- 1Department of Respiratory and Critical Care Medicine, Shandong Public Health Clinical Center, Jinan, China
- 2Department of Chest Surgery, Shandong Public Health Clinical Center, Jinan, China
- 3Department of Respiratory and Critical Care Medicine, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
Background: Pulmonary aspergillosis is a rare but serious complication following lung cancer surgery, increasing the risk of mortality. The incidence of pulmonary aspergillosis and its risk factors among lung cancer patients is unknown. This study systematically investigates the incidence and associated risk factors of pulmonary aspergillosis in lung cancer patients.
Methods: The databases PubMed, Web of Science, Scopus, Embase, CINAHL, and the Cochrane Library were comprehensively searched from their inception to March 2025. The overall incidence of pulmonary aspergillosis among lung cancer patients was analyzed using a random-effects model with logit transformation. Risk factors for pulmonary aspergillosis in lung cancer patients were presented as odds ratios (ORs) with 95% confidence intervals (CIs), calculated using a random-effects model.
Results: Nine retrospective studies involving 20,138 patients with lung cancer were selected for the final analysis. The overall incidence of pulmonary aspergillosis in lung cancer patients was 2.4% (95% confidence interval [CI]: 1.5–3.2%). Subgroup analyses revealed higher incidences of pulmonary aspergillosis than corresponding subgroups in the following categories: Asia (2.8%; 95% CI: 2.0–3.7%), diagnosis by serological test (11.7%; 95% CI: 8.0–15.4%), patients with both non-small cell lung cancer and small cell lung cancers (3.6%; 95% CI: 2.0–5.2%), patients treated with chemoradiotherapy (5.7%; 95% CI: 1.6–9.7%), and pooled studies with moderate quality (2.9%; 95% CI: 1.7–3.2%). Moreover, the risk factors for pulmonary aspergillosis in lung cancer patients included male sex (OR: 1.96; p = 0.008), current or past smoking (odds ratio [OR]: 2.92; p < 0.001), chronic obstructive pulmonary disease (OR: 1.88; p = 0.011), interstitial lung disease (OR: 3.71; p < 0.001), pulmonary tuberculosis (OR: 2.79; p = 0.028), and treatment with double lobectomy (OR: 2.74; p < 0.001).
Conclusion: Our study highlights pulmonary aspergillosis as a significant complication in lung cancer patients, with an overall incidence of 2.4%. The identified risk factors provide crucial insights for targeted screening and intervention in this patient population. Future research should focus on validating these findings in prospective studies and exploring the underlying biological mechanisms to develop more effective preventive and treatment strategies.
Systematic review registration: This study was registered in the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY) platform (number: INPLASY2024100066).
1 Introduction
Lung cancer is the leading cause of cancer-related deaths globally, with over a million fatalities annually (1). Based on tissue characteristics, it can be categorized into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) (2). The development of lung cancer is a complex, multifaceted process involving numerous risk factors (3). Currently, the primary treatment approaches for lung cancer encompass surgical resection, chemotherapy, radiation therapy, and biological therapies (4). Nonetheless, despite the use of these multimodal strategies, a high rate of treatment failure and disease recurrence remain crucial factors contributing to poor patient outcomes (5).
Pulmonary aspergillosis is a severe yet frequently overlooked fungal lung infection, with an estimated global patient population exceeding three million; however, the epidemiological data concerning pulmonary aspergillosis remain inadequate (6). Currently, clinicians’ understanding of the risk factors contributing to the progression of pulmonary aspergillosis is often limited and incomplete (7). Pulmonary aspergillosis predominantly affects individuals with preexisting underlying respiratory disease, particularly those with tuberculosis (TB) (8, 9). A history of lung cancer treatment or previous thoracic surgery has been reported as a potential trigger for the development of pulmonary aspergillosis (10). Due to its relatively low incidence rate, current longitudinal studies on lung cancer patients provide limited data regarding the progression of pulmonary aspergillosis and its associated risk factors. The existing literature has reported variable incidences and inconsistent risk factors for pulmonary aspergillosis in lung cancer patients, mainly due to differences in study design, patient populations, and diagnostic criteria. Our study aims to bridge these knowledge gaps by conducting a comprehensive systematic review and meta-analysis. By pooling data from multiple studies, we aim to provide a more accurate estimate of the incidence of pulmonary aspergillosis in lung cancer patients. In addition, identifying consistent risk factors will enable clinicians to better stratify patients at high risk, facilitating targeted preventive strategies and early interventions. This, in turn, has the potential to improve patient outcomes, reduce healthcare costs associated with the management of this fungal infection, and contribute to a deeper understanding of the complex relationship between lung cancer and pulmonary aspergillosis. Thus, our research holds great promise for enhancing clinical practice and future research in this area.
2 Methods
2.1 Data sources, search strategy, and selection criteria
This review adheres to the requirements and reporting guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (11). This study was registered in the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY) platform (number: INPLASY2024100066). Studies reporting the incidence and risk factors associated with pulmonary aspergillosis in lung cancer patients were included. No restrictions were applied regarding language or publication status. PubMed, Web of Science, Scopus, Embase, CINAHL, and the Cochrane Library were searched for literature up to 26 March 2025, using the search terms ‘lung cancer’ and ‘Pulmonary aspergillosis’ [PubMed: (“lung cancer”[MeSH Terms] OR “lung cancer”[All Fields]) AND (“Pulmonary aspergillosis”[MeSH Terms] OR “Pulmonary aspergillosis”[All Fields]); Web of Science: “TS = [‘lung cancer’ AND ‘Pulmonary aspergillosis’ AND (‘incidence’ OR ‘risk factors’)]”; Scopus: “TITLE-ABS-KEY [‘lung cancer’ AND ‘Pulmonary aspergillosis’ AND (‘incidence’ OR ‘risk factors’)]”; Embase: (“lung cancer”: ab,ti OR “lung neoplasm”: ab,ti) AND (“Pulmonary aspergillosis”: ab,ti); CINAHL: “[‘lung cancer’ AND ‘Pulmonary aspergillosis’ AND (‘incidence’ OR ‘risk factors’)]”; Cochrane Library: (“lung cancer” AND “Pulmonary aspergillosis”)]. Furthermore, we manually examined the reference lists of all relevant original studies and reviews to identify and include any studies that met our inclusion criteria but may have been missed during the screening process, ensuring the comprehensiveness of our search.
