- 1Department of Pathology and Forensic Medicine, Pham Ngoc Thach University of Medicine, Ho Chi Minh City, Vietnam
- 2Department of Pathology, Ho Chi Minh City Oncology Hospital, Ho Chi Minh City, Vietnam
- 3University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam
- 4Department of Pathology, Ho Chi Minh City University Medical Center, Ho Chi Minh City, Vietnam
- 5Department of Pathology, Ditmanson Medical Foundation Chia-Yi Christian Hospital, Chiayi, Taiwan
- 6Department of Cosmetic Science, Chia Nan University of Pharmacy and Science, Tainan, Taiwan
- 7Doctoral Program in Translational Medicine, National Chung Hsing University, Taichung, Taiwan
- 8Department of Biotechnology and Bioindustry Sciences, College of Bioscience and Biotechnology, National Cheng Kung University, Tainan, Taiwan
Background: High-grade serous ovarian carcinoma (HGSOC), an aggressive cancer associated with pathogenic BRCA variants, causes genomic instability and sensitivity to poly (ADP-ribose) polymerase inhibitors. Identifying pathogenic BRCA variants is crucial for the treatment of HGSOC; however, genetic testing is expensive and time-consuming. This study aimed to explore pathological features, particularly the presence of tumor-infiltrating lymphocytes (TILs), as potential surrogates to streamline patient selection for genetic testing.
Methods: We retrospectively analyzed 58 cases of HGSOC with known BRCA variant profiles. Tumors were categorized as TIL-positive or TIL-negative based on the presence of > 40 or ≤ 40 intraepithelial lymphocytes in a single high-power field (HPF), respectively. Key pathological features, including solid, endometrioid, and transitional (SET) architecture patterns; necrosis; and mitotic activity, were evaluated within these subgroups. Statistical analyses were used to determine the associations between these features and BRCA variant status.
Results: In TIL-negative HGSOCs, SET patterns were strongly associated with pathogenic or likely pathogenic BRCA variants (p = 0.028), emerging as the most reliable morphological marker in this group. In TIL-positive HGSOCs, low mitotic activity (≤7 mitotic figure per 10 HPFs) was significantly correlated with pathogenic BRCA variants (p = 0.0002), underscoring its diagnostic significance. Necrosis and mitotic activity in TIL-negative cases and SET patterns in TIL-positive cases were not significantly associated with pathogenic BRCA variants. Combined analysis of both TIL subgroups diluted these associations, underscoring the significance of stratifying cases by the immune context.
Discussion: The presence of TILs affects the diagnostic value of pathological features for BRCA variant status in HGSOC. Regarding pathogenic BRCA variants, SET patterns and low mitotic activity were identified as critical markers in TIL-negative tumors and TIL-positive tumors, respectively. These associations likely stem from interactions among genomic instability, immune response, and tumor growth. Our framework leverages these insights to prioritize high-risk cases for genetic testing, thereby optimizing resource allocation.
Conclusion: The presence of TILs is critical for understanding the association between pathological features and pathogenic BRCA variants in HGSOC. To improve pathogenic BRCA variant prediction, optimize genetic testing, and guide tailored intervention, our framework integrates immune context and morphological markers. This approach is especially useful in resource-limited settings and can enhance diagnostic efficiency and clinical decision-making.
1 Introduction
Ovarian cancer is the third most prevalent cancer among females of childbearing age (1). Among its subtypes, the most common and deadliest form is high-grade serous ovarian carcinoma (HGSOC), which is responsible for most ovarian cancer-related deaths (2, 3). The aggressive nature of HGSOC, along with its frequent late-stage diagnosis, highlights the significance of refined diagnostic and prognostic tools for guiding therapeutic interventions (4).
BRCA1 and BRCA2 play a significant role in the homologous recombination repair of DNA double-strand breaks (5). Approximately 20–25% of patients with HGSOC exhibit germline or somatic mutations in BRCA1 and BRCA2 (6, 7). The pathogenic variants of these genes cause homologous recombination deficiency (HRD), subsequently leading to genetic instability, increased mutation burden, and sensitivity to DNA-damaging agents, including platinum-based chemotherapy and poly (ADP-ribose) polymerase (PARP) inhibitors (8, 9). PARP inhibitors have been reported to significantly improve progression-free survival in patients with HGSOC with pathogenic BRCA variants (10–12). Current guidelines recommend BRCA1/2 and HRD testing at the time of primary diagnosis, ideally before completing first-line chemotherapy, because up to 50% of patients with ovarian cancer demonstrate HRD (13). Early detection is crucial for precision medicine. Despite its essential clinical implication, identifying BRCA variant status can be challenging in resource-limited settings, where next-generation sequencing (NGS) may be costly and inaccessible to several patients (14, 15). Although females with ovarian cancer are recommended to undergo testing (16, 17), stratifying patients into risk categories for pathogenic BRCA variants is an alternative approach (18, 19). Individuals with a higher risk profile should be prioritized for genetic testing. Morphological features offer a cost-effective way to identify patients at high risk for pathogenic BRCA variants; this approach has been well established in breast cancer research (20–23). Such approaches prioritize genetic testing for patients with a high likelihood of benefiting from the test, enabling better allocation of resources (19).
The tumor microenvironment profoundly impacts tumor growth and treatment response (24). Tumor-infiltrating lymphocytes (TILs) are key factors in the tumor microenvironment. The presence of TILs reflects the immune system’s ability to recognize and respond to tumor cells. TILs are frequently associated with better outcomes in cancer, including HGSOC (25, 26), reflecting more robust immune surveillance and stronger antitumor response. Simultaneously, BRCA1/2-regulated autophagy affects immune response by modulating MHC class II expression and hindering immune recognition of clonal neoantigens (27). Considering the cellular immunity TILs predict prognosis and may indicate underlying BRCA1/2 alterations that facilitate immune evasion. Furthermore, we hypothesize that TILs can affect the expression of specific morphological features. Stratifying HGSOCs by the presence of TILs may offer an opportunity to more systematically explore these relationships and refine their use in predicting BRCA variant status.
Several studies have been conducted to identify pathogenic BRCA variant-related pathological features in ovarian cancer (18, 28–30). These studies have identified solid, endometrioid, and transitional architecture (SET) patterns; mitotic activity; and necrosis as key morphological features in pathogenic BRCA variant-related HGSOC. However, the significance of these pathological features varies across studies, and their predictive value in the context of TIL stratification remains underexplored. Therefore, the present study investigated the relationship between BRCA variant status and critical morphological features (SET patterns, necrosis, and mitotic activity) across TIL-positive and TIL-negative HGSOC tumors. We also determined whether stratification according to the presence of TILs improved the diagnostic utility of these features, thereby refining patient selection for genetic testing and enhancing clinical decision-making in settings where comprehensive BRCA sequencing may not be readily available.
2 Materials and methods
2.1 Ethical approval and patient recruitment
The Ho Chi Minh City Medicine and Pharmacy University Ethics Committee approved this study (approval number: IRB-VN01002). All procedures were conducted following institutional and international ethical standards. Data of patients with HGSOC were retrospectively collected from Ho Chi Minh City Oncology Hospital over 20 months (August 2022–April 2024), with prior targeted NGS testing for BRCA1/2 variant profiles. This study used archived formalin-fixed paraffin-embedded (FFPE) tissue blocks, which were fully anonymized before analysis to ensure patient confidentiality. Patients with HGSOC were included in this study when their samples (tissue FFPE blocks and slides) were available and of good quality to undergo morphological feature analysis. Additional clinical data, including age at diagnosis and disease stage, were collected for each case.
2.2 Sequencing for BRCA1/2 variants
Regarding targeted NGS testing for BRCA1/2, all recruited patients with HGSOC were tested at the Ho Chi Minh City Oncology Hospital. In brief, genomic DNA was extracted from tumor-bearing FFPE tissues using the GeneRead™ DNA FFPE Kit (Qiagen, Hilden, Germany). NGS was performed on the Illumina MiSeqDx platform using the reagent kit BRCAaccuTest™ Plus (NGeneBio Co., Ltd., Seoul, South Korea) to produce libraries targeting BRCA1 and BRCA2. Sequencing data were processed and analyzed using the automated NGeneAnalySys™ Software (NGeneBio Co., Ltd., Seoul, South Korea), with variants classified according to the American College of Medical Genetics and Genomics guidelines into the following categories: pathogenic, likely pathogenic, uncertain significance, likely benign, and benign variants (31). In the present study, cases with pathogenic or likely pathogenic variants were classified into the pathogenic group, whereas cases with benign or likely benign variants were categorized into the benign group. Variants of uncertain significance were excluded from the analysis.
