- 1Department of Pathology, University of Kansas Medical Center, Kansas City, KS, United States
- 2Department of Urology, University of Kansas Medical Center, Kansas City, KS, United States
Oncocytomas are clinically benign tumors composed of cells with abundant granular eosinophilic cytoplasm due to a high mitochondrial content. While typically non-metastatic, rare cases of metastatic oncocytomas have been documented. This report describes a unique case involving transcriptome analysis to identify genes associated with the oncocytoma signature. A 57-year-old woman presented to the emergency department with COVID-19 pneumonia. Incidentally, a CT chest scan revealed a large mass in the left upper quadrant. Further imaging of the abdomen and pelvis identified a 14 cm left renal mass and multiple low-density hepatic lesions. A liver biopsy confirmed a PAX-8 and CD10 positive carcinoma, consistent with metastatic renal cell carcinoma. Following neoadjuvant therapy, the patient underwent a left radical nephrectomy, partial hepatectomy, and cholecystectomy. This case emphasizes the rarity of metastatic oncocytoma and the importance of genomic testing in elucidating its molecular underpinnings. By identifying specific genetic alterations linked to the oncocytoma signature, genomic analysis offers critical insights into potential mechanisms of metastasis. These findings could enhance diagnostic accuracy and guide the development of targeted therapeutic strategies in rare metastatic cases of oncocytoma.
Introduction
In the current 5th edition of the WHO Classification of Urinary and Male Genital Tumors, renal oncocytoma (RO) is classified as a subtype of oncocytic and chromophobe renal tumors. It is a benign neoplasm and typically asymptomatic (1, 2). Microscopically, RO displays nested or tubulocystic structures lined with eosinophilic cells containing granular cytoplasm. Although RO cells exhibit benign histological features, the presence of mitotic figures is still consistent with an oncocytoma diagnosis (3, 4). The nuclei are round with uniform chromatin and a single prominent nucleolus, though occasional areas of cellular atypia with large, irregularly contoured nuclei and smudged chromatin may be observed (5).
RO diagnosis is generally based on morphology, but distinguishing it from the eosinophilic variant of chromophobe renal cell carcinoma (eo-ChRCC) can be difficult. In these cases, immunohistochemistry aids in differentiation. CK7 is commonly employed, with focal staining in < 5% of cells supporting a diagnosis of RO (4). Additional markers such as kidney-specific cadherin and S100A1 may also be used, showing cytoplasmic and nuclear/cytoplasmic staining patterns, respectively, in RO (5, 6). For ambiguous cases or rare instances of metastatic RO, molecular analysis can provide further diagnostic clarity. Notable molecular signatures in RO include 11q13 rearrangements and losses of chromosomes 1, X, or Y (7). Genomic and transcriptomic analyses have demonstrated potential in distinguishing RO from chromophobe RCC (8, 9). The classification of oncocytic renal tumors remains a subject of ongoing refinement and debate. The Genitourinary Pathology Society (GUPS) recently proposed the term “oncocytic renal neoplasm of low malignant potential, not further classified” for solitary, sporadic tumors with equivocal RO/eo-ChRCC features but an indolent clinical course (10).
Although RO is generally benign, rare cases of metastasis have been reported in the literature (2, 11, 12). One notable biopsy-confirmed case reported by Perez-Ordonez et al. in 1997 described a patient with stable metastatic disease 58 months after diagnosis, managed expectantly (2). Additional reports by Oxley et al. (11) and others highlight similar occurrences, though some were published before chromophobe RCC was widely recognized, and not all were biopsy-confirmed. This raises uncertainty regarding whether reported metastases originated from the renal tumor or a separate primary oncocytoma. Molecular analysis of both primary and metastatic lesions presents a promising approach to clarify this distinction.
Case presentation
A 57-year-old woman presented to the emergency department with COVID-19 pneumonia. Her past medical history included basal cell carcinoma. Incidentally, a chest CT revealed a large mass in the left upper quadrant. A follow-up CT of the abdomen and pelvis identified a 14.8 cm left renal mass and multiple low-density hepatic lesions. A liver biopsy confirmed PAX-8 positive carcinoma, consistent with metastatic renal cell carcinoma.
The patient began first-line systemic chemotherapy with combination Nivolumab and Ipilimumab, followed by Nivolumab monotherapy, aiming for tumor debulking and subsequent surgical resection. Despite stable hepatic lesions, a lack of significant therapeutic response led to second-line chemotherapy with Axitinib. After 8 weeks, follow-up imaging showed a slight reduction in the renal mass and stable hepatic lesions. However, subsequent scans indicated poor overall response, with mild progression of liver metastases.
