- 1Department of Respirology, Kashiwa Kousei General Hospital, Kashiwa, Japan
- 2Department of Hematology, Kashiwa Kousei General Hospital, Kashiwa, Japan
The management of persistent coronavirus disease 2019 (COVID-19) in patients with hematological malignancies who are immunocompromised because of underlying disease or iatrogenic immunosuppression remains clinically challenging. Herein, we report an 84-year-old man with stage 3 diffuse large B-cell lymphoma treated with rituximab and epcoritamab who subsequently developed persistent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, despite having received seven doses of COVID-19 mRNA vaccine and remdesivir. The patient was treated with a combination of remdesivir, sotrovimab, and nirmatrelvir/ritonavir, and recovered clinically. SARS-CoV-2 polymerase chain reaction and antigen tests eventually turned negative, and he was discharged after 28 days of hospitalization. This case highlights the challenges associated with managing persistent SARS-CoV-2 infection in immunocompromised patients with hematological malignancies. Combined treatment with antivirals and monoclonal antibodies may be an effective strategy.
1 Introduction
Immunocompromised individuals, particularly those with hematological malignancies are susceptible to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Despite the effectiveness of vaccines and antiviral treatment, the risk of coronavirus disease 2019 (COVID-19)-related morbidity and mortality persists, even in the era of the Omicron variant (1). Patients with humoral immunodeficiency, such as those with B-cell malignancies and those receiving anti-CD20 therapy, are particularly susceptible to developing persistent COVID-19. Persistent SARS-CoV-2 infection can lead to decreased viral clearance, prolonged viral shedding, and an increased risk of viral mutations (2–4). The management of persistent COVID-19 in patients who are immunocompromised because of underlying disease or iatrogenic immunosuppression remains clinically challenging. Herein, we report the case of a patient with advanced diffuse large B-cell lymphoma (DLBCL) who developed persistent SARS-CoV-2 infection following treatment with rituximab and epcoritamab.
2 Case report
An 84-year-old Japanese man with stage 3 DLBCL received rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone therapy, followed by polatuzumab vedotin, bendamustine, and rituximab therapy. Owing to tumor progression, the patient was treated with epcoritamab in June 2024. Although it had been approved for prophylactic use against SARS-CoV-2 infection in Japan, tixagevimab/cilgavimab was not available at the time. The patient had received seven doses of an mRNA-based COVID-19 vaccine according to the schedule recommended in Japan, with the last dose administered in December 2023.
In July 2024, after the first cycle of epcoritamab, the patient developed a high fever and mild cough. The SARS-CoV-2 rapid antigen test (QuickNavi-COVID19 Ag; Denka Co., Ltd., Tokyo, Japan) was positive, and chest computed tomography (CT) revealed ground-glass opacities in the right lower lobe, left lingual lobe, and left lower lobe of his lungs (Figure 1). The patient was diagnosed with COVID-19 pneumonia and treated with remdesivir for 8 days (200 mg on the first day and 100 mg on the subsequent 7 days), tocilizumab (400 mg on the first day), and cefepime (2 g twice daily for 6 days), before being discharged from hospital on day 9. Nine days after the last administration of epcoritamab in the second cycle (i.e., 40 days after the previous COVID-19 onset), the patient presented with recurrent high fever, cough, and anorexia. Physical examination revealed no evidence of heart failure, including no peripheral edema, weight gain, or wheezing. SARS-CoV-2 antigen and polymerase chain reaction (PCR) tests (Xpert Xpress SARS-CoV-2 Cepheid; Beckman Coulter, Inc., Brea, CA, USA) were positive (cycle threshold [Ct]: 22.8). Chest CT revealed ground-glass opacities in both upper lobes and the right middle lobe of his lungs, although the previously identified abnormal shadows had almost disappeared (Figure 1). The patient was diagnosed with persistent SARS-CoV-2 infection. He had no history of foreign travel, sexually transmitted infections, or inhalational exposures. He was taking fluconazole (100 mg/day) for prophylaxis against fungal infections.