2.3 Assessment of pathological features and TILs in the tumor microenvironment
We retrospectively retrieved tumor-containing slides from each case to assess pathological characteristics. For cases where existing slides were faded or unsuitable for assessment, new hematoxylin and eosin (H&E)-stained slides were subsequently prepared from the corresponding tissue blocks to ensure diagnostic quality. We collected 184 slides of tissue samples from all 58 cases of HGSOC analyzed by experienced pathologists to independently evaluate pathological features. The number of slides per case ranged from 1 to 9, with the mean number of slides per case being 3.17. Furthermore, we employed validation methods to minimize interobserver bias for morphological assessments. Before the evaluation, we reached a consensus on the morphology assessment as this study is an integrative teamwork. Each case was independently reviewed by at least two pathologists. In the event of a disagreement, our central review board would hold a consensus meeting to discuss the findings and reach a joint decision.
To assess architectural patterns and other key morphological features, including TILs and comedo-like or geographic necrosis, H&E slides were analyzed under low magnification (40×). Architectural patterns were categorized as solid, endometrioid, transitional cell carcinoma-like, papillary, or micropapillary (Figure 1). Tumors were classified as SET-positive when ≥ 30% of tumor cells displayed solid, endometrioid, or transitional cell carcinoma-like patterns.
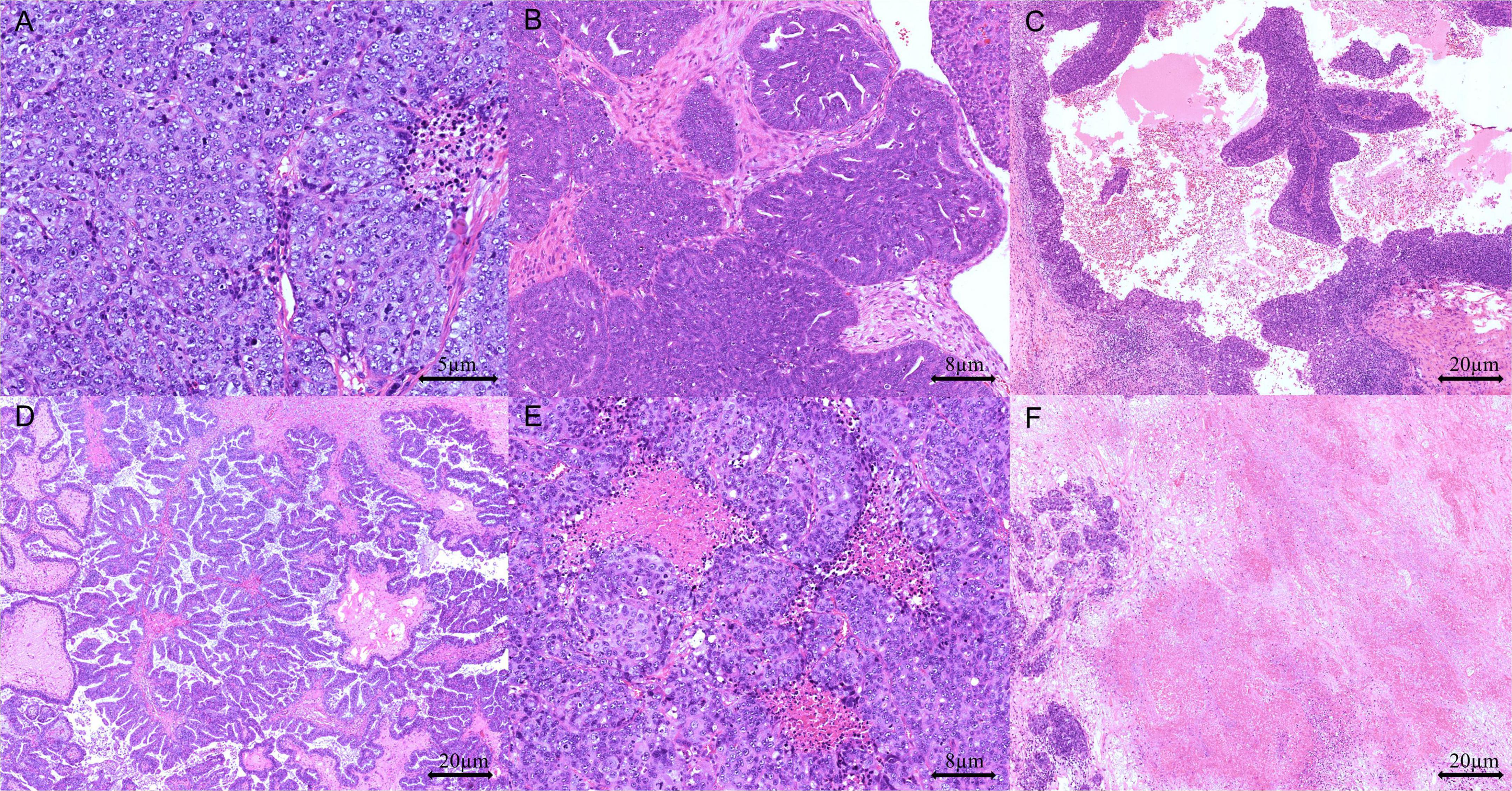
Figure 1. Representative pathological features in high-grade serous ovarian carcinoma (HGSOC): (A) solid growth pattern (H&E stain, 200 × magnification), (B) endometrioid growth pattern (H&E stain, 100 × magnification), (C) transitional cell-like growth pattern (H&E stain, 40 × magnification), (D) papillary growth pattern (H&E stain, 40 × magnification), (E) comedo-like necrosis (H&E stain, 100 × magnification), and (F) geographic necrosis (H&E stain, 40 × magnification).
Areas with the highest concentration of TILs or mitotic figures, termed “hot spots,” were identified for evaluation. Mononuclear cells were considered TILs when located within the tumor margins or in the intercellular spaces and the cores of papillary tumor structures. Cells outside the tumor border, those associated with necrotic regions, and those within blood vessels were excluded. TILs were considered positive when the tumor contained more than 40 intraepithelial lymphocytes in at least one high-power-field (HPF) (Figure 2). Furthermore, we classified mitotic activity using the Nottingham Grading System (32), which was originally developed for breast cancer histopathological grading. Using our microscope (Olympus BX43) with a 0.5-mm field diameter, we counted the number of mitotic figures in 10 consecutive HPFs within the most mitotically active area of the tumors and subsequently categorized tumors as low (≤ 7 mitotic figures/10 HPFs), moderate (8–14 mitotic figures/10 HPFs), or high (≥ 15 mitotic figures/10 HPFs) (33).
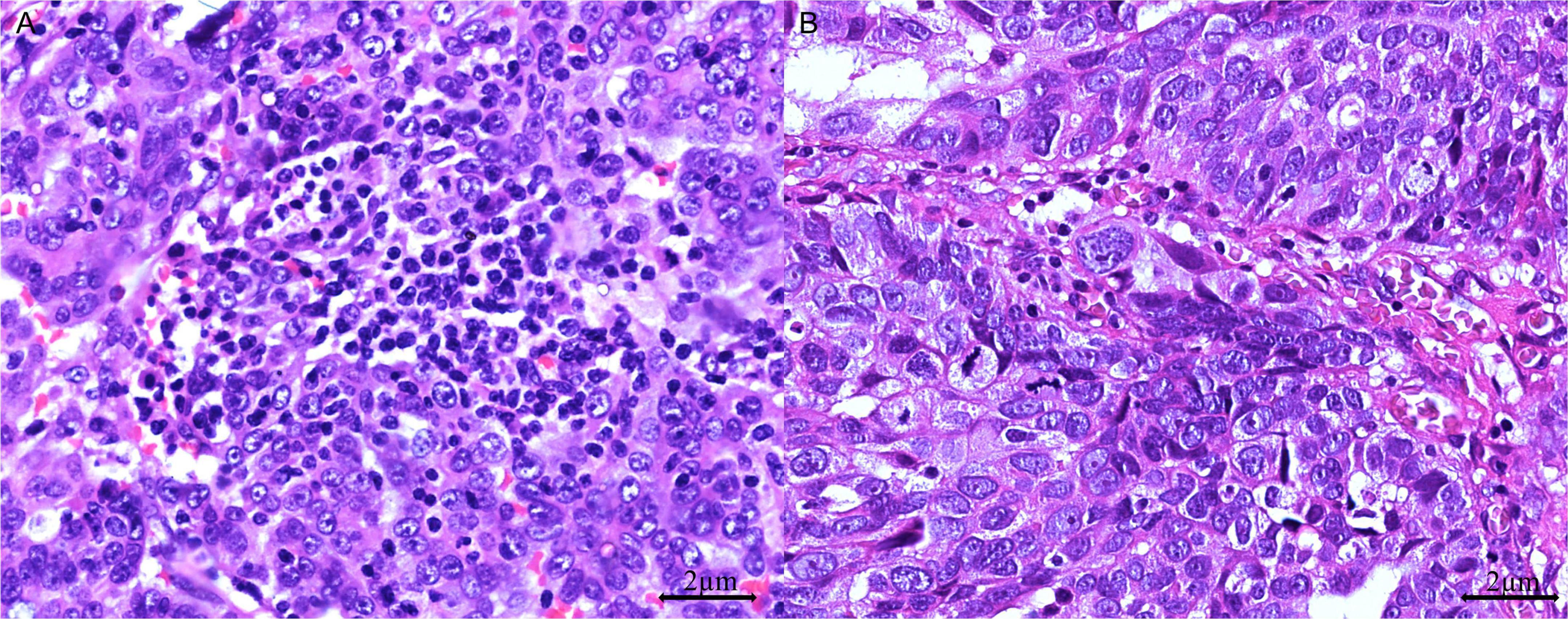
Figure 2. Tumor-infiltrating lymphocytes (TILs) in HGSOC. (A) TIL-positive: > 40 dense intraepithelial infiltrates of TILs (H&E stain, 400 × magnification); (B) TIL-negative: ≤ 40 sparse infiltrates of TILs (H&E stain, 400 × magnification).
