- Department of Anesthesiology, Shengli Clinical Medical College of Fujian Medical University, Fujian Provincial Hospital, Fuzhou University Affiliated Provincial Hospital, Fuzhou, Fujian, China
Background: Postoperative nausea and vomiting (PONV) is a common complication following surgery. Despite various preventive measures, satisfactory outcomes have not been achieved. This study explores the potential of gut microbiota interactions with the host in understanding and preventing PONV, using 16S absolute quantitative sequencing technology to uncover new insights.
Methods: Patients who experienced nausea and vomiting within 24 h after surgery were divided into a PONV group (n = 22) and a non-PONV group (n = 22). Microbial communities linked to PONV were assessed through bioinformatics analysis. Fecal samples from both groups were transplanted into rats, which were then anesthetized with isoflurane for 100 min. Pica behavior was monitored over the next 24 h to assess nausea and vomiting in the rats.
Results: Significant differences in α- and β-diversity were observed between the PONV and non-PONV groups. Six key microorganisms were identified, with Bifidobacterium, Bilophila, and Oscillibacter showing a negative correlation with PONV severity. Receiver operating characteristic (ROC) analysis demonstrated that Bifidobacterium could reliably predict PONV. Rats receiving feces from the PONV group exhibited significantly higher kaolin consumption within 24 h post-anesthesia compared to those receiving feces from the non-PONV group.
Conclusion: These results suggest a potential new mechanism for PONV involving gut microbiota, offering a theoretical basis for preoperative prediction of PONV based on gut microbial composition.
1 Introduction
Postoperative nausea and vomiting (PONV) is a common gastrointestinal issue that typically occurs within 24 h after surgery, affecting approximately 30% of general surgical patients and up to 80% in high-risk populations (1). Surveys have shown that vomiting and nausea are among the least tolerated postoperative reactions, with vomiting ranking first and nausea fourth in terms of patient discomfort (2). PONV negatively impacts patient comfort, recovery, and lengthens hospital stays (2). Despite various preventive measures, PONV continues to be inadequately controlled, highlighting the need for new approaches in its prevention.
The physiological mechanisms behind nausea and vomiting are complex, involving five main afferent pathways: (1) the central chemoreceptor trigger zone (CTZ) (3), (2) the vagus nerve in the gastrointestinal system (3), (3) neuronal pathways from the vestibular system (4), (4) the reflex afferent pathway of the cerebral cortex (5) and (5) the midbrain afferent pathway (6). Recent studies have proposed that the gut-brain axis may also play a role in PONV. One cohort study observed that patients undergoing esophagectomy and gastrectomy with vagotomy had a reduced risk of PONV, suggesting the vagus nerve-dependent gut-brain axis may contribute significantly to this condition (7). Additionally, research by Peng Cao and colleagues indicated that when the gastrointestinal tract is exposed to enterotoxins, enterochromaffin cells release serotonin, which signals the CTZ via the vagus nerve, triggering nausea and vomiting (8). However, the involvement of gut microbiota in PONV has yet to be explored.
Most microbiota analyses rely on relative quantitative sequencing, which only provides data on the relative abundance of microbial communities. This method can miss important information about the absolute number of microorganisms, potentially leading to misleading results (9, 10). Therefore, absolute quantitative sequencing was performed by adding a certain amount of artificial spiked-in reference standard sequences to the sample DNA as well as during amplicon library construction and when performing next-generation high-throughput sequencing. In contrast, absolute quantitative sequencing involves spiking the sample DNA with a reference standard, allowing for accurate calculation of microbial abundance based on standard curves and the absolute copy numbers of operational taxonomic units (OTUs)/amplicon sequence variants (ASVs) (11).
Various perioperative factors-such as emotions (12) (including anxiety and fear), sleep (13), antibiotics, stress (14), inhaled anesthetics (15) and opioids (16) can influence gut microbiota diversity. However, whether changes in microbiota composition contribute to PONV remains unclear. Therefore, this study aims to investigate the potential relationship between gut microbiota composition and PONV by assessing microbial diversity and abundance before surgery.
2 Materials and methods
2.1 Patients
This was a prospective, observational clinical study involving patients with thyroid cancer undergoing radical surgery admitted to the Fujian Provincial Hospital from June 2020 to February 2021. This study was approved by the Ethics Review Committee of Fujian Provincial Hospital (K2019-12-019, December 24, 2019) and written informed consent was obtained from all subjects participating in the trial. The trial was registered prior to patient enrollment at chictr.org.cn (ChiCTR2000029084, Principal investigator: Yi-Jie Tang and Xiao-dan Wu, Date of registration: January 13, 2021). Female patients aged 18–65 years, with American Society of Anesthesiologists (ASA) I-II status, not undergoing chemoradiotherapy, and scheduled for radical thyroid cancer surgery were included. Exclusion criteria were: (1) body mass index (BMI) > 28 kg/m2, (2) use of antibiotics or microecological regulators within 2 months prior to surgery, (3) absence of a preoperative fecal sample, (4) history of cognitive disorders, digestive diseases, allergies to anesthetics, or serious illnesses that may affect the intervention, outcomes, or ethical safety of the study (including heart failure, severe coronary artery disease and hypertension, chronic obstructive pulmonary disease, and hepatic or renal dysfunction), (5) vestibular labyrinthine dysfunction. All participants provided informed consent and agreed to take part in the study.
2.2 Fecal sample collection and clinical data
The study researcher, specifically trained for this purpose, guided patients in fecal sample collection and defecation 1 day before surgery. A trained clinical anesthesiologist was responsible for gathering data during the patients’ preoperative and postoperative visits, as well as conducting scale assessments.
2.2.1 Sample collection
Patients were provided with disposable fecal collection boxes. Before defecation, they were asked to empty their bladder. A sterile spoon was used to collect the portion of feces that had not come into contact with the air or the collection box, and it was placed in a disposable sterile storage tube. After collection, the specimen tubes were immediately frozen in liquid nitrogen for 2 h, then transferred to a freezer for storage at −80°C.
2.2.2 Assessment for nausea/vomiting and the basis for groupings
Nausea was defined as an unpleasant sensation accompanied by the awareness of the urge to vomit, while vomiting was described as the forceful expulsion of stomach contents through the mouth. The severity of nausea and vomiting was assessed using the World Health Organization (WHO) classification (17): 0, no nausea or vomiting; 1, nausea without vomiting; 2, vomiting 1–2 times per day; 3, vomiting 3–5 times per day, requiring pharmacological control; 4, vomiting more than six times per day, difficult to control with medications.
Patients were evaluated for the severity of nausea and vomiting 24 h after surgery. Those with a score ≥1 were placed in the PONV group, while the remaining patients were categorized into the non-PONV group. If the score was ≥3, patients received 5 mg of intravenous ropivacaine for antiemetic treatment.
2.2.3 Demographic and clinical variables
The Self-Rating Anxiety Scale (SAS) was used to assess the preoperative anxiety level of patients (18). The Pittsburgh Sleep Quality Index (PSQI) measured baseline subjective sleep quality 24 h before surgery (19). Additionally, the St. Mary’s Hospital Sleep Questionnaire (SMHSQ) was employed to record subjective sleep quality during the same period (20). Rest pain intensity was evaluated 24 h post-surgery using the Visual Analog Scale (VAS) (21). Information such as age, BMI, Apfel score (22), and other relevant details were recorded during the patients’ preoperative visits.
