- 1Department of Oncology, The First Affiliated Hospital with Nanjing Medical University, Nanjing, China
- 2Department of Nuclear Medicine, The First Affiliated Hospital with Nanjing Medical University, Nanjing, China
- 3Central Laboratory, The Friendship Hospital of Ili Kazakh Autonomous Prefecture, Ili & Jiangsu Joint Institute of Health, Yining, China
- 4Department of Hematology and Oncology, Department of Geriatric Lung Cancer Research Laboratory, Jiangsu Province Geriatric Hospital, Nanjing, China
- 5Department of Pathology, The First Affiliated Hospital with Nanjing Medical University, Nanjing, China
Anaplastic lymphoma kinase (ALK) gene rearrangements have been increasingly detected in mesenchymal neoplasms. ALK-rearranged mesenchymal neoplasms occur mainly in superficial tissues but rarely in internal organs. Herein, we firstly report a primary lung lesion presenting as a rare ALK-rearranged mesenchymal neoplasm. The patient diagnosed with primary pulmonary ALK-rearranged mesenchymal neoplasm (PPAMN) received ensartinib as postoperative adjuvant therapy, achieving a disease-free survival of 10 months. Continuation of ensartinib as first-line treatment enabled him to benefit from a partial response, with a progression-free survival of 11 months. Second next-generation sequencing (NGS) revealed elevated HMBOX1::ALK abundance along with secondary NF2 mutation. After local radiotherapy combined with ensartinib continuation, his disease was temporarily stable for 7 months. Unfortunately, this disease became uncontrolled with an overall survival (OS) of 34 months. This is the first case of ALK-rearranged mesenchymal neoplasm manifested as a primary lung lesion and a novel HMBOX1::ALK fusion was identified by NGS. The family of ALK-rearranged mesenchymal neoplasms is expanding and ensartinib could be a potential treatment option for patients with HMBOX1::ALK. Repeated biopsy and NGS detection are critical to guide treatment selection at disease progression.
1 Introduction
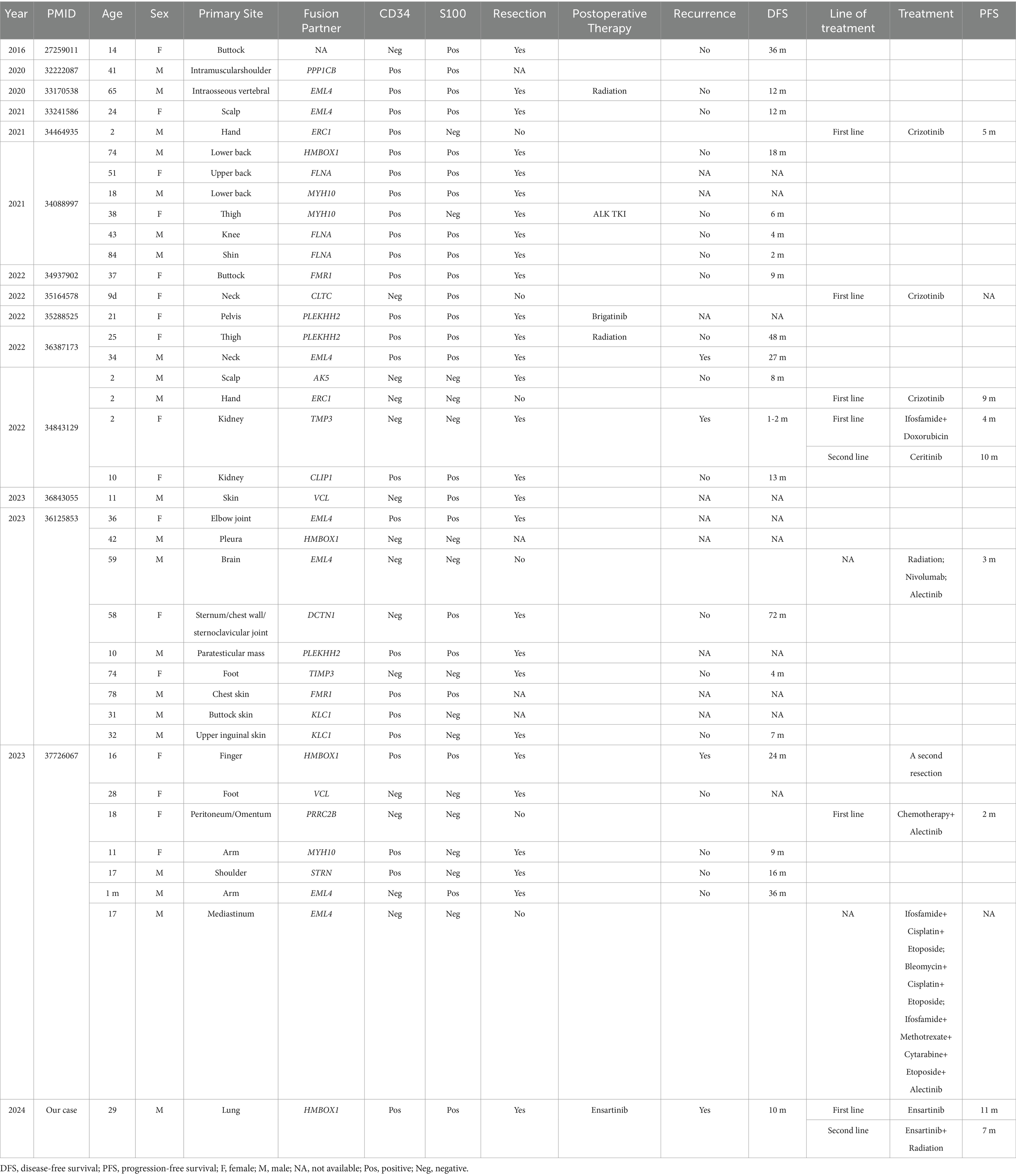
Anaplastic lymphoma kinase (ALK) gene rearrangements have been progressively identified in numerous tumors (1, 2), including non-small cell lung cancer (NSCLC) (3), inflammatory myofibroblastic tumor (IMT) (4), and recently reported ALK-rearranged mesenchymal neoplasms (5–8). The earliest in 2016, Agaram et.al reported the first ALK-rearranged soft tissue tumor in a cohort of provisionally named