Despite several studies focusing on TILs and mitotic rates in ovarian cancer, universally accepted evaluation criteria are not available. The methodologies applied here were adapted from established frameworks to ensure consistency and reliability in assessing these critical features (29, 30).
2.4 Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (version 25, IBM, Armonk, NY, United States) and GraphPad Prism 8 software to compare morphological and pathological features between the pathogenic and benign BRCA variant groups. Chi-square test was used for evaluating qualitative variables, whereas t-test was used for evaluating quantitative variables. When the expected value in any contingency table cell was < 5, Fisher’s exact test was used instead of the chi-square test. For quantitative variables, when normality assumptions were violated (assessed using the Shapiro–Wilk test), the Mann–Whitney U test was applied as a non-parametric alternative. Statistical significance was set at p < 0.05 (two-tailed).
3 Results
3.1 Patients’ age at diagnosis and Federation Internationale de Gynecologie et d’Obstetrique (FIGO) stage
Overall, 58 patients with HGSOC were retrospectively enrolled, and the age at diagnosis was 33–78 years, with a mean age of 55.8 years (standard deviation = 9.1) and a median age of 55.0 years. Patients with benign BRCA variants had a mean age of 55.0 years (standard deviation = 9.3) and a median age of 51.0 years, whereas those with pathogenic BRCA variants had a mean age of 57.0 years (standard deviation = 8.8) and a median age of 58.0 years. Patients with pathogenic and benign BRCA variants showed no statistically significant differences in terms of age (p = 0.163; Mann–Whitney U test) (Supplementary Figure 1).
Regarding the FIGO stage, most cases were diagnosed with FIGO IIIC, comprising 72.4% (42/58) of the cohort. Among the patients with benign BRCA variants, 24 cases were assigned to FIGO IIIC, whereas 18 cases with pathogenic BRCA variants were assigned to this stage. Early stage disease (FIGO IC or IIB) was observed only in cases with benign BRCA variants. In contrast, no cases with pathogenic BRCA variants were diagnosed at a stage earlier than stage IIIC. Both groups encompassed cases diagnosed at an advanced stage (FIGO IVB).
3.2 BRCA1/2 variant profiles and TILs
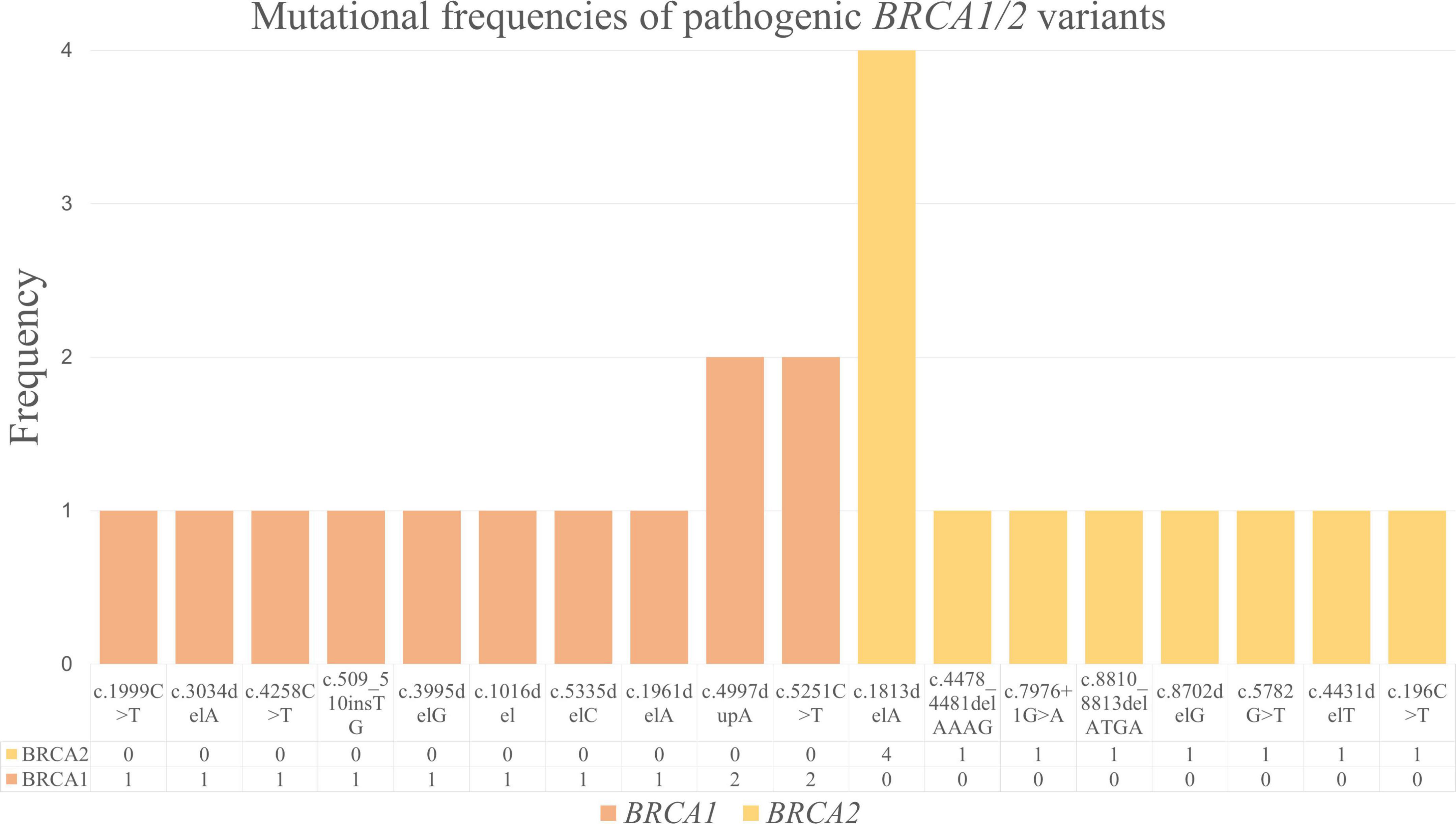
We detected 10 distinct BRCA1 and 8 distinct BRCA2 pathogenic variants (Figure 3). Among the pathogenic BRCA1 variants, c.4997dupA and c.5251C > T were the most frequent; each was observed in 2 of 23 cases. The remaining pathogenic BRCA1 variants, including c.1999C > T, c.3034delA, c.4258C > T, c.509_510insTG, c.3995delG, c.1016del, c.5335delC, and c.1961delA, were noted in 1 of 23 cases each. Considering the pathogenic BRCA2 variants, we detected c.1813delA in 4 of 23 cases, making it the most frequent pathogenic variant in our cohort. The other pathogenic BRCA2 variants, including c.4478_4481delAAAG, c.7976 + 1G > A, c.8810_8813delATGA, c.8702delG, c.5782G > T, c.4431delT, and c.196C > T, were detected in 1 of 23 cases each.
Among the 58 cases of HGSOC analyzed, 30 and 28 were classified as TIL-negative and TIL-positive, respectively. The information and analyses of demographic and clinical characteristics between the two groups are shown in Supplementary Table 1. In the TIL-negative group, 16 cases (53.3%) had benign BRCA variants, whereas 14 (46.7%) harbored pathogenic BRCA variants. Similarly, in the TIL-positive group, 19 cases (67.9%) had benign BRCA variants, whereas 9 (32.1%) had pathogenic BRCA variants. No statistical significance in pathogenic BRCA variant distribution was observed between the two groups (p = 0.29).
3.3 Pathological features in the TIL-negative group
In the TIL-negative group (30 cases of HGSOC), we evaluated the relationship between pathogenic BRCA variants and three key morphological features: SET patterns, necrosis, and mitotic activity. Without dominant infiltrates of intraepithelial TILs, we noted a statistically positive association between SET-positive tumors and pathogenic BRCA variants. This association was evaluated using the chi-square test, which yielded a relative risk of 2.200 and a p-value of 0.028 (Figure 4). Our findings suggest that SET-positive tumors are particularly significant in TIL-negative HGSOCs, where SET patterns are a key morphological marker for identifying pathogenic BRCA variants.