2.3 General anesthesia
Upon entering the operating room, patients were routinely monitored for invasive arterial blood pressure, heart rate, oxygen saturation, and respiratory rate. The depth of anesthesia was monitored using a BIS EEG Vista anesthesia depth monitor. Anesthesia induction included 0.02 mg/kg midazolam, 0.5 μg/kg sufentanil, and 0.15 mg/kg cisatracurium, along with a target-controlled infusion of propofol, set to a plasma target concentration of 3.0 μg/mL. During anesthesia maintenance, the target concentration of propofol was maintained at 3–6 μg/mL, while the remifentanil effect chamber concentration was kept between 4 and 6 ng/mL, and BIS values were maintained between 40 and 60. If intraoperative blood pressure dropped by more than 20% from baseline or heart rate fell below 40 beats/min, ephedrine or atropine was administered to stabilize the patient’s blood pressure and heart rate. Postoperatively, 0.25% ropivacaine was used for local infiltration analgesia, and patients with VAS scores ≥4 were given 100 mg of flurbiprofen axetil for additional pain relief.
2.4 16S absolute quantitative sequencing
Genomic DNA was extracted and amplified from the fecal samples. Sample DNA (10 ng/μL) and spike-in internal reference DNA were used as templates for PCR amplification, both added to the same PCR system. An appropriate proportion of spike-in reference DNA was included in each sample for the target region, and a positive control was also prepared. DNA libraries were constructed, and sequencing was conducted using the Illumina NovaSeq system (Genesky Biotechnologies Inc., Shanghai, China).
2.5 Animals
Twelve male Sprague–Dawley rats, aged 6 weeks, were obtained from the Laboratory Animal Center of Fujian Medical University. They were housed in a clean laboratory at 24 ± 2°C with 50–70% humidity, simulating a standard 12-h light/dark cycle (ZT0-ZT12 light, ZT13-ZT24 dark), and had ad libitum access to food and water. The rats acclimated for 1 week prior to the experiment. All procedures were approved by the Experimental Animal Ethics Review and Use Committee of Fujian Medical University (IACUC FJMU 2022-0889) and followed the institution’s animal welfare regulations.
2.6 Fecal microbiota transplantation
Initially, antibiotics (ABX) were administered to both groups of fecal microbiota transplantation (FMT) recipient rats to create a pseudo germ-free model. The antibiotic cocktail, consisting of ampicillin sodium (1 mg/mL), neomycin sulfate (1 mg/mL), metronidazole (1 mg/mL), and vancomycin hydrochloride (0.5 mg/mL) (23), was given to the rats for 7 days after preparation. Fecal pellets were pooled, homogenized with 1 mL PBS and 1 mL skimmed milk per pellet (0.2 g) (24), and allowed to settle for 5 min. The supernatant (1 mL) was then administered to the rats via oral gavage daily for 14 days. Based on the source of the fecal solution, rats were randomly divided into two groups: ① FMTnon-PONV group, receiving feces from the non-PONV group; ② FMTPONV group, receiving feces from the PONV group. The recipients were gavaged daily with the microbiota and were sacrificed after 2 weeks of FMT.
2.7 Anesthesia for animals
Rats were anesthetized in an induction chamber with 3% isoflurane. Once they lost the righting reflex, the isoflurane concentration was reduced to 1.5% for maintenance over 100 min. An isoflurane-specific calibrated vaporizer was used to administer 50% oxygen at a flow rate of 3 L/min (15). The loss and recovery of the righting reflex (LORR and RORR) were used as indicators of consciousness. LORR was defined as the inability of the rat to right itself within 30 s, while recovery was confirmed when the rat could turn onto all four limbs (25). After regaining the righting reflex, the rats were returned to their cages.
2.8 Rat pica model
Rats lack a central nervous system mechanism for controlling nausea and vomiting, which is primarily observed through pica behavior (26, 27). Kaolin was placed in a separate compartment of the food hopper 3 days before anesthesia to allow the rats to acclimate to its presence. Kaolin and food intake were measured 24 h after anesthesia. Following the experiment, the rats were housed in a clean laboratory at the Laboratory Animal Center of Fujian Medical University.
2.9 Statistical analysis
Statistical analyses of clinical data were conducted using SPSS 26.0 software (IBM Corp., Armonk, New York, United States). Descriptive statistics are presented as means ± standard deviations or percentages. Normality was assessed with the Shapiro–Wilk test, and normally distributed data were analyzed using the independent-samples t test. Non-normally distributed data are presented as the median [IQR] and analyzed using the Mann–Whitney U test. Correlation analysis was performed using Pearson’s correlation. Strain composition, α-diversity, β-diversity, and functional analyses were conducted using QIIME (V1.9.1) and R packages (ggplot2 [3.3.6], stats [4.2.1], car [3.1–0], vegan [2.6.4], and ape [5.6.2]). p-value < 0.05 was considered statistically significant.
3 Results
3.1 Demographic data
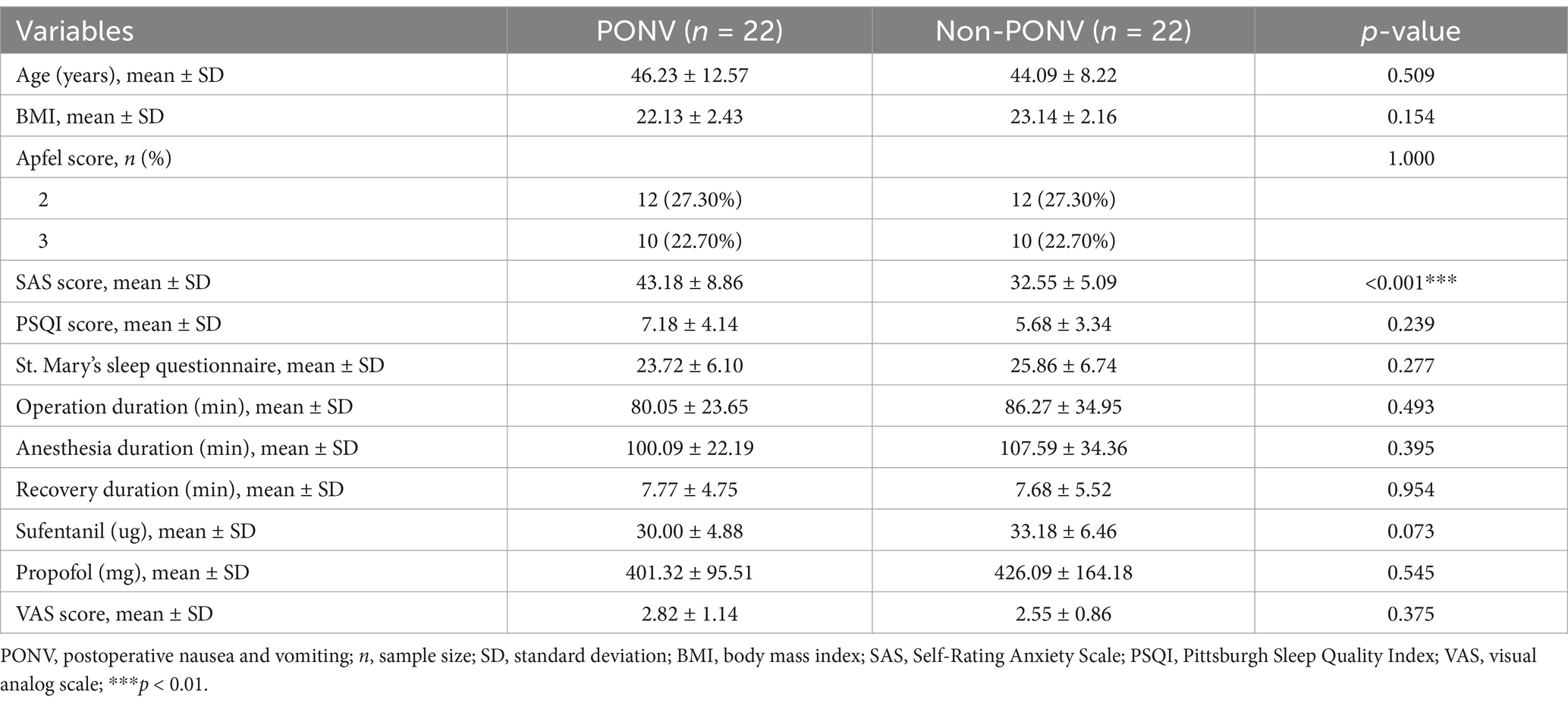
The study adhered to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines. As shown in Supplementary Figure 1, 82 patients were assessed for eligibility, and 72 were included in the study. Ten patients were excluded due to the lack of fecal samples, four had received antibiotics, six had gastrointestinal diseases, and eight refused to participate. Ultimately, 44 patients were included, with 22 assigned to the PONV group (experiencing nausea and vomiting) and the remaining patients assigned to the non-PONV group. The PONV group had a significantly higher mean SAS score compared to the non-PONV group (non-PONV: 32.55 ± 5.09, PONV: 43.18 ± 8.86, p < 0.001) (Figure 1A). There were no significant differences in other demographic factors, including age, BMI, Apfel score, PSQI score, St. Mary’s sleep questionnaire, VAS scores, and the duration of the operation, anesthesia, recovery, or the administration of sufentanil and propofol (Table 1).