lipofibromatosis-like neural tumors with recurrent NTRK1 gene fusions, among which one tumor presented with ALK gene rearrangement replacing NTRK gene (9). Subsequently, mesenchymal neoplasms with ALK gene rearrangement were successively reported, showing spindle or eptheloid cell morphology, myxoid to myxohyaline stroma, some hyalinized vessels, occasional concentric whorls, and variable CD34 and S100 expression (5–8). Given their wide spectrum of morphology and immunophenotype, these tumors were reported with various names, such as lipofibromatosis-Like neural tumors (9), S100 and CD34 co-expressing mesenchymal neoplasms (10), infantile fibrosarcoma-like spindle cell tumors (6), and superficial ALK-rearranged myxoid spindle cell neoplasms (7, 11). They typically occur in cutaneous and subcutaneous tissues of trunk, limbs, head and neck. While only few cases demonstrated the origination of visceral organs, including brain (5), kidney (6), mediastinum (8), pleura (5), peritoneum (8), and bone (5, 12).
ALK-rearranged mesenchymal neoplasms typically present as superficial nodules or deep masses, and surgical resection with negative margins is the preferred approach. However, margin-positive cases show high rates of locoregional recurrence and metastasis, highlighting the need for other treatment strategies. In recent years, emerging ALK inhibitors have made significant advancements in targeted therapy for NSCLC and IMT. In 2022, crizotinib has been approved by the Food and Drug Administration in unresectable ALK-positive IMT patients. Ensartinib, an oral ALK tyrosine kinase inhibitor (TKI), exhibits superior efficacy to crizotinib in both systemic and central nervous system disease in ALK-positive NSCLC patients (13). Herein, we presented the first example manifested as a primary pulmonary ALK-rearranged mesenchymal neoplasm, who harbored a novel homeobox containing 1 (HMBOX1)::ALK fusion and sensitive to ensartinib.
2 Case description
A 29-year-old male, non-smoker, presented to our hospital following the identification of a mass in the left lung during a routine physical examination. Further examination revealed a mass in the left lung, measuring 14.1*10.5 cm, supposing a solitary fibrous tumor. Then, he underwent surgical resection of the mass and the left lower lobe. During intraoperative exploration, the mass was observed tightly adherent to the diaphragm and pericardium, accompanied by invasion of the pulmonary pleura.