The literature search and study selection were independently performed by two reviewers following a standardized procedure, and disagreements between reviewers were resolved through group discussion until a consensus was reached. The inclusion criteria are as follows: (1) Patients: all lung cancer patients, irrespective of cancer type or stage; (2) Exposure: patients infected with pulmonary aspergillosis; (3) Control: patients not infected with pulmonary aspergillosis; (4) Outcomes: the incidence of pulmonary aspergillosis or its predictors for pulmonary aspergillosis in lung cancer patients; and (5) Study design: prospective and retrospective observational studies.
2.2 Data collection and quality assessment
Two reviewers independently extracted the following information from the included studies: first author’s surname, publication year, country, study design, sample size, age, male proportion, body mass index (BMI), smoking proportion, diagnostic criteria for pulmonary aspergillosis, disease status, treatments, and investigated effect estimates. Then, these two reviewers independently assessed the quality of the included studies using the Newcastle–Ottawa Scale (NOS), which primarily comprises sections on selection (four items), comparability (one item), and outcome (three items) (12). Discrepancies in the results between reviewers regarding data collection and quality assessment were resolved by an additional reviewer referring to the original article.
2.3 Statistical analysis
The overall incidence of pulmonary aspergillosis in lung cancer patients was pooled using a random-effects model with logit transformation, and all models were fitted using restricted maximum likelihood estimation. For studies with zero events, a continuity correction of 0.5 was applied (13). Then, the predictors for pulmonary aspergillosis in lung cancer patients were assigned as odds ratios (ORs) with 95% confidence intervals (CIs), and the pooled analyses were calculated using the random-effects model (13, 14). Heterogeneity across included studies was assessed using I2 and Q-statistic tests, and significant heterogeneity was defined as I2 > 50.0% or p < 0.10 (15, 16). Sensitivity analyses were performed to assess the robustness of the pooled conclusion by sequentially removing individual studies (17). Subgroup analyses for the incidence of pulmonary aspergillosis in lung cancer patients according to country, diagnostic criteria, lung cancer, treatments, and study quality, and the differences between subgroups were compared using an interaction t-test, assuming the data met normal distribution (18). Publication bias was assessed using both qualitative and quantitative methods, including funnel plots, Egger’s test, and Begg’s test (19, 20). All reported p-values were derived from two-tailed tests, with combined results having a p-value < 0.05 considered statistically significant. The data analysis was conducted using STATA software, version 12.0 (StataCorp, College Station, TX, USA). All STATA commands used are shown in the Supplementary material.
3 Results
3.1 Literature search
A total of 742 articles were identified through electronic searches; all the retrieved articles were imported into a reference management software. The software’s duplicate-detection function was used to identify and remove exact duplicates based on title, author list, and publication details, resulting in the retention of 413 studies after removing the duplicate records. Then, 374 articles were excluded during title and abstract screening because they reported irrelevant topics. The remaining 39 studies were retrieved for full-text evaluation, of which 30 were excluded for the following reasons: case reports (n = 19), other disease statuses (n = 8), and reviews (n = 3). Reviewing the reference lists of relevant articles did not yield any new eligible studies. The remaining nine studies were selected for the final meta-analysis (21–29), and the details of the literature search and study selection process are shown in Figure 1.
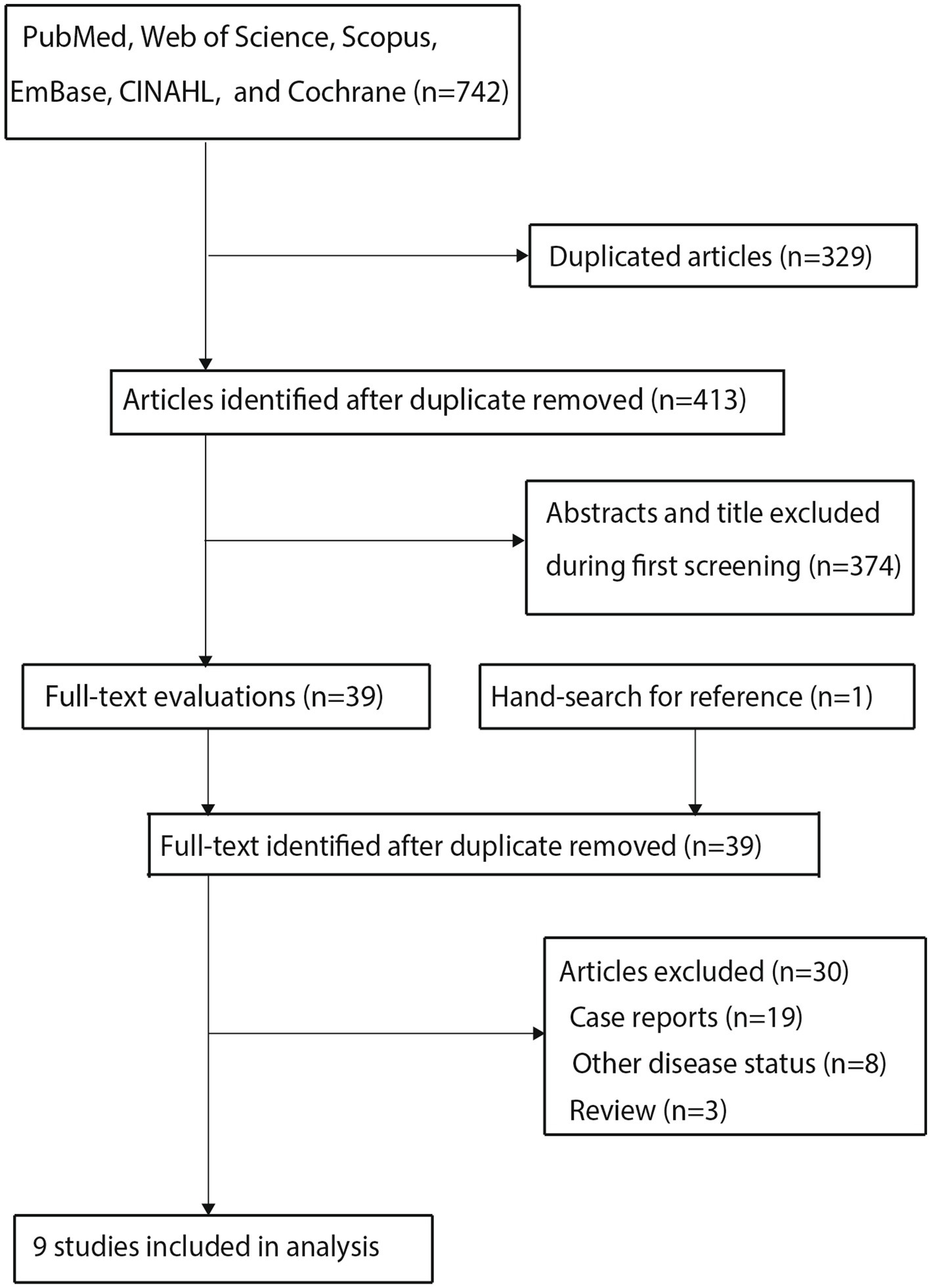
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart regarding the study selection process.