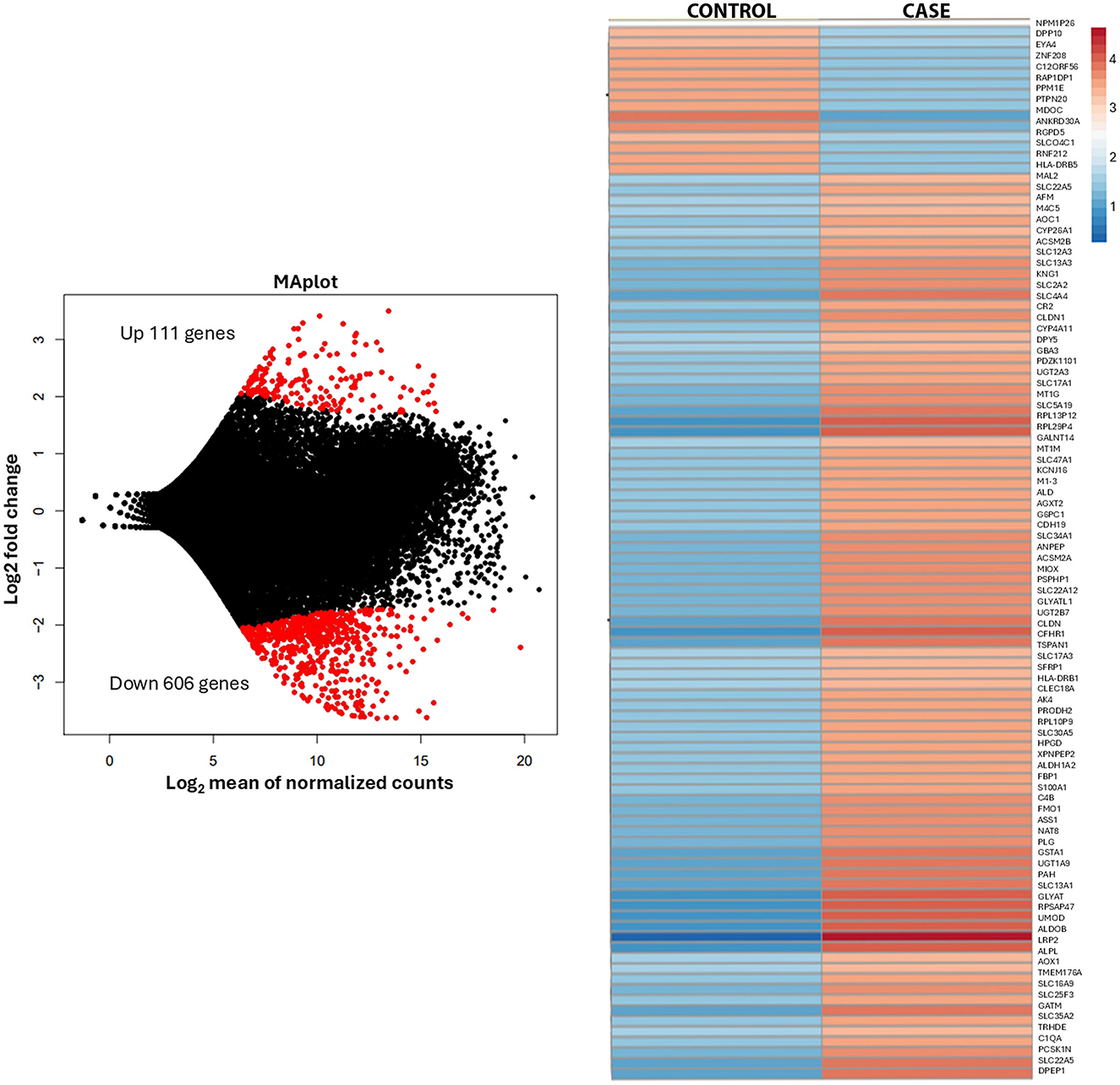
The patient underwent left radical nephrectomy, partial hepatectomy, and cholecystectomy, with good surgical tolerance despite post-operative left lower lobe pneumonia. Histological analysis confirmed metastatic oncocytoma, with no evidence of lymphovascular invasion. The tumor showed solid nests of oncocytic cells with granular eosinophilic cytoplasm and areas of hypocellular myxoid-to-fibrous stroma (Figure 1). Immunohistochemistry showed positivity for PAX-8, CD117, patchy AMACR, and rare CK7-positive cells Intact SDHB and FH expression was noted. Electron microscopy on paraffin-embedded tissue revealed numerous poorly preserved intracellular organelles resembling mitochondria (Supplementary Figure 1). Transcriptome analysis of the renal mass identified 27,715 genes (performed at Admera Health, LLC, South Plainfield, NJ, USA). Compared to oncocytoma control specimens with no history of metastasis, the renal mass demonstrated 182 upregulated genes enriched in 32 pathways, with key genes of interest including RGPD6, RGPD5, RGPD8, RGPD2, NUP210L, and PLCL1. Additionally, 606 genes were downregulated, enriched in 20 pathways (Figure 2).

