorectal cancer is primarily prevented through the endoscopic removal of polyps (24). However, even after endoscopic polypectomy or treatment, preventive measures are necessary to reduce the risk of recurrence or malignant transformation (22). The clinical field is actively seeking strategies tailored to individuals with varying risk levels. Preventing the development and progression of polyps by targeting risk factors is one of the urgent challenges in current clinical research.
Our study identified male gender as a risk factor for colorectal polyp recurrence. Males were found to have a significantly higher recurrence odds compared to females. This elevated odds may be associated with lifestyle choices, hormonal levels, and metabolic factors. Research suggests that men often consume higher levels of fat and lower amounts of dietary fiber, which may increase intestinal inflammation and polyp formation. Androgens may further influence gut microbiota and inflammation, promoting polyp recurrence (25). A positive correlation was observed between older age and polyp recurrence. Elderly patients (> 60 years old) require enhanced postoperative monitoring. Age-related cumulative genetic and epigenetic changes, such as mutations in the APC, KRAS, and TP53 genes, contribute to the adenoma-carcinoma sequence (26, 27). Additionally, immunosenescence and gut microenvironment alterations, such as dysbiosis, further exacerbate the recurrence risk in older patients (28).
Gallbladder diseases, especially chronic cholecystitis, are associated with chronic inflammation in the biliary system. This inflammatory response may alter bile composition, impact intestinal bile acid metabolism, and induce local intestinal inflammation and mucosal hyperplasia, thereby promoting polyp recurrence and malignant transformation (29, 30). Our founding indicate that among the 108 patients with a history of cholecystitis, the recurrence rate was significantly higher compared to those without prior cholecystitis. Patients with food allergy had a higher odds of recurrence compared to non-allergic individuals. Food allergy result in overactivation of the immune system, particularly Th2-mediated immune responses, leading to chronic inflammation and impaired intestinal mucosal barrier function. Cytokines such as IL-4 and IL-13 released during immune activation may promote mucosal hyperplasia, accelerating polyp formation and recurrence (31).
Pathological classification emerged as the strongest predictor of polyp recurrence. Adenomatous polyps were significantly more odds to recur than non-adenomatous polyps. Adenomatous polyps exhibit higher proliferation and dysplasia, and their progression is closely linked to activation of the Wnt/β-catenin signaling pathway. Additionally, high-grade adenomas are prone to abnormal cell proliferation and genomic instability, increasing the odds of recurrence or malignant transformation (32). H. pylori infection was significantly associated with polyp recurrence, consistent with findings from other studies (33). H. pylori induces chronic gastritis, disrupts the gastrointestinal mucosal barrier, and leads to abnormal gastric acid secretion, altering the gut microenvironment. Furthermore, H. pylori infection is linked to the release of gastrointestinal inflammatory mediators such as TNF-α and IL-8, which may promote polyp formation and recurrence (34, 35). Interestingly, larger polyps (≥2 cm) were associated with a lower odds of recurrence (36). This may be due to the slower growth cycle of larger polyps. Given the 1-year follow-up period in this study, smaller polyps may have a higher odds of recurrence within this timeframe. These findings provide important evidence for risk stratification and targeted interventions aimed at reducing recurrence rates and improving patient outcomes. Strategies such as eradication therapy for H. pylori infection and dietary adjustments for food allergy patients may help mitigate recurrence risks. In the present study, no significant association was found between parathyroid hormone (PTH), gastrin, or serum lipid levels and the recurrence of colorectal polyps, despite existing literature reporting correlations between these factors and colorectal polyp recurrence (37–39). This discrepancy may stem from our methodological approach of dichotomizing these parameters (normal vs. abnormal) rather than incorporating continuous numerical values, which consequently excluded these biomarkers from the final analytical model.
Despite the rigorous statistical analysis in this study, certain limitations remain. First, as a retrospective study, there is a potential for selection bias. Second, unmeasured confounding factors, such as genetic predisposition, may have influenced the results. Future research should focus on prospective validation of these findings in larger cohorts. Integrating genetic and molecular biomarkers with clinical factors may enhance the accuracy of recurrence prediction models.
5 Conclusion
Elderly (60–80 years) male patients with adenomatous colorectal cancer and the history of Helicobacter pylori (Hp) infection, gallbladder disease and food allergy have higher odds to experience recurrence after surgical resection. On the contrary, those patients with a larger polyp size (≥2 cm) are less odds to experience recurrence. When the colorectal polyp patients with predicted model value is greater than or equal to 0.44, they have higher odds to experience postoperative recurrence and need to be paid more attentions.