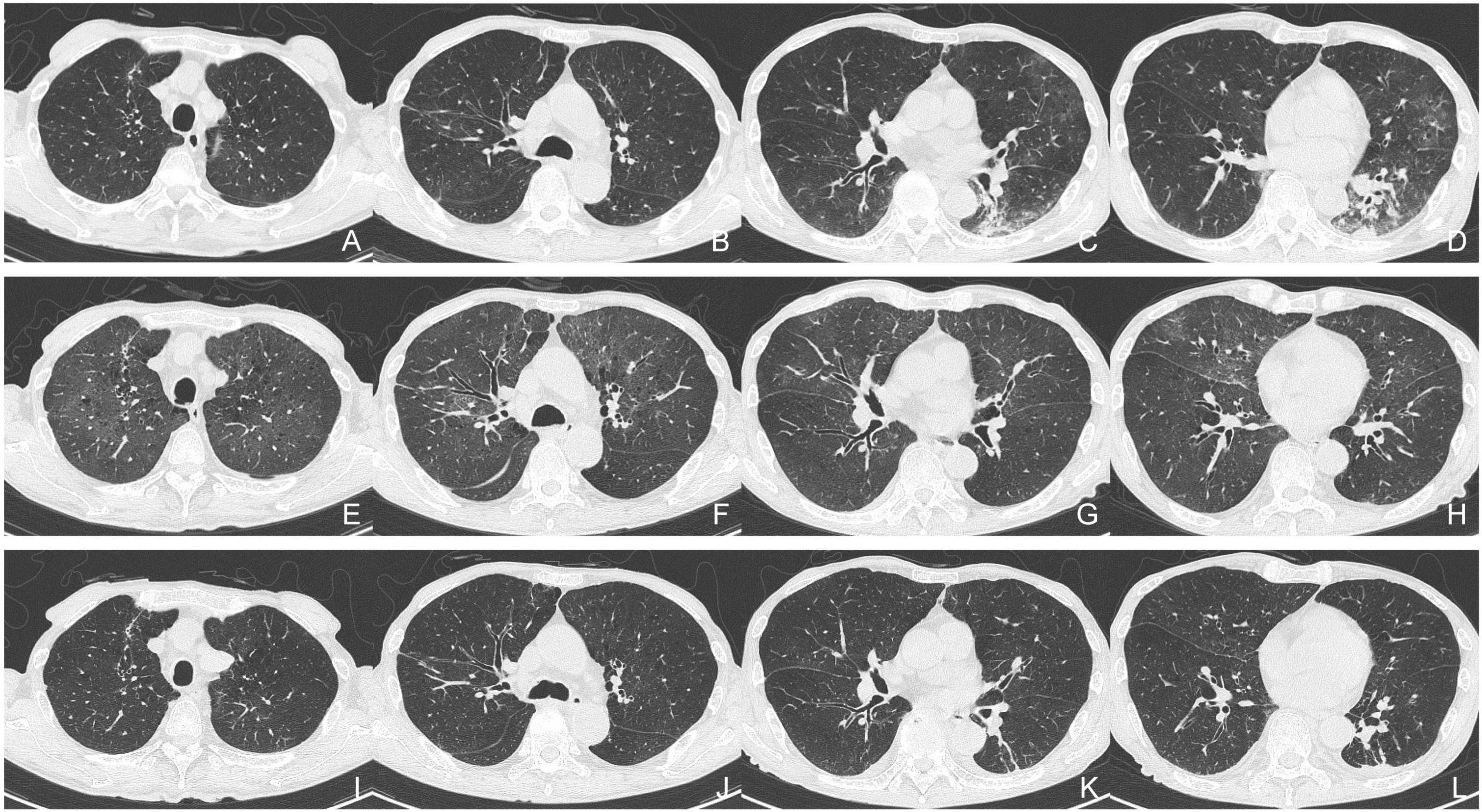
Figure 1. Serial chest computed tomography (CT). (A–D) Initial CT scan obtained during the patient’s first episode of COVID-19. (E–H) Second CT scan obtained on the patient’s readmission in August 2024. (I–L) Follow-up CT scan obtained before the patient’s discharge from hospital.