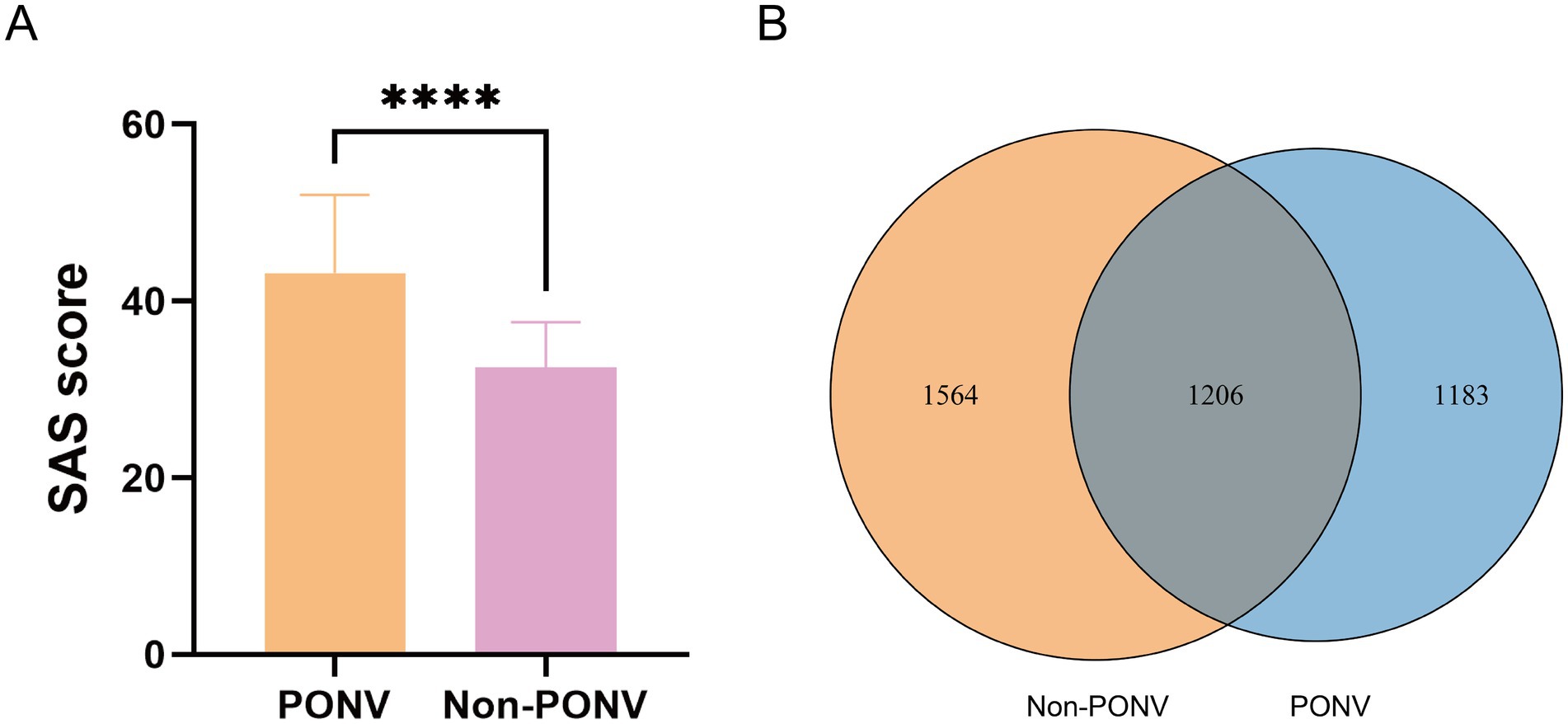
Figure 1. (A) Compared with the non-PONV group, the mean SAS score was significantly higher in the PONV group (non-PONV group vs. PONV group: 32.55 ± 5.09 vs. 43.18 ± 8.86, respectively, p < 0.0001). (B) As shown in the Venn diagram, a relatively low number of shared OTUs between the two groups, with 1,206 OTUs. The PONV group had a significantly lower gut microbiome abundance compared to the non-PONV group, with 2,389 OTUs.
3.2 Diversity analysis of the gut microbiota between the groups
The OTU Venn diagram revealed a relatively low number of shared OTUs between the two groups, with 1,206 OTUs. The PONV group had a significantly lower gut microbiome abundance compared to the non-PONV group, with 2,389 OTUs (Figure 1B). The α-diversity index, which measures species abundance and diversity, showed that the PONV group had significantly lower Chao1 and Shannon indexes, and a significantly higher Simpson index, compared to the non-PONV group (Figures 2A–C). These results indicate significant differences in α-diversity between the two groups, with lower microbiome abundance and diversity in the PONV group. To analyze β-diversity, principal coordinate analysis (PCoA) and partial least squares-discriminant analysis (PLS-DA) were performed, and PERMANOVA was applied. The results revealed significant differences in microbial community composition between the groups, with a dispersed distribution (p = 0.0495; Figures 2D,E).