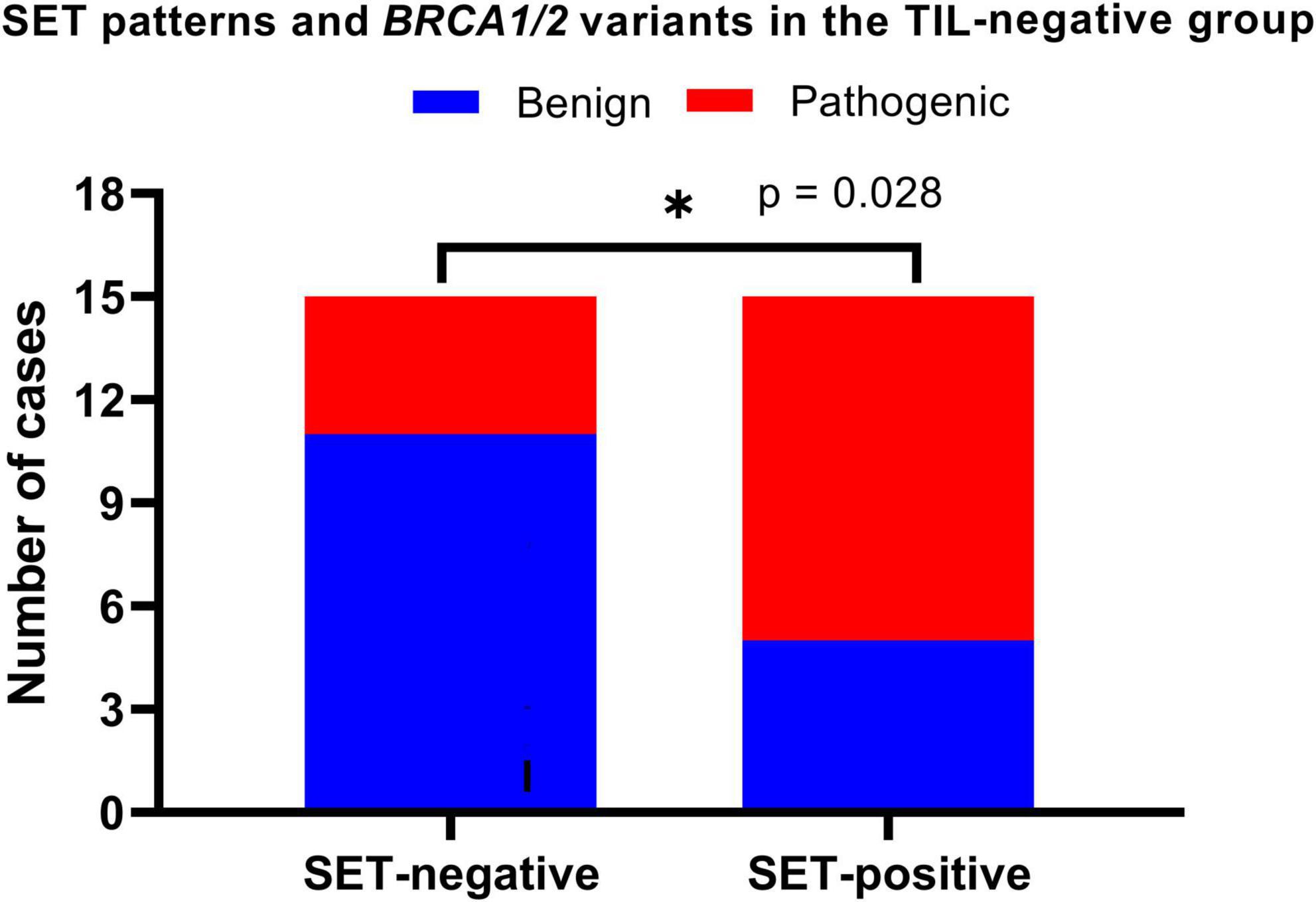
Figure 4. Solid, endometrioid, and transitional (SET) patterns and BRCA variants in TIL-negative HGSOCs. SET-positive was defined if ≥ 30% of the tumor components demonstrated solid, endometrioid, or transitional cell carcinoma-like patterns. We found SET-positive tumors positively associated with pathogenic BRCA variants (p = 0.028). *p < 0.05.
Combining comedo and geographic necrosis, we observed necrosis in 19 of the 30 cases (63.3%). However, statistical analysis of necrosis patterns revealed no significant association with BRCA variants (p = 0.389). Regarding mitotic activity, high mitotic activity was predominant in 10 cases (71.4%) with pathogenic BRCA variants, whereas 3 (21.4%) and 1 (7.1%) cases were in the moderate and low categories, respectively. Similarly, among the 16 benign BRCA cases, 11 (68.8%), 2 (12.5%), and 3 (18.8%) demonstrated high, low, and moderate activities, respectively. Fisher’s exact test for mitotic activity categories and BRCA variants revealed no significant association (p = 0.733). Our results indicate that mitotic activity and necrosis do not aid in distinguishing between benign and pathogenic BRCA variants in TIL-negative HGSOCs.
3.4 Pathological features in the TIL-positive group
The TIL-positive group comprised 28 cases of HGSOC, and we evaluated the relationships between BRCA variants and three pathological features (SET patterns, necrosis, and mitotic activity) as we did in the TIL-negative group. In the TIL-positive group, 15 of 28 cases (53.6%) met the criteria for SET-positive tumors. Among the nine cases with pathogenic BRCA variants, five were SET-positive cases (55.6%). Similarly, 10 of 19 cases (52.6%) with benign BRCA variants were SET-positive. No significant difference in BRCA variants was noted between the SET-positive and SET-negative groups, as revealed using Fisher’s exact test (p = 1). Our results suggest that SET patterns are unreliable for differentiating BRCA variants within the TIL-positive group.
Necrosis was detected in 14 of the 28 cases (50.0%). Among the 9 cases with pathogenic BRCA variants, 4 (44.4%) exhibited necrosis, whereas 10 (52.6%) had necrosis in 19 cases with benign BRCA variants. Fisher’s exact test was used to evaluate the relationship between necrosis and BRCA variant status in TIL-positive tumors. The test yielded a p-value of 1, indicating that necrosis and BRCA variants were not significantly associated. These findings emphasize that although frequently observed, necrosis patterns do not reliably differentiate between benign and pathogenic BRCA variants in TIL-positive tumors.
Interestingly, in TIL-positive tumors, mitotic activity was significantly associated with BRCA variant status (Figure 5). Among the nine cases with pathogenic BRCA variants, seven (77.8%) exhibited low mitotic activity, one (11.1%) showed high mitotic activity, and one (11.1%) showed moderate mitotic activity. Conversely, in the 19 cases with benign BRCA variants, 2 cases (10.5%) exhibited low mitotic activity, 1 case (5.3%) showed moderate mitotic activity, and 16 cases (84.2%) had high mitotic activity.