The patient’s vital signs on admission revealed fever (body temperature, 38.0°C), tachycardia (125 beats/min), and hypoxia (SpO2: 92% breathing ambient air), with a normal blood pressure (127/76 mmHg). His blood test results showed elevated inflammatory marker levels and hypogammaglobulinemia (Table 1). D-dimer levels exhibited a slight elevation, remaining essentially unchanged from the baseline prior to admission. He was treated with intravenous remdesivir for 10 days (200 mg on the first day, followed by 100 mg for 9 days), dexamethasone (6.6 mg/day for 5 days), ceftriaxone (2 g/day for 5 days), and azithromycin (500 mg/day for 3 days) (Figure 2), and oxygen was administered through a nasal cannula. Anticoagulant thromboprophylaxis was not implemented. Tests for autoantibodies associated with connective tissue diseases, serum anti-neutrophil cytoplasmic antibodies, Mycoplasma antibody, Aspergillus antigen, and cytomegalovirus immunoglobulin M (IgM), and an interferon-γ release assay to screen for tuberculosis were all negative. As the patient had a moderately elevated serum (1→3)-β-D-glucan level, sulfamethoxazole/trimethoprim (3,600/720 mg per day) was administered to treat possible Pneumocystis jirovecii pneumonia; however, a sputum PCR test for P. jirovecii DNA was negative.
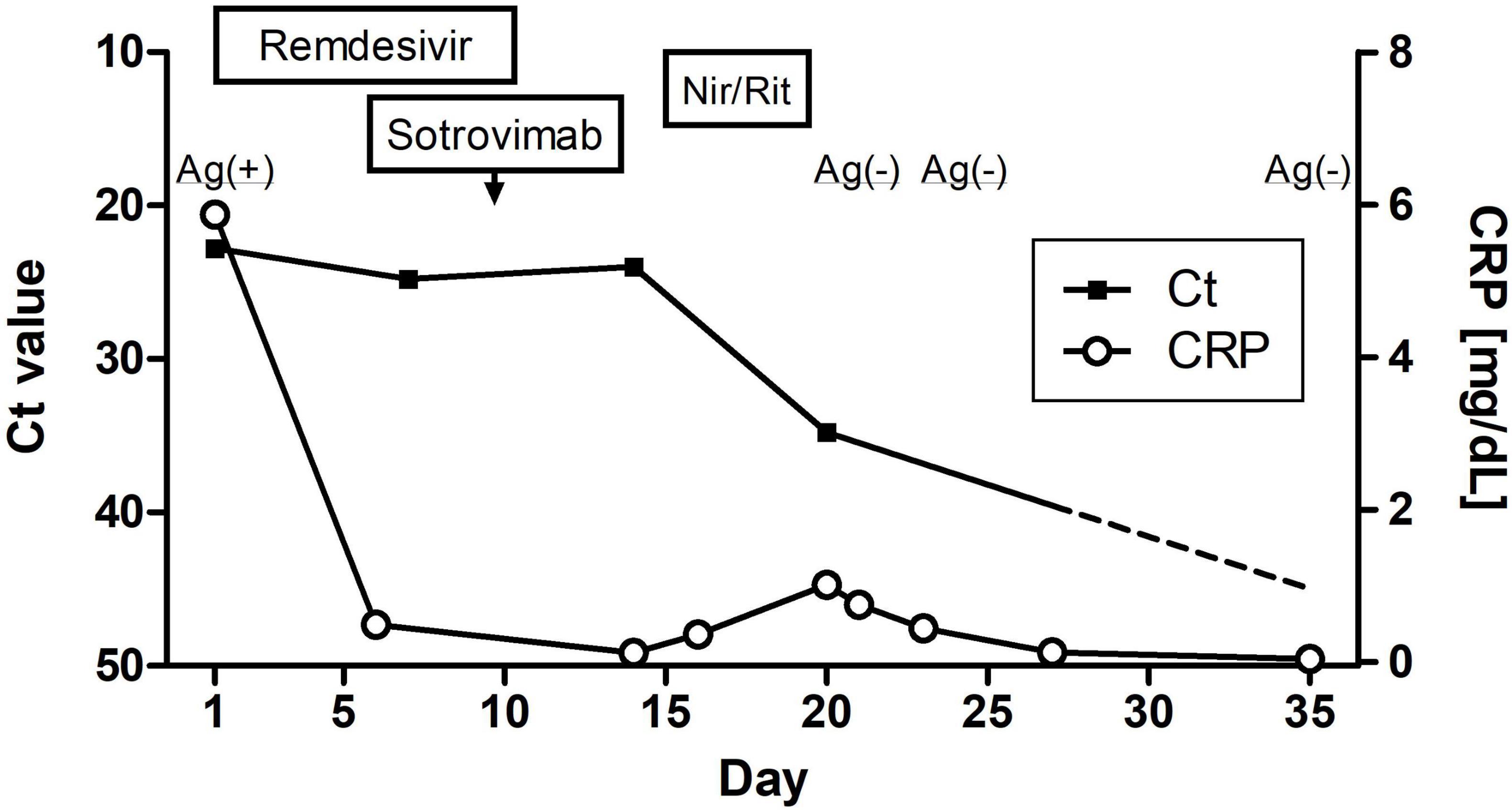
Figure 2. Clinical course of this case showing the changes in SARS-CoV-2 nucleic acid and C-reactive protein levels. Nir/Rit, nirmatrelvir/ritonavir.