3.2 Study characteristics
The baseline characteristics of the included studies and involved patients are summarized in Table 1. A total of 20,138 patients with lung cancer were identified from the nine studies, and the sample size ranged from 187 to 6,777. All included studies were retrospective cohorts. Two studies were conducted in Europe, and the remaining seven studies were conducted in Asia. Four of the included studies included both NSCLC and SCLC, while the remaining five studies included patients with NSCLC. Study quality was assessed using the NOS scale; four studies received seven stars, three studies received six stars, and the remaining two studies received five stars (Supplementary Table S1).
3.3 Incidence of pulmonary aspergillosis in patients with lung cancer
After pooling all included studies, we noted the incidence of pulmonary aspergillosis in patients with lung cancer was 2.4% (95% CI: 1.5–3.2%; p < 0.001; Figure 2), and significant heterogeneity was observed across included studies (I2 = 95.9%; p < 0.001). Sensitivity analysis revealed that the pooled incidence of pulmonary aspergillosis ranged from 2.0% (95% CI: 1.2–2.8%) to 2.7% (95% CI: 1.6–3.9%) when a single study was sequentially removed (Supplementary Figure S1). Subgroup analysis further suggested higher incidences of pulmonary aspergillosis than corresponding subgroups in the following categories: Asia (2.8%; 95% CI: 2.0–3.7%), diagnosis by serological test (11.7%; 95% CI: 8.0–15.4%), patients with NSCLC and SCLC (3.6%; 95% CI: 2.0–5.2%), patients treated with chemoradiotherapy (5.7%; 95% CI: 1.6–9.7%), and pooled studies of moderate quality (2.9%; 95% CI: 1.7–3.2%) (Table 2).
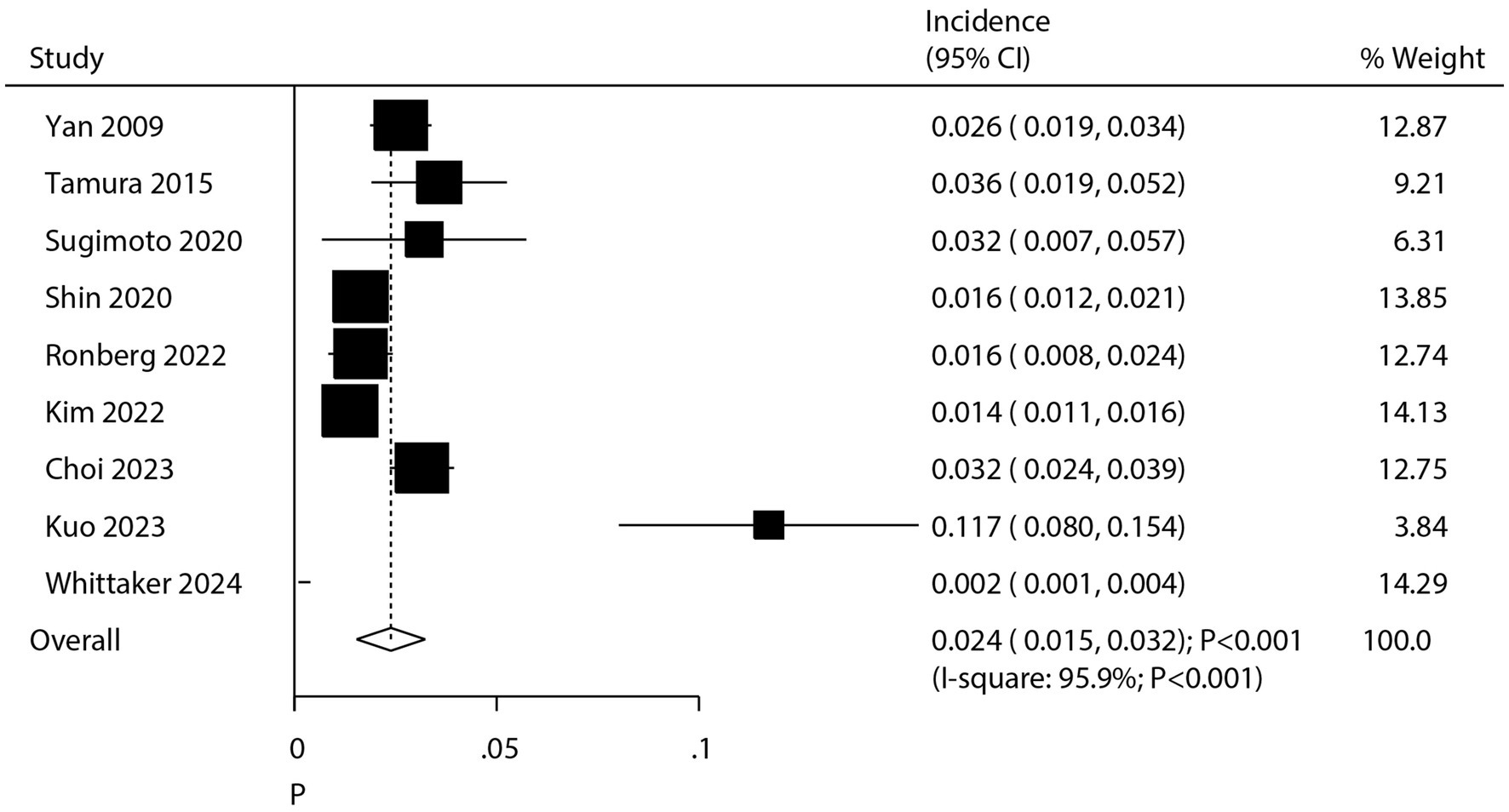
Figure 2. Summary incidence of chronic pulmonary aspergillosis in patients with lung cancer. CI, confidence interval. Incidence was calculated by the number of pulmonary aspergillosis/the number of patients with lung cancer.
