- 1Neurology Department of Perpignan Hospital, Perpignan, France
- 2Commission of Clinical Research and Innovation, Perpignan, France
- 3Regional Health Agency of Occitanie, Montpellier, France
- 4Clinique Beausoleil, Montpellier, France
- 5Neurology Department, University Hospital Hassan II, University of Fes, Fes, Morocco
- 6Department of Intensive Care, Montreuil Hospital, Montreuil, France
- 7Department of Visceral and Digestive Surgery, Monastir University Hospital, University of Monastir, Monastir, Tunisia
Background: Patients with atrial fibrillation and a history of intracranial hemorrhage (ICH) face a dilemma when resuming anticoagulation therapy due to the risk of ICH recurrence versus the need for Ischemic stroke (IS) prevention. This study aims to evaluate the safety and efficacy of direct oral anticoagulants (DOAC) compared to no therapy or antiplatelets in these patients.
Methods: We conducted a systematic review and meta-analysis following PRISMA 2020 guidelines. Electronic searches were performed in multiple databases (Cochrane, PubMed, Web of Science, Embase, Google Scholar, Scopus) up to March 1, 2024. We included randomized controlled trials (RCTs) and controlled clinical trials (CCTs) involving patients with atrial fibrillation and prior ICH. Studies compared the group with no therapy or antiplatelets (no-DOAC group). Outcomes assessed included mortality, IS, ICH recurrence, and major bleeding events.
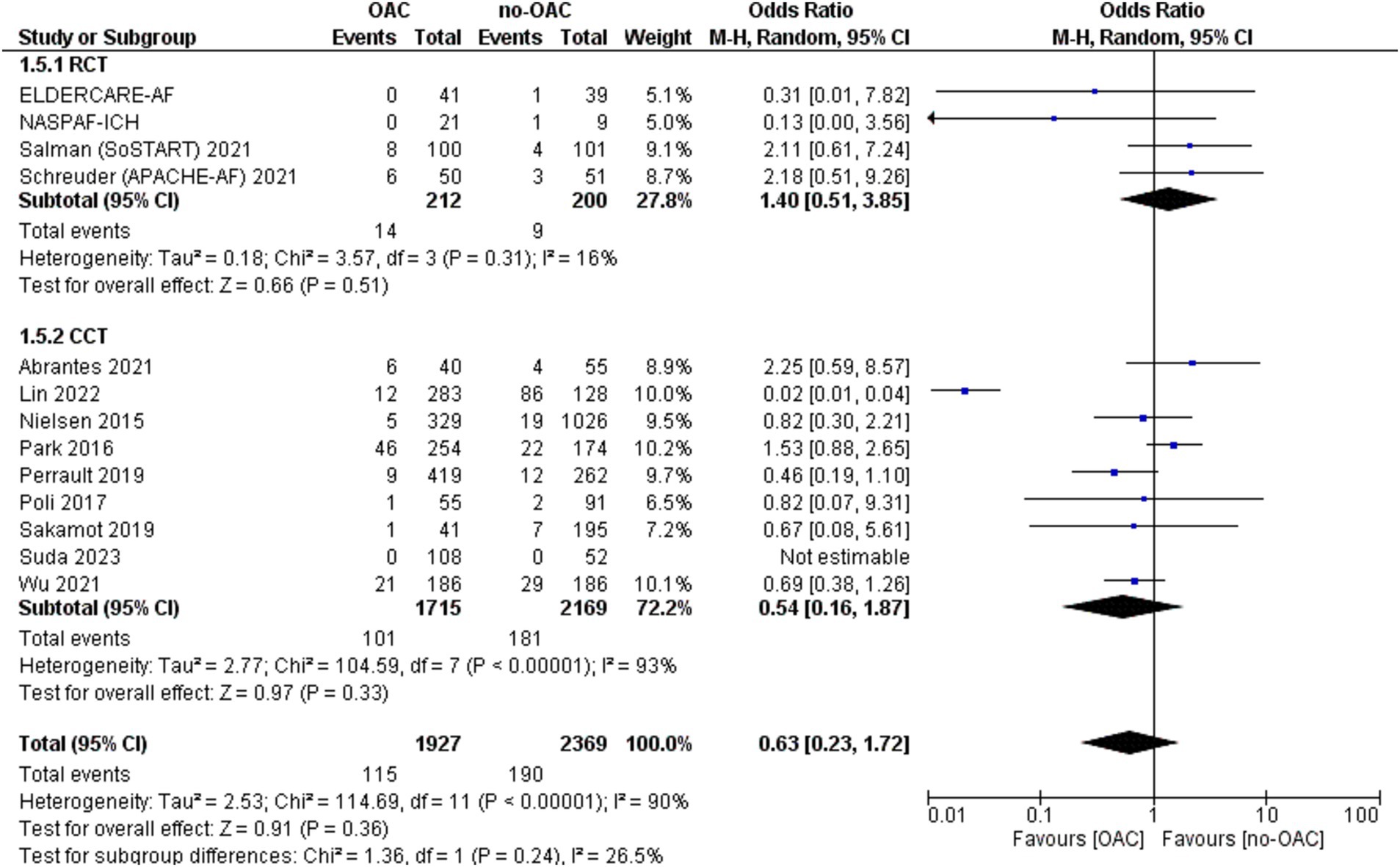
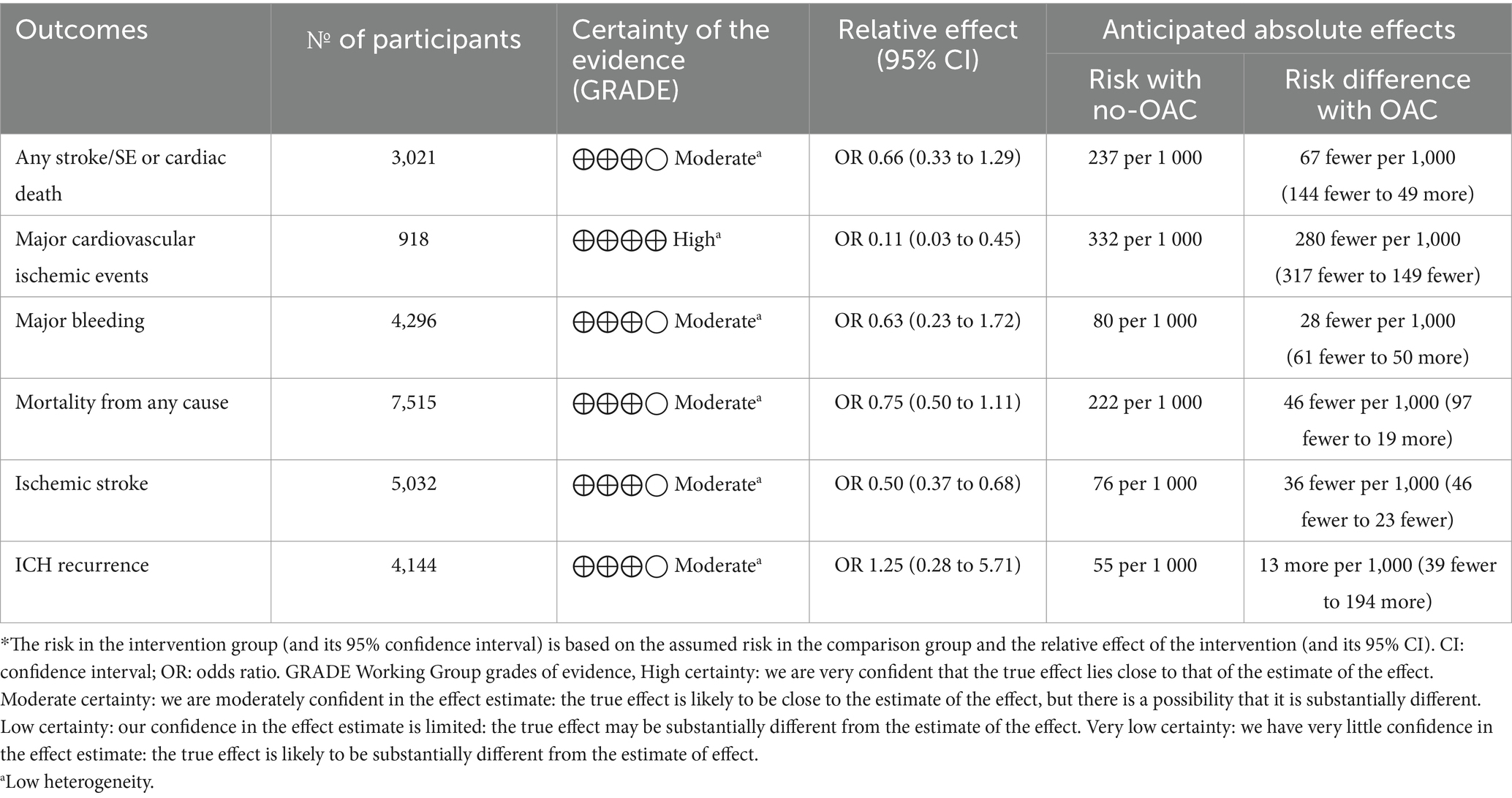
Results: Fifteen studies (8,318 patients) met the inclusion criteria, including 2,226 patients in the DOAC group and 5,936 in the no-OAC group. The major cardiovascular ischemic event was significantly lower in the DOAC group [OR = 0.11; CI 95% (0.03, 0.45); p = 0.002]. Ischemic stroke was lower in the DOAC group [OR = 0.53, 95% CI (0.39–0.72), p < 0.001]. There was no difference in ICH recurrence [OR = 1.25, 95% CI (0.28–5.71), p = 0.77] or major bleeding [OR = 0.63, 95% CI (0.23–1.72), p = 0.36]. Mortality rates were similar between groups [OR = 0.75, 95% CI (0.50–1.11), p = 0.15], while Heterogeneity was low for most outcomes.