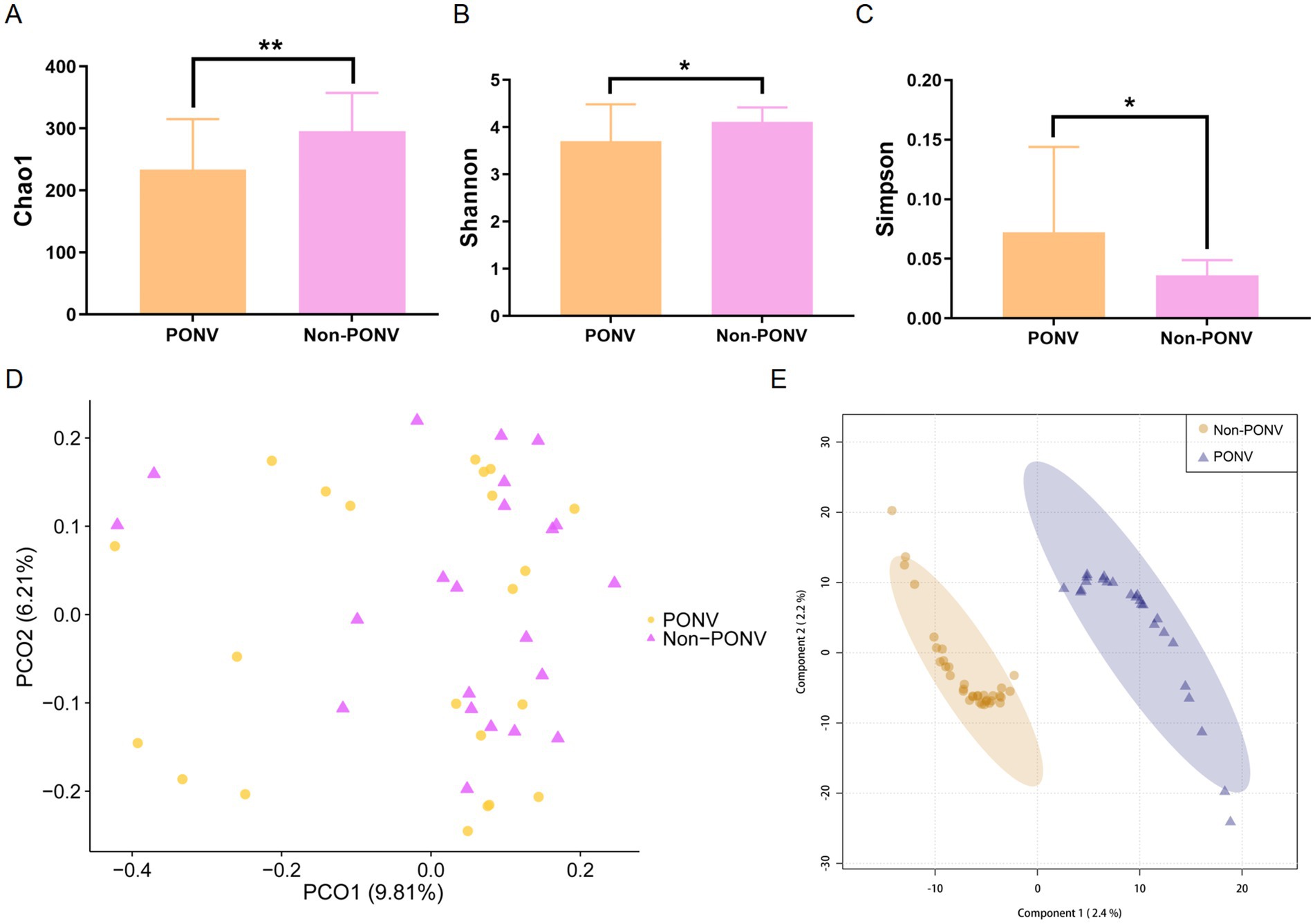
Figure 2. (A–C) α-diversity between the two groups as estimated by the Chao1, Shannon and Simpson indexes (p = 0.0071, p = 0.0283, p = 0.0254, respectively). (D,E) β-diversity reflecting the significant differences in community composition between the two groups (p = 0.0495).
3.3 Composition and absolute abundance of gut microbiota between the groups
At the phylum level, the PONV group exhibited a lower absolute abundance of Verrucomicrobia (p = 0.036) compared to the non-PONV group (Figures 3A,D). At the family level, Bifidobacteriaceae abundance was also lower in the PONV group than in the non-PONV group (p < 0.001) (Figures 3B,E). At the genus level, the non-PONV group showed higher levels of Acidaminococcus, Anaerovorax, Bifidobacterium, Bilophila, Oscillibacter, and Saccharibacteria (Figures 3C,F). These differences were further validated by LEfSe analysis using linear discriminant analysis (LDA), which suggested that Verrucomicrobia (phylum), Bifidobacteriales (order), Bifidobacteriaceae (family), Bifidobacterium (genus), and Bacteroides plebeius and Prabacteroides distasonis (species) may serve as key biomarkers differentiating the PONV and non-PONV groups (Figure 3G). Functional enrichment analysis revealed that the microbiota in the PONV group were significantly associated with pathways like lipoic acid metabolism, nitrotoluene degradation, and apoptosis (Figure 3H).
Figure 3. (A–F) The significant differences in gut microbiota between the two groups. At the phylum level, the PONV group had a lower absolute abundance of Verrucomicrobia than the non-PONV group. At the family level, the absolute abundance of Bifidobacteriaceae in the PONV group was lower than that in the non-PONV group. At the genus level, the non-PONV group had a higher absolute abundance of Acidaminococcus, Anaerovorax, Bifidobacterium, Bilophila, Oscillibacter and Saccharibacteria. (G) According to the LDA score size, the phylum Verrucomicrobia, the order Bifidobacteriales, the family Bifidobacteriaceae and the genus Bifidobacterium as well as the species Bacteroides plebeius and Parabacteroides distasonis may be the main biomarkers that lead to the difference between the PONV and non-PONV groups. (H) Enrichment of the different functions between the two groups. *p < 0.05, **p < 0.01, ****p < 0.0001.
3.4 Correlation analysis of gut microbiota and clinical data
The SAS score showed a positive correlation with the severity of PONV (r = 0.5448, p < 0.001). In contrast, the genera Bifidobacterium, Bilophila, and Oscillibacter were negatively correlated with PONV severity (r = −0.6919, p < 0.001; r = −0.3259, p = 0.030; r = −0.3034, p = 0.045, respectively; Figure 4A). Bilophila was positively correlated with age (r = 0.4594, p = 0.002), and Acidaminococcus was positively correlated with BMI (r = 0.3471, p = 0.021). Bifidobacterium and Oscillibacter had negative associations with the SAS scores (r = −0.4943, p < 0.001; r = −0.3099, p = 0.041, respectively), while Saccharibacteria was negatively associated with the VAS score (r = −0.3074, p = 0.042; Figure 4B). No correlations were found between age, BMI, Apfel score, PSQI score, St. Mary’s sleep questionnaire score, operation duration, sufentanil, VAS score, and the abundances of Acidaminococcus, Anaerovorax, and Saccharibacteria with PONV severity.