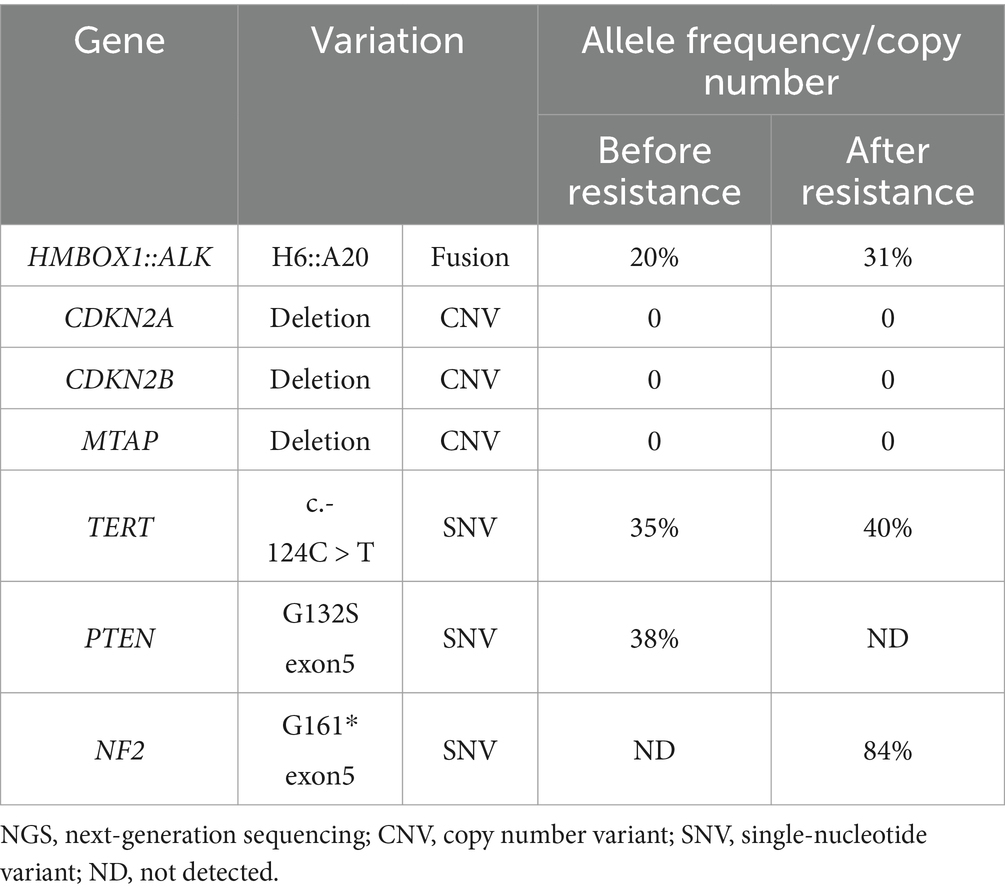
Microscopically, the tumor was composed of sheets of round, oval to epithelioid cells with background staghorn or hemangiopericytoma-like vessels punched in (Figure 1A). Most tumor cells showed mild atypia, abundant eosinophilic to clear cytoplasm, round to polygonal nuclei, delicate chromatin, and inconspicuous nucleoli. Focally, hyalinizaed wire-like filament was presented around neoplasm cells (Figure 1B). The mitotic figures were about 1–2/10HPFs. In some areas adjoined to the pleura, the tumor cells were more cellular with increased atypia and brisk mitosis (Figure 1C). Focal hemorrhage and necrosis were also observed. Immunohistochemically, the tumor cells were positive for CD34 (Figure 1D), S100 (Figure 1E), ALK-D5F3 (Figure 1F), H3K27 me3, vimentin, and CD99 (paranuclear dot-like staining), while they were negative for STAT6, CK-pan, EMA, desmin, SMA, CD31, WT-1, and pan-TRK. The average Ki-67 index of celluar area was around 20%. Break-apart ALK fluorescent in situ hybridization (FISH) probe exhibited a positive red-green-yellow signal in over 20% of the tumor cells, suggesting ALK rearrangement (Figure 1G). The resected tumor tissue underwent DNA&RNA-based next-generation sequencing (NGS) (OrigiMed, Shanghai, China), revealing the fusion of HMBOX1::ALK (H6::A20) fusion (Figures 1H,I; Table 1). Ultimately, he was diagnosed with primary pulmonary ALK-rearranged mesenchymal neoplasm.