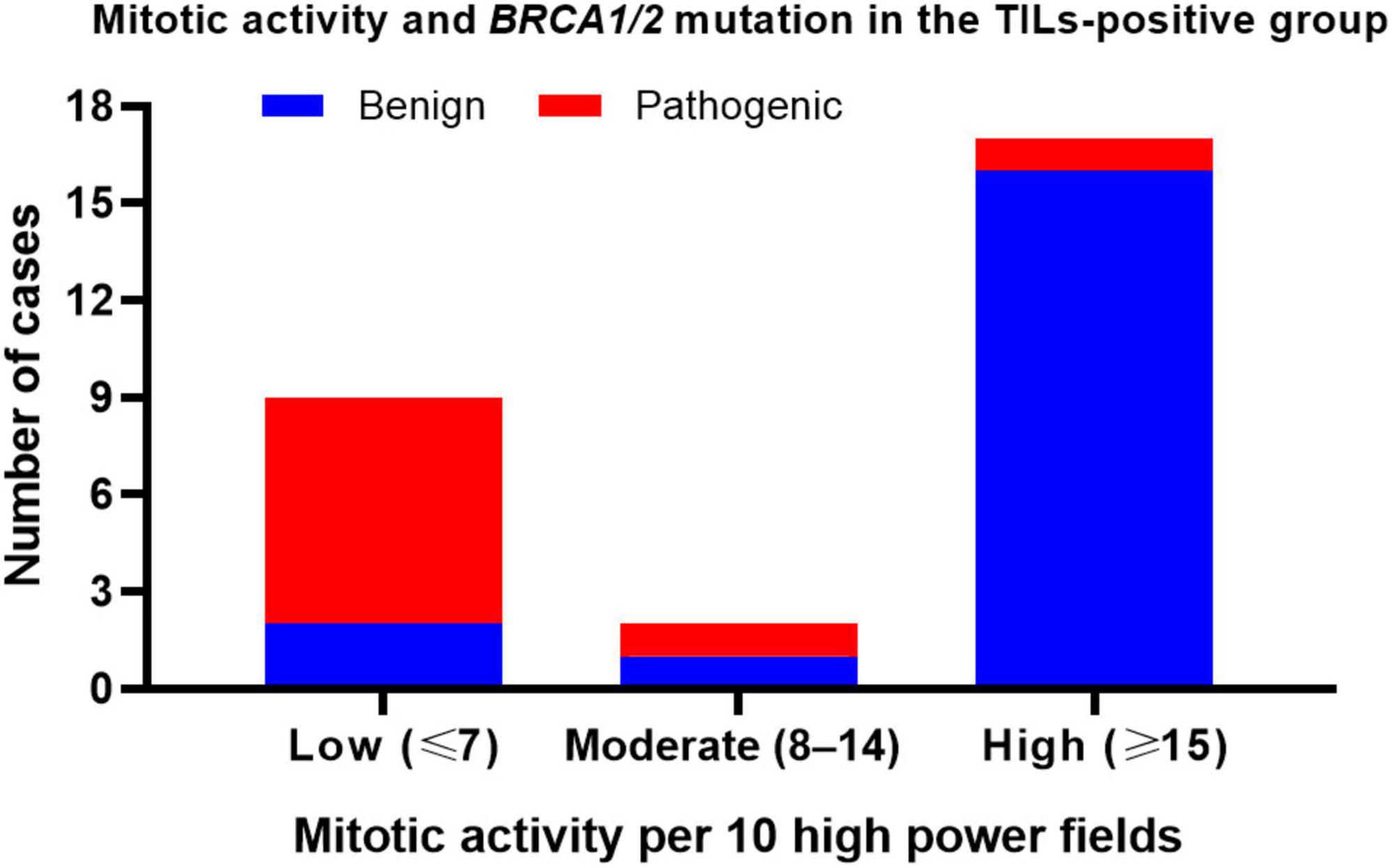
Figure 5. Mitotic activity and BRCA variants in TIL-positive HGSOCs. Low mitotic activity is strongly associated with pathogenic BRCA variants in TIL-positive HGSOCs.
Classifying tumors with moderate or high mitotic activity as the same group and comparing them to tumors with low mitotic activity using Fisher’s exact test revealed that low mitotic activity was significantly and positively associated with pathogenic BRCA variants in TIL-positive tumors (p = 0.0002). The relative risk was 4.722. Our results suggest that low mitotic activity is a distinguishing feature of pathogenic BRCA variants in TIL-positive tumors.
In summary, among TIL-positive tumors, the most significant morphological predictor of BRCA variant status was mitotic activity. Low mitotic activity was strongly associated with pathogenic BRCA variants, whereas moderate-to-high mitotic activities were more prevalent in benign cases. In contrast, neither SET patterns nor necrosis was significantly associated with BRCA variant status.
3.5 Pathological features in the complete dataset
We also assessed the relationships between BRCA variant status and key pathological features (SET patterns, necrosis, and mitotic activity) in the complete dataset, including TIL-positive and TIL-negative HGSOCs. Our findings showed a higher frequency of SET positivity in cases with pathogenic BRCA variants than in those with benign variants. However, no statistically significant difference was observed (p = 0.096). Our findings suggested that although SET patterns exhibit some diagnostic value, their predictive value appears less pronounced when analyzing TIL-positive and TIL-negative tumors together.
Similarly, in the complete dataset, necrosis showed no significant association with BRCA variant status (p = 0.620), suggesting that necrosis patterns cannot differentiate between benign and pathogenic BRCA variants across a mixed population of TIL-positive and TIL-negative tumors. In addition, mitotic activity did not significantly affect BRCA variant status in the complete dataset (p = 0.075), indicating that the diagnostic value of mitotic activity observed in specific TIL subgroups is not maintained when combined.
The lack of significant associations in the complete dataset highlights the significance of stratifying tumors by dense intraepithelial infiltrates of TILs when evaluating pathological features. The relationships observed in the separate analyses of TIL-positive and TIL-negative groups, including the strong association between SET patterns and pathogenic BRCA variants in TIL-negative cases and that between low mitotic activity and pathogenic BRCA variants in TIL-positive cases, are obscured when these groups are combined. These data suggest that the diagnostic value of morphological features is context-dependent, and stratifying tumors by immune infiltration is the first step before using other pathological factors as predictive features of BRCA variant status. The histopathological features of the cases included in our study in correlation with the BRCA variant status are summarized in Table 1.
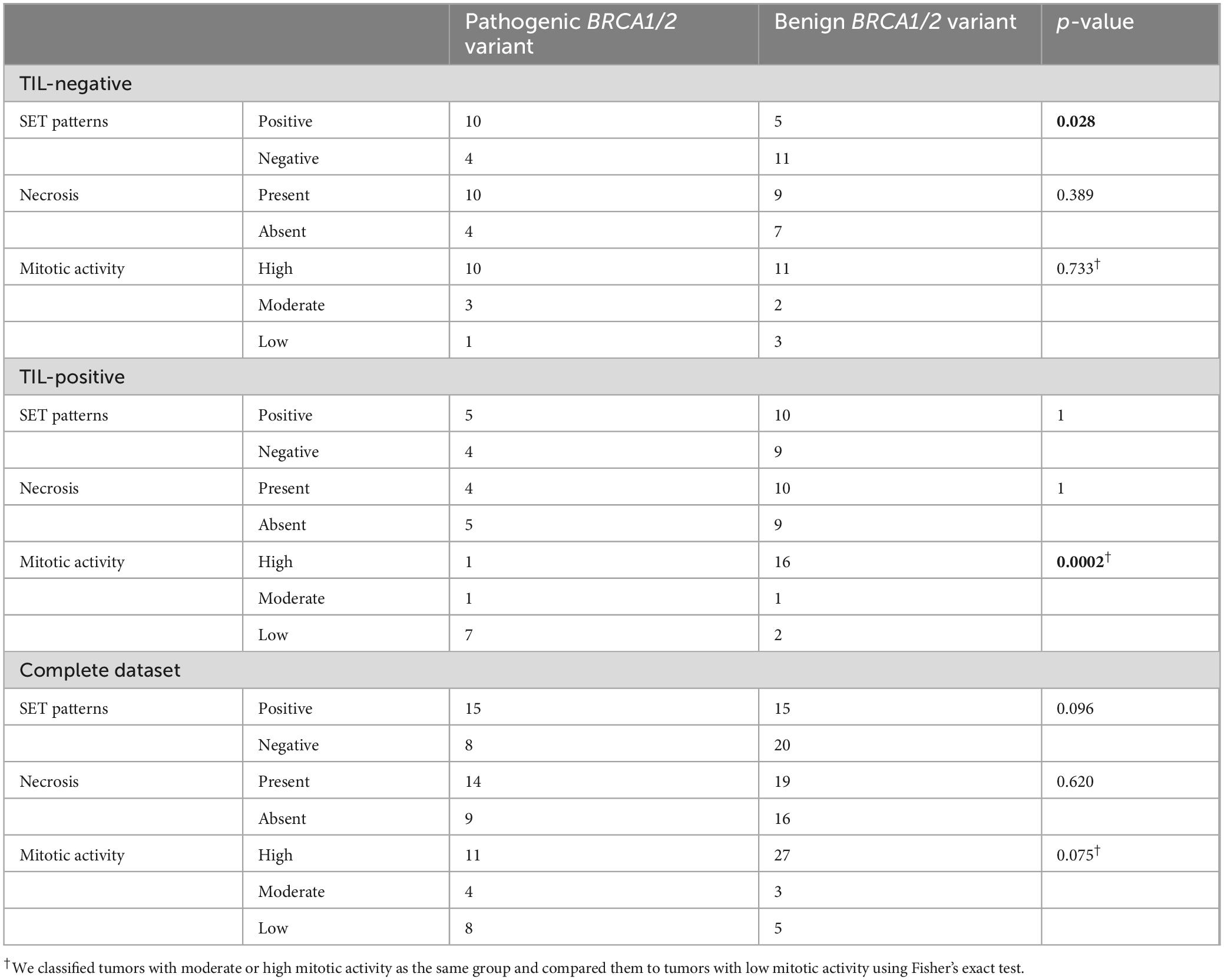
Table 1. Correlation of histopathological features with BRCA1/2 variant status in high-grade serous ovarian carcinoma (HGSOC)
4 Discussion
4.1 General demographic characteristics
In this study, the age range at the time of HGSOC diagnosis was 33–78 years, with a mean of 55.8 years. The mean age aligned with global data (34, 35), suggesting that our cohort is representative of individuals affected by HGSOC. In addition, the FIGO stage distribution supports the representativeness of this cohort. Notably, 72.4% of our cases were diagnosed as stage IIIC, reflecting the aggressive nature of the disease, which is frequently identified at advanced stages owing to its asymptomatic early progression (35). Herein, the typical age distribution and FIGO stage pattern enhance the applicability of our findings to other populations with HGSOC.