Conclusion: DOACs appear to reduce the risk of IS without increasing mortality or major bleeding in patients with atrial fibrillation and prior ICH. However, the risk of ICH recurrence remains uncertain. These findings suggest a potential role for DOACs in this high-risk population, but further RCTs are needed to confirm these results.
Systematic review registration: Identifier CRD42024587511.
Introduction
Survivors of intracranial hemorrhage (ICH) with atrial fibrillation (AF) face the highest risk of major adverse cardiovascular events and notably ischemic stroke (IS), with their risk appearing to exceed that predicted by the CHA2DS2-VASc score for individuals with AF who have not experienced a prior ICH (1, 2). As a result, preventing recurrent IS in patients with AF and a history of ICH is a key priority in cerebrovascular care. Closing the auricle can be a favored alternative for these patients (3–5), but in current practice, this is not retained or contraindicated in many cases, and the choice is then limited between resuming anticoagulant treatment, an antiplatelet agent, or therapeutic abstention. Therefore, oral anticoagulation decreases the risk of IS in individuals with AF by nearly two-thirds compared to controls, despite an increased risk of bleeding, while antiplatelet therapy offers a lesser degree of risk reduction (6, 7). However, individuals with a history of intracranial hemorrhage were excluded from the trials that demonstrated these benefits. In addition, adjusted-dose warfarin is effective in preventing strokes in these patients. Its use is constrained by its narrow therapeutic range, interactions with food and other medications, the need for lifelong coagulation monitoring, and mainly, a high risk of both intracranial and systemic bleeding (7). Compared with warfarin, direct oral anticoagulants (DOACs) may be linked to a reduction in stroke or systemic embolic events (8) but, above all, demonstrated a decreased risk of intracranial hemorrhage (6–11). Evaluating the risk–benefit profile of DOACs is essential for patients with AF and a history of intracranial hemorrhage (ICH), as these individuals face a heightened risk of both recurrent stroke and bleeding complications from anticoagulation therapy, especially ICH.
So, this study aims to evaluate the safety and efficacy of DOAC compared to no therapy or antiplatelets in these patients.
Methods
We conducted this meta-analysis following the PRISMA 2020 (Preferred Reporting Items for Systematic Review and Meta-analysis) guidelines (12). To evaluate its quality, we employed the AMSTAR 2 (A Measurement Tool to Assess systematic Reviews) tool (13). The study protocol was duly registered in PROSPERO under the identification number CRD42024587511.
Electronic database searches
We performed a bibliographic electronic literature search and trial registries without language restrictions on March 1st, 2024. The search included multiple databases: the Cochrane Library, PubMed, Web of Science, Embase, Google Scholar, and Scopus. The search strategy included the following keywords: “Randomized Controlled Trials” AND “clinical controlled trials” AND “intracranial hemorrhage” AND “oral anticoagulation” AND “atrial fibrillation.” To identify relevant clinical trials, we manually reviewed the retrieved articles’ reference lists to find additional trials. The research strategies in the different databases are listed in Supplementary File 1.
Eligibility criteria
Studies
We included only RCTs and CCTs, including assigned patients with AF and spontaneous intracranial hemorrhage to long-term use or not of an oral anticoagulant to prevent cardiovascular events. If a subgroup of eligible patients was reported, we retained them for analysis. We excluded review articles, clinical control trials, non-comparative studies, letters to editors, editorials, and case series.
Population
The study focused on adults of any gender with AF and a history of spontaneous intracranial hemorrhage (ie, intraventricular hemorrhage, intracerebral hemorrhage, non-aneurysmal subarachnoid hemorrhage).
Intervention group
The use of oral anticoagulants was the intervention group (OAC group).
Control group
The non-use of oral anticoagulants, or use of antiplatelet or placebo was the control group (no-OAC group).
Outcomes
The different outcomes assessed in our study were mortality, cardiovascular mortality, ischemic complications, and bleeding. Cardiovascular mortality was considered if it occurred within 30 days after the onset of a cardiovascular event. We considered the definition of major bleeding proposed by the International Society on Thrombosis and Haemostasis, which includes fatal bleeding, symptomatic bleeding in a critical area or organ (such as intracranial bleeding), bleeding resulting in a hemoglobin drop of ≥2 g/dL, or requiring transfusion of ≥2 units of blood.
Study selection
Following independent literature searches conducted by two authors, all abstracts were independently reviewed. The inclusion criteria considered RCTs and CCTs. The full texts of studies that met these criteria were recovered and any disagreements were resolved with a third author. We included ongoing RCTs if they shared the results of a group or subgroup of included patients.
Evaluation of study quality and risk of bias
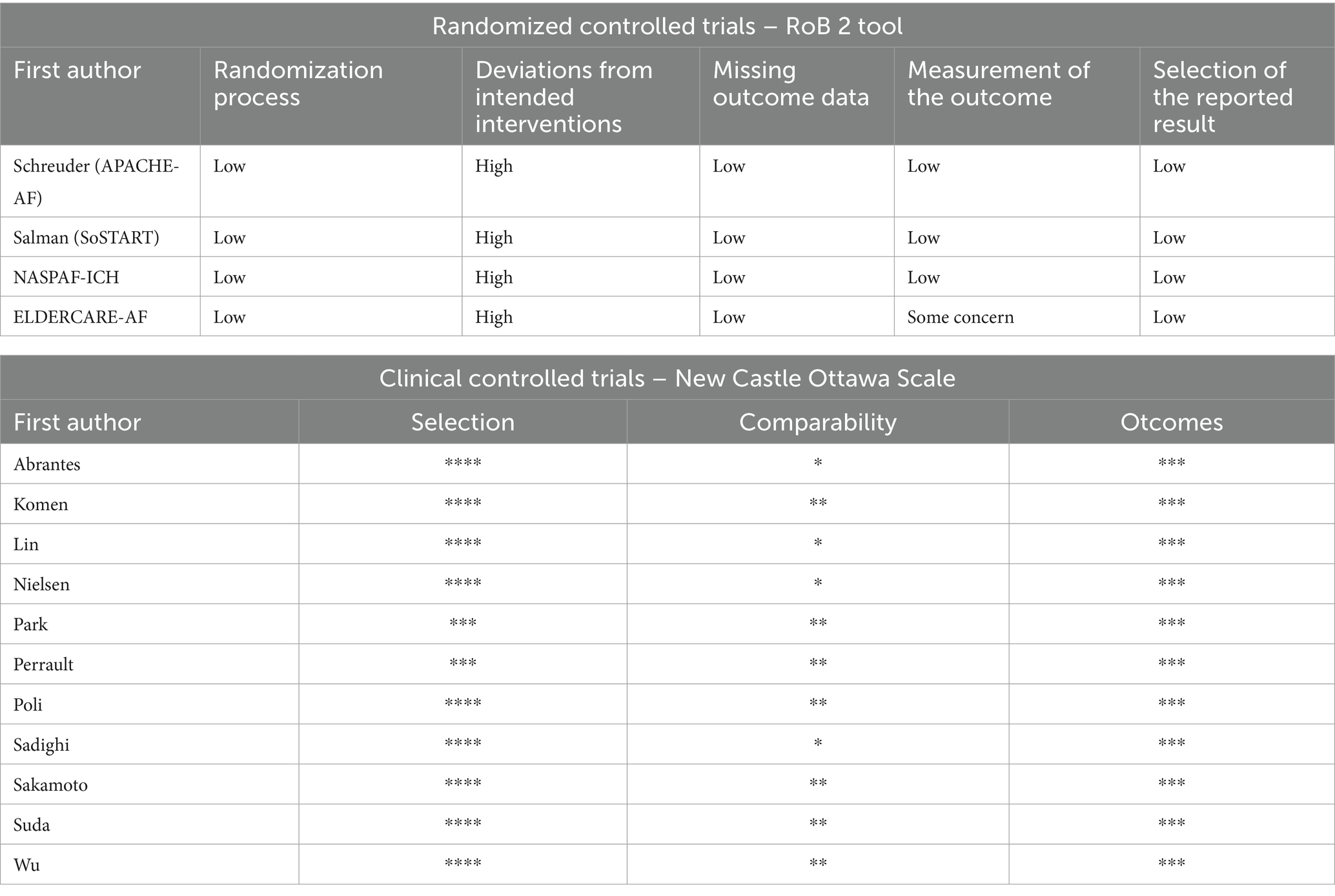
Two authors independently evaluated the selected studies. For the different retained RCTs, we used the Statement of Revision of Consolidated Standards of Reporting Trials (CONSORT) (14). Studies scoring below 13 out of 25 were excluded due to fair quality. The risk of bias was assessed using the Cochrane tool for bias assessment (RoB2) (15). For the retained CCTs, we used the Newcastle-Ottawa scale (NOS) (16) and the Methodological Index For Non-Randomized Studies (MINORS) scale (17).