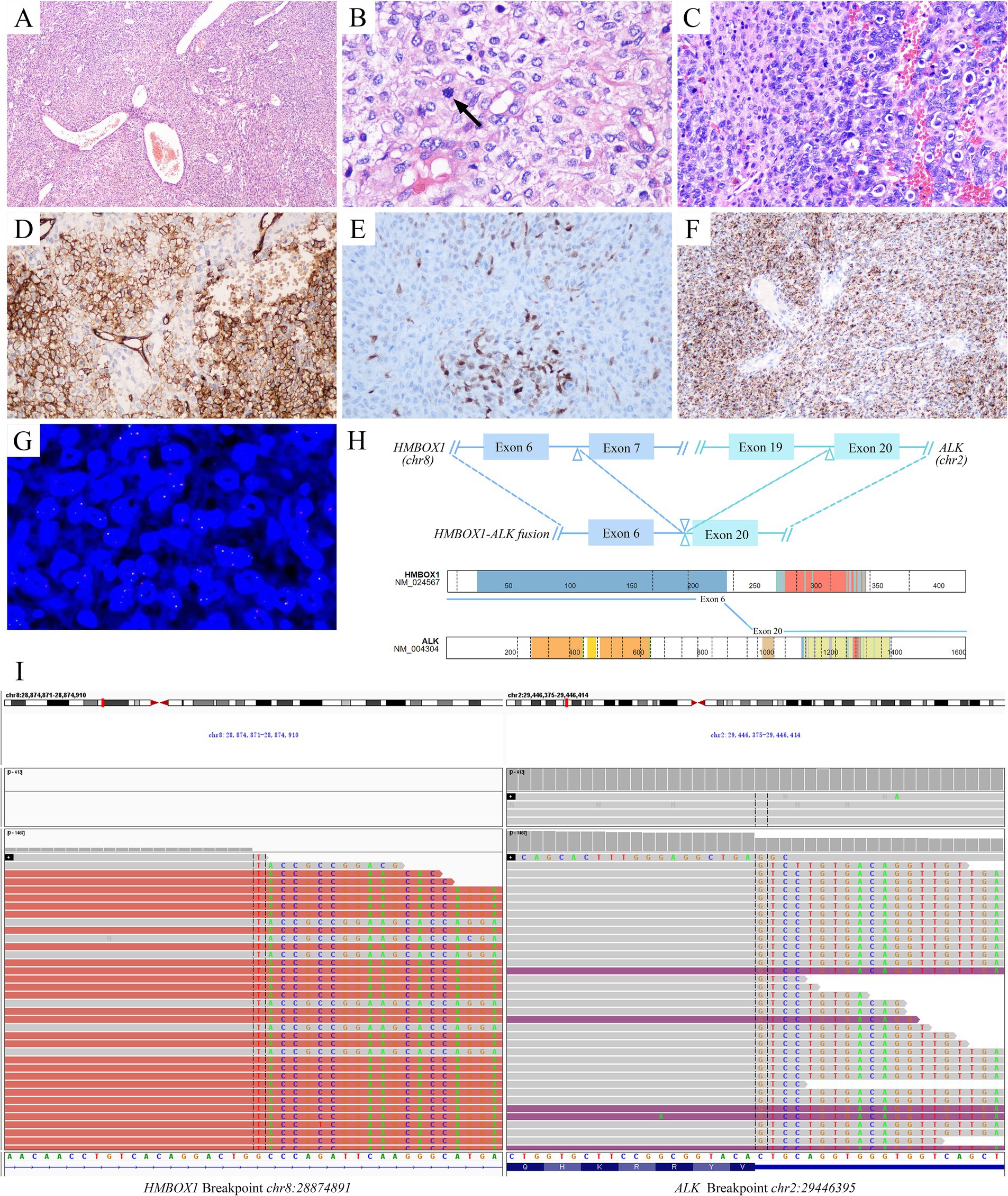
Figure 1. Pathological diagnosis of primary pulmonary ALK-rearranged mesenchymal neoplasm. Histopathologically, the tumor was composed of sheets of round, oval to epithelioid cells with background staghorn vessels (A). Most of the tumor cells were mild atypia, with focal wire-like filament hyalinization around neoplasm cells. The mitosis was seen (arrow) (B). In some area, the tumor cells were more cellular with increased atypia and brisk mitosis (C). Immunohistochemically, the tumor cells were positive for CD34 (D), S100 (E), ALK-D5F3 (F). More than 20% of tumor cells were signaled by break-apart ALK FISH assays (G). Identification of HMBOX1::ALK fusion and schematic of predicted fusion protein encoded by it [adapted from https://proteinpaint.stjude.org/ (25)] (H). IGV visualization of HMBOX1::ALK fusion (I). ALK, anaplastic lymphoma kinase; FISH, fluorescent in situ hybridization; HMBOX1, homeobox containing 1; IGV, Itegrative Genomics Viewer.