4.2 TILs in the tumor microenvironment are a key indicator of pathological features
This study investigated the pathological features of HGSOCs concerning BRCA variant status, focusing on stratification by the presence of dense intraepithelial TILs. By examining pathological features, including SET patterns, necrosis, and mitotic activity, within the TIL-positive and TIL-negative groups and in the complete dataset, we aimed to identify morphological markers that could differentiate pathogenic BRCA variants from benign variants.
In the TIL-negative group, SET patterns were the most reliable predictor for pathogenic BRCA variants. A strong association between SET-positive tumors and pathogenic BRCA variants was observed (p = 0.028), consistent with previous studies that reported associations between SET patterns and BRCA-mutated tumors (29, 30, 36, 37). In contrast, necrosis and mitotic activity were not significantly correlated with BRCA variant status in this group. These results suggest that SET patterns should be prioritized for evaluation in TIL-negative tumors.
In the TIL-positive group, low mitotic activity was strongly associated with pathogenic BRCA variants. Conversely, SET patterns and necrosis were not significantly correlated with BRCA variant status. Our results emphasize the significance of mitotic activity as a crucial factor for distinguishing tumors with immune infiltration. Moreover, we analyzed the relationship between the evaluated pathological features (SET patterns, necrosis, and mitotic activity) and pathogenic BRCA variants in the entire cohort. No statistically significant associations were observed in the complete dataset, underscoring the significance of stratifying cases by TILs.
4.3 Proposed framework for predicting BRCA variant status in individual cases
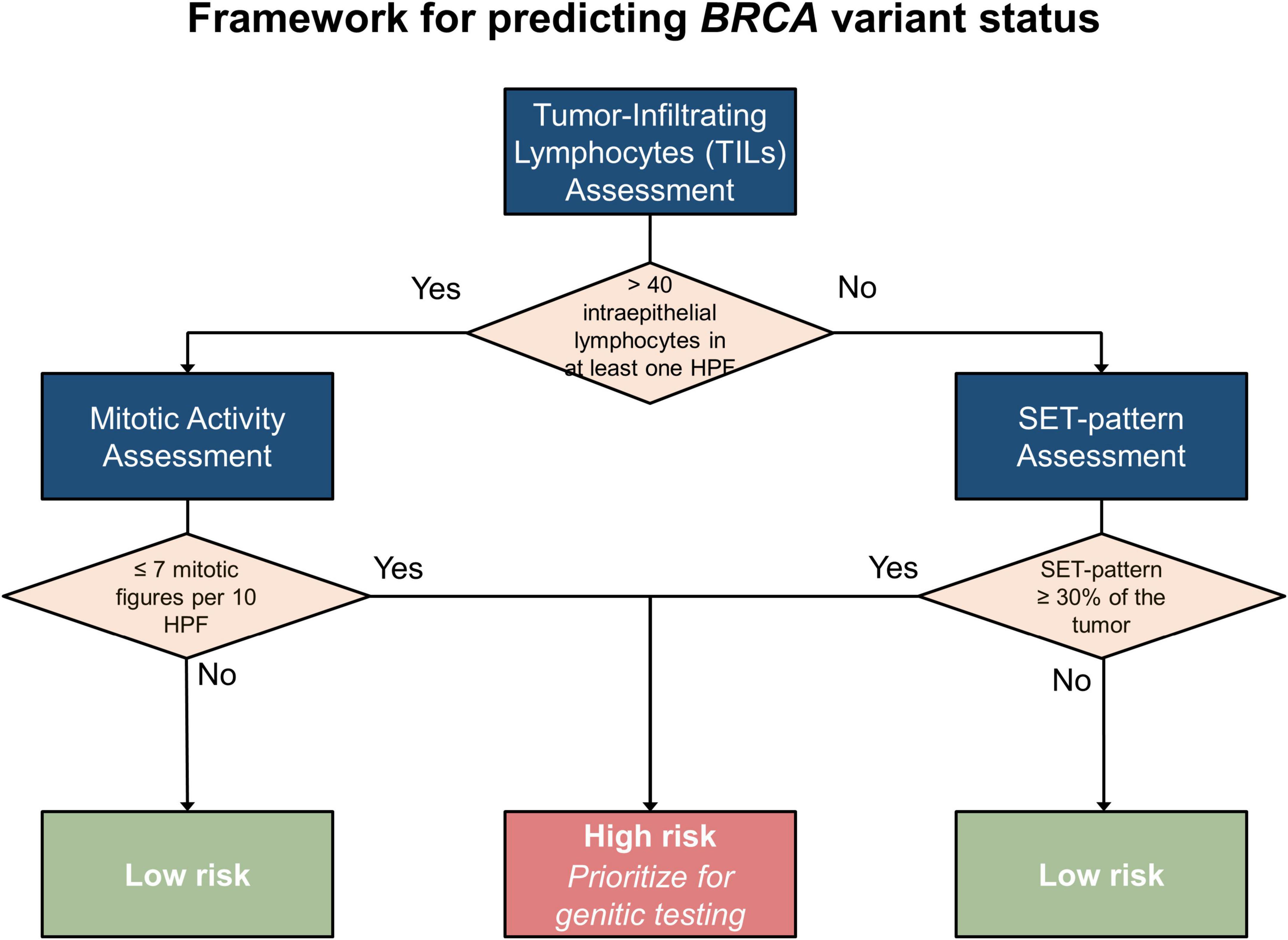
To guide the stratification of cases and prioritize individuals for genetic testing, particularly in resource-limited settings, we developed a framework for predicting the likelihood of pathogenic BRCA variants in individual cases of HGSOC based on our findings. The proposed framework (Figure 6) synthesizes the immune context, classified according to TILs, alongside essential pathological characteristics such as SET patterns, necrosis, and mitotic activity.
4.3.1 Step 1: evaluate TILs
To identify hot spots for TILs, the entire tumor should be examined at low-power magnification. Subsequently, the identified areas should be evaluated at high-power magnification. When a single HPF with more than 40 intraepithelial TILs is noted, the tumor should be categorized as TIL-positive. Otherwise, it will be classified as TIL-negative. This stratification is crucial because the diagnostic relevance of specific pathological features varies between TIL-positive and TIL-negative tumors.
4.3.2 Step 2: analyze pathological features by TIL subgroups
Once the immune context is established, specific pathological features can be assessed according to the tumor’s TIL classification:
In TIL-positive tumors:
- Primary marker: mitotic activity is the most reliable indicator of pathogenic BRCA variants in TIL-positive cases.
+ Low mitotic activity (≤ 7 mitotic figures/10 HPFs with a 0.5-mm field diameter) is strongly associated with pathogenic BRCA variants.
+ Moderate or high mitotic activity (> 7 mitotic figures/10 HPFs with a 0.5-mm field diameter) suggests a lower likelihood of pathogenic BRCA variants.
- Secondary features: although less predictive, SET patterns in ≥ 30% of the tumor or necrosis may provide supportive evidence but should not be relied upon as primary markers in this subgroup. These features are supportive markers because previous studies have identified their correlation with pathogenic BRCA variants (29, 30, 36).
In TIL-negative tumors:
- Primary marker: SET patterns in ≥ 30% of the tumor strongly predict pathogenic BRCA variants in TIL-negative tumors.
- Secondary features: necrosis and mitotic activity provide Supplementary Information as previous studies have shown their correlation with pathogenic BRCA variants (29, 30, 36).
4.3.3 Step 3: synthesize findings
The integration of immune context and pathological features enables stratifying cases into the following two categories:
- High likelihood of pathogenic BRCA variant
+ TIL-positive: cases with low mitotic activity and supportive features (e.g., necrosis or SET patterns)
+ TIL-negative: SET-positive cases, particularly when combined with other supportive markers
- Low likelihood of pathogenic BRCA variant
+ TIL-positive: cases with moderate or high mitotic activity
+ TIL-negative: SET-negative cases
4.3.4 Step 4: prioritize cases for genetic testing
This framework enables prioritizing cases for genetic testing:
- High-risk cases: these should be prioritized for NGS or other genetic testing modalities to confirm BRCA variant status.
- Low-risk cases: testing should be considered only when additional risk factors, including family history and clinical presentation, are present or as resources allow.