Figure 1. (A) Abdominal CT image showing a large, enhancing left upper pole renal mass measuring 14.8 cm and an enhancing liver lesion measuring 3 cm at its greatest dimension. (B) Histologic section of the renal mass displaying solid nests of oncocytic cells with granular, eosinophilic cytoplasm, interspersed with areas of hypocellular, myxoid to fibrous edematous stroma. (C) Histologic section of the liver mass revealing similar morphology and adjacent normal liver tissue.
Three months post-operation, imaging revealed two new liver metastases, later determined to have likely been present for 5 months and slow-growing. Biopsy of these lesions showed no evidence of neoplasm. The patient underwent microwave ablation, though one hepatic lesion persisted, requiring two rounds of bland embolization. After 4 months, imaging showed slight growth of the lesion, and repeat microwave ablation is now planned.
Discussion
This case highlights a rare instance of metastatic oncocytoma, challenging the conventional understanding of these typically benign tumors. The presence of metastatic liver lesions in a patient with renal oncocytoma underscores the importance of further research into the molecular mechanisms that may drive metastasis in oncocytomas.
The rarity of metastatic oncocytomas presents significant clinical management challenges, as no standardized treatment guidelines currently exist, and therapeutic options remain limited. In this case, the patient underwent first-line treatment with a combination of Nivolumab and Ipilimumab, followed by second-line Axitinib, along with surgical intervention. Incomplete tumor resection necessitated additional procedures, including embolization and microwave ablation.
Transcriptome analysis revealed distinct gene expression alterations compared to control oncocytoma samples. Notably, multiple genes encoding structural extracellular matrix glycoproteins, collagens, and proteoglycans were upregulated, including RGPD6, RGPD5, RGPD8, RGPD2, NUP210L, and PLCL1. The RGPD gene family, encoding RanBP2-like and GRIP domain-containing proteins, is emerging as a key regulator of extracellular matrix (ECM) remodeling, nuclear-cytoplasmic transport, and mechanotransduction, all of which are crucial for tumor metastasis. Alongside RGPD genes, NUP210—another nuclear pore complex component—plays a critical role in facilitating metastatic potential by maintaining nuclear pore integrity, modulating chromatin architecture, and enabling tumor cells to respond to mechanical stress (13). Upregulation of RGPD6, RGPD5, RGPD8, RGPD2, NUP210L, and PLCL1 has been observed in invasive tumors, implicating this functional network in the remodeling of ECM stiffness and integrin signaling, both of which enhance cancer cell migration (14). Nuclear pore dysregulation, including that mediated by RGPD proteins, alters the export and import of transcriptional regulators involved in epithelial-mesenchymal transition (EMT), enabling cells to transition toward a more mesenchymal, invasive phenotype (15). In addition, altered nuclear mechanics mediated by NUP210 and RGPD family members contributes to invadopodia formation, a key mechanism driving metastatic spread (13). Together, these findings position the RGPD-NUP210 axis as a critical driver of metastatic competence, highlighting it as a potential therapeutic target in metastatic cancer. Immunohistochemical staining did not show marked expression differences compared to the surrounding normal renal parenchyma.
In contrast, the downregulated pathways were associated with retinol, lipid, fatty acid, and amino acid metabolism, as well as xenobiotic metabolism via cytochrome P450 and proximal tubule transport. These findings suggest a distinct metabolic profile in metastatic oncocytomas, potentially distinguishing them from typical benign cases. Further analysis of these genes is currently in progress.