Despite remarkable advancements in ALK TKIs for NSCLC, their utilization as postoperative adjuvant therapy remains in clinical trials. Nevertheless, given his locally advanced stage and involvement of pulmonary pleura, he received ensartinib as postoperative adjuvant therapy based on economic affordability. Ensartinib was administered orally at a daily dosage of 225 mg from December 2021. After 5-month postoperative adjuvant targeted therapy, no recurrence was witnessed and then he discontinued ensartinib. Unfortunately, recurrence occurred 3 months later and metastatic lesions were detected in the left pleura, pericardium, and bilateral cervical lymph nodes using positron emission tomography imaging (Figure 2). Ensartinib was reintroduced as first-line therapy from August 2022. Following 1-month treatment, the enlarged lymph node sharply shrank from 4.9 × 2.6 cm to 2.9 × 1.2 cm, and partial response (PR) persisted for 11 months (Figure 2). However, a reoccurrence of mediastinal lymph node enlargement was observed along with ineffectiveness of anti-infective therapy. Regrettably, the disease progression was confirmed following a mediastinal biopsy and subsequent NGS analysis unveiled the increase of HMBOX1::ALK fusion (from 20 to 31% abundance) and the presence of Neurofibromin 2 (NF2) variant (84% abundance) (Table 1). Then he received 1-month local radiotherapy with ensartinib continuation and then a follow-up computed tomography scan revealed his disease was his disease was stable for 7 months. The patient tolerated ensartinib well, with no toxic symptoms observed during therapy. Regrettably, this disease exhibited aggressive behavior and refractoriness. Despite the administration of two cycles of chemotherapy in conjunction with anti-angiogenic therapy, his disease continued its progression unabated due to drug resistance. Subsequently, this patient was unable to tolerate anti-tumor treatment and died in August 2024.
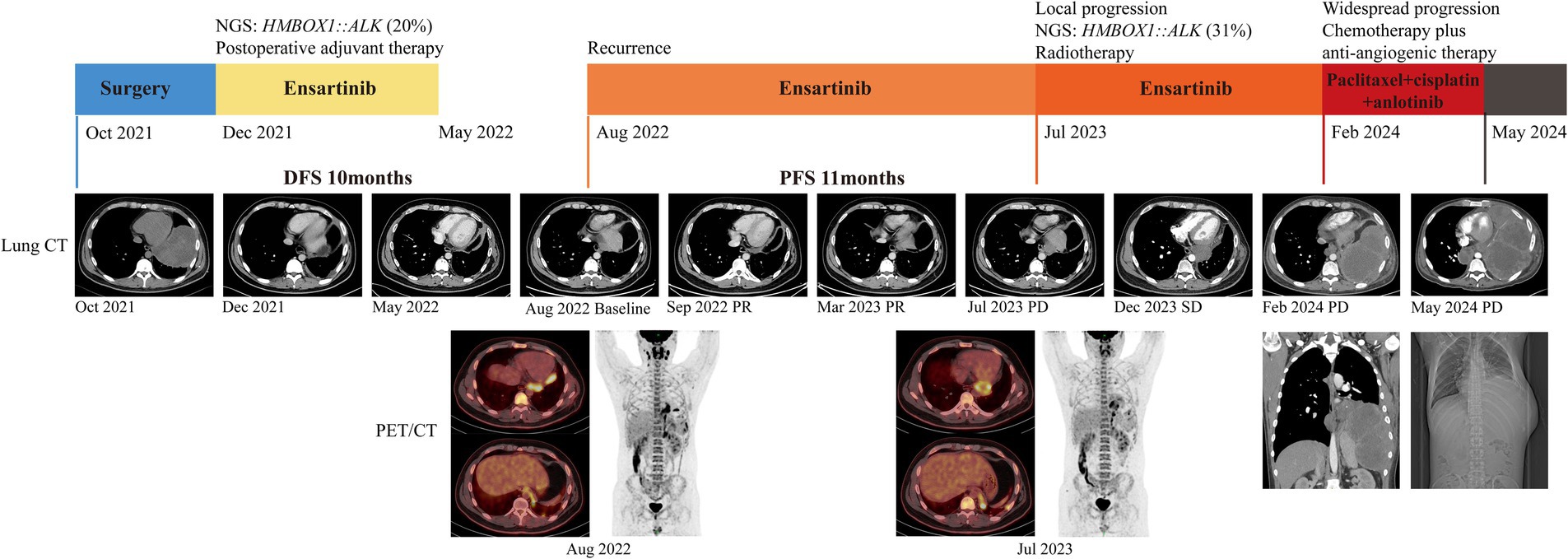
Figure 2. Clinical treatment history and imaging data of the patient. NGS, next-generation sequencing; CT, computed tomography; PET, positron emission tomography.
3 Discussion
With the promotion of NGS the family of ALK-rearranged mesenchymal neoplasms is expanding. Thus far, there are around 40 cases of ALK-rearranged mesenchymal neoplasms and nearly 20 distinct fusion partners of ALK are identified among them (Table 2). Here, we provided a unique case of lung-originating ALK-rearranged mesenchymal neoplasm. To our knowledge, this is the first case with primary pulmonary ALK-rearranged mesenchymal neoplasm harboring a novel HMBOX1::ALK fusion and sensitive to ensartinib.