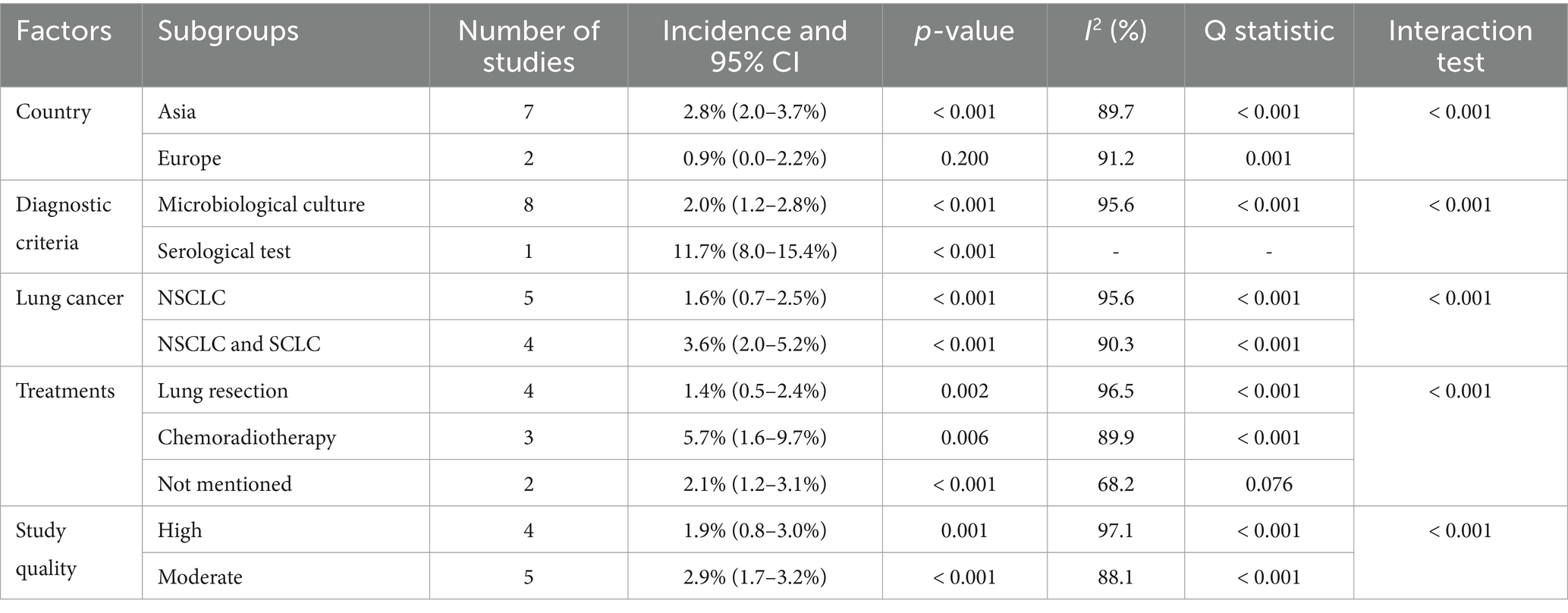
Table 2. Subgroup analyses for the incidence of pulmonary aspergillosis in patients with lung cancer.
3.4 Predictors for pulmonary aspergillosis in patients with lung cancer
As shown in Figure 3 and Supplementary Figures S2–S18, we investigated the factors influencing the occurrence of pulmonary aspergillosis in lung cancer patients. The summary results indicated that male sex (OR: 1.96; 95% CI: 1.19–3.24; p = 0.008), current or past smoking (OR: 2.92; 95% CI: 1.71–4.99; p < 0.001), chronic obstructive pulmonary disease (COPD) (OR: 1.88; 95% CI: 1.15–3.07; p = 0.011), interstitial lung disease (ILD) (OR: 3.71; 95% CI: 2.12–6.47; p < 0.001), pulmonary tuberculosis (OR: 2.79; 95% CI: 1.12–6.99; p = 0.028), treatment with double lobectomy (OR: 2.74; 95% CI: 1.63–4.61; p < 0.001), and postoperative pulmonary complications (OR: 3.42; 95% CI: 1.89–6.19; p < 0.001) were all associated with an increased risk of pulmonary aspergillosis. However, diabetes mellitus (DM), cardiovascular disease (CVD) history, stroke, cancer type, cancer location, clinical stage, and whether patients received chemotherapy or radiotherapy were not associated with the risk of pulmonary aspergillosis. There were significant heterogeneity for male sex (I2 = 61.7%; p = 0.011), COPD (I2 = 61.2%; p = 0.024), pulmonary tuberculosis (I2 = 79.9%; p = 0.002), CVD (I2 = 68.0%; p = 0.014), cancer type (I2 = 74.2%; p = 0.021), clinical stage (I2 = 88.2%; p < 0.001), radiotherapy (I2 = 87.7%; p < 0.001), and postoperative pulmonary complications (I2 = 66.6%; p = 0.084). The sensitivity analysis revealed that the pooled conclusion regarding the association of COPD with the risk of pulmonary aspergillosis in patients with lung cancer was variable. In contrast, the pooled conclusions regarding other predictors were stable.