The findings of this study provide a valuable predictive tool for clinically estimating the probability of recurrence in postoperative colon cancer patients. The results will guide gastroenterologists in evaluating the long-term outcomes of patients after colon cancer surgery and better inform postoperative rehabilitation strategies, demonstrating significant application value in clinical practice.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Guanghua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this study was a retrospective analysis of clinical data.
Author contributions
FL: Data curation, Formal Analysis, Investigation, Supervision, Validation, Writing – original draft. ML: Data curation, Formal Analysis, Investigation, Supervision, Validation, Writing – original draft. BX: Data curation, Formal Analysis, Investigation, Supervision, Validation, Writing – original draft. ZX: Data curation, Formal Analysis, Validation, Writing – original draft. YZ: Data curation, Formal Analysis, Validation, Writing – original draft. BC: Data curation, Formal Analysis, Validation, Writing – review and editing. XL: Data curation, Investigation, Validation, Writing – original draft. YW: Data curation, Investigation, Validation, Writing – review and editing. RZ: Data curation, Investigation, Validation, Writing – original draft. QC: Data curation, Investigation, Validation, Writing – review and editing. JS: Data curation, Formal Analysis, Investigation, Writing – review and editing. PX: Data curation, Formal Analysis, Investigation, Writing – original draft. LX: Funding acquisition, Investigation, Supervision, Writing – review and editing. YB: Funding acquisition, Investigation, Supervision, Writing – review and editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This study was supported by the Youth Research Project of Shanghai Municipal Health Commission (20214Y0166), Training Program for High-caliber Talents of Clinical Research at Affiliated Hospital of SHUTCM (2023LCRC24), and National Master of Traditional Chinese Medicine Studio of Changning District Health Commission.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1553194/full#supplementary-material
References
1. Shaukat A, Kaltenbach T, Dominitz J, Robertson D, Anderson J, Cruise M, et al. Endoscopic recognition and management strategies for malignant colorectal polyps: Recommendations of the US multi-society task force on colorectal cancer. Gastroenterology. (2020) 159:1916–34.e2. doi: 10.1053/j.gastro.2020.08.050
2. Winawer S, Zauber A, Ho M, O’Brien M, Gottlieb L, Sternberg S, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. (1993) 329:1977–81. doi: 10.1056/NEJM199312303292701
3. van Toledo D, IJspeert J, Bossuyt P, Bleijenberg A, van Leerdam M, van der Vlugt M, et al. Serrated polyp detection and risk of interval post-colonoscopy colorectal cancer: A population-based study. Lancet Gastroenterol Hepatol. (2022) 7:747–54. doi: 10.1016/S2468-1253(22)00090-5
4. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. (2013) 63:11–30. doi: 10.3322/caac.21166
5. Bonithon-Kopp C, Piard F, Fenger C, Cabeza E, O’Morain C, Kronborg O, et al. Colorectal adenoma characteristics as predictors of recurrence. Dis Colon Rectum. (2004) 47:323–33. doi: 10.1007/s10350-003-0054-1
6. Chi Z, Lin Y, Huang J, Lv M, Chen J, Chen X, et al. Risk factors for recurrence of colorectal conventional adenoma and serrated polyp. Gastroenterol Rep. (2022) 10:goab038. doi: 10.1093/gastro/goab038
7. Viel J, Studer J, Ottignon Y, Hirsch J. Predictors of colorectal polyp recurrence after the first polypectomy in private practice settings: A cohort study. PLoS One. (2012) 7:e50990. doi: 10.1371/journal.pone.0050990