The emergence of ALK-rearranged mesenchymal neoplasms has brought pathologists with significant diagnostic challenges due to their diverse and distinctive morphological and immunophenotypic features. They exhibit epithelioid to round cell or spindle cell, arranged in a myxoid to hyaline or collagenous stroma, with a positive rate of 63% for CD34 and 61% for S100 (Table 2). In our case, the tumor was composed of predominantly round to epitheliod cells, with staghorn vessels and focal hyalinized stroma. Immunohistochemically, the tumor cells co-expressed CD34 and S100. Genetically, ALK gene rearrangement was verified by FISH and NGS. All the above evidences support the diagnosis of ALK-rearranged mesenchymal neoplasm. Furthermore, the tumor showed transition of low-grad area to aggressive area with increased atypia and brisk mitosis, explaining the progression of tumor and the agressiveness of biological behavior. Consistent with the findings of Gestrich et al. (8), ALK-rearranged epithelioid mesenchymal neoplasms in a deep location with higher-grade features follow a more aggressive clinical course.
In the clinical setting, ALK-rearranged mesenchymal neoplasms encompass a spectrum ranging from indolent tumors to aggressive sarcomas that are prone to local recurrence or distant metastasis. Surgical resection benefits over 70% of patients with no disease recurrence or progression (5). However, our case recurred 10 months after surgery, with metastasis involving the left pleura, pericardium, and bilateral cervical lymph nodes. Since mediastinal lymph nodes were not dissected during surgery, the potential tumor involvement remained uncertain. In addition, intrathoracic adhesions and invasion of the pulmonary pleura seem to interpretate the occurrence of metastasis to a certain extent.
HMBOX1 gene located on chromosome 8, a member of the homeobox family (14), plays a positive role in telomere length regulation by facilitating the interaction between telomerase and telomeres (15). Degradation of HMBOX1 mRNAs leads to progressive telomere shortening, p53 signaling inactivation, and subsequent genomic instability in cancer cells. This process drives malignancy and even progression to a more aggressive phenotype (16). To date, only three articles have mentioned the presence of HMBOX1::ALK fusion in ALK-rearranged mesenchymal neoplasms, with two cases of soft tissue (7, 8) and one case of diffuse pleural (5).
As increasing ALK fusions are being detected, ALK TKIs are revolutionizing the treatment landscape and significantly improving outcomes in ALK-rearranged patients (17–19). Recently, a systematic review and network meta-analysis has confirmed that ensartinib confers the most substantial progression-free survival (PFS) benefit among Asian patients with NSCLC (20). Furthermore, ensartinib is emerging as a promising therapeutic option for ALK-rearranged IMT, yielding favorable outcomes (21–23). Encouragingly, our case achieved a disease-free survival of 10 months, while PR was obtained with a PFS of 11 months under the administration of ensartinib.
Unfortunately, our case exhibited disease progression and subsequently acquired a mutation in NF2. Merlin (Moesin-ezrin-radixin-like protein) is a tumor suppressor protein encoded by NF2 gene and plays a crucial role in tumorigenesis and metastasis (24). In our case, the glycine at position 161 of NF2 was replaced with a stop codon, potentially impairing NF2 function and promoting tumor proliferation. We further speculated that NF2 mutation secondary to ensartinib may lead to drug resistance.
ALK TKIs offer a promising and well-tolerated treatment approach for patients with ALK-rearranged solid tumors, including ALK-rearranged mesenchymal neoplasms (Table 2). The ALK fusion appears to be a histology-independent target, thus future studies should consider a molecular-defined cohort approach. Given the acknowledged efficacy of ALK TKIs in diverse cancer types, more large-scale clinical trials are warranted to identify applicable population of ALK TKIs, such as patients with ALK-rearranged mesenchymal neoplasms. Meanwhile, it is urgent to further explore the mechanisms of resistance and explore subsequent treatment strategies.