Missing data
The authors were contacted by email in the case of:
• Unclear bias domains or missing primary outcome.
• Specific data extraction or additional analysis is required to obtain an outcome.
Information was extracted from the figures if the data were not numerically reported.
Handling continuous data
Continuous data were analyzed using the Cochrane Collaboration 5.3.5 statistical package Review Manager for meta-analysis (18). When the mean and standard deviation (SD) were not provided, these were estimated from the interquartile range (IQR) and median, following the formula described by Hozo et al. (19).
Assessment of heterogeneity
To assess heterogeneity, three strategies were used:
1. The Cochrane Chi2 test (Q test), Tau2, is the variance of true effects (20).
2. Graphical exploration with funnel plots (21).
3. Sensitivity analysis with a subgroup analysis when applicable.
Summary of findings
Two authors independently evaluated the certainty of evidence using The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) (22). Factors considered included study limitations, constancy of effect, imprecision, indirectness, and publication bias. Certainty of evidence was classified as high, moderate, low, or very low. Criteria to improve certainty included a large effect, dose–response gradient, and plausible confounding effect. The Cochrane Handbook for Systematic Reviews of Interventions (Sections 8.5 and 8.7, and Chapters 11 and 12) and GRADEpro GDT software were used to prepare a summary of Findings’ tables, providing explanations for downgrading or upgrading certainty using footnotes with comments.
Evaluation of effect size
Meta-analysis was performed using the RevMan 5.3.5 statistical package from the Cochrane Collaboration (23). The standard mean difference (SMD) was selected as the effective measure for continuous data, while odds ratios (OR) with 95% confidence intervals (95% CI) were calculated for dichotomous variables. A random-effects model was applied, with significance set at 0.05.
Results
Literature search results
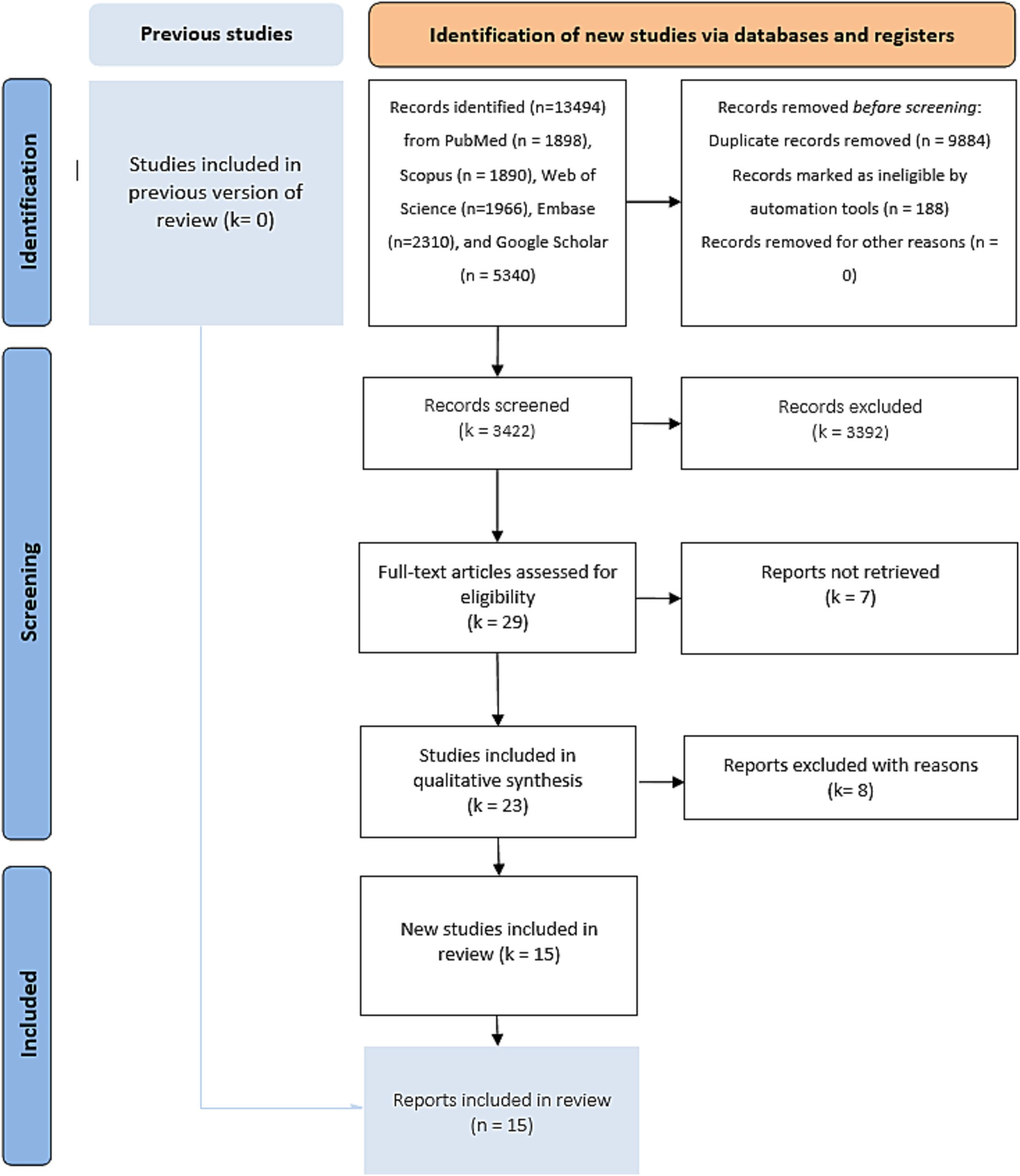
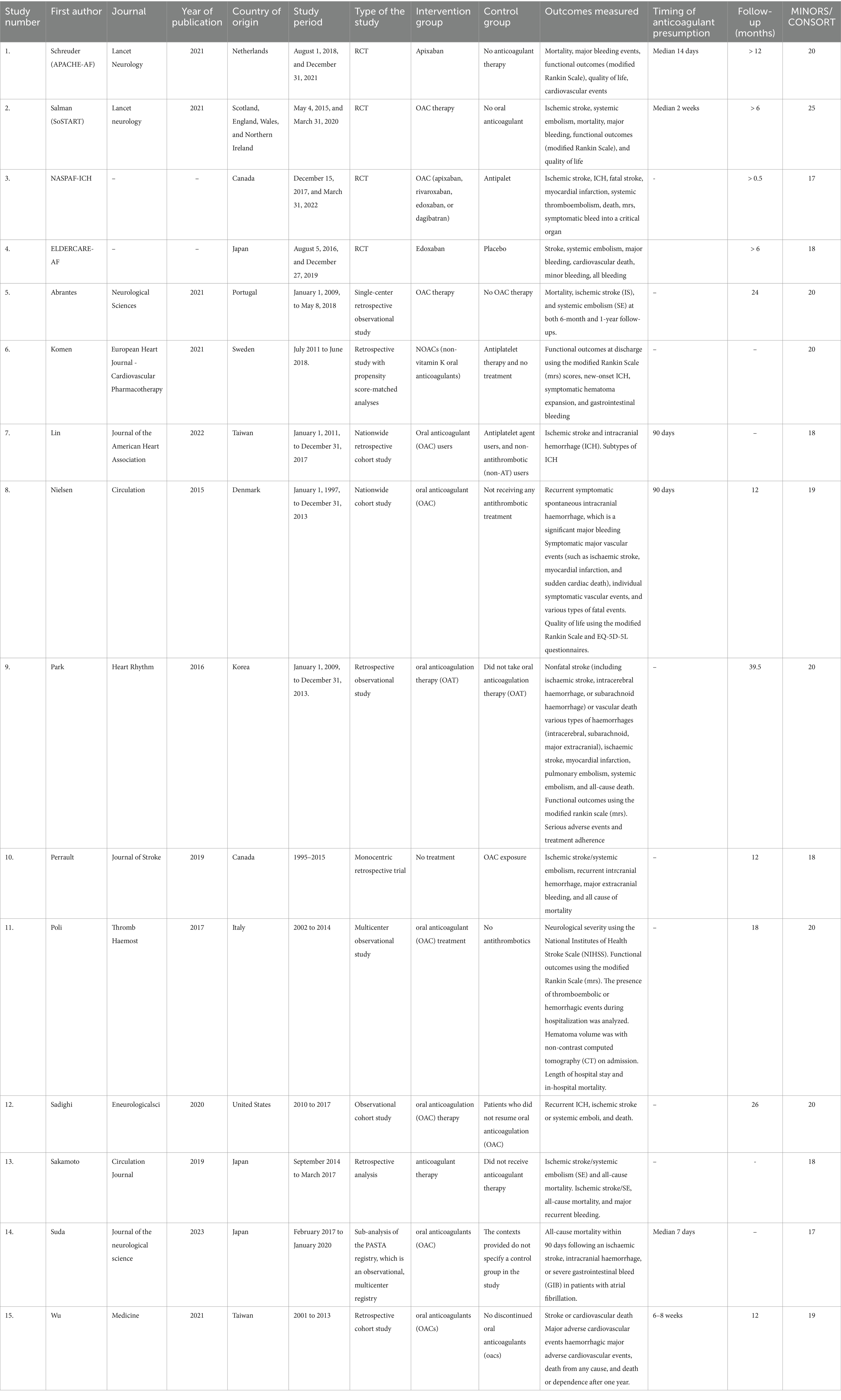
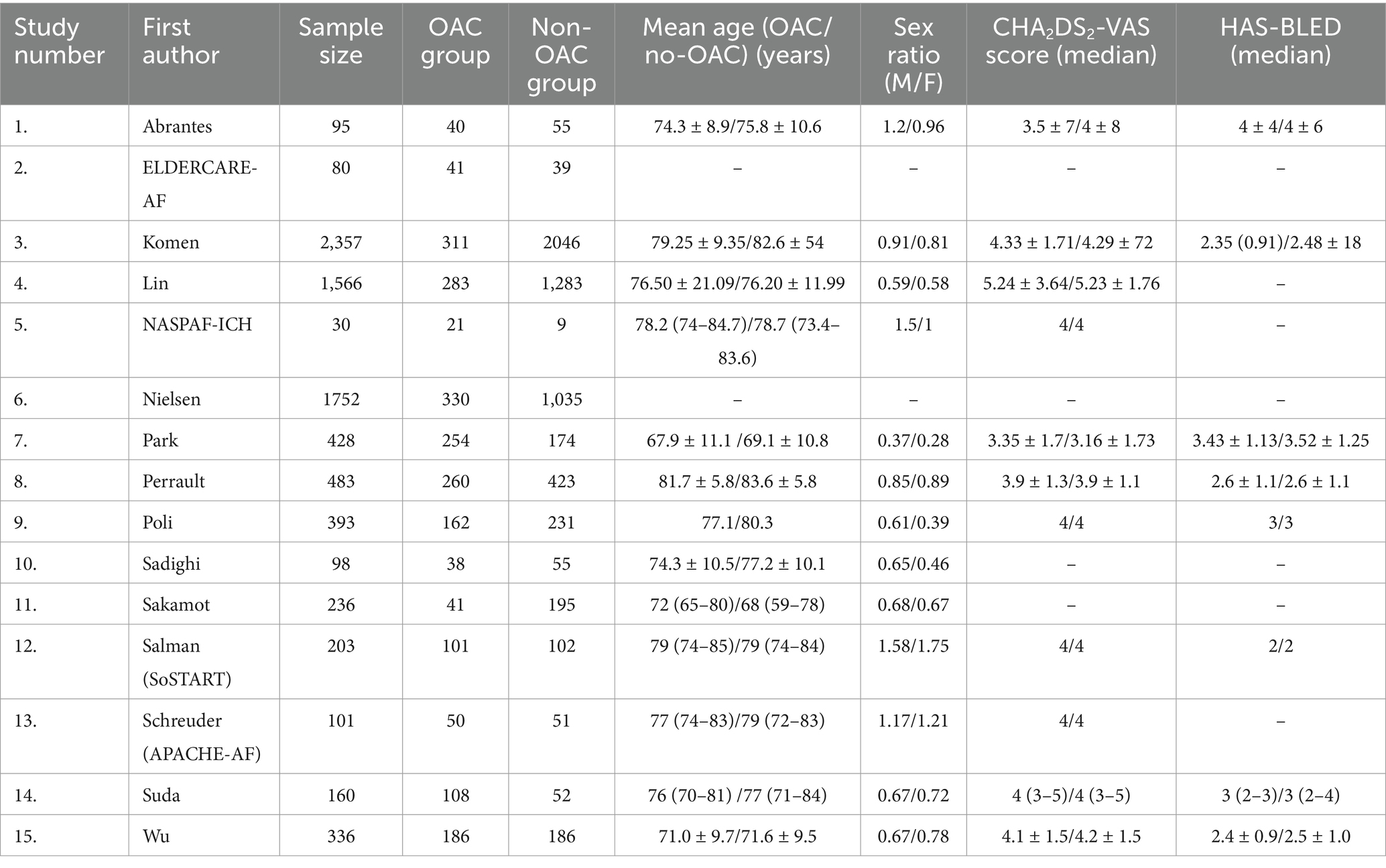