4.4 Applications of the framework
PARP inhibitors have transformed ovarian cancer treatment, with the SOLO-1, PRIMA, ATHENA-MONO, and PAOLA-1 trials demonstrating significant survival benefits, particularly in patients with BRCA mutation and HRD positivity (13). To optimize treatment, early biomarker testing is essential; however, access remains a challenge. By applying our framework, pathologists and oncologists could refine the selection of patients for genetic testing. In particular, histopathological evaluation of TILs and other morphological features may be a cost-effective triage tool in resource-limited settings where universal genetic testing is scarce. Identifying high-risk cases enables efficient use of limited resources, focusing on those most likely to benefit from BRCA1/2 testing and subsequent interventions. Ultimately, integrating our framework into routine pathological assessments could enhance diagnostic accuracy and guide tailored therapeutic approaches.
4.5 Comparison of our proposed framework with other methods for predicting BRCA variant status
Several methods for predicting BRCA variant status in HGSOC have been explored, including BRCA1/2 immunostaining and the use of characteristic histopathological features, such as SET patterns, high mitotic activity, necrosis, and TILs (29, 30, 36–38). BRCA1/2 immunohistochemistry, which has the capacity to screen for protein expression loss due to mutation (38), requires specialized reagents and infrastructure. Although some studies have reported a correlation between histopathological features and BRCA variant status, the results have been inconsistent, and the association between these features has not been clearly established (18, 28–30).
In contrast, our method solely relies on routine H&E-stained slides, making it more cost-effective than approaches requiring additional immunohistochemical staining. Furthermore, by using a structured framework that combines TIL assessment with other morphological features, such as SET patterns and mitotic activity, our approach more effectively captures the association among tumor morphology, immune microenvironment, and BRCA variant status than previous studies.
4.6 Limitations and future directions
Our study had several limitations that warrant caution when interpreting the results. First, the sample size was relatively small, especially within the TIL-positive and TIL-negative subgroups, which may restrict the statistical power and generalizability. In addition, this was a retrospective single-center study, potentially introducing selection bias and limiting the applicability of our conclusions to other clinical settings. To validate and ensure that our findings are generalizable to various patient populations, further multi-institutional or prospective studies with standardized protocols for tissue sampling and data collection are warranted. Such a study would help confirm the reliability of the framework and more effectively address confounding factors.
Second, we relied on semiquantitative methods to assess certain morphological features. Although the methods were based on previously published criteria, the lack of a universally accepted and standardized scoring system may result in interobserver variability, thereby affecting reproducibility. Implementing more objective measurement tools, including digital image analysis or standardized scoring guidelines, could improve consistency across different centers and pathologists.
Third, while our study emphasized on associations between pathogenic BRCA variants and morphological features, it did not investigate the potential influence of other genetic alterations commonly involved in HGSOC. Genes such as TP53 (39), which is almost universally mutated in HGSOC, as well as PTEN (40), CCNE1 (41), BRAF (42), and other homologous recombination repair-related genes may also shape tumor morphology and promote immune infiltration. The unavailability of molecular data beyond BRCA1/2 in our cohort represents a limitation in understanding the broader genomic context. Future studies incorporating comprehensive genomic profiling would be valuable in determining how these genetic alterations contribute to specific tumor morphology within immune infiltration.
Finally, although our study emphasized on associations among pathogenic BRCA variants, TILs, and mitotic activity, we could not identify a clear relationship among these factors in the existing literature, highlighting a gap in current knowledge. This disparity makes the full contextualization of our results challenging. Further research is warranted to explore the molecular and pathological mechanisms linking immune infiltration, morphological changes, and BRCA genetic alterations.
By acknowledging the limitations of our study, we hope to provide context for our findings and encourage future studies that address the noted gaps in methodologies, study design, and mechanistic understanding.
5 Conclusion
This study underscores the significance of stratifying HGSOCs by dense or sparse infiltrates of TILs to evaluate the relationship between pathological features and pathogenic BRCA variant status. In particular, SET patterns and mitotic activity emerged as critical markers for distinguishing pathogenic BRCA variants in TIL-negative tumors and TIL-positive tumors, respectively. These results demonstrate that histopathological evaluation offers a practical and cost-effective method for identifying high-risk patients for molecular analysis, particularly in resource-limited settings. Our method ensures that patients at the highest risk of harboring pathogenic BRCA variants receive timely genetic testing and benefit from tailored interventions, including PARP inhibitors, leading to enhanced treatment outcomes.
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ho Chi Minh City Medicine and Pharmacy University Ethics Committee (IRB-VN01002). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
DN-P: Project administration, Supervision, Visualization, Writing – review & editing, Conceptualization, Methodology, Resources, Writing – original draft. TD: Resources, Investigation, Writing – review & editing. AD: Investigation, Resources, Writing – review & editing. LH: Writing – review & editing, Investigation, Resources. PN: Investigation, Resources, Writing – review & editing. VT: Investigation, Resources, Writing – review & editing. HN: Resources, Supervision, Writing – review & editing, Project administration, Validation. TD: Resources, Supervision, Writing – review & editing. TT: Resources, Supervision, Writing – review & editing. C-CC: Data curation, Formal Analysis, Funding acquisition, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was partly supported by the research grant from C-CC from the National Science and Technology Council of Taiwan (NSTC-113-2320-B-705-002). The funder had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgments
We would like to express their gratitude to all staff members in the Department of Pathology at the Ho Chi Minh City Oncology Hospital, Ho Chi Minh City, Vietnam, for their assistance throughout this study. We also sincerely thank Oanh T.T. Pham, M.Sc., and Huong T.Q. Nguyen, MBA, Pharm., for their significant contributions to establishing the NGS system at Ho Chi Minh City Oncology Hospital, which provided the molecular data essential for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1555883/full#supplementary-material
References
1. Wei Y, Ning L, Xu Y, Ma J, Li D, Feng Z, et al. Worldwide patterns and trends in ovarian cancer incidence by histological subtype: A population-based analysis from 1988 to 2017. EClinicalMedicine. (2024) 79:102983. doi: 10.1016/j.eclinm.2024.102983
2. Kurman R, Shih I. The dualistic model of ovarian carcinogenesis. Am J Pathol. (2016) 186:733–47. doi: 10.1016/j.ajpath.2015.11.011
3. Bowtell D, Böhm S, Ahmed A, Aspuria P, Bast R, Beral V, et al. Rethinking ovarian cancer II: Reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer. (2015) 15:668–79. doi: 10.1038/nrc4019
4. Millstein J, Budden T, Goode E, Talhouk A, Intermaggio MP, Leong HS, et al. Prognostic gene expression signature for high-grade serous ovarian cancer. Ann Oncol. (2020) 31:1240–50. doi: 10.1016/j.annonc.2020.05.019
5. Prakash R, Zhang Y, Feng W, Jasin M. Homologous recombination and human health: The roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harb Perspect Biol. (2015) 7:a016600. doi: 10.1101/cshperspect.a016600
6. Bell D, Berchuck A, Birrer M, Chien J, Cramer D, Dao F, et al. Integrated genomic analyses of ovarian carcinoma. Nature. (2011) 474:609–15. doi: 10.1038/nature10166
7. Eoh K, Kim H, Lee J, Kim S, Kim S, Kim Y, et al. Mutation landscape of germline and somatic BRCA1/2 in patients with high-grade serous ovarian cancer. BMC Cancer. (2020) 20:204. doi: 10.1186/s12885-020-6693-y