Although the patient’s high fever and hypoxia resolved, a follow-up SARS-CoV-2 PCR test remained positive (Ct: 24.8) (Figure 2), and his symptoms of cough, fatigue, and anorexia persisted. On day 9 of hospitalization, he received sotrovimab (500 mg) monoclonal antibody treatment. On day 14, another SARS-CoV-2 PCR test was again positive (Ct: 24.0). Nirmatrelvir/ritonavir was administered on day 15. On day 21, the SARS-CoV-2 antigen and PCR test results were negative (Ct: 34.8). By day 24, the CT abnormalities had almost disappeared (Figure 1), and the patient was discharged on day 28 of hospitalization. One month after discharge, a follow-up PCR test for SARS-CoV-2 remained negative (Ct > 45.0) and there was no evidence of recurrent abnormalities on the CT scan. The CT severity of lung abnormalities was evaluated by three clinicians using a semi-quantitative scoring method as described by Pan et al. (5). The mean severity scores were 10.7, 11.0, 7.3, and 4.0 in the initial CT, second CT (admission in this report), pre-discharge follow-up, and 1-month post-discharge follow-up, respectively. The scores for each lung lobe are shown in Supplementary Table 1.
3 Discussion
Anti-CD20 therapy is frequently used to treat B-cell hematological malignancies. However, anti-CD20 agents can lead to prolonged immunosuppression that makes patients more susceptible to infection, including SARS-CoV-2 infection (2, 3). Rituximab, a commonly used immunosuppressive drug targeting B-cells, is associated with reduced response to SARS-CoV-2 vaccination (6). Patients with hematological malignancies who receive anti-CD20 therapy may have a reduced immune response to SARS-CoV-2 infection for as long as 12 months, which increases their risk of persistent COVID-19 (7). Epcoritamab is a novel bispecific antibody that targets both CD20 and CD3 and activates T-cells to target and eliminate CD20-expressing cells (8). The effect of epcoritamab-induced CD20 depletion in SARS-CoV-2 infection remains unclear, and there are only a few reports on the clinical course of persistent SARS-CoV-2 infection following rituximab and epcoritamab treatment. Faxén and Edvinsson (9) described a case of persistent COVID-19 treated using a combination therapy of remdesivir, tixagevimab/cilgavimab, and nirmatrelvir/ritonavir. Longo et al. (10) reported three cases of persistent COVID-19 and highlighted the effectiveness of combination therapy with antiviral agents and monoclonal antibodies. In our case, despite receiving rituximab and epcoritamab, which may have exerted an additive immunosuppressive effect, the patient recovered after sequential administration of remdesivir, sotrovimab, and nirmatrelvir/ritonavir.
Identifying patients who are most likely to experience persistent COVID-19 and establishing appropriate diagnostic criteria and management strategies for patients at high risk remain major clinical challenges. As humoral immunity plays a crucial role in the clearance of SARS-CoV-2, patients with B-cell depletion caused by conditions such as hematological malignancy, anti-CD20 therapy, hematopoietic stem cell/solid organ transplant, and common variable immunodeficiency, are more susceptible to persistent SARS-CoV-2 infection (1, 2). Although local protocols for the management of prolonged COVID-19 in patients with severe immunosuppression have been introduced (11), the optimal diagnostic evaluation and therapeutic strategies for patients with different degrees of immunodeficiency remain unclear. Moreover, although the US National Institutes of Health COVID-19 treatment guidelines acknowledge the occurrence of prolonged viral shedding in immunocompromised individuals (12), standardized clinical terminology for this phenomenon has not yet been established. Given our patient’s clinical course, prolonged viral shedding, symptoms, and CT findings, we diagnosed him with persistent COVID-19 based on the proposed criteria for persistent COVID-19 (11, 13).