After bibliographic research, we screened 13,483 publications. We found 23 potentially eligible studies (Figure 1). We retained 15 studies after assessing the different available full texts. Seven studies were excluded for the following reasons: one systematic review and meta-analysis (24), three studies because the control group was the percutaneous left atrial appendage (3–5), and three studies because the control group was the use of vitamin K antagonists (8–11). Three RCTs were eligible, but the recruitment of patients was ongoing (ASPIRE “NCT03907046” with an expected completion in April 2027, ENRICH-AF “NCT03950076” with an anticipated completion in November 2026, and STROKECLOSE “NCT02830152” with a scheduled study completion in May 2030). Two studies completed patient recruitment, and the follow-up is ongoing (STATICH “NCT03186729” with an estimated primary completion date of December 2026 and A3ICH “NCT03243175” with a primary completion date of June 2023, but no results have been posted yet, and the full study is expected to conclude in 2031). Of the 15 studies included in this systematic review and meta-analysis, two were published RCTs (25, 26), two were RCTs with shared available data in the COCROACH prospective individual participant data meta-analysis of RCTs (26), and 11 were CCTs (27–37). The list of the retained studies and the demographic data of these studies were reported in Tables 1, 2, respectively. The retained studies were published between 2015 and 2023 and conducted between 1995 and 2022. These studies included 8,168 patients: 2226 in the OAC group and 5,936 in the no-OAC group. The mean age ranged from 67.9 to 81.7 years in the OAC group and from 69.1 to 83.6 years in the no-OAC group.
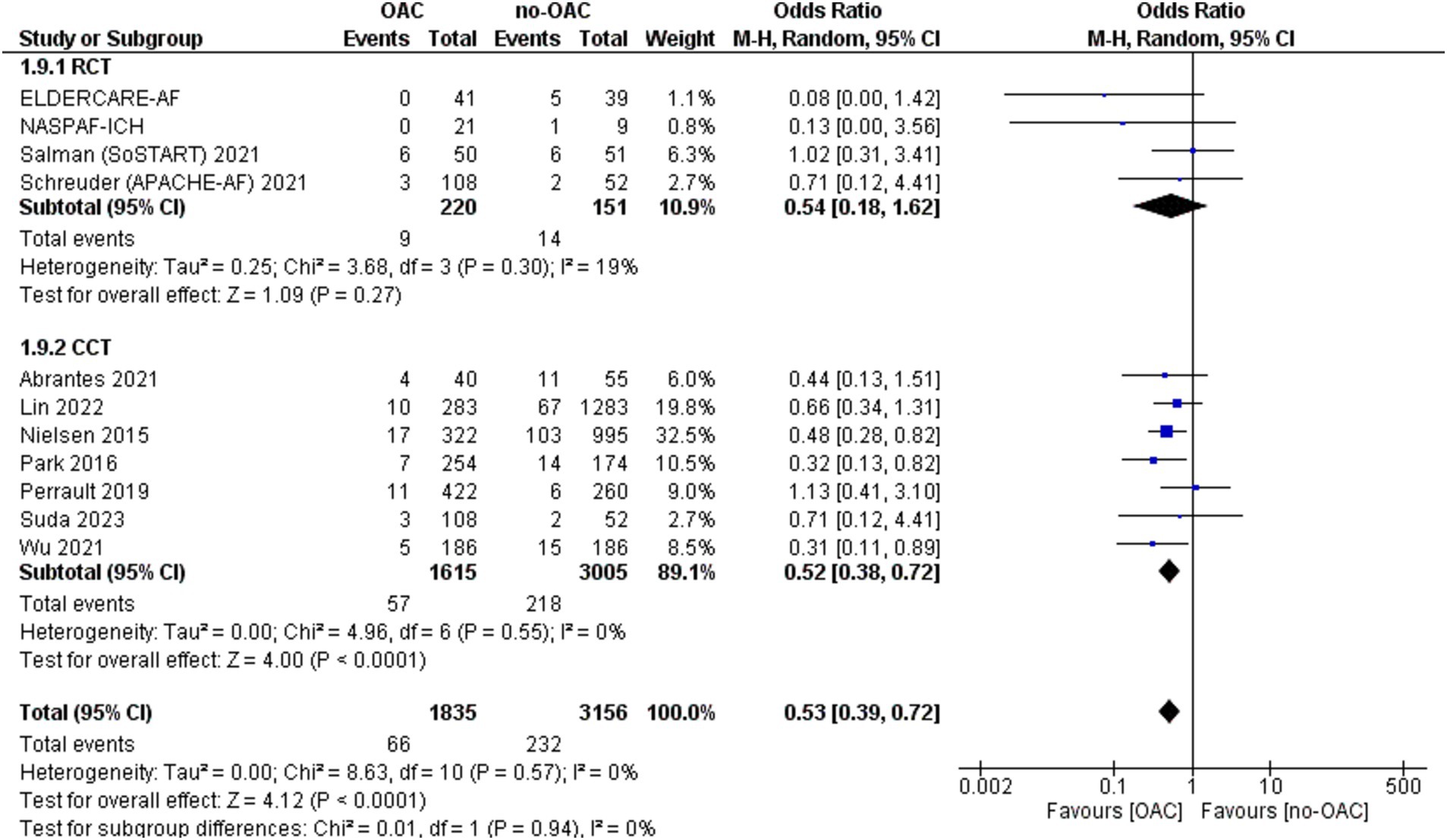
Ischemic stroke
Ischemic stroke was reported in 11 included studies (Figure 2). It was counted in 66 out of 1835 patients in the OAC group and 232 out of 3,156 patients in the no-OAC group. There was a lower rate of ischemic stroke in the OAC group [OR = 0.53; CI 95% (0.39, 0.72); p < 0.001]. In the subgroup analysis, there was no difference in the RCT subgroup [OR = 0.54; CI 95% (0.18, 1.62); p = 0.72] and statistically lower ischemic stroke rate in the CCT subgroup [OR = 0.52; CI 95% (0.38, 0.72); p < 0.001]. There was no heterogeneity among the different studies.