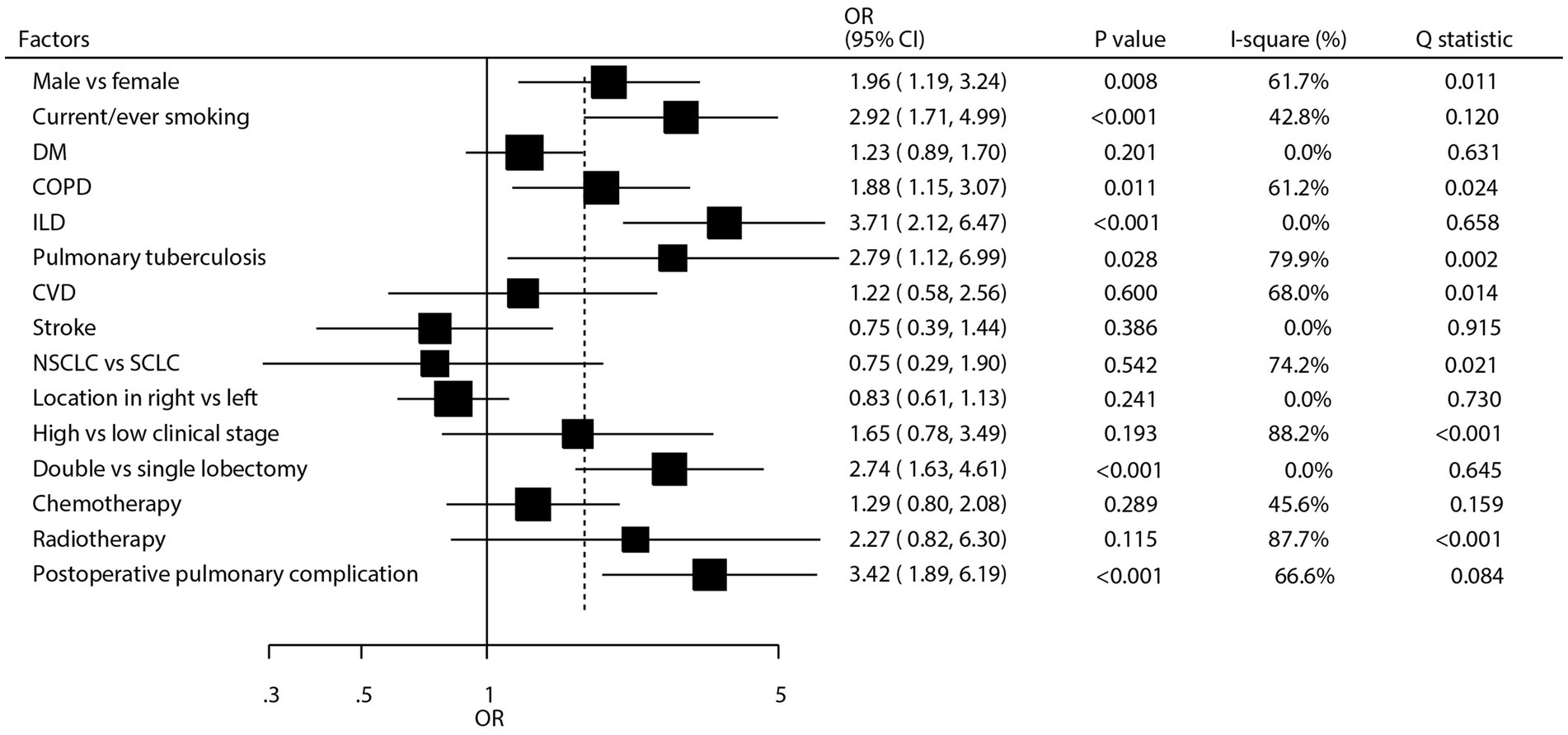
Figure 3. Summary predictors for pulmonary aspergillosis in patients with lung cancer. CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DM, diabetes mellitus; ILD, interstitial lung disease; NSCLC, non-small cell lung cancer; OR, odds ratio; SCLC, small cell lung cancer.
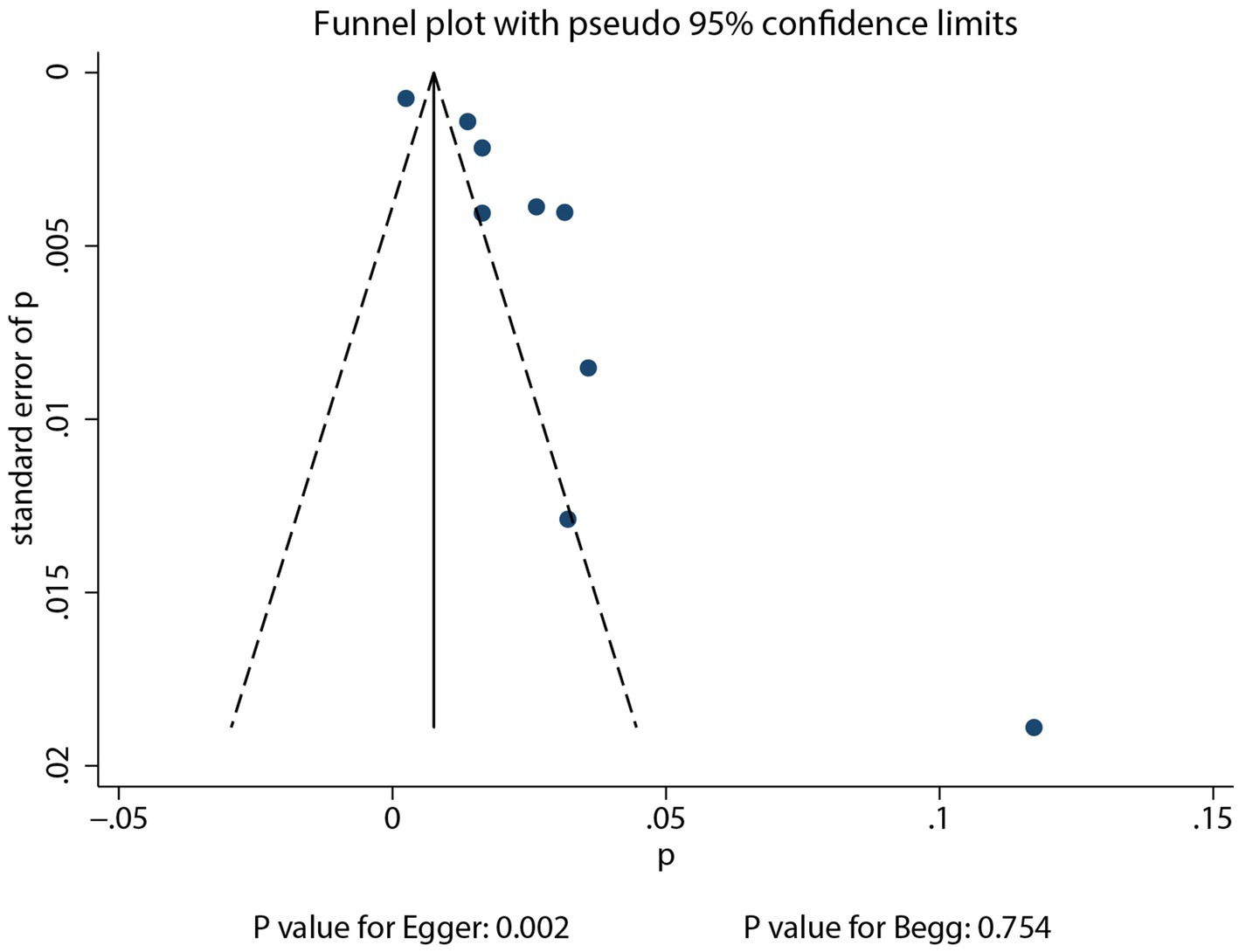
3.5 Publication bias
Reviewing the funnel plots did not rule out potential publication bias for the incidence of pulmonary aspergillosis in patients with lung cancer (Figure 4). Begg’s test (p = 0.754) indicated no significant publication bias, while Egger’s test (p = 0.002) suggested potential publication bias for pulmonary aspergillosis in patients with lung cancer. After adjusting for potential confounding factors, the conclusion indicated stability using the trim and fill method (30). There was no significant publication bias for gender (Egger’s p = 0.637; Begg’s p = 0.174), current or past smoking (Egger’s p = 0.921; Begg’s p = 1.000), DM (Egger’s p = 0.236; Begg’s p = 0.707), and COPD (Egger’s p = 0.800; Begg’s p = 1.000) with respect to the risk of pulmonary aspergillosis. However, potential publication bias was observed for the association between clinical stage and the risk of pulmonary aspergillosis (Egger’s p = 0.041; Begg’s p = 0.133). The conclusion was not altered after adjusting for publication bias using the trim and fill method (30).