Consensus regarding the optimal treatment strategy for persistent SARS-CoV-2 infection in patients who are immunocompromised, such as those undergoing B-cell depletion therapy, is lacking. Remdesivir suppresses viral replication by blocking the RNA-dependent RNA polymerase, which is essential for SARS-CoV-2 replication (14). Similarly, nirmatrelvir inhibits 3CL protease, an enzyme necessary for SARS-CoV-2 viral replication, and ritonavir inhibits its degradation. Dexamethasone, in combination with remdesivir is effective for treating COVID-19 (15). However, the potential for adverse outcomes due to steroid-induced suppression of interferon production and prolonged viral shedding has concerns (16). Therefore, cautious use of dexamethasone in combination with remdesivir with attention to dosage and duration is warranted in patients with hematological malignancies. The use of single antiviral agents for persistent SARS-CoV-2 infection is associated with a risk of drug resistance and promotes viral evolution (4). Prolonged use of nirmatrelvir/ritonavir treatment has been reported to be effective for treating persistent SARS-CoV-2 infection (17). However, clinically significant resistance to remdesivir and nirmatrelvir/ritonavir has been reported, particularly in patients with hematological malignancies and persistent SARS-CoV-2 infection (14). Recent reviews and case series have highlighted the effectiveness of therapies involving a combination of antiviral agents and monoclonal antibodies in patients who are immunocompromised with persistent COVID-19 (9, 10, 18–20). Both sequential and simultaneous combination therapies have been reported to be safe and effective (10). The Japanese guidelines for COVID-19 do not mention simultaneous combination therapy with antiviral agents (21). In this case, we used sequential combination therapy, which resulted in a favorable outcome. Intravenous immunoglobulin (IVIg) boosts and modulates the immune system and helps to fight infection in patients with immunodeficiency. Although the effectiveness of IVIg against persistent COVID-19 has not been confirmed, Maruki et al. (22) reported successful use of IVIg and antiviral agents to treat persistent COVID-19 in an immunocompromised patient who was receiving CD20-depleting therapy for follicular lymphoma. Convalescent plasma may offer therapeutic benefits for persistent COVID-19 (18), but its availability in general medical practice is limited. Optimizing personalized patient care and the choice and prioritization of drug combinations is challenging owing to the heterogeneity of immunosuppression according to the type and severity of the underlying disease. Further research in this area is warranted.
Previous studies have demonstrated the effectiveness of neutralizing antibodies for treating persistent COVID-19 (9, 10, 18–20). Although sotrovimab has been reported to be less effective at neutralizing SARS-CoV-2 variants BA.2.12.1, BA.4, and BA.5 in vitro (23), in real-world settings, it continued to be effective at preventing severe disease and complications during the period when the BA.2 and BA.5 variants were predominant (24). In patients with hematological malignancy, sotrovimab substantially boosts neutralizing antibody titers, even in those with inadequate humoral immune responses to COVID-19 vaccination (25). These studies suggest that sotrovimab may be a valuable therapeutic option against SARS-CoV-2 Omicron subvariants. In our patient, sotrovimab was administered sequentially owing to the unavailability of casirivimab/imdevimab.
Persistent COVID-19 can present with or without abnormal pulmonary findings, and the severity of respiratory decompensation varies widely (9, 10, 19). Frequently, multiple ground-glass opacities are observed on CT scans, and migration of airspace opacities, as demonstrated in this case, may also be observed (26) (Supplementary Table 1). In cases of ground-glass opacities that appear after the onset of COVID-19, the differential diagnosis includes persistent SARS-CoV-2 infection, as well as infections induced by a broad spectrum of pathogens, non-infectious diseases, pulmonary edema, and exacerbation complicating pre-existing interstitial lung disease. The wide range of conditions to consider in the differential diagnosis contributes to the diagnostic challenge. Challenges to diagnosing persistent COVID-19 include the positioning of imaging patterns and predicting cases of severe or refractory disease that require close monitoring. Even cases with a relatively mild but persistent clinical course of COVID-19 pneumonia can be fatal (10, 27), highlighting the need for further research in this field.