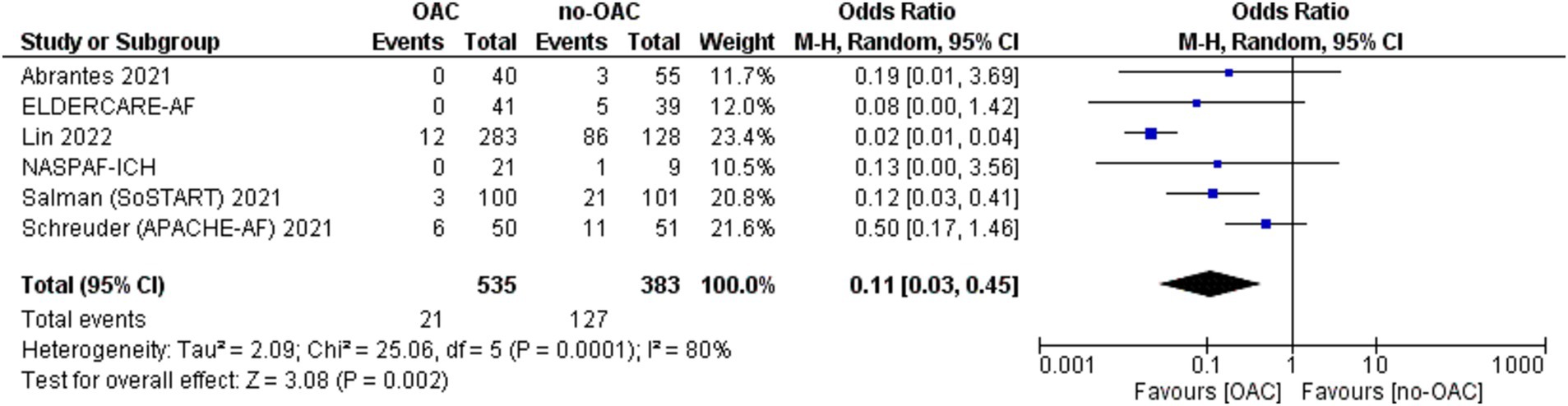
Major cardiovascular ischemic events
A major cardiovascular ischemic event was reported in six of the included studies (Figure 3). It was counted in 21 out of 535 patients in the OAC group and 127 out of 383 patients in the no-OAC group. There was a lower major cardiovascular ischemic event in the OAC group [OR = 0.11; CI 95% (0.03, 0.45); p = 0.002]. There was a low heterogeneity among the different studies (Tau2 = 2.53).
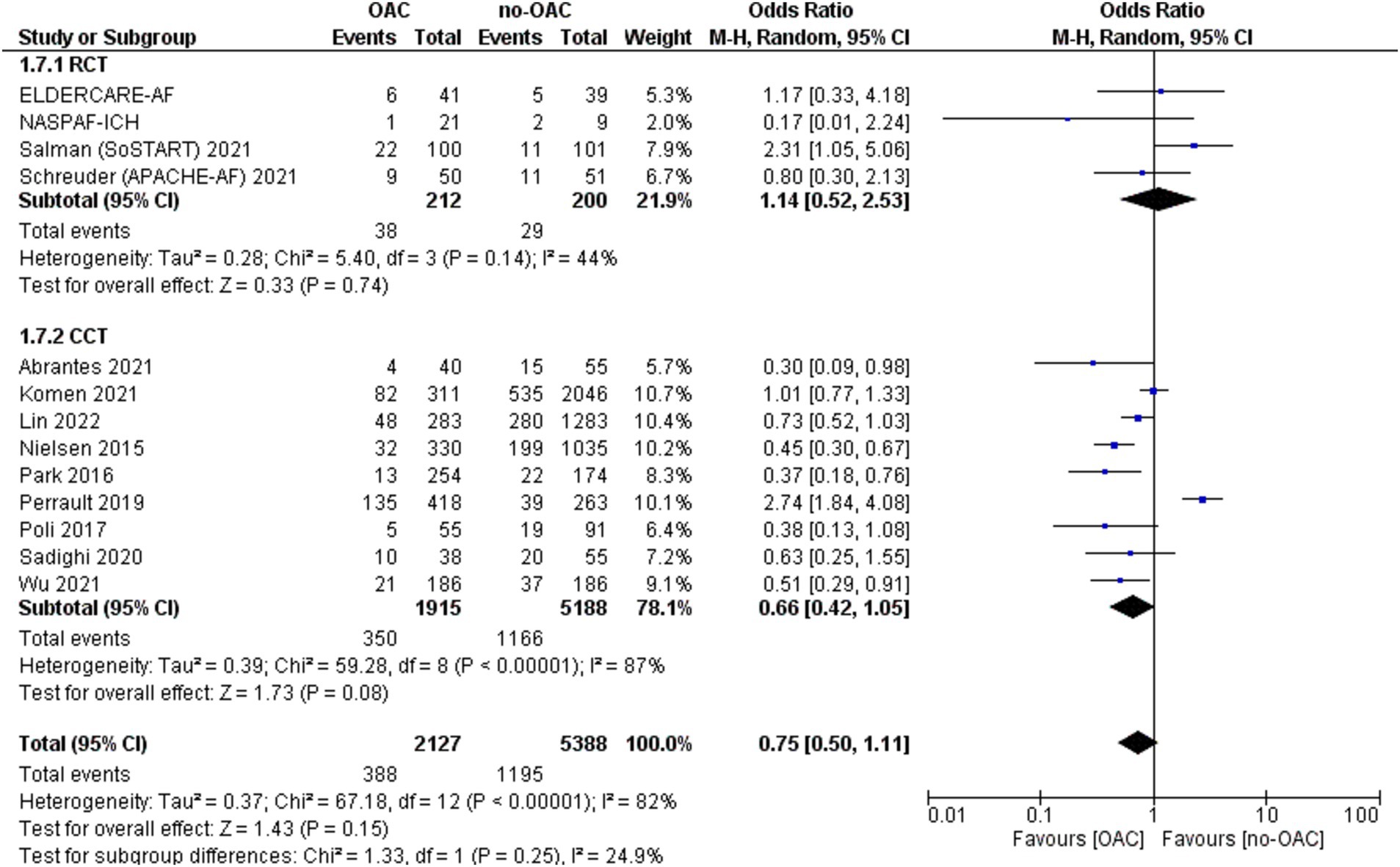
Mortality
The mortality rate was reported in 13 studies (Figure 4). It was counted 388 out of 2,127 patients in the OAC group and 1,166 out of 8,188 in the no-OAC group. There was no difference between the two groups in terms of mortality [OR = 0.75; CI 95% (0.50, 1.11); p = 0.15], even in the subgroup analysis of RCT (p = 0.74) and CCT (p = 0.08). There was a low heterogeneity among the different studies (Tau2 = 0.37). There was no difference according to the location of ICH in terms of mortality.