A literature review revealed 22 documented cases of metastatic oncocytoma (Supplementary Table 1) (20), six of which were surgically confirmed using various diagnostic methods, including immunohistochemistry, electron microscopy, FISH, SNP-based karyotyping, multiregion sequencing, and expression array analysis. Notably, in five of these six cases (83.3%), liver involvement was observed. While there is limited data on hepatic metastasis in renal oncocytomas, uveal melanoma serves as a prototypical neoplasm with a strong predilection for liver metastases, occurring in up to 95% of systemic cases (16). Studies by Ye et al. (17), Economou et al. (18), and Li et al. (19) have demonstrated that pathways involving HGF, c-Met, PI3K/Akt, and CXCR4 contribute to liver tropism in uveal melanoma. However, in our case, there was no evidence of increased expression of HGF, CXCR4, or activation of the PI3K/Akt pathway.
Our findings underscore the critical role of genomic testing in guiding clinical management and the potential for developing targeted therapies. Identifying specific genetic alterations in metastatic oncocytomas could pave the way for personalized treatment strategies and improved outcomes.
In conclusion, this case provides valuable insights into the genetic landscape of metastatic oncocytomas and emphasizes the importance of comprehensive genomic analyses. Identifying genetic alterations linked to metastasis offers promising avenues for future research and may lead to the development of targeted therapies for this rare and difficult-to-treat condition.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving humans were approved by the Institutional Review Board, University of Kansas Medical Center Research Administration. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
IA-k: Writing – original draft, Writing – review & editing. CM: Writing – original draft, Writing – review & editing. AH: Writing – original draft, Writing – review & editing. BL: Writing – original draft, Writing – review & editing. DZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was supported by the department of pathology, KUMC.
Acknowledgments
We sincerely appreciate our patient's gracious consent to publish this case report and willingness to support our academic mission to advance the understanding of disease pathogenesis, improve patient care, and contribute to medical knowledge.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1558224/full#supplementary-material
Supplementary Figure 1 | Electron microscopy performed on paraffin-embedded tissue revealed numerous poorly preserved intracellular organelles resembling mitochondria.
Supplementary Table 1 | Literature review of previously reported metastatic renal oncocytoma cases.
References
1. Klein MJ, Valensi QJ. Proximal tubular adenomas of kidney with so-called oncocytic features. A clinicopathologic study of 13 cases of a rarely reported neoplasm. Cancer. (1976) 38:906–14.
2. Perez-Ordonez B, Hamed G, Campbell S, Erlandson RA, Russo P, Gaudin PB, et al. Renal oncocytoma: a clinicopathologic study of 70 cases. Am J Surg Pathol. (1997) 21:871–83. doi: 10.1097/00000478-199708000-00001
3. Wobker SE, Williamson SR. Modern pathologic diagnosis of renal oncocytoma. J Kidney Cancer VHL. (2017) 4:1–12. doi: 10.15586/jkcvhl.2017.96
4. Williamson SR, Gadde R, Trpkov K, Hirsch MS, Srigley JR, Reuter VE, et al. Diagnostic criteria for oncocytic renal neoplasms: a survey of urologic pathologists. Hum Pathol. (2017) 63:149–56. doi: 10.1016/j.humpath.2017.03.004
5. Trpkov K, Yilmaz A, Uzer D, Dishongh KM, Quick CM, Bismar TA, et al. Renal oncocytoma revisited: a clinicopathological study of 109 cases with emphasis on problematic diagnostic features. Histopathology. (2010) 57:893–906. doi: 10.1111/j.1365-2559.2010.03726.x
6. Mazal P, Exner M, Haitel A, Krieger S, Thomson RB, Aronson PS, et al. Expression of kidney-specific cadherin distinguishes chromophobe renal cell carcinoma from renal oncocytoma. Hum Pathol. (2005) 36:22–8. doi: 10.1016/j.humpath.2004.09.011