This study has some limitations. First, genetic sequencing of the SARS-CoV-2 virus was not performed in this case. According to Japanese nationwide surveillance data, the Omicron variant JN.1 and its subvariants accounted for approximately 95% of SARS-CoV-2 strains circulating in Japan in July 2024 (28), consistent with global trends (29). Second, no lower respiratory tract specimens (e.g., bronchoalveolar lavage fluid) were collected from the patient; therefore, the possible involvement of other respiratory pathogens cannot be ruled out in this case. However, except for elevated (1→3)-β-D-glucan levels, no other laboratory findings suggested the presence of other infections. Decisions regarding further testing should be personalized based on each patient’s unique immune status and the invasiveness of the proposed diagnostic procedures.
This case highlights key aspects of persistent COVID-19 in immunocompromised patients. Although patient backgrounds and treatment strategies vary, most cases are mild, with favorable outcomes from antiviral monotherapy or combination therapy. Notably, viral load appears to rapidly decrease following clinical improvement (10). Few reports are available of persistent infection following rituximab and epcoritamab treatment (9, 10, 30, 31). Among them, Bay et al. (30) suggested that intermittent remdesivir monotherapy may induce resistance through ORF1b:C455F mutation. As with our patient, most patients improve with prolonged (sequential or simultaneous) nirmatrelvir/ritonavir-based therapy. Although Breeden et al. (31) reported pneumonia associated with persistent SARS-CoV-2 infection, to our knowledge, our report is the first report migratory ground-glass opacities observed on serial high-resolution CT scans. Persistent COVID-19 in immunocompromised patients is diverse and clinically challenging to manage. More clinical research is needed for optimal practice.
In conclusion, in immunocompromised patients, particularly those with B-cell hematological disorders who are undergoing chemotherapy, are at risk of persistent SARS-CoV-2 infection. Although no standardized guidelines are available for treatment of persistent SARS-CoV-2 infection, combination therapy should be considered in immunocompromised patients to enhance the effectiveness of treatment and reduce the risk of prolonged viral shedding and the emergence of new SARS-CoV-2 mutations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics committee in Kashiwa Kosei General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MS: Investigation, Resources, Supervision, Writing – original draft, Writing – review and editing. IF: Investigation, Resources, Writing – original draft, Writing – review and editing. TM: Investigation, Resources, Supervision, Writing – original draft, Writing – review and editing.
Funding
The authors declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the medical staff for the management of the patient. We thank Editage (https://www.editage.jp/) for English language editing of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1554100/full#supplementary-material
Abbreviations
COVID-19, coronavirus disease 2019; Ct, cycle threshold; CT, computed tomography; DLBCL, diffuse large B-cell lymphoma; IgM, immunoglobulin M; IVIg, intravenous immunoglobulin; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SpO2, peripheral oxygen saturation.
References
1. Ward I, Robertson C, Agrawal U, Patterson L, Bradley D, Shi T, et al. Risk of COVID-19 death in adults who received booster COVID-19 vaccinations in England. Nat Commun. (2024) 15:398. doi: 10.1038/s41467-023-44276-x
2. Li Y, Choudhary M, Regan J, Boucau J, Nathan A, Speidel T, et al. SARS-CoV-2 viral clearance and evolution varies by type and severity of immunodeficiency. Sci Transl Med. (2024) 16:eadk1599. doi: 10.1126/scitranslmed.adk1599
3. Nussenblatt V, Roder A, Das S, de Wit E, Youn J, Banakis S, et al. Yearlong COVID-19 infection reveals within-host evolution of SARS-CoV-2 in a patient with B-cell depletion. J Infect Dis. (2022) 225:1118–23. doi: 10.1093/infdis/jiab622