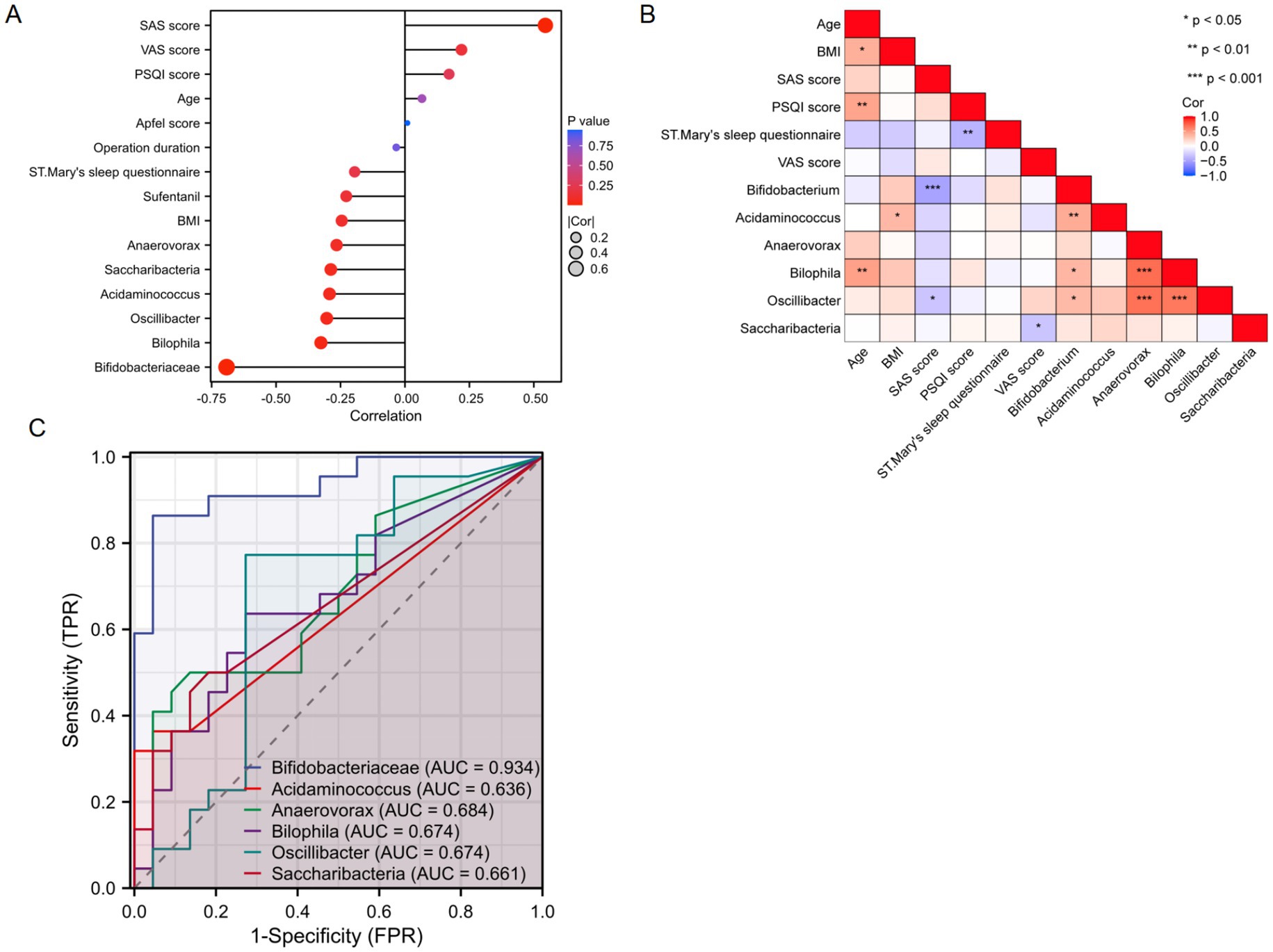
Figure 4. (A,B) Correlation analysis between the gut microbiota and clinical indicators. (C) ROC analysis of representative differential gut microorganisms for predicting PONV.
3.5 Potential predictive functions of gut microbiota
The predictive ability of representative gut microbiota for PONV was assessed using the receiver operating characteristic (ROC) curve, a common analytical method for evaluating model performance. The results indicated that Bifidobacterium, a key differential microbiota, could effectively predict PONV (AUC = 0.934, p < 0.01; Figure 4C).
3.6 PONV is mediated by gut microbiota in rats
Animal experiments were needed to verify that gut microbiota is one of the potential mechanisms of PONV. As shown in Figure 5A, fecal samples from PONV and non-PONV patients were transplanted into the gut tract of the rats. After 14 days of colonization, the rats were anesthetized with isoflurane for 100 min. Pica behavior was observed within 24 h after anesthesia to reflect nausea and vomiting of rats. The level of kaolin intake was similar between the two groups before anesthesia (Figure 5B). Rats that received feces from patients in the PONV group consumed significantly greater amounts of kaolin within 24 h after anesthesia compared to those received feces from patients in the non-PONV group (Figure 5C).
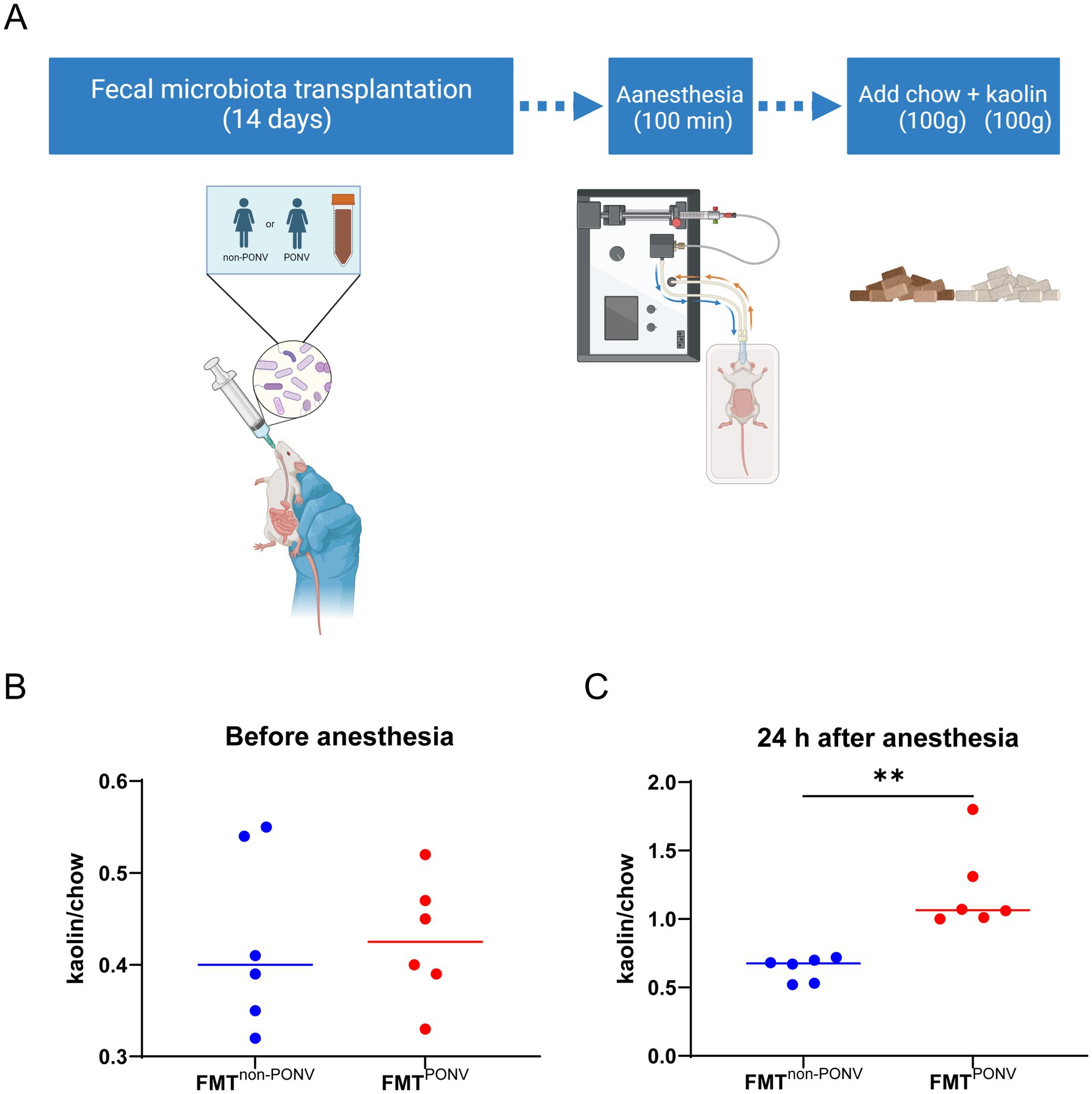
Figure 5. (A) Procedures for animal experiments; (B) The level of kaolin intake was similar between the two groups before anesthesia. (C) Rats that received feces from patients in the PONV group consumed significantly greater amounts of kaolin within 24 h after anesthesia compared to those received feces from patients in the non-PONV group.