Major bleeding
The major bleeding was reported in 13 included studies (Figure 5). It was counted in 115 out of 1927 patients in the OAC group and 190 out of 2,369 patients in the no-OAC group. There was no difference between the two groups in terms of major bleeding [OR = 0.63; CI 95% (0.23, 1.72); p = 0.36], even in the subgroup analysis of RCT (p = 0.0.51) and CCT (p = 0.33). There was a low heterogeneity among the different studies (Tau2 = 2.53).
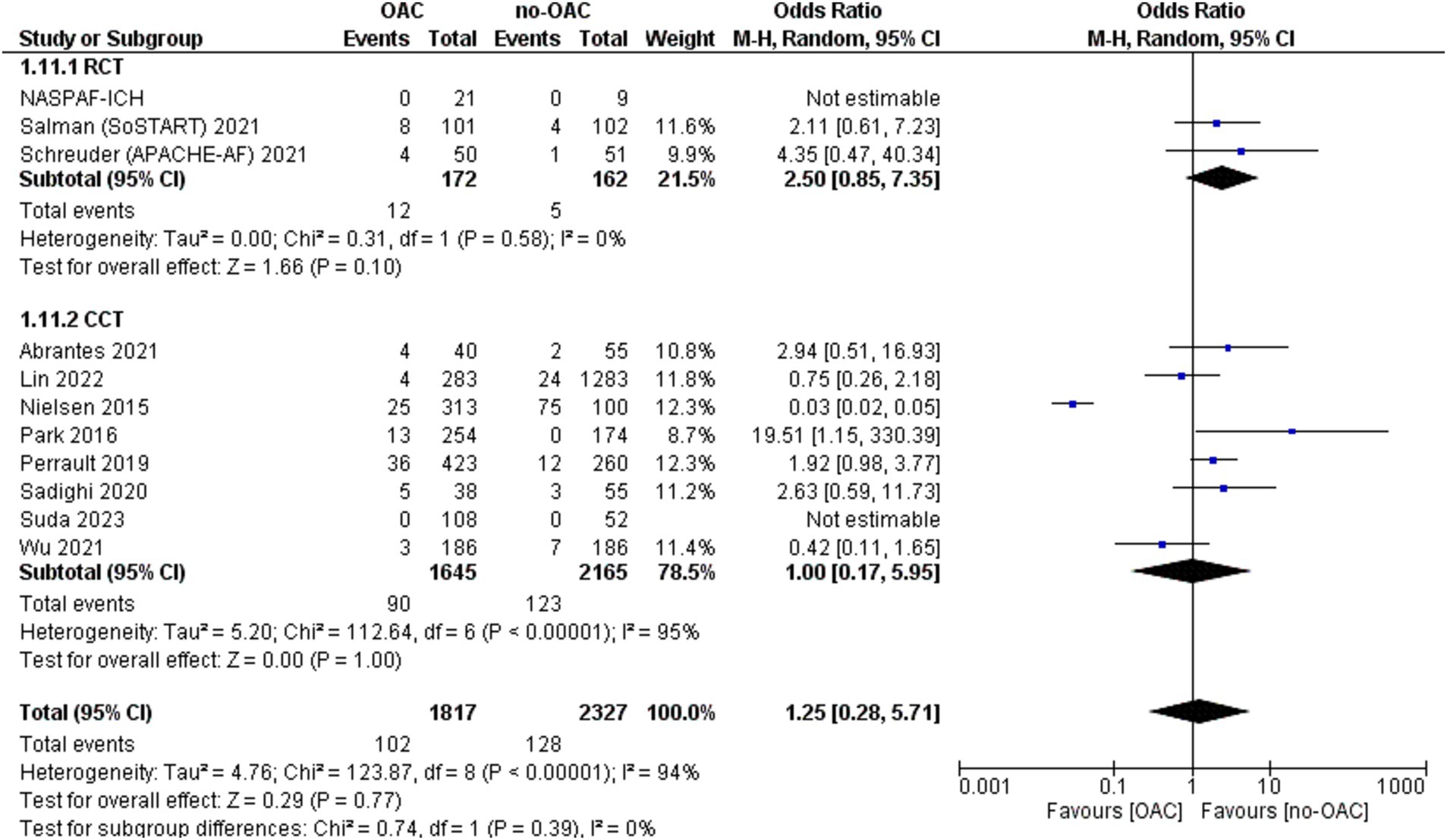
Any stroke/SE or cardiac death
This outcome was reported in nine studies (Figure 6). It was counted in 219 out of 1,013 patients in the OAC group and 186 out of 903 patients in the no-OAC group. There was no difference between the two groups [OR = 0.66; CI 95% (0.33, 1.33); p = 0.25], even in the subgroup analysis of RCT (p = 0.13) and CCT (p = 0.41). There was a low heterogeneity among the different studies (Tau2 = 0.83).
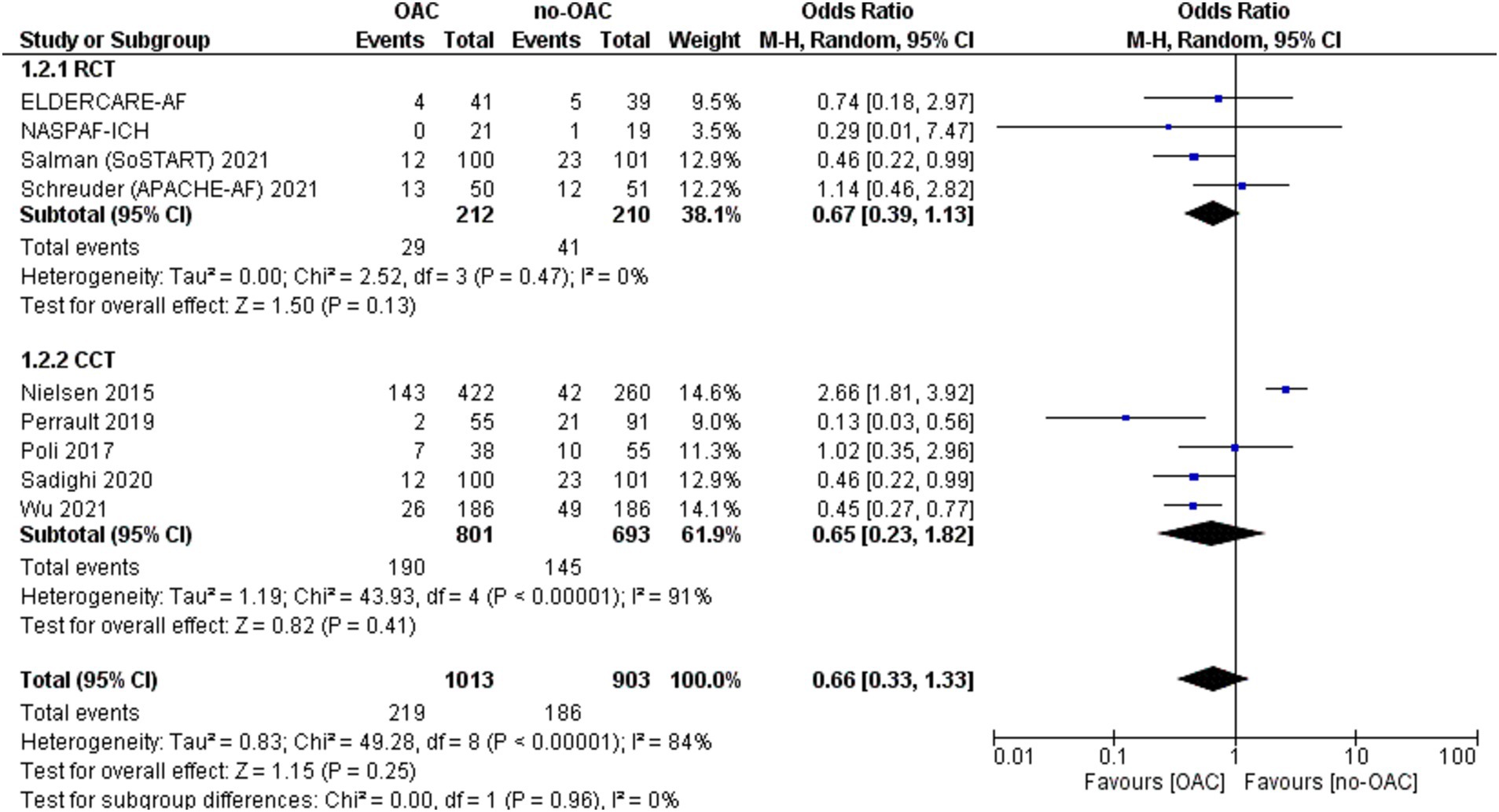
ICH recurrence
The ICH recurrence was reported in 11 included studies (Figure 7). It was counted in 102 out of 1817 patients in the OAC group and 128 out of 2,327 patients in the no-OAC group. There was no difference between the two groups in terms of ICH recurrence [OR = 1.25; CI 95% (0.28, 5.71); p = 0.77] even in the subgroup analysis of RCT (p = 0.10) and CCT (p = 1.00). There was a low heterogeneity among the different studies (Tau2 = 4.76). There was no difference in the location of ICH in terms of mortality.