8. Cai S, Shi H, Fan M, Zhang Q, Lin R. Risk of adenoma recurrence after polypectomy in patients younger than 50 years vs. 50 years old and over with diminutive or small adenomas. Front Oncol. (2022) 12:823263. doi: 10.3389/fonc.2022.823263
9. Cavestro G, Mannucci A, Balaguer F, Hampel H, Kupfer S, Repici A, et al. Delphi Initiative for Early-Onset Colorectal Cancer (DIRECt) International Management Guidelines. Clin Gastroenterol Hepatol. (2023) 21:581–603.e33. doi: 10.1016/j.cgh.2022.12.006
10. Low E, Demb J, Liu L, Earles A, Bustamante R, Williams C, et al. Risk factors for early-onset colorectal cancer. Gastroenterology. (2020) 159:492–501.e7. doi: 10.1053/j.gastro.2020.01.004
11. Stoffel EM, Murphy CC. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology. (2020) 158:341–53. doi: 10.1053/j.gastro.2019.07.055
12. Montminy E, Zhou M, Maniscalco L, Penrose H, Yen T, Patel S, et al. Trends in the incidence of early-onset colorectal adenocarcinoma among black and white US Residents Aged 40 to 49 Years, 2000-2017. JAMA Netw Open. (2021) 4:e2130433. doi: 10.1001/jamanetworkopen.2021.30433
13. Hur J, Otegbeye E, Joh H, Nimptsch K, Ng K, Ogino S, et al. Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women. Gut. (2021) 70:2330–6. doi: 10.1136/gutjnl-2020-323450
14. Joh H, Lee D, Hur J, Nimptsch K, Chang Y, Joung H, et al. Simple sugar and sugar-sweetened beverage intake during adolescence and risk of colorectal cancer precursors. Gastroenterology. (2021) 161:128–42.e20. doi: 10.1053/j.gastro.2021.03.028
15. Lei X, Song S, Li X, Geng C, Wang C. Excessive body fat at a young age increases the risk of colorectal cancer: A systematic review and meta-analysis. Nutr Cancer. (2021) 73:1601–12. doi: 10.1080/01635581.2020.1804951
16. Li H, Boakye D, Chen X, Jansen L, Chang-Claude J, Hoffmeister M, et al. Associations of body mass index at different ages with early-onset colorectal cancer. Gastroenterology. (2022) 162:1088–97.e3. doi: 10.1053/j.gastro.2021.12.239
17. O’Sullivan D, Sutherland R, Town S, Chow K, Fan J, Forbes N, et al. Risk factors for early-onset colorectal cancer: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. (2022) 20:1229–40.e5. doi: 10.1016/j.cgh.2021.01.037
18. Puzzono M, Mannucci A, Di Leo M, Zuppardo R, Russo M, Ditonno I, et al. Diet and lifestyle habits in early-onset colorectal cancer: A pilot case-control study. Dig Dis. (2022) 40:710–8. doi: 10.1159/000521932
19. McDowell R, Perrott S, Murchie P, Cardwell C, Hughes C, Samuel L. Oral antibiotic use and early-onset colorectal cancer: Findings from a case-control study using a national clinical database. Br J Cancer. (2022) 126:957–67. doi: 10.1038/s41416-021-01665-7
20. Kim H, Lipsyc-Sharf M, Zong X, Wang X, Hur J, Song M, et al. Total vitamin D intake and risks of early-onset colorectal cancer and precursors. Gastroenterology. (2021) 161:1208–17.e9. doi: 10.1053/j.gastro.2021.07.002
21. Shaukat A, Kahi C, Burke C, Rabeneck L, Sauer B, Rex D. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am J Gastroenterol. (2021) 116:458–79. doi: 10.14309/ajg.0000000000001122
22. Ferlitsch M, Hassan C, Bisschops R, Bhandari P, Dinis-Ribeiro M, Risio M, et al. Colorectal polypectomy and endoscopic mucosal resection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2024. Endoscopy. (2024) 56:516–45. doi: 10.1055/a-2304-3219
23. Sung J, Ng S, Chan F, Chiu H, Kim H, Matsuda T, et al. An updated Asia Pacific Consensus Recommendations on colorectal cancer screening. Gut. (2015) 64:121–32. doi: 10.1136/gutjnl-2013-306503
24. Chan F, Goh K, Reddy N, Fujimoto K, Ho K, Hokimoto S, et al. Management of patients on antithrombotic agents undergoing emergency and elective endoscopy: Joint Asian Pacific Association of Gastroenterology (APAGE) and Asian Pacific Society for Digestive Endoscopy (APSDE) practice guidelines. Gut. (2018) 67:405–17. doi: 10.1136/gutjnl-2017-315131