4 Conclusion
This study was novel in reporting the case of a patient with primary pulmonary ALK-rearranged mesenchymal neoplasm harboring a HMBOX1::ALK (H6::A20) fusion. Additionally, we demonstrated the sensitivity of ensartinib and highlighted its potential therapeutic efficacy in ALK-rearranged mesenchymal neoplasms. Repeated biopsy and NGS testing are essential for guiding treatment selection, but secondary resistance remains to be addressed. We strongly believe in the immense potential of NGS-guided precision targeted therapy to deliver substantial benefits to more ALK-rearranged patients in the future.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding authors.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QL: Visualization, Writing – original draft. XJ: Writing – original draft. ZZ: Writing – original draft. YJ: Writing – review & editing. SN: Writing – review & editing. TZ: Writing – review & editing. XZ: Writing – review & editing. HX: Visualization, Writing – review & editing. QG: Visualization, Writing – review & editing. LL: Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China [81472782], National Clinical Key Specialty Department (Oncology) of China [YWC-ZKJS-2023-01], Research Fund of Yili Institute of Clinical Medicine [yl2021 ms02] and Postgraduate Research & Practice Innovation Program of Jiangsu Province [SJCX24_0776].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1572632/full#supplementary-material
References
1. Stransky, N, Cerami, E, Schalm, S, Kim, JL, and Lengauer, C. The landscape of kinase fusions in cancer. Nat Commun. (2014) 5:4846. doi: 10.1038/ncomms5846
2. Shaw, AT, Hsu, PP, Awad, MM, and Engelman, JA. Tyrosine kinase gene rearrangements in epithelial malignancies. Nat Rev Cancer. (2013) 13:772–87. doi: 10.1038/nrc3612
3. Soda, M, Choi, YL, Enomoto, M, Takada, S, Yamashita, Y, Ishikawa, S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. (2007) 448:561–6. doi: 10.1038/nature05945
4. Bridge, JA, Kanamori, M, Ma, Z, Pickering, D, Hill, DA, Lydiatt, W, et al. Fusion of the ALK gene to the clathrin heavy chain gene, CLTC, in inflammatory myofibroblastic tumor. Am J Pathol. (2001) 159:411–5. doi: 10.1016/S0002-9440(10)61711-7
5. Dermawan, JK, DiNapoli, SE, Mullaney, KA, Sukhadia, P, Agaram, NP, Dickson, BC, et al. ALK-rearranged mesenchymal neoplasms: a report of 9 cases further expanding the Clinicopathologic Spectrum of emerging kinase fusion positive Group of Tumors. Genes Chromosomes Cancer. (2023) 62:75–84. doi: 10.1002/gcc.23097
6. Tan, SY, Al-Ibraheemi, A, Ahrens, WA, Oesterheld, JE, Fanburg-Smith, JC, Liu, YJ, et al. ALK rearrangements in infantile fibrosarcoma-like spindle cell tumours of soft tissue and kidney. Histopathology. (2022) 80:698–707. doi: 10.1111/his.14603
7. Dermawan, JK, Azzato, EM, Goldblum, JR, Rubin, BP, Billings, SD, and Ko, JS. Superficial ALK-rearranged myxoid spindle cell neoplasm: a cutaneous soft tissue tumor with distinctive morphology and immunophenotypic profile. Mod Pathol. (2021) 34:1710–8. doi: 10.1038/s41379-021-00830-w
8. Gestrich, CK, Davis, JL, Biederman, L, John, I, Alaggio, R, Giovannoni, I, et al. ALK-rearranged epithelioid mesenchymal neoplasm: expanding the Spectrum of tyrosine kinase-altered mesenchymal tumors. Mod Pathol. (2023) 36:100334. doi: 10.1016/j.modpat.2023.100334
9. Agaram, NP, Zhang, L, Sung, Y-S, Chen, CL, Chung, CT, Antonescu, CR, et al. Recurrent NTRK1 gene fusions define a novel subset of locally aggressive Lipofibromatosis-like neural tumors. Am J Surg Pathol. (2016) 40:1407–16. doi: 10.1097/PAS.0000000000000675
10. Coppock, JD, Schneider, MA, Surrey, LF, Karakousis, GC, Maki, RG, and Cooper, K. S100 and CD34 expressing mesenchymal neoplasm with rare PLEKHH2::ALK fusion and response to ALK inhibition. Am J Surg Pathol. (2022) 46:1309–13. doi: 10.1097/PAS.0000000000001887
11. Kao, Y-C, Lee, P-H, Wu, C-L, Yu, S-C, and Huang, H-Y. Superficial ALK-rearranged myxoid spindle cell neoplasm with a novel FMR1-ALK fusion gene. Mod Pathol. (2022) 35:438–41. doi: 10.1038/s41379-021-00952-1