Quality assessment of the included studies and reporting on the effects of OAC use
The quality assessment and risk of bias are presented in Table 1. The risk of bias assessment is presented in Table 3. A Summary of the evidence is presented in Table 4. This review shows that when OAC was used after ICH in patients with AF:
• It reduces major cardiovascular ischemic events
• It may be associated with a lower ischemic stroke rate.
• We do not know if it leads to additional mortality, major bleeding, ICH recurrence, or any stroke death because the evidence is very uncertain.
Discussion
This systematic review and meta-analysis explored the complex balance of risks and benefits associated with the use of DOACs in patients with AF who also have a history of ICH. Our findings indicate that DOACs significantly reduce ischemic stroke risk compared to no therapy or antiplatelet agents without a corresponding increase in mortality or major bleeding events. These results align with prior observational studies suggesting that DOACs may offer a more favorable safety profile than traditional anticoagulants like vitamin K antagonists in this high-risk group. Thus, our findings may support the use of DOACs as a viable strategy for stroke prevention despite prior ICH, especially in those with high stroke risk scores.
Patients with AF are at a heightened risk of IS correlated to the CHA2DS2-VASc score, notably those with a history of stroke or transient ischemic attack being the strongest predictor of future strokes (7, 38). Consequently, preventing recurrent strokes in individuals with AF and a prior ischemic stroke or TIA is a critical priority in cerebrovascular care. Closing the auricle can be an alternative for these patients (3–5), but in current practice, this is not retained or contraindicated in many cases. Thus, the choice is then limited between resuming Warfarin, DOACs, antiplatelet agents, and therapeutic abstention. In numerous countries, before the availability of DOACs, only 50 to 66% of patients with AF were treated with warfarin (39). Besides, reluctance to prescribe warfarin for patients with AF may sometimes be justified, as intracranial hemorrhage during follow-up is a significant predictor of poor long-term functional outcomes following an ischemic stroke or transient ischemic attack (40). The possibility of another intracranial hemorrhage remains a concern and deciding to resume anticoagulation therapy is a challenging one for physicians. However, a Danish nationwide cohort study showed that Oral anticoagulant therapy has been shown to significantly reduce rates of ischemic stroke and all-cause mortality, suggesting that reintroducing anticoagulants after intracranial hemorrhage is a viable option (32). This finding may support the use of DOACs as a viable strategy for stroke prevention despite prior ICH, especially in those with high stroke risk scores. However, our analysis did not show a significant difference in ICH recurrence or major bleeding rates between the DOAC and non-therapy/antiplatelet groups, suggesting that the anticipated bleeding risk associated with anticoagulation may be less substantial than previously thought for DOACs. A meta-analysis published in 2023 by Al-Shahi Salman et al. (26), assessed the effects of starting versus avoiding DOACs in patients with spontaneous intracranial hemorrhage. The study demonstrated that DOACs lowered the risk of ischemic major adverse cardiovascular events. However, it had several limitations, including the inclusion of only four trials with a total of 412 patients in the final analysis. Furthermore, the researchers were unable to conclude the risk of hemorrhagic major adverse cardiovascular events, mortality, or functional outcomes.
This absolute reduction in the risk of ischemic major adverse cardiovascular events appears to outweigh the potential increase in the risk of hemorrhagic major adverse cardiovascular events. Yet, the overall net benefit of oral anticoagulation has to be fully established. Additionally, hemorrhagic events are more likely to lead to death or disability compared to ischemic events. However, it remains uncertain whether an overall reduction in the absolute risk of major adverse cardiovascular events translates into a net reduction in mortality or long-term dependence.
The main critical limitation of this study is the absence of stratification based on the type and location of intracranial hemorrhage. We were not able to distinguish patients with lobar intracerebral hemorrhage or non-aneurysmal convexity subarachnoid hemorrhage—subgroups associated with a higher risk of recurrence, often due to cerebral amyloid angiopathy—from those with deep hemorrhages across all endpoints. Although our meta-analysis included a subset of 103 participants from these higher-risk groups and found no clear interaction between ICH location and the net effect of oral anticoagulation, our findings contrast with preliminary safety concerns raised in the ENRICH-AF trial. In that trial, enrollment of patients with lobar ICH was halted due to an unacceptable rate of recurrent hemorrhagic stroke in the edoxaban group. These emerging findings, though unpublished, raise uncertainty that underscores the need for ongoing trials to further clarify the role of anticoagulation in this subgroup. Additionally, our analysis is limited by the lack of patient-level data on important confounders such as comorbidities, hypertension control, and prior use of combination antiplatelet-anticoagulant therapy. The inclusion of both randomized controlled trials and controlled clinical trials introduces methodological variability and potential bias in patient selection and outcome reporting. Data availability also varied significantly across outcomes: for example, ischemic stroke was reported in only a portion of the total cohort due to inconsistent outcome reporting across studies. Moreover, definitions of comparator groups differed; some control groups included antiplatelet therapy, and others had no therapy or placebo, which may affect the comparability of outcomes. Although a stratified analysis of OAC vs. antiplatelet and OAC vs. no therapy would be insightful, the available data did not consistently support such distinctions. Finally, follow-up duration was heterogeneous, and long-term data, especially from ongoing trials such as ASPIRE, ENRICH-AF, and STROKECLOSE, are still lacking. While DOACs showed promise in reducing ischemic events, uncertainty remains regarding their safety profile, particularly with respect to bleeding and ICH recurrence, as evidenced by the wide confidence intervals. Future research should prioritize individualized risk stratification, standardized outcome reporting, and longer follow-up to better inform anticoagulation strategies after intracranial hemorrhage.
Conclusion
To conclude, our findings suggest that DOACs substantially lower the risk of ischemic stroke compared to no treatment or antiplatelet therapy without a notable rise in mortality or major bleeding events. These outcomes are consistent with previous observational studies indicating that DOACs may provide a safer alternative to traditional anticoagulants, such as vitamin K antagonists, for this high-risk population. These findings should serve as motivation to support the recruitment efforts and the successful completion of ongoing clinical trials.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
DS: Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. NG: Data curation, Formal analysis, Writing – original draft. FB: Investigation, Software, Writing – original draft. SL: Resources, Visualization, Writing – original draft. AD: Methodology, Supervision, Writing – original draft. CP: Conceptualization, Data curation, Writing – original draft. MD: Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Validation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1570809/full#supplementary-material
References
1. Lip, GYH, Nieuwlaat, R, Pisters, R, Lane, DA, and Crijns, HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. (2010) 137:263–72. doi: 10.1378/chest.09-1584
2. Li, L, Poon, MTC, Samarasekera, NE, Perry, LA, Moullaali, TJ, Rodrigues, MA, et al. Risks of recurrent stroke and all serious vascular events after spontaneous intracerebral haemorrhage: pooled analyses of two population-based studies. Lancet Neurol. (2021) 20:437–47. doi: 10.1016/S1474-4422(21)00075-2
3. Aarnink, EW, Maarse, M, Fierro, N, Mazzone, P, Beneduce, A, Tondo, C, et al. Left atrial appendage occlusion in patients with anticoagulation failure vs anticoagulation contraindication. JACC Cardiovasc Interv. (2024) 17:1311–21. doi: 10.1016/j.jcin.2024.04.012
4. Masjuan, J, Salido, L, DeFelipe, A, Hernández-Antolín, R, Fernández-Golfín, C, Cruz-Culebras, A, et al. Oral anticoagulation and left atrial appendage closure: a new strategy for recurrent cardioembolic stroke. Eur J Neurol. (2019) 26:816–20. doi: 10.1111/ene.13894
5. Galloo, X, Carmeliet, T, Ea, P, Lochy, S, Scott, B, Verheye, S, et al. Left atrial appendage occlusion in recurrent ischaemic stroke, a multicentre experience. Acta Clin Belg. (2022) 77:255–60. doi: 10.1080/17843286.2020.1821494
6. Ruff, CT, Giugliano, RP, Braunwald, E, Hoffman, EB, Deenadayalu, N, Ezekowitz, MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. (2014) 383:955–62. doi: 10.1016/S0140-6736(13)62343-0
7. Hart, RG, Benavente, O, McBride, R, and Pearce, LA. Antithrombotic therapy to prevent stroke in patients with atrial fibrillation. Ann Intern Med. (1999) 131:492–501. doi: 10.7326/0003-4819-131-7-199910050-00003
8. Connolly, SJ, Ezekowitz, MD, Yusuf, S, Eikelboom, J, Oldgren, J, Parekh, A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. (2009) 361:1139–51. doi: 10.1056/NEJMoa0905561
9. Patel, MR, Mahaffey, KW, Garg, J, Pan, G, Singer, DE, Hacke, W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. (2011) 365:883–91. doi: 10.1056/NEJMoa1009638
10. Granger, CB, Alexander, JH, McMurray, JJV, Lopes, RD, Hylek, EM, Hanna, M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. (2011) 365:981–92. doi: 10.1056/NEJMoa1107039
11. Giugliano, RP, Ruff, CT, Braunwald, E, Murphy, SA, Wiviott, SD, Halperin, JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. (2013) 369:2093–104. doi: 10.1056/NEJMoa1310907
12. Page, MJ, Moher, D, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. Explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2020) 2021:n160. doi: 10.1136/bmj.n160
13. Shea, BJ, Reeves, BC, Wells, G, Thuku, M, Hamel, C, Moran, J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
14. Altman, DG, Schulz, KF, Moher, D, Egger, M, Davidoff, F, Elbourne, D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. (2001) 134:663–94. doi: 10.7326/0003-4819-134-8-200104170-00012
15. Yang, ZR, Sun, F, and Zhan, SY. Risk on bias assessment:(2) revised Cochrane risk of bias tool for individually randomized, parallel group trials (RoB2. 0). Zhonghua Liu Xing Bing Xue Za Zhi. (2017) 38:1285–91. doi: 10.3760/cma.j.issn.0254-6450.2017.09.028
16. Peterson, J, Welch, V, Losos, M, and Tugwell, P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ott Ott Hosp Res Inst. (2011) 2:1–12.