4 Discussion
This study was the first to employ a meta-analysis approach to investigate the incidence of pulmonary aspergillosis in lung cancer patients and its associated risk factors. Through a systematic review, we included a total of 9 studies, encompassing 20,138 lung cancer patients, and 337 patients diagnosed with pulmonary aspergillosis. Although we did not restrict the study design during the initial search, only retrospective studies met our inclusion criteria. The rarity of pulmonary aspergillosis in lung cancer patients has made it difficult to conduct prospective studies, which typically require substantial resources, extended follow-up periods, and large sample sizes to yield meaningful results. Notably, there was significant variability in the severity of illness and baseline characteristics among the patients included in these studies. We noted the incidence of pulmonary aspergillosis in lung cancer patients was 2.4% (95% CI: 1.5–3.2%). Moreover, the risk factors for pulmonary aspergillosis in patients with lung cancer included male sex, current or past smoking, COPD, ILD, pulmonary tuberculosis, double lobectomy, and postoperative pulmonary complications.
Our research revealed an incidence rate of 2.4% for pulmonary aspergillosis among lung cancer patients, with rates in the included studies ranging from 0.2 to 11.7%. The majority of studies reported incidence rates between 1 and 3%. A primary reason contributing to the higher reported incidence, particularly in one study, was the administration of a galactomannan test on 290 high-risk patients out of 2,543 with advanced lung cancer, leading to a relatively higher reported incidence of pulmonary aspergillosis (28). The subgroup analysis revealed that the incidence of pulmonary aspergillosis was higher in Asia, among patients diagnosed with pulmonary aspergillosis using serological tests, in those with both NSCLC and SCLC, in patients treated with chemoradiotherapy, and in pooled studies of moderate quality than corresponding subgroups. The potential reasons for this include variations in diagnostic practices and accessibility to medical resources across regions, which can impact disease identification and reporting rates. In addition, Asian patients may be more prone to developing pulmonary aspergillosis due to immune suppression resulting from specific treatment regimens (31). Environmental factors play a crucial role in the regional differences in Aspergillus species distribution. In Asia, the climate is often more humid and warmer in many areas than in Europe. Differences in air pollution levels can impact fungal survival and distribution. Polluted air can damage the respiratory mucosa of lung cancer patients, making them more susceptible to Aspergillus infection. The pollutants may also interact with the fungal spores, affecting their viability and the likelihood of causing infection. Demographic factors can also contribute to regional differences. Population density varies significantly between regions. In highly populated Asian cities, the proximity of individuals can lead to increased exposure to Aspergillus spores. Moreover, differences in lifestyle and occupation can influence exposure. In some Asian countries, certain occupations such as farming or working in traditional markets may expose individuals to a higher concentration of Aspergillus spores. Genetic factors also play a role, and these genetic differences could make Asian lung cancer patients more or less susceptible to certain Aspergillus species compared to European patients. Serological tests are more likely to detect early-stage or subclinical infections, as they can detect the presence of fungal antigens in the blood. This allows for the detection of cases before the development of obvious clinical symptoms or before the fungus can be cultured. As a result, serological tests contribute to higher incidence estimates compared to diagnostic methods that rely on more advanced disease manifestations. SCLC is often more aggressive, and patients with SCLC may receive more intensive chemotherapy and radiotherapy regimens. These treatments can cause profound immunosuppression, including a decrease in the number and function of lymphocytes. The combined effect of the disease-related immune suppression and the intensive treatment-induced immunosuppression in patients with both NSCLC and SCLC makes them more prone to pulmonary aspergillosis (32). Finally, chemoradiotherapy can induce oxidative stress in the body. High levels of reactive oxygen species generated during treatment can damage cells and tissues, including immune cells. Increased oxidative stress can also enhance the inflammatory response in the lungs. This chronic inflammation can create a microenvironment that is more conducive to Aspergillus growth and infection (33).
Our study found that risk factors for pulmonary aspergillosis in lung cancer patients included male sex, current or past smoking, COPD, ILD, pulmonary tuberculosis, double lobectomy, and postoperative pulmonary complications. Several potential reasons could explain these results: (1) Males and females exhibit physiological differences in their immune responses, with males potentially having reduced resistance to Aspergillus infections due to hormonal levels, genetic backgrounds, or other physiological traits (34); (2) long-term smoking suppresses the immune system, particularly weakening the body’s defense mechanisms against pathogens, including reducing the functionality of macrophages and lymphocytes, thereby decreasing resistance to Aspergillus infections. Furthermore, smoking can lead to imbalances in pulmonary cytokines (proteins involved in immune responses), which may disrupt normal immune responses and create conditions conducive to the development of aspergillosis (35); (3) COPD-induced airway inflammation, mucus hypersecretion, structural alterations, and lung damage create a susceptible environment for Aspergillus, facilitating local infections (36); (4) ILD leads to inflammation and fibrosis of the lung interstitium, impairing gas exchange and also disrupting the typical structure of lung tissue, thereby providing a conducive environment for colonization by microorganisms (37); (5) inflammation and tissue damage caused by tuberculosis can lead to the formation of scars and cavities within the lung parenchyma. These structural alterations create physical spaces that facilitate colonization and growth of microorganisms (38); (6) double lobectomy significantly reduces the total lung tissue volume, leading to severe impairment of lung function, including diminished capacity for gas exchange and a decline in the respiratory tract’s clearance mechanisms. This renders the remaining lung tissue more vulnerable to infection by pathogens (22), and (7) lung cancer surgeries often entail the resection of lung tissue, which may inflict damage to the surrounding healthy lung tissue, leading to a decline in lung function. Postoperative atelectasis, reduced lung compliance, and impaired gas exchange and respiratory mechanics collectively contribute to a condition that facilitates colonization by microorganisms.