4 Discussion
This study explored the connection between gut microbiota and PONV, revealing significant differences between female patients with and without PONV. We found a notable reduction in microbiota richness in PONV patients, along with distinct differences in community composition compared to non-PONV patients. Currently, no similar studies have been reported. Through fecal microbiota transplantation experiments, it was confirmed that the gut microbiota is a potential mechanism for the occurrence of PONV.
This study found that patients with PONV had higher anxiety scores compared to those without PONV. Several prospective studies have shown a link between preoperative anxiety and PONV, with heightened anxiety potentially serving as a predictor for PONV risk (28). Reducing anxiety and improving sleep quality could also help lower PONV incidence (29). Roh et al. (30) similarly found that patients who experienced PONV had higher preoperative anxiety scores. These findings align with our results. Previous research also indicated that PONV patients had higher Apfel scores (22), longer surgery durations (31) and greater use of sufentanil (32) than those without PONV. The discrepancies in our study may be attributed to the following factors: (1) The Apfel score was based on four independent risk factors: female sex, history of PONV and/or motion sickness, nonsmoking status, and postoperative opioid use. Due to our study’s inclusion criteria, all participants were nonsmoking females who did not use opioids post-surgery, and (2) all patients in our study underwent radical, straightforward thyroid cancer surgeries, with no significant difference in operation time between the two groups.
Bifidobacterium, a genus that mainly produces SCFAs such as acetic acid and lactic acid, which can improve gastrointestinal function, was negatively associated with the severity of PONV and the state of anxiety in our study. 5-HT enhances anxiety by acting on forebrain structures (33). Supplementation with Bifidobacterium reduced plasma and central 5-HT levels in individuals (34). The abundance of Bifidobacterium in people (35) or mice (36) with anxiety and depression is significantly reduced, which is consistent with findings from this study. Therefore, we consider that patients with a reduced abundance of Bifidobacterium are more likely to develop PONV, which may be related to the improvement of anxiety state by 5-HT reduction. Oscillospira is a gram-positive bacterium. From the metagenomic and metabolic profiles, the finding that Oscillospira possesses a butyrate kinase-mediated pathway can produce butyric acid (37). A variety of exogenous factors affect the abundance of Oscillospira, such as probiotics, heavy metals, naturally active products, pharmacological interventions, exercise and diet and diseases (38). The genus was found to be negatively associated with preoperative symptoms of anxiety in the present study, which is consistent with the results of many studies. Oscillibacter was also found to be negatively associated with the severity of PONV, and this may be related to the reuptake of 5-HT caused by these organisms (39). We performed functional prediction analysis for different species and found that the involvement of the lipoic acid metabolic pathway in PONV may be related to 5-HT. This further supported our previous conjecture. Freitas et al. (40) found that lipoic (also known as thioctic) acid, which inhibited the hyperactivity of the 5-HTergic neuronal system during pilocarpine-induced seizures, could normalize the levels of dopamine (DA) and 5-HT (39). Another study found that coadministration of lipoic acid and a 5-HT reuptake inhibitor improved anxiolytic and antidepressant responses (41). However, further studies are needed to clarify the role of the lipoic acid pathway in PONV.
Our study found that the preoperative anxiety state was positively correlated with the severity of PONV. Previous studies found a correlation between preoperative anxiety and PONV, but the correlation was weak (42), which was consistent with our results. However, another study found no correlation between anxiety and PONV (43). The differences in the above results may be due to the differences in anxiety assessment and anesthesia levels as well as medication and many other factors. Many studies suggest that the susceptibility to motion sickness decreases with age, so patients younger than 50 years old were considered to be more likely to develop PONV (44). Our study, however, found no correlation between age and the occurrence of PONV. This discrepancy may be due to the fact that the patients in our study were primarily from medium- and high-risk populations, where age might not have a significant impact on the development of PONV.
FMT is an extremely important and highly effective experimental approach for investigating host–microbe interactions. The humanized mouse model, which is established by transplanting single bacteria, multiple bacteria or flora into germ-free (GF) mice, or by transplanting human feces into GF mice after treatment, can achieve the transfer and translocation of intestinal flora between different animals. Subsequently, it can be used to explore the causal relationship between intestinal flora and disease phenotype, thus serving as an important method for studying the relationship between the human microbiome and disease (45, 46). In animal experiments, GF mice are ideal recipients for microbiota transplantation. By introducing specific gut microbiota at different developmental stages of GF mice, the underlying mechanisms and their effects on health and disease can be explored. However, the high price of sterile animals, strict feeding environment and technical requirements make it difficult to carry out experimental operation in ordinary laboratories. Therefore, most researchers tend to choose ABX, which has the effect of eliminating gut microbes and is cheap, to construct pseudo-germ-free mice to study the role of gut microbiota (47). As a result, many researchers use antibiotics (ABX) to create pseudo-germ-free mice, as they are more affordable and effective in eliminating gut microbes. Studies have shown that broad-spectrum ABX “cocktails” can eliminate up to 90% of intestinal microorganisms in mice (47, 48). In this study, pseudo-sterile rats were used as recipients for fecal transplants from patients in both the non-PONV and PONV groups. The results showed that pica occurred within 24 h after anesthesia in the FMTPONV group, effectively replicating the disease phenotype of postoperative nausea and vomiting.
TDespite the promising findings, our study had some limitations. Clinical trials to validate the role of Bifidobacterium in predicting and managing PONV were not conducted, and we plan to address this in future research. This study is based on a small sample size, which may not fully capture inter-individual variability and lacks population representativeness. Further validation with larger cohorts is needed to explore the underlying mechanisms and identify potential intervention targets in greater depth. Additionally, the study controlled for patient sex and surgical type, meaning the results are specific to nonsmoking female patients undergoing radical thyroid cancer surgery. Future studies should explore these mechanisms in more depth. Nonetheless, this preliminary study provides initial evidence of a link between gut microbiota and PONV.
5 Conclusion
The gut microbiota of patients in the non-PONV and PONV groups was analyzed using 16S absolute quantitative sequencing. The findings revealed significant differences in gut microbiota between the two groups, with six key microbial species showing distinct correlations with PONV occurrence. ROC curve analysis further demonstrated that Bifidobacterium could potentially predict PONV versus non-PONV status. The FMT experiment suggested a novel mechanism for PONV from the perspective of gut microbiota, providing a theoretical basis for predicting PONV based on preoperative gut microbiota. This aligns with the medical concept of enhanced recovery after surgery.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving humans were approved by the Ethics Review Committee of Fujian Provincial Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by the Experimental Animal Ethics Review and Use Committee of Fujian Medical University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YT: Writing – original draft, Writing – review & editing. XX: Writing – original draft, Writing – review & editing. YG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. YC: Conceptualization, Data curation, Investigation, Methodology, Resources, Supervision, Visualization, Writing – review & editing. XH: Conceptualization, Data curation, Methodology, Supervision, Validation, Visualization, Writing – review & editing. DD: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Writing – review & editing. XW: Formal analysis, Funding acquisition, Project administration, Resources, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the National Natural Science Foundation of China (82271238) awarded to XW and the QIHANG Funding of Fujian Medical University (2021QH2050) granted to YT.