12. Mantilla, JG, Cheung, H, Ha, AS, Hoch, BL, Liu, YJ, and Ricciotti, RW. Spindle cell neoplasm with EML4-ALK gene fusion presenting as an intraosseous vertebral mass. Genes Chromosomes Cancer. (2021) 60:282–6. doi: 10.1002/gcc.22917
13. Horn, L, Wang, Z, Wu, G, Poddubskaya, E, Mok, T, Reck, M, et al. Ensartinib vs Crizotinib for patients with anaplastic lymphoma kinase-positive non-small cell lung Cancer: a randomized clinical trial. JAMA Oncol. (2021) 7:1617–25. doi: 10.1001/jamaoncol.2021.3523
14. Chen, S, Saiyin, H, Zeng, X, Xi, J, Liu, X, Li, X, et al. Isolation and functional analysis of human HMBOX1, a homeobox containing protein with transcriptional repressor activity. Cytogenet Genome Res. (2006) 114:131–6. doi: 10.1159/000093328
15. Kappei, D, Butter, F, Benda, C, Scheibe, M, Draškovič, I, Stevense, M, et al. HOT1 is a mammalian direct telomere repeat-binding protein contributing to telomerase recruitment. EMBO J. (2013) 32:1681–701. doi: 10.1038/emboj.2013.105
16. Lee, JH, Hong, J, Zhang, Z, de la Peña Avalos, B, Proietti, CJ, Deamicis, AR, et al. Regulation of telomere homeostasis and genomic stability in cancer by N 6-adenosine methylation (m6A). Sci Adv. (2021) 7:7. doi: 10.1126/sciadv.abg7073
17. Kauffmann-Guerrero, D, Kahnert, K, and Huber, RM. Treatment sequencing for anaplastic lymphoma kinase-rearranged non-small-cell lung Cancer. Drugs. (2021) 81:87–100. doi: 10.1007/s40265-020-01445-2
18. Fischer, M, Moreno, L, Ziegler, DS, Marshall, LV, Zwaan, CM, Irwin, MS, et al. Ceritinib in paediatric patients with anaplastic lymphoma kinase-positive malignancies: an open-label, multicentre, phase 1, dose-escalation and dose-expansion study. Lancet Oncol. (2021) 22:1764–76. doi: 10.1016/S1470-2045(21)00536-2
19. Mossé, YP, Voss, SD, Lim, MS, Rolland, D, Minard, CG, Fox, E, et al. Targeting ALK with Crizotinib in pediatric anaplastic large cell lymphoma and inflammatory Myofibroblastic tumor: a Children's oncology group study. J Clin Oncol. (2017) 35:3215–21. doi: 10.1200/JCO.2017.73.4830
20. Peng, Y, Zhao, Q, Liao, Z, Ma, Y, and Ma, D. Efficacy and safety of first-line treatments for patients with advanced anaplastic lymphoma kinase mutated, non-small cell cancer: a systematic review and network meta-analysis. Cancer. (2023) 129:1261–75. doi: 10.1002/cncr.34664
21. He, W, Ji, X, Song, C, Song, S, and Liu, L. Case report: efficacy of ensartinib treatment in pulmonary inflammatory myofibroblastic tumor with a rare GCC2-ALK fusion. Front Oncol. (2022) 12:934887. doi: 10.3389/fonc.2022.934887
22. Li, X, Zheng, J, Li, X, Chen, YY, Liu, K, Li, FC, et al. Case report: Ensartinib for gastric epithelioid inflammatory myofibrosarcoma with STRN-ALK fusion. Front Oncol. (2023) 13:1252221. doi: 10.3389/fonc.2023.1252221
23. Li, M, Xing, R, Huang, J, Shi, C, Wei, C, and Wang, H. Case report: epithelioid inflammatory myofibroblastic sarcoma treated with an ALK TKI ensartinib. Front Oncol. (2023) 13:1084456. doi: 10.3389/fonc.2023.1084456
24. Zhang, N, Zhao, Z, Long, J, Li, H, Zhang, B, Chen, G, et al. Molecular alterations of the NF2 gene in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Oncol Rep. (2017) 38:3650–8. doi: 10.3892/or.2017.6055
Keywords: anaplastic lymphoma kinase (ALK), homeobox containing 1 (HMBOX1), ALK-rearranged mesenchymal neoplasm, ensartinib, case report
Citation: Liang Q, Jiang X, Zhou Z, Jiang Y, Ni S, Zhao T, Zhang X, Xu H, Gong Q and Liu L (2025) Ensartinib in a primary pulmonary ALK-rearranged mesenchymal neoplasm harboring a novel HMBOX1::ALK fusion: a case report and literature review. Front. Med. 12:1572632. doi: 10.3389/fmed.2025.1572632
Edited by:
Beatrice Aramini, University of Bologna, ItalyReviewed by:
Umamaheswaran Gurusamy, Nationwide Children’s Hospital, United StatesZheng Jin Tu, Cleveland Clinic, United States
Copyright © 2025 Liang, Jiang, Zhou, Jiang, Ni, Zhao, Zhang, Xu, Gong and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huanhuan Xu, eGhoOTUxMjIyQDE2My5jb20=; Qixing Gong, Z29uZ3FpeGluZ0Bob3RtYWlsLmNvbQ==; Lingxiang Liu, bGx4bGF1QDE2My5jb20=
†These authors have contributed equally to this work
 Qi Liang
Qi Liang Xingyu Jiang
Xingyu Jiang Ziwei Zhou2†
Ziwei Zhou2† Siqi Ni
Siqi Ni Huanhuan Xu
Huanhuan Xu Qixing Gong
Qixing Gong Lingxiang Liu
Lingxiang Liu