7. Anderson CB, Lipsky M, Nandula SV, Freeman CE, Matthews T, Walsh CE, et al. Cytogenetic analysis of 130 renal oncocytomas identify three distinct and mutually exclusive diagnostic classes of chromosome aberrations. Genes Chromosomes Cancer. (2020) 59:6–12. doi: 10.1002/gcc.22766
8. Wu H, Fan L, Liu H, Guan B, Hu B, Liu F, et al. Identification of key genes and prognostic analysis between chromophobe renal cell carcinoma and renal oncocytoma by bioinformatic analysis. Biomed Res Int. (2020) 2020:4030915. doi: 10.1155/2020/4030915
9. Satter KB, Tran PMH, Tran LKH, Ramsey Z, Pinkerton K, Bai S, et al. Oncocytoma-related gene signature to differentiate chromophobe renal cancer and oncocytoma using machine learning. Cells. (2022) 11:287. doi: 10.3390/cells11020287
10. Hes O, Trpkov K. Do we need an updated classification of oncocytic renal tumors?: Emergence of low-grade oncocytic tumor (LOT) and eosinophilic vacuolated tumor (EVT) as novel renal entities. Mod Pathol. (2022) 35:1140–50. doi: 10.1038/s41379-022-01057-z
11. Oxley JD, Sullivan J, Mitchelmore A, Gillatt DA. Metastatic renal oncocytoma. J Clin Pathol. (2007) 60:720–2. doi: 10.1136/jcp.2006.044198
12. Webster BR, Ricketts CJ, Vocke CD, Gamble D, Crooks DR, Yang Y, et al. Molecular characterization of metastatic oncocytoma with exceptional response to treatment: a case report. JCO Precis Oncol. (2024) 8:e2400188. doi: 10.1200/PO.24.00188
13. Amin R, Shukla A, Zhu JJ, Kim S, Wang P, Tian SZ, et al. Nuclear pore protein NUP210 depletion suppresses metastasis through heterochromatin-mediated disruption of tumor cell mechanical response. Nat Commun. (2021) 12:7216. doi: 10.1038/s41467-021-27451-w
14. Prakash J, Shaked Y. The interplay between extracellular matrix remodeling and cancer therapeutics. Cancer Discov. (2024) 14:1375–88. doi: 10.1158/2159-8290.CD-24-0002
15. Kai F, Drain AP, Weaver VM. The extracellular matrix modulates the metastatic journey. Dev Cell. (2019) 49:332–46. doi: 10.1016/j.devcel.2019.03.026
16. Carvajal RD, Schwartz GK, Tezel T, Marr B, Francis JH, Nathan PD. Metastatic disease from uveal melanoma: treatment options and future prospects. Br J Ophthalmol. (2017) 101:38–44. doi: 10.1136/bjophthalmol-2016-309034
17. Ye M, Hu D, Tu L, Zhou X, Lu F, Wen B, et al. Involvement of PI3K/Akt signaling pathway in hepatocyte growth factor-induced migration of uveal melanoma cells. Invest Ophthalmol Vis Sci. (2008) 49:497–504. doi: 10.1167/iovs.07-0975
18. Economou MA, All-Ericsson C, Bykov V, Girnita L, Bartolazzi A, Larsson O, et al. Receptors for the liver synthesized growth factors IGF-1 and HGF/SF in uveal melanoma: intercorrelation and prognostic implications. Acta Ophthalmol. (2008) 4:20–5. doi: 10.1111/j.1755-3768.2008.01182.x
19. Li H, Yang W, Chen PW, Alizadeh H, Niederkorn JY. Inhibition of chemokine receptor expression on uveal melanomas by CXCR4 siRNA and its effect on uveal melanoma liver metastases. Invest Ophthalmol Vis Sci. (2009) 50:5522–8. doi: 10.1167/iovs.09-3804
20. Reuter VE, Argani P, Zhou M, Delahunt B. Members of the ISUP immunohistochemistry in diagnostic urologic pathology group. Best practices recommendations in the application of immunohistochemistry in the kidney tumors: report from the international society of urologic pathology consensus conference. Am J Surg Pathol. (2014) 38:e35–49. doi: 10.1097/PAS.0000000000000258
Keywords: oncocytoma, genetic profile, RGPD, NUP210L, PLCL1
Citation: Al-kharouf I, Miller C, Hamza A, Li B and Zhang D (2025) Renal oncocytoma with liver metastasis: a case report with genetic analysis and literature review. Front. Med. 12:1558224. doi: 10.3389/fmed.2025.1558224
Received: 09 January 2025; Accepted: 17 March 2025;
Published: 30 April 2025.
Edited by:
Volker Nickeleit, The University of North Carolina School of Medicine, United StatesReviewed by:
Harsharan Singh, University of North Carolina at Chapel Hill, United StatesAlexei Mikhailov, Wake Forest Baptist Medical Center, United States
Copyright © 2025 Al-kharouf, Miller, Hamza, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Da Zhang, ZHpoYW5nQGt1bWMuZWR1; Benyi Li, YmxpQGt1bWMuZWR1
 Issa Al-kharouf
Issa Al-kharouf Carmen Miller
Carmen Miller Ameer Hamza
Ameer Hamza Benyi Li
Benyi Li Da Zhang
Da Zhang