Considering the identified risk factors for pulmonary aspergillosis in lung cancer patients, routine screening using biomarkers such as galactomannan or β-D-glucan could be considered in high-risk populations. Patients with risk factors such as male sex, current or past smoking, COPD, interstitial lung disease, pulmonary tuberculosis, double lobectomy, or postoperative pulmonary complications are at increased risk. Galactomannan, a cell-wall component of Aspergillus, can be detected in serum or other body fluids. Its measurement has shown potential in the early detection of invasive aspergillosis. Similarly, β-D-glucan, a polysaccharide present in the cell walls of many fungi, including Aspergillus, can also be used as a screening biomarker. Routine screening with these biomarkers in high-risk lung cancer patients could lead to earlier diagnosis and potentially more effective treatment. However, it is important to note that both tests have limitations, such as false-positive results, especially in patients with other conditions that can cause cross-reactivity. Further research is needed to determine the optimal screening intervals and cutoff values for these biomarkers in the context of lung cancer patients at high risk of pulmonary aspergillosis.
Since the included studies were retrospective and the majority of them did not comprehensively adjust for potential confounders, unmeasured confounding is a significant concern. Unmeasured factors, such as lifestyle factors (e.g., diet and exercise), environmental exposures (e.g., exposure to other fungi or pollutants in the living or working environment), and genetic factors, may influence both the development of lung cancer and the susceptibility to pulmonary aspergillosis. These unmeasured confounders could potentially distort our observed associations between risk factors and the development of pulmonary aspergillosis.
Several shortcomings of this study should be acknowledged. First, this study’s analysis is based on a retrospective cohort study, which inherently limits our ability to establish causation and is inevitably subject to selection and recall biases. Second, the lung cancer patients included in the study had varying histological types and disease severity and received different treatments, all of which could influence the risk of developing pulmonary aspergillosis. Third, the investigation of risk factors was based on crude data, and adjusted effect estimates were not obtainable. Fourth, the use of limited databases for the search represents a significant limitation of this study, potentially obscuring the actual disease burden and introducing publication bias. Fifth, variations in the diagnostic approaches for pulmonary aspergillosis across different studies may impact the accuracy of reported incidence rates among lung cancer patients. Sixth, the incidence and risk factors for pulmonary aspergillosis in lung cancer patients may be influenced by lung cancer type, but the incidence rates and associated risk factors for pulmonary aspergillosis by lung cancer type were not available. Seventh, the potential confounding effects of differences in lung cancer stage and histological subtypes were not fully addressed in the exploratory analysis. Eighth, although identifying the specific Aspergillus spp. causing pulmonary aspergillosis in lung cancer patients is important, the included studies did not provide this information. Future research should focus on using more advanced diagnostic techniques, such as molecular identification methods, to determine the species of Aspergillus involved. This could potentially provide more insights into the pathogenesis and treatment of pulmonary aspergillosis in this patient population. Finally, the analysis of this study, based on published articles, was limited by the unpublished data and restricted detailed analyses.
5 Conclusion
This systematic review and meta-analysis represents one of the first comprehensive attempts to explore the incidence and predictors of pulmonary aspergillosis in lung cancer patients. Despite the limitations associated with the retrospective nature of the included studies, our findings provide valuable insights into the field. The overall incidence of pulmonary aspergillosis in lung cancer patients, estimated at 2.4%, highlights that this is a non-negligible complication. The identification of male sex, current or past smoking, COPD, ILD, pulmonary tuberculosis, double lobectomy, and postoperative pulmonary complications as risk factors for pulmonary aspergillosis in lung cancer patients provides a basis for targeted screening and preventive measures. These factors, either directly or indirectly, compromise the immune system or the integrity of the lung tissue, creating a conducive environment for Aspergillus infection. However, our study has limitations, primarily due to the retrospective nature of the included studies, potential unmeasured confounding, and variations in diagnostic criteria. Future prospective studies using standardized diagnostic methods and carefully adjusting for confounders are urgently needed to validate and expand upon our findings. These studies should also explore the underlying biological mechanisms linking these risk factors to the development of pulmonary aspergillosis, potentially leading to more effective preventive and treatment strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
GT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. FJ: Data curation, Investigation, Methodology, Validation, Writing – review & editing. HZ: Data curation, Investigation, Methodology, Supervision, Writing – review & editing. MZ: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Shandong Provincial Science and Technology Development Program for Traditional Chinese Medicine (No: M-2023185).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1560288/full#supplementary-material
References
1. Miller, KD, Nogueira, L, Devasia, T, Mariotto, AB, Yabroff, KR, Jemal, A, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. (2022) 72:409–36. doi: 10.3322/caac.21731
2. Reck, M, Heigener, DF, Mok, T, Soria, JC, and Rabe, KF. Management of non-small-cell lung cancer: recent developments. Lancet. (2013) 382:709–19. doi: 10.1016/S0140-6736(13)61502-0
3. Chen, S, and Wu, S. Identifying lung Cancer risk factors in the elderly using deep neural networks: quantitative analysis of web-based survey data. J Med Internet Res. (2020) 22:e17695. doi: 10.2196/17695
4. Molina, JR, Yang, P, Cassivi, SD, Schild, SE, and Adjei, AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. (2008) 83:584–94. doi: 10.1016/S0025-6196(11)60735-0
5. Mountzios, G, Naidoo, J, Wang, C, Creelan, BC, Trotier, DC, Campbell, TC, et al. Beyond Chemoimmunotherapy in advanced non-small cell lung Cancer: new Frontiers, new challenges. Am Soc Clin Oncol Educ Book. (2024) 44:e432526. doi: 10.1200/EDBK_432526
6. Denning, DW, Cadranel, J, Beigelman-Aubry, C, Ader, F, Chakrabarti, A, Blot, S, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. (2016) 47:45–68. doi: 10.1183/13993003.00583-2015
7. Kosmidis, C, and Denning, DW. The clinical spectrum of pulmonary aspergillosis. Thorax. (2015) 70:270–7. doi: 10.1136/thoraxjnl-2014-206291
8. Denning, DW, Pleuvry, A, and Cole, DC. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ. (2011) 89:864–72. doi: 10.2471/BLT.11.089441
9. Page, ID, Byanyima, R, Hosmane, S, Onyachi, N, Opira, C, Richardson, M, et al. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J. (2019) 53:1801184. doi: 10.1183/13993003.01184-2018
10. Smith, NL, and Denning, DW. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur Respir J. (2011) 37:865–72. doi: 10.1183/09031936.00054810
11. Page, MJ, McKenzie, JE, Bossuyt, PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
12. Wells, G, Shea, B, and O’Connell, D. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, ON: Ottawa Hospital Research Institute (2009).