Acknowledgments
We would like to thank Dev Sooranna from Imperial College London for his assistance in editing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1563329/full#supplementary-material
References
1. Hari, Y, Satomi, S, Murakami, C, Narasaki, S, Morio, A, Kato, T, et al. Remimazolam decreased the incidence of early postoperative nausea and vomiting compared to desflurane after laparoscopic gynecological surgery. J Anesth. (2022) 36:265–9. doi: 10.1007/s00540-022-03041-y
2. Shaikh, SI, Nagarekha, D, Hegade, G, and Marutheesh, M. Postoperative nausea and vomiting: a simple yet complex problem. Anesth Essays Res. (2016) 10:388–96. doi: 10.4103/0259-1162.179310
3. Cangemi, DJ, and Kuo, B. Practical perspectives in the treatment of nausea and vomiting. J Clin Gastroenterol. (2019) 53:170–8. doi: 10.1097/MCG.0000000000001164
4. Horn, CC. Why is the neurobiology of nausea and vomiting so important? Appetite. (2008) 50:430–4. doi: 10.1016/j.appet.2007.09.015
5. Grunberg, SM. Advances in the management of nausea and vomiting induced by non-cisplatin containing chemotherapeutic regimens. Blood Rev. (1989) 3:216–21. doi: 10.1016/0268-960X(89)90029-5
6. Babic, T, and Browning, KN. The role of vagal neurocircuits in the regulation of nausea and vomiting. Eur J Pharmacol. (2014) 722:38–47. doi: 10.1016/j.ejphar.2013.08.047
7. Li, N, Liu, L, Sun, M, Wang, R, Jin, W, Liu, C, et al. Predominant role of gut-vagus-brain neuronal pathway in postoperative nausea and vomiting: evidence from an observational cohort study. BMC Anesthesiol. (2021) 21:234. doi: 10.1186/s12871-021-01449-9
8. Xie, Z, Zhang, X, Zhao, M, Huo, L, Huang, M, Li, D, et al. The gut-to-brain axis for toxin-induced defensive responses. Cell. (2022) 185:4298–4316.e21. doi: 10.1016/j.cell.2022.10.001
9. Feng, Y, Zhang, M, Liu, Y, Yang, X, Wei, F, Jin, X, et al. Quantitative microbiome profiling reveals the developmental trajectory of the chicken gut microbiota and its connection to host metabolism. iMeta. (2023) 2:e105. doi: 10.1002/imt2.105
10. Vieira-Silva, S, Sabino, J, Valles-Colomer, M, Falony, G, Kathagen, G, Caenepeel, C, et al. Quantitative microbiome profiling disentangles inflammation- and bile duct obstruction-associated microbiota alterations across PSC/IBD diagnoses. Nat Microbiol. (2019) 4:1826–31. doi: 10.1038/s41564-019-0483-9
11. Rao, C, Coyte, KZ, Bainter, W, Geha, RS, Martin, CR, and Rakoff-Nahoum, S. Multi-kingdom ecological drivers of microbiota assembly in preterm infants. Nature. (2021) 591:633–8. doi: 10.1038/s41586-021-03241-8
12. Andriolo, IRL, Longo, B, de Melo, DM, de Souza, MM, Prediger, RD, and da Silva, LM. Gastrointestinal issues in depression, anxiety, and neurodegenerative diseases: a systematic review on pathways and clinical targets implications. CNS Neurol Disord Drug Targets. (2024) 23:1371–91. doi: 10.2174/0118715273289138240306050532
13. Sen, P, Molinero-Perez, A, O'Riordan, KJ, McCafferty, CP, O'Halloran, KD, and Cryan, JF. Microbiota and sleep: awakening the gut feeling. Trends Mol Med. (2021) 27:935–45. doi: 10.1016/j.molmed.2021.07.004
14. Gao, X, Cao, Q, Cheng, Y, Zhao, D, Wang, Z, Yang, H, et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc Natl Acad Sci USA. (2018) 115:E2960–e2969. doi: 10.1073/pnas.1720696115
15. Serbanescu, MA, Mathena, RP, Xu, J, Santiago-Rodriguez, T, Hartsell, TL, Cano, RJ, et al. General anesthesia alters the diversity and composition of the intestinal microbiota in mice. Anesth Analg. (2019) 129:e126–9. doi: 10.1213/ANE.0000000000003938
16. Shakhsheer, BA, Versten, LA, Luo, JN, Defazio, JR, Klabbers, R, Christley, S, et al. Morphine promotes colonization of anastomotic tissues with collagenase—producing enterococcus faecalis and causes leak. J Gastrointest Surg. (2016) 20:1744–51. doi: 10.1007/s11605-016-3237-5
17. DREAMS Trial Collaborators and West Midlands Research Collaborative. Dexamethasone versus standard treatment for postoperative nausea and vomiting in gastrointestinal surgery: randomised controlled trial (DREAMS trial). BMJ. (2017) 357:j1455. doi: 10.1136/bmj.j1455
18. Dunstan, DA, and Scott, N. Norms for Zung's self-rating anxiety scale. BMC Psychiatry. (2020) 20:90. doi: 10.1186/s12888-019-2427-6
19. Zitser, J, Allen, IE, Falgàs, N, Le, MM, Neylan, TC, Kramer, JH, et al. Pittsburgh sleep quality index (PSQI) responses are modulated by total sleep time and wake after sleep onset in healthy older adults. PLoS One. (2022) 17:e0270095. doi: 10.1371/journal.pone.0270095
20. Murphy, F, Bentley, S, Ellis, BW, and Dudley, H. Sleep deprivation in patients undergoing operation: a factor in the stress of surgery. Br Med J. (1977) 2:1521–2. doi: 10.1136/bmj.2.6101.1521-a
21. He, S, Renne, A, Argandykov, D, Convissar, D, and Lee, J. Comparison of an emoji-based visual analog scale with a numeric rating scale for pain assessment. JAMA. (2022) 328:208–9. doi: 10.1001/jama.2022.7489
22. Apfel, CC, Läärä, E, Koivuranta, M, Greim, CA, and Roewer, N. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. (1999) 91:693–700. doi: 10.1097/00000542-199909000-00022
23. Josefsdottir, KS, Baldridge, MT, Kadmon, CS, and King, KY. Antibiotics impair murine hematopoiesis by depleting the intestinal microbiota. Blood. (2017) 129:729–39. doi: 10.1182/blood-2016-03-708594
24. Zhao, L, Huang, Y, Lu, L, Yang, W, Huang, T, Lin, Z, et al. Saturated long-chain fatty acid-producing bacteria contribute to enhanced colonic motility in rats. Microbiome. (2018) 6:107. doi: 10.1186/s40168-018-0492-6
25. Guo, N, Zhang, Z, Han, C, Chen, L, Zheng, X, Yu, K, et al. Effects of continuous intravenous infusion of propofol on intestinal flora in rats. Biomed Pharmacother. (2021) 134:111080. doi: 10.1016/j.biopha.2020.111080
26. Liao, X, Ye, B, Hu, W, Han, J, Zhao, Y, Dai, Y, et al. Xiaobanxia decoction alleviates chemotherapy-induced nausea and vomiting by inhibiting GSDME-mediated pyroptosis. J Ethnopharmacol. (2024) 318:116970. doi: 10.1016/j.jep.2023.116970