4. Yamamoto C, Taniguchi M, Furukawa K, Inaba T, Niiyama Y, Ide D, et al. Nirmatrelvir resistance in an immunocompromised patient with persistent coronavirus disease 2019. Viruses. (2024) 16:718. doi: 10.3390/v16050718
5. Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology. (2020) 295:715–21. doi: 10.1148/radiol.2020200370
6. Mrak D, Tobudic S, Koblischke M, Graninger M, Radner H, Sieghart D, et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis. (2021) 80:1345–50. doi: 10.1136/annrheumdis-2021-220781
7. Kakkassery H, Carpenter E, Patten P, Irshad S. Immunogenicity of SARS-CoV-2 vaccines in patients with cancer. Trends Mol Med. (2022) 28:1082–99. doi: 10.1016/j.molmed.2022.07.006
8. Thieblemont C, Phillips T, Ghesquieres H, Cheah C, Clausen M, Cunningham D, et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell–engaging antibody, in relapsed or refractory large B-cell lymphoma: Dose expansion in a phase I/II trial. J Clin Oncol. (2023) 41:2238–47. doi: 10.1200/JCO.22.01725
9. Faxén L, Edvinsson M. Persistent SARS-CoV-2 infection in patients with B-cell deficiency: A case series of successful antiviral treatment of four patients. Ups J Med Sci. (2023) 128:9807. doi: 10.48101/ujms.v128.9807
10. Longo B, Venuti F, Gaviraghi A, Lupia T, Ranzani F, Pepe A, et al. Sequential or combination treatments as rescue therapies in immunocompromised patients with persistent SARS-CoV-2 infection in the omicron era: A case series. Antibiotics (Basel). (2023) 12:1460. doi: 10.3390/antibiotics12091460
11. Blennow O, Vesterbacka J, Tovatt T, Nowak P. Successful combination treatment for persistent severe acute respiratory syndrome coronavirus 2 infection. Clin Infect Dis. (2023) 76:1864–5. doi: 10.1093/cid/ciad085
12. US National Institutes of Health. Coronavirus Disease; 2019 (COVID-19) Treatment Guidelines, Last Update. Bethesda, MD: US National Institutes of Health (2025).
13. Belkin A, Leibowitz A, Shargian L, Yahav D. The unique presentation of SARS-CoV-2 infection in patients with B-cell depletion: Definition of ‘persistent inflammatory sero-negative COVID’. Clin Microbiol Infect. (2023) 29:1–3. doi: 10.1016/j.cmi.2022.10.007
14. Meyerowitz E, Li Y. Review: The landscape of antiviral therapy for COVID-19 in the era of widespread population immunity and Omicron-lineage viruses. Clin Infect Dis. (2024) 78:908–17. doi: 10.1093/cid/ciad685
15. Marrone A, Nevola R, Sellitto A, Cozzolino D, Romano C, Cuomo G, et al. Remdesivir plus dexamethasone versus dexamethasone alone for the treatment of coronavirus disease 2019 (COVID-19) patients requiring supplemental O2 therapy: A prospective controlled nonrandomized study. Clin Infect Dis. (2022) 75:e403–9. doi: 10.1093/cid/ciac014
16. Aiello T, Salmanton-Garcia J, Marchesi F, Weinbergerova B, Glenthoj A, Praet J, et al. Dexamethasone treatment for COVID-19 is related to increased mortality in hematologic malignancy patients: Results from the EPICOVIDEHA registry. Haematologica. (2024) 109:2693–700. doi: 10.3324/haematol.2023.284678
17. Snell L, McGreal-Bellone A, Nye C, Gage S, Bakrania P, Williams T, et al. A multinational case series describing successful treatment of persistent severe acute respiratory syndrome coronavirus 2 infection caused by omicron sublineages with prolonged courses of nirmatrelvir/ritonavir. Open Forum Infect Dis. (2024) 11:ofad612. doi: 10.1093/ofid/ofad612
18. Focosi D, Maggi F, D’Abramo A, Nicastri E, Sullivan D. Antiviral combination therapies for persistent COVID-19 in immunocompromised patients. Int J Infect Dis. (2023) 137:55–9. doi: 10.1016/j.ijid.2023.09.021
19. D’Abramo A, Vita S, Beccacece A, Navarra A, Pisapia R, Fusco F, et al. B-cell-depleted patients with persistent SARS-CoV-2 infection: Combination therapy or monotherapy? A real-world experience. Front Med (Lausanne). (2024) 11:1344267. doi: 10.3389/fmed.2024.1344267