13. DerSimonian, R, and Laird, N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
14. Ades, AE, Lu, G, and Higgins, JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Mak. (2005) 25:646–54. doi: 10.1177/0272989X05282643
15. Deeks, JJ, Higgins, JPT, and Altman, DG. Analyzing data and undertaking meta-analyses In: J Higgins and S Green, editors. Cochrane handbook for systematic reviews of interventions 5.0.1. Oxford, UK: The Cochrane collaboration (2008)
16. Higgins, JP, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
17. Tobias, A. Assessing the influence of a single study in meta-analysis. Stata Tech Bull. (1999) 47:15–7.
18. Altman, DG, and Bland, JM. Interaction revisited: the difference between two estimates. BMJ. (2003) 326:219. doi: 10.1136/bmj.326.7382.219
19. Egger, M, Davey Smith, G, Schneider, M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
20. Begg, CB, and Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
21. Yan, X, Li, M, Jiang, M, Zou, LQ, Luo, F, and Jiang, Y. Clinical characteristics of 45 patients with invasive pulmonary aspergillosis: retrospective analysis of 1711 lung cancer cases. Cancer. (2009) 115:5018–25. doi: 10.1002/cncr.24559
22. Tamura, A, Suzuki, J, Fukami, T, Matsui, H, Akagawa, S, Ohta, K, et al. Chronic pulmonary aspergillosis as a sequel to lobectomy for lung cancer. Interact Cardiovasc Thorac Surg. (2015) 21:650–6. doi: 10.1093/icvts/ivv239
23. Sugimoto, S, Soh, J, Suzawa, K, Miyoshi, K, Otani, S, Yamamoto, H, et al. Pulmonary aspergillosis as a late complication after surgery for locally advanced non-small cell lung cancer treated with induction chemoradiotherapy. Surg Today. (2020) 50:863–71. doi: 10.1007/s00595-020-01960-5
24. Shin, SH, Kim, BG, Kang, J, Um, SW, Kim, H, Kim, H, et al. Incidence and risk factors of chronic pulmonary Aspergillosis development during long-term follow-up after lung Cancer surgery. J Fungi. (2020) 6:271. doi: 10.3390/jof6040271
25. Rønberg, R, Davidsen, JR, Salzer, HJF, van Braeckel, E, Rosenvinge, FS, and Laursen, CB. Prevalence of chronic pulmonary Aspergillosis in patients suspected of chest malignancy. J Fungi. (2022) 8:297. doi: 10.3390/jof8030297
26. Kim, BG, Choi, YS, Shin, SH, Lee, K, Um, SW, Kim, H, et al. Mortality and lung function decline in patients who develop chronic pulmonary aspergillosis after lung cancer surgery. BMC Pulm Med. (2022) 22:436. doi: 10.1186/s12890-022-02253-y
27. Choi, Y, Noh, JM, Shin, SH, Lee, K, Um, SW, Kim, H, et al. The incidence and risk factors of chronic pulmonary infection after radiotherapy in patients with lung Cancer. Cancer Res Treat. (2023) 55:804–13. doi: 10.4143/crt.2022.1305
28. Kuo, CW, Lin, CY, Wei, SH, Chou, YT, Chen, CW, Tsai, JS, et al. Navigating the challenges of invasive pulmonary aspergillosis in lung cancer treatment: a propensity score study. Ther Adv Med Oncol. (2023) 15:17588359231198454. doi: 10.1177/17588359231198454
29. Whittaker, G, Taylor, M, Chamula, M, Granato, F, Balata, H, and Kosmidis, C. Chronic pulmonary Aspergillosis after surgical treatment for non-small cell lung Cancer-an analysis of risk factors and clinical outcomes. J Fungi. (2024) 10:335. doi: 10.3390/jof10050335
30. Duvall, S, and Tweedie, R. A nonparametric “trim and fill” method for assessing publication bias in meta-analysis. J Am Stat Assoc. (2000) 95:89–98. doi: 10.1080/01621459.2000.10473905
31. Waser, N, Vo, L, McKenna, M, Penrod, JR, and Goring, S. Real-world treatment patterns in resectable (stages I-III) non-small-cell lung cancer: a systematic literature review. Future Oncol. (2022) 18:1519–30. doi: 10.2217/fon-2021-1417
32. Kang, MS, Noh, GY, Choe, DH, et al. A newly developed lung nodule during chemotherapy for small cell lung cancer. Chronic necrotising pulmonary aspergillosis. Thorax. (2009) 64:682–97. doi: 10.1136/thx.2008.106260
33. Watanabe, H, Shirai, T, Saigusa, M, Asada, K, and Arai, K. Subacute invasive pulmonary aspergillosis after chemoradiotherapy for lung cancer. Respirol Case Rep. (2020) 8:e00523. doi: 10.1002/rcr2.523
34. Tarka, P, Nitsch-Osuch, A, Gorynski, P, Tyszko, P, Bogdan, M, and Kanecki, K. Epidemiology of pulmonary Aspergillosis in hospitalized patients in Poland during 2009-2016. Adv Exp Med Biol. (2019) 1160:73–80. doi: 10.1007/5584_2019_347
35. Dai, Y, Pu, Q, Hu, N, Zhu, J, Han, Y, Shi, P, et al. The dose-response relationship between smoking and the risk factor for invasive pulmonary aspergillosis in patients with severe fever with thrombocytopenia syndrome. Front Microbiol. (2023) 14:1209705. doi: 10.3389/fmicb.2023.1209705
36. Sun, KS, Tsai, CF, Chen, SC, et al. Clinical outcome and prognostic factors associated with invasive pulmonary aspergillosis: an 11-year follow-up report from Taiwan. PLoS One. (2017) 12:e0186422. doi: 10.1371/journal.pone.0186422
37. Shadrach, BJ, Deokar, K, Agarwal, V, Jain, A, and Goel, R. Systemic sclerosis-interstitial lung disease with coexistent subacute invasive pulmonary aspergillosis: a rare association. Adv Respir Med. (2021) 89:75–6. doi: 10.5603/ARM.a2020.0183
Keywords: incidence, predictors, pulmonary aspergillosis, lung cancer, systematic review, meta-analysis
Citation: Teng G, Jin F, Zhang H and Zhang M (2025) Incidence and predictors of pulmonary aspergillosis in patients with lung cancer: a systematic review and meta-analysis. Front. Med. 12:1560288. doi: 10.3389/fmed.2025.1560288
Edited by:
Mehdi Razzaghi-Abyaneh, Pasteur Institute of Iran (PII), IranReviewed by:
Javad Javidnia, Mazandaran University of Medical Sciences, IranOmid Raiesi, Medical University of Ilam, Iran
Copyright © 2025 Teng, Jin, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Geling Teng, dGVuZ2dlbGluZ0AxNjMuY29t; Min Zhang, emhhbmdtaW43MTJAc2luYS5jb20=
 Geling Teng
Geling Teng Feng Jin2
Feng Jin2 Hua Zhang
Hua Zhang