27. Nakajima, S. Pica caused by emetic drugs in laboratory rats with kaolin, gypsum, and lime as test substances. Physiol Behav. (2023) 261:114076. doi: 10.1016/j.physbeh.2023.114076
28. Laufenberg-Feldmann, R, Müller, M, Ferner, M, Engelhard, K, and Kappis, B. Is 'anxiety sensitivity' predictive of postoperative nausea and vomiting?: a prospective observational study. Eur J Anaesthesiol. (2019) 36:369–74. doi: 10.1097/EJA.0000000000000979
29. Lu, D, Wang, Y, Zhao, T, Liu, B, Ye, L, Zhao, L, et al. Successful implementation of an enhanced recovery after surgery (ERAS) protocol reduces nausea and vomiting after infratentorial craniotomy for tumour resection: a randomized controlled trial. BMC Neurol. (2020) 20:150. doi: 10.1186/s12883-020-01699-z
30. Hadzic, A, Arliss, J, Kerimoglu, B, Karaca, PE, Yufa, M, Claudio, RE, et al. A comparison of infraclavicular nerve block versus general anesthesia for hand and wrist day-case surgeries. Anesthesiology. (2004) 101:127–32. doi: 10.1097/00000542-200407000-00020
31. Wallenborn, J, Gelbrich, G, Bulst, D, Behrends, K, Wallenborn, H, Rohrbach, A, et al. Prevention of postoperative nausea and vomiting by metoclopramide combined with dexamethasone: randomised double blind multicentre trial. BMJ. (2006) 333:324. doi: 10.1136/bmj.38903.419549.80
32. Bhakta, P, Karim, HMR, O'Brien, B, and Mugawar, M. Comment on: "impact of opioid-free anaesthesia on postoperative nausea, vomiting and pain after gynaecological laparoscopy—a randomised controlled trial". J Clin Anesth. (2021) 75:110492. doi: 10.1016/j.jclinane.2021.110492
33. Fernandez, SP, and Gaspar, P. Investigating anxiety and depressive-like phenotypes in genetic mouse models of serotonin depletion. Neuropharmacology. (2012) 62:144–54. doi: 10.1016/j.neuropharm.2011.08.049
34. Kao, AC, Spitzer, S, Anthony, DC, Lennox, B, and Burnet, PWJ. Prebiotic attenuation of olanzapine-induced weight gain in rats: analysis of central and peripheral biomarkers and gut microbiota. Transl Psychiatry. (2018) 8:66. doi: 10.1038/s41398-018-0116-8
35. Okubo, R, Koga, M, Katsumata, N, Odamaki, T, Matsuyama, S, Oka, M, et al. Effect of bifidobacterium breve A-1 on anxiety and depressive symptoms in schizophrenia: a proof-of-concept study. J Affect Disord. (2019) 245:377–85. doi: 10.1016/j.jad.2018.11.011
36. Jang, HM, Lee, KE, and Kim, DH. The preventive and curative effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on immobilization stress-induced anxiety/depression and colitis in mice. Nutrients. (2019) 11:819. doi: 10.3390/nu11040819
37. Zhang, S, Wu, P, Tian, Y, Liu, B, Huang, L, Liu, Z, et al. Gut microbiota serves a predictable outcome of short-term low-carbohydrate diet (LCD) intervention for patients with obesity. Microbiol Spectr. (2021) 9:e0022321. doi: 10.1128/Spectrum.00223-21
38. Konikoff, T, and Gophna, U. Oscillospira: a central, enigmatic component of the human gut microbiota. Trends Microbiol. (2016) 24:523–4. doi: 10.1016/j.tim.2016.02.015
39. Shen, W, Tao, Y, Zheng, F, Zhou, H, Wu, H, Shi, H, et al. The alteration of gut microbiota in venlafaxine-ameliorated chronic unpredictable mild stress-induced depression in mice. Behav Brain Res. (2023) 446:114399. doi: 10.1016/j.bbr.2023.114399
40. de Freitas, RM, de Oliveira Silva, F, Saldanha, GB, and Jordán, J. Lipoic acid alters amino acid neurotransmitters content in rat hippocampus after pilocarpine-induced seizures. Fundam Clin Pharmacol. (2011) 25:485–492. doi: 10.1111/j.1472-8206.2010.00862.x
41. Abdelkader, NF, El-Batal, AI, Amin, YM, Hawas, AM, Hassan, SHM, and Eid, NI. Neuroprotective effect of gold nanoparticles and alpha-Lipoic acid mixture against radiation-induced brain damage in rats. Int J Mol Sci. (2022) 23:640. doi: 10.3390/ijms23179640
42. Van den Bosch, JE, Moons, KG, Bonsel, GJ, and Kalkman, CJ. Does measurement of preoperative anxiety have added value for predicting postoperative nausea and vomiting? Anesth Analg. (2005) 100:1525–32. doi: 10.1213/01.ANE.0000149325.20542.D4
43. Kovac, AL. Postoperative nausea and vomiting in pediatric patients. Paediatr Drugs. (2021) 23:11–37. doi: 10.1007/s40272-020-00424-0
44. Andrew, BY, Holmes, R, Taicher, BM, and Habib, AS. The Association of Guideline-Directed Prophylaxis with Postoperative Nausea and Vomiting in adult patients: a single-center, retrospective cohort study. Anesth Analg. (2024) 139:1006–16. doi: 10.1213/ANE.0000000000006855
45. Bokoliya, SC, Dorsett, Y, Panier, H, and Zhou, Y. Procedures for fecal microbiota transplantation in murine microbiome studies. Front Cell Infect Microbiol. (2021) 11:711055. doi: 10.3389/fcimb.2021.711055
46. Wang, JW, Kuo, CH, Kuo, FC, Wang, YK, Hsu, WH, Yu, FJ, et al. Fecal microbiota transplantation: review and update. J Formos Med Assoc. (2019) 118:S23–s31. doi: 10.1016/j.jfma.2018.08.011
47. Lundberg, R, Toft, MF, August, B, Hansen, AK, and Hansen, CH. Antibiotic-treated versus germ-free rodents for microbiota transplantation studies. Gut Microbes. (2016) 7:68–74. doi: 10.1080/19490976.2015.1127463
Keywords: gut microbiota, 16S absolute quantitative sequencing, postoperative nausea and vomiting, Bifidobacterium , prevention
Citation: Tang Y, Xie X, Guo Y, Chen Y, Huang X, Dai D and Wu X (2025) Exploring correlation between preoperative gut microbiota and PONV using 16S absolute quantitative sequencing: a prospective observational study. Front. Med. 12:1563329. doi: 10.3389/fmed.2025.1563329
Edited by:
Zhangran Chen, Xiamen University, ChinaReviewed by:
Veronica Ueckermann, University of Pretoria, South AfricaChristian Bohringer, UC Davis Medical Center, United States
Copyright © 2025 Tang, Xie, Guo, Chen, Huang, Dai and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaodan Wu, d3hpYW9kYW5Ac2luYS5jb20=
†These authors have contributed equally to this work
 Yijie Tang
Yijie Tang Xiyuan Xie
Xiyuan Xie Yu Guo
Yu Guo Yu Chen
Yu Chen Xiaodan Wu
Xiaodan Wu