25. Araghi M, Soerjomataram I, Jenkins M, Brierley J, Morris E, Bray F, et al. Global trends in colorectal cancer mortality: Projections to the year 2035. Int J Cancer. (2019) 144:2992–3000. doi: 10.1002/ijc.32055
26. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. (1990) 61:759–67. doi: 10.1016/0092-8674(90)90186-i
27. Lieberman D, Rex D, Winawer S, Giardiello F, Johnson D, Levin T. Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. (2012) 143:844–57. doi: 10.1053/j.gastro.2012.06.001
28. Zauber A. The impact of screening on colorectal cancer mortality and incidence: Has it really made a difference? Dig Dis Sci. (2015) 60:681–91. doi: 10.1007/s10620-015-3600-5
29. Coussens LM, Werb Z. Inflammation and cancer. Nature. (2002) 420:860–7. doi: 10.1038/nature01322
30. Stergios K, Damaskos C, Frountzas M, Nikiteas N, Lalude O. Can gallbladder polyps predict colorectal adenoma or even neoplasia? A systematic review. Int J Surg. (2016) 33(Pt A):23–7. doi: 10.1016/j.ijsu.2016.06.048
31. Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. (2014) 133:291–307; quiz 308. doi: 10.1016/j.jaci.2013.11.020
32. Winawer S, Zauber A, Fletcher R, Stillman J, O’brien M, Levin B, et al. Guidelines for colonoscopy surveillance after polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin. (2006) 56:143–59; quiz 184–5. doi: 10.3322/canjclin.56.3.143
33. Liu Y, Yang D-Q, Jiang J-N, Jiao Y. Relationship between Helicobacter pylori infection and colorectal polyp/colorectal cancer. World J Gastrointest Surg. (2024) 16:1008–16. doi: 10.4240/wjgs.v16.i4.1008
34. Polk DB, Peek RM. Helicobacter pylori: Gastric cancer and beyond. Nat Rev Cancer. (2010) 10:403–14. doi: 10.1038/nrc2857
35. Wong B, Lam S, Wong W, Chen J, Zheng T, Feng R, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: A randomized controlled trial. JAMA. (2004) 291(2):187–94. doi: 10.1001/jama.291.2.187
36. Saito M, Yamamura T, Nakamura M, Maeda K, Sawada T, Ishikawa E, et al. Real-world local recurrence rate after cold polypectomy in colorectal polyps less than 10 mm using propensity score matching. World J Gastroenterol. (2021) 27:8182–93. doi: 10.3748/wjg.v27.i47.8182
37. Zhu X, Shrubsole M, Ness R, Hibler E, Cai Q, Long J, et al. Calcium/magnesium intake ratio, but not magnesium intake, interacts with genetic polymorphism in relation to colorectal neoplasia in a two-phase study. Mol Carcinog. (2016) 55:1449–57. doi: 10.1002/mc.22387
38. Ferrand A, Sandrin M, Shulkes A, Baldwin G. Expression of gastrin precursors by CD133-positive colorectal cancer cells is crucial for tumour growth. Biochimica Biophy Acta. (2009) 1793:477–88. doi: 10.1016/j.bbamcr.2009.01.004
Keywords: risk factors, recurrence, a cross-sectional retrospective cohort study, risk prediction model, colorectal polyps
Citation: Li F, Lu M, Xu B, Xiang Z, Zhang Y, Cao B, Li X, Wu Y, Zheng R, Cai Q, Shen J, Xin P, Xiao L and Bian Y (2025) Study on the risk factors for colorectal polyp recurrence: a cross-sectional retrospective cohort study. Front. Med. 12:1553194. doi: 10.3389/fmed.2025.1553194
Received: 30 December 2024; Accepted: 02 June 2025;
Published: 18 June 2025.
Edited by:
Fu Shen, Naval Medical University, ChinaReviewed by:
Mehmet Sait Ozsoy, Medeniyet Üniversitesi Göztepe Eğitim ve Araştırma Hastanesi, TürkiyeXinyu Yan, University of Florida, United States
Copyright © 2025 Li, Lu, Xu, Xiang, Zhang, Cao, Li, Wu, Zheng, Cai, Shen, Xin, Xiao and Bian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lianbo Xiao, eGlhb19saWFuYm9AMTYzLmNvbQ==; Yanqin Bian, eGlhb2JpYW41MDRAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Fang Li
Fang Li Mengge Lu1,2†
Mengge Lu1,2† Boran Cao
Boran Cao Jun Shen
Jun Shen Pengfei Xin
Pengfei Xin Lianbo Xiao
Lianbo Xiao Yanqin Bian
Yanqin Bian