8. Van Wilpe S, Tolmeijer S, Koornstra R, De Vries I, Gerritsen W, Ligtenberg M, et al. Homologous recombination repair deficiency and implications for tumor immunogenicity. Cancers. (2021) 13:2249. doi: 10.3390/cancers13092249
9. Mangogna A, Munari G, Pepe F, Maffii E, Giampaolino P, Ricci G, et al. Homologous recombination deficiency in ovarian cancer: From the biological rationale to current diagnostic approaches. J Pers Med. (2023) 13:284. doi: 10.3390/jpm13020284
10. Tomasik B, BieńKowski M, Jassem J. PARP inhibitors beyond BRCA-mutated cancers: Precision medicine at the crossroads. Precision Cancer Medicine. (2021) 4:19. doi: 10.21037/pcm-21-8
11. Bound N, Vandenberg C, Kartikasari A, Plebanski M, Scott C. Improving PARP inhibitor efficacy in high-grade serous ovarian carcinoma: A focus on the immune system. Front Genet. (2022) 13:886170. doi: 10.3389/fgene.2022.886170
12. Evans T, Matulonis U. PARP inhibitors in ovarian cancer: Evidence, experience and clinical potential. Ther Adv Med Oncol. (2017) 9:253–67. doi: 10.1177/1758834016687254
13. Tonti N, D’Augè T, Cuccu I, De Angelis E, D’Oria O, Perniola G, et al. The role of tumor biomarkers in tailoring the approach to advanced ovarian cancer. Int J Mol Sci. (2024) 25:11239. doi: 10.3390/ijms252011239
14. Morganti S, Tarantino P, Ferraro E, D’Amico P, Viale G, Trapani D, et al. Role of next-generation sequencing technologies in personalized medicine. In: G Pravettoni, S Triberti editors. eHealth: An Agenda for the Health Technologies of the Future. Cham: Springer (2019). 5 p. doi: 10.1007/978-3-030-27994-3_8
15. Saj F, Nag S, Nair N, Sirohi B. Management of BRCA-associated breast cancer patients in low and middle-income countries: A review. Ecancermedicalscience. (2024) 18:1744. doi: 10.3332/ecancer.2024.1744
16. Grafodatskaya D, O’Rielly D, Bedard K, Butcher D, Howlett C, Lytwyn A, et al. Practice guidelines for BRCA1/2 tumour testing in ovarian cancer. J Med Genet. (2022) 59:727–36. doi: 10.1136/jmedgenet-2021-108238
17. Slade E, Berg L, Dworzynski K, Manchanda R. Ovarian cancer: Identifying and managing familial and genetic risk—Summary of new NICE guidance. BMJ. (2024) 385:q807. doi: 10.1136/bmj.q807
18. Fujiwara M, McGuire V, Felberg A, Sieh W, Whittemore A, Longacre T. Prediction of BRCA1 germline mutation status in women with ovarian cancer using morphology-based criteria. Am J Surg Pathol. (2012) 36:1170–7. doi: 10.1097/pas.0b013e31825d9b8d
19. Quesada S, Penault-Llorca F, Matias-Guiu X, Banerjee S, Barberis M, Coleman R, et al. Homologous recombination deficiency in ovarian cancer: Global expert consensus on testing and a comparison of companion diagnostics. Eur J Cancer. (2024) 215:115169. doi: 10.1016/j.ejca.2024.115169
20. Hodgson A, Turashvili G. Pathology of hereditary breast and ovarian cancer. Front Oncol. (2020) 10:531790. doi: 10.3389/fonc.2020.531790
21. Premnath N, O’Reilly E. BReast CAncer (BRCA) gene mutations as an emerging biomarker for the treatment of gastrointestinal malignancies. Chin Clin Oncol. (2020) 9:64. doi: 10.21037/cco-2019-ddp-05
22. Eerola H, Heikkilä P, Tamminen A, Aittomäki K, Blomqvist C, Nevanlinna H. Histopathological features of breast tumours in BRCA1, BRCA2 and mutation-negative breast cancer families. Breast Cancer Res. (2004) 7:R93–100. doi: 10.1186/bcr953
23. Honrado E, Benítez J, Palacios J. Histopathology of BRCA1- and BRCA2-associated breast cancer. Crit Rev Oncol Hematol. (2006) 59:27–39. doi: 10.1016/j.critrevonc.2006.01.006
24. Sanegre S, Lucantoni F, Burgos-Panadero R, De La Cruz-Merino L, Noguera R, Naranjo TÁ. Integrating the tumor microenvironment into cancer therapy. Cancers (Basel). (2020) 12:1677. doi: 10.3390/cancers12061677
25. Hao J, Yu H, Zhang T, An R, Xue Y. Prognostic impact of tumor-infiltrating lymphocytes in high grade serous ovarian cancer: A systematic review and meta-analysis. Ther Adv Med Oncol. (2020) 12:175883592096724. doi: 10.1177/1758835920967241
26. Fanale D, Dimino A, Pedone E, Brando C, Corsini L, Filorizzo C, et al. Prognostic and predictive role of tumor-infiltrating lymphocytes (TILs) in ovarian cancer. Cancers (Basel). (2022) 14:4344. doi: 10.3390/cancers14184344
27. Morand S, Stanbery L, Walter A, Rocconi R, Nemunaitis J. BRCA1/2 mutation status impact on autophagy and immune response: Unheralded target. JNCI Cancer Spectr. (2020) 4:kaa077. doi: 10.1093/jncics/pkaa077
28. Howitt B, Hanamornroongruang S, Lin D, Conner J, Schulte S, Horowitz N, et al. Evidence for a dualistic model of high-grade serous carcinoma. Am J Surg Pathol. (2015) 39:287–93. doi: 10.1097/pas.0000000000000369
29. Soslow R, Han G, Park K, Garg K, Olvera N, Spriggs D, et al. Morphologic patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod Pathol. (2011) 25:625–36. doi: 10.1038/modpathol.2011.183
30. Aköz G, Özge ÖK, Özdemir TR, Çakır İ, Canan KT. The histopathological findings of high-grade serous ovarian carcinomas in patients with BRCA germline mutations, single center experience. Anatol J Med. (2024) 34:50–6. doi: 10.4274/anatoljmed.2023.92609
31. Richards C, Bale S, Bellissimo D, Das S, Grody W, Hegde M, et al. ACMG recommendations for standards for interpretation and reporting of sequence variations: Revisions 2007. Genet Med. (2008) 10:294–300. doi: 10.1097/gim.0b013e31816b5cae
32. Elston C, Ellis I. pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology. (1991) 19:403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x
33. Royal College of Pathologists. Pathology Reporting of Breast Disease in Surgical Excision Specimens Incorporating the Dataset for Histological Reporting of Breast Cancer. (2016). Available online at: https://www.rcpath.org/static/693db661-0592-4d7e-9644357fbfa00a76/G148_BreastDataset-lowres-Jun16.pdf (accessed December 29, 2024).
34. Webb P, Jordan S. Global epidemiology of epithelial ovarian cancer. Nat Rev Clin Oncol. (2024) 21:389–400. doi: 10.1038/s41571-024-00881-3
35. Torre L, Trabert B, DeSantis C, Miller K, Samimi G, Runowicz C, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. (2018) 68:284–96. doi: 10.3322/caac.21456
36. Gilks C, Soslow R, Campbell I, Malpica A, Brenton J, Vang R, et al. High-grade serous carcinoma of the ovary. WHO Classification of Tumours Editorial Board. Female Genital Tumours [Internet]. Lyon: International Agency for Research on Cancer (2020).
37. Kime A, Bataillon G, Treilleux I, Callens C, Selle F, Heitz F, et al. Can morphology and immune infiltration predict the homologous recombination deficiency status in newly diagnosed high-grade serous ovarian carcinoma? Arch Pathol Lab Med. (2024): doi: 10.5858/arpa.2024-0081-oa Online ahead of print.
38. Manchana T, Tantbirojn P, Pohthipornthawat NB. RCA immunohistochemistry for screening of BRCA mutation in epithelial ovarian cancer patients. Gynecol Oncol Rep. (2020) 33:100582. doi: 10.1016/j.gore.2020.100582
39. Saleh A, Perets R. Mutated p53 in HGSC-from a common mutation to a target for therapy. Cancers (Basel). (2021) 13:3465. doi: 10.3390/cancers13143465
40. Zhang X, Wang A, Han L, Liang B, Allard G, Diver E, et al. deficiency in tubo-ovarian high-grade serous carcinoma is associated with poor progression-free survival and is mutually exclusive with CCNE1 amplification. Mod Pathol. (2023) 36:100106. doi: 10.1016/j.modpat.2023.100106
41. Kang E, Weir A, Meagher N, Farrington K, Nelson G, Ghatage P, et al. CCNE1 and survival of patients with tubo-ovarian high-grade serous carcinoma: An ovarian tumor tissue analysis consortium study. Cancer. (2023) 129:697–713. doi: 10.1002/cncr.34582
Keywords: BRCA, histology, high-grade serous ovarian carcinoma, mitosis, ovary, tumor infiltrating lymphocyte
Citation: Nguyen-Phan DH, Dang T, Dang AN, Huynh LTH, Nguyen PTB, Tran VQ, Ngo HTT, Doan TTP, Thai TA and Chen C-C (2025) Tumor-infiltrating lymphocytes are the key determinants of pathological features associated with pathogenic BRCA variants in high-grade serous ovarian carcinoma. Front. Med. 12:1555883. doi: 10.3389/fmed.2025.1555883
Received: 05 January 2025; Accepted: 29 April 2025;
Published: 03 June 2025.
Edited by:
Yu Ligh Liou, The First Affiliated Hospital of Guangdong Pharmaceutical University, ChinaReviewed by:
Dmitry Aleksandrovich Zinovkin, Gomel State Medical University, BelarusYongbin Liu, Houston Methodist Research Institute, United States
Ilaria Cuccu, Sapienza University of Rome, Italy
Tullio Golia D’Augè, Sapienza University of Rome, Italy
Copyright © 2025 Nguyen-Phan, Dang, Dang, Huynh, Nguyen, Tran, Ngo, Doan, Thai and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanh T. T. Ngo, bmdvdGhpdHV5ZXRoYW5oQHVtcC5lZHUudm4=; Chien-Chin Chen, aGxtYXJrY0BnbWFpbC5jb20=
 Dang H. Nguyen-Phan
Dang H. Nguyen-Phan Tin Dang2
Tin Dang2 Chien-Chin Chen
Chien-Chin Chen