20. Mikulska M, Sepulcri C, Dentone C, Magne F, Balletto E, Baldi F, et al. Triple combination therapy with 2 antivirals and monoclonal antibodies for persistent or relapsed severe acute respiratory syndrome coronavirus 2 infection in immunocompromised patients. Clin Infect Dis. (2023) 77:280–6. doi: 10.1093/cid/ciad181
21. Yamakawa K, Yamamoto R, Terayama T, Hashimoto H, Ishihara T, Ishimaru G, et al. Special committee of the Japanese clinical practice guidelines for the management of sepsis and septic shock 2020 (J-SSCG 2020), the COVID-19 task force. Japanese rapid/living recommendations on drug management for COVID-19: Updated guidelines (July 2022). Acute Med Surg. (2022) 9:e789. doi: 10.1002/ams2.789
22. Maruki T, Nomoto H, Iwamoto N, Yamamoto K, Kurokawa M, Iwatsuki-Horimoto K, et al. Successful management of persistent COVID-19 using combination antiviral therapy (nirmatrelvir/ritonavir and remdesivir) and intravenous immunoglobulin transfusion in an immunocompromised host who had received CD20 depleting therapy for follicular lymphoma. J Infect Chemother. (2024) 30:793–5. doi: 10.1016/j.jiac.2024.01.008
23. Takashita E, Yamayoshi S, Simon V, van Bakel H, Sordillo E, Pekosz A, et al. Efficacy of antibodies and antiviral drugs against Omicron BA. 2.12. 1, BA. 4, and BA. 5 subvariants. N Engl J Med. (2022) 387:468–70. doi: 10.1056/NEJMc2207519
24. Drysdale M, Berktas M, Gibbons D, Rolland C, Lavoie L, Lloyd E. Real-world effectiveness of sotrovimab for the treatment of SARS-CoV-2 infection during Omicron BA.2 and BA.5 subvariant predominance: A systematic literature review. Infection. (2024) 52:1839–61. doi: 10.1007/s15010-024-02245-6
25. Wu M, Shepherd S, Fendler A, Carr E, Au L, Harvey R, et al. Sotrovimab restores neutralization against current Omicron subvariants in patients with blood cancer. Cancer Cell. (2023) 41:821–3. doi: 10.1016/j.ccell.2023.04.005
26. Beck K, Yoon J, Yoon S. Radiologic abnormalities in prolonged SARS-CoV-2 infection: A systematic review. Korean J Radiol. (2024) 25:473–80. doi: 10.3348/kjr.2023.1149
27. Kambe R, Sato M, Uehara D, Iizuka Y, Kakizaki S. Prolonged SARS-CoV-2 infection during obinutuzumab and Bendamustine treatment for follicular lymphoma: A case report. Clin Case Rep. (2023) 11:e7861. doi: 10.1002/ccr3.7861
28. The National Institute of Infectious Diseases. Infectious Diseases Weekly Report (IDWR). (2025). Available online at: https://www.niid.go.jp/niid/en/survaillance-data-table-english.html (accessed April 8, 2025).
29. US Centers for Disease Control and Prevention. COVID Data Tracker. (2025). Available online at: https://covid.cdc.gov/covid-data-tracker/ (accessed April 8, 2025).
30. Bay A, Clausen M, Røge B, Sydenham T, Steinke K, Pedersen R, et al. Antiviral combination treatment of SARS-CoV-2 after repeated treatment failures of remdesivir monotherapy: A case report. IDCases. (2024) 38:e02118. doi: 10.1016/j.idcr.2024.e02118
31. Breeden M, Aitken S, Baang J, Gravelin M, Kaul D, Lauring A, et al. Successful treatment of prolonged severe acute respiratory syndrome coronavirus 2 infection in patients with immunodeficiency with extended nirmatrelvir/ritonavir: Case series. Open Forum Infect Dis. (2023) 10:ofad189. doi: 10.1093/ofid/ofad189
Keywords: persistent COVID-19, diffuse large B-cell lymphoma, rituximab, epcoritamab, SARS-CoV-2
Citation: Suzuki M, Fujioka I and Matsushima T (2025) Case Report: Persistent COVID-19 in a fully vaccinated Japanese man being treated with rituximab and epcoritamab for diffuse large B-cell lymphoma. Front. Med. 12:1554100. doi: 10.3389/fmed.2025.1554100
Received: 31 December 2024; Accepted: 14 April 2025;
Published: 30 April 2025.
Edited by:
Alessandro Perrella, Hospital of the Hills, ItalyReviewed by:
Luca Rinaldi, University of Molise, ItalySushil Selvarajan, Christian Medical College and Hospital, India
Copyright © 2025 Suzuki, Fujioka and Matsushima. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masaki Suzuki, c3ouc3V6dS5tYXNhQGdtYWlsLmNvbQ==
 Masaki Suzuki
Masaki Suzuki Isao Fujioka2
Isao Fujioka2