17. Slim, K, Nini, E, Forestier, D, Kwiatkowski, F, Panis, Y, and Chipponi, J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
18. Higgins, JP. Cochrane handbook for systematic reviews of interventions version 5.0. 1. England: The Cochrane collaboration (2008).
19. Hozo, SP, Djulbegovic, B, and Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:1–10. doi: 10.1186/1471-2288-5-13
20. Higgins, JPT, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
21. Egger, M, Smith, GD, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
22. Balshem, H, Helfand, M, Schünemann, HJ, Oxman, AD, Kunz, R, Brozek, J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
23. Higgins, JP, and Green, S. (eds). Cochrane handbook for systematic reviews of interventions. (2008). The Cochrane Collaboration and John Wiley & Sons Ltd.
24. Liu, M, Hou, Y, Liu, W, and Liu, M. Effect of oral anticoagulation therapy in atrial fibrillation patients with a history of intracranial hemorrhage: a systematic review and meta-analysis. Ann Palliat Med. (2022) 11:3063–74. doi: 10.21037/apm-22-582
25. Schreuder, FHBM, Van Nieuwenhuizen, KM, Hofmeijer, J, Vermeer, SE, Kerkhoff, H, Zock, E, et al. Apixaban versus no anticoagulation after anticoagulation-associated intracerebral haemorrhage in patients with atrial fibrillation in the Netherlands (APACHE-AF): a randomised, open-label, phase 2 trial. Lancet Neurol. (2021) 20:907–16. doi: 10.1016/S1474-4422(21)00298-2
26. Al-Shahi Salman, R, Stephen, J, Tierney, JF, Lewis, SC, Newby, DE, Parry-Jones, AR, et al. Effects of oral anticoagulation in people with atrial fibrillation after spontaneous intracranial haemorrhage (COCROACH): prospective, individual participant data meta-analysis of randomised trials. Lancet Neurol. (2023) 22:1140–9. doi: 10.1016/S1474-4422(23)00315-0
27. Sadighi, A, Wasko, L, DiCristina, H, Wagner, T, Wright, K, Capone, K, et al. Long-term outcome of resuming anticoagulation after anticoagulation-associated intracerebral hemorrhage. eNeurologicalSci. (2020) 18:100222. doi: 10.1016/j.ensci.2020.100222
28. Perreault, S, Côté, R, White-Guay, B, Dorais, M, Oussaïd, E, and Schnitzer, ME. Anticoagulants in older patients with Nonvalvular atrial fibrillation after intracranial hemorrhage. J Stroke. (2019) 21:195–206. doi: 10.5853/jos.2018.02243
29. Sakamoto, Y, Nito, C, Nishiyama, Y, Suda, S, Matsumoto, N, Aoki, J, et al. Safety of anticoagulant therapy including direct Oral anticoagulants in patients with acute spontaneous intracerebral hemorrhage. Circ J. (2019) 83:441–6. doi: 10.1253/circj.CJ-18-0938
30. Poli, L, Grassi, M, Zedde, M, Marcheselli, S, Silvestrelli, G, Sessa, M, et al. Anticoagulants resumption after warfarin-related intracerebral Haemorrhage: the multicenter study on cerebral hemorrhage in Italy (MUCH-Italy). Thromb Haemost. (2018) 118:572–80. doi: 10.1055/s-0038-1627454
31. Abrantes, CS, Pintalhão, M, Tavares, S, Fonseca, L, and Chaves, PC. Anticoagulation after intracerebral hemorrhage in patients with atrial fibrillation: between Scylla and Charybdis. Neurol Sci. (2022) 43:2441–8. doi: 10.1007/s10072-021-05602-7
32. Nielsen, PB, Larsen, TB, Skjøth, F, Gorst-Rasmussen, A, Rasmussen, LH, and Lip, GYH. Restarting anticoagulant treatment after intracranial hemorrhage in patients with atrial fibrillation and the impact on recurrent stroke, mortality, and bleeding: a Nationwide cohort study. Circulation. (2015) 132:517–25. doi: 10.1161/CIRCULATIONAHA.115.015735
33. Komen, JJ, Forslund, T, Mantel-Teeuwisse, AK, Klungel, OH, Von Euler, M, Braunschweig, F, et al. Association of preceding antithrombotic therapy in atrial fibrillation patients with ischaemic stroke, intracranial haemorrhage, or gastrointestinal bleed and mortality. Eur Heart J Cardiovasc Pharmacother. (2021) 7:3–10. doi: 10.1093/ehjcvp/pvz063
34. Lin, S, Chang, Y, Lin, F, Tang, S, Dong, Y, and Wang, C. Post-intracranial hemorrhage antithrombotic therapy in patients with atrial fibrillation. J Am Heart Assoc. (2022) 11:e022849. doi: 10.1161/JAHA.121.022849
35. Park, YA, Uhm, JS, Pak, HN, Lee, MH, and Joung, B. Anticoagulation therapy in atrial fibrillation after intracranial hemorrhage. Heart Rhythm. (2016) 13:1794–802. doi: 10.1016/j.hrthm.2016.05.016
36. Wu, VCC, Huang, YC, Chen, SW, Liu, CH, Chang, CW, Chen, CC, et al. Resuming anticoagulation in patients with atrial fibrillation experiencing intracranial hemorrhage. Medicine (Baltimore). (2021) 100:e26945. doi: 10.1097/MD.0000000000026945
37. Suda, S, Iguchi, Y, Yagita, Y, Kanzawa, T, Okubo, S, Fujimoto, S, et al. Resumption of oral anticoagulation in patients with non-valvular atrial fibrillation after intracerebral hemorrhage: a sub-analysis of the PASTA registry study. J Neurol Sci. (2023) 453:120810. doi: 10.1016/j.jns.2023.120810
38. The Stroke Risk in Atrial Fibrillation Working Group. Independent predictors of stroke in patients with atrial fibrillation. Neurology. (2007) 69:546–54. doi: 10.1212/01.wnl.0000267275.68538.8d
39. Bungard, TJ, Ghali, WA, Teo, KK, McAlister, FA, and Tsuyuki, RT. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. (2000) 160:41–6. doi: 10.1001/archinte.160.1.41
40. Hobeanu, C, Lavallée, PC, Charles, H, Labreuche, J, Albers, GW, Caplan, LR, et al. Risk of subsequent disabling or fatal stroke in patients with transient ischaemic attack or minor ischaemic stroke: an international, prospective cohort study. Lancet Neurol. (2022) 21:889–98. doi: 10.1016/S1474-4422(22)00302-7
Keywords: atrial fibrillation, intracranial hemorrhage, direct oral anticoagulants, stroke prevention, meta-analysis, antiplatelet therapy
Citation: Sablot D, Gaillard N, Belahsen F, Lamelo SR, Dumitrana A, Plantard C, Daghmouri MA and Chaouch MA (2025) Direct oral anticoagulation versus no therapy or antiplatelet for stroke prevention in patients with atrial fibrillation and history of intracranial hemorrhage: a systematic review and meta-analysis. Front. Med. 12:1570809. doi: 10.3389/fmed.2025.1570809
Edited by:
Lei Qin, University of International Business and Economics, ChinaReviewed by:
Janhavi Modak, Baptist Health Medical Center, Little Rock, United StatesArianna Pannunzio, Sapienza University of Rome, Italy
Copyright © 2025 Sablot, Gaillard, Belahsen, Lamelo, Dumitrana, Plantard, Daghmouri and Chaouch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed Ali Chaouch, ZG9jbWVkYWxpY2hhb3VjaEBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Denis Sablot1,2,3†
Denis Sablot1,2,3† Mohamed Ali Chaouch
Mohamed